Prophylaxis for Deep Vein Thrombosis and Pulmonary Embolism in the Surgical Patient
Guidelines for venous thromboembolism (VTE) prevention in the surgical patient have been published by the American College of Chest Physicians (ACCP), the American College of Physicians, the American Academy of Orthopaedic Surgery, and the International Society of Angiology [1–3]. The ongoing challenge is to balance the risk of bleeding versus the benefit of VTE prevention because studies have suggested that there is an increased bleeding risk associated with more effective pharmacologic prophylaxis. The purpose of this article is to review the cause and risk factors for VTE as well as to discuss the methods of prophylaxis for various procedures as recommended by the guidelines. The article concludes with a more detailed overview of the pharmacology and clinical trial results of the new oral anticoagulants that have already been approved in Europe and Canada for VTE prevention in the orthopedic patient population.
Cause of VTE in the surgical patient
When assessing the cause of deep vein thrombosis (DVT) and pulmonary embolism (PE) in the surgical patient, the triad of stasis, intimal injury, and hypercoagulability contributes to thrombosis. The first arm of the triad is stasis resulting from the supine position and the effects of anesthesia. Nicolaides and coworkers [4] reported delayed clearing of venographic contrast media from the soleal sinuses of the calf in supine patients. Concomitant with this pooling is the vasodilatory effect of anesthesia, which results in increased venous capacitance and decreased venous return from the lower extremities [5,6]. Venous thrombi composed of platelets, fibrin, and red blood cells develop behind the venous valve cusps or the intramuscular sinuses of the calf secondary to decreased blood flow and stasis [7].
The second arm of the triad is intimal injury resulting from excessive vasodilation caused by vasoactive amines (histamine, serotonin, bradykinin) and anesthesia. Studies using scanning and transmission electron microscopy have shown focal tears in the venous endothelium of dogs around valves and branch vessels with accumulation of leukocytes, erythrocytes, and platelets after injection of vasoactive amines, and similar findings were documented after sham abdominal surgery in these animals [8–10].
Hypercoagulability is the third risk factor in the surgical patient. Stasis and surgery set up the conditions conducive to clot formation. The impaired venous blood flow results in a decreased clearance of activated clotting factors, which subsequently set up clot formation on areas of intimal injury and low flow areas such as the posterior valve cusp [11]. Reperfusion of these transiently hypoxic regions of the vessel with oxygenated blood induces thrombus, impairing venous valve function and promoting growth of thrombus beyond this localized area [12]. Other factors have been assessed such as fibrinopeptide A, platelet factor 4, b-thromboglobulin, d-dimers, antithrombin (AT), a2-antiplasmin, factor VIII activity, von Willebrand factor antigen, thrombin/antithrombin ratio, fragments 1 + 2, tissue plasminogen activator inhibitor, and decreased plasmin activity [13–17]. None of these factors has been shown to be sensitive and specific in predicting which patients are at risk for the development of DVT.
VTE risk factor assessment before surgery
The ACCP advocates a unified approach to VTE risk assessment by assigning risk according to the type of surgery, mobility, and individual risk factors (Box 1, Table 1) [1]. The patient can be classified as being at low, moderate, or high risk for the development of VTE. Low-risk patients are those who are mobile and are having minor surgery. Medical patients who are fully ambulatory are also considered to be at low risk. Based on studies using objective, diagnostic screening for asymptomatic DVT in patients not receiving prophylaxis, the approximate DVT risk is less than 10% in patients assigned to the low-risk category. Moderate-risk patients are those undergoing general, open gynecologic, or urological surgery. The approximate incidence of DVT risk without thromboprophylaxis in this group is 10% to 40%. The high-risk group includes patients having hip or knee replacement, fractured hip surgery, major trauma, and acute spinal cord injury. The DVT risk without thromboprophylaxis in this category is between 40% and 80%.
Box 1 Risk factors for VTE
Data from Geerts WH, Bergqvist, Pineo G, et al. Prevention of venous thromboembolism. Chest 2008;133:381S–453S.
Table 1 Classification of the risk of postoperative venous thrombosis and PE
| Level of risk | Approximate DVT risk No prophylaxis (%) | Prophylaxis options |
|---|---|---|
| High Risk | 40–80 | |
| Total hip or knee arthroplasty | LMWH, fondaparinux, warfarin | |
| Hip fracture | ||
| Major trauma | ||
| Spinal cord injury | ||
| High VTE risk plus high bleeding risk | Intermittent pneumatic compression | |
| Moderate Risk | 10–40 | |
| Most general, open gynecologic, or urological surgery patients, medical patients, bed rest or sick, | LMWH, fondaparinux, UFH (2 or 3 times a day) | |
| Moderate VTE risk plus high bleeding risk | Intermittent pneumatic compression | |
| Low Risk | <10 | |
| Minor surgery in mobile patients, | No specific thromboprophylaxis | |
| Medical patients who are fully mobile | Early and aggressive ambulation |
Data from Geerts WH, Bergqvist, Pineo G, et al. Prevention of venous thromboembolism. Chest 2008;133:381S–453S.
