Chapter 3 Fluoroscopy, Ultrasonography, Computed Tomography, and Radiation Safety
 At the present time, multiple forms of radiological imaging devices are used to evaluate and confirm the diagnosis of clinical conditions.
At the present time, multiple forms of radiological imaging devices are used to evaluate and confirm the diagnosis of clinical conditions. Safety is still a very real concern, and awareness of risk that is taken with each procedure is important.
Safety is still a very real concern, and awareness of risk that is taken with each procedure is important. Interventional pain procedures are now required to be performed with the use of radiological guidance in most situations. Without imaging, patients may be placed at greater risk unnecessarily.
Interventional pain procedures are now required to be performed with the use of radiological guidance in most situations. Without imaging, patients may be placed at greater risk unnecessarily. Minimizing radiation exposure is still most important. MRI or US allows the performance of evaluations without radiation exposure, and may be considered in specific situations.
Minimizing radiation exposure is still most important. MRI or US allows the performance of evaluations without radiation exposure, and may be considered in specific situations. Careless use of and overexposure to radiation can compromise the health and well-being of both the operator and the patient being examined. Careful attention to lead apron quality, sizing, and regular safety inspections of all equipment are important.
Careless use of and overexposure to radiation can compromise the health and well-being of both the operator and the patient being examined. Careful attention to lead apron quality, sizing, and regular safety inspections of all equipment are important.Ultrasound
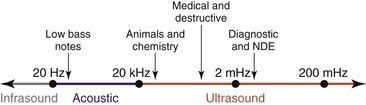
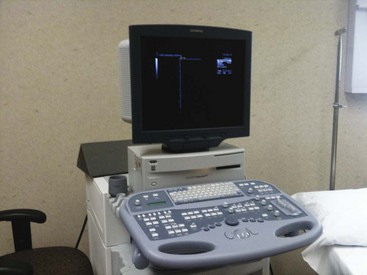
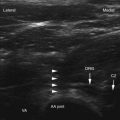
Ultrasound is cyclic sound pressure with a frequency greater than the upper limit of human hearing. Although this limit varies from person to person, it is approximately 20 kHz (20,000 Hz) in healthy, young adults, and thus 20 kHz serves as a useful lower limit in describing ultrasound (Fig. 3-1).
Safety Concerns
Most infants now born in the United States are exposed to ultrasonography before birth, and in Germany, Norway, Iceland, and Austria, all pregnant women are screened with ultrasonography. To date, researchers have not identified any adverse biological effects clearly caused by ultrasonography, even though 3 million babies born each year have had ultrasound scans in utero. This is an enviable safety record. However, the National Council on Radiation Protection and Measurements advocates continued study of ultrasound safety, improvements in the safety features of ultrasound systems, and more safety education for ultrasound system operators.1 Because of the sheer number of people exposed to ultrasonography, any possibility of a harmful effect must be investigated thoroughly.
Ultrasound gel is intended only for external use. If a needle becomes contaminated with gel, every effort should be made to remove the needle and replace it with a sterile new one. Even though the gel initially is sterile, the substance itself may irritate structures either in the epidural space or even intrathecally. Either way, one should err toward needle replacement. Remember, ultrasound gel contains propylene glycol, glycerine, phenoxyethanol, and FD&C Blue #1. For properties and side effects of ultrasound gel, see Box 3-1.
Computed Tomography
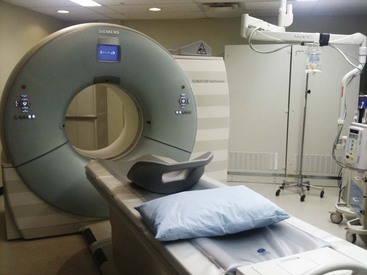
The CT scanner is an expensive yet sophisticated way to guide needle placement (Fig. 3-2). It is somewhat expensive for the routine use of image-guided procedures, especially in an office-based practice or even an ambulatory surgery center. One could justify the use of such a device if looking at a study or working in a hospital with access to a scanner. Most scanners are used daily for diagnostic workups but not for pain management procedures. They allow for excellent needle placement and biopsies that are performed.
