Chapter 9 Fundamentals of Regenerative Medicine and Its Applications to Electrophysiology
The adult human heart has long been accepted as an end organ having no regenerative properties. In contrast, nonmammalian species such as zebrafish recover completely after ventricular apical resection thereby manifesting cardiac regeneration.1 Regenerative medicine builds on such observations, with the aim to replace or regenerate cells, tissues, and organs to restore or establish normal function.
Despite previous wisdom, recent evidence suggests that (1) adult human cardiomyocytes have mitotic potential, (2) cardiac progenitor cells can be isolated, (3) cardiomyocyte turnover occurs, and (4) human embryonic stem cells can differentiate into cardiomyocyte-like cells in culture.2–7 These discoveries have sparked excitement about the idea of repopulating the heart with healthy cardiomyocytes after a myocardial infarction, an idea that only recently was considered science fiction.4 Part of the effort in regenerative medicine has focused on cardiac arrhythmias. This effort has drawn on knowledge of the molecular and biophysical properties of the ion channels and signaling molecules that contribute to the initiation and propagation of the action potential. The effort has gained impetus from continued disappointment with the performance of antiarrhythmic drugs.
Gene Transfer by Viral Vectors
A successful gene therapy strategy must be safe, easy to deliver, and predictable in expression, efficacy, and duration of effect. Viral vectors have been widely used for gene transfer. Several factors need to be considered in choosing a viral vector: (1) the size of the gene it can incorporate; (2) the ease of genetic manipulation; (3) the ability to infect the target cell type; (4) replication deficiency; (5) lack of inflammatory and oncogenic potential; and (6) reliability of expression. Adenovirus and adeno-associated virus are favored for proof of concept studies because they are easily manipulated and have high expression levels. Durability of expression is the major limitation of adenovirus. Adeno-associated virus can mediate expression in the heart for months, if not longer, but the packaged gene size is limited to under five kilobases. Retroviruses such as lentivirus are incorporated into the genome and have the potential for long-term expression of the therapeutic gene.8–10
Stem Cell–Based Therapy
The multipotency of progenitor cells and pluripotency of embryonic stem cells are the fundamental properties that make them attractive for regenerative medicine, but they also raise safety questions. Undesirable differentiation and proliferation of stem cells may cause tumor formation. Also, stem cells migrate and home to specific biochemical signals. These homing properties can be exploited to target them to specific areas of disease.11 Alternatively, stem cells may migrate and lose their effect if they detect a chemoattractant located elsewhere. While autologous progenitor cells are ideal for limiting rejection, some stem cells (e.g., mesenchymal stem cells) appear to be immunoprivileged and have potential application in allogeneic therapy. Genetic engineering of stem cells can be accomplished by various techniques that use viruses, electroporation, or liposomes. Stem cells also represent a platform for “designer” therapeutics.
Treatment Strategies for Bradyarrhythmias: Biologic Pacemakers
Properties of an Ideal Biologic Pacemaker
Advances in microcircuitry and battery technology have miniaturized the modern electronic pacemaker such that implantation is now a routine procedure done outside a surgical operating suite. However, electronic pacemaker therapy has some shortcomings, such as the requirement for permanent hardware implantation, limited battery life, potential for malfunction, and a foreign body that may serve as a nidus for infections. The extraction of an infected pacemaker (especially an infected lead) is a complex undertaking that has a significant risk of mortality. The placement of pacemaker leads and the activation of the myocardium may impact unfavorably on cardiac contractility and electrophysiology. Furthermore, electronic pacemakers are not responsive to autonomic stimulation, especially that related to a physical activity or an emotional state. In the pediatric patient, electronic pacemaker hardware must be selected taking physical growth into consideration. Lastly, the function of the electronic pacemaker is prone to interference from common consumer electronic devices as well as medical equipment such as magnetic resonance imaging (MRI) equipment. These limitations have led to interest in the development of biologic pacemakers.12,13
Strategies to Create Biologic Pacemakers
Gene Therapy
Overexpression of β-Adrenergic Receptors
Glycoprotein (G-protein)–coupled β-adrenergic receptors regulate chronotropic and ionotropic responses to circulating catecholamines. The first successful gene transfer experiment resulting in biologic pacemaking used plasmids to overexpress the human β2-adrenergic receptor in the murine atrium.14 Edelberg et al then demonstrated that gene transfer was feasible by using catheter-based injection of plasmid into the procine right atrium. Overexpressing the human β2-adrenergic receptor was shown to increase heart rate by about 50% 2 days after plasmid injection.15 Further application of these studies was limited because the plasmid-based gene delivery system conferred only short-lived expression.
