General Principles of Orthopedic Injuries
Management Principles
1. Most orthopedic injuries can be predicted by understanding the chief complaint, the age of the patient, the mechanism of injury, and an estimate of the amount of energy delivered.
2. A careful history and physical examination predict radiographic findings with a high degree of accuracy. A presumptive diagnosis before a radiographic study may prompt the physician to order special views necessary to correctly diagnose an injury. Many fractures were accurately described before the advent of roentgenology (Table 49-1).
Table 49-1
Common Fracture Names and Their Origins
| FRACTURE EPONYM OR NAME | DESCRIPTION | COMMENT |
| Aviator’s | Vertical fracture of the neck of the talus with subtalar dislocation and backward displacement of the body. | First described in flyers during World War I. Arises from forced dorsiflexion of the foot in flying accidents and in traffic accidents after a head-on collision. |
| Barton’s | Intra-articular fracture-dislocation of the wrist. | Considered complicated and unstable. Requires surgical reduction in most cases. Described by Barton in 1838 before the advent of radiography. |
| Dorsal Barton’s | Oblique intra-articular fracture of the dorsal rim of the distal radius with displacement of the carpus along with the fracture fragment. | Results from high-velocity impact across the articular surface of the radiocarpal joint, with the wrist in dorsiflexion at the moment of impact. |
| Volar Barton’s | Wedge-shaped articular fragment sheared off the volar surface of the radius (volar rim fracture), displaced volarly along with the carpus. | Similar mechanism as dorsal Barton’s but with wrist in volar flexion at time of injury. Also referred to as reverse Barton’s fracture. Much rarer than dorsal Barton’s fracture. |
| Bennett’s | Oblique fracture through base of the first metacarpal with dislocation of the radial portion of the articular surface. | Usually produced by direct force applied to the end of the metacarpal. Dorsal capsular structures disrupted by the dislocation. Marked tenderness along medial base of thumb. |
| Bosworth | Fracture-dislocation of the ankle resulting in the fibula being entrapped behind the tibia. | Rare injury, produced by a severe external rotation force applied to the foot. Physical examination reveals foot severely externally rotated in relation to the tibia. |
| Boxer’s | Fracture of the neck of the fourth or fifth metacarpal. | Results from striking a clenched fist into an unyielding object, usually during an altercation, or against a wall, out of frustration or anger. |
| Chance’s | Vertebral fracture, usually lumbar, involving the posterior spinous process, pedicles, and vertebral body. | Caused by simultaneous flexion and distraction forces on the spinal column, usually associated with use of lap seat belts. Anterior column fails in tension along with the middle and posterior columns. May be misdiagnosed as a compression fracture. |
| Chauffeur’s | Solitary fracture of radial styloid. | Occurs from tension forces sustained during ulnar deviation and supination of the wrist. Name derives from occurrence in chauffeurs who suffered violent, direct blows to the radius incurred while turning the crank on a car, only to have it snap back, during previous eras. |
| Clay shoveler’s | Fracture of the tip of the spinous process of the sixth or seventh cervical vertebra. | First described in Australian clay shovelers who sustained a fracture of the spinous process by traction as they lifted heavy loads of clay. |
| Colles’ | Fracture of the distal radius with dorsal displacement and volar angulation, with or without an ulnar styloid fracture. | Most common wrist fracture in adults, especially in the elderly. Results from fall on an outstretched hand. Also known as silver fork deformity, which accurately describes the gross appearance in the lateral view. First described by Colles in 1814, before the advent of radiography. |
| Cotton’s | Trimalleolar fracture. | Fracture of the lateral malleolus, fracture of the posterior malleolus, and either a fracture of the medial malleolus or a disruption of the deltoid ligament with visible widening of the mortise on ankle radiograph. |
| Dashboard fracture | Fracture of the posterior rim of the acetabulum. | Named for mechanism of injury: a seated passenger striking the knee on a dashboard, driving the head of the femur into the acetabulum. |
| Dupuytren’s | Fracture-dislocation of the ankle. | Results from a similar mechanism as the better known Maisonneuve fracture (i.e., external rotation of the ankle), resulting in either deltoid ligament rupture or medial malleolus fracture, diastasis of the inferior tibiofibular joint, and indirect fracture of the fibular shaft. Maisonneuve was the student of Dupuytren. |
| Essex-Lopresti | Fracture of radial head with dislocation of distal radioulnar joint. | Results from longitudinal (axial) compression of the forearm. |
| Galeazzi’s | Fracture of the shaft of the radius with dislocation of the distal radioulnar joint. Ligaments of inferior radioulnar joint are ruptured and head of ulna displaced from ulnar notch of the radius. | Results from fall on outstretched hand, with the wrist in extension and the forearm forcibly pronated. Inherently unstable with tendency to redisplace after reduction. |
| Hangman’s | Fracture-dislocation of atlas and axis, specifically of pars interarticularis of C2 and disruption of C2-3 junction. Separation occurs between second and third vertebral bodies from anterior to posterior side. | Results from extreme hyperextension during abrupt deceleration. Most common cause is the forehead striking the windshield of a car during a collision. A bit of a misnomer in that hanging usually produces death by strangulation rather than cord damage. |
| Hume’s | Fracture of the proximal ulna associated with forward dislocation of the head of the radius. | Essentially high Monteggia’s injury. |
| Jefferson’s | Burst fracture of ring of C1, or atlas. | Axial loading results in a shattering of the ring of the atlas. Decompressive type of injury. Associated with disruption of transverse ligament; an unstable injury. |
| Jones | Transverse fracture of the metatarsal base, occurring at least 15 mm distal to the proximal end of the bone, distal to the insertion of the peroneus brevis. | Should not be confused with the more common avulsion fracture of fifth metatarsal styloid, produced by avulsion at the insertion of the peroneus brevis. Jones described the fracture that bears his name in 1902, after sustaining the injury himself while dancing. |
| Le Fort | Maxillary fracture. | Types I, II, and III (see Chapter 42). |
| Le Fort-Wagstaffe | Avulsion fracture of the anterior cortex of the lateral malleolus. | Rare pull-off injury of the fibular attachment of the anterior tibiofibular ligament. |
| Lisfranc’s | Fracture located around the tarsometatarsal (Lisfranc’s) joint, usually associated with dislocation of this joint. | Lisfranc, a field surgeon in Napoleon’s army, described an amputation performed through the tarsometatarsal joint in a soldier who caught his foot in a stirrup when he fell off his horse. Since then, the joint has borne his name. |
| Maisonneuve | Fracture of proximal third of fibula associated with rupture of the deltoid ligament or fracture of the medial malleolus and disruption of the syndesmosis. | Results from external rotation of the ankle with transmission of forces through syndesmosis; proximally the force is relieved by fracture of the fibula. Described experimentally in 1840, before radiography. |
| Malgaigne’s | Fracture of the ilium near the sacroiliac joint with displacement of the symphysis, or a dislocation of the sacroiliac joint with fracture of both ipsilateral pubic rami. | Resultant pelvic injury is unstable. Described by Malgaigne, based on clinical findings, in 1847. |
| March | Fatigue, or stress, fracture of the metatarsal. | Arises from long marches or other repetitive use trauma (e.g., marathon running) or less commonly from single stumbling movements. |
| Monteggia’s | Fracture of the junction of the proximal and middle thirds of the ulna associated with anterior dislocation of the radial head. | Usually caused by fall on outstretched hand along with forced pronation of forearm or by a direct blow on the posterior aspect of the ulna. Reported by Monteggia in 1814. |
| Nightstick | Fracture of either ulna or radius, or both. | Name derived from a citizen’s attempt to protect himself from a police officer’s baton or “nightstick” by offering the forearm. |
| Piedmont | Closed fracture of the radius at the middle third–distal third junction, without associated ulnar fracture. | Named for a series of cases presented at the Piedmont Orthopaedic Society of Durham, North Carolina. |
| Pott’s | Definitions vary (see comment); most commonly a bimalleolar fracture or a fracture of the distal fibula, 4-7 cm above the lateral malleolus. | The exact fracture Pott described in 1769 is uncertain; clearly it referred to a fracture of the lower fibula, usually associated with other fractures or dislocations about the ankle. |
| Rolando’s | Intra-articular fracture at base of metacarpal. Frequently Y– or T-shaped, or may be severely comminuted. | Produced by an axial load with the metacarpal in partial flexion. Worse prognosis than a Bennett’s fracture and, fortunately, rarer. |
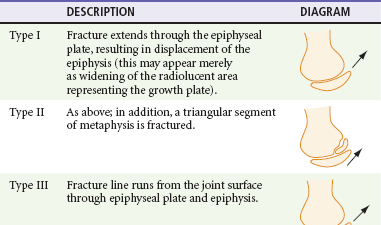
| Salter-Harris | An epiphyseal fracture occurring in children or adolescents. | Graded I-V, depending on degree of involvement and/or displacement of epiphysis and metaphysis (see text dealing with Salter-Harris fractures and also Figure 46-1). |
| Smith’s | Extra-articular fracture of the distal radius with volar displacement of distal fragment. | Reverse of the Colles’ fracture but much more uncommon. Sometimes referred to as a “garden spade” deformity. Usually results from fall with force to back of hand. First described by Smith in 1847. |
| Stener | Avulsion of the ulnar corner of the base of the proximal phalanx of the thumb. | Bony equivalent of rupture of the ulnar collateral ligament, or “gamekeeper’s thumb.” |
| Teardrop | Wedge-shaped fracture of the anteroinferior portion of the vertebral body, displaced anteriorly. | Commonly involves a ligamentous injury and may produce neurologic injury. |
| Thurston Holland’s fragment | Triangular metaphyseal fragment that accompanies the epiphysis in Salter-Harris type II fractures. | Described by Thurston Holland in 1929. The name is commonly hyphenated, although technically it should not be. |
| Tillaux | Isolated avulsion fracture of the anterolateral aspect of the distal tibial epiphysis. | Occurs in older adolescents (12-15 years) after the medial parts of the epiphyseal plates close but before the lateral part closes. External rotation force places stress on anterior talofibular ligament. Described by Tillaux in 1872. |
3. If a fracture is suggested clinically, but radiographic films appear negative, the patient should initially be treated with immobilization as though a fracture were present.
4. Criteria for adequate radiographic studies exist; inadequate studies should not be accepted.
5. Radiographic studies should be performed before most reductions are attempted, except when a delay could be potentially harmful to the patient or in some field situations.
6. Neurovascular competence should be checked and recorded before and after all reductions and after application of immobilization.
7. Patients must be checked for the ability to safely ambulate before discharge from the ED and should not be discharged unless this can be established.
8. Patients should receive explicit aftercare instructions before leaving the ED, covering such areas as monitoring for signs of neurovascular compromise or increasing compartment pressure, cast care, weightbearing, crutch use, and an explicit plan and timing for follow-up.
9. In a patient with multiple trauma, noncritical orthopedic injuries should be diagnosed and treated only after more threatening injuries have been addressed.
10. All orthopedic injuries should be described precisely and according to established conventions. When communicating with an orthopedic consultant, this may affect decisions regarding disposition of a patient and operative versus nonoperative management.
Fractures
Describing orthopedic injuries with precise language according to established convention enables accurate, clear communication with other parties. Terms commonly used to describe a fracture are listed in Box 49-1. A fracture is a break in the continuity of bone or cartilage. Clinically, a history of loss of function, pain, tenderness, swelling, abnormal motion, and deformity suggests a fracture. Radiographic studies are the mainstay of diagnosis and are usually, although not always, confirmatory. At times, use of special views, radionuclide bone scans, computed tomography (CT), or magnetic resonance imaging (MRI) is necessary to confirm a clinical impression. These studies should be considered when the clinical evidence is at odds with the findings of routine radiography.
General Descriptors
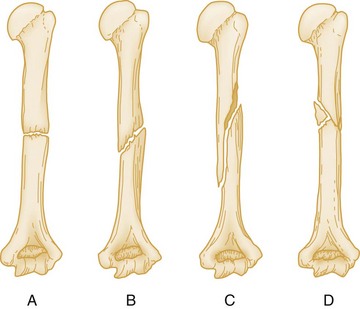
An additional modifier describes the direction of the fracture line in relation to the long axis of the bone in question. A transverse fracture occurs at a right angle to the long axis of the bone (Fig. 49-1A), whereas an oblique fracture runs oblique to the long axis of the bone (Fig. 49-1B). A spiral fracture results from a rotational force and encircles the shaft of a long bone in a spiral fashion (Fig. 49-1C). A fracture with more than two fragments is termed comminuted (Fig. 49-1D).