Safety Concerns
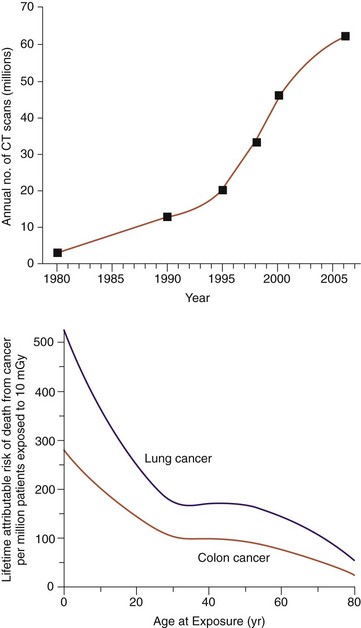
The individual risk from radiation associated with a CT scan is quite small compared with the benefits that accurate diagnosis and treatment can provide. Still, unnecessary radiation exposure during medical procedures should be avoided. This is particularly important when the patient is a child because children exposed to radiation are at a relatively greater risk than adults. The American College of Radiology has noted, “Because they have more rapidly dividing cells than adults and longer life expectancy, the odds that children will develop cancers from x-ray radiation may be significantly higher than adults”3(Fig. 3-3). Unnecessary radiation may be delivered when CT scanner parameters are not appropriately adjusted for patient size. When a CT scan is performed on a child or small adult with the same technique factors used for a typically sized adult, the small patient receives a significantly larger effective dose than the full-sized patient.
A recent study by Academic Emergency Medicine4 confirms what many doctors already believed; people may be receiving doses of radiation, sometimes unnecessarily, that puts them at a heightened risk for cancer. Researchers found that a typical patient who visited the emergency department received a cumulative radiation dose of 40 mSv over a 5-year period. Ten percent of patients ended up with a staggering 100 or more mSv. Both levels are well above the safety threshold for lifetime radiation exposure. Exposure above the threshold leaves patients vulnerable to increased long-term risk of cancer. As a point of comparison, one chest CT is around 10 mSv of radiation, and a traditional chest radiograph is only 0.02 mSv (Table 3-1).
Table 3-1 Typical Organ Radiation Doses from Various Radiologic Studies
| Relevant Organ Dose* | Study Type | Organ (mGy or mSv) |
|---|---|---|
| Dental radiography | Brain | 0.005 |
| Posterior-anterior chest radiography | Lung | 0.01 |
| Lateral chest radiography | Lung | 0.15 |
| Screening mammography | Breast | 3 |
| Adult abdominal CT | Stomach | 10 |
| Barium enema | Colon | 15 |
| Neonatal abdominal CT | Stomach | 20 |
* The radiation dose, a measure of ionizing energy absorbed per unit of mass, is expressed in grays (Gy) or milligrays (mGy); 1 Gy = 1 joule per kilogram. The radiation dose is often expressed as an equivalent dose in sieverts (Sv) or millisieverts (mSv). For x-ray radiation, which is the type used in computed tomography (CT) scanners, 1 mSv = 1 mGy.
Organ doses from CT scanning are considerably larger than those from corresponding conventional radiography (Table 3-2). For example, a conventional anterior-posterior abdominal radiographic examination results in a dose to the stomach of approximately 0.25 mGy, which is at least 50 times smaller than the corresponding stomach dose from an abdominal CT scan.
Table 3-2 Total Radiation for Body Parts
| Body Part | Dose |
|---|---|
| Whole body, critical organs | 5 rems in any year |
| Gonads, lens of eye | (Prospective annual limit) 10-15 rems in a year |
| Bone marrow | 10-15 rems in any 1 year (Retrospective annual limit) (N-18) × 5 rems (long-term accumulation) |
| Skin | 15 rems in any 1 year |
| Hands | 75 rems in any 1 year (25/qtr) |
| Forearms | 30 rems in any 1 year (10/qtr) |
| Other organs, tissues, and organ systems | 15 rems in any 1 year |
qtr, Quarter; rem, roentgen-equivalent-man.
Radiation doses vary by operator, radiology technician, and even the radiologist who may request additional images to verify areas of concern. Because there is no universal policy as to how many images should be used per examination, concerns among radiologists are on the rise. Precautions for radiation safety are presented in Box 3-2.
Box 3-2 Basic Radiation Safety
 Distance: Doubling the distance from the exposure means reducing the dose by one-fourth; tripling the distance means only one-ninth the dose.
Distance: Doubling the distance from the exposure means reducing the dose by one-fourth; tripling the distance means only one-ninth the dose. Shielding: Lead barriers, aprons, sliding walls, and eyewear, along with hand and arm protectors, all add improved safety and reduce the total dose of radiation exposure.
