Chapter 107 Gamma Surgery for Functional Disorders
Gamma Surgery for Pain
The lack of anatomic and pathophysiologic background knowledge of the mechanisms of pain makes management of pain by open or closed stereotactic techniques largely unsatisfactory. Early results using gamma surgery to produce thalamotomies for pain control were published by Steiner et al.1 All 52 patients treated suffered from terminal cancer and were treated prior to the advent of computed tomography (CT) or magnetic resonance imaging (MRI). Pneumoencephalography was used to target the thalamic centrum medianum-parafasciculus (CM-Pf) complex. Good pain relief was obtained in 8 patients and moderate pain relief in 18. The patients had in general only temporary relief of pain. Of those with good pain relief, 5 died without recurrence of pain between 1 and 13 months after the procedure, and 3 had recurrence of pain at 3, 6, and 9 months. Doses between 100 and 250 Gy were tested. The collimators used were 3 × 5 and 3 × 7 mm. Observation of an actual lesion was only possible in 21 of 36 patients who had a postmortem examination. Not surprisingly, the presence of a lesion was associated with relief. Lesions were only reliably created with doses greater than 160 Gy. The most effective lesions were more medially located near the wall of the third ventricle, and the greatest relief was for face or arm pain.
These early results were not very encouraging. However, with improvements in neuroimaging and alternate target selection, it is possible that more effective lesions can be produced. Recent reports seem to support this expectation. Hayashi et al. reported significant pain reduction in patients with severe cancer pain and post-stroke thalamic pain after gamma knife lesioning of the hypophysis.2,3 Using the 4-mm collimator and doses of 140 to 180 Gy, Young et al. have published effective pain relief in patients with chronic, intractable pain following medial thalamotomy with the gamma knife.4,5 In a series of 15 patients followed for more than 3 months after a radiosurgical medial thalamotomy, 4 (27%) were pain free, and 5 others (33%) had greater than 50% pain relief.4 Additional investigation must be conducted before the role of the gamma knife for pain treatment can be fully defined.
Gamma Surgery for Trigeminal Neuralgia
The first time radiosurgery was used to treat trigeminal neuralgia, Leksell treated two patients with the stereotactic technique using orthovoltage x-rays.16 The patients treated with this method were followed up for 17 years, during which time both remained pain free. With the introduction of the gamma knife, a series of 46 patients were treated in Stockholm with less encouraging results.6 The target in these cases was the Gasserian ganglion, and targeting was by bony landmarks or cisternography. In the first 24 patients, where stereotactic cranial x-rays were used for targeting, 33% of patients were pain free at 6 months and 8.3% at a mean follow-up of 26 months. Transoral cisternography with tantalum dust suspended in glycerol was used in a group of 22 patients and 59% of the patients became pain free at 6 months and 18% at 26 months. A later report by Lindquist et al. stated that approximately 50% of the patients initially became pain free, but neuralgia recurred for most of them several years after radiosurgery.7 With advances in neuroimaging, most notably MRI, gamma surgery for trigeminal neuralgia was revisited. However, the focus of treatment shifted from the ganglion to the nerve root entry zone or the cisternal segment of trigeminal nerve.8
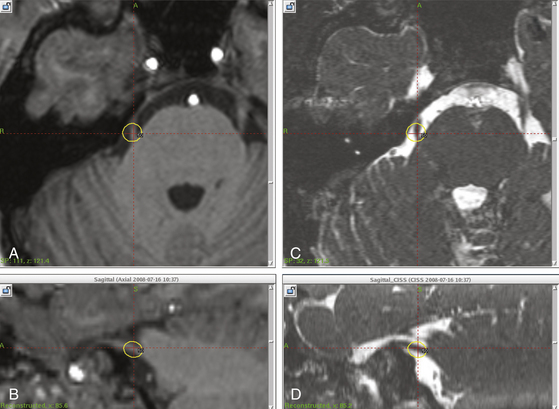
A number of centers have since shown the safety and at least short-term pain relief with gamma surgery for trigeminal neuralgia (Fig. 107-1). Maesawa et al. reported complete pain relief without medication in 47.7% of patients at the initial follow-up and in 40% of patients at the last follow-up in 220 patients with a median follow-up of 22 months (range 6–78 months).9 In a series of 117 consecutive patients who were followed up for an average of 26 months (range 1–48 months), Pollock et al. reported an actuarial rate of freedom from pain without medication in 57% and 55% of patients at 1 and 3 years, respectively.10 Tawk et al. in a series of 38 patients followed up for a median of 24 months observed pain relief without medication in 44% at the 3-month evaluation but in only 16% of patients at the 24-month follow-up visit.11 Regis et al. reported a series of 100 patients with a minimum follow-up of 12 months. Fifty-eight patients were pain free without medication.8
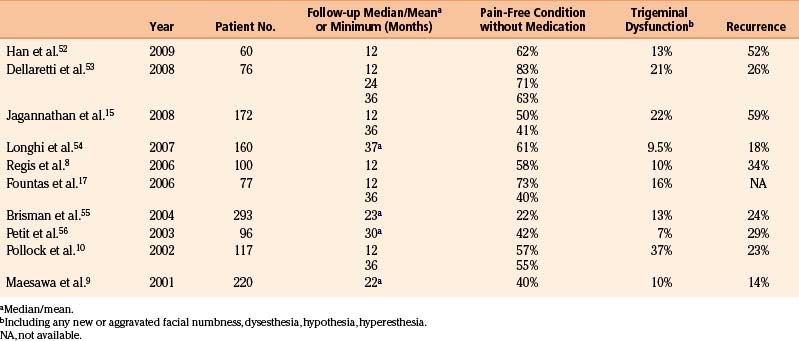
The reported rates of recurrence following radiosurgery for trigeminal neuralgia have ranged from 5% to 42% and probably are related to incomplete radiation effects on the targeted tissues (Table 107-1).
