CHAPTER 36 GAIT DISTURBANCES AND FALLS
Gait, the action of walking from one place to another on two legs, is one of the most fundamental human motor tasks. Unlike all other mammals, it takes about 1 year for a human baby to start walking independently on two legs and 2 to 3 additional years until gait is fully coordinated and the response to external restrictions is well developed. Many congenital or perinatal psychomotor disturbances first manifest as a delayed initiation of walking, and this underscores how much normal gait depends on mature and intact central and peripheral nervous systems. Furthermore, gait also requires intact musculoskeletal and cardiovascular systems, from the very first year of life. For the next six or seven decades of their lives, humans generally walk independently and largely automatically, without any apparent need for paying special attention to this essential daily task. Remarkably, healthy adults fall only rarely, despite multiple and often unexpected challenges in the everyday environment, such as slippery floors or doorsteps.
LOCOMOTION, EQUILIBRIUM, AND THE SUPPORT SYSTEMS
Central Locomotion Generators
Locomotion is controlled by several centers at different levels of the CNS. There is strong evidence that the most basic rhythmic, bipedal, synchronized stepping of mammalian quadrupeds originates in centers in the spinal cord referred to as the central pattern generators. Indeed, decerebrate cats can produce a rhythmic stepping pattern if they are put on a treadmill. Although anatomical demonstration of the existence of such centers is lacking in humans, central pattern generator-like centers probably exist in humans as well. The human central pattern generator also controls the timed coupling between arm and leg movements during normal walking.1 In primates, including humans, the central pattern generator is probably located within the spinal cord, as is suggested by the production of gaitlike movements in paraplegic patients who, when placed with support on a moving treadmill, can also produce rhythmic stepping. Such spinal central pattern generators are under the influence of brainstem control mechanisms, which are most probably situated at the level of the mesencephalon in the brain. In humans, the caudal cholinergic mesencephalic nucleus, also referred to as the pedunculopontine nucleus (PPN), is considered as the human mesencephalic locomotion center. It is believed to control spinal pattern generators or to play a similar role as the spinal central pattern generator in animals and is considered the human mesencephalic locomotion center.2 The PPN receives afferent GABAergic projections (secreting the neurotransmitter gamma-amino-butyric acid [GABA]) from the internal globus pallidus, subthalamic nucleus, and the substantia nigra pars reticulata. It sends efferent cholinergic and glutamatergic projections (secreting the neurotransmitters acetylcholine and glutamic acid) to the substantia nigra pars compacta and downward to the brainstem and spinal cord. The PPN is the only nucleus associated with the basal ganglia that has a direct connection with the spinal cord and, as such, it is believed to play a major role in motor control of gait and posture. PPN stimulation can induce stepping in primates while a lesion at the PPN causes severe akinesia and parkinsonism in primates that is reversed by blocking GABAergic stimulation of the PPN. Furthermore, a focal stroke within the dorsal mesencephalon (presumably involving the PPN) has been shown to cause severe gait initiation problems in a human patient.
The Gait Cycle
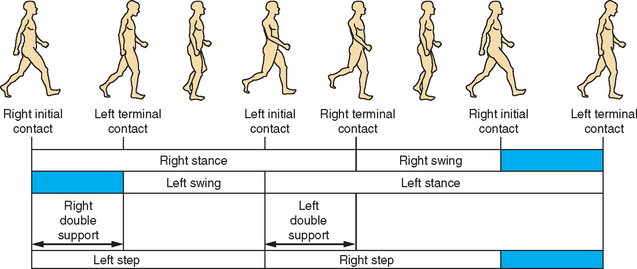
Walking can be regarded as a composite of numerous small and similar gait cycles, each based on an alternating single step accomplished by both legs. A full cycle can be calculated from any given point because of the rhythmic and stereotypic manner of locomotion. Classically, spatial description of the gait cycle starts when the right heel touches the ground while the right knee is stretched (locked) and the right foot is dorsiflexed. The foot rolls on the ground and carries most or all of the body mass as part of the stance phase. As the body mass moves forward, the heel leaves the ground. After a forced plantar flexion that pushes the body center of mass forward, the toes also leave the ground. When the toes of the right foot leave the ground, the swing phase of the right leg starts. In the swing phase, the right leg swings forward after the right hip is pulled up (flexed) while the knee initially will be flexed and later extended to reach the ground in a locked position. During the swing phase, the foot is dorsiflexed to avoid any contact of the toes with the ground. The cycle ends when the right heel again comes in contact with the ground (Fig. 36-1).
Three additional spatiotemporal features of locomotion are stride length, cadence, and step width. Stride length is the distance between the places where the right heel first touches the ground at the initiation of one gait cycle to the place where it touches the ground again at the beginning of the next gait cycle. Stride length is also the summation of the distances of two steps (left plus right). Cadence is the step rate or the number of steps in a given time (e.g., steps per minute), and step width describes the distance between the two feet along the perpendicular axis to the walking direction for a given step.
