Gastrointestinal Disorders and Therapeutic Management
Acute Gastrointestinal Hemorrhage
Description
GI hemorrhage is a potentially life-threatening emergency that remains a common complication of critical illness and results in over 300,000 hospital admissions yearly.1 Despite advances in medical knowledge and nursing care, the mortality rate for patients with acute GI bleeding remains at 10% per annum in the United States.1
Etiology
GI hemorrhage occurs from bleeding in the upper or lower GI tract. The ligament of Treitz is the anatomic division used to differentiate between the two areas. Bleeding proximal to the ligament is considered to be from the upper GI tract, and bleeding distal to the ligament is considered to be from the lower GI tract.1–3 The various causes of acute GI hemorrhage are listed in Box 30-1.4 Only the three main causes of GI hemorrhage commonly seen in the critical care unit are discussed further.
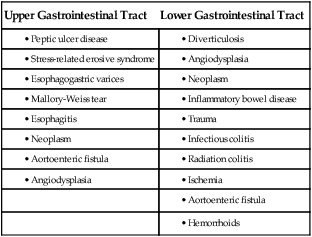
Peptic Ulcer Disease
Peptic ulcer disease (i.e., gastric and duodenal ulcers), which results from the breakdown of the gastromucosal lining, is the leading cause of upper GI hemorrhage, accounting for approximately 40% of cases.5,6 Normally, protection of the gastric mucosa from the digestive effects of gastric secretions is accomplished in several ways. First, the gastroduodenal mucosa is coated by a glycoprotein mucous barrier that protects the surface of the epithelium from hydrogen ions and other noxious substances present in the gut lumen.6–8 Adequate gastric mucosal blood flow is necessary to maintain this mucosal barrier function. Second, gastroduodenal epithelial cells are protected structurally against damage from acid and pepsin because they are connected by tight junctions that help prevent acid penetration. Third, prostaglandins and nitric oxide protect the mucosal barrier by stimulating the secretion of mucus and bicarbonate and inhibiting the secretion of acid.6–8
Peptic ulceration occurs when these protective mechanisms cease to function, allowing gastroduodenal mucosal breakdown. After the mucosal lining is penetrated, gastric secretions autodigest the layers of the stomach or duodenum, leading to injury of the mucosal and submucosal layers. This results in damaged blood vessels and subsequent hemorrhage. The two main causes of disruption of gastroduodenal mucosal resistance are the bacterial action of Helicobacter pylori and nonsteroidal anti-inflammatory drugs (NSAIDs).9
Stress-Related Mucosal Disease
Stress-related mucosal disease (SRMD) is an acute erosive gastritis that covers both types of mucosal lesions that are often found in the critically ill patient: stress-related injury and discrete stress ulcers.10–12 Additional terms used to describe this condition include stress ulcers, stress erosions, stress gastritis, hemorrhagic gastritis, and erosive gastritis. These abnormalities develop within hours of admission.11 They range from superficial mucosal erosions to deep focal lesions and usually affect the upper GI tract.11 SRMD occurs by means of the same pathophysiologic mechanisms as peptic ulcer disease, but the main cause of disruption of gastric mucosal resistance is increased acid production and decreased mucosal blood flow, resulting in ischemia and degeneration of the mucosal lining.11 Patients at risk include those in situations of high physiologic stress, as occurs with mechanical ventilation, extensive burns, severe trauma, major surgery, shock, sepsis, coagulopathy, or acute neurologic disease.13 SRMD is decreasing in incidence because of advances in therapeutic techniques and prevention of hypoperfusion of the mucosa.14
Esophagogastric Varices
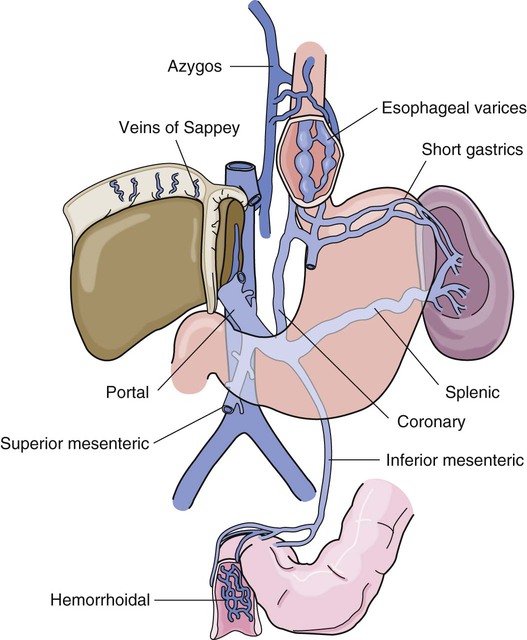
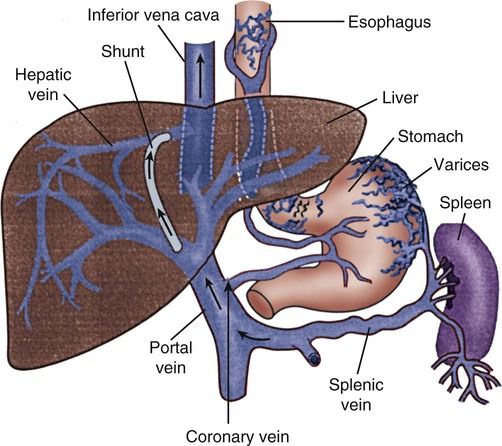
Esophagogastric varices are engorged and distended blood vessels of the esophagus and proximal stomach that develop as a result of portal hypertension caused by hepatic cirrhosis, a chronic disease of the liver, which results in damage to the liver sinusoids (Fig. 30-1). Without adequate sinusoid function, resistance to portal blood flow is increased, and pressures within the liver are elevated. This leads to increased portal venous pressure (portal hypertension), causing collateral circulation to divert portal blood from areas of high pressure within the liver to adjacent areas of low pressure outside the liver, such as into the veins of the esophagus, the spleen, the intestines, and the stomach. The tiny, thin-walled vessels of the esophagus and proximal stomach that receive this diverted blood lack sturdy mucosal protection. The vessels become engorged and dilated, forming esophagogastric varices that are vulnerable to damage from gastric secretions and that may result in subsequent rupture and massive hemorrhage.15 The risk of variceal bleeding increases with disease severity and variceal size, but overall, bleeding occurs in 25% to 30% of patients within 2 years of diagnosis, and 20% to 30% mortality from each bleeding episode.16,17
Pathophysiology
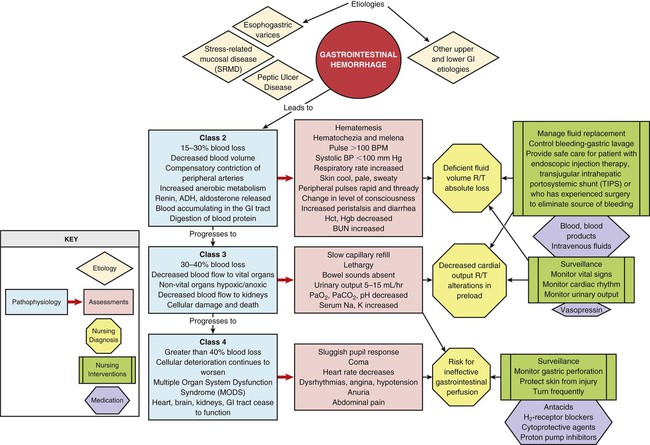
GI hemorrhage is a life-threatening disorder that is characterized by acute, massive bleeding. Regardless of the cause, acute GI hemorrhage results in hypovolemic shock, initiation of the shock response, and development of multiple organ dysfunction syndrome if left untreated (see Concept Map, Fig. 30-2).7 However, the most common cause of death in cases of GI hemorrhage is exacerbation of the underlying disease, not intractable hypovolemic shock.
Assessment and Diagnosis
The initial clinical presentation of the patient with acute GI hemorrhage is that of a patient in hypovolemic shock, and the clinical presentation depends on the amount of blood lost (Table 30-1).7 Hematemesis (bright red or brown, “coffee grounds” emesis), hematochezia (bright red stools), and melena (black, tarry, or dark red stools) are the hallmarks of GI hemorrhage.5,18.
TABLE 30-1
CLINICAL CLASSIFICATION OF HEMORRHAGE
| CLASS | BLOOD LOSS (%) | CLINICAL SIGNS AND SYMPTOMS |
| 1 | ≤15 | Pulse rate: normal or <100 beats/min (supine) |
| Capillary refill <3 seconds | ||
| Urine output: adequate (30-35 mL/hr) | ||
| Orthostatic hypotension | ||
| Apprehensive | ||
| 2 | 15-30 | Pulse rate: increased (>100 beats/min) |
| Capillary refill: sluggish | ||
| Pulse pressure: decreased | ||
| Blood pressure: normal (supine) | ||
| Tachypnea | ||
| Urine output: low (25-30 mL/hr) | ||
| 3 | 30-40 | Pulse rate: 120+ beats/min (supine) |
| Hypotension | ||
| Skin: cool, pale | ||
| Confused | ||
| Hyperventilating | ||
| Urine output: low (5-15 mL/hr) | ||
| 4 | ≥40 | Profoundly hypotensive |
| Pulse rate: 140+ beats/min | ||
| Confused, lethargic | ||
| Urine output minimal |
From Klein DG. Physiologic response to traumatic shock. AACN Clin Issues Crit Care Nurs. 1990;1:505.
