Chapter 3 Functional Tractography, Diffusion Tensor Imaging, Intraoperative Integration of Modalities, and Neuronavigation
Diffusion tensor imaging (DTI) with functional tractography is a noninvasive MRI modality which depicts the probable location and orientation of subcortical white matter tracts in vivo. DTI offers a variety of possible applications for neurosurgeons and neuroscientists to help further the understanding of neurologic organization and function and to advance patient care which explains the enthusiasm and optimism with which it has been received. Potential clinical applications for individual patients include prediction of neurologic outcome from tumor1 and stroke,2–4 targeting for functional and stereotactic neurosurgery5–7 and pre- and intra-operative planning for the surgical resection of space-occupying lesions. This chapter shall focus on the application of DTI in the surgical resection of intra-axial brain tumors.
Surgery occupies a vital place in the management of intra-axial brain tumors by virtue of providing symptom relief, recovery of pathologic tissue for diagnosis and beneficial influence on long-term outcome. The ultimate aim of resection of intra-axial brain tumors is to achieve as complete excision of neoplastic tissue as possible. A substantial body of evidence exists to suggest that a greater extent of resection results in extended mean survival time in low-grade and high-grade glioma.8–17 However, the neurosurgeon is limited in the scope of surgical resection possible by the imperative to avoid injury to eloquent brain tissue and therefore the development of post-operative neurologic deficits. Knowledge of which tissue is functionally important in the individual patient is therefore crucial in pre-operative and intra-operative decision making. Although imaging in the form of computerized tomography (CT) and MRI can define structural anatomy, they do not provide reliable information on functional anatomy in the individual. Other modalities need to be employed by the neurosurgeon to delineate areas of functional importance. This functional mapping can be performed by invasive and non-invasive methods. Invasive examinations include pre-operative cortical electrode grid recordings and intra-operative cortical and subcortical stimulation. Non-invasive examinations include functional MRI and magnetoencephalography (MEG). Of those studies performed pre-operatively, none provide information on subcortical functional anatomy. Despite great care taken by the neurosurgeon to avoid injury to eloquent cortex through careful pre-operative and intra-operative functional mapping and meticulous surgical technique, straying into critical subcortical white matter tracts can still result in devastating deficits. There is concern that localization using subcortical white matter stimulation is less reliable and safe than cortical stimulation.18,19 By visually representing white matter tracts to the surgeon, DTI promises to improve the safety of tumor resections, especially when involving subcortical areas.
Scientific Principles of DTI
Diffusion MRI scans image the molecular diffusion of water at the same scale as cellular dimensions and therefore allow the microarchitecture of the brain to be investigated. The constant random motion of molecules is described by Brownian motion and is exploited by diffusion imaging to specifically detect the displacement of water molecules through the brain tissue medium. Diffusion-weighted scanning consists of a T2-weighted spin-echo sequence with the addition of two diffusion-sensitizing gradients applied before and after the 180o refocusing pulse, through an identical axis. Therefore, there is a loss of signal intensity as a result of incomplete rephasing of water proton spins after they have moved during the time elapsed between the two diffusion-sensitizing gradients.20 Diffusion times in the region of 10 to 50 ms are used which provides microscopic detail, capturing average molecular displacements of 10 μm.20 Scan acquisition using standard MRI systems takes 3 to 10 min,21 and therefore is minimally burdensome on patient, radiographer, or scanner time.
The direction of the passage of water is different depending on the nature of tissue in which it is found. Where no structural boundaries exist nearby, the molecular motion of water is unimpeded and equal in all directions. This is known as isotropic diffusion. This is exhibited within the cerebrospinal fluid spaces of the brain, with the exception of sites of bulk flow such as the aqueduct of Sylvius or foramen of Munro.20 Isotropic diffusion is also believed to occur in grey matter.22,23 In contrast, myelinated white matter fiber tracts are arranged into parallel, densely packed bundles that impede the diffusion of water molecules perpendicular to the fibers’ direction. Therefore, diffusion of water molecules in this situation is not equal in all directions and is defined as anisotropic diffusion. Detection of water molecule anisotropy is the basis of diffusion tensor imaging and tractography.
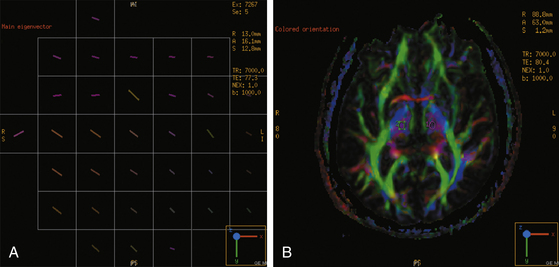
The diffusion tensor is a 3 × 3 matrix of vectors which mathematically describes the three-dimensional (3D) directionality and magnitude, or diffusion anisotropy, of water molecules.20,21,24 The three principal axes of the diffusion tensor are termed eigenvectors. When plotted as an ellipsoid, isotropic diffusion is a sphere whereas anisotropic diffusion forms an elongated ellipsoid, becoming a prolate (cigar) shape when the eigenvector of greatest magnitude is much larger than the other two. Prolate diffusion within a brain voxel is assumed to represent a white matter fiber bundle where the primary eigenvector is aligned with the axonal axis. Tracing of white matter tracts to produce functional tractograms uses each voxel’s diffusion tensor to link it to adjacent voxels and in this way trace out the likely path of a fiber bundle in 3D space (Fig. 3-1).
