CHAPTER 288 Flat Back and Sagittal Plane Deformity
History, Etiology, Incidence, and Rate of Progression
Sagittal plane deformity as described by Doherty1 in 1973 is a fixed forward inclination of the trunk because of loss of normal lumbar lordosis after posterior spinal fusion for scoliosis. The term flat back syndrome is also known as kyphotic decompensation syndrome and flat buttock syndrome.2,3 There are other causes of sagittal plane deformity as defined by Errico and colleagues, such as ankylosing spondylitis, diffuse idiopathic skeletal hyperostosis, Scheuermann’s disease, congenital kyphosis, postlaminectomy kyphosis, and kyphosis secondary to radiation therapy and trauma.4
Iatrogenic flat back syndrome is classified into two types. Type 1 is segmental (previous fusion levels) hypolordosis or kyphosis of the lumbar spine with the body of the C7 vertebral body remaining centered over the lumbosacral disk (i.e., a compensated deformity). A defining characteristic of type 1 on standing lateral radiographs is that anterior disk height is 5 mm greater than posterior disk height because of compensatory hyperextension to maintain sagittal balance. A noteworthy goal on postoperative radiographic assessment is to have the anterior disk height be reduced to less than 2 mm greater than posterior height on standing radiographs.5 Type 2 flat back syndrome is present when the plumb line from C7 falls more than 5 cm anterior to the lumbosacral disk.6
Clinical Findings
Lee and colleague7 and Hasday and associates8 discussed the significance of hip contractures in these patients leading to an abnormal pelvic tilt. This abnormal tilt increases the chance for a suboptimal postoperative result despite correction of the lordosis and should therefore be assessed preoperatively.
Positive sagittal balance is the most reliable predictor of clinical symptoms in patients with spinal deformity.9,10 Sagittal imbalance greater than 4 cm results in deterioration of pain and function scores over time in most unoperated patients. Restoration of normal sagittal balance should therefore be one of the main goals of any deformity reconstruction procedure.10
These patients also exhibit decreased step, stride length, and gait velocity. Sarwahi and coworkers prospectively analyzed the gait function of 21 patients with postsurgical flat back deformity.11 The authors concluded that the patients’ gait was slower than that of normal controls and that several compensatory mechanisms were used by the patients. The compensatory mechanisms adversely affected the hip and knee joints.11
Prevention of Flat Back and Sagittal Plane Deformity
A common cause of flat back syndrome is previous spinal fusion. Potter and associates mentioned four essential methods for prevention of this iatrogenic condition12: (1) thorough preoperative assessment of sagittal alignment, (2) limitation of the caudad extent of fusion when possible, (3) use of segmental instrumentation and avoidance of distraction with preservation or improvement of physiologic lumbar lordosis and sagittal balance, and (4) intraoperative positioning of the hips in an extended fashion.
Preoperatively, the surgeon must thoroughly evaluate the patient’s deformity and its overall impact. Multiple clinic visits are recommended to fully evaluate gait, pain levels, the severity of the deformity, and radiographic evidence. Maintaining the current normal curves of the patient while addressing correction of the deformity should be a leading consideration in formulating the surgical plan. For degenerative short-segment fusion in the lumbar spine, increasing lordosis in anticipation of loss of lordosis over time as a result of ongoing degenerative changes is a preferred strategy.13,14
Limitation of caudal fusion levels to L3 or lower to avoid decompensation or progression of the curve during scoliosis surgery and thus prevent flat back syndrome should be evaluated on a case-by-case basis. Although several studies have indicated benefit,15–17 it must be noted that these studies were undertaken with nonsegmental and currently rarely used Harrington rod instrumentation.
Most early cases of flat back syndrome secondary to loss of lumbar lordosis arose from the use of distraction via Harrington instrumentation in the lumbar spine.1,15–17 The evolution of posterior instrumentation to segmental pedicle screws from Harrington instrumentation and Luque segmental wiring has produced greater correction of curves with better construct rigidity and has thus decreased the incidence of sagittal plane deformity.12
Intraoperative positioning is vital in preventing sagittal plane deformity during long-segment spinal fusion. In a study of 13 anesthetized patients, Benfanti and Geissele demonstrated that 95% of lordosis was maintained on a Wilson frame when the patients were positioned with the hips in full extension.18 With the use of a Jackson table (our preferred choice for instrumented fusion), Stephens and colleagues proved that positioning patients in hip extension resulted in a minor increase in lumbar lordosis.19 It is therefore prudent to position patients for prone posterior lumbar fusion with the hips in extension to preserve physiologic lumbar lordosis.