Pain free outcomes were usually achieved within several weeks after the gamma procedure. Fountas et al. reported that most patients with no previous surgeries responded within 4 weeks after treatment.12 Pollock et al. reported that complete pain relief occurred within a median of 3 weeks (range 1–20 weeks).10 According to Regis et al., initial pain relief occurred after a median delay of 10 days (range 2–5 weeks) in 94% of patients.8
The incidence of new trigeminal dysfunction varies in the literature from 6% to 66%. Pollock et al. reported new trigeminal dysfunction in 43 patients (37%) and demonstrated an association between greater radiation doses and the risk of trigeminal nerve dysfunction—90 Gy as a maximum dose caused numbness in 50% of patients, whereas in only 15% of patients who received 70 Gy.13 They also reported a strong correlation between the development of new facial sensory loss and achievement and maintenance of pain relief—excellent pain-free outcome 4.5 times more likely in patients with new trigeminal deficits compared with those who had normal postradiosurgical trigeminal nerve function. Regis et al. reported that six patients in their series of 100 cases presented with facial paresthesia and four with facial hyperesthesia.8 In Young’s series, facial numbness occurred in 12%, 20%, and 29% of cases when a maximum dose of 70, 80, and 90 Gy, respectively, was used.14
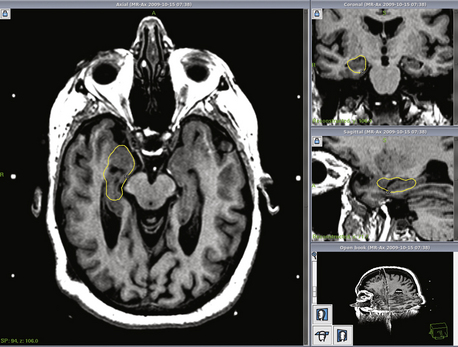
At the University of Virginia, we have recently reviewed our treatment of 170 cases of typical trigeminal neuralgia with gamma surgery.15 There were 67 males and 93 females with a mean age of 63.8 years (range 23–95 years). Prior to gamma surgery, 24 patients had prior microvascular decompression, 44 had one or more glycerol injections, radio-frequency thermocoagulation, balloon compression or neurectomy prior to radiosurgery. In each case, the radiosurgical target chosen was 2 to 4 mm anterior to the entry zone of the trigeminal nerve into the pons. A prescription dose of between 30 and 45 Gy to the 50% isodose line was used. The maximum dose ranged from 60 to 90 Gy (median 80 Gy). Excellent outcome was defined as complete pain-free condition without medication. Pain relief of a different degree with or without medication was considered palliative outcome. Follow-up ranged from 6 months to 12 years (mean 4.2 years) (Fig. 107-2).
Thirty-eight patients (22%) developed postradiosurgical facial numbness. Two patients considered the numbness to be worse than the original facial pain. The fact that they did not have pain relief after gamma surgery presumably contributed to their dissatisfaction. We also observed the association of facial numbness with higher rate of pain free outcome as reported by Pollock et al.13 Forty-five patients (26.4%) including 32 who were pain free without medication and 13 who had palliative results after gamma surgery eventually had a recurrence of pain at some point during the follow-up. Twenty-three patients underwent a second gamma procedure. Of 17 patients who were completely pain free for two to 60 months following the initial procedure, 12 became pain free without medication again after retreatment. Four of six patients who had only palliative outcome after the initial procedure became pain free without medication following the second treatment.
Gamma Surgery for Movement Disorders
Thalamotomy for tremor in Parkinson’s disease remains one of the most gratifying procedures in functional neurosurgery and defends its place in the therapeutic armory for those common cases in which drugs fail to stop the tremor. To avoid the potential risks of open thalamotomies, the prototype of the gamma knife was used by Leksell16 to produce thalamic lesions in five cases of tremor between 1968 and 1970. At that time, the intended target could not be visualized but was indirectly determined by using derived coordinates relative to the anterior and posterior commisures visualized by pneumoencephalography. Verification that a lesion had been produced could not be obtained because neither CT nor MRI was available. The fixation of the head of the patient for the radiosurgical procedure was also unsatisfactory, because the stereotactic frame used for target localization was too large to fit into the collimator helmet. Instead, fixation devices were applied onto a plaster-of-Paris helmet previously molded on the patient’s head. It is therefore not surprising that beneficial results were lacking.
