Foreign Body Removal
Evaluation and Diagnosis
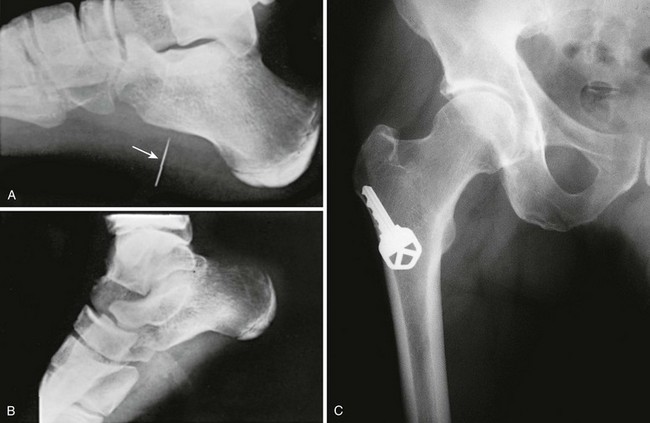
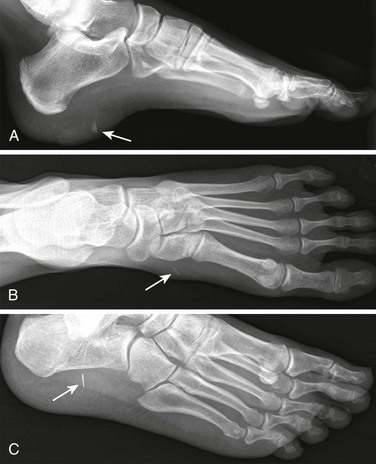
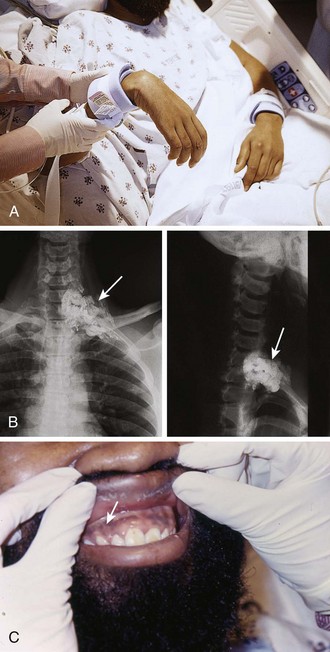
A foreign body (FB) is any substance that is not naturally part of the body. These cases are common in the clinical setting. An FB should be suspected whenever the skin is broken. A thorough history and physical examination are essential to assess the risk for an FB. During assessment, remember that FBs may not be obvious and that the wound may appear closed, but they should be considered whenever the history is particularly concerning. For example, a patient who is walking without shoes and experiences a sharp, sudden pain in the foot may have a needle, toothpick, or any other similar type of FB, even when a “sprained foot” seems obvious (Fig. 36-1). Certain mechanisms of injury, such as punching or kicking out a window or stepping on an unknown object while walking in a field or stream, are associated with a retained FB. Mechanisms that make FBs less likely include lacerations from metal objects; however, if considerable force was applied during the injury and the object is not available for inspection, radiographic imaging may be warranted since bone may have been encountered and a small section might have splintered off the offending object. The history should also include asking whether the patient perceives or suspects an FB. Steele and associates found that the negative predictive value of patient perception was 89% but that the positive predictive value was just 31%.1 Importantly, the patient’s past medical history should be explored for allergies to local anesthetics, bleeding diatheses, diabetes mellitus, vascular disease, uremia, immunocompromised state, or other diseases that would affect wound healing or management.
Exploration is an important part of the bedside evaluation, whether done initially or after further imaging studies (Fig. 36-2). This requires adequate pain control, good lighting, proper equipment, a bloodless field, a cooperative patient, and appropriate positioning to visualize as much of the wound as possible (Figs. 36-3 and 36-4). A metal probe may help identify the FB by feel or sound. Glass, as an example, is difficult to identify by sight in soft tissue, but touching it with metal causes a characteristic grating sound. Probing a wound with a gloved finger to locate or identify an FB is strongly discouraged because of the risk of the FB penetrating the glove and exposing the clinician to human immunodeficiency virus (HIV) and hepatitis (Fig. 36-5). Alternatively, some authors have suggested injecting the entrance wound with methylene blue to outline the track of the FB.2 The blue line of injected dye is followed into the deeper tissues. This technique is of limited value because the track of the FB often closes tightly and does not allow passage of the methylene blue.
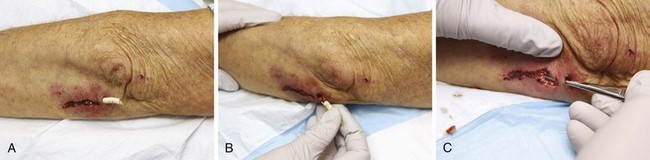
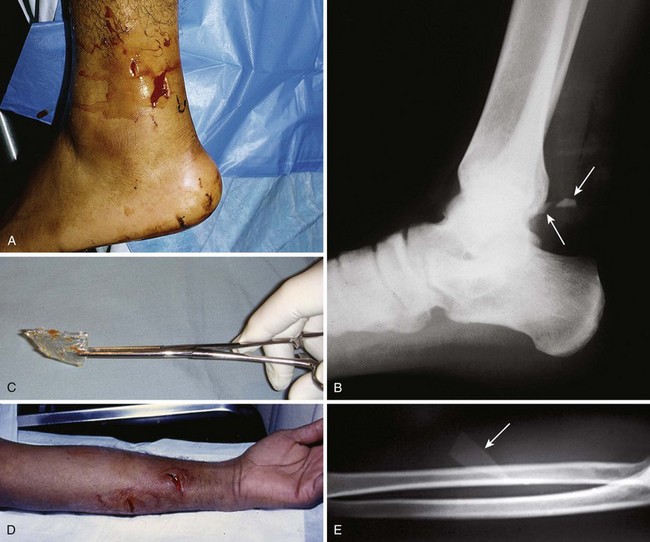
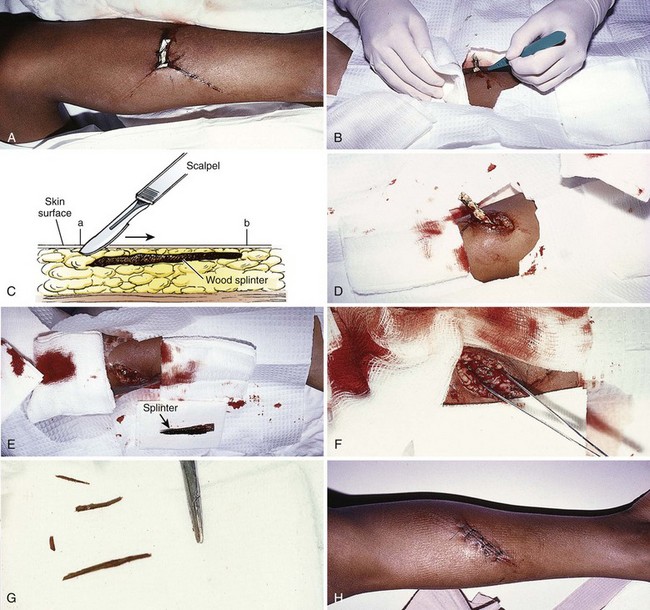
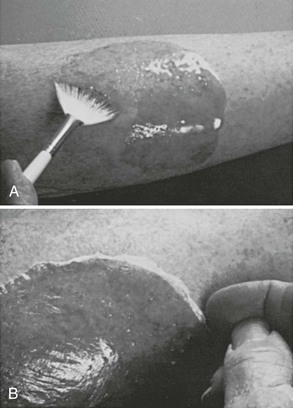
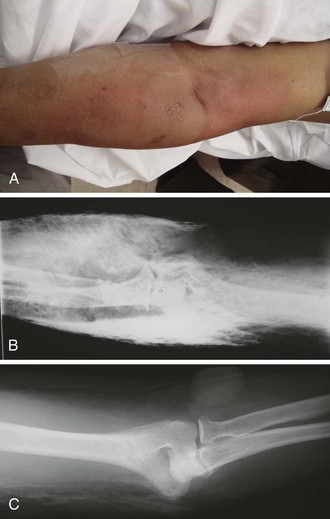
Figure 36-3 A, This patient has an obvious wooden foreign body on the dorsal aspect of the proximal part of the forearm. B, Manual removal of the object was not difficult. C, However, thorough exploration of the wound cavity via an incision over the entire length of the FB tract revealed multiple small wooden fragments. Proper exploration requires adequate analgesia, good lighting, hemostasis, and a cooperative patient. Use of a metal probe may help identify the foreign object by feel or sound (see Fig. 36-17).
Augmenting the Physical Examination: Imaging Techniques
Many emergency clinicians mistakenly believe that in the absence of adipose tissue, if the base of the wound can be clearly visualized and explored, an FB can always be ruled out. Orlinsky and Bright found the reliability of exploration to be related to the depth of the wound.3 In their study, only 2 of 133 superficial wounds, deemed adequately explored, had an FB on plain films, but 10 of 130 wounds had FBs beyond the subcutaneous fat despite negative explorations. Anderson and coworkers reported that 37.5% of the foreign bodies were initially missed by the treating physicians.4 In many of these cases, a radiograph of the injured area was not taken. Avner and Baker detected glass with routine radiographs in 11 of 160 wounds (6.9%) that were inspected and believed by the clinician to be free of glass.5 Clinicians evaluating for FBs should maintain a low threshold for ordering or performing imaging studies. Options for imaging include plain radiographs, ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI), and fluoroscopy. See Table 36-1 for a summary.
TABLE 36-1
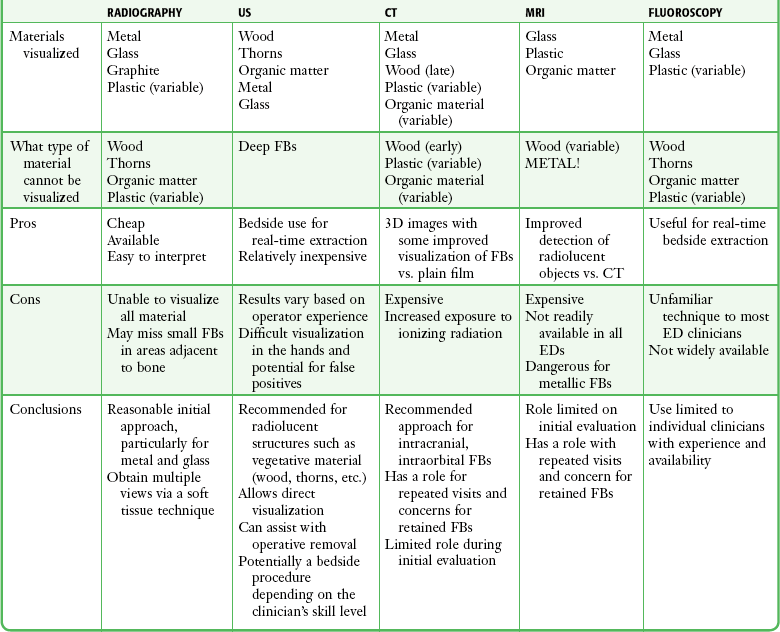
A reasonable initial approach for localizing nonvisualized FBs in the ED is to obtain multiple-projection plain radiographs with a soft tissue technique. This technique will visualize the majority of FBs, especially metal and glass. US should be considered for objects known to be radiolucent, such as wood or thorns. The role of CT and MRI for evaluation of FBs in the ED is limited, but they are the definitive imaging tests in confusing cases. For suspected intraorbital or intracranial FBs, CT is recommended. CT or MRI is also warranted when a previously negative explored wound exhibits recurrent infection, poor healing, or persistent pain. CT or MRI may be the appropriate initial test for patients with nonspecific swelling to define FBs and possible alternative diagnoses such as abscesses, masses, or other inflammatory processes.6 Bedside US and fluoroscopy (for radiopaque FBs) may be used to guide difficult FB extractions if initial attempts at removal are unsuccessful and the proper equipment and experienced personnel are available.
