Chapter 5 Functional Anatomy of the Spine
Overview of the Vertebral Column and Spinal Cord
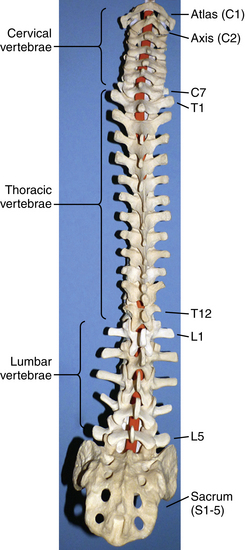
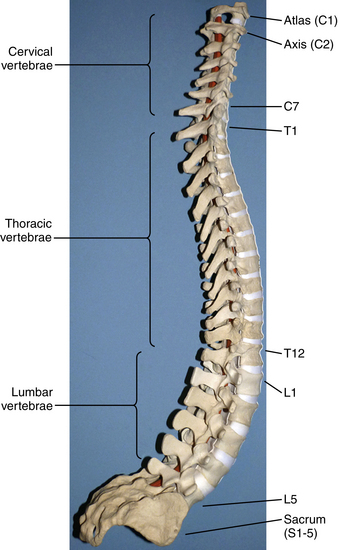
The human spinal column consists of 33 vertebrae separated into five anatomic regions. These regions include 7 cervical (C1-7), 12 thoracic (T1-12), 5 lumbar (L1-5), 5 sacral (S1-5), and 4 coccygeal bones (Fig. 5-1). In utero development plays a large part in the formation of the adult spine, contributing to the primary curvatures: kyphosis in the thoracic and sacral regions. Late in utero, after the development of the primary curvatures and continuing through early childhood, the secondary curvatures of the spine develop (Fig. 5-2). The cervical and lumbar lordosis becomes significant because of the gravitational forces created by the weight of the head and upright posture.1 The positions taken by the cervical and lumbar spine allow for horizontal gaze while standing in an upright posture.
The flexibility of the spine varies from region to region and is based on anatomic constraints. The cervical spine offers the greatest flexibility because of the requisite mobility of the head. This is in contrast to the rigidity of the thoracic spine due to its association with the chest wall.2 Unique articulations in the cervical region afford flexibility such as those found between the skull-atlas and atlas-axis (C1-2). Flexibility at other regions of the spine is influenced by cartilaginous discs between vertebral bodies and apophyseal joints found dorsal to the vertebral arches, all developed in a manner to provide optimum stability, flexibility, and mobility. The center of gravity of the spinal column and that of the body do not pass through the same points. The former begins cranially at the odontoid process of the axis and passes caudally through the sacral promontory.3 The latter passes ventral to the sacral promontory caudally. Disability may occur if the center of gravity deviates from the normal position. Studies have found that when the center of gravity passes too far ventral to the sacrum so the work required to maintain erect posture is significantly increased, lumbar muscle fatigue and pain result.
Normal physiologic function is supported by the ligaments and joint capsules of the spine. The ligaments of the spinal column are composed of elastin and collagen3,4 and may span several segments. The anterior longitudinal ligament (ALL) spans the entire length of the spinal column, extending from the ventral border of the foramen magnum (basion), where it is known as the anterior atlanto-occipital membrane, to the sacrum. The ALL spans 25% to 33% of the ventral surface of the vertebral bodies and intervertebral discs, supporting the annulus fibrosus and preventing hyperextension. The ALL is arranged in three layers: the outermost spanning four to five levels, the middle layer spanning three levels and connecting vertebral bodies and intervertebral discs, and the innermost layer binding adjacent vertebral discs. The posterior longitudinal ligament (PLL) begins as the tectorial membrane at C2 and extends to the sacrum. The PLL runs within the vertebral canal and flares at the level of the intervertebral disc where it is interwoven with the annulus fibrosus and narrows at the vertebral bodies, where it is loosely attached. The layers of the PLL are similar to the ALL but function to prevent hyperflexion.