In 1986, MRI was introduced at Karolinska Hospital, and better anatomic visualization of the target volume became possible. A new stereotactic frame compatible with MRI that also served as the fixation device in the gamma knife was introduced.16 These improvements paved the way for new attempts to relieve Parkinsonian tremor by gammathalamotomy, and two cases were treated using this improved methodology.7 The first procedure was performed using an 8-mm collimator, and the volume of the resulting lesion was much larger than intended (1.5 cm3). The tremor began to dwindle after 2 months, but a transient hemiparesis and mild speech disturbance ensued secondary to edema. The eventual outcome was, however, satisfactory, and 4 years after the treatment the patient returned free of tremor contralateral to the side of the thalamic lesion, asking for a second procedure to stop the tremor that had developed on the other side. The second patient was treated using a 4-mm collimator, which gave a smaller volume to the thalamic lesion. In this case, the clinical result was not satisfactory. The patient was treated a second time without relief of tremor. It is not clear whether the lack of effect was due to the atypical clinical picture in this patient or to the lack of physiologic corroboration of the target. In spite of experience from centers active in this field indicating that modern imaging techniques, especially MRI, may obviate the need for physiologic target definition, this assertion remains controversial.
Pioneering work in neuroanatomy and neurophysiology by Hirai, Jones, and Ohye has shed much light on the position, anatomic organization, and physiologic significance of the thalamus as it pertains to tremor, rigidity, and dyskinesia.17–19 MRI guidance for selective thalamotomy in the treatment of Parkinsonian or essential tremors has been well established. The correlations between neuroanatomic and electrophysiologic findings in the human ventrolateral thalamic nuclei (e.g., VLa, VLp, VPLa, VPLc) are better understood. For gamma surgery, the difficulty arises in identifying the VLp and VLa nuclei in the human thalamus purely by radiologic methods. As such, thin-slice MRI, Surgiplan, and a neuroanatomic atlas may be required to treat these nuclei with the gamma knife.
Ohye (C. Ohye, personal communications, 2004) has performed gammathalamotomies on 56 patients for Parkinson’s disease, 21 with essential tremor, and 6 with intention tremor. Thalamotomies were performed using a single 4-mm shot and 130-Gy dose. Follow-up MRI revealed two different types of thalamic changes. One type was a round, punched-out lesion with a volume of less than 100 mm3, and the other was an irregular high-signal zone (volume up to 800 mm3) that may extend into the internal capsule and streak along the border of the thalamus. The efficacy of the procedure did not seem to correlate with the type of postoperative imaging observed. Ohye noted improvements in tremor and/or rigidity in 85% of patients. Hirai (T. Hirai, personal communications, 2004) has treated 14 patients with gamma surgery for movement disorders. Of these 14 patients, 8 had tremor-dominant Parkinson’s disease, 4 rigidity and dyskinesia-dominant Parkinson’s disease, and 2 had essential tremors. Hirai’s target points were the VLp nucleus for control of tremor and the VLa nucleus for control of rigidity and dyskinesia. The maximum dose varied from 130 to 150 Gy; a single 4-mm isocenter was used to make each lesion. At the last follow-up, 13 out of 14 patients noted subjective improvement in their symptomatology. In 9 of these patients who had at least 1 year of follow-up, Hirai noted symptomatic improvement by 50% to 90% in the patients’ Unified Parkinson’s Disease Rating Scale for tremor, rigidity, and dyskinesia scores. On follow-up MRI, Hirai observed T2-weighted changes 3 months after gamma surgery, and these lesions gradually increased to 5 to 8 mm in diameter. In another series, Young et al. reported significant improvements in Unified Parkinson’s Disease Rating Scale tremor and rigidity scores in 74 of 102 Parkinson’s patients (73%) at 4 years or longer post–gamma knife thalamotomy; in 52 patients with essential tremor, 88.2% remained tremor free at 4 years or more postoperatively.20 Two patients had permanent hemiparesis and facial paresthesias. One patient experienced transient deficit.
In general the outcome of gamma pallidotomy is less satisfactory and a high risk of complication has been reported.18,21 The gamma knife was occasionally used to create a lesion in subthalamic nucleus, however, its efficacy and safety remain to be investigated.22
A significant change in the surgical management of movement disorders, particularly Parkinson’s disease, was the introduction of deep brain stimulation (DBS), which allows the amelioration of symptoms without a destructive lesion. Good results have been obtained with this technique, and, at present, DBS has supplanted destructive lesions as the surgical procedure of choice in most patients.23,24 The enthusiasm for deep brain stimulation may be lessened by the high rate of complications and cost.19,25–27 As the benefits and risks for DBS become better defined, neurosurgeons will be able to counsel patients and select the more appropriate neurosurgical tool (i.e., gamma knife or DBS).
Gamma Surgery for Obsessive-Compulsive Neurosis
Despite therapeutic progress in recent years, conventional treatment of anxiety disorders fails or has only a temporary effect in 20% of patients. These disorders are often severely disabling and are associated with rates of suicide comparable to those of depression. First described by Leksell,28 psychosurgery targeting the frontolimbic connections in both anterior internal capsules (capsulotomy) occasionally may be useful for selected severe cases. The first cases using the gamma knife to create the lesions were also performed by Leksell.