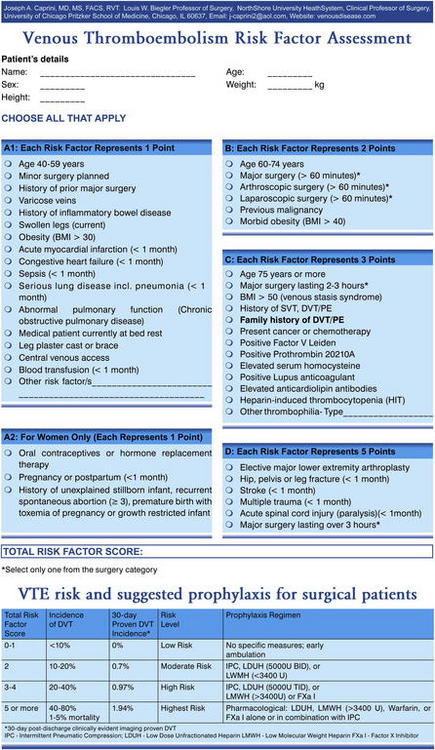
Another approach to risk assessment is the Caprini Risk Assessment Model (Fig. 1) [18]. This method consists of a list of exposing risk factors (genetic and clinical characteristics), each with an assigned relative risk score. The scores are summed to produce a cumulative score, which is used to classify the patient into 1 to 4 risk levels and determines the type and duration of VTE prophylaxis. This risk assessment tool was validated by Bahl and colleagues [19].
Modalities of prophylaxis
Unfractionated heparin
Heparin inhibits thrombin, factor Xa, and other serine proteases through its activation of antithrombin (Table 2) [1]. It has been shown to reduce the incidence of VTE by 50% to 70% in moderate-risk general surgery and medical patients. In double-blind trials, the incidence of major hemorrhagic events was 1.8% versus 0.8% in the controls and was not statistically significant [20,21]. The incidence of minor bleeding, such as injection site and wound hematomas, has been reported to be significant, with a rate of 6.3% in the low-dose heparin group and 4.1% in the controls. Rare complications include skin necrosis, thrombocytopenia, and hyperkalemia. A potential advantage of this medication over others is its short half-life, reversibility with protamine, and its lack of a contraindication in patients with renal impairment. Heparin has not been proved to decrease the incidence of VTE in patients undergoing major knee surgery or in patients with hip fractures. Although it has been shown to be effective in patients undergoing elective hip surgery, other prophylactic modalities have been shown to be more efficacious in reducing the incidence of VTE in this patient population [22]. Thus, it is indicated in patients undergoing moderate-risk general surgery and is also typically used in those whose bleeding risk is considered high, such as neurosurgical patients. It is administered subcutaneously (SC) at 5000 units beginning 2 hours before surgery. This treatment is followed postoperatively by the administration of 5000 units SC every 8 to 12 hours until the patient is fully ambulatory or discharged.
| Agent | Dose and schedule |
|---|---|
Continue until discharge
Dalteparin: 5000 units SC every 24 hours (initiated evening of surgery). Fondaparinux: 2.5 mg, SC beginning 6 hours after surgery then once daily. Enoxaparin: orthopedic surgery 30 mg SC every 12 hours (initiated evening of surgery) and all other surgeries 40 mg SC every 24 hours (initiated evening of surgery).
Abbreviations: THA, total hip arthroplasty; TKA, total knee arthroplasty.
Low-molecular-weight heparin and pentasaccharide
Low-molecular-weight heparins (LMWHs) also catalyze the activation of antithrombin (see Table 2). However, this group of heparins has been observed to have a more significant inhibitory effect on factor Xa than factor IIa as well as a lower bleeding risk than standard heparin [23]. These agents are not bound to plasma proteins (histidine-rich glycoprotein, platelet factor 4, vitronectin, fibronectin, and von Willebrand factor), endothelial cells, or macrophages like standard heparin [16,24]. This lower affinity contributes to a longer plasma half-life, more complete plasma recovery at all concentrations, and a clearance that is independent of dose and plasma concentration. They have been shown to be safe and effective for the prevention of postoperative VTE in orthopedic and general surgery [25,26]. Currently, 6 LMWH preparations are approved for use in Europe, whereas in the United States enoxaparin and dalteparin are available for orthopedic and general surgery, respectively. Each of these drugs has a different molecular weight, antiXa to anti-IIa activity, rates of plasma clearance, and recommended dosage regimens [24].
Both LMWH and fondaparinux are renally excreted and are contraindicated in patients with renal impairment. Protamine partially reverses the anticoagulant effects of LMWH and is ineffective as an antidote to fondaparinux [1]. Enoxaparin is initiated 12 to 24 hours after orthopedic surgery at 30 mg SC every 12 hours. For all other surgeries enoxaparin is administered at 40 mg SC once daily. Dalteparin is administered 2 hours before general abdominal surgery at 2500 units SC and then once daily at 2500 units or 5000 units. Fondaparinux is given at 2.5 mg SC once daily beginning 6 hours after surgery.
Warfarin
Warfarin has been studied and approved for use in patients undergoing orthopedic surgery (see Table 2) [1]. It can be administered by 2 methods. The first approach is to begin this medication on the evening before the day of surgery, whereas the second method involves the initiation of this drug on the day of the procedure. The usual starting dose of warfarin is 5 mg and the dose is adjusted for a goal international normalized ratio (INR) between 2 and 3. A loading dose of coumadin in excess of 5 mg is generally not recommended and lower starting doses may be considered for patients who are elderly, have impaired nutrition, or who have liver disease or congestive heart failure [1]. The duration of prophylaxis is maintained for up to 35 days at an INR goal of 2 to 3 with some studies using an INR of 1.8 to 2.5. The rare complication of warfarin-induced skin necrosis has never been reported in studies using this agent as prophylaxis for DVT and PE.