ASSESSMENT OF GAIT
The quantitative assessment of gait and balance can be stratified into three levels: no tech, low tech, and high tech.3 Each has an appropriate time and place. The decision to use one or the other of these approaches partly depends on the purpose of the evaluation, the degree of sensitivity and specificity required, and trade-offs of cost, time, and convenience. Many volumes have been written on each of these approaches.4 Here, we briefly describe some of the more salient features of each of them and common approaches.
History Taking
Interviewing patients about their problems with gait or balance can be a challenge. When asking about gait problems, the clinician has to understand the amount of walking a person does in daily life. In addition, the nature of the questions themselves should try to help the patient describe his difficulties in terms of walking speed, amount of effort required, confidence or degree of fear from falling, and whether any assistance or walking aids are used. It is informative to look at the patient’s step length as a good general marker and to note if one can hear the soles dragging on the floor during each step as a practical marker for the height and duration of the swing phase. For patients presenting with gait disorders, try to distinguish between consistently present walking difficulties versus an episodic gait disorder, such as freezing of gait, festination, or sensory ataxia in the dark. When asking about possible freezing of gait, one must specifically inquire about the characteristic subjective feeling of the feet “being glued to the ground.” A specific Freezing of Gait Questionnaire has been developed that may assist in screening for and rating the severity of freezing of gait.5 To make sure that the patient understands what is meant by freezing of gait, it may be useful to demonstrate an imitation of a typical freezing of gait episode.
Falls are one of the most serious complications of gait disturbances and should receive special attention. Many elderly persons tend to forget their falls, particularly when there is concurrent cognitive impairment, as is often the case. Some persons may even purposely deny the occurrence of falls because they fear being admitted to a nursing home or another form of sheltered care. Many elderly persons find it extremely difficult to indicate under which circumstances their falls occur. Nevertheless, obtaining a careful fall history is important, because the presence of a prior fall in the preceding 6 to 12 months consistently emerges as an excellent, simple predictor for repeated falls in the future.6 Patients should be asked not only about falls but also about the presence of “near-falls.” These are at least as common and can be debilitating in their own right. In light of the characteristic amnesia for falls, eyewitness reports are often indispensable, and every effort must be made to retrieve the accounts of bystanders who witness falls.
Table 36-1 can serve as a guideline for the interview. Several important elements of this clinical approach are discussed in more detail next.
TABLE 36-1 Elements of History Taking in Persons With Gait Disorders or Falls
* If needed, evaluate domestic situation by community nurse, physiotherapist, or occupational therapist.
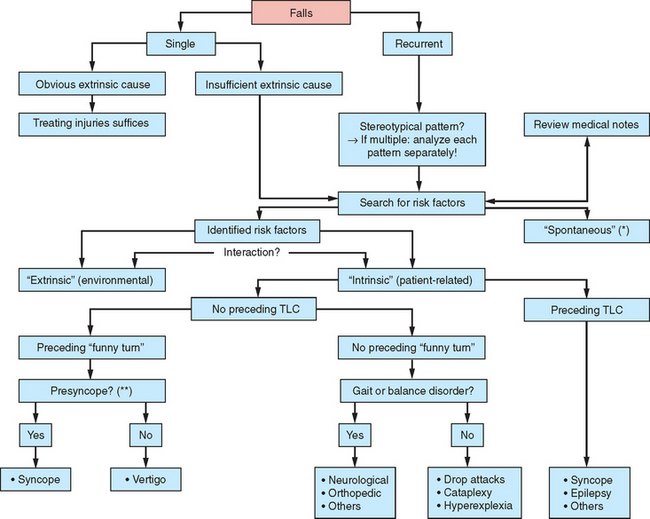
For any patient presenting with falls, the first thing to do is to clarify the circumstances surrounding the fall and, in particular, to identify any specific pattern, because this may reveal the underlying pathophysiology and offer opportunities for prevention. The second step involves a distinction between single versus recurrent (two or more) falls and between “internal” and “external” falls, features that are important for secondary prevention. One should therefore evaluate whether any environmental factors were obviously responsible for the falls. When the role of environmental factors is unclear or frankly denied, the fall can then be classified as “intrinsic” (usually defined as a fall that was caused by gait or balance disorders or misperception of the environment). The third step is to inquire whether any transient loss of consciousness preceded the fall. The absence of a preceding transient loss of consciousness indicates a probable involvement of an underlying gait disorder and/or balance deficit. This will become evident during physical examinations between falling episodes. If the gait or balance disorder is severe enough, even small movements can be destabilizing and the patient may start to fall almost spontaneously. In patients with apparently unprovoked falls, care must be taken not to miss a possible preceding transient loss of consciousness. Explicit inquiry into the use of medication is vitally important, because this is one of the most common risk factors for gait disorders, falls, and hip fractures in elderly persons. In particular, benzodiazepines and antidepressants are associated with falls in the elderly, but other psychotropic medications (e.g., neuroleptics) and drugs causing orthostatic hypotension or arrhythmia also increase the risk of falls. Dopaminergic medication may paradoxically increase the fall frequency of patients with Parkinson’s disease by causing excessive dyskinesias, orthostatic hypotension, or confusion. The risk of falls increases dramatically if different drugs are used at the same time (Fig. 36-2).