Radiographic Findings
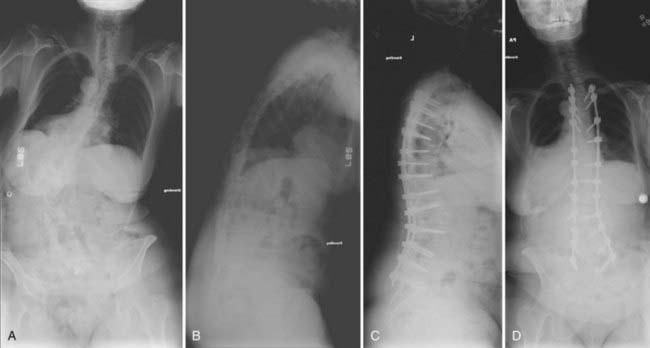
Global assessment of spinal balance is required in all patients with deformity, particularly if any surgical intervention is anticipated. This radiographic work-up begins with upright, full-length 36- by 14-inch posteroanterior and lateral scoliosis films to determine coronal and sagittal balance. This may be complemented with additional studies, including supine, flexion, extension, and side-bending radiographs, which can be used to better evaluate dynamic pathologies such as degenerative spondylolisthesis. The “clavicle position” should optimized to visualize the entire spine on the lateral scoliosis radiograph. In this position, the patient fully flexes the elbows with the hands in a relaxed fist, wrists flexed, and the proximal interphalangeal joints placed comfortably up into the supraclavicular fossa while passively flexing the humerus forward. This maneuver produces an overall visualization of critical vertebral landmarks that is significantly better than that achieved with positions in which the arm is either positioned straight out or partially flexed. Ideally, on the lateral radiograph one should be able to visualize C2 to the pelvis, including the femoral heads, to assess the global sagittal balance of the spinal column (Fig. 288-1A to D). Similarly, on the posteroanterior view, the margins of the rib cage and the pelvis along with the femoral heads can be clearly visualized. Assessment of the hips helps determine whether a leg length discrepancy, hip arthritis, or pelvic pathology is present. Visualization of the ribs helps in diagnosing the presence of any associated thoracic cage deformity. Either congenital fusion of the ribs or a significant chest wall deformity can be associated with rigid or fused spinal segments. After assessing spinal balance in the sagittal and coronal planes, Cobb’s measurements are obtained for each area of the spine, including the cervical, proximal thoracic, main thoracic, thoracolumbar, and lumbar areas. Vertebral body rotation at the apex of the coronal plane is a factor in determining the rigidity of the curve. The greater the vertebral body rotation, the greater the rigidity of the coronal curve. If the curve and clinical characteristics warrant serious consideration of surgical intervention, the flexibility of the curve can be further assessed with dynamic side-bending radiographs.
Nonoperative Treatment
Farcy and Schwab reported poor outcomes in symptomatic patients initially managed with intensive conservative therapy.2,3 Only 27% were considered to have long-term success (13 of 48 patients). Overall, conservative management has been disappointing in the treatment of flat back and sagittal plane deformity.
Operative Treatment of Sagittal Plane Deformity
Surgical Options
Pedicle Subtraction Osteotomy
Pedicle resection plus transpedicular wedge resection of the vertebral body to restore sagittal balance was first reported in patients with ankylosing spondylolysis in 1985.20 Since Thomasen’s initial description, the procedure has been used for the management of flat back deformity and lumbar kyphosis secondary to other causes. PSO directly involves performing two Smith-Petersen–type (extension) osteotomies, as well as resection of the intervening pedicles and a portion of the vertebral body from a posterior approach. It accomplishes approximately as much correction as three SPO procedures. This aggressive osteotomy results in removal of the posterior elements, including the pedicle and transverse process. With this technique, removal of up to 6 cm of bone is possible with resultant sagittal plane correction of up to 60 degrees. By performing asymmetrical removal of the posterior elements, correction of both sagittal and coronal plane deformities can be achieved. PSO is a technically demanding procedure, and substantial blood loss can occur from the epidural venous plexus or from cancellous bone. With removal of the pedicle bilaterally, two nerve roots exit through the reconstructed neural foramina at the level of the osteotomy and are at risk for injury. During closure of the osteotomy, care must be taken so that impingement of the thecal sac or nerve roots does not occur.
