Functional neurosurgery
Implantation of deep brain stimulators
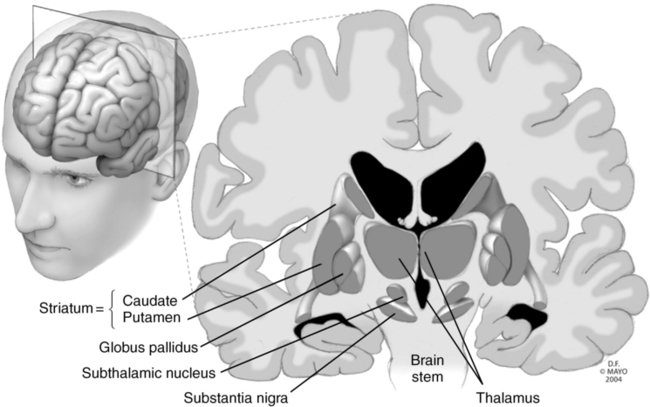
Deep brain stimulation (DBS) involves the implantation of electrodes into select regions of the brain, allowing for electrical stimulation of the area to modulate brain activity, resulting in attenuation, if not elimination, of the symptoms and signs of a number of disease states (Table 133-1). The specific site of electrode implantation depends on the disorder for which the patient requires treatment (Figure 133-1). Despite the use of DBS for many decades, the exact mechanism or mechanisms accounting for its clinical efficacy are not well understood, but the electric current is believed to somehow modulate abnormal neuronal function, either by acting directly on neuronal action potentials or altering neurotransmitter release. Use of DBS has generally replaced ablative procedures, such as pallidotomy or thalamotomy (i.e., thermal, mechanical, or electrical destruction of a region of the globus pallidus or thalamus, respectively), for the treatment of Parkinson disease. Unlike these earlier procedures, DBS is reversible.
Table 133-1
Disease States and Potential Anatomic Targets for Deep Brain Stimulation
| Disease | Potential Deep Brain Stimulation Target(s) |
| Parkinson disease and essential tremor | Subthalamic nucleus Globus pallidus |
| Dystonia | Globus pallidus |
| Cerebellar tremor from multiple sclerosis | Thalamic ventral intermediate nucleus |
| Pantothenate kinase–associated neurodegeneration | Globus pallidus |
| Medically refractory depression | Subgenual cingulate region |
| Tourette syndrome | Anterior limb of the internal capsule Thalamic centromedian-parafascicular complex |
| Obsessive-compulsive disorder | Nucleus accumbens Anterior limb of the internal capsule |
| Central pain syndromes | Motor cortex Periaqueductal gray matter Periventricular gray matter Thalamus |
| Medically refractory epilepsy | Anterior nucleus of the thalamus Centromedian nucleus of the thalamus Subthalamic nucleus |
| Cluster headaches | Posterior hypothalamus |
| Obesity | Lateral hypothalamus Ventromedial hypothalamus Nucleus accumbens |
Modified, with permission, from Siddiqui MS, Ellis TL, Tatter SB, Okun MS. Deep brain stimulation: Treating neurological and psychiatric disorders by modulating brain activity. NeuroRehabilitation. 2008;23:105-113.