Chapter 10 Gait disorders
Pathophysiology and clinical syndromes
Balance
It is important to distinguish between balance and stance. Stance is the posture of standing. It is normally upright on two legs with a distance between the feet approximately equal to pelvic width. An example of abnormal stance would be the stooped posture seen in Parkinson disease. Balance is the ability to maintain stance without falling or excessive lurching (Benvenuti, 2001). If balance is poor, sometimes persons modify their stance; the most common manifestation is increasing the distance between the feet to widen the base of support (BOS).
From standard Newtonian physics, for an object to maintain its position, its center of mass (COM) must be over its BOS. In the case of a human standing, the COM is generally somewhere in the abdomen and the BOS is the area between and including the feet. Since the BOS is relatively small and the COM relatively high above the BOS, it takes considerable skill to maintain the BOS above the COM. Recently, it was recognized that the ability to remain upright cannot be explained best by just the simple need to maintain the BOS above the COM. This is a static requirement, and the body or body parts are constantly moving. This movement must be taken into account. A “dynamic model” of balance includes consideration of the velocity of COM (Pai et al., 1998). The general principle is that the balance mechanisms of the body (such as the postural muscles) must be able to counteract any large velocities of the COM, even if the COM is currently over the BOS, since if they cannot, the COM will soon be beyond the BOS.
The body is constantly moving and this movement is called sway. Each body part can have its own movement, but the important “summary” of these movements would be movement of the COM for the reasons already described. Neurologists are used to doing a visual analysis of sway, but this is not very exact, of course. Instrumental methods have been developed. The position of the COM can be estimated although it is generally somewhat difficult. The position of the different body parts can be determined with video methods, and if their individual masses are estimated, then the COM can be calculated. This is generally done only in research laboratories. An alternative method, generally called posturography, comes from the ability to record forces that the body exerts on the floor with devices called force plates (Kaufman et al., 2001). The devices measure force in three directions, vertical, forward/backward, and side-to-side, and the two-dimensional center of action of the forward/backward and side-to-side force is called the center of pressure (COP). In the static situation, the COM is directly over the COP. Movements of the COM cause deviance of the matching of the COM and COP, but if the movements are small and slow, the deviance is not much. For this reason, sway is often measured with movements of the COP.
Generally it is thought that more sway means less good balance. This is often but not always true. In some circumstances, because the balance is bad, patients stiffen up, voluntarily or involuntarily, and the sway might decrease. This might be true in Parkinson disease, for example (Panzer et al., 1990). When a patient is “off,” balance is poor and patients are very stiff, often swaying little. When the patient is “on,” balance is better, the patient is more relaxed and sway might increase. Sway markedly increases with dyskinesias (characteristic of the “on” state and good balance). In most circumstances, however, increased sway does indeed indicate poor balance and posturography can be employed to make this assessment quantitatively. A surer way to assess balance is to see what happens with a perturbation to stance. If patients can remain upright, then balance is good; if they fall, balance is defective. This is the logic of the pull test.
The pull test is done by having the patient stand and having the investigator stand behind the patient and give a sudden pull backward. The patient is told to maintain balance. The best ability is to deal with the perturbation by body movements without moving the feet. A second-level ability is to maintain balance by taking a timely step backward. Failure, of course, is a fall, and that is the reason that the test is a pull rather than a push; the patient can fall into the examiner. Balance is virtually always better in the forward direction than the backward direction. With poor balance, patients tend to fall backward. All the reasons for this are not clear, but one is likely that when standing, the COM is closer to the back of the foot than the front. In any event, because of this, the pull test is often only done in the backward direction. A related test called the push and release test has been suggested, and it might be more sensitive and consistent than the pull test, but it has not been much utilized (Jacobs et al., 2006).
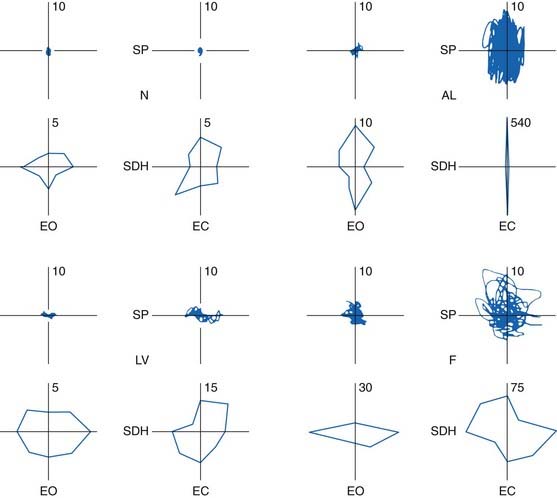
Abnormalities in posturography have been reported in many disorders. The best studied has been cerebellar disturbances (Diener et al., 1984; Gatev et al., 1996); the findings are reviewed in detail in Chapter 2 and illustrated as an example here (Fig. 10.1).
Gait
Human gait is a complex, rhythmic, cyclic movement (Winter, 1991). The movements are generated to some extent by a locomotor generator in the spinal cord, but they are under control by supraspinal mechanisms. The spinal cord generator can produce only simple, primitive stepping and react to perturbations in stereotypic fashion (Burke, 2001). Supraspinal mechanisms are required for a person to go in desired directions, with desired velocities and to deal well with perturbations. An important supraspinal control center is the mesencephalic locomotor region, which includes the pedunculopontine nucleus (PPN). The PPN is an important integrator of activity from basal ganglia, cerebellum, and motor cortex and projects to reticular nuclei in the brainstem. The fastigial nucleus of the cerebellum seems also important (Mori et al., 2001). Supraspinal control signals are conveyed to the spinal cord by reticulospinal and vestibulospinal tracts.
There are a number of terms that are useful in describing gait, and many of them are defined below.
A gait cycle for the right leg is illustrated in Figure 10.2. Note that the gait cycle for the left leg is not exactly 180° out of phase. For this reason, and because stance phase is longer than swing phase, there are several periods where both feet are on the ground, and these are called double support. (In normal walking, there are no periods of simultaneous swing; that is, no “flying,” but this may occur with running.) With normal gait, when the foot contacts the ground, the heel contacts first and then the foot rotates to flat with the heel as the point of rotation. When the foot leaves the floor for swing, the foot rotates over the toe and the heel leaves the ground first.