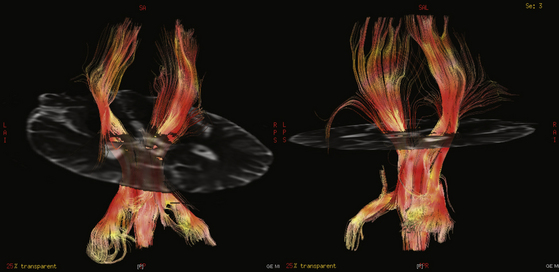
DTI fiber tract data can be presented in two forms. Functional anisotropy maps provide information on fiber anatomy in cross-sectional two-dimensional (2D) images with color-coded axes where the brightness is proportional to the degree of anisotropy (see Fig. 3- 1). By convention, the anteroposterior axis is represented by green, left-right by red, and up-down by blue. Therefore, the corpus callosum will appear red, for example. Alternatively, deterministic or probabilistic25 functional tractography performs a 3Ddimensional reconstruction and portrayal of the fiber pathways based on following a white matter tract from voxel to voxel as described above (Fig. 3-2). Specified anatomic points, known as “seeds” (see Fig. 3-1), can be selected by the user from where the tractogram can be plotted by the processing software to delineate proposed neural connectivity with the selected site. Alternatively, larger volumes of brain can be selected as regions of interest or “masks.” To reduce dependence on the user and therefore the inherent subjectivity of seed selection while also increasing the likelihood of depicting functionally relevant tracts, Schonberg et al. used functional MRI to define where seed points should be sited.26 Although this represents an extra stage of patient assessment, they found that it enabled a more comprehensive mapping of fiber systems such as the pyramidal tract and the superior longitudinal fasciculus. See glossary of terms in Table 3-1.
| Isotropic diffusion | Motion of Molecules Being Equal in all Directions |
|---|---|
| Anisotropic diffusion | Motion of molecules not being equal in all directions |
| Fractional anisotropy | Directionally averaged diffusion of water molecules within a voxel measured as its deviation from isotropic diffusion |
| Diffusion tensor | Matrix of vectors which mathematically describe anisotropic diffusion within a 3D space |
| Tractography | Representation of white matter fiber tracts produced by following eigenvectors of adjacent voxels in 3D space |
Preoperative Planning Applications
DTI shows the surgeon the relationship of the intra-axial tumor to local white matter tracts in multiple planes. A variety of aspects of the tumor–tract relationship can therefore be demonstrated. The identity of the tract can be surmised from its position and course, such as the corticospinal tract and optic radiations. The proximity of the tumor to the tract can be appreciated. Also the position of the tumor can be seen in relation to the tract, for example superior, lateral, medial etc., allowing optimal approach to be determined to highly eloquent and complex areas such as the pons.27 Displacement of the tract by the tumor or edema can also be demonstrated.28,29 This is crucial information when planning a surgical trajectory in order to avoid eloquent tissue. DTI has been found to provide important preoperative warning of this surgical hazard in situations where a precisely planned trajectory is imperative such as during resection of thalamic juvenile pilocytic astrocytoma with displacement of the posterior limb of the internal capsule.30 Incorporation of white matter fibers within the tumor mass, seen especially in low-grade tumors,31 and destruction of white matter fibers by the tumor can also be depicted. These features will have profound implications for the extent of resection amenable for the individual tumor.
DTI can also help elucidate the anatomy of poorly described pathways in vivo in the human to inform and advance established surgical strategies. Resection strategies that aim to excise normal as well as neoplastic tissue with a view to minimizing the likelihood of recurrence such as frontal and temporal lobectomy can be enhanced by DTI to maintain safety. The anatomico-functional connectivity of the dominant temporal lobe, for example, was reviewed by Duffau et al. using a combination of DTI and subcortical intraoperative stimulation studies to elucidate the white matter pathways, which should represent the resection boundaries of temporal lobectomy such as the pyramidal tract and the anterior wall of the temporal part of the superior longitudinal fasciculus.32 Indeed, DTI has been proposed as the preoperative investigation to assess individual patients’ risk of visual field defect prior to anterior temporal lobe resection as it images the Meyer loop of the optic radiation as it courses anteriorly from the lateral geniculate nucleus and around the tip of the temporal horn before projecting to the visual cortex. The individual variation of this white matter pathway33 increases the risk of a deficit which can permanently disqualify the patient from holding a driving license. Therefore, preoperative warning of a more ventral position of the Meyer loop along its course anterior to the temporal horn should identify those with a higher likelihood of postoperative deficit.34,35
Although DTI visualizes white matter tracts, it is possible to extrapolate these projections and therefore visualize their grey matter cortical projections/origins. Kamada et al. applied this technique to map the primary motor area (PMA) preoperatively in thirty patients with supratentorial lesion affecting the motor system.36 By selecting seed points within the corticospinal tract at the cerebral peduncle, plus the medial lemniscus to differentiate from somatosensory projections, a PMA map was produced that was successfully validated against subsequent intraoperative cortical somatosensory evoked potentials. Indeed, fMRI failed to identify the PMA in eight patients. The reasons for this were inherent in the patients’ pathology through its effect on the motor system in that they were incapable of successfully completing the self-paced finger tapping task required to elicit the blood oxygenation level dependent signal that fMRI detects. In contrast, DTI requires no patient tasks to acquire its data and therefore offers an important alternative for preoperative non-invasive cortical mapping in patients who, for whatever reason, cannot complete them.
