Gastrointestinal tract
Introduction to contrast media
Barium
There are many varieties of barium suspensions in use. Ingredients are designed to optimise mucosal coating and to make it palatable. In most situations the preparation will be diluted with water to give a lower density (Table 3.1) and must be shaken well immediately before use.
Table 3.1
Barium suspensions and dilutions with water to give a lower density
| Proprietary name | Density (w/v) – use |
| Baritop 100 | 100% – all parts gastrointestinal tract |
| EPI-C | 150% – large bowel |
| E-Z-Cat | 1–2% – computed tomography of gastrointestinal tract |
| E-Z HD | 250% – oesophagus, stomach and duodenum |
| E-Z Paque | 100% – small intestine |
| Micropaque DC | 100% – oesophagus, stomach and duodenum |
| Micropaque liquid | 100% – small and large bowel |
| Micropaque powder | 76% – small and large bowel |
| Polibar | 115% – large bowel |
| Polibar rapid | 100% – large bowel |
1. Barium swallow, e.g. Baritop® 100% w/v or E-Z HD® 200–250% 100 ml (or more, as required).
2. Barium meal, e.g. E-Z HD® 250% w/v. A high-density, low-viscosity barium is required for a double-contrast barium meal to give a good thin coating that is still sufficiently dense to give satisfactory opacification. It also contains simethicone (an anti-foaming and coating agent) and sorbitol (a coating agent).
3. Barium follow-through, e.g. E-Z Paque® 60–100% w/v 300 ml (150 ml if performed after a barium meal). This preparation contains sorbitol, which produces an osmotic hurrying and is partially resistant to flocculation.
4. Small bowel enema, e.g. either one 300 ml can of Baritop 100% w/v or two tubs of E-Z Paque made up to 1500 ml (60% w/v). N.B. As the transit time through the small bowel is relatively short in this investigation, there is a reduced chance of flocculation. This enables the use of barium preparations which are not flocculation-resistant. Gastrografin can be added to the mixture as this may help reduce the transit time still further.
5. Barium enema, e.g. Polibar 115% w/v 500 ml or more, as required. Reduced density between 20% and 40% w/v for single contrast examinations.
Disadvantages
1. Subsequent abdominal CT is rendered difficult (if not impossible) to interpret. Patients may need to wait for up to 2 weeks to allow satisfactory clearance of the barium. There may also be some reduction in US quality. It is advised that CT and/or US are performed before the barium study.
2. High morbidity associated with barium in the peritoneal cavity.
Complications
1. Perforation. The escape of barium into the peritoneal cavity is rare. If large amounts enter the peritoneal cavity it is extremely serious and will produce pain and severe hypovolaemic shock. Treatment should consist of aggressive intravenous fluid resuscitation, emergency surgery with copious washout and antibiotics. A 50% mortality rate is quoted and of those that survive, 30% will develop granulomata and peritoneal adhesions. Intramediastinal barium also has a significant mortality rate. It is imperative that a water-soluble contrast medium be the initial agent used for any investigation in which there is a risk or suspicion of perforation.
2. Aspiration. Aspirated barium is relatively harmless. Sequelae include pneumonitis and granuloma formation. Physiotherapy is the only treatment required (for both aspirated barium and low osmolar contrast material (LOCM)), and should be arranged before the patient leaves hospital.
3. Intravasation. This very rare complication may result in a barium pulmonary embolus, which carries a mortality of 80%.
For further complications (e.g. constipation and impaction), see the specific procedure involved.
Water-soluble contrast agents
Indications
Complications
1. HOCM can precipitate pulmonary oedema if aspirated (not LOCM).
2. HOCM can cause hypovolaemia and electrolyte disturbance due to the hyperosmolality of the contrast media drawing fluid into the bowel (not LOCM).
3. May precipitate in hyperchlorhydric gastric acid (i.e. 0.1 M HCl) – not non-ionic.
Gases
1. Oesophagus, stomach and duodenum – Carbon dioxide and, less often, air are used in conjunction with barium to achieve a ‘double contrast’ effect. For the upper gastrointestinal tract, CO2 is administered orally in the form of gas-producing granules/powder (sodium bicarbonate) which when mixed with fluid (citric acid) produces gas. The requirements of these agents are as follows:
(a) Production of an adequate volume of gas – 200–400 ml
(b) Non-interference with barium coating
2. Large bowel – For the large bowel, room air is administered per rectum via a hand pump attached to the enema tube. Carbon dioxide can also be administered by hand pump and is said to be resorbed more quickly, cause less abdominal pain, but produce inferior bowel distension when compared to air.1 CO2 insufflating pumps are in common usage in CT colonography.
Pharmacological agents
Hyoscine-N-butylbromide (Buscopan®)
Adult dose
The advantages of hyoscine include its immediate onset of action, relatively short duration of action (approx. 5–10 min) and its relatively low cost. Disadvantages include short-lived antimuscarinic side effects which include blurring of vision, a dry mouth, transient bradycardia followed by tachycardia and rare side effects said to include urinary retention and acute gastric dilatation. A particular side effect is that hyoscine can precipitate acute-angle closure glaucoma (AACG) in those patients who are susceptible to this because it dilates the pupil. In the UK patients who have AACG are almost always treated surgically in both eyes to prevent any recurrence. Pupillary dilatation has no role to play in the most common sort of glaucoma, open angle glaucoma, which accounts for 90% cases. Denying patients hyoscine on the basis of previous history of glaucoma is now not thought justified. Instead the following precautions are thought sufficient for the administration of hyoscine.1
Do
• Ask clinicians to identify patients who have unstable cardiac disease
• Ask whether there is a history of allergy to hyoscine
• Warn patients to expect blurred vision and not to drive until this has worn off
• Patient information leaflets should include ‘in the rare event that following the examination you develop painful, blurred vision in one or both eyes, you must attend hospital immediately for assessment.’
Glucagon
Disadvantages
Metoclopramide (Maxolon®)
This dopamine antagonist stimulates gastric emptying and small-intestinal transit.
References
1. Holemans, JA, Matson, MB, Hughes, JA, et al. A comparison of air, carbon dioxide and air/carbon dioxide mixture as insufflation agents for double contrast barium enema. European Radiology. 1998; 8:274–276.
1. Dyde, R, Chapman, AH, Gale, R, et al. Precautions to be taken by radiologists and radiographers when prescribing hyoscine-N-butylbromide. Clinical Radiology. 2008; 63:739–743.
2. Froehlich, JM, Daenzer, M, von Weymarn, C, et al. A peristaltic effect of hyoscine N-butylbromide versus glucagon on the small bowel assessed by magnetic resonance imaging. European Radiology. 2009; 19:1387–1393.
3. van Harten, PN, Hoek, HW, Kahn, RS. Acute dystonia induced by drug treatment. British Medical Journal. 1999; 319:623–626.