In some institutions, a debate exists regarding the most appropriate VTE prophylaxis in orthopedic patients that adequately balances the risk of bleeding with efficacy. A meta-analysis by Mismetti and colleagues [27] concluded that LMWH is more effective in reducing the risk of venographically detected and proximal DVT compared with vitamin K antagonists. However, there was no difference in the rate of PE between these 2 classes of medications with a similar to slightly greater risk of bleeding associated with LMWH. The ACCP guidelines have also acknowledged a greater efficacy of LMWH and, by indirect comparisons, fondaparinux in preventing both asymptomatic and symptomatic VTE in orthopedic patients at a cost of a slight increase in surgical site bleeding [1]. The postulated reason for this finding is a quicker onset of action with LMWH and fondaparinux compared with warfarin.
Mechanical prophylaxis modalities
Various forms of mechanical prophylaxis exist and include intermittent pneumatic compression, graduated compression stockings, and venous foot pumps. The main advantage of these products is the lack of a potential for bleeding with their use. Studies have shown them to be effective in reducing the rate of DVT, but not PE or death, in various surgical populations and they may provide additive efficacy when combined with anticoagulants. However, they have generally been found to be less effective than the pharmacologic prophylactic modalities and have not been so vigorously studied as the anticoagulants. A lack of compliance with these devices has been observed and should be taken into account, along with their respective costs, before their use [1].
External pneumatic compression sleeves are mechanical methods of improving venous return from the lower extremities [28]. They reduce stasis in the gastrocnemius-soleus pump. They are placed on the patient on the morning of surgery and are worn throughout the surgical procedure and continuously in the postoperative period until the patient is ambulatory or an anticoagulant is started. The most common complaints pertain to local discomfort caused by increased warmth, sweating, or disturbance of sleep. If a patient has been at bed rest or immobilized for more than 72 hours without any form of prophylaxis, it is our practice to perform lower extremity noninvasive testing to ensure that the patient does not have a DVT before the application of the sleeves.
Aspirin
There is a lack of consensus on the role of aspirin for the prevention of VTE in the orthopedic population. The American Association of Orthopedic Surgeons (AAOS) endorses the use of aspirin for certain patients who undergo nontraumatic hip or knee arthroplasty whereas the ACCP recommends against the use of aspirin for any patient undergoing a joint replacement procedure [1,2]. The AAOS places an emphasis on reducing the risk of symptomatic PE and cites a lack of a clear correlation between the presence of a lower extremity DVT and the risk of subsequently developing a symptomatic PE. On the other hand, the ACCP endorses the presence of a lower extremity DVT as a marker for an increased risk of PE and, thus, places an emphasis on reducing the risk of developing a lower extremity thrombus. Warfarin, LMWH, and the synthetic pentasaccharide have been shown to more effectively reduce this risk and, thus, are recommended by the ACCP. However, the AAOS recommends the use of aspirin in patients with a standard risk of PE and major bleeding or in those with an increased risk of major bleeding with a standard risk of PE because there is evidence to suggest a decrease in the rate of symptomatic events with the use of aspirin. There are no recommendations for aspirin use in the other surgical groups.
VTE prophylaxis for surgery
Orthopedic surgery
Prophylaxis for VTE in orthopedic surgery patients was strongly advocated by the ACCP Consensus Conference on Antithrombotic Therapy 2008 (Box 2) [1]. Joint replacement procedures and hip fracture repair comprise the predominant procedures performed in patients with degenerative joint disease or rheumatoid arthritis. The incidence of fatal PE in patients undergoing joint replacements who have not received prophylaxis has been reported to be to 5% [1]. This high incidence of fatal PE is not an acceptable outcome in patients undergoing these procedures. To understand the approach to prophylaxis, joint replacement procedures and fractured hip repair is reviewed.
Box 2 Prophylaxis orthopedic surgery
Total Hip Replacement (THR) Prophylaxis
Fractured Hip
Total Knee Replacement
Without prophylaxis, the overall incidence of DVT after total hip replacement (THR) procedures has ranged from 42% to 57% and this complication has been reported to occur in 41% to 85% of patients undergoing total knee replacement (TKR). The rate of proximal DVT has ranged from 18% to 36% in THR and 5% to 22% in TKR. Fatal PE has occurred in 0.1% to 2.0% in the THR patient group, whereas the incidence of this complication has ranged from 0.1% to 1.7% in patients undergoing TKR. Without VTE prophylaxis, the incidence of total DVT in patients with hip fracture has ranged from 46% to 60%, with 23% to 30% of these thrombotic events located proximally [1]. In a study by Eriksson and colleagues [29], 1711 patients with hip fracture were randomized to receive enoxaparin 40 mg once daily beginning 12 to 24 hours postoperatively or fondaparinux 2.5 mg once daily, starting 4 to 8 hours after surgery. The rates of VTE by postoperative day 11 were 19.1% in the enoxaparin group and 8.3% in the fondaparinux cohort (P<.001). Proximal DVT occurred in 4.3% of those taking enoxaparin versus 0.9% in the fondaparinux group (P<.001). There was no difference in major bleeding between the 2 groups. The fatal PE rate has ranged from 0.3% to 7.5% [1]. LMWH, fondaparinux, and warfarin are currently the pharmacologic agents of choice for DVT prophylaxis according to the ACCP for the aforementioned procedures and should be administered as described earlier. Intermittent pneumatic compression sleeves can be used in combination with an anticoagulant in those patients considered to have a high risk of developing a VTE [1].