DTI has been applied in neuro-oncology beyond not only functional mapping but for noninvasive assessment of tumor architecture in terms of cell density, white matter invasion and even histologic discrimination such as the distinction between primary and secondary intra-axial tumors.37–41 It has been proposed that DTI can distinguish between vasogenic edema and tumor-infiltrated edema. Edematous tissue surrounding glioma is generally accepted to be infiltrated by tumor cells. In contrast, edema surrounding cerebral metastases or meningioma is considered to be vasogenic.40,42 Therefore, hyperintensity surrounding tumor on T2-weighted MRI may reflect any of glioma, metastasis or meningioma. However, as the FA at the voxels corresponding to the site of edema has been shown in some studies to be of a lower value in infiltrative pathologies such as glioma, a tumor infiltration index was derived by Lu et al. to help distinguish against pathologies producing only vasogenic edema.39 There have been contradictory reports including a PET-labeling study questioning whether this DTI analysis is specific enough to differentiate between tumor-infiltrated edema and vasogenic edema.41,43 Further investigation will determine whether DTI can fulfill this potential and provide reliable presurgical histologic tumor characterization.
Therefore, DTI provides advanced warning of potential intraoperative misadventures to help surgeons adapt their approach subsequently in theater to minimize these. Even prior to this stage, DTI can inform the surgeon of how amenable the tumor is to surgery by virtue of its relationship to eloquent brain and even potentially its histologic nature, and therefore what surgery can offer in terms of likely benefits and associated risk of adverse effects.
Intraoperative Neuronavigation
The feasibility of intraoperative guidance by incorporation of DTI fiber tracking into neuronavigation systems has been demonstrated by a number of investigators within the last decade.44–49 White matter pathways such as the pyramidal tract and the optic radiation were successfully portrayed in relation to intra-axial tumors such as cavernoma and glioma. Coenen et al. were the first to report the use of intraoperative neuronavigation with 3D tract reconstruction to assist the resection of glioblastoma associated with the pyramidal tract. They found fiber tract navigation to be a helpful adjunct to resection in the four patients in whom it was applied. Subsequent studies have also underlined its potential for efficiency and patient safety.47,48 The ability of DTI to reliably predict the true location of critical white matter pathways intraoperatively is crucial for the technique to be applied with confidence during surgery. Investigators have evaluated intraoperative DTI’s accuracy in depicting motor pathways by comparing it to intraoperative electrophysiologic methods, in particular cortical stimulation.50,51 One particular study of 13 patients employed electrical motor cortex stimulation to verify the location of the precentral gyrus and indirectly the pyramidal tract, DTI neuronavigation correctly predicted the principal motor pathways’ position in 92%.51 DTI has not only been applied to surgery for supratentorial tumors but also to the resection of brainstem lesions such as cavernoma with promise of improving operative safety.52
The perceived benefits of fiber tract neuronavigation need to be translated into objective improvements in aspects of patient care; however, few studies have addressed this rigorously with objective endpoints. Notably, Wu et al. performed a prospective, randomized controlled trial to attend to this deficiency in the literature.53 They studied 238 patients undergoing resection for high- and low-grade supratentorial glioma involving the pyramidal tracts over 4 years. A total of 118 patients underwent preoperative DTI scanning to aid preoperative planning and integration by rigid registration into the neuronavigation system for intraoperative image guidance. This cohort was compared to 120 similar controls undergoing resection aided by standard neuronavigation. Multiple outcome measures were improved by the implementation of fiber tracking. Gross total resection of high-grade glioma in the DTI group was achieved in more than twice the number of cases than in the control group (74.4% vs. 33.3%). Median survival was 21.2 months in the DTI group compared to 14 months and DTI neuronavigation estimated hazard ratio was 0.570, conferring a 43% reduction in mortality risk. With respect to neurologic function, 6-month Karnofsky Performance Scale score was significantly better in the DTI group (32.8% vs. 15.3%). A criticism of this investigation was the lack of physician blinding in the nonradiologic assessments. However, it provides Class I evidence that fiber tract neuronavigation can improve patient mortality and morbidity in glioma surgery, and that this technology can be successfully integrated into a routine neurosurgical practice.
Limitations of DTI
Although DTI promises to be an effective tool in the surgeon’s armamentarium, its limitations must be borne in mind so that it is appropriately interpreted in individual patients. DTI does not directly trace fibers unlike tracer injection studies, which remain the gold standard for defining neural connectivity. Rather it produces representations of white matter tracts based on the fundamental assumption that the dominant direction of water movement is aligned with the predominant direction of white matter fiber bundles within each voxel.21,54 This is closer to the biological reality in some circumstances more than others. Neuronal axons are micrometers wide but the voxels used are in the order of a few millimeters in each plane; therefore, one voxel may contain some tens of thousands of axons. If a voxel contains groups of nearby axons with differing longitudinal axes such as are found at sites where different tracts cross or axons whose path is tortuous and change course within a very short distance, their anatomy will be misrepresented by current DTI methods. Advances in resolution and modeling of water diffusion are improving this limitation such that complex fiber architectural relationships can be depicted more reliably and accurately in the future.55–61 In larger, densely packed parallel fiber bundles such as the corpus callosum, this is much less of a problem. Other limitations include the inability to decipher whether a tract is projecting retrograde or anterograde,55 which hampers neuroscientific investigation. However, in the context of surgical planning and intraoperative neuronavigation, the presence and location of major white matter tracts is the critical information rather than the direction in which they project.
A further limitation is that tractography is a user-dependent technique. The ultimate results of fiber tracking reflect the chosen thresholding of the functional anisotropy, the site and size of selected seed areas, and which algorithm is used.62 A threshold value of 0.15 to 0.2 has been suggested by a rigorous, albeit retrospective, comparison of DTI tract representation and stereotactic biopsy histologic findings, although the functionality of the tracts was not evaluated.40 Therefore, interuser variation can produce important differences in the tractogram generated.