Smith-Petersen (Extension) Osteotomy
Smith-Petersen and associates were the first to describe a posterior osteotomy for correction of fixed sagittal deformity in patients with rheumatoid arthritis.21 The osteotomy involves removing the posterior elements, undercutting the adjacent spinous processes, and then closing the osteotomy to create an opening in the spine (extension) anteriorly through the disk space. The posterior aspect of the disk space is the axis of rotation. Sagittal correction is then achieved by posterior compression with instrumentation, which results in anterior osteoclasis through the vertebral body or distraction through rupture of the anterior longitudinal ligament and disk space. The extension osteotomy creates hyperextension by closing the posterior elements and opening the anterior elements. SPO achieves correction by creating a sharply lordotic angle and elongation of the anterior column, which may result in significant complications. The risk for morbidity and even mortality results from distraction injury to the anterior vascular structures or neural elements or the development of superior mesenteric artery syndrome. This technique may result in decompensation of the spine if the instrumentation fails before fusion. As a general rule, approximately 1 degree of correction can be expected for each millimeter of posterior bone resected during the procedure. Lagrone and coauthors reported an average initial correction of 22 degrees in lumbar lordosis and 9 degrees in kyphosis at the thoracolumbar junction, and an 8.1-cm improvement in the sagittal vertical axis, when using this technique. A number of complications were reported, including pseudarthrosis, implant failure, inadequate initial correction, loss of correction, and a need for further surgical intervention.17
Polysegmental Osteotomies
In 1949, Wilson and Turkell reported on the use of multiple osteotomies to correct sagittal balance in a patient with ankylosing spondylitis.22 Sagittal balance was achieved through polysegmental posterior lumbar osteotomies. This technique involves removing the facet joints at several levels and then compressing the posterior elements to create lordosis. The correction is achieved through deformation of the disk spaces without rupture of the anterior longitudinal ligament with the use of transpedicular instrumentation. Polysegmental osteotomies achieve less correction per level but permit more gradual correction of sagittal plane deformity than possible with either PSO or SPO, both of which cause an abrupt angular correction. Currently, this technique may be used for the correction of milder forms of sagittal imbalance or in conjunction with PSO for additional correction in patients with severe sagittal imbalance. The amount of correction achieved with polysegmental osteotomies is less than that achieved with the other methods described. One series found an average correction of 9.5 degrees per level with this technique.
Indications and Contraindications
PSO is indicated when significant correction is needed because of prominent sagittal imbalance. Patients who require approximately 30 degrees of additional lumbar lordosis will need to undergo PSO to restore sagittal balance. Bridwell and colleagues noted an average increase of 34 degrees and an average improvement in the sagittal plumb line of 13.5 cm after PSO in patients with fixed sagittal plane deformity.23,24 Numerous other studies have reported angular correction in the range of 32 to 38.8 degrees. Clinical conditions for which PSO is an effective procedure include ankylosing spondylitis, progressive adult lumbar idiopathic scoliosis, degenerative scoliosis, infectious deformity, and progressive kyphosis after fracture or iatrogenic flat back syndrome. It may also be beneficial in the group of patients who have already undergone anterior surgery, which increases the risk for vascular injury with a repeat anterior procedure or classic SPO.
Preoperative Planning
Bernhardt and Bridwell and coworkers have shown that the average thoracic kyphosis is 30 degrees, or roughly 2.5 degrees per segment,5,23 whereas the average lumbar lordosis is 60 degrees. Sagittal alignment dictates that there should be 30 degrees more lumbar lordosis than thoracic kyphosis.5 The angle of osteotomy is the total angular correction achieved to restore sagittal balance. The angle at which the spine is redirected at the osteotomy site (the osteotomy angle) depends on the level of the osteotomy. When the osteotomy is performed at a higher level, the angle of osteotomy must be greater than that of an osteotomy performed at the lower levels of the spine to achieve the same degree of correction to restore sagittal balance.25 Preoperative planning can determine with relative accuracy the extent of osteotomy required at the vertebral body level to achieve the predicted angular correction. The inverse tangent of the angle of correction at the vertebral body level will determine the amount of bone removal needed at the initial pedicle–vertebral body interface. This measurement is the perpendicular distance, and it diminishes as the osteotomy is developed in a wedge fashion along the vertebral body toward the anterior cortical surface.