Interspinous and supraspinous ligaments provide stability to the dorsal elements of the spinal column. Ligamenta flava connect spinal laminae in a discontinuous fashion and are intertwined with the facet joint capsule. The proximal insertion of the ligamentum flavum is the ventral part of the cranial lamina extending to the dorsal part of the caudal lamina. Laterally, the ligamentum flavum is in contact with the ventral capsule of the facet joint. These attachments are significant when excision of the ligamentum flavum is necessary to alleviate spinal stenosis. The microscopic anatomy of the ligamentum flavum is unique due to its approximately 80% elastin content. This is the source of the yellow appearance and the nickname “yellow ligament.” The elasticity of the ligamentum flavum allows it to stretch during flexion without limiting motion and allows it to become taut when returning to neutral and during extension. As a person ages, the elastin is replaced with a higher percentage of collagen, causing it to become less elastic, which may lead to buckling into the spinal canal.
Spinal canal dimensions provide adequate space for the spinal cord in all segments except for the midthoracic region. Here, the risk of neural tissue impingement during surgical instrumentation is increased. The lumbar region has a consistent spinal canal size and, along with the cauda equina, the anatomy functions to limit nervous tissue damage due to trauma or degenerative changes.5
Decreases in canal dimensions may result in radiographic and clinical spinal stenosis. These decreases in canal size may be either congenital, generally presenting at a younger age, or acquired, presenting at a later age due to degenerative changes. Stenosis is a self-perpetuating loop often beginning with disc degeneration leading to alterations in mechanical stress that cause facet joint degeneration and ligamentous changes, with an end result of a decrease in the canal space. Spinal stenosis may lead to changes in intradural pressure that diminish the blood flow to nerve roots and alter axonoplasmic flow. Acute nerve root constrictions have substantial edema, which can slow electrical conduction and nutrient transport and which are more substantial than chronic conditions. There is also an inflammatory component to stenosis that alters neuropeptide concentrations.
Vertebrae
Atlas (C1) and Axis (C2)
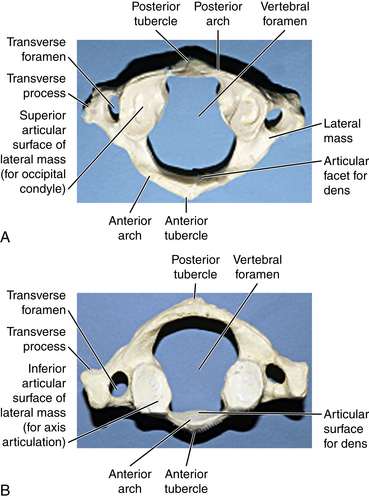
The first cervical vertebra is unique in its articulation with the occipital condyle of the cranium. This articulation is the basis for significant flexion and extension of the head. Another unique aspect of the atlas is that although it lacks a true ventral body it still supports the cranium by the superior facet surfaces of the lateral masses (Fig. 5-3A). The caudal facet surfaces of the lateral mass articulate with the superior facets of the axis (Fig. 5-3B). The transverse process of the atlas houses the vertebral artery within the transverse foramina. Superior and inferior oblique muscles attach to the transverse process. The atlas is hydrostatically held between the cranium and axis. The anterior and posterior occipital membranes attach to the atlas and also contribute to stability. They are continuations of the anterior longitudinal ligament and ligamentum flavum, respectively.