Physical Examination
Table 36-2 lists several important elements of the physical examination. The examination should include a battery of functional tests to capture the full repertoire of gait and balance abnormalities. Ideally, physicians should examine the patients in the environment where they walk and function in daily life (i.e., home and surroundings), but this is not practical. A homemade video can sometimes be extremely informative, especially for episodic gait disturbances. Similarly, examining the patient immediately after a fall can help with the identification of its cause(s) and consequences, but this is rarely possible. A problem with the inevitably “interictal” examinations is that the observed abnormalities may not be causally related to the gait disturbance or the fall. Physical examinations can appear to be entirely normal in between freezing of gait or fall episodes.
TABLE 36-2 Elements of Physical Examination in Patients With Gait Disorders or Falls
† Speed of performance less important than safety.
‡ Instruct to execute slowly and abruptly; note occurrence of freezing.
§ Often difficult to assess during routine clinical examination; preferentially use standardized trajectory (from Schaafsma JD, Balash Y, Gurevich T, et al: Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease. Eur J Neurol 2003; 10:391-398.)
¶ Elements to be noted include the trunk (stooped; marked lateroflexion, or Pisa syndrome) and neck (retrocollis, anterocollis, or laterocollis).
¶¶ Difficult to standardize and score.
** As a measure of cardiopulmonary condition.
†† Specific rating scales are available for some disorders, but there is a general lack of validated measures for posture, balance, and gait.
Modified from Bloem and Bhatia 2004, with permission.
Gait
Most examination rooms are too small to assess gait properly. It is therefore helpful to monitor the patient while walking to and from the examination room or even follow him down the corridor. The reduced step height observed in mildly affected patients such as those with mild spasticity or parkinsonism may be better heard than seen. Inspecting their shoes may reveal excessive wear on the medial and anterior side of the sole on the side of a spastic leg. A reduced support phase on one leg suggests an antalgic gait (e.g., that of someone with a twisted ankle). Dystonic gait disorders can be task specific (e.g., evident during regular walking but disappearing during walking backward or running). Freezing of gait is particularly difficult to elicit in the physician’s examination room and is best examined as patients walk along a specifically developed trajectory, periodically passing through narrow paths and performing turns of 180 or 540 degrees. Abnormalities to be noted during turning movements include the “pivoting” strategy, where the trunk rotates stiffly (“en bloc”) with the legs, shuffling of the feet with multiple small steps or even overt freezing of gait. Providing patients with one or more secondary “distracting” tasks while walking can be informative because impaired multiple task performance may detect balance problems that might otherwise remain unnoticed. Furthermore, multiple task performance provides insight into actual falling risks because falls in daily life commonly occur when subjects attempt to do more than one thing at the same time. Cognitively impaired patients may try to perform all tasks equally well, but pay a price in terms of poor gait or balance. The simplest test is to start a routine conversation while walking beside the patient. An inability to walk and talk at the same time (usually manifested by a stop in the conversation) has a good predictive value for falls in cognitively impaired elderly persons, but probably not in patients without cognitive impairment. Other examples of secondary tasks include avoiding obstacles or carrying objects such as a tray while walking. Various different tasks can be combined to challenge balance and gait even further.
Timed Tests
Some clinicians advocate the use of timed tests because they produce a quantifiable result. One example is the Timed Up and Go test (TUaG), which is a reliable measure of functional performance that captures transfers, gait, and turning movements.7 In this test, the subject is asked to rise from a chair, walk 3 m, turn around, return to the chair, and sit down. In its original form, this was a subjective test in which the performance of this task was assessed qualitatively. It has since become timed, however, to lessen its subjective nature. The TUaG test predicts falls and may be used to screen for fall risk. Part of the reason that the TUaG test is used so widely is that it evaluates different important mobility functions (e.g., transfers, gait, and turning), it takes only a very short time to complete, and it requires minimal expertise or training. Subjects who perform the TUaG test in more than 13.5 seconds are believed to have an increased risk of falls.8 The TUaG test could be classified as a low-tech measure of gait.
Rating Scales
Standardized rating scales are comprised of combinations of different tests and this helps to systematically rate most aspects of gait and balance. A widely used example is the standardized version of Tinetti’s test9 (also known as the Performance Oriented Mobility Assessment [POMA]). It includes an evaluation of balance under challenging conditions (e.g., while rising from a chair, after a nudge to the sternum, standing with eyes closed, and while turning) and an evaluation of gait characteristics (including gait initiation, step height, length, continuity and symmetry, trunk sway, and path deviation). Scoring is based on clinical judgement and takes only about 15 minutes. Poor performance on this scale is associated with an increased risk of falls in the elderly population. One disadvantage is that very unstable patients cannot (or refuse to) perform all the elements of these rating scales. Another disadvantage is that the rating scales lack validity in general and have never been validated for specific disorders of balance or gait.