Contrast swallow
Contrast medium
1. E-Z HD 200–250% or Baritop 100% w/v, 100 ml (or more, as required)
2. Water-soluble contrast agent if perforation is suspected (e.g. Conray, Gastrografin)
3. LOCM (approx. 300 mg I ml−1) is safest if there is a risk of aspiration.
1. Gastrografin should NOT be used for the investigation of a tracheo-oesophageal fistula or when aspiration is a possibility. Use LOCM instead.
2. Barium should NOT be used initially if perforation is suspected. If perforation is not identified with a water-soluble contrast agent then a barium examination should be considered.
Technique
1. Start with the patient in the erect position, right anterior oblique (RAO) position to project the oesophagus clear of the spine. An ample mouthful of barium is swallowed and this bolus is observed under fluoroscopy for dynamic assessment to assess the function of the oesophagus. Then further mouthfuls with spot exposure(s) to include the whole oesophagus with dedicated AP views of the gastro-oesophageal junction.
2. Coned views of the hypopharynx should be obtained with a frame rate of 3–4 per second to include AP, lateral and oblique views whilst the patient swallows contrast.
3. The patient is placed semi-prone in a ‘recovery position’ in a left posterior oblique (LPO) position with their right arm by their side behind their back and the left arm used to support the cup containing contrast. One further swallow with a single bolus is observed under fluoroscopy to assess motility with the effect of gravity eliminated. A distended single-contrast view should be obtained as the patient rapidly sips and swallows contrast as this best identifies hernias, subtle mucosal rings and varices.
4. Modifications may be required depending on the clinical indication.
(a) If dysmotility is suspected barium should be mixed with bread or marshmallow bolus and observed under fluoroscopy correlating symptoms with the passage of the bolus in the erect position.
(b) If perforation is suspected a control film may be useful to identify pneumomediastinum and ideally the patient should be examined in four positions (prone/supine/left lateral/right lateral) with water-soluble contrast first, and if this is negative then with barium contrast.
(c) To demonstrate a tracheo-oesophageal fistula in infants, a ‘pull back’ nasogastric tube oeosphagogram may be performed if the standard oesophagogram is negative.1 This technique is particularly useful in patients known to aspirate or in ventilated patients. Suction and nursing support should be available should aspiration occur. The patient is positioned prone with the arms up and the table may be tilted slightly head down. A nasogastric tube is introduced into stomach and then withdrawn to the level of the lower oesophagus under lateral screening guidance. Ten to 20 ml of LOCM is syringed in to distend the oesophagus which will force the contrast medium through any small fistula which may be present. The process is repeated for the upper and mid oesophagus. It is important to watch for aspiration into the airway from overspill which can lead to diagnostic confusion.
Barium meal
Indications
Technique
The double contrast method (Fig. 3.1):
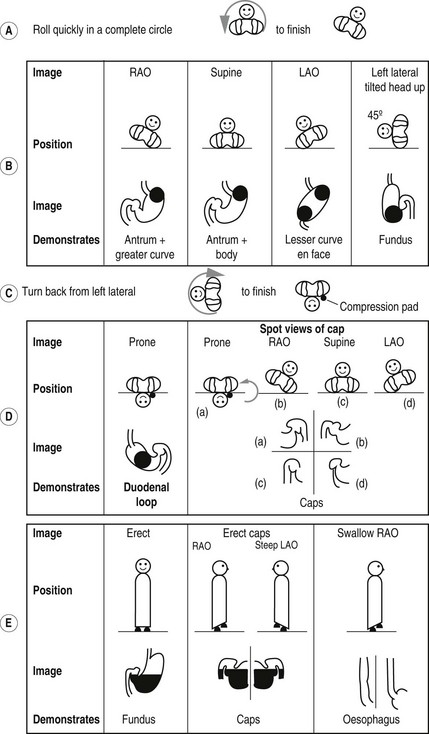
Figure 3.1 Barium meal sequence. Note in a, b, c and d, the patient position is depicted as if the operator were standing at the end of the screening table looking towards the patient’s head.
1. A gas-producing agent is swallowed.
2. The patient then drinks the barium while lying on the left side, supported by their elbow. This position prevents the barium from reaching the duodenum too quickly and so obscuring the greater curve of the stomach.
3. The patient then lies supine and slightly on the right side, to bring the barium up against the gastro-oesophageal junction. This manoeuvre is screened to check for reflux, which may be revealed by asking the patient to cough or to swallow water while in this position (the ‘water siphon’ test). The significance of reflux produced by tipping the patient’s head down and simultaneously drinking water is debatable, as this is non-physiological – 24-hour pH probe monitoring is the best current investigation. If reflux is observed, images are taken to record the level to which it ascends.
4. An i.v. injection of a smooth muscle relaxant (Buscopan 20 mg or glucagon 0.3 mg) may be given to better distend the stomach and to slow down the emptying of contrast into duodenum. The administration of Buscopan has been shown not to affect the detection of gastro-oesophageal reflux or hiatus hernia.
5. The patient is asked to roll onto the right side and then quickly over in a complete circle, to finish in an RAO position. This roll is performed to coat the gastric mucosa with barium. Good coating has been achieved if the areae gastricae in the antrum are visible.
Images
There is a great variation in views recommended. One scheme is:
1. Spot exposures of the stomach (lying):
(a) RAO – to demonstrate the antrum and greater curve
(b) Supine – to demonstrate the antrum and body
(c) LAO – to demonstrate the lesser curve en face
(d) Left lateral tilted, head up 45°– to demonstrate the fundus.
2. Spot image of the duodenal loop (lying):
(a) Prone – the patient lies on a compression pad to prevent barium from flooding into the duodenum.
3. Spot images of the duodenal cap (lying):
(b) RAO – the patient attains this position from the prone position by rolling first onto the left side, for the reasons mentioned above
4. Additional views of the fundus in an erect position may be taken at this stage, if there is suspicion of a fundal lesion.
5. Spot images of the oesophagus are taken, while barium is being swallowed, to complete the examination.
Modification of technique for young children
1. Single-contrast technique using 30% w/v barium sulphate and no paralytic agent.
2. A relatively small volume of barium – enough to just fill the fundus – is given to the infant in the supine position. An image of the distended oesophagus is exposed.
3. The child is turned semi-prone into a LPO or RAO position. An image is taken as barium passes through the pylorus. The pylorus is shown to even better advantage if 20–40° caudocranial angulation can be employed with an overhead screening unit. Gastric emptying is prolonged if the child is upset. A dummy coated with glycerine is a useful pacifier.
4. Once barium enters the duodenum, the infant is returned to the supine position, and with the child perfectly straight a second image is taken as barium passes around the duodenojejunal flexure. This image should just include the lower chest to verify that the child is straight.
