Chapter 64 Genetic Diseases
Short QT Syndrome
Historical Context
It has long been recognized that long QT syndrome (LQTS) is a clinical entity with a significant risk of SCD. When analyzing retrospective Holter monitor strips, Algra et al first recognized that short Q-T intervals had an increased risk of SCD. Gussak and associates were the first to propose SQTS as a unique clinical entity in a case series of three patients from one family with similar electrocardiogram (ECG) findings in the setting of atrial and ventricular arrhythmias.1 In 2003, the definitive link was demonstrated between SQTS and SCD. Gaita et al studied several members of two families who exhibited Q-T intervals less than 280 ms and presented with syncope, palpitations, and a family history of SCD.2
Pathophysiology
Furthermore, the shortening of the refractory period is heterogeneous within the myocardial cells, leading to nonuniform dispersion of refractoriness.3 This, in turn, may lead to VF through the mechanism of wavebreak through re-entry initiation, wavelet formation and, ultimately, fibrillation.3–5
Molecular Genetics
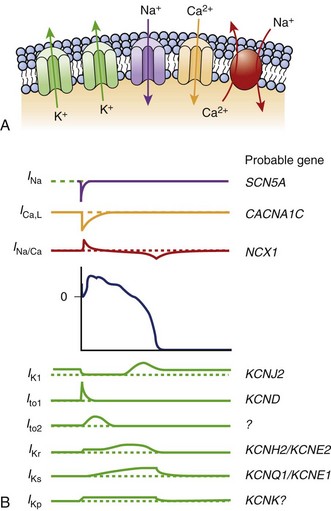
The KCNH2 gene, or HERG, encodes a transmembrane protein responsible for the rapidly activating delayed rectifier potassium channel IKr. Two missense mutations have been described that lead to “gain in function” and shortening of the APD leading to SQT1.6,7 This potassium channel is the most commonly implicated in LQTS as well.
The KCNQ1 gene encodes the ion channel responsible for the slowly activating delayed rectifier potassium channel IKs. A missense mutation in the KCNQ1 gene results in accelerated activation kinetics consistent with a gain of function in the outward current. This mutation also leads to shortening of the APD and is considered the sporadic form of SQTS, called SQT2.8,9
The KCNJ2 gene encodes for a protein responsible for the inward potassium rectifier current (IK1). A mutation in this gene generates electrical currents that do not decrease to the extent of normal potassium channels. This corresponds to the end of phase 3 of repolarization, leading to acceleration of late repolarization, thereby shortening the APD. This mutation leads to a unique ECG finding of asymmetric tall T waves with a rapid descending portion that is now called SQT3.10
Antzelevitch et al were the first to report loss-of-function mutations in genes encoding the cardiac L-type calcium channel to be associated with a familial SCD, in which a Brugada syndrome phenotype is combined with shorter than normal Q-T intervals. Among 82 probands with a clinically robust diagnosis of Brugada syndrome in the present registry, 6% (5 patients) presented with a shorter than normal Q-T interval, specifically mutations in CACNA1C and CACNB2b. Although the QTc intervals (330 to 360 ms) are longer than those encountered in KCNH2 and KCNQ1 mutations, they do overlap within the range found in patients with KCNJ2 mutations (SQT3) (Figure 64-1).11
Clinical Manifestations
SQTS has been characterized by major and minor cardiac events or symptoms. Major events include SCD, syncope, and VF. Minor events include palpitations, lightheadedness or dizziness, and paroxysmal AF. SCD is the most frequent first symptom and first clinical presentation. Most patients have a significant family history of SCD, occurring in relatives with a variable age distribution, ranging from 3 months to 70 years. The mean age of diagnosis is 30 years.12 It has been suggested that sudden infant death syndrome may, in some cases, be attributed to SQTS.12,13 Paroxysmal AF was first reported in 2000 as being linked to SQTS in a young woman who developed rapid AF during surgery. She subsequently underwent cardioversion to sinus rhythm that uncovered a Q-T interval of 280 ms. It was found that she had multiple family members with paroxysmal AF in the setting of short Q-T intervals.14 It is vital to exclude SQTS in a young patient with lone AF. SCD was definitively linked to SQTS in a case series in which five of six members were inducible for VF during EPS, and all six received an implantable cardioverter-defibrillator (ICD).2
