Chapter 4. Fluid and electrolytes
This chapter covers the common problems of fluid balance and management of infants with electrolyte disturbances that are faced on a daily basis. Other topics covered in this chapter are problems with glucose metabolism and inborn errors of metabolism.
QUESTION 1
The following maternal drugs cause acute renal failure in a baby (answer true or false):
i) Ibuprofen
ii) Aspirin
iii) Losartan
iv) Celecoxib
v) Gentamicin
vi) Captopril.
QUESTION 2
A baby born at 24 weeks gestation is being nursed on a platform with an overhead radiant heater. His birth weight is 600 g and he is receiving a total fluid of 1.5 mL/h. He is now 24 hours old and his blood results are as follows:
| Na | 149 mmol/L |
| K | 4.5 mmol/L |
| Urea | 9.4 mmol/L |
| Creat | 91 μmol/L |
| SBR | 85 μmol/L |
| CRP | 10 mg/L |
His urine output has been 3.5 mL since birth.
i) What is the most likely cause for this result?
a. Postnatal diuresis
b. Inappropriate ADH secretion
c. Inadequate water intake
d. Excessive water losses
e. Acute renal failure
f. Sepsis.
ii) What action would you take to improve the situation? Give two answers.
The next day, his blood results are as follows:
| Na | 145 mmol/L |
| K | 3.7 mmol/L |
| Urea | 8.4 mmol/L |
| Creat | 80 μmol/L |
| SBR | 160 μmol/L |
At this point the baby is on 120 mL/kg/day of 10% dextrose. His urine output has been 8 mL over the last 24 hours.
iii) Which of the following actions do you take next? Choose one answer.
a. Restrict fluid intake
b. Increase fluid intake to 150 mL/kg/day
c. Add additional sodium
d. Challenge with fluid bolus and diuretics
e. Observe and repeat U+E in 12 hours.
The next day his fluids are increased to 180 mL/kg/day. A loud systolic murmur becomes audible and pulses are bounding. Echocardiography shows evidence of a large ductus.
Urine output has been 18 mL over the last 24 hours. The following electrolytes are obtained:
| Na | 139 mmol/L |
| K | 3.9 mmol/L |
| Urea | 6.4 mmol/L |
| Creat | 70 μmol/L |
iv)
a. What changes would you make to his fluid regime?
b. Would you add sodium and potassium to his fluids?
Over the next 24 hours the baby’s respiratory condition deteriorates. A chest x-ray suggests moderate enlargement of the heart and a degree of pulmonary oedema. It is felt that the PDA is contributing significantly and the decision is made to commence indomethacin 0.6 mg/kg for 3 days. 24 hours later, the baby is thought to be more oedematous and the urine output has fallen to 0.4 mL/kg/hour with the following electrolytes:
| Na | 130 mmol/L |
| K | 4.2 mmol/L |
| Urea | 8.1 mmol/L |
| Creat | 92 μmol/L |
v) What do you think the most likely cause is for the current results?
vi) What action would you take?
vii) Which of the following measures of renal function could be helpful in distinguishing between prerenal and ischaemic acute renal failure?
a. Sodium
b. Potassium
c. Urea
d. Creatinine
e. Urine output
f. Urine osmolality
g. Urine microscopy
h. Urine sodium
i. Fractional excretion of sodium.
viii) At the age of 72 hours, you notice that his urine output is now 2.7 mL/kg/hour and his ventilation has significantly improved. Why is this?
QUESTION 3
You are asked to review a baby on the postnatal wards who is now 3 days old. Mum has had a caesarean section for failure to progress and is breast feeding the baby. The midwives are concerned that the baby is jaundiced. Birth weight 3.6 kg. On examination the baby is obviously jaundiced and is quiet.
i) What investigations/observations would you request?
The midwife has performed a test feed and thinks that a reasonable feed intake was achieved. She has weighed the baby before and after the feed.
ii) Does this help?
The investigations from a capillary blood sample reveal:
| Na | 157 mmol/L |
| K | 5.6 mmol/L |
| Urea | 12.8 mmol/L |
| Creat | 95 μmol/L |
| SBR | 286 μmol/L (unconjugated 6) |
| CRP | 8 mg/L |
| WBC | 11.6×10 9 (neutrophils 9.1) |
| Platelets | 242×10 9 |
| Hb | 21.5×10 9 |
| Film | normal |
| HCT | 68% |
| Weight | 2.9 kg |
iii) What is your first action?
Venous sample has comparable results with an HCT of 68%.
iv) What is the most important immediate action? Choose one answer.
a. Dilutional exchange transfusion
b. Glucose and insulin
c. ECG monitoring
d. Lumbar puncture
e. Intravenous fluids
f. NG feed.
v) How would you treat the baby? Which fluids would you consider using in immediate rehydration?
a. 10% dextrose
b. 5% dextrose
c. 0.9% saline
d. 0.45% saline
e. 0.18% saline / 5% dextrose.
vi) What complications can occur if this is not treated? List four.
QUESTION 4
You are called to see a baby on transitional care who was born at 36 weeks gestation, weighing 1.8 kg.The baby is now 4 hours old and had a bottle feed of 40 mL of formula milk an hour ago. The blood glucose is 1.8 mmol/L.
i) What action would you take?
a. Do nothing and reassure mum
b. Give another bottle feed and repeat blood glucose measurement
c. Carry out true laboratory glucose
d. Carry out full hypoglycaemia screen
e. Give bolus of intravenous dextrose
f. Give intramuscular glucagon.
Another bottle feed is offered and the infant takes a further 30 mL. A repeat blood glucose an hour later is 1.4 mmol/L.
ii) What action would you take?
