Chapter 45 Gastric/GE Junction Cancer
Epidemiology and Etiology
Epidemiology
In the United States, gastric cancer now ranks 14th in incidence among the major types of malignancy. Over the past 60 years there has been a significant decline in the incidence of gastric cancer among both sexes in Western countries1 (see web-only Fig. 45-1![]() ). In the United States, from 1930 to 1980, the incidence decreased from 38 to 10 (per 100,000) for men and from 30 to 5 (per 100,000) for women. In 2010, it was estimated that 21,000 new cases and approximately 10,570 deaths will occur.2 The disease rarely occurs before the age of 40 years, but its incidence increases steadily thereafter and peaks in the seventh decade. African Americans, Hispanic Americans, and Native Americans are twice as likely to develop gastric cancer as are whites.3 There has been a slight improvement in 5-year (OS) among U.S. patients: 11% (1900 to 1963), 15% (1974 to 1976), 18% (1980 to 1982), and 21% (1989 to 1994).3
). In the United States, from 1930 to 1980, the incidence decreased from 38 to 10 (per 100,000) for men and from 30 to 5 (per 100,000) for women. In 2010, it was estimated that 21,000 new cases and approximately 10,570 deaths will occur.2 The disease rarely occurs before the age of 40 years, but its incidence increases steadily thereafter and peaks in the seventh decade. African Americans, Hispanic Americans, and Native Americans are twice as likely to develop gastric cancer as are whites.3 There has been a slight improvement in 5-year (OS) among U.S. patients: 11% (1900 to 1963), 15% (1974 to 1976), 18% (1980 to 1982), and 21% (1989 to 1994).3
Although gastric cancer incidence has decreased significantly in the United States, on a worldwide scale gastric cancer has a very high incidence and is still a leading cause of cancer death.2,4 Of the 45 countries in which age-adjusted death rates for gastric cancer were compared for 2000 (see web-only Fig. 45-2![]() ), the United States ranked 45th for both males and females.2 Kyrgyzstan ranked first for both males (47.0/100,000) and females (18.9/100,000).
), the United States ranked 45th for both males and females.2 Kyrgyzstan ranked first for both males (47.0/100,000) and females (18.9/100,000).
The site of origin within the stomach has changed in frequency in the United States over recent decades, with proximal lesions now being diagnosed and treated much more often than previously. The largest percentage of gastric cancers still arises within the antrum or distal stomach (~40%), are least common in the body of the stomach (~25%), and are of intermediate frequency in the fundus, cardia, and esophagogastric junction (~35%). In 1930, most cases of gastric carcinoma originated in the distal stomach (body and antrum). The reduction in incidence of gastric cancer from 1930 to 1980 primarily is attributable to a decline in distal lesions.5
Since the 1970s there has been a steady rise in the incidence of adenocarcinoma of the gastroesophageal (GE) junction and proximal stomach. For both sites the rate of increase during the 1970s and 1980s surpasses that of any other cancer, including melanoma, lung cancer, and non-Hodgkin’s lymphoma.6 Similar trends in the increased incidence of gastric cardia cancer have also been reported in Denmark, where rates were much higher in men, and in the United Kingdom, where rates were highest among those in higher socioeconomic classes.7
Etiology
Although the precise cause is unknown, environmental factors are likely to be important. The incidence of gastric cancer in first- and second-generation Japanese who have moved to the United States is much lower than in native Japanese citizens.8 Studies of immigrant populations from Japan and Eastern Europe show that the risk of the disease declines markedly in the second and third generations.9 The excess risk of first-generation immigrants is largely restricted to the intestinal type.10 The risk of gastric cancer is inversely associated with socioeconomic status throughout the world,11 and the marked decline in the incidence of gastric cancer in industrialized countries, including the United States, suggests that environmental factors play an important role. These may be related to poor nutrition, inadequate sanitation facilities, and substandard quality of food preservation.
Diets rich in fruits or vegetables are associated with a reduced risk of gastric cancer,12 whereas diets containing abundant quantities of smoked, heavily salted, or poorly preserved foods are associated with an increased risk of this disease.12,13 Excessive dietary salt has been associated with atrophic changes in the gastric mucosa.14 Chronic atrophic gastritis and the associated abnormality, intestinal metaplasia, are most closely linked to the intestinal type of gastric adenocarcinoma. These lesions can progress to dysplasia and carcinoma.15 The prevalence of atrophic gastritis and intestinal metaplasia is highest in regions of the world that have the highest rates of gastric cancer.16
It has been postulated that endogenous formation of N-nitroso compounds (amine or amides) can occur in the stomach when both an amino compound and a nitrating agent such as nitrate are present.13 Anaerobic bacteria that colonize stomachs in which achlorhydria occurs can convert nitrates and nitrites to potentially carcinogenic nitroso compounds.12
Epidemiologic studies have demonstrated an association between infection with Helicobacter pylori and risk of gastric cancer.17 Serologic studies have reported that persons with H. pylori infection have a threefold to sixfold higher risk of distal gastric cancer than those without this infection.18,19 The exact role of H. pylori in gastric carcinogenesis is still to be determined, although infection is associated with the development of chronic atrophic gastritis and decreased acid production.12 However, only a minority of infected patients develop gastric cancer, and data do not yet exist on the effect of treatment of the H. pylori infection on subsequent malignancy. Proximal gastric cancers (gastroesophageal junction, cardia) do not appear to be related to H. pylori infection, atrophic gastritis, or decreased acid production, although it is possible that an inverse relationship exists.
There has been a dramatic increase in the incidence of GE and gastric cardia carcinoma over the past few decades, similar to the increase in adenocarcinomas of the distal esophagus, suggesting that they may have similar causes.20,21 Although the reasons for these changes are unknown, they may be related to the increased incidence of esophageal reflux and Barrett’s esophagus.
In summary, the worldwide decline in distal, predominantly intestinal-type gastric adenocarcinoma may be the result of improved food handling (refrigeration and storage). In contrast, proximal (mostly diffuse-type) gastric cancer, which is equally prevalent in both high-risk and low-risk regions, is probably attributable to other, as yet unrecognized, factors.20 The exact role of H. pylori in gastric carcinogenesis remains to be defined.14
Prevention And Early Detection
Early detection would markedly improve prognosis of gastric cancer, because surgical resection has a high cure rate with lesions limited to the mucosa or submucosa. In the United States, however, the incidence of early gastric cancer is less than 5% in most series. In Japan, the incidence of carcinoma confined to the mucosa or submucosa was only 3.8% in the 1955 to 1956 period but by 1966 the incidence of early lesions had increased to 34.5% because of vigorous screening procedures (5-year OS of 90.9% in this cohort of patients). The value of screening is evidenced by the 5-year OS of 53% for all gastric cancers in Japan versus 21% worldwide.4
Germline mutations in the CDH1 gene, which encodes the E-cadherin protein, have been recognized in families with hereditary diffuse gastric adenocarcinoma. Carriers of these mutations have a 70% lifetime risk of developing gastric cancer. Several reports of prophylactic gastrectomy have demonstrated the routine presence of microscopic intraepithelial carcinomas for patients having regular endoscopic surveillance with multiple random biopsies.22–24 Early total gastrectomy has been recommended for this small patient population because of the lack of effective early tumor detection. Microscopic evaluation of the proximal and distal resection margins for complete removal of the gastric mucosa is necessary, because residual gastric mucosa can degenerate and result in a gastric cancer.24
Biologic Characteristics and Molecular Biology
Prognostic Factors
The most valuable prognostic indicators relate to tumor extent. With either blood-borne metastasis or peritoneal seeding, cure is rare to nonexistent. Survival decreases with progressive direct tumor extension both within and beyond the wall of the stomach.25,26,27,28,29 Lymph node involvement, per se, is not as important as the number and location of nodes.26,30 Minimal lymph node involvement adjacent to the primary lesion is the most favorable.31 The solitary finding of either involved lymph nodes or extension beyond the gastric wall is usually not as ominous as the presence of both.26,27,28,29,30
Tumor grade and gross and microscopic pathologic appearance of the primary malignancy appear to provide some prognostic information, but none of these factors is an independent prognostic variable relative to tumor stage. Prognosis is generally worse with higher-grade and diffuse-type carcinomas, which usually present with a higher stage of disease. Borrmann’s type I and II carcinomas have relatively favorable 5-year OS, whereas patients with type IV tumors (linitis plastica) have a very poor prognosis.31,32
Prognostic factors evaluated in a British study group trial33 included tumor size, macroscopic aspect, number of sites involved, wall invasion, involvement of surgical resection limits, nodal stage, and histologic grade. Multivariate analyses established as prognostic features the depth of wall invasion, nodal involvement, and involvement of margins of surgical resection.
Some investigators have suggested that tumors of the gastric cardia may have different epidemiologic factors than cancers of the distal stomach34,35 and may exhibit different tumor biology.36 Prognosis appears worse for cardia lesions,37,38 and flow cytometry reveals a greater incidence of aneuploidy when compared with tumors of the antrum and body.39
Molecular Biology
The molecular biology of gastric cancer reflects the heterogeneity of its causes and its histologic subtypes. Identification of the genetic and phenotypic variables existing among gastric cancers may lead to more directed therapeutic approaches and a more accurate prediction of clinical outcome. Changes that may affect the behavior of gastric tumor cells involve four major types of alterations. Loss of tumor-suppressor gene function, especially inactivation of the TP53 gene, certainly plays a critical role. The TP53 gene is located on the short arm of chromosome 17 and plays a key role in tumor suppression and cell-cycle regulation.40 This gene puts a brake on DNA replication and triggers programmed cell death in response to DNA damage.41 Loss of TP53 function is associated with the development of malignancy, impacts the effectiveness of chemotherapy and irradiation,42,43 and predisposes cells to genetic instability. The latter is particularly important because TP53 mutations typically occur early in carcinogenesis.
A second major aberration affecting gastric epithelial cells is the impact of alterations in mismatch repair genes. Two such genes, MSH2 and MLH1, on chromosomes 2 and 3, respectively, account for replication errors throughout the genome. Mutations in these genes are implicated in cancer family syndromes and hereditary nonpolyposis colorectal cancer, a syndrome associated with an increased tendency for the development of colorectal and gastric tumors.44 Mutations in these genes generate genetic instability and have the potential to lead to further alterations in oncogenes.
Two proto-oncogenes, MET and FGFR2, are associated with scirrhous carcinoma of the stomach. The former encodes hepatocyte growth factor, which is a potent endogenous promoter of gastric epithelial cell growth.45 Its overexpression correlates with tumor progression and metastasis.47 The latter encodes a tyrosine kinase receptor family.46 In scirrhous carcinoma, MET and FGFR2 amplification may occur independently. There is a tendency for FGFR2 to be activated in women with gastric cancer younger than 40 years of age and for MET to be amplified in men older than 50 years of age.47,48
Flow cytometry provides valuable prognostic information for gastric cancer and may be an independent prognostic factor.39 As noted previously, aneuploidy is associated with unfavorable tumor location such as the cardia39,49 but is also associated with lymph node metastasis49–51 and direct tumor extension.28 Unfavorable DNA flow cytometry characteristics seem to relate to an unfavorable prognosis.39,49,50 In one series in which multivariate analysis of DNA ploidy was analyzed with other known prognostic factors such as stage, age, and sex, DNA ploidy carried statistically significant independent prognostic information.51
The presence of several peptides including estrogen receptor,52 epidermal growth factor receptor,53 and the ERBB2 protein54 appears to affect prognosis adversely. The expression of epidermal growth factor receptor and high levels of epidermal growth factor correlate with a higher incidence of primary tumor infiltration, poor histologic differentiation, and linitis plastica. The pathophysiologic relationship between these peptide receptors and poor patient prognosis is not clear.
ERBB2 overexpression has been shown to occur in approximately 20% of patients with gastric cancer and is associated with a poorer prognosis. Perhaps more importantly, the use of the anti-ERBB2 antibody trastuzumab has been demonstrated to improve survival by approximately 2.5 months in patients with metastatic disease.55
Modern molecular biology observations confirm the heterogeneity of human gastric cancer. Genetic alterations, detected and potentially associated with worse prognosis, included CD44 expression, telomerase reactivation, TP53 gene inactivation, dysfunction of repair genes such as MSH2 and MLH1, overexpression of proto-oncogenes such as ERBB2, BCL2, MET, and FGFR2, estrogenic receptor expression, and presence of viral genome.56 Gastric cancers with class II major histocompatibility complex antigen expression (HLA-DR) have a better prognosis, but the loss of expression is not an independent prognostic factor.57
Pathology And Pathways Of Spread
Pathology
Adenocarcinoma (ACA) histology accounts for 90% to 95% of all gastric malignancies, and the terms gastric cancer and stomach cancer usually refer to such tumors. Other histologies include lymphoma (usually unfavorable histology), leiomyosarcoma, carcinoid, adenoacanthoma, and squamous cell carcinoma.58
Gastric carcinomas have been categorized with regard to both microscopic and gross pathologic features. The Lauren classification system includes an intestinal type with improved prognosis (that predominates in regions with high prevalence of gastric cancer) and a diffuse histologic type with poor prognosis (that occurs more commonly in countries with low prevalence).59 Diffuse carcinomas occur more often in young patients and develop throughout the stomach but especially in the cardia. Intestinal-type lesions are frequently ulcerative and occur in the distal stomach more commonly than the diffuse type. Grossly, gastric cancers can be categorized according to Borrmann’s five types: I, polypoid or fungating; II, ulcerating lesions surrounded by elevated borders; III, ulceration with invasion of the gastric wall; IV, diffusely infiltrating (linitis plastica); and V, not classifiable.60 The Japanese Research Society for Gastric Cancer has a classification system that divides lesions into protruded (I), superficial (II) (with elevated [IIa], flat [IIb], and depressed [IIc] subtypes), and excavated (III) types.58
Pathways of Spread
Lymphatics
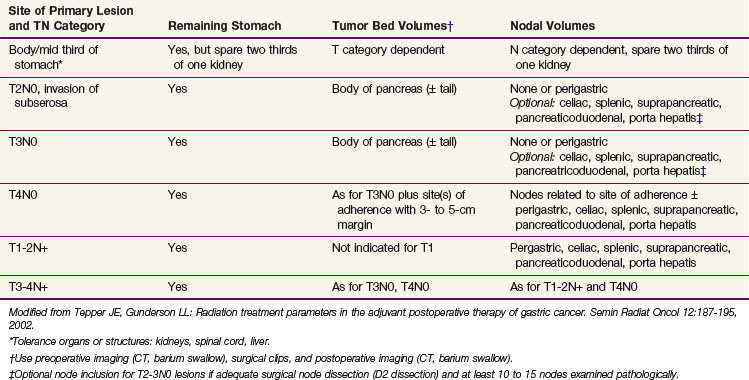
Because of the numerous pathways of lymphatic drainage in the stomach, it is difficult to perform a complete nodal dissection (see web-only Fig. 45-3![]() ). Although initial drainage is usually to lymph nodes along the lesser and greater curvatures (perigastric or N1 nodes using the Japanese Research Society for Gastric Cancer designation), primary nodal drainage includes nodes along all three branches of the celiac (left gastric, common hepatic, splenic) and the celiac itself (N2).58 Node groups that are more distal include hepatoduodenal, peripancreatic, root of mesentery (N3), periaortic, and middle colic (N4). When proximal gastric lesions extend into the distal esophagus, that nodal system is at risk.
). Although initial drainage is usually to lymph nodes along the lesser and greater curvatures (perigastric or N1 nodes using the Japanese Research Society for Gastric Cancer designation), primary nodal drainage includes nodes along all three branches of the celiac (left gastric, common hepatic, splenic) and the celiac itself (N2).58 Node groups that are more distal include hepatoduodenal, peripancreatic, root of mesentery (N3), periaortic, and middle colic (N4). When proximal gastric lesions extend into the distal esophagus, that nodal system is at risk.
Clinical Manifestations, Patient Evaluation, And Staging
Patient Evaluation
The diagnosis of gastric cancer is usually confirmed by upper gastrointestinal endoscopy or radiographs (Table 45-1). Double-contrast radiographs may reveal small lesions limited to the superficial (inner) layers of the gastric wall. Endoscopy is now the preferred diagnostic study, because it allows direct tumor visualization, cytology, and histologic biopsy that yield the diagnosis in 90% or more of patients with exophytic lesions. Ulcerated cancers and linitis plastica lesions may be harder to diagnose endoscopically, but multiple biopsies and washings enhance the probability of accurate diagnosis. Endoscopic ultrasonography (EUS) has a high degree of accuracy in determining depth of tumor invasion before resection (i.e., whether the lesion extends beyond the muscularis propria) but is less accurate in detecting regional nodal metastasis.61,62 Needle biopsies of suspicious nodes can be performed at the time of endoscopy with EUS. Although EUS may not affect therapy decisions with most current management approaches, its use may lead to selective management strategies based on tumor stage.
TABLE 45-1 Diagnostic Algorithm: Gastric/Gastroesophageal (GE) Junction Cancer
| Diagnostic Procedure | Diagnosis and Staging Capability | Recommend Routine Use |
|---|---|---|
| Primary Tumor/Regional Nodes* | ||
| Single-contrast UGI | Useful in detecting and defining primary lesions in stomach | Optional; consider along with double-contrast UGI |
| Gastroscopy | Very accurate modality to detect and define primary lesion, ≈90% confirmation rate | Yes; use to confirm lesion detected in UGI series and to screen high-risk patients |
| Ultrasound-endoscopy | Most accurate method of determining extension within and beyond gastric wall | Yes; biopsy node(s), if feasible |
| Double-contrast UGI | Useful in detecting early gastric cancers | Consider along with single-contrast UGI |
| CT: abdomen + chest | Most valuable modality to determine degree of extragastric extension and distant metastases | Yes; include CT chest for GE junction cancers |
| Metastatic Tumors | ||
| Chest films | Good for detecting metastases | Yes |
| Laparoscopy | May allow visualization of small serosal implants or liver metastases | Optional; recommended if planning preoperative chemotherapy or chemoradiation |
| PET | Excellent for detecting unsuspected metastases | Optional; recommended if planning preoperative chemotherapy or chemoradiation |
UGI, upper gastrointestinal study.
* Laboratory studies: complete blood cell count, creatinine level, liver function studies (alkaline phosphate, bilirubin, aspartate transaminase, lactate dehydrogenase), albumin level.
The value of laparoscopy in the staging of gastric cancer is still being defined. In 71 patients with CT criteria of resectable disease, 69 completed a laparoscopic evaluation.63 Laparoscopy confirmed disease in 16 of the 17 patients with peritoneal metastasis (avoiding 12 laparotomies) and in 1 of 3 patients with small liver metastases and negative CT scan. The combination of CT and laparoscopy staging information yielded a resectability rate of 93% for patients defined as potential candidates for curative gastric cancer surgery.
Treatment planning in gastric cancer patients could be improved by accurate preoperative classifications of depth of invasion (T category) and lymph node involvement (N category). A prospective study in 108 consecutive patients evaluated EUS, CT, and intraoperative surgical assessment for T and N classification.64 Staging of the T category was correct with CT in 43% of the cases, in 86% with endoscopic ultrasonography, and in 56% with intraoperative assessment. Staging of N1 and N2 lymph nodes was correct with CT in 51% of the cases, with endoscopic ultrasonography in 74%, and with intraoperative assessment in 54%. Advanced gastric tumors tended to be more accurately staged with CT; CT, in general, overstaged the T category and understaged the N category. Endoscopic ultrasonography showed high and similar accuracy for all the T categories, although it also tended to understage N categories. Finally, intraoperative assessment was equally accurate for all N categories but tended to overstage early T stages and to understage N categories.
Staging
Table 45-2 shows the current American Joint Commission on Cancer (AJCC) TNM (tumor, lymph nodes, metastasis) staging system.65 The TNM system is the standard system for reporting outcomes in gastric and gastroesophageal junction cancer.
Primary Therapy
Surgical Method
Although only one small prospective randomized trial has been performed with regard to extent of the gastric resection,66 extensive experience exists with various surgical procedures, and appropriate generalizations can be made. The preferred treatment for lesions arising in the body or antrum of the stomach is a radical distal subtotal resection. This removes approximately 80% of the stomach along with the first portion of the duodenum, the gastrohepatic and gastrocolic omenta, and the nodal tissue adjacent to the three branches of the celiac axis. Extensive or proximal cancers will require a total gastrectomy to achieve an adequate proximal gastric margin. However, total gastrectomy is not necessary when subtotal gastrectomy will provide a 5-cm clearance of the gross tumor.67,68 The propensity for gastric carcinoma to spread via submucosal and subserosal lymphatics dictates the need for a 5-cm surgical resection margin of normal stomach beyond the visible lesion. It may be necessary to extend the resection to include some (or additional) esophagus or duodenum if frozen section pathologic evaluation of the surgical margins fails to confirm the adequacy of proximal and distal resection margins. If total gastrectomy is necessary, a splenectomy is sometimes performed, particularly in gastric cancers of the proximal third of the stomach and tumors of the body near the greater curvature. Cancers in these locations are more likely to metastasize to lymph nodes in the splenic hilum that cannot be completely excised without a splenectomy. Although the value of routine splenectomy has not been addressed in prospective randomized trials, retrospective Japanese and American data do not provide evidence of a survival benefit with the routine use of this procedure.69,70
The optimal extent of lymph node dissection for gastric cancer remains controversial. Several studies have shown that the presence and extent of lymph node metastasis correlate with the depth of primary tumor invasion.71–73 Although several nonrandomized clinical trials have suggested that extended lymphadenectomies may improve survival,70,74–78 other nonrandomized79,80 and randomized trials81–83,84–87 have not demonstrated an advantage. A large multicenter phase III study in the Netherlands accrued 996 evaluable patients and provides additional objective data on the lack of value of extended lymphadenectomy in gastric carcinoma.82 Among the 711 patients having curative resections, both morbidity and mortality were significantly higher with the more extensive nodal dissection.83,84,85 A randomized study of 400 patients conducted by the Medical Research Council (MRC) in the United Kingdom has also demonstrated higher morbidity and mortality in the extended lymphadenectomy cohort.86,87 No benefit in disease-free survival (DFS) or OS was found in either the Dutch or British trials (Table 45-3). Any apparent survival benefit seen with the extended node dissection performed in Japan may be caused by the phenomenon of a shift in stage rather than superior surgery. Although most patients with five or more lymph node metastases or node metastasis not adjacent to the primary tumor have a very poor outcome, extended node dissection seems reasonable when it can be performed by experienced surgeons without significant additional surgical morbidity or mortality. A recent systematic review and meta-analysis88,89 demonstrate the safety and feasibility of D2 resection with or without para-aortic nodal dissection but fail to show a benefit in OS.
Endoscopic laser surgery has been used in selected patients with early gastric cancer.90 Small lesions (≤3 cm) that are pedunculated, do not invade the submucosa, and are well differentiated infrequently have lymph node metastasis (<5%). Nearly 75% of these select tumors can be completely removed endoscopically. Although early gastric cancer may have a long natural history before progression, standard surgical resection rather than endoscopic removal is still preferable.91 Irradiation plus chemotherapy should be considered as adjuvant therapy when the tumor is endoscopically treated.