Urological surgery
A review of the prophylaxis studies in urological surgery has shown that the average patient was a man in the 50-year-old to 70-year-old age group (Box 3). The incidence of DVT has varied in these studies, with a reported rate between 31% and 51% in open prostatectomies to 7% to 10% in transurethral resections of the prostate [22]. The subject population of these studies had a mixture of benign and malignant diseases. This factor could have potentially introduced bias into the outcome of these studies. A clinical trial by Soderdahl and colleagues [30] in major urological surgery randomized 90 patients to receive thigh-length or calf-length intermittent pneumatic compression stockings. Venous compression ultrasound was the trial end point. One patient in the thigh-length group developed a PE, whereas only 1 patient in the calf-length group developed a proximal thrombotic event. Thus, both mechanical methods were effective. However, the optimal prophylactic modality for VTE in urological surgery is not known, because of the lack of well-controlled trials. Box 3 outlines the current recommendations for VTE prophylaxis in urological surgery.
Box 3 Prophylaxis for urological surgery
Neurosurgery
Craniotomies and spinal surgeries have been the predominant neurosurgical procedures evaluated for prophylaxis (Box 4). In several randomized clinical trials, which included a variety of neurosurgical procedures, the rate of DVT detected by fibrinogen uptake testing among the control subjects was 22% with 5% of thrombotic events located proximally [22]. The 2 largest studies performed in neurosurgical patients compared gradient compression stockings (GCS) alone versus GCS with LMWH initiated after procedure with venography as the end point of the trial. There was a significant reduction in DVT in GCS plus LMWH compared with GCS alone [31,32]. Goldhaber and colleagues [33] randomized 150 patients with brain tumor undergoing craniotomy to receive IPC plus either UFH (5000 U twice a day) or enoxaparin (40 mg daily). The UFH group had a 7% incidence of DVT, whereas the enoxaparin cohort had 12% DVT. Proximal DVT was found in 3% of patients in both groups. There was no difference in major bleeding between the groups. Although the reported incidence of major bleeding was not increased, clinicians hesitate to use pharmacologic prevention. The pooled rates of intracranial hemorrhage in randomized trials of neurosurgery patients were 2.1% for postoperative LMWH and 1.1% for mechanical or no thromboprophylaxis [31,32]. Most of these bleeds occurred within the first 2 days after surgery. However, a meta-analysis for intracranial hemorrhage did not show significant differences for comparisons of LMWH versus UFH, or between LMWH and no heparin [34]. Box 4 outlines the recommendations for DVT prophylaxis in neurosurgery patients.
Box 4 Prophylaxis for neurosurgery
Gynecologic surgery
The incidence of DVT, PE, and fatal PE in major gynecologic surgery is similar to those after general surgical procedures (Box 5). A Cochrane Database review by Oates-Whitehead and colleagues [35] identified 11 studies, 6 of which were randomized controlled trials. The trials included a total of 7431 patients. Compared with compression alone, the use of combined modalities reduced significantly the incidence of both symptomatic PE (from about 3% to 1%; odds ratio [OR] 0.39, 95% confidence interval [CI] 0.25–0.63) and DVT (from about 4% to 1%; OR 0.43, 95% CI 0.24–0.76). Compared with pharmacologic prophylaxis alone, the use of combined modalities significantly reduced the incidence of DVT (from 4.21% to 0.65%; OR 0.16, 95% CI 0.07–0.34) but the included studies were underpowered with regard to PE. The comparison of compression plus pharmacologic prophylaxis versus compression plus aspirin showed a nonsignificant reduction in PE and DVT in favor of the former group. Four randomized clinical trials compared UFH given 3 times daily versus LMWH in gynecologic cancer surgery. Both agents were effective and safe in preventing postoperative VTE [36–39]. The current recommended options for DVT prophylaxis are UFH, LMWHs, and intermittent pneumatic compression. The issue of extended VTE prevention in the outpatient setting was studied by Bergqvist and colleagues [40]. In this double-blind multicenter trial, 322 patients undergoing abdominal or pelvic surgery were randomized to receive enoxaparin (40 mg once daily) versus placebo for 25 to 31 days after the initial procedure. Venography at the completion of the trial was the end point of the study. The enoxaparin group had a 5% incidence of DVT, whereas the placebo cohort had a 12% incidence (OR 0.36, P = .02). The rate of proximal DVT was low in both groups, with calf vein thrombosis being the predominant finding.
