CHAPTER 71 Flaccid Dysfunction of the Elbow
GENERAL PRINCIPLES
TIMING
Timing of surgical reconstruction varies depending on the etiology of the injury that caused loss of elbow flexion. Historically, the literature is often confusing with respect to timing of surgical procedures. Older literature is often in direct conflict with the newer literature. For example, for traumatic brachial plexus injuries, the recommendation was to wait at least 12 months for spontaneous return of function before embarking on tendon transfer. Often a locking-hinge elbow brace was applied, particularly for patients who have good hand function.249 However, with the advent of microsurgical reconstructive techniques, intervention before 6 months from injury is now recommended.7,54,73,176,214
In tetraplegic patients, reconstructive surgery to replace triceps function should not be undertaken less than a year after the injury. In a child with arthrogryposis, transfers to gain elbow flexion can be done after the age of 5 years. Usually, reconstruction should be performed on one side only so that one extremity can be used for activities that require flexion, while the unoperated elbow is used for those that require extension. After the traumatic loss of a flexor or extensor muscle compartment, it is advisable to wait until the tissues about the elbow joint are supple before proceeding with tendon transfer or free muscle transfers.
Any use of the hand will be wasted without elbow function to maintain it in a useful position. Therefore, the first goal in the treatment of a flail arm is the restoration of elbow flexion by primary direct nerve surgery or secondary reconstructive surgery.17,172,176 An exception is in polio-type brachial plexus paralysis, in which priority should be given to restoration of shoulder abduction rather than elbow flexion. In these cases, early nerve transfers within 1 year of paralysis achieve significantly more shoulder abduction than late nerve transfer or palliative reconstruction with muscle/tendon transfer or shoulder arthrodesis, whereas elbow flexion may spontaneously recover in up to 90% of cases.142 In obstetric brachial plexus palsy, timing of surgical intervention is highly controversial. Although most authors agree that surgical intervention is indicated when no recovery of biceps function occurs, some have recommended intervention at 3 months92 or as late as 9 months.60
In all timing of reconstructive procedures for neurologically disabled patients, achieving stability of neurologic status, mental status (acceptance of the permanency of the disorder), and social status (clearly established goals of rehabilitation) is an essential prerequisite before any surgery is undertaken that is not itself concerned with stability.110 Conditions that interfere with progression toward the goals of rehabilitation before neurologic stability has been achieved include flexion contractures of the elbow and supination contractures of the forearm, which must be corrected before further reconstruction is undertaken.
CORRECTION OF PRE-EXISTING JOINT CONTRACTURE
Reasonably functional passive motion of the elbow must be obtained before nerve, tendon, or muscle transfers are performed. This often requires the joint and muscle contractures to be stretched, and as a result, the functioning muscles are strengthened by progressive resistance exercises. Passive flexion of at least 120 degrees is desirable before tendon transfer is carried out. Static adjustable splints (see Chapter 11) are useful in regaining passive extension.95 Occasionally, surgery is required to release a severely damaged elbow flexor, extensor muscle, or joint capsule before the joint can be mobilized. A preliminary or concurrent V to Y release of a contracted triceps, in conjunction with flexorplasty, may be necessary to correct an extension contracture in an arthrogrypotic child. If soft tissue cover is necessary, it usually can be provided as a skin paddle on the transposed latissimus dorsi flap.
When contractures are greater than 60 degrees and interfere with or are exaggerated during ADLs, surgical release is indicated.126 The contracture correction can consist of combinations of Z-lengthening of the biceps tendon, myofascial release of the brachialis, and release at the origin of the wrist flexor-pronators. Capsular release is necessary only in severe, long-standing contracture. The contracture that occurs in Erb’s palsy is paradoxical in nature, given the normal triceps function in the face of weak or absent biceps function. A dynamic splint126 or serial casting, followed by biceps tenotomy with further casting,84 may also be used. The latter method is associated with improved joint range of motion without loss of elbow flexion strength in 80% of cases. Drawbacks of this treatment are pressure sores and stiffness, and the limitations in correcting pronation as the elbow reaches full extension. When an upper motor neuron unit is involved in the contracture, botulism toxin or selective crush of the musculocutaneous nerve is used with success concurrent with casting, especially in contractures developing early after spinal cord injury. These patients are especially prone to developing a pronation contracture due to biceps weakness. Pronation and supination contractures should also be addressed before fixed bony changes occur in the radius and ulna.
CHOOSING AN APPROPRIATE MUSCLE FOR TENDON TRANSFER
The type of muscle transfer depends on the muscle groups that are available.172 The original functional properties between the donor and recipient muscles must be matched.86 A transferred muscle must provide both adequate strength and excursion in its new role.