5. Once malrotation has been diagnosed or excluded, a further volume of barium is administered until the stomach is reasonably full and barium lies against the gastro-oesophageal junction. The child is gently rotated through 180° in an attempt to elicit gastro-oesophageal reflux.
In newborn infants with upper intestinal obstruction, e.g. duodenal atresia, the diagnosis may be confirmed if 20 ml of air is injected down the nasogastric tube (which will almost certainly have already been introduced by the medical staff). If the diagnosis remains in doubt, it can be replaced by a positive contrast agent (dilute barium or LOCM if the risk of aspiration is high).
Aftercare
1. The patient must not drive until any blurring of vision produced by the Buscopan has resolved.
2. The patient should be warned that their bowel motions will be white for a few days after the examination and may be difficult to flush away.
3. The patient should be advised to eat and drink normally but with extra fluids to avoid barium impaction. Occasionally laxatives may also be required.
Complications
1. Leakage of barium from an unsuspected perforation
3. Conversion of a partial large bowel obstruction into a complete obstruction by the impaction of barium
4. Barium appendicitis, if barium impacts in the appendix (exceedingly rare)
N.B. It must be emphasized that there are many variations in technique, according to individual preference, and that the best way of becoming familiar with the sequence of positioning is actually to perform the procedure oneself.
Small bowel follow-through
Contrast medium
In general water-soluble small bowel contrast studies are avoided as contrast becomes diluted in small bowel fluid resulting in poor mucosal detail compared with barium. An exception is in adhesional small bowel obstruction where conservative investigation and ‘treatment’ with water-soluble contrast agents, frequently Gastrografin, may reduce the need for surgical intervention.1 In this case limited images are usually acquired at 1, 4 and 24 h, stopping once contrast is seen in the colon.
Preliminary image
Plain abdominal film is used if high-grade small bowel obstruction is thought possible.
Images
1. Prone PA images of the abdomen are taken every 15–20 min during the first hour, and subsequently every 20–30 min until the colon is reached. The prone position is used because the pressure on the abdomen helps to separate the loops of small bowel.
2. Each image should be reviewed and spot supine fluoroscopic views, using a compression device or pad if appropriate, may be considered.
3. Dedicated spot views of the terminal ileum are routinely acquired.
Additional images
1. To separate loops of small bowel:
(a) compression with fluoroscopy
(b) with X-ray tube angled into the pelvis
(c) obliques – in particular with the right side raised for terminal ileum views, or
(d) occasionally with the patient tilted head down.
(e) pneumocolon.2 Gaseous insufflation of the colon via a rectal tube after barium arrives in the caecum often results in good-quality double-contrast views of the terminal ileum.
2. Erect image – occasionally used to reveal any fluid levels caused by contrast medium retained within diverticula.
References
1. Catena, F, Di Saverio, S, Kelly, MD, et al. Bologna Guidelines for Diagnosis and Management of Adhesive Small Bowel Obstruction (ASBO): 2010 Evidence-Based Guidelines of the World Society of Emergency Surgery. World J Emerg Surg. 2011; 6:5.
2. Pickhardt, PJ. The peroral pneumocolon revisited: a valuable fluoroscopic and CT technique for ileocecal evaluation. Abdom Imaging. 2012; 37(3):313–325.
Small-bowel enema
Contrast medium
1. Single contrast: e.g. E-Z Paque 70% w/v diluted; or Baritop 100% w/v (one 300 ml can made up to 1500 ml with water).
2. 600 ml of 0.5% methylcellulose after 500 ml of 70% w/v barium.1
It may be difficult to obtain good distension and double-contrast effect of the distal small bowel and terminal ileum.
Equipment
A choice of tubes is available:
1. Bilbao-Dotter tube (Cook Ltd) with a guidewire (the tube is blind ending). Comes in various sizes and modifications including one variant with an inflatable balloon at the end to prevent reflux into the stomach.
2. Silk tube (E. Merck Ltd). This is a 10-F, 140-cm-long tube. It is made of polyurethane and the stylet and the internal lumen of the tube are coated with a water-activated lubricant to facilitate the smooth removal of the stylet after insertion.
Technique
1. The patient sits on the edge of the X-ray table. If a per-nasal approach is planned, the patency of the nasal passages is checked by asking the patient to sniff with one nostril occluded. The pharynx is anaesthetized with lidocaine spray or Xylocaine gel instilled into a nostril. The Silk tube should be passed with the guidewire pre-lubricated and fully within the tube, whereas for the Bilbao-Dotter tube the guidewire is introduced after the tube tip is in the stomach.
2. The tube is then passed through the nose or the mouth, and brief lateral screening of the neck may be helpful in negotiating the epiglottic region. The patient is asked to swallow with the neck flexed, as the tube is passed through the pharynx. The tube is then advanced into the gastric antrum.
3. The patient then lies down and the tube is passed into the duodenum. Various manoeuvres may be used alone, or in combination, to help this part of the procedure, which may be difficult:
(a) Lay the patient on his left side so that the gastric air bubble rises to the antrum, thus straightening out the stomach.
(b) Advance the tube whilst applying clockwise rotational motion (as viewed from the head of the patient looking towards the feet).
(c) Get the patient to sit up, to try to overcome the tendency of the tube to coil in the fundus of the stomach.
4. When the tip of the tube has been passed through the pylorus, the guidewire tip is maintained at the pylorus as the tube is passed over it along the duodenum to the level of the ligament of Treitz. The tube is ideally passed beyond the duodenojejunal flexure to diminish the risk of aspiration due to reflux of barium into the stomach.
5. Barium is then run in, ideally with a controllable mechanical pump, or by gravity. Initially start at 50 ml min−1 and, with regular initial screening, aim to ‘chase’ the leading edge of the barium distally to maintain an unbroken column of contrast within the small bowel. The infusion can usually be increased rapidly to 100 ml min−1 depending on the progress of the barium through the bowel. If methylcellulose is used, it is infused continuously, after an initial bolus of 500 ml of barium, until the barium has reached the colon.
6. The tube is then withdrawn, aspirating any residual fluid in the stomach. This is to decrease the risk of aspiration.
7. If the terminal ileum is obscured at the end of the examination it can be helpful to further re-screen the patient after an interval when barium has emptied into the colon as better views may be then obtained. A pneumocolon, as per small bowel follow-through, may also help.
Minordi, LM, Vecchioli, A, Guidi, L, et al. Multidetector CT enteroclysis versus barium enteroclysis with methylcellulose in patients with suspected small bowel disease. Eur Radiol. 2006; 16(7):1527–1536.
Nolan, DJ. The true yield of the small intestinal barium study. Endoscopy. 1997; 29(6):447–453.
Barium enema
Contraindications
Patient preparation
Many regimens for bowel preparation exist. A suggested regimen is as follows:
Technique
1. The patient lies on their left side, and the catheter is inserted gently into the rectum. It is taped firmly in position. Connections are made to the barium reservoir and the hand pump for injecting air.