Acquisition and processing of DTI images is affected by multiple sources of spatial inaccuracy therefore allowances need to be made when interpreting tractograms during surgical resection to maintain patient safety. During the scanning process, static and encoding direction-dependent distortions occur due to factors such as magnetic field inhomogeneity, imperfections in gradient waveforms, and eddy currents.52 Although progress has been made in minimizing the impact of these by correcting for the resulting misrepresentations,63,64 some inaccuracy remains in the current DTI technique. The integration of DTI images with neuronavigation systems produces further discrepancy with an image registration error in the region of 2 to 3 mm, although this is comparable to the error encountered when integrating functional MRI data.48
Neuronavigation using functional tractography also suffers from the same limitations as conventional neuronavigation. Patient registration error must be factored in to the overall inaccuracy.48 Further, any change in patient position with respect to the registration landmarks will severely diminish accuracy. As neuronavigation images are acquired preoperatively, they do not respond in real-time to the changing anatomy and brain shifts of cranial surgery. Therefore, head positioning, continuing resection, cerebrospinal fluid loss, breach of the ventricles and brain retraction, for example, will diminish the accuracy. However, with the advent of intraoperative MRI, this limitation could in future be overcome. Nimsky et al.47 successfully applied preoperative and intraoperative DTI and stereotactic MRI acquisition with a 1.5-Tesla scanner in 37 patients undergoing glioma surgery. They first demonstrated that it was feasible to perform intraoperative DTI in all patients and that the encountered brain shift was reflected in DTI white matter tract shifts of up to 15 mm.
The multiple sources of spatial inaccuracy described above must be taken into consideration when employing DTI during tumor resection in patients. Animal, phantom, and patient studies have attempted to quantify this discrepancy.65–67 Berman et al. compared the location of DTI-imaged fiber tracts to intraoperative subcortical stimulation and found a mean distance between stimulation sites and imaged tracts of 8.7 mm.65 Investigators therefore advise a safety margin of up to 1 cm to be maintained around the depicted white matter pathways, such as the corticospinal tract, during surgery.31,48,65
Intraoperative Integration of Modalities
The term “functional neuronavigation” has been coined to describe the incorporation of MEG and fMRI data into frameless stereotactic neuronavigation systems.48 This has been an important adjunct to modern tumor surgery and has been demonstrated to reduce morbidity during resection of lesions adjacent to eloquent brain.68 These modalities provide truly functional information generated preoperatively during series of patient tasks, whereas DTI provides only structural information. All three imaging modalities can be incorporated into the neuronavigation system to provide simultaneous representations of the cortical and subcortical functionally important tissue which is then displayed on the workstation or heads-up display. Successful incorporation and navigation using fMRI and DTI together has been reported to be user-friendly and suitable for routine use within a neurosurgical service69 and to help facilitate maximal tumor resection,70 although there has been no control group to compare outcomes with. Other imaging modalities such as CT or MR angiography can also be incorporated within the neuronavigation system providing the surgeon with the optimal anatomic and functional representation of the intracranial cavity.
If preoperative imaging, such as MEG, fMRI, and angiography, are integrated with DTI and stereotactic images and intraoperative MRI is performed then registration will be lost due to the brain shift associated with surgery.71 There are potential solutions to this problem whereby the preoperative data are registered with the intraoperative data; however, as brain shift is complex and difficult to predict reliably,47 suitable algorithms to make this correction are some way off. Therefore, as intraoperative DTI has been shown to be a more reliable possibility in responding to brain shift,47 this may be the most accurate intraoperative functional imaging modality once the resection has advanced.
An alternative solution to the brain shift problem during surgery is the use of intraoperative 3D ultrasound scanning (3D USS). It has the advantage over intraoperative MRI of not requiring modifications to the operating theater and it is much less expensive. 3D USS acquired intraoperatively can be used to update preoperative stereotactic MRI images, allowing continued navigation with fMRI and DTI data throughout the resection.72 Two studies of patients undergoing resection of cavernoma or glioma have found that intraoperative updates of fMRI and DTI neuronavigation by 3D USS has been feasible,69,70 and that the combination facilitates maximal tumor resection.70
Subcortical stimulation has been used in trials to verify the reliability of depicted DTI fiber tracts; however, it can be used in tandem with DTI to confirm functional tissue location. Bello et al. reported the accurate identification of eloquent fiber tracts using a combination of DTI neuronavigation and subcortical electrical stimulation during resection of low- and high-grade glioma in 64 patients.31 They concluded that surgery safety was improved with a shorter operative time and fewer intraoperative seizures. The use of combined intraoperative recorded motor-evoked potentials with neuronavigation has been supported by other investigators with reported benefits such as real-time demonstration of spatial relationships, less injury to eloquent tracts and optimal tumor resections.73,74 Again, no controlled trials currently exist; however, the addition of direct functional monitoring would be expected to increase the likelihood of identifying eloquent pathways. Intraoperative DTI has been successfully coregistered with the electrical stimulation probe to facilitate both navigation and stimulation of cortical and subcortical tracts during resection of recurrent glioblastoma beside eloquent brain.75 Therefore, it is possible for both of these modalities to be truly integrated in the operating theater.
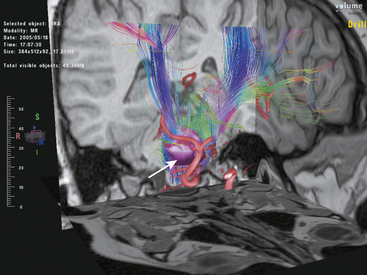
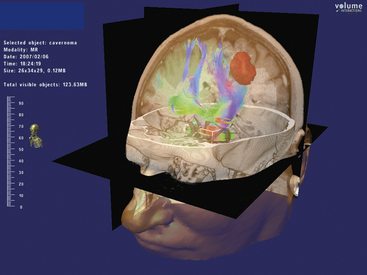
Virtual reality technology in neurosurgery has emerged during the last decade as a viable complement to surgical planning and intraoperative performance.76–79 It is possible to integrate the variety of imaging modalities including fiber tracking into the virtual display (Figs. 3-3 and 3-4). Intraoperative neuronavigation using this technology has also been achieved.80 This therefore allows a preoperative “dry run” of surgery in the virtual world using multiple anatomic and functional image data followed by the retracing of the same operative steps stereotactically in the operating theater. Virtual neurosurgery may be an important platform in which DTI fiber tracking is employed in the future.