You are unable to establish an intravenous infusion. The infant starts to vomit and repeat blood glucose is 0.8 mmol/L.
The baby is admitted to the neonatal unit and an infusion of 12.5% dextrose at 120 mL/kg/day is required to maintain the blood glucose above 2.6 mmol/L.
vi) What practical action should you take in regard to the baby’s high concentration of dextrose solution?
vii) Is this glucose infusion rate abnormally high?
viii) What is the most likely reason for this infant’s hypoglycaemia?
QUESTION 5
A term baby has suffered an asphyxial episode requiring full resuscitation at birth. Spontaneous respiration was not seen for 36 hours after birth although the heart rate had returned within 8 minutes of resuscitation.
A markedly abnormal CFAM was recorded and fits were treated with phenobarbitone, phenytoin and a midazolam infusion. The baby is now semi-comatose and breathing spontaneously and fitting has stopped. The baby has both a UVC and a UAC in situ.
Initial fluid replacement was 40 mL/kg/day of 10% dextrose with no additives and has been increased on day 3 to 90 mL/kg/day. Routine U+E analysis gives the following results.
| Na | 124 mmol/L |
| K | 3.6 mmol/L |
| Urea | 4.1 mmol/L |
| Creat | 35 μmol/L |
i) What explanation may account for this result? Choose the best answer.
a. Acute renal failure
b. Iatrogenic fluid overload
c. SIADH
d. Diabetes insipidus
e. Iatrogenic electrolyte depletion
f. Abnormal maternal electrolytes.
ii) What in the history supports your favoured diagnosis?
iv) Which of the following investigations would be most helpful? Choose two.
a. Urinary sodium
b. Urinary osmolality
c. Fractional excretion of sodium
d. Serial plasma sodium
e. Renin-angiotensin-aldosterone measurements
f. Renal ultrasound
g. Serum ADH levels
h. Plasma osmolality.
QUESTION 6
A 2-day-old term baby has a total plasma calcium of 1.7 mmol/L; ionised calcium is 0.65 mmol/L. The baby is well.
i) Which of the following is the most likely? Choose one answer.
a. Normal phenomenon
b. Pseudohyperparathyroidism
c. Infant of diabetic mother
d. Maternal elevated vitamin D intake
e. Exchange transfusion
f. Diuretic therapy
g. Hypoalbuminaemia
h. Maternal hypoparathyroidism
i. Low calcium intake
j. Perinatal asphyxia
k. PTH resistance
l. Hypoparathyroidism
m. IUGR
n. Maternal anticonvulsants
o. Maternal anti-TB therapy.
ii) Explain why you feel the other diagnoses are less likely.
iii) How do you treat the baby?
QUESTION 7
A preterm infant born at 28 weeks received one week of diuretic therapy following diagnosis of a PDA. The clinical course thereafter was uneventful. A renal ultrasound performed at 36 weeks corrected gestational age (as part of the screen for suspected UTI) revealed bilateral nephrocalcinosis.
The parents want to know how this has happened and what the long-term consequences are for their baby. What will you tell them?
QUESTION 8
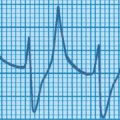
A term baby is born following a severe antepartum haemorrhage and requires full resuscitation. A diagnosis of hypoxic–ischaemic encephalopathy is made. At 48 hours the baby is still ventilated because of a lack of respiratory effort. The following ECG is obtained.
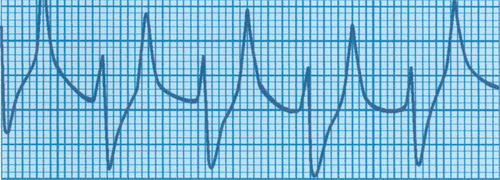 |
| Figure 4.1. |
i) What does this show?
ii) What is the underlying cause?
iii) What is the treatment? Give six key elements.
QUESTION 9
A baby suddenly collapses on the postnatal ward at the age of 36 hours. Prior to collapse, his feeding had deteriorated and he had started to vomit. On examination the baby is lethargic and tachypnoeic. Examination is unremarkable.
i) What is your differential diagnosis? Give four possibilities.
You bring the baby round to the neonatal unit and commence intravenous fluids and start antibiotics. Basic investigations are performed and results are as follows:
| CXR | Normal |
| Hb | 17.4g/dL |
| WCC | 9.4×10 9/L |
| Plat | 351×10 9/L |
| CRP | 11.3 mg/L |
| Blood glucose | 3.2 mmol/L |
The baby deteriorates and becomes more lethargic and drowsy.
ii) What urgent investigations would you now consider? Give four.
While awaiting the results of these investigations the baby becomes more tachypnoeic with marked recession. There is a sudden dramatic deterioration. Oxygen saturations fall to <40% and heart rate to <30.
iii) What differential diagnoses do you consider?
iv) What does it show and what would you do?
While dealing with this problem the results of your other investigations return; the results obtained are as follows:
| Echocardiogram | Normal |
| Ammonia | 350 μmol/L |
| Lactate | 3.4 mmol/L |
| Capillary blood gas | pH 7.48 |
| PCO 2 | 2.1 kPa |
| PO 2 | 3.2 kPa |
| BE | −8.0 mmol/L |
| Bic | 15.5 mEq/L |
v) What is the most likely diagnosis? Choose one answer.
a. Sepsis
b. Transient hyperammonaemia of the newborn
c. Organic acid defect
d. Fatty acid oxidation defect
e. Urea cycle defect
f. Congenital heart disease.
vi) What would be the basis of your management? Explain your decisions.
vii) What would you say to the parents?