Shielding: Lead barriers, aprons, sliding walls, and eyewear, along with hand and arm protectors, all add improved safety and reduce the total dose of radiation exposure.
The use of a CT-guided image to perform most interventional pain procedures does expose a patient and staff to small doses of radiation. Very few procedures actually require a CT scanner to be able to correctly place needles in clinically difficult areas.5 Waldman5 notes that celiac plexus blockade and sacroiliac joint blocks have been performed with CT guidance, but each of these procedures can be performed with fluoroscopy guidance.
Fluoroscopy
The beginning of fluoroscopy can be traced back to November 8, 1895, when Wilhelm Röntgen noticed a barium platinocyanide screen fluorescing as a result of exposure to what he would later call x-rays.6 Within months of this discovery, the first fluoroscopes were created. Early fluoroscopes were simply cardboard funnels, open at the narrow end for the eyes of the observer. The wide end was closed with a thin cardboard piece that had been coated on the inside with a layer of fluorescent metal salt. The fluoroscopic image obtained in this way was rather faint. Thomas Edison quickly discovered that calcium tungstate screens produced brighter images, and he is credited with designing and producing the first commercially available fluoroscope.
Over the past several years, the use of fluoroscopy has allowed interventional pain physicians to accurately perform injections with precision guidance. What was once considered the gold standard (performing injections blind) is now taboo. A typical injection is performed with either a local anesthetic to confirm the cause of a pain source or in combination with a corticosteroid to help reduce the inflammatory effect caused by an injury or a chronic condition. Confirmation of a painful etiology is necessary to aid in the diagnosis of suspected painful areas. (Fig. 3-4).
Safety Concerns
As fluoroscopy became increasingly useful as an interventional imaging tool, concerns about increasing exposure times increased scrutiny regarding radiation safety for patients and radiology professionals.7,8 In 1994, the U.S. Food and Drug Administration entered the picture, issuing public health advisories dealing with serious radiation-related skin injuries resulting from some fluoroscopic procedures.9 Today’s newer techniques and equipment have contributed to lower dose rates, but fluoroscopy procedures still produce the greatest radiation exposures in diagnostic radiology. Investigators continue to study methods to further reduce exposure rates.7,10
Image Storage
A typical portable fluoroscope used today is versatile and mobile and occupies less space in confined quarters than fixed units (Fig. 3-5). These units also allow one to store and archive images for scanning, reprinting, or illustrating details as to where needle placements are located. PACS (picture archiving and communication system) is a combination of hardware and software dedicated to the short- and long-term storage, retrieval, management, distribution, and presentation of images. Electronic images and reports are transmitted digitally via PACS; this eliminates the need to manually file, retrieve, or transport film jackets. The universal format for PACS image storage and transfer is DICOM (digital imaging and communication in medicine). Non-image data, such as scanned documents, may be incorporated using consumer industry standard formats such as PDF (portable document format) after being encapsulated in DICOM.
1 National Council on Radiation Protection and Measurements. Basic radiation protection. NCRP Report No. 39. Washington, DC: The Council. 1971. p 106
2 GF Health Products. Material Safety Data Sheet, Rev No: A. accessed from http://www.grahamfield.com/pdfs/MSDS%20Aerosil%20(AQ106-12)%2007.19.06.pdf, August 2003.
3 Feigal DW, Jr: Letter regarding CT scan safety.
4 Dill CE, Uraneck K. Radiation Screening. Acad Emerg Med. 2011;18(1):105.
5 Waldman S. Interventional pain management, ed 2. Leawood, KS: Headache and Pain Center; 2011.
6 Hall EJ. Radiobiology for the radiologist, ed 5. Philadelphia: Lippincott Williams & Wilkins; 2002.
7 Mahesh M. The AAPM/RSNA physics tutorial for residents. Fluoroscopy: patient radiation exposure issues. Radiographics. 2001;21:1033-1045.
8 King JN, Champlin AM, Kelsey CA, Tripp DA. Using a sterile disposable protective drape for reduction of radiation exposure to interventionalists. AJR Am J Roentgenol. 2002;178:153-157.
9 Fajardo LF, Berthrong M, Anderson RE. Radiation pathology. New York: Oxford University Press; 2001.
10 Curry TS, Dowdey JE, Murry RC. Christensen’s physics of diagnostic radiology, ed 2. Malvern, PA: Lea & Febiger; 1990.