Quality of life after gastrectomy is being analyzed.92,93 In a University of Heidelberg report of 104 patients,94 12 months after gastrectomy, postoperative symptoms of heartburn or dumping in the group undergoing total gastrectomy with pouch reconstruction did not differ significantly from the group having only distal gastric resection. In a recent prospective trial of 120 patients requiring total gastrectomy plus pouch who were randomized to two different types of reconstruction (jejunal interposition versus Roux-en-Y),95 there were no differences in food passage, body weight, or quality of life parameters. However, for all patients there are significant quality of life issues related to decreased absorption of fats, iron, and calcium, as well as the loss of intrinsic factor, and thus vitamin B12 deficiency. These losses need to be addressed with dietary supplements (iron, calcium), monitoring for osteoporosis, and possible monthly vitamin B12 injections.
Survival Results of Surgery Alone
Overall survival with surgery alone remains poor, despite improved perioperative care that has resulted in a substantial decline in postoperative mortality.96 A large review from Europe97 reported excellent 5-year survival for early gastric cancer patients (83%) but a marked diminution in survival for more invasive cancers (31% for tumors of the antrum, 24% for the midstomach, and 16% for the cardia). Survival in excess of 90% has been achieved throughout the world with resection of lesions confined to the mucosa or submucosa.98–102 In reports with lower 5-year survivals for the early lesions, most of the mortality is from noncancer causes.99,103 For gastric cancers with deeper invasion or nodal involvement, survival decreases proportional to the degree of invasion or nodal involvement. When N1 or N2 nodes are involved, Western reports continue to show 5-year OS of 10% to 30%,104,105 whereas Japanese authors report 5-year OS of 25% to 60% (vs. <10% with N3 or N4 involvement)70,106–109 (see web-only Table 45-1)![]() . Although pathologic staging differences, different tumor biology, and more radical surgical extirpation have been proposed as explanations, the cause of the difference in U.S. and Japanese results with N1 or N2 disease remains uncertain. Even in the Far East, half of all patients with more invasive gastric cancer do not survive their disease, a fact that underlies the need for new nonsurgical therapies. Results of phase III trials, to be discussed subsequently, demonstrate improvements in survival with the addition of adjuvant chemoradiation to surgical resection.
. Although pathologic staging differences, different tumor biology, and more radical surgical extirpation have been proposed as explanations, the cause of the difference in U.S. and Japanese results with N1 or N2 disease remains uncertain. Even in the Far East, half of all patients with more invasive gastric cancer do not survive their disease, a fact that underlies the need for new nonsurgical therapies. Results of phase III trials, to be discussed subsequently, demonstrate improvements in survival with the addition of adjuvant chemoradiation to surgical resection.
Relapse Patterns after “Curative Resection”
Local relapse or failure in the tumor bed and regional lymph nodes or distant failures via hematogenous or peritoneal routes are all common mechanisms of relapse after “curative resection” in clinical, reoperative, and autopsy series.* For lesions of the GE junction, both the liver and lungs are common sites of distant metastases. With gastric lesions that do not extend to the esophagus, the initial site of distant metastases is usually the liver, and many relapses could be prevented if an effective “abdominal” or liver therapy could be combined with treatment of the primary tumor and regional lymph nodes.
Locoregional failures occur commonly within the region of the gastric bed and nearby lymph nodes (Table 45-4 and Fig. 45-1). Tumor relapse in anastomoses, the gastric remnant, or the duodenal stump is also frequent. In a University of Minnesota reoperative analysis,28,29 locoregional failure occurred as the only evidence of relapse in 29% of the 86 patients with relapse (23% of the 105 evaluable patients at risk) and as any component of failure in 88%. More extensive operative procedures including routine splenectomy, omentectomy, and radical lymph node dissection neither improved survival116 nor decreased the incidence of locoregional failure28,29 in the reoperative analysis. Subsequent relapse within the scope of the initial node dissection occurred in a high percentage of the patients even when radical node dissections were performed (removal of N1 and N2 with or without N3 nodes)9 (see web-only Table 45-2)![]() . These data indicate the difficulty of performing a complete lymph node and lymphatic resection in this anatomic location and provide a partial explanation for the lack of survival benefit with a D2 versus D1 node dissection in phase III surgical trials discussed previously. In a more recent clinical analysis of patterns of relapse from Memorial Sloan-Kettering Cancer Center, 50% of patients with relapse had a locoregional component.118
. These data indicate the difficulty of performing a complete lymph node and lymphatic resection in this anatomic location and provide a partial explanation for the lack of survival benefit with a D2 versus D1 node dissection in phase III surgical trials discussed previously. In a more recent clinical analysis of patterns of relapse from Memorial Sloan-Kettering Cancer Center, 50% of patients with relapse had a locoregional component.118
TABLE 45-4 Gastric Cancer: Patterns of Locoregional Relapse in Clinical, Reoperation, and Autopsy Series
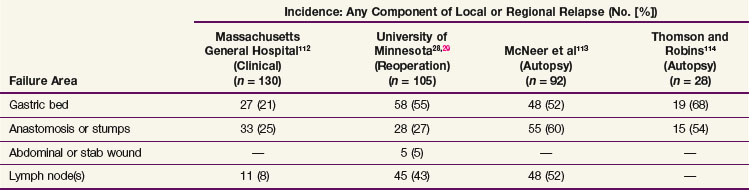
WEB-ONLY TABLE 45-2 Extent of Gastrectomy and Node Dissection versus Type of Local Regional Relapse: Reoperation Series
Patterns of relapse by stage were analyzed in detail in a series of 130 patients who underwent resection with curative intent at the Massachusetts General Hospital.112 Locoregional relapse occurred as any component of failure in 49 patients (38%) and as the sole failure in 21 (16% of 130 patients at risk and 24% of the 88 with disease progression). The incidence of locoregional relapse by stage was in excess of 35% for modified Astler-Coller stages B2 (T3N0), B3 (T4N0), C2 (T3N1-3), and C3 (T4N1-3). The sites at higher risk for locoregional relapse included the gastric bed (27 of 130 patients, 21%) and the anastomosis or gastric remnant (33 of 130 patients, 25%). The true incidence of gastric bed, regional lymph node, and peritoneal failures may be higher because this was neither a reoperative nor an autopsy series (see comparative findings in Table 45-4). Additional information on patterns of relapse by stage exist in both the University of Minnesota reoperation analysis28,29 and the University of Washington autopsy analysis.115 Although patterns of relapse data are more accurate in such analyses, patient selection is biased.
Adjuvant Chemotherapy
The role of adjuvant chemotherapy in gastric cancer has been extensively studied over the past decades, being the first attempt to improve the prognosis of resected gastric cancer.* Results of randomized clinical trials of adjuvant single-agent chemotherapy after curative resection in gastric cancer generally have not shown any survival benefit when compared with surgical resection alone. For an expanded discussion of this topic, the reader is referred to the Expert Consult website![]() .
.
Two phase III clinical trials evaluated the impact of combination chemotherapy with 5-FU, doxorubicin (Adriamycin), and mitomycin C (the FAM regimen) after curative surgery in resected high-risk gastric cancer.119,120 Coombs and associates119 included 315 patients, and no difference was observed in either OS (FAM 45% vs. control 35%) or relapse rates (FAM 56% vs. control 56%). In a Southwest Oncology Group study,120 no advantage to adjuvant treatment with the FAM regimen was reported either.
A Spanish trial121 compared high-dose mitomycin C after surgical resection (33 patients) versus surgery alone (37 patients). This small trial showed a significant 5-year OS advantage (p = .001) in the group receiving chemotherapy (76% vs. 30%), and a low rate of relapse in the treated arm (7/33 patients or 21%) versus the control arm (23/37 patients or 62%). This trial was extended to 134 patients with median follow-up of 8.75 years and still showed a survival advantage for postoperative mitomycin (p = .025). A subsequent randomized Spanish trial122 observed a significantly better 5-year OS for mitomycin plus tegafur (67%) versus mitomycin alone (44%) in resected high-risk gastric cancer, suggesting a strong benefit in patients with node-negative and early-stage disease.
Neri and colleagues123 treated 68 surgically resected, node-positive patients with the addition of adjuvant epirubicin, 5-FU, and leucovorin (the EFL regimen) and compared them with 69 surgery-alone control patients. The authors noted an improvement in 5-year OS from 13% to 30% for those receiving adjuvant chemotherapy.
Adjuvant chemotherapy with FAM2, seven cycles, was compared with surgery alone in a European Organization for Research and Treatment of Cancer (EORTC) randomized phase II trial that included 314 patients with stage II and III gastric adenocarcinoma.124 Five-year OS was 70% for stage II and 32% for stage III disease with no significant differences between groups. Toxicity was high with the FAM2 regimen, but time to disease progression was significantly longer in this group. DFS increased to a borderline significant value. Similarly, an adjuvant FAM randomized trial done by the Southwest Oncology Group, failed to show improved survival results.125
The EORTC and the International Collaborative Cancer Group (ICCG) conducted independent randomized phase III studies of either adjuvant FAMTX or FEMTX (5-FU, epirubicin, and MTX) compared with surgery alone.126 Both studies were closed when they failed to reach accrual after being open for 7 years. A pooled analysis of the two studies demonstrated that they both caused substantial toxicity but had no significant effect on OS, which was the primary endpoint of each study.
Perioperative Chemotherapy
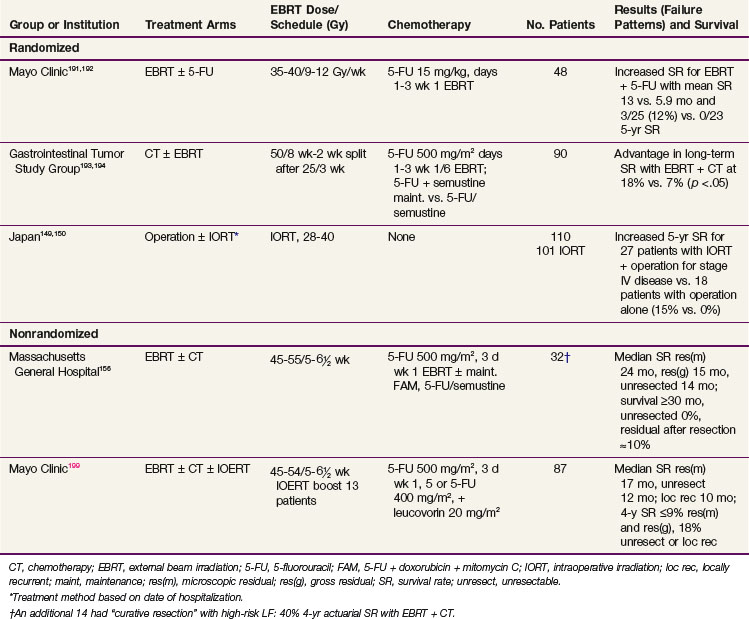
In the British MAGIC trial, 503 patients with resectable adenocarcinoma of the stomach (372 patients), GE junction (58 patients), or lower esophagus (73 patients) were randomized to receive either surgery alone or surgery plus three cycles of preoperative epirubicin, cisplatin, and infusional 5-FU (ECF regimen) and three cycles of postoperative ECF therapy.127 The use of perioperative chemotherapy was associated with significant improvement in survival compared with surgery alone (median survival 24 vs. 20 months, 5-year OS 36% vs. 23%, p = .009). Overall, 103 patients completed all six cycles of chemotherapy (Table 45-5).
Meta-Analysis of Adjuvant Trials
Because many of the trials conducted to date have been underpowered to adequately assess potential differences between the control and treatment arms in regard to OS, seven meta-analyses have been performed since 1990 to better assess the use of adjuvant chemotherapy.128,129,130–133,134 Three recent meta-analyses showed a significant survival advantage to the use of chemotherapy after surgical resection of gastric cancer. Seventeen randomized clinical trials, involving 3118 patients, were included in the meta-analysis by Panzini and associates.128 The pooled odds ratio was 0.72 (95% confidence interval [CI], 0.62 to 0.84). The meta-analysis by Janunger and coauthors129 included 21 randomized clinical trials, involving 3962 patients, a slightly larger and somewhat different group of studies. The pooled odds ratio from this study was 0.84 (95% CI, 0.74 to 0.96). In a subanalysis, Western and Asian studies were assessed separately. The meta-analysis of the Western studies did not show a benefit to adjuvant therapy (pooled odds ratio of 0.96; 95% CI, 0.83 to 1.12), whereas the Asian studies did show evidence of benefit (pooled odds ratio of 0.58; 95% CI, 0.44 to 0.76). In 2010, a meta-analysis by Paoletti and colleagues134 identified 17 trials with individual patient data availability (3838 patients) and a median follow-up exceeding 7 years. There were 1000 deaths in the 1924 patients assigned to chemotherapy and 1067 deaths in 1857 patients assigned to surgery only. Adjuvant chemotherapy achieved a significant benefit in 5-year OS (49.6% vs. 55.3%, p = .001).
Adjuvant Irradiation
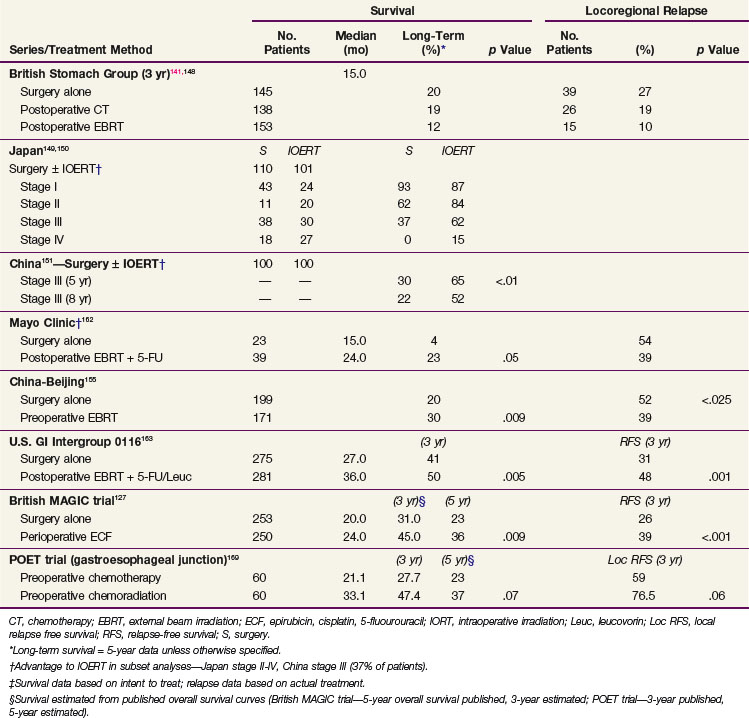
Irradiation has been only minimally evaluated as the sole adjuvant treatment after complete surgical resection in randomized phase III trials. Adjuvant EBRT reduced locoregional failures when compared with the surgery-alone control arm in the British adjuvant trial noted later, but no survival benefits were found.141,148 Although phase III trials from Japan149,150 and China151 suggest some survival benefit for IORT versus a surgery-alone control arm, the advantage was found only in subset analyses.
Postoperative External Beam Irradiation
The British Stomach Cancer Group (BSCG) completed a prospectively randomized trial of surgery alone versus postoperative FAM or EBRT (45 Gy in 25 fractions ± 5-Gy boost).141,148 A total of 436 patients were randomized and observed for a minimum of 12 months; the study arms were well balanced with regard to prognostic factors. No survival differences by treatment arm were seen (median, 15 months) (see Table 45-5). However, locoregional failure was documented in only 15 of 153 patients (10%) in the adjuvant irradiation arm versus 39 of 145 (27%) in the surgery-alone arm and 26 of 138 (19%) in the FAM group. Interpretation of the results is complicated by the inclusion of 93 patients with resection but gross residual disease and of 78 patients (18%) with gross total resection but microscopically positive resection margins. Neither group of patients would be candidates for current gastric surgical adjuvant trials in the United States. In addition, nearly one third of the patients randomized to receive adjuvant treatment did not receive the assigned therapy. Of 153 patients randomized to the irradiation study arm, only 104 (68%) received a dose of 40.5 Gy or more. Only 62% of the patients received six or more cycles of chemotherapy. The results of this study are similar to results seen in the adjuvant treatment of rectal cancer, in which adjuvant preoperative and postoperative irradiation improve local control but did not increase patient survival unless combined with chemotherapy.
Preoperative External Beam Irradiation
Three prospective randomized Russian trials have evaluated preoperative irradiation in potentially resectable gastric cancer.152–154 The first trial randomly assigned 293 patients to receive either surgery alone, surgery after preoperative EBRT (20 Gy in four fractions), or surgery after the same EBRT plus daily hyperthermia. The survival rates at 3 and 5 years were improved in both irradiation study arms compared with surgery alone, and the improvement with combined EBRT and hyperthermia was statistically significant at both 3 and 5 years.157 The second trial compared preoperative EBRT (20 Gy) versus surgery alone in 279 patients. Three- and 5-year survival rates were increased, and no increase in operative morbidity was observed.158 The third trial compared surgery alone versus preoperative EBRT (32 Gy with concomitant inhalation of oxygen) plus surgery. A survival advantage was observed with preoperative treatment, and the resection rate was increased by 17%.159 There are some methodologic uncertainties with all three of these trials, and their applicability to Western gastric carcinoma is not clear.
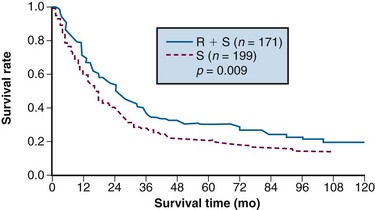
A double-blind, randomized trial from Beijing, conducted from 1978 to 1989, compared a surgery-alone control arm (n = 199) with preoperative EBRT plus surgery (n = 171) for patients with adenocarcinoma of the gastric cardia.155 Irradiation was given with 8-MV photons or cobalt with anterior-posterior/posterior-anterior fields to a dose of 40 Gy in 20 fractions of 2 Gy over 4 weeks. Surgery was performed 2 to 4 weeks after completion of irradiation. The addition of preoperative EBRT produced downstaging of disease and improvements in radical resection rates (radical resection rates of 80% vs. 62% with preoperative EBRT vs. surgery alone).
Survival and locoregional disease control were improved in the patients assigned to preoperative EBRT versus surgery alone. The 5- and 10-year OS were 30% versus 20% and 20% versus 13%, respectively (Fig. 45-2; p = .009 Kaplan-Meier log-rank) (see Table 45-5). The divergence in survival curves began in the first year of follow-up and persisted through 9 years. Locoregional disease control was also improved with combined modality treatment with local relapse rates of 39% versus 52% (p <.025) and regional node relapse rates of 39% versus 54% (p <.005). The rates of distant metastases were the same at 24% versus 25%. The improvements in survival and disease control (locoregional) were accomplished with no increase in treatment-related morbidity or mortality (operative mortality 0.6% vs. 2.5% with or without preoperative EBRT; intrathoracic leak rates were 1.8% and 4.2%, respectively).
Adjuvant Irradiation Plus Chemotherapy
Postoperative EBRT Plus Chemotherapy
Phase II Trials
Phase II single-institution gastric cancer trials that showed promise for postoperative adjuvant chemoradiation included series from Massachusetts General Hospital (MGH),156 Israel (Hadassah Medical Center),157 Thomas Jefferson University Hospital,158,159 the University of Pennsylvania,160 and the Mayo Clinic161 (see web-only Table 45-3)![]() . Gunderson and associates156 reported a median survival of 24 months and a 4-year survival of 43% in 14 patients from MGH who had complete resection of tumors with extension beyond the wall, nodal involvement, or both. Patients received postoperative EBRT (45 to 52 Gy, 1.8 Gy/day) plus concurrent 5-FU–based chemotherapy. Subsequent locoregional relapse was documented in 2 of 14 patients (14%), in contrast to a 42% incidence in similar high-risk patients treated with surgery alone.112 Information on the other phase II trials is provided in detail on the Expert Consult website.
. Gunderson and associates156 reported a median survival of 24 months and a 4-year survival of 43% in 14 patients from MGH who had complete resection of tumors with extension beyond the wall, nodal involvement, or both. Patients received postoperative EBRT (45 to 52 Gy, 1.8 Gy/day) plus concurrent 5-FU–based chemotherapy. Subsequent locoregional relapse was documented in 2 of 14 patients (14%), in contrast to a 42% incidence in similar high-risk patients treated with surgery alone.112 Information on the other phase II trials is provided in detail on the Expert Consult website. ![]()
In a gastric series from Thomas Jefferson University Hospital, 120 patients had surgical resection but were at high risk for relapse because of extension beyond the gastric wall, nodal metastases, or positive margins of resection.158 Seventy patients had surgery alone, and 50 received adjuvant therapy. Apparent improvements in local control as well as median and 5-year survival were noted with additional therapy. For patients with negative resection margins, 2-year local control with surgery alone was 55% versus 93% with adjuvant irradiation with or without chemotherapy (p = .03). For patients with T3 and T4 tumors and lymph node involvement, median survival was 9 months versus 13 months (surgery alone or plus adjuvant treatment) and 5-year OS was 4% versus 22% (p = .03). In a separate analysis at the same institution of 55 patients with cancers of the GE junction, local relapse with surgery alone was 74% versus 36% in patients with adjuvant EBRT ± chemotherapy (p = .0014).159 Survival trends for node-positive patients appeared better in patients who received adjuvant treatment (5-year OS 15% versus 0%, p >.01). In a University of Pennsylvania analysis, treatment intensification appeared to improve both disease control and survival. The incidence of local relapse with surgery alone was 75% (31 of 40) versus 24% with adjuvant irradiation (4 of 17) and 15% with adjuvant irradiation plus chemotherapy (4 of 27).160 Five-year survival trends favored adjuvant chemoradiation over surgery alone at 55% versus 31%. In a retrospective Mayo Clinic analysis, 63 patients received postoperative EBRT ± 5-FU after resection of carcinoma of the stomach or GE junction.161 Twenty-five of the 63 patients had complete resection with no residual disease but had high-risk factors for disease relapse (extension beyond gastric wall, 92% of the patients; involved nodes, 92%; both high-risk factors, 84%). Concurrent 5-FU ± leucovorin was given with EBRT in 84% of the 25 adjuvantly treated patients, but maintenance chemotherapy was given in only 20%. Locoregional control was achieved in 20 of the 25 (80%), with median survival of 19 months. Four-year OS was 31% in spite of the very poor prognostic factors in these 25 patients.