Inhibition of Diastolic Repolarization Current, IK1
Mutations within the pore regions of channels can dramatically affect channel conductance. Miake et al introduced an adenovirus packaged with the Kir2.1AAA mutant and green fluorescent protein (GFP) into the guinea pig left ventricle cavity.16 Transfected myocytes showed 80% suppression of IK1. The Kir2.1AAA-expressing myocytes exhibited two electrophysiological behaviors: (1) They lacked spontaneous activity with elicited prolonged action potentials, or (2) they expressed spontaneous activity remarkably similar to that of sinoatrial pacemaker cells. The electrocardiograms (ECGs) of transfected animals showed that half of them remained in sinus rhythm with QT prolongation, and the other half showed spontaneous ventricular rhythms that were at times faster than sinus.
Overexpression of If
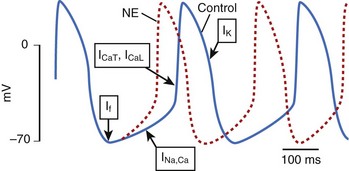
Ion channels encoded by the HCN (hyperpolarization-activated, cyclic nucleotide–gated) gene family underlie the pacemaker current, If, which initiates depolarization during phase 4 of the sinoatrial action potential (Figure 9-1). Because If only activates on hyperpolarization, it does not have the potential to prolong the duration of the action potential and initiate proarrhythmia on this basis. Qu et al injected adenovirus carrying the mouse HCN2 gene (one of four HCN isoforms), into the canine left atrial appendage.17 A spontaneous cardiac rhythm originated from the left atrium in all four dogs studied during sinus arrest (induced by right vagal stimulation). Patch-clamping of isolated HCN2-expressing atrial myocytes showed If current magnitude 500 times greater than that in control atrial myocytes.
Plotnikov et al used catheter-based endocardial injection to deliver the adenovirus expressing HCN2 into the canine proximal left bundle branch, an ideal site for providing organized left ventricular activation when the distal conducting system is functional.18 Two days later, a left ventricular rhythm was observed during sinus arrest induced by vagal stimulation. Subsequently, stable pacemaker function was demonstrated following HCN2 injection into the left bundle branch of dogs with complete heart block.19 Expression with this adenoviral construct lasted 2 weeks.
Ion Channel Mutations
Structure-function studies of HCN2 channels have revealed that certain amino acids are critical to defining the channel’s operating characteristics. A point mutation (glutamic acid to alanine at position 324, E324A) in mHCN2 positively shifted the voltage dependence of activation and deactivation gating kinetics. The positive shift in voltage dependence of E324A channels generates a faster pacemaker rate and increased sensitivity to catecholamines than native HCN2. When adenovirus expressing E324A was injected into the canine left bundle branch, dogs receiving E324A were significantly more responsive to catecholamines.19 During epinephrine infusion, all E324A-injected dogs had their heart rate increase by at least 50%, whereas only a third of the HCN2-injected dogs and a fifth of the control dogs had a similar response. The E324A study also illustrates that gene therapy is not limited to using endogenous genes but that mutations can be tailored to function.
A chimeric approach to creating an HCN-based biologic pacemaker with faster basal rates was undertaken by Plotnikov et al.20 A channel with the N and C terminals of HCN2 and the transmembrane domains of HCN1 was created (HCN212) that would have HCN2’s superior catecholamine response with the favorable activation kinetics of HCN1. The HCN212 chimera had similar electrophysiological characteristics to HCN2 when expressed in isolated ventricular myocytes; however, the mean time constant of activation was faster in HCN212. An HCN212-based biologic pacemaker would likely result in a faster basal rate than an HCN2-based one, as more current would pass earlier during diastolic depolarization. Expression of HCN212 into the left bundle of dogs with complete heart block resulted in rapid ventricular tachycardia originating from the adenoviral injection site that was responsive to the If-blocking drug, ivabradine. Additional work to fine tune an HCN-based biologic pacemaker is needed. If If-associated arrhythmias occur with HCN-based pacemakers, If-blocking drugs maybe useful in the suppression of these arrhythmias.
