Gastrointestinal Bleeding
More than 300,000 annual hospitalizations in the United States are attributed to GI bleeding.1 Upper GI bleeding accounts for most of these hospitalizations, with an incidence rate of 100 cases per 100,000,1 whereas the incidence of lower GI bleeding is estimated at 20 to 27 cases per 100,000.2,3 Mortality rates vary depending on the source of the bleeding. Most nonvariceal upper GI bleeding studies document mortality rates approximating 10%.4,5 These rates have not changed over the past 2 decades despite the evolution of acid suppression therapy, which is probably explained by an aging population with increased comorbid diseases. Mortality rates for lower GI bleeding are usually in the range of 5%.6,7
Clinical Presentation
Initial Evaluation and Resuscitation
The clinical findings in a patient with GI bleeding are crucial in determining the site, cause, and rate of bleeding. The first step in clinical evaluation is to assess the severity of the bleeding. This is done primarily by measuring hemodynamic parameters in an attempt to quantify the amount of blood volume lost (Table 76.1).8 Because bleeding can represent a dynamic ongoing situation, continuous monitoring of hemodynamics is necessary to guide resuscitation efforts and provide key prognostic information. In patients with GI bleeding, two large-bore intravenous catheters should be placed immediately on arrival to restore euvolemia.
Table 76.1
Hemodynamics, Vital Signs, and Blood Loss
| Hemodynamics and Vital Signs | % Blood Loss (Fraction of Intravascular Volume) | Bleeding Type |
| Shock (resting hypotension) | 20-25 | Massive |
| Postural (orthostatic tachycardia or hypotension) | 10-20 | Moderate |
| Normal | <10 | Minor |
From Rockey DC: Gastrointestinal bleeding. Gastroenterol Clin North Am 2005;34:581-588.
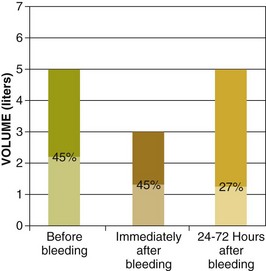
The hematocrit at initial evaluation may not reflect the severity of the bleeding because very recent loss of both plasma and red blood cells leads to a percentage of red blood cells in the remaining blood (which defines the hematocrit) that is close to the same value. The hematocrit drops when hemodilution occurs as extravascular fluid enters the vascular space to restore volume, a process that may take up to 72 hours (Fig. 76.1).9
The rapidity of blood and colloid infusion depends in part on the patient’s cardiovascular condition. Placement of a central venous pressure catheter can help one base decisions on more objective findings. Several recent studies found that transfusion was associated with higher risk for nosocomial infection, multiorgan dysfunction, acute respiratory distress syndrome, and death.10 Current transfusion trends are to administer blood to patients with hemoglobin less than 7 mg/dL and to avoid transfusion when it’s above 10 mg/dL. However, the threshold for blood transfusion should take into consideration the patient’s underlying condition, hemodynamic status, and markers of tissue hypoxemia.11 In patients with conditions associated with defects in platelet or coagulation factors, these substances should be replaced. Patients requiring massive transfusion of packed red blood cells require fresh frozen plasma and platelets. Hypocalcemia can develop in these patients because of the large amount of citrate received with massive transfusion, and thus calcium replacement should be considered, especially in individuals with end-stage liver disease and heart failure, in whom citrate metabolism may be impaired.12 The initial evaluation of patients with nonvariceal upper GI bleeding should include risk stratification of patients into low- and high-risk categories for rebleeding and death based on clinical, laboratory, and when available, endoscopic criteria.10 This allows appropriate intervention and minimizes morbidity and mortality risks. The most extensively validated scores for risk stratification are the Blatchford and Rockall scores13,14 (Tables 76.2 and 76.3). Similarly, risk stratification in lower GI bleeding helps in guiding the management of these patients. Hemodynamic instability, ongoing hematochezia, and comorbid illnesses have been consistently associated with poor outcome in lower GI bleeding.15
Table 76.2
Blatchford Score for Gastrointestinal Bleeding*
| Admission Parameter | Score Value |
| Urea (mg/dL) | |
| ≥6.5 to <8.0 | 2 |
| ≥8 to <10 | 3 |
| ≥10 to <25 | 4 |
| ≥25 | 6 |
| Hemoglobin (g/dL) | |
| Men | |
| ≥12 to <13 | 1 |
| ≥10 to <12 | 3 |
| <10 | 6 |
| Women | |
| ≥10 to <12 | 1 |
| <10 | 6 |
| Systolic blood pressure (mm Hg) | |
| 100 to 109 | 1 |
| 90 to 99 | 3 |
| <90 | 3 |
| Other parameters | |
| Pulse >100 | 1 |
| Melena at presentation | 1 |
| Syncope | 2 |
| Hepatic disease | 2 |
| Cardiac failure | 2 |
*The score is calculated by adding the points from each variable. A score of zero is associated with a low risk of the need for endoscopic intervention
From Blatchford O, Murray WR, Blatchford M: A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet 2000;356:1318-1321.
Table 76.3
Rockall Scoring System for Risk of Rebleeding and Death in Acute Gastrointestinal Bleeding*
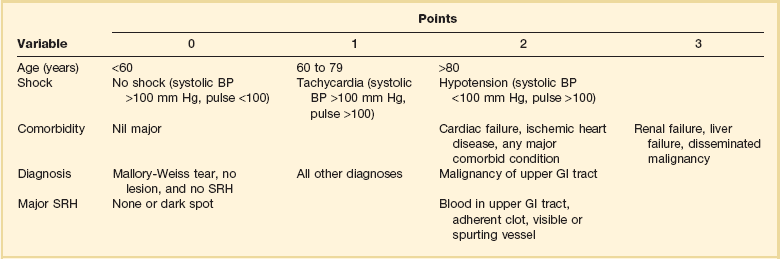
BP, blood pressure; GI, gastrointestinal; SRH, stigma(ta) of recent hemorrhage.
*The score is calculated by adding the points from each variable. A score of 2 or less is associated with a low risk of further bleeding or death.
From Rockall TA, Logan RF, Delvin HB, et al: Risk assessment after acute upper gastrointestinal haemorrhage. Gut 1996;38:316-321.
History and Clinical Findings
After hemodynamic stabilization is achieved, a careful history and clinical examination are imperative to make a preliminary assessment of the location and cause of the bleeding. Important historical features in the evaluation of GI bleeding are shown in Box 76.1.8
Hematemesis typically points to an upper source of bleeding, proximal to the ligament of Treitz. In rare cases, hematemesis can be a sign of swallowed blood from oral, pharyngeal, or nasal bleeding. Melena is defined as black tarry stool with a glistering sheen and results from degradation of blood in the GI tract. At least 50 mL of blood in the upper GI tract is required to cause melena, although volumes up to 100 mL may be clinically silent.16 Melena usually indicates upper GI bleeding, but its source may be the small bowel and sometimes even the proximal part of the colon when the volume of blood is too small to cause hematochezia. Coffee-ground emesis is typically a sign of recent, but currently inactive upper GI bleeding, and its appearance is caused by the acid’s effect on blood in the lumen. Hematochezia is generally associated with colonic bleeding but can also be caused by more proximal bleeding. Proximal bleeding in association with hematochezia is usually more hemodynamically significant.
The use of a nasogastric tube in patients presenting with GI bleeding is a common practice but at the same time controversial. The presumed benefits of nasogastric lavage include confirmation of an upper GI source of bleeding, better visualization during endoscopy, and prediction of high-risk lesions such as an oozing or spurting lesion and nonbleeding visible vessel. A bloody aspirate suggests, with some degree of certainty, an upper source of bleeding because false-positive results are rare and generally related to nasogastric trauma.17 In one study looking at patients presenting with suspected upper GI bleeding, 45% of cases with a bloody aspirate were associated with a high-risk lesion on endoscopy versus 15% when the aspirate was clear or bilious.18 Identifying patients with high-risk lesions is important because those patients have the worst outcome and early endoscopic therapy may have the most benefit. However, the positive and negative predictive values of a bloody aspirate for high-risk lesions are only 45% and 85%, respectively, which makes basing the decision of an early endoscopy on the information obtained by the nasogastric lavage controversial. In a recent retrospective study looking at the impact of performance of nasogastric lavage, this practice was associated with earlier time to endoscopy but had no effect on mortality rate, length of hospital stay, surgery, or transfusion requirements.19 In our opinion, if an upper endoscopy is planned in the next 12 to 24 hours after presentation of a patient with suspected upper GI bleeding, nasogastric lavage should be avoided owing to lack of proven benefit and major discomfort and pain inflicted to the patient. In the setting of hematochezia with hemodynamic instability, extremely brisk upper GI bleeding should be suspected, and a positive nasogastric aspirate can confirm this suspicion.
Diagnostic Tests
The initial laboratory evaluation in patients with GI bleeding should include a complete blood count, liver enzymes, prothrombin time, blood urea nitrogen (BUN), and creatinine. As mentioned earlier, the first hematocrit level may be falsely reassuring, so management decisions should rely on other parameters such as hemodynamics and the nature of the bleeding. A high white blood cell count should alert one to the presence of ischemia or infarction. Thrombocytopenia can be a sign of portal hypertension, and a critically low platelet count, as well as a high prothrombin time, should be addressed immediately by transfusion of platelets and fresh frozen plasma. An elevation in the BUN level out of proportion to creatinine is compatible with upper GI bleeding but may also be seen with intravascular volume depletion from any source of bleeding. In one study, this ratio was significantly higher in patients with upper GI bleeding than in those with lower GI bleeding (22.5 ± 11.5 vs. 15.9 ± 8.2; p = 0.001); however, the degree of overlap shows the poor discriminatory value of this ratio.20 In another study, a ratio greater than 36 had a specificity of 27% and a sensitivity of 90% for upper GI bleeding.21
Upper Gastrointestinal Bleeding
Differential Diagnosis
Nonvariceal Bleeding
Nonvariceal upper GI bleeding remains a significant cause of death and morbidity despite recent advances in pharmacologic and endoscopic therapy. The cause of nonvariceal bleeding encompasses a large array of diagnoses involving multiple organs above the ligament of Treitz and at times outside the GI tract. The causes and frequency of nonvariceal bleeding are listed in Table 76.4.22
Table 76.4
Causes of Nonvariceal Upper Gastrointestinal Bleeding
| Diagnosis | Incidence (%) |
| Peptic ulcer | 30-50 |
| Mallory-Weiss tear | 15-20 |
| Erosive gastritis or duodenitis | 10-15 |
| Esophagitis | 5-10 |
| Malignancy | 1-2 |
| Angiodysplasia or vascular malformations | 5 |
| Other | 5 |
From Ferguson CB, Mitchell RM: Nonvariceal upper gastrointestinal bleeding: Standard and new treatment. Gastroenterol Clin North Am 2005;34:607-621.