2. An i.v. injection of Buscopan (20 mg) or glucagon (1 mg) is given. Some radiologists choose to give the muscle relaxant half way through the procedure at the end of step (3).
3. The infusion of barium is commenced. Intermittent screening is required to check the progress of the barium. The barium is run to splenic flexure in the left lateral position and then the patient is turned prone. Contrast is then run to the hepatic flexure and is stopped when it tips into the right colon. Gentle puffs of air may be needed to encourage the barium to flow. The patient rolls onto their right and quickly onto their back. An adequate amount of barium in the right colon is confirmed with fluoroscopy. The column of barium within the distal colon is run back out by either lowering the infusion bag to the floor or tilting the table to the erect position.
4. The catheter tube is occluded and air is gently pumped into the bowel to produce the double-contrast effect. CO2 gas has been shown to reduce the incidence of severe, post enema pain.
Exposures
A suggested sequence of positioning and films in a standard over couch image intensifier include:
• Left lateral rectum; then roll patient half way back
• RAO sigmoid; then roll patient prone and insufflate to distend transverse colon. The patient lifts left side up to obtain
• LPO sigmoid, then turn supine
• Raise patient to erect position with dedicated views of both flexures with some LAO positioning for splenic flexure and RAO positioning for hepatic flexure. Return patient to supine position
• Dedicated views of caecum and right colon, often with some RAO positioning (sometimes prior to the decubitus films)
Aftercare
1. The patient must not drive until any blurring of vision produced by Buscopan has resolved; usually within 30 minutes.
2. Patients should be warned that their bowel motions will be white for a few days after the examination. They may eat normally and should drink extra fluids to avoid barium impaction.
Antibiotic prophylaxis in barium enema2
Complications3 (all are rare)
1. Cardiac arrhythmias induced by Buscopan or the procedure itself. This is the most frequent cause of death after barium enema.
2. Perforation of the bowel. The second most common cause of death after barium enema. Often associated with the rectal catheter balloon
4. Side effects of the pharmacological agents used (see p. 51).
6. Venous intravasation. This may result in a barium pulmonary embolus, which carries an 80% mortality risk.
References
1. Low, VH. What is the current recommended waiting time for performance of a gastrointestinal barium study after endoscopic biopsy of the upper or lower gastrointestinal tract. Am J Roentgenol. 1998; 170(4):1104–1105.
2. NICE. NICE guidance. Antimicrobial prophylaxis against infective endocarditis in adults and children undergoing interventional procedures. NICE Clinical Guidelines. (64):2008.
3. Blakeborough, A, Sheridan, MB, Chapman, AH. Complications of barium enema examinations: a survey of UK Consultant Radiologists 1992 to 1994. Clin Radiol. 1997; 52(2):142–148.
Enema variants
Reduction of an intussusception
This procedure should only be attempted in full consultation with the surgeon in charge of the case and when an anaesthetist with appropriate paediatric training and experience, and with paediatric anaesthetic equipment, is available.1,2
Methods
1. Using air and fluoroscopy.3 Barium is no longer used in the majority of centres as air reduction has the following advantages:
(a) more rapid reduction, because the low viscosity of air permits rapid filling of the colon
(b) reduced radiation dose because of the above
(d) in the presence of a perforation, air in the peritoneal cavity is preferable to barium, and gut organisms are not washed into the peritoneal cavity
(e) there is more accurate control of intraluminal pressure
Contraindications
1. Peritonitis, perforation or shock.
2. The pneumatic method should probably not be used in children over 4 years of age as there is a higher incidence of significant lead points which may be missed.4
3. Successful reduction is less likely in patients with prolonged symptoms or radiographic signs of obstruction, but this does not preclude an attempt at reduction if the patient is well hydrated and stable.5
Patient preparation
1. Sedation is of questionable value, but analgesia is important (e.g. morphine 50 µg kg−1 if <1 year or 50–100 µg kg−1 if >1 year).
2. Some institutions perform the examination under general anaesthesia. This has the advantage of greater muscle relaxation, which may increase the likelihood of successful reduction, and also enables the child to go to surgery quickly in the event of a failed radiological reduction.
3. Correction of fluid and electrolyte imbalance. The child requires an i.v. line in situ.
Preliminary investigations
1. Plain abdominal film – to assess bowel distension and to exclude perforation. A right-side-up decubitus film is often helpful in confirming the diagnosis by showing a failure of caecal filling with bowel gas because of the presence of the soft-tissue mass of the intussusception. Normal plain films do not exclude the diagnosis, and the clinical findings and/or history should be sufficient indications.
2. US – should confirm or exclude the diagnosis. Absence of blood flow within the intussusceptum on colour-flow Doppler should lead to cautious reduction.6 US may identify a lead point; if so, even attempted reduction (to facilitate surgery) should be followed by surgery. If US identifies fluid trapped between the intussusceptum and intussuscipiens, the success rate is significantly reduced.7
Technique
Pneumatic reduction
1. The child is placed in the prone position so that it is easier to maintain the catheter in the rectum and the child is disturbed as little as possible during the procedure.
2. Air is instilled by a hand or mechanical pump and the intussusception is pushed back by a sustained pressure of up to 80 mmHg. Pressure should be monitored at all times and there should be a fail-safe pressure-release valve in the system to ensure that excessive pressures are not delivered.
3. Reduction is successful when there is free flow of air into the distal ileum.
4. If the intussusception does not move after 3 min of sustained pressure, the pressure is reduced and the child rested for 3 minutes. Two further attempts are made increasing the pressure to 120 mmHg for 3 minutes with a 3-minute rest. If the intussusception is still immovable it is considered irreducible and arrangements are made for surgery.
5. The intussusception is only considered completely reduced when the terminal ileum is filled with air. However, it is not uncommon for there to be a persisting filling defect in the caecum at the end of the procedure, with or without reflux of air into the terminal ileum. This is often due to an oedematous ileocaecal valve. In the presence of a soft-tissue caecal mass, a clinically well and stable child should be returned to the ward to await a further attempt at reduction after a period of 2–8 h rather than proceed to surgery. A second enema is often successful at complete reduction or showing resolution of the oedematous ileocaecal valve.8
6. When air (or barium) dissects between the two layers of the intussusception – the dissection sign – reduction is less likely.9
Ultrasound reduction10
1. To facilitate scanning the child must be supine.
2. The intussusception can be identified with US. Rectal saline or Hartmann’s solution is run as far as the obstruction and its passage around the colon and the reducing head of the intussusception is monitored by US. The intussusception is reduced when reflux into the terminal ileum is seen.
3. The points regarding failed or incomplete reduction discussed above also apply to this technique.
Barium reduction (now rarely used)
1. Patient positioning is as for the pneumatic method.
2. The bag containing barium is raised 100 cm above the table top and barium run in under hydrostatic pressure. Progress of the column of barium is monitored by intermittent fluoroscopy.