Quantitative Assessment of Gait
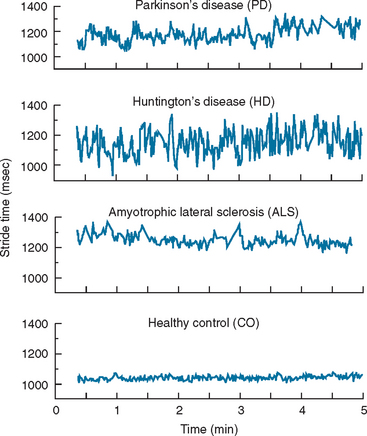
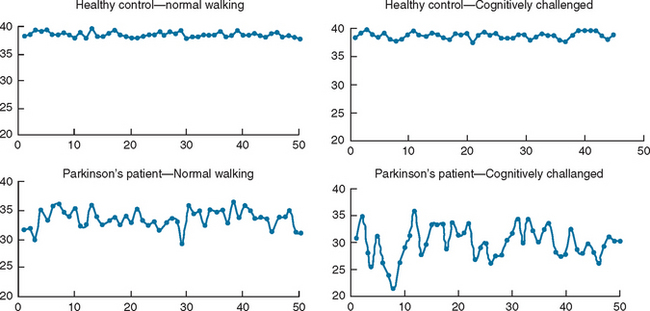
Ambulatory monitoring systems enable the quantitative measurement of the timing of gait. The subject might be equipped with accelerometers, foot switches, or other wearable sensors. A key advantage of such systems is that they are not tethered to any computer or stationary system and therefore allow the measurement of multiple strides in almost any environment. This, in turn, enables evaluation of gait rhythmicity and the stride-to-stride fluctuations of gait timing (Fig. 36-3). Several studies have shown that such measures of gait variability or dynamics are sensitive to subtle changes that occur with aging and neurodegenerative diseases, such as Parkinson’s disease, Huntington’s disease, and Alzheimer’s disease10–13 (see, for example, Fig. 36-4). Stride-to-stride fluctuations reflect the consistency or steadiness of gait and the ability of the locomotor system to regulate gait from one stride to the next. Thus, it is easy to understand how such measures have clinical efficacy in the identification and prospective prediction of elderly fallers14 (see, for example, Fig. 36-5).
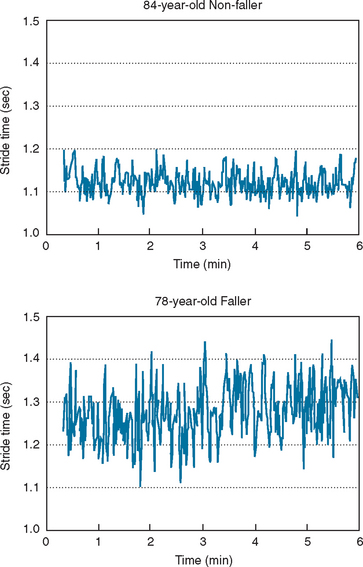
Figure 36-5 Stride-to-stride fluctuations in the stride time, were much larger in this subject who subsequently experienced a fall during the 12-month follow-up period than in the non-faller. Group results were similar. Measures of the stride-to-stride variability prospectively predicted falls; older adults with increased variability were more likely to fall during the 12-month follow-up period compared with those with a lower variability. (See Hausdorff et al.14 for further details.)
Gait Disturbances
Etiology and Pathophysiology
Gait disturbances can be the result of a lesion or functional derangement at any level, from the toes to all areas of the cortex. In many instances, it is easy and straightforward to identify the etiology of a gait disturbance just by observing the patient walking and by performing physical and neurological examinations. The origin of gait disturbances in a person with orthopedic problems as seen in feet, joint, or spinal diseases can be easily diagnosed similar to the way that visual, vestibular, cardiovascular, respiratory, or muscular disturbances can often be readily identified. The effect of those and other peripheral neuropathies on gait is secondary. As a result, any therapeutic intervention should be focused on the primary etiology. Due to the critical role of gait in normal daily function, however, a compensatory approach frequently has to be taken, even before solving the primary problem, simply in order to promote mobility and independence.