Hematemesis
The patient who is vomiting blood is usually bleeding from a source above the duodenojejunal junction; reverse peristalsis is seldom sufficient to cause hematemesis if the bleeding point is below this area. The hematemesis may be bright red or look like coffee grounds, depending on the amount of gastric contents at the time of bleeding and the length of time the blood has been in contact with gastric secretions. Gastric acid converts bright red hemoglobin to brown hematin, accounting for the coffee grounds appearance of the emesis. Bright red emesis results from profuse bleeding with little contact with gastric secretions.19
Laboratory Studies
Laboratory tests can help determine the extent of bleeding, although the patient’s hemoglobin level and hematocrit are poor indicators of the severity of blood loss if the bleeding is acute. As whole blood is lost, plasma and red blood cells are lost in the same proportion; if the patient’s hematocrit is 45% before a bleeding episode, it will be 45% several hours later.7 It may take 24 to 72 hours for the redistribution of plasma from the extravascular space to the intravascular space to occur and cause the patient’s hemoglobin level and hematocrit value to decrease.19
Diagnostic Procedures
To isolate and treat the source of bleeding, an urgent fiberoptic endoscopy is usually undertaken.20 Before endoscopy, the patient must be hemodynamically stabilized.21 Tagged red blood cell scanning, angiography, or both, may be done to assist with localizing and treating a bleeding lesion in the GI tract when it is impossible to clearly view the GI tract because of continued active bleeding.19
Medical Management
To reduce mortality related to GI hemorrhage, patients at risk should be identified early, and interventions should be implemented to reduce gastric acidity and support the gastric mucosal defense mechanisms. Management of the patient at risk for GI hemorrhage should include prophylactic administration of pharmacologic agents for neutralization of gastric acids. These agents include antacids, histamine-2 (H2) antagonists, cytoprotective agents, and proton pump inhibitors (PPIs).5,22 Priorities in the medical management of the patient with GI hemorrhage include airway protection, fluid resuscitation to achieve hemodynamic stability, correction of co-morbid conditions (e.g., coagulopathy), therapeutic procedures to control or stop bleeding, and diagnostic procedures to determine the exact cause of the bleeding.5,21
Stabilization
The initial treatment priority is the restoration of adequate circulating blood volume to treat or prevent shock. This is accomplished with the administration of intravenous infusions of crystalloids, blood, and blood products.22 Hemodynamic monitoring can help guide fluid replacement therapy, particularly in patients at risk for heart failure.7 Supplemental oxygen therapy is initiated to increase oxygen delivery and improve tissue perfusion.7,22 A large nasogastric tube may be inserted to confirm the diagnosis of active bleeding, to facilitate gastric lavage, decrease the risk for aspiration and to prepare the esophagus, stomach, and proximal duodenum for endoscopic evaluation.5
Controlling the Bleeding
Interventions to control bleeding are the second priority for the patient with GI hemorrhage.
Peptic Ulcer Disease.
In the patient with GI hemorrhage related to peptic ulcer disease, bleeding hemostasis may be accomplished by endoscopic injection therapy in conjunction with thermal or hemostatic clips.20 Endoscopic thermal therapy uses heat to cauterize the bleeding vessel, and endoscopic injection therapy uses a variety of agents such as hypertonic saline, epinephrine, ethanol, and sclerosants to induce localized vasoconstriction of the bleeding vessel.6 Intra-arterial infusion of vasopressin into the gastric artery or intra-arterial injection of an embolizing agent (e.g., Gelfoam pledgets, polyvinyl alcohol particles, coils) can be performed during arteriography to control bleeding after the site has been identified.19
Esophagogastric Varices.
In acute variceal hemorrhage, control of bleeding may be initially accomplished through the use of pharmacologic agents and endoscopic therapies. Intravenous vasopressin, somatostatin, and octreotide can reduce portal venous pressure and slow variceal hemorrhaging by constricting the splanchnic arteriolar bed.16 Two commonly used endoscopic therapies are endoscopic injection sclerotherapy (EIS) and endoscopic variceal ligation (EVL).23 EIS controls bleeding by the injection of a sclerosing agent in or around the varices. This creates an inflammatory reaction that induces vasoconstriction and results in the formation of a venous thrombosis. During EVL, bands are placed around the varices to create an obstruction to stop the bleeding.24
If these initial therapies fail, transjugular intrahepatic portosystemic shunting (TIPS) may be necessary. In a TIPS procedure, a channel between the systemic and portal venous systems is created to redirect portal blood, thereby reducing portal hypertension and decompressing the varices to control bleeding (Fig. 30-3).23,24
Surgical Intervention
The patient who remains hemodynamically unstable despite volume replacement may need urgent surgery.
Peptic Ulcer Disease.
Surgical intervention is required to control bleeding in a minority of patients.25 The operative procedure of choice to control bleeding from peptic ulcer disease is a vagotomy and pyloroplasty. During this procedure, the vagus nerve to the stomach is severed, eliminating the autonomic stimulus to the gastric cells and reducing hydrochloric acid production. Because the vagus nerve also stimulates motility, a pyloroplasty is performed to provide for gastric emptying.26
Esophagogastric Varices.
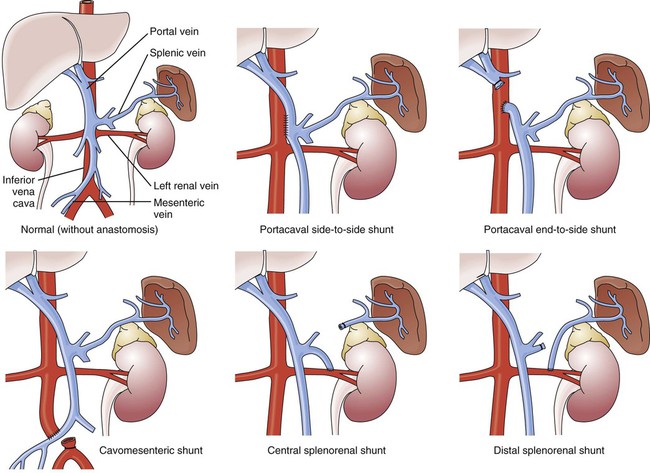
If medical treatment is unsuccessful and angiographic interventional TIPS procedure is not available, operative procedures to control bleeding gastroesophageal varices may be undertaken. Though rarely performed, operative interventions focus on some form of shunting (Fig 30-4).25 These shunt procedures are also referred to as decompression procedures because they result in the diversion of portal blood flow away from the liver and decompression of the portal system. The portacaval shunt procedure has two variations: (1) an end-to-side portacaval shunt procedure, which involves the ligation of the hepatic end of the portal vein with subsequent anastomosis to the vena cava; and (2) a side-to-side portacaval shunt procedure, during which the side of the portal vein is anastomosed to the side of the vena cava. A mesocaval shunt procedure involves the insertion of a graft between the superior mesenteric artery and the vena cava. During a distal splenorenal shunt procedure, the splenic vein is detached from the portal vein and anastomosed to the left renal vein.27
Nursing Management
All critically ill patients should be considered at risk for stress ulcers and, therefore, GI hemorrhage. Routine assessment of gastric fluid pH monitoring is controversial.12,28 Maintaining the pH between 3.5 and 4.5 is a goal of prophylactic therapy. Gastric pH measurements made with litmus paper or direct nasogastric tube probes may be used to assess gastric fluid pH and the effectiveness or need for prophylactic agents.28 Patients at risk also should be assessed for the presence of bright red or coffee grounds emesis, bloody nasogastric aspirate, and bright red, black, or dark red stools.4 Any signs of bleeding should be promptly reported to the physician.
Nursing management of a patient experiencing acute GI hemorrhage incorporates a variety of nursing diagnoses (Box 30-2). Nursing interventions include administering volume replacement, controlling the bleeding, providing comfort and emotional support, maintaining surveillance for complications, and educating the patient and family.
Administering Volume Replacement.
Measures to facilitate volume replacement include obtaining intravenous access and administering prescribed fluids and blood products. Two large-diameter peripheral intravenous catheters should be inserted to facilitate the rapid administration of prescribed fluids.1
Controlling the Bleeding.