Peptic Ulcer Disease
PUD traditionally refers to gastric and duodenal ulcers, gastritis, and duodenitis. A number of population-based and prospective studies rank PUD as the most common source of acute upper GI bleeding, with PUD representing up to 50% of all such cases.23 However, recent analysis from the Clinical Outcomes Research Initiative (CORI) database reported that the most common endoscopic finding in persons with acute upper GI bleeding was “mucosal abnormality” (40%), and gastric or duodenal ulcers were found in 20.6%.24 Eradication of Helicobacter pylori and extensive use of PPIs are probably responsible for this observed decline in the frequency of PUD.
The most important factors predisposing to ulcer disease include acid, H. pylori infection, and NSAIDs; however, the role of some of these risk factors in inducing ulcer bleeding remains unclear. Indirect evidence regarding the role of acid in ulcer bleeding comes from data showing that acid suppression by PPIs in patients with active or recent bleeding reduces the risk for rebleeding.25 Evidence of the association between NSAIDs and ulcer bleeding is strong and comes from both placebo-controlled and case-control studies.26,27
The pathogenesis of bleeding from an ulcer involves aneurysmal dilation with an intense arteritis associated with a marked inflammatory response.28 When eroding into large vessels, ulcers can cause catastrophic bleeding. This most commonly occurs in the posterior portion of the duodenal bulb, where ulcers can erode directly into the pancreaticoduodenal artery.
Other Sources of Nonvariceal Upper Gastrointestinal Bleeding
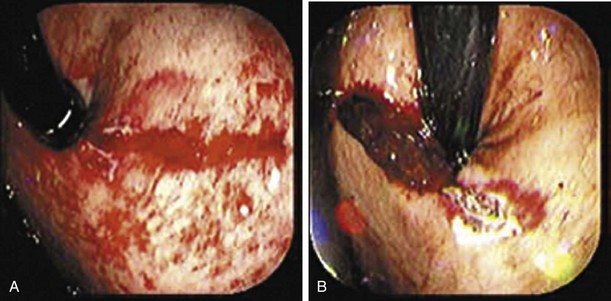
Mallory-Weiss tears (MWTs) are mucosal and occasionally submucosal lacerations caused by sudden increases in pressure within the cardia and lower esophagus produced by retching. MWTs are responsible for 5% to 15% of upper GI bleeding.23 The majority of upper GI bleeding caused by MWTs stops spontaneously and does not require any blood transfusion or endoscopic treatment. However, some cases are severe enough to require endoscopic hemostasis and occasionally angiography with embolization or even surgery. A history of retching on arrival at the emergency department is not always present.29 Endoscopically, tears are usually 1.5 to 2 cm in length and occur at the gastroesophageal junction or, most commonly, in the proximal part of the stomach (Fig. 76.2).
Angiodysplasia, also referred to as arteriovenous malformation or vascular ectasia, is another source of upper GI bleeding and accounts for approximately 5% to 10% of cases.23 It occurs in inherited syndromes such as hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu syndrome) and blue rubber bleb nevus syndrome, but most cases are acquired.30 The association of angiodysplasia with medical conditions such as renal failure, aortic stenosis, connective tissue disease (scleroderma), and von Willebrand disease has been recognized, but the evidence for these associations is limited.23,31
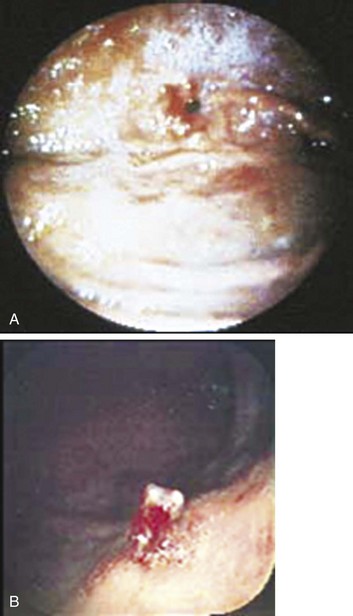
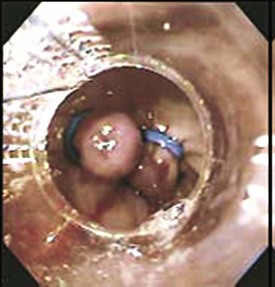
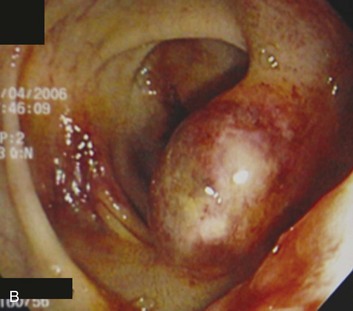
Dieulafoy’s lesion is an underrecognized cause of upper GI bleeding and is described as a visible vessel protruding from a small mucosal defect without any underlying ulcer.23 It has also been called “caliber-persistent artery” in submucosal tissue. Bleeding from Dieulafoy’s lesion represents less than 5% of cases of upper GI bleeding.32,33 The lesion is generally located on the lesser curvature within 6 cm of the gastroesophageal junction and can be difficult to detect because of its small size and normal surrounding mucosa (Fig. 76.3).31
Benign and malignant neoplasms are responsible for less than 5% of upper GI bleeding.34 Bleeding can be the initial symptom of tumors originating from the esophagus, stomach, or small intestine. These neoplasms can be primary malignancies, such as adenocarcinoma of the esophagus, stomach, or duodenum; squamous cell carcinoma of the esophagus; and gastric or duodenal lymphomas. Gastrointestinal stromal cell tumors (GISTs), carcinoid tumors, and lipomas are examples of benign tumors that can cause upper GI bleeding.23
Upper GI bleeding from an aortoenteric fistula is very rare but should be considered in the appropriate clinical setting (such as a patient with a history of aortic aneurysm repair). Fistulas are generally located in the third portion of the duodenum. Patients will frequently have a small hemorrhage first, known as a “herald bleed,” that occurs before a major hemorrhage.35
Hemosuccus pancreaticus is an uncommon cause of upper GI bleeding that occurs secondary to pseudoaneurysm formation and rupture into the pancreatic duct as a complication of a pancreatic pseudocyst in patients with chronic pancreatitis.36
Bleeding Secondary to Portal Hypertension
Esophageal Varices
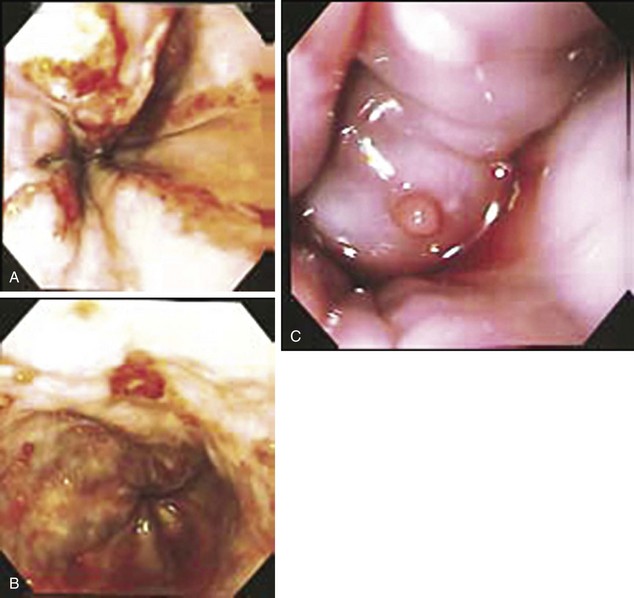
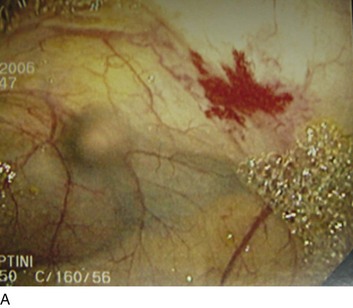
The incidence of varices among all cirrhotic patients is around 50%. However, if monitored long enough, varices eventually develop in most cirrhotics.37 Variceal hemorrhage occurs in a third of cirrhotic patients with varices. Although portal hypertension is defined by an HVPG greater than 5 mm Hg, Garcia-Tsao and colleagues showed that varices do not develop and hence do not bleed as long as the HVPG is less than 12 mm Hg.38 However, once this threshold is reached, there is poor correlation between the pressure gradient and the risk for bleeding. Other risk factors associated with variceal bleeding include the degree of liver disease (provided by Child’s classification), variceal location and size (higher risk near the gastroesophageal junction and with large varices), and the presence of particular endoscopic signs (red wale markings suggestive of dilated longitudinal venules, cherry-red spots, and hematocystic spots consisting of small red dots or a reddish blister-like formation on the variceal surface and the white or purple nipple sign on a varix) (Fig. 76.4).37,39
Gastric Varices
Sarin and colleagues classified gastric varices into two groups: (1) isolated gastric varices (IGVs) and (2) gastroesophageal varices (GEVs) that are continuous with esophageal varices and extending to the cardia (GEV 1) or to the fundus (GEV 2).40 The authors found that GEV 1 was the most common type of gastric varix but IGV was the type that bleeds the most. Although the incidence of bleeding from gastric varices is in the range of 10% to 36%, it is usually massive and associated with high mortality rates.41
Diagnostic Evaluation
Endoscopic Examination
After the initial evaluation, resuscitation, and stabilization of a patient with upper GI bleeding, effort is directed at localization and treatment of the hemorrhage. Endoscopy has become the preferred diagnostic and therapeutic modality for upper GI bleeding because of its accuracy and low complication rate. With its use, a specific diagnosis can be achieved in 95% of patients. The use of promotility agents such as metoclopramide and intravenous erythromycin (250 mg by intravenous bolus or 3 mg/kg over a 30-minute period) before the endoscopic examination can significantly improve mucosal visibility by promoting gastric motility and emptying of gastric contents.42–44 An analysis of data from erythromycin trials showed that pre-endoscopic erythromycin was cost effective.45
The optimal timing of endoscopy remains a balance between clinical need and resources and is still subject to controversy.22 Recent guidelines recommend the performance of upper endoscopy within 24 hours in patients with suspected nonvariceal upper GI bleeding.11 Endoscopy has to be delayed or deferred in selected high-risk patients such as patients with bowel perforation or acute coronary syndrome. In a meta-analysis looking at timing of endoscopy, no significant reduction in rebleeding, surgery, or mortality rate was found with urgent endoscopy (1 to 12 hours) compared with later endoscopy (more than 12 hours).11 In a subset of patients with bloody gastric lavage, length of hospital stay and blood tranfusion requirements were significantly lower in the urgent endoscopy group (within 12 hours).46
One major role of endoscopy is to identify stigmata of recent hemorrhage known to correlate well with an increased risk for rebleeding and thus a need for endoscopic therapy. Rates of rebleeding with these specific endoscopic findings are summarized in Table 76.5.47
Table 76.5
Stigmata of Ulcer Hemorrhage and Risk for Recurrent Bleeding without Endoscopic Therapy
| Endoscopic Finding | Risk for Recurrent Bleeding without Therapy |
| Active arterial (spurting) bleeding | Approaches 100% |
| Nonbleeding visible vessel (“pigmented protuberance”) | Up to 50% |
| Nonbleeding adherent clot | 30-35% |
| Ulcer oozing (without other stigmata) | 10-27% |
| Flat spots | <8% |
| Clean-based ulcers | <3% |
From ASGE Standards of Practice Committee: ASGE guideline: The role of endoscopy in acute nonvariceal upper GI-hemorrhage. Gastrointest Endosc 1999;49:145-152; and from ASGE Standards of Practice Committee: ASGE guideline: The role of endoscopy in acute nonvariceal upper GI-hemorrhage. Gastrointest Endosc 1999;49:145-152.