QUESTION 10
QUESTION 11
Which of the following could cause an elevation in lactic acid in a term baby?
a. Hypoxia
b. Cardiac disease
c. Infection
d. Convulsions
e. Breath holding
f. Pyruvate dehydrogenase deficiency
g. Fructose-1,6-bisphosphatase deficiency
h. Mitochondrial defects
i. Urea cycle defect
j. Ornithine transcarbamylase deficiency.
QUESTION 12
A term baby weighing 5.2 kg is born by caesarean section. Hairy ears are noted and the infant appears macrosomic. Blood glucose is measured and is 0.1 mmol/L one hour after birth.
The baby is brought round to the neonatal unit for further management. Several attempts are made at siting a peripheral cannula with no success.
i) What action do you take?
ii) There is a suggestion to give a bolus of 10% dextrose. Should you give this?
After one hour of the dextrose infusion, the blood glucose is checked again and is still 0.1 mmol/L. The baby remains asymptomatic.
iii) What do you do now?
By the age of 4 hours, the baby is on 15% dextrose at a rate of 7 mL/kg/hr and the blood glucose remains low at 0.5 mmol/L.
iv) What action do you take? Give three answers.
v) What other medications may help?
vi) What is the most likely diagnosis in this baby?
ANSWER 1
i) True – non-steroidal anti-inflammatory drugs are known to cause acute renal failure in neonates secondary to vascular damage.
ii) False – Aspirin is the most commonly ingested drug in pregnancy either as a single drug or in combination with other drugs. It may cause problems with increased perinatal mortality, intrauterine growth restriction and decreased albumin binding capacity, and can also affect the clotting ability of the newborn with reports of increased incidence of intracranial haemorrhage. There are however no data reporting renal failure in neonates after use in the mother.
iii) True – losartan is an angiotensin II receptor antagonist that has many similar properties to those of ACE inhibitors. It is used for the treatment of hypertension. Unlike ACE inhibitors, it does not inhibit the breakdown of bradykinin and other kinins which cause the persistent dry cough which commonly complicates ACE inhibitor therapy.
iv) True – COX-2 inhibitors are newly developed drugs for inflammation that selectively block the COX-2 enzyme. Blocking this enzyme impedes the production of prostaglandins. During renal development, immunoreactive COX-2 is first observed in mid-gestation embryonic stages, notably in cells undergoing induction and/or morphogenesis and for the duration of nephrogenesis.
It has been shown in animal models of the postnatal kidney that COX-2 expression is relatively low at birth, increases in the first two postnatal weeks, and then gradually declines to low levels. This expression pattern of COX-2 in the developing kidney is of interest because of the evidence that COX metabolites play important functional and developmental roles in the fetal kidney. Although a full set of glomeruli is achieved by 34 weeks of pregnancy, glomerular and tubular maturation goes on up to 2 months into postnatal life. COX-2 inhibitors used in the last part of pregnancy can adversely affect the maturation of tubules and cause renal failure which can be irreversible.
v) False – although gentamicin can be nephrotoxic in the neonatal period, requiring monitoring of levels, no problems have been reported as a result of maternal gentamicin. Gentamicin is an aminoglycoside antibiotic that can cross the placenta and enter the fetal circulation. No toxicity has been seen in newborns whose mothers received gentamicin, and there have been no links with congenital defects.
vi) True – captopril, which is an angiotensin-converting enzyme inhibitor, also causes ARF. Use of ACEIs during the second and third trimesters of pregnancy has been associated with a pattern of defects known as ACEI fetopathy. The predominant feature of the fetopathy is renal tubular dysplasia. Other associated conditions include intra-uterine growth retardation (IUGR) and patent ductus arteriosus (PDA). These features may be related to fetal hypotension secondary to ACEI-induced decreases in fetal angiotensin or increased bradykinin.
ANSWER 2
i) Inadequate water intake is the most likely explanation for these results. The majority of babies become oliguric in the early postnatal period and a diuresis is unlikely in the first 24 hours. This urine output is less than 0.25 mL/kg/h, signifying oliguria. Normal value would be greater than 1 mL/kg/h. Inappropriate ADH secretion does occur but is an uncommon phenomenon and usually is found in association with other significant problems (see below).A fluid intake of 60 mL/kg/day is insufficient for a preterm baby who has high insensible losses. Nursing on a platform is almost certainly the most serious contributory factor in this baby. Insensible losses will be extremely high and impossible to quantify. Transepidermal water loss may be as high as 60 g/m 2/h in a baby born at 24 weeks gestation.
Acute renal failure may well develop in this infant if fluid balance is not corrected but is unlikely to be the primary cause at present. Sepsis is always a possibility but most preterm babies would be on prophylactic antibiotics in view of preterm delivery.
ii)
a. Nursing the baby in an incubator with high humidity is the best method of reducing insensible losses.
b. Increasing daily fluid intake to 80–100 mL/kg/day or higher, with frequent monitoring of electrolytes, urine output and, wherever possible, weight.
iii) Answer b is correct. The baby’s urine output is still low and his electrolytes suggest that he is still receiving an inadequate amount of water. Further restriction of fluid intake will almost certainly precipitate acute prerenal failure. Adding additional sodium would exacerbate the hypernatraemia that is already present. Although a fluid bolus might be beneficial in this situation, the combination with a diuretic would be likely to further exacerbate renal problems. Action must be taken at this stage and waiting for 12 hours to repeat the electrolytes will almost certainly show a further increase in the sodium levels.
iv) It is likely that this baby is starting to enter the polyuric phase that many preterm babies have after their initial oliguria. Urine output is reasonable at 1.4 mL/kg/h; urea and electrolytes are normalising. Appropriate management would be to maintain the same fluid intake but carefully monitor urine output and U+E. It is likely that sodium and potassium will continue to fall and it would therefore be appropriate to commence supplementation at 1–2 mmol/kg/day.
v) This baby was already in a degree of heart failure which will have compromised renal perfusion. Indomethacin may well have lead to a marked decrease in renal perfusion and has precipitated acute renal failure. It is however also possible that this baby has become dehydrated because urine losses during the polyuria were not being matched by replacement.
vi) It would be prudent to discontinue indomethacin as there is a strong possibility that this is responsible for the deterioration. However, if it was felt that cardiac function was being seriously compromised by the PDA, the alternatives of low dose indomethacin or ibuprofen could be used.