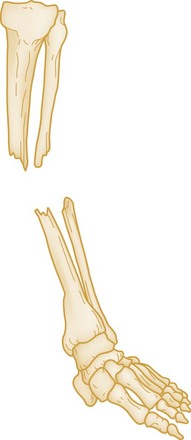
The position and alignment of the fracture fragments (i.e., their relationship to one another) should be described. Fragments are described relative to their normal position, and any deviation from normal is termed displacement. By convention, the position of the distal fragment is described relative to the proximal one. Displacement may be described as a quantitative measurement (i.e., in millimeters) or as a percentage of the bone width. Figure 49-2 shows a dorsal displacement of the fractured radius, and Figure 49-3 shows lateral, or valgus, displacement of the distal tibia and fibula.
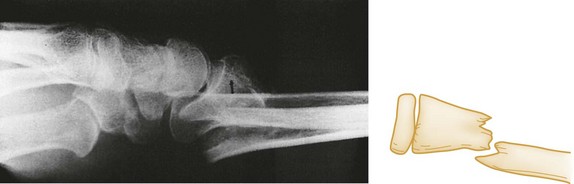
Figure 49-2 Dorsal displacement of distal radius.
The terms valgus and varus are sometimes confusing. Valgus denotes a deformity in which the described part is angled away from the midline of the body. Conversely, varus denotes a deformity in which the angulation of the part is toward the midline. Alignment refers to the relationship of the longitudinal axis of one fragment to another; deviation from the normal alignment is termed angulation. The direction of angulation is determined by the direction of the apex of an angle formed by the two fracture fragments (Fig. 49-4). This angle is opposite to the direction of displacement of the distal fragment. The relative position or angulation of the distal fragment of a fracture may also be described with terms such as radial or ulnar, dorsal or volar, anterior or posterior, and lateral or medial. One should also be aware of rotational deformity, present when the distal fragment of a fracture is rotated to some degree along the axis of the bone itself. Especially in the digits of the hand, radial or ulnar deviation of a flexed finger can occur, and radiographs often underestimate the degree of clinical deformity and rotation present.
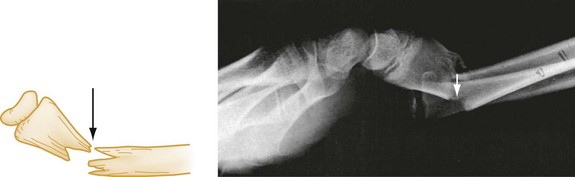
Figure 49-4 Volar angulation of a fractured radius.
Descriptive Modifiers
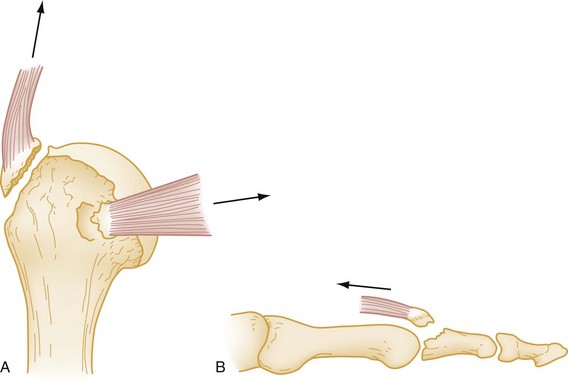
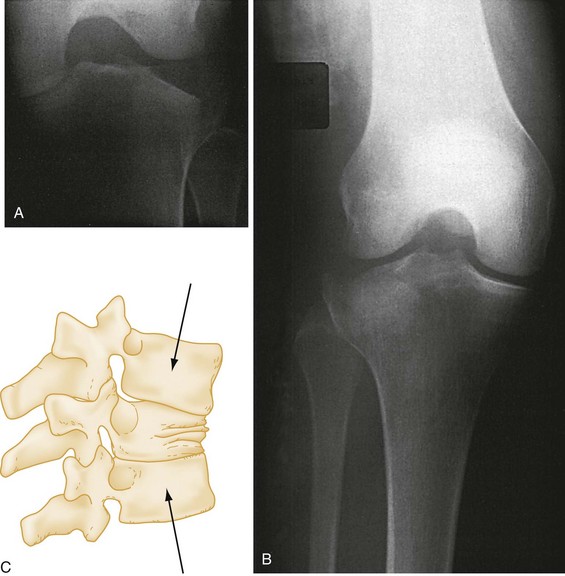
Avulsion fracture refers to a bone fragment that is pulled away from its normal position by either the forceful contraction of a muscle (Fig. 49-5A) or the resistance of a tendon or ligament to a force in the opposite direction (Fig. 49-5B). Impaction refers to the forceful collapse of one fragment of bone into or onto another. In the proximal humerus, this collapse typically occurs in a telescoping manner, particularly in elderly patients, whose bones are soft and brittle. In the tibial plateau, impaction occurs frequently in the form of a depression (Fig. 49-6A and B), and in the vertebral bodies, impaction frequently occurs in the form of compression (Fig. 49-6C).
A fracture that occurs through abnormal bone is termed pathologic. A pathologic fracture is suggested whenever a fracture occurs from seemingly trivial trauma. Diseases that cause structural weakness predisposing to injury include primary or metastatic malignancies, cysts, enchondromata, and giant-cell tumors. In addition, osteomalacia, osteogenesis imperfecta, scurvy, rickets, and Paget’s disease all weaken bones, making them susceptible to fracture. The term pathologic also is applied to fractures through osteoporotic bone when the demineralization is a result of disease, as in polio. Fractures through osteoporotic bone of the elderly usually are not described as pathologic. When fractures occur in normal bones and a history of “trivial trauma” is elicited, violence or battering should be suspected. Repeated low-intensity forces may lead to resorption of normal bone, resulting in a stress fracture. Other names for this condition are fatigue fracture and march fracture (see Table 49-1). Most stress fractures occur in the lower extremities and commonly affect individuals involved in activities such as running, basketball, aerobics, and dancing. Extrinsic factors such as training regimens, type of equipment used, and nutrition habits, as well as intrinsic factors such as anatomic variation, muscle endurance, and hormonal factors have all been associated with stress fractures. These injuries may not be recognizable on initial plain films; therefore management should be based on clinical diagnosis.1 The tibia, fibula, metatarsals, navicular, cuneiform, calcaneus, femoral neck, or femoral shaft may be involved.2,3
Fracture Eponyms
Many fractures were described before the advent of radiography and are described by an eponym rather than the exact bony injury. These eponyms reflect the rich history of orthopedic care and, despite the objections of some, are still commonly used to describe orthopedic injuries (see Table 49-1).
Fractures in Children
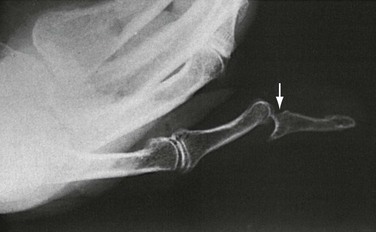
Certain features of children’s bones distinguish pediatric fractures from adult fractures. Bones of children are necessarily soft and resilient and sustain numerous incomplete fractures. Greenstick fractures are incomplete angulated fractures of long bones. The resultant bowing of the bone causes an appearance resembling a moist, immature branch that breaks in a similar fashion when bent (Fig. 49-7A). A torus fracture is another form of incomplete fracture, characterized by a wrinkling or buckling of the cortex. In Greek architecture a torus is a bump at the base of a column, and these fractures, occurring at the end of long bones, take on such an appearance. These fractures may be extremely subtle on radiographs (Fig. 49-7B).
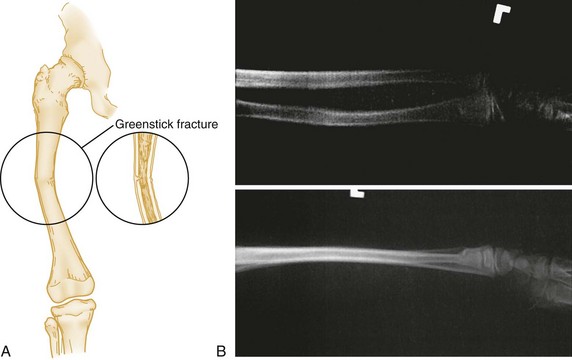
Figure 49-7 A, Greenstick fracture of midshaft of femur. B, Torus fracture of distal third of radius.
Another feature of growing long bones that is a frequent source of trouble and confusion is the presence of epiphyses, cartilaginous centers at or near the ends of bone that give rise to growth of the bone. Figure 49-8 is a schematic review of the anatomy of a growing bone. Because cartilage is radiolucent, the cartilaginous portion of an epiphysis is not visualized on radiographs. A tendency exists to consider only the ossified nucleus and to ignore the cartilaginous structure that bridges to the metaphysis. Cartilage is present even before an ossified nucleus is seen. Because the epiphyseal growth plate is represented by a radiolucent line, confusion may exist as to whether a fracture line is present. These complexities in interpreting radiographs in children sometimes, but not always, require comparison radiographic views of the uninjured side. Injuries to the epiphyses may result from either compressive or shearing forces. These injuries are relatively common during childhood as opposed to sprains or shaft fractures and should be considered in children with a “sprained ankle” because of the relative weakness of the cartilaginous growth zone, which separates before stronger ligaments and bones are torn or broken. Epiphyseal injuries should be described according to the Salter-Harris classification (Table 49-2).
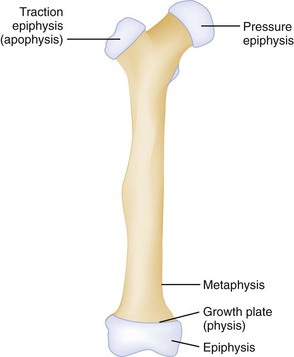
Figure 49-8 Anatomy of growing bone.
Type II injuries are similar to type I injuries, with a fracture extending into the metaphysis. The triangular metaphyseal fragment sometimes is referred to as the Thurston Holland sign (see Table 49-1). Type II injuries account for approximately three fourths of all epiphyseal fractures. Because the germinal layer is not involved, growth disturbance usually does not occur with type I and II injuries. These injuries are amenable to closed reduction and immobilization without internal fixation.
Type V fractures are crush injuries of the epiphyseal plate, usually produced by a compressive force.3 This type of injury usually occurs in joints that move in one plane, most commonly the knee and ankle. Because this injury occurs in a radiolucent area, the injury may be difficult to diagnose on radiograph, but it is suggested by mechanism of injury and pain over the epiphysis. The diagnosis can be established by MRI if hemorrhage or a hematoma is identified within the growth plate immediately after injury.3–5 Also reported is loss of MRI signal from the cartilage.6 Rarely are type V injuries diagnosed acutely, and growth arrest manifested by shortening or angulation is the rule with this injury.
Physeal injuries occur twice as often in boys as in girls and are most common in boys aged 12 to 15 years and in girls aged 9 to 12 years.7 Distal physes are injured more often than proximal physes, and the most common anatomic locations include the distal radius, phalanges, and distal tibia. Distal radius fractures account for two thirds of fractures in pediatric patients in the ED.8 The incidence of these fractures has increased by 40% over the past three decades, possibly because of a change in recreational activities, such as increased skiing and skating among boys and increased basketball, soccer, and skating among girls.9 Most distal radius type I and II fractures can be treated through closed reduction, though this may be more technically difficult with completely displaced both-bone fractures. Similar practice has been applied to distal tibia type I and II fractures, though the incidence of premature physeal closure was shown to be 3.5 times higher if the residual fracture displacement was greater than 3 mm in postreduction radiographs.10 Growth arrest as a complication of physeal fracture is most likely to be seen at the distal femur, distal and proximal tibia, and distal radius.7
Diagnostic Modalities for Fracture Diagnosis
Stress views of joints are used in some instances to evaluate the degree of ligamentous injury. Some authors argue against the use of stress views, citing a risk of further injuring an already traumatized structure, additional radiation exposure to the patient and the technologist, and the possibility that pain may not allow sufficient stress to be applied. For these reasons, stress views should be used judiciously in circumstances when other methods of evaluating ligamentous injuries are not available. Comparison views are useful in selected situations but should not routinely be performed in all pediatric examinations.11 If a fracture is definitely present on the affected side, the comparison view exposes the child to radiation and adds expense with no benefit. Similarly, an experienced physician generally is able to read a normal film with reasonable certainty. It is reasonable to use comparison views in instances when radiographs are inconclusive and when the confusion arises specifically out of the need to distinguish between a possible fracture and normal developmental anatomy. Obtaining a wide field of the affected extremity is more useful than routine comparison views for a young child because the child often does not localize the pain well; this is especially true with regard to complaints of knee pain in cases of hip injury or wrist complaints in forearm and elbow injury. Comparison views sometimes are helpful in adults when a question of accessory ossicles or nonfused bones (e.g., bipartite patella) exists because these anomalies are usually bilateral. The bleeding that inevitably accompanies fractures may produce soft tissue swelling, which may impinge on or obliterate overlying muscle planes. Fat pads, such as in the elbow, may be displaced. Another useful sign is the fat-fluid level, which may accompany fractures extending into the knee joint. The fat-fluid level is visible, however, only if the cross-table technique is used.