Therapeutic Alternatives
Nonvariceal Bleeding
Pharmacotherapy
Treatment with PPI before the performance of upper endoscopy has been shown to down-stage the endoscopic lesion and decrease the need for endoscopic intervention.11 However, a meta-analysis including studies assessing oral, intravenous, and high-dose PPI failed to show significant differences in rates of mortality, rebleeding, or surgery between, the pre-endoscopic PPI therapy and control groups.48
Once endoscopy is performed, the role of PPI therapy becomes more obvious. In patients found to have high-risk lesions treated endoscopically, an intravenous bolus followed by continuous-infusion PPI therapy is indicated.11 The use of a continuous infusion is supported by data showing that maintenance of intragastric pH at greater than 6 after endoscopic hemostasis is associated with the lowest rebleeding rates and that this goal can be achieved only by bolus administration of a PPI followed by a constant infusion.49 Strong data from several studies showed significant benefit in rebleeding, surgery, and mortality rates with high-dose intravenous PPI therapy after endoscopic treatment.50 Other reports suggest similar benefits from low-dose intravenous and high-dose oral PPI therapy but these remain to be confirmed in larger studies.
Other agents with different mechanisms of action have been studied in patients with upper GI bleeding. A reduction in splanchnic blood flow with somatostatin and its analogs (such as octreotide) is an attractive measure in patients with upper GI bleeding and has been the subject of several trials, with conflicting results.51–53 To date, the available data are not convincingly in favor of using these agents in patients with nonvariceal upper GI bleeding, even though some authors find them useful in patients who are bleeding uncontrollably while awaiting endoscopy or surgery or in whom surgery is contraindicated.51
Endoscopic Therapy
Endoscopic therapy for nonvariceal upper GI bleeding in patients with high-risk lesions has been shown to reduce the rate of rebleeding, need for surgery, and mortality rate.54 It is indicated in patients with active bleeding, spurting arterial vessels, and nonbleeding visible vessels in an underlying ulcer.47 When a clot is found in an ulcer bed, targeted irrigation in an attempt at dislodgement with treatment of the underlying lesion is indicated. If the clot remains adherent after irrigation, endoscopic therapy (injection followed by snaring of the clot) may be considered but intensive PPI therapy alone may be sufficient.11 Different forms of endoscopic therapy include injection technique, thermal technique, and mechanical technique. In many cases a combination of these modalities is used to treat a single bleeding lesion or one at high risk of bleeding.
Injection therapy results in hemostasis, probably from a combination of vascular tamponade and pharmacologic effect of the injected agent. Agents used for injection include normal saline solution, epinephrine, and sclerosants such as ethanol, ethanolamine, and polidocanol. In addition to the local tamponade effect of normal saline solution and epinephrine, the latter has a vasoconstricting effect. Sclerosant agents work by causing direct tissue injury and thrombosis.47 Another class of agents used during injection therapy includes thrombin, fibrin, and cyanoacrylate glue. These agents create a primary tissue seal at the bleeding site. Epinephrine at a concentration of 1:10,000 or 1:20,000 is the most commonly used agent, sometimes in conjunction with other agents or techniques.
Thermal energy can be delivered through noncontact techniques such as the neodymium:yttrium-aluminum-garnet (Nd:YAG) and argon lasers and argon plasma coagulation (APC). The laser technique has been abandoned because of its high cost and the high risk of complications associated with its use. In APC, the argon gas forms a plasma and acts as an electrical conductor of the current, thereby resulting in coagulation of superficial tissues. Despite comparable results with the heater probe technique when used for ulcer bleeding,55 APC is used primarily for the treatment of superficial lesions such as vascular abnormalities.47
Mechanical therapy refers to the use of a device that causes physical tamponade of a bleeding site. Such devices include metallic clips, sewing devices, rubber band ligation, and endoloops. Metallic clips are the most studied of these devices and have shown variable success, probably because of the difficulty encountered with their placement. Some authors have suggested the use of clips in combination with other endoscopic treatments.51
The choice of endoscopic therapy depends on the stigmata of ulcer hemorrhage and the experience of the endoscopist. Monotherapy with epinephrine injection has been shown to be more effective than medical therapy in patients with high-risk lesions. However, it is inferior to other monotherapies (thermal or mechanical) or to combination therapy that uses two or more methods.11 Epinephrine plus a second method significantly reduces rates of rebleeding, surgery, and mortality when compared to epinephrine alone.56
Angiographic Therapy
Angiographic or “transcatheter” intervention in cases of upper GI bleeding involves two techniques: infusion of vasoconstricting medication and mechanical occlusion (embolization) of the arterial supply responsible for the hemorrhage. Vasopressin infusion induces vasoconstriction and results in cessation of bleeding in 70% to 80% of cases initially, but rebleeding occurs in up to 20%. Complications can range from problems related to arterial access to pulmonary edema and myocardial depression.57 Vasopressin infusion has lost favor with the advent of embolization. Transcatheter embolization has become the mainstay for the radiographic treatment of nonvariceal upper GI bleeding and comes second after endoscopic therapy in most centers.58 The embolic material used can be temporary (such as a gelatin sponge and autologous clot) or permanent (such as coils). Clinical success with embolization ranges from 52% to 91%. Potential complications of embolization include ischemia and perforation.58
Variceal Bleeding
Pharmacotherapy
Vasopressin, terlipressin, somatostatin, and somatostatin analogs (octreotide and lanreotide) all induce constriction of the splanchnic vasculature and result in a decrease in portal blood flow. Vasopressin causes systemic vasoconstriction, as well as splanchnic vasoconstriction, and can lead to adverse effects such as myocardial ischemia and infarction and cerebrovascular accidents.59 These dangerous side effects led to the combination of vasopressin with other agents and the search for analogs with a better safety profile.
In one study, the combination of vasopressin with nitroglycerin resulted in a reduction in side effects and improved efficacy over vasopressin alone.59 Nitroglycerin, a potent venous dilator, decreases the hemodynamic side effects of vasopressin and at the same time reduces intrahepatic resistance, thereby resulting in a further reduction in portal pressure.
Terlipressin is a synthetic analog of vasopressin with a longer duration of action and fewer side effects than vasopressin. Unlike other vasoactive agents, terlipressin has been shown to reduce mortality rate when compared with placebo.60 At present, terlipressin is not available in the United States.
Somatostatin is a naturally occurring peptide that induces splanchnic vasoconstriction without affecting the systemic circulation and thus results in a decrease in portal pressure. When compared with vasopressin, somatostatin was equivalent in bleeding control but had significantly fewer side effects.61 Further data suggested that somatostatin has the greatest effect when used in conjunction with endoscopic therapy.62,63 Octreotide, a somatostatin analog, is approved for use in variceal bleeding in the United States. Meta-analyses looking at the efficacy of octreotide led to controversial results.61,64 One meta-analysis of trials of somatostatin analogs in general showed a negligible effect.65 However, octreotide appears to be useful as an adjunct to endoscopic therapy.66,67 At present, because of its excellent safety profile and easy availability, octreotide (50-µg bolus followed by a 50-µg/hour continuous infusion for 3 to 5 days) is almost always used in conjunction with endoscopic therapy.
The use of prophylactic antibiotics in patients with variceal bleeding has been shown to decrease the mortality rate in several randomized controlled trials.62 The rationale behind antibiotic prophylaxis derives from the fact that bacterial infection is present in up to 20% of patients with cirrhosis who are admitted with variceal bleeding and that infection develops in as many as 50% of patients while hospitalized.68 The mortality rate is significantly higher in infected cirrhotic patients than in noninfected cirrhotics.69,70 Furthermore, infected cirrhotic patients have a higher rate of variceal rebleeding.71 This is probably due to the presence of cytokines and endotoxins that induce hematologic abnormalities such as platelet dysfunction and activation of the coagulation and fibrinolytic systems.62 A recently updated meta-analysis showed that antibiotic prophylaxis significantly reduced the all-cause mortality rate, bacterial infection mortality rate, rebleeding events, and length of hospital stay.72 Prophylaxis benefits were observed regardless of the type of antibiotic used. Nonabsorbable antibiotics, quinolones, and more recently cephalosporins have all been used with good success. Recent concerns have been raised about bacterial resistance and potential decreased benefit with quinolones in some geographic areas.72
The use of recombinant factor VIIa in cirrhotic patients with upper GI bleeding has been attractive because cirrhotic patients will often have deficiencies in coagulation factors and factor VII in particular. In a randomized controlled trial investigating the use of this factor, Bosch and coworkers73 demonstrated benefit in the rate of rebleeding only in the subgroup of patients with advanced cirrhosis (Child-Pugh grades B and C). A 2010 retrospective study (Flower and colleagues) showed no benefits in mortality rate reduction when recombinant factor VII was used in patients with upper GI bleeding.74 Further studies are needed before the routine use of this expensive therapy can be recommended.
Endoscopic Therapy
Endoscopic sclerotherapy and endoscopic band ligation are the mainstays of therapy for acute variceal bleeding. These procedures are successful in achieving hemostasis in 80% to 90% of patients.62
Sclerotherapy consists of intravariceal or paravariceal injection of a sclerosant agent such as sodium tetradecyl sulfate or ethanolamine. These agents provoke a severe inflammatory reaction within or around the varix that leads to variceal thrombosis and obliteration. Despite its high effectiveness in controlling bleeding, the complication rate with sclerotherapy can be as high as 40%.75 Complications range from local effects, such as deep ulcers causing rebleeding, odynophagia, and in some cases perforation to bacteremia and systemic complications such as pleural and pericardial effusion. Esophageal stricture is the most common long-term side effect of sclerotherapy. Once the acute bleeding is controlled, repeated sessions of sclerotherapy are required to eradicate the varices and prevent rebleeding.