Urine output has decreased almost certainly due to poor renal perfusion and it would therefore be sensible to reduce fluid intake to prevent further haemodilution.
A more detailed assessment of renal function may help differentiate between the two causes:
Urinalysis is usually normal in prerenal failure and although it may be normal in established acute renal failure, cellular debris and granular casts may be seen.
Urine volume is always decreased in prerenal failure and is likely to change from decreased to normal or even raised as acute renal failure becomes established.
Urine sodium is normally low (<20 mmol/L) in prerenal failure and may increase above this in established renal failure.
Fractional excretion of sodium is less than 2% in prerenal failure and greater than 2–3% in acute renal failure.
Urine osmolality is elevated to above 350 mOsmol/L in prerenal failure and is reduced due to water retention in established renal failure.
These observations reflect the fact that the renal tubules are still functioning in prerenal failure and are able to retain some salt and excrete some water. In acute renal failure these functions are lost and therefore sodium losses will be higher and haemoconcentration will be poor. In a very premature baby, these results may be more difficult to interpret as the underlying renal function may well have been poor before renal failure intervened.
viii) Premature infants often enter a diuretic phase associated with an increase in GFR and a transient increase in the fractional excretion of sodium. The polyuric phase usually starts between 24 and 48 hours although a range of 12–100 hours has been quoted. This is then followed by an adaptive phase during which the GFR decreases and urine output parallels fluid intake. 1
The surfactant-deficient lung, with damaged epithelial and endothelial barriers, is susceptible to water accumulation. Lung gas exchange in babies with hyaline membrane disease is further compromised when total body water intake exceeds renal and insensible losses. The onset of diuresis heralds an improvement in lung function and oxygenation.
ANSWER 3
i) U+E, SBR (split), CRP, FBC and film, blood glucose and blood cultures would be the investigations of choice. The weight should also be requested to compare with birth weight.
ii) No, this does not help. In the past, this was a frequently performed practice. It is now no longer widely used and there are reports suggesting accuracy and precision are such that it cannot be relied upon. 2 This has been disputed however, and there are authorities who claim that the use of accurate scales may provide reliable information. 3
iii) Repeating the blood test urgently with a venous sample. A capillary sample may give misleadingly high values particularly if there is a degree of dehydration.
iv) This baby is suffering from hypernatraemic dehydration and is at considerable risk of developing problems. This is a serious complication of breast feeding and results from the combination of inadequate fluid intake and persistence of high milk sodium concentrations secondary to poor milk drainage from the breast. 4 A recent paper has shown an incidence of 1.9% in term or near term babies that was increased in primiparous women. 5 The most common presenting symptom was jaundice in over 80% of cases, and lethargy and fever are also common. It can be difficult in these babies to pick up dehydration clinically as hypernatraemic dehydration leads to better preservation of extracellular volume and therefore less pronounced signs of dehydration. Weight loss is a much easier way to pick up inadequate feeding and dehydration. Although a dilutional exchange transfusion may be warranted for an elevated haematocrit, particularly if the infant is symptomatic, it is appropriate to increase fluid intake first and reassess the effect on the haematocrit.
v) Rehydration should commence with normal saline. The elevated plasma sodium in the baby will have been accompanied by natriuresis and elevated urinary sodium. Although plasma sodium is high, the baby will be sodium deficient. Electrolytes should be monitored closely and the amount of sodium chloride in the resuscitation fluid adjusted accordingly. Rehydration with fluids without sodium chloride will lead to hyponatraemia that may be sudden in onset. Fluid replacement should not be performed at an abnormally high rate as this may precipitate cerebral oedema due to rapid shifts in extracellular water. A resuscitation volume of 20–30 mL/kg of normal saline over 60 minutes should be used. Subsequent rehydration should be with either normal saline or a saline/dextrose mixture so as to reduce the serum sodium at a rate that should not exceed 12 mmol/L/day. 6
vi) Seizures, intracranial haemorrhage, vascular thrombosis, death.
ANSWER 4
i) Answer b is correct.
ii) It is unlikely that the baby will take any more milk as he has just taken 70 mL. Therefore the best treatment would be to give a bolus of intravenous dextrose. Commence intravenous 10% dextrose at a rate of at least 3 mL/kg/hr.
iii) Give glucagon 100 μg/kg intramuscularly.
iv) Establish intravenous access and start a dextrose infusion. The glucagon will result in utilisation of whatever glycogen is available and once this is used, profound hypoglycaemia will occur. Glycogen stores are extremely limited.
v) Samples should have been taken for the investigation of severe hypoglycaemia. Blood should be sent to confirm the hypoglycaemia, pH and lactate should also be measured and samples should be stored for intermediary metabolites, ketone bodies and fatty acids, insulin, c-peptide, glucagon, catecholamine, corticosteroids and growth hormone. The next urine sample should be stored for amino and organic acids profiles. Although these investigations may not be required at present, samples should be stored for later analysis when indicated. Different laboratories may have different protocols for the samples to be taken.
vii) Yes. Normal glucose infusion rates providing 4–6 mg/kg/min will usually maintain normoglycaemia. In this infant the infusion is giving 10.4 mg/kg/min (120 mL/kg of 12.5% dextrose=15 g glucose/24 hours/kg=15,000 mg/day=10.4 mg/kg/min).
viii) This infant has a weight of 1.8 kg which is on the 2nd centile at 26 weeks. Infants who are significantly growth retarded may have transient hyperinsulinism in combination with reduced glycogen stores, and may develop severe hypoglycaemia that persists for several days. It is normally manageable by glucose infusion and additional treatment is rarely needed.