Mindus et al. at the Karolinska Institute29 reported the effects of such procedures on the anxiety symptoms and personality characteristics presented in conjunction with results of imaging studies performed by MRI and PET. The patient material comprised two series of patients with a 15-year mean duration of psychiatric illness, in all of whom various extensive treatment trials had previously been made. One series consisted of 24 patients subjected to capsulotomy by a conventional thermocoagulation technique and followed for 1 year. The other series comprised 7 patients treated by gamma surgery and followed for 7 years. The clinical effects of these treatments were evaluated subjectively by two independent observers and also rated on the Comprehensive Psychopathological Rating Scale (CPRS). Ratings were performed 10 days before and 2, 6, and 12 months after surgery. The effects on the personality were evaluated by the Karolinska Scales of Personality (KSP). These scales have been developed to measure traits related to frontal lobe dysfunction and to reflect different dimensions of anxiety proneness. At the 12-month follow-up, statistically and clinically significant improvement was noted in all assessments of symptomatic and psychosocial function. Freedom from symptoms or considerable improvement was noted in 79% of patients, and none were worse after the operation. At the 1-year follow-up, seven patients reported fatigue, four poor memory, two carelessness, and two long-windedness. Behind these numbers are numerous examples of dramatic improvements in individual lifestyles. A number of patients were preoperatively unable to work or function socially owing to such problems as preoccupation with personal cleanliness and the inability to use public transportation, with resulting domestic confinement, aggravated psychological problems, deterioration of family relationships, and devastation of personal economy. Postoperatively, these patients could return to their previous occupation and to a normal social function. The results of gamma capsulotomy were found to be comparable to those of capsulotomy performed by the thermocoagulation technique. Only in five of the seven patients could a lesion be demonstrated by MRI, and those were the patients who benefited from the procedure. The lowest effective target dose was 160 Gy, whereas 100, 120, and 152 Gy failed to produce lesions.
Ruck et al. in 2008 reported 25 obsessive-compulsive disorder (OCD) patients undergoing gamma- or thermocapsulotomy between 1988 and 2000 at the Karolinska Institute.30 Among these patients were nine cases treated with bilateral gamma capsulotomy with long-term follow-up. Two hundred Gy with three 4-mm shots were used in five cases and 180 Gy with a single 4-mm shot at each side were performed in five (two of them had repeat bilateral gamma capsulotomy and one had retreatment with thermocapsulotomy). The mean Yale-Brown Obsessive-Compulsive Scale used to rate OCD severity decreased significantly from 33.4 preoperatively to 17 at 1-year follow-up and 14.2 at long-term follow-up. Fifty percent of patients display signs of apathy and dysexecutive function behavior. Cases showed these neurologic deficits were those who received high radiation doses or underwent a repeat procedure. There was no difference in outcome between patients who underwent gamma or thermocapsulotomy and the incidence of apathy and executive dysfunction were similar in both groups. The authors suggested that a small lesion would achieve an adequate outcome with fewer complications.
Gamma capsulotomy offers several important clinical advantages over capsulotomy via an open technique.31 The most important is patient tolerance. This psychologically vulnerable group of patients seems to be much more willing to undergo a closed stereotactic procedure in contrast to open surgery. Theoretically, the gradual development of the radiolesion may also allow the patient better psychological adjustment to his new situation. The psychological rehabilitation phase is an important part of any psychosurgical procedure.
If it would be ethically acceptable, a control group of patients could be subjected to a sham gamma treatment. In a later stage, if this sham procedure is proven to give no result in comparison with the real procedure, the patients would receive the appropriate gamma treatment. Such a controlled study would probably be necessary before the real effect of gamma capsulotomy can be established. Further efforts should also be made to study the biology of the developing lesions. Important questions are: When does the functional effect of the radiation start and what are the characteristics of the MRI and CT images at this time? Even the issue of dose–volume relationships needs to be addressed further. PET or SPECT imaging may help to answer some of these questions, and pre- and post-treatment evaluation should be carried out before further series of patients are treated. The experience from multiple centers suggests a degree of optimism for the use of radiosurgery in the treatment of intractable OCD, and future research in psychiatric neurosurgery is proceeding in a cautious fashion.32 Any such work necessitates the coordination and effort of a multidisciplinary team.
Gamma Surgery for Epilepsy
When the gamma knife was installed in Stockholm, Leksell always invited his colleagues and pupils to suggest indications for using the “gadget.” None of them mentioned epilepsy as a possible indication. The interest in using gamma surgery for epilepsy was triggered by an early report that seizures were alleviated in a series of arteriovenous malformation (AVM) cases. In 59 of the 247 AVM patients with seizure as the presenting symptom treated by Steiner et al. using gamma surgery between 1970 and 1984, the treatment resulted in relief of some or all seizures in 52 of these patients.33 Eleven were successfully taken off anticonvulsant medication. Interestingly, in three patients seizure disorder symptoms were eliminated, although the AVM itself was unaffected by the radiation. These observations and the observations made by others34–36 prompted the idea of testing focal irradiation as a treatment modality for focal epilepsy.
At the University of Virginia, basic science research was done on changes in neuroexcitability after irradiation. The hippocampal slices from rats treated with the gamma knife were found to have a higher seizure threshold than those of controls when placed in solutions of varying concentrations of penicillin. This effect was lost at high concentrations (S.L. Henson, personal communication, 2001). Using single doses of either 20 or 40 Gy to the hippocampus in a rat model of chronic spontaneous limbic epilepsy, a reduction in both the frequency and duration of spontaneous seizures was observed.37 Histologic evaluation of the targeted region revealed no signs of necrosis and hippocampal slice recordings revealed intact synaptically driven neuronal firing.37 Subsequent work by the University of Pittsburgh group using a kainic acid–induced hippocampal epilepsy rat model revealed similarly efficacious results in terms of seizure control and the absence of behavioral impairment with subnecrotic doses of radiosurgery.38,39
Biochemical analysis of changes in rat brains after gamma surgery showed changes in the concentrations of excitatory and inhibitory amino acids, particularly gamma-amino butyric acid (GABA).40 Warnke et al. showed that patients with low-grade astrocytomas and associated epilepsy had significant relief from seizures following interstitial radiosurgery. SPECT scanning showed a reduced number of GABA receptors prior to treatment in both the tumors and surrounding brain. Levels of these receptors increased following therapy.41 These early studies show that functional changes may occur at the cellular level without gross structural damage. The implications of this for functional neurosurgery are intriguing.