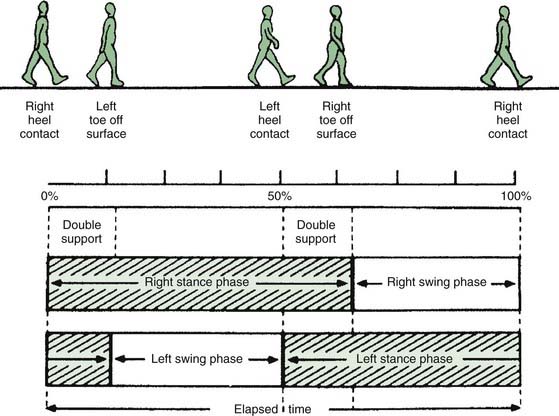
Figure 10.2 Diagram of the gait cycle; see text for detailed description.
From Sudarsky L. Geriatrics: gait disorders in the elderly. N Engl J Med 1990;322(20):1441–1446, with permission.
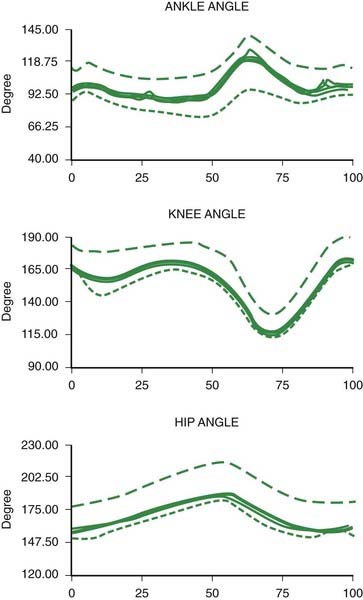
The joint angles of a normal gait cycle are illustrated in Figure 10.3. The ankle shows a brief plantar flexion at heel strike, followed by a gentle dorsiflexion in stance as the body moves over the foot. The ankle then shows a brisk plantar flexion producing push-off lasting from heel off to toe off. During swing there is a dorsiflexion of the ankle to avoid hitting the toe on the ground and to prepare for heel strike. The knee shows a slight flexion during stance and a more pronounced flexion during swing. The hip extends in stance and flexes during swing.
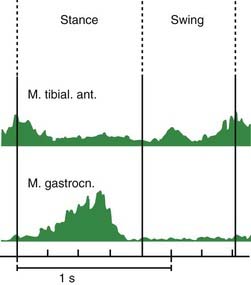
Muscle activities during gait are illustrated in Figure 10.4. During the gait cycle, the triceps surae (gastrocnemius-soleus) is active primarily at the end of stance to push off for the swing phase. The tibialis anterior muscle is active in the beginning of stance to slow the plantarflexion of the foot so that it does not slap onto the floor. Then it is active again during swing to produce the dorsiflexion of the ankle mentioned above.
Gait initiation
The initiation of gait is a special problem (Elble et al., 1994). Not only does it pose a unique biomechanical problem, it also causes some patients particular difficulty (Mancini et al., 2009). The task is to get the body moving forward and to get the first foot off the ground into a “swing phase.” In quiet standing, there is a very slight tonic activation of the triceps surae. This prevents the body from falling forward since the COM is anterior to the ankle joints. The first event is a diminution of activity in the triceps surae muscles, more prominent on the side of the leg that will become the stance leg for the first step. This by itself would cause the body to start moving forward, rotating around the ankle. Then, the tibialis anterior muscles contract, which actively rotates the body forward around the ankle. These events get the body moving.
Gait disorders
Epidemiology
Gait problems are a major neurologic problem, particularly in elderly people (Sudarsky, 2001). In this population, common causes for problems are stroke, peripheral neuropathy, brain or spinal cord trauma, and Parkinson disease. The term “senile gait” is sometimes used, but it likely does not exist as a distinct entity. Multiple medical problems accumulate with age, including visual difficulties and arthritis, that become additive.
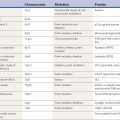
Sudarsky (1997) evaluated a series of patients referred for an unknown gait disorder. After careful neurologic evaluation, he was able to make a diagnosis in most (Table 10.1). The most frequent entities were sensory deficits, myelopathy, multiple infarcts, parkinsonism, and unknown.
Table 10.1 Frequency of etiologies of neurologically referred undiagnosed gait disorders
| Sensory deficits | 18.3% |
| Myelopathy | 16.7% |
| Multiple infarcts | 15.0% |
| Unknown | 14.2% |
| Parkinsonism | 11.7% |
| Cerebellar degeneration | 6.7% |
| Hydrocephalus | 6.7% |
| Psychogenic | 3.3% |
| Other | 7.5% |
“Other” etiologies include metabolic encephalopathy, antidepressant and sedative drugs, toxic disorders, brain tumor, and subdural hematoma.
From Sudarsky L. Clinical approaches to gait disorders of aging. In: Masdeu J, Sudarsky L, Wolfson L, eds. Gait Disorders of Aging: Falls and Therapeutic Strategies. Philadelphia: Lippincott-Raven; 1997, pp. 147–158, with permission.
Evaluation of gait
There are a number of important observations that have implications for the differential diagnosis (Nutt et al., 1993):
Veering
Deviations from a direct line of progression are due to either vestibular or cerebellar disorders.
Freezing
Freezing is also known as motor blocks and is characterized by lack of movement with the feet looking like they are glued to the floor (Snijders et al., 2008; Browner and Giladi, 2010). Patients often look like they are trying to move, but they cannot. This may be due to inability to generate sufficient postural shifting to initiate forward movement (Elble et al., 1996; Mancini et al., 2009). Freezing can occur when trying to initiate gait, in which circumstance it has also been called “start hesitation.” Freezing can also interrupt walking, and in this circumstance is sometimes precipitated by a sensory stimulus such as a doorway, the ring of a doorbell, or a street light changing color. Curiously, sensory stimuli can also be used to improve freezing. They appear to act in this regard by providing external triggers for movement.