The classic sign on ECG is a very short Q-T interval. A cutoff of 320 ms should raise suspicion for SQTS because the two families first identified by Gaita et al had Q-T intervals less than 280 ms.2 The T waves are tall, peaked, and symmetrical except in patients with the form of SQTS involving a mutation in the KCNJ2 gene (SQT3).10 The T wave is upright, and the interval of the T wave is not usually prolonged. The ST segment is often noted to be very short or completely absent. An appearance of a U wave has been reported in a few cases. Occasionally, the PR segment may be depressed, consistent with abnormal atrial repolarization.15 A slight reduction in the Q-T interval accompanies a physiological increase in the heart rate, although it can often be difficult to make a diagnosis of SQTS when the heart rate is above 100 beats/min. It is important to exclude other potential etiologies of acquired short Q-T interval, such as sinus tachycardia, hyperthermia, hypocalcemia, acidosis, hyperkalemia, and digoxin therapy.16
EPS uniformly reveals short atrial and ventricular refractory periods with easily inducible AF and VF by programmed stimulation. In a study by Giustetto et al, 18 patients with SQTS underwent EPS to determine effective refractory periods and inducibility of VT or VF. The effective refractory period (ERP), determined from the apex of the right ventricle at a drive cycle length of 500 to 600 ms, varied between 140 and 180 ms. The ERP from the high right atrium with drive cycle length of 600 ms varied between 120 and 180 ms. The significance of ventricular programmed stimulation as a risk stratifier in patients with inherited channelopathies is unclear. In the study by Giustetto et al, VF was induced in only 11 of 18 patients, of whom only three had a prior history of resuscitated cardiac arrest. Only three of six patients with prior documented VF could be induced by EPS.12
Prognosis
Patients with SQTS are at considerable risk for SCD. In the largest group of patients evaluated in a single study, 29 patients were diagnosed with SQTS. Of these, three patients died before clinical evaluation and six other patients had resuscitated cardiac arrest. In eight of the nine patients, sudden cardiac arrest was the initial presentation, and six other patients had documented syncope; 62% of patients reported symptoms at the time of diagnosis.17 In a Kaplan-Meier estimate of cumulative survival free from cardiac arrest from birth to age 40 years, only 14 of 29 patients are expected to survive, free of cardiac arrest, to the age of 40 years.12
Therapy
The clinical syndrome is extremely heterogeneous, with variations in symptoms and risk of SCD within an individual genotype. It currently is difficult to link genotypes with definite phenotypic expression.18 Given the high incidence of SCD, an ICD is recommended unless an absolute contraindication to implantation exists.19 Implantation of an ICD in young children remains a technical challenge for a number of reasons: a body too small to accommodate the generator, a right ventricle too small to accommodate a lead, difficulty of vascular access, and the need to account for body growth.19 Transvenous leads may need to be replaced multiple times in a child or adolescent throughout the person’s lifetime, which further complicates the treatment process. The psychological impact of this in a young child cannot be understated, especially if the child experiences therapy from the device, which can lead to anxiety and depression. Research is being conducted on the role of pharmacologic therapy in in overcoming this difficulty until the minimum body size is attained.
Patients with SQTS are at an increased risk for inappropriate therapy caused by the detection of short coupled and prominent T waves. Schimpf et al observed inappropriate shocks in three of five patients who received an ICD for SQTS. This occurred soon after implantation and was caused by oversensing of the shortly coupled T waves.20 This constitutes a significant proarrhythmic risk and the potential for psychological stress. Even with true bipolar sensing, inappropriate shocks still occurred during sinus rhythm in two of three patients with bipolar leads. Increased T-wave amplitude in SQTS, in combination with reduced R wave, accounts for the majority of inappropriate shocks. Device algorithms to allow for decreased sensitivity and linear or programmable decay after the R wave to avoid oversensing of the T wave may address this particular dilemma. However, to avoid detection problems with ventricular arrhythmias, it is important to be careful with programming.