Clinical and electromyographic testing of various muscles should be performed before transfer. A muscle to be transferred should show an adequate electromygraphic trace and have appropriate strength. The extent of a brachial plexus lesion dictates which muscles will have retained their innervation. The most common cause of failure in tendon transfers about the elbow, as elsewhere, is overestimating the strength of the transferred muscle. The muscle to be transferred should contract against gravity resistance (grade 4, “M4”) if significant function is to be anticipated (Table 71-1). Preoperative electromyography (EMG) of the muscle proposed for transfer is a good idea, but mild denervation changes do not preclude the transfer.226
TABLE 71-1 Muscle Strength Grading System According to the British Medical Research Council161
| Grade | Definition | Explanation |
|---|---|---|
| 0 | Absent | No function |
| 1 | Trace | Slight contraction, no motion |
| 2 | Poor | Complete motion, gravity eliminated |
| 3 | Fair | Complete motion against gravity |
| 4 | Good | Complete motion against gravity and some resistance |
| 5 | Normal | Apparently normal strength |
In addition to muscle strength, which is determined by clinical examination, it is helpful to consider the comparative work capacities of the various muscles available for transfer.245 The work capacity is determined by multiplying the power of the muscle (3.65 kg/cm2 of physiologic cross section) by its amplitude (the distance through which the tendon moves when its muscle contracts). The work capacity of the biceps for elbow flexion is 4.8 kg/m, and for forearm supination, it is 1.1 kg/m. The sternocostal head of the pectoralis major muscle has nearly three times as much work capacity at 10.4 kg/m. By contrast, the work capacity of all forearm flexors1 (pronator teres, flexor carpi radialis and ulnaris, and palmaris longus) is 3.8 kg/m.116
RESTORATION OF ELBOW FLEXION
INTRODUCTION
Neurotization or brachial plexus reconstruction is the treatment of choice in early cases of the flail upper extremity.* In chronic cases or failed early reconstruction, several tendon transfer procedures to restore elbow flexion are available49: Steindler flexorplasty and its modifications,28,36,40,76,146,158,222 anterior transposition of the triceps tendon,43 pectoralis-to-biceps transfer, with or without the pectoralis minor,† pectoralis minor alone,200 sternomastoid-to-biceps transfer with or without shoulder arthrodesis,40,44 transfer of the flexor carpi ulnaris,1 and unipolar or bipolar transposition of the latissimus dorsi muscle.11,15,33,48,117,207,237,255 Free muscle transfer‡ is fast becoming a mainstay of the armamentarium of upper extremity reconstructive surgeons and has proved useful in both early and chronic cases of brachial plexus and traumatic injuries. Each of these treatment modalities has its advocates, and knowledge of a number of different options will allow treatment to be tailored to the needs and expectations of the patient.
The muscle groups chosen for tendon transfer depend on those that are available. Triceps-to-biceps transfer is advocated when there are biceps-triceps co-contractions, and Steindler’s procedure when the elbow flexors reach only grade 2.7 The latter may be contraindicated when the elbow flexors are grade 0, when the wrist flexors are weak, or when wrist and finger extensors are paralyzed.172 The Steindler flexorplasty can also be augmented by a pectoralis minor transfer. In children with obstetric brachial plexus palsy at the C5, C6 level, flexorplasties are indicated when there is a functional hand but with weak elbow flexion and inability to position the hand.113 In these patients, the Steindler procedure is reserved for elbows with excellent forearm muscles for wrist and digit flexion. When these are not all M5, the triceps-to-biceps transfer is advocated, by some authors using only a normal triceps,113 and by others,202 even with a weak triceps. When neither the triceps nor the forearm flexors are judged as being of sufficient strength, the pectoralis major or minor transfer can be used, or preferably, a free-functioning muscle transfer can be performed.
NEUROTIZATION
Neurotization is the technique of nerve transfer used to restore motor or sensory function after brachial plexus injuries when the nerve damage is too far proximal for standard nerve-grafting techniques to be feasible. In 1903, Harris and Low104 inserted half of the distal fascicles of the C5 root, which was damaged at the foramen, into the healthy spinal nerve C6 or C7 in three cases of Erb’s palsy. No results were recorded. In 1913, Tuttle241 mentioned one case of neurotization using the spinal accessory nerve. In 1963, Seddon211 first reported neurotization of the musculocutaneous nerve using intercostal nerves in two patients, both times obtaining active elbow flexion.
Subsequently, many early cases of neurotization using intercostal nerves have reported a modicum of success.§ Early reports on neurotization of the musculocutaneous nerve using parts of the cervical plexus35 and spinal accessory nerve5,122,181 also mention some limited success.98 Later series report satisfactory restoration of elbow flexion after intercostal nerve transfer to the musculocutaneous nerve without intercalated grafts.68,128,174 The goal is to provide the musculocutaneous nerve with the best motor donors.233 This can be achieved by reconstruction using intraplexus donors such as nerve grafts from the proximal stumps of avulsed C5 roots. It must be stressed, however, that nerve transpositions are effective only when they are performed within 6 to 9 months of injury.5,24,51,128,163,181,201,217
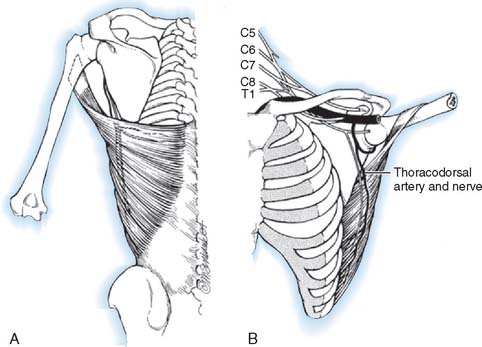
Currently used extraplexus transpositions for elbow flexion, in general order of decreasing frequency, are the intercostal nerve,88,179 spinal accessory nerve,6 thoracodorsal nerve,204 pectoral nerve,31 phrenic nerve,53,98 hypoglossal nerve,177 platysma motor branch,22 and high cervical nerve roots.252 The ulnar186 and median nerves148,150,151 can be used as intraplexus donors, with their vascular supply left intact. This is recommended only for global plexopathy or tetraplegia involving C7, C8 and T1 so as not to sacrifice any remaining or potential nerve function.139,186 Other intraplexus donors can be obtained by the selective contralateral C7 method56,97,235 or by transfer of the medial pectoral nerve.205,246 Cross-chest radial nerve transfer has been proposed and is feasible anatomically but requires tendon transfer reconstruction of radial nerve innervated muscles in the contralateral arm.21 Another rarely used intraplexus donor is the long thoracic nerve,177 which provides the sole innervation for the serratus anterior, causes scapular winging after transfer, and therefore, is not recommended.144