One measure to control active bleeding is gastric lavage. It is used to decrease gastric mucosal blood flow and evacuate blood from the stomach. Gastric lavage is performed by inserting a large-bore nasogastric tube into the stomach and irrigating it with normal saline or water until the returned solution is clear. It is important to keep accurate records of the amount of fluid instilled and aspirated to ascertain the true amount of bleeding.1 Historically, iced saline was favored as a lavage irrigant. Research has shown, however, that low-temperature fluids shift the oxyhemoglobin dissociation curve to the left, decrease oxygen delivery to vital organs, and prolong bleeding time and prothrombin time. Iced saline also may further aggravate bleeding; therefore, room-temperature water or saline is the preferred irrigant for use in gastric lavage.29
Maintaining Surveillance for Complications.
The patient should be continuously observed for signs of gastric perforation. Although a rare complication, gastric perforation constitutes a surgical emergency. Signs and symptoms include sudden, severe, generalized abdominal pain with significant rebound tenderness and rigidity. Perforation should be suspected when fever, leukocytosis, and tachycardia persist despite adequate volume replacement.30
Educating the Patient and Family.
Early in the hospital stay, the patient and family should be taught about acute GI hemorrhage and its causes and treatments. As the patient moves toward discharge, teaching should focus on the interventions necessary for preventing the recurrence of the precipitating disorder. If an alcohol abuser, the patient should be encouraged to stop drinking and be referred to an alcohol cessation program (Box 30-3).
Collaborative management of the patient with acute GI hemorrhage is outlined in Box 30-4.
Acute Pancreatitis
Description
Acute pancreatitis is an inflammation of the pancreas that produces exocrine and endocrine dysfunction that may also involve surrounding tissues, remote organ systems, or both. The clinical course can range from a mild, self-limiting disease to a systemic process characterized by organ failure, sepsis, and death. In approximately 80% of patients, it takes the milder form of edematous interstitial pancreatitis, whereas the other 20% develop severe acute necrotizing pancreatitis.31 Reported mortality rates for acute pancreatitis range from 2% to 15% overall, with a steadily increasing rate of about 20,000 deaths per year in the United States.32,33 Several prognostic scoring systems have been developed to predict the severity of acute pancreatitis. One of the most commonly used is Ranson criteria34 (Box 30-5). If the patient has 0 to 2 factors present, the predicted mortality rate is 2%; with 3 to 4 factors, the rate is 15%; with 5 to 6 factors, the rate is 40%; and with 7 to 8 factors, predicted mortality rate is 100%.34–36
Etiology
The two most common causes of acute pancreatitis are gallstone migration and alcoholism.36 Together, they account for approximately 80% of cases.19 Less common causes are quite diverse and include surgical trauma, hypercalcemia, various toxins, ischemia, infections, and the use of certain medications (Box 30-6). In up to 20% of patients with acute pancreatitis, no etiologic factor can be determined.19,31
Pathophysiology
In acute pancreatitis, the normally inactive digestive enzymes become prematurely activated within the pancreas itself, leading to autodigestion of pancreatic tissue. The enzymes become activated through various mechanisms, including obstruction of or damage to the pancreatic duct system, alterations in the secretory processes of the acinar cells, infection, ischemia, and other unknown factors.7,36
Trypsin is the enzyme that becomes activated first. It initiates the autodigestion process by triggering the secretion of proteolytic enzymes such as kallikrein, chymotrypsin, elastase, phospholipase A, and lipase. Release of kallikrein and chymotrypsin results in increased capillary membrane permeability, leading to leakage of fluid into the interstitium and the development of edema and relative hypovolemia. Elastase is the most harmful enzyme in terms of direct cell damage. It dissolves the elastic fibers of blood vessels and ducts, leading to hemorrhage. Phospholipase A, in the presence of bile, destroys the phospholipids of cell membranes, causing severe pancreatic and adipose tissue necrosis. Lipase flows into the damaged tissue and is absorbed into the systemic circulation, resulting in fat necrosis of the pancreas and surrounding tissues.7,37
The extent of injury to the pancreatic cells determines the type of acute pancreatitis that develops. If injury to the pancreatic cells is mild and without necrosis, edematous pancreatitis develops. The acinar cells appear structurally intact, and blood flow is maintained through small capillaries and venules. This form of acute pancreatitis is self-limiting. If injury to the pancreatic cells is severe, acute necrotizing pancreatitis develops.32,37 Cellular destruction in pancreatic injury results in the release of toxic enzymes and inflammatory mediators into the systemic circulation and causes injury to vessels and other organs distant from the pancreas; this may result in systemic inflammatory response syndrome (SIRS), multiorgan failure, and death.19,36 Local tissue injury results in infection, abscess and pseudocyst formation, disruption of the pancreatic duct, and severe hemorrhage and shock.19
Assessment and Diagnosis
The clinical manifestations of acute pancreatitis range from mild to severe and often mimic those of other disorders (Box 30-7). Acute onset of abdominal pain, nausea, and vomiting are hallmark symptoms.19,32,37 Epigastric to periumbilical pain may vary from mild and tolerable to severe and incapacitating. Many patients report a twisting or knifelike sensation that radiates to the low dorsal region of the back. The patient may obtain some comfort by leaning forward or assuming a semifetal position. Other clinical findings include fever, diaphoresis, weakness, tachypnea, hypotension, and tachycardia. Depending on the extent of fluid loss and hemorrhage, the patient may exhibit signs of hypovolemic shock.19,37
Physical Examination
The results of physical assessment usually reveal hypoactive bowel sounds and abdominal tenderness, guarding, distention, and tympany. Findings that may indicate pancreatic hemorrhage include Grey Turner sign (gray-blue discoloration of the flanks) and Cullen sign (discoloration of the umbilical region); however, they are rare and usually seen several days into the illness.19 A palpable abdominal mass indicates the presence of a pseudocyst or abscess.19
Laboratory Studies
Assessment of laboratory data usually demonstrates elevated levels of serum amylase and lipase. Serum lipase is more pancreas specific than amylase and a more accurate marker for acute pancreatitis. Amylase is present in other body tissues, and other disorders (e.g., intra-abdominal emergencies, renal insufficiency, salivary gland trauma, liver disease) may contribute to an elevated level. Unlike other serum enzymes, however, amylase is excreted in urine, and this clearance increases with acute pancreatitis. Measurement of urinary versus serum amylase should be considered in light of the patient’s creatinine clearance. The serum amylase level may be elevated for only 3 to 5 days; if the patient delays seeking treatment, a normal level (false-negative result) may be detected. A marker of severity may be determined with a serum cross-reactive (C-reactive) protein level.19,38 Leukocytosis, hypocalcemia, hyperglycemia, hyperbilirubinemia, and hypoalbuminemia may also be present (Table 30-2).19,36,38
TABLE 30-2
LABORATORY TESTS AND DIAGNOSTIC PROCEDURES FOR ACUTE PANCREATITIS
| STUDY | FINDING IN PANCREATITIS |
| Laboratory Studies | |
| Serum amylase | Elevated |
| Serum isoamylase | Elevated |
| Urine amylase | Elevated |
| Serum lipase (if available) | Elevated |
| Serum triglycerides | Elevated |
| Cross-reactive protein | Elevated |
| Glucose | Elevated |
| Calcium | Decreased |
| Magnesium | Decreased |
| Potassium | Decreased |
| Albumin | Decreased or increased |
| White blood cell count | Elevated |
| Bilirubin | May be elevated |
| Liver enzymes | May be elevated |
| Prothrombin time | Prolonged |
| Arterial blood gases | Hypoxemia, metabolic acidosis |
| Diagnostic Procedures | |
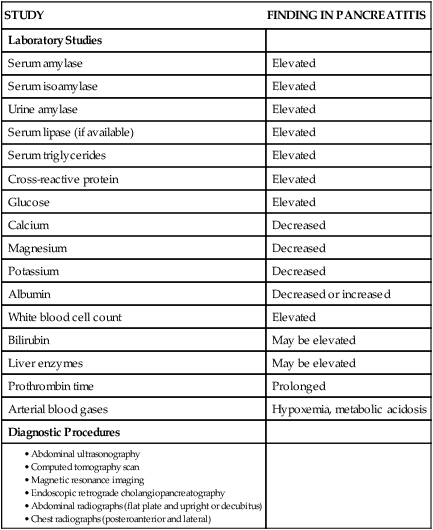
Modified from Krumberger JM. Acute pancreatitis. Crit Care Nurs Clin North Am. 1993;5:185.
Diagnostic Procedures
An abdominal ultrasound scan is obtained as part of the diagnostic evaluation to determine the presence of biliary stones. Contrast-enhanced computed tomography (CT) is considered the gold standard for diagnosing pancreatitis and for ascertaining the overall degree of pancreatic inflammation and necrosis.19,32
Medical Management
Initial management of the patient with severe acute pancreatitis includes ensuring adequate fluid and electrolyte replacement, providing nutritional support, and correcting metabolic alterations.35 Careful monitoring for systemic and local complications is critical.