The bones themselves should be examined systematically. Normal adult bones possess a smooth unbroken contour. A distinct angle is highly suggestive of a fracture. In an adult the typical fracture is represented by a lucent line that interrupts the smooth contour and usually extends to the opposite side. Nutrient arteries may be confused with fractures but have different radiographic characteristics: They are fine, are sharply marginated, extend obliquely through the cortex, and are less radiolucent than fractures. Pseudofractures can be created by soft tissue folds, bandages or other overlying material, or a radiographic artifact called the Mach effect. If lucencies extend beyond the bones, the line is highly unlikely to represent a fracture. Anomalous bones and calcified soft tissue likewise may be mistaken for fractures. Avulsions and small fracture fragments have an irregular surface that lacks well-corticated margins and a defect in the adjacent bone is present, whereas anomalous ossification centers (accessory ossicles) and sesamoids are characterized by smooth cortical margins. Reference texts are useful in identifying and confirming these anomalies because they tend to occur in predictable locations.12 Compression fractures are represented by increased density rather than a lucency. Finally, the most commonly missed fracture is the second fracture. One should be diligent in searching for additional fractures after discovering the first fracture on a study. In particular, certain paired fractures, such as the distal tibia and proximal fibula, should be sought out.
Special Imaging Techniques
Radionuclide Bone Scanning.: In the past, radionuclide bone scanning was used to detect skeletal abnormalities not radiographically evident in children and adults.13 Occult lesions, especially stress fractures, acute osteomyelitis, and tumors, can be detected on these scans, although there are problems with specificity and sensitivity. This modality has been largely supplanted by CT and MRI and now is seldom used.
Computed Tomography.: Computed (digital) radiography is now in widespread use. Although conventional radiography remains the initial imaging study of choice for skeletal trauma, CT offers a more detailed and diagnostically sensitive evaluation of bones and joints. With improved resolution and speed, multidetector-row CT captures large volumetric data sets from which two- and three-dimensional images can be created.14,15 Workstation postprocessing has become an integral part of the examination. Two-dimensional multiplanar reconstruction in any chosen plane, and three-dimensional surface rendering techniques provide images with unprecedented quality, even in the presence of metallic implants or fixation devices.16
CT is used to confirm possible fractures or to better define displacement, alignment, or fragmentation of fractures. It is also useful in trauma to rule out cervical spine fracture when plain films are equivocal and in noncompressive vertebral fractures to assess the number of fragments and their spatial relationship to the spinal canal. CT is used frequently to define the integrity of articular surfaces in the acetabulum, knee, wrist, or ankle and in Salter-Harris type IV fractures.4 In the multiple trauma patient requiring thoracic, abdominal, and pelvic CT imaging to rule out visceral injury, the soft tissue protocols may be adapted to acquire diagnostic bone images, as well.17 During imaging of the chest, abdomen, or pelvis, data sets are created from which the thoracolumbar spine and bony pelvis can be derived.
Magnetic Resonance Imaging.: MRI constitutes the most advanced noninvasive examination of orthopedic structures, delineating lesions of bone, cartilage, ligaments, and other structures, such as menisci, disks, and epiphyseal structures. MRI is expensive and time-consuming and should be reserved for instances in which the diagnosis is in doubt and specific findings would alter the treatment.
Ultrasound Imaging.: Point-of-care ultrasound may be an effective tool for the diagnosis of fractures when conventional radiography is unavailable. Through use of bedside ultrasonography, fractures are visualized as an interruption of the linear bony cortex and may be clinically correlated during the physical examination of the affected area. Ultrasound can be effective in the diagnosis of long bone fractures, orbital floor fractures, rib fractures, and occult fractures of the foot and ankle.18 Ultrasound can be used in “real-time” during fracture reduction to confirm proper reduction and alignment of bony fragments.19
Complications of Fractures
Any fracture communicating with the surface of the skin is termed an open fracture. Open fractures are treated as true orthopedic emergencies because of the risk of infection; the dreadful nature of the complication of osteomyelitis dictates that no time should be wasted in initiating therapy (Box 49-2). Wounds should be irrigated of gross debris and covered with a sterile dressing, and parenteral antibiotics should be instituted as early as possible.20
Currently, suggested therapy includes a first-generation cephalosporin, such as cefazolin, for all open fractures, with the addition of an aminoglycoside for grade II or III fractures.21,22 Early versus delayed treatment of open fractures and its subsequent effect on rates of infection has been a source of debate. Historic guidelines recommending débridement of open fracture wounds within 6 hours of injury were based on animal experiments conducted in the 1890s.23 Current human studies suggest no clear advantage to performing surgical débridement within 6 hours of injury. The timing of débridement—less than 6 hours versus more than 6 hours after injury—had no effect on clinical or functional outcome in a prospective multicenter study of severe lower-extremity fractures.24 Other retrospective studies and literature reviews advocate early débridement and irrigation of the wound within the first 24 hours of injury to prevent infection.
Certain open fractures of the finger present a notable exception to the previous recommendation. Such injuries, especially an open distal tuft fracture, are common when the phalanx of a finger is subject to crush injury (e.g., by a door) and there exists a skin defect overlying a fractured bone. In a prospective randomized, placebo-controlled study of 193 patients with open fracture of the finger, flucloxacillin or placebo was administered to randomized patients with open phalangeal fractures, and both groups were treated with aggressive surgical irrigation and débridement. No significant difference was found in the infection rate between the groups, and no patient developed osteomyelitis. The data suggest that vigorous irrigation and débridement are adequate primary treatment for open phalangeal fractures in fingers with intact digital arteries.25 Such injuries might be repaired by the emergency physician without consultation.
Hemorrhage
Because of the rich blood supply to the skeleton, fractures can result in large amounts of blood loss, shock, and death from exsanguination. In particular, certain pelvic fractures can cause great blood loss as adequate tamponade is not possible. In adults, blood loss can range from 100 mL from a forearm fracture to 3 L from a pelvic fracture (Table 49-3).
Table 49-3
Blood Loss Associated with Fracture in Adults
| FRACTURE SITE | AMOUNT OF BLOOD LOSS (mL) |
| Radius and ulna | 150-250 |
| Humerus | 250 |
| Tibia and fibula | 500 |
| Femur | 1000 |
| Pelvis | 1500-3000 |
Vascular Injuries
Vascular injuries characteristically are associated with certain fractures and may be limb-threatening. Fractures and dislocations (at the femorotibial articulation) of the knee result from tremendous force that often injures the popliteal artery. Fracture of the femoral neck requires emergent reduction and fixation to protect the blood supply to the femoral head.23 In the extremities, assessment of vascular injuries may be difficult. The initial survey should note the presence or absence of pulses and the state of capillary filling. If an end artery is completely disrupted, the tissue distal to the injury may exhibit the classic five Ps: pain, pallor, pulselessness, paresthesias, and paralysis. Incomplete and subclinical injuries occasionally occur that initially may be asymptomatic and undetectable. Likewise, in an unconscious, multiply injured patient, major vascular injuries may not be obvious. The mechanism of injury and anatomy dictate the need to assess the possibility of an injured vessel. If pulses cannot be palpated, a Doppler stethoscope should be used to listen for blood flow. Even palpable pulses may be misleading, however, because it has been shown that in 10 to 20% of significant arterial injuries, distal pulses may initially be normal. When pulses are present but the mechanism of injury suggests the possibility of vascular injury, additional diagnostic studies or surgical exploration may be necessary. If a limb is clearly not perfused, operative vascular exploration and repair should take place promptly. Late complications of undiagnosed vascular injuries include thrombosis, arteriovenous fistulae, aneurysm, false aneurysm, and tissue ischemia with limb dysfunction. Delay of vascular injury repair is a risk factor for consequent amputation.26
Traditionally, conventional arteriography has been the diagnostic modality of choice in evaluating vascular injuries; however, it is invasive and costly. Alternative methods of vascular assessment include arterial pressure indices and serial physical examinations. Recent advances in computed tomographic angiography have proven it an effective alternative to conventional arteriography, and its advantages include immediate availability and noninvasiveness.27
Nerve Injuries
Nerves can be injured by either blunt or penetrating trauma. Neurapraxia is the contusion of a nerve, with disruption of the ability to transmit impulses. Paralysis, if present, is transient, and sensory loss is slight. Normal function usually returns to a neurapraxic nerve in weeks to months. Axonotmesis is a more severe crush injury to a nerve. The injury to nerve fibers occurs within their sheaths. Because the Schwann tubes remain in continuity, spontaneous healing is possible but slow. Neurotmesis is the severing of a nerve, usually requiring surgical repair. Age, site, injured nerve, and delay between injury and repair have all been shown to influence prognosis after microsurgical repair.28 Because of proximity, specific nerve injuries characteristically accompany certain fractures (Table 49-4). For example, in the upper extremity, a distal radius fracture caused by a high-energy insult can be associated with acute dysfunction of the median nerve. Deteriorating neurologic function may necessitate temporary or definitive stabilization of a fracture.
Table 49-4
Nerve Injuries Accompanying Orthopedic Injuries
| ORTHOPEDIC INJURY | NERVE INJURY |
| Distal radius | Median nerve |
| Elbow injury | Median or ulnar |
| Shoulder dislocation | Axillary |
| Sacral fracture | Cauda equina |
| Acetabulum fracture | Sciatic |
| Hip dislocation | Femoral nerve |
| Femoral shaft fracture | Peroneal |
| Knee dislocation | Tibial or peroneal |
| Lateral tibial plateau fracture | Peroneal |
When the nerve is completely severed, all functions are absent, including superficial sensation to touch, pain, and temperature; deep sensation to muscle and joint movements, position, deep pressure, and vibration; motor supply and deep tendon reflexes (to distally innervated muscle groups); and response to electrical stimulation. For less severe injuries, any subjective change in sensation should be noted. Light touch is a good screening test. Two-point discrimination is a more sensitive examination and should be used routinely in evaluating digital nerves. Separating the ends of a paper clip a few millimeters apart and asking the patient to discriminate between one or two points may easily accomplish this. The discrimination on the injured digit is then compared with the uninjured ones. Two-point discrimination may be of limited value in children in whom a subjective response may be misleading; this is also true in patients with calloused fingertips and in patients who are uncooperative, comatose, in severe pain, or intoxicated. Testing for sympathetic nerve function with the O’Riain wrinkle test may be helpful.29,30 Soaking the normally innervated digits in warm saline for 20 minutes causes the digital pulps to wrinkle through a mechanism that is not understood. The presence of wrinkling probably indicates the nerve is intact, whereas absence of wrinkling may be more difficult to interpret. The Ninhydrin test, another sudomotor test used to assess peripheral nerve integrity, is reportedly more reliable than the O’Riain test but is not practical to perform in the ED.