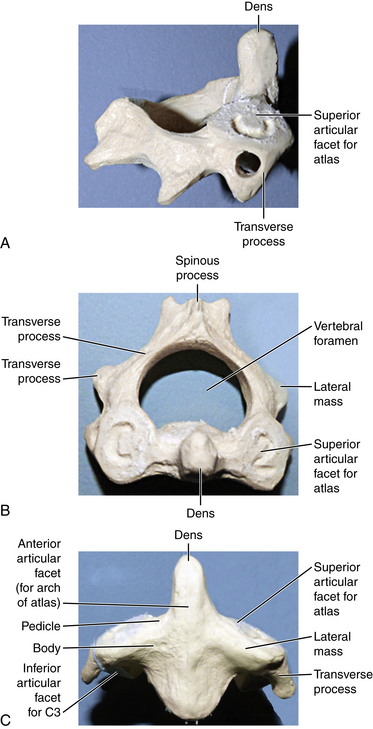
The axis is the second cervical vertebra. The articulation between the atlas and axis, known as the atlantoaxial joint, contributes to the majority of cervical rotation and stability to the upper cervical region. Unlike the atlas, the axis does have a true vertebral body and a unique structure known as the odontoid process projecting cranially from its dorsal aspect (Figs. 5-4A–C). The alar, cruciform, and transverse ligaments are anchored to the odontoid process. Further stability of the cervical region is contributed to by the muscular attachments at the spinous process of the axis, which include the rectus major and inferior oblique muscles. Like the atlas, a transverse foramen encases the vertebral artery.
Ligamentous anatomy of the cervical spine is unique, providing support for the head and maintaining stability despite the tremendous flexibility of this region. Ligaments exist both within and outside of the spinal canal. Much of the stability of the craniocervical region is provided by the ligaments within the spinal canal, which are ventral to the spinal cord. These are arranged in three layers. The tectorial membrane is the most dorsal of these ligaments and is a continuation of the PLL, attaching dorsally to the cruciate ligament at the basiocciput. The cruciate ligament is the middle layer and functions to constrain ventral translation between C1 and C2. It is a complex ligament with both horizontal and vertical bands. The odontoid ligament, or apical ligament, is the most ventral of the inner ligaments and extends from the lateral aspect of the odontoid to the medial aspect of the occipital condyles. Outside of the spinal canal are fibroelastic bands extending from the foramen magnum to C1. From the ventral portion of the foramen magnum extends the anterior atlanto-occipital membrane. From the dorsal foramen magnum arises the posterior atlanto-occipital membrane. Because these are thin bands, their contribution to the strength of the cervical spine is limited.
Subaxial Cervical Vertebrae (C3-7)
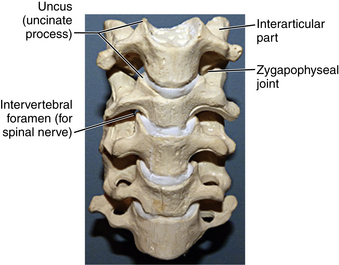
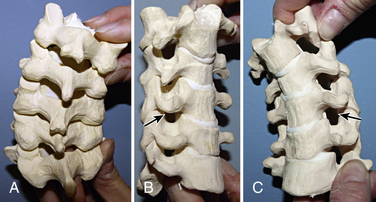
The remaining cervical vertebrae share anatomic features and may be considered separately from the atlas and axis. They are the smallest in size compared with all other regions of the spine and begin the trend of gradually increasing in size with each successively lower level. Descending down the spine, more body weight is supported, which is why the vertebrae increase in size. The end plates of the vertebrae in this region are concave superiorly and convex inferiorly, and they articulate to form the uncovertebral joints (joints of Luschka) (Fig. 5-5). These joints are often the site of arthritic changes, which can cause nerve root impingement. The position of the subaxial cervical spine affects the relative size of the neural foramen (Figs. 5-6A–C). Clinically, this is demonstrated by the Spurling maneuver. If the volume of the neural foramen is compromised by an osteophyte or disc fragment, pain can be elicited by tilting the head toward the affected side, which further reduces the foramen volume.