Fluid Management
Because pancreatitis if often associated with massive fluid shifts, intravenous crystalloids and colloids are administered immediately to prevent hypovolemic shock and maintain hemodynamic stability. Electrolytes are monitored closely, and abnormalities such as hypocalcemia, hypokalemia, and hypomagnesemia are corrected.37 If hyperglycemia develops, exogenous insulin may be required.
Nutritional Support
Over the past three decades, nutritional support has shifted. Previously, conventional nutritional management was to place the patient on a nothing-by-mouth (NPO) regimen and institute intravenous hydration. The rationale was to rest the inflamed pancreas and prevent enzyme release. Enteral or parental support should be initiated if oral intake is withheld more than 5 to 7 days.39 Randomized clinical trials have demonstrated that enteral feeding (gastric or jejunal) is safe and cost effective and that it is associated with fewer septic and metabolic complications than other methods.40–42 Enteral feeding enhances immune modulation and maintenance of the intestinal barrier, and it avoids complications associated with parental nutrition. Early initiation of enteral feeding is preferred over TPN.39 However, TPN still has a role for the critically ill patient with acute pancreatitis who does not tolerate enteral feeding or when nutritional goals are not reached within 2 days.37,41,42 In the past, nasogastric suction was also recommended, but this intervention has not been shown to be beneficial and should be instituted only if the patient has persistent vomiting, obstruction, or gastric distention.37
Systemic Complications
Acute pancreatitis can affect every organ system, and recognition and treatment of systemic complications are crucial to management of the patient (Box 30-11). The most serious complications are hypovolemic shock, acute respiratory distress syndrome (ARDS), acute kidney injury (AKI), and GI hemorrhage. Hypovolemic shock is the result of relative hypovolemia resulting from third spacing of intravascular volume and vasodilation caused by the release of inflammatory immune mediators. These mediators also contribute to the development of ARDS and AKI. Other possible pulmonary complications include pleural effusions, atelectasis, and pneumonia.37
Local Complications
Local complications include the development of infected pancreatic necrosis and pancreatic pseudocyst.19,32,38 The necrotic areas of the pancreas can lead to development of a widespread pancreatic infection (infected pancreatic necrosis), which significantly increases the risk of death.
Prophylactic antibiotics may not reduce mortality in patients suspected of having necrotizing pancreatitis.43 The use of IV antibiotics should not be used prophylactically but if sepsis, abscess, or biliary calculi is evident.37 After the patient develops infected necrosis, however, surgical débridement is necessary.36 The procedure of choice is a minimal invasive necrosectomy, which entails careful débridement of the necrotic tissue in and around the pancreas. A pancreatic pseudocyst is a collection of pancreatic fluid enclosed by a nonepithelialized wall. Cyst formation may result from liquefaction of a pancreatic fluid collection or from direct obstruction in the main pancreatic duct.37 A pancreatic pseudocyst may (1) resolve spontaneously; (2) rupture, resulting in peritonitis; (3) erode a major blood vessel, resulting in hemorrhage; (4) become infected, resulting in abscess; or (5) invade surrounding structures, resulting in obstruction. Treatment involves drainage of the pseudocyst surgically, endoscopically, or percutaneously.36,37,44
Nursing Management
Nursing management of the patient with pancreatitis incorporates a variety of nursing diagnoses (Box 30-8). Nursing interventions include providing pain relief and emotional support, maintaining surveillance for complications, and educating the patient and family.
Providing Comfort and Emotional Support
Pain management is a major priority in acute pancreatitis. Administration of around-the-clock analgesics to achieve pain relief is essential. Morphine, fentanyl, or hydromorphine are the commonly used narcotics for pain control.37 Relaxation techniques and the knee-chest position can also assist in pain control.
Maintaining Surveillance for Complications
The patient must be routinely monitored for signs of local or systemic complications (Box 30-10). Intensive monitoring of each of the organ systems is imperative because organ failure is a major indicator of the severity of the disease.19 The patient must be closely monitored for signs and symptoms of pancreatic infection, which include increased abdominal pain and tenderness, fever, and increased white blood cell count (see Box 30-10).19
Educating the Patient and Family
Early in the patient’s hospital stay, the patient and family should be taught about acute pancreatitis and its causes and treatment. As the patient moves toward discharge, teaching should focus on the interventions necessary for preventing the recurrence of the precipitating disorder. If sustained, permanent damage to the pancreas has occurred, the patient will require teaching specific to diet modification and supplemental pancreatic enzymes. Diabetes education may also be necessary. If an alcohol abuser, the patient should be encouraged to stop drinking and be referred to an alcohol cessation program (Box 30-9). Collaborative management of the patient with pancreatitis is outlined in Box 30-12.
Acute Liver Failure
Description
Acute liver failure (ALF), is a life-threatening condition characterized by severe and sudden liver cell dysfunction, coagulopathy, and hepatic encephalopathy.45 Although uncommon, ALF is associated with a mortality rate as high as 40%, and it usually occurs in patients without pre-existing liver disease.45 Because liver transplantation is one of the few definitive treatments, the patient with ALF should be transferred to a critical care unit and strongly considered for referral to a major medical center where transplantation services are available.45
Etiology
The causes of ALF include infections, medications, toxins, hypoperfusion, metabolic disorders, and surgery (Box 30-13); however, viral hepatitis and medication-induced liver damage are the predominant causes in North America. Patients are usually healthy before the onset of symptoms because ALF tends to occur in patients with no known liver history. A thorough medication and health history is imperative to determine a possible cause. The patient should be questioned about exposure to environmental toxins, hepatitis, intravenous drug use, sexual history, viral hepatitis, medication toxicity, and poisoning. Additional vascular causes such as thrombosis, ischemia, and Budd-Chiari syndrome and metabolic disorders such as Reye syndrome, Wilson disease, galactosemia, and fructose intolerance should be considered.45
Pathophysiology
ALF is a syndrome characterized by the development of acute liver failure over 1 to 3 weeks, followed by the development of hepatic encephalopathy within 8 weeks, in a patient with a previously healthy liver. The interval between the failure of the liver and the onset of hepatic encephalopathy usually is less than 2 weeks. The underlying cause is massive necrosis of the hepatocytes.46
Acute liver failure results in a number of derangements, including impaired bilirubin conjugation, decreased production of clotting factors, depressed glucose synthesis, and decreased lactate clearance. This results in jaundice, coagulopathies, hypoglycemia, and metabolic acidosis. Other effects of acute liver failure include increased risk of infection and altered carbohydrate, protein, and glucose metabolism. Hypoalbuminemia, fluid and electrolyte imbalances, and acute portal hypertension contribute to the development of ascites.45 Hepatic encephalopathy is thought to result from failure of the liver to detoxify various substances in the bloodstream, and it may be worsened by metabolic and electrolyte imbalances.46
The patient may experience a variety of other complications, including cerebral edema, cardiac dysrhythmias, acute respiratory failure, sepsis, and AKI. Cerebral edema and increased intracranial pressure (ICP) develop as a result of breakdown of the blood–brain barrier and astrocyte swelling. Circulatory failure that mimics sepsis is common in ALF and may exacerbate low cerebral perfusion pressure (CPP).47 Hypoxemia, acidosis, electrolyte imbalances, and cerebral edema can precipitate the development of cardiac dysrhythmias. Acute respiratory failure, progressing to ARDS, intrapulmonary shunting, ventilation–perfusion mismatch, sepsis, and aspiration may attribute to the universal arterial hypoxemia.47
Assessment and Diagnosis
Early recognition of ALF is essential. The diagnosis should include potentially reversible conditions (e.g., autoimmune hepatitis) and should differentiate ALF from decompensating chronic liver disease. Prognostic indicators such as coma grade, serum bilirubin, prothrombin time, coagulation factors, and pH should be assessed and potential causes investigated.45
Signs and symptoms of ALF include headache, hyperventilation, jaundice, mental status changes, palmar erythema, spider nevi, bruises, and edema. The patient should be evaluated for the presence of asterixis, or “liver flap,” best described as the inability to voluntarily sustain a fixed position of the extremities. Asterixis is best recognized by downward flapping of the hands when the patient extends the arms and dorsiflexes the wrists. Hepatic encephalopathy is assessed by using a grading system that stages the encephalopathy according to the patient’s clinical manifestations (Box 30-14). Diagnostic findings include prolonged prothrombin times, elevated levels of serum bilirubin, aspartate aminotransferase (AST), alkaline phosphatase, and serum ammonia and decreased levels of serum albumin.46 Arterial blood gases (ABGs) reveal respiratory alkalosis, metabolic acidosis, or both. Hypoglycemia, hypokalemia, and hyponatremia also may be present.46,47
Factors I (fibrinogen), II (prothrombin), V, VII, IX, and X are produced exclusively by the liver. Prothrombin time may be the most useful of tests of these in the evaluation of acute ALF because levels may be 40 to 80 seconds above control values. Test results show decreased levels of plasmin and plasminogen and increased levels of fibrin and fibrin-split products. Platelet counts may be less than 100,000/mm3.46
Medical Management
Medical interventions are directed toward management of the multiple system impact of ALF.