Plain Radiography
Plain radiographs are readily available, are easily interpreted, and cost significantly less than CT, formal US, and MRI do.7 The ability of plain films to detect FBs in soft tissue depends on the object’s composition (relative density), configuration, size, and orientation (Fig. 36-6). Detection of FBs on plain films can be enhanced by requesting an underpenetrated soft tissue technique and by obtaining multiple views to prevent FBs from being obscured by superimposed bone or soft tissue folds.8 Plain films are often sufficient; however, digitized radiographs may be manipulated to enhance identification of a suspected FB. Indirect evidence of an FB may include trapped or surrounding air, a radiolucent filling defect, or secondary bony changes such as periosteal elevation, osteolytic or osteoblastic alterations, or pseudotumors of bone.8
Metallic objects are readily visualized on radiographs. Despite a common misconception that glass must contain lead to be visualized on a plain radiograph, almost all glass objects in soft tissue (bottles, windshield glass, lightbulbs, microscope cover slips, laboratory capillary tubes) are at least somewhat radiopaque and can be detected by plain radiographs unless they are obscured by bone or are very small (<1 mm) (Fig. 36-7).7,9,10 The absence of a glass FB on multiple projections is strong, though not absolute evidence that glass is not contained in a wound. Other nonmetallic objects readily visualized include teeth, bone, pencil graphite, asphalt, and gravel.7,11 Aluminum, which has traditionally been deemed radiolucent, can occasionally be visualized on plain films if the object is projected away from the underlying bone. Ellis demonstrated that pure aluminum fragments as small as 0.5 × 0.5 × 1 mm could be identified in a chicken wing model simulating a human hand or foot.12 Ellis cautioned that other aluminum FBs, such as pull tabs from cans, may not be visualized in other parts of the body such as the esophagus or stomach.12
Certain FBs such as vegetative material (thorns, wood, splinters, and cactus spines) are radiolucent and not readily visualized on plain radiographs. These materials absorb body fluids as they sit in situ and become isodense with the surrounding tissue. Because of their varying chemical composition and density, plastics may or may not be visible on plain films.13
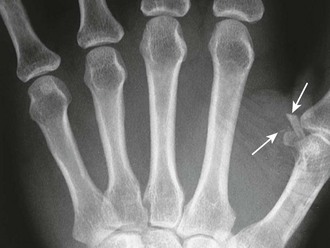
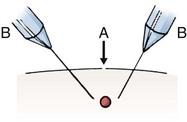
Besides simply diagnosing FBs, radiographs can also be used to estimate the general location, depth, and structure of radiopaque FBs. If one strategically attaches a marker (needle or paper clip) to the skin surface at the wound entrance before taking a radiograph, the FB will be seen in relation to the entrance wound (Fig. 36-8). This also helps identify the path that leads to the FB and the relative distance from the surface to the FB.14 Needles at two angles may also be used to aid in localization (Fig. 36-9).
Liberal use of plain film radiography makes sound medicolegal practice. A review of 54 wound FB claims against 32 physicians from 22 institutions found glass, a radiopaque substance, to be the most common material. However, in only 35% of the cases involving glass were plain films taken. Cases with a glass FB without a radiograph ordered were associated with unsuccessful defense (60%) and higher indemnity payments.15
US
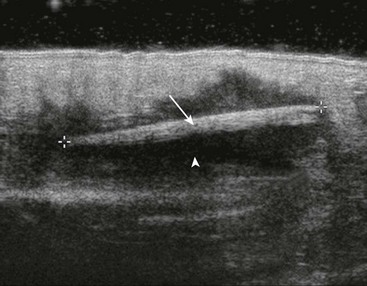
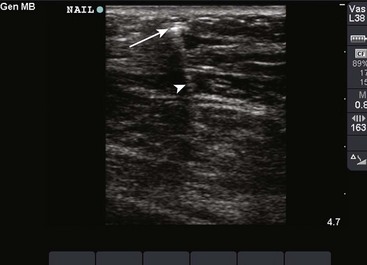
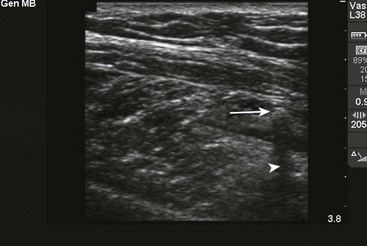
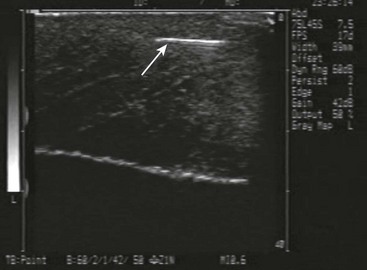
US has become the modality of choice for imaging radiolucent FBs such as wood and thorns because most soft tissue FBs are hyperechoic on US. In addition, if in place for more than 24 hours, most FBs will be surrounded by a hypoechoic area corresponding to granulation tissue, edema, or hemorrhage, which may aid in making the diagnosis.16 Metal will leave a linear trail of echoes deep to the FB, referred to as the “comet tail artifact.” A wooden object leaves an acoustic shadow without artifact.17,18
US may be performed at the bedside. To do so, use a high-frequency transducer (at least 7.5 MHz, such as a high-frequency linear vascular probe) since most FBs are small and superficial. A lower frequency may also be necessary if a deeper FB is suspected, but it may miss small FBs.19 A spacer may be needed to adjust the “focal zone”—where the beam is the narrowest and signal intensity is the highest. US may be particularly difficult in the hand or foot, which have many echogenic structures and web spaces that may limit visualization when the FB is adjacent to bone.16,18,20 Keep in mind that the presence of air, scars, calcification, sutures, and sesamoid bones in surrounding tissue may lead to false-positive results.20,21
Advantages of US include low cost, no ionizing radiation, the ability to define the object in three dimensions, and real-time imaging, which may be used during removal.8 Many studies report that US is highly sensitive for the detection of FBs by adequately trained personnel, either radiologists, technicians, or emergency physicians.3 It is difficult to cite a precise sensitivity or specificity because of the wide variation in these studies with regard to FB size, material, and location; operator experience; and the models used in these studies.21–23
CT
CT depends on x-ray absorption; thus, it generally visualizes the same material detected on plain films, but subtle differences in soft tissue densities may help identify FBs not seen on radiographs.7,11 Also, CT produces a better three-dimensional image than plain films do and may visualize objects embedded in or behind bone. However, CT is more costly and exposes the patient to more ionizing radiation than radiography or US does, and its use should therefore be judicious.
As an example, wood is unlikely to be visible initially on CT, but after 1 week, the wood absorbs surrounding blood products and may become higher in attenuation than muscle and fat. It may then appear on CT as a linear area of increased attenuation on a wide window setting such as a bone window.6
MRI
Although MRI is expensive and not as readily available to emergency clinicians as plain films and CT are, it may be superior to CT in detecting small, nonmetallic, radiolucent FBs such as plastic, particularly in the orbit.24 MRI may not visualize wood, which may appear as a linear signal with associated inflammation and looks hypointense with respect to muscle on T1- and T2-weighted sequences, on which it appears as a signal void.6 Plastic is more easily visualized with MRI than with CT. MRI cannot be used for metallic objects and gravel, which contain various ferromagnetic particles that produce signal artifacts and a theoretical risk of shifting within the magnetic field and causing structural damage.7 This is particularly important when evaluating FBs in the eye, brain, or deep structures of the neck, face, or extremities. FBs may be difficult to differentiate from other low-signal structures on MRI, such as tendon, scar tissue, and calcium.25
Fluoroscopy
More recently, portable, low-power, C-arm fluoroscopy has become available in some emergency departments (EDs), particularly for orthopedic reductions. Its use has also been reported for the removal of BB pellets, metal, glass, and coins from patients.26 Like radiography, fluoroscopy can visualize objects that are radiopaque but not radiolucent such as wood and plastic.26,27 By using correct technique and shielding, radiation scatter to imaging personnel is minute, less than 0.0001 R/hr.28 Fluoroscopy also offers the advantage of real-time bedside imaging.26,28 Fluoroscopic image-intensifying equipment may be used to follow a wound’s entrance, localize the material, grasp the FB, and remove it without making a larger incision. Ariyan described a technique in which two needles are placed in the soft tissue from opposite directions and pointing toward the FB.29 The extremity is rotated while the clinician watches the image under the image intensifier to obtain a three-dimensional effect. An incision is made perpendicular to the plane of the needles, and the object is removed. Although the technique to use fluoroscopy is relatively easy to learn, the lack of instruction and availability is the major limitation to its use in the ED.28–30
FB Removal
When reviewing the decision regarding when and how to remove the FB, the possibility of the FB migrating to involve vital structures, though quite remote, should be discussed with the patient. Cases of reported missile embolization in the vascular system are influenced by missile caliber, impact velocity, physical wound characteristics, point of vessel entrance, body position and movement, and velocity of blood flow.31 Retained bullets usually remain in soft tissue but can rarely make their way into the vascular system. Schurr and colleagues reported a paradoxical bullet embolization from the left external iliac vein to the left iliac artery via a patent foramen ovale.32 When clinicians first examined the patient, a bullet was noted on the chest radiograph, and an isolated chest wound was suspected. However, the bullet had apparently entered the chest, traversed the abdomen to the iliac vein, and then embolized back to the chest and arterial system.
Equipment and Preparation
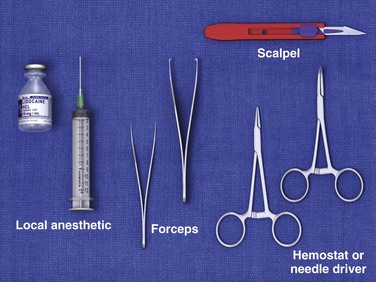
Good space and lighting, a comfortable patient and operator position, a standard suture tray, and a scalpel are usually adequate equipment for the removal of most simple FBs (Fig. 36-10). Tissue retractors, special pickups, and loupes may be added if needed.