The selected lumbar PSO level should generally be below the level of the conus medullaris to minimize the risk for injury to the spinal cord. It also facilitates retraction of the thecal sac to perform the osteotomy safely. The level chosen is approximately at L2, L3, and occasionally L4. It is preferable to perform pedicle subtraction through areas of previous fusion to reduce the risk for pseudarthrosis.2 The apex of the lumbar lordosis in a sagittally balanced spine should be at the level of the L3-4 disk space. Thus, the maximum physiologic lordotic curvature that can be achieved will be in this area. Asymmetrical osteotomy can also be performed to address any coronal plane deformity.
PSO does not always obviate the need for an anterior procedure. An anterior procedure may involve anterior release with interbody bone grafting to reduce the risk for pseudarthrosis, particularly when the instrumentation extends past the lumbosacral junction. Anterior and posterior surgery can be performed in stages, depending on surgeon preference, the extent of surgery needed, and the overall clinical status of the patient. Anterior release should be considered in patients who have a very severe deformity and when PSO alone may not restore spinal balance. This may include patients with greater than 10 cm of sagittal imbalance.24 Another indication for anterior release would be multiple levels of narrow anterior ossified disk spaces or previous anterior spinal instrumentation.
PSO may restore sagittal balance. However, if the fusion is extended across the lumbosacral junction, an anterior procedure may be warranted because of the difficulty in achieving solid arthrodesis in this area. Anterior structural bone grafting can achieve arthrodesis when a long construct is extended to the sacrum.26,27 Reconstruction of the anterior column may also be indicated in patients with poor biology, when adequate stable fixation points are not available posteriorly, and if the plumb line still fails to lie posterior to the area of the osteotomy. Bridwell and coworkers also recommended anterior surgery if more spinal segments are added proximal to the previous fusion.
Surgical approaches can be planned as a staged or same-day surgery. Proponents of the staged procedure attribute its safety to reduction of hemodynamic stress, fluid shifts, surgeon fatigue, pressure sores, and neurological risk. In contrast, others argue that same-day anterior and posterior procedures are associated with less overall blood loss, shorter hospitalization, fewer pulmonary complications, and early mobilization. Rhee and colleagues reviewed their results in 42 consecutive adult patients with deformity who underwent staged posterior surgery.9 In adult patients with severe deformity, one complex prolonged posterior surgery was divided into two smaller procedures. It was performed successfully in high-risk patients with low blood loss and no major medical complications.
If PSO is performed in conjunction with a long construct and the lumbosacral junction is included in the construct, consideration should be given to protection of the sacral screws with additional iliac screw fixation. Iliac screw fixation with tricortical sacral screw placement minimizes distal construct failure and pseudoarthrosis.6
Close attention should be paid to positioning of the patient during spinal deformity surgery because the patient will be prone for an extended period. A Jackson table is suitable because it slightly increases hip extension and thus accentuates the lumbar lordosis.19 The abdomen should be loose and freely hanging, which diminishes venous pressure and helps minimize excess blood loss. Intraoperative use of cell savers also helps preserve the patient’s blood and may decrease the need for transfusion. The patient should also be placed in a slight reverse Trendelenburg position because it may theoretically reduce the risk for intraoperative blindness secondary to ischemia during a prolonged procedure.
Surgical Technique
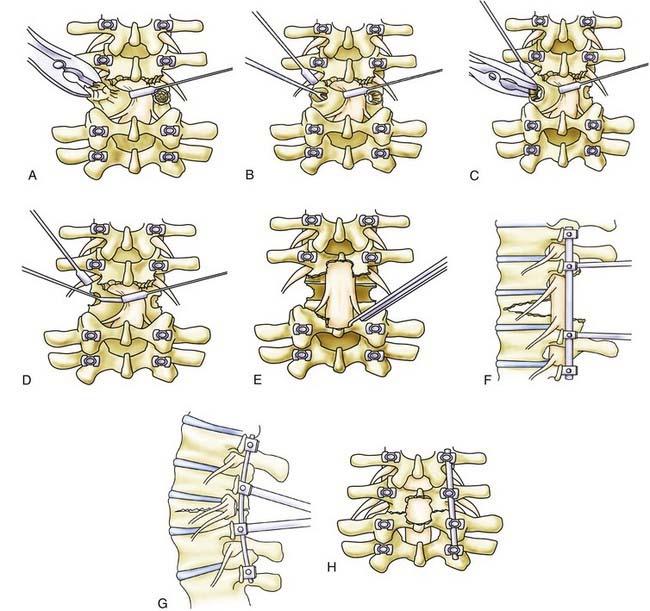
We always perform a generous laminectomy at the level of the osteotomy and even at times perform a partial or total facetectomy above and below the level of the osteotomy (Fig. 288-2A). In some cases, partial laminectomies are also performed at the levels above and below the planned osteotomy. The bone surrounding each pedicle is then removed completely to expose the nerve roots running inferior and superior to the pedicle to be removed for the PSO (Fig. 288-2B). In essence, circumferential bone removal involves excising the superior facet, inferior facet, and pars interarticularis and disconnecting the transverse process at the level of the PSO. Thus, an SPO is performed cephalad and caudal to the pedicle, and bone removal is performed from the level of the pars above and below.