Endoscopic band ligation consists of the placement of a ligating device on the tip of the endoscope, and elastic rings are placed over the targeted varix (Fig. 76.5). With the availability of multiband ligating devices, 5 to 10 bands can be deployed in one session. Band ligation has a lower rate of complication (including the rebleeding rate in some but not all studies76) than sclerotherapy does, but it can be difficult to perform during acute bleeding.62 Superficial mucosal ulceration occurs when the elastic ring and underlying tissue slough off. Transient chest discomfort and dysphagia are common after band ligation. Band ligation also has the advantage of achieving eradication of varices with fewer endoscopic sessions than are typically needed for sclerotherapy.76
A meta-analysis of 10 randomized clinical trials showed an almost significant benefit of endoscopic band ligation over sclerotherapy in the initial control of bleeding.77 A practice guideline by the American Association for the Study of Liver Diseases recommends the use of endoscopic band ligation as the preferred form of endoscopic therapy and the use of sclerotherapy when band ligation is not technically feasible.67
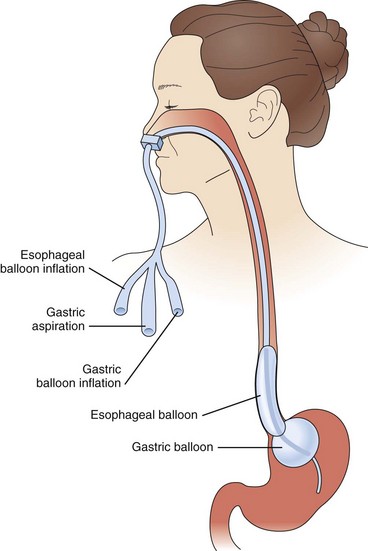
Balloon Tamponade
The use of balloon tamponade in the control of active variceal bleeding comes as a last resort when other forms of therapy are not available or fail to achieve hemostasis. Three balloons have been used for this purpose: the Minnesota tube, the Sengstaken-Blakemore tube, and the Linton-Nachlas tube. Control of bleeding with these tubes depends on patient selection, the concomitant use of other therapies, and the experience of the staff using them. With the advent of other therapies and the increasing experience of endoscopists in sclerotherapy and endoscopic banding, experience in the use of tamponade balloons decreased substantially. A major concern with the use of these tubes is the high risk of rebleeding after deflation of the balloon, in addition to the risk of esophageal rupture. Balloon tamponade should be used only as a temporary means of stabilization and as a bridge to a more definitive form of therapy. Figure 76.6 illustrates the steps used to control bleeding with a balloon. The use of a fully covered self-expandable metallic stent has shown some promise in control of variceal bleeding.78
Transjugular Intrahepatic Portosystemic Shunt
The transjugular intrahepatic portosystemic shunt (TIPS) procedure involves the creation of a low-resistance channel between the portal vein and the hepatic vein through which blood is shunted from the portal to the systemic circulation. Via an angiographic technique, an expandable metal stent is deployed across this channel and results in a significant decrease in portal pressure. This technique allows the creation of a portosystemic shunt without requiring general anesthesia and major surgery. TIPS is indicated in situations in which acute variceal bleeding is refractory to endoscopic and pharmacologic therapy. It has been shown to control bleeding in more than 90% of patients, with a rebleeding rate of less than 20%.79,80 In 2010 data (Garcia-Tsao and colleagues) showed that early TIPS (within 24 to 48 hours of admission) was associated with significant decrease in mortality rate in high-risk patients (with HVPG >20 mm Hg or with Child-Pugh class C disease).81 However, the mortality rate after the TIPS procedure in the setting of acute uncontrollable variceal bleeding is between 30% and 40%.62 A major complication associated with this procedure is the development of encephalopathy in 10% to 20% of patients. Stenosis and complete occlusion of the shunt occur in 5% to 15% of the cases, requiring revision.
Surgical Therapy
Surgical creation of portosystemic shunts has been used for decompression of the portal system to treat and prevent variceal bleeding. The various forms of surgical shunts include portacaval shunts, distal splenorenal shunts, and partial shunts. Revascularization of the esophagus is another surgical option for the treatment of variceal bleeding. Interest in surgery has declined over the years because of the advent of the less invasive endoscopic and TIPS procedures. At present, surgery is considered only when medical and endoscopic therapy fails to control bleeding and TIPS is not available.62
Stress-Related Mucosal Disease
The incidence of endoscopically documented gastric lesions in patients in the ICU ranges from 74% to 100%.82 The lesions are typically, but not exclusively, located in the fundus and body of the stomach. However, most of these lesions are of little significance because healing occurs rapidly. Clinically evident bleeding, defined as blood in a nasogastric aspirate, hematemesis, and melena, occurs in 5% to 25% of patients in the ICU. Clinically important bleeding, however, is defined as overt bleeding associated with one of the following within 24 hours of the onset of the bleeding: a drop in systolic blood pressure of greater than 20 mm Hg, an increase in the pulse rate of more than 20 beats per minute and a 10-mm Hg drop in systolic blood pressure, or a decrease in hemoglobin of greater than 2 g/dL requiring blood transfusion and failure of hemoglobin to increase by the number of units transfused minus 2 g/dL.83 Clinically important bleeding affects only 3% to 6% of critically ill patients.84
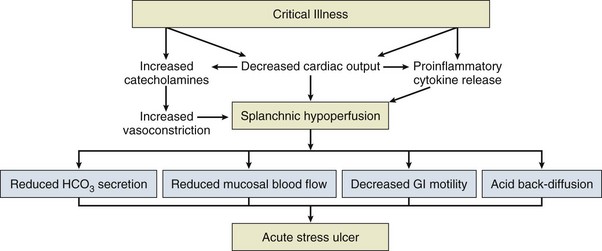
The pathogenesis of SRMD involves a disruption in the balance between aggressive and protective factors that leads to gastric mucosal damage. Adequate microcirculation in the upper GI tract is the most important defense mechanism against luminal aggressors such as acid and enzyme secretion, as well as infection. The role of the microcirculation is to provide nutrients and eliminate toxic oxygen-derived free radicals. In the presence of severe physiologic stress, disruption of intramucosal blood flow leads to a number of events, including the formation of toxic oxygen-derived radicals, a decrease in the synthesis of cytoprotective prostaglandins and mucus production, and a reduction in bicarbonate secretion resulting in an increase in intramural acidity (Fig. 76.7).82,84 These events create a favorable setting for mucosal injury and ulceration.85,86 Subsequent reperfusion also plays a major role in the development of lesions.86
Identification of critically ill patients at risk for significant bleeding from SRMD has been the subject of several studies. A landmark study by Cook and associates83 demonstrated a significant increase in the risk for bleeding from SRMD in patients requiring mechanical ventilation for more than 48 hours and in those with coagulopathy (defined as thrombocytopenia [platelet count <50,000/mL3], an international normalized ratio above 1.5, or a partial thromboplastin time more than 2.0 times the control value). Other risk factors such as shock, sepsis, renal failure, liver failure, and glucocorticoids were identified, but the association with bleeding was not statistically significant. Risk factors associated with the presence of SRMD (not necessarily bleeding) include recent major surgery, major trauma, severe burns (Curling’s ulcers), head trauma or coma (Cushing’s ulcers), and multiple organ failure.87–89 Further understanding of the risk factors associated with bleeding from SRMD will help in directing prophylactic therapy to patients who will benefit most from this therapy.90
The reported mortality rates in critically ill patients with bleeding from SRMD ranges from 46% to 77%.83,91,92 These high rates are most likely due to the underlying disease and not directly related to GI bleeding. However, they reveal the importance of significant bleeding as a sign of the degree of severity of the primary illness.
Prophylaxis
The role of enteral nutrition in prophylaxis for SRMD is controversial.93,94 The rationale behind the use of enteral nutrition derives from its ability to improve blood flow to the stomach and sustain mucosal immunity and integrity.95 However, experimental data in hemodynamically compromised animals showed an increase in tissue hypoxia when enteral nutrition was administered.96 Furthermore, in one study the degree of acid suppression was reduced in patients receiving H2 receptor antagonists (H2RAs) or PPIs with concomitant enteral feeding.97
When compared with placebo, antacids have been shown to decrease bleeding in patients with SRMD.98 However, antacids require frequent dosing and monitoring of pH and are not practical to use, especially with the advent of other effective agents for prophylaxis.
Sucralfate works by coating the gastric mucosa and forming a thin protective layer between the mucosa and the gastric acid in the lumen without affecting acid secretion and intragastric pH. The use of sucralfate for the prevention of bleeding in patients with SRMD gained interest because of theoretical concern about gram-negative bacterial overgrowth when the pH of the stomach is increased by the use of H2RAs or PPIs, which can potentially lead to nosocomial pneumonia. Conflicting results were reported regarding this issue, but a large trial by Cook and colleagues in which ranitidine and sucralfate were compared showed no significant difference in the development of nosocomial pneumonia.99 In this same trial, the incidence of clinically important bleeding was significantly lower with ranitidine. Other studies demonstrated comparable efficacy between sucralfate and antacids and H2RAs in terms of prevention of SRMD.100,101 One drawback to the use of sucralfate is that it may decrease the absorption of other concomitantly administered oral drugs.
Intravenous H2RAs are the most widely used pharmacologic agents for the prophylaxis of bleeding in patients with SRMD. A meta-analysis of studies looking at the role of H2RAs in SRMD demonstrated a decrease in the incidence of clinically important bleeding.98 Furthermore, H2RAs were more effective than antacids in preventing bleeding.98,102 Despite better control of gastric pH with continuous infusion of H2RAs, bolus infusion has been shown to be as effective in preventing bleeding.98,103 One major concern is the development of tolerance to H2RAs when administered for a prolonged period.
In recent years PPIs have gained wider use in the prevention of bleeding from SRMD because of their ease of dosing, effective and predictable acid suppression, lack of need for regular gastric pH monitoring, and lack of tolerance as encountered with H2RAs.95 A recent meta-analysis compared the efficacy of PPIs versus H2RAs in the prophylaxis of stress-related mucosal bleeding in critically ill patients at risk of bleeding. It demonstrated that PPI prophylaxis significantly decreased rates of clinically significant bleeding compared with H2RAs without affecting the development of nosocomial pneumonia or mortality rates.104
Treatment
Endoscopy is the principal diagnostic and therapeutic procedure used in patients with bleeding from SRMD. The same endoscopic techniques used for hemostasis in peptic ulcer bleeding are used in SRMD when an actively bleeding lesion is found. Surgery is reserved for patients with uncontrolled hemorrhage and usually involves vagotomy, oversewing of bleeding sites, and in rare cases, subtotal or total gastrectomy.95 In poor surgical candidates with severe uncontrolled hemorrhage, angiography may be a useful diagnostic and therapeutic option.
Lower Gastrointestinal Bleeding
Differential Diagnosis
Lower GI bleeding is defined as bleeding that originates from a source distal to the ligament of Treitz. It is caused by a diverse range of bleeding sources and can range from trivial to life-threatening bleeding. It can also be acute, arbitrarily defined as less than 3 days’ duration and possibly resulting in hemodynamic compromise, or chronic, extending over a period of several days or longer and usually less severe in terms of the amount of blood loss.105 Sources of lower GI bleeding and their incidence from several large studies are listed in Table 76.6.106–113
Table 76.6
Sources of Lower Gastrointestinal Bleeding
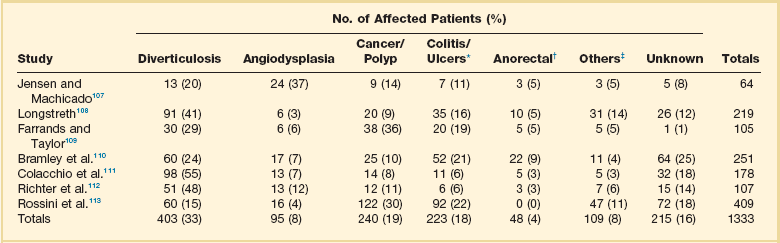
*Includes inflammatory bowel disease, infectious colitis, ischemic colitis, radiation colitis, vasculitis, and inflammation of unknown origin.