The pathophysiology of freezing is not clear, but there are a number of associated abnormalities. Patients show defective bilateral coordination of stepping (Plotnik et al., 2008). There is an association of freezing with loss of frontal lobe executive function (Amboni et al., 2008). Another possible factor is the sequence effect where sequential movements become progressively smaller (Iansek et al., 2006; Chee et al., 2009). One study showed that freezing would occur when the demands for rapid stepping were high (Moreau et al., 2008a). Yet another factor contributing to freezing is the excessive dependence on external stimuli in patients with PD (Hallett, 2008). Imaging studies suggest that freezing is associated with abnormalities of the mesencephalic locomotor region (Snijders et al., 2011).
In addition to the absence of movement, another form of freezing is characterized by rapid, side-to-side shifting of weight, but no lifting of the feet and no forward progression. This has been called the “slipping clutch syndrome.” In this situation, physiologic studies show co-contraction activity in antagonist muscles, apparently not permitting effective forward movement (Yanagisawa et al., 2001).
Freezing is very common in idiopathic PD, but is also seen in other parkinsonian states such as progressive supranuclear palsy, vascular parkinsonism, and normal pressure hydrocephalus. It seems less common in multiple system atrophy and drug-induced parkinsonism (Giladi, 2001).
Anatomic/physiologic classification
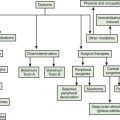
Gait disorders, like gait itself, are very complex. This chapter will follow the classification of Nutt, Marsden, and Thompson, proposed in 1993, since that has been the most commonly used (Nutt et al., 1993). There have been some more recent refinements suggested (Jankovic et al., 2001; Nutt, 2001).
Lowest level
This refers to elemental disorders such as those resulting from muscle, nerve, or root disorders. This would also include the consequences of sensory deficits such as peripheral neuropathy, vestibular or visual disorder. Severe sensory loss can produce a gait similar to that of cerebellar ataxia; these patients have particular difficulty walking in the dark. An example is the GALOP syndrome, characterized by gait “ataxia” and peripheral neuropathy associated with a monoclonal IgM specific for galopin, a central nervous system white matter antigen (Alpert, 2004).
Middle level
Hemiparetic gait
Because of a unilateral lesion of the corticospinal tract, most commonly seen with stroke, there is a stiff extended leg that circumducts during swing with scraping of the toe (Video 10.1). Typically, of course, there should be some weakness in a “pyramidal” distribution and increased reflexes. The earliest sign might be reduced knee flexion during swing (Kerrigan et al., 2001). ![]()
Treatment of the spasticity might be useful, but this must be done carefully since without spasticity, the leg might collapse and not provide support. Oral agents or intrathecal baclofen can be used. Botulinum toxin directed to the triceps surae has been used very successfully, particularly in the setting of cerebral palsy (Simpson, 1997; Flett et al., 1999).
Ataxic gait
Patients with cerebellar ataxia have difficulty with motor control by virtue of dysmetria, dyssynergia, variability of performance, and poor balance. All these features contribute to their disorder of gait (Ilg et al., 2008). Clinically, the gait is characterized by irregularity of stepping, in direction, distance, and timing. Patients may lurch in different directions. Stability of upright stance is poor and patients may fall. Just as with standing balance, the base, or distance between the feet, is said to be broad (Video 10.4). ![]()
Palliyath et al. (1998) studied the gait pattern in 10 patients with cerebellar degenerations. Gait at natural speed was studied using a video-based kinematic data acquisition system for measuring body movements. Patients showed a reduced step and stride length with a trend to reduced cadence. Heel off time, toe off time, and time of peak flexion of the knee in swing were all delayed. Range of rotation of ankle, knee, and hip were all reduced, but only ankle range of rotation reached significance. Multijoint coordination was impaired as indicated by a relatively greater delay of plantar flexion of the ankle compared with flexion of the knee and a relatively late knee flexion compared with hip flexion at the onset of swing. The patients also showed increased variability of almost all measures. While some of the deviations from normal were due simply to the slowness of walking, the gait pattern of patients with cerebellar degeneration showed incoordination similar to that previously described for their multijoint limb motion. A wide base was not seen in this study, but it was seen in another one (Hudson and Krebs, 2000).
Patients with essential tremor have a mild gait abnormality that is ataxic in type (Stolze et al., 2001b). This forms part of the evidence that essential tremor results from cerebellar dysfunction.
Parkinsonian gait
Parkinson patients often have a stooped posture and stand and walk on a narrow base (Morris et al., 2001a, 2001b). Sometimes there is marked flexion of the trunk, called camptocormia, a condition also seen in dystonia and in psychogenic conditions (Azher and Jankovic, 2005). Balance is poor and patients often fall (Kerr et al., 2010). They have short, shuffling steps, the marché à petit pas, and this can be associated with festination. They turn en bloc, and important associated signs are lack of arm swing and tremor of the hands. Freezing is common and can occur in both the “off” and “on” states. Freezing is seen in about one-quarter of patients by 4 years (Rascol et al., 2000). Abnormalities of EMG pattern have been described (Nieuwboer et al., 2004).
Gait speed in Parkinson disease is slow. If trying to walk faster, patients increase step rate proportionately more than stride length (compared with normal subjects) (Morris et al., 1994, 1998). EMG and kinematic studies have illuminated the pathophysiology (Albani et al., 2003; Svehlik et al., 2009; Nanhoe-Mahabier et al., 2011).
Variability of stride length is a gait feature that has been associated with falls. Stride time and variability were studied in patients with Parkinson disease and compared with other clinical measures (Schaafsma et al., 2003). Variability was independent of tremor, rigidity, and bradykinesia, but somewhat responsive to levodopa. Gait variability markedly increases with a simultaneous cognitive task, and this certainly would make patients more prone to falls (Hausdorff et al., 2003a). Those patients with more freezing also have more variability, suggesting that this might be a factor in the etiology of freezing (Hausdorff et al., 2003b).