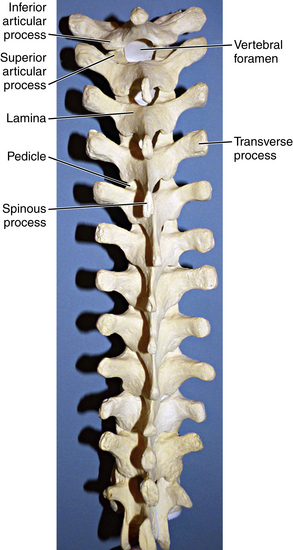
Pedicles are short and arise from the midpoint of the vertebral bodies. The laminae are fairly narrow. The spinous processes are bifid, with C7 being the largest (Fig. 5-7). The transverse processes, like the atlas and axis, have vertebral foramen that transmit the vertebral artery. The majority of individuals have vertebral arteries passing through the transverse foramen of C1-6, but in 5% of cases these arteries pass through the foramen at C7.6 The facet joints are horizontal, and the facet capsule is weak, which allows for the mobility of the cervical spine.
The ligamentum nuchae is the primary ligamentous structure in the dorsal cervical spine outside of the spinal canal with attachments to the spinous processes. Descending past C7, the ligamentum nuchae transitions into the supraspinous ligament, which extends to the lumbosacral region, ending between L3 and L5.
The range of motion measure at the cervical spine can vary due to age, gender, and method of measurement. Visual estimation and radiography are the primary methods used to measure cervical range of motion. Studies of these measurement techniques on active cervical range of motion have found that, on average, the cervical spine has a total of 151 degrees of rotation, a total of 86 degrees of lateral bending, and a total of 126 degrees of flexion-extension. When considering motion in only one direction, leftward and rightward rotation and lateral bending are, on average, half the total values, whereas the cervical spine has, on average, a greater range of extension than flexion.7
Thoracic Vertebrae
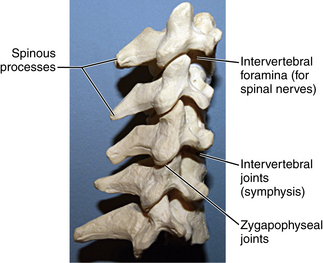
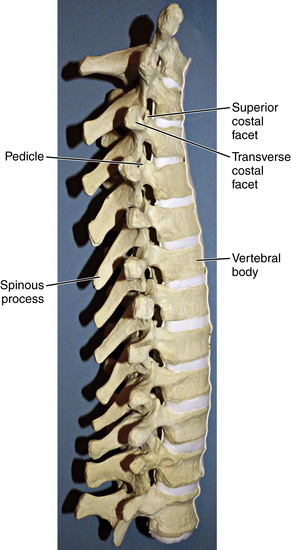
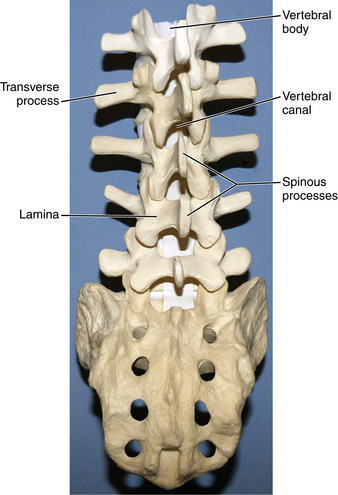
The thoracic region of the spine contains the largest number of vertebrae, which continue to increase in size from T1 to T12. The first four thoracic vertebrae maintain some cervical features, and the last four possess some lumbar features, maintaining a smooth transition between the adjacent regions. The superior vertebral notch is the cervical feature of T1, and the lumbar features of T12 include lateral direction and inferior articular processes. Laminae in the thoracic region are broader than in the cervical spine and overlap, whereas the transverse processes increase in size as they progress down the thoracic spine8 (Fig. 5-8). The spinous processes of the thoracic vertebrae are variably arranged in horizontal, oblique, or overlapping vertical arrangements. Horizontal spinous processes are found at T1-2 and T11-12 and oblique spinous processes at T3-4 and T9-10, with the rest of the thoracic vertebrae possessing overlapping, vertical spinous processes (Fig. 5-9). Thoracic facets are primarily arranged in a coronal plane but develop a sagittal orientation near the junction of the lumbar vertebrae. There is less free space in the spinal canal in the thoracic region compared with both cervical and lumbar regions.