Tse et al working with an HCN1 mutant (HCN1-ΔΔΔ) with a deletion in the S3-S4 linker (position 235 to 237) created a biologic pacemaker in the procine atrium.21 The HCN1-ΔΔΔ mutation favors channel opening, and its expression in ventricular myocytes has been shown to result in automaticity with rates greater than 200 beats/min. In a porcine model of sick sinus syndrome, HCN1-ΔΔΔ was transduced with an adenoviral vector into the left atrium, and an electronic pacemaker was implanted. The HCN1-ΔΔΔ–injected pigs exhibited atrial pacemaking activity originating from the left atrium, which increased with catecholamines. The approach of Tse et al relies on normal atrioventricular (AV) nodal conduction to activate the ventricle. In a heart with impaired AV conduction, biologic pacemakers in the atrium will not effectively pace the ventricle.
Kv1.4 is a member of the Shaker K channel gene family. When expressed in heterologous systems, Kv1.4 channels express depolarization-activated delayed rectifier potassium currents. Furthermore, Kv1 gene family members are not expressed significantly in cardiac tissue. Kashiwakura et al created an ion channel based on Kv1.4, but functionally similar to HCN channels.22 This synthetic If-like channel was made via three point mutations within the voltage sensor in S4 (R447N, L448A, R453I): The channel was hyperpolarization activated and had a point mutation in the pore region (G528S), conferring permeability to K+ and Na+. Because the genes of Kv and HCN channels produce tetrameric ion channel complexes, hetero-multimerization with products from genes of the same family can occur. One advantage of a Kv1.4-based, If-like channel is that hetero-multimerization with native If channels (HCN genes) will not occur. A disadvantage of the synthetic Kv channel is that the cyclic adenosine monophosphate (cAMP) binding that is essential to the autonomic responsiveness of HCN channels is not replicated. Three to five days after injecting the synthetic Kv1.4 channel into guinea pig hearts, pacemaker function was detected. Patch clamp records of isolated myocytes revealed a robust hyperpolarization-activated inward current, and the myocytes also exhibited spontaneous action potentials.
Cell Therapy Approaches
Human Mesenchymal Stem Cells Overexpressing HCN2
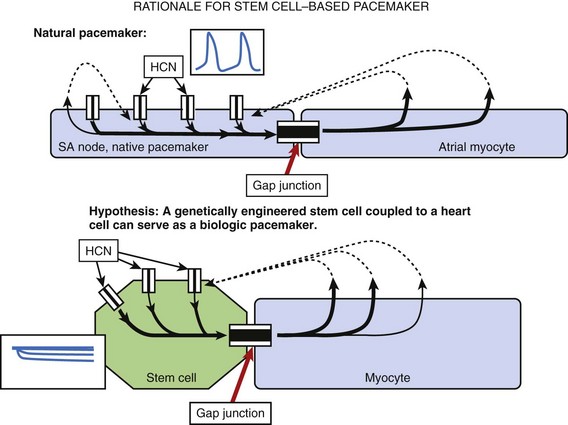
Human mesenchymal stem cells (hMSC) are readily available, are easily harvested, and can be maintained in culture. They appear to be immunoprivileged, a property that facilitates allogeneic transplantation without significant rejection. The rationale for using hMSC to build a biologic pacemaker is shown in Figure 9-2.