Phase III Trials: Mayo Clinic, U.S. GI Intergroup 0116
A prospective randomized trial was conducted at the Mayo Clinic162 and included 62 patients with a poor prognosis after complete resection of gastric cancers (Table 45-5). Patients were randomized to either surgery alone or surgery followed by EBRT plus concomitant 5-FU (37.5 Gy in 24 fractions over 4 to 5 weeks; 5-FU, 15 mg/kg/day 1 to 3 by IV bolus). A nonstratified, prerandomization scheme was used with a 2 : 3 ratio favoring treatment. Informed consent was requested only of the 39 patients randomized to treatment. Ten of the 39 patients refused further therapy and were observed. When analyzed by intent to treat, the adjuvant arm had statistically significant improvement in both relapse-free (RFS) and OS (5-year OS 23% versus 4%; p <.05). When patient outcome was compared by actual treatment received (29 adjuvant treatment, 33 surgery alone), 5-year OS still favored the adjuvant group (20% vs. 12%), but the differences were not statistically significant. The 10 patients who refused assignment to adjuvant treatment had more favorable prognostic findings than the other two groups of patients. When the two groups with equally poor prognostic factors were compared, 5-year OS was 20% versus 4%, with an advantage to those receiving adjuvant treatment. When analyzed by treatment delivered, locoregional relapse was decreased with adjuvant treatment (54% incidence with surgery alone vs. 39% with irradiation plus 5-FU).
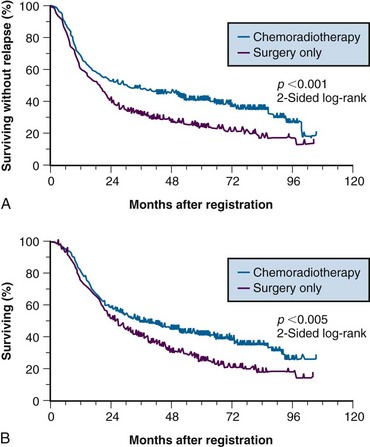
Because of conflicting results in prior small phase III studies, a U.S. GI Intergroup trial (INT 0116) was initiated to evaluate postoperative combined 5-FU–based chemotherapy and irradiation to the gastric bed and regional nodes versus surgery only in resected but high-risk gastric cancer patients.163 Eligible patients had completely resected ACA of the stomach or GE junction with complete penetration of the muscularis propria (T2-4N0) or involved nodes (T1-4N1-3). After an en bloc resection, 556 patients were randomized to either surgery alone or postoperative combined modality therapy. This consisted of one 5-day cycle of 5-FU and leucovorin followed by concurrent chemoradiation (45 Gy in 25 fractions plus concurrent 5-FU and leucovorin, 4 days/week 1, 3 days/week 5) followed by two additional 5-day cycles of 5-FU and leucovorin given at 1-month intervals. Nodal metastases were present in 85% of patients. With median follow-up of 5 years, RFS at 3 years is 48% for adjuvant treatment and 31% for observation (p = .001); 3-year OS is 50% for treatment and 41% for observation (p = .005) (see Table 45-5). The median survival in the surgery-only group was 27 months, as compared with 36 months in the chemoradiation group. The median duration of RFS was 30 months in the chemoradiation group and 19 months in the surgery-only group (Fig. 45-3).
The results of this large randomized phase III U.S. GI Intergroup trial demonstrate a clear survival advantage to the use of postoperative chemoradiation in high-risk patients who have undergone resection of their disease.163 Furthermore, the results strongly support the integration of postoperative chemoradiation into the routine care of patients with curatively resected high-risk carcinoma of the stomach and GE junction. This approach is now viewed by many as the standard of care in the United States.
Quality control of irradiation field design in the INT 0116 trial was conducted during the cycle of chemotherapy given before the start of concurrent chemoradiation.164 The upfront quality control provided the mechanism to correct most of the major or minor deviations (35% incidence) in field design before the start of treatment and resulted in only a 6.5% final major deviation rate. Utilization of upfront quality control may have been a key factor in achieving a positive survival advantage for adjuvant chemoradiation.
Postoperative Chemoradiation Plus D2 Resection
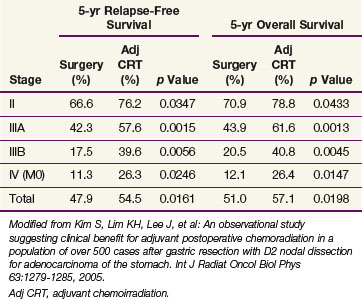
The potential role of postoperative chemoradiation in D2 resected patients was evaluated in a series of 990 patients treated in Seoul, South Korea165 (Table 45-6 and see web-only Table 45-3)![]() . Surgery alone was used in 446 patients, and postoperative chemoradiation was given to 544 patients (disease and patient characteristics and method of chemoradiation paralleled the INT 0116 trial). Both disease control and survival were improved in patients who received trimodality treatment (median survival 95.3 vs. 62.6 months, 5-year OS 57% vs. 51%, p = .02; 5-year RFS 54.5% vs. 47.9%, p = .016; locoregional relapse 14.9% vs. 21.7%, p = .005). Five-year RFS and OS were consistently better with trimodality treatment for each stage grouping.
. Surgery alone was used in 446 patients, and postoperative chemoradiation was given to 544 patients (disease and patient characteristics and method of chemoradiation paralleled the INT 0116 trial). Both disease control and survival were improved in patients who received trimodality treatment (median survival 95.3 vs. 62.6 months, 5-year OS 57% vs. 51%, p = .02; 5-year RFS 54.5% vs. 47.9%, p = .016; locoregional relapse 14.9% vs. 21.7%, p = .005). Five-year RFS and OS were consistently better with trimodality treatment for each stage grouping.
Preoperative EBRT Plus Chemotherapy
Although no randomized trials testing preoperative EBRT plus chemotherapy for gastric cancer have yet been published, three phase III trials for patients with esophagus cancer (alone or combined with GE junction or cardia lesions) included patients with adenocarcinoma. This includes studies by Walsh and associates,166 Urba and coauthors,167 and the U.S. GI Intergroup trial published by Tepper and colleagues.168
In the Dublin trial by Walsh and associates,166 patients with ACA of the esophagus and gastric cardia were randomized to either immediate surgery (control arm) or preoperative EBRT plus 5-FU and cisplatin (40 Gy in 15 fractions; 5-FU, 15 mg/kg/day for 5 days weeks 1 and 6 as a continuous infusion or approximately 600 mg/m2/day for 5 days; cisplatin 75 mg/m2 on the first day of each 5-FU infusion), followed by surgical resection 8 weeks after completion of EBRT plus chemotherapy. A highly significant difference in survival was observed with combined modality therapy (intent to treat: median survival 16 vs. 11 months; 3-year OS, 32% vs. 6%; p = .01; actual treatment: median survival 32 vs. 11 months; p = .001; 3-year OS, 37% vs. 7%; p = .006). Survival rates for the control group of patients were inferior to those from other historical data.
Urba and coauthors167 at the University of Michigan found a borderline survival advantage for patients treated with preoperative chemoradiation versus surgery alone. Preoperative treatment involved 5-FU, cisplatin, and vinblastine given concurrently with EBRT (45 Gy/1.5 Gy twice daily for 3 weeks). Patients with either squamous cell carcinoma (SCC) or ACA were eligible, but 75 of the 100 randomized patients had ACA. Survival differences favoring trimodality treatment versus surgery alone did not appear until several years of follow-up (3-year OS, 30 vs. 16%; p = .15 Cox regression; 0.09 log-rank multivariate analysis).
A confirmatory U.S. GI Intergroup trial (CALGB 9781) included patients with either esophagus or GE junction cancers (either SCC or ACA); this trial was stopped prematurely because of poor accrual. Despite the low accrual, patients randomized to trimodality treatment had a survival benefit when compared with patients randomized to surgery alone (median survival 54 vs. 21.6 months; 5-year OS 39% vs. 16%, p = .008).168
Summary: Adjuvant EBRT Alone or Plus Chemotherapy
In summary, postoperative chemoradiation163,164 has been demonstrated to be superior to surgery alone for resectable gastric and GE junction cancers in randomized phase III trials. There are also randomized phase III data to suggest a benefit to preoperative irradiation that need to be confirmed.155 Future preoperative irradiation trials should evaluate the addition of concurrent and maintenance chemotherapy. Postoperative chemoradiation trials are evaluating more aggressive chemotherapy both as concurrent and maintenance components of treatment.
GE Junction: Preoperative Chemoradiation versus Chemotherapy (POET Trial)
The POET phase III trial by Stahl and colleagues169 included 120 eligible patients with T3-4 ACA of the GE junction treated with either preoperative chemoradiation (n = 60) or chemotherapy alone (n = 60). All patients received two cycles of a cisplatin/leucovorin/5-FU (PLF) regimen of chemotherapy on weeks 1 to 6/7 to 13: cisplatin 50 mg/m2/hour on days 1, 15, and 29; leucovorin 500 mg/m2/2 hours and 5-FU 2 g/m2/24 hours infusion on days 1, 8, 15, 22, 29, and 36, followed by either PLF alone (weeks 14 to 17) or chemoradiation with 30 Gy EBRT/15 × 2 Gy/3 wk (weeks 14 to 17) plus a concurrent regimen of cisplatin and etoposide on week 1 of EBRT (cisplatin 50 mg/m2/hour on days 2 and 8; etoposide 80 mg/m2/hour on days 3 to 5). Outcomes analyses trends favored preoperative chemoradiation over chemotherapy alone with regard to both OS (p = .07) and freedom from local relapse (p = .06).
SEER Analysis, Gastric Cancer: Adjuvant Irradiation or Chemoradiation
Coburn and colleagues170 evaluated the impact of postoperative adjuvant EBRT or chemoradiation in 4041 patients who had resection of nonmetastatic gastric ACA from May, 2000 to December, 2003 (SEER database). Patients treated with postoperative EBRT (alone or plus concurrent chemotherapy) versus surgery alone had improved median OS for stage III (31 vs. 24 months; p = .005) and stage IV M0 (20 vs. 15 months; p <.001), and the difference approached significance for stage II (p = .0535). Adjusted analysis using a propensity score suggested that the benefit of adjuvant irradiation was similar for stage II and III patients (hazard ratio of death was 0.733 and 0.773, respectively).
Meta-Analyses, Gastroesophageal Cancer: EBRT, Chemoradiation, Chemotherapy
A meta-analysis by Fiorica and colleagues171 evaluated the impact of adjuvant EBRT or chemoradiation in the reduction of all-cause mortality in patients with resectable gastric ACA (nine published phase III trials—four with preoperative EBRT, five with postoperative chemoradiation). For patients treated with preoperative EBRT, the hazard ratio for all-cause mortality was 0.62 (p = .002). In patients treated with postoperative chemoradiation, the hazard ratio for all-cause mortality was 0.45 (p <.00001).
An Australian meta-analysis published by Gebski and associates172 evaluated the impact of either preoperative chemoradiation or preoperative chemotherapy in patients with resectable esophageal ACA or SCC. For patients treated with preoperative chemoirradiation, the hazard ratio for all-cause mortality was 0.81 (p = .002) with similar results for patients with ACA (0.75, p = .02) and SCC (0.84; p = .04). In patients treated with preoperative chemotherapy, the hazard ratio for all-cause mortality was 0.90 (p = .05) with significant benefit found for patients with ACA (0.78; p = .014) but no benefit for those with SCC (0.88; p = .12).
IORT Alone or Plus EBRT
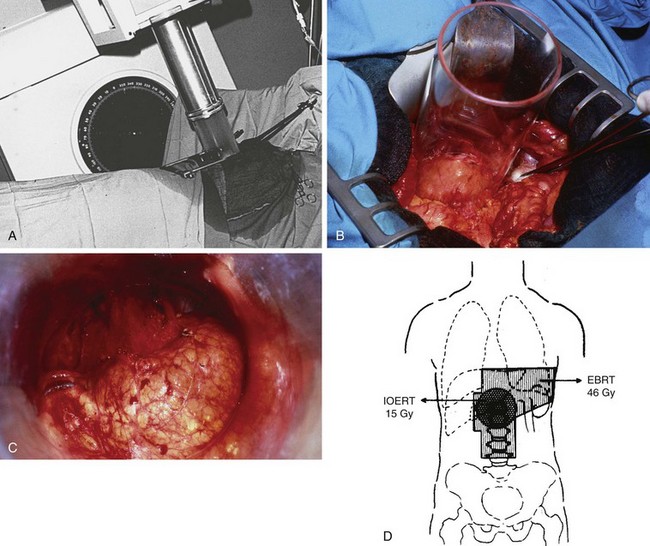
The pioneer work of Abe and Takahashi at Kyoto University, Japan, in the 1970s, fostered a renewed interest in the old idea of irradiating tumor-bearing areas under direct vision during laparotomy.149,150 Although their work triggered gastric IORT trials around the world, only a few investigators have favored the use of IORT as the only adjuvant treatment after surgical resection.173,174–178 Most Western IORT protocols included the delivery of EBRT preoperatively or postoperatively and used IORT doses considerably lower than those advised by Abe and Takahashi, because of fear of severe toxicity.179–187 This followed a tendency in the design of IORT trials in other anatomic locations, where IORT doses, in the range of 10 to 20 Gy, were combined with adjuvant EBRT doses of 45 to 50 Gy in 1.8- to 2.0-Gy fractions (Fig. 45-4).
Survival and Locoregional Relapse Outcomes
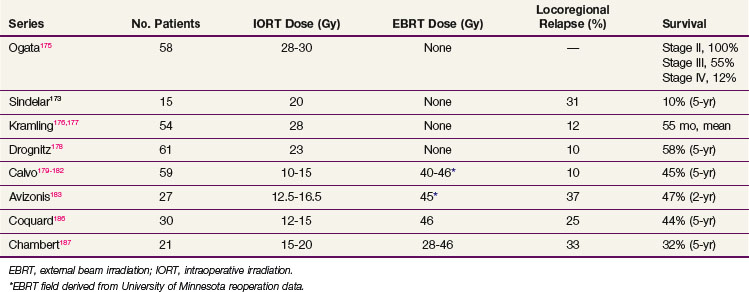
Survival results of IORT (± EBRT) are reviewed in Table 45-7.
IORT Alone
Ogata and colleagues reported a study from the Kochi Medical School, Japan, with 178 gastric cancer patients, Japan Radiological Society stages II through IV, treated with surgery alone (120 patients) or surgery plus IORT (58 patients) from August 1983 to July 1992.175 The patients were not randomized, but a surgery-alone group served as controls. In general, the IORT patients had more unfavorable features. The IORT results by stage (see Table 45-7) demonstrated a survival advantage in stages III and IV that was not statistically significant; the survival in stage II patients was surprisingly high but did not reach statistical significance.
Takahashi and Abe150 reported results from a large Japanese trial in which 211 patients were selected, on the basis of day of hospital admission, to receive surgery only or surgery plus IORT (28 to 35 Gy). Five-year OS for Japanese stages II to IV were improved 15% to 25% in the IORT group versus those treated with surgery alone (see Table 45-5; stage II, 84% vs. 62%; stage III, 62% vs. 37%; stage IV, 15% vs. 0%). This magnitude of survival improvement correlates nicely with the approximately 20% of patients who experience failure of their treatment only locoregionally after complete surgical resection. However, this method of treatment selection is susceptible to bias, and the trial failed to stratify for important prognostic factors.
In an analysis from Beijing, patients with stage III (serosal involvement or node-positive tumors) or stage IV (unresectable metastasis or adjacent organ involvement) disease were randomized to surgery alone or IORT (single dose, 25 to 40 Gy).151 In the most recent report of 200 patients, a survival advantage with IORT was demonstrated for patients with stage III disease (see Table 45-5; 65% vs. 30% 5-year OS; 52% vs. 22% 8-year OS; p <.01).
IORT versus EBRT
At the National Cancer Institute (NCI), Sindelar and associates173 performed a small randomized trial of IORT versus EBRT after complete surgical resection that demonstrated improved local control with IORT but no survival benefit. The incidence of locoregional recurrence was lower in the IORT versus control group, at 31% versus 80% (p <.01). The 5-year OS was 10% for 15 patients with resected gastric cancer stages III and IV treated with radical surgery plus IORT.173 Median survival time for this subset of patients was 25 months. However, for the total group of patients there were no differences in survival between the IORT and the EBRT study arms. The median survival of the surgery + EBRT arm of 25 patients was 21 months, and the 5-year OS was 20%. No patient with stage III or IV disease in the control group survived after a median follow-up of 7 years, whereas 3 of 15 (20%) in the IORT group were alive with no evidence of disease at the time of the analysis (p = .06).
IORT Plus EBRT
Calvo and coauthors179–182 described a 5-year OS of 39% and a locoregional failure rate of 10.4% in 48 patients treated with IORT plus EBRT in Pamplona, Spain. This report included 16 patients with AJCC stages I and II and 8 patients with anastomotic or nodal recurrences. The proportion of patients with serosal involvement was 70% and of nodal involvement was 56%. An update of the series by Martinez-Monge and colleagues181 included only the 28 patients with serosal (89%) and/or lymph node involvement (63%) treated with IORT 15 Gy and EBRT 40 to 46 Gy in 1.8- to 2-Gy fractions. The update revealed a 10-year OS of 38% and a locoregional failure rate of 11%.
Avizonis and coauthors reported the Radiation Therapy Oncology Group series of 27 patients treated with surgery plus IORT 12.5 to 16.5 Gy ± EBRT 45 Gy.183 Seventy percent of the patients had AJCC stages III and IV tumors. The 2-year OS was 47%, 2-year DFS was 27%, and median survival was 19.3 months.
Gilly and associates reported on 45 patients treated in Lyon, France, with surgical resection, IORT 15 Gy, and EBRT 44 Gy.184 The 5-year OS for N1/N2+ patients was 51%. In the last update of the Lyon experience with 82 patients,185 8-year OS among the 49 patients with pT3 and/or pN+ disease treated with surgery plus IORT plus EBRT was 50% versus 28% in patients with similar-stage disease treated with surgery alone during the same time period.
Locally Advanced Disease, Palliation
Locally Advanced Disease
Surgery
The extent of a surgical procedure must be tempered by the knowledge that cure is at best improbable. Patients with symptomatic obstruction, hemorrhage, and ulceration, and some with perforation, can be successfully relieved of symptoms by even limited gastric resection. Radical subtotal or total gastrectomy may be indicated in some patients whose lesions cannot be completely resected with negative pathologic margins to achieve symptomatic palliation. Results with total gastrectomy in locally advanced gastric cancer showed good quality of life when this procedure was indicated for bulky or proximal tumors, but symptom relief was less likely for patients with linitis plastica.188 Although adjacent organ resection can be undertaken if all gross tumor can be removed, it is rarely justified if gross residual tumor (visible or palpable) would remain. Because total (or near total) gastrectomy has the risk of both acute and long-term morbidity (severe early satiety), it should be used sparingly. If sites of adherence or residual disease are marked with surgical clips, postoperative irradiation plus chemotherapy can be delivered with greater accuracy.
Irradiation ± Chemotherapy
Irradiation
The available literature suggests that ACA of the stomach is radioresponsive. Wieland and Hymmen189 used 60 Gy when feasible (1.5 to 2.0 Gy daily) with 11% (9 of 82) 3-year and 7% (5 of 72) 5-year OS. Takahashi190 compared historical controls with patients whose tumors were unresectable or who had palliative procedures and received postoperative radiation (unknown if chemotherapy was also used). The average survival for irradiated patients was 9 to 10 months longer, with 74% 1-year (32 of 43) and 27% 2.5-year survival (12 of 43).
Abe and Takahashi149,150 reported 15% 5-year OS with a single dose of IORT after resection (28 to 33 Gy) in a group of 27 patients with stage IV disease. Three of the four long-term survivors had residual disease after maximal resection. In the same study, 18 patients with stage IV disease were randomized to a surgery-alone control arm; the 5-year OS was 0% (see Table 45-5).
Irradiation Plus Chemotherapy
Most reports of combined irradiation and chemotherapy for gastric cancer involve patients with residual and unresectable disease, and most phase III trials in this setting show an advantage for combined-modality treatment over single-modality treatment. In a randomized series from the Mayo Clinic,191,192 5-FU was used during the first 3 days of EBRT in half of the patients (EBRT, 35 to 37.5 Gy in 4 to 5 weeks; 5-FU 15 mg/kg for 3 days, week 1 of EBRT). For the combined treatment group, mean and OS was improved (13 vs. 5.9 months and 3 of 25 or 12% vs. 0 of 23 for 5-year survival) (Table 45-8). In a randomized study by the GI Tumor Study Group (GITSG),193,194 the combination of EBRT and 5-FU followed by maintenance 5-FU plus semustine resulted in statistically superior long-term survival when compared with 5-FU and semustine alone (3- and 4-year OS of 18% vs. 6% to 7%; p <.05). These researchers performed a second trial in which combined irradiation plus chemotherapy did not produce a survival advantage when compared with chemotherapy alone.195 Because 46% of the patients on the combined study arm either did not receive full-course EBRT or had a major deviation in the delivery of the EBRT, the results are difficult to interpret. In a randomized EORTC trial of EBRT with or without 5-FU,196 residual disease after resection was identified in 22 patients. The three long-term survivors (14%) received EBRT plus 5-FU.
Data from nonrandomized single-institution or group analyses also suggest that the combination of EBRT and chemotherapy may have an impact on disease control and survival (see Table 45-8). In published series from the Mayo Clinic197 and MGH,156 long-term survival of 10% or more was demonstrated in patients who received EBRT plus chemotherapy after subtotal surgical resection with residual disease or with unresectable lesions. In a University of Pennsylvania analysis of patients with unresected adenocarcinoma of the GE junction or esophagus, local control was better with combined- versus single-modality treatment (irradiation, 1 of 23, or 4%; chemotherapy, 0 of 8; irradiation plus chemotherapy, 11 of 21, or 52%). Median survival with the combined-modality treatment was 10 months compared with 5 months for irradiation alone. In a Mayo Clinic/North Central Cancer Treatment Group (NCCTG) dose escalation pilot study, EBRT was combined with 5-FU plus low-dose leucovorin (400 mg/m2 and 20 mg/m2, respectively for 3 to 4 days, week 1, or week 1 plus 5 days of irradiation).198 Two of six patients with locally advanced gastric cancer were alive and disease free beyond 3 years.
Published analyses from both GITSIG and MGH suggest an improvement in survival if partial resection with gross residual disease or gross total resection with microscopic residual disease can be accomplished. In the former series 3-year OS was about 25% versus 10% in patients with partial resection versus no resection.193,194 In the latter analysis, median survival with irradiation plus chemotherapy was 24 months for microscopic residual disease, 15 months with gross residual tumor, and 14 months in patients whose tumor was not resected.156 Four-year OS was 0% in patients without resection versus 10% in those with residual disease after maximal resection.