A characteristic feature of the thoracic vertebrae is the relationship with the ribs.9 The ribs articulate with unique costal facets—found where the vertebral body and pedicle meet—as well as on transverse processes, with T10-12 being exceptions to facets on transverse processes. The thoracic spine has maximum stiffness relative to all regions of the spine, which is a function of the relationship between the rib and vertebrae combined with support from accessory ligaments.9 The “junctional” regions of the spine, such as C7-T1 and T12-L1, are sites of transition from a rigid spinal region to one with maximal spinal motion. These junctional sites are often the sites of natural and iatrogenic pathology.
Lumbar Vertebrae
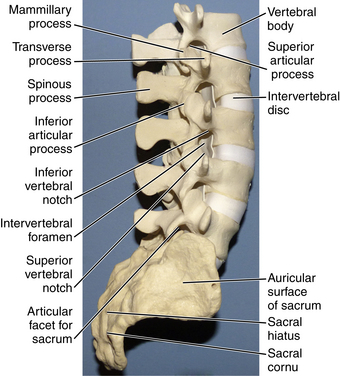
Descending down the spinal column, we come to the largest vertebral bodies—the lumbar vertebrae (Fig. 5-10). These vertebrae progressively increase in diameter when approaching the sacrum and are greater in transverse width relative to anteroposterior diameter. Within the lumbar region are subregional variations in the anatomy of the vertebrae. These are attributed to the greater weight and forces that these vertebrae must distribute as the spinal column transitions into the pelvis. The L1-2 vertebral bodies have greater depth dorsally, whereas L4-5 vertebral bodies have greater depth ventrally (Fig. 5-11). The two subregions are balanced by the L3 vertebral body, which provides a transitional point between the two. Vertebral body angulation and translation are affected by these locoregional differences in anatomy during flexion and extension. These variations produce changes in intervertebral disc height and foramen cross-sectional area, which are functionally linked to motion during flexion and extension. The variations may be associated with susceptibility at lumbar regions for disc herniation, spinal canal stenosis, and other pathology. Cadaveric studies have shown that in the L4-5 region, flexion results in a greater dorsal disc bulge than in the L1-2 region. The cross-sectional area of the foramen in the lumbar region shows that compared with a neutral position, flexion increases the area by 12% (15 mm2) and extension decreases the area by 15% (19 mm2). The vertebral bodies move closer ventrally and further apart dorsally during flexion, which increases the dimensions of the spinal canal; the opposite occurs during extension.10
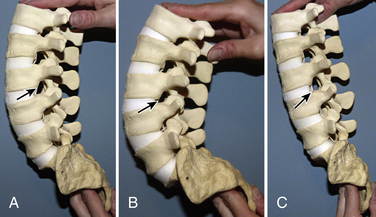
The cross-sectional area of the nerve root is linked to flexion and extension as well. The nerve roots traverse beneath the lateral recess of the pedicles and articular facets through the intervertebral foramina. Ventral borders of the nerve root are the vertebral body and intervertebral disc, dorsal borders are lamina and facets, and both superior and inferior borders are adjacent pedicles. Because of the locoregional differences in anatomy of the lumbar region, flexion and extension movements alter these borders and result in changes in nerve root cross-sectional area (Figs. 5-12A–C). These changes can be associated with susceptibility to nerve root impingement. Cadaveric studies have found that the neutral cross-sectional area of the L1-2 nerve root is 28.31 ± 10.48 mm; in flexion it increases to 32.37 ± 9.92 mm, and in extension it decreases to 22.97 ± 7.52 mm.10
Pedicles in the lumbar region arise from the rostral aspect of the vertebral body. They can be visualized behind the facet of the named vertebra and supra-adjacent vertebra. The diameter of the L1 pedicle is approximately 9 mm with a medial angle of 12 degrees,11 which requires consideration with screw placement. Lumbar facets have a sagittal orientation, which limits axial rotation. The L5-S1 facet is unique, with a coronal orientation that resists anteroposterior translation.2
Intervertebral Discs
The nucleus pulposus is bounded peripherally by the annulus fibrosus and both superiorly and inferiorly by the end plates. It is made up of negatively charged proteoglycan molecules and collagen. The negative charge makes the nucleus hydrophilic, which contributes to the extensive water component of the disc. Height and resistance to axial loads is maintained by the hydraulic properties of the fluid surrounded by end plates and the annulus fibrosus. Maintenance of disc height keeps the ligaments and capsules of the spine at optimal length and allows them to function physiologically. With aging, the distinct regions of the intervertebral disc are no longer present, and the proteoglycan content and hydration decreases. As disc height diminishes, increased demands are placed on the annulus fibrosus, thus increasing susceptibility to tears and subsequent herniation. Dorsal structures are also affected by the loss of disc height, including facet subluxation and hypertrophy, which may predispose an individual to nerve root compression.