Pharmacologic therapy is a potential alternative to ICDs in patients in whom ICD implantation is not feasible. Gaita et al evaluated multiple antiarrhythmic agents to determine the potential of these drugs to prolong the Q-T interval and reduce the risk of ventricular arrhythmias. Six patients were tested with flecainide, sotalol, ibutilide, and hydroquinidine. The class IC and class III agents did not produce a significant prolongation of the Q-T interval. Hydroquinidine was the only agent to cause prolongation of the Q-T interval, which increased from a mean of 263 ms to a mean of 362 ms. When performing ventricular programmed stimulation after administration of hydroquinidine, the ventricular ERP increased to 200 ms or more, and VF was no longer inducible.21,22 Quinidine blocks slow and rapid delayed rectifying potassium channels, which explains the prolongation of the APD and the Q-T interval. It has been found to be effective in the SQT1 variant.23
Wolpert et al showed that a mutation in the KCNH2 gene (N588K mutation) resulted in a 5.8-fold decrease in blocking potency in the IKs channel. Class III agents such as sotalol or ibutilide may not be effective secondary to a mutation in the IKs channel, which reduces the affinity of the IKs channel for a class III agent. Sotalol was found to have a 20-fold attenuation with N588K mutation in its ability to block the IKr channel. Disopyramide was shown, in vitro, to be less affected by the blocking of the IKr channel by N588KL mutation (1.5-fold decrease in blocking potency) and may be a rational treatment option for SQTS.24 Propafenone was shown to be effective in preventing paroxysms of AF in a small study of two patients with SQTS for more than 1 year.
Conclusion
Key References
Antzelevitch C, Oliva A. Amplification of spatial dispersion of repolarization underlies sudden cardiac death associated with catecholaminergic polymorphic VT, long QT, short QT and Brugada syndromes. J Intern Med. 2006;259(1):48-58.
Antzelevitch C, Pollevick GD, Cordeiro JM, et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115:442-449.
Bellocq C, van Ginneken AC, Bezzina CR, et al. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation. 2004;109:2394-2397.
Boriani G, Biffi M, Valzania C, et al. Short QT syndrome and arrhythmogenic cardiac diseases in the young: The challenge of implantable cardioverter-defibrillator therapy for children. Eur Heart J. 2006;27(20):2382-2384.
Brugada R, Hong K, Dumaine R, et al. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation. 2004;109(1):30-35.
Gaita F, Giustetto C, Bianchi F, et al. Short QT syndrome: a familial cause of sudden death. Circulation. 2003;108(8):965-970.
Gaita F, Giustetto C, Bianchi F, et al. Short QT syndrome: pharmacological treatment. J Am Coll Cardiol. 2004;43:1494-1499.
Giustetto C, Di Monte F, Wolpert C, et al. Short QT syndrome: Clinical findings and diagnostic-therapeutic implications. Eur Heart J. 2006;27(20):2440-2447.
Gussak I, Brugada P, Brugada J, et al. Idiopathic short QT interval: a new clinical syndrome? Cardiology. 2000;94:99-102.
Hong K, Bjerregaard P, Gussak I, et al. Short QT syndrome and atrial fibrillation caused by mutation in KCNH2. J Cardiovasc Electrophysiol. 2005;16:394-396.
Morphet JA. The short QT syndrome and sudden infant death syndrome. Can J Cardiol. 2007;23(2):105.
Wolpert C, Schimpf R, Giustetto C, et al. Further insights into the effect of quinidine in short QT syndrome caused by a mutation in HERG. J Cardiovasc Electrophysiol. 2005;16(1):54-58.
1 Gussak I, Brugada P, Brugada J, et al. Idiopathic short QT interval: a new clinical syndrome? Cardiology. 2000;94:99-102.
2 Gaita F, Giustetto C, Bianchi F, et al. Short QT syndrome: a familial cause of sudden death. Circulation. 2003;108(8):965-970.