Epilepsy has been treated with the gamma knife at many centers but there have been few published long-term results. Barcia Salorio et al. treated 11 patients with idiopathic epilepsy. Preoperative invasive electrodiagnostic confirmation of the epileptogenic focus was performed and treatment was with low-dose (10–20 Gy) radiosurgery.34 Complete relief from seizures was obtained in four patients and significant reduction in seizure activity in five. The effect of the treatment was not seen for several months in most instances. Regis et al. reported the first radiosurgical amygadohippocampectomy in a case of mesial temporal lobe epilepsy (MTLE) treated with gamma surgery.42 They used 25 Gy given to the 50% isodose line. The patient was seizure free after the treatment. At 10 months a lesion was evident on both CT and MRI that conformed to the 50% isodose line (amygdala and hippocampus). Whether actual gross structural lesioning with this method is associated with better results or more complications is unknown. Further results of 25 patients with medically intractable MTLE treated by Regis et al. (2000) showed that of the 16 patients with more than 2 years of follow-up, 13 are seizure free and two are improved.43 In addition, they noted minimal morbidity (only three cases of nonsymptomatic visual field deficit) and no mortality associated with the gamma surgery.
The potential of a less invasive, nondestructive therapy to treat epilepsy prompted the creation of prospective European and a National Institutes of Health (NIH)–sponsored multicenter studies of gamma surgery for temporal lobe epilepsy. In the European study, three centers enrolled 21 patients with MTLE. The anterior parahippocampal cortex, the basal and lateral portions of the amygdala, and the anterior hippocampus were targeted, and patients received a mean dose of 24 Gy. At 2 years postradiosurgery, 65% of the patients were seizure free. However, nine patients developed visual field deficits, and five suffered transient side effects, including depression, headache, nausea, vomiting, and imbalance.44 The NIH sponsored study, in which our center also participated, was recently reported.45 Thirty patients were randomized to have gamma surgery with a prescription dose of 20 or 24 Gy targeting the amygdala, hippocampus, and parahippocampus. At the 3-year follow-up, 67% were free of seizure. Ten of 13 patients (76.9%) receiving the high dose and 10 of 17 patients (58.8%) receiving low dose were seizure free. “A wide range of responses on serial MRI” was reported; however, when followed up over time, the edema resolved. One patient did require an urgent anterior temporal resection due to headache and visual field defect caused by brain edema. Twenty-one patients experienced new headaches at least once during the follow-up and 15 developed superior quadrantanopsias similar to those observed after temporal lobectomy. Verbal memory impairment was noticed in four of 26 patients (15%). The authors concluded that radiosurgery for MTLE offers seizure remission rates comparable with those reported for open microsurgery and there are no major safety concerns.
The long-term outcomes of a series of 14 patients who underwent gamma surgery for MTLE reported by Vojtech et al. are not promising.46 A prescription dose of 18 Gy was used in six patients, 20 Gy in two, and 25 Gy in six. In the control group of seven patients, no patient was seizure free after a follow-up of 96 to 138 months. In the second group of seven patients, none of the patients became seizure free 40 to 105 months following radiosurgery and subsequently underwent microsurgical amygdalohippocampectomy. At 11 to 74 months following open surgery, four were seizure free, one had rare seizures, and one had nondisabling nocturnal seizures. Edema was observed in nine patients, of whom three presented signs of increased intracranial pressure. Two developed permanent visual field defects related to radiosurgery. Two had repeated psychotic episodes and two developed status epilepticus following radiosurgery. No significant memory changes occurred.
The safety and demonstrated short-term efficacy of gamma surgery for the treatment of epilepsies arising from space occupying lesions (e.g., low-grade gliomas, hypothalamic hamartomas, cavernous malformations, and arteriovenous malformations) make it an attractive option.47,48 However, the gamma knife’s long-term feasibility and effectiveness for MTLE need to be proved. Also, it is unclear what underlying mechanisms are responsible for amelioration of seizures following radiosurgery. Some have suggested a “neuromodulation” phenomenon following gamma surgery with accompanying glial cell reduction, stem cell migration, neuronal plasticity and sprouting, and biochemical changes.49 Rigorous scientific studies evaluating the cellular and subcellular mechanisms responsible for improvements in epilepsy post-gamma surgery are thus far lacking. Furthermore, the need for physiologic monitoring (e.g., depth electrodes, cortical grids) to determine conclusively the epileptogenic focus cannot be entirely discarded.
Conclusions
The indications and usefulness of gamma surgery for functional disorders are being defined more carefully with the passage of time. The advances of neuroimaging in the past several decades have offered the identification of discrete thalamic, subthalamic, and basal ganglia nuclei with acceptable confidence, and allowed the use of gamma surgery in a wider variety of functional disorders. This will require a better understanding of the relationship of anatomy and function as well as improved spatial definition of these nuclei. The ongoing developments of noninvasive physiologic monitoring will influence the development of gamma surgery for epilepsy.50,51 When everything is considered, one may contend that while gamma surgery may be used for functional diseases of the brain, the current efficacy of alternative methods significantly limits the role of gamma surgery in the management of these diseases.