Postoperative Evaluation
There is scope for DTI to answer important surgical questions postoperatively. Duffau et al. suggest postoperative check DTI to compare with preoperative DTI to help establish whether eloquent tracts have been interrupted.32 In the case of new deficits occurring postoperatively, interruption of pre-existing functional tracts would imply that direct surgical injury had been the cause. Alternatively, an intact pathway in the presence of a novel deficit may suggest that there had been indirect injury to eloquent tissue from interruption of the white matter bundles’ blood supply such as the perforators to the pyramidal tract.81
Just as DTI has been shown to predict functional outcome after stroke,2–4 it may provide prognostic information with regard to postoperative deficits. If relevant tracts are spared this may suggest a more favorable prognosis with function potentially returning in some degree with time. It is important to bear in mind, however, that intact tract anatomy alone does not guarantee functional recovery as DTI describes the tissue architecture but does not provide information on the level of physiologic performance.
Behrens T.E., Johansen-Berg H., Woolrich M.W., et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750-757.
Berman J.I., Berger M.S., Chung S.W., et al. Accuracy of diffusion tensor magnetic resonance imaging tractography assessed using intraoperative subcortical stimulation mapping and magnetic source imaging. J Neurosurg. 2007;107:488-494.
Berman J.I., Berger M.S., Mukherjee P., et al. Diffusion-tensor imaging-guided tracking of fibers of the pyramidal tract combined with intraoperative cortical stimulation mapping in patients with gliomas. J Neurosurg. 2004;101:66-72.
Ciccarelli O., Catani M., Johansen-Berg H., et al. Diffusion-based tractography in neurological disorders: concepts, applications, and future developments. Lancet Neurol. 2008;7:715-727.
Coenen V.A., Huber K.K., Krings T., et al. Diffusion-weighted imaging-guided resection of intracerebral lesions involving the optic radiation. Neurosurg Rev. 2005;28:188-195.
Coenen V.A., Krings T., Mayfrank L., et al. Three-dimensional visualization of the pyramidal tract in a neuronavigation system during brain tumor surgery: first experiences and technical note. Neurosurg. 2001;49(1):84-92. (discussion 92)
Duffau H., Thiebaut de Schotten M., Mandonnet E. White matter functional connectivity as an additional landmark for dominant temporal lobectomy. J Neurol Neurosurg Psychiatry. 2008;79(5):492-495.
Ganslandt O., Fahlbusch R., Nimsky C., et al. Functional neuronavigation with magnetoencephalography outcome in 50 patients with lesions around the motor cortex. J Neurosurg. 1999;91(1):73-79.
Gasco J., Tummala S., Mahajan N.M., et al. Simultaneous use of functional tractography, neuronavigation-integrated subcortical white matter stimulation and intraoperative magnetic resonance imaging in glioma surgery: technical note. Stereotact Funct Neurosurg. 2009;87:395-398.
Gulati S., Berntsen E.M., Solheim O., et al. Surgical resection of high-grade gliomas in eloquent regions guided by blood oxygenation level dependent functional magnetic resonance imaging, diffusion tensor tractography, and intraoperative navigated 3D ultrasound. Minim Invasive Neurosurg. 2009;52:17-24.
Johansen-Berg H., Behrens T.E.J. Just pretty pictures? What diffusion tractography can add in clinical neuroscience. Curr Opin Neurol. 2006;19:379-385.
Kamada K., Todo T., Masutani Y., et al. Combined use of tractography-integrated functional neuronavigation and direct fiber stimulation. J Neurosurg. 2005;102:664-672.
Kinoshita M., Hashimoto N., Goto T., et al. Fractional anisotropy and tumor cell density of the tumor core show positive correlation in diffusion tensor magnetic resonance imaging of malignant brain tumors. Neuroimage. 2008;43:29-35.
Kinoshita M., Yamada K., Hashimoto N., et al. Fiber-tracking does not accurately estimate size of fiber bundle in pathological condition: initial neurosurgical experience using neuronavigation and subcortical white matter stimulation. Neuroimage. 2005;25:424-429.
Mukherjee P., Berman J.I., Chung S.W., et al. Diffusion tensor MR imaging and fiber tractography: theoretic underpinnings. Am J Neuroradiol. 2008;29:632-641.
Nimsky C., Ganslandt O., Fahlbusch R. Implementation of fiber tract navigation. Neurosurgery. 58(ONS Suppl 2), 2006. ONS-292–304
Nimsky C., Ganslandt O., Hastreiter P., et al. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery. 2005;56(1):130-138.
Nimsky C., Ganslandt O., Merhof D., et al. Intraoperative visualization of the pyramidal tract by diffusion-tensor-imaging-based fiber tracking. Neuroimage. 2006;30(4):1219-1229.
Okada T., Mikuni N., Miki Y., et al. Corticospinal tract localization: integration of diffusion-tensor tractography at 3-T MR imaging with intraoperative white matter stimulation mapping — preliminary results. Radiology. 2006;240(3):849-857.
Rasmussen I.-A.Jr., Lindseth F., Rygh O.M., et al. Functional neuronavigation combined with intra-operative 3D ultrasound: initial experiences during surgical resections close to eloquent brain areas and future directions in automatic brain shift compensation of preoperative data. Acta Neurochir (Wien). 2007;149:365-378.