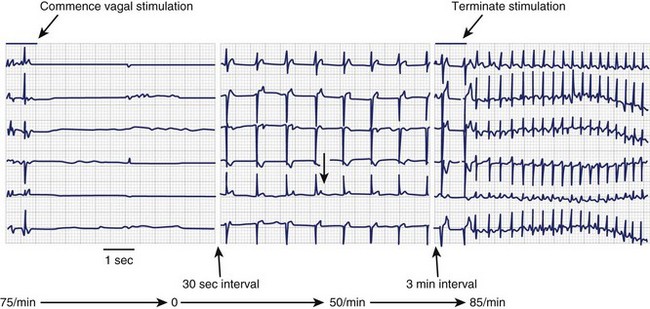
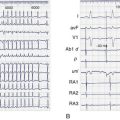
Potapova et al showed that robust If is present in hMSC transfected via electroporation with the murine HCN2 gene and that they possess the cellular machinery required to respond to neurohumors.23 In co-cultures of HCN2-hMSC with canine ventricular myocytes, dual whole-cell recording of hMSC and myocyte pairs demonstrated electrical coupling. Furthermore, ventricular myocytes co-cultured with HCN2-hMSC had more positive maximum diastolic potentials and faster spontaneous rates than had myocytes cultured with hMSC expressing GFP alone. Dogs implanted with HCN2-hMSC had significantly faster idioventricular rates originating from the implant site than did controls (Figure 9-3). hMSC were identified histologically, and immunostaining revealed connexin43 in junctions between the hMSC and canine ventricular myocytes in vivo. No inflammation or rejection was seen, underscoring the immunoprivileged status of hMSC. Moreover, HCN2-hMSC provided reliable biologic pacing for up to 6 weeks without wandering, rejection, or apoptosis.24
Human Embryonic Stem Cell–Derived Cardiac Pacemaker
Human embryonic stem cells (hESC) are derived from blastocysts, are pluripotent, and can be maintained in culture in the undifferentiated state for prolonged periods. hESC can be directed down the cardiomyocyte lineage in culture, raising the possibility of producing biologic pacemakers.7 These cells have spontaneous action potentials, large sodium current, and If. Kehat et al injected the spontaneously beating embryoid bodies into the left ventricular wall in 13 pigs with complete heart block.25 Eleven had ventricular rhythms that originated from the injection site. Histologic examination revealed the hESC-derived cardiomyocytes and gap junction formation with neighboring porcine cardiomyocytes. Xue et al had similar findings using an hESC line stably expressing GFP after lentiviral gene transduction.26 Following implantation of hESC into guinea pig heart, optical mapping revealed membrane depolarizations propagating from the injection site to the surrounding myocardium.
Chemically Induced Fusion of HCN1 Expressing Fibroblast with Cardiomyocytes
An alternative approach to couple biologic pacemakers to the myocardium is direct fusion of the cell-based pacemaker with resident cardiomyocytes. Cho et al explored chemically induced fusion between a cell-based pacemaker and the host myocardium to achieve electrical integration.27 In this study, a guinea pig lung fibroblast cell line stably expressing HCN1 channels was established. These fibroblasts were mixed with isolated guinea pig ventricular myocytes in the presence of polyethylene glycol in vitro. Cell fusion occurred rapidly and formed heterokaryons, which exhibited spontaneous action potentials having phase 4 depolarization. HCN1-expressing fibroblasts suspended in polyethylene glycol (PEG) solution were then directly injected into the cardiac apex of the guinea pigs. More than a third of them exhibited an idioventricular rhythm pace mapped to the apex for up to 3 weeks. Such use of a local chemical fusion agent could be expected to anchor biologic pacemaker therapy at a specific site and minimize pacemaker cell migration.
Treatment Strategies for Tachyarrhythmias
Atrioventricular Nodal Conduction Inhibition
Rapid ventricular rates during atrial fibrillation can often be difficult to control medically. AV nodal modification by gene-based and cell-based methodologies has been explored as an alternative therapy. In the AV node, β-adrenergic effects on adenylyl cyclase speed conduction and are countered by Gαi, which is coupled to muscarinic M2 and adenosine A1 receptors. Gαi binds and inactivates adenylyl cyclase counteracting the actions of β-adrenergic stimulation and slowing AV nodal conduction. In a porcine model of atrial fibrillation (AF), adenovirus encoding Gαi2 was arterially infused into the AV nodal region; it reduced ventricular rates during acutely induced AF. A follow-up study using an adenovirus carrying a constitutively active mutant of Gαi2 (Gαi2-Q205L) provided continuous heart rate control; similar results were obtained when a Ca2+ channel–inhibiting G-protein, GEM, was delivered by gene transfer to the AV node.28,29
A study with a cell-based approach to modifying AV nodal conduction used autologous fibroblasts pretreated with transforming growth factor-β (TGF-β), a fibroblast stimulant.30 Injections were targeted at the peri-AV node. Marked increases in AH interval and average RR interval during pacing-induced AF were noted in the fibroblast+TGF-β group with lesser increases in a fibroblast-alone group, compared with saline controls and a TGF-β–alone group. Complete heart block was never observed. Histologic examination showed the presence of fibroblastic proliferation in all the fibroblast-alone group as well as in the fibroblast+TGF-β–injected dogs.