Compartment Syndrome
Compartment syndrome is a serious acute emergency complication that should be considered whenever pain and paresthesias occur in an extremity after a fracture within an enclosed osseofascial space (Table 49-5). The immediate threat is to the viability of nerve and muscle tissue within the involved compartment, but infection, gangrene, myoglobinuria, and renal failure also may ensue. Compartment syndrome is associated most commonly with a closed long bone fracture of the tibia, but it also is well described in the thigh, forearm, arm, hand, and foot.31–35 In addition, compartment syndrome can occur with soft tissue trauma alone and even with open fractures. It also has been described in a host of unusual situations, including prolonged procedures in the lithotomy position, the tuck position (knees tucked to the chest for lumbar surgery), coma, spontaneous hemorrhage, intravenous injections, and the application of excessive traction in treatment of a fracture.36,37 Acute surgical stabilization of a fracture is also associated with higher rates of compartment syndrome in the postoperative period.38
Table 49-5
Life-Threatening or Limb-Threatening Emergencies
| CONDITION | POSSIBLE ADVERSE OUTCOME |
| Open fracture | Osteomyelitis |
| Fracture or dislocation with major vascular disruption (especially popliteal) | Amputation |
| Major pelvic fracture | Exsanguination |
| Hip dislocation | Avascular necrosis of femoral head |
| Compartment syndrome | Ischemic contracture; myoglobinuria, renal failure |
Pathophysiology.: Increased pressure in a closed nonexpandable compartment essentially represents a mismatch between the volume of that space and its contents. As such, it may arise from one of three circumstances: (1) increased compartment contents, (2) decreased compartment volume, or (3) external pressure (Box 49-3). As tissue pressure increases, so does venous pressure, resulting in compromise of the local circulation and tissue hypoxia; this is believed to occur at pressures that are above normal diastolic pressure but below systemic arterial pressure because of a reduced arteriovenous gradient at the tissue level. The body responds by releasing histamine in an attempt to dilate capillaries and increase blood flow to the affected area. Histamine also increases capillary membrane permeability, resulting in a leak of proteins and fluid into the surrounding tissue, further increasing compartment pressure.
Normal compartment pressure is 0 mm Hg. Microcirculation generally is impaired when tissue pressures reach 30 mm Hg or more; however, some patients can tolerate much higher compartment pressures without development of compartment syndrome. Controversy exists over attempts to define compartment syndromes on the basis of specific tissue pressure. The tolerance to tissue ischemia varies among individuals because of shock, compensatory hypertension, altered tone in resistance vessels, preexisting vascular disease, and other unknown factors. Inadequate perfusion and relative ischemia begin when tissue pressure within a closed compartment increases to within 20 mm Hg of a patient’s diastolic pressure or, more accurately, within 30 mm Hg of the mean arterial pressure.39 When tissue pressure equals or exceeds the patient’s diastolic pressure, tissue perfusion effectively ceases. The development of muscle ischemia depends not only on the magnitude but also on the duration of elevated pressure. Intracompartmental pressures do not measure muscle and nerve ischemia but rather suggest the existence of the proper setting for compartment syndrome.
Anatomic Considerations and Risk Factors.: Compartment syndrome theoretically can develop in any location where neuromuscular tissue is contained in a limiting envelope. The condition has been reported in the leg, thigh, buttock, arm, forearm, and hand (Box 49-4). By virtue of its location and higher likelihood of sustaining high-energy trauma, the leg, particularly the anterior compartment, is most commonly involved. Higher rates of compartment syndrome are seen with open fractures than with closed fractures, despite the fascial rents that accompany open fractures. The higher energy of injury observed with open fractures, with resultant tissue trauma, swelling, and bleeding, may account for this observation.
In a study of 164 patients treated over an 8-year period in the United Kingdom, a fracture was present in 69%, and half of these involved the tibial shaft.31 Perhaps more significant is that 31% of patients had only soft tissue injury without fracture. Most patients were men younger than age 35. Ten percent of patients either had a bleeding disorder or were taking anticoagulants. Traffic accidents and sports activities were the most common mechanisms of injury.
Clinical Presentation.: Compartment syndrome is a clinical diagnosis. In a conscious and fully oriented patient, pain that is disproportionate to the injury or physical findings is a hallmark finding in compartment syndrome. Pain often is characterized as deep, burning, and unrelenting and is difficult to localize. The need for increasing amounts of analgesics should not lead the clinician automatically to the conclusion that the patient is drug-seeking; rather, it should serve as a prompt to the possibility that a compartment syndrome is developing or is present.
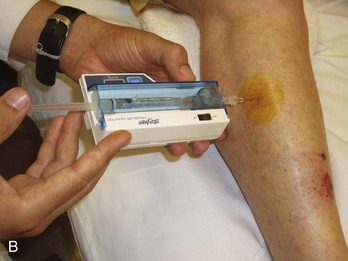
Diagnostic Tests.: If the history and examination suggest compartment syndrome, compartment pressures should be measured with any of the commercially available monitors (Fig. 49-9).40,41 The two most common methods of determining compartment pressures are the slit-catheter techniques and the side-port needle. The Stryker Intra-Compartmental Pressure Monitor system is a hand-held digital device that is easy to use with minimal training. Care should be taken to zero the monitor in on the plane in which it will be inserted to account for the effects of gravity. It is also paramount that the appropriate compartment be measured. Pressures of less than 30 mm Hg generally do not produce compartment syndrome. Pressures exceeding 30 mm Hg or within 30 mm Hg of the patient’s mean arterial pressure are an indication for fasciotomy. Serial or continuous pressure measurements should be performed in cases that are not clear-cut. A rising or sustained elevated compartment pressure is superior to a single measurement as an indicator of acute compartment syndrome or the need for fasciotomy. Compartment syndrome can occur at pressures significantly below systemic pressure. Doppler ultrasound is not useful in evaluating these patients because excellent arterial blood flow may be documented even in the presence of a significant compartment syndrome. Newer devices based on near-infrared spectroscopy (NIRS) measurement of tissue oxygenation have proven effective experimentally in detecting compartment syndrome but will require validation in the clinical setting before widespread application.42,43
Treatment, Complications, and Disposition.: Complete fasciotomy is the only treatment that can reliably normalize elevated compartment pressure. Preparation for surgery is done as quickly as possible. Delaying fasciotomy for more than 12 hours often results in irreversible myonecrosis and nerve damage. While the patient is awaiting definitive treatment, the affected part should not be elevated above the level of the heart because this maneuver does not improve venous outflow and reduces arterial inflow. Slight dependency has been suggested to maximize the pressure head in the extremity.
Because tissue compartments are more pliable soon after injury, the rate of extremity swelling is greater in the immediate postinjury period, and tends to peak at 36 to 48 hours after injury.44 Extremity swelling decreases over a similar duration. Rhabdomyolysis, hyperkalemia, and myoglobinuria may occur and should be managed aggressively to avoid renal failure. Lactic acid also is released from necrotic muscle tissue. Other complications include infection and tissue loss.
Delayed treatment results in loss of nerve and muscle function and eventual contracture formation. The magnitude of these disasters may be measured by the fact that in 2004, the average indemnity award in cases of missed compartment syndrome was nearly $426,000. Awards were proportional to the delay beyond 8 hours in performing fasciotomy and the number of the five Ps indicating ischemia that were present during the initial missed diagnosis.45 For these reasons, when the diagnosis of compartment syndrome is confirmed, fasciotomy should be done without delay.
Avascular Necrosis
Because of their blood supply, certain bones may undergo avascular necrosis after fracture, especially if fractures are comminuted and go untreated for any length of time. The femoral head, talus, scaphoid, lunate, and capitate are particularly prone to this complication.46–49 These injuries are described in subsequent chapters.
Complex Regional Pain Syndromes (Reflex Sympathetic Dystrophy and Causalgia)
Definitions.: The terms reflex sympathetic dystrophy (RSD) and causalgia have been used to describe pain syndromes that sometimes follow fractures, orthopedic surgery, soft tissue injuries, and other unrecognized trauma to the limbs and their appendages. Other names previously used for this spectrum of post-traumatic injuries include Sudeck‘s atrophy, shoulder-hand syndrome, and postinfarction sclerodactyly. In an attempt to reduce misunderstanding about their etiology and treatment, the International Association for the Study of Pain (IASP) issued a consensus statement renaming the syndromes formerly called reflex sympathetic dystrophy and causalgia.50,51 Complex regional pain syndrome type 1 (CRPS-1), replacing the term reflex sympathetic dystrophy, is a pain syndrome that develops after an initiating noxious event, extends beyond the distribution of a single peripheral nerve, and is usually disproportionate to the inciting event. The site is most often the distal end of the affected extremity, with a distal-to-proximal gradient. It is associated with edema, changes in blood flow to the skin, abnormal sudomotor activity in the region of the pain, allodynia (pain resulting from non-noxious stimulation to the skin), hyperpathia (pain persisting or increasing after mild or light pressure), or hyperalgesia. The presence of a condition that otherwise would explain the degree of pain and dysfunction excludes the diagnosis of CRPS-1. Because so much of the literature still refers to RSD, this chapter still uses the terms RSD and CRPS-1 interchangeably. The definition of CRPS-2 is the same as for CRPS-1 except that there is demonstrable peripheral nerve injury. This term replaces causalgia under the IASP taxonomy.
Pathogenesis and Etiology.: The pathogenesis of CRPS-1 has not been elucidated. Current research suggests that central and peripheral sensitization after a noxious event, inflammation, alteration of sympathetic and catecholaminergic function, altered limb representation in the somatosensory cortex, genetic factors, and psychophysiologic interactions may all play a role.52 Cases of CRPS-1 have been reported after fractures and as iatrogenic complications of surgery or after minor procedures, including subcutaneous excision and intravenous injection. Forceful manipulations and tight casts also are alleged to have produced the syndrome. In 10 to 26% of patients, no inciting event is identified.53,54
Although malingering and secondary gain are suspected in some patients, they are not the causes in most patients, as evidenced by pathologic tissue changes in patients who actually have CRPS-1.55 CRPS-1 occurs in children and adolescents.56 Girls are affected three times as often as boys, and the median age is 12 years. The lower limb is affected twice as often as the upper, and history of inciting trauma can be identified in only half the children with the disorder.
Diagnosis.: No correlation exists between the severity of the original trauma and the incidence, severity, and cause of the symptoms, making early diagnosis a challenge, especially after trivial injury. Early diagnosis is crucial because the earlier treatment is initiated, the better the response. Although consensus-based diagnostic criteria were proposed by the IASP in 1994, because of their low specificity, there is no agreement on the best method for diagnosis of CRPS, and several sets of diagnostic criteria exist.57 Bruehl’s and Veldman’s criteria sought to improve the IASP criteria, but there is no reason to recommend one set of criteria over another (Table 49-6).57
Table 49-6
Diagnostic Criteria for Complex Regional Pain Syndrome (CRPS) Type 1*
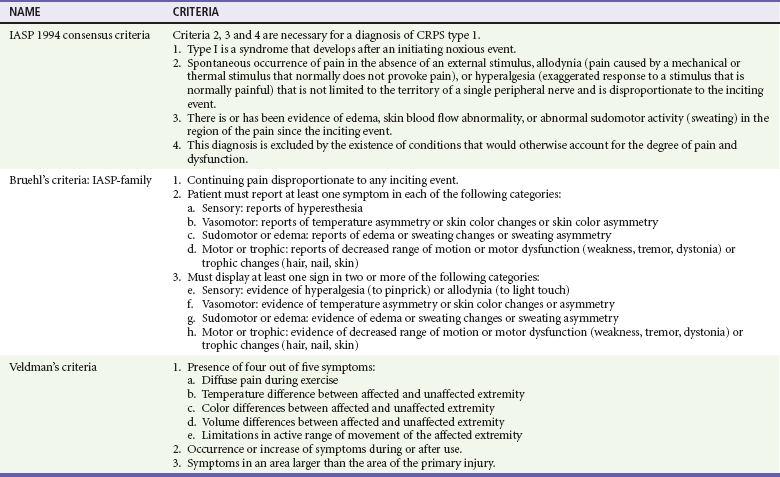
IASP, International Association for the Study of Pain.
*IASP definition of CRPS-1: A variety of painful conditions following injury that appear regionally, having a distal predominance of abnormal findings, exceeding in both magnitude and duration the expected clinical course of the inciting event and often resulting in significant impairment of motor function, and showing variable progression over time. (All three criteria sets use this definition).
From Quisel A, Gill JM, Witherell P: Complex regional pain syndrome underdiagnosed. J Fam Pract 54:524-532, 2005.
Treatment.: Treatment of CRPS is controversial. Debate arises because published randomized controlled trials provide limited evidence to formulate treatment recommendations; individual response to treatment varies; and experts disagree regarding the pathogenesis of the disease. A multidisciplinary approach, including physical therapy and psychological counseling, is often necessary for treatment of CRPS.58 For some patients, definitive treatment involves sympathetic blockade, usually with regional anesthesia and occasionally by surgical sympathectomy. Oral medications, including bisphosphonates, calcitonin, indomethacin, corticosteroids, tricyclic antidepressants, gabapentin, acupuncture, spinal cord stimulation, regional nerve blocks, and other medications and methods, have been used to treat RSD with variable success.59,60 In one study, vitamin C was shown to reduce the incidence of RSD after wrist fracture.61
Fat Embolism Syndrome
Fat embolism refers to the presence of fat globules in the lung parenchyma and peripheral circulation after a long bone fracture or major trauma.62 The phenomenon of fat embolization is probably common as a subclinical event after long bone fracture. Intravascular fat droplets appear in nearly one of five patients admitted with major trauma, although not all patients are symptomatic or require treatment.