ANSWER 5
i) Answer c is the best answer – SIADH. This infant has significant hyponatraemia. This, in combination with the other results, also supports the possibility of haemodilution. Iatrogenic fluid overload is particularly unlikely in this situation where there has been fluid restriction as part of the management. If anything, one would expect an elevated sodium in this situation. Diabetes insipidus would lead to hypernatraemia and although this has been reported in infants, it is exceptionally rare. Failure to adequately replace electrolytes may lead to significant hyponatraemia but again is unlikely in this particular situation in view of the postnatal age and fluid restriction. Acute renal failure would lead to hypernatraemia, with an elevation in both urea and creatinine.
ii) The most relevant part of the history is the clinical evidence of a significant brain injury. The principle associations of SIADH are pneumothorax, positive pressure ventilation, acute brain injury and central nervous system infection. It has also been reported following maternal substance misuse. 7
iii) Answers b, d and h are correct – blood pressure, CRT and temperature differential. SIADH is characterised by hyponatraemia in the presence of normovolaemia, normal blood pressure, and normal renal and cardiac function. Blood pressure would be a useful observation but although a normal BP is suggestive of a good circulating volume, the range of normal BP is wide and correlates poorly with circulating blood volume. It is essential that you establish some other measure of circulating volume in combination with BP measurements such as core–toe differential, capillary refill time and Doppler echocardiography. ECG will offer little additional help and urine output will be of limited assistance as both oliguria and polyuria could be due to a variety of different complications. Skin turgor is notably unreliable as a clinical sign in neonates.
iv) The two most helpful investigations would be b (urinary) and h (plasma) osmolality. SIADH results from an elevation of anti-diuretic hormone that is inappropriate for osmolality, extracellular fluid volume and blood volume (the factors that normally regulate ADH secretion). SIADH is manifested by hyponatraemia and corresponding hypo-osmolality and a concentrated urine. The urinary osmolality is generally greater than the plasma osmolality in SIADH. Plasma osmolality less than 280 mOsm/kg is abnormally low and the urine osmolality will be inappropriately high for this value. Urine osmolality will usually be greater than 100 mOsm/L but may be difficult to interpret in extremely premature babies.
ANSWER 6
i) Answer a is correct. This baby has early hypocalcaemia. The definition of hypocalcaemia varies widely, because of the lack of clinical signs in many babies and the lack of consensus of a lower limit of normal (values of 1.75–2.0 mmol/L have been quoted). It is therefore better to use a level of ionised calcium, as this is the metabolically active form of calcium and changes in the ionised component are more likely to have a physiological effect. Hypocalcaemia is defined as an ionised calcium of less than 1.22–1.4 mM. 8
Early hypocalcaemia is defined as a low calcium level in the first 4 days of life. Exaggeration of the normal physiological fall of serum calcium within the first 3 days is a common occurrence. At birth, maternal calcium supply ends, and the infant’s level is maintained by a flux of increased calcium from trabecular bone or increased gut absorption from good oral intake. In term infants, there is a physiological decline in serum calcium after birth usually reaching a nadir within 24–48 hours. It then rises again over the following few days. Approximately 3% of healthy term infants have a total calcium below 2 mmol/L at 24 hours of age. 8
ii) Pseudohypoparathyroidism is a rare cause of hypocalcaemia in infancy due to peripheral lack of response to the action of PTH. It has not been documented in infancy but minimal adult data describes it to cover both ectopic hyperparathyroidism and the more commonly encountered non-parathyroid humoral hypercalcaemia of malignancy, metastatic breast cancer being the classic example.
Hyperparathyroidism is associated with hypercalcaemia, not hypocalcaemia.
Hypocalcaemia is well described in infants of diabetic mothers, and is a combination of an exaggerated normal postnatal fall, a degree of PTH resistance and increased calcium demands due to the high metabolic rate in these infants. The hypocalcaemia appears to be related to hypomagnesaemia which is secondary to maternal urinary loss of magnesium. The extent of hypocalcaemia in these infants is influenced by the severity of maternal disease.
Maternal vitamin D deficiency may lead to vitamin D deficiency and hypocal-caemia in the newborn infant. This is commoner in certain ethnic groups and reflects maternal diet and exposure to sunlight.
Exchange transfusion has been associated with hypocalcaemia and appears to be due to the use of calcium-chelating anticoagulants. Newer anticoagulants do not have this effect and this complication is now very unlikely.
Diuretic therapy may induce hypercalciuria and thus hypocalcaemia, but is unlikely in this situation as the infant is well.
Hypoalbuminaemia may be associated with a low total calcium, as in this case, but ionised calcium should be within the normal range.
Maternal hyperparathyroidism may be associated with significant neonatal hypocalcaemia, but hypoparathyroidism leads to neonatal hyperparathyroidism and a normally self-limiting hypercalcaemia.
Low calcium intake may be associated with hypocalcaemia but would normally reflect a prolonged period of poor dietary intake rather than a coincidental finding soon after birth.
iii) Although hypocalcaemia is self-limiting, infants with symptoms or an abnormal ECG with prolonged QTc should be treated with oral or intravenous calcium. The baby in this question is asymptomatic and well, and therefore no treatment is indicated, but calcium levels should be monitored to ensure that they return to normality.