In a Mayo Clinic analysis of irradiation with or without chemotherapy for gastric or GE junction cancers, an improvement in median survival was also suggested for patients with gross total resection but microscopic residual disease when compared with higher-risk subsets of patients.199 In these analyses the results of irradiation or chemoradiation were evaluated in 87 patients with either locally advanced primary or locally recurrent ACA of the stomach or GE junction treated from 1980 through 1996. Of those with primary lesions, 28 had unresectable disease and 39 had resection but residual disease (microscopic, 28; gross, 11). An additional 21 presented with a local or regional relapse with no evidence of abdominal (liver, peritoneal) or extra-abdominal metastasis (lung, other). Chemotherapy with 5-FU (± leucovorin) was given during or after EBRT in 75% of the patients with microscopic residual disease and 92% of the other subgroups (concomitant with EBRT in 84%). An intraoperative electron irradiation (IOERT) supplement to EBRT was given in 13 patients. Median survival in primary cancer patients with microscopic residual was 16.7 months versus 9.6 months for patients with subtotal resection and gross residual disease or 12 months in those with unresectable disease. Patients who presented with local or regional relapse had a median survival of 10 months.
Prognostic factor analyses showed that long-term survival appeared slightly poorer in patients who had resection before irradiation or chemoradiation in the Mayo Clinic analysis.199 Four-year OS was 0% versus 9% in patients with gross residual disease after partial resection (1 of 11 patients was alive with no evidence of disease 2 years after treatment), 9% in those with microscopic residual disease after gross total resection, and 18% in patients with unresectable primary or locally recurrent cancers. The survival trends may be a reflection of both treatment sequence and higher irradiation dose, because 12 of 13 patients with EBRT plus IOERT had unresectable primary or locally recurrent cancers. In the 21 patients with local or regionally recurrent cancers, irradiation dose greater than 54 Gy had a trend for improved survival (median survival 25.6 vs. 5.5 months, p = .06). If patients with microscopic residual disease are excluded, an increase in the number of cycles of chemotherapy appeared to correlate with an improvement in median survival (fewer than two cycles, median 5.2 months vs. 11.5 months with two or three cycles and 14.5 months with four or more cycles, p = .014).
Although problems with excess toxicity were encountered in the GITSIG study,193,194 such problems were minimal or nonexistent in the MGH series of 46 patients.156 In the latter series, shaped radiation portals and single fraction size of 1.8 Gy were used; 43 of 46 patients received both irradiation and chemotherapy.
Neoadjuvant Chemotherapy
The use of adjuvant preoperative (neoadjuvant) chemotherapy has been less well studied compared with adjuvant postoperative therapy. Because of the inability of adjuvant (postoperative) systemic therapy to prolong survival in surgically managed gastric cancer, several investigators have pursued the approach of neoadjuvant chemotherapy in an attempt to increase resectability and improve survival. These studies involve a mix of patients including those with clinically operable, “locally advanced,” or unresectable lesions. All but one of these studies are phase II protocols.200–214 The results of these reports are summarized in web-only Table 45-4![]() on the Expert Consult website, along with more information on neoadjuvant chemotherapy for patients with locally advanced or potentially resectable disease.
on the Expert Consult website, along with more information on neoadjuvant chemotherapy for patients with locally advanced or potentially resectable disease.
One of the few randomized trials to assess the potential benefit of preoperative chemotherapy to surgery alone in potentially resectable patients failed to show any benefit.213 In this Dutch study, patients were randomized to FAMTX followed by surgery or to surgery alone. The study was closed early because of poor accrual, but analysis of the enrolled patients showed no benefit to rate of curative resectability, rate of relapse, or median survival.
Unresectable Disease
Wilke and colleagues examined the role of etoposide, doxorubicin, and cisplatin (EAP regimen) in a group of 34 patients with laparotomy-determined unresectable stomach cancer.200 This study was prompted by the promising results with this regimen in advanced disease (21% complete remission and 73% overall response rate).215 After exploratory laparotomy, patients were begun on this regimen. Twenty patients (59%) who achieved clinical response went on to a second-look operation followed by two additional courses of chemotherapy. Fifteen patients (44%) of the original cohort were able to have a resection, and 5 patients were pathologic complete responders (15% of the original 34). Median survival in this phase II trial was 18 months for the entire study group. In an update of these data, results were reported in 21 patients who had total resection after EAP chemotherapy for locally unresectable disease.216 Fourteen of 21 patients experienced relapse, and 11 of 14 had a locoregional component of disease (79% of relapses, 52% of group at risk).
Verschueren and associates201 evaluated 17 patients with unresectable gastric cancer. Fifteen of them were defined as unresectable at laparotomy, whereas 2 were deemed unresectable on the basis of CT. After receiving up to four courses of sequential 5-FU and high-dose methotrexate, 13 patients (76%) underwent attempted resection. Although 7 patients had resectable tumors (41%), locoregional relapse occurred in 5 of these patients.
Locally Advanced Disease
Two studies have tested the use of preoperative systemic treatment for patients with “locally advanced” stomach cancer. Ducreux and colleagues202 treated 30 patients with two or three cycles of cisplatin and 5-FU before surgery. Twenty-eight (93%) patients subsequently underwent laparotomy, and 23 (77%) had their tumor resected; the pathologic response rate was not reported. Median survival was 16 months. Kang and colleagues203,204 randomized 107 patients with locally advanced gastric cancer to receive two or three cycles of etoposide, 5-FU, and cisplatin (EFP regimen) followed by surgery versus surgery alone. Of the 53 patients randomized to preoperative treatment, 47 (89%) underwent surgical exploration and 37 (70%) had a complete resection. A complete pathologic response rate of 7% was noted. More patients were able to undergo a curative resection in the treated group when compared with surgery alone (70% vs. 61%). Median survival was 43 versus 30 months in favor of neoadjuvant treatment, but this did not achieve statistical significance (p = .114).
Potentially Resectable Disease
Ajani and colleagues206,207 reported two phase II studies of preoperative chemotherapy (with EFP and EAP regimens) in patients thought clinically to have potentially resectable disease. Patients were treated with two or three cycles of EFP/EAP preoperatively plus an additional two or three cycles postoperatively if a positive response to neoadjuvant treatment could be detected. No pathologic complete responses were seen with either EFP (n = 25 patients) or EAP (n = 48 patients), and median survival was 15 and 15.5 months, respectively. The impressive results obtained with neoadjuvant EAP in Wilke and coauthors’ study200 could not be reproduced in this group of patients who had potentially smaller tumor burdens.
Other investigators have examined the utility of combining preoperative chemotherapy and postoperative treatment with intraperitoneal chemotherapy in view of the high peritoneal failure rate after resection of gastric cancer.208–211 Schwartz and colleagues208,209 delivered three cycles of neoadjuvant FAMTX (sequential 5-FU and high-dose methotrexate followed by doxorubicin) plus postoperative intraperitoneal 5-FU and cisplatin along with infusional 5-FU. No complete pathologic responses were observed. Leichman and colleagues210,211 delivered two cycles of 5-FU, leucovorin, and cisplatin preoperatively and two cycles of intraperitoneal floxuridine and cisplatin postoperatively. In the updated report of 59 potentially resectable patients, 96% underwent exploration and 68% had complete resection.211 The pathologic complete response was only 9%, but median survival was about 52 months.
Summary
The high response rates achieved with neoadjuvant chemotherapy are of interest, and this form of treatment will undoubtedly be the subject of further investigation. The roles of neoadjuvant systemic treatment in increasing resectability rates and improving survival in localized gastric cancer are unknown. Although resectability rates seem higher than the median rate of 40% from several studies, the patients in the neoadjuvant studies were highly selected. Except for the two trials in which tumors were unresectable on the basis of exploratory laparotomy, the successful resection in these reports may not have been influenced by neoadjuvant chemotherapy. In general, pathologic complete response rates are low (≤15%), and no proof exists that primary lesions are made more resectable by such treatment. The impact of preoperative systemic treatment on survival is even less clear. The one randomized trial that has been reported shows a nonsignificant improvement in survival for neoadjuvant chemotherapy in borderline resectable locally advanced disease.203,204 Newer technologies such as EUS may identify patients who will do poorly with standard therapy alone and would be reasonable candidates for adjuvant studies.217
In view of the high incidence of locoregional relapse in several series of patients whose tumors were resected after neoadjuvant chemotherapy for initially unresectable lesions, irradiation should be incorporated into the study design of future trials for patients with high-risk factors. Patients with unresectable lesions who do not respond to neoadjuvant chemotherapy may still respond to preoperative EBRT plus chemotherapy. The survival advantage of preoperative chemoradiation versus surgery alone for carcinomas of the esophagus and proximal stomach/GE junction was noted in phase III trials by Walsh and others166 and Tepper and colleagues168 (confirmatory U.S. GI Intergoup study).
Palliation for Metastatic Disease
Surgery
Surgical intervention in the patient with metastatic gastric cancer requires sound judgment. The underlying health and function (performance status) of the patient, the estimated duration of patient survival, and the nature of the symptoms must all be taken into account before deciding to proceed with an operation. Resection for palliation is generally better than bypass or intubation in appropriately selected patients, leading to better symptomatic relief and often longer survival.188 Obstructing lesions may be resected with excellent palliation, but endoluminal stents, endoscopic laser treatments, or gastrostomy tube placement should be considered for poor operative candidates.219 Although significant hemorrhage from an ulcerating or necrotic polypoid tumor may be temporarily controlled by endoscopic techniques, stabilization and urgent surgical intervention should be undertaken when appropriate. A perforated gastric cancer usually presents as an emergency and may be unrecognized preoperatively. Aggressive treatment with gastric resection should be carried out in the fit patient; pain control and hydration alone are preferable for the moribund or unfit patient.
Chemotherapy
Traditional chemotherapy agents with 20% or more response rates include doxorubicin,220,221 cisplatin,222 5-FU,223 etoposide,224 and mitomycin C.225 More recently a variety of chemotherapy drugs have shown promising response rates in advanced gastric cancer, including docetaxel (23%), irinotecan (23%), and paclitaxel (17% to 21%).226–228 Complete remissions with single-agent chemotherapy are exceedingly rare, and remission duration is usually 3 to 5 months. For patients with measurable disease, survival is usually only 3 to 6 months. Single-agent chemotherapy therefore offers little practical benefit to the patient.
Combination chemotherapy regimens, which almost universally incorporate a fluorinated pyrimidine, obtain reliable responses in 25% to 50% of patients.225–234,235,236,237
Combination chemotherapy regimens that have produced response rates of 30% to 45% include the following: the FAM regimen225; 5-FU plus carmustine229; 5-FU plus semustine230; 5-FU plus mitomycin C221; 5-FU, doxorubicin, and semustine (FAMe)220,230; 5-FU, doxorubicin, and carmustine (FAB)231; 5-FU, doxorubicin, and cisplatin (FAP)232–234; etoposide, doxorubicin, and cisplatin (EAP)235; 5-FU, doxorubicin, and methotrexate (FAMTX)234,236; and etoposide, leucovorin, and 5-FU (ELF).237
See Expert Consult website for supplemental text and tables on additional chemotherapy trials.![]()
The NCCTG reported a prospective randomized phase III trial comparing 5-FU versus 5-FU + doxorubicin versus the FAM regimen. Overall responses were quite similar, and no differences were seen in survival.238 A large Korean study randomized 324 patients to 5-FU, 5-FU plus cisplatin, or the FAM regimen. Responses were observed more often in the patients treated with 5-FU plus cisplatin, but OS was similar in all three treatment arms.239 Others have reported similar problems in demonstrating improved survival for multiple-agent regimens in phase III trials.240–241 Several trials have also used pharmacologic manipulation of 5-FU with leucovorin in gastric carcinoma in attempts to follow up on the apparent benefit that this combination achieved in patients with colorectal carcinoma.242 Responses varied between 11% and 50%, but median survival rates were almost identical to those of 5-FU alone at 5.5 months.
Many of the combination chemotherapy regimens, which achieved response rates of 30% to 50% in single-institution trials, have often failed to reproduce the initially high response rates when subjected to confirmatory trials. A case in point is the EAP regimen with etoposide, doxorubicin, and cisplatin. The initial phase II trial reported overall response rates of 64% (complete and partial) and 21% (complete) and median survival of 9 months.215 The studies that followed did not confirm such high response rates, and toxicity was sometimes substantial.243
Anthracycline-Based Regimens
The combination of 5-FU, doxorubicin, and methotrexate (FAMTX) was a widely used regimen in the 1990s. Klein first reported a 58% response rate for the combination in a phase II study.244 The EORTC group performed a randomized phase II trial of FAM versus FAMTX in 213 patients with advanced measurable or nonmeasurable gastric cancer.235 FAMTX was found to have a significantly superior response rate (41% vs. 9%; p <.0001) and survival (median 42 vs. 29 weeks; p = .004). The authors considered FAMTX the reference chemotherapy for future advanced gastric trials.
Kelsen and colleagues236 randomly compared FAMTX and EAP for advanced disease in 60 patients. Response rates were similar, at 33% versus 20% (advantage to FAMTX; p = .24), and tolerance was better for FAMTX with a significantly higher toxic death rate for EAP. Accordingly, the trial was stopped.
In 1991, ELF was introduced as an alternative to more aggressive regimens for patients with cardiac or poor medical conditions or advanced age.245 It achieved a response rate of 53% and a median survival time of 11 months. Multiple clinical trials have now evaluated the activity and tolerability of the ELF regimen and have generally shown median survival in the range of 8 to 10 months and response rates of about 30%.246–248
FAMTX has been compared with ELF and infusional 5-FU/cisplatin (FUP) in a phase III EORTC study. No apparent differences in response rates or survival have been demonstrated to date.249
Platinum Regimens
The Italian Group for the Study of Digestive Tract Cancer reported an intensive, multiple-agent regimen including the most active agents in gastric cancer: cisplatin, epirubicin, leucovorin, and 5-FU (PELF regimen).250 Administered weekly with growth factor support, this regimen produced an overall response rate of 62%, a complete response rate of 17%, and a median survival time of 11 months.
The PELF regimen was subsequently compared with the FAMTX regimen in a randomized phase III trial.251 Although both the overall and complete response rates with PELF were significantly higher (overall, 39% vs. 22%; complete, 13% vs. 2%), only a nonsignificant improvement in OS was seen with PELF.
A regimen combining epirubicin, cisplatin, and infusional 5-FU (ECF) was introduced by investigators at the Royal Marsden Hospital for patients with advanced gastroesophageal ACA. An overall response rate of 71% was obtained with a complete response rate of 12%.252 A subsequent randomized phase III trial, which compared FAMTX with ECF, showed that ECF was associated with a superior response rate (45% vs. 21%) and OS (median 9 vs. 6 months).253 In view of the promising results of this trial, ECF has become the standard regimen to which other regimens are compared.
A recent phase III trial for gastroesophageal cancer was conducted to assess the potential benefit of replacing cisplatin with oxaliplatin and infusional 5-FU with capecitabine using a 2 × 2 design.254 This trial enrolled 1002 patients from 61 centers. Using a noninferiority statistical design for the trial, the combination of epirubicin, oxaliplatin, and capecitabine (EOX) was shown to be the most active of the four regimens evaluated. When compared with those on an ECF regimen, patients receiving EOX had both an improved 1-year survival (46.8% vs. 37.7%) and a statistically improved median survival (11.2 vs. 9.9 months; hazard ratio 0.80, 95% CI 0.65-0.97).
Taxane Regimens
Several recent phase II studies have evaluated non–5-FU-containing combinations.255–257 The response to combinations such as docetaxel and cisplatin, paclitaxel and cisplatin, and irinotecan and mitomycin C have been comparable to those of 5-FU-containing regimens.
The combination of docetaxel and cisplatin has been evaluated in a number of clinical trials as either the two drug combination or in combination with other chemotherapy agents. As a two- drug combination in phase II trials the reported response rate is 28% to 46% with a median survival of 10.4 to 11.5 months.258–261 Given the apparent promising activity of this combination, other trials explored the added benefit of 5-fluorouracil and reported response rates of 40% to 43% and median survivals of 9.0 to 9.7 months.262–263
A multicenter randomized phase II trial, involving 158 patients, assessed docetaxel and cisplatin with or without 5-FU as potential support for a phase III trial.264 In this trial the combination of docetaxel and cisplatin gave a response rate of 26% and a median survival of 10.5 months. The addition of 5-FU increased the response rate to 43%, but the median survival was only 9.6 months.
In a subsequent phase III trial, the combination of docetaxel, cisplatin, and 5-FU was compared with cisplatin and 5-FU.265 Overall survival was significantly longer with docetaxel, cisplatin, and 5-FU (p = .02). The response rate was also higher with this combination (69% vs. 59%, p = .01). However, the combination of docetaxel, cisplatin, and 5-FU was also more toxic, with 69% of patients developing treatment-related grade 3 or 4 adverse events compared with 59% of patients receiving cisplatin and 5-fluorouracil. Neutropenia and neutropenic fever were both significantly higher with the three-drug combination.
Supportive Care versus Chemotherapy
In 1993, Murad and associates266 published results of a randomized trial in which a modified FAMTX regimen was compared with best supportive care. The trial was interrupted after entry of 22 patients, because the treated patients were enjoying a significantly better outcome. The next 18 patients were assigned directly to treatment. Median survival for all the treated patients was 10 months versus 3 months for the untreated controls (p = .001).
Pyrhonen and coauthors267 reported the results of 41 patients randomized to FEMTX plus vitamins A and E versus the same vitamins and best supportive care. In the FEMTX patients, 29% had an objective response and 33% had stable disease for greater than 2 months. In control patients, 20% had stable disease. Median time to progression (5.4 vs. 1.7 months, p = .0013) and median survival (12.3 vs. 3.1 months, p = .0006) both favored treatment with FEMTX.
Glimelius and colleagues268 studied, in a group of 61 patients with inoperable gastric cancer, the cost-effectiveness of palliative therapy by randomly assigning patients to primary chemotherapy with ELF or 5-FU + leucovorin versus best supportive care. Improved or prolonged high quality of life was documented in patients receiving chemotherapy versus supportive care alone. Chemotherapy patients had a significantly longer median survival at 8 months versus 5 months (p = .003, adjusted) and quality of life (p <.05) also favored the treated patients.
Park and colleagues269 performed a retrospective analysis of 409 patients with incurable gastric cancer in which 202 patients were treated with a modified FAM regimen and 207 were not treated. The 1-year survival was 34.1% for patients treated with FAM versus 22.5% for the control group. Details on how treatment decisions were made were not in the report.
A 2010 meta-analysis reviewed the efficacy of chemotherapy versus best supportive care, combination versus single-agent therapy, and different combinations of regimens in advanced gastric cancer. A total of 5726 patients from 35 trials were analyzed. Survival benefit was observed in favor of chemotherapy versus supportive care, combination chemotherapy versus single-agent therapy (but with an increased toxicity), and regimens containing cisplatin.270
Irradiation Techniques AND Tolerance
Irradiation Field Design
For patients who receive postoperative EBRT after surgical resection or exploratory laparotomy, the irradiation field should include unresected or residual tumor or the tumor bed plus major nodal regions (lesser and greater curvature; celiac axis including pancreaticoduodenal; suprapancreatic, splenic, and porta hepatis; paraesophageal with proximal lesions) (Figs. 45-5 to 45-9; see also web-only Figs. 45-4 to 45-6![]() ). The pattern of tumor bed and nodal failures in the reoperative series from the University of Minnesota is demonstrated in Figure 45-1A. This represents an idealized, shaped irradiation field that incorporates the areas of locoregional relapse.28 The tumor bed and nodal volumes are reconstructed with the aid of preoperative and postoperative imaging studies and surgical clip placement. In Figure 45-1B the idealized field is superimposed on the organs and structures that define irradiation tolerance.
). The pattern of tumor bed and nodal failures in the reoperative series from the University of Minnesota is demonstrated in Figure 45-1A. This represents an idealized, shaped irradiation field that incorporates the areas of locoregional relapse.28 The tumor bed and nodal volumes are reconstructed with the aid of preoperative and postoperative imaging studies and surgical clip placement. In Figure 45-1B the idealized field is superimposed on the organs and structures that define irradiation tolerance.
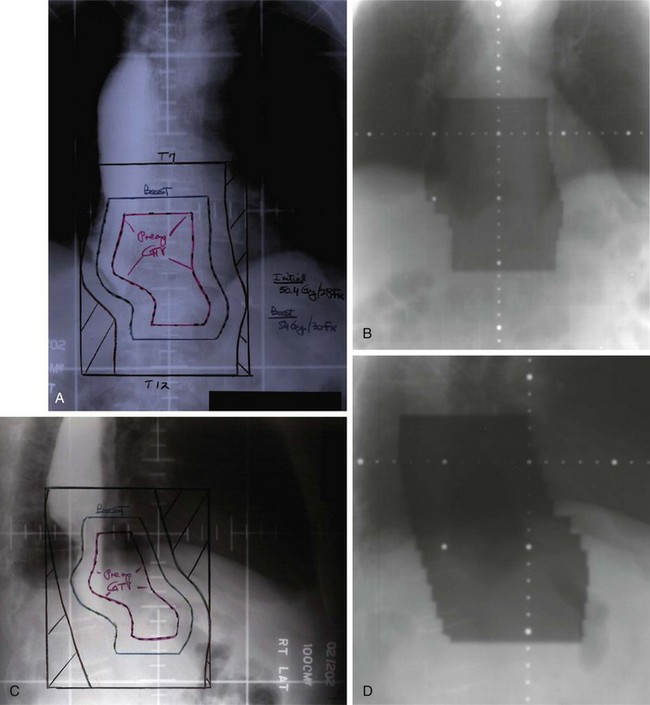
Figure 45-7 Radiation and port films on a 65-year-old patient with Ivor Lewis esophagogastrectomy (transthoracic plus abdominal approach) for a T3N0 adenocarcinoma of the gastroesophageal junction (see Table 45-10). Postoperative chemoradiation, as per INT 0116, was recommended. Simulation films (A and C) were obtained after administration of a contrast agent in the remaining stomach, and a treatment planning CT scan was obtained for the purpose of reconstructing tumor bed volumes. The patient was concerned about treatment-related morbidity, so the decision was made to treat with a more involved treatment field approach in view of pathologic findings of 12 negative nodes and adequate proximal and distal resection margins (~5 cm). The involved fields included the tumor bed (preoperative GTV), as generated from preoperative CT imaging but excluded the remaining stomach and nodal sites to decrease normal tissue morbidity. The initial anterior-posterior/posterior-anterior fields (A and B) included tumor bed with 3-cm superior-inferior and 2-cm lateral margin to block edge (CTV/PTV). Lateral fields (C and D) were generated with an anterior margin of 2 cm to block edge and demonstrate sparing of heart and spinal cord. Initial fields were treated to 45 Gy/25 fractions; boost fields (A and C) received an additional 9 Gy/5 fractions to GTV plus 1-cm margin to block edge (total 54 Gy/30 fractions. The patient received 5-fluorouracil plus leucovorin before, during, and after EBRT as per INT 0116. There was no evidence of disease and he was without symptoms at his 4-year post-treatment evaluation.