Fat embolism syndrome is a serious manifestation of fat embolism, occurring most commonly after long bone fractures (usually tibia and fibula) in young adults and after hip fractures in elderly patients. Symptoms usually appear 1 to 2 days after an acute injury or after intramedullary nailing. Respiratory distress and hypoxemia are the earliest, most common manifestations. Acute respiratory distress syndrome (ARDS) may occur and is the usual cause of death. Neurologic involvement, manifesting as restlessness, confusion, or deteriorating mental status, also is an early sign, as are thrombocytopenia and a petechial rash. Fever, tachycardia, jaundice, retinal changes, and renal involvement may occur. Fat is seen in the urine in 50% of patients within 3 days of the injury. The incidence of full-blown fat embolism syndrome varies from 0.5 to 2% in patients with isolated long bone fractures to 5 to 10% in patients with multiple fractures. Management of fat embolism syndrome is primarily supportive, usually in an intensive care unit. The mortality rate is 20%, but most patients recover without severe sequelae.63 No specific therapy has shown benefit.
Fracture Blisters
Fracture blisters are tense blisters or bullae that accompany high-energy injuries in areas of relatively little skin coverage over a fracture site. The ankle, elbow, foot, and knee (in that order) are the most common sites; all of these contain fewer hair follicles and sweat glands to anchor together the epidermal-dermal junction than do other limb locations.64 Fracture blisters are believed, in many cases, to occur in the setting of increased underlying tissue pressure and may be a harbinger of compartment syndrome.
Early surgical intervention reduces the incidence of fracture blister formation.64 In addition, the presence of a fracture blister requires an alteration of the surgical approach or a delay in surgery. Most experts discourage incisions through a fracture blister because such incisions seem to increase infection and skin breakdown. Measures to perform early surgery after high-energy injuries and to minimize increases in tissue pressures might reduce the incidence of this complication. Intact blisters should be covered with povidone-iodine solution and a sterile dressing. Unroofing the blister and applying coverage with silver sulfadiazine paste has been reported to decrease the incidence of complications.64
Complications of Immobilization
Fractures frequently result in long periods of immobilization. Immobility may lead to multiple medical problems, especially in elderly patients, including pneumonia, deep venous thrombophlebitis, pulmonary embolism, urinary tract infection, wound infection, decubitus ulcers, muscle atrophy, stress ulcers, gastrointestinal hemorrhage, and psychiatric disorders (Box 49-5). Early ambulation is a major goal of optimal orthopedic care.
Damage Control Orthopedic Surgery
Over the past few decades, the management of the multiply injured trauma patient has changed considerably. Historically, patients with multiple injuries were treated nonoperatively as it was believed they were too ill to tolerate surgery. In the 1970s, literature began to appear suggesting adverse outcomes as a result of prolonged recumbency. In addition, operative fracture fixation techniques were evolving, and this lead to the advent of early fracture stabilization and the notion of early total care of the polytrauma patient.65 During the 1990s, the concept of damage control surgery came to the forefront of trauma surgical care. In a landmark paper, Rotondo and colleagues reported on the successful use of an abbreviated operation in patients with penetrating abdominal trauma to avoid the lethal triad of hypothermia, acidosis, and coagulopathy, and coined the phrase “damage control.”66 Similar principles were found to be applicable to the management of pelvic and long bone fractures in the polytrauma patient. Early total care with immediate definitive fixation of all major fractures saw a gradual move toward early temporary fracture stabilization, resuscitation of the patient to a stable physiologic state, then definitive fixation at a later time once the patient’s physiology had been stabilized.65 Temporary fracture stabilization is usually accomplished by application of external fixation devices to aid in hemorrhage control and tissue oxygenation, but the timing and optimal type of fracture surgery in the multiply injured trauma patient are still subjects of research.67
Subluxation and Dislocations
Abnormal forces applied to joints may result in the loss of continuity between two articulating surfaces. Partial loss of continuity is termed subluxation, and complete loss is termed dislocation. In general, dislocations are named for the major joint involved, as in a dislocated shoulder or hip. In three-bone joints, the injury is named for the joint involved if the disturbance involves the two major bones, or, if the lesser bone is involved, the disturbance is named for that bone. Separation of the femur from the tibia is termed dislocation of the knee, whereas displacement of the patella from its normal articulation is termed dislocation of the patella (Fig. 49-10). At the elbow, separation of the olecranon from the humerus is a dislocation of the elbow, whereas separation of the radius from the humerus is termed radial head dislocation.
Figure 49-10 Dislocation of the patella.
Dislocations and subluxations should be described according to the direction of the distal segment relative to the proximal segment or of the displaced bone relative to the normal structures. The injury shown in Figure 49-11 is termed dorsal dislocation of the interphalangeal joint of the thumb. Disruption of articulation also may occur in combination with a fracture. The term fracture-dislocation is used to describe this combination. If the overlying skin is broken in any way, dislocations, subluxations, or fracture-dislocations are described as open and constitute the same emergency as does an open fracture alone.
Treatment
Methods of relocating specific joints are reviewed in subsequent chapters, but a few principles apply. In general, the sooner a joint is relocated, the better. Later, swelling and muscle spasm make reduction more difficult. Also, pain is not adequately relieved until the dislocation is reduced. In the hip, early reduction is mandatory to restore vascular supply and to avert the complication of avascular necrosis. Before relocation is attempted, adequate analgesia or conscious sedation should be used. Nerve blocks are especially useful on the digits. The general principle of reducing a dislocation is to re-create and reverse the mechanism of injury, pulling the proximal end of the dislocated bone out and away from whatever is trapping it in its final resting place. This technique helps prevent interposition of soft tissues in the joint that can preclude reduction. As this maneuver is accomplished, the disarticulated surface is manipulated back or may snap back spontaneously toward its normal anatomic position. If the reduction is difficult, it should not be forced. A single good attempt is better than repeated attempts in an inadequately relaxed patient. Some joints cannot be reduced in the ED because (1) the opposing muscles are contracting too forcefully and general anesthesia is necessary to overcome these forces or (2) mechanical obstruction by a bony fragment or a torn piece of cartilage, tendon, joint capsule, or skin requires surgical removal for reduction to occur.68
Soft Tissue Injuries
Assessment
The clinical presentation of a sprain of the extremity may be indistinguishable from that of a fracture. The injury frequently occurs during vigorous athletic activity when forces applied in opposite directions result in a joint being stressed in an abnormal or exaggerated direction. The patient may complain of hearing a “snap” or a “pop” at the moment of injury and conclude that a fracture is present. Other patients report “seeing stars” or “almost passing out” at the moment the injury occurred and may still be in extreme pain, appearing pale and diaphoretic if seen shortly after the injury. Analgesia should be provided to these patients. Evaluation should include a careful history of the exact sequence of events at the time of the injury and ascertaining the position of the extremity and the forces applied to it at that moment. A history of any sounds that accompanied the injury should be elicited. Examination of the joint should include stressing it to show abnormal motion. If radiographs are planned to rule out a fracture anyway or if exquisite pain is produced by mild attempts to apply stress, it is probably better to delay stressing until films have verified the absence of a significant fracture. Plain radiography is indicated in some, but not all, cases to rule out a fracture. It has been well demonstrated that clinical decision rules can reduce the number of radiographic studies without missing significant fractures.69–72
Avulsion fractures may occur concomitantly with sprains. In children, epiphyseal fractures occur more commonly than ligamentous disruption because of the relative ligamentous strength compared with the ease of disrupting the epiphyses. Arthroscopy or MRI is indicated in the follow-up evaluation of some of these injuries (e.g., for suspected cruciate ligament tears) when significant pain or disability is present.73–75
Treatment and Disposition
Specific management of sprains varis depending on the location and severity of the injury. In general, initial measures should include the traditional recommendations of ice, elevation, and analgesia. Nonsteroidal anti-inflammatory drugs (NSAIDs) are effective analgesics in many patients. Several studies have found a more rapid decrease in swelling, increased exercise endurance, and earlier return to work with use of NSAIDs.76
Immobilization through use of one of the following methods provides protection and comfort in the initial management of most injuries. Because the severity of injury is sometimes difficult to establish at the first visit, it is reasonable to immobilize the affected joint for the first 48 to 72 hours, after which the extent of injury can be better determined. At that time, early mobilization is often desirable, particularly in lateral ankle injuries because this leads to earlier return to work and athletic activities and better preservation of proprioceptive neuromuscular function.77 Use of an inflatable “air cast” alone or in conjunction with an elastic ankle wrap has been shown to be effective in decreasing the symptomatic period.78 For lower extremity injuries, protected weightbearing with crutches provides patients with comfort and avoids motion of the impaired part. In elderly patients, safe ambulation sometimes cannot be accomplished, and a short hospitalization or admission to a skilled nursing facility may be necessary.
Strains
A first-degree (mild) strain is a minor tearing of the musculotendinous unit, characterized by minor swelling, local tenderness, and minimal restriction of movement. Findings increase along a continuum such that in a second-degree (moderate) strain, more fibers are torn, but without complete disruption; swelling, ecchymosis, and loss of strength are more marked. In a third-degree (severe) strain, the muscle or tendon is completely disrupted, with resultant separation of muscle from muscle, muscle from tendon, or tendon from bone.79 An accompanying avulsion fracture may be present on radiographs in either second- or third-degree injuries.
Assessment
Signs and symptoms include pain, ecchymosis, swelling, and loss of function. A force applied to the muscle, either passive stress or active contraction, produces sharp pain at the site of injury even as the injured muscle may be relatively comfortable at rest. A palpable defect sometimes is present at the site of a complete rupture, which usually involves the region of the muscle-tendon junction, or a bunching up of the muscle may be appreciated. Ultrasound is increasingly being used to diagnose an assortment of soft tissue injuries, including rotator cuff tears, tendon ruptures, and muscle tears.80,81 Among nonathletes, strains commonly are seen in patients who have either overstressed a muscle group or tried to generate excessive force in an unconditioned muscle. Examples are the weekend gardener or mover who experiences lower back strain on Monday morning, the aerobics student who strains the rectus muscles, and the weightlifter with chest wall pain resulting from pectoralis major strain. These are usually first-degree injuries, and the onset is slow. Rapid acceleration (e.g., in a tennis player) may result in a third-degree gastrocnemius or plantaris tear, whereas pushing off to jump is a common cause of ruptures of the Achilles tendon in a basketball player. A sudden violent attempt at lifting in an older individual can result in a complete biceps brachii disruption. Sudden generation of forces of which the thighs are capable results in second-degree strain of the hamstrings, quadriceps, or thigh abductor muscles.
Treatment and Disposition
Treatment depends on the degree of disruption, location, and functional loss. Most first-degree injuries respond in a few days to rest, application of ice, and, for some patients, analgesics. NSAIDs commonly are recommended and prescribed, although their efficacy for other than analgesic purposes is unproven. Second-degree strains are treated similarly, with protection against aggravating activity required for longer periods. Third-degree strains receive similar initial treatment in the ED plus early orthopedic consultation. Some of these injuries are amenable to surgical repair, whereas others may be treated with immobilization. The muscle affected and the age, occupation, and activity level of the patient all are factors in deciding whether surgical intervention is appropriate. Early mobilization is an important tenet in the treatment of muscle strains; its timing may be based on the ability to stretch the injured muscle as much as the uninjured contralateral muscle, and the use of the injured muscle without pain during basic movements.79 Many athletes and their trainers believe, and it is universally espoused, that many strains can be prevented by proper preseason conditioning, warm-up, stretching exercises, and avoidance of overexertion, although limited scientific data exist to support these recommendations.82
Tendinitis and Tendinosis
Tendinitis is classically described as an inflammatory condition characterized by pain at tendinous insertions into bone, occurring in the setting of overuse.83,84 It is now believed that the pathophysiology of this condition is more complex than mere overuse, with the roles of load and use affecting cell-matrix interaction.85 Causative factors are believed to include aging, with decreased blood supply and decreased tensile strength; muscle weakness and imbalance; insufficient flexibility; male gender; obesity (in weight-bearing joints); smoking; malalignments; training errors; and improper equipment.86,87 In addition, certain systemic diseases, including diabetes mellitus, chronic renal failure, rheumatoid arthritis, and systemic lupus erythematosus; steroid use; and occasionally fluoroquinolone use are associated with the development of tendinopathy.88
The histopathology of tendinitis is characterized by degeneration and disorganization of collagen fibers; infiltration by macrophages, plasma cells, and lymphocytes rather than leukocytes; and increased vascularity. Inflammatory changes are not a principal finding in tendinitis.89 This evolving understanding of tendinitis in the future should allow for more logical treatment of these injuries aimed at the underlying pathophysiology. It also has led some authors to propose that chronic painful conditions of the tendon should be referred to as tendinosis rather than tendinitis or other terms previously used to describe this condition, including tendonitis, degenerative changes, chronic tendinopathy, or partial rupture.86,90 In this chapter, tendinitis and tendinosis are used interchangeably.