Schonberg T., Pianka P., Hendler T., et al. Characterization of displaced white matter by brain tumors using combined DTI and fMRI. Neuroimage. 2006;30:1100-1111.
Stadlbauer A., Nimsky C., Buslei R., et al. Diffusion tensor imaging and optimized fiber tracking in glioma patients: histopathologic evaluation of tumor-invaded white matter structures. Neuroimage. 2007;34:949-956.
Wu J.-S., Mao Y., Zhou W.-J., et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery. 2007;61(5):935-948. (discussion 948-949)
Yogarajah M., Focke N.K., Bonelli S., et al. Defining Meyer’s loop-temporal lobe resections, visual field deficits and diffusion tensor tractography. Brain. 2009;132:1656-1668.
1. Lui Y.W., Law M., Chacko-Mathew J., et al. Brainstem corticospinal tract diffusion tensor imaging in patients with primary posterior fossa neoplasms stratified by tumor type: a study of association with motor weakness and outcome. Neurosurgery. 2007;61(6):1199-1208.
2. Cho S.-H., Kim S.H., Choi B.Y., et al. Motor outcome according to diffusion tensor tractography findings in the early stage of intracerebral hemorrhage. Neurosci Lett. 2007;421:142-146.
3. Jang S.H., Bai D., Son S.M., et al. Motor outcome prediction using diffusion tensor tractography in pontine infarct. Ann Neurol. 2008;64:460-465.
4. Yoshioka H., Horikoshi T., Aoki S., et al. Diffusion tensor tractography predicts motor functional outcome in patients with spontaneous intracerebral hemorrhage. Neurosurgery. 2008;62(1):97-103.
5. Muthusamy K.A., Aravamuthan B.R., Kringelbach M.L., et al. Connectivity of the human pedunculopontine nucleus region and diffusion tensor tractography in surgical targeting. J Neurosurg. 2007;107:814-820.
6. Owen S.L.F., Heath J., Kringelbach M., et al. Pre-operative DTI and probabilistic tractography in four patients with deep brain stimulation for chronic pain. J Clin Neurosci. 2008;15(7):801-805.
7. Sillery E., Bittar R.G., Robson M.D., et al. Connectivity of the human periventricular-periaqueductal gray region. J Neurosurg. 2005;103:1030-1034.
8. Sanai N., Berger M.S. Operative techniques for gliomas and the value of extent of resection. Neurotherapeutics. 2009;6:478-486.
9. Ito S., Chandler K.L., Prados M.D., et al. Proliferative potential and prognostic evaluation of low-grade astrocytomas. J Neurooncol. 1994;19:1-9.
10. Gorlia T., Van den Bent M.J., Hegi M.E., et al. Normograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol. 2008;9:29-38.
11. Nicloato A., Gerosa M.A., Fina P., et al. Prognostic factors in low-grade supratentorial astrocytomas: a uni-multivariate statistical analysis in 76 surgically treated adult patients. Surg Neurol. 1995;44:208-221.
12. Whitton A.C., Bloom H.J. Low-grade glioma of the cerebral hemispheres in adults: a retrospective analysis of 88 cases. Int J Radiat Oncol Biol Phys. 1990;18:783-786.
13. Scerrati M., Roselli R., Iacoangeli M., et al. Prognostic factors in low grade (WHO grade II) gliomas of the cerebral hemispheres: the role of surgery. J Neurol Neurosurg Psychiatry. 1996;61:291-296.
14. Lote K., Egeland T., Hager B., et al. Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: a retrospective study in 379 patients. J Clin Oncol. 1997;15:3129-3140.
15. Peraud A., Ansari H., Bise K., et al. Clinical outcome of supratentorial astrocytoma WO grade II. Acta Neurochir (Wien). 1998;140:1213-1222.
16. Bauman G., Pahapill P., Macdonald D., et al. Low grade glioma: a measuring radiographic response to radiotherapy. Can J Neurol Sci. 1999;26:18-22.
17. Johannesen T.B., Langmark F., Lote K. Progress in long-term survival in adult patients with supratentorial low-grade gliomas: a population-based study of 993 patients in whom tumors were diagnosed between 1970 and 1993. J Neurosurg. 2003;99:854-862.
18. Holodny A.I., Schwartz T.H., Ollenschleger M., et al. Tumor involvement of the corticospinal tract: diffusion magnetic resonance tractography with intraoperative correlation. J Neurosurg. 2001;95(6):1082.
19. Lang F.F., Olansen N.E., DeMonte F., et al. Surgical resection of intrinsic insular tumors: Complication avoidance. J Neurosurg. 2001;95(4):638-650.
20. Mukherjee P., Berman J.I., Chung S.W., et al. Diffusion tensor MR imaging and fiber tractography: theoretic underpinnings. Am J Neuroradiol. 2008;29:632-641.
21. Ciccarelli O., Catani M., Johansen-Berg H., et al. Diffusion-based tractography in neurological disorders: concepts, applications, and future developments. Lancet Neurol. 2008;7:715-727.
22. Pierpaoli C., Jezzard P., Basser P.J., et al. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637-648.
23. Shimony J.S., McKinstry R.C., Akbudak E., et al. Quantitative diffusion-tensor anisotropy brain MR imaging: normative human data and anatomic analysis. Radiology. 1999;212:770-784.
24. Basser P.J. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333-344.
25. Behrens T.E., Johansen-Berg H., Woolrich M.W., et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750-757.
26. Schonberg T., Pianka P., Hendler T., et al. Characterization of displaced white matter by brain tumors using combined DTI and fMRI. Neuroimage. 2006;30:1100-1111.