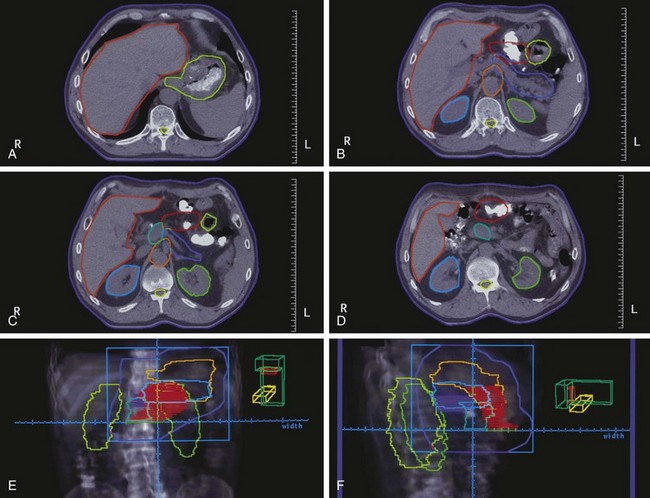
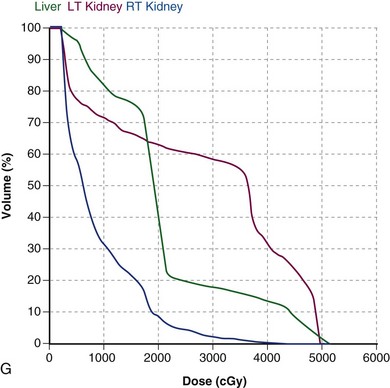
Figure 45-8 Optimized postoperative radiation fields for patient with T4N0 adenocarcinoma of the posterior wall of the body of the stomach (middle third—see Table 45-12). CT simulation was performed and structures of interest were delineated based on preoperative and postoperative CT imaging and operative/pathologic findings (A to D: tumor bed; gastric remnant; tolerance organs/structures-kidneys, liver, spinal cord). A, Gastric remnant and distal esophagus (yellow-green) are adjacent to liver (red-orange) and spleen. B, CT image demonstrates tumor bed (red), body/tail of pancreas (blue), celiac artery (orange), and kidneys (right—light blue; left—green). C, Head of pancreas (blue-green) and splenic artery/body of pancreas are shown at level of midkidney. D, Tumor bed and head of pancreas on more distal CT image. Radiation fields were designed with the aid of digitally reconstructed radiographs (E and F) and a dose-volume histogram (DVH) was performed with regard to tolerance organs/structures (G). A four-field technique of anterior-posterior/posterior-anterior (AP/PA) and paired lateral fields was selected (field margins in medium blue). The fields included the gastric remnant (yellow-orange), tumor bed (red), and nodal regions at risk based on sites of adherence of the primary lesion (celiac [blue-purple cross-hatched]), suprapancreatic [body of pancreas—light blue], superior mesenteric, pancreaticoduodenal [head of pancreas, blue-green]). E, The AP/PA field includes approximately two thirds of the left kidney and a portion of the left lobe of the liver but excludes most of the right kidney and the right lobe of the liver. F, The lateral field demonstrates exclusion of the spinal cord and posterior kidneys. G, DVH demonstrates a dose of 20 Gy to more than 60% of the left kidney versus less than 10% of the right kidney.
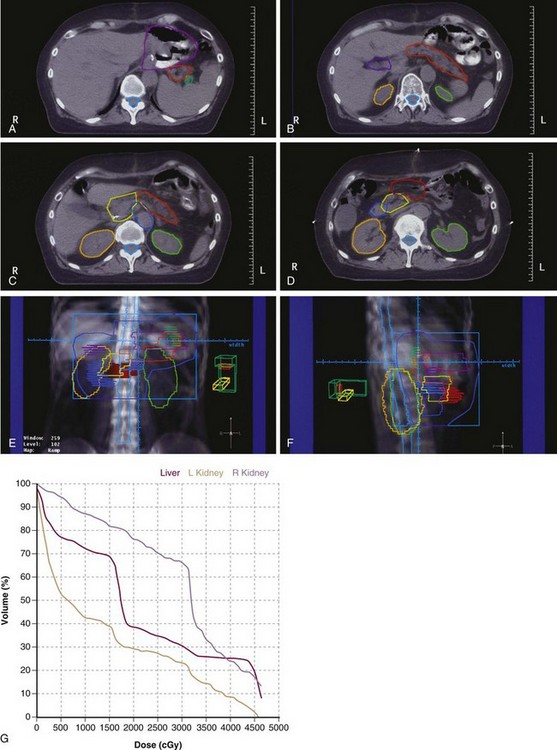
Figure 45-9 Optimized postoperative radiation fields for patient with T3N2 antral primary (distal third, see Table 45-13). Structures of interest were delineated at the time of CT simulation (A to D). A, Gastric remnant (lavender) is demonstrated along with the body/tail of pancreas (red-orange), and splenic hilum (light green). B, Porta hepatis (blue-purple) and kidneys (left—yellow green, right—yellow orange) are delineated. C, Head of pancreas (yellow) and celiac versus superior mesenteric artery (light blue) are shown. D, Antral tumor bed (red) and duodenum (medium blue) are delineated. Irradiation fields were designed with the aid of digitally reconstructed radiographs (E and F) and a dose-volume histogram (DVH) (G) was performed with regard to dose-limiting structures (liver, kidneys, spinal cord). A four-field technique of anterior-posterior/posterior-anterior (AP/PA) and paired lateral fields was chosen (field margins shown in medium blue). Treatment fields include the gastric remnant, tumor bed, head of pancreas, first and second part of the duodenum (medium blue cross-hatched), pertinent nodal volumes (perigastric, pancreaticoduodenal [head of pancreas], porta [blue-purple cross-hatched], celiac, suprapancreatic [body/tail pancreas]) and the optional nodal volume of splenic hilum (light green cross-hatched). E, AP/PA field (field margins exclude approximately two thirds of the left kidney and include about 90% of the right kidney). Exclusion of optional splenic hilar nodes would not have allowed additional sparing of the left kidney in view of the adjacency of the gastric remnant and splenic hilum. F, Lateral field demonstrates exclusion of the spinal cord (turquoise) and substantial portions of both kidneys. G, DVH, combining doses from all four fields, demonstrated that the dose volume of 20 Gy included about 30% of the left kidney versus about 75% of the right kidney. With regard to the liver, about 30% receives a dose of 30 Gy and about 25% receives a dose of 35 to 40 Gy.
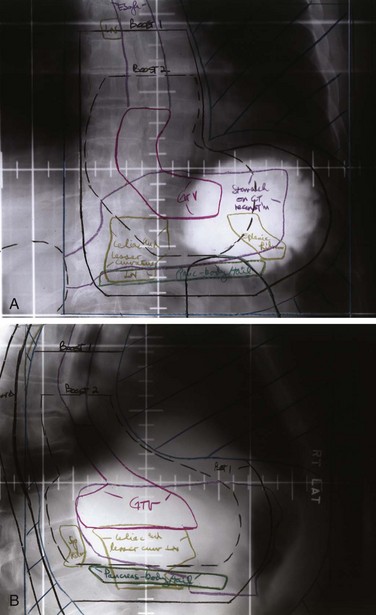
Web-Only Figure 45-5 The patient is a 73-year-old man with T3N0 adenocarcinoma of the gastroesophageal junction (see Table 45-10) who preferred primary chemoradiation treatment with surgery reserved for salvage, as indicated. Baseline PET/CT and CT of the chest were negative for nodal or blood-borne metastases. Radiation fields were generated using treatment planning CT and beam’s eye reconstruction. The patient was treated with a four-field technique of anterior-posterior/posterior-anterior (A) and paired lateral (B) fields. The initial fields included a greater than 5-cm margin of uninvolved esophagus superiorly, about a 5-cm margin of stomach in all directions, and nodal groups, including celiac artery and lesser curvature nodes, suprapancreatic (body/tail of pancreas), and splenic hilum. The patient received external beam radiation therapy (EBRT) plus one cycle of concurrent carboplatin/paclitaxel and infusion 5-fluorouracil during the first week of EBRT but did not receive further concurrent chemotherapy in view of intolerance. The extended fields received 39.6 Gy in 22 fractions of 1.8 Gy; boost 1 received an additional 4.5 Gy in 3 fractions (total 45 Gy/25 fractions) and boost 2 received an additional 9 Gy in 5 fractions for a total dose of 54 Gy/30 fractions for 6 weeks. There was no evidence of relapse on the basis of history, physical examination, imaging (CT chest/abdomen; PET/CT) or endoscopy at the time of ongoing evaluation 14 months later.
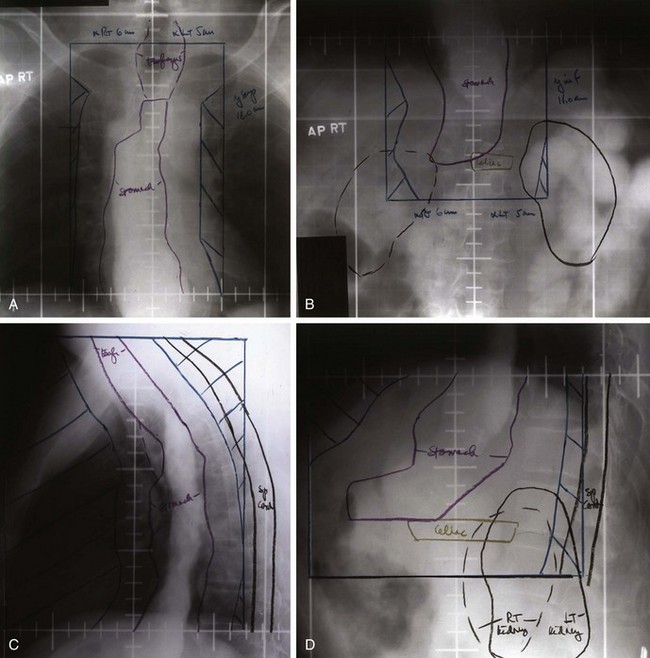
Web-Only Figure 45-6 Radiation fields in a patient with a T2N1 adenocarcinoma of the gastroesophageal junction who had a transhiatal esophagectomy with cervical esophagogastrostomy (see Table 45-10). Postoperative chemoirradiation was recommended, and the patient was placed on the current phase III U.S. Gastrointestinal Intergroup trial. At the time of simulation, oral contrast medium was used to define the esophagogastric anastomosis at the thoracic inlet (A and C), the residual stomach within the mediastinum, and the duodenal sweep inferiorly (B and D). A combined treatment planning/diagnostic CT was obtained to define the celiac artery and dose-limiting structures including heart, lungs, spinal cord, kidneys, and liver. Irradiation fields were defined to include the distal cervical esophagus and supraclavicular nodes superiorly, the remaining stomach, and perigastric nodes and nodal groups in the mediastinum and celiac axis (A to D). Lateral simulation films (C and D) demonstrate the ability to spare heart and spinal cord with appropriate use of blocks (cross-hatched blocks). The patient received 45 Gy/25 fractions plus concurrent protracted venous infusion 5-FU (200 mg/m2/24 hr) in addition to systemic multidrug chemotherapy before and after concurrent chemoradiation.
For individual patients, the idealized field needs to be modified depending on the surgical or pathologic extent of disease (primary site, tumor/node extent of disease) and the adjacency of tolerance organs or structures.164,271,272 The relative risk of nodal metastases at a specific nodal location is dependent on both the site of origin of the primary tumor and other factors, including width and depth of invasion of the gastric wall. Tumors that originate in the proximal portion of the stomach and the GE junction have a higher propensity to spread to nodes in the mediastinum and pericardial region but a lower likelihood of involvement of nodes in the region of the gastric antrum, periduodenal area, and porta hepatis. Tumors that originate in the body of the stomach can spread to all nodal sites but have the highest likelihood of spreading to nodes along the greater and lesser curvature, near the location of the primary tumor mass. Tumors that originate in the distal stomach have a high likelihood of spread to the periduodenal, peripancreatic, and porta hepatis nodes but a lower likelihood of spread to nodes near the cardia of the stomach, the periesophageal and mediastinal nodes, or splenic hilar nodes.271 Any tumor originating in the stomach has a high propensity to spread to nodes along the greater and lesser curvature, although they are most likely to spread to those sites in close anatomic proximity to the primary tumor mass.
With preoperative or primary chemoradiation for GE junction or proximal gastric cancers, owing to the risk of submucosal or subserosal lymphatic spread, a 5-cm or greater margin of uninvolved esophagus and proximal stomach should be included proximally and the distal/lateral field extent should include a 5-cm or greater margin of uninvolved stomach (see Figs. 45-5 and 45-6 and web-only Fig. 45-5![]() ). If the lesion extends beyond the gastric/GE junction wall, a major portion of the left hemidiaphragm should be included. Cerrobend blocks or multiple-leaf collimators should be used to decrease the volume of irradiated heart and lung, when technically feasible (see Figs. 45-5 and 45-6; see also web-only Fig. 45-5
). If the lesion extends beyond the gastric/GE junction wall, a major portion of the left hemidiaphragm should be included. Cerrobend blocks or multiple-leaf collimators should be used to decrease the volume of irradiated heart and lung, when technically feasible (see Figs. 45-5 and 45-6; see also web-only Fig. 45-5![]() ). Preoperative EBRT fields usually can be much more conservative than postoperative fields with regard to both heart and lung volumes and are preferred if preoperative imaging and EUS demonstrate indications for preoperative adjuvant treatment. Intensity-modulated irradiation (IMRT) may reduce heart and lung exposure when dose-volume histograms are compared with three-dimensional (3-D) conformal techniques for patients with GE junction cancers and reduce kidney doses for more distal lesions (see Fig. 45-6), but there is uncertainty whether this will improve short- or long-term treatment tolerance.
). Preoperative EBRT fields usually can be much more conservative than postoperative fields with regard to both heart and lung volumes and are preferred if preoperative imaging and EUS demonstrate indications for preoperative adjuvant treatment. Intensity-modulated irradiation (IMRT) may reduce heart and lung exposure when dose-volume histograms are compared with three-dimensional (3-D) conformal techniques for patients with GE junction cancers and reduce kidney doses for more distal lesions (see Fig. 45-6), but there is uncertainty whether this will improve short- or long-term treatment tolerance.
With postoperative irradiation of GE junction cancers (see web-only Fig. 45-6![]() ), the irradiation field may include the anastomotic site and some or all of the remaining stomach. Postoperative fields are larger than preoperative fields unless an involved field approach is chosen for select patients with T3N0 disease (see Fig. 45-7).
), the irradiation field may include the anastomotic site and some or all of the remaining stomach. Postoperative fields are larger than preoperative fields unless an involved field approach is chosen for select patients with T3N0 disease (see Fig. 45-7).
Dose-Limiting Organs/Structures
Dose-limiting organs and structures in the upper abdomen are numerous (stomach, small intestine, liver, kidneys, and spinal cord). With properly shaped fields, doses of 45 to 50.4 Gy in 1.8- to 2.0-Gy fractions can be delivered to the stomach and small intestine with a 5% or less risk of severe toxicity.260 In most patients a portion of both kidneys will be within the treatment fields, but at least two thirds or three fourths of one kidney should be excluded (the entirety of both kidneys can be included to a dose of 20 Gy if necessary).
For patients with GE junction or proximal to mid gastric cancers (see Figs. 45-5 to 45-7; see also web-only Figs. 45-4 to 45-6![]() ), half to two thirds of the left kidney can often be spared as a result of accurate field definition, which is aided by preoperative and postoperative imaging studies and surgical clip placement. The pancreaticoduodenal nodes can be included, if indicated, while sparing 75% to 90% of the right kidney.
), half to two thirds of the left kidney can often be spared as a result of accurate field definition, which is aided by preoperative and postoperative imaging studies and surgical clip placement. The pancreaticoduodenal nodes can be included, if indicated, while sparing 75% to 90% of the right kidney.
In patients with distal gastric lesions and narrow or positive duodenal resection margins, the duodenal circumference may need to be included as target volume (see Fig. 45-9). In such instances 50% or more of the right kidney is within the field and two thirds to three fourths of the left kidney should be spared. Chronic renal problems are infrequent when these techniques are utilized.273
Three-dimensional conformal irradiation with CT-based planning is recommended in most patients owing to the ability to spare more normal organs/structures (heart, lung, liver, kidneys, spinal cord). IMRT may optimize sparing of normal organs and structures274–280 (see Fig. 45-7). As noted by Wysocka and colleagues,281 planning target volume margins need to account for respiratory motion during conformal EBRT for gastric cancer.
Routine use of multiple field techniques should be considered even for postoperative irradiation, when preoperative imaging exists to allow accurate reconstruction of target volumes (see Figs. 45-7 to 45-9; see also web-only Figs. 45-4 and 45-6![]() ). Single institution data suggest that multiple field arrangements may produce less toxicity.161 When patients are treated preoperatively, paired lateral fields can be combined with anterior-posterior/posterior-anterior fields to achieve improved dose distributions on normal tissue (see web-only Fig. 45-5)
). Single institution data suggest that multiple field arrangements may produce less toxicity.161 When patients are treated preoperatively, paired lateral fields can be combined with anterior-posterior/posterior-anterior fields to achieve improved dose distributions on normal tissue (see web-only Fig. 45-5)![]() . Depending on the posterior extent of the gastric fundus, either oblique or lateral fields can be used to deliver a 10- to 20-Gy component of radiation to spare spinal cord and kidney. Liver and kidney tolerance limits the use of lateral fields to 20 Gy or less for patients with gastric cancer; for patients with gastroesophageal junction cancers, the contribution from lateral fields is generally limited to 10 to 15 Gy owing to lung tolerance issues.
. Depending on the posterior extent of the gastric fundus, either oblique or lateral fields can be used to deliver a 10- to 20-Gy component of radiation to spare spinal cord and kidney. Liver and kidney tolerance limits the use of lateral fields to 20 Gy or less for patients with gastric cancer; for patients with gastroesophageal junction cancers, the contribution from lateral fields is generally limited to 10 to 15 Gy owing to lung tolerance issues.
Idealized Treatment Field Guidelines
Guidelines for defining the clinical target volume for postoperative radiation fields have been developed by Tepper and Gunderson based on location and extent of the primary tumor (T category) and location and extent of known nodal involvement (N category).271 Table 45-9 presents general guidelines on the impact of T and N category on inclusion of the remaining stomach (gastric remnant), tumor bed, and nodal sites, whereas Tables 45-10 to 45-13 present treatment guidelines based on T and N category for each of the four primary sites (GE junction and proximal, mid, and distal stomach). In general, for patients with node-positive disease, there should be wide coverage of tumor bed, remaining stomach, resection margins, and nodal drainage regions. For node-negative disease, if there is a good surgical resection with pathologic evaluation of at least 10 to 15 nodes, and there are wide surgical margins on the primary tumor (at least 5 cm), treatment of the nodal beds is optional (see Figure 45-7). Treatment of the remaining stomach should depend on a balance of the likely normal tissue morbidity and the perceived risk of local relapse in the residual stomach.
TABLE 45-9 General Guidelines of Impact of T and N Category on Inclusion of Remaining Stomach, Tumor Bed, and Nodal Sites within Radiation Fields
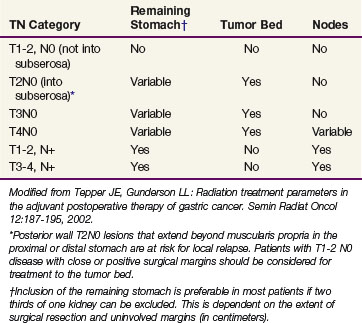
TABLE 45-10 Impact of Site of Primary Lesion and TN Category on Irradiation Treatment Volumes: Gastroesophageal Junction
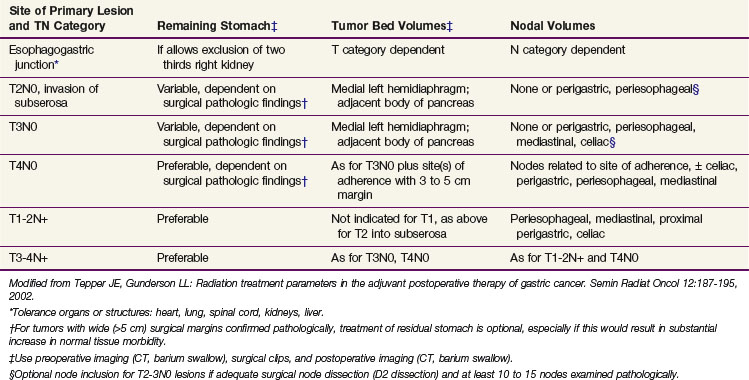
TABLE 45-11 Impact of Site of Primary Lesion and TN Category on Irradiation Treatment Volumes: Cardia/Proximal Third of Stomach
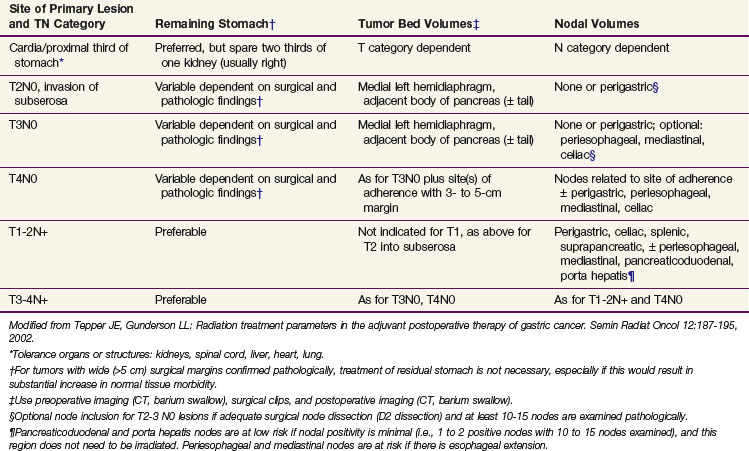
Treatment Algorithm, Challenges, And Future Possibilities
Completely Resected Lesions
Many patients with gross complete resection of their gastric cancer are not cured with surgery alone. The final results of the Dutch and British multicenter trials evaluating the value of extended lymphadenectomy demonstrated that the procedure increased morbidity but did not benefit survival. Because experienced surgeons can perform extended node dissection without significant additional surgical morbidity or mortality, use of the procedure is still reasonable in node-positive patients; however, existing studies do not define the optimal extent of surgical resection. Patients with extended resection are still at high risk for locoregional and systemic relapse,117 however, and should receive postoperative chemoradiation (see treatment algorithm by TNM extent, Fig. 45-10 and Table 45-14). The nonrandomized Samsung Medical Center analysis by Kim and colleagues165 appeared to demonstrate an advantage in disease control and survival in the 544 patients who received postoperative chemoradiation after D2 resection versus the 446 surgery-alone patients (5-year OS 57% vs. 51%, p = .02; 5-year RFS 54.4% vs. 47.9%, p = .016).