Before initiation of the decancellation procedure, the medial pedicle wall is delineated and the thecal sac is protected with a nerve root retractor. Gentle traction may be applied with the retractor on the thecal sac if the level of the osteotomy is below the conus. The nerve root above the pedicle is also protected with a retractor or a No. 4 Penfield instrument (Fig. 288-1C).
After circumscribing the pedicle, decancellation is performed through the residual pedicle stump (Fig. 288-2D). The residual pedicle stump is removed with a Leksell rongeur, and both sides are now made flush with the level of the vertebral body (Fig. 288-2E). The extent of decancellation depends on the amount of bone to be removed as determined during preoperative planning. Straight and curved curets are then used to perform decancellation through the pedicles and extended in wedge fashion into the vertebral body with the apex at the anterior cortex. The decancellation is performed until one side connects to the other. The extent of vertebral body removal should include the inferior dimension of the pedicle without violating the anterior cortical wall. Use of the curet should be controlled and meticulous with close attention paid to the surrounding neural structures. Bleeding during the decancellation procedure is managed with the intermittent use of hemostatic agents and tamponade with cottonoids. The cancellous bone behind the posterior vertebral cortical wall is removed thoroughly to make the wall as thin as possible. This cancellous bone should be saved for the future posterolateral fusion portion of the procedure.
Before closure of the osteotomy, a Kerrison rongeur is used to further enlarge the central canal and remove any bone fragments that may interfere with the exiting nerve roots. The opposing bone surfaces are also made symmetrical with a Leksell or Kerrison rongeur. On one side, an appropriately sized rod is contoured and fixed to the screws loosely with caps. The compressor is placed along the head of the pedicle screws on each side and gently compressed to close the osteotomy defect. While maintaining compressive force, the caps are tightened and the rod is secured to the screw. Multiple sequential compression steps may be required for complete bone apposition (Fig. 288-2F). If the osteotomy is not completely closed, one should check for intervening residual bone fragments, inadequate rod contouring, or subluxation of the proximal elements. Bone fragments may be removed with a curet or Kerrison rongeur. In situ benders may be used to recontour the rod and compressive forces then reapplied. The proximal spinal elements may sublux dorsally with respect to the distal elements. Such subluxation needs to be reduced to achieve anatomic alignment before final tightening of the implant (Fig. 288-2G). Finally, a Woodson elevator is used to facilitate inspection of the thecal sac and the nerve roots to make certain that there is no bone compression or any compromise of the neural structures. Midline buckling of the thecal sac is expected after closure of the osteotomy. The procedure is completed by posterolateral decortication with a drill and placement of the bone graft harvested from the osteotomy procedure. A subfascial drain is placed and the wound is closed in standard multilayer fashion.
Conclusion
Aaro S, Ohlen G. The effect of Harrington instrumentation on the sagittal configuration and mobility of the spine in scoliosis. Spine. 1983;8:570-575.
Benfanti PL, Geissele AE. The effect of intraoperative hip position on maintenance of lumbar lordosis: a radiographic study of anesthetized patients and unanesthetized volunteers on the Wilson frame. Spine. 1997;22:2299-2303.
Bernhardt M, Bridwell KH. Segmental analysis of the sagittal plane alignment of the normal thoracic and lumbar spines and thoracolumbar junction. Spine. 1989;14:717-721.
Booth KC, Bridwell KH, Lenke LG, et al. Complications and predictive factors for the successful treatment of flatback deformity (fixed sagittal imbalance). Spine. 1999;24:1712-1720.
Bridwell KH, Lewis SJ, Lenke LG, et al. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance. J Bone Joint Surg Am. 2003;85:454-463.