27. Kashimura H., Inoue T., Ogasawara K., et al. Pontine cavernous angioma resected using the subtemporal, anterior transpetrosal approach determined using three-dimensional anisotropy contrast imaging: technical case report. Neurosurgery. 2006;58(Suppl 1):ONS-E175. (discussion ONS-E175)
28. Niizuma K., Fujimura M., Kumabe T., et al. Surgical treatment of paraventricular cavernous angioma: fibre tracking for visualizing the corticospinal tract and determining surgical approach. J Clin Neurosci. 2006;13(10):1028-1032.
29. Yu C.S., Li K.C., Xuan Y., et al. Diffusion tensor tractography in patients with cerebral tumors: a helpful technique for neurosurgical planning and postoperative assessment. Eur J Radiol. 2005;56(2):197-204.
30. Moshel Y.A., Elliott R.E., Monoky D.J., et al. Role of diffusion tensor imaging in resection of thalamic juvenile pilocytic astrocytoma. J Neurosurg Paediatr. 2009;4:495-505.
31. Bello L., Gambini A., Castellano A., et al. Motor and language DTI fiber tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. Neuroimage. 2008;39:369-382.
32. Duffau H., Thiebaut de Schotten M., Mandonnet E. White matter functional connectivity as an additional landmark for dominant temporal lobectomy. J Neurol Neurosurg Psychiatry. 2008;79(5):492-495.
33. Ebeling U., Reulen H.J. Neurosurgical topography of the optic radiation in the temporal lobe. Acta Neurochir (Wien). 1988;92:29-36.
34. Powell H.W.R., Parker G.J.M., Alexander D.C., et al. MR tractography predicts visual field defects following temporal lobe resection. Neurology. 2005;65:596-599.
35. Yogarajah M., Focke N.K., Bonelli S., et al. Defining Meyer’s loop-temporal lobe resections, visual field deficits and diffusion tensor tractography. Brain. 2009;132:1656-1668.
36. Kamada K., Sawamura Y., Takeuchi F., et al. Functional identification of the primary motor area by corticospinal tractography. Neurosurgery. 56(Suppl ONS 1), 2005. ONS-98-ONS-109
37. Goebell E., Paustenbach S., Vaeterlein O., et al. Low-grade and anaplastic gliomas: differences in architecture evaluated with diffusion-tensor MR imaging. Radiology. 2006;239:217-222.
38. Kinoshita M., Hashimoto N., Goto T., et al. Fractional anisotropy and tumor cell density of the tumor core show positive correlation in diffusion tensor magnetic resonance imaging of malignant brain tumors. Neuroimage. 2008;43:29-35.
39. Lu S., Ahn D., Johnson G., et al. Diffusion-tensor MR imaging of intracranial neoplasia and associated peritumoral edema: introduction of the tumor infiltration index. Radiology. 2004;232:221-228.
40. Stadlbauer A., Nimsky C., Buslei R., et al. Diffusion tensor imaging and optimized fiber tracking in glioma patients: histopathologic evaluation of tumor-invaded white matter structures. Neuroimage. 2007;34:949-956.
41. Wang S., Kim S., Chawla S., et al. Differentiation between glioblastomas and solitary brain metastases using diffusion tensor imaging. Neuroimage. 2009;44:653-660.
42. Kinoshita M., Goto T., Okita Y., et al. Diffusion tensor-based tumor infiltration index cannot discriminate vasogenic edema from tumor-infiltrated edema. J Neurooncol. 2010;96(3):409-415.
43. Kinoshita M., Hashimoto N., Goto T., et al. Use of fractional anisotropy for determination of the cut-off value in (11)C-methionine positron emission tomography for glioma. Neuroimage. 2009;45:7.
44. Coenen V.A., Krings T., Mayfrank L., et al. Three-dimensional visualization of the pyramidal tract in a neuronavigation system during brain tumor surgery: first experiences and technical note. Neurosurgery. 2001;49(1):84-92. (discussion 92)
45. Coenen V.A., Huber K.K., Krings T., et al. Diffusion-weighted imaging-guided resection of intracerebral lesions involving the optic radiation. Neurosurg Rev. 2005;28:188-195.
46. Hlatsky R., Jackson E.F., Weinberg J.S., et al. Intraoperative neuronavigation using diffusion tensor MR tractography for the resection of a deep tumor adjacent to the corticospinal tract. Stereotact Funct Neurosurg. 2005;83(5-6):228-232.
47. Nimsky C., Ganslandt O., Hastreiter P., et al. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery. 2005;56(1):130-138.
48. Nimsky C., Ganslandt O., Fahlbusch R. Implementation of fiber tract navigation. Neurosurgery. 58(ONS Suppl 2), 2006. ONS-292–304
49. Nimsky C., Ganslandt O., Merhof D., et al. Intraoperative visualization of the pyramidal tract by diffusion-tensor-imaging-based fiber tracking. Neuroimage. 2006;30(4):1219-1229.
50. Berman J.I., Berger M.S., Mukherjee P., et al. Diffusion-tensor imaging-guided tracking of fibers of the pyramidal tract combined with intraoperative cortical stimulation mapping in patients with gliomas. J Neurosurg. 2004;101:66-72.
51. Coenen V.A., Krings T., Axer H., et al. Intraoperative three-dimensional visualization of the pyramidal tract in a neuronavigation system (PTV) reliably predicts true position of principal motor pathways. Surg. Neurol. 2003;60(5):381-390. (discussion 390)
52. Chen X., Weigel D., Ganslandt O., et al. Diffusion tensor-based fiber tracking and intraoperative neuronavigation for the resection of a brainstem cavernous angioma. Surg. Neurol. 2007;68(3):285-291. (discussion 291)
53. Wu J.-S., Mao Y., Zhou W.-J., et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery. 2007;61(5):935-948. (discussion 948-949)
54. Le Bihan D., Mangin J.F., Poupon C., et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534-546.
55. Johansen-Berg H., Behrens T.E.J. Just pretty pictures? What diffusion tractography can add in clinical neuroscience. Curr Opin Neurol. 2006;19:379-385.