Ammonia Levels
Antibiotics such as neomycin, metronidazole, rifaximin, or lactulose, which is the gold standard, are administered to remove or decrease production of nitrogenous wastes in the large intestine. Antibiotics reduces bacterial flora of the colon. This aids in decreasing ammonia formation by decreasing bacterial action on the protein in feces. Side effects include renal toxicity and hearing impairment. Lactulose, a synthetic ketoanalogue of lactose split into lactic acid and acetic acid in the intestine, is given orally through a nasogastric tube or as a retention enema. The result is the creation of an acidic environment that results in ammonia being drawn out of the portal circulation. Lactulose has a laxative effect that promotes expulsion.46,47
Complications
Bleeding is best controlled through prevention. If an invasive procedure (e.g., central line placement, ICP monitor) will be performed or the patient develops active bleeding, vitamin K, fresh-frozen plasma (to maintain a reasonable prothrombin time), and platelet transfusions are necessary.25 Metabolic disturbances such as hypoglycemia, metabolic acidosis, hypokalemia, and hyponatremia should be monitored and treated appropriately. Prophylactic antibiotic administration may be initiated because the patient is at high risk for an infection.25 The development of cerebral edema necessitates ICP monitoring. Treatment with mannitol has been shown to be of benefit in managing ICP in the patient with ALF, but it must be used with caution in patients with renal failure to avoid hyperosmolarity.47 Other interventions to control ICP include elevating the head of the bed (HOB) to 30 degrees, treating fever and hypertension, minimizing noxious stimulation, and correcting hypercapnia and hypoxemia.25 Renal failure develops in 70% of patients with ALF and continuous renal replacement therapy (CRRT) provides renal support.25 Hemodynamic instability is a common complication necessitating fluid administration and vasoactive medications to prevent prolonged episodes of hypotension. A pulmonary artery catheter may be used to guide clinical management.46
If ALF continues and the patient shows no immediate signs of improvement or reversal, the patient should be considered for a liver transplantation. Prompt referral to a transplantation center should be a high priority for patients experiencing ALF.25,45
Nursing Management
Nursing management of the patient with ALF incorporates a variety of nursing diagnoses (Box 30-15). Nursing interventions include protecting the patient from injury, providing comfort and emotional support, maintaining surveillance for complications, and educating the patient and family.
Protecting the Patient from Injury
Use of benzodiazepines and other sedatives is discouraged in the patient with ALF because pertinent neurologic changes may be masked and hepatic encephalopathy may be exacerbated.47 These patients are often very difficult to manage because they may be extremely agitated and combative. Physical restraint may be necessary to prevent injury to the patient.
Educating the Patient and Family
Early in the patient’s hospital stay, the patient and family should be taught about ALF and its causes and treatment. As patient discharge is imminent, teaching should focus on the interventions necessary for preventing the recurrence of the precipitating cause. If the patient is considered a candidate for liver transplantation, the patient and family will need specific information regarding the procedure and care. Evaluation for liver transplantation may include screening for medical contraindications, human immunodeficiency virus (HIV) serology, anticipated compliance, and assessment of the social support system. Psychiatric and other specialty team consultations are necessary for a thorough evaluation of the patient’s suitability for a liver transplantation (Box 30-16).
Collaborative management of the patient with ALF is outlined in Box 30-17.
Gastrointestinal Surgery
Types of Surgery
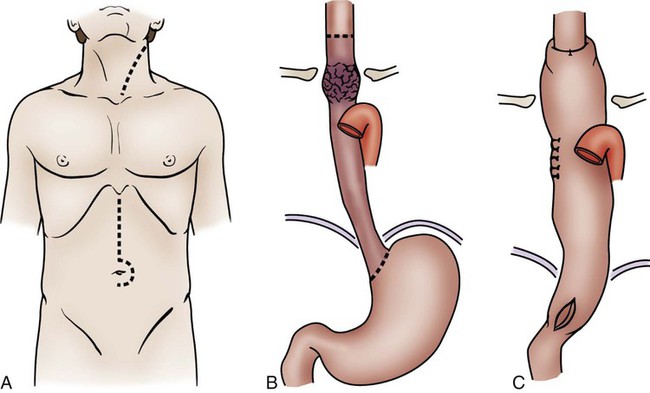
Esophagectomy
Esophagectomy is usually performed for cancer of the distal esophagus and gastroesophageal junction. The technically difficult procedure involves the removal of part or all of the esophagus, part of the stomach, and lymph nodes in the surrounding area. The stomach is then pulled up into the chest and connected to the remaining part of the esophagus. If the entire esophagus and stomach must be removed, part of the bowel may be used to form the esophageal replacement (Figs. 30-5 and 30-6).48
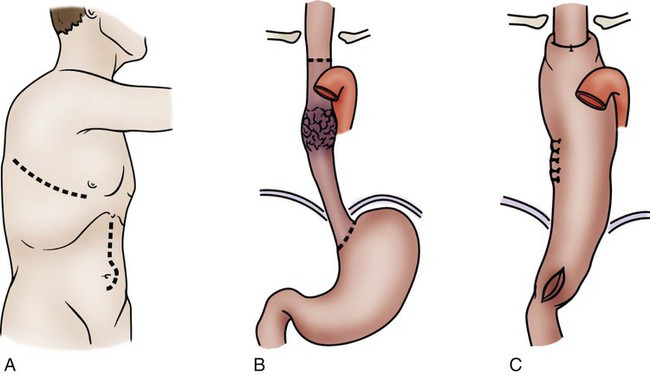
Pancreaticoduodenectomy
The standard operation for pancreatic cancer is a pancreaticoduodenectomy, also called the Whipple procedure. In the Whipple procedure, the pancreatic head, the duodenum, part of the jejunum, the common bile duct, the gallbladder, and part of the stomach are removed. The continuity of the GI tract is restored by anastomosing the remaining portion of the pancreas, the bile duct, and the stomach to the jejunum (Fig. 30-7).48
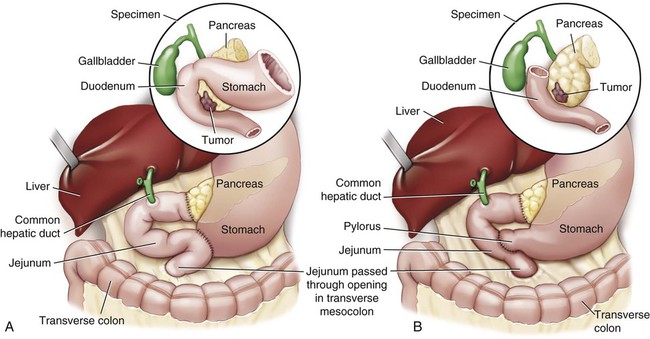
A, The standard Whipple procedure involves resection of the gastric antrum, head of pancreas, distal bile duct, and entire duodenum with reconstruction as shown. B, The pylorus-preserving Whipple procedure does not include resection of the distal stomach, pylorus, or proximal duodenum.
Bariatric Surgery
Bariatric surgery refers to surgical procedures of the GI tract that are performed to induce weight loss. Bariatric procedures are divided into three broad types: (1) restrictive, (2) malabsorptive, and (3) combined restrictive and malabsorptive.49 Restrictive procedures such as vertical banded gastroplasty (VBG) (Fig. 30-8A) and gastric banding (see Fig. 30-8B) reduce the capacity of the stomach and limit the amount of food that can be consumed. Malabsorptive procedures such as the biliopancreatic diversion (BPD) (see Fig. 30-8C) alter the GI tract to limit the digestion and absorption of food. The Roux-en-Y gastric bypass (RYGBP) (see Fig. 30-8D) combines both strategies by creating a small gastric pouch and anastomosing the jejunum to the pouch. Food then bypasses the lower stomach and duodenum, resulting in decreased absorption of digestive materials.49
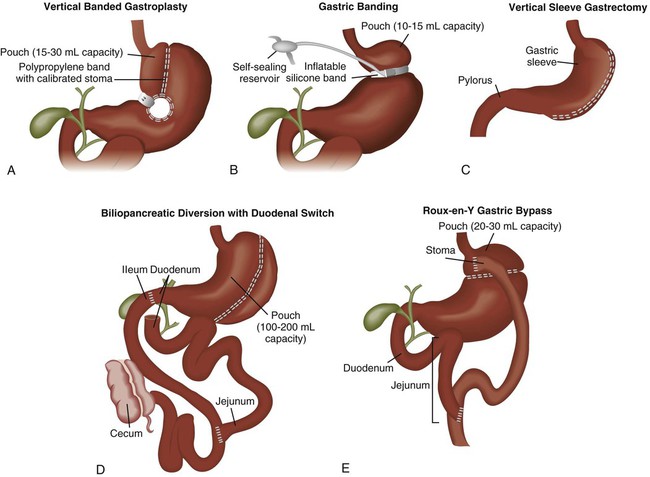
A, Vertical banded gastroplasty involves creating a small gastric pouch. B, Adjustable gastric banding uses a band to create a gastric pouch. C, Vertical sleeve gastrectomy involves creating a sleeve-shaped stomach by removing about 80% of the stomach. D, Biliopancreatic diversion with duodenal switch procedure creates an anastomosis between the stomach and intestine. E, Roux-en-Y gastric bypass procedure involves constructing a gastric pouch whose outlet is a Y-shaped limb of small intestine. (From Lewis SL, et al. Medical-Surgical Nursing: Assessment and Management of Clinical Problems. 8th ed. St. Louis: Mosby; 2011.)