Operative Technique
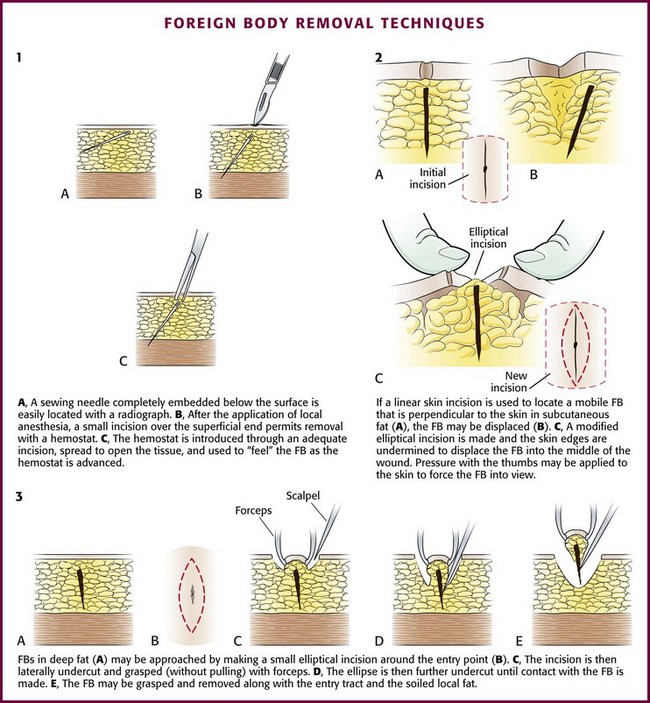
After obtaining appropriate informed consent and following sterile preparation, consider enlarging the entrance wound with an adequate skin incision, which can be advantageous. Numerous techniques are used, depending on the clinical scenario (Fig. 36-11). Attempting to remove an FB through a puncture wound or an inadequate skin incision is a common error that is both frustrating and self-defeating. After a proper skin incision, explore the wound carefully by spreading the soft tissue with a hemostat. Frequently, the FB can be felt with an instrument before it can be seen (see Fig. 36-11, plate 1). After placing a tourniquet on an extremity, follow the track of the FB, although it often cannot be identified when surrounded by muscle or fat. If the FB, such as one that is made of fiberglass or plastic, is difficult to visualize, is located in the superficial soft tissue, or has contaminated the surrounding soft tissue, excising a small block of tissue rather than removing the FB alone may be necessary. Excise the block of tissue only under direct vision and after nerves, tendons, and vessels have been identified and excluded from the excision area.
If an FB such as a thorn or needle enters the skin perpendicularly, a linear incision may pass to one side or the other of the FB, and it may be difficult to determine where the FB lies in relation to this incision (see Fig. 36-11, plate 2). For this reason the search must then be extended into the walls of the incision rather than simply through the skin.33 In such cases, excise a small ellipse of skin and undermine the skin for 0.5 to 1.0 cm in all directions. Next, compress the tissue from the sides in the hope that the FB will extrude and can then be grasped with a hemostat.
After removal of the FB, it is important to irrigate and cleanse the wounds. If a small incision has been made in a noncosmetic area (such as the bottom of the foot), leave the incision open and bandaged. The area may be periodically soaked in hot water for a few days. A return visit is necessary only if signs of infection develop. If a large incision has been made, the skin may be sutured primarily as long as no other contraindications are present. In cases in which gross contamination has occurred or there is significant tissue injury, do not close the wound on the initial visit. Leave the wound open but packed. Suture the skin after 3 to 5 days if the wound is free of inflammation or infection (known as delayed primary closure; see Chapters 34 and 35 for details).
Special Scenarios and Techniques
Puncture Wounds in the Sole of the Foot
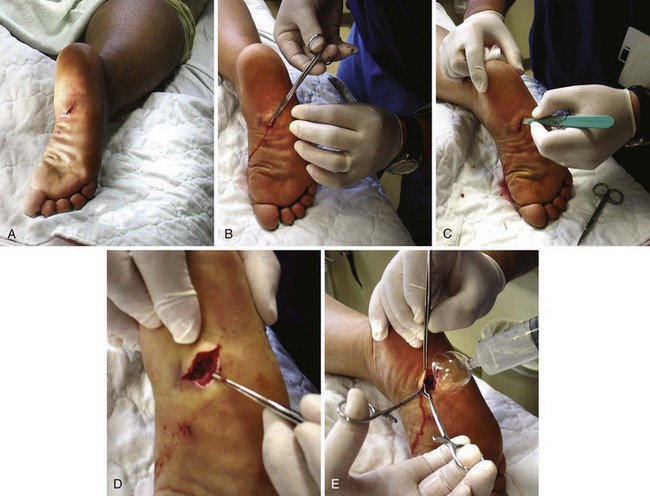
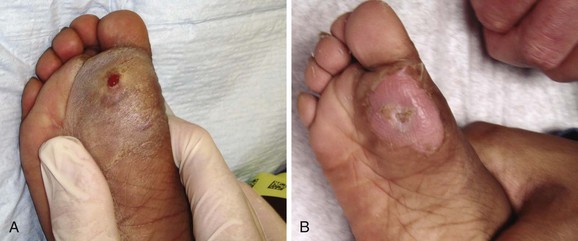
Puncture wounds in the feet from unknown objects and under unknown circumstances present problems to the clinician. In general, puncture wounds in the feet are at risk for retained FBs and infection. It is impossible to adequately explore a puncture wound. In noncosmetic areas, therefore, a puncture wound can be converted to a laceration to adequately explore and clean it (Fig. 36-12). Under most circumstances, delayed primary closure is recommended (see Chapter 34). This topic, including stepping on a nail and puncture wounds in the sole of the foot, are discussed extensively in Chapter 51.
Subungual FBs
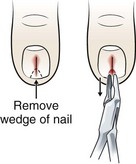
Once a subungual FB has been identified, perform a digital block before manipulation of the nail or nail bed. Pay special attention to deeply embedded subungual FBs.34 Some cases may require removing a small portion of the nail with double-pointed heavy scissors to expose the FB and grasp the foreign material with splinter forceps (Fig. 36-13).
One technique is to bend the tip of a sterile hypodermic needle and slide it under the nail. Hook the FB and then remove it. Alternatively, slide a 19-gauge hypodermic needle under the nail to surround a small splinter. Bring the needle tip against the underside of the nail and secure the splinter. Remove the needle and splinter as a unit.35 Another possible technique is to shave the overlying nail plate with a No. 15 scalpel blade via light strokes in a proximal-to-distal direction. This creates a U-shaped defect in the nail and exposes the entire length of the sliver.36
Wooden splinters are commonly embedded under the fingernail. Such FBs must be removed completely because subsequent infection is almost certain. If complete removal cannot be achieved with the techniques described earlier, remove the entire nail (see Chapter 35). This allows all the fragments to be visualized and removed. The proximity of the distal phalanx to the subungual area is a constant concern for the development of osteomyelitis and requires follow-up.
Metallic Fragments and Bullets
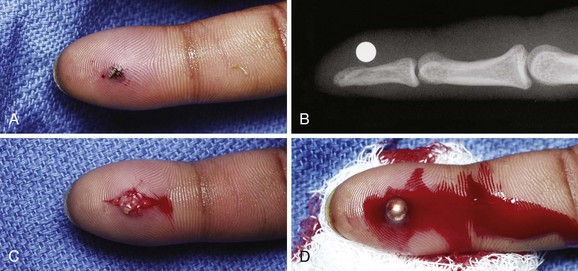
High-velocity fragments (e.g., bullets, BBs) are easy to visualize radiographically and relatively simple to remove if embedded in areas that are anatomically accessible (Fig. 36-14). Before removal, assess the area in which the fragments are embedded to determine which structures are involved and which structures might be encountered during removal. Defer removal of deeply embedded metallic FBs unless symptoms of infection develop. Infection is rare because such FBs usually become encysted over time.
A caveat is lead toxicity from bullet fragments. If a bullet is bathed in synovial, pleural, peritoneal, or cerebrospinal fluid, the lead may leach out over time and produce a significant elevation in blood lead levels. Though rare, lead fragments in contact with synovium are a reason for concern and potentially a reason for removal (see the previous discussion). In one study, Farrell and associates found that patients with retained lead FBs had statistically significant elevated blood lead levels.37 The study did not differentiate the location of the FB and whether patients were, in fact, symptomatic from plumbism. Nevertheless, it may be prudent for the patient’s primary care provider to monitor blood lead levels in patients with known lead FBs to prevent the future development of lead toxicity. The value of routine prophylactic antibiotics for metallic FBs left in soft tissue has not been proved.
For superficial metallic FBs, a sterile magnet can be used to facilitate the removal of small metallic fragments.38 Introduce the magnet into the entry site, with a small scalpel and hemostat used to extend and open the wound as needed. When the magnet comes in contact with the metal, a click is heard and the FB is removed while attached to the magnet. If resistance to extraction occurs, exploration can be done with the FB attached to the magnet. Sometimes, instead of introducing the magnet directly into the wound it can be placed on the overlying skin to guide superficial small FBs out of the wound.39 Make an effort to not grasp or touch the bullet because it can interfere with ballistics evidence during any subsequent legal investigation.
FBs in Fatty Tissue
Remove FBs in fatty tissue by making an elliptical incision around the entrance wound. Grasp the incised skin loosely with Allis forceps. Undercut the incision until the FB is contacted. Remove the FB, skin, and entrance track in one block. Remove a small portion of subcutaneous fat along with the FB to minimize infection. FBs in fat are very mobile, and probing may displace them even further. FBs that are embedded in fat and are perpendicular to the skin can also be removed, as shown in Figure 36-11.