†Includes hemorrhoids, anal fissure, and idiopathic rectal ulcers.
‡Includes postpolypectomy bleeding, aortocolonic fistula, trauma from fecal impaction, and anastomotic bleeding.
From Zuckerman GR, Prakash C: Acute lower intestinal bleeding. Part II: Etiology, therapy, and outcomes. Gastrointest Endosc 1999;49:228-238.
Diverticular Bleeding
Diverticular bleeding, the most common source of lower GI bleeding, accounts for up to 40% of cases.105 Diverticular disease is more common in the elderly. It affects up to two thirds of people older than 80 years. Only 3% to 15% of patients with diverticulosis experience diverticular bleeding.105
Patients with diverticular bleeding typically have painless hematochezia. Most patients will stop bleeding spontaneously, but the bleeding can recur in 10% to 40% of cases.114
The bleeding is thought to occur after repetitive trauma to the vasa recta (nutrient arteries) that stretch over the diverticular dome.115 NSAIDs have been associated with increased risk for diverticular bleeding.116
Ischemic Colitis and Other Forms of Colitis
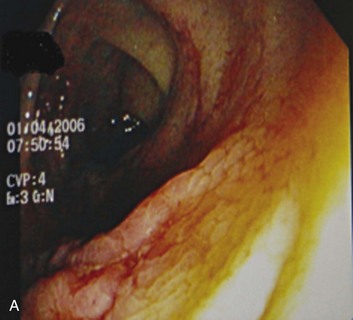
Ischemic colitis accounts for up to 19% of lower GI bleeding.105 It results from a sudden reduction in mesenteric blood flow. As opposed to acute mesenteric ischemia, this reduction is transient and reversible. The typical regions affected are the “watershed” areas of the colon: the splenic flexure, the rectosigmoid junction, and the right colon. Ischemia is precipitated by any event that compromises colonic blood flow. Clinically, patients have a sudden onset of abdominal pain, followed by hematochezia within 24 hours. Endoscopically, ischemia is suspected in the presence of bluish hemorrhagic nodules from submucosal bleeding, cyanotic or necrotic mucosa with hemorrhagic ulcerations, or segmental distribution with an abrupt transition between injured and normal mucosa (Fig. 76.8).105 Most cases of ischemic colitis resolve spontaneously with supportive treatment. Rare cases require surgery (if peritoneal signs are present) or develop chronic ischemic colitis with stricture formation. Ischemic colitis is a common cause of lower GI bleeding in patients in the ICU because of the frequent hemodynamic instability in this setting.117
Angiodysplasia
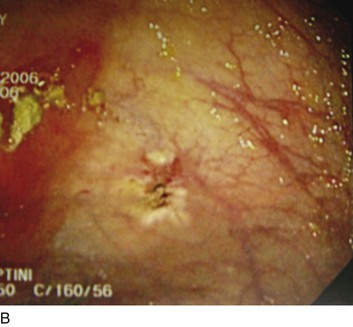
Estimates of angiodysplasia as a source of lower GI bleeding vary widely and reach 37% in some reports.108 Current data suggest that acute bleeding caused by angiodysplasia is less frequent than previously thought, with most cases demonstrating iron deficiency anemia and occult blood loss.115 These lesions are seen predominantly in the elderly. They appear endoscopically as red, flat lesions with ectatic blood vessels radiating from a central feeding vessel, usually in the right colon (Fig. 76.9). Overt bleeding from angiodysplasia is usually brisk and painless and cannot be distinguished from diverticular bleeding. Endoscopic treatment is highly effective and safe and prevents bleeding episodes from recurring.
Other Sources of Lower Gastrointestinal Bleeding
Acute significant bleeding from colonic neoplasia and polyps is uncommon despite reports in which it accounts for up to 36% of cases.112 Cancer and polyps are thought to bleed from erosions on the surface.
Postpolypectomy bleeding occurs in 1% to 6% of patients undergoing colonoscopic polypectomy.115 Bleeding can be immediate or delayed up to 3 weeks after the procedure. Risk factors for postpolypectomy bleeding include large size, sessile morphologic appearance, and right colonic location.118
Anorectal sources of lower GI bleeding include hemorrhoids, anorectal fissures, stercoral ulcers, and proctitis. Hemorrhoids can cause significant bleeding and may sometimes require endoscopic or surgical intervention. Bleeding from rectal ulcers is common in elderly bedridden patients and in critically ill patients.106
Small bowel sources of lower GI bleeding include angiodysplasia (most frequent), lymphoma, small bowel ulcers, and Crohn’s disease.115 Small bowel lesions are typically more difficult to identify and require more diagnostic procedures.119
Diagnostic Evaluation
Colonoscopy
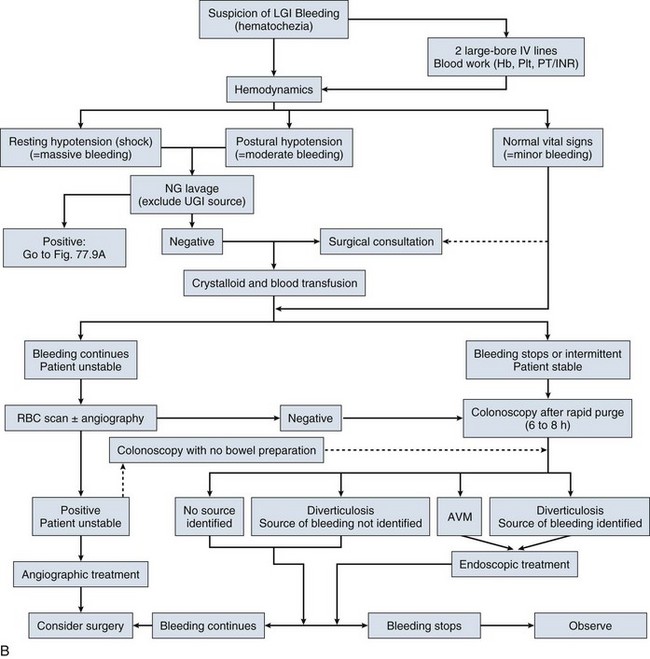
After initial evaluation, stabilization, and exclusion of an upper GI source, management shifts to localization of the lower GI bleeding site. Colonoscopy is an attractive choice in this setting because it offers the best opportunity for early diagnosis and subsequent management. In the past, colonoscopy used to be performed in an expectant manner, after cessation of bleeding and colonic preparation. Concern about poor visibility, the potential for complications, and the adverse effects of bowel preparation in the setting of bleeding is behind the reluctance in performing “urgent” colonoscopy. Over the past decade, early colonoscopy has gained interest in the management of lower GI bleeding. Several reports have shown that early (or urgent) colonoscopy is safe and has high diagnostic yield in patients with hematochezia.120–124 The definition of “urgent” varied in these reports, from within 8 hours to within 24 hours of initial evaluation. This approach was also associated with shorter hospital stay.125 Bowel preparation is generally recommended before urgent colonoscopy to improve visibility and prevent complications related to poor visibility. Polyethylene glycol lavage solution by mouth or through a nasogastric tube is commonly used in these cases. It can be administered at a rate of approximately 1 L every 30 to 45 minutes.105 Some authors have proposed the use of colonoscopy without bowel preparation and have shown high diagnostic yield with this approach.126 Despite the fact that urgent colonoscopy improves the diagnostic yield and leads to more frequent endoscopic therapy, there is still controversy regarding its impact on important outcomes such as mortality rate, rebleeding, and the need for surgery.122,124 Jensen and colleagues122 showed that urgent colonoscopy with endoscopic treatment in a group of patients with active diverticular bleeding (versus a historical group that did not receive endoscopic treatment) may prevent recurrent bleeding and the need for surgery. Another randomized trial compared urgent colonoscopy with a standard care algorithm in which radionuclide scanning, followed, if positive, by angiography, was used in patients with suspected active bleeding versus expectant colonoscopy in those without active bleeding. This trial showed that a definite source of bleeding was found more often in urgent colonoscopy patients. However, no difference in mortality rate, surgery, and rebleeding was found in the two groups.124 A more recent trial looking at the benefit of urgent versus elective colonoscopy (<12 hours vs. 36 to 60 hours from presentation) showed no evidence of improving clinical outcomes such as rebleeding, transfusion requirements, and hospital length of stay. A major limitation of this trial was that the study was terminated before reaching the prespecified sample size.127 In conclusion, the optimal timing of colonoscopy in patients with lower GI bleeding has not been determined and it is unknown if early performance of colonoscopy affects major outcomes such as rebleeding and mortality rate.15
Radionuclide Scanning
The use of radionuclide scanning (also known as a technetium-labeled red blood cell scan) in patients with lower GI bleeding is highly controversial. It has been used for several decades as a method for localization of the bleeding source.115 A main advantage of radionuclide scanning is its sensitivity for bleeding at a rate as low as 0.05 to 0.1 mL/minute, in addition to its noninvasive nature.115 It also can be repeated in cases of intermittent bleeding. On the other hand, this diagnostic modality lacks therapeutic capability, has variable accuracy, and may delay other diagnostic and therapeutic procedures. A review of data from several studies of radionuclide scanning for lower GI bleeding in which radionuclide scintigraphy findings were confirmed by a different test found the accuracy of a positive test to be 66%.15 Localization improves when scans are positive within 2 hours of injection. A common practice in the setting of ongoing lower GI bleeding is to perform radionuclide scanning as a screening test before angiography and in some cases as a guide to surgery. The problem with this approach is the inconsistent and widely variable accuracy and the high rate of false-positive results in different reports.115,128,129
Angiography
Angiography is less sensitive than radionuclide scanning in the detection of active bleeding. It can detect bleeding at a rate of 0.5 to 1.0 mL/minute. In addition to its role in accurate localization of bleeding lesions, angiography offers therapeutic possibilities. As with radionuclide scanning, the ability of angiography to detect the bleeding source varies widely among studies, from 20% to 70%.115 This variation is due to several factors, including patient selection (higher diagnostic yield in patients with positive radionuclide scanning), the severity and intermittent nature of the bleeding, procedural delay, and venous or small vessel bleeding.115 In one study, a systolic blood pressure of less than 90 mm Hg and a requirement of at least 5 units of blood within a 24-hour period have been shown to predict a positive angiography (85% when both criteria were present).130 Angiography is usually performed in patients with ongoing lower GI bleeding with or without a positive radionuclide scan. This sequence in management was the standard approach to ongoing bleeding up until recently, when colonoscopy started gaining interest in the setting of acute bleeding (as opposed to expectant elective colonoscopy). When angiographic therapy fails or is not available after localization of the bleeding, the findings are used to guide surgical resection. Angiography can cause serious complications such as contrast reactions, arterial thrombosis and dissection, and catheter site infection and bleeding. Thus, it should be used in carefully selected patients.