Barbaro N.M., Quigg M., Broshek D.K., et al. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Ann Neurol. 2009;65:167-175.
Chen Z.F., Kamiryo T., Henson S.L., et al. Anticonvulsant effects of gamma surgery in a model of chronic spontaneous limbic epilepsy in rats. J Neurosurg. 2001;94:270-280.
Fountas K.N., Smith J.R., Lee G.P., et al. Gamma knife stereotactic radiosurgical treatment of idiopathic trigeminal neuralgia: long-term outcome and complications. Neurosurg Focus. 2007;23:E8.
Friehs G.M., Park M.C., Goldman M.A., et al. Stereotactic radiosurgery for functional disorders. Neurosurg Focus. 2007;23:E3.
Han J.H., Kim D.G., Chung H.T., et al. Long-term outcome of gamma knife radiosurgery for treatment of typical trigeminal neuralgia. Int J Radiat Oncol Biol Phys. 2009;75:822-827.
Hayashi M., Chernov M.F., Taira T., et al. Outcome after pituitary radiosurgery for thalamic pain syndrome. Int J Radiat Oncol Biol Phys. 2007;69:852-857.
Hayashi M., Taira T., Chernov M., et al. Role of pituitary radiosurgery for the management of intractable pain and potential future applications. Stereotact Funct Neurosurg. 2003;81:75-83.
Jagannathan J., Yen C.P., Steiner L. Gamma knife radiosurgery for idiopathic trigeminal neuralgia. Contemp Neurosurg. 2008;30:1-8.
Keep M.F., Mastrofrancesco L., Erdman D., et al. Gamma knife subthalamotomy for Parkinson disease: the subthalamic nucleus as a new radiosurgical target. Case report. J Neurosurg. 2002;97:592-599.
Lindquist C., Kihlstrom L., Hellstrand E. Functional neurosurgery—a future for the gamma knife? Stereotact Funct Neurosurg. 1991;57:72-81.
Longhi M., Rizzo P., Nicolato A., et al. Gamma knife radiosurgery for trigeminal neuralgia: results and potentially predictive parameters. Part I: idiopathic trigeminal neuralgia. Neurosurgery. 2007;61:1254-1260. discussion 1260-1261
Maesawa S., Kondziolka D., Dixon C.E., et al. Subnecrotic stereotactic radiosurgery controlling epilepsy produced by kainic acid injection in rats. J Neurosurg. 2000;93:1033-1040.
Maesawa S., Salame C., Flickinger J.C., et al. Clinical outcomes after stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2001;94:14-20.
Mori Y., Kondziolka D., Balzer J., et al. Effects of stereotactic radiosurgery on an animal model of hippocampal epilepsy. Neurosurgery. 2000;46:157-165. discussion 165-168
Pollock B.E., Phuong L.K., Foote R.L., et al. High-dose trigeminal neuralgia radiosurgery associated with increased risk of trigeminal nerve dysfunction. Neurosurgery. 2001;49:58-62. discussion 62-64
Pollock B.E., Phuong L.K., Gorman D.A., et al. Stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2002;97:347-353.
Regis J., Kerkerian-Legoff L., Rey M., et al. First biochemical evidence of differential functional effects following gamma knife surgery. Stereotact Funct Neurosurg. 1996;66(suppl 1):29-38.
Regis J., Metellus P., Hayashi M., et al. Prospective controlled trial of gamma knife surgery for essential trigeminal neuralgia. J Neurosurg. 2006;104:913-924.
Regis J., Rey M., Bartolomei F., et al. Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia. 2004;45:504-515.
Ruck C., Karlsson A., Steele J.D., et al. Capsulotomy for obsessive-compulsive disorder: long-term follow-up of 25 patients. Arch Gen Psychiatry. 2008;65:914-921.
Steiner L., Forster D., Leksell L., et al. Gammathalamotomy in intractable pain. Acta Neurochir (Wien). 1980;52:173-184.
Vojtech Z., Vladyka V., Kalina M., et al. The use of radiosurgery for the treatment of mesial temporal lobe epilepsy and long-term results. Epilepsia. 2009;50:2061-2071.
Young R.F., Jacques D.S., Mark R., et al. Gamma knife radiosurgery for treatment of trigeminal neuralgia: long-term results. Neurosurgery. 2001;49:533-534.
Young R.F., Jacques S., Mark R., et al. Gamma knife thalamotomy for treatment of tremor: long-term results. J Neurosurg. 2000;93(suppl 3):128-135.
Young R.F., Vermeulen S.S., Grimm P., et al. Gamma knife thalamotomy for the treatment of persistent pain. Stereotact Funct Neurosurg. 1995;64(suppl 1):172-181.
1. Steiner L., Forster D., Leksell L., et al. Gammathalamotomy in intractable pain. Acta Neurochir (Wien). 1980;52:173-184.
2. Hayashi M., Chernov M.F., Taira T., et al. Outcome after pituitary radiosurgery for thalamic pain syndrome. Int J Radiat Oncol Biol Phys. 2007;69:852-857.