Pencil Lead/Graphite
Use careful judgment in removing pencil graphite when it is lodged in the skin. Graphite invariably leaves a pigmented tattoo in the soft tissue, and it is preferable to excise the material en bloc (see Fig. 36-11) when pencil lead is found in a cosmetic area. The graphite specks cannot be irrigated or scrubbed off, and tattooing results if they are not removed. Furthermore, a pencil lead FB may resemble a malignant melanoma over time. US has been shown to be useful in differentiating between pencil lead FBs and melanoma.40
Fishhooks
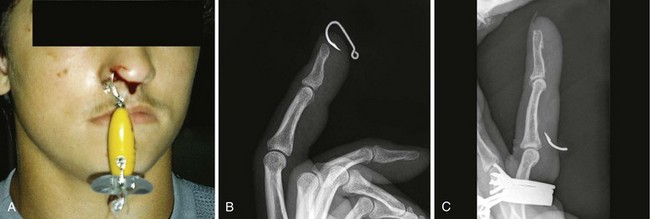
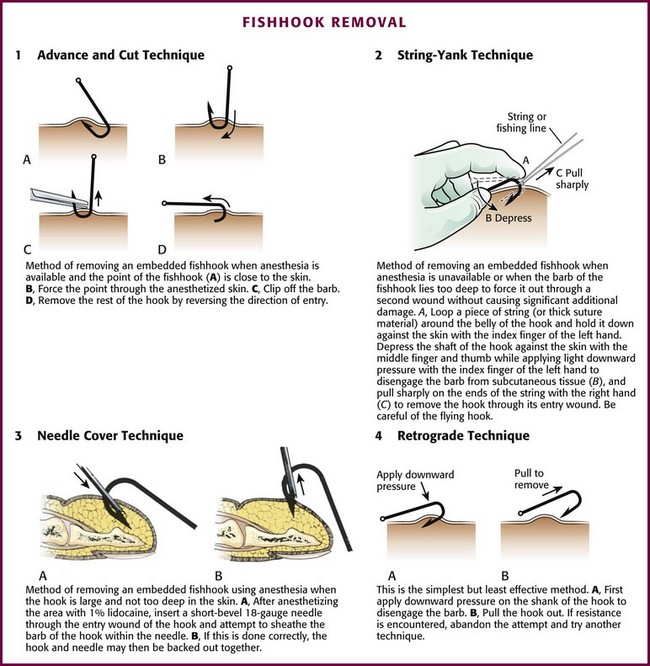
Traditionally, four fishhook removal techniques have been described: advance and cut, string-yank, needle cover, and retrograde.41 The preferred method depends on the location, depth of penetration, and conditions under which the removal is to take place (Fig. 36-15).42–44 Initially, note whether the fishhook is single or multiple. In addition, note the number and location of barbs. Remove or cover any remaining exposed hooks to prevent subsequent injury. As with most injuries, document the patient’s vascular and neurologic status before and after removal. As with all wounds, administer tetanus prophylaxis if indicated. Prophylactic antibiotics are not generally necessary.
Advance and Cut Technique: To perform the advance and cut technique for removal, advance the fishhook and cut proximal to the barb (Fig. 36-16, plate 1). This method is particularly well suited for superficially embedded fishhooks. Generally, infiltrate local anesthetic (1% lidocaine) into the tissue overlying the barb. Force the barb through the anesthetized skin and clip it off. Move the rest of the hook retrogradely, along the direction of entry. Because this technique is almost always successful, it can be considered a first-line option.
String-Yank Technique: In the field or stream, removal of a fishhook may be accomplished without local anesthesia by using the string-yank technique. This technique may be used in the ED as well. Some clinicians prefer to use local 1% lidocaine to facilitate removal. For the “stream” technique (see Fig. 36-16, plate 2), pass a looped string or fishing line around the belly of the hook at the point where it enters the skin. Wrap approximately 1 ft of string around the dominant hand to provide strong traction. Hold the shank of the hook parallel to and in approximation to the skin with the index finger of the opposite hand. Use the thumb and middle finger of the opposite hand to stabilize and depress the barb, which helps the index finger disengage the barb from the subcutaneous tissue. When the barb has been disengaged, give a sharp pull (i.e., a quick tug with a snapping motion) with the dominant hand to remove the hook. Take care to keep bystanders out of the expected path of the hook because it often flies out of the patient. A commercial fishhook extractor device is available that is based on this method. It is used to grasp the hook during removal (Minto Research and Development, Inc., Redding, CA).
Needle Cover Technique: For this technique use an 18-gauge needle to cover the barb (see Fig. 36-16, plate 3). After adequate local anesthesia has been achieved, pass the needle through the entrance wound of the hook parallel to the shank of the hook to sheath the barb and allow the hook to be backed out while the barb is covered. An alternative to this procedure is to insert a No. 11 blade parallel to the shank of the hook down to the barb and use the point of the blade to free the subcutaneous tissue that is engaged on the barb. Cover the barb with the point of the No. 11 blade and back the hook out, with the blade protecting the barb.
Retrograde Technique: This is the simplest, but least effective method. By applying downward pressure on the shank of the hook, disengage the barb to allow successful removal (see Fig. 36-16, plate 4). If resistance is encountered, abandon the procedure and attempt removal with another technique. If the barb is not already protruding from the skin, the retrograde technique may cause less tissue trauma.
Wooden Splinters
Removal of wooden splinters is a common FB removal procedure. By simply grasping the end of a superficial, protruding splinter, it may be adequately removed, but care should be taken to not leave small pieces of material in the wound. Some splinters cannot be visualized at the point of entry but can easily and readily be palpated beneath the skin. When a wood FB is in subcutaneous tissue, it is advisable to cut down on the long axis of the FB to remove it via a skin incision rather than pulling it out through the entrance wound (Figs. 36-17 and 36-18). Although an incision may seem extensive and creates a laceration where only a puncture wound existed, opening the track allows thorough cleaning and removal of all the small pieces of the splinter that might otherwise remain. If the incision is linear, it may be sutured. Occasionally, the fastest method of removing small wooden splinters is to completely excise the entrance track and the FB en bloc, followed by linear closure. Particular mention should be made of certain wood splinters that are pliable and reactive, such as California redwood and northwest cedar. Any wood that is easily fragmented requires meticulous care to ensure removal of all material.
Traumatic Tattooing
Ground-in foreign material or tattooing of the skin is a difficult problem because permanent disfigurement may occur (Fig. 36-19). These injuries occur most often from falls on blacktop surfaces or asphalt or falls from bicycles or motorcycles on a variety of surfaces. Many cases may be managed with adequate local anesthesia and meticulous débridement with a sponge, scrub brush, or toothbrush. If all foreign material cannot be removed with these methods, give careful consideration to secondary excision of the tattooed area and primary closure with subsequent plastic surgery to repair the defect. However, it is usually impossible to completely remove traumatic tattooing in the ED. Referral for more extensive surgical treatment after local wound care is quite acceptable. Dermabrasion may be an acceptable delayed treatment when the tattooing is superficial.45 An alternative is to refer patients to a dermatologist for laser removal of traumatic tattoos. Certain lasers, such as a yttrium-aluminum-garnet (YAG) laser, have proved to be an excellent alternative.46 The process is more specific in removing embedded material without harming the surrounding tissue. The type of material will determine the number of treatments required. Not all tattoos can be removed completely.47
Marine FBs
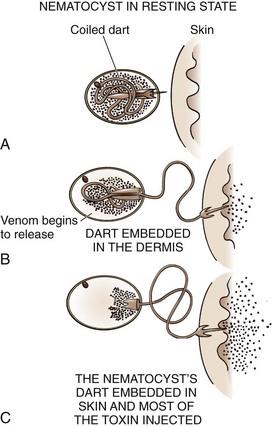
Coelenterates: Coelenterates, including the Portuguese man-of-war, true jellyfish, fire coral, box jellyfish, and sea anemones, inject several different toxins that are responsible for many marine envenomations by embedding venom-containing organelles, called nematocysts, into the victim’s skin (Fig. 36-20). The tentacles of coelenterates may contain thousands of nematocysts, which allows many to be deposited by even minor skin contact. After deposition, nematocysts discharge their venom. Reactions may be local or systemic, and the pain may be severe and is often described as “shocklike,” “itching,” “burning,” or “throbbing.” Substances such as tetramine, histamine, and 5-hydroxytryptamine are thought to be responsible for this localized reaction, whereas proteinaceous substances are implicated in the systemic response. Systemic reactions usually consist of fever, chills, and muscle spasm, but severe reactions may result in neurologic sequelae ranging from malaise and headache to paralysis and coma. Potential cardiopulmonary manifestations include dysrhythmias, hypotension, syncope, bronchospasm, laryngeal edema, and cardiorespiratory failure.48,49
Initial wound care should focus on decontamination and removal of unfired nematocysts, which will decrease the pain and systemic reactions. Vinegar (5% acetic acid) is the initial decontaminating agent of choice because it will inactivate the unfired nematocysts of most (but not all) species of jellyfish, Portuguese man-of-war, and sea anemones.48,50,51 Apply it continuously for 30 minutes or until the pain is gone.49 Do not apply fresh water to the area because the osmotic shock will activate any remaining unfired nematocysts and cause them to discharge toxin and increase pain and toxicity. Other suggested, but unproven remedies include meat tenderizer, ammonia, baking soda, urine, olive oil, sugar, and papaya latex.
After the wound has been decontaminated, remove any remaining tentacle fragments. Extract large fragments with forceps; however, individual nematocysts are very small (<1 mm in length) and are not easily visible. To remove the remaining nematocysts, scrape the skin with a hard edge, such as a credit card held perpendicular to the skin. An alternative is to apply shaving cream and shave the area gently to eliminate the remaining fragments.49 Do not used sand to remove nematocysts because sand can increase the discharge of venom. If the pain persists, it should be assumed that organelles still remain and further cleansing is required.
After decontamination, use topical anesthetics or steroids, but prophylactic antibiotics are not necessary. Follow routine wound care. Treat allergic and systemic reactions appropriately. The application of warm or cold packs to the area has not been shown to reduce pain.50 Antivenin is available only for box jellyfish envenomation, but its use remains incompletely understood and controversial.
Coral: Coral is composed of a calcium carbonate core and thousands of small marine animals. Fire coral, a specific type of coral, is another type of coelenterate that produces toxicity with stinging nematocysts. After contact, a burning and intense pruritus may occur along with a series of skin eruptions. Within minutes of contact, pruritus, erythema, and urticaria-like lesions may appear, and blister formation may result within hours (Fig. 36-21). Eventually, the lesions will become lichenoid, but complete resolution may not take place for 15 weeks after contact. Ultimately, hyperpigmented areas will form at the point of initial contact. Immediate care with oral antihistamines and topical steroids tends to reduce, but not prevent the symptoms.
“Coral cuts,” which can be deep lacerations, occur in divers and snorkelers exploring coral reefs. With these wounds, delayed healing may take place with the secondary development of cellulitis or ulceration, perhaps as a result of contamination of the wound with bacteria or microparticles of coral.48 Treatment consists of saline irrigation. Hydrogen peroxide may be used to help remove small coral particles from the wound.48 Do not close the wound; wet-to-dry dressings are advised instead.
Sponges: Sponges produce both an irritant and a contact dermatitis. The irritant dermatitis occurs as a result of sponge spicules embedded in the victim’s skin. Remove the spicules with adhesive tape (applied to the skin and then peeled back), and then bathe the area with vinegar.48 Contact dermatitis, which is believed to be caused by a toxin, produces erythema, pruritus, and vesicles similar to those with poison oak.48 Treatment is initial immersion in vinegar followed by local steroid creams.