The phrenic nerve can be transferred without pulmonary complications,98 even with concurrent intercostal nerve transfer,101 although the risk of reduced vital capacity is greater when the right phrenic nerve is used.149 Its full length can be harvested using video-assisted thoracic surgery techniques.251 Transfer of intercostal nerves alone have been associated with a nonsignificant mild decline in pulmonary function, without a subjective effect on respiratory status or stamina.128
Intraoperative somatosensory evoked potential (SEP) recording is mandatory to ascertain the level of injury—even when a root appears to have sustained a pos-ganglionic injury, nerve grafting is never indicated in a root with a negative SEP.175
TECHNIQUE
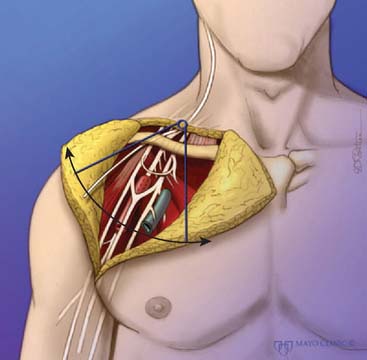
The exposure of the brachial plexus as performed by Sedel is described in this section.19 The patient, under general anesthesia, is given no neuromuscular blocking agents. An incision is made parallel to the clavicle, with an extension parallel to the sternocleidomastoid muscle. The skin flaps are elevated, and the external jugular vein is ligated. The sternocleidomastoid is retracted, allowing dissection to proceed in the prescalene area. The phrenic nerve is identified on the scalenus anterior. The fifth cervical root is identified at the point where the phrenic nerve crosses the lateral aspect of the scalenus anterior proximally. The sixth cervical root is more distal and medial with a more horizontal direction. The seventh cranial nerve root is more horizontal still and more posterior; more posteriorly and medially lie the more difficult to identify eighth cervical and first thoracic nerve roots. The scalenus anterior is divided, the subclavian artery is retracted ventrally, and the pleural dome is retracted dorsally. The infraclavicular incision is extended onto the deltopectoral groove, sometimes extending over the arm. The axillary artery is dissected and the lateral, posterior and medial cords of the brachial plexus are identified. If exposure is required behind the clavicle, this is divided and repaired with an AO plate later. A supraclavicular or infraclavicular approach can be used.
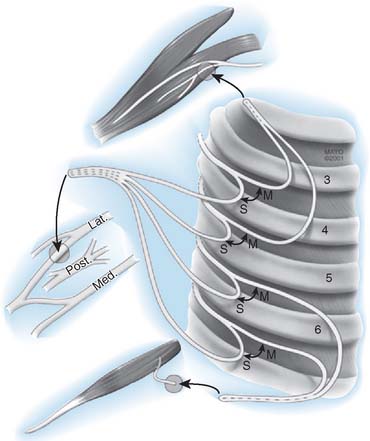
In accordance with the goals of reconstruction of the flail upper limb, the first priority in neurotization is to attach a motor nerve donor to the musculocutaneous nerve. Grafts used for this purpose include the suprascapular and spinal accessory nerve, alone or in conjunction with two or three intercostal nerves (Fig. 71-1). In decreasing order of priority, the radial nerve (after dissection and removal of the triceps or posterior cord branch), median nerve, and axillary nerves are reconstructed in a similar fashion. The operative strategy depends on the type and extent of the lesion and the number, quality and length of available grafts, as well as the aspect of the proximal neuroma. If there is a neuroma in continuity, there is controversy as to whether it should be resected or treated by simple neurolysis.250 In any case, conduction across the neuroma is best assessed using nerve action potential rather than muscle action potential, because the former requires 40 to 50 times as many fibers of reasonable caliber to elicit a response. There is discussion as to the advantage of using interposition nerve grafting when performing intercostal nerve transfers. It has been demonstrated that intercostal nerves lose up to 10% of motor axons per 10 cm measured from the midaxillary line to the sternum.163 Coaptation of the transfer closer to the motor endplates, avoidance of tension on the nerve repair, and earlier shoulder mobilization are other theoretical advantages. This includes coaptation directly to the dominant motor branch of the biceps muscle rather than to the musculocutaneous nerve.31,128 There is no consensus on the optimum number of intercostal nerves to be transferred to the musculocutaneous nerve to obtain optimal biceps function. Also, there is no consensus on using the spinal accessory nerve over intercostal nerves. A theoretical advantage of the spinal accessory nerve for transfer is the high number and ratio of motor fibers (1500-3000) compared with an intercostal nerve (500-700 motor fibers per nerve).5,88,244 However, a clear disadvantage cited by most authors is the need for an interpositional graft when using the spinal accessory nerve. If necessary, the spinal accessory nerve can be harvested through a posterior approach.12,99,194 An average extra length of 12 cm can be gained compared with the standard anterior approach, allowing the terminal branches of the brachial plexus to be neurotized without the need for an interposition graft (Fig. 71-2).244 Vathana et al.244 measured an average of 817 myelinated axons at the most distal level; however, these authors warn that the size mismatch with terminal branches in the brachial plexus (varying between 5000 and 14000 axons) may predict poorer outcome and that this approach may be more suitable for reinnervation of the suprascapular nerve at the level of the supraclavicular notch. When interposition grafts are unavoidable, results may be improved with the use of vascularized nerve grafts.53 The vascularized ulnar nerve graft has been shown to maintain its blood supply and viability even in a scarred bed.235 Pedicled on the superior ulnar collateral vessels and ulnar vessels, this yields better results than including the superior ulnar collateral vessels alone.97 This intraplexus donor has a greater number of axons than any extraplexus donor, potentially increasing the probability for successful neurotization.235
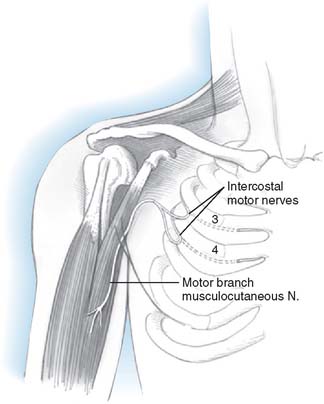
FIGURE 71-1 Intercostal nerve neurotization of the musculocutaneous nerve.