ANSWER 7
Nephrocalcinosis is a common finding in preterm infants with an incidence of 16–64% in infants with a birth weight below 1500 g. There are many sources, including text books, which suggest a strong association with diuretic therapy, but this association does not appear so clear cut when detailed analysis of risk factors has been performed. Studies suggest that it is only seen in those with severe respiratory disease who have been ventilated and progress to develop chronic lung disease (CLD). Other factors which appear associated are gestational age, male sex, duration and frequency of gentamicin therapy, gentamicin and vancomycin toxicity and postnatal dexamethasone. Interestingly, in one study, diuretic therapy did not appear relevant and duration of oxygen was found to be the strongest clinical indicator of renal calcification. It is possible that the much quoted association between nephrocalcinosis and diuretics is only a reflection of the presence of CLD. 9
Preterm infants with lung disease are reported to have decreased urinary citrate, which may predispose them to nephrocalcinosis because citrate is a known inhibitor of renal calcification in adults and children. Follow-up studies have shown that progressive resolution of nephrocalcinosis occurs in the majority of cases (with figures quoted between 85% by 30 months and 75% at a median of 6.75 years). In one paper, in the 25% of patients in whom nephrocalcinosis persisted, there was no evidence to suggest an association with renal dysfunction or long-term symptoms or persisting abnormalities in calcium metabolism or excretion. 10 Renal function does not appear to be adversely affected into childhood. There is no reliable information on the consequence of neonatal nephrocalcinosis persisting into adult life. Although the natural history of nephrocalcinosis is that it resolves, some infants will develop renal calculi although it is not proven that this is a direct result of the nephrocalcinosis.
ANSWER 8
i) The ECG shows tall peaked T waves, and widening of the QRS complexes.
ii) The cause is hyperkalaemia due to acute renal failure secondary to perinatal asphyxia.
iii) The treatment options are:
a. Maintenance of normocalcaemia using 10% calcium gluconate, 0.1–0.2 mL/kg. Hypocalcaemia and hypomagnesaemia potentiate the toxic effect of hyperkalaemia on the heart.
c. Intravenous glucose and insulin infusion (5 mL/kg of a solution of 12 units insulin in 100 mL 25% glucose given over 30 minutes). This also promotes influx of potassium into cells and has an additive effect with salbutamol.
d. Intravenous sodium bicarbonate 1 mmoL/kg. This also may result in a shift of potassium from extracellular to intracellular compartments.
e. Oral/rectal calcium-chelating agents such as resonium will help to remove potassium from the body. They are, however, associated with bowel obstruction and perforation, and should therefore only be used where there are no concerns about gut integrity.
f. Dialysis. Potassium can be effectively removed by dialysis and should be considered early if aggressive management is thought to be appropriate.
ANSWER 9
i) With the amount of information available at this moment it is difficult to give specific diagnoses. However, given the age and history the following possibilities should be considered:
a. Sepsis – this must always be top of the list
b. Congenital heart disease – not uncommon to present in this way at this age
c. Inborn error of metabolism – rare but very important to detect
d. Hypoglycaemia
e. Intra-abdominal pathology – volvulus for example
f. Electrolyte disturbance
g. Endocrine problem – congenital adrenal hyperplasia for example.
ii) As all the ‘routine’ bloods have been unremarkable it is appropriate to start screening for some of the less likely but serious possibilities. Further investigation should include an echocardiogram to exclude congenital heart disease, electrolytes to exclude acute disturbances and a capillary or arterial blood gas. Lactate is essential and should be requested separately if it is not a part of the routine blood gas analysis. Plasma ammonia should be measured urgently as hyperammonaemia is suggestive of a narrow range of serious conditions that will require urgent intervention.
iii) Common things are common. In a baby who is struggling to breathe and showing marked recession before, during and after the deterioration, a pneumothorax must be top of the list. Babies with congenital heart disease may show sudden deterioration but rarely as acutely as this. Although metabolic conditions may lead to babies who are very unwell it would be rare to deteriorate as rapidly as this. Babies with severe hypoglycaemia can deteriorate very quickly, but in this case a blood glucose within the normal range has been recorded already. Fits may lead to a sudden alteration in conscious state and are not always accompanied by obvious seizure activity but the respiratory signs in this case are not usually associated with fits.
iv) Tension pneumothorax with midline shift to the left. Insertion of a chest drain is essential. The pneumothorax must be drained before assessing the need for ventilation. Commencing positive pressure ventilation pre-drainage of an air leak may worsen the tension effect due to alterations in the intra-thoracic pressure, while at the same time detracting from the urgency of chest drain insertion.
v) Answer e is the most likely. Normal ammonia concentrations in neonates should be less than 65 μmol/L, but research has frequently shown concentrations of up to 180 μmol/L in sick newborns who do not have a primary metabolic disturbance. Higher ammonia concentrations warrant thorough investigation for metabolic causes. A level greater than 150 μmol/L (or persistently greater than 100 μmol/L) is an inborn error of metabolism until proven otherwise. If hyperammonaemia is not recognised and treated, the illness progresses rapidly to coma, seizures and death.
The onset of hyperammonaemia 24 hours after birth is characteristic of the primary urea cycle defects and several of the organic acidaemias (propionic, methylmalonic and isovaleric acidaemia) defects. In either case respiratory alkalosis may be the initial acid–base disturbance. In the absence of a severe acidosis, ketosis or hypoglycaemia, a provisional diagnosis of a urea cycle defect should be made.