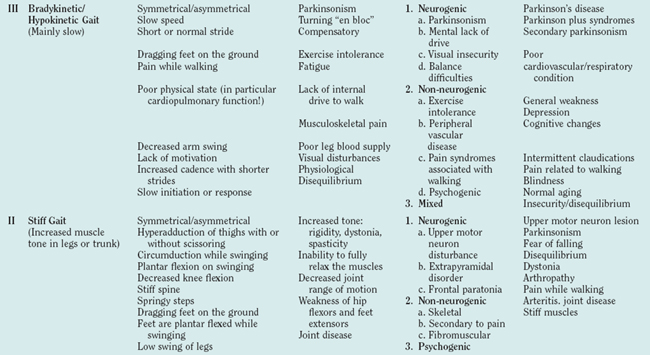
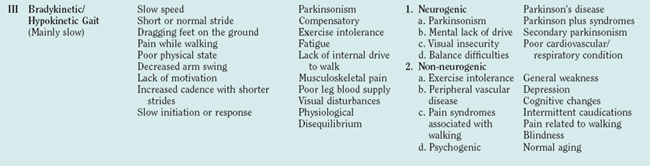
Due to the complex nature of the central gait network, different lesions at multiple levels of the CNS can cause gait disturbances. In some instances, such as stroke and hemiparesis or cerebellar disease and ataxia, the association between focal brain dysfunction and a distinct gait disturbance is clear-cut and easily diagnosed. When it comes to disturbances in the extrapyramidal-limbic-prefrontal locomotion network, the clinical-pathological relationships may be less clear. The gait disturbance also becomes more difficult to describe, even from a phenomenological perspective. The pathophysiology of those disturbances, which were first described by Nutt and colleagues as “high level gait disturbances,”15 is not completely defined. Nonetheless, there is justification for associating those gait disturbances with the frontal lobe in general and, more specifically, with the prefrontal-limbic-anterior basal ganglia network. (See Table 36-3 for gait classification.) Frequently, the patient complains about a gait disturbance or fear of falling but the formal examination may not detect any specific cause. As a result, frontal gait disturbances are often mistakenly associated with the normal aging process or with parkinsonism just because the patient walks slowly and with a stooped posture. In addition, the frontal gait disorders may look bizarre and be suggestive of “apraxia” of gait. A major symptomatic aspect of the frontal gait disorders is a feeling of insecurity and of disequilibrium associated with anxiety, fear, and depression that might not be associated with a history of falls or proportional to the degree of imbalance or postural disturbances.16 This group of gait disturbances poses a real challenge from the diagnostic and therapeutic points of view. General atrophy with enlarged lateral ventricles may be seen on brain imaging. Many such patients undergo surgery in which a ventriculoperitoneal shunt is inserted, occasionally with partial and transient improvement. Based on clinical and neuroimaging techniques, it is very difficult to differentiate between normal pressure hydrocephalus and frontal gait disorders: only those cases with a very long-term and clear-cut response to ventriculoperitoneal shunting can be diagnosed—retrospectively—as having typical normal pressure hydrocephalus.
* The etiology of many gait syndromes is not always readily attributable to a single cause. For example, fear of falling may be due to changes in frontal and/or extrapyramidal function.
From the functional point of view, one of the most important features of gait disturbances is that it has a characteristic time course. Abnormal gait that develops slowly over a period of weeks or months can be incorporated into the daily routine with a relatively low risk of falls. For example, a person who develops continuous locomotion disturbances, such as slow gait, unsecured gait, hemiparetic gait, or even spastic gait, can use a walking aid, compensate for the difficulty by slowing the walking speed, and, very often, broaden the walking base. In contrast, the episodic gait disturbances (Table 36-4) are characterized by their unexpected and transient appearance. As a result, episodic gait disturbances often lead to falls or fear of falling and are associated with self-imposed restrictions of daily activities. As mentioned earlier, freezing of gait refers to the phenomenon where patients experience brief and sudden moments where the feet subjectively become “glued to the floor.” Freezing of gait most commonly appears when patients make turns (thereby explaining why patients with Parkinson’s disease frequently fall while turning), but it may also occur spontaneously during straight walking, while crossing narrow spaces, when trying to initiate gait (“start hesitation”), or when reaching a target. Freezing of gait is a particularly common source of falls, because the legs fail to follow through with the forward motion of the trunk. An objective demonstration of freezing of gait episode is demonstrated in Figure 36-5 using the ambulatory gait assessment technique.
TABLE 36-4 Examples of Episodic Gait Disturbances
It is helpful to distinguish between those gait disturbances that occur continuously, whenever walking occurs (e.g., bradykinetic gait) and those that are transient and episodic in nature. Examples of the latter are listed in this table.