Sea Urchins and Starfish: Sea urchins and starfish are free-living echinoderms covered with venomous, sharp, brittle spines and with venom-secreting pincers located near the mouth. If sea urchins or starfish are handled or inadvertently stepped on, these spines may become embedded in the patient and a severe local reaction may result from venom in the spines. Systemic symptoms occur and include muscle weakness; paralysis of the lips, tongue, and face; hypotension; abdominal pain; and respiratory distress. Local pain responds quite well to immersion in hot water (43.3°C to 46.1°C [110°F to 115°F]) for 30 to 90 minutes. Retained spines may become infected or cause delayed (≤1 to 2 months) FB granulomas. This reaction is not adequately understood, but it may be due to an intense and persistent inflammatory reaction. Spines that penetrate joints may induce synovitis. Echinoderm spines may discharge a purple dye that may be mistaken for a retained spine (Fig. 36-22).49 Spines are usually visible on radiographs and should be removed if possible, although they are brittle and can break off in the skin. A YAG laser may be an effective alternative to remove sea urchin spines. If spines are located in a joint or near a nerve, surgical extraction using an operative microscope may be necessary.48 Otherwise, if removal is difficult, leave the spine in place until it is resorbed or a local reaction takes place. Open the wound and drain it to allow it to close by secondary intention.
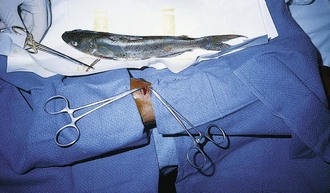
Catfish: Several species of catfish in North America contain toxic venom, and a sting from the dorsal or pectoral spines can embed an FB. The spine secretes venom from an epidermal gland at the base of the spine.52 The pain is usually ephemeral, and because no specific antitoxin exists, treatment consists of local care and analgesics. Immerse the affected part in hot water (≈43.3°C [110°F]) for at least 30 minutes if the pain is severe. This is believed to provide relief of symptoms by decreasing vascular and muscle spasm.53 Local injection of the wound with alkalized bupivacaine provides local analgesia and may also neutralize the toxin.52 Inspect the wound and remove any remaining spines (Fig. 36-23). A radiograph may be taken to confirm the absence of FBs, but catfish spines and cartilage may be radiolucent. Bedside US may be helpful, depending on the clinician’s level of experience. Thoroughly clean and irrigate the wound. Update the patient’s tetanus prophylaxis if necessary. Some authors recommend empirical antibiotic therapy to cover gram-negative bacilli (e.g., Aeromonas hydrophila), but infection is quite rare and routine antibiotic prophylaxis is not standard.52
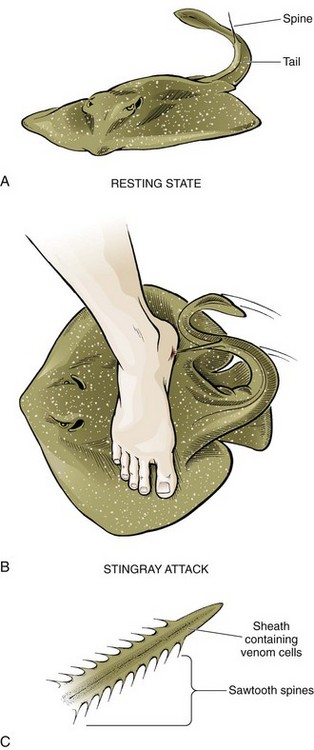
Stingrays: Stingray envenomation usually occurs in a person who accidentally steps on a creature that is resting on the bottom in shallow water and covered by sand (Fig. 36-24). This causes the stingray to lash out its whiplike caudal appendage, or tail, which contains one to four venom-containing serrated spines. Portions of the spine may become buried in the victim’s skin. Each spine is covered with a sheath containing venom glands, and in addition to immediate toxin-induced pain, pieces of the spine or sheath may remain embedded in the wound. These fragments, though often difficult to locate, do not dissolve and must therefore be removed. Persistent pain and inflammation, even weeks to months after the attack, mandate consideration of a retained FB, but a persistent and difficult-to-treat irritative process can occur in the absence of a retained spine or sheath. Immediate local and systemic reactions develop as a result of injection of a complex toxin. Systemic reactions may be severe and can include muscle cramps, vomiting, seizures, hypotension, arrhythmias, and (rarely) death.54
Treatment consists of irrigation with saline followed by immersion in hot water at 42°C to 45°C for 30 to 90 minutes to inactivate the heat-labile toxin. Local digital blocks without vasoconstrictors provide effective analgesia for hand wounds. Explore and débride all wounds, and remove all remnants of the spine and integumentary sheath.48 Wounds should heal by secondary intention. The venom can cause significant local tissue necrosis, and surgical débridement may be required.
Tetanus and Antibiotic Therapy: Prophylactic antibiotic therapy for marine injuries is common, although there are no convincing data to support or refute this practice. Unlike other soft tissue infections, marine injuries become infected with unusual gram-negative organisms, particularly Vibrio species. Although few studies have evaluated the effects of specific antibiotics, it is recommended that quinolones, trimethoprim-sulfamethoxazole, tetracyclines, third-generation cephalosporins, or aminoglycosides be used in lieu of penicillin, ampicillin, erythromycin, or first-generation cephalosporins.49 It is always difficult to differentiate chronic inflammation caused by toxins and foreign material from true infection, and surgical exploration is often required in persistent cases. Administer tetanus prophylaxis as per routine recommendations.
Cactus Spines
The size of cactus spines fluctuates considerably. The difficulty of removal is generally inversely proportional to the size of the FB.55 Larger embedded cactus spines are managed like wood splinters and sea urchin spine FBs. More advanced imaging techniques (US, CT, or MRI) may be required for localization of deeply embedded spines.
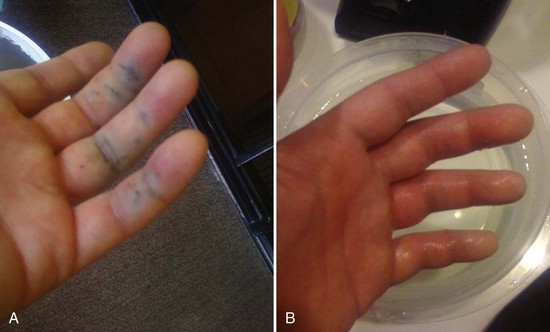
Deeply embedded cactus spines generally produce granulomatous reactions, but infections are rare.56 Dermatitis from embedded cactus spines is a well-described phenomenon. Hence, make an effort to remove deeply embedded spines after carefully weighing the benefit and potential harm related to deep exploration, especially in a sensitive location.55 Using forceps, remove superficially embedded, medium to large cactus spines by direct axial traction on each spine. Smaller spines (glochids) may be difficult and tedious to remove individually. Apply an adherent facial mask gel to remove the spines en masse with the gel (Fig. 36-25). Depilatory wax melted in a microwave oven and applied warm, commercial facial gels, and household glue (Elmer’s Glue-All, Borden, Inc., Columbus, OH) have all been recommended for this purpose.55,57–59 Over-the-counter “home use” facial mask gels are not adherent enough to be effective without multiple (eight or more) applications.
Ring Removal
String-Wrap Method: An occasional patient can remain calm during this procedure, but if the swelling is significant or the digit has been traumatized, anesthesia is necessary. Perform a proximal digital or metacarpal block to provide sufficient anesthesia and to minimize tissue distention at the ring site. Before removal of the ring, wrap a wide Penrose drain circumferentially in a distal-to-proximal direction to reduce the soft tissue swelling (Fig. 36-26). Leave the wrap in place for a few minutes to reach the maximum effect. Some nonanesthetized patients panic during the procedure because of increasing pain from compression and unwinding.60
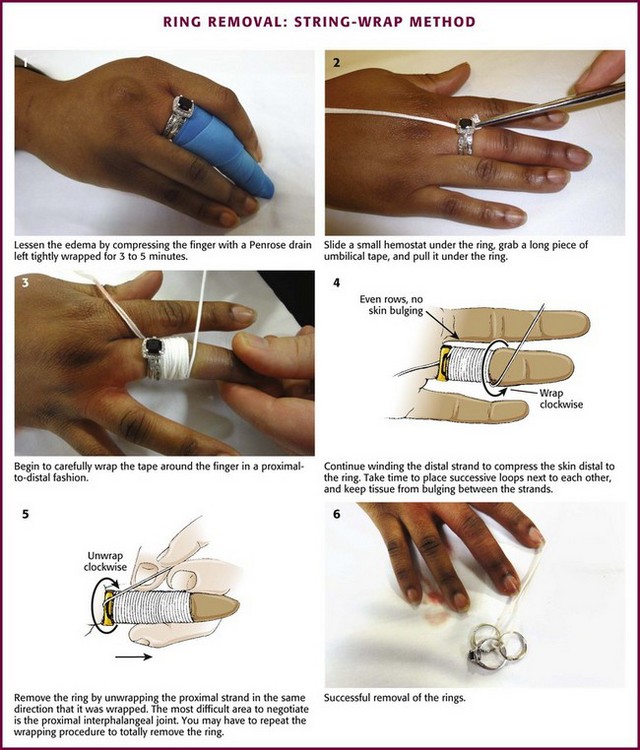
Figure 36-26 Ring removal: string-wrap method.
Ring Cutter: A ring cutter should be used when the swelling is excessive or other methods fail (Fig. 36-27). A ring cutter has a small hook that fits under the ring and serves as a guide for the saw-toothed wheel that cuts the metal. The cut ends of the ring are spread with large hemostats (e.g., Kelly clamps), and the ring is removed. If the tension is too great to spread the ring, another cut 180 degrees apart from the original ring cut can be performed. This will allow the ring to fall off in two pieces. A jeweler can subsequently repair cut rings.
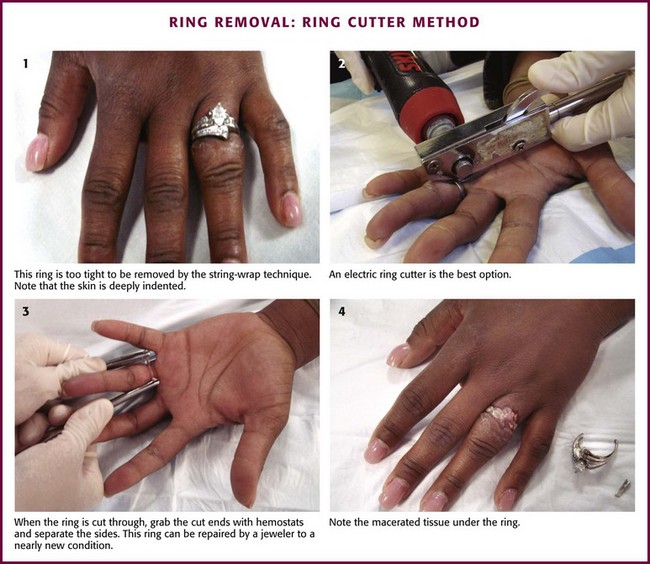
Figure 36-27 Ring removal: ring cutter method.