Common sites for tendinitis are the rotator cuff of the shoulder, the Achilles tendon, the radial aspect of the wrist (de Quervain’s tenosynovitis), and the insertion of the hand extensors on the lateral humeral epicondyle (tennis elbow). Also commonly involved in athletes are the patellar tendon, particularly in athletes engaged in jumping sports; the biceps femoris, semitendinosus, and semimembranosus (hamstring syndrome); the posterior tibial tendon (shin splint syndrome); the iliotibial band; and the common wrist flexors (medial epicondylitis) (involvement is seen in little league pitchers and golfers).85 In some locations, most commonly the shoulder, calcium deposition occurs along the course of the tendon, resulting in a painful condition termed calcific tendinitis. This condition also may occur in the wrist, hand, neck, hip, knee, ankle, or foot.91
Physical examination reveals pain with motion and limitation of function and may include point tenderness and palpable crepitus over the involved tendon with motion. In general, a clinical test can be performed by forcible flexion of the involved muscle while keeping the point of insertion fixed or by operating the involved muscle against resistance. Either test should intensify the discomfort. Radiographs are usually negative. A small fleck of bone may suggest an avulsion, or the surface of the bone at the attachment may be roughened, indicating periostitis. As mentioned, there also may be calcium deposits along the course of the tendon, which should not be confused with an avulsion fracture. Ultrasound is sometimes useful in confirming the diagnosis of tendinitis. Although a normal tendon is characterized by a relatively homogeneous pattern, tendinitis is characterized by one or more of the following features: loss of the fibrillar echotexture, focal tendon thickening, diffuse thickening, focal hypoechoic area, irregular or ill-defined borders, or microruptures.92
There is little evidence to support any specific treatment for tendinosis. The classic approach consists of rest, ice, and NSAIDs initially, followed by rehabilitation and training and control of force loads to prevent recurrences. Although NSAIDs may be useful for a brief period at the onset of symptoms for their analgesic effects, no evidence exists that they significantly alter the pathophysiology of this condition, and no rationale exists for ordering them in patients at any risk for complications from this class of drugs.93,94 Peritendinous local infiltrations of anesthetics and corticosteroids may be useful but should not be repeated too often because tendon rupture may occur. Injection therapy is especially useful in calcific tendinitis around the shoulder. Injection of steroids directly into the Achilles tendon should be avoided because of reports of partial or complete rupture after even a single injection. Some cases of calcific tendinitis that do not respond to conservative therapy may require either arthroscopic or open surgery.95
Bursitis
Bursitis is a painful inflammation of the bursa that may be traumatic, infectious, or related to systemic illness. Commonly involved sites include the olecranon, the greater trochanter of the femur, and the prepatellar and anserine bursae around the knee. Physical findings are tenderness and swelling over the involved bursa, whereas warmth and overlying erythema may signal infection. If infection is suggested, aspiration of the bursal fluid and Gram’s staining and culture are recommended. Otherwise, treatment may be conservative and is similar to treatment for tendinitis, with ice, NSAIDs, or steroid injections. Most patients can be treated as outpatients.96
Treatment Modalities
Field Care
Splinting should begin in the field because it reduces the risk of further neurovascular compromise, prevents a closed injury from potentially being converted to an open one during transport, reduces the patient’s pain, and facilitates subsequent ED assessment and imaging. Numerous commercial devices are available, and most ambulances carry an assortment of immobilization devices (Fig. 49-12). Minimal equipment includes long and short backboards, cervical collars, sandbags, and extremity splints. A half-ring traction splint also is essential. Inflatable air splints are favored by some authors because they are convenient, easy to apply, transparent, and radiolucent and because they tamponade low-pressure bleeding. Other authors prefer to avoid these devices because theoretically they could contribute to the development of a compartment syndrome. If used, inflatable splints should be inflated only by mouth and to the point that still permits indentation by gentle finger pressure.
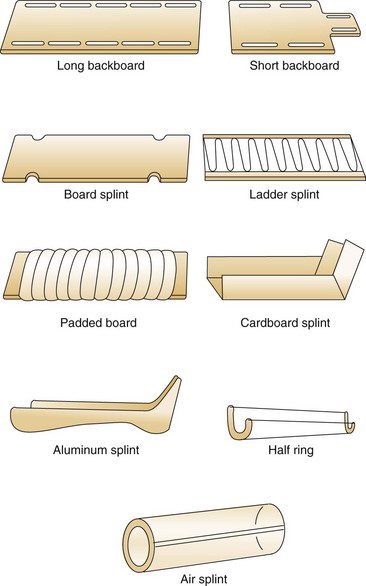
Figure 49-12 Commercial splints.
Upper Extremity
Sling-and-Swathe and Velpeau Bandages.: Sling-and-swathe and Velpeau bandages are useful in immobilizing the shoulder, humerus, and elbow. They are commonly used after reduction of dislocated shoulders and to treat impacted fractures of the humeral neck. The axillae should be padded and powdered to avoid skin maceration. A commercial shoulder immobilizer also is available and is useful after reduction of a shoulder dislocation. Its advantages are ease of application and ease of removal and reapplication by the patient for bathing.
Clavicle Splint.: Historically, middle third fractures of the clavicle routinely were initially treated with a figure-of-eight clavicle strap with or without the addition of a sling. This device is commercially available or can be fashioned from tubular stockinette. If used, a clavicle splint should be applied snugly enough to keep the shoulders back in the “at-attention” position, but not so tight as to compress the axillary artery or brachial plexus. Chafing of the skin can be avoided by padding and powdering the axillae. Superiority of the figure-of-eight clavicle strap over a simple sling has not been shown.
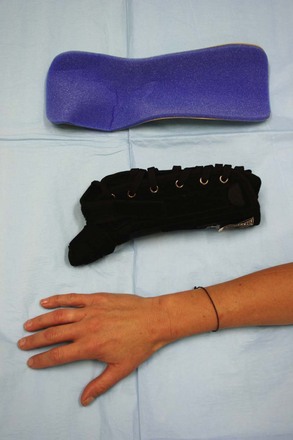
Plaster and Fiberglass Splints.: Well-fitting, customized plaster splints can be fashioned easily to immobilize the elbow, forearm, wrist, and hand. The advantage of these splints is the ability to mold them to an exact size and shape (e.g., along the ulnar side of the forearm and hand to immobilize a midshaft fourth or fifth metacarpal fracture, the so-called “gutter splint”). Several commercially available products consist of multiple layers of plaster or fiberglass strips, inside a covering of foam and flannel, on a continuous roll that can be applied to any length. While the splint is still wet, a bandage is wrapped over it, and the splint is molded and held in the desired position as the plaster or fiberglass resin hardens.
Lower Extremity
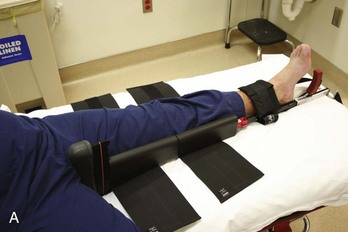
Femur and Hip.: Fractures of the femoral shaft can be immobilized with a traction device, such as the Hare traction splint or a similar appliance (Fig. 49-14). These devices should be applied in the field if possible; most ambulances carry them. The principle is that a proximal ring engages the ischial tuberosity for countertraction while the longitudinal traction is applied through the ankle by means of an ankle hitch. A commercial ankle hitch is recommended, but if one is not available, an improvised hitch can be fashioned with a triangular bandage or a wide piece of cloth tied in a Collins hitch. The patient’s ankle bones, Achilles tendon, and arch of the foot should be padded, and the circulation should be checked to ensure it is intact. A properly applied splinting device relieves pain from a fracture rather than exacerbating it.
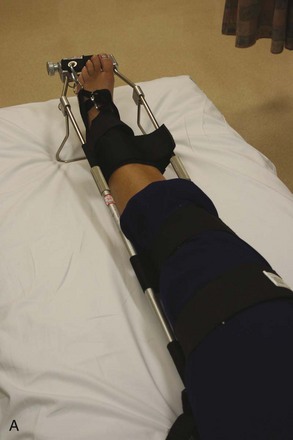
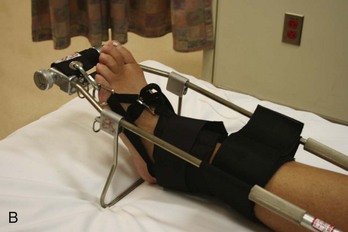
Figure 49-14 A, Hare traction splint. B, The commercial hitch is designed to protect the ankle and heel during traction.
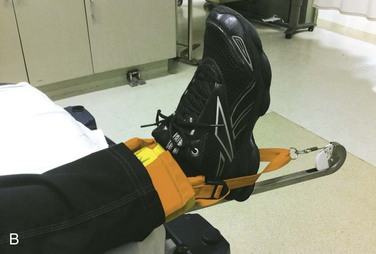
The Sager splint might offer advantages over other appliances in that it is applied to the medial and lateral aspect of the thigh rather than having a half-ring posteriorly (Fig. 49-15). The Sager splint is more acceptable for use in the presence of pelvic fractures and avoids compression of the sciatic nerve. Because the half-ring devices may produce angulation at the fracture site, the Sager device is purported to result in better alignment, although this has never been measured. The Sager splint is shorter and more compact than the Hare traction splint, rendering it more acceptable for certain transport helicopters and body scanners. Also, the amount of traction is metered at the ankle, and overtraction can be avoided.
Knee.: Commercially available knee immobilizers can be used after acute injuries to provide firm but not rigid stabilization of the knee. The device is essentially a foam cylinder with medial and lateral aluminum stays, attached by Velcro straps, and spanning the upper thigh to upper ankle. This device is commonly used after trauma to let the knee “cool off” until a better physical examination or diagnostic study can be performed in a few days.
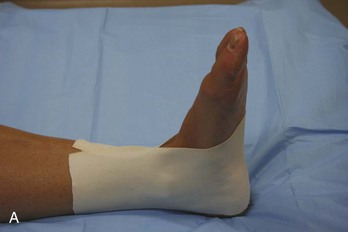
Ankle.: Immobilization of the ankle can be accomplished by numerous means. Plaster splints can be used temporarily for the treatment of nondisplaced ankle fractures or for the treatment of severe sprains. These can be fashioned in the same manner as described for the upper extremity. An alternative method is to apply a full circular cast, bivalve it on either side, discard the anterior piece, and affix the posterior mold with an elastic bandage or bias-cut stockinette. Most ankle injuries should be splinted with the patient’s ankle in neutral position. Injuries to the Achilles tendon, plantaris muscle, or gastrocnemius muscle initially should be treated with the foot held in slight equinus (plantar flexion) for comfort. The toes should be free to move distal to the metatarsophalangeal joints, and the proximal border should end below the tibial tubercle to avoid pressure on the peroneal nerve.
Adhesive strapping is an alternative method of ankle immobilization that provides good support and limitation of motion. Taping reportedly loses its “protective properties” with cyclic loading and sweating; although this is cited as a disadvantage, it may actually be helpful in encouraging and allowing early mobilization. This method is lightweight and not bulky, and a shoe can be worn over the material. Tape is applied in a noncontinuous manner, which allows for swelling and avoids constriction. First, the hair is shaved. Next, strips of tape are measured and torn off;  -inch or 2-inch cloth-backed adhesive tape or Elastoplast is used. Elastoplast is an elastic-backed tape, constructed to stretch only in the longitudinal direction; this serves to spring the foot back automatically to a neutral position if the foot is plantar-flexed for any reason. The tape should be applied directly to the skin after a skin adherent, such as tincture of benzoin, is applied. The tape should lie flat because wrinkles may damage the skin (Fig. 49-16). The use of tape is associated with dermatologic complications, including itching or contact dermatitis, owing to adhesion of the tape to the skin.