It may be difficult to distinguish freezing of gait from festination, another episodic gait disorder that commonly leads to falls. Festination is characterized by a sudden change in locomotion rhythm because of an inability to maintain the base of support (the feet) beneath the forward moving trunk during gait, forcing the patient to rapidly take increasingly small steps (hastening) to avoid a fall. Festination leads to propulsion when patients walk forward or to retropulsion when patients lose their balance and take corrective steps backward. Festination or freezing of gait can also be embarrassing due to their unusual and bizarre nature, leading to the avoidance of situations in which they might occur. For example, patients may try to avoid crowds, walking in the street, crossing through doorways, or entering elevators. These episodic gait disturbances may be especially responsive to external cues like rhythmic auditory stimulation, lines on the floor, or changing the normal gait pattern to marching or walking sideway.17
Classification
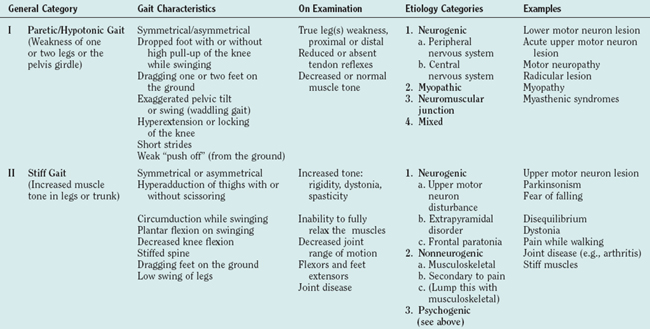
There are many ways to classify gait disturbances. It is, however, important to have a common understanding of terms when describing changes in walking patterns, to facilitate communication between clinicians and investigators. Classification of gait should give phenomenological information to form a faithful picture of the nature of the individual’s gait, as well as temporal data about its course and linkage to possible etiologies. Table 36-5 was developed to provide a practical stepwise classification of gait disturbances. The classification moves from phenomenology to examination to etiological options. Gait disturbances are divided into six general categories that are based on observation of a patient walking and should help describe gait at a given moment. It is important to note that a patient might have one or more types of general gait disturbances. For example, a parkinsonian patient can have bradykinetic, dyskinetic, and episodic (freezing) gait disturbances, with each type probably reflecting a different pathophysiology; each type should therefore be discussed (and treated) separately. The second column of Table 36-5 presents a more detailed but again observational characterization of gait. The observational description is followed by the physical and neurological examination. Based on the results of detailed and focused observation and examination (with or without the help of more advanced methods, such as motion analysis imaging, etc.), one can move to etiological categorization. The last column of Table 36-5 provides examples of the different types of gait disturbances in the different categories. The main reason for classifying a patient’s gait is the need to understand the pathophysiology behind the gait disturbance to plan appropriate therapeutic strategies.
GAIT AND MENTAL FUNCTION
Epidemiological evidence indicates that among older adults, fall risk increases in those with impaired cognitive function.8 This highlights the importance of cognitive function in gait and walking steadiness. In addition, the stability of gait and balance declines in certain populations when subjects are asked to walk and perform a second task (dual tasking).18 If gait were really automatic, one would anticipate that the performance of an additional task would have no influence on gait. An exacerbated dual-task decrement in walking ability has, however, been observed in idiopathic elderly fallers, in patients with Parkinson’s disease (primarily a motor disorder) (Fig. 36-6), and in patients with Alzheimer’s disease (primarily a cognitive disorder).19–23 Moreover, patients with dementia have an impaired gait and an extremely high, not fully explained, risk of falls,24 despite only minimal involvement of their disease in the classic motor control systems. Walking and the maintenance of upright balance during gait clearly require more than “auto-pilot” control. Instead, walking and the proper integration of the many steps that are involved in walking should be viewed as a complex motor task that needs higher-level cognitive input.
With this in mind, one can better appreciate the ground-breaking study by Lundin-Olson and associates.25 They investigated “stops walking when talking” in a mixed group of community-dwelling older adults, some of whom had dementia or depression or were poststroke or a combination. About 80% of the subjects who were unable to walk and talk fell at least once during a 6-month follow-up period, whereas about 80% of the subjects who were able to walk and talk did not fall. In other words, subjects who were unable to coordinate walking and talking such that they had to completely stop one task in order to perform the second task were at increased risk of falls, a further demonstration of the reliance of safe walking and falls on cognitive function.
This idea that higher-level cognitive function is critical to walking also partially accounts for the intriguing results of prospective studies that reported impaired mobility that was not attributable to a specific disease predicts future dementia as much as 6 years into the future.26,27 If walking is a complex task that requires harmonic function of multiple brain networks, one can understand why even minor changes in locomotion reflect brain dysfunction, which may predict the future development of dementia. Furthermore, this concept suggests the possibility that interventions designed to improve cognitive function28 might enhance gait and reduce the fall risk of certain patient populations. To ensure that gait disturbances are truly minimized and that walking and safety are optimized, it is essential to keep in mind that there is much more to gait than automated musculoskeletal movements.
Rehabilitation Strategies
Physical Therapy and Exercise
Exercises, strength training, and gait or balance rehabilitation can help to improve the subject’s fitness and reduce the number of falls (Table 36-6). There is good evidence to suggest that a home exercise program can prevent injuries in older people.29 Because leg weakness is associated with falls in the elderly, fall prevention programs often include strength training. Balance training using Tai Chi Quan techniques may also help to reduce fall risk in this population. Gait or balance training will not cure the underlying neurological deficits, but patients can be taught to cope better with existing mobility problems. For example, patients can learn to make safer transfers by using alternative motor strategies. Physiotherapists could also play a role in restoring balance confidence and reduce the fear of falls. Furthermore, physiotherapists can train patients to avoid hazardous activities, such as sudden changes of posture or the performance of simultaneous tasks. By training and promoting regular exercise, physical therapy can lead to improvement of general fitness and reduce cardiovascular comorbidity and mortality.30 Regular exercises also help to arrest the development or progression of osteoporosis and increase bone mass, density, and strength. When exercise, such as daily walking, is combined with oral bisphosphonates, the risk of bone fracture as the result of a fall can be decreased. Although the effects of exercise on bone strength are most prominent when initiated at a young age, beneficial effects can also be seen in elderly persons.