Body Piercing and Removal
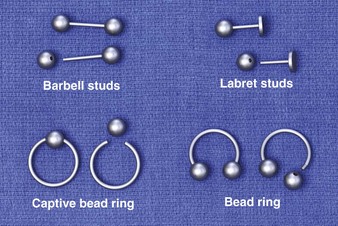
The art of body piercing predates most history books. Over the last decade an enormous increase in the practice of body piercing has occurred. Accordingly, so have the complications associated with the practice.61 For centuries the ears were the most common place. Today, the lips, tongue, eyebrow, nose, navel, nipples, and genital areas have become sites of body piercing. To date, there are only a limited number of studies on infection after piercings in areas other than the ears. Three major types of jewelry are used: (1) barbell studs, which are straight bars with a ball threaded onto both ends; (2) labret studs, which are straight bars with a ball threaded on one end and a disk permanently fixed on the other end (more commonly used on the lips); and (3) a captive bead ring, which consists of a bead with small dimples on opposite sides and an incomplete ring with rounded ends to fit into the dimples (Fig. 36-28). The bead is held “captive” by tension from both sides of this incomplete ring. The bead ring is a variation of this: one bead is permanently fixed to one end, and an opening is made by removing the free end of the ring.62
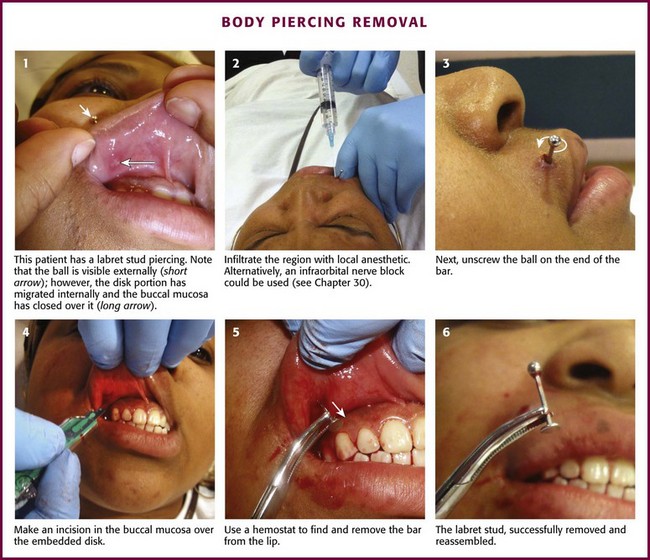
The most common reason for removal is infection (Fig. 36-29). Other symptoms such as bleeding, edema, allergic reaction, and keloid formation may also prompt removal. Occasionally, tongue piercings must be removed to permit intubation. To remove barbell- and labret-type studs, hold the bar with forceps and unscrew the bead on the other end. To remove a captive bead ring, hold the ring on both sides of the captive bead to release tension on the bead. This will dislodge the bead from the ring, which is holding it in place. If the jewelry is near the mouth or nose, take care to prevent aspiration of the bead. The complete microbiology of infections related to body piercing has not yet been determined. However, organisms such as Staphylococcus epidermidis and Staphylococcus aureus, along with Pseudomonas aeruginosa, have been commonly implicated pathogens. Other infectious complications from body piercing such as septic arthritis, endocarditis, hepatitis B and C, and HIV have been reported.63,64
Tick Removal
It is important to remove ticks early because the hard tick of the Ixodid family is likely to transmit disease. Rocky Mountain spotted fever, Lyme disease, tularemia, and ascending paralysis are among the many infections identified as tick-borne diseases. It is important to note that the rate of disease transmission before 48 hours of attachment is exceedingly low.65 Removal of Ixodid ticks is difficult because the mouthparts become cemented within 5 to 30 minutes of contact with the host’s skin. Removal will become more difficult the longer the tick is attached. Inadequate or partial removal of the tick may cause infection or chronic granuloma formation. Removal by mechanical means is recommended.66 Nonmechanical, traditional, and folk methods of forcing the tick to disengage (e.g., the use of petroleum jelly, fingernail polish, a hot match, or alcohol) are not advised and can cause the tick to regurgitate, thereby increasing the possibility of transmission of infection.
The use of straight or curved forceps or tweezers is the recommended method of removal. If these instruments are not available, use a gloved hand. Grasp the tick as close to the patient’s skin surface as possible and gently apply steady axial traction (Fig. 36-30). Take care to not squeeze, crush, twist, or jerk the tick’s body because this may expel infective agents or leave mouthparts in the skin. If mouthparts are left behind after removal of the body, they may be removed with tweezers. If one is still unable to remove the mouthparts, consider excision under local anesthesia to prevent local infection.
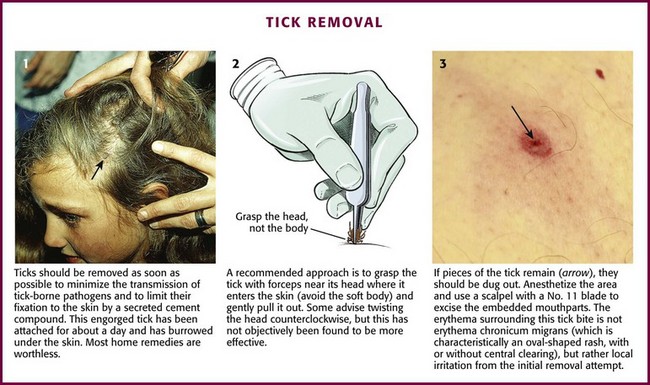
Figure 36-30 Tick removal.
Many patients have great anxiety about the development of tick-borne diseases after tick removal. Some studies have demonstrated that single-dose doxycycline (200 mg) may prevent the development of Lyme disease.65 Children younger than 8 years may be given a single dose of doxycycline (4 mg/kg). However, prophylactic antibiotic treatment of all tick bites is not recommended. Amoxicillin prophylaxis is not effective. When a patient is in an area where the incidence of Lyme disease is high or when a partially engorged deer tick in the nymphal stage is discovered on the body, the patient is more likely to benefit from prophylaxis. Regardless of whether prophylaxis is given, instruct patients about the symptoms and signs of Lyme disease and encourage them to return or seek medical evaluation if these symptoms develop.
Zipper Entrapment
The skin of the penis may become painfully entangled in a zipper mechanism (Fig. 36-31). Unzipping the zipper frequently lacerates the skin and increases the amount of tissue caught in the mechanism. Although the clinician may anesthetize the skin and excise the entrapped tissue, a less invasive method can be considered.

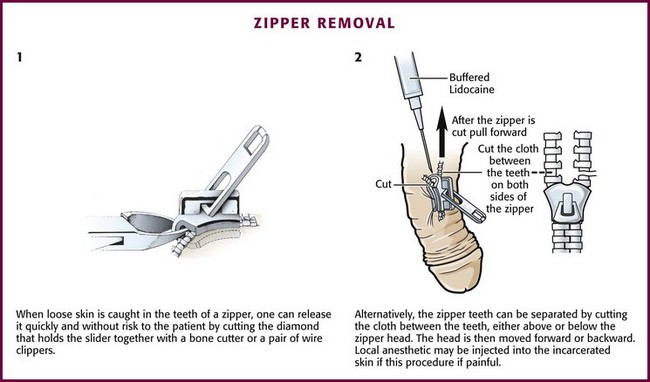
Figure 36-31 Penile skin caught in a zipper creates a rather painful and embarrassing situation for the patient. Instead of anesthetizing and excising the skin, consider cutting the zipper as demonstrated in Figure 36-32.
Cutting the median bar between the faceplates of the zipper mechanism remains the most common method. The interlocking teeth of the zipper then fall apart when the median bar (diamond or bridge) of the zipper is cut in half (Fig. 36-32), and the skin is subsequently freed. A bone cutter or wire clippers and a moderate amount of force may be required to break the bar. The addition of mineral oil followed by traction has been demonstrated to achieve some success. Patients with penile lacerations warrant urologic follow-up to assess for urethral injury.
Infiltration of Radiographic Contrast Material
The infiltration of high-osmolality intravenous contrast material has the potential to cause skin necrosis, but the use of low-osmolality dye, which is well tolerated in soft tissues, has essentially eliminated this problem (Fig. 36-33). The use of high-pressure power injectors, with the technologist out of the room, calls for careful inspection of the intravenous site before injection. However, because of the high-pressure rapid auto-injection of contrast material and the absence of a technician in the room, extravasation of contrast material is not a rare event and occurs even with meticulous technique. Infiltration occurs in about 0.5% to 1% of auto-injections. Once contrast material has infiltrated, no intervention has been demonstrated to ameliorate the local reactions, which are usually mild. Swelling, erythema, and mild discomfort at the infiltration site may occur. It is best to resist the temptation to use excessive heat or cold. Elevation of an extremity is appropriate but of unproven value. Injection of steroids and other agents has no known benefit. Low-osmolality dye is usually totally resorbed in a few days, with no serious consequences in the vast majority of cases. Progress of the dye’s egress may be followed with radiographs. The majority of contrast material extravasations resolve with no long-term consequences with conservative management. If large volumes of contrast material are injected (>75 to 100 mL), especially in confined areas such as the dorsum of the hand, it is possible that skin necrosis from the pressure or a compartment syndrome may develop, but this is rare. The clinician should resist attempts to routinely remove even large volumes of extravasated contrast material by incision or aspiration. Although some contrast material may be removed with surgical techniques during fasciotomy, the actual benefit is unknown. Surgical intervention is rarely required and is based on measurement of compartment pressure and clinical evaluation. Patients with small-volume and minimally symptomatic extravasations may be discharged. Large-volume extravasations warrant consultation or further observation.
TASER Darts
The Thomas A. Swift Electric Rifle, or “TASER,” is a conducted electrical weapon used in many areas by police to subdue violent patients (see Chapter 70). The TASER fires two barbed electrodes on long copper wires (Fig. 36-34). The barbs attach to skin or clothing and create an arc that delivers an electrical jolt that causes overwhelming pain and involuntary muscle contraction and incapacitates the subject. The electricity is of such high frequency that it is believed to stay near the surface and not penetrate to the depth of internal organs. The barbs are designed to not penetrate deeper than 4 mm, and police are taught to remove them by stretching the surrounding skin and tugging sharply. If this fails, cutting down on the dart after local anesthesia should facilitate removal.67,68 Patients may need medical evaluation after ED removal of the darts for the underlying state of agitation that required the use of a TASER, complications of electrical injury, injury from the fall after incapacitation, and injury from the barb, especially if struck in the mouth, eye, neck, or groin. Most patients do well with minimal intervention and proper wound management if the patient is not unduly agitated and the TASER did not involve the critical areas of the body just mentioned.