Since gait does require some attention, if patients are asked to do a second task while walking, the gait performance can deteriorate (Plotnik et al., 2009). Patients can even fall. Patients realize this, at least subconsciously, and may well stop walking to answer a question. This is the basis of the “stops walking when talking” test (Lundin-Olsson et al., 1997).
Treatment of the parkinsonism with dopaminergic therapy or deep brain stimulation (DBS) improves gait, but often not as much as other motor symptoms (Lubik et al., 2006; Ferraye et al., 2008). Freezing particularly is generally difficult to treat, but off-freezing may well improve with dopaminergic medication. Another approach is the use of methylphenidate (Auriel et al., 2006; Devos et al., 2007; Pollak et al., 2007) although there is some negative evidence (Espay et al., 2011). On-freezing is a particularly difficult problem, and may even respond to lowering the dopaminergic medication. It was suggested that botulinum toxin might improve gait freezing (Giladi et al., 2001), but this was not confirmed in a double-blind trial (Wieler et al., 2005). Parkinsonian gait can also be improved by rhythmic visual or auditory clues (Suteerawattananon et al., 2004; Ledger et al., 2008; Arias and Cudeiro, 2010). Mental singing while walking may also improve gait performance (Satoh and Kuzuhara, 2008). Physical therapy can help, but may have only a short-term benefit (Ellis et al., 2005; Kwakkel et al., 2007; Frazzitta et al., 2009; Morris et al., 2010). One component of therapy should have the patients concentrate on making “big” steps (Werner and Gentile, 2010). Action observation may improve freezing of gait in Parkinson patients (Pelosin et al., 2010). Treadmill training can be helpful as well (Bello et al., 2010; Mehrholz et al., 2010). Dance therapy may have some longer lasting effects (Hackney et al., 2007; Hackney and Earhart, 2009). Transcranial magnetic stimulation may have benefit (Lomarev et al., 2006).
Indeed, it is now often the case that problems with balance and gait are continuing despite improvements in bradykinesia after DBS. This has led to attempts to improve DBS. One approach is to change the stimulation parameters (Moreau et al., 2008b). There has also been considerable interest in the pedunculopontine nucleus (PPN) as a DBS target (Stefani et al., 2007; Pereira et al., 2008).
Highest level
The highest-level disorders come from malfunction of the cerebral hemispheres, and include disorders arising from psychiatric origin, including cautious gait and psychogenic gait. These disorders are not completely distinct from each other; patients may have characteristics of more than one or may progress from one to another (Jankovic et al., 2001; Nutt, 2001; Thompson, 2001).
Subcortical disequilibrium
This is a severe impairment of balance (Masdeu, 2001). The disorder arises from dysfunction at midbrain, basal ganglia, or thalamic levels, and is often a feature of parkinsonism-plus disorders. It has been called thalamic astasia. This entity, by its etiology, is really misplaced and belongs in the middle-level category. Perhaps it really represents a combined basal ganglionic and cerebellar balance disorder.
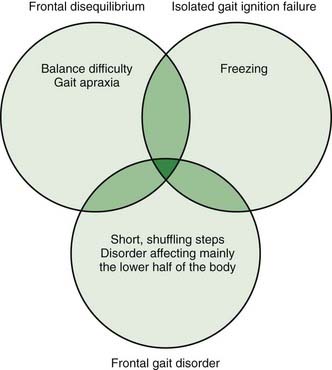
The next three conditions, frontal disequilibrium, isolated gait ignition failure, and frontal gait disorder, are frequently difficult to separate from each other and might be considered varied presentations of a frontal lobe dysfunction of gait (Figure 10.5).
Frontal disequilibrium
This is also characterized by a loss of balance, but is accompanied by disordered stepping that is difficult to describe. Some have called the stepping disorder “apraxia” (Gerstmann and Schilder, 1926) and others have called it “ataxia” (Bruns, 1892). The concept of apraxia comes from the observation that leg movements unrelated to walking seem reasonably good. It is also associated with frontal lobe signs, and it is this feature that suggests the frontal origin.
Isolated gait ignition failure
This is the particular difficulty in initiating and sustaining locomotion. The patient has freezing, particularly of the type getting started. The term primary progressive freezing gait has also been used (Factor et al., 2002). Balance can be good, and while steps begin short, they gradually increase in amplitude. Hence, the patient may say that they walk normally once they get started. This gradual increase differs from festination in that with festination, the stepping is never really normal looking.
Frontal gait disorder
These patients at first look like they have a parkinsonian gait, with short, shuffling steps, poor balance, initiation failure, and hesitations on turns (Video 10.8). Differentiating features are a more upright stance, lack of tremor, frontal lobe signs, and apparent involvement of only the lower part of the body (Thompson, 2001). This last feature gives rise to the term “lower half parkinsonism” or “lower body parkinsonism.” The disorder can evolve to a frontal disequilibrium with the difficult-to-describe stepping disorder. The disorder does not respond to levodopa. ![]()
As noted, frontal disequilibrium, isolated gait ignition failure, and frontal gait disorder often overlap. Their etiologies are also similar and include subcortical arteriosclerotic encephalopathy (Binswanger disease), multi-infarct state, anterior cerebral artery stroke, normal pressure hydrocephalus, Pick disease, Alzheimer disease, frontotemporal dementia, subdural hematoma, brain tumor, multiple sclerosis, progressive supranuclear palsy (PSP), and corticobasal degeneration (Thompson, 2001). Older patients are more likely to have gait dysfunction if they have more white matter lesions on MRI (Franch et al., 2009). Two patients have been described with lesions predominant in the supplementary motor area (SMA) (Nadeau, 2007).
The gait in normal pressure hydrocephalus has been the most studied. These patients have freezing and gait apraxia. Quantitative gait studies show decreased stride length and reduced foot floor clearance (Stolze et al., 2000, 2001a). It is, of course, critical to make this diagnosis since it is a treatable disorder (McGirt et al., 2008). The diagnosis can be tricky at times and laboratory data must be used thoughtfully (Relkin et al., 2005). An evidence-based review suggested that the best test is prolonged external lumbar drainage in excess of 300 mL, which is associated with high sensitivity (50–100%) and high positive predictive value (80–100%) (Marmarou et al., 2005). The features of gait most responsive to lumbar drainage are walking speed, number of steps for turning, and tendency for falls (Ravdin et al., 2008). MRI features can be helpful, but are not as good as lumbar drainage (Lee et al., 2010). Identification of increased pulsatility of intracranial pressure might well also be of value (Eide and Sorteberg, 2010). One study has shown that the white matter lesions in patients will improve with shunting, suggesting that this aspect may play a role in the pathophysiology of the disorder (Akiguchi et al., 2008).