Benzel E. Stability and instability of the spine. In: Benzel E., editor. Biomechanics of spine stabilization: principles and clinical practice. New York: McGraw Hill, Inc; 1995:25-40.
Panjabi M.M., Duranceau J., Goel V., et al. Cervical human vertebrae. Quantitative three-dimensional anatomy of the middle and lower regions. Spine (Phila Pa 1976). 1991;16:861-869.
Panjabi M.M., Takata K., Goel V., et al. Thoracic human vertebrae. Quantitative three-dimensional anatomy. Spine (Phila Pa 1976). 1991;16:888-901.
Vollmer D.G., Banister W.M. Thoracolumbar spinal anatomy. Neurosurg Clin North Am. 1997;8(4):443-453.
White A.A., Panjabi M.M. The basic kinematics of the human spine. A review of past and current knowledge. Spine (Phila Pa 1976). 1978;3:12-20.
Woodburne R.T. Essentials of human anatomy, ed 7. Oxford, England: Oxford University Press; 1982.
1. Benzel E. Stability and instability of the spine. In: Benzel E., editor. Biomechanics of spine stabilization: principles and clinical practice. New York: McGraw Hill, Inc; 1995:25-40.
2. An H.S., editor. Principles and techniques of spine surgery. Baltimore: Williams and Wilkins, 1998.
3. Woodburne R.T. Essentials of human anatomy, ed 7. Oxford, England: Oxford University Press; 1982.
4. White A.A., Panjabi M.M. The basic kinematics of the human spine: a review of past and current knowledge. Spine (Phila Pa 1976). 1978;3:12-20.
5. Reynolds A.F., Roberts A., Pollay M., et al. Quantitative anatomy of the thoracolumbar epidural space. Neurosurgery. 1985;17:905-907.
6. Panjabi M.M., Duranceau J., Goel V., et al. Cervical human vertebrae: quantitative three-dimensional anatomy of the middle and lower regions. Spine (Phila Pa 1976). 1991;16:861-869.
7. Chen J., Solinger A.B., Poncet J.F., et al. Meta-analysis of normative cervical motion. Spine (Phila Pa 1976). 1999;24:1571-1578.
8. Berry J.L., Morgan J.M., Berg W.S., et al. A morphometric study of human lumbar and selected thoracic vertebrae. Spine (Phila Pa 1976). 1987;12:362-366.
9. Panjabi M.M., Takata K., Goel V., et al. Thoracic human vertebrae: quantitative three-dimensional anatomy. Spine (Phila Pa 1976). 1991;16:888-901.
10. Inufusa A., An H.S., Lim T.H., et al. Anatomic changes of the spinal canal and intervertebral foramen associated with flexion-extension movement. Spine (Phila Pa 1976). 1996;21:2412-2420.
11. Zindrick M.R., Wiltse L.L., Doornick A., et al. Analysis of the morphometric characteristics of the thoracic and lumbar pedicles. Spine (Phila Pa 1976). 1987;12:160-166.