-inch or 2-inch cloth-backed adhesive tape or Elastoplast is used. Elastoplast is an elastic-backed tape, constructed to stretch only in the longitudinal direction; this serves to spring the foot back automatically to a neutral position if the foot is plantar-flexed for any reason. The tape should be applied directly to the skin after a skin adherent, such as tincture of benzoin, is applied. The tape should lie flat because wrinkles may damage the skin (Fig. 49-16). The use of tape is associated with dermatologic complications, including itching or contact dermatitis, owing to adhesion of the tape to the skin.
For mild-to-moderate sprains, functional treatment options including elastic bandaging, soft cast, taping, or orthoses were found to be statistically better than immobilization for multiple outcome measures.97 Lace-up supports may be more effective than elastic bandaging and result in less persistent swelling in the short term as compared with semirigid ankle supports, elastic bandaging, and tape.98
For moderate-to-severe lateral ankle sprains, a commercial mechanical support composed of molded plastic with Velcro straps (e.g., AirCast Air-Stirrup) is more effective than elastic bandaging alone (Fig. 49-17).77,99 This product may be worn in the patient’s shoe and permits early weightbearing and return to activity. It is designed to permit dorsiflexion and plantar flexion but to limit inversion and eversion, a concept referred to as functional bracing. Some authors also find this device useful for athletic activities instead of adhesive taping to prevent recurrences.78,100 Although these orthoses are relatively expensive, the cost might be more than offset by their ease of application, their reusability, and the benefit derived from earlier return to work.
Casts
Plaster or synthetic (fiberglass) casts perform a function similar to splints in that they provide stability and pain relief. Casts are not mandatory for all fractures, and in situations in which they are, application is usually not an immediate necessity. Because they are circumferential, casts provide more effective immobilization of a fracture, but they require more skill and time to apply. Swelling and subsequent pressure under the cast are highest during the first 24 hours after injury. Complications of casting include compartment syndrome, thermal injury, pressure sores, bacterial and fungal infections (especially if a wound is present under the casted area), and pruritic dermatitis.101 Plaster is applied as strips or rolls of cloth that are impregnated with a hemihydrate of calcium sulfate. When this cloth is dipped in lukewarm water, a creamy paste is formed that can be molded into a cast. An exothermic reaction takes place that causes the plaster to harden and can burn the skin.102 Factors that have been shown experimentally to increase skin temperatures during plaster application are dip-water temperatures greater than 24° C, cast thickness greater than eight plies, and inadequate ventilation of the newly applied cast.103 Immersing the plaster in water for too short a time or squeezing too much water out also may lead to generation of excess heat. To avoid pressure on the skin and over bony prominences, stockinette and layers of cotton sheet wadding (Webril) are snugly applied first. Padding that migrates under a formed cast can be uncomfortable and result in pressure sores. Padding alone does not prevent burns.
Variations of the basic cast exist. A window may be placed in the cast, and the cutout area may be used for access to skin wounds that require care during immobilization. Walking heels may be worked onto a lower extremity cast and should be placed in the center of the foot. Synthetic casts (fiberglass and other materials) are lightweight, durable, and water-resistant.104 In addition, their setting temperatures are significantly lower, and they are less likely to produce burns. However, they are more expensive and more difficult to apply.
The need for mandatory routine cast checks 1 day after initial application has been questioned.105 In a retrospective study of 250 patients, none experienced problems from neurovascular compression, although 24% required some alteration of the cast. In the study, it may be simply that the casts were applied incorrectly in the first place. It is probably advisable to continue routine cast checks if casts are applied in the ED.
Thermal Therapy
Some confusion exists as to the role of cryotherapy versus heat therapy in the treatment of acute orthopedic injuries. Part of this confusion arises because heat may be more soothing to the patient. Cold causes vasoconstriction, limiting blood flow and hemorrhage into the traumatized area. Metabolic requirements are reduced in cooled tissues, as is histamine, and less capillary breakdown occurs as a result. The sooner postinjury ice therapy is initiated, the more beneficial the reduction in metabolism will be.106 Reduced blood flow also limits edema formation. Lower extravascular fluid pressure allows for better lymphatic drainage of injured areas. Cryotherapy produces three, and perhaps four, stages of sensation of which the physician and the patient should be aware. In the first stage, a cold sensation is noted for 1 to 3 minutes. The second stage consists of a burning or aching sensation for 2 to 7 minutes after the application of cold. This stage is uncomfortable but needs to be endured if the benefits of the next two stages are to be received. Heat therapy, by contrast, is soothing, and patients are likely to prefer this to the second stage of cryotherapy. The third stage begins 5 to 12 minutes after the application of ice and produces local numbness or anesthesia. The pain-spasm cycle is interrupted. While the patient is under this anesthetic effect, passive exercise may be desirable. This exercise helps to prevent atrophy, mobilize edema, clear injury debris, and reduce adhesions. However, several reviews of cryotherapy for soft tissue injury all reflect the lack of adequate science in establishing recommendations for cryotherapy. Application of ice appears to be effective in reducing pain, but there is no credible evidence that it accelerates healing.107–109
A fourth stage sometimes occurs 12 to 15 minutes after intense cryotherapy is begun, consisting of reflex deep tissue vasodilation without a corresponding increase in metabolism (reminiscent of the situation in rewarming shock). Because of this, a maximum of 10 to 15 minutes of cold application per treatment usually is advised. A systematic review of small, limited-quality studies assessed cryotherapy in the management of soft tissue injuries noted wide variation on the optimal mode, duration, and frequency of ice application.109 Therefore recommendations for an optimal cryotherapy protocol cannot be made.
Autologous Blood Product and Growth Factors
In recent years, growth factors have been used for the management of soft tissue injury related to acute trauma and overuse injury. Platelets contain growth factors in their alpha-granules, including insulinlike growth factor-1, basic fibroblast growth factor, platelet-derived growth factor, epidermal growth factor, vascular endothelial growth factor, and transforming growth factor-β1, and these are thought to help the regeneration of tissues that otherwise have low healing potential.110,111 Growth factors can be delivered as a preparation of platelet rich plasma (PRP), autologous blood injection, and autologous conditioned serum. PRP injections have been described in patients with patellar and elbow tendinosis, Achilles tendon injury, and acute ligamentous and muscle injuries, and they have also been administered intraoperatively during anterior cruciate ligament reconstruction and rotator cuff repair.112–114 Theoretic advantages to the use of PRP include faster recovery and improved functional outcome, but evidence supporting the use of PRP in different tissues is limited and further studies are required before conclusions are drawn.
References
1. Raasch, WG, Hergan, DJ. Treatment of stress fractures: The fundamentals. Clin Sports Med. 2006;25:29.
2. Dugan, SA, Weber, KM. Stress fractures and rehabilitation. Phys Med Rehabil Clin North Am. 2007;18:401.
3. Datir, AP. Stress related bone injury with emphasis on MRI. Clin Radiol. 2007;62:828.
4. de Sancis, N, Della Corte, S, Pempinello, C. Orthopaedics distal tibial and fibular epiphyseal fractures in children: Prognostic criteria and long-term results in 158 patients. J Pediatr Orthop. 2000;9:40.
5. Sferopoulos, NK. Type V physeal injury. J Trauma. 2007;63:E121.
6. Close, BJ, Strouse, PJ. MR of physeal fractures of the adolescent knee. Pediatr Radiol. 2000;30:756.
7. Green, NE, Swiontkowski, MF. Skeletal Trauma in Children, 4th ed. Philadelphia: Elsevier; 2009.
8. Laine, JC, Kaiser, SP, Diab, M. High-risk pediatric orthopedic pitfalls. Emerg Med Clin North Am. 2010;28:85–102.
9. Khosla, S, et al. Incidence of childhood distal forearm fractures over 30 years: A population based study. JAMA. 2003;290:1479–1485.
10. Barmada, A, Gaynor, T, Mubarak, SJ. Premature physeal closure following distal tibia physeal fractures: A new radiographic predictor. J Pediatr Orthop. 2003;23:733–739.
11. Dowling, S, Farion, K, Clifford, T. Comparison views to diagnose elbow injuries in children: A survey of Canadian non-pediatric emergency physicians. CJEM. 2005;7:237.
12. Keats, TE, Anderson, MW. Atlas of Normal Roentgen Variants That May Simulate Disease, 7th ed. St Louis: Mosby; 2001.
13. Diagnostic imaging of child abuse. Pediatrics. 2000;105:1345.
14. El-Khoury, GY, Bennet, DL, Ondr, GJ. Multidetector-row computed tomography. J Am Acad Orthop Surg. 2004;12:1–5.
15. Buckwalter, KA, Rydberg, J, Kopecky, KK, Crow, K, Yang, EL. Musculoskeletal imaging with multislice CT. AJR Am J Roentgenol. 2001;176:979–986.
16. You, JS, et al. The usefulness of CT for patients with carpal bone fractures in the emergency department. Emerg Med J. 2007;24:248–250.
17. Watura, R, Cobby, M, Taylor, J. Multislice CT in imaging of trauma of the spine, pelvis and complex foot injuries. Br J Radiol. 2004;77:S46–S63.
18. Legome, E, Pancu, D. Future applications for emergency ultrasound. Emerg Med Clin North Am. 2004;22:817–827.
19. McManus, JG, et al. Use of ultrasound to assess acute fracture reduction in emergency care settings. Am J Disaster Med. 2008;3:241–247.
20. Gosselin, RA, Roberts, I, Gillespie, WJ. Antibiotics for preventing infection in open limb fractures. Cochrane Database Syst Rev. 2004;1:CD003764.
21. Sorger, JI, et al. Once daily, high dose versus divided, low dose gentamicin for open fractures. Clin Orthop Relat Res. 1999;366:197.
22. Stewart, DG, Jr., Kay, RM, Skaggs, DL. Open fractures in children. Principles of evaluation and management. J Bone Joint Surg Am. 2005;87:2784–2798.
23. Schmidt, AH, Anglen, J, Nana, AD, Varecka, TF. Adult trauma: Getting through the night. J Bone Joint Surg Am. 2010;92:490–505.
24. Webb, LX, Bosse, MJ, Castillo, RC, LEAP Study Group. Analysis of surgeon-controlled variables in the treatment of limb-threatening type-III open tibial diaphyseal fractures. J Bone Joint Surg Am. 2007;89:923–928.
25. Stevenson, J, McNaughton, G, Riley, J. The use of prophylactic flucloxacillin in the treatment of open fractures of the distal phalanx within an accident and emergency department: A double-blind randomized placebo controlled trial. J Hand Surg Br. 2003;28:388.
26. Hollis, JD, Daley, BJ. 10-year review of knee dislocations: Is arteriography always necessary? J Trauma. 2005;59:672–675.
27. Peng, PD, et al. CT angiography effectively evaluates extremity vascular trauma. Am Surg. 2008;74:10.
28. Ruijs, AC, Jaquet, JB, Kalmijn, S, Giele, H, Hovius, SE. Median and ulnar nerve injuries: A meta-analysis of predictors of motor and sensory recovery after modern microsurgical nerve repair. Plast Reconstr Surg. 2005;116:484.
29. Tindall, A, Dawood, R, Povlsen, B. Case of the month: The skin wrinkle test: A simple nerve injury test for paediatric and uncooperative patients. Emerg Med J. 2006;23:883–886.
30. Vasudevan, TM, van Rij, AM, Nukada, H, Taylor, PK. Skin wrinkling for the assessment of sympathetic function in the limbs. Aust N Z J Surg. 2000;70:57–59.
31. McQueen, MM, Gaston, P, Court-Brown, CM. Acute compartment syndrome. Who is at risk? J Bone Joint Surg Br. 2000;82:200–203.
32. Konstantakos, EK, Dalstrom, DJ, Nelles, ME, Laughlin, RT, Prayson, MJ. Diagnosis and management of extremity compartment syndromes: An orthopaedic perspective. Am Surg. 2007;73:1199.
33. Rozycki, GS, Tremblay, LN, Feliciano, DV, McClelland, WB. Blunt vascular trauma in the extremity: Diagnosis, management and outcome. J Trauma. 2003;55:814.
34. Andermahr, J, Helling, HJ, Tsironis, K, Rehm, KE, Koebke, J. Compartment syndrome of the foot. Clin Anat. 2001;14:184.