3. If the intussusception does not move after 3 min of sustained pressure, the bag of barium is lowered to table-top height and the child rested for 3 min. If, after three similar attempts, the intussusception is still immovable it is considered irreducible and arrangements are made for surgery.
4. The points regarding failed or incomplete reduction discussed above also apply to hydrostatic reduction.
References
1. The Royal College of Anaesthetists. Guidelines for the Provision of Anaesthetic Services. Guidance on the Provision of Paediatric Anaesthesia. London: Royal College of Anaesthetists; 1999.
2. The British Association of Paediatric Surgeons. A Guide for Purchasers and Providers of Paediatric Surgeons. London: British Association of Paediatric Surgeons; 1994.
3. Kirks, DR. Air intussusception reduction: ‘The winds of change’. Pediatric Radiology. 1995; 25:89–91.
4. Stringer, DA, Ein, SH. Pneumatic reduction: advantages, risks and indications. Pediatr Radiol. 1990; 20:475–477.
5. Daneman, A, Navarro, O. Intussusception Part 2: An update on the evolution of management. Paediatr Radiol. 2004; 34:97–108.
6. Lim, HK, Bae, SH, Lee, KH, et al. Assessment of reducibility of ileocolic intussusception in children: usefulness of color Doppler sonography. Radiology. 1994; 191:781–785.
7. Britton, I, Wilkinson, AG. Ultrasound features of intussusception predicting outcome of air enema. Pediatr Radiol. 1999; 29:705–710.
8. Gorenstein, A, Raucher, A, Serour, F, et al. Intussusception in children: reduction with repeated, delayed air enema. Radiology. 1998; 206:721–724.
9. Barr, LL, Stansberry, SD, Swischuk, LE. Significance of age, duration, obstruction and the dissection sign in intussusception. Pediatr Radiol. 1990; 20:454–456.
10. Khong, PL, Peh, WC, Lam, CH, et al. Ultrasound-guided hydrostatic reduction of childhood intussusception: technique and demonstration. RadioGraphics. 2000; 20:E1.
Contrast enema in neonatal low intestinal obstruction
Patient preparation
The baby should already have i.v. access and be well hydrated prior to the procedure.
Contrast medium
Dilute ionic contrast medium as is used for cystography, e.g. Urografin 150. This has the advantage of not provoking large fluid shifts and being dense enough to provide satisfactory images. Non-ionic contrast media and barium offer no advantages (and the latter is contraindicated with perforation as a possibility). Infants with meconium ileus or functional immaturity will benefit from a water-soluble contrast enema and so their therapeutic enema commences with the diagnostic study. The non-operative treatment of meconium ileus was first described using the hypertonic agent Gastrografin, which dislodged the sticky meconium by drawing water into the bowel lumen. However, most paediatric radiologists now believe that a hypertonic agent is not necessary for successful treatment.1
Sinogram
1. A water-soluble contrast medium should be used (e.g. Urografin 150).
2. A preliminary film is taken to exclude the presence of a radio-opaque foreign body.
3. An appropriate-size Foley catheter is then inserted into the orifice of the sinus. The balloon may be inflated inside the sinus to prevent retrograde flow. Alternatively the balloon may be inflated outside the sinus and pushed to the skin using a Spencer-Wells forceps or similar to secure a seal.
4. The contrast medium is injected carefully under fluoroscopic control.
5. Fluoroscopic ‘grab’ images supplemented by proper exposures are taken as required, including tangential views.
Retrograde ileogram
Technique
1. Cannulate the ileostomy with an appropriate (16–22-F) Foley catheter. Carefully inflate the balloon at or just inside the stoma.
2. Inject contrast. Dilute barium (Baritop) or water-soluble contrast can be used as a single contrast or as double contrast with either air or water/methyl cellulose (see small bowel enema).
3. Tangential views of the spout of the stoma may be obtained at the end of the examination upon deflation of the balloon.
Herniogram
Technique
1. The patient lies supine on the X-ray table.
2. An aseptic technique is employed.
3. 5–10 ml of 1% lidocaine is injected into the skin, subcutaneous tissues and peritoneum. The injection site varies between operators; the midline just below the umbilicus is most common, the midpoint of the left lateral rectus muscle is one variation.
4. An 18–22 G spinal needle is introduced into the peritoneal cavity until a ‘popping’ sensation is felt (as the needle tip passes into peritoneal cavity. Contrast is injected. If the needle is in the correct position a ‘spider web’ of intraperitoneal contrast outlining the outside of bowel loops is seen. If needle tip is in abdominal wall a bleb is seen and the needle requires repositioning.
Images
The patient is asked to cough and perform the Valsalva manoeuvre while these are taken. The tube may be angled 25° caudally.
Evacuating proctogram
Technique
1. One can of Baritop or equivalent given orally 1 hour prior to examination to opacify small bowel.
2. For female patients, approximately 20–30 ml of water-soluble contrast mixed with US gel are introduced into the vagina through a Foley catheter (not always required).
3. Approximately 120 ml of barium paste (or enough to fill the rectum) are instilled via a bladder syringe.
Coeliac axis, superior mesenteric and inferior mesenteric arteriography
Indications
Technique
1. Femoral artery puncture – Seldinger technique
2. Bowel movement causing subtraction artefact can be reduced by using a smooth-muscle relaxant (e.g. Buscopan 10–20 mg) and abdominal compression
3. When performed for gastrointestinal bleeding, provided that the patient is actively bleeding at the time, a blood loss of 0.5–0.6 ml min−1 can be demonstrated. The site of active bleeding is revealed by extravasated contrast medium remaining in the bowel on the late films, when intravascular contrast has cleared. Vascular malformations, tumours and varices may be demonstrated.
Late-phase visceral arteriography
N.B. If splenic vein opacification is required, then a late-phase splenic arteriogram is necessary.
Ultrasound of the gastrointestinal tract
Endoluminal examination of the oesophagus and stomach
Equipment
‘Radial’ echoendoscope with 5.0–10-MHz 360° transducer for diagnosis.
‘Linear’ echoendoscope with 5.0–10-MHz transducer for diagnosis and to allow biopsies.
Hypertrophic pyloric stenosis
The typical patient is a 6-week-old male infant presenting with non-bilious projectile vomiting.
Technique
1. The right upper quadrant is scanned with the patient supine. If the stomach is very distended, the pylorus will be displaced posteriorly and the stomach should be decompressed with a nasogastric tube. If the stomach is collapsed, the introduction of some dextrose, by mouth or via a nasogastric tube, will distend the antrum and differentiate it from the pylorus.