Box 5 Prophylaxis for gynecologic surgery
General surgery
The incidence of DVT in general surgery has been documented to be 15% to 30%, whereas the rates of fatal PE ranged between 0.2% and 0.9% (Box 6) [1]. These studies evaluated a wide age group of patients undergoing a variety of procedures, and studies without VTE prophylaxis are no longer performed. A meta-analysis of 46 randomized clinical trials in general surgery compared thromboprophylaxis using UFH (5000 U every 8 hours or every 12 hours) with no thromboprophylaxis or with placebo [41]. The rate of DVT was significantly reduced from 22% to 9% (OR 0.3; number needed to treat [NNT] 7) as were the rates of symptomatic PE from 2.0% to 1.3% (OR 0.5; NNT 143), fatal PE 0.8% to 0.3% (OR 0.4; NNT 182), and all-cause mortality from 4.2% to 3.2% (OR 0.8; NNT 97). The rates of bleeding were reported as 3.8% in the UFH group and 5.9% in the nontreated or placebo cohorts, most of which were not major bleeding (OR 1.6; NNT 47). This meta-analysis concluded that, based on indirect comparisons, UFH 5000 U every 8 hours was more efficacious than 5000 U every 12 hours and there was no increase in the incidence of bleeding. There are no head-to-head studies comparing UFH 5000 U every 8 hours versus every 12 hours. In evaluating LMWHs in general surgery, a meta-analysis reported a reduction in asymptomatic DVT and symptomatic VTE by greater than 70% compared with patients not receiving prophylaxis [42]. When UFH and LMWHs were compared, there was no difference in the rates of symptomatic VTE. A large randomized trial in major abdominal surgery compared fondaparinux (2.5 mg started 6 hours postoperatively and then once daily) with dalteparin (5000 U given preoperatively then once daily) [43]. There were no significant differences between the groups in the rates of VTE (4.6% vs 6.1%), major bleeding (3.4% vs 2.4%), or death (1.6% vs 1.4%). The mechanical methods of prophylaxis are recommended for patients with a high perioperative bleeding risk and are replaced with a pharmacologic agent once the bleeding risk subsides. As stated earlier, the combined use of mechanical and pharmacologic prophylaxis may be considered for patients considered to have a high VTE risk. The recommended prophylactic agents in order of preference are UFH, LMWHs, external pneumatic compression, and gradient elastic stockings. Box 6 outlines the recommendations for VTE prophylaxis in the general surgery population.
Box 6 VTE prophylaxis: general surgery
Extended prophylaxis for DVT and PE
Despite our most effective DVT and PE prophylaxis regimens, the incidence of DVT has not been reduced to zero (Box 7). The duration of risk for the development of DVT after release from the hospital after surgery has become an important issue. The topic of extended VTE prevention in the outpatient setting was studied by Bergqvist and colleagues [40]. In this double-blind, multicenter trial of 322 patients undergoing abdominal or pelvic surgery, patients were randomized to receive enoxaparin (40 mg once daily) versus placebo for 25 to 31 days after the initial procedure. Venography at the completion of the trial was the end point of the study. The enoxaparin group had a 5% incidence of DVT, whereas the placebo cohort had 12% (OR 0.36, P = .02). The rate of proximal DVT was low in both groups, with calf vein thrombosis being the predominant finding.
Box 7 Extended VTE prophylaxis: general, gynecologic, and orthopedic surgery
In another open-label study conducted in 233 patients undergoing major abdominal surgery, LMWH (dalteparin 5000 IU every 24 hours) was administered once daily for 1 or 4 weeks [44]. All patients completed bilateral lower extremity venography at day 28 ± 2 days. DVT was detected in 16% of patients who had 7 days of prophylaxis versus 6% in those receiving LMWH for 4 weeks (P = .09). The proximal DVT incidence was 9% in the former and 0% the latter group.
More recently, 2 studies evaluated patients with THR for 21 days after discharge [45,46]. Both studies were randomized, double-blind, placebo-controlled trials using enoxaparin (40 mg daily). All study patients underwent bilateral lower extremity venography at the completion of 21 days of prophylaxis. Planes and colleagues [45] reported a 19.3% incidence of DVT in the placebo group and a 7.1% incidence in the patients receiving enoxaparin. Bergqvist and coworkers [46] showed a 39% incidence in the placebo-treated patients and an 18% incidence in those receiving enoxaparin. Three meta-analyses of patients undergoing THR and total knee arthroplasty (TKA) found that posthospital discharge VTE prophylaxis was both effective and safe [47–49]. Major bleeding did not occur in any groups receiving extended prophylaxis with LMWH. Those who underwent THR derived greater protection from symptomatic VTE using extended prophylaxis (pooled OR, 0.33; 95% CI, 0.19–0.56; NNT 62) than patients who underwent TKA (pooled OR, 0.74; 95% CI, 0.26–2.15; NNT, 250). A recent double-blinded clinical trial treated 656 patients undergoing hip fracture surgery with fondaparinux or placebo for an additional 3 weeks after discharge [29]. Venography documented DVT in 1.4% of the extended prophylaxis group and 35% in the placebo cohort. The major bleeding rates were the same in both groups. The recent Chest guidelines have defined the risk period after discharge to be up to 35 days [1]. It is recommended that prophylaxis with LMWH or warfarin be provided for this period in patients undergoing major orthopedic procedures (see Box 7). As for the nonorthopedic surgery population, those who have undergone surgery for a malignancy are considered high risk for VTE and should be considered for extended VTE prophylaxis for 21 to 30 days after the procedure.
New oral anticoagulants
Apixaban
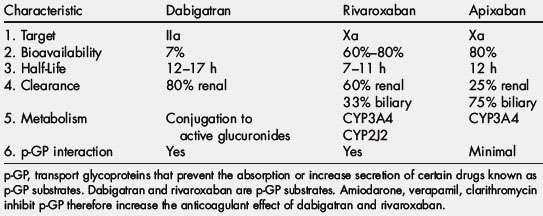
Apixaban is a selective, reversible, direct inhibitor of factor Xa. Its time to maximum plasma concentration is 30 minutes to 2 hours (Tables 3–5). The half-life of this drug is 8 to 15 hours [50]. This agent is metabolized by CYP3A4 in the CYP450 system, and the route of elimination is 30% renal and 70% fecal [50]. Apixaban showed moderate selectivity for clot-bound over free factor Xa and also inhibits thrombin generation [50]. In addition, apixaban is a substrate for the transport protein p-glycoprotein (p-GP), which functions as an efflux pump to prevent the absorption or increase the renal secretion of certain drugs known as p-GP substrates [51,52]. Apixaban has not been reported to have any food interactions. In healthy volunteers, activated partial thromboplastin time (aPTT) and modified PT were dose dependently prolonged and correlated with the determined plasma concentrations of apixaban [53]. Apixaban has a minimal impact on the prothrombin time (internationalized normalized ratio [INR]) and aPTT at therapeutic concentrations, but factor Xa inhibition seems sensitive to detect its presence. There are no specific reversing agents for this medication.