Increased ventricular dilation, even independent of a formal diagnosis of normal pressure hydrocephalus, is associated with gait impairment (Palm et al., 2009). An increased ratio of ventricular volume compared with sulcal cerebrospinal fluid volume predicted gait speed; this was independent of white matter hyperintensity volume.
In the series of 30 patients reported by Factor et al. (2002) under the name of primary progressive freezing gait, they found a clinically distinct, progressive neurologic disorder that primarily affected gait, initially resulting in freezing and later in postural instability. A wheelchair-bound state often developed within 5 years. It was accompanied by other parkinsonian features, particularly bradykinesia, but was unresponsive to dopaminergic medications. Because of its stereotyped manner, they suggested that it be considered a specific entity within the parkinsonism-plus disorders. Such patients may well have progressive supranuclear palsy (Williams et al., 2007).
Another classification of frontal gait disorders has been proposed: three main types based on presumptive sites of anatomical damage: (a) ignition apraxia, where damage is predominantly in the supplementary motor area and its connections, with good responses to external clues; (b) equilibrium apraxia, where damage is predominantly in the premotor area in its connections, with poor responses to external cues; and (c) mixed gait apraxia (Liston et al., 2003). The authors of this proposal studied 13 patients with cerebral multi-infarct states and higher-level gait disorder and diagnosed 7 with ignition apraxia and 6 with equilibrium apraxia.
In some patients with frontal gait disorder at least one component of the difficulty may be due to orthostatic myoclonus–jerking of the legs only when standing (Glass et al., 2007). In 13 of 15 patients with postural unsteadiness due to this type of myoclonus, there was also a frontal gait disorder. Some of these patients improved with clonazepam.
While the term frontal gait disorder is used, it is not completely clear where the relevant pathology is. There is, in fact, some evidence for frontal dysfunction. For example, relevant white matter lesions leading to poor gait were largely in the frontal lobe (Srikanth et al., 2010). One study looked at SPECT scanning in patients with subcortical chronic vascular encephalopathy with and without walking. In those patients with gait dysfunction, there was less activation of the medial frontal gyrus bilaterally (as well as the anterior lobes of the cerebellum) (Carboncini et al., 2009). Dysfunction of the basal ganglio-thalamo-cortical loop may also play a role (Iseki et al., 2010).
Cautious gait
This is a common disorder, particularly in the elderly or with patients who have already experienced a fall for some reason (Kurlan, 2005; Sudarsky, 2006). The term “space phobia” has been used. Patients become cautious because of anxiety that they will fall. The gait is like what it would be for normal persons who are walking on ice. There is a wide base with slow, short steps; turns are en bloc. Arms are tense. Patients try to find support and when they have it, there is marked improvement. These individuals do not have overt freezing or shuffling.
Psychogenic gait
A gait disorder is a common way for psychogenic movement disorders to present (Lempert et al., 1991; Hayes et al., 1999; Bhatia, 2001; Morris et al., 2006; Sudarsky, 2006; Baik and Lang, 2007) (Video 10.9) This is also called astasia–abasia or acrobatic gait. There are unusual patterns of stance and gait, often inconsistent, often dramatic, with lurching, but only rarely falls (and then without hurting themselves). Sudden knee buckling without falling is a common pattern (Lempert et al., 1991). Extreme slow motion can be seen, and there is a sense that energy is being wasted. Camptocormia is one of the patterns that is commonly psychogenic (Skidmore et al., 2007). Careful observation will show that the person typically demonstrates excellent balance. There may well be wide fluctuations over short periods of time. As with other psychogenic movement disorders, positive psychiatric features are frequent and can be important in making a clear diagnosis. A possible clue to this is a suffering or strained facial expression, with moaning and hyperventilation (Lempert et al., 1991). ![]()
Akiguchi I., Ishii M., Watanabe Y., et al. Shunt-responsive parkinsonism and reversible white matter lesions in patients with idiopathic NPH. J Neurol. 2008;255(9):1392-1399.
Albani G., Sandrini G., Kunig G., et al. Differences in the EMG pattern of leg muscle activation during locomotion in Parkinson’s disease. Funct Neurol. 2003;18(3):165-170.
Alpert J.N. GALOP syndrome: case report with 7-year follow-up. South Med J. 2004;97(4):410-412.
Amboni M., Cozzolino A., Longo K., et al. Freezing of gait and executive functions in patients with Parkinson’s disease. Mov Disord. 2008;23(3):395-400.
Arias P., Cudeiro J. Effect of rhythmic auditory stimulation on gait in Parkinsonian patients with and without freezing of gait. PLoS ONE. 2010;5(3):e9675.
Auriel E., Hausdorff J.M., Herman T., et al. Effects of methylphenidate on cognitive function and gait in patients with Parkinson’s disease: a pilot study. Clin Neuropharmacol. 2006;29(1):15-17.
Azher S.N., Jankovic J. Camptocormia: pathogenesis, classification, and response to therapy. Neurology. 2005;65(3):355-359.
Baik J.S., Lang A.E. Gait abnormalities in psychogenic movement disorders. Mov Disord. 2007;22(3):395-399.
Bello O., Marquez G., Camblor M., Fernandez-Del-Olmo M. Mechanisms involved in treadmill walking improvements in Parkinson’s disease. Gait Posture. 2010;32(1):118-123.
Benvenuti F. Physiology of human balance. Adv Neurol. 2001;87:41-51.
Bhatia K.P. Psychogenic gait disorders. Adv Neurol. 2001;87:251-254.