2. The pylorus is scanned in its longitudinal and transverse planes and images will resemble an olive and a doughnut, respectively. The poorly echogenic muscle is easily differentiated from the bright mucosa. Antral peristalsis can be seen and the volume of fluid passing through the pylorus with each antral wave can be assessed.
3. A number of measurements can be made. These include muscle thickness, canal length, pyloric volume and muscle thickness/wall diameter ratio, but there is no universal agreement as to which is the most discriminating parameter.
Small bowel
Indications
1. In expert hands ultrasound has a useful role in the evaluation of small bowel Crohn’s disease, including whether it is present and assessing disease activity. Contrast-enhanced ultrasound and Doppler flow ultrasound may help to evaluate disease activity.
2. In children ultrasound is useful to assess for intussusception.
3. Malrotation of the small bowel may be suspected by alteration of the normal relationship between the superior mesenteric artery and vein. The vein should normally lie anterior and to the right of the artery.
Technique
1. Patient in supine position, fasting for >6 hours helps but is not mandatory.
2. Oral fluids may usefully reduce the air content in the intestine. Graded compression in which the examiner uses the US transducer to squeeze the air away from the area of interest helps.
3. Initially with a 3.5–5-MHz transducer to get an overview, then switch to a curved or linear transducer with frequencies in the range 7.5–14-MHz to focus on pathology.
Endoluminal examination of the rectum and anus
Equipment
5–7-MHz radially scanning transducer. A linear transducer can be used but is less satisfactory.
Technique
1. The patient is placed in the left lateral position.
2. A careful digital rectal examination is carried out.
3. The probe is covered with a latex sheath containing contact jelly, and all air bubbles are expelled.
4. More jelly is placed over the latex sheath and the probe is introduced into the rectum.
American Society for Gastrointestinal Endoscopy. Role of endoscopic ultrasonography. Gastrointest Endosc. 2000; 52(6):852–859.
Weber, WA, Ott, K. Imaging of esophageal and gastric cancer. Seminars in Oncology. 2004; 31(4):530–541.
King, SJ. Ultrasound of the hollow gastrointestinal tract in children. Eur Radiol. 1997; 7(4):559–565.
Misra, D, Akhter, A, Potts, SR, et al. Pyloric stenosis. Is over reliance on ultrasound scans leading to negative explorations. Eur J Ped Surg. 1997; 7(6):328–330.
Munden, MM, Hill, JG. Ultrasound of the acute abdomen in children. Ultrasound Clin. 2010; 5(1):113–135.
CT of the gastrointestinal tract
Computed tomographic colonography
Indications
1. Incomplete colonoscopy – can be performed the same day if no biopsies taken. If the patient has had a colonic biopsy then CT colonography should be deferred – a minimum of 1 week though some suggest up to 4 weeks. Tagging the residual colon fluid with, e.g. 30 ml gastrografin 2+ hours before the CTC, helps with interpretation.
2. Patients in high-risk group or who have aversion to colonoscopy.
3. Patients on warfarin when the clinician preference is not to discontinue anticoagulants for colonoscopy.
4. Patients with an obstructing lesion preventing full colonoscopy who require evaluation of the entire colon.
5. It has an evolving role in colorectal cancer screening and in some centres has replaced optical colonoscopy.
Bowel preparation
1. ‘Standard’ purgative large-bowel preparation as for barium enema (see above). Diabetic patients may need to be admitted onto the ward for the duration of the bowel preparation.
2. ‘Faecal tagging’ using water-soluble contrast material in addition to the standard preparation (e.g. an additional 50 ml Gastrografin on the evening before the scan).
Technique
1. Patient to go to toilet immediately before procedure.
2. 20 mg i.v. Buscopan is given (glucagon is not recommended).
3. Patient positioned on their left side and a thin (e.g. Foley) catheter is placed in rectum and gas insufflated. The gas may be air but CO2 is better tolerated by patients. Gas is best administered by a dedicated pump. If a pump is available then 1.5–2 l of CO2 is initially administered, the patient is turned supine and further gas (typically up to 4–6 l) administered. Pumps limit the administered pressure to 25 psi and deliver further gas to maintain this pressure. Manual inflation with a bulb-sized hand pump or alternatively an empty enema bag filled with air/CO2 and gentle pressure on the enema bag are other options.
4. CT scout performed to check satisfactory gaseous distension of large bowel.
5. CT parameters will depend upon the type of CT scanner available but collimation thickness should be between 1–3 mm. Symptomatic patients typically receive i.v. contrast but asymptomatic patients from cancer screening programmes should not receive i.v. contrast. CT scans may be performed using a low-dose technique (e.g. 80 mA) without i.v. contrast, or using usual diagnostic CT parameters with i.v. iodinated contrast performed in portal phase (70 s delay).
6. Patient is turned prone; further insufflation is usually needed (e.g. 2 l CO2) and a further low-dose scan performed. If the patient is unable to turn prone they can be scanned in the left lateral position. The supine and prone scans should be reviewed to ensure all areas of the colon are distended on at least one of the acquisitions and if not then consideration of a further low-dose scan after repositioning in a lateral position and extra gas should be made.
Domjan, J, Blaquiere, R, Odurny, A. Is minimal preparation CT comparable with barium enema in elderly patients with colonic symptom. Clin Radiol. 1998; 53(12):894–898.
Geffroy, Y, Rodallec, MH, Boulay-Coletta, I, et al. Multidetector CT angiography in acute gastrointestinal bleeding: why, when, and how. RadioGraphics. 2011; 31(3):E35–E46.
Graça, BM, Freire, PA, Brito, JB, et al. Gastroenterologic and radiologic approach to obscure gastrointestinal bleeding: how, why, and when? RadioGraphics. 2010; 30(1):235–252.
Ilangovan, R, Burling, D, George, A, et al. CT enterography: review of technique and practical tips. Br J Radiol. 2012; 85(1015):876–886.
Silva, AC, Pimenta, M, Guimarães, LS. Small bowel obstruction: what to look for. RadioGraphics. 2009; 29(2):423–439.
Slater, A, Planner, A, Bungay, HK, et al. Three-day regimen improves faecal tagging for minimal preparation CT examination of the colon. Br J Radiol. 2009; 82(979):545–548.
Wittenberg, J, Harisinghani, MG, Jhaveri, K, et al. Algorithmic approach to CT diagnosis of the abnormal bowel wall. RadioGraphics. 2002; 22(5):1093–1107.
Burling, D, International Collaboration for CT Colonography Standards. CT colonography standards. Clin Radiol. 2010; 65(6):474–480.
Pickhardt, PJ. Missed lesions at CT colonography: lessons learned. Abdom Imaging. 2013; 38(1):82–97.
Tolan, DJ, Armstrong, EM, Chapman, AH. Replacing barium enema with CT colonography in patients older than 70 years: the importance of detecting extracolonic abnormalities. Am J Roentgenol. 2007; 189(5):1104–1111.