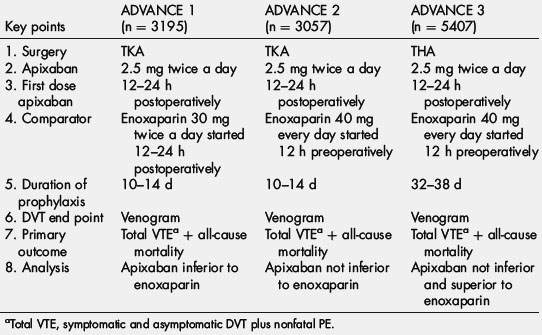
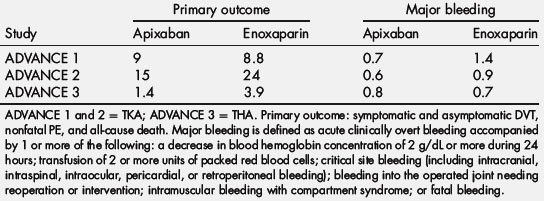
From the results of a phase II study in patients undergoing knee arthroplasty, the phase III Apixaban for the Prevention of Thrombosis-Related Events (ADVANCE) program compared a 2.5-mg twice-daily dose of apixaban (started in the morning of the day after surgery) with enoxaparin in patients undergoing knee arthroplasty. Tables 4 and 5 outline the design and outcomes of the 3 trials in the program. For both trials, the primary efficacy outcome (total event rate) was a composite of asymptomatic and symptomatic DVT, nonfatal PE, and death from any cause during treatment. In ADVANCE 1, which involved 3195 patients, a 10-day to 14-day course of apixaban was compared with a similar duration of enoxaparin (30 mg twice daily). Apixaban had efficacy similar to enoxaparin, with total event rates of 9.0% and 8.8%, respectively [54]. Major bleeding rates were 0.7% with apixaban and 1.4% with enoxaparin (P = .05). Despite similar efficacy, apixaban did not meet the prespecified noninferiority goal because the event rates were lower than expected. The ADVANCE 2 trial, which included 3057 patients, compared the same apixaban regimen with an equal duration of treatment with enoxaparin at a dose of 40 mg once daily [55]. In this trial, apixaban significantly reduced total event rates compared with enoxaparin (15.1% and 24.4%, respectively; P<.0001) and was associated with a trend for less major bleeding (0.6% and 0.9%, respectively; P = .3). ADVANCE 3 treated 5407 patients undergoing total hip arthroplasty (THA) for 32 to 38 days with apixaban (2.5 mg twice daily) versus enoxaparin (40 mg once daily). Apixaban (1.4%) was superior to enoxaparin (3.9%) for the primary outcome. Major bleeding rates were the same in apixaban (0.8%) and enoxaparin (0.7%) [56].
Rivaroxaban
Rivaroxaban is a selective, reversible direct inhibitor of factor Xa (see Table 3; Tables 6 and 7). The time to maximum plasma concentration is 30 minutes to 3 hours (see Table 3). The half-life of rivaroxaban has been reported to be 3 to 9 hours [57,58]. Three aspects of the pharmacodynamics of rivaroxaban are its concentration-dependent inhibition of factor Xa with high potency and selectivity, its inhibition of thrombin generated from prothrombin, and a dose-dependent inhibition of tissue factor [59]. This agent is metabolized by CYP3A4 in the CYP450 system and the route of elimination is 70% renal and 30% fecal [60]. Rivaroxaban does interact with the CYP450 system with specific interactions with CYP3A4 and CYP2J2 [61]. In addition, this agent is a substrate for transport p-GP and subject to interaction with drugs that interact with this protein. Studies reported the lack of any clinically relevant interaction of rivaroxaban with salicylic acid or naproxen [61]. The bioavailability of rivaroxaban was increased by about 2.5 fold on coadministration of CYP3A4/p-GP inhibitors such as ketoconazole or ritonavir and decreased by about 50% after administration of the CYP3A4 inducer rifampicin [57]. Concomitant food intake only marginally increased the bioavailability of rivaroxaban in healthy subjects [62]. Changes in gastric pH by antacids or ranitidine did not significantly affect absorption. There have not been any relevant effects of extreme body weight, age, or gender on the pharmacologic profile of this drug, which has facilitated fixed-dose prescribing recommendations. Rivaroxaban prolongs the prothrombin time (INR) with the sensitivity dependent on the reagent being used. Factor Xa inhibition may be a more appropriate surrogate marker for evaluating the plasma concentration of rivaroxaban. There are no specific reversing agents for this medication.