* For example, learning to direct the fall toward bed or chair.
CUEING
One of the most powerful modes of intervention to improve gait initiation and walking is the cueing technique. Despite even severe locomotion disturbances, patients (classically those with Parkinson’s disease) can walk almost normally by adopting special behavioral techniques, by being exposed to special sensory stimulations, or by using learned cognitive tricks.17 The challenge lies in enabling patients to access this ability at will and to apply it in their normal, daily function.
Different cueing techniques have been used, and the population that most dramatically benefits from them are patients with Parkinson’s disease. All cueing techniques, in essence, intervene with the automatic walking brain network through behavioral sensory or cognitive techniques. The rationale behind these powerful behavioral modification techniques is that the normal locomotion network is dysfunctional and the cues “force” the brain to use other alternative locomotion programs by partly bypassing the primary network. Two common examples of behavioral cues are marching and climbing stairs. Lifting the knees and thinking about each step can enable a patient to walk almost normally when a regular walking pattern is impossible due to severe freezing of gait. Although stairs may pose a challenge to many older adults, patients (mainly those with Parkinson’s disease) who struggle to put together continuous steps on level ground can easily manage stair climbing. This is because the stairs act as an external cue. Another powerful external cue is rhythmic auditory stimulation, which intervenes with a deficient internal gait rhythm system. Rhythmic auditory stimulation has been shown to be very effective, not only for improving general walking but also for enhancing internal gait rhythmicity and stride-to-stride fluctuations (gait dynamics).17 When walking with rhythmic auditory stimulation, patients with Parkinson’s disease are able to match their cadence to a beat set at 10% faster than their baseline values, thereby significantly improving their velocity, cadence, and stride length.31–33 These improvements remained evident in the immediate short term even after the cues were removed. The fact that the improvements persisted suggests that rhythmic auditory stimulation may also provide some sort of rhythmic training mechanism. Walking on a treadmill can also be considered an external pacemaker for providing an external cue and enhancing gait.34 Another commonly used and very effective group of external cues is that of working through the visual system to set the proper stride length, thus providing external information to help augment the defective scaling motor set, which is commonly downregulated in extrapyramidal disorders.35 Placement of floor markers is one technique that can be very effective in regulating stride length and demonstrating to the patient the potential value of cues. Floor markers were reported to be effective for improving gaits of patients with Parkinson’s disease as early as 1967.36 Some studies found that these patients retained a positive “carryover” effect even after the visual cues were removed.37,38 This suggests that a certain degree of training had taken place, even with only a short exposure to the visual cues. Visual cues have also been found to be helpful in alleviating freezing of gait episodes. The cognitive tricks are all attentional cues that have been used to focus attention on walking and thus remove it from the automatic mode of action.
Medical Treatment
SUMMARY
Bloem BR, Visser JE, Allum JH. Posturography. In: Hallett M, editor. Handbook of Clinical Neurophysiology. Amsterdam: Elsevier Science BV; 2003:295-336.
Emere M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med. 2004;351:2509-2518.
Herman T, Giladi N, Gurevich T, et al. Gait instability and fractal dynamics of older adults with a “cautious” gait: why do certain older adults walk fearfully? Gait Posture. 2005;21:178-185.
Nutt JG, Carter JH, Sexton GJ. The dopamine transporter: importance in Parkinson’s disease. Ann Neurol. 2004;55:766-773.
1 Wannier T, Bastiaanse C, Colombo G, et al. Arm to leg coordination in humans during walking, creeping and swimming activities. Exp Brain Res. 2001;141:375-379.
2 Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson’s disease. Brain. 2000;123:1767-1783.
3 Alexander NB. Clinical evaluation of gait disorders: no-tech and low-tech. In: Hausdorff JM, Alexander NB, editors. Evaluation and Management of Gait Disorders. New York: Marcel Dekker Inc, 2005.
4 Bloem BR, Visser JE, Allum JH. Posturography. In: Hallett M, editor. Handbook of clinical neurophysiology. Amsterdam: Elsevier Science BV; 2003:295-336.
5 Giladi N, Shabtai H, Simon ES, et al. Construction of Freezing of Gait Questionnaire for patients with parkinsonism. Parkinson Relat D. 2000;6:165-170.
6 Tinetti ME. Clinical practice. Preventing falls in elderly persons. N Engl J Med. 2003;348:42-49.
7 Podsiadlo D, Richardson S. The timed “Up & Go:” a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142-148.
8 American Geriatrics Society, British Geriatrics Society, American Academy of Orthopedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J Am Geriatr Soc. 2001;49:664-672.
9 Tinetti ME. Performance-oriented assessment of mobility problems in elderly persons. J Am Geriatr Soc. 1986;34:119-126.