Magnetic resonance imaging of the gastrointestinal tract
Contrast agents
Contrast agents that can be used to alter the signal intensity within the bowel can be classified as positive contrast agents (high signal on T1 and T2 weighting) or negative agents (low signal on T1 and T2 weighting) but are usually biphasic (high signal on one sequence, low signal on the other). Water is a biphasic agent but is typically resorbed quickly, so a variety of formulations to increase osmolality are employed as discussed earlier on page 83. They include Klean-Prep® or 250 ml of mannitol 10% solution made up to 1 l with water. Air within bowel is a natural contrast agent and is usually sufficient for rectal and anal MRI.
Techniques
Suspected peri-anal fistula
1. No special patient preparation required, patient scanned supine in MRI scanner.
2. Buscopan not routinely used.
3. The anal canal is angulated forward from the vertical by about 45o. Initial midline sagittal T2 scan to identify the orientation of the canal. Oblique axial and coronal high-resolution scans at right angles, and parallel, to the anal canal complex are obtained to facilitate interpretation. T2W SE (with fat saturation) or short tau inversion recovery (STIR) are particularly useful sequences but T1W SE and occasionally scans following intravenous gadolinium using T1W with fat saturation may also assist.
Local staging of anorectal cancer
1. Patient scanned supine; Buscopan not routinely used.
2. Sagittal T2-weighted SE sequence of central pelvic structures. Large field of view axial T2 scan of the whole pelvis. Use these scans to plan high-resolution 3-mm axial T2W images perpendicular to and also parallel to the long axis of the tumour. Coronal high-resolution scans are useful for low rectal tumours. Diffusion weighted scans may also be useful.
Small-bowel magnetic resonance enteroclysis
1. Pass Bilbao Dotter tube to DJ flexure using fluoroscopy.
2. Transfer patient to MR scanner and obtain venous access.
3. Scan prone (though some centres scan supine) and obtain ‘scout’ localizer.
4. Connect Bilbao Dotter tube to enteroclysis pump (situated in control room if not MR compatible). Infuse (80–100 ml min−1) oral contrast under MR fluoroscopy (coronal thick-slab single-shot sequence, e.g. half Fourier acquisition single-shot turbo spin echo (HASTE)) to monitor filling of small bowel to ileocaecal valve. Check for reflux to stomach and slow/stop if significant. Stop infusion when contrast reaches colon.
5. Give Buscopan 20 mg intravenously.
6. Obtain sequences in coronal and transverse axial planes using HASTE, FISP sequences to include one fat-saturated sequence.
7. 3D T1W fat suppressed, e.g. volumetric interpolated breath-hold examination (VIBE) sequences pre-and post-intravenous gadolinium.
Small-bowel magnetic resonance enterography
1. Steadily drink oral contrast (ideally 1.5 l) over 30–45 min and scan immediately.
2. Obtain venous access and give Buscopan 20 mg i.v.
3. Obtain sequences in coronal and axial planes using HASTE, FISP sequences (or manufacturer’s equivalent) to include one fat-saturated sequence. Coronal pre-contrast 3D T1W fat suppressed (e.g. VIBE).
4. Give gadolinium-based contrast agent i.v.
5. Obtain fast 3D T1W fat-suppressed (e.g. VIBE) sequences in coronal and axial planes.
Halligan, S, Stoker, J. Imaging of fistula in ano. Radiology. 2006; 239(1):18–33.
Shihab, OC, Moran, BJ, Heald, RJ, et al. MRI staging of low rectal cancer. Eur Radiol. 2009; 19(3):643–650.
Tolan, DJ, Greenhalgh, R, Zealley, IA, et al. MR enterographic manifestations of small bowel Crohn disease. RadioGraphics. 2010; 30(2):367–384.
Radionuclide gastro-oesophageal reflux study
Radiopharmaceuticals
99mTc-colloid or 99mTc-DTPA mixed with:
1. Adults and older children: 150–300 ml orange juice acidified with an equal volume of 0.1 M hydrochloric acid.
Typical adult dose is 10–20 MBq, max. 40 MBq (0.9 mSv ED).
Technique
Physiological test1 – adults and older children
1. The liquid containing the tracer is given and washed down with unlabelled liquid to clear residual activity from the oesophagus.
2. The patient lies semi-recumbent with the camera centred over the stomach and lower oesophagus.
3. Dynamic imaging is commenced with 5-s 64 × 64 frames for 30–60 min.
Milk scan1 – infants and younger children
1. The milk feed is divided into two parts and one mixed with the tracer.
2. The radiolabelled milk is given and washed down with the remaining unlabelled milk.
3. After eructation, the child is placed either supine or prone, according to natural behaviour (although reflux appears to occur more readily in the supine position), with the camera anterior over stomach and oesophagus.
4. Dynamic imaging is commenced with 5-s 64 × 64 frames for 30–60 min.
5. If pulmonary aspiration of feed is suspected, later imaging at 4 h may be performed. The test is specific but not very sensitive for this purpose.
Provocation with abdominal compression2 – adults and older children
1. The abdominal binder is placed around the upper abdomen.
2. The radiolabelled liquid is given as above.
3. The patient lies supine with the camera centred over the stomach and lower oesophagus.
5. The pressure in the binder is increased in steps of 20 mmHg up to 100 mmHg, being maintained at each step for 30 s while an image is taken.
6. The test is terminated as soon as significant reflux is seen.
References
1. Guillet, J, Basse-Cathalinat, B, Christophe, E, et al. Routine studies of swallowed radionuclide transit in paediatrics: experience with 400 patients. Eur J Nucl Med. 1984; 9:86–90.
2. Martins, JC, Isaacs, PE, Sladen, GE, et al. Gastro-oesophageal reflux scintigraphy compared with pH probe monitoring. Nucl Med Commun. 1984; 5:201–204.
Radionuclide gastric-emptying study
Radiopharmaceuticals
Both liquid and solid studies may be performed, separately or simultaneously, as a dual isotope study. Liquids have generally shorter emptying times than solids, and tend to follow an exponential emptying pattern. Solids tend to empty linearly after a lag phase. Prolonged solid emptying is highly correlated with prolonged liquid emptying, and there is debate, therefore, as to whether both studies are routinely necessary.1 Examples of meals used are:
1. Liquid meal: Max. 12 MBq 99mTc-tin colloid (0.3 mSv ED) mixed with 200 ml orange juice, or with milk or formula feed for infants.
2. Solid meal: Scrambled egg prepared with max. 12 MBq 99mTc-colloid (0.3 mSv ED) or 99mTc-DTPA. Bulk is made up with other non-labelled foods such as bread and milk.