35. King, TW, Lerman, OZ, Carter, JJ, Warren, SM. Exertional compartment syndrome of the thigh: A rare diagnosis and literature review. J Emerg Med. 2010;39:93–99.
36. Newton, EJ, Love, J. Acute complications of extremity trauma. Emerg Med Clin North Am. 2007;25:751.
37. Dua, RS, Bankes, MJ, Down, GS, Lewis, AA. Compartment syndrome following pelvic surgery in the lithotomy position. Ann R Coll Surg Engl. 2002;84:170–171.
38. Nassif, JM, Gorczyca, JT, Cole, JK, Pugh, KJ, Pienkowski, D. Effect of acute reamed versus unreamed intramedullary nailing on compartment pressure when treating closed tibial shaft fractures: A randomized prospective study. J Orthop Trauma. 2000;14:554–558.
39. Olson, SA, Glasgow, RR. Acute compartment syndrome in lower extremity musculoskeletal trauma. J Am Acad Orthop Surg. 2005;13:436.
40. Elliott, KG, Johnstone, AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br. 2003;85:625–632.
41. Perron, AD, Brady, WJ, Keats, TE. Orthopedic pitfalls in the ED: acute compartment syndrome. Am J Emerg Med. 2001;19:413–416.
42. Boody, AR, Wongworawat, MD. Accuracy in the measurement of compartment pressures: A comparison of three commonly used devices. J Bone Joint Surg Am. 2005;87:2415–2422.
43. Tobias, JD, Hoernschyer, DG. Near infrared spectroscopy identifies compartment syndrome in an infant. J Pediatr Orthop. 2007;27:311.
44. Tull, F, Borrelli, J, Jr. Soft-tissue injury associated with closed fractures: Evaluation and management. J Am Acad Orthop Surg. 2003;11:431–438.
45. Battcharya, T, Vrahas, MS. The medical legal aspects of compartment syndrome. J Bone Joint Surg Am. 2004;86:864.
46. Lauder, AJ, Trumble, TE. Idiopathic avascular necrosis of the scaphoid: Preiser’s disease. Hand Clin. 2006;22:475.
47. Kerachian, MA, Harvey, EJ, Cournoyer, D, Chow, TY, Séguin, C. Avascular necrosis of the femoral head: Vascular hypotheses. Endothelium. 2006;13:237.
48. Rammelt, S, Winkler, J, Grass, R, Zwipp, H. Reconstruction after talar fractures. Foot Ankle Clin. 2006;11:61.
49. Niesten, JA, Verhaar, JA. Idiopathic avascular necrosis of the capitate—a case report and review of the literature. Hand Surg. 2002;7:159.
50. Stanton-Hicks, M, et al. Reflex sympathetic dystrophy: Changing concepts and taxonomy. Pain. 1995;63:127–133.
51. Albazaz, R, Wong, YT, Homer-Vanniasinkam, S. Complex regional pain syndrome: A review. Ann Vasc Surg. 2008;22:297.
52. Bruehl, S. Modifying diagnostic criteria for complex regional pain syndrome. Pain. 2010;150:217–218.
53. Marinus, J, et al. Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol. 2011;10:637–648.
54. Schott, GD. Complex? Regional? Pain? Syndrome? Pract Neurol. 2007;7:145–157.
55. Bruehl, S. An update on the pathophysiology of complex regional pain syndrome. Anesthesiology. 2010;113:713–725.
56. Low, AK, Ward, K, Wines, AP. Pediatric complex regional pain syndrome. J Pediatr Orthop. 2007;27:567–572.
57. Quisel, A, Gill, JM, Witherell, P. Complex regional pain syndrome underdiagnosed. J Fam Pract. 2005;54:524–532.
58. Oerlemans, HM, Oosteodorp, RA, de Boo, T, Goris, RJ. Pain and reduced mobility in complex regional pain syndrome I: Outcome of a prospective randomized, controlled clinical trial of adjuvant physical therapy versus occupational therapy. Pain. 1999;83:77.
59. Perez, RS, et al. Evidence based guidelines for complex regional pain syndrome type 1. BMC Neurol. 2010;10:20.
60. van de Vusse, AC, Stomp-van den Berg, SG, Kessels, AH, Weber, WE. Randomised controlled trial of gabapentin in complex regional pain syndrome type 1. BMC Neurol. 2004;4:13.
61. Zollinger, PE, Tuinebreijer, WE, Kreis, RW, Breederveld, RS. Effect of vitamin C on frequency of reflex sympathetic dystrophy in wrist fractures: A randomised trial. Lancet. 1999;354:2025.
62. Imberti, R, Scagnelli, P, Amatu, A. Post-traumatic pulmonary and cerebral fat embolism. Anaesth Int Care. 2008;36:619.
63. Memtsoukis, SG, Rosenberger, P, Walz, JM. Critical care issues in the patient after major joint replacement. J Int Care Med. 2007;22:92.
64. Strauss, EJ, Petrucelli, G, Bong, M, Koval, KJ, Egol, KA. Blisters associated with lower extremity fracture: Results of a prospective treatment protocol. J Orthop Trauma. 2006;20:618.
65. Bose, D, Tejwani, NC. Evolving trends in the care of polytrauma patients. Injury. 2006;37:20–28.
66. Rotondo, MF, et al. ‘Damage control’: An approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35:375–383.
67. Pape, HC, Giannoudis, P, Krettek, C. The timing of fracture treatment in polytrauma patients: Relevance of damage control orthopedic surgery. Am J Surg. 2002;183:622–629.
68. Worth, C, Harris, A. Non-reducible hip dislocation in the ED. Emerg Med J. 2009;26:42.
69. Stiell, IG, et al. A study to develop clinical decision rules for the use of radiography in acute ankle injuries. Ann Emerg Med. 1992;21:384.
70. Stiell, IG, et al. Decision rules for the use of radiography in acute ankle injuries: Refinement and prospective validation. JAMA. 1993;269:1127.
71. Stiell, IG, et al. Implementation of the Ottawa ankle rules. JAMA. 1994;271:827.
72. Bachmann, LM, Kolb, E, Koller, MT, Steurer, J, ter Riet, G. Accuracy of Ottawa ankle rules to exclude fractures of the ankle and mid-foot: Systematic review. BMJ. 2003;326:417.
73. Anderson, SJ. Lower extremity injuries in youth sports. Pediatr Clin North Am. 2002;49:627–641.
74. Schub, D, Saluan, P. Anterior cruciate ligament injuries in the young athlete: Evaluation and treatment. Sports Med Arthrosc. 2011;19:34–43.
75. Emery, KH. Imaging of sports injuries of the upper extremity in children. Clin Sports Med. 2006;25:543–568.
76. Ivins, D. Acute ankle sprain: An update. Am Fam Physician. 2006;15:1714.
77. Jones, MH, Amendola, AS. Acute treatment of inversion ankle sprains: Immobilization versus functional treatment. Clin Orthop Relat Res. 2007;455:169.
78. Boyce, SH, Quigley, MA, Campbell, S. Management of ankle sprains: A randomized controlled trial of the treatment of inversion injuries using an elastic support bandage or an Aircast ankle brace. Br J Sports Med. 2005;39:91.
79. Järvinen, TA, Kääriäinen, M, Järvinen, M, Kalimo, H. Muscle strain injuries. Curr Opin Rheumatol. 2000;12:155–161.
80. Lew, HL, Chen, CP, Wang, TG, Chew, KT. Introduction to musculoskeletal diagnostic ultrasound: Examination of the upper limb. Am J Phys Med Rehabil. 2007;86:310.
81. Bianchi, S, Poletti, PA, Martinoli, C, Abdelwahab, IF. Ultrasound appearance of tendon tears. Part 2: Lower extremity and myotendinous tears. Skeletal Radiol. 2006;35:63.
82. Heidt, RS, Jr., Sweeterman, LM, Carlonas, RL, Traub, JA, Tekulve, FX. Avoidance of soccer injuries with preseason conditioning. Am J Sports Med. 2000;28:659–662.
83. Rettig, AC. Wrist and hand overuse syndromes. Clin Sports Med. 2001;20:591.
84. Sharma, P, Maffuli, N. Biology of tendon injury: Healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6:1818.
85. Scott, A, Ashe, MC. Common tendinopathies in the upper and lower extremities. Curr Sports Med Rep. 2006;5:233.
86. Alfredson, A, Lorentzon, R. Chronic Achilles tendinosis: Recommendations for treatment and prevention. Sports Med. 2000;29:135.
87. Fenwick, SA, Hazleman, BL, Graham, PR. The vasculature and its role in the damaged and healing tendon. Arthritis Res. 2002;4:252.
88. Corrao, G, et al. Evidence of tendinitis provoked by fluoroquinolone treatment: A case-control study. Drug Saf. 2006;8:889.
89. Khan, KM, Cook, JL, Bonar, F, Harcourt, P, Astrom, M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393–408.
90. Maffulli, N, Wong, J, Almekinders, LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22:675.
91. Harvie, P, Pollard, TC, Carr, AJ. Calcific tendinitis: Natural history and association with endocrine disorders. J Shoulder Elbow Surg. 2007;16:169.
92. Grassi, W. Sonographic imaging of tendons. Arthritis Rheum. 2000;43:969.
93. Andres, BM, Murrell, GA. Treatment of tendinopathy: What works, what does not and what is on the horizon. Clin Orthop Relat Res. 2008;466:1539.
94. Feucht, CL, Patel, D. Analgesics and anti-inflammatory medications in sports: Use and abuse. Pediatr Clin North Am. 2010;57:751–774.
95. Shrier, I, Matheson, GO, Kohl, HW. Achilles tendonitis: Are corticosteroid injections useful or harmful? Clin J Sports Med. 1996;6:245–250.
96. Stell, I. Management of acute bursitis: Outcome study of a structured approach. J R Soc Med. 1999;92:5216.
97. Seah, R, Mani-Babu, S. Managing ankle sprains in primary care: What is best practice? A systematic review of the last 10 years of evidence. Br Med Bull. 2011;97:105–135.
98. Kerkhoffs, GM, et al. Functional treatments for acute ruptures of the lateral ankle ligament: A systematic review. Acta Orthop Scand. 2003;74:69–77.
99. Lamb, SE, et al. Mechanical supports for acute, severe ankle sprain: A pragmatic, multicentre, randomised controlled trial. Lancet. 2009;373:575–581.
100. Handoll, HH, Rowe, BH, Quinn, KM, de Bie, R. Interventions for preventing ankle ligament injuries. Cochrane Database Syst Rev. 2001;3:CD000018.
101. Boyd, AS, Benjamin, HJ, Asplund, C. Principles of casting and splinting. Am Fam Physician. 2009;79:16–22.
102. Unal, S, Aksoy, A, Yilmaz, C, Arslan, E, Demirkan, F. Third-degree burn after plaster of Paris brace. Plast Reconstr Surg. 2004;114:1686.
103. Halanski, MA, Halanski, AD, Oza, A. Thermal injury with contemporary cast-application techniques and methods to circumvent morbidity. J Bone Joint Surg Am. 2007;89:2369–2377.
104. Smith, GD, Hart, RG, Tsai, TM. Fiberglass cast application. Am J Emerg Med. 2005;23:347–360.
105. Riding, G, Edgel, M, James, M. Plaster checks: A waste of resources? J Accid Emerg Med. 1994;11:266.
106. Knight, KL, et al. Muscle injury management with cryotherapy. Athl Ther Today. 2000;5:26–30.
107. Hubbard, TJ, Denegar, CR. Does cryotherapy improve outcomes with soft tissue injury? J Athlet Train. 2004;39:278.
108. Collins, NC. Is ice right? Does cryotherapy improve outcome for acute soft tissue injury? Emerg Med J. 2008;25:65.
109. Bleakley, C, McDonough, S, McCauley, D. The use of ice in the treatment of acute soft-tissue injury: A systematic review of randomized controlled trials. Am J Sports Med. 2004;32:251.
110. Creaney, L, Hamilton, B. Growth factor delivery methods in the management of sports injuries: The state of play. Br J Sports Med. 2008;42:314–320.
111. Taylor, DW, Petrera, M, Hendry, M, Theodoropoulos, JS. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med. 2011;21:344–352.
112. Mishra, A, Pavelko, T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34:1774–1778.
113. Mishra, A, Woodall, J, Jr., Vieira, A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28:113–125.
114. Sánchez, M, et al. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35:245–251.