Human and Animal Bite FBs
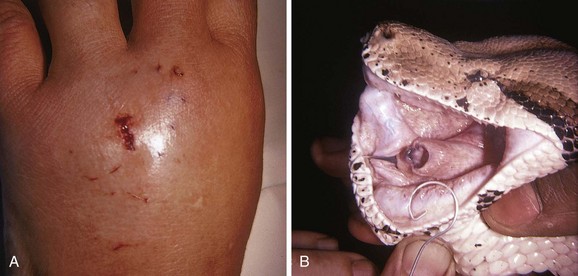
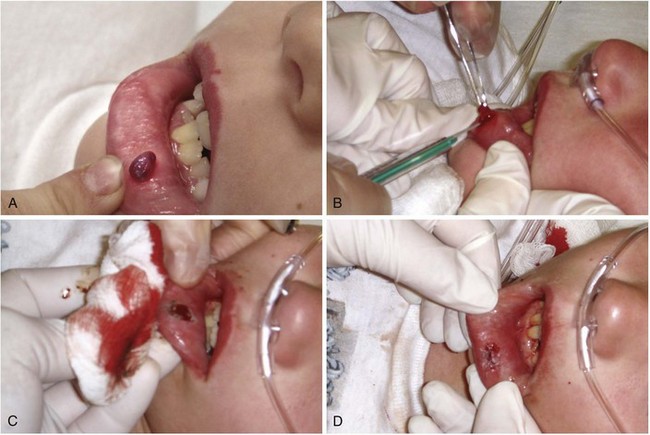
The most common FB after a human bite is a piece of tooth. These FBs can be difficult to find and may not be appreciated if the patient does not admit or disclose the bite wound. Usually, the bite puncture is small and not easily cleaned or visualized. The puncture can be widened with a formal incision to aid in cleaning and evaluation for tendon integrity, injury to the joint capsule, fracture, or an FB (Fig. 36-35). Occasionally, small pieces of teeth can become embedded in a wound, such as with dog, cat, or snake bites (Fig. 36-36). Radiographic detection is variable, and exploration is often the only alternative.
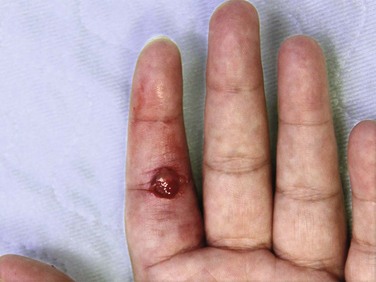
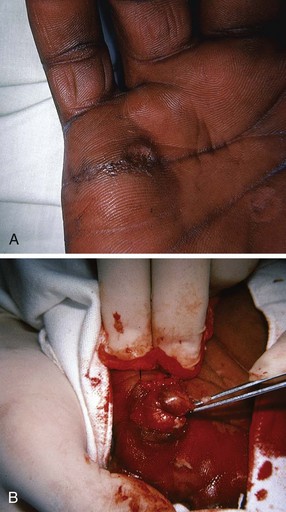
Pyogenic Granuloma (Lobar Capillary Hemangioma)
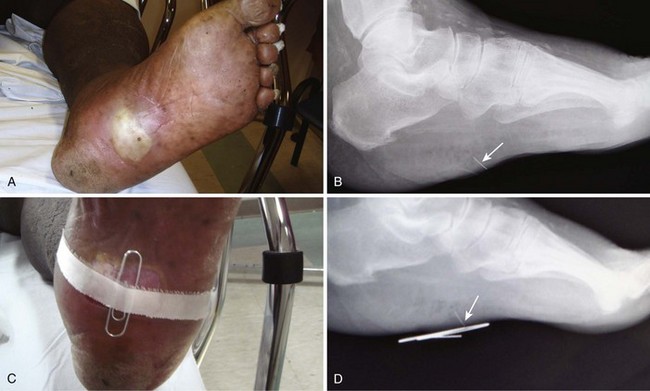
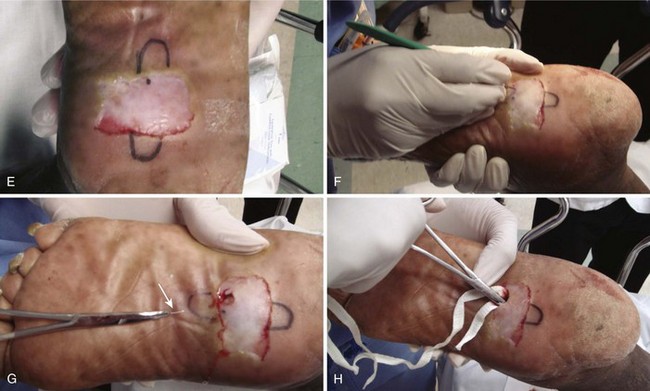
A pyogenic granuloma is a benign acquired polypoid, friable vascular lesion of the skin (hand, neck, foot, fingers, and trunk) and mucous membranes (Fig. 36-37). They are common in children; in pregnancy the lesion is termed epulis gravidarum (pregnancy tumor). The cause is unknown, but there is some association with topical retinoids and the protease inhibitor indinavir; they are not due to infection, and they are not granulomas. They grow rather rapidly over a period of a few weeks, are occasionally associated with minor trauma, have a glistening dark red appearance, and may bleed. Histologically, a pyogenic granuloma is a hemangioma. A variety of topical therapies (silver nitrate, cryotherapy), laser, or cautery is available, but removal by sharp dissection with primary suturing is usually curative (Fig. 36-38). The recurrence rate may be as high as 40% with nonsurgical intervention.69
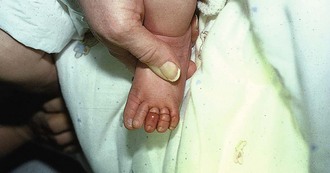
Hair-Thread Tourniquet
Hair or thread fibers adherent to infants’ clothing occasionally become tightly wrapped around a child’s digits or genitals (Fig. 36-39).70 If they are left in place, amputation may eventually occur. The offending fibers may be difficult to visualize, and the child is often brought for evaluation only after signs of distal ischemia appear. Occasionally, the fiber can be grasped with toothless forceps or a small hemostat and then be unwrapped. More commonly, fibers cannot be identified because they are deeply embedded in swollen tissue. Removal not requiring minor surgery includes use of a chemical depilatory, such as the over-the-counter product Nair (calcium thioglycolate), to dissolve or weaken the hair. A generous amount of depilatory cream is worked into the involved area and allowed to dissolve or weaken the hair, which is then removed mechanically. If a depilatory is not successful in 30 to 60 minutes, surgical removal is indicated.
A No. 11 blade can be used to cut the constricting bands under a regional nerve block.71 It may be difficult to identify individual hairs that are deeply embedded in a swollen digit and even more difficult to assess the success of the intervention. Frequently, multiple hairs are involved. Because the bands may be quite deep, the incision should avoid known neurovascular tracts. Barton and coworkers recommend a dorsal, rather than a lateral incision on the digits.70 If the soft tissue on the distal end of the digit has been rotated after a circumferential dermal laceration from the tourniquet, the distal tissue can be realigned with the proximal tissue and two dorsolateral sutures placed or tissue adhesive glue applied to maintain the digit in alignment.
Disposition Management
Antibiotics
If prophylactic antibiotics are prescribed, a first-generation cephalosporin or penicillinase-resistant penicillin has traditionally been the first-line choice. In patients with contraindications to penicillins and cephalosporins and with the increasing incidence of community-acquired methicillin-resistant S. aureus in many areas, clindamycin, trimethoprim-sulfamethoxazole, or tetracycline may provide alternative coverage.72 However, infections associated with FBs are not likely to be from methicillin-resistant S. aureus. Under certain circumstances, alternative antibiotics may be indicated. For infected plantar punctures through a shoe, a fluoroquinolone to cover P. aeruginosa is appropriate coverage, although most Pseudomonas infections are complex and may need extensive débridement and intravenous antibiotics. Saltwater marine FBs may be contaminated with Vibrio species, which are usually sensitive to tetracyclines, aminoglycosides, or third-generation cephalosporins. Freshwater FBs, on the other hand, are more likely to harbor A. hydrophila, which can be treted with tetracyclines.
FB Reactions
A purulent bacterial infection may develop in the presence of any FB; therefore, any abscess or cellulitis that recurs or wounds that do not heal as expected should always be investigated for retained FBs.73,74 Karpman and coworkers found a 15% rate of infection (S. aureus and Enterobacteriaceae) in a series of 25 patients treated for cactus thorn injuries on the extremities.56 Certain thorns (black thorns, rose thorns), redwood and northwest cedar splinters, toothpicks, hair, and stingray or sea urchin spines are noted for their ability to initiate chronic FB reactions. Sea urchin spines and other marine FBs are covered with slime, calcareous material, and other debris that commonly initiate an FB granuloma. The inflammatory reaction seen with cactus thorns may be an allergic reaction to fungus found on the cactus plant.
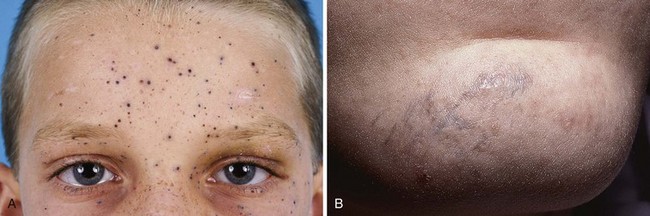
Many FB reactions are thought to result from an inflammatory response to organic material, or they may represent infection from bacteria introduced at the time of the wound. Clinically evident reactions may be delayed for weeks or even years after injury (Fig. 36-40). The chronic infection or inflammatory reaction may not be accompanied by the production of pus, but it may be quite painful or result in loss of function. FBs may also be associated with the formation or development of a chronic pseudotumor, a sinus tract, or an osteomyelitis-like lesion of bone and soft tissue.73 In addition, organic material has been noted to induce chronic tenosynovitis, chronic monarticular synovitis, and chronic bursitis.
Rapidly traveling projectiles with considerable inherent heat (e.g., bullets) are less likely to cause infection but are more apt to cause other difficulties. Damage to surrounding areas can occur during passage through tissue. Rarely do retained lead FBs, such as bullets or shotgun pellets, leach out lead into the general circulation and produce systemic lead poisoning unless they are in contact with synovium (Fig. 36-41). If this process does occur, it may take years to develop and can result in vague or nondescript symptoms (e.g., fatigue, arthralgia, headache, or abdominal pain) many years after the initial injury. Elevated blood lead levels are more likely to occur if body fluids such as joint, pleural, peritoneal, or cerebrospinal fluid bathe the lead. Bullets retained in muscle or other soft tissue are not likely to produce any sequelae related to their lead content. However, Farrell and colleagues reported unsuspected elevated lead levels in patients with retained lead fragments who were seen in the ED with a variety of complaints.75 Lead levels of up to 50 µg/dL were reported. Levels greater than 45 µg/dL are generally considered an indication for chelation therapy. The relationship between the retained lead and the symptoms was unclear, but this report verifies the observations of others that retained lead FBs in selected areas can significantly elevate blood lead levels and may produce symptomatic plumbism.