Browner N., Giladi N. What can we learn from freezing of gait in Parkinson’s disease? Curr Neurol Neurosci Rep. 2010;10(5):345-351.
Bruns L. Uber storugen des gleichgewichtes bei stirnhirntumoren. Dtsch Med Wochensch. 1892;18:138-140.
Burke R.E. The central pattern generator for locomotion in mammals. Adv Neurol. 2001;87:11-24.
Carboncini M.C., Volterrani D., Bonfiglio L., et al. Higher level gait disorders in subcortical chronic vascular encephalopathy: a single photon emission computed tomography study. Age Ageing. 2009;38(3):302-307.
Chee R., Murphy A., Danoudis M., et al. Gait freezing in Parkinson’s disease and the stride length sequence effect interaction. Brain. 2009;132(Pt 8):2151-2160.
Conrad B., Meinck H.M., Benecke R. Motor patterns in human gait: adaptation to different modes of progression. In: Bles W., Brandt T., editors. Disorders of Posture and Gait. Amsterdam: Elsevier Science Publishers BV; 1986:53-67.
Devos D., Krystkowiak P., Clement F., et al. Improvement of gait by chronic, high doses of methylphenidate in patients with advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78(5):470-475.
Diener H.C., Dichgans J., Bacher M., Gompf B. Quantification of postural sway in normals and patients with cerebellar diseases. Electroencephalog Clin Neurophysiol. 1984;57:134-142.
Eide P.K., Sorteberg W. Diagnostic intracranial pressure monitoring and surgical management in idiopathic normal pressure hydrocephalus: a 6-year review of 214 patients. Neurosurgery. 2010;66(1):80-91.
Elble R.J., Cousins R., Leffler K., Hughes L. Gait initiation by patients with lower-half parkinsonism. Brain. 1996;119(Pt 5):1705-1716.
Elble R.J., Moody C., Leffler K., Sinha R. The initiation of normal walking. Mov Disord. 1994;9(2):139-146.
Ellis T., de Goede C.J., Feldman R.G., et al. Efficacy of a physical therapy program in patients with Parkinson’s disease: a randomized controlled trial. Arch Phys Med Rehabil. 2005;86(4):626-632.
Espay A.J., Owivedi A.K., Payne M., et al. Methylphenidate for gait impairment in Parkinson disease: a randomized clinical trial. Neurology. 2011;76(14):1256-1262.
Factor S.A., Jennings D.L., Molho E.S., Marek K.L. The natural history of the syndrome of primary progressive freezing gait. Arch Neurol. 2002;59(11):1778-1783.
Ferraye M.U., Debu B., Fraix V., et al. Effects of subthalamic nucleus stimulation and levodopa on freezing of gait in Parkinson disease. Neurology. 2008;70(16 Pt 2):1431-1437.
Flett P.J., Stern L.M., Waddy H., et al. Botulinum toxin A versus fixed cast stretching for dynamic calf tightness in cerebral palsy. J Paediatr Child Health. 1999;35(1):71-77.
Franch O., Calandre L., Alvarez-Linera J., et al. Gait disorders of unknown cause in the elderly: Clinical and MRI findings. J Neurol Sci. 2009;280(1–2):84-86.
Frazzitta G., Maestri R., Uccellini D., et al. Rehabilitation treatment of gait in patients with Parkinson’s disease with freezing: A comparison between two physical therapy protocols using visual and auditory cues with or without treadmill training. Mov Disord. 2009;24(8):1139-1143.
Gatev P., Thomas S., Lou J.-S., et al. Effects of diminished and conflicting sensory information on balance in patients with cerebellar deficits. Mov Disord. 1996;11(6):654-664.
Gerstmann J., Schilder P. Uber eine besondere gangstorieng bei stirnhirnerkankhungen. Wien Med Wschr. 1926;76:97-107.
Giladi N. Freezing of gait. Clinical overview. Adv Neurol. 2001;87:191-197.
Giladi N., Gurevich T., Shabtai H., et al. The effect of botulinum toxin injections to the calf muscles on freezing of gait in parkinsonism: a pilot study. J Neurol. 2001;248(7):572-576.
Glass G.A., Ahlskog J.E., Matsumoto J.Y. Orthostatic myoclonus: a contributor to gait decline in selected elderly. Neurology. 2007;68(21):1826-1830.
Hackney M.E., Earhart G.M. Effects of dance on movement control in Parkinson’s disease: a comparison of Argentine tango and American ballroom. J Rehabil Med. 2009;41(6):475-481.
Hackney M.E., Kantorovich S., Levin R., Earhart G.M. Effects of tango on functional mobility in Parkinson’s disease: a preliminary study. J Neurol Phys Ther. 2007;31(4):173-179.
Hallett M. The intrinsic and extrinsic aspects of freezing of gait. Mov Disord. 2008;23(Suppl 2):S439-S443.
Hausdorff J.M., Balash J., Giladi N. Effects of cognitive challenge on gait variability in patients with Parkinson’s disease. J Geriatr Psychiatry Neurol. 2003;16(1):53-58.
Hausdorff J.M., Schaafsma J.D., Balash Y., et al. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp Brain Res. 2003;149(2):187-194.
Hayes M.W., Graham S., Heldorf P., et al. A video review of the diagnosis of psychogenic gait: appendix and commentary. Mov Disord. 1999;14(6):914-921.
Hudson C.C., Krebs D.E. Frontal plane dynamic stability and coordination in subjects with cerebellar degeneration. Exp Brain Res. 2000;132(1):103-113.
Iansek R., Huxham F., McGinley J. The sequence effect and gait festination in Parkinson disease: contributors to freezing of gait? Mov Disord. 2006;21(9):1419-1424.
Ilg W., Giese M.A., Gizewski E.R., et al. The influence of focal cerebellar lesions on the control and adaptation of gait. Brain. 2008;131(Pt 11):2913-2927.
Iseki K., Hanakawa T., Hashikawa K., et al. Gait disturbance associated with white matter changes: a gait analysis and blood flow study. Neuroimage. 2010;49(2):1659-1666.
Jacobs J.V., Horak F.B., Van Tran K., Nutt J.G. An alternative clinical postural stability test for patients with Parkinson’s disease. J Neurol. 2006;253(11):1404-1413.