Preoperative Care
A thorough preoperative evaluation should be conducted to evaluate the patient’s physical status and identify risk factors that may affect the postoperative course. Because obesity is associated with a higher incidence of co-morbidities such as cardiovascular disease, hypertension, diabetes, gastroesophageal reflux, obstructive sleep apnea, and heart failure, an extensive workup may be required for the patient who underwent bariatric surgery.48,50
Surgical Considerations
Two approaches may be used for esophageal resection: transhiatal or transthoracic (see Figs. 30-5 and 30-6). In both approaches, the stomach is mobilized through an abdominal incision and then transposed into the chest. The anastomosis of the stomach to the esophagus is performed in the chest (transthoracic) or in the neck (transhiatal). The approach selected depends on the location of the tumor, the patient’s overall health and pulmonary function, and the experience of the surgeon. After surgery, the patient has a nasogastric tube in place, and it should not be manipulated because of the potential to damage the anastomosis. Those who undergo transthoracic esophagectomy have chest tubes.48
Most bariatric procedures can be performed using an open or laparoscopic surgical technique. Although laparoscopic approaches are more technically difficult to perform, they have largely replaced open procedures because they are associated with decreased pulmonary complications, less postoperative pain, reduced length of hospital stay, fewer wound complications (e.g., infections, incisional hernia), and an earlier return to full activity.48,49 Open procedures are performed on patients who have had prior upper abdominal surgery, are morbidly obese, or who may not be able to tolerate the increased abdominal pressure associated with laparoscopic procedures.48
Complications and Medical Management
Several complications are associated with GI surgery, including respiratory failure, atelectasis, pneumonia, anastomotic leak, deep vein thrombosis, pulmonary embolus, and bleeding. The morbidly obese patient is at even greater risk for many postoperative complications.49
Pulmonary Complications
The risk for pulmonary complications is substantial after GI surgery, and adverse respiratory events such as atelectasis and pneumonia are twice as likely to occur in the patient who is obese.50 Aggressive pulmonary exercise should be initiated in the immediate postoperative period. Early ambulation and adequate pain control assist in reducing the risk of atelectasis development. Suctioning, chest physiotherapy, or bronchodilators may be needed to optimize pulmonary function. Patients should be closely monitored for the development of oxygenation problems. Treatment should be aimed at supporting adequate ventilation and gas exchange. Mechanical ventilation may be required in the event of respiratory failure.
Anastomotic Leak
An anastomotic leak is a severe complication of GI surgery. It occurs when there is a breakdown of the suture line in a surgical anastomosis and results in leakage of gastric or intestinal contents into the abdomen or mediastinum (transthoracic esophagectomy).48 The clinical signs and symptoms of a leak can be subtle and often go unrecognized. They include tachycardia, tachypnea, fever, abdominal pain, anxiety, and restlessness.48 In the patient who had an esophagectomy, a leak of the esophageal anastomosis may manifest as subcutaneous emphysema in the chest and neck.48 If undetected, a leak can result in sepsis, multiorgan failure, and death. Patients with progressive tachycardia and tachypnea should have a radiologic study (upper GI study with Gastrografin or CT scan with contrast) to rule out an anastomotic leak.50 The type of treatment depends on the severity of the leak. If the leak is small and well contained, it may be managed conservatively by maintaining the NPO status, administering antibiotics, and draining the fluid percutaneously. If the patient is deteriorating rapidly, an urgent laparotomy is indicated to repair the defect.48,50
Deep Vein Thrombosis and Pulmonary Embolism
Pulmonary embolism (PE) is a very serious complication of any surgical procedure. Deep vein thrombosis (DVT) prophylaxis should be initiated before surgery and continued until the patient is fully ambulatory to reduce the risk of clot development. Typically, a combination of sequential compression devices and subcutaneous unfractionated heparin or low–molecular-weight heparin is used. Patients determined to be at high risk for PE may benefit from prophylactic inferior vena cava filter placement.48
Bleeding
Upper GI bleeding is an uncommon but life-threatening complication of GI surgery. Early bleeding usually occurs at the site of the anastomosis and can usually be treated through endoscopic intervention. Surgical revision may be needed for persistent, uncontrolled bleeding. Late bleeding is usually a result of ulcer development. Medical therapy is aimed at the prevention of this complication through administration of histamine 2 (H2)–antagonists or PPIs.5
Postoperative Nursing Management
Nursing care of the patient who has had GI surgery incorporates a number of nursing diagnoses (Box 30-18). Nursing management involves interventions aimed at optimizing oxygenation and ventilation, preventing atelectasis, providing comfort and emotional support, and maintaining surveillance for complications.
Pain Management
It is imperative to appropriately manage the patient’s pain after GI surgery. Adequate analgesia is necessary to promote the mobility of the patient and decrease pulmonary complications. Initial pain management may be accomplished by intravenous opioid (morphine, hydromorphone) administration by means of a patient-controlled analgesia (PCA) pump, or through continuous epidural infusion of an opioid and local anesthetic (bupivacaine).48 Oral pain medications can be started after an anastomosis leak is ruled out. Nonpharmacologic interventions such as positioning, application of heat or cold, and distraction may also be used. If the patient’s pain is not being sufficiently relieved, the pain management service should be consulted.48,50
Therapeutic Management
Gastrointestinal Intubation
Because GI intubation is used so often in critical care units, it is important for nurses to know the clinical indications and responsibilities inherent in tube use. The three categories of GI tubes are based on function: (1) nasogastric suction tubes, (2) long intestinal tubes, and (3) feeding tubes (Box 30-19).