Suppression of Myocardial Infarction–Related Ventricular Arrhythmia
Dominant Negative KCNH2
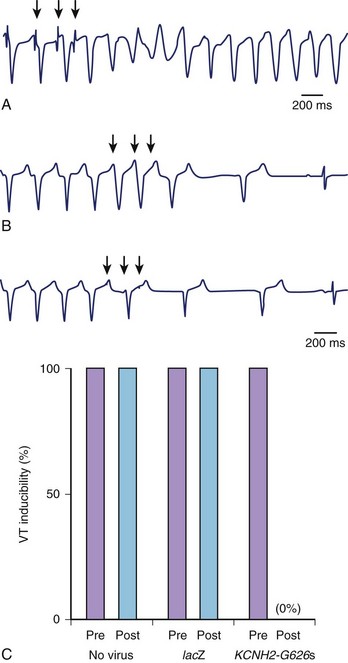
In their study, Sasano et al created myocardial infarctions in pigs and assessed VT inducibility by programmed stimulation after 3 weeks of recovery. Monomorphic VT was induced in all the pigs.31 Adenovirus expressing a dominant negative KCNH2 (hERG) K+ channel (G628S) was locally infused into the mid left anterior descending artery. VT was no longer inducible in all pigs treated with G628S (Figure 9-4). The duration of the monophasic action potential and the effective refractory period increased only in the anterior septum (gene transfer zone) but not in other areas of the heart. Patch clamping of isolated myocytes from the anterior septum also exhibited prolonged action potential durations. G628S gene transfer was not proarrhythmic. Three pigs with infarcts were treated with dofetilide, a known KCNH2-blocking drug. Unlike G628S, dofetilide increased the QT interval and prolonged the effective refractory period (ERP) globally, and the pigs still had inducible VT.
Overexpression of SkM1
The native cardiac Na+ channel, SCN5a, has a V1/2 of inactivation of –84 mV. In the relatively depolarized cardiomyocyte of the myocardial infarct border zone, inactivated SCN5A channels accumulate and contribute to low action potential upstroke, slow conduction, and re-entry. A Na+ channel with a more positive V1/2 of inactivation, such as the skeletal muscle Na+ channel, SkM1 (V1/2 inactivation –62 mV), operates more efficiently at depolarized diastolic membrane potentials. Modeling studies have demonstrated that SkM1 expression permits action potential generation at diastolic membrane potentials as positive as –60mV but that SCN5A overexpression does not.32
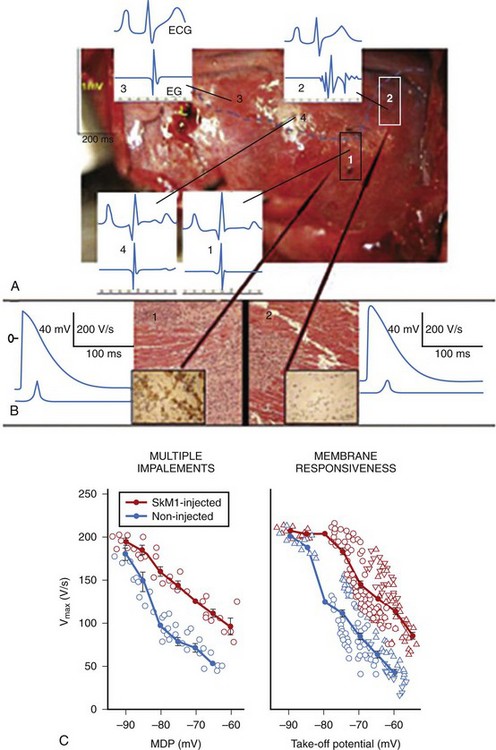
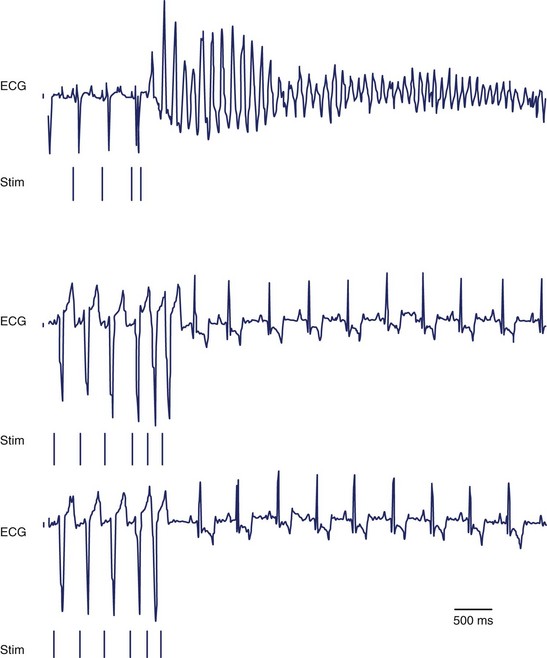
Adenovirus-expressing SkM1 was injected into the epicardial border zone after left anterior descending artery ligation of canine hearts. A week after the injection, local electrograms from the epicardial border zone were significantly broader and fragmented in the controls (adenovirus-expressing GFP) compared with SkM1-injected dogs (Figure 9-5, A). The infarct sizes were similar between SkM1-injected and control dogs. Programmed premature stimulation induced sustained VT or VF in 6 of 8 controls versus 2 of 12 SkM1-injected dogs (Figure 9-6). No difference was seen in the duration of the maximum diastolic potential or of the action potential between the SkM1-expressing myocytes and control myocytes in microelectrode studies of myocardium isolated from the canine hearts. However, action potentials recorded from SkM1-injected sites had significantly higher Vmax than did those of the controls at all membrane potentials tested (Figure 9-5, B).