References
1. Steele, MT, Tran, LV, Watson, WA, et al. Predictive value of wound characteristics, patient perception, and wound exploration. Am J Emerg Med. 1998;16:627.
2. Bhavsar, MS. Technique of finding a metallic foreign body. Am J Surg. 1981;141:305.
3. Orlinsky, M, Bright, AA. The utility of routine x-rays in all glass-caused wounds. Am J Emerg Med. 2006;24:233.
4. Anderson, A, Newmeyer, WL, Kilgore, ES. Diagnosis and treatment of retained foreign bodies of the hand. Am J Surg. 1982;144:63.
5. Avner, J, Baker, MD. Lacerations involving glass—the role of routine roentgenograms. Am J Dis Child. 1992;146:600.
6. Peterson, JJ, Bancroft, LW, Kransdorf, MJ. Wooden foreign bodies: imaging appearance. AJR Am J Roentgenol. 2002;178:557.
7. Russell, RC, Williamson, DA, Sullivan, JW, et al. Detection of foreign bodies in the hand. J Hand Surg [Am]. 1991;16:2.
8. Lammers, RL, Magill, T. Detection and management of foreign bodies in soft tissue. Emerg Med Clin North Am. 1992;10:767.
9. Tandberg, D. Glass in the hand and foot: will an x-ray film show it? JAMA. 1982;248:1872.
10. Courter, BJ. Radiographic screening for glass foreign bodies—what does a “negative” foreign body series really mean? Ann Emerg Med. 1990;19:997.
11. Oikarinem, KS, Nieminen, TM, Makarainem, H, et al. Visibility of foreign bodies in soft tissue in plain radiographs, computed tomography, magnetic resonance imaging and ultrasound. Int J Oral Maxillofac Surg. 1993;22:119.
12. Ellis, GL. Are aluminum foreign bodies detectable radiographically? Am J Emerg Med. 1993;11:12.
13. Roobottom, CA, Weston, MJ. The detection of foreign bodies in soft tissue—comparison of conventional and digital radiography. Clin Radiol. 1994;49:330.
14. Gahlos, F. Embedded objects in perspective. J Trauma. 1985;24:340.
15. Kaiser, CW, Slowick, T, Spurling, KP, et al. Retained foreign bodies. J Trauma. 1997;43:107.
16. Boyse, TD, Fessell, DP, Jacobson, JA, et al. US of soft-tissue foreign bodies and associated complications with surgical correlation. Radiographics. 2001;21:1251.
17. Horton, LK, Jacobson, JA, Powell, A, et al. Sonography and radiography of soft-tissue foreign bodies. AJR Am J Roentgenol. 2001;176:1155.
18. Lyon, M, Brannam, L, Johnson, D, et al. Detection of soft tissue foreign bodies in the presence of soft tissue gas. J Ultrasound Med. 2004;23:677.
19. Dean, AJ, Gronczewski, CA, Costantino, TG. Technique for emergency medicine bedside ultrasound identification of a radiolucent foreign body. J Emerg Med. 2003;24:303.
20. Shiels, WE, Babcock, DS, Wilson, JL, et al. Localization and guided removal of soft tissue foreign bodies with sonography. AJR Am J Roentgenol. 1990;155:1277.
21. Jacobson, JA, Powell, A, Craig, JG, et al. Wooden foreign bodies in soft tissue: detection at US. Radiology. 1998;206:45.
22. Hill, R, Conron, R, Greissinger, P, et al. Ultrasound for the detection of foreign bodies in human tissue. Ann Emerg Med. 1997;29:353.
23. Manthey, DE, Storrow, AB, Milbourn, JM, et al. Ultrasound versus radiography in the detection of soft-tissue foreign bodies. Ann Emerg Med. 1996;28:7.
24. Specht, CS, Varga, JH, Jalali, MM, et al. Orbital-cranial wooden foreign bodies diagnosed by magnetic resonance imaging. Dry wood can be isodense with air and orbital fat by computed tomography. Surv Ophthalmol. 1992;36:341.
25. Dumarey, A, De Maeseneer, M, Ernst, C. Large wooden foreign body in the hand: recognition of occult fragments with ultrasound. Emerg Radiol. 2004;10:337.
26. Cohen, DM, Garcia, CT, Dietrich, AM, et al. Miniature C-arm imaging: an in vitro study of detecting foreign bodies in the emergency department. Pediatr Emerg Care. 1997;13:247.
27. Wyn, T, Jones, J, McNinch, D, et al. Bedside fluoroscopy for detection of foreign bodies. Acad Emerg Med. 1995;2:979.
28. Levine, MR, Yarnold, PR, Michelson, EA. A training program in portable fluoroscopy for the detection of glass in soft tissues. Acad Emerg Med. 2002;9:858.
29. Ariyan, S. A simple stereotactic method to isolate and remove foreign bodies. Arch Surg. 1977;112:857.
30. Levine, MR, Gorman, SM, Yarnold, PR. A model for teaching bedside detection of glass in wounds. Emerg Med J. 2007;24:413.
31. Chapman, AJ, McClain, J. Wandering missiles: autopsy study. Trauma. 1984;24:634.
32. Schurr, M, McCord, S, Croce, M. Paradoxical bullet embolism: case report and literature review. J Trauma. 1996;40:1034.
33. Rees, CE. The removal of foreign bodies: a modified incision. JAMA. 1939;113:35.
34. Swischuk, LE, Jorgenson, F, Jorgenson, A, et al. Wooden splinter induced pseudo-tumor and osteomyelitis-like lesions of bone and soft tissue. AJR Am J Roentgenol. 1974;122:176.
35. Davis, LJ. Removal of subungual foreign bodies. J Fam Pract. 1980;11:714.
36. Schwartz, GR, Schwen, SA. Subungual splinter removal. Am J Emerg Med. 1997;15:330.
37. Farrell, SE, Vandevander, P, Schoffstall, JM, et al. Blood lead levels in emergency department patients with retained lead bullets and shrapnel. Acad Emerg Med. 1999;6:208.
38. Bocka, JJ, Godfrey, J. Emergency department use of an eye magnet for removal of soft tissue foreign body. Ann Emerg Med. 1994;23:350.
39. Cakir, B, Akan, M, Yildirim, S, et al. Localization and removal of ferromagnetic foreign bodies by magnet. Ann Plast Surg. 2002;49:541.
40. Hatano, Y, Asada, Y, Komada, S, et al. A case of pencil core granuloma with an unusual temporal profile. Dermatology. 2000;201:151.
41. Gammons, MG, Jackson, E. Fishhook removal. Am Fam Physician. 2001;63:2231.
42. Barnett, RC. Three useful techniques for removing imbedded fishhooks. Hosp Med. 1982;18:72.
43. Friedenberg, S. How to remove an imbedded fishhook in 5 seconds without really trying. N Engl J Med. 1971;284:733.
44. Rose, JD. Removing the imbedded fishhook. Aust Fam Physician. 1981;10:33.
45. Alt, TH. Technical aids for dermabrasion. J Dermatol Surg Oncol. 1987;13:638.
46. Achauer, BM, Nelson, JS, Vander Kam, VM, et al. Treatment of traumatic tattoos by Q-switched ruby laser. Plast Reconstr Surg. 1994;93:318.
47. Troilius, AM. Effective treatment of traumatic tattoos with Q-switched Nd:YAG laser. Lasers Surg Med. 1998;22:103.
48. Rosson, CL, Tolle, SW. Management of marine stings and scrapes. West J Med. 1989;150:97.
49. Auerbach, PS. Marine envenomations. N Engl J Med. 1991;325:486.
50. Thomas, CS, Scott, SA, Galanis, DJ, et al. Box jellyfish (Carybdea alata) in Waikiki: their influx cycle plus the analgesic effect of hot and cold packs on their stings to swimmers at the beach: a randomized, placebo-controlled, clinical trial. Hawaii Med J. 2001;60:100.
51. Tibballs, J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48:830.
52. Mann, JW, 3rd., Werntz, JR. Catfish stings to the hand. J Hand Surg [Am]. 1991;16:318.
53. Shepard, S, Thomas, SH, Stone, K. Catfish envenomation. J Wilderness Med. 1994;5:67.
54. Renner, PJ, Williamson, JA, Skinner, RA. Fatal and nonfatal stingray envenomation. Med J Aust. 1989;151:621.
55. Lindsey, D, Lindsey, WE. Cactus spine injuries. Am J Emerg Med. 1988;6:362.
56. Karpman, RR, Sparks, RP, Fried, M. Cactus thorn injuries to the extremities: their management and etiology. Ariz Med. 1980;37:849.
57. Schunk, JE, Corneli, HM. Cactus spine removal. J Pediatr. 1987;110:667.
58. Putnam, MH. Simple cactus spine removal. J Pediatr. 1981;98:333.
59. Martinez, TT, Jerome, M, Barry, RC, et al. Removal of cactus spines from the skin: a comparative evaluation of several methods. Am J Dis Child. 1987;141:1291.
60. Mizrahi, S, Lunski, I. A simplified method for ring removal from an edematous finger. Am J Surg. 1986;151:412.
61. Meltzer, DI. Complications of body piercing. Am Fam Physician. 2005;72:2029.
62. Khanna, R, Kumar, SS, Raju, BS, et al. Body piercing in the accident and emergency department. J Accid Emerg Med. 1999;16:418.
63. Tweeten, SS, Rickman, LS. Infectious complications of body piercing. Clin Infect Dis. 1998;26:735.
64. Marcoux, D. Dermatologic aspects of cosmetics: appearance, cosmetics and body art in adolescents. Dermatol Clin. 2000;18:667.
65. Nadelman, RB, Nowakowski, JN, Fish, D, et al. Prophylaxis with single dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345:79.
66. Needham, GR. Evaluation of five popular methods for tick removal. Pediatrics. 1985;75:997.
67. Koscove, EM. The Taser weapon: a new emergency medicine problem. Am J Emerg Med. 1985;14:1205.
68. Ordog, GJ, Wasserberger, J, Schlater, T, et al. Electronic gun (Taser) injuries. Ann Emerg Med. 1987;16:73.
69. Patrice, SJ, Wiss, K, Mulliken, JB. Pyogenic granuloma (lobar capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267.
70. Barton, DJ, Sloan, GM, Nitcher, LS, et al. Hair-thread tourniquet syndrome. Pediatrics. 1988;82:925.
71. Peckler, B, Hsu, C. Tourniquet syndrome: a review of constricting band removal. J Emerg Med. 2001;20:253.
72. Moran, G, Krishnadasan, A, Gorwitz, R, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666.
73. MacDowell, RT. Unsuspected foreign bodies in puncture wounds. J Musculoskelet Med. 1986;7:33.
74. Lammers, RL. Soft tissue foreign bodies. Ann Emerg Med. 1988;17:1336.
75. Farrell, SE, Vandevander, P, Lee, D, et al. Elevated serum lead levels in ED patients with retained lead foreign bodies [abstract]. Acad Emerg Med. 1996;3:418.