Urea cycle defects are generally associated with a respiratory alkalosis in response to the increased respiratory rate because of the hyperammonaemia. They can be associated with a mild acidosis, but the acidosis is rarely the main presenting feature. Acidosis may subsequently develop as decompensation occurs.
vi) Management. 11. and 12.
a. Resuscitate as for any collapsed infant. Management of respiratory problems as appropriate and establish vascular access. All feeds should be discontinued.
b. Discuss with regional metabolic expert immediately. These conditions have very poor outlook with or without treatment and involvement of a specialist from the outset is essential.
c. Discuss with local paediatric intensive care facility. Haemofiltration or haemodialysis may be required and it is important that the availability of such treatment is established early on. Haemodialysis is reported to be the most efficient treatment but peritoneal dialysis and haemofiltration are options. Exchange transfusion can be used but is much less efficient and should only be regarded as a temporary treatment when access to dialysis is not immediately available.
d. Intravenous dextrose should be administered to restore hydration as the majority of these infants are dehydrated as a consequence of vomiting and poor intake. Maintenance electrolytes should be added and adjusted according to frequent biochemical monitoring. Tissue perfusion increases with adequate hydration and catabolism is reduced.
e. Arginine. The use of other elements in management (see below) reduces available nitrogen and there is thus a decrease in arginine synthesis. Arginine thus becomes an essential amino acid in all urea cycle defects except arginase deficiency.
f. Sodium phenylbutyrate and/or sodium benzoate at a dose of 250–500 mg/kg/day. These compounds help excrete nitrogen waste through alternative pathways. If benzoate is used amino acid nitrogen is excreted as hippuric acid. If phenylbutyrate is used phenyl glutamine is excreted.
g. Carnitine. This enhances fatty acid oxidation by transport into the mitochondria. This is most appropriate for organic acid disorders and is normally given until the diagnosis is established.
vii) Parental discussion. The prognosis for babies with urea cycle disorders should be guarded. Without treatment they will die. Even with treatment many will die and the long-term outcome for survivors is largely unknown. Growth and development are likely to be affected and there may be recurrent life-threatening metabolic crises. Liver transplant is an option but there is little information on long-term quality of life. Gene therapy may be the ultimate treatment but is not a possibility at present.
ANSWER 10
Answers a and b are correct. A normal anion gap is 12–16 mmol/L and is calculated by the following formula (all units mmol/L):
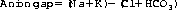
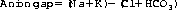
In reality, the total cations and total anions in a solution balance exactly. The gap is therefore due to unmeasured anions, mainly albumin, phosphate and small amounts of organic anions including lactate. In patients with acidosis, the calculation of the anion gap can be particularly useful. If the anion gap is increased, there must be either an increase in one of the ‘normal’ unmeasured anions (e.g. lactate) or a substantial increase in the level of an organic anion, which is normally only present in tiny amounts (e.g. ketoacids, complex organic anions). This helps determine whether the metabolic acidosis is caused by an accumulation of organic acids or a decrease in bicarbonate.
A normal anion gap with acidosis is likely to be due to renal tubular acidosis or intestinal bicarbonate loss. If the anion gap is increased, an organic acidaemia is very likely.
a. Propionic acidaemia is an organic acid disorder and presents with severe metabolic acidosis, poor feeding and drowsiness. There is hyperammonaemia and hypoglycaemia. A high anion gap is present.
b. Phenylketonuria leads to accumulation of phenylalanine which does not add to the anion gap.
c. Renal tubular acidosis leads to a metabolic acidosis with a normal anion gap. This is due to a hyperchloraemic metabolic acidosis secondary to renal loss of bicarbonate.
d. Medium chain acyl-CoA dehydrogenase deficiency leads to accumulation of medium-chain fatty acids or partially degraded fatty acids in tissues and these may cause liver and brain damage. As medium-chain fatty acids from food and from fats stored in the body cannot be metabolised they are not converted into energy, leading to characteristic signs and symptoms such as lethargy and hypoglycaemia. A high anion gap is present.
e. Maple syrup disease is a defect in the metabolism of the branched chain amino acids (valine, leucine and isoleucine) due to a deficiency of one of three enzyme systems, of which the commonest is branched chain 2-ketoacid dehydrogenase complex. Babies develop severe ketoacidosis and are often hypoglycaemic. Although ketosis is prominent, metabolic acidosis is not often present until later in the course of disease. It usually presents at the end of the first week of extra-uterine life with vomiting, seizures and dystonia. It derives its name from the characteristic sweet, burnt-sugar smell of the urine.
f. Total villous atrophy does not lead to an increased anion gap. It leads to a metabolic acidosis with a normal anion gap due to gastrointestinal loss of bicarbonate.
ANSWER 11
All are correct. Infants with lactic acidosis present a difficult diagnostic problem. A high plasma lactate can be secondary to hypoxia, cardiac disease, infection or convulsions. Although the lactate may be elevated it is normally not to the same levels as seen in the more worrying congenital lactic acidoses.
Primary lactic acidosis may be caused by inborn errors of metabolism (IEM), e.g. disorders of pyruvate metabolism (leading to inability to convert lactic acid back to pyruvate to enter the Krebs cycle), gluconeogenesis disorder (leading the body to scavenge pyruvate which is converted to lactic acid with ATP production), respiratory chain defects (causing inability to produce ATP during the Krebs cycle), or a mitochondrial disorder (e.g. error in oxidative phosphorylation).
Venous obstruction by tourniquet for blood sampling, crying or breath holding may increase plasma lactate concentrations and it is therefore wise to obtain an arterial blood sample to confirm the lactic acidosis.
As a rule of thumb, a persistent increase of plasma lactate above 3 mmol/L in a non-asphyxiated infant who has no evidence of multi-organ failure, should lead to further investigations for an IEM.
As discussed earlier, other IEMs (fatty acid oxidation disorders, organic acidaemias and urea cycle defects) may be associated with a lactic acidosis, although this is usually mild and not the presenting feature.