(By permission of Mayo Foundation for Medical Education and Research. All rights reserved.)
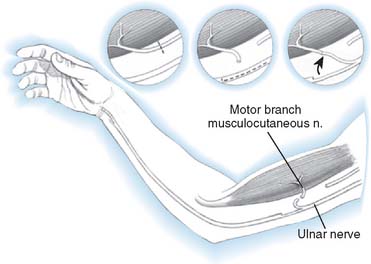
Distal nerve-to-nerve transfers are possible, however, only when the lower plexus is intact. Possible transfers include ulnar nerve to the biceps branch of the musculocutaneous nerve or to biceps and brachialis, medial pectoral, and thoracodorsal nerves (Fig. 71-3).147,150,151,187 A shorter distance and time to reinnervation is provided by these transfers.
RESULTS
Intraplexus donors provide better results than extraplexus donors, although direct neurotization of the musculocutaneous nerve from ipsilateral intercostal nerves produces results comparable to intraplexus neurotizations,233 as does direct neurotization of the suprascapular nerve with the spinal accessory nerve for shoulder function.235 A meta-analysis of the English literature (26 studies, 965 nerve transfers) showed M3 biceps strength in 72% of direct intercostal-to-musculocutaneous transfers versus 47% with interposition grafts (P < 0.001), with a trend observed at ≥M4 biceps strength (41% vs 32%).163 Spinal accessory nerve–to–musculocutaneous nerve transfers for elbow flexion were comparable to intercostal nerves without interposition grafts at ≥M3 strength (77% vs 72%). Intercostal nerves, however, were significantly better at providing ≥M4 biceps strength relative to spinal accessory nerve (41% vs 29%; P < 0.001). The same authors were unable to demonstrate a difference when two versus three or four intercostal nerves were used for restoration of elbow flexion to ≥M3 strength.
This contrasts with a prospective study involving 205 patients performed by Waikakul et al.,247 comparing spinal accessory to intercostal nerve transfer. These authors found that spinal accessory nerve transfers produced significantly better motor outcomes, but patients with intercostal nerve transfers had significantly better protective sensation and pain relief. Also, the shoulder subluxation in the spinal accessory group required more frequent shoulder fusion. Spinal accessory nerve transfer is preferred by these authors in isolated biceps paralysis, whereas intercostal transfer is recommended for global plexus palsy, particularly those with deafferentation pain.247 A beneficial effect of neurotization that frequently precedes functional return by months is the alleviation of deafferentation pain and an improvement in protective sensation.181 A lack of pain allows patients to concentrate on rehabilitation and improve their function and dexterity.235
The contrasting results between different studies may be due to the use of an interposition graft with the spinal accessory nerve that may offset the advantage of containing more motor fibers.163
Oberlin’s method of transfer of two ulnar nerve fascicles show promising results for restoration of elbow flexion,139,186,232 as does the double fascicular nerve transfer from the ulnar and median nerves to biceps and brachialis branches of the musculocutaneous nerves.147,151
Several nerve transfers may be combined to decrease the time to functional return and provide better results. Leechavengvongs and colleagues140 reported the results of 15 combined spinal accessory–to–suprascapular nerve, ulnar nerve–to–biceps motor branch, and long head of the triceps branch–to–the axillary nerve transfers for C5 and C6 avulsion injuries. Elbow flexion strength was M4 in 13 patients and M3 in two patients at an average follow-up of 24 months.
TENDON TRANSFERS
Steindler’s Flexorplasty
Steindler first described his simple but ingenious concept of proximally shifting the origin of the flexor-pronator muscle group to increase its lever arm in flexing the elbow in 1918.220,221 His technique consisted of the subperiosteal dissection of the origin of the flexor-pronator muscles of the forearm from the medial epicondyle. The muscle flap was then transposed proximally between the brachialis and the triceps, and was sutured to the medial epicondylar ridge through two drill holes 2 inches above the epicondyle. Steindler recommended the procedure only if the wrist flexors were normal or only slightly weakened.
Subsequent authors differed on the prerequisites for Steindler’s flexorplasty. Mayer and Green158 believed that the epicondylar muscle group strength should be grade 3 or better, and they re-emphasized the need for strong wrist flexors if the operation is to succeed. They described this simple test:
The arm is abducted to 90 degrees to eliminate gravity. Any patient who can flex the elbow in this position (using the epicondylar muscle group) is a candidate for the operation. Nyholm believed that the criteria for forearm muscle strength should be liberalized because all of his patients achieved 90 degrees of elbow flexion, even though a relatively large number had forearm muscles that were “primarily paretic.”183 Dutton and Dawson76 performed the operation if the strength of the forearm flexor-pronator group was rated fair or better. Alnot7 believed that the shoulder flexorplasty was most indicated to reinforce a biceps that had recovered to M2 strength. It seems that there is considerable variability in the preoperative muscle strength needed for active flexion, and the axiom that the muscle loses a grade in transferring it does not necessarily apply in this particular situation.