Computed Tomography
CT gained interest in the setting of GI bleeding after the recent introduction of multidetector row CT. It enables the accurate acquisition of arterial images, which can show contrast extravasation in areas of active bleeding.15 It can detect bleeding rates as low as 0.3 to 0.5 mL/minute. The yield for lower GI bleeding ranges from 25% to 95% and is highest in severe ongoing bleeding. The advantages of CT are its wide availability and the added diagnostic yield of cross-sectional imaging, but exposure to radiation and contrast and, like radionuclide scanning, the inability to deliver therapy constitute some of the disadvantages of this technique.15
Therapeutic Alternatives
Endoscopic Therapy
Data on the effectiveness of endoscopic therapy for lesions causing lower GI bleeding are limited. However, the experience from published series suggests that this form of therapy is likely to be beneficial. Methods of endoscopic hemostasis are similar to those used for upper GI bleeding and include injection therapy, thermal therapy (APC and heater and electrocautery probes), and mechanical therapy (hemoclips).131 Several studies showed that clipping is very effective modality for diverticular bleeding with a low rate of early rebleeding.15
Angiographic Therapy
When angiography identifies the bleeding site in cases of lower GI bleeding, hemostasis can be achieved by the intra-arterial infusion of vasopressin or superselective embolization. Infusion of vasopressin can control bleeding in up to 91% of cases,131 but complications develop in 10% to 20% and include arrhythmia, pulmonary edema, and ischemia.131 Furthermore, rebleeding occurs in as many as 50% of cases.131 In early studies, transcatheter embolization carried a high risk for bowel infarction, but current superselective techniques using smaller catheters with various agents (gelatin sponge, microcoils, and polyvinyl alcohol particles) appear to be more effective and safer than the old techniques. Review of several studies using superselective techniques showed that immediate hemostasis was achieved in 96% of cases and rebleeding within 30 days occurred in 22%.15 Since the advent of these new techniques, embolization has become the preferred modality when angiographic therapy is contemplated.
Surgical Therapy
Surgery is indicated in cases of recurrent bleeding (especially diverticular) and massive ongoing bleeding with high transfusion requirements (generally more than 6 units of packed red blood cells in a 24-hour period).105 Accurate localization of the site of bleeding preoperatively is crucial so that segmental rather than subtotal colectomy can be performed. Unfortunately, the accuracy of radionuclide scanning in localization of the bleeding is variable, and hence it should not be used as a guide for surgery. Colonoscopy and angiography may be used as a guide for surgery in cases in which bleeding is initially identified and possibly treated through these modalities and then recurs and requires surgery.
Obscure Gastrointestinal Bleeding
Differential Diagnosis
Missed lesions on upper and lower endoscopy should be considered first in the workup of obscure bleeding. Causes difficult to identify on routine endoscopy include hemosuccus pancreaticus, hemobilia, aortoenteric fistula, Dieulafoy’s ulcer, and extraesophageal varices. Small bowel lesions are another source of obscure bleeding and include tumors (lymphomas, carcinoids, adenocarcinomas), vascular ectasia, NSAID-induced ulcers, Meckel’s diverticulum, and other less common causes.132
Diagnostic Evaluation
Small bowel follow-through (SBFT) and enteroclysis (which is a modified form of SBFT) should be performed only when Crohn’s disease or malignancy is suspected. These modalities have lost favor with the advent of capsule endoscopy and device-assisted enteroscopy (except in cases in which there may be narrowing of the small bowel). Small bowel push enteroscopy can be very helpful in identifying and, in some cases, treating lesions in the small bowel that are responsible for obscure bleeding. Unfortunately, it can examine only 50 to 150 cm of small bowel.133 Device-assisted enteroscopy (double and single balloon enteroscopy and spiral enteroscopy) has comparable diagnostic yield to capsule endoscopy but has the advantage of performing therapeutic interventions and obtaining biopsies.134
Capsule endoscopy gained large interest in evaluation of the small bowel because of its ability to examine the entire small bowel and its noninvasive nature. It consists of swallowing a pill-sized camera with sufficient battery life to image the entire small bowel. Studies have supported the role of capsule endoscopy in the evaluation of obscure GI bleeding with an overall diagnostic yield of 55% to 70%.132 The diagnostic yield is even higher when it is administered to patients with ongoing bleeding (87% to 92%).135,136 In our opinion, efforts should be made to perform capsule endoscopy in patients with obscure overt bleeding while still in the hospital instead of waiting until discharge (which is the current standard practice in most centers). In patients who can tolerate an invasive procedure, device-assisted enteroscopy can be used when available and has the advantage of therapeutic ability. However, this procedure only achieves total enteroscopy in 29% of patients. Other limitations include limited availability, time, and sedation requirements. The procedure’s risk of complications is low (less than 1%).134
Exploratory laparotomy is generally the last option in the evaluation of obscure GI bleeding. When combined with intraoperative enteroscopy, the diagnostic yield reaches 50% to 100%.132
Conclusion
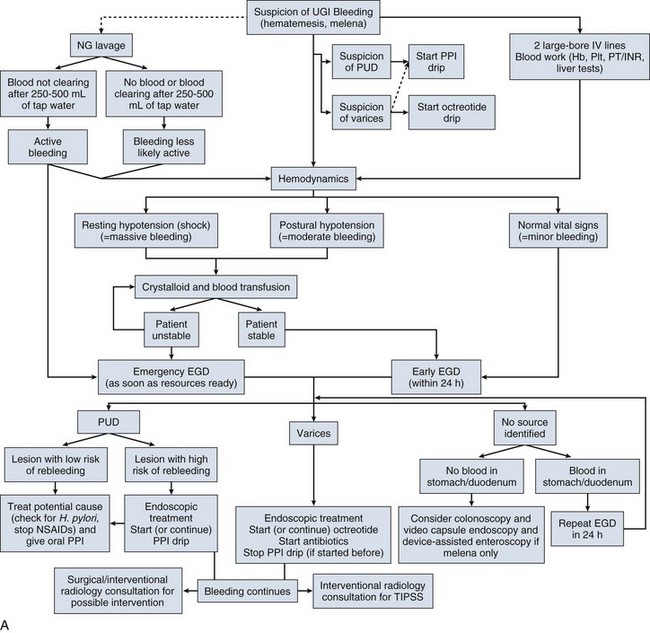
In summary, acute GI bleeding is a life-threatening emergency that requires a quick response and a coordinated team approach. Acute management is the same regardless of the source of bleeding (Fig. 76.10) and should be orchestrated by a critical care team. The prime direction in resuscitation should be restoration of euvolemia. Once a source is suspected, specific management and therapeutic options are considered and may require communication with gastroenterologists, surgeons, and interventional radiologists. Prevention of bleeding from SRMD is indicated in a specific population of critically ill patients. Endoscopy plays a major role in the diagnosis and management of acute GI bleeding. Endoscopic therapy with standard and innovative techniques has been shown to have a major impact on the prognosis of bleeding patients.
References
1. Longstreth, GF. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: A population based study. Am J Gastroenterol. 1995; 90:206–210.
2. Longstreth, GF. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: A population based study. Am J Gastroenterol. 1997; 92:419–424.
3. Bramley, PN, Masson, JW, McKnight, G, et al. The role of an open-access bleeding unit in the management of colonic hemorrhage. A 2-year prospective study. Scand J Gastroenterol. 1996; 31:764–769.
4. Yavorski, RT, Wong, RK, Maydonovitch, C, et al. Analysis of 3,294 cases of upper gastrointestinal bleeding in military medical facilities. Am J Gastroenterol. 1995; 90:568–573.
5. Rockall, TA, Logan, RF, Devlin, HB, et al. Variation in outcome after acute upper gastrointestinal haemorrhage. The National Audit of Acute Upper Gastrointestinal Haemorrhage. Lancet. 1995; 346:346–350.
6. Zuckerman, GR, Prakash, C. Acute lower intestinal bleeding, Part I: Clinical presentation and diagnosis. Gastrointest Endosc. 1998; 48:606–617.
7. Zuckerman, GR, Prakash, C. Acute lower intestinal bleeding, Part II: Etiology, therapy, and outcomes. Gastrointest Endosc. 1999; 49:228–238.
8. Rockey, DC. Gastrointestinal bleeding. Gastroenterol Clin North Am. 2005; 34:581–588.
9. Rockey, DC. Gastrointestinal bleeding. In: Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 7th ed. Philadelphia: WB Saunders; 2002:221–248.
10. Marik, PE, Corwin, HL. Efficacy of red blood cell transfusion in the critically ill: A systematic review of the literature. Crit Care Med. 2008; 36:2667–2674.
11. Barkun, AN, Bardou, M, Kuipers, EJ. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010; 152:101–113.
12. Kruskall, MS, Mintz, PD, Bergin, JJ, et al. Transfusion therapy in emergency medicine. Ann Emerg Med. 1988; 17:327–335.
13. Blatchford, O, Murray, WR, Blatchford, M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000; 356:1318–1321.
14. Rockall, TA, Logan, RF, Delvin, HB, et al. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996; 38:316–321.
15. Strate, LL, Naumann, CR. The role of colonoscopy and radiological procedures in the management of acute lower intestinal bleeding. Clin Gastroenterol Hepatol. 2010; 8:333–343.
16. Schiff, L, Stevens, RJ, Shapiro, N, et al. Observations on the oral administration of citrate blood in man. Am J Med Sci. 1942; 203:409.
17. Luk, GD, Bynum, TE, Hendrix, TR. Gastric aspiration in localization of gastrointestinal hemorrhage. JAMA. 1979; 241:576–578.
18. Aljebreen, AM, Fallone, CA, Barkun, AN. Nasogastric aspirate predicts high-risk endoscopic lesions in patients with acute upper-GI bleeding. Gastrointest Endosc. 2004; 59:172–178.
19. Huang, ES, Karsan, S, Kanwal, F, et al. Impact of nasogastric lavage on outcomes in acute GI bleeding. Gastrointest Endosc. 2011; 74:971–980.
20. Chalasani, N, Clark, WS, Wilcox, CM. Blood urea nitrogen to creatinine concentration in gastrointestinal bleeding: A reappraisal. Am J Gastroenterol. 1997; 92:1796–1799.
21. Ernst, AA, Haynes, ML, Nick, TG, et al. Usefulness of the blood urea nitrogen/creatinine ratio in gastrointestinal bleeding. Am J Emerg Med. 1999; 17:70–72.