ANSWER 12
i) Establishing central access in a macrosomic baby, as in this case, is crucial. It is notoriously difficult to get a peripheral cannula sited and an umbilical venous catheter would be easy and quick to insert. In the rare occurrence where this is not possible, it would be more appropriate to use an intra-osseous route rather than continue to strive to achieve venous access. With access secured an infusion of 10% dextrose should be started at 3 mL/kg/h.
iii) The rate of infusion should be increased according to the blood glucose measurements. If there are other factors that mean that fluid intake should be restricted, the concentration of the dextrose solution should be increased.
iv)
a. As severe hypoglycaemia is persisting, investigations must be carried out to ascertain the cause. This will include pH and lactate, and blood samples should be stored for intermediary metabolites, ketone bodies and fatty acids, insulin, c-peptide, glucagon, catecholamines, corticosteroids and growth hormone. The next urine sample should be stored for amino and organic acid profiles.
b. Specialist advice should be sought at an early stage in these cases as hyperinsulinism, which is not self-limiting and is resistant to very high glucose concentration, has a risk of precipitating heart failure, especially if there is co-existing hypertrophic cardiomyopathy.
c. Diazoxide should be given at a dose of 10–20 mg/kg/day. This suppresses pancreatic insulin release. It is usually given with chlorthiazide, which enhances the hyperglycaemic effect and may help prevent the fluid retention which is one of the side effects of diazoxide.
v) Octreotide could be given. This is an analogue of somatostatin, the hypothalamic release-inhibiting hormone. It also suppresses insulin release and can be given either by subcutaneous or intravenous injection at a dose of 10 μg/kg/day. There are some concerns about long-term tolerance and the possible effects on other hormones. To reduce this risk, glucagon is also given at the same time.
Glucagon has been used to treat hypoglycaemia as it has glycogenolytic properties. It should however only be used when a brief period of hypoglycaemia needs to be prevented, e.g. when a drip needs re-siting, as prolonged use may cause further release of insulin, thus worsening the situation.
Nifedipine has also been used to treat hyperinsulinism. In 30–40% of cases of persistent hyperinsulinaemic hypoglycaemia of infancy (PHHI) there are gene mutations leading to functional loss in K +-ATP channels leading to disregulation of calcium fluxes and unregulated insulin release. Blood pressure monitoring is crucial.
vi) This baby is likely to have persistent hyperinsulinaemic hypoglycaemia of infancy (previously known as nesidioblastosis). PHHI leads to recurrent and persisting hypoglycaemia which can be difficult to treat. It occurs in macrosomic babies with extremely high insulin levels and high glucose requirements. The risk of neurological damage is high and thus treatment must be initiated immediately. There are several different histological forms, focal and diffuse, and several different underlying pathologies. Early referral and treatment in a specialist centre is essential.
Self-limiting hyperinsulinism may occur in infants born to mothers with poorly controlled diabetes, or where there has been antenatal administration of thiazide diuretics. It is also encountered in Beckwith–Wiedemann syndrome where it is a common feature – in this case there are normally other abnormalities detected. In these cases the hypoglycaemia is usually less severe and easier to manage. 13
REFERENCES
1. Bidiwala, KS; Lorenz, JM; Kleinman, LI, Renal function correlates of postnatal diuresis in preterm infants, Pediatrics 82 (1988) 50–58.
2. Savenije, OEM; Brand, PLP, Accuracy and precision of test weighing to assess milk intake in newborn infants, Arch Dis Child Fetal Neonatal Ed 91 (2006) F330–F332.
3. Meier, PP; Engstrom, JL, Test weighing for term and premature infants is an accurate procedure, Arch Dis Child Fetal Neonatal Ed 92 (2007) F155–F156.
4. Morton, JA, The clinical usefulness of breast milk sodium in the assessment of lactogenesis, Pediatrics 93 (5) ( 1994) 802–806.
5. Moritz, ML; Manole, MD; Bogen, DL; Ayus, JC, Breastfeeding-associated hypernatremia: are we missing the diagnosis?Pediatrics 116 (3) ( 2005) e343–e347.
6. Laing, IA; Wong, CM, Hypernatraemia in the first few days: is the incidence rising?Arch Dis Child Fetal Neonatal Ed 87 (2002) F158–F162.
7. Winrow, AP; Kovar, IZ; Jani, BR; Gatzoulis, M, Early hyponatraemia and neonatal drug withdrawal, Acta Paediatrica 81 (1992) 847–848.
8. Hsu, SC; Levine, MA, Perinatal calcium metabolism: physiology and pathophysiology, Semin Neonatol 9 (1) ( 2004) 23–36.
9. Narendra, A; White, MP; Rolton, RA; et al., Nephrocalcinosis in preterm babies, Arch Dis Child Fetal Neonatal Ed 85 (2001) F207–F213.
10. Porter, E; McKie, A; Beattie, TJ; et al., Neonatal nephrocalcinosis: long term follow up, Arch Dis Child Fetal Neonatal Ed 91 (2006) F333–F336.
11. Chakrapani, A; Cleary, MA; Wraith, JE, Detection of inborn errors of metabolism in the newborn, Arch Dis Child Fetal Neonatal Ed 84 (2001) F205–F210.
12. Chakrapani, A; Wraith, JE, Principles of management of the more common metabolic disorders, Curr Paediatr 12 (2002) 117–124.
13. Rahier, J; Guiot, Y; Sempoux, C, Persistent hyperinsulinaemic hypoglycaemia of infancy: a heterogeneous syndrome unrelated to nesidioblastosis, Arch Dis Child Fetal Neonatal Ed 82 (2000) F108–F112.