56. Nunes R.G., Jezzard P., Behrens T.E., et al. Self-navigated multishot echoplanar pulse sequence for high-resolution diffusion-weighted imaging. Magn Reson Med. 2005;53:1474-1478.
57. Parker G.J., Alexander D.C. Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue. Phil Trans R Soc Lond B Biol Sci. 2005;360:893-902.
58. Tournier J.D., Calamante F., Gadian D.G., et al. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage. 2004;23:1176-1185.
59. Tuch D.S., Reese T.G., Wiegell M.R., et al. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48:577.
60. Tuch D.S. Q-ball imaging. Magn Reson Med. 2004;52:1358-1372.
61. Wedeen V.J., Hagmann P., Tseng W.Y., et al. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med. 2005;54:1377-1386.
62. Kinoshita M., Yamada K., Hashimoto N., et al. Fiber-tracking does not accurately estimate size of fiber bundle in pathological condition: initial neurosurgical experience using neuronavigation and subcortical white matter stimulation. Neuroimage. 2005;25:424-429.
63. Cusack R., Huntley J., Goldrein H.T. Improved noise-immune phase-unwrapping algorithm. Appl Optics. 1995;34(5):781-789.
64. Fast Jenkinson M. automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49(1):193-197.
65. Berman J.I., Berger M.S., Chung S.W., et al. Accuracy of diffusion tensor magnetic resonance imaging tractography assessed using intraoperative subcortical stimulation mapping and magnetic source imaging. J Neurosurg. 2007;107:488-494.
66. Lazar M., Alexander A.L. An error analysis of white matter tractography methods: synthetic diffusion tensor field simulations. Neuroimage. 2003;20:1140-1153.
67. Lin C.P., Tseng W.Y., Cheng H.C., et al. Validation of diffusion tensor magnetic resonance axonal fiber imaging with registered manganese-enhanced optic tracts. Neuroimage. 2001;14:1035-1047.
68. Ganslandt O., Fahlbusch R., Nimsky C., et al. Functional neuronavigation with magnetoencephalography outcome in 50 patients with lesions around the motor cortex. J Neurosurg. 1999;91(1):73-79.
69. Rasmussen I.-A.Jr., Lindseth F., Rygh O.M., et al. Functional neuronavigation combined with intra-operative 3D ultrasound: initial experiences during surgical resections close to eloquent brain areas and future directions in automatic brain shift compensation of preoperative data. Acta Neurochir (Wien). 2007;149:365-378.
70. Gulati S., Berntsen E.M., Solheim O., et al. Surgical resection of high-grade gliomas in eloquent regions guided by blood oxygenation level dependent functional magnetic resonance imaging, diffusion tensor tractography, and intraoperative navigated 3D ultrasound. Minim Invasive Neurosurg. 2009;52:17-24.
71. Nimsky C., Ganslandt O., Hastreiter P., et al. Intraoperative compensation for brain shift. Surg Neurol. 2001;56:357-365.
72. Nagelhus Hernes T.A., Lindseth F., Selbekk T., et al. Computer-assisted 3D ultrasound-guided neurosurgery: technological contributions, including multimodal registration and advanced display, demonstrating future perspectives. Int J Med Robotics. 2006;2(1):45-59.
73. Okada T., Mikuni N., Miki Y., et al. Corticospinal tract localization: integration of diffusion-tensor tractography at 3-T MR imaging with intraoperative white matter stimulation mapping—preliminary results. Radiology. 2006;240(3):849-857.
74. Kamada K., Todo T., Masutani Y., et al. Combined use of tractography-integrated functional neuronavigation and direct fiber stimulation. J Neurosurg. 2005;102:664-672.
75. Gasco J., Tummala S., Mahajan N.M., et al. Simultaneous use of functional tractography, neuronavigation-integrated subcortical white matter stimulation and intraoperative magnetic resonance imaging in glioma surgery: technical note. Stereotact Funct Neurosurg. 2009;87:395-398.
76. Anil S.M., Kato Y., Hayakawa M., et al. Virtual 3-dimensional preoperative planning with the dextroscope for excision of a 4th ventricular ependymoma. Minim Invasive Neurosurg. 2007;50(2):65-70.
77. Kockro R.A., Serra L., Tseng-Tsai Y., et al. Planning and simulation of neurosurgery in a virtual reality environment. Neurosurgery. 2000;46(1):118-135. (discussion 135-137)
78. Kockro R.A., Stadie A., Schwandt E., et al. A collaborative virtual reality environment for neurosurgical planning and training. Neurosurgery. 2007;61(5 Suppl 2):379-391.
79. Wong G.K., Zhu C.X., Ahuja A.T., et al. Craniotomy and clipping of intracranial aneurysm in a stereoscopic virtual reality environment. Neurosurgery. 2007;61(3):564-568. (discussion 568-569)
80. Kockro R.A., Tsai Y.T., Ng I., et al. Dex-ray: augmented reality neurosurgical navigation with a handheld video probe. Neurosurgery. 2009;65(4):795-807.
81. Berger M.S., comment on Nimsky C., Ganslandt O., Hastreiter P., et al. Preoperative and intraoperative diffusion-tensor imaging-based fiber tracking in glioma surgery. Neurosurgery. 2005;56(1):130-138.