22. Ferguson, CB, Mitchell, RM. Nonvariceal upper gastrointestinal bleeding: Standard and new treatment. Gastroenterol Clin North Am. 2005; 34:607–621.
23. Esrailian, E, Gralnek, IM. Nonvariceal upper gastrointestinal bleeding: Epidemiology and diagnosis. Gastroenterol Clin North Am. 2005; 34:589–605.
24. Lieberman, D, Fennerty, MB, Morris, CD, et al. Endoscopic evaluation of patients with dyspepsia: Results from the national endoscopic data repository. Gastroenterology. 2004; 127:1067–1075.
25. Hasselgren, G, Lind, T, Lundell, L, et al. Continuous intravenous infusion of omeprazole in elderly patients with peptic ulcer bleeding. Results of a placebo-controlled multicenter study. Scand J Gastroenterol. 1997; 32:328–333.
26. Roderick, PJ, Wilkes, HC, Meade, TW. The gastrointestinal toxicity of aspirin: An overview of randomised controlled trials. Br J Clin Pharmacol. 1993; 35:219–226.
27. Bjorkman, DJ. Current status of nonsteroidal anti-inflammatory drug (NSAID) use in the United States: Risk factors and frequency of complications. Am J Med. 1999; 107:3–8.
28. Swain, CP, Storey, DW, Bown, SG, et al. Nature of the bleeding vessel in recurrently bleeding gastric ulcers. Gastroenterology. 1986; 90:595–608.
29. Schmulewitz, N, O’Connor, JB. Esophageal disease caused by medication, radiation and internal trauma. In: DiMarino AJ, Benjamin S, eds. Gastrointestinal Disease: An Endoscopic Approach. 2nd ed. Thorofare, NJ: Slack; 2002:245–262.
30. Machicado, GA, Jensen, DM. Upper gastrointestinal angiomata: Diagnosis and treatment. Gastrointest Endosc Clin North Am. 1991; 1:241–262.
31. Bashir, RM, Al-Kawas, FH. Vascular lesions of the stomach. In: DiMarino AJ, Benjamin S, eds. Gastrointestinal Disease: An Endoscopic Approach. 2nd ed. Thorofare, NJ: Slack; 2002:535–550.
32. Romaozinho, JM, Pontes, JM, Lerias, C, et al. Dieulafoy’s lesion: Management and long-term outcome. Endoscopy. 2004; 36:416–420.
33. Park, CH, Joo, YE, Kim, HS, et al. A prospective, randomized trial of endoscopic band ligation versus endoscopic hemoclip placement for bleeding gastric Dieulafoy’s lesions. Endoscopy. 2004; 36:677–681.
34. Savides, TJ, Jensen, DM, Cohen, J, et al. Severe upper gastrointestinal tumor bleeding: Endoscopic findings, treatment, and outcome. Endoscopy. 1996; 28:244–248.
35. Busuttil, RW, Rees, W, Baker, JD, et al. Pathogenesis of aortoduodenal fistula: Experimental and clinical correlates. Surgery. 1979; 85:1–13.
36. Elton, E, Howell, DA, Amberson, SM, et al. Combined angiographic and endoscopic management of bleeding pancreatic pseudoaneurysms. Gastrointest Endosc. 1997; 46:544–549.
37. Luketic, VA, Sanyal, AJ. Esophageal varices. I. Clinical presentation, medical therapy, and endoscopic therapy. Gastroenterol Clin North Am. 2000; 29:337–385.
38. Garcia-Tsao, G, Groszmann, RJ, Fisher, RL, et al. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology. 1985; 5:419–424.
39. Sheikh, RA, Prindiville, TP, Trudeau, W. Gastrointestinal bleeding in portal hypertension. In: DiMarino AJ, Benjamin S, eds. Gastrointestinal Disease: An Endoscopic Approach. 2nd ed. Thorofare, NJ: Slack; 2002:605–644.
40. Sarin, SK, Lahoti, D, Saxena, SP, et al. Prevalence, classification and natural history of gastric varices: Long-term follow up study in 568 patients with portal hypertension. Hepatology. 1992; 16:1343–1349.
41. Hashizume, M, Akahoshi, T, Tomikawa, M. Management of gastric varices. J Gastroenterol Hepatol. 2011; 26:102–108.
42. Frossard, JL, Spahr, L, Queneau, PE, et al. Erythromycin IV bolus infusion in acute upper gastrointestinal bleeding: A randomized, controlled, doubleblind trial. Gastroenterology. 2002; 123:17–23.
43. Coffin, B, Pocard, M, Panis, Y, et al. Erythromycin improves the quality of EGD in patients with acute upper GI bleeding: A randomized controlled study. Gastrointest Endosc. 2002; 56:174–179.
44. Sussman, D, Deshpande, A, Parra, J, et al. Intravenous metoclopramide to increase mucosal visualization during endoscopy in patients with acute upper gastrointestinal bleeding: A randomized, controlled study. Gastrointest Endosc. 2008; 67:AB247.
45. Winstead, NS, Wilcox, CM. Erythromycin prior to endoscopy for acute upper gastrointestinal haemorrhage: A cost-effectiveness analysis. Aliment Pharmacol Ther. 2007; 26:1371–1377.
46. Lin, HJ, Wang, K, Perng, CL, et al. Early or delayed endoscopy for patients with peptic ulcer bleeding. A prospective randomized study. J Clin Gastroenterol. 1996; 22:267–271.
47. ASGE Standards of Practice Committee. ASGE guideline: The role of endoscopy in acute nonvariceal upper-GI hemorrhage. Gastrointest Endosc. 2004; 60:497–504.
48. Lau, JY, Leung, WK, Wu, JC, et al. Omeprazole before endoscopy in patients with gastrointestinal bleeding. N Engl J Med.. 2007; 356:1631–1640.
49. Julapalli, VR, Graham, DY. Appropriate use of intravenous proton pump inhibitors in the management of bleeding peptic ulcer. Dig Dis Sci. 2005; 50:1185–1193.
50. Laine, L, McQuaid, KR. Endoscopic therapy for bleeding ulcers: An evidence-based approach based on meta-analyses of randomized controlled trials. Clin Gastroenterol Hepatol. 2009; 7:33–47.
51. Barkun, A, Bardou, M, Marshall, JK. Nonvariceal Upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003; 139:843–857.
52. Imperiale, TF, Birgisson, S. Somatostatin or octreotide compared with H2 antagonists and placebo in the management of acute nonvariceal upper gastrointestinal hemorrhage: A meta-analysis. Ann Intern Med. 1997; 127:1062–1071.
53. Lin, HJ, Wang, K, Perng, CL, et al. Octreotide and heater probe thermocoagulation for arrest of peptic ulcer hemorrhage. A prospective, randomized, controlled trial. J Clin Gastroenterol. 1995; 21:95–98.
54. Cook, DJ, Guyatt, GH, Salena, BJ, et al. Endoscopic therapy for acute nonvariceal upper gastrointestinal hemorrhage: A meta-analysis. Gastroenterology. 1992; 102:139–148.
55. Cipolletta, L, Bianco, MA, Rotondano, G, et al. Prospective comparison of argon plasma coagulator and heater probe in the endoscopic treatment of major peptic ulcer bleeding. Gastrointest Endosc. 1998; 48:191–195.
56. Vergara, M, Calvet, X, Gisbert, JP. Epinephrine injection versus epinephrine injection and a second endoscopic method in high risk bleeding ulcers. Cochrane Database Syst Rev. 2007.
57. Sherman, LM, Shenoy, SS, Cerra, FB. Selective intraarterial vasopressin: Clinical efficacy and complications. Ann Surg. 1979; 189:298–302.
58. Miller, M, Jr., Smith, TP. Angiographic diagnosis and endovascular management of nonvariceal gastrointestinal hemorrhage. Gastroenterol Clin North Am. 2005; 34:735–752.
59. D’Amico, G, Pagliaro, L, Bosch, J. The treatment of portal hypertension: A meta-analytic review. Hepatology. 1995; 22:332–354.
60. Ioannu, GN, Doust, J, Rockey, D. Systematic review: Terlipressin in acute oesophageal variceal hemorrhage. Aliment Pharmacol Ther. 2003; 17:53–64.
61. D’Amico, G, Pagliaro, L, Bosch, J. Pharmacologic treatment of portal hypertension: An evidenced approach. Semin Liver Dis. 1999; 19:475–505.
62. Zaman, A, Chalasani, N. Bleeding caused by portal hypertension. Gastroenterol Clin North Am. 2005; 34:623–642.
63. Villanueva, C, Ortiz, J, Sabat, M, et al. Somatostatin alone or combined with emergency sclerotherapy in the treatment of acute esophageal variceal bleeding: A prospective randomized trial. Hepatology. 1999; 30:384–389.
64. Corley, DA, Cello, JP, Adkisson, W, et al. Octreotide for acute esophageal variceal bleeding: Meta-analysis. Gastroenterology. 2001; 120:946–954.
65. Gotzsche, PC, Hrobjartsson, A. Somatostatin analogues for acute bleeding oesophageal varices. Cochrane Database Syst Rev. (1):2005.
66. Bañares, R, Albillos, A, Rincon, D, et al. Endoscopic treatment versus endoscopic plus pharmacologic treatment for acute variceal bleeding: A meta-analysis. Hepatology. 2002; 305:609–615.
67. Garcia-Tsao, G, Sanyal, AJ, Norman, GD, Carey, W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007; 46:922–938.
68. Soares-Weiser, K, Brezis, M, Tur-Kaspa, R, et al. Antibiotic prophylaxis for cirrhotic patients with gastrointestinal bleeding. Cochrane Database Syst Rev. 2002.
69. Bleichner, G, Boulanger, R, Squara, P, et al. Frequency of infections in cirrhotic patients presenting with acute gastrointestinal hemorrhage. Br J Surg. 1986; 73:724–726.
70. Caly, WR, Strauss, E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993; 18:353–358.
71. Bernard, B, Cadranell, JF, Valla, D, et al. Prognostic significance of bacterial infection in bleeding cirrhotic patients: A prospective study. Gastroenterology. 1995; 108:1828–1834.
72. Chavez-Tapia, NC, Barrientos-Gutierrez, T, Tellez-Avila, F, et al. Meta-analysis: Antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding—an updated Cochrane review. Aliment Pharmacol Ther. 2011; 34(5):509–518.
73. Bosch, J, Thiabut, D, Bendtsen, F, et al. Recombinant factor VIIa for upper gastrointestinal bleeding in patients with cirrhosis: A randomized, doubleblind trial. Gastroenterology. 2004; 127:1123–1130.
74. Flower, O, Phillips, LE, Cameron, P, et al. Recombinant activated factor VII in liver patients: A retrospective cohort study from Australia and New Zealand. Blood Coagul Fibrinolysis. 2010; 21(3):207–215.
75. Heaton, ND, Howard, ER. Complications and limitations of injection sclerotherapy in portal hypertension. Gut. 1993; 34:7–10.