Nasogastric Suction Tubes
Nasogastric tubes remove fluid regurgitated into the stomach, prevent accumulation of swallowed air, may partially decompress the bowel, and reduce the patient’s risk for aspiration. Nasogastric tubes also can be used for collecting specimens, assessing the presence of blood, and administering tube feedings. The most common nasogastric tubes are the single-lumen Levin tube and the double-lumen Salem sump. The Salem sump has one lumen that is used for suction and drainage and another that allows air to enter the patient’s stomach and prevents the tube from adhering to the gastric wall and damaging the mucosa. The tube is passed through the nose into the nasopharynx and then down through the pharynx into the esophagus and stomach. The length of time the nasogastric tube remains in place depends on its use. The tube is then placed to gravity, low intermittent suction, or low continuous suction, and in rare instances, it is clamped.51
Nursing management focuses on preventing complications common to this therapy, for example, ulceration and necrosis of the nares, esophageal reflux, esophagitis, esophageal erosion and stricture, gastric erosion, and dry mouth and parotitis from mouth breathing. Interference with ventilation and coughing, aspiration, and loss of fluid and electrolytes can be critical problems. Interventions include irrigating the tube every 4 hours with normal saline, ensuring the blue air vent of the Salem sump is patent and maintained above the level of the patient’s stomach, and providing frequent mouth and nares care (Box 30-20).51
Long Intestinal Tubes
Interventions used in the care of the patient with a long intestinal tube are similar to those with a nasogastric tube. The patient should be observed for (1) gaseous distention of the balloon section, which makes removal difficult; (2) rupture of the balloon; (3) overinflation of the balloon, which can lead to intestinal rupture; and (4) reverse intussusception if the tube is removed rapidly. Intestinal tubes should be removed slowly; usually 6 inches of the tube is withdrawn every hour.51
Feeding Tubes
Small-diameter (8- to 12-Fr [French]) flexible feeding tubes, such as Dobhoff tubes, are commonly placed at the bedside for patients who cannot take nourishment orally. The feeding tube may be inserted orally or nasally so that the tip ends up in the stomach or duodenum. To facilitate passage into the GI tract, these tubes have a weighted tungsten tip, and a guidewire is needed to prevent them from curling up in the back of the patient’s throat. A radiograph must be obtained to verify correct placement of the tube before initiating feeding.52 The tube should also be marked with indelible ink where it exits the mouth or nares so that tube location can be checked at 4-hour intervals.52
Nursing management of the patient with a feeding tube includes prevention of complications and monitoring the tolerance of feeding. Tracheobronchial aspiration of gastric contents is a serious potential complication.52–54 Before administering medications or feedings, it is important to ensure that the tube is in the patient’s stomach or duodenum. Assessing the exit point marked on the tube helps determine whether the tube has maintained the same position. Looking for coiling in the mouth or throat can help detect upward displacement that may have occurred as a result of vomiting. The traditional practice of confirming placement by auscultating air inserted through the tube over the epigastrium is not reliable and is not recommended.52–55 If any doubt exists about the tube’s position, a repeat radiograph should be obtained. During feedings, the head of bed should be elevated at least 30 degrees to minimize the risk of aspiration, and gastric residuals should be checked at least every 4 to 6 hours.53,54 Large gastric residuals, cramping, and abdominal distention may indicate intolerance of feeding, and the physician should be notified.54 Other interventions include nares and oral care and flushing the tube with water before and after medication administration to maintain patency.54
Endoscopic Injection Therapy
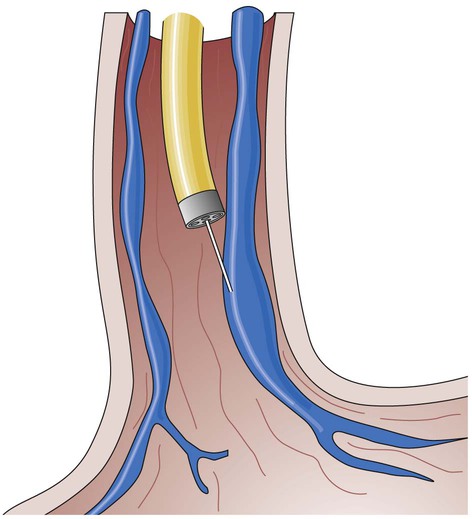
Endoscopic injection therapy is used to control bleeding of ulcers. It may be performed emergently, electively, or prophylactically. An endoscope is introduced through the patient’s mouth, and endoscopy of the esophagus and stomach is performed to identify the bleeding varices or ulcers. An injector with a retractable 23- to 25-gauge needle is introduced through the biopsy channel of the endoscope. The needle then is inserted in or around the varices or into the area around the ulcer, and a liquid agent is injected (Fig. 30-9). The most commonly used agent is epinephrine, which results in localized vasoconstriction and enhanced platelet aggregation. Sclerosing agents such as ethanolamine, alcohol, and polidocanol also may be used. These agents cause an inflammatory reaction in the vessel that results in thrombosis and eventually produces a fibrous band. Repeated sclerotherapy results in the development of supportive scar tissue around the varices. Other embolic agents are used, including fibrinogen and thrombin, which when injected together react to form an active fibrin clot, and “glues” (n-butyl cyanoacrylate), which are used as a sealant to stop the bleeding.20,24,56
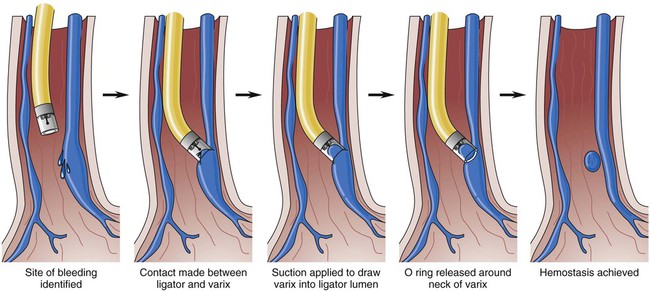
Endoscopic Variceal Ligation
Endoscopic variceal ligation (EVL) involves applying bands or metal clips around the circumference of the bleeding varices to induce venous obstruction and control bleeding. EVL has replaced endoscopic sclerotherapy of variceal hemorrhage (Fig. 30-10). Between 1 and 2 days after the procedure, necrosis and scar formation promote band and tissue sloughing. Fibrinous deposits within the healing ulcer potentiate vessel obliteration. Band ligation is accomplished through endoscopy, with multiple bands placed per session.16 The procedure may be repeated on an inpatient or outpatient basis every 1 to 2 weeks until all the varices are obliterated.56 Endoscopic variceal ligation controls bleeding in approximately 80% to 90% of the time.56 The most common complication of endoscopic variceal ligation is the development of superficial mucosal ulcers. Varices may reoccur as local banding does not affect portal pressure.16,57
Transjugular Intrahepatic Portosystemic Shunt
TIPS is an angiographic interventional procedure for decreasing portal hypertension. TIPS is advocated for (1) patients with portal hypertension who are also experiencing active bleeding or have poor liver reserve, (2) transplant recipients, and (3) patients with other operative risks.25,57 The TIPS procedure is usually performed by a gastroenterologist, vascular surgeon, or interventional radiologist.
Portal hypertension is confirmed by direct measurement of the pressure in the portal vein (gradient greater than 10 mm Hg). Cannulation is achieved through the internal jugular vein, and an angiographic catheter is advanced into the middle or right hepatic vein. The midhepatic vein is then catheterized, and a new route is created connecting the portal and hepatic veins using a needle and guidewire with a dilating balloon. A polytetrafluoroethylene (PTFE)–coated stent is then placed in the liver parenchyma to maintain that connection (Fig. 30-11). The increased resistance in the liver is bypassed.25,58 TIPS may be performed on patients with bleeding varices, with refractory bleeding varices, or as a bridge to liver transplantation if the candidate becomes hemodynamically unstable. Postprocedural care should include observation for overt (cannulation site) or covert (intrahepatic site) bleeding, hepatic or portal vein laceration (resulting in rapid loss of blood volume), and inadvertent puncture of surrounding organs. Other complications include hepatic encephalopathy, liver failure, bacteremia, and stent stenosis.25,58
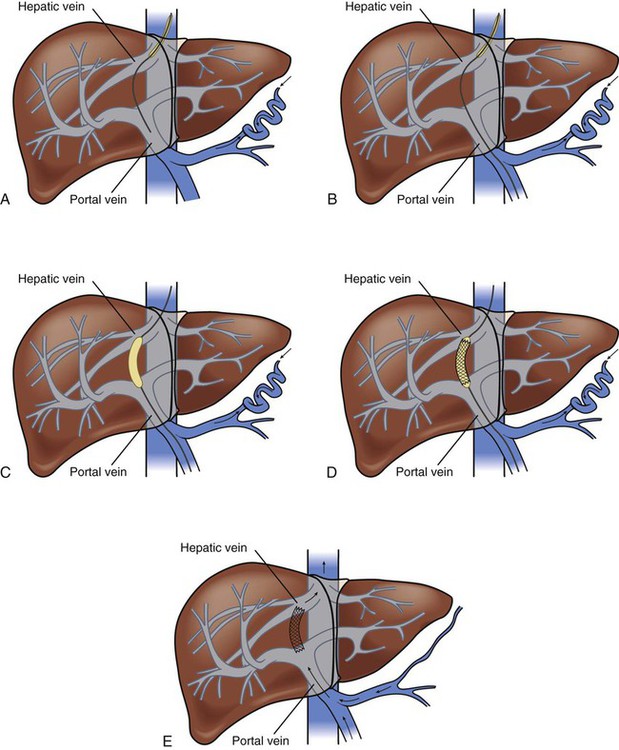
A, Needle directed though liver parenchyma to portal vein. B, Needle and guidewire passed down to midportal vein. C, Balloon dilation. D, Deployment of stent. E, Intrahepatic shunt from portal to hepatic vein. (From Urder LU, et al. Priorities in Critical Care Nursing. 6th ed. St. Louis: Mosby; 2012.)
Pharmacologic Agents
Many pharmacologic agents are used in the care of patients with GI disorders. Table 30-3 reviews the various agents and any special considerations necessary for administering them.