The successor U.S. GI Intergroup phase III randomized postoperative chemoradiation trial CALGB 80101 was designed to build on the positive results of the INT 0116 trial and tested ECF chemotherapy versus 5-FU plus leucovorin as the maintenance chemotherapy. Results presented at ASCO 2011 show no survival advantage using ECF chemotherapy versus bolus 5-FU/LV before and after PVI 5FU/EBRT.283 This trial showed no advantage to the use of ECF chemotherapy compared to 5-FU with leucovorin. The irradiation fields in both the phase II and successor phase III trials were based on optimized field design related to site of the primary lesion and T and N category of disease.271
In view of encouraging results with preoperative chemotherapy or chemoradiation169 for locally advanced or borderline resectable disease and the survival advantage for perioperative ECF versus surgery alone for resectable gastric/distal esophageal cancers in the MAGIC trial,127 these treatment approaches merit further evaluation. Possible treatment arms for patients with potentially resectable high-risk lesions include preoperative chemotherapy (alone or followed by postoperative chemoradiation) and preoperative chemoradiation followed by resection as done in the POET trial for patients with GE junction cancers. Another randomization design could compare two preoperative chemoradiation arms, keeping the EBRT components constant while randomly comparing alternate systemic chemotherapy regimens (ECF, EOX) or taxane-based regimens).
Although some investigators and institutions may prefer to simply replace postoperative adjuvant chemoradiation with perioperative ECF chemotherapy, it would seem advantageous to attempt to combine the advances in disease control and survival found with both approaches, because neither approach by itself has resulted in optimal relapse or survival outcomes.284
Locally Advanced Disease
For patients with locally advanced disease, it seems reasonable to build on existing positive treatment data (EBRT plus chemotherapy, IORT, preoperative chemotherapy, perioperative chemoradiation) plus patterns of relapse information (see Fig. 45-8 and Table 45-14). It would be of interest to merge these components of treatment.
Treatment Algorithm by TNM Disease Extent
Total surgical resection of the adenocarcinoma with a radical subtotal gastrectomy and reconstruction with gastrojejunostomy is recommended as standard treatment (see Table 45-14 and Fig. 45-10). Patients with posterior wall T2N0M0 lesions should be evaluated for postoperative adjuvant chemoradiation (see T1-2N1-3M0, T3N0-3M0, next).
Postoperative chemoradiation is the preferred treatment. For GE junction cancers found to be either T1-2N1-3M0 or T3N0-3M0 on EUS, preoperative chemoradiation is preferable, because safer EBRT fields can be designed for preoperative rather than postoperative chemoradiation.21,283 If transhiatal resection is performed, keeping the reconstructed stomach in the mediastinal midline, postoperative chemoradiation is facilitated.
28 Gunderson LL, Sosin H. Adenocarcinoma of the stomach areas of failure in a reoperation series (second or symptomatic looks). Clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1982;8:1.
59 Lauren P. The two histologic main types of gastric carcinomas. Diffuse and so-called intestinal type carcinoma—an attempt at a histological classification. Acta Pathol Microbiol Scand. 1965;64:31.
84 Bonnenkamp JJ, Songum I, Hermans J, et al. Randomized comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345:745-748.
85 Bonnenkamp JJ, Hermans J, Sasako M, Vande Velde CJH for the Dutch Gastric Cancer Group. Extended lymph node dissection for gastric cancer. N Engl J Med. 1999;340:908-914.
86 Cuschieri A, Fayers P, Fielding J, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer. Preliminary results of the MRC randomized controlled surgical trial. Lancet. 1996;347:995-999.
87 Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer. Long-term results of the MRC randomized controlled surgical trial. Br J Cancer. 1999;79:1522.
112 Landry J, Tepper J, Wood W, et al. Analysis of survival and local control following surgery for gastric cancer. Int J Radiat Oncol Biol Phys. 1990;19:1357.
113 McNeer G, Vandenberg H, Donn FY, Bowden LA. A critical evaluation of subtotal gastrectomy for the cure of cancer of the stomach. Ann Surg. 1957;134:2.
114 Thomson FB, Robins RE. Local recurrence following subtotal resection for gastric carcinoma. Surg Gynecol Obstet. 1952;91:341.
115 Wisbek WA, Becker EM, Russell AH. Adenocarcinoma of the stomach. Autopsy observations with therapeutic implications for the radiation oncologist. Radiother Oncol. 1986;7:13.
116 Gilbertson VA. Results of treatment of stomach cancer. An appraisal of efforts for more extensive surgery and a report of 1,938 cases. Cancer. 1969;23:1305.
117 Gunderson LL. Gastric cancer-patterns of relapse after surgical resection. Semin Rad Oncol. 2002;12:150-161.
118 D’Angelica M, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808-816.
127 Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone in resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20.
128 Panzini I, Gianni L, Fattori PP, et al. Adjuvant chemotherapy in gastric cancer. A meta-analysis of randomized trials and a comparison with previous meta-analyses. Tumori. 2002;88:21.
129 Janunger KG, Hafstrom L, Nygren P, et al. A systematic overview of chemotherapy effects in gastric cancer. Acta Oncol. 2001;40:309.
134 Paoletti X, Oba K, Burzykowsky T, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer. A meta-analysis. JAMA. 2010;303:1729-1737.
148 Allum WH, Hallissey MT, Ward LC, Hockey MS. A controlled prospective and randomized trial of adjuvant chemotherapy or radiotherapy in resectable gastric cancer. Interim report. British Stomach Cancer Group. Br J Cancer. 1989;60:739.
149 Abe M, Takahashi M. Intraoperative radiotherapy. The Japanese experience. Int J Radiat Oncol Biol Phys. 1981;5:683.
150 Takahashi M, Abe M. Intraoperative radiotherapy for carcinoma of the stomach. Eur J Surg Oncol. 1986;12:247.
151 Chen G, Song S. Evaluation of intraoperative radiotherapy for gastric carcinoma: analysis of 247 patients. In: Abe M, Takahashi M, editors. Proceedings of Third International IORT Symposium. Kyoto, Japan. New York: Pergamon Press; 1991:190.
155 Zhang ZX, Gu XZ, Yin WB, et al. Randomized clinical trial combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of the gastric cardia (AGC). Report on 370 patients. Int J Radiat Oncol Biol Phys. 1998;42:929-934.
156 Gunderson LL, Hoskins B, Cohen AM, et al. Combined modality treatment of gastric cancer. Int J Radiat Oncol Biol Phys. 1983;9:965.
158 Regine WF, Mohuidden M. Impact of adjuvant therapy on locally advanced adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys. 1992;24:921.
159 Mehta K, Mohuidden M. Improved local control with adjunctive therapy for cancers of the gastroesophageal junction. Int J Radiat Oncol Biol Phys. 1994;30:272.
160 Whittington R, Coia L, Haller DG, et al. Adenocarcinoma of the esophagus and esophagogastric junction. The effects of single and combined modalities in the survival and patterns of failure following treatment. Int J Radiat Oncol Biol Phys. 1990;19:593.
161 Henning GT, Schild SF, Stafford SL, et al. Results of irradiation or chemoirradiation following resection of gastric adenocarcinoma. Int J Radiat Oncol Biol Phys. 2000;46:589-598.
162 Moertel CG, Childs DS, O’Fallon JR, et al. Combined 5-fluorouracil and radiation therapy as a surgical adjuvant for poor prognosis gastric carcinoma. J Clin Oncol. 1984;2:1249.
163 MacDonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725.
164 Smalley SS, Gunderson L, Tepper J, et al. Gastric surgical adjuvant radiotherapy consensus report. Rationale and treatment implementation. Int J Radiat Oncol Biol Phys. 2002;52:282.
165 Kim S, Lim KH, Lee J, et al. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys. 2005;63:1279-1285.
166 Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462-467.
167 Urba S, Orringer M, Turrisi A, et al. Randomized trial of preoperative chemoradiation vs surgery alone in patients with locoregional esophageal cancer. J Clin Oncol. 2001;19:305-313.
168 Tepper JE, Krasna M, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy and surgery compared with surgery alone for esophageal cancer. CALGB 9781. J Clin Oncol. 2008;26:1086-1092.
169 Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851-856.
170 Coburn NG, Govindaragan A, Law CHL, et al. Stage-specific effect of adjuvant therapy following gastric cancer resection. A population-based analysis of 4,041 patients. Ann Surg Oncol. 2008;15:500-507.
171 Fiorica F, Cartei F, Enea N, et al. The impact of radiotherapy on survival in resectable gastric carcinoma. A meta-analysis of literature data. Cancer Treat Rev. 2007;33:729-740.
172 Gebski V, Burneister B, Smithers BM, et al. for the Australasian Gastro-Intestinal Trials Group: Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma. A meta-analysis. Lancet Oncol. 2007;8:226-234.
173 Sindelar WF, Kinsella TJ, Tepper JE, et al. Randomized trial of intraoperative radiotherapy in carcinoma of the stomach. Am J Surg. 1993;165:178.
191 Moertel CG, Childs DSJr, Reitemeier RJ, et al. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet. 1969;2:865.
192 Holbrook MA. Radiation therapy. Current concepts in cancer. Gastric cancer. Treatment principles. JAMA. 1974;228:1289.
193 Schein PS, Novak J, for the Gastrointestinal Tumor Study Group. Combined modality therapy (XRT-chemo) versus chemotherapy alone for locally unresectable gastric cancer. Cancer. 1982;49:1771.
194 Chevalier TL, Smith FP, Harter WK, Schein PS. Chemotherapy and combined modality therapy for locally advanced and metastatic gastric carcinoma. Semin Oncol. 1985;12:46.
235 Wils JA, Klein HO, Wagener DJT, et al. Sequential high-dose methotrexate and fluorouracil combined with doxorubicin—a step ahead in the treatment of advanced gastric cancer. A trial of the European Organization for Research and Treatment of Cancer. J Clin Oncol. 1991;9:827.
253 Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261-267.
254 Cunningham D, Rao S, Starling T, et al. Randomised multicentre phase III study comparing capecitabine with fluorouracil and oxaliplatin with cisplatin in patients with advanced oesophagogastric cancer. The REAL 2 trial [abstract LBA4017]. J Clin Oncol. 2006;24:183s.
266 Murad AM, Santiago FF, Petroianu A, et al. Modified therapy with 5-fluorouracil, doxorubicin and methotrexate in advanced gastric cancer. Cancer. 1993;72:37.
267 Pyrhonen S, Kuitunen T, Nyandoto P, et al. Randomized comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995;71:587.
268 Glimelius B, Ekstrom K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163.
271 Tepper JE, Gunderson LL. Radiation treatment parameters in the adjuvant postoperative therapy of gastric cancer. Semin Radiat Oncol. 2002;12:187-195.
1 Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300.
2 Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23-47.
3 Wiggins CL, Becker TM, Key CR, et al. Stomach cancer among New Mexico’s American Indians, Hispanic whites and non-Hispanic whites. Cancer Res. 1989;49:1595-1599.
4 Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33-64.
5 Blot WJ, Devesa SS, Kneller RW, et al. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287-1289.
6 Ries GA, Hanley BF, Edwards BK, editors. Cancer Statistics Review 1973-1987, vol 90. Department of Health and Human Services, National Institutes of Health, Bethesda, MD, 1990;2789.
7 Powell J, McConkey CC. Increasing incidence of adenocarcinoma of the gastric cardia and adjacent sites. Br J Cancer. 1990;59:440-443.
8 Haenzel W, Kurihara M, Segi M, et al. Stomach cancer among Japanese in Hawaii. J Natl Cancer Inst. 1972;49:969-988.
9 Correa P, Cuello C, Duque E. Carcinoma and intestinal metaplasia of the stomach in Colombian migrants. J Natl Cancer Inst. 1970;44:297-306.
10 Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;353:32-42.
11 You WC, Blot WJ, Chang YS, et al. Diet and high risk of stomach cancer in Shandong, China. Cancer Res. 1988;48:3518-3523.
12 Nomura A. Stomach cancer. In: Schottenfeld D, Fraumeni JFJr, editors. Cancer Epidemiology and Prevention. Oxford, United Kingdom: Oxford University Press; 1996:707-724.
13 Wadström T. An update on Helicobacter pylori. Curr Opin Gastroenterol. 1995;11:69-75.
14 Neugut AI, Hayeh M, Howe G. Epidemiology of gastric cancer. Semin Oncol. 1996;23:281-291.
15 Correa P. Human gastric carcinogenesis. Multistep and multifocal process. Cancer Res. 1992;52:6735-6740.
16 Correa P, Haenzel W, Cuello C, et al. Gastric precancerous process in a high-risk population. Cross-sectional studies. Cancer Res. 1990;50:4731-4736.
17 The Eurogast Study Group. An international association between H. pylori infection and gastric cancer. Lancet. 1993;341:1359-1362.
18 Nomura A, Stemmerman GN, Chyou PH, et al. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132-1136.
19 Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131.
20 Levin B. Gastric Cancer. New Insights into Epidemiology and Etiology. 1997. American Society of Clinical Oncology Educational Books, pp 273-274
21 Apisarnthanarax S, Tepper J. Crossroads in the combined-modality management of gastroesophageal junction carcinomas. Gastrointest Cancer Res. 2008;2:235-243.
22 Chun YS, Lindor NM, Smyrk TC, et al. Germline E-cadherin gene mutations. Is prophylactic total gastrectomy indicated? Cancer. 2001;92:181.
23 Huntsman DG, Carneiro F, Lewis FR, et al. Early gastric cancer in young, asymptomatic carriers of germ-line E-cadherin mutations. N Engl J Med. 2001;344:1904.
24 Lewis FR, Mellinger JD, Hayashi A, et al. Prophylactic total gastrectomy for familial gastric cancer. Surgery. 2001;130:612.
25 Thompson GB, van Heerden J, Sarr MC. Adenocarcinoma of the stomach. Are we making progress? Lancet. 1993;342:713-718.
26 Dockerty MB. Pathologic aspects of primary malignant neoplasms of the stomach. In: ReMine WH, Priestley JT, Berkson J, editors. Cancer of the Stomach. Philadelphia: WB Saunders; 1964:173.
27 Kennedy BJ. TNM classification for stomach cancer. Cancer. 1970;26:971.
28 Gunderson LL, Sosin H. Adenocarcinoma of the stomach areas of failure in a reoperation series (second or symptomatic looks). Clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1982;8:1.
29 MacDonald JS, Cohn I, Gunderson LL. Carcinoma of the stomach. In: DeVita V, Hellman S, Rosenberg S, editors. Principles and Practice of Oncology. ed 2. Philadelphia: Lippincott; 1985:659-690.
30 Nagatomo T, Mukarami E, Kondo K. Histologic criteria of serosal rupture and prognosis in gastric carcinoma. Cancer. 1969;29:180.
31 Dent DM, Werner ID, Novis B, et al. Prospective randomized trial of combined oncological therapy for gastric carcinoma. Cancer. 1979;44:385.
32 Tsukivama J, Akine Y, Kajiura Y, et al. Radiation therapy for advanced gastric cancer. Int J Radiat Oncol Biol Phys. 1988;15:123.
33 Yu CC, Levison DA, Dunn JA, et al. Pathological prognostic factors in the second British Stomach Cancer Group trial of adjuvant therapy in resectable gastric cancer. Br J Cancer. 1995;71:1106-1110.
34 MacDonald WC, MacDonald JB. Adenocarcinoma of the esophagus or gastric cardia. Cancer. 1987;60:33.
35 Meyers WC, Damiano RJ, Postlethwait RW, Rotolo FS. Adenocarcinoma of the stomach: Changing patterns over the last 4 decades. Ann Surg. 1987;205:1.
36 Yamada Y, Kato Y. Greater tendency for submucosal invasion in fundic area gastric carcinomas than those arising in the pyloric area. Cancer. 1989;63:1757.
37 Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer. 1985;56:2512.
38 Hartley LC, Evans E, Windsor CJ. Factors influencing prognosis in gastric cancer. Aust NZ J Surg. 1987;57:5.
39 Nanus DM, Kelsen DP, Niedzwiecki D, et al. Flow cytometry as a predictive indicator in patients with operable gastric cancer. J Clin Oncol. 1989;7:1105.
40 Finlay CA, Hinds PW, Levine AJ. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989;57:1083-1093.
41 Lane DP. Worrying about p53. Curr Biol. 1989;2:581-583.
42 Kastan MB, Oyekwere O, Sidransky D, et al. Participation of p53 in the cell response to DNA damage. Cancer Res. 1991;51:6304-6311.
43 O’Connor PM, Jackman J, Jondle D, et al. Role of p53 tumor suppressor gene in cell cycle arrest and radiosensitivity of Burkitt’s lymphoma cell lines. Cancer Res. 1993;53:4776-4780.
44 Lynch HT, Smyrk TC, Watson P, et al. Genetics, natural history, tumor spectrum and pathology of hereditary non-polyposis colon cancer. An updated review. Gastroenterology. 1993;10:1535-1549.
45 Takahashi M, Ota S, Shimada T, et al. Hepatocyte growth factor is the most potent endogenous stimulant of rabbit gastric epithelial cell proliferation and migration in primary culture. J Clin Invest. 1995;95:1994-2003.
46 Tahara E. Molecular mechanism of stomach carcinogenesis. J Cancer Res Clin Oncol. 1993;119:265-272.
47 Tahara E, Yokozaki H, Yasui W. Growth factors in gastric cancer. In: Nishi M, Tahara E, editors. Gastric Cancer. Tokyo: Springer-Verlag; 1993:209-217.
48 Fenoglio-Preiser CM. The Effect of Oncogenes on Biology and Prognosis of Surgically Resected Gastric Cancer. 1997. American Society of Clinical Oncology Educational Books, pp 275-277
49 Tushima K, Nagorney DM, Cha SS, Reiman HM. Correlation of DNA ploidy, histopathology, stage, and clinical outcome in gastric carcinoma. Surg Oncol. 1992;1:17.
50 Baba H, Korenaga D, Okamura T, et al. Prognostic significance of DNA content with special reference to age in gastric cancer. Cancer. 1989;63:1768.
51 Korenaga D, Okamura T, Saito A, et al. DNA ploidy is closely linked to tumor invasion, lymph node metastasis, and prognosis in clinical gastric cancer. Cancer. 1988;62:309.
52 Harrison JD, Morris DL, Ellis IO, et al. The effect of tamoxifen and estrogen receptor status on survival in gastric carcinoma. Cancer. 1989;64:1007.
53 Sugiyama K, Yomemura Y, Miyazaki I. Immunohistochemical study of epidermal growth factor and epidermal growth factor receptor in gastric carcinoma. Cancer. 1989;63:1557.
54 Yonemura Y, Ninimiya I, Ohoyama S, et al. Expression of C-erbB-2 oncoprotein in gastric carcinoma. Immunoreactivity for C-erbB-2 protein is independent of poor short-term prognosis in patients with gastric carcinoma. Cancer. 1991;62:2914.
55 Van Cutsem E, Kang Y, Chung H, et al. Efficacy results from the ToGA trial. A phase III study of trastuzumab added to standard chemotherapy in first-line human epidermal growth factor rector 2 (HER2)-positive advanced gastric cancer. 2009 ASCO Proceedings. J Clin Oncol. 2009;27(18S):LBA4509.
56 Hiton DA, West KP. An evaluation of the prognostic significance of HLA-DR expression in gastric carcinoma. Cancer. 1990;66:1154.
57 Tahara E, Semba S, Tahara H. Molecular biological observations in gastric cancer. Semin Oncol. 1996;23:307-315.
58 Japanese Research Society for Gastric Cancer. The general rules for the gastric cancer study in surgery and pathology. I. Clinical classification. Jpn J Surg. 1981;11:127.
59 Lauren P. The two histologic main types of gastric carcinomas. Diffuse and so-called intestinal type carcinoma—an attempt at a histological classification. Acta Pathol Microbiol Scand. 1965;64:31.
60 Borrmann R. Geschwulste des Magens und Duodenums. In: Henke F, Lanbarsch O, editors. Handbuch der Spaziellen Pathologischen Anatomie and Histologie, vol 4. Berlin: Julius Springer; 1926.
61 Wang J-Y, Hsieh J-S, Juang Y-S, et al. Endoscopic ultrasonography for preoperative locoregional staging and assessment of resectability in gastric cancer. Clin Imaging. 1998;22:355.
62 Willis S, Truong S, Gribritz S, et al. Endoscopic ultrasonography in the preoperative staging of gastric cancer. Accuracy and impact on surgical therapy. Surg Endosc. 2000;14:951.
63 Loury AM, Mansfield PF, Leach SD, et al. Laparoscopic staging for gastric cancer. Surgery. 1996;119:611-614.
64 Ziegler K, Sanft C, Zimmer T, et al. Comparison of computed tomography, endosonography, and intraoperative assessment in TN staging of gastric carcinoma. Gut. 1993;34:604-610.
65 Edge S, Compton C, et al. AJCC Cancer Staging Manual, ed 7. New York: Springer-Verlag; 2010.
66 Robertson CS, Chung SCS, Woods SDS, et al. A prospective randomized trial comparing R1 subtotal gastrectomy with R3 total gastrectomy for antral cancer. Ann Surg. 1994;220:176-182.
67 Dupont JBJr, et al. Adenocarcinoma of the stomach. Review of 1497 cases. Cancer. 1978;41:941.
68 Serlin O, Keehn RJ, Higgins GA, et al. Factors related to survival following resection for gastric carcinoma. Cancer. 1977;40:1318.
69 Sugimachi K, Kodama Y, Kumashito R, et al. Critical evaluation of prophylactic splenectomy in total gastrectomy for stomach cancer. Gann. 1980;71:704.
70 Noguchi Y, Imada T, Matsumoto A, et al. Radical surgery for gastric cancer. A review of the Japanese experience. Cancer. 1989;64:2053.
71 Iriyama K, Asukawa T, Koike H, et al. Is extensive lymphadenectomy necessary for surgical treatment of intramucosal carcinoma of the stomach? Arch Surg. 1989;124:309.
72 Bolen T, Nakane Y, Okusa T, et al. Strategy for lymphadenectomy of gastric cancer. Surgery. 1989;105:585.
73 Maruyama K, Gunven P, Okabayashi K, et al. Lymph node metastases of gastric cancer. General pattern in 1931 patients. Ann Surg. 1989;210:596.
74 Douglas HO, Nava HR. Gastric adenocarcinoma. Management of the primary disease. Semin Oncol. 1985;12:32.
75 Kodama Y, Sugimachi K, Soejima K, et al. Evaluation of extensive lymph node dissection for carcinoma of the stomach. World J Surg. 1981;5:241.
76 Okajima K. Surgical treatment of gastric cancer with specific reference to lymph node removal. Acta Med Okayama. 1977;31:369.
77 Shiu MH, Moore E, Sanders M, et al. Influence of the extent of resection on survival after curative treatment of gastric carcinoma. Arch Surg. 1987;122:1347.
78 Soja J, Ohyama S, Miyashita K, et al. A statistical evaluation advancement in gastric cancer surgery with special reference to the significance of lymphadenectomy for cure. World J Surg. 1988;12:398.
79 Kern KA. Gastric cancer. A neoplastic enigma. J Surg Oncol. 1989;1(Suppl):34-39.
80 Has CD, Mansfield CM, Leichman LP, et al. Combined non-simultaneous radiation therapy and chemotherapy with 5-FU, doxorubicin and mitomycin for residual localized gastric adenocarcinoma. A Southwest Oncology Group pilot study. Cancer Treat Rep. 1983;67:421.