76. Avgerinos, A, Armonis, A, Manolakpoulos, S, et al. Endoscopic sclerotherapy versus variceal ligation in the long-term management of patients with cirrhosis after variceal bleeding: A prospective randomized study. J Hepatol. 1997; 26:1034–1041.
77. Garcia-Pagan, JC, Bosch, J. Endoscopic band ligation in the treatment of portal hypertension. Nat Clin Pract Gastroenterol Hepatol. 2005; 2:526–535.
78. Hubmann, R, Bodlaj, G, Czompo, M, et al. The use of self-expanding metal stents to treat acute variceal bleeding. Endoscopy. 2006; 38:896–901.
79. Burroughs, AK, Patch, D. Transjugular intrahepatic portosystemic shunt. Semin Liver Dis. 1999; 19:457–473.
80. Vangeli, M, Patch, D, Burroughs, AK. Salvage TIPS for uncontrolled variceal bleeding. J Hepatol. 2003; 37:703–704.
81. Garcia-Tsao, G, Bosch, J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010; 362(9):823–832.
82. Mutlu, GM, Mutlu, EA, Factor, P. GI complications in patients receiving mechanical ventilation. Chest. 2001; 119:1222–1241.
83. Cook, DJ, Fuller, HD, Guyatt, GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. N Engl J Med. 1994; 330:377–381.
84. Martindale, RG. Contemporary strategies for the prevention of stress-related mucosal bleeding. Am J Health Syst Pharm. 2005; 62(Suppl 2):S11–S17.
85. Itoh, M, Guth, PH. Role of oxygen-derived free radicals in hemorrhagic shock-induced gastric lesions in the rat. Gastroenterology. 1985; 88:1162–1167.
86. Yasue, N, Guth, PH. Role of exogenous acid and retransfusion in hemorrhagic shock-induced gastric lesions in the rat. Gastroenterology. 1988; 94:1135–1143.
87. Czaja, AJ, McAlhany, JC, Pruitt, BA, Jr. Acute gastroduodenal disease after thermal injury. An endoscopic evaluation of incidence and natural history. N Engl J Med. 1974; 291:925–929.
88. Goodman, AA, Frey, CF. Massive upper gastrointestinal hemorrhage following surgical operations. Ann Surg. 1968; 167:180–184.
89. Brown, TH, Davidson, PF, Larson, GM. Acute gastritis occurring within 24 hours of severe head injury. Gastrointest Endosc. 1989; 35:37–40.
90. Schuster, DP. Stress ulcer prophylaxis: In whom? With what? Crit Care Med. 1993; 21:4–6.
91. Cook, DJ, Griffith, LE, Walter, SD, et al. The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients. Crit Care. 2001; 5:368–375.
92. Zuckerman, GR, Shuman, R. Therapeutic goals and treatment options for prevention of stress ulcer syndrome. Am J Med. 1987; 83:29–35.
93. Stollman, N, Metz, DC. Pathophysiology and prophylaxis of stress ulcer in intensive care unit patients. J Crit Care. 2005; 20:35–45.
94. MacLaren, R, Jarvis, CL, Fish, DN. Use of enteral nutrition for stress ulcer prophylaxis. Ann Pharmacother. 2001; 35:1614–1623.
95. Harty, RF, Ancha, HB. Stress ulcer bleeding. Curr Treat Options Gastroenterol. 2006; 9:157–166.
96. Kles, KA, Wellig, MA, Tappenden, KA. Luminal nutrients exacerbate intestinal hypoxia in the hypoperfused jejunum. J Parenter Enteral Nutr. 2001; 25:246–253.
97. Smith, JS, Karlstadt, R, Blatcher, et al. Gastric pH from NPO to enteral-fed period with intermittent intravenous (IV) pantoprazole (P) vs continuously infused cimetidine (C). Am J Gastroenterol. 2002; 97:S47–S48.
98. Cook, DJ, Reeve, BK, Guyatt, GH, et al. Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta-analysis. JAMA. 1996; 275:308–314.
99. Cook, D, Guyatt, G, Marshall, J, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med. 1998; 388:791–797.
100. Szabo, S, Hollander, D. Pathways of gastrointestinal protection and repair: Mechanisms of action of sucralfate. Am J Med. 1989; 86:23–31.
101. Tryba, M. Sucralfate versus antacids or H2-antagonists for stress ulcer prophylaxis: A meta-analysis on efficacy and pneumonia rate. Crit Care Med. 1991; 19:942–949.
102. Macdougall, BRD, Bailey, RJ, Williams, R. H2-receptor antagonists and antacids in the prevention of acute gastrointestinal hemorrhage in fulminant hepatic failure: Two controlled trials. Lancet. 1977; 19:617–619.
103. Baghaie, AA, Mojtahedzadeh, M, Levine, RL, et al. Comparison of the effect of intermittent administration and continuous infusion of famotidine on gastric pH in critically ill patients: Results of a prospective randomized, crossover study. Crit Care Med. 1995; 23:687–691.
104. Barkun, AN, Bardou, M, Pham, CQ, Martel, M. Proton pump inhibitors vs. histamine 2 receptor antagonists for stress-related mucosal bleeding prophyaxis in critically ill patients: A meta-analysis. Am J Gastroenterol. 2012; 107(4):507–520.
105. Davila, RE, Rajan, E, Adler, DG, et al. ASGE guideline: The role of endoscopy in the patient with lower-GI bleeding. Gastrointest Endosc. 2005; 62:656–660.
106. Zuckerman, GR, Prakash, C. Acute lower intestinal bleeding. Part II: Etiology, therapy, and outcomes. Gastrointest Endosc. 1999; 49:228–238.
107. Jensen, DM, Machicado, GA. Diagnosis and treatment of severe hematochezia: The role of urgent colonoscopy after purge. Gastroenterology. 1988; 95:1569–1574.
108. Longstreth, GF. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: A population-based study. Am J Gastroenterol. 1997; 92:419–424.
109. Farrands, PA, Taylor, I. Management of acute lower gastrointestinal haemorrhage in a surgical unit over a 4-year period. J R Soc Med. 1987; 80:79–82.
110. Bramley, PN, Masson, JW, McKnight, G, et al. The role of an open-access bleeding unit in the management of colonic hemorrhage: A 2-year prospective study. Scand J Gastroenterol. 1996; 31:764–769.
111. Colacchio, TA, Forde, KA, Patsos, TJ, et al. Impact of modern diagnostic methods on the management of active rectal bleeding—Ten year experience. Am J Surg. 1982; 143:607–610.
112. Richter, JM, Christensen, MR, Kaplan, LM, et al. Effectiveness of current technology in the diagnosis and management of lower gastrointestinal hemorrhage. Gastrointest Endosc. 1995; 41:93–98.
113. Rossini, FP, Ferrari, A, Spandre, M, et al. Emergency colonoscopy. World J Surg. 1989; 13:190–192.
114. McGuire, HH, Jr. Bleeding colonic diverticula. A reappraisal of natural history and management. Ann Surg. 1994; 220:653–656.
115. Strate, LL. Lower GI bleeding: Epidemiology and diagnosis. Gastroenterol Clin North Am. 2005; 34:643–664.
116. Laine, L, Connors, LG, Reicin, A, et al. Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology. 2003; 124:288–292.
117. Lin, CC, Lee, YC, Lee, H, et al. Bedside colonoscopy for critically ill patients with acute lower gastrointestinal bleeding. Intensive Care Med. 2005; 31:743–746.
118. Sorbi, D, Norton, I, Conio, M, et al. Postpolypectomy lower GI bleeding: Descriptive analysis. Gastrointest Endosc. 2000; 51:690–696.
119. Prakash, C, Zuckerman, GR. Acute small bowel bleeding: A distinct entity with significantly different economic implications compared with GI bleeding from other locations. Gastrointest Endosc. 2003; 58:330–335.
120. Chaudhry, V, Hyser, MJ, Gracias, VH, et al. Colonoscopy: The initial test for acute lower gastrointestinal bleeding. Am Surg. 1998; 64:723–728.
121. Kok, KY, Kum, CK, Goh, PM. Colonoscopic evaluation of severe hematochezia in an Oriental population. Endoscopy. 1998; 30:675–680.
122. Jensen, DM, Machicado, GA, Jutabha, R, et al. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. 2000; 342:78–82.
123. Angtuaco, TL, Reddy, SK, Drapkin, S, et al. The utility of urgent colonoscopy in the evaluation of acute lower gastrointestinal tract bleeding: A 2-year experience from a single center. Am J Gastroenterol. 2001; 96:1782–1785.
124. Green, BT, Rockey, DC, Portwood, G, et al. Urgent colonoscopy for evaluation and management of acute lower gastrointestinal hemorrhage: A randomized controlled trial. Am J Gastroenterol. 2005; 100:2395–2402.
125. State, LL, Syngal, S. Timing of colonoscopy: Impact on length of hospital stay in patients with acute lower intestinal bleeding. Am J Gastroenterol. 2003; 98:317–322.
126. Chaudhry, V, Hyser, MJ, Gracias, VH, et al. Colonoscopy: The initial test for acute lower gastrointestinal bleeding. Am Surg. 1998; 64:723–728.
127. Laine, L, Shah, A. Randomized trial of urgent vs. elective colonoscopy in patients hospitalized with lower GI bleeding. Am J Gastroenterol. 2010; 105:2636–2641.
128. Hunter, JM, Pezim, ME. Limited value of technetium 99m-labeled red cell scintigraphy in localization of lower gastrointestinal bleeding. Am J Surg. 1990; 159:504–506.
129. Voeller, GR, Bunch, G, Britt, LG. Use of technetium-labeled red blood cell scintigraphy in the detection and management of gastrointestinal hemorrhage. Surgery. 1991; 110:799–804.
130. Abbas, SM, Bissett, IP, Holden, A, et al. Clinical variables associated with positive angiographic localization of lower gastrointestinal bleeding. Aust N Z J Surg. 2005; 75:953–957.
131. Green, BT, Rockey, DC. Lower gastrointestinal bleeding management. Gastroenterol Clin North Am. 2005; 34:665–678.
132. Lin, S, Rockey, DC. Obscure gastrointestinal bleeding. Gastroenterol Clin North Am. 2005; 34:679–698.
133. Carey, EJ, Fleischer, DE. Investigation of the small bowel in gastrointestinal bleeding—Enteroscopy and capsule endoscopy. Gastroenterol Clin North Am. 2005; 34:719–734.
134. Liu, K, Kaffes, AJ. Review article: The diagnosis and investigation of obscure gastrointestinal bleeding. Aliment Pharmacol Ther. 2011; 34:416–423.
135. Pennazio, M, Santucci, R, Rondonotti, E, et al. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: Report of 100 consecutive patients. Gastroenterology. 2004; 126:643–653.
136. Carey, EJ, Leighton, JA, Heigh, RI, et al. Single center outcomes of 260 consecutive patients undergoing capsule endoscopy for obscure GI bleeding. Gastroenterology. 2004; 126:A96.