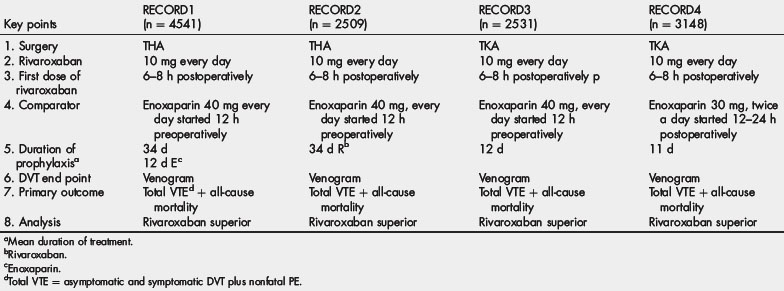
The phase II Oral Direct Factor Xa Inhibitor (ODIXa) VTE prevention studies established the dose for rivaroxaban that was used in the phase III RECORD trial program [63–65]. This program evaluated the efficacy and safety of rivaroxaban compared with enoxaparin in more than 12,000 patients undergoing hip or knee arthroplasty. Tables 6 and 7 outline the design of these trials as well as the primary outcomes. The dose of rivaroxaban in all 4 RECORD trials was 10 mg once daily started 6 to 8 hours after wound closure. The European-approved dose of enoxaparin (40 mg once daily, with the first dose given in the evening before surgery) was used as the comparator in the first 3 RECORD trials, whereas the North American approved dose of enoxaparin (30 mg twice daily, starting 12 to 24 hours after surgery) was the comparator in the RECORD 4 trial [66–69]. The primary efficacy outcome (total event rate) in all of the trials was the composite of DVT (either symptomatic or detected by bilateral venography if the patient was asymptomatic), nonfatal PE, or death from any cause.
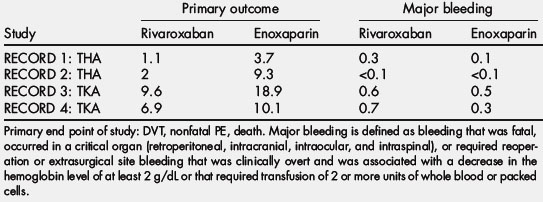
In the RECORD 1 trial, which included 4541 patients undergoing hip arthroplasty, a 31-day to 39-day course of rivaroxaban significantly reduced the total event rate compared with an equal duration of treatment with enoxaparin (1.1% and 3.7%, respectively; P<.001) [66]. In the RECORD 2 trial involving 2509 patients undergoing THA, a 31-day to 39-day course of rivaroxaban significantly reduced the total event rate compared with a 10-day to 14-day course of enoxaparin followed by 21 to 25 days of placebo (2.0% and 9.3%, respectively; P<.0001) [67]. The RECORD 3 trial included 2531 patients undergoing knee arthroplasty. A 10-day to 14-day course of treatment with rivaroxaban significantly reduced the total event rate compared with an equal duration of treatment with enoxaparin (9.6% and 18.9%, respectively, P<.001) [68]. In the RECORD 4 trial involving 3148 patients undergoing knee arthroplasty, a 10-day to 14-day course of treatment with rivaroxaban significantly reduced the total event rate compared with an equal duration of enoxaparin at the higher 30-mg twice-daily dose (6.9% and 10.1%, respectively; P<.012) [69]. In both the RECORD 2 and 3 trials, rivaroxaban significantly reduced the incidence of symptomatic VTE compared with enoxaparin [66,68]. Rivaroxaban did not increase major bleeding in any of the trials, but a pooled analysis performed by the US Food and Drug Administration of the 4 RECORD trials revealed a small but significant increase in major plus clinically relevant nonmajor bleeding with rivaroxaban. From these results, rivaroxaban is approved in Europe and Canada for the prevention of VTE in patients undergoing elective hip or knee arthroplasty.
Dabigatran
Dabigatran etexilate is the prodrug of dabigatran that selectively and reversibly inhibits both free and clot-bound thrombin by binding to the active site of the thrombin molecule (see Table 3; Tables 8 and 9). The time to maximum plasma concentration is 1.25 to 1.5 hours, with maximum effect in 2 hours [70]. Its half-life is about 12 hours. In human studies, more than 90% to 95% of systemically available dabigatran was eliminated unchanged via renal excretion, with the remaining 5% to 10% excreted in bile [71]. A unique aspect of this drug is that it is neither metabolized by nor induced or inhibited by the cytochrome P450 drug-metabolizing enzymes. Because this drug exhibits low plasma protein binding (35%), it is a dialyzable agent, with few displacement interactions to affect its pharmacodynamics [72]. In cases of overdose or severe bleeding, where more rapid reversal of the anticoagulant effects is required, hemodialysis could be effective in accelerating plasma clearance of dabigatran, especially in patients with renal impairment [72].
Food prolongs the time to peak plasma dabigatran levels by approximately 2 hours without significantly influencing overall bioavailability in healthy volunteers [71,73]. There have been no reported food interactions with dabigatran. Dabigatran is a substrate for transporter p-GP that could lead to changes in bioavailability of the drug. Drug interaction studies of dabigatran etexilate in combination with atorvastatin (CYP3A4 and p-GP substrate), diclofenac (CYP2C9 substrate), and digoxin (p-GP substrate) did not result in any significant pharmacokinetic changes of dabigatran or coadministered drugs [70,71,74–76]. Amiodarone, a p-GP inhibitor, increased the bioavailability of dabigatran by about 50% to 60%, which may require an appropriate reduction in dosing [72]. In contrast, the bioavailability of dabigatran was about 20% to 30% lower when pantoprazole was coadministered, indicating its decreased oral bioavailability at increased gastric pH [71,73]. Both the thrombin clotting time and ecarin clotting time are highly sensitive tests for quantitating the anticoagulant effects of dabigatran [77]. The prothrombin time (INR) is prolonged by dabigatran, but it is not sensitive enough to detect clinically relevant changes in drug concentration, and the aPTT is prolonged but not in a dose-dependent manner. Thus, the aPTT may serve as a qualitative test because it is less sensitive at supratherapeutic concentrations of dabigatran. There are no specific reversing agents for dabigatran.