Bridwell KH, Lewis SJ, Rinella A, et al. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance. Surgical technique. J Bone Joint Surg Am. 2004;86(Suppl 1):44-50.
Casey MP, Asher MA, Jacobs RR, et al. The effect of Harrington rod contouring on lumbar lordosis. Spine. 1987;12:750-753.
Diedrich O, Luring C, Pennekamp PH, et al. Effect of posterior lumbar interbody fusion on the lumbar sagittal spinal profile. Z Orthop Ihre Grenzgeb. 2003;141:425-432.
Doherty JH. Complications of fusion in lumbar scoliosis. Proceedings of the Scoliosis Research Society. J Bone Joint Surg Am. 1973;55:438.
Farcy JP, Schwab F. Posterior osteotomies with pedicle substraction for flat back and associated syndromes. Technique and results of a prospective study. Bull Hosp Jt Dis. 2000;59:11-16.
Farcy JP, Schwab FJ. Management of flatback and related kyphotic decompensation syndromes. Spine. 1997;22:2452-2457.
Glassman SD, Berven S, Bridwell K, et al. Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine. 2005;30:682-688.
Glassman SD, Bridwell K, Dimar JR, et al. The impact of positive sagittal balance in adult spinal deformity. Spine. 2005;30:2024-2029.
Hasday CA, Passoff TL, Perry J. Gait abnormalities arising from iatrogenic loss of lumbar lordosis secondary to Harrington instrumentation in lumbar fractures. Spine. 1983;8:501-511.
Herkowitz HN. International Society for Study of the Lumbar Spine. The Lumbar Spine. Philadelphia: Lippincott Williams & Wilkins; 2004.
Lagrone MO, Bradford DS, Moe JH, et al. Treatment of symptomatic flatback after spinal fusion. J Bone Joint Surg Am. 1988;70:569-580.
Lee CS, Lee CK, Kim YT, et al. Dynamic sagittal imbalance of the spine in degenerative flat back: significance of pelvic tilt in surgical treatment. Spine. 2001;26:2029-2035.
Potter BK, Lenke LG, Kuklo TR. Prevention and management of iatrogenic flatback deformity. J Bone Joint Surg Am. 2004;86:1793-1808.
Rauzzino MJ, Shaffrey CI, Wagner J, et al. Surgical approaches for the management of idiopathic thoracic scoliosis and the indications for combined anterior-posterior technique. Neurosurg Focus. 1999;6(5):E6.
Sansur CA, Fu KM, Oskouian RJJr, et al. Surgical management of global sagittal deformity in ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E8.
Sarwahi V, Boachie-Adjei O, Backus SI, et al. Characterization of gait function in patients with postsurgical sagittal (flatback) deformity: a prospective study of 21 patients. Spine. 2002;27:2328-2337.
Smith-Petersen MN, Larson CB, Aufranc OE. Osteotomy of the spine for correction of flexion deformity in rheumatoid arthritis. Clin Orthop Relat Res. 1969;66:6-9.
Stephens GC, Yoo JU, Wilbur G. Comparison of lumbar sagittal alignment produced by different operative positions. Spine. 1996;21:1802-1806.
Thomasen E. Vertebral osteotomy for correction of kyphosis in ankylosing spondylitis. Clin Orthop Relat Res. 1985;194:142-152.
Umehara S, Zindrick MR, Patwardhan AG, et al. The biomechanical effect of postoperative hypolordosis in instrumented lumbar fusion on instrumented and adjacent spinal segments. Spine. 2000;25:1617-1624.
Van Royen BJ, De Gast A, Smit TH. Deformity planning for sagittal plane corrective osteotomies of the spine in ankylosing spondylitis. Eur Spine J. 2000;9:492-498.
Wilson MJ, Turkell JH. Multiple spinal wedge osteotomy; its use in a case of Marie-Strümpell spondylitis. Am J Surg. 1949;77:777-782.
1 Doherty JH. Complications of fusion in lumbar scoliosis. Proceedings of the Scoliosis Research Society. J Bone Joint Surg Am. 1973;55:438.
2 Farcy JP, Schwab FJ. Management of flatback and related kyphotic decompensation syndromes. Spine. 1997;22:2452-2457.
3 Farcy JP, Schwab F. Posterior osteotomies with pedicle substraction for flat back and associated syndromes. Technique and results of a prospective study. Bull Hosp Jt Dis. 2000;59:11-16.