Others have modified and refined Steindler’s original concept to increase flexor strength and decrease the tendency toward development of a pronation deformity. Bunnell40 described extending the common tendon of origin with a fascia lata graft that would reach 2 inches up the lateral border of the humerus. This resulted in moderate but not complete correction of the pronation tendency. Mayer and Green158 detached the flexor-pronator origin with a portion of the medial epicondyle and attached it through a window cut in the anterior cortex of the humerus 5 to 7.5 cm proximal to the joint. Their description of the technical details of the Steindler flexorplasty, which includes careful dissection of the ulnar and median motor branches, is the best in the literature. Lindholm and Einola145 used a screw to fix the epicondylar fragment to the humerus in two cases. Eyler79 advocated omitting the flexor carpi ulnaris to allow the surgeon to work in the “internervous plane” between the flexor sublimis, anteriorly, and the flexor profundus and flexor carpi ulnaris, dorsally.
Efforts have been made to augment the strength of elbow flexion, especially in patients who have weakness of the flexor-pronator muscle group. Initially, Steindler recommended proximal transfer of the radial wrist extensor muscle origins off the lateral epicondyle in conjunction with the medial transfer, but he did not comment on it in his later communications.222 Mayer and Green reported having difficulty mobilizing the lateral epicondylar muscle group without damaging the nerve supply158 and gave up the procedure after two unsatisfactory results. Lindholm and Einola146 were unable to detect any increase in flexion strength in the six patients who had the additional lateral transfer.
Technique (modified from Mayer and Green)
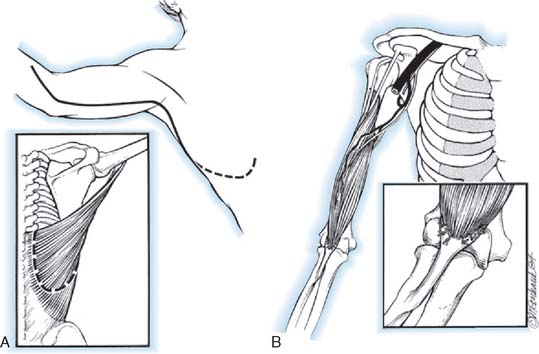
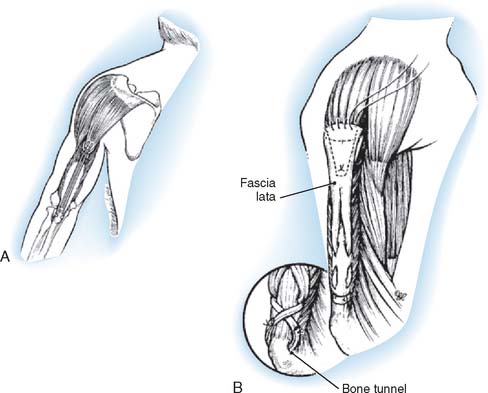
A sandbag is placed under the opposite hip (Fig. 71-4). A tourniquet usually is not used. The incision begins on the anterior aspect of the arm about 7.5 cm above the elbow and swings gently in a medial direction. At the elbow, it runs just posterior to the epicondyle. It then curves anteriorly, following the direction of the pronator teres, ending about 10 cm below the elbow. The ulnar nerve is isolated and freed distally to the branches of the flexor carpi ulnaris. Preserving these motor branches, the surgeon continues the dissection distally for 5 cm. The lacertus fibrosus is divided, and the median nerve is exposed above the elbow and dissected distally, exposing the motor branches (all of which leave the medial aspect of the nerve) to the common flexor-pronator muscle group. The common flexor-pronator muscle origin is then detached with a flake of epicondyle (cartilage in children and bone in adults). The flake of bone or cartilage is then grasped with a clamp, and while traction is exerted in distal and anterior directions, the muscles are stripped from the anterior surface of the joint and from the coronoid process of the ulna. As the ulnar head of the flexor carpi ulnaris is detached from the ulna with an elevator, the assistant puts gentle traction on the median and ulnar nerves, demonstrating the motor twigs that must be carefully avoided. Dissection is continued distally as far as the anatomic distribution of the nerves permits. The common tendon is then transfixed with a modified pull-out suture of No. 1 prolene. The elbow is flexed to 120 degrees, and traction is exerted on the transfer to determine how far above the elbow the transfer will reach. This is usually between 5 and 7.5 cm. The ulnar nerve often seems to have less tension on it if it is transferred anterior to the epicondyle. The atrophic fibers of the brachialis are slit longitudinally, the periosteum is incised, and the anterior humerus is exposed subperiosteally. An opening in the anterior cortex of the humerus nearer to the lateral than to the medial border is made at the point to which the transplant reaches when the elbow is flexed. Two small drill holes are made from anterior to posterior through this cortical window. The prolene suture ends are then threaded through the holes and out through the triceps muscle and skin. To be sure that they are not subjected to undue tension on twisting, the nerves are inspected as the transfer is pulled into the cortical window. The distal portion of the wound is closed, up to the bend in the elbow. The sutured ends are drawn tight with the elbow in maximal flexion and tied over a button under which a thick piece of felt has been placed. Several auxiliary sutures between the periosteum and the transplanted epicondylar tissues are placed, and the wound is closed with a drain.

FIGURE 71-4 Steindler’s flexorplasty.221 A, The incision. B, The ulnar nerve is mobilized proximally and distally, and its motor branches to the flexor carpi ulnaris are identified and protected. C, The common flexor-pronator origin is detached with a flake of medial epicondyle. The motor branches of the median nerve to the flexor-pronator group are identified and protected. D, The detached flexor-pronator group is mobilized distally as far as the motor branches of the medial and ulnar nerves will permit. The brachialis muscle is divided. E, The distal humerus is prepared, and a prolene pull-out suture is used to anchor the transferred muscles to the anterolateral surface of the humerus 5 to 7.5 cm above the elbow.