3. Hayashi M., Taira T., Chernov M., et al. Role of pituitary radiosurgery for the management of intractable pain and potential future applications. Stereotact Funct Neurosurg. 2003;81:75-83.
4. Young R.F., Jacques D.S., Rand R.W., Copcutt B.R. Medial thalamotomy with the Leksell gamma knife for treatment of chronic pain. Acta Neurochir Suppl. 1994;62:105-110.
5. Young R.F., Vermeulen S.S., Grimm P., et al. Gamma knife thalamotomy for the treatment of persistent pain. Stereotact Funct Neurosurg. 1995;64(suppl 1):172-181.
6. Hakanson S. Transoval trigeminal cisternography. Surg Neurol. 1978;10:137-144.
7. Lindquist C., Kihlstrom L., Hellstrand E. Functional neurosurgery—a future for the gamma knife? Stereotact Funct Neurosurg. 1991;57:72-81.
8. Regis J., Metellus P., Hayashi M., et al. Prospective controlled trial of gamma knife surgery for essential trigeminal neuralgia. J Neurosurg. 2006;104:913-924.
9. Maesawa S., Salame C., Flickinger J.C., et al. Clinical outcomes after stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2001;94:14-20.
10. Pollock B.E., Phuong L.K., Gorman D.A., et al. Stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2002;97:347-353.
11. Tawk R.G., Duffy-Fronckowiak M., Scott B.E., et al. Stereotactic gamma knife surgery for trigeminal neuralgia: detailed analysis of treatment response. J Neurosurg. 2005;102:442-449.
12. Fountas K.N., Smith J.R., Lee G.P., et al. Gamma knife stereotactic radiosurgical treatment of idiopathic trigeminal neuralgia: long-term outcome and complications. Neurosurg Focus. 2007;23:E8.
13. Pollock B.E., Phuong L.K., Foote R.L., et al. High-dose trigeminal neuralgia radiosurgery associated with increased risk of trigeminal nerve dysfunction. Neurosurgery. 2001;49:58-62. discussion 62-64
14. Young R.F., Jacques D.S., Mark R., et al. Gamma knife radiosurgery for treatment of trigeminal neuralgia: long-term Results. Neurosurgery. 2001;49:533-534.
15. Jagannathan J., Yen C.P., Steiner L. Gamma knife radiosurgery for idiopathic trigeminal neuralgia. Contemp Neurosurg. 2008;30:1-8.
16. Leksell L., Lindquist C., Adler J.R., et al. A new fixation device for the Leksell stereotaxic system. Technical note. J Neurosurg. 1987;66:626-629.
17. Fountas K.N., Lee G.P., Smith J.R. Outcome of patients undergoing gamma knife stereotactic radiosurgery for medically refractory idiopathic trigeminal neuralgia: medical College of Georgia’s experience. Stereotact Funct Neurosurg. 2006;84:88-96.
18. Friehs G.M., Park M.C., Goldman M.A., et al. Stereotactic radiosurgery for functional disorders. Neurosurg Focus. 2007;23:E3.
19. Kondziolka D., Whiting D., Germanwala A., Oh M. Hardware-related complications after placement of thalamic deep brain stimulator systems. Stereotact Funct Neurosurg. 2002;79:228-233.
20. Young R.F., Jacques S., Mark R., et al. Gamma knife thalamotomy for treatment of tremor: long-term results. J Neurosurg. 2000;93(suppl 3):128-135.
21. Duma C.M. Movement disorder radiosurgery—planning, physics and complication avoidance. Prog Neurol Surg. 2007;20:249-266.
22. Keep M.F., Mastrofrancesco L., Erdman D., et al. Gamma knife subthalamotomy for Parkinson disease: the subthalamic nucleus as a new radiosurgical target. Case report. J Neurosurg. 2002;97:592-599.
23. Duff J., Sime E. Surgical interventions in the treatment of Parkinson’s disease (PD) and essential tremor (ET): medial pallidotomy in PD and chronic deep brain stimulation (DBS) in PD and ET. Axone. 1997;18:85-89.
24. Tasker R.R. Deep brain stimulation is preferable to thalamotomy for tremor suppression. Surg Neurol. 1998;49:145-153. discussion 153-154
25. Beric A., Kelly P.J., Rezai A., et al. Complications of deep brain stimulation surgery. Stereotact Funct Neurosurg. 2001;77:73-78.
26. Eskandar E.N., Flaherty A., Cosgrove G.R., et al. Surgery for Parkinson disease in the United States, 1996 to 2000: practice patterns, short-term outcomes, and hospital charges in a nationwide sample. J Neurosurg. 2003;99:863-871.
27. Umemura A., Jaggi J.L., Hurtig H.I., et al. Deep brain stimulation for movement disorders: morbidity and mortality in 109 patients. J Neurosurg. 2003;98:779-784.
28. Bingley T., Leksell L., Meyerson B.A. Long-term results of stereotactic anterior capsulotomy in chronic obsessive-compulsive neurosis. In: Sweet W.H., editor. Neurosurgical Treatment in Psychiatry, Pain, and Epilepsy. Baltimore: University Park Press; 1977:287-299.
29. Mindus P. Capsulotomy in anxiety disorders, a multidisciplinary study, in. Stockholm. Karolinska Institute; 1991.
30. Ruck C., Karlsson A., Steele J.D., et al. Capsulotomy for obsessive-compulsive disorder: long-term follow-up of 25 patients. Arch Gen Psychiatry. 2008;65:914-921.