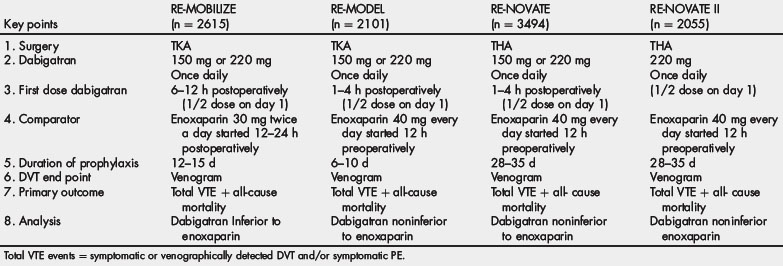
Based on results from phase II studies, 2 doses of dabigatran were investigated in the phase III trials for thromboprophylaxis after hip or knee arthroplasty: 220 or 150 mg (both given once daily), which was initiated at half the usual dose on the first day. The European-approved dose of enoxaparin (40 mg once daily, with the first dose given in the evening before surgery) was used as the comparator in the RE-MODEL study after TKR and RE-NOVATE and RE-NOVATE II studies after THR. The North American approved dose of enoxaparin (30 mg twice daily, starting 12 to 24 hours after surgery) was the comparator in the RE-MOBILIZE study after TKR [78–80]. In all 3 trials, the primary efficacy end point (total event rate) was a composite of venographically detected or symptomatic DVT, nonfatal PE, and all-cause mortality. Tables 8 and 9 outline the design of these trials as well as the primary outcomes.
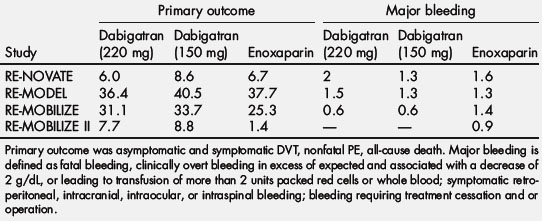
In the RE-MODEL trial involving 2076 patients undergoing knee arthroplasty, 6 to 10 days of either dose of dabigatran etexilate had efficacy similar to that of enoxaparin (dabigatran 220 mg, 36.4%; dabigatran 150 mg, 40.5%; enoxaparin, 37.7%). The incidence of major bleeding did not differ significantly among the 3 groups (1.5%, 1.3%, and 1.3%, respectively) [78]. In the RE-NOVATE trial involving 3494 patients undergoing hip arthroplasty, treatment with either dose of dabigatran etexilate for 28 to 35 days had efficacy similar to that of enoxaparin (dabigatran 220 mg, 6.0%; dabigatran 150 mg, 8.6%; enoxaparin, 6.7%). The incidence of major bleeding did not differ significantly among the 3 groups (2.0%, 1.3%, and 1.6%, respectively) [79]. In the RE-MOBILIZE study of 2615 patients undergoing knee arthroplasty, treatment with either dose of dabigatran etexilate for 12 to 15 days was statistically inferior to a similar duration of treatment with enoxaparin (dabigatran 220 mg, 31%; dabigatran 150 mg, 34%; enoxaparin, 25%). The incidence of major bleeding did not differ significantly among the 3 groups (0.6%, 0.6%, and 1.4%, respectively) [80]. The RE-NOVATE II study evaluated 2055 patients undergoing THA treated with dabigatran (220 mg once daily) versus enoxaparin (40 mg once daily) for 28 to 35 days [81]. Dabigatran (7.7%) was not inferior to enoxaparin (8.8%) for the primary outcome [81]. The incidence of major bleeding did not differ significantly among the 2 groups (1.4% dabigatran, 0.9% enoxaparin) [81].
References
[4] A. Nicolaides, V. Kakkar, J. Renney. Soleal sinuses and stasis. Br J Surg. 1970;57:307.
[12] P.C. Malone, P.S. Agutter. The aetiology of deep venous thrombosis. QJM. 2006;99(9):581-593.
[24] J. Hirsh, M. Levine. Low molecular weight heparin. Blood. 1992;79:1-17.
[72] European Medicines Agency (EMEA). European public assessment report: pradaxa. Available at: http://www/emea.europa.eu/humandocs/PFFs/EPAR/pradaxa/H-829-PI-en.pdf Accessed December 5, 2010
[75] J. Stangier, H. Stahle, K. Rathgen, et al. Coadministration of the oral direct thrombin inhibitor dabigatran etexilate and diclofenac has little impact on the pharmacokinetics of either drug (abstract) XXIst Congress of the International Society of Thrombosis and Haemostasis 2007; P-T-677. Available at: http://isth2007.abstractsondemand.com Accessed June 19, 2011
[76] J. Stangier, H. Stahle, K. Rathgen, et al. No interaction of the oral direct thrombin inhibitor dabigatran etexilate and digoxin (abstract) XXI st Congress of the International Society of Thrombosis and Haemostasis 2007;P-W-672. Available at: http://isth2007.abstractsondemand.com Accessed June 19, 2011