Jankovic J., Nutt J.G., Sudarsky L. Classification, diagnosis, and etiology of gait disorders. Adv Neurol. 2001;87:119-133.
Kaufman K.R., Shaughnessy W.J., Noseworthy J.H. Use of motion analysis for quantifying movement disorders. Adv Neurol. 2001;87:71-81.
Kerr G.K., Worringham C.J., Cole M.H., et al. Predictors of future falls in Parkinson disease. Neurology. 2010;75(2):116-124.
Kerrigan D.C., Karvosky M.E., Riley P.O. Spastic paretic stiff-legged gait: joint kinetics. Am J Phys Med Rehabil. 2001;80(4):244-249.
Kurlan R. “Fear of falling” gait: a potentially reversible psychogenic gait disorder. Cogn Behav Neurol. 2005;18(3):171-172.
Kwakkel G., de Goede C.J., van Wegen E.E. Impact of physical therapy for Parkinson’s disease: a critical review of the literature. Parkinsonism Relat Disord. 2007;13(Suppl 3):S478-S487.
Ledger S., Galvin R., Lynch D., Stokes E.K. A randomised controlled trial evaluating the effect of an individual auditory cueing device on freezing and gait speed in people with Parkinson’s disease. BMC Neurol. 2008;8:46.
Lee W.J., Wang S.J., Hsu L.C., et al. Brain MRI as a predictor of CSF tap test response in patients with idiopathic normal pressure hydrocephalus. J Neurol. 2010;257(10):1675-1681.
Lempert T., Brandt T., Dieterich M., Huppert D. How to identify psychogenic disorders of stance and gait. A video study in 37 patients. J Neurol. 1991;238(3):140-146.
Liston R., Mickelborough J., Bene J., Tallis R. A new classification of higher level gait disorders in patients with cerebral multi-infarct states. Age Ageing. 2003;32(3):252-258.
Lomarev M.P., Kanchana S., Bara-Jimenez W., et al. Placebo-controlled study of rTMS for the treatment of Parkinson’s disease. Mov Disord. 2006;21(3):325-331.
Lubik S., Fogel W., Tronnier V., et al. Gait analysis in patients with advanced Parkinson disease: different or additive effects on gait induced by levodopa and chronic STN stimulation. J Neural Transm. 2006;113(2):163-173.
Lundin-Olsson L., Nyberg L., Gustafson Y. “Stops walking when talking” as a predictor of falls in elderly people. Lancet. 1997;349(9052):617.
Mancini M., Zampieri C., Carlson-Kuhta P., et al. Anticipatory postural adjustments prior to step initiation are hypometric in untreated Parkinson’s disease: an accelerometer-based approach. Eur J Neurol. 2009;16(9):1028-1034.
Marmarou A., Bergsneider M., Klinge P., et al. The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57(3 Suppl):S17-S28. discussion ii–v
Masdeu J.C. Central disequilibrium syndromes. Adv Neurol. 2001;87:183-189.
McGirt M.J., Woodworth G., Coon A.L., et al. Diagnosis, treatment, and analysis of long-term outcomes in idiopathic normal-pressure hydrocephalus. Neurosurgery. 2008;62(Suppl 2):670-677.
Mehrholz Mehrholz, J., Friis, R., Kugler, J., et al., 2010. Treadmill training for patients with Parkinson’s disease. Cochrane Database Syst Rev (1), CD007830.
Moreau C., Defebvre L., Bleuse S., et al. Externally provoked freezing of gait in open runways in advanced Parkinson’s disease results from motor and mental collapse. J Neural Transm. 2008;115(10):1431-1436.
Moreau C., Defebvre L., Destee A., et al. STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology. 2008;71(2):80-84.
Mori S., Matsuyama K., Mori F., Nakajima K. Supraspinal sites that induce locomotion in the vertebrate central nervous system. Adv Neurol. 2001;87:25-40.
Morris J.G., de Moore G.M., Herberstein M. Psychogenic gait: An example of deceptive signaling. In: Hallett M., Fahn S., Jankovic J., et al, editors. Psychogenic Movement Disorders: Neurology and Neuropsychiatry. Philadelphia: Lippincott Williams & Wilkins; 2006:69-75.
Morris M.E., Huxham F., McGinley J., et al. The biomechanics and motor control of gait in Parkinson disease. Clin Biomech (Bristol, Avon). 2001;16(6):459-470.
Morris M.E., Huxham F.E., McGinley J., Iansek R. Gait disorders and gait rehabilitation in Parkinson’s disease. Adv Neurol. 2001;87:347-361.
Morris M.E., Iansek R., Matyas T.A., Summers J.J. Ability to modulate walking cadence remains intact in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1994;57(12):1532-1534.
Morris M., Iansek R., Matyas T., Summers J. Abnormalities in the stride length-cadence relation in parkinsonian gait. Mov Disord. 1998;13(1):61-69.
Morris M.E., Martin C.L., Schenkman M.L. Striding out with Parkinson disease: evidence-based physical therapy for gait disorders. Phys Ther. 2010;90(2):280-288.
Nadeau S.E. Gait apraxia: further clues to localization. Eur Neurol. 2007;58(3):142-145.
Nanhoe-Mahabier W., Snijders A.H., Delval A., et al. Walking patterns in Parkinson’s disease with and without freezing of gait. Neuroscience. 2011. [epub ahead of print]
Nieuwboer A., Dom R., De Weerdt W., et al. Electromyographic profiles of gait prior to onset of freezing episodes in patients with Parkinson’s disease. Brain. 2004;127(Pt 7):1650-1660.
Nutt J.G. Classification of gait and balance disorders. Adv Neurol. 2001;87:135-141.
Nutt J.G., Marsden C.D., Thompson P.D. Human walking and higher-level gait disorders, particularly in the elderly. Neurology. 1993;43(2):268-279.
Pai Y.C., Rogers M.W., Patton J., et al. Static versus dynamic predictions of protective stepping following waist-pull perturbations in young and older adults. J Biomech. 1998;31(12):1111-1118.