4 Herkowitz HN. International Society for Study of the Lumbar Spine. The Lumbar Spine. Philadelphia: Lippincott Williams & Wilkins; 2004.
5 Bernhardt M, Bridwell KH. Segmental analysis of the sagittal plane alignment of the normal thoracic and lumbar spines and thoracolumbar junction. Spine. 1989;14:717-721.
6 Booth KC, Bridwell KH, Lenke LG, et al. Complications and predictive factors for the successful treatment of flatback deformity (fixed sagittal imbalance). Spine. 1999;24:1712-1720.
7 Lee CS, Lee CK, Kim YT, et al. Dynamic sagittal imbalance of the spine in degenerative flat back: significance of pelvic tilt in surgical treatment. Spine. 2001;26:2029-2035.
8 Hasday CA, Passoff TL, Perry J. Gait abnormalities arising from iatrogenic loss of lumbar lordosis secondary to Harrington instrumentation in lumbar fractures. Spine. 1983;8:501-511.
9 Glassman SD, Berven S, Bridwell K, et al. Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine. 2005;30:682-688.
10 Glassman SD, Bridwell K, Dimar JR, et al. The impact of positive sagittal balance in adult spinal deformity. Spine. 2005;30:2024-2029.
11 Sarwahi V, Boachie-Adjei O, Backus SI, et al. Characterization of gait function in patients with postsurgical sagittal (flatback) deformity: a prospective study of 21 patients. Spine. 2002;27:2328-2337.
12 Potter BK, Lenke LG, Kuklo TR. Prevention and management of iatrogenic flatback deformity. J Bone Joint Surg Am. 2004;86:1793-1808.
13 Umehara S, Zindrick MR, Patwardhan AG, et al. The biomechanical effect of postoperative hypolordosis in instrumented lumbar fusion on instrumented and adjacent spinal segments. Spine. 2000;25:1617-1624.
14 Diedrich O, Luring C, Pennekamp PH, et al. Effect of posterior lumbar interbody fusion on the lumbar sagittal spinal profile. Z Orthop Ihre Grenzgeb. 2003;141:425-432.
15 Aaro S, Ohlen G. The effect of Harrington instrumentation on the sagittal configuration and mobility of the spine in scoliosis. Spine. 1983;8:570-575.
16 Casey MP, Asher MA, Jacobs RR, et al. The effect of Harrington rod contouring on lumbar lordosis. Spine. 1987;12:750-753.
17 Lagrone MO, Bradford DS, Moe JH, et al. Treatment of symptomatic flatback after spinal fusion. J Bone Joint Surg Am. 1988;70:569-580.
18 Benfanti PL, Geissele AE. The effect of intraoperative hip position on maintenance of lumbar lordosis: a radiographic study of anesthetized patients and unanesthetized volunteers on the Wilson frame. Spine. 1997;22:2299-2303.
19 Stephens GC, Yoo JU, Wilbur G. Comparison of lumbar sagittal alignment produced by different operative positions. Spine. 1996;21:1802-1806.
20 Thomasen E. Vertebral osteotomy for correction of kyphosis in ankylosing spondylitis. Clin Orthop Relat Res. 1985;194:142-152.
21 Smith-Petersen MN, Larson CB, Aufranc OE. Osteotomy of the spine for correction of flexion deformity in rheumatoid arthritis. Clin Orthop Relat Res. 1969;66:6-9.
22 Wilson MJ, Turkell JH. Multiple spinal wedge osteotomy; its use in a case of Marie-Strumpell spondylitis. Am J Surg. 1949;77:777-782.
23 Bridwell KH, Lewis SJ, Lenke LG, et al. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance. J Bone Joint Surg Am. 2003;85:454-463.
24 Bridwell KH, Lewis SJ, Rinella A, et al. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance. Surgical technique. J Bone Joint Surg Am. 2004;86(suppl 1):44-50.
25 Van Royen BJ, De Gast A, Smit TH. Deformity planning for sagittal plane corrective osteotomies of the spine in ankylosing spondylitis. Eur Spine J. 2000;9:492-498.
26 Rauzzino MJ, Shaffrey CI, Wagner J, et al. Surgical approaches for the management of idiopathic thoracic scoliosis and the indications for combined anterior-posterior technique. Neurosurg Focus. 1999;6(5):E6.
27 Sansur CA, Fu KM, Oskouian RJJr, et al. Surgical management of global sagittal deformity in ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E8.