Current antiarrhythmic strategies focus on slowing conduction, prolonging refractoriness, or inducing conduction block by ablation, but SkM1 expression preserves conduction at depolarized membrane potentials and can be delivered focally to areas of slow conduction. The SMASH-VT study showed a significant decrease in ICD discharges in patients with ischemic cardiomyopathy, who underwent left ventricular ablation at endocardial sites showing fractionated electrograms.33 SkM1 gene therapy may be targeted to areas with fractionated electrograms, to normalize activation without inducing myocardial injury with ablation.
Overexpression of SERCA2a
Abnormal Ca2+ handling occurs during ischemia and may lead to after-depolarizations with significant consequences in the development of arrhythmias. SERCA2a, the sacroplasmic reticulum (SR) Ca2+ adenosine triphosphatase (ATPase), plays an important role in intracellular Ca2+ regulation by pumping Ca2+ from the cytosol into the SR. Prunier et al hypothesized that overexpression of SERCA2a in the porcine heart may be antiarrhythmic.34 Adenovirus-expressing SERCA2a was delivered into the porcine heart by intracoronary infusion. Seven days after gene delivery, the pigs were subjected to transient left anterior descending artery (LAD) balloon occlusion (ischemia-reperfusion) or complete LAD occlusion and observed over 24 hours for the development of spontaneous ventricular arrhythmias. In the complete occlusion group, no difference was seen in VT or VF incidence between the SERCA2a-injected pigs compared with control pigs. However, among the ischemia-reperfusion pigs, SERCA2a-overexpressing pigs had substantially reduced episodes of VT. Regulation of post-ischemia Ca2+ handling may be an antiarrhythmic strategy.
Fibroblasts Expressing Kv1.3
In a fibroblast-based approach to modify ventricular excitability, Kv1.3, a voltage-gated K+ channel with very slow deactivation kinetics, was stably transfected into a fibroblast cell line.35 Computer simulations showed that depolarizing a cardiomyocyte would depolarize the gap junction–coupled, Kv1.3-expressing fibroblast and activate the Kv1.3 K channel. The outward Kv1.3 current would hyperpolarize the fibroblast; through electrotonic interaction, the fibroblast, acting as a current sink, would decrease phase 0 depolarization of the myocyte, hyperpolarize the diastolic membrane potential, increase the refractory period, and depress conduction. This outcome was clearly demonstrated in neonatal cardiomyocyte cultures focally seeded with Kv1.3 fibroblasts. In vivo studies grafting the Kv1.3 fibroblasts into rat and pig ventricles demonstrated significant prolongation of local event-related potential 1 week after implantation. No ventricular arrhythmias were noted with Kv1.3 fibroblast grafting; programmed premature stimulation did not induce any ventricular arrhythmias in four pigs.