Palliyath S., Hallett M., Thomas S.L., Lebiedowska M.K. Gait in patients with cerebellar ataxia. Mov Disord. 1998;13(6):958-964.
Palm W.M., Saczynski J.S., van der Grond J., et al. Ventricular dilation: association with gait and cognition. Ann Neurol. 2009;66(4):485-493.
Panzer V.P., Zeffiro T.A., Hallett M. Kinematics of standing posture associated with aging and Parkinson’s disease. In: Brandt T., Paulus W., Bles W., et al, editors. Disorders of Posture and Gait. Stuttgart: Georg Thieme; 1990:390-393.
Pelosin E., Avanzino L., Bove M., et al. Action observation improves freezing of gait in patients with Parkinson’s disease. Neurorehabil Neural Repair. 2010;24(8):746-752.
Pereira E.A., Muthusamy K.A., De Pennington N., et al. Deep brain stimulation of the pedunculopontine nucleus in Parkinson’s disease. Preliminary experience at Oxford. Br J Neurosurg. 2008;22(Suppl 1):S41-S44.
Plotnik M., Giladi N., Hausdorff J.M. Bilateral coordination of walking and freezing of gait in Parkinson’s disease. Eur J Neurosci. 2008;27(8):1999-2006.
Plotnik M., Giladi N., Hausdorff J.M. Bilateral coordination of gait and Parkinson’s disease: the effects of dual tasking. J Neurol Neurosurg Psychiatry. 2009;80(3):347-350.
Pollak L., Dobronevsky Y., Prohorov T., et al. Low dose methylphenidate improves freezing in advanced Parkinson’s disease during off-state. J Neural Transm Suppl. 2007;72:145-148.
Rascol O., Brooks D.J., Korczyn A.D., et al. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. 056 Study Group. N Engl J Med. 2000;342(20):1484-1491.
Ravdin L.D., Katzen H.L., Jackson A.E., et al. Features of gait most responsive to tap test in normal pressure hydrocephalus. Clin Neurol Neurosurg. 2008;110(5):455-461.
Relkin N., Marmarou A., Klinge P., et al. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57(3 Suppl):S4-S16. discussion ii–v
Satoh M., Kuzuhara S. Training in mental singing while walking improves gait disturbance in Parkinson’s disease patients. Eur Neurol. 2008;60(5):237-243.
Schaafsma J.D., Giladi N., Balash Y., et al. Gait dynamics in Parkinson’s disease: relationship to Parkinsonian features, falls and response to levodopa. J Neurol Sci. 2003;212(1–2):47-53.
Simpson D.M. Clinical trials of botulinum toxin in the treatment of spasticity. Muscle Nerve Suppl. 1997;6(75):S169-S175.
Skidmore F., Anderson K., Fram D., Weiner W. Psychogenic camptocormia. Mov Disord. 2007;22(13):1974-1975.
Snijders A.H., Nijkrake M.J., Bakker M., et al. Clinimetrics of freezing of gait. Mov Disord. 2008;23(Suppl 2):S468-S474.
Snijders A.H., Leunissen I., Bakker M., et al. Gait-related cerebral alterations in patients with Parkinson’s disease with freezing of gait. Brain. 2011;134(Pt 1):59-72.
Srikanth V., Phan T.G., Chen J., et al. The location of white matter lesions and gait – a voxel-based study. Ann Neurol. 2010;67(2):265-269.
Stefani A., Lozano A.M., Peppe A., et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007;130(Pt 6):1596-1607.
Stolze H., Kuhtz-Buschbeck J.P., Drucke H., et al. Gait analysis in idiopathic normal pressure hydrocephalus – which parameters respond to the CSF tap test? Clin Neurophysiol. 2000;111(9):1678-1686.
Stolze H., Kuhtz-Buschbeck J.P., Drucke H., et al. Comparative analysis of the gait disorder of normal pressure hydrocephalus and Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2001;70(3):289-297.
Stolze H., Petersen G., Raethjen J., et al. The gait disorder of advanced essential tremor. Brain. 2001;124(Pt 11):2278-2286.
Sudarsky L. Geriatrics: gait disorders in the elderly. N Engl J Med. 1990;322(20):1441-1446.
Sudarsky L. Clinical approaches to gait disorders of aging. In: Masdeu J., Sudarsky L., Wolfson L., editors. Gait Disorders of Aging: Falls and Therapeutic Strategies. Philadelphia: Lippincott-Raven; 1997:147-158.
Sudarsky L. Gait disorders: prevalence, morbidity, and etiology. Adv Neurol. 2001;87:111-117.
Sudarsky L. Psychogenic gait disorders. Semin Neurol. 2006;26(3):351-356.
Suteerawattananon M., Morris G.S., Etnyre B.R., et al. Effects of visual and auditory cues on gait in individuals with Parkinson’s disease. J Neurol Sci. 2004;219(1–2):63-69.
Svehlik M., Zwick E.B., Steinwender G., et al. Gait analysis in patients with Parkinson’s disease off dopaminergic therapy. Arch Phys Med Rehabil. 2009;90(11):1880-1886.
Thompson P.D. Gait disorders accompanying diseases of the frontal lobes. Adv Neurol. 2001;87:235-241.
Werner W.G., Gentile A.M. Improving gait and promoting retention in individuals with Parkinson’s disease: a pilot study. J Neurol. 2010;257(11):1841-1847.
Wieler M., Camicioli R., Jones C.A., Martin W.R. Botulinum toxin injections do not improve freezing of gait in Parkinson disease. Neurology. 2005;65(4):626-628.
Williams D.R., Holton J.L., Strand K., et al. Pure akinesia with gait freezing: a third clinical phenotype of progressive supranuclear palsy. Mov Disord. 2007;22(15):2235-2241.
Winter D.A. The Biomechanics and Motor Control of Human Gait: Normal, Elderly and Pathological, second ed. Waterloo: University of Waterloo Press; 1991.
Yanagisawa N., Hayashi R., Mitoma H. Pathophysiology of frozen gait in Parkinsonism. Adv Neurol. 2001;87:199-207.