31. Lippitz B.E., Mindus P., Meyerson B.A., et al. Lesion topography and outcome after thermocapsulotomy or gamma knife capsulotomy for obsessive-compulsive disorder: relevance of the right hemisphere. Neurosurgery. 1999;44:452-458. discussion 458-460
32. Greenberg B.D., Price L.H., Rauch S.L., et al. Neurosurgery for intractable obsessive-compulsive disorder and depression: critical issues. Neurosurg Clin North Am. 2003;14:199-212.
33. Steiner L., Lindquist C., Adler J.R., et al. Clinical outcome of radiosurgery for cerebral arteriovenous malformations. J Neurosurg. 1992;77:1-8.
34. Barcia Salorio J.L., Roldan P., Hernandez G., Lopez Gomez L. Radiosurgical treatment of epilepsy. Appl Neurophysiol. 1985;48:400-403.
35. Elomaa E. Focal irradiation of the brain: an alternative to temporal lobe resection in intractable focal epilepsy? Med Hypotheses. 1980;6:501-503.
36. Rossi G.F., Scerrati M., Roselli R. Epileptogenic cerebral low-grade tumors: effect of interstitial stereotactic irradiation on seizures. Appl Neurophysiol. 1985;48:127-132.
37. Chen Z.F., Kamiryo T., Henson S.L., et al. Anticonvulsant effects of gamma surgery in a model of chronic spontaneous limbic epilepsy in rats. J Neurosurg. 2001;94:270-280.
38. Maesawa S., Kondziolka D., Dixon C.E., et al. Subnecrotic stereotactic radiosurgery controlling epilepsy produced by kainic acid injection in rats. J Neurosurg. 2000;93:1033-1040.
39. Mori Y., Kondziolka D., Balzer J., et al. Effects of stereotactic radiosurgery on an animal model of hippocampal epilepsy. Neurosurgery. 2000;46:157-165. discussion 165-168
40. Regis J., Kerkerian-Legoff L., Rey M., et al. First biochemical evidence of differential functional effects following gamma knife surgery. Stereotact Funct Neurosurg. 1996;66(suppl 1):29-38.
41. Warnke P.C., Berlis A., Weyerbrock A., Ostertag C.B. Significant reduction of seizure incidence and increase of benzodiazepine receptor density after interstitial radiosurgery in low-grade gliomas. Acta Neurochir Suppl. 1997;68:90-92.
42. Regis J., Peragui J.C., Rey M., et al. First selective amygdalohippocampal radiosurgery for ‘mesial temporal lobe epilepsy’. Stereotact Funct Neurosurg. 1995;64(suppl 1):193-201.
43. Regis J., Bartolomei F., Rey M., et al. Gamma knife surgery for mesial temporal lobe epilepsy. J Neurosurg. 2000;93(suppl 3):141-146.
44. Regis J., Rey M., Bartolomei F., et al. Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia. 2004;45:504-515.
45. Barbaro N.M., Quigg M., Broshek D.K., et al. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Ann Neurol. 2009;65:167-175.
46. Vojtech Z., Vladyka V., Kalina M., et al. The use of radiosurgery for the treatment of mesial temporal lobe epilepsy and long-term results. Epilepsia. 2009;50:2061-2071.
47. Regis J., Bartolomei F., Kida Y., et al. Radiosurgery for epilepsy associated with cavernous malformation: retrospective study in 49 patients. Neurosurgery. 2000;47:1091-1097.
48. Regis J., Scavarda D., Tamura M., et al. Gamma knife surgery for epilepsy related to hypothalamic hamartomas. Semin Pediatr Neurol. 2007;14:73-79.
49. Regis J., Bartolomei F., Hayashi M., Chauvel P. Gamma knife surgery, a neuromodulation therapy in epilepsy surgery!. Acta Neurochir Suppl. 2002;84:37-47.
50. Shibasaki H., Ikeda A., Nagamine T. Use of magnetoencephalography in the presurgical evaluation of epilepsy patients. Clin Neurophysiol. 2007;118:1438-1448.
51. Vulliemoz S., Lemieux L., Daunizeau J., et al. The combination of EEG source Imaging and EEG-correlated functional MRI to map epileptic networks. Epilepsia. 2009.
52. Han J.H., Kim D.G., Chung H.T., et al. Long-term outcome of gamma knife radiosurgery for treatment of typical trigeminal neuralgia. Int J Radiat Oncol Biol Phys. 2009;75:822-827.
53. Dellaretti M., Reyns N., Touzet G., et al. Clinical outcomes after gamma knife surgery for idiopathic trigeminal neuralgia: review of 76 consecutive cases. J Neurosurg. 2008;109(suppl):173-178.
54. Longhi M., Rizzo P., Nicolato A., et al. Gamma knife radiosurgery for trigeminal neuralgia: results and potentially predictive parameters. Part I: idiopathic trigeminal neuralgia. Neurosurgery. 2007;61:1254-1260. discussion 1260-1261
55. Brisman R. Gamma knife surgery with a dose of 75 to 76.8 gray for trigeminal neuralgia. J Neurosurg. 2004;100:848-854.
56. Petit J.H., Herman J.M., Nagda S., et al. Radiosurgical treatment of trigeminal neuralgia: evaluating quality of life and treatment outcomes. Int J Radiat Oncol Biol Phys. 2003;56:1147-1153.