Arrhythmic Potential of Stem Cell–Based Cardiac Regeneration Strategies
Thus far, we have focused on the use of cell therapy to carry constructs to an arrhythmic and diseased heart. However, an ultimate therapy would replace diseased, arrhythmogenic tissues with healthy tissue and normal rhythm. Regenerative approaches to heart disease have mainly focused on the improvement of ventricular function after myocardial infarction. Preclinical studies have used skeletal myoblasts, embryonic stem cells, mesenchymal stem cells, and early cardiomyocytes derived from stem cells.5 Early clinical testing with skeletal myoblasts has underscored the potential for arrhythmic consequences of certain cell-based regenerative therapies.36 In a study where ischemic cardiac patients received intracardiac skeletal myoblasts, the myoblast group did not differ in ventricular function or mortality at 4-year follow-up compared with the placebo group. However, 87% of the skeletal-myoblast group had appropriate automatic ICD discharges compared with 13% of controls.37 In contrast, the Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial of skeletal myoblasts injected around ventricular scar tissue at the time of coronary artery bypass surgery but did not report a significantly higher arrhythmia incidence.38
The clinical problem encountered with skeletal myoblast therapies is that they do not appear to form gap junctions with host cardiomyocytes in vivo. Moreover, studies mapping the co-cultures of skeletal myoblasts with rat cardiomyocytes showed a lack of electrical coupling between the two cell types and resultant spiral-like waves of depolarization.37 When skeletal myoblasts were transfected with connenix43, the co-cultures revealed functional coupling, more homogenous wavefronts of activation, and less re-entrant activity.39
Functional electrical integration does occur when fetal cardiomyocytes and stem cell–derived cardiomyocytes are introduced into the adult myocardium. Cardiomyocytes derived from embryoid bodies co-cultured with neonatal cardiomyocytes exhibit synchronous contraction.25 Furthermore, the embryoid body–derived cardiomyocytes provide biologic pacemaking. Evidence of electrical coupling between fetal cardiomyocytes grafted to adult myocardium has been obtained by microelectrode recording and with two-photon microscopy visualizing Ca2+ transients.40,41 Mesenchymal stem cells have also been shown to express sufficient levels of gap junctional proteins to couple to host myocardium and provide a delivery platform.23,42
1 Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188-2190.
2 Beltrami AP, Urbanek K, Kajstura J, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750-1757.
3 Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763-776.
4 Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845-856.
5 Smith RR, Barile L, Messina E, Marbán E. Stem cells in the heart: What’s the buzz all about? Part 1: Preclinical considerations. Heart Rhythm. 2008;5(5):749-757.
6 Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98-102.
7 Kehat I, Kenyagin-Karsenti D, Snir M, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407-414.
8 Chu D, Sullivan CC, Weitzman MD, et al. Direct comparison of efficiency and stability of gene transfer into the mammalian heart using adeno-associated virus versus adenovirus vectors. J Thorac Cardiovasc Surg. 2003;126(3):671-679.
9 Palomeque J, Chemaly ER, Colosi P, et al. Efficiency of eight different AAV serotypes in transducing rat myocardium in vivo. Gene Ther. 2007;14(13):989-997.
10 Lyon AR, Sato M, Hajjar RJ, et al. Gene therapy: Targeting the myocardium. Heart. 2008;94(1):89-99.
11 Kraitchman DL, Tatsumi M, Gilson WD, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451-1461.
12 Rosen MR, Brink PR, Cohen IS, Robinson RB. Genes, stem cells and biological pacemakers. Cardiovasc Res. 2004;64(1):12-23.
13 Robinson RB, Brink PR, Cohen IS, Rosen MR. I(f) and the biological pacemaker. Pharmacol Res. 2006;53(5):407-415.
14 Edelberg JM, Aird WC, Rosenberg RD. Enhancement of murine cardiac chronotropy by the molecular transfer of the human β2-adrenergic receptor cDNA. J Clin Invest. 1998;101(2):337-343.
15 Edelberg JM, Huang DT, Josephson ME, Rosenberg RD. Molecular enhancement of porcine cardiac chronotropy. Heart. 2001;86(5):559-562.
16 Miake J, Marbán E, Nuss HB. Biological pacemaker created by gene transfer. Nature. 2002;419:132-133.
17 Qu J, Plotnikov AN, Danilo PJr, et al. Expression and function of a biological pacemaker in canine heart. Circulation. 2003;107(8):1106-1109.
18 Plotnikov AN, Sosunov EA, Qu J, et al. Biological pacemaker implanted in canine left bundle branch provides ventricular escape rhythms that have physiologically acceptable rates. Circulation. 2004;109(4):506-512.
19 Bucchi A, Plotnikov AN, Shlapakova I, et al. Wild-type and mutant HCN channels in a tandem biological-electronic cardiac pacemaker. Circulation. 2006;114(10):992-999.