(After Mayer, L., and Green, W.: Experiences with Steindler flexorplasty of the elbow. J. Bone Joint Surg. 36A:775, 1954.)
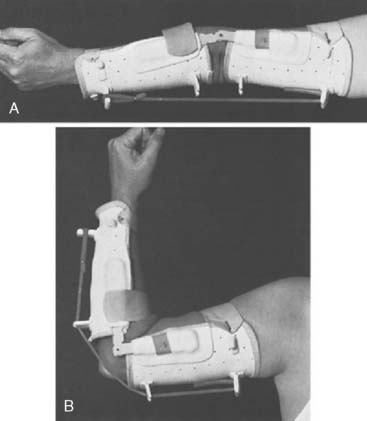
A posterior splint is then applied with the elbow in about 120 degrees’ flexion and the forearm in full supination. Four weeks after surgery, the pull-out suture is removed. A removable Orthoplast splint is applied, and active flexion and supination and extension exercises are begun. No special retraining is required because the muscles transferred functioned as an accessory elbow flexor before transfer.58 Splinting is discontinued 6 to 8 weeks after surgery, the longer time being necessary for patients with normal triceps function. A dynamic extension splint is often useful if the patient has no triceps function (Fig. 71-5).
In patients with C5-7 palsy in which the wrist and finger extensors are weakened or paralyzed, the forearm flexor-pronators are strong but the triceps is not available or co-contracts with the biceps, the Steindler procedure should be complemented with wrist arthrodesis.172 Furthermore, success of the procedure relies on sufficient power of the flexor-pronator muscles.76,124,145,146,155,158
Results
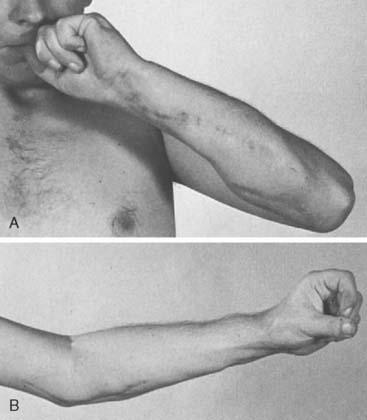
Published results of the Steindler flexorplasty reflect a high degree of success in achieving a functional range of elbow flexion against gravity. Most transfers reported have been performed for poliomyelitis. Steindler achieved 79.5% good results (flexion against gravity of not less than 90 degrees) in 39 cases.224 Fifteen good results (useful range of flexion with good to fair power) in 27 flexorplasties were reported by Carroll and Gartland.42 Using strict criteria for success, including subtracting from the total score for flexion contracture of more than 15 degrees and for supination of less than 45 degrees, Mayer and Green recorded 11 excellent results, five good, four fair, and two poor results among the 22 flexorplasties they followed up (Fig. 71-6).158 Segal and associates compared the results of 13 Steindler flexorplasties (transplantation of both flexor and extensor origins) and 17 Clark pectoralis major transfers.213 The flexorplasty results were better than the pectoralis transfers, but the average flexion contracture in the flexorplasty group was 60 degrees, whereas it rarely exceeded 15 degrees in the Clark transfer group. Kettelkamp and Larson, evaluating 15 flexorplasties using Mayer and Green’s scoring system, noted eight excellent or good results, six fair results, and one poor one.124 They also measured the carry-lift strength (flexion against gravity, plus weight in 1-pound increments) and found that nine of the 14 patients who were able to flex the elbow 110 degrees or more against gravity were able to flex through this range with 1 pound or more. The maximum weight lifted was 6 pounds by one patient.
Nyholm183 reported on 24 patients, six of whom achieved a normal degree of elbow flexion after flexorplasty. Five patients (three who had brachial plexus trauma and two polio) recovered some biceps function, which did not seem to be compromised by the previous flexorplasty. Nyholm noted that all of the patients except two preferred to flex the elbow with the forearm pronated and hand clenched.
Hoffer and Phipps113 reported on three Steindler flexorplasties in children with obstetric brachial plexus palsies (C5, C6). They achieved flexion strength M4 with 20 to 30 degrees of extension deficit.
One of the largest series of flexorplasties was reported by Lindholm and Einola,145 who found that 50 of their 61 patients achieved a range of flexion of at least 90 degrees. Eleven were able to lift a weight heavier than 1 kg at right angles. Dutton and Dawson76 noted that 20 patients had excellent or good function of the transferred muscles. Eighteen could lift at least 1 to 2 kg through an arc of full elbow motion.
Finally, several investigators have noted the beneficial effect of arthrodesis of the flail shoulder in patients who have had flexorplasties.76,124,145,223 Shoulder arthrodesis permits abduction at the scapulothoracic joint that decreases the gravitational forces that must be overcome by the transferred flexor-pronator muscle group. Stabilizing the humerus also maximizes the power of the transfer in preventing backward movement of the elbow, which diminishes the advantage of the flexion contracture by increasing the length of the arc through which the reconstructed flexor muscle must move the weight against gravity.124
A retrospective review of 12 patients with C5-7 brachial plexus palsy and extremely weak elbow flexion (M2 or less) were treated by Steindler flexorplasty and wrist arthrodesis. Eleven patients were found to have good or very good outcomes based on the criteria established by Alnot and Abols,8 and one patient had mild active flexion of the elbow.172 These results emphasize that despite recent advances in neurolysis nerve grafting and neurotization procedures, a direct approach to the neurologic lesion can give incomplete results, and for this reason, tendon transfers still play an important role. Chen49 reported results in eight patients, in four of whom one or several flexor-pronator tendons had been used in tendon transfer procedures to restore wrist and finger extension. The results after modified Steindler flexorplasty were not negatively affected by these previous procedures.