3 Extramiana F, Antzelevitch C. Amplified transmural dispersion of repolarization as the basis for arrhythmogenesis in a canine ventricular-wedge model of short-QT syndrome. Circulation. 2004;110(24):3661-3666.
4 Cerrone M, Noujaim S, Jalife J. The short QT syndrome as a paradigm to understand the role of potassium channels in ventricular fibrillation. Intern Med. 2006;259(1):24-38.
5 Antzelevitch C, Oliva A. Amplification of spatial dispersion of repolarization underlies sudden cardiac death associated with catecholaminergic polymorphic VT, long QT, short QT and Brugada syndromes. J Intern Med. 2006;259(1):48-58.
6 Brugada R, Hong K, Dumaine R, et al. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation. 2004;109(1):30-35.
7 Cordeiro JM, Brugada R, Wu YS, et al. Modulation of I(Kr) inactivation by mutation N588K in KCNH2: a link to arrhythmogenesis in short QT syndrome. Cardiovasc Res. 2005;67(3):498-509.
8 Bellocq C, van Ginneken AC, Bezzina CR, et al. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation. 2004;109:2394-2397.
9 Hong K, Piper DR, Diaz-Valdecantos A, et al. De novo KCNQ1 mutation responsible for atrial fibrillation and short QT syndrome in utero. Cardiovasc Res. 2005;68(3):433-440.
10 Priori SG, Pandit SV, Rivolta I, et al. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res. 2005;96(7):800-807.
11 Antzelevitch C, Pollevick GD, Cordeiro JM, et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115:442-449.
12 Giustetto C, Di Monte F, Wolpert C, et al. Short QT syndrome: Clinical findings and diagnostic-therapeutic implications. Eur Heart J. 2006;27(20):2440-2447.
13 Morphet JA. The short QT syndrome and sudden infant death syndrome. Can J Cardiol. 2007;23(2):105.
14 Hong K, Bjerregaard P, Gussak I, et al. Short QT syndrome and atrial fibrillation caused by mutation in KCNH2. J Cardiovasc Electrophysiol. 2005;16:394-396.
15 Bjerregaard P, Gussak I. Short QT syndrome. Ann Noninvasive Electrocardiol. 2005;10(4):436-440.
16 Brugada R, Hong K, Cordeiro JM, Dumaine R. Short QT syndrome. CMAJ. 2005;173(11):1349-1354.
17 Lu LX, Zhou W, Zhang X, et al. Short QT syndrome: A case report and review of literature. Resuscitation. 2006;71(1):115-121.
18 Borggrefe M, Wolpert C, Antzelevitch C, et al. Short QT syndrome. Genotype-phenotype correlations. J Electrocardiol. 2005;38:75-80.
19 Boriani G, Biffi M, Valzania C, et al. Short QT syndrome and arrhythmogenic cardiac diseases in the young: The challenge of implantable cardioverter-defibrillator therapy for children. Eur Heart J. 2006;27(20):2382-2384.
20 Schimpf R, Wolpert C, Bianchi F, et al. Congenital short QT syndrome and implantable cardioverter defibrillator treatment: inherent risk for inappropriate shock delivery. J Cardiovasc Electrophysiol. 2003;14:1273-1277.
21 Gaita F, Giustetto C, Bianchi F, et al. Short QT syndrome: pharmacological treatment. J Am Coll Cardiol. 2004;43:1494-1499.
22 McPate MJ, Duncan RS, Witchel HJ, Hancox JC. Disopyramide is an effective inhibitor of mutant HERG K+ channels involved in variant 1 short QT syndrome. J Mol Cell Cardiol. 2006;41(3):563-566.
23 Milberg P, Tegelkamp R, Osada N, et al. Reduction of dispersion of repolarization and prolongation of postrepolarization refractoriness explain the antiarrhythmic effects of quinidine in a model of short QT syndrome. J Cardiovasc Electrophysiol. 2007;18(6):658-664.
24 Wolpert C, Schimpf R, Giustetto C, et al. Further insights into the effect of quinidine in short QT syndrome caused by a mutation in HERG. J Cardiovasc Electrophysiol. 2005;16(1):54-58.