3. Dual isotope combined liquid and solid meal:
(a) Liquid: 12 MBq 99mTc-colloid (0.3 mSv ED) mixed with 200 ml orange juice
(b) Solid: 2 MBq 111In-labelled resin beads (0.7 mSv ED) incorporated into a pancake containing 27 g fat, 18 g protein, 625 calories. Bulk is made up with other non-labelled foods. Only 2 MBq of 111In is suggested (ARSAC max. is 12 MBq, 4 mSv ED) in order to minimize the downscatter into the 99mTc energy window.
Patient preparation
2. No smoking or alcohol from midnight before test
3. Where practical, stop medications affecting gastric motility such as dopaminergic agonists (e.g. metoclopramide, domperidone), cholinergic agonists (e.g. bethanechol), tricyclic antidepressants and anticholinergics for 24 h or more prior to the study, depending upon their biological half-life.
Technique
1. The patient ingests the meal as quickly as they comfortably can. (If dumping syndrome is suspected, the meal should be eaten in front of the camera with a fast-frame dynamic acquisition running, or the dumping episode may be missed.)
2. The patient is positioned standing.
3. Every 5 min a pair of 1-min anterior and posterior 128 × 128 images is obtained.
4. The patient sits and relaxes between images.
5. A liquid study should be continued for up to 60 min and a solid study for up to 90 min. If it can be seen that the majority of the meal has emptied inside this time, the study may be terminated.
6. If emptying is very slow, later pairs of images may be acquired at intervals of 30–60 min.
Analysis
1. Stomach region of interest is drawn.
2. Stomach time–activity curve is produced, using geometric mean if anterior and posterior imaging performed.
3. Half-emptying time is calculated.
4. Other parameters may be calculated, e.g. lag-phase duration for solid studies, or percentage left in the stomach at various time points.
Additional techniques
1. The small-bowel transit time (SBTT) can be ascertained by continuing imaging at intervals until the caecum is seen. Since the position of the caecum is often not obvious and may be overlain by small bowel, a 12–24-h image can be useful to determine the position of the large bowel.
2. Frequency analysis of fast dynamic scans (1-s frame time) can be used to characterize antral contraction patterns.2
Radionuclide meckel’s diverticulum scan
Contraindications
1. Barium study in previous 2–3 days (barium causes significant attenuation of gamma photons and may mask a diverticulum).
2. In vivo, labelled red blood cell study in previous few days (due to likelihood of pertechnetate adhering to red cells).
3. Precautions and contraindications to any pre-administered drugs should be observed.
Patient preparation
1. Nil by mouth for 6 h, unless emergency
2. It may be possible to enhance detection by prior administration of drugs, e.g. pentagastrin, cimetidine or ranitidine aimed at increasing the uptake of 99mTc-pertechnetate into gastric mucosa and inhibiting its release into the lumen of the stomach and progression into the bowel.
Technique
1. The bladder is emptied – a full bladder may obscure the diverticulum.
2. The patient lies supine with the camera over the abdomen and pelvis. The stomach must be included in the field of view because diagnosis is dependent on demonstrating uptake of radionuclide in the diverticulum concurrent with uptake by gastric mucosa.
Radionuclide imaging of gastrointestinal bleeding
Radiopharmaceuticals
1. 99mTc-labelled red blood cells, 400 MBq max (4 mSv ED). Red cells are pre-treated with a stannous agent. 99mTc-pertechnetate is added and is reduced by the stannous ions, causing it to be retained intracellularly. Labelling efficiency is important, as false-positive scans can result from accumulations of free pertechnetate. In vitro preparation gives the best labelling efficiency, but is complex and time-consuming. However, commercial kits are available which can reduce the preparation time to around 30 min. In vivo labelling is least efficient, and there is also a compromise in vivo/vitro method where the labelling occurs in the syringe as blood is withdrawn from the patient.1
2. 99mTc-colloid, 400 MBq max (4 mSv ED). This used to be a commonly used alternative to labelled red cells, but studies showed it to be a less sensitive tracer for detecting bleeding sites,2 hence it is not recommended. Colloids are rapidly extracted from the circulation, so bleeding occurring only within 10 min or so of injection can be detected. It also localizes intensely in liver and spleen, masking upper gastrointestinal bleeding sites.
Patient preparation
1. In vivo or in vivo/vitro methods: ‘Cold’ stannous agent (15 g kg−1 tin) is administered directly into a vein 20–30 min before the 99mTc-pertechnetate injection. (Injection via a plastic cannula will result in a poor label.)
2. The patient is asked to empty their bladder before each image is taken. Catheterization is ideal if appropriate.
Technique
2. The camera is positioned over the anterior abdomen with the symphysis pubis at the bottom of the field of view.
3. 99mTc-pertechnetate (in vivo method) or 99mTc-labelled red cells (in vitro or in vivo/vitro methods) are injected i.v.
4. A 128 × 128 dynamic acquisition is begun immediately with 2-s images for 1 min to help to demonstrate vascular blood pool anatomy, followed by 1-min images up to 45 min. Dynamic imaging permits cinematic viewing of images to detect bleed sites and movement through the bowel.3
5. Further 15 × 1-min dynamic image sets are acquired at 1, 2, 4, 6, 8 and 24 h or until bleeding site is detected (imaging much beyond 24 h is limited by radioactive decay).
6. Oblique and lateral views may help to localize any abnormal collections of activity.
References
1. Chaudhuri, TK. Radionuclide methods of detecting acute gastrointestinal bleeding. Int J Rad Appl Instrum. 1991; 18(Part B):655–661.
2. Bunker, SR, Lull, RJ, Tanasescu, DE, et al. Scintigraphy of gastrointestinal hemorrhage: superiority of 99mTc red blood cells over 99mTc sulfur colloid. Am J Roentgenol. 1984; 143:543–548.
3. Maurer, AH. Gastrointestinal bleeding and cine-scintigraphy. Semin Nucl Med. 1996; 26:43–50.
4. Laing, CJ, Tobias, T, Rosenblum, DI, et al. Acute gastrointestinal bleeding: emerging role of multidetector CT angiography and review of current imaging techniques. RadioGraphics. 2007; 27:1055–1070.

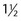 l drunk gradually over 30 minutes to fill small bowel is usually required, with a larger volume required in some and less in others. Patient encouragement and supervision are important to achieve this. ‘Enterography’ is the term used to describe this protocol but the same contrast may also be administered via an NJ tube when it is termed ‘enteroclysis’. In high-grade intestinal obstruction oral contrast is not necessary as fluid within dilated bowel will act as a negative contrast agent and allow definition of the transition point.
l drunk gradually over 30 minutes to fill small bowel is usually required, with a larger volume required in some and less in others. Patient encouragement and supervision are important to achieve this. ‘Enterography’ is the term used to describe this protocol but the same contrast may also be administered via an NJ tube when it is termed ‘enteroclysis’. In high-grade intestinal obstruction oral contrast is not necessary as fluid within dilated bowel will act as a negative contrast agent and allow definition of the transition point.