81 Dent DM, Madden MV, Price SK. Randomized comparison of R1 and R2 gastrectomy for gastric carcinoma. Br J Surg. 1988;75:110.
82 Sasako M, Maruyama K, Kinoshita T. Quality control of surgical technique in a multicenter, prospective, randomized, controlled study on the surgical treatment of gastric cancer. Jpn J Clin Oncol. 1992;22:41.
83 Burt AMG, Hermans J, Boon MC, et al. Evaluation of the extent of lymphadenectomy in a randomized trial of Western versus Japanese-type of surgery in gastric cancer. J Clin Oncol. 1994;12:417-422.
84 Bonnenkamp JJ, Songum I, Hermans J, et al. Randomized comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345:745-748.
85 Bonnenkamp JJ, Hermans J, Sasako M, Vande Velde CJH for the Dutch Gastric Cancer Group. Extended lymph node dissection for gastric cancer. N Engl J Med. 1999;340:908-914.
86 Cuschieri A, Fayers P, Fielding J, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer. Preliminary results of the MRC randomized controlled surgical trial. Lancet. 1996;347:995-999.
87 Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer. Long-term results of the MRC randomized controlled surgical trial. Br J Cancer. 1999;79:1522.
88 Wang Z, Chen JQ, Cao YF, et al. Systematic review of D2 lymphadenectomy vs D2 with para-aortic nodal dissection for advanced gastric cancer. World J Gastroenterol. 2010;16:1138-1149.
89 Chen XZ, Hu JK, Zhon ZG, et al. Meta-analysis of effectiveness and safety of D2 plus para-aortic lymphadenectomy for resectable gastric cancer. J Am Coll Surg. 2010;21:100-105.
90 Fukutomi H, Nakahara A. Endoscopic therapy of gastrointestinal cancer and its curability. Gan To Kagaku Ryoho. 1988;4:1132.
91 Adachi Y, Mori M, Sugimachi K. Persistence of mucosal gastric carcinomas for 8 and 6 years in two patients. Arch Pathol Lab Med. 1990;114:1046.
92 Bozzeti F, Bonfanti G, Castellani R, et al. Comparing reconstruction with Roux-en-Y to pouch following total gastrectomy. J Am Coll Surg. 1996;183:243-248.
93 Chareton B, Landen S, Manganas D, et al. Prospective randomized trial comparing Billroth I and II procedures for carcinoma of the gastric antrum. J Ann Coll Surg. 1996;183:190-194.
94 Buhl K, Lehnert T, Schlag P, et al. Reconstruction after gastrectomy and quality of life. World J Surg. 1995;19:558-564.
95 Fuchs KH, Thiede A, Engemam R, et al. Reconstruction of the food passage after total gastrectomy. Randomized trial. World J Surg. 1995;19:698-706.
96 Macintyre DMC, Akoh JA. Improving survival in gastric cancer. Review of operation mortality in English language publications from 1970. Br J Surg. 1981;68:773.
97 Heberer G, Teichmann RK, Kramling HJ, Gunther B. Results of gastric resection for carcinoma of the stomach. The European experience. World J Surg. 1988;12:374.
98 Green PH, O’Toole KM, Slonin D, et al. Increasing incidence and excellent survival of patients with early gastric cancer. Expression in a United States Medical Center. Am J Med. 1988;85:658.
99 Itoh H, Oohata Y, Nakamura K, et al. Complete ten-year post-gastrectomy follow-up of early gastric cancer. Am J Surg. 1989;158:14.
100 Endo M, Habu H. Clinical studies of early gastric cancer. Hepatogastroenterology. 1990;37:408.
101 de Dombral FT, Price AB, Thompson H, et al. The British Society of Gastroenterology early gastric cancer/dysplasia survey. An interim report. J Gut. 1990;3:115.
102 Gentsch HH, Grould H, Gerdl J. Results of surgical treatment of early gastric cancer in 113 patients. World J Surg. 1981;5:103.
103 Farley DR, Donohue JH, Nagorney DM, et al. Early gastric cancer. Br J Surg. 1992;79:539.
104 Majus WC, Damiano RJJr, Rotolo FS, et al. Adenocarcinoma of the stomach. Changing patterns over the last four decades. Ann Surg. 1987;205:1.
105 Cady B, Rossi RL, Silverman ML, et al. Gastric adenocarcinoma. A disease in transition. Arch Surg. 1989;124:303.
106 Kim JP, Kim YW, Yang HK, et al. Significant prognostic factors by multivariate analyses of 3,926 gastric cancer patients. World J Surg. 1994;18:872-878.
107 Kajiyama Y, Tsurumaru M, Udagawa H, et al. Prognostic factors in adenocarcinoma of the gastric cardia. Pathologic stage analysis and multivariate regression analysis. J Clin Oncol. 1997;15:2015-2021.
108 Jatzko GR, Lisborg PH, Denk H, et al. A 10-year experience with Japanese-type radical lymph node dissection for gastric cancer outside Japan. Cancer. 1995;76:1302-1312.
109 Bunt TMG, Bonnenkamp HJ, Hermans J, et al. Factors influencing the noncompliance and contamination in a randomized trial of “Western” (R1) versus “Japanese” (R2) types of surgery in gastric cancer. Cancer. 1994;73:1544-1551.
110 Papachristou DN, Fortner JG. Local recurrence of gastric adenocarcinomas after gastrectomy. J Surg Oncol. 1981;18:47.
111 Nakamura K, Keyama T, Yao T, et al. Pathology and prognosis of gastric carcinoma. Findings in 10,000 patients who underwent primary gastrectomy. Cancer. 1992;70:1030.
112 Landry J, Tepper J, Wood W, et al. Analysis of survival and local control following surgery for gastric cancer. Int J Radiat Oncol Biol Phys. 1990;19:1357.
113 McNeer G, Vandenberg H, Donn FY, Bowden LA. A critical evaluation of subtotal gastrectomy for the cure of cancer of the stomach. Ann Surg. 1957;134:2.
114 Thomson FB, Robins RE. Local recurrence following subtotal resection for gastric carcinoma. Surg Gynecol Obstet. 1952;91:341.
115 Wisbek WA, Becker EM, Russell AH. Adenocarcinoma of the stomach: Autopsy observations with therapeutic implications for the radiation oncologist. Radiother Oncol. 1986;7:13.
116 Gilbertson VA. Results of treatment of stomach cancer: An appraisal of efforts for more extensive surgery and a report of 1,938 cases. Cancer. 1969;23:1305.
117 Gunderson LL. Gastric cancer—patterns of relapse after surgical resection. Semin Radiat Oncol. 2002;12:150-161.
118 D’Angelica M, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808-816.
119 Coombs RC, Schein PS, Chilvers CE, et al. A randomized trial comparing adjuvant fluorouracil, doxorubicin, and mitomycin with no treatment in operable gastric cancer. International Collaborative Cancer Group. J Clin Oncol. 1990;8:1362-1369.
120 Gagliano R, McCracken J, Chen T. Adjuvant chemotherapy with FAM in gastric cancer. A SWOG study. Proc Am Soc Clin Oncol. 1983;2:114.
121 Estape J, Grau J, Alcobendas F, et al. Mitomycin C as an adjuvant treatment to resected gastric cancer. A 20-year follow-up. Ann Surg. 1991;213:219-221.
122 Grau JJ, Estape J, Fuster J, et al. Randomized trial of adjuvant chemotherapy with mitomycin plus ftorafur versus mitomycin alone in resected locally advanced gastric cancer. J Clin Oncol. 1998;16:1036-1039.
123 Neri B, Cini G, Andreoli F, et al. Randomized trial of adjuvant chemotherapy versus control after curative resection for gastric cancer. 5-Year follow-up. Br J Cancer. 2001;84:878.
124 Lise M, Nitti D, Marchet A, et al. Final results of a phase II clinical trial of adjuvant chemotherapy with the modified fluorouracil, doxorubicin, and mitomycin regimen in resectable gastric cancer. J Clin Oncol. 1995;23:2757-2763.
125 MacDonald JS, Fleming TR, Peterson RF, et al. Adjuvant chemotherapy with 5-FU, Adriamycin, and mitomycin C (FAM) versus surgery alone for patients with locally advanced gastric adenocarcinoma. A Southwest Oncology Group Study. Ann Surg Oncol. 1995;2:488-494.
126 Wils J, Nitti D, Guimaraes dos Santos J, et al. Randomized phase III studies of adjuvant chemotherapy with FAMTX or FEMTX in resected gastric cancer. Pooled results of studies from the EORTC GI-group and the ICCG. Proc Am Soc Clin Oncol. 2002;21:131a.
127 Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone in resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20.
128 Panzini I, Gianni L, Fattori PP, et al. Adjuvant chemotherapy in gastric cancer. A meta-analysis of randomized trials and a comparison with previous meta-analyses. Tumori. 2002;88:21.
129 Janunger KG, Hafstrom L, Nygren P, et al. A systematic overview of chemotherapy effects in gastric cancer. Acta Oncol. 2001;40:309.
130 Gianni L, Panzini I, Tassinari D, et al. Meta-analyses of randomized trials of adjuvant chemotherapy in gastric cancer. Ann Oncol. 2001;12:1179.
131 Hermans J, Bonenkamp H. Meta-analysis of adjuvant chemotherapy in gastric cancer. A critical reappraisal. J Clin Oncol. 1994;12:878.
132 Earle CC, Maroun JA. Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients. Revisiting a meta-analysis of randomised trials. Eur J Cancer. 1999;35:1059.
133 Mari E, Floriani I, Tinazzi A, et al. Efficacy of adjuvant chemotherapy after curative resection for gastric cancer. A meta-analysis of published randomised trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente). Ann Oncol. 2000;11:837.
134 Paoletti X, Oba K, Burzykowsky T, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer. A meta-analysis. JAMA. 2010;303:1729-1737.
135 Serlin O, Wolkoff JS, Amadeo JM, Keehn RJ. Use of 5-fluorodeoxyuridine (FUDR) as an adjuvant to the surgical management of carcinoma of the stomach. Cancer. 1969;24:223.
136 Dixon WJ, Longmire WP, Holden J. Use of triethylenethiophosphoramide as an adjuvant to the surgical treatment of gastric and colorectal carcinoma. Ten-year follow-up. Ann Surg. 1971;173:26.
137 The Gastrointestinal Tumor Study Group. Adjuvant chemotherapy following resection for gastric cancer. Cancer. 1982;49:1116.
138 Higgins GA, Amadeo JH, Smith DE, et al. Efficacy of prolonged intermittent therapy with combined 5-FU and methyl CCNU following resection for gastric carcinoma. Veterans Administration Surgical Oncology Group report. Cancer. 1983;52:1105.
139 Engstrom PF, Lavin PT, Douglas HO, Brunner KW. Postoperative adjuvant 5-FU and methyl CCNU therapy for gastric cancer patients. Eastern Cooperative Oncology Study Group 3275. Cancer. 1985;55:1868.
140 Italian Gastrointestinal Tumor Study Group. Adjuvant treatments following curative resection for gastric cancer. Br J Surg. 1988;75:1100.
141 Hallissey MT, Dunn JA, Ward LC, et al. The Second British Stomach Cancer Group trial of adjuvant radiotherapy or chemotherapy in resectable gastric cancer. Five-year follow-up. Lancet. 1989;343:1309-1312.
142 MacDonald JS, Gagliano R, Fleming T, et al. A phase II trial of FAM (5-fluorouracil, Adriamycin, and mitomycin C) chemotherapy versus control as adjuvant treatment for resected gastric cancer. Proc Am Soc Clin Oncol. 1992;11:488.
143 Krook JE, O’Connell MJ, Wieand HS, et al. A prospective, randomized evaluation of intensive-course 5-fluorouracil plus doxorubicin as surgical adjuvant chemotherapy for resected gastric cancer. Cancer. 1991;67:2454.
144 Allum WH, Hallissey MT, Kelly KA. Adjuvant chemotherapy in operable gastric cancer. Five-year follow-up of first British Stomach Cancer Group trial. Lancet. 1989;1:571.
145 Jakesz R, Dittrich C, Funovics J, et al. The effect of adjuvant chemotherapy in gastric carcinoma is dependent on tumor histology. 5-Year results of a prospective randomized trial. Recent results. Cancer Res. 1988;110:44.
146 Carrato A, Diaz-Rubio E, Medrano J, et al. Phase III trial of surgery versus adjuvant chemotherapy with mitomycin C and tegafur plus uracil, starting within the first week after surgery for gastric carcinoma. Proc Am Soc Clin Oncol. 1995;14:198.
147 Neri B, De Leonardis V, Romano S, et al. Adjuvant chemotherapy after gastric resection in node-positive cancer patients. A multicenter randomized study. Br J Cancer. 1996;73:549-552.
148 Allum WH, Hallissey MT, Ward LC, Hockey MS. A controlled prospective and randomized trial of adjuvant chemotherapy or radiotherapy in resectable gastric cancer. Interim report. British Stomach Cancer Group. Br J Cancer. 1989;60:739.
149 Abe M, Takahashi M. Intraoperative radiotherapy: The Japanese experience. Int J Radiat Oncol Biol Phys. 1981;5:683.
150 Takahashi M, Abe M. Intraoperative radiotherapy for carcinoma of the stomach. Eur J Surg Oncol. 1986;12:247.
151 Chen G, Song S. Evaluation of intraoperative radiotherapy for gastric carcinoma: Analysis of 247 patients. In: Abe M, Takahashi M, editors. Proceedings of Third International IORT Symposium. Kyoto, Japan. New York: Pergamon Press; 1991:190.
152 Shchepotin IB, Evans SRT, Chorny V, et al. Intensive preoperative radiotherapy with local hyperthermia for the treatment of gastric carcinoma. Surg Oncol. 1994;3:37.
153 Talaev MI, Starinskii VV, Kovalev BN, et al. Results of combined treatment of cancer of the gastric antrum and gastric body. Vopr Onkol. 1990;36:1485.
154 Kosse VA. Combined treatment of gastric cancer using hypoxic radiotherapy. Vopr Onkol. 1990;36:1349.
155 Zhang ZX, Gu XZ, Yin WB, et al. Randomized clinical trial combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of the gastric cardia (AGC). Report on 370 patients. Int J Radiat Oncol Biol Phys. 1998;42:929-934.
156 Gunderson LL, Hoskins B, Cohen AM, et al. Combined modality treatment of gastric cancer. Int J Radiat Oncol Biol Phys. 1983;9:965.
157 Gez E, Sulkes A, Yablonski-Peretz T, Weshler Z. Combined 5-fluorouracil (5-FU) and radiation therapy following resection of locally advanced gastric carcinoma. J Surg Oncol. 1986;31:139.
158 Regine WF, Mohuidden M. Impact of adjuvant therapy on locally advanced adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys. 1992;24:921.
159 Mehta K, Mohuidden M. Improved local control with adjunctive therapy for cancers of the gastroesophageal junction. Int J Radiat Oncol Biol Phys. 1994;30:272.
160 Whittington R, Coia L, Haller DG, et al. Adenocarcinoma of the esophagus and esophagogastric junction. The effects of single and combined modalities in the survival and patterns of failure following treatment. Int J Radiat Oncol Biol Phys. 1990;19:593.
161 Henning GT, Schild SF, Stafford SL, et al. Results of irradiation or chemoirradiation following resection of gastric adenocarcinoma. Int J Radiat Oncol Biol Phys. 2000;46:589-598.
162 Moertel CG, Childs DS, O’Fallon JR, et al. Combined 5-fluorouracil and radiation therapy as a surgical adjuvant for poor prognosis gastric carcinoma. J Clin Oncol. 1984;2:1249.
163 MacDonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725.
164 Smalley SS, Gunderson L, Tepper J, et al. Gastric surgical adjuvant radiotherapy consensus report. Rationale and treatment implementation. Int J Radiat Oncol Biol Phys. 2002;52:282.
165 Kim S, Lim KH, Lee J, et al. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys. 2005;63:1279-1285.
166 Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462-467.
167 Urba S, Orringer M, Turrisi A, et al. Randomized trial of preoperative chemoradiation vs surgery alone in patients with locoregional esophageal cancer. J Clin Oncol. 2001;19:305-313.
168 Tepper JE, Krasna M, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy and surgery compared with surgery alone for esophageal cancer. CALGB 9781. J Clin Oncol. 2008;26:1086-1092.
169 Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851-856.
170 Coburn NG, Govindaragan A, Law CHL, et al. Stage-specific effect of adjuvant therapy following gastric cancer resection. A population-based analysis of 4,041 patients. Ann Surg Oncol. 2008;15:500-507.
171 Fiorica F, Cartei F, Enea N, et al. The impact of radiotherapy on survival in resectable gastric carcinoma. A meta-analysis of literature data. Cancer Treatment Reviews. 2007;33:729-740.
172 Gebski V, Burneister B, Smithers BM, et al. for the Australasian Gastro-Intestinal Trials Group: Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8:226-234.
173 Sindelar WF, Kinsella TJ, Tepper JE, et al. Randomized trial of intraoperative radiotherapy in carcinoma of the stomach. Am J Surg. 1993;165:178.
174 Abe M, Takahashi M, Yabumoto E, et al. Clinical experiences with intraoperative radiotherapy for locally advanced cancers. Cancer. 1980;45:40-48.
175 Ogata T, Araki K, Matsuura K, et al. A 10-year experience of intraoperative radiotherapy for gastric carcinoma and a new surgical method of creating a wider irradiation field for cases of total gastrectomy patients. Int J Radiat Oncol Biol Phys. 1995;32:341-347.
176 Kramling HJ, Grab J, Zaspel J, et al. Experimental study of vascular sequelae of combined upper abdominal intraoperative and external radiation therapy. In Vaeth J (ed): Intraoperative Radiation Therapy in the Treatment of Cancer. Front Radiat Ther Oncol. 1997;31:36-40.
177 Kramling HJ, Willich N, Cramer C, et al: Results of IORT in the treatment of gastric cancer. ISIORT 2002 Proceedings. Aachen, Germany, abstract 4.3.
178 Drognitz O, Henne K, Weissenberger C, et al. Long-term results after intraoperative radiation therapy for gastric cancer. Int J Radiat Oncol Biol Phys. 2008;70:715-721.
179 Calvo FA, Henriquez I, Santos M, et al. Intraoperative and external beam radiotherapy in advanced resectable gastric cancer. Technical description and preliminary results. Int J Radiat Oncol Biol Phys. 1989;17:183-189.
180 Calvo FA, Aristu JJ, Azinovic I, et al. Intraoperative and external radiotherapy in resected gastric cancer. Updated report of a phase II trial. Int J Radiat Oncol Biol Phys. 1992;24:729-736.
181 Martinez-Monge R, Calvo FA, Azinovic I, et al. Patterns of failure and long-term results in high-risk resected gastric cancer treated with postoperative radiotherapy ± intraoperative electron boost. J Surg Oncol. 1997;66:24-29.
182 Calvo FA, Flaquer A, Gonzalez C, et al. Esophageal and gastric cancer treated with IORT containing adjuvant (neo) treatments. ISIORT 2008 Proceedings. Rev Cancer. 2008;22(Suppl):24-25.
183 Avizonis VN, Buzydlowski J, Lanciano R, et al. Treatment of adenocarcinoma of the stomach with resection, intraoperative radiotherapy and adjuvant external beam radiation. A phase II study from Radiation Therapy Oncology Group 85-04. Ann Surg Oncol. 1995;2:295-302.
184 Gilly FN, Gerard JP, Braillon G, et al. Intraoperative radiotherapy in gastric adenocarcinomas. Apropos of 45 cases. Ann Chir. 1993;47:234-239.
185 Gilly FN, Gerard JP, Braillon G, et al: Surgery, IORT and postoperative radiotherapy in the treatment of gastric cancer. The long term experience in Lyon. ISIORT 2002 Proceedings. Aachen, Germany, abstract 4.4.
186 Coquard R, Ayzac L, Gilly FN, et al. Intraoperative radiation therapy combined with limited lymph node resection in gastric cancer. An alternative to extended dissection? Int J Radiat Oncol Biol Phys. 1997;39:1093-1098.
187 Chambert M, Schmitt T, Soglu M, et al: Intraoperative radiation therapy (IORT) for locally advanced gastric cancer. 6th International IORT Symposium and 31st San Francisco Cancer Symposium [abstracts]. San Francisco, September 23-25, 1996.
188 Monson JRT, Donohue JH, McIlrath DC, et al. Total gastrectomy for advanced cancer. A worthwhile palliative procedure. Cancer. 1991;68:1863.
189 Wieland C, Hymmen U. Megavoltage therapy for malignant gastric tumors. Strahlentherapie. 1970;40:20.
190 Takahashi T. Studies on preoperative and postoperative telecobalt therapy in gastric cancer. Nipon Acta Radiol. 1964;24:129.
191 Moertel CG, Childs DSJr, Reitemeier RJ, et al. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet. 1969;2:865.
192 Holbrook MA. Radiation therapy. Current concepts in cancer. Gastric cancer. Treatment principles. JAMA. 1974;228:1289.
193 Schein PS. Novak J, for the Gastrointestinal Tumor Study Group: Combined modality therapy (XRT-chemo) versus chemotherapy alone for locally unresectable gastric cancer. Cancer. 1982;49:1771.
194 Chevalier TL, Smith FP, Harter WK, Schein PS. Chemotherapy and combined modality therapy for locally advanced and metastatic gastric carcinoma. Semin Oncol. 1985;12:46.
195 The Gastrointestinal Tumor Study Group. The concept of locally advanced gastric cancer. Cancer. 1990;66:2324.
196 Bleiberg H, Goffin JC, Dalesie O, et al. Adjuvant radiotherapy and chemotherapy in resectable gastric cancer. Eur J Surg Oncol. 1989;15:535.
197 O’Connell MJ, Gunderson LL, Moertel CG, et al. A pilot study of intensive combined therapy for locally unresectable gastric cancer. Int J Radiat Oncol Biol Phys. 1985;11:1827.
198 Moertel CG, Gunderson LL, Malliard JA, et al. Early evaluation of combined fluorouracil and leucovorin as a radiation enhancer for locally unresectable, residual, or recurrent gastrointestinal carcinoma. J Clin Oncol. 1994;12:21.
199 Henning GT, Schild SF, Stafford SL, Gunderson LL. Results of irradiation or chemoirradiation for primary unresectable, locally recurrent or grossly incomplete resection of gastric adenocarcinomas. Int J Radiat Oncol Biol Phys. 2000;46:109-118.
200 Wilke H, Preusser P, Fink U, et al. Preoperative chemotherapy in locally advanced or non-resectable gastric cancer. A phase II study with etoposide, doxorubicin, and cisplatin. J Clin Oncol. 1989;7:1318.
201 Verschueren R, Willemse P, Sleijfer D, et al. Combined chemotherapeutic-surgical approach of locally advanced gastric cancer. Proc Am Soc Clin Oncol. 1988;7:355.