TABLE 30-3
PHARMACOLOGIC MANAGEMENT
Gastrointestinal Disorders
| MEDICATION | DOSAGE | ACTIONS | SPECIAL CONSIDERATIONS |
| Antacids | 30-90 mL q1-2h PO or NG; possibly titrated to NG pH | Used to buffer stomach acid and raise gastric pH | Can cause diarrhea or constipation and electrolyte disturbances Irrigate NG tube with water after administration because antacids can clog tube |
| Histamine2 (H2) Antagonists | |||
| Cimetidine (Tagamet) | 300 mg q6h IV or PO | Used to reduce volume and concentration of gastric secretions | Side effects include CNS toxicity (confusion or delirium) and thrombocytopenia Separate administration of antacids and PO histamine blocking agents by 1 hour Dosage adjustments recommended for patients with moderate (creatinine clearance <50 mL/min) or severe (creatinine clearance <10 mL/min) renal insufficiency |
| Ranitidine (Zantac) | 150 mg q12h PO or 50 mg q8h IV | ||
| Famotidine (Pepcid) | 40 mg daily PO or 20 mg q12h IV | ||
| Nizatidine (Axid) | 150 mg q12h PO or 300 mg q24h | ||
| Gastric Mucosal Agents | |||
| Sucralfate (Carafate) | 1 g q6h NG or PO, given 1 hour before meals and at bedtime | Forms an ulcer-adherent complex with proteinaceous exudates Covers the ulcer and protects against acid, pepsin, and bile salts |
Requires an acid medium for activation; do not administer within 30 minutes of an antacid May cause severe constipation May cause decreased absorption of certain medications |
| Gastric Proton-Pump Inhibitors | |||
| Omeprazole (Prilosec) | 20-40 mg q12h PO | Inactivates acid, or hydrogen, acid pump, blocking secretion of hydrochloric acid by gastric parietal cells | Capsules should be swallowed intact May increase levels of phenytoin, diazepam, warfarin May administer concomitantly with antacids |
| Lansoprazole (Prevacid) | 15-30 mg q24h PO 30 mg over 30 min q24h IV |
||
| Rabeprazole (Aciphex) | 20-40 mg q24h PO | ||
| Esomeprazole (Nexium) | 40 mg q12-24h PO 20-40 mg q24h IV |
||
| Pantoprazole (Protonix) | 20-40 mg q24h PO 80 mg q8-12h IV |
||
| Vasopressin | |||
| (Pitressin Synthetic) | Loading dose of 20 units over 20 min IV, followed by 0.2-0.4 unit/min IV infusion Doses can be increased to 0.9 unit/min, if necessary |
Decreases splanchnic blood flow, reducing portal pressure | Side effects include coronary, mesenteric, and peripheral vasoconstriction May be administered concurrently with nitroglycerin to minimize side effects |
| Somatostatin | |||
| Octreotide (Sandostatin) | Bolus dose of 25-50 mcg followed by IV infusion of 25-50 mcg/hr for 48 hours | Decreases splanchnic blood flow, reducing portal pressure | May cause hyperglycemia or hypoglycemia when initiating the drip and changing dosages |
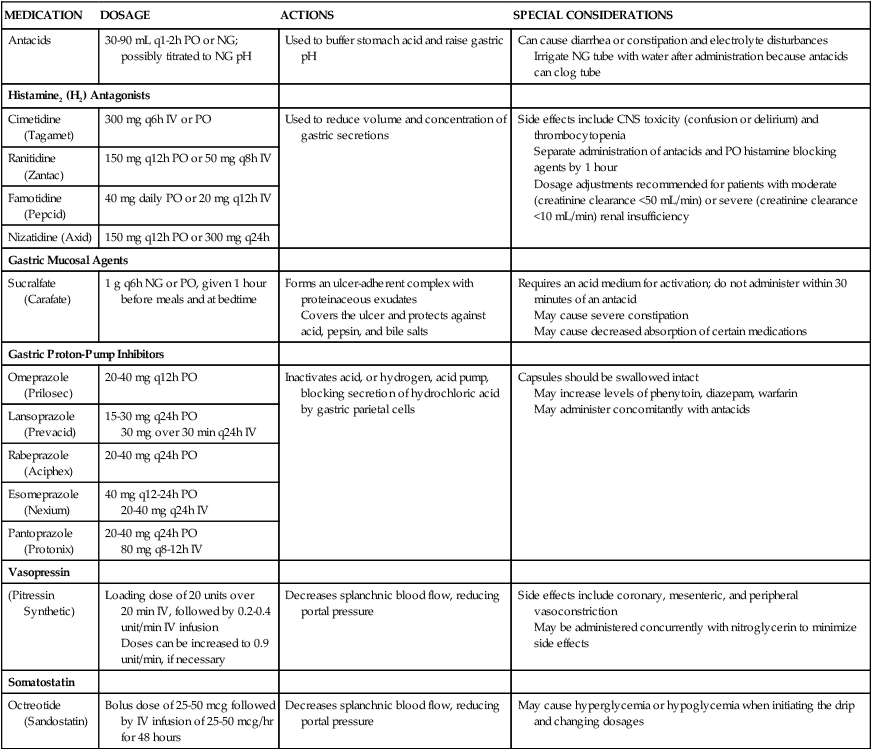
CNS, Central nervous system; IV, intravenous; NG, nasogastric; PO, by mouth.
From Gold Standard. Available at: http://www.mdconsult.com/das/pharm/lookup/340530070-12?type=alldrugs. Accessed June 14, 2012.
Antiulcer Agents
A number of different antiulcer agents are commonly used in the critical care setting, including H2-antagonists, gastric PPIs, and gastric mucosal agents. H2-antagonists are used to decrease the volume and concentration of gastric secretions and control gastric pH, decreasing the incidence of stress-related upper GI bleeding. These agents work by blocking histamine stimulation of the H2-receptors on the gastric parietal cells, reducing acid production.59 Although these medications may be administered orally, intramuscularly, or intravenously, they usually are given intravenously in the critical care setting.
PPIs decrease gastric acid secretion by binding to the proton pump, blocking the release of acid from the gastric parietal cells. PPIs are potent acid inhibitors and have greater suppressive ability and efficacy to consistency reduce rebleeding rates than the H2-agonists.59–61 Esomeprazole, pantoprazole, and lansoprazole are available for intravenous administration. The oral PPIs are formulated as enteric-coated tablets or as delayed-release capsules containing enteric-coated granules. Absorption occurs in an alkaline environment and begins only after the granules leave the stomach and enter the duodenum.
Unlike H2-antagonists or PPIs, sucralfate does not affect gastric acid concentration but rather exerts its action locally. Sucralfate reacts with hydrochloric acid to form a sticky, pastelike substance that adheres to the surface of the ulcer and shields it from pepsin, acid, and bile. Sucralfate predominantly binds to damaged GI mucosa, with minimal adherence to normal tissue.59 It is administered orally or through a gastric tube. Sucralfate should not be crushed but may be dissolved in 10 mL of water to form a slurry. It is also available as a suspension.
Vasopressin
Vasopressin is used to control gastric ulcer and variceal bleeding. It is administered intra-arterially, through a catheter inserted into the right or left gastric artery (through the femoral artery, aorta, and celiac trunk) or intravenously. It causes splanchnic and systemic vasoconstriction, subsequently reducing portal blood flow and pressure.24,62–64
A major side effect of the medication is systemic vasoconstriction, which can result in cardiac ischemia, chest pain, hypertension, acute heart failure, dysrhythmias, phlebitis, bowel ischemia, and cerebrovascular accident. These side effects can be offset with concurrent administration of nitroglycerin.24,62,63 Other complications include bradycardia and fluid retention. Nursing responsibilities associated with the use of this therapy include maintenance of a patent infusion line and continuous monitoring for vasoconstrictive complications of therapy. Because of the known adverse effects, vasopressin is not the first choice of treatment.62,63
Octreotide
Octreotide, a long-acting synthetic analog of somatostatin, is a peptide that is administered parenterally in the patient with acute bleeding and cirrhosis. It reduces splanchnic vasodilation and portal pressure through the inhibition of secretion of various vasodilator hormones, and it is as effective as vasopressin in treating variceal bleeding with minimal side effects. Combined with endoscopic therapy, octreotide is the preferred treatment for achieving hemostasis.16
Summary
Acute Gastrointestinal Hemorrhage
• Acute GI hemorrhage can be caused by peptic ulcer disease or stress-related erosive syndrome, and it can result in hypovolemic shock.
• Medical management focuses on restoration of hemodynamic stability and control of bleeding. Nursing actions include administering volume replacement, controlling the bleeding, and maintaining surveillance for complications.
Acute Pancreatitis
• Acute pancreatitis can be caused by gallstones and alcoholism, and it can result in autodigestion of the pancreas.
• Medical management focuses on fluid management, nutritional support, and control of systemic and local complications.
• Nursing actions include providing comfort and emotional support and maintaining surveillance for complications.
Acute Liver Failure
• ALF is a life-threatening condition characterized by severe and sudden liver cell dysfunction, coagulopathy, and hepatic encephalopathy.
• Medical management focuses on treatment of elevated ammonia levels and control of complications such as bleeding, metabolic disturbances, and cerebral edema.
• Nursing actions include protecting the patient from injury and maintaining surveillance for complications.
Therapeutic Management
• Nasogastric suction tubes and long intestinal tubes are often used in the management of GI disorders.
• Endoscopic procedures to control bleeding include endoscopic injection therapy and endoscopic variceal ligation.
• TIPS is an angiographic interventional procedure for decreasing portal hypertension.
• Common pharmacologic agents used in the management of GI disorders include antacids, H2-antagonists, gastric mucosal agents, PPIs, vasopressin, and octreotide.