20 Plotnikov AN, Bucchi A, Shlapakova I, et al. HCN212-channel biological pacemakers manifesting ventricular tachyarrhythmias are responsive to treatment with I(f) blockade. Heart Rhythm. 2008;5(2):282-288.
21 Tse HF, Xue T, Lau CP, et al. Bioartificial sinus node constructed via in vivo gene transfer of an engineered pacemaker HCN channel reduces the dependence on electronic pacemaker in a sick-sinus syndrome model. Circulation. 2006;114(10):1000-1011.
22 Kashiwakura Y, Cho HC, Barth AS, et al. Gene transfer of a synthetic pacemaker channel into the heart: A novel strategy for biological pacing. Circulation. 2006;114(16):1682-1686.
23 Potapova I, Plotnikov A, Lu Z, et al. Human mesenchymal stem cells as a gene delivery system to create cardiac pacemakers. Circ Res. 2004;94:952-959.
24 Plotnikov AN, Shlapakova I, Szabolcs MJ, et al. Xenografted adult human mesenchymal stem cells provide a platform for sustained biological pacemaker function in canine heart. Circulation. 2007;116(7):706-713.
25 Kehat I, Khimovich L, Caspi O, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22(10):1282-1289.
26 Xue T, Cho HC, Akar FG, et al. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: Insights into the development of cell-based pacemakers. Circulation. 2005;111(1):11-20.
27 Cho HC, Kashiwakura Y, Marbán E. Creation of a biological pacemaker by cell fusion. Circ Res. 2007;100(8):1112-1115.
28 Bauer A, McDonald AD, Nasir K, et al. Inhibitory G protein overexpression provides physiologically relevant heart rate control in persistent atrial fibrillation. Circulation. 2004;110(19):3115-3120.
29 Murata M, Cingolani E, McDonald AD, et al. Creation of a genetic calcium channel blocker by targeted GEM gene transfer in the heart. Circ Res. 2004;95(4):398-405.
30 Bunch TJ, Mahapatra S, Bruce GK, et al. Impact of transforming growth factor-β1 on atrioventricular node conduction modification by injected autologous fibroblasts in the canine heart. Circulation. 2006;113(21):2485-2494.
31 Sasano T, McDonald AD, Kikuchi K, Donahue JK. Molecular ablation of ventricular tachycardia after myocardial infarction. Nature Med. 2006;12:1256-1258.
32 Lau DH, Clausen C, Sosunov EA, et al. Epicardial border zone overexpression of skeletal muscle sodium channel SkM1 normalizes activation, preserves conduction, and suppresses ventricular arrhythmia: An in silico, in vivo, in vitro study. Circulation. 2009;119(1):19-27.
33 Reddy VY, Reynolds MR, Neuzil P, et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007;357(26):2657-2665.
34 Prunier F, Kawase Y, Gianni D, et al. Prevention of ventricular arrhythmias with sarcoplasmic reticulum Ca2+ ATPase pump overexpression in a porcine model of ischemia reperfusion. Circulation. 2008;118(6):614-624.
35 Yankelson L, Feld Y, Bressler-Stramer T, et al. Cell therapy for modification of the myocardial electrophysiological substrate. Circulation. 2008;117(6):720-731.
36 Smits PC, van Geuns RJ, Poldermans D, et al. Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: Clinical experience with six-month follow-up. J Am Coll Cardiol. 2003;42(12):2063-2069.
37 Veltman CE, Soliman OI, Geleijnse ML, et al. Four-year follow-up of treatment with intramyocardial skeletal myoblasts injection in patients with ischaemic cardiomyopathy. Eur Heart J. 2008;29(11):1386-1396.
38 Menasche P, Alfieri O, Janssens S, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial. Circulation. 2008;117:1189-1200.
39 Abraham MR, Henrikson CA, Tung L, et al. Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ Res. 2005;97(2):159-167.
40 Halbach M, Pfannkuche K, Pillekamp F, et al. Electrophysiological maturation and integration of murine fetal cardiomyocytes after transplantation. Circ Res. 2007;101(5):484-492.
41 Rubart M, Pasumarthi KB, Nakajima H, et al. Physiological coupling of donor and host cardiomyocytes after cellular transplantation. Circ Res. 2003;92(11):1217-1224.
42 Valiunas V, Doronin S, Valiuniene L, et al. Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. J Physiol. 2004;555:617-626.