202 Ducreux M, Rougier P, Lasser P, et al. Neoadjuvant chemotherapy in locally advanced gastric carcinoma. Does it increase long-term survival? Proc Am Soc Clin Oncol. 1993;12:670.
203 Kang YK, Choi DW, Kim CM, et al. The effect of neoadjuvant chemotherapy on the surgical outcome of locally advanced gastric adenocarcinoma. Interim report of a randomized controlled arm. Proc Am Soc Clin Oncol. 1992;11:505.
204 Kang YK, Choi DW, Im YH, et al. A phase III randomized comparison of neoadjuvant therapy followed by surgery versus surgery for locally advanced stomach cancer. Proc ASCO. 1996;15:215.
205 Rougier PH, Mahjoubi M, Lasser PH, et al. Neoadjuvant chemotherapy in locally advanced gastric carcinoma—a phase II trial with combined continuous intravenous 5-fluorouracil and bolus cisplatin. Eur J Cancer. 1994;30A:1269-1275.
206 Ajani JA, Ota DM, Jessup JM, et al. Resectable gastric carcinoma. An evaluation of preoperative and postoperative chemotherapy. Cancer. 1991;68:1501.
207 Ajani JA, Mayer RJ, Ota DM, et al. Preoperative and postoperative combination chemotherapy for potentially resectable gastric carcinoma. J Natl Cancer Inst. 1993;85:1839.
208 Schwartz G, Kelsen D, Christman K, et al. A phase II study of neoadjuvant FAMTx (5-fluorouracil, Adriamycin, methotrexate) and postoperative intraperitoneal 5-FU and cisplatin in high risk patients with gastric cancer. Proc Am Soc Clin. 1993;12:572.
209 Kelsen D, Karpeh M, Schwartz G, et al. Neoadjuvant therapy of high risk gastric cancer. A phase II trial of preoperative FAMTx and postoperative intraperitoneal fluorouracil-cisplatin plus intravenous fluorouracil. J Clin Oncol. 1996;14:1818-1828.
210 Leichman L, Silberman H, Leichman CG. Preoperative systemic chemotherapy followed by adjuvant postoperative intraperitoneal therapy for gastric cancer. A University of Southern California pilot program. J Clin Oncol. 1992;10:1933.
211 Crookes P, Leichman GC, Leichman L, et al. Systemic chemotherapy for gastric carcinoma followed by postoperative intraperitoneal therapy. A final report. Cancer. 1997;79:1767-1775.
212 Fink U, Schumacher C, Stein HJ, et al. Preoperative chemotherapy for stage III-IV gastric carcinoma. Feasibility, response and outcome after complete resection. Br J Surg. 1995;82:1248-1275.
213 Songun I, Keizer HJ, Hermans J, et al. Chemotherapy for operable gastric cancer. Results of the Dutch randomised FAMTX trial. The Dutch Gastric Cancer Group (DGCG). Eur J Cancer. 1999;35:558.
214 Yano M, Shiozaki H, Inoue M, et al. Neoadjuvant chemotherapy followed by salvage surgery. Effect on survival of patients with primary noncurative gastric cancer. World J Surg. 2002;26:1155.
215 Preusser P, Wilke H, Achterrath W, et al. Phase II study with the combination etoposide, doxorubicin, and cisplatin in advanced measurable gastric cancer. J Clin Oncol. 1989;7:1310-1317.
216 Wilke H, Preusser P, Fink U, et al: Neoadjuvant chemotherapy of primarily unresectable gastric cancer. In Proceedings of the International Conference on Biology and Treatment of Gastrointestinal Malignancies, Frankfurt, Germany, 1992.
217 Smith JW, Brennan MF, Botet JF, et al. Preoperative endoscopic ultrasound can predict the risk of recurrence after operation for gastric carcinoma. J Clin Oncol. 1993;11:2380.
218 Ajani JA, Mansfield PF, Janjan N, et al. Preoperative chemoradiation therapy in patients with potentially resectable gastric carcinoma. A multi-institutional pilot. Proc ASCO. 1998;17:283a.
219 Ell C, Hochberger J, May A, et al. Coated and uncoated self-expanding metal stents for malignant stenosis in the upper GI tract. Preliminary clinical experiences with wall stents. Am J Gastroenterol. 1994;89:1496-1500.
220 Gastrointestinal Tumor Study Group. Phase II-III chemotherapy studies in advanced gastric cancer. Cancer Treat Rep. 1979;63:1871.
221 Moertel CG, Lavin PT. Phase II-III chemotherapy studies in advanced gastric cancer. Cancer Treat Rep. 1979;63:1863.
222 Schein PS, Smith FP, Woolley PV, Ahlgren JD. Current management of advanced and locally unresectable gastric carcinoma. Cancer. 1982;50:2590.
223 Comis RL, Carter SK. Integration of chemotherapy into combined modality treatment of solid tumors. III. Gastric cancer. Cancer Treat Rev. 1974;1:221.
224 MacDonald JS, Havlin KA. Etoposide in gastric cancer. Semin Oncol. 1992;19:59.
225 MacDonald JS, Schein PS, Woolley PV, et al. 5-Fluorouracil, mitomycin C and Adriamycin (FAM). A new combination chemotherapy program for advanced gastric carcinoma. Ann Intern Med. 1980;93:533.
226 Kelsen D. The use of chemotherapy in the treatment of advanced gastric cancer and pancreatic cancer. Semin Oncol. 1994;21(Suppl 7):58-66.
227 Sulkes A, Cavalli F, van Oosterom A, et al. Taxotere is active in advanced gastric carcinoma. Results of a phase II clinical trial. Eur J Cancer. 1993;23A(Suppl 6):S101.
228 Kambe M, Wakui A, Nakao I, et al. A late phase II study of irinotecan (CPT-11) in patients with advanced gastric cancer. Proc Am Soc Clin Oncol. 1993;12:198.
229 Kovach JS, Moertel CG, Schutt AJ. A controlled study of combined 1.3-bis-2-chloroethyl-nitrosourea and 5-fluorouracil therapy for advanced gastric and pancreatic cancer. Cancer. 1974;33:563.
230 The Gastrointestinal Tumor Study Group. A comparative clinical assessment of combination of chemotherapy in the management of advanced gastric carcinoma. Cancer. 1982;49:1362.
231 Levi JA, Dalley DN, Aronev RS. Improved combination chemotherapy in advanced gastric cancer. BMJ. 1979;2:1471.
232 Moertel CG, Rubin J, O’Connell MJ, et al. A phase II study of combined 5-fluorouracil, doxorubicin, and cisplatin in the treatment of advanced upper gastrointestinal adenocarcinomas. J Clin Oncol. 1986;4:1053.
233 Rougier P, Droz JP, Theodore C, et al. A phase II trial of combined 5-fluorouracil plus doxorubicin plus cisplatin (FAP regimen) in advanced gastric carcinoma. Cancer Treat Rep. 1987;71:1301.
234 Woolley PV, Smith F, Estevez R. A phase II trial of 5-FU, Adriamycin, and cisplatin (FAP) in advanced gastric carcinoma. Proc Am Soc Clin. 1981;22:455.
235 Wils JA, Klein HO, Wagener DJT, et al. Sequential high-dose methotrexate and fluorouracil combined with doxorubicin—a step ahead in the treatment of advanced gastric cancer. A trial of the European Organization for Research and Treatment of Cancer. J Clin Oncol. 1991;9:827.
236 Kelsen D, Atiq O, Saltz L, et al. FAMTx versus etoposide, doxorubicin, and cisplatin. A random assignment trial in gastric cancer. J Clin Oncol. 1992;10:541-548.
237 Wilke H, Preusser P, Stahl M, et al. Etoposide, folinic acid, and 5-fluorouracil in carboplatin-pretreated patients with advanced gastric cancer. Cancer Chemother Pharmacol. 1991;29:83.
238 Cullinan S, Moertel CG, Fleming T, et al. A randomized comparison of 5-FU alone (F), 5-FU + Adriamycin (FA), and 5-FU + Adriamycin + mitomycin C (FAM) in gastric and pancreatic cancer. Proc Am Soc Clin Oncol. 1984;3:137.
239 Kim NK, Park YS, Heo DS, et al. A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer. 1993;71:3813.
240 Findlay M, Cunningham D. Chemotherapy of carcinoma of the stomach. Cancer Treat Rev. 1993;19:29.
241 Delasi V, Coconi G, Tonato M, et al. Randomized comparison of 5-FU alone or combined with carmustine, doxorubicin, and mitomycin (BAFM1) in the treatment of advanced gastric cancer. A phase III trial of the Italian Clinical Research Oncology Group (GOIRC). Cancer Treat Rep. 1986;70:481.
242 Machover D, Goldschmidt E, Chollet P, et al. Treatment of advanced colorectal and gastric adenocarcinomas with 5-fluorouracil and high-dose folinic acid. J Clin Oncol. 1986;4:685.
243 Wils JA. Perspectives in chemotherapy of advanced gastric cancer. Anti-Cancer Drugs. 1991;2:133.
244 Klein HO. Long-term results with FAMTx (5-fluorouracil, Adriamycin, methotrexate) in advanced gastric cancer. Anticancer Res. 1989;9:1025-1026.
245 Stahl M, Wilke H, Preusser P, et al. Etoposide, leucovorin and 5-fluorouracil (ELF) in advanced gastric carcinoma—final results of a phase II study in elderly patients or patients with cardiac risk. Onkologie. 1991;14:314-318.
246 Van Cutsem E, Filez L, Dewyspelaere J, et al. Etoposide, leucovorin and 5-fluorouracil in advanced gastric cancer. A phase II study. Anticancer Res. 1995;15:1079.
247 Chiou TJ, Tung SL, Hsieh RK, et al. Phase II study of the modified regimen of etoposide, leucovorin and 5-fluorouracil for patients with advanced gastric cancer. Jpn J Clin Oncol. 1998;28:318.
248 Au E, Koo WH, Tan EH, et al. A phase II trial of etoposide, leucovorin and 5-fluorouracil (ELF) in patients with advanced gastric cancer. J Chemother. 1996;8:300.
249 Wilke H, Wils J, Rougier P, et al. Preliminary analysis of a randomized phase III trial of FAMTx versus ELF versus cisplatin/FU in advanced gastric cancer. A trial of the EORTC Gastrointestinal Tract Cancer Cooperative Group and the AIO (Arbeitsgemeinschaft Internistische Onkologie). Proc ASCO. 1995;14:206.
250 Cascinu S, Labianca R, Alessandroni P, et al. Intensive weekly chemotherapy for advanced gastric cancer using fluorouracil, cisplatin, epi-doxorubicin, 6s-leucovorin, glutathione, and filgrastim. A report from the Italian Group for the Study of Digestive Tract Cancer. J Clin Oncol. 1997;15:3313-3319.
251 Cocconi G, Carlini P, Gamboni A, et al. Cisplatin, epirubicin, leucovorin and 5-fluorouracil (PELF) is more active than 5-fluorouracil, doxorubicin and methotrexate (FAMTX) in advanced gastric cancer. Ann Oncol. 2003;14:1258.
252 Findlay M, Cunningham D, Scarffe JH, et al. A phase II study in advanced gastroesophageal cancer using epirubicin and cisplatin in combination with continuous infusion 5-fluorouracil (ECF). Ann Oncol. 1994;5:609-616.
253 Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261-267.
254 Cunningham D, Rao S, Starling T, et al. Randomised multicentre phase III study comparing capecitabine with fluorouracil and oxaliplatin with cisplatin in patients with advanced oesophagogastric cancer: The REAL 2 trial (abstract LBA4017). J Clin Oncol. 2006;24:183s.
255 Ridwelski K, Gebauer T, Fahlke J, et al. Combination chemotherapy with docetaxel and cisplatin for locally advanced and metastatic gastric cancer. Ann Oncol. 2001;12:47.
256 Kornek GV, Raderer M, Schull B, et al. Effective combination chemotherapy with paclitaxel and cisplatin with or without human granulocyte colony-stimulating factor and or erythropoietin in patients with advanced gastric cancer. Br J Cancer. 2002;86:1858.
257 Yamao T, Shirao K, Matsumura Y, et al. Phase I-II study of irinotecan combined with mitomycin-C in patients with advanced gastric cancer. Ann Oncol. 2001;12:1729.
258 Park KW, Ahn JS, Park YS, et al. Phase II study of docetaxel and cisplatin combination chemotherapy in metastatic gastric cancer. Cancer Chemother Pharmacol. 2007;59:17.
259 Schull B, Kornek GV, Schmid K, et al. Effective combination chemotherapy with bimonthly docetaxel and cisplatin with or without hematopoietic growth factor support in patients with advanced gastroesophageal cancer. Oncology. 2003;65:211.
260 Saitoh S, Sakata Y. Docetaxel and cisplatin in patients with advanced gastric cancer. Results of Japanese phase I/II study. Gastric Cancer. 2002;5(Suppl 1):23.
261 Ridwelski K, Gebauer T, Fahlke J, et al. Combination chemotherapy with docetaxel and cisplatin for locally advanced and metastatic gastric cancer. Ann Oncol. 2001;12:47.
262 Oh DY, Kim TY, Kwon JH, et al. Docetaxel + 5-fluorouracil + cisplatin 3-day combination chemotherapy as a first-line treatment in patients with unresectable gastric cancer. Jpn J Clin Oncol. 2005;35:380.
263 Park SR, Chun JH, Kim YW, et al. Phase II study of low-dose docetaxel/fluorouracil/cisplatin in metastatic gastric carcinoma. Am J Clin Oncol. 2005;28:433.
264 Ajani JA, Fodor MB, Tjulandin SA, et al. Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol. 2005;23:5660.
265 Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer. A report of the V325 study group. J Clin Oncol. 2006;24:4991.
266 Murad AM, Santiago FF, Petroianu A, et al. Modified therapy with 5-fluorouracil, doxorubicin and methotrexate in advanced gastric cancer. Cancer. 1993;72:37.
267 Pyrhonen S, Kuitunen T, Nyandoto P, et al. Randomized comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995;71:587.
268 Glimelius B, Ekstrom K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163.
269 Park JO, Chung HC, Cho JY, et al. Retrospective comparison of infusional 5-fluorouracil, doxorubicin, and mitomycin-C (modified FAM) combination chemotherapy versus palliative therapy in treatment of advanced gastric cancer. Am J Clin Oncol. 1997;20:484.
270 Wagner AD, Unvergzagt S, Grothe W, et alChemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 17(3):CD004064, 2010.
271 Tepper JE, Gunderson LL. Radiation treatment parameters in the adjuvant postoperative therapy of gastric cancer. Semin Radiat Oncol. 2002;12:187-195.
272 Gunderson LL, Martenson JA. Gastrointestinal tract radiation tolerance. In: Vaeth JM, Meyer JL, editors. Radiation Tolerance of Normal Tissues, vol 23. Basel: Karger; 1989:277.
273 Willett CG, Tepper JE, Orlow EL, Shipley WU. Renal complications secondary to radiation treatment of upper abdominal malignancies. Int J Radiat Oncol Biol Phys. 1986;12:1601.
274 Wieland P, Dobler B, Mai S, et al. IMRT for postoperative treatment of gastric cancer. Covering large target volumes in the upper abdomen. A comparison of a step-and-shoot and an arc therapy approach. Int J Radiat Oncol Biol Phys. 2004;59:1236-1244.
275 Ringash J, Perkins F, Brierley J, et al. IMRT for adjuvant radiation in gastric cancer. A preferred plan? Int J Radiat Oncol Biol Phys. 2005;63:732-738.
276 Milano MT, Garofalo MC, Chmura SJ, et al. Intensity-modulated radiation therapy in the treatment of gastric cancer. Early clinical outcome and dosimetric comparison with conventional techniques. Br J Radiol. 2006;79:497-503.
277 Chung HT, Lee B, Park E, et al. Can all centers plan intensity-modulated radiotherapy (IMRT) effectively? An external audit of dosimetric comparisons between three-dimensional conformal radiotherapy and IMRT for adjuvant chemoradiation for gastric cancer. Int J Radiat Oncol Biol Phys. 2008;71:1167-1174.
278 Alani S, Soyfer V, Strauss N, et al. Limited advantages of intensity-modulated radiotherapy over 3D conformal radiation therapy in the adjuvant management of gastric cancer. Int J Radiat Oncol Biol Phys. 2009;74:562-566.
279 Dahele M, Skinner M, Schultz B, et al. Adjuvant radiotherapy for gastric cancer. A dosimetric comparison of 3-dimensional conformal radiotherapy, tomotherapy and conventional intensity modulated radiotherapy treatment plans. Med Dosim. 2010;35:115-121.
280 Callister MD, Gunderson LL. Advancements in radiation techniques for gastric cancer. J Natl Comp Cancer Network. 2010;8:428-435.
281 Wysocka B, Kassam Z, Lockwood G, et al. Interfraction and respiratory organ motion during conformal radiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys. 2010;77:53-59.
282 Fuchs C, Fitzgerald TJ, Mamon H, et al. Postoperative adjuvant chemoradiation for gastric or gastroesophageal adenocarcinoma using epirubicin, cisplatin and infusional 5-fluorouracil (ECF) before and after 5-FU and radiotherapy. A multicenter pilot study. 2003 ASCO Proceedings. J Clin Oncol. 2003;22(Suppl):257.
283 Fuchs C, Tepper J, Niedzwiecki D, et al. Postoperative adjuvant chemoradiation for gastric or gastroesophageal junction (GEJ) adenocarcinoma using epirubicin, cisplatin, and infusional (CI) 5-FU (ECF) before and after CI 5-FU and radiotherapy (CRT) compared with bolus 5-FU/LV before and after CRT: Intergroup trial CALGB 80101. J Clin Oncol. 2011;29(15S):256.
284 Gunderson LL, Callister MD, Jaroszewski DE, et al. Localized gastric or gastroesophageal cancer—chemoradiation is a pertinent component of adjuvant treatment for patients at high risk of relapse. Gastroint Cancer Res. 2009;3(Suppl):24-30.

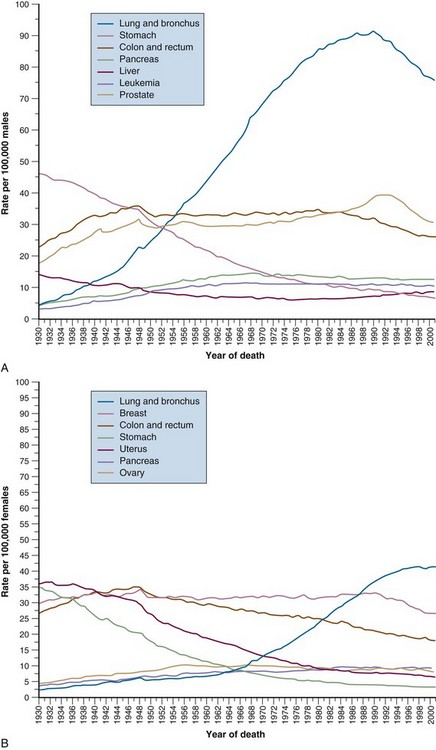
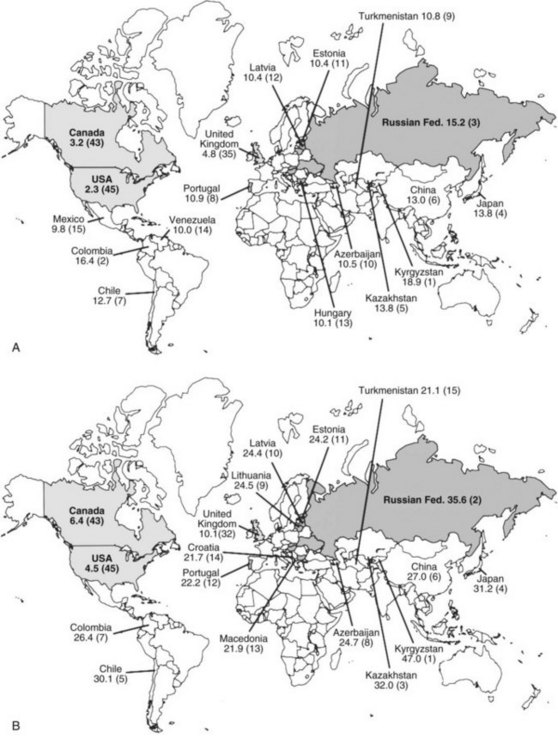
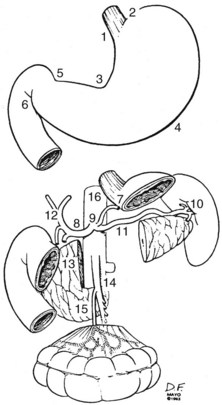
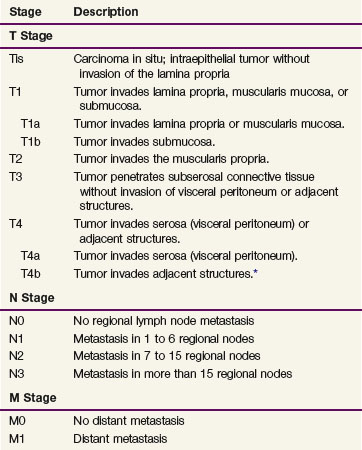
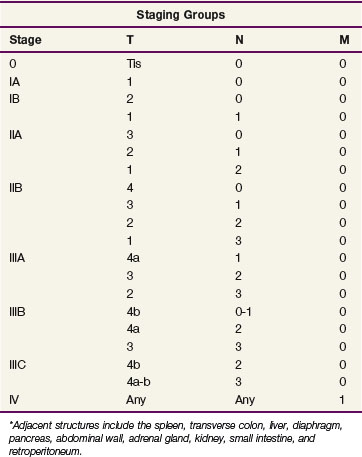
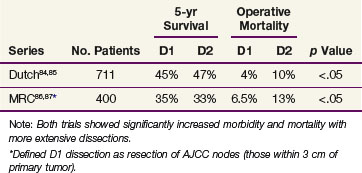
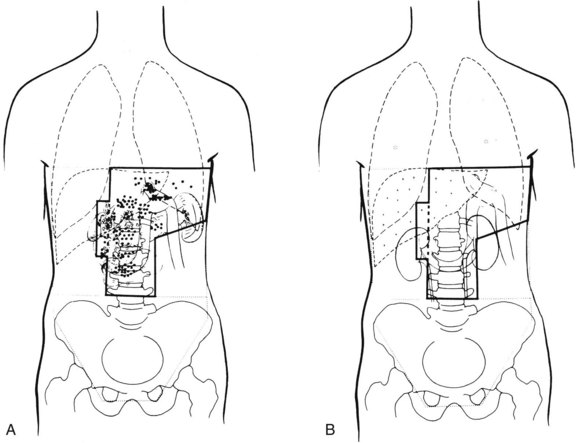
 , lymph node failures; *, lung metastases; +, liver metastases.
, lymph node failures; *, lung metastases; +, liver metastases.