10 Hausdorff JM, Cudkowicz M, Firtion R, et al. Gait variability and basal ganglia disorders: stride-to-stride variations of gait cycle timing in Parkinson’s disease and Huntington’s disease. Mov Disord. 1997;13:428-437.
11 Herman T, Giladi N, Gurevich T, et al. Gait instability and fractal dynamics of older adults with a “cautious” gait: why do certain older adults walk fearfully? Gait Posture. 2005;21:178-185.
12 Nakamura T, Meguro K, Sasaki H. Relationship between falls and stride length variability in senile dementia of the Alzheimer type. Gerontology. 1996;42:108-113.
13 Hausdorff JM, Schaafsma JD, Balash Y, et al. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp Brain Res. 2003;149:187-194.
14 Hausdorff JM, Rios D, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82:1050-1056.
15 Nutt JG, Marsden CD, Thompson PD. Human walking and higher-level gait disorders, particularly in the elderly. Neurology. 1993;43:268-279.
16 Giladi N, Herman T, Rieder I, et al. Clinical characteristics of the idiopathic gait disorders of the elderly: evidence suggestive of a distinct neurodegenerative syndrome. J Neurol. 2005;252:300-306.
17 Rubenstein TC, Giladi N, Hausdorff JM. The power of cueing to circumvent dopamine deficits: a review of physical therapy treatment of gait disturbances in Parkinson’s disease. Mov Disord. 2002;17:1148-1160.
18 Woollacott M, Shumway-Cook A. Attention and the control of posture and view of an emerging area of research. Gait Posture. 2002;16:1-14.
19 Shumway-Cook A, Brauer S, et al. Predicting the probability for falls in community-dwelling older adults using the timed Get Up and Go test. Phys Ther. 2000;80:896-903.
20 Bloem BR, Valkenburg VV, Slabbekoorn M, et al. The multiple tasks test. Strategies in Parkinson’s disease. Exp. Brain Res. 2001;137:478-486.
21 Sheridan PL, Solomont J, Kowall N, et al. Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer’s disease. J Am Geriatr Soc. 2003;51:1633-1637.
22 Yogev G, Giladi N, Peretz C, et al. Dual tasking, gait rhythmicity, and Parkinson’s disease: which aspects of gait are attention demanding? Eur J Neurosci. 2005;22:1248-1256.
23 Hausdorff JM, Balash J, Giladi N. Effects of cognitive challenge on gait variability in patients with Parkinson’s disease. J Geriatr Psychol Neurol. 2003;16:53-58.
24 Shaw FE, Bond J, Richardson DA, et al. Multifactorial intervention after a fall in older people with cognitive impairment and dementia presenting to the accident and emergency department: randomised controlled trial. BMJ. 2003;326:73.
25 Lundin-Olsson L, Nyberg L, et al. “Stops walking when talking” as a predictor of falls in elderly people. Lancet. 1997;349:617.
26 Verghese J, Lipton RB, Hall CB, et al. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med. 2002;347:1761-1768.
27 Marquis S, Moore MM, Howieson DB, et al. Independent predictors of cognitive decline in healthy elderly persons. Arch Neurol. 2002;59:601-606.
28 Emere M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med. 2004;351:2509-2518.
29 Robertson MC, Campbell AJ, Gardner MM, et al. Preventing injuries in older people by preventing falls: a metaanalysis of individual-level data. J Am Geriatr Soc. 2002;50:905-911.
30 Hakim AA, Petrovitch H, Burchfiel CM, et al. Effects of walking on mortality among nonsmoking retired men. N Engl J Med. 1998;338:94-99.
31 Cunnington R, Iansek R, Bradshaw J, et al. Movement-related potentials in Parkinson’s disease: presence and predictability of temporal and spatial cues. Brain. 1995;118:935-950.
32 McIntosh GC, Brown SH, Rice RR, et al. Rhythmic auditory-motor facilitation of gait patterns in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1997;62:22-26.
33 McIntosh G, Thaut M, Rice R, et al. Stride frequency modulation in Parkinsonian gait using rhythmic auditory stimulation. Ann Neurol. 1994;36:316.
34 Frenkel-Toledo S, Giladi N, Peretz C, et al. Treadmill walking as an external cue to improve gait rhythm and stability in Parkinson’s disease. Mov Disord. 2005;20:1109-1114.
35 Morris ME, Iansek R, Matyas TA, et al. Ability to modulate walking cadence remains intact in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1994;57:1532-1534.
36 Stern GM, Lander CM, Lees AJ. Akinetic freezing and trick movements in Parkinson’s disease. J Neural Transm Suppl. 1980;16:137-141.
37 Morris ME, Iansek R, Matyas TA, et al. Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain. 1996;119:551-568.
38 Bagley S, Kelly B, Tunnicliffe N, et al. The effect of visual cues on the gait of independently mobile Parkinson’s disease patients. Physiotherapy. 1991;77:415-420.
39 Nutt JG, Carter JH, Sexton GJ. The dopamine transporter: importance in Parkinson’s disease. Ann Neurol. 2004;55:766-773.