Liu and associates146 reported on the largest cohort of 71 patients with a follow-up of 4 to 15 years. The mean range of motion during active flexion against gravity was 114 degrees (range: 80 to 140 degrees). A mean extension lag of 28 degrees (range: 0 to 50 degrees) was created. Fourteen patients (20%) had significant loss of supination, for 11 of whom flexor carpi ulnaris–to–extensor carpi radialis brevis tendon transfers were performed to regain supination. Using Mayer and Green’s scoring system,158 results were excellent in 32%, good in 47%, fair in 13%, and poor in 8%. A clear improvement was seen over time; the results in operations performed between 1981 and 1987 were significantly better than those performed between 1970 and 1980 (57% versus 16% excellent results). This difference was attributed by the authors to the addition of shoulder arthrodesis as a stabilizing supplementary procedure in 45 cases, and the aforementioned tendon transfers in patients without active supination.
Andrisano9 reported on the long-term follow up (3-13 years, average 8.6 years) in 22 patients. These authors found good results in 50%, fair in 19% and poor results in 38%. These results were independent of the etiology of the elbow paralysis and the patient’s age, but were strongly dependent on surgical factors, such as the correct tension and placement of the epitrochlear mass.
Brunelli and coworkers36 have modified the procedure such that during transfer of the flexor carpi ulnaris, flexor carpi radialis, and palmaris longus, they are carefully separated from the flexor digitorum superficialis, which is left in place. Together with placement of the transfer on the anterior aspect of the humerus, the so-called Steindler effect (pronation and simultaneous elbow and finger flexion) could be avoided in 28 of 32 cases.
Complications
The main complications of Steindler’s transfer are pronation and flexion contractures. Pronation contractures are related to (1) preoperative absence of active supination in most of these patients and (2) tightening of the pronator teres as a result of its proximal shift. Inserting the transfer more anteriorly and laterally on the humerus diminishes this tendency, but if no active supination is present, pronation deformity still may be a problem. Segal and associates213 and Nyholm183 did not notice any great change in passive supination after Steindler flexorplasty in their patients. Carroll and Gartland42 noted that approximately half of the 23 patients who were improved by the flexorplasty had pronation defects. Lindholm and Einola145 encountered pronation contracture in 17 of 61 patients who had had a flexorplasty, but 14 of them had had no active supination preoperatively. Dutton and Dawson noted an average loss of supination of 39 degrees in the 25 patients on whom they reported.76
The strength of the transferred muscles, the duration of immobilization, and the strength of the opposing triceps muscle affect the extent of the flexion contracture. A flexion contracture of the elbow does increase the mechanical advantage of the transferred muscle, and this is especially important in patients who have significant weakness of the transferred muscle group. The degree of fixed flexion contracture of the elbow that is considered acceptable varies a good deal among surgeons. Steindler believed that a 60-degree flexion contracture was acceptable,223 and Carroll and Gartland accepted 40 degrees.42 Kettlekamp and Larson124 observed that, when strength rather than appearance was important (typically for men and patients with severe paralysis of the contralateral arm), flexorplasties are more satisfactory with a flexion contracture between 30 and 60 degrees. Mayer and Green158 were not satisfied if the flexion contracture exceeded 15 degrees, and they used turnbuckle splints postoperatively to minimize the flexion deformity. Dutton and Dawson76 preferred a flexion contracture of 15 to 30 degrees, which allows the arm to “hang at the side” more normally in patients concerned with appearance, and a 30- to 45-degree contracture in patients who were more concerned with function.
Dutton and Dawson76 reported transient ulnar nerve paresthesias in two patients. Lindholm and Einola145 reported on two patients with postoperative ulnar nerve paresthesias, one of whom was still having symptoms at follow-up. Description of the transferred muscle occasionally occurs with variable impact. Steindler reported an excellent result after reinsertion,223 whereas Kettelkamp and Larson recorded a poor result in a patient with an untreated description.124
Inherent in the Steindler flexorplasty is the increased pronatory effect on the forearm and the associated risk of pronation deformity (Steindler effect). The incidence in the literature varies from 28% to 50%.42,76,145 Theoretically, inserting the flexor mass more anteriorly and laterally on the humerus will decrease this effect.146 The flexor carpi ulnaris can be transferred to the flexor carpi radialis40 or extensor carpi radialis brevis146 primarily or secondarily, or the flexor digitorum superficialis origin can be left in place.36
Latissimus Dorsi Transfer
History
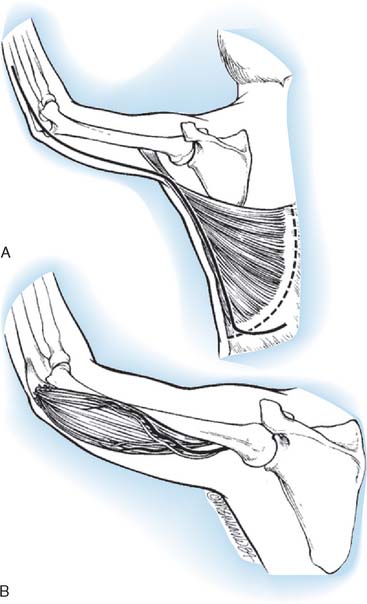
Schottstaedt and colleagues207 were the first investigators to report successful restoration of elbow flexion by rotating the entire latissimus dorsi muscle on its neurovascular pedicle, reattaching its insertion to the coracoid and its origin to the distal biceps muscle and tendon. One patient could lift 4 pounds. They noted that the procedure was contraindicated when the latissimus dorsi was the only muscle capable of elevating the pelvis for a forward step. Zancolli and Mitre refined the technique described by Schottstaedt and associates and coined the term bipolar transfer.254 Brones and colleagues33 reported that the bipolar myocutaneous latissimus dorsi flap restored contour and function in a post-traumatic condition.
Restoration of elbow flexion by transferring only the origin of the latissimus dorsi to the biceps insertion (unipolar transfer) was reported by Hovnanian in 1956.117 He noted that the cross-sectional area, fiber length, and excursion of the latissimus dorsi muscle compared favorably with those of the biceps and brachialis. Axer11 and others modified the unipolar technique by using only the upper third of the latissimus dorsi, transferring its origin off several spinous processes to the biceps tendon. Excursion of 7 cm was obtained by stimulating this part of the muscle. According to these authors, transferring only a portion of the muscle avoids the sometimes bulky enlargement of the arm that results when the whole muscle is used. Preservation of some function in the undisturbed portion of the latissimus dorsi was noted in one of the patients. Botte and Wood28 decided whether to do bipolar or unipolar transfer at the time of surgery. If the line of pull stabilized in the plane of elbow motion and there was no tension on its neurovascular pedicle, the transfer was completed as a unipolar transfer. If the line of pull deviated outside the plane of elbow flexion or there was tension on the neurovascular pedicle, the transfer was converted to a bipolar transfer.
The latissimus muscle flap is widely used as a pedicled muscle flap for restoration of elbow flexion.53,55,155,182
Anatomy
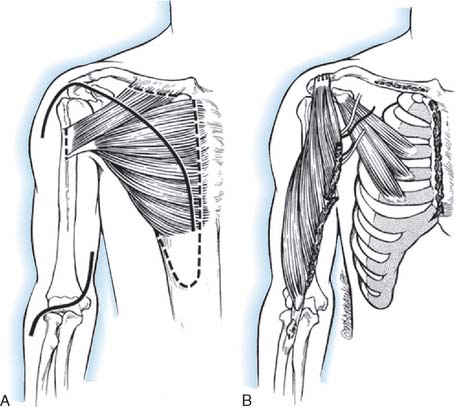
The latissimus dorsi is a broad, thin muscle (Fig. 71-7) with a wide origin from the lower six thoracic vertebrae, from the spinous processes of the lumbar and sacral vertebrae by an aponeurosis from the iliac crest, and by muscle slips from the lower four ribs. It inserts into the medial wall and floor of the intertubercular groove of the humerus. The major vascular pedicle of the muscle is the thoracodorsal artery, a terminal branch of the subscapular artery. In 94% of the 114 specimens, a bifurcation of the common neurovascular trunk into lateral (parallel to the lateral border of the muscle) and medial (parallel to the upper border) branches has been documented.237 Bartlett and coworkers15 studied 50 latissimus dorsi muscles and noted the same bifurcation in 56% of the dissections. These studies provide the anatomic basis for the clinical findings noted by Axer and colleagues.11 The thoracodorsal nerve (C6-8) is derived from the posterior cord, and enters the muscle with the artery and its vein on its deep surface about 10 cm from its insertion. There are one to three branches from the thoracodorsal artery to the serratus muscle that have to be ligated to allow complete mobilization of the latissimus during a bipolar transfer. In the study by Bartlett and coworkers,15 the vascular pedicle to the latissimus dorsi had an average length of 11 cm and the thoracodorsal nerve a mean length of 12.3 cm.
Bipolar Transplantation
This description follows that of Zancolli and Mitre (Fig. 71-8).254 The operation is carried out in four steps:
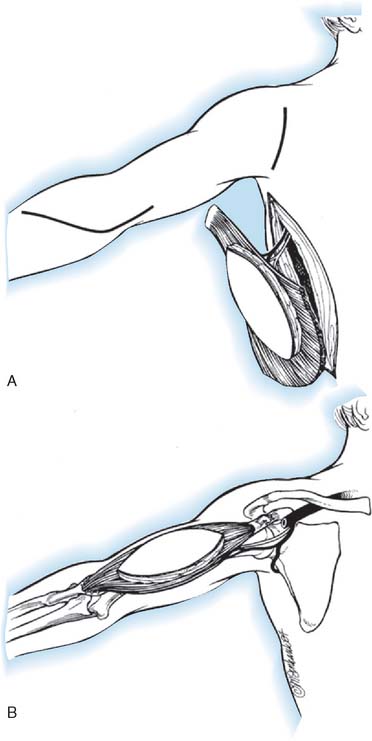
The patient is in the lateral position, and the upper extremity is draped free. A longitudinal incision is made parallel to the lateral border of the latissimus dorsi muscle, extending from the posterior border of the axilla to the iliac crest. The dissection of the muscle is begun along its lateral border and leads from distal to proximal. The neurovascular pedicle must be freed up to its origin in the axilla, and this requires the ligation of any branches of the thoracodorsal artery that enter the serratus anterior. Once the neurovascular pedicle is freed, the origin and insertion of the latissimus dorsi are sectioned. When the muscle is small, it is transplanted completely, but when it is entirely normal, it may be necessary to transplant only its lateral half. It is also possible to fold over the vertebral border of the muscle to make it more tubular in shape. Initially, a pocket is created in the anterior aspect of the upper arm, big enough to fit the muscle. Then the muscle is dissected from its origin to its insertion and transferred to the anterior arm based on its neurovascular pedicle. The muscle is shaped to simulate a biceps muscle and is fixed proximally at the clavicle and distally to the radial tuberosity.218
A deltopectoral incision is used to expose the coracoid process and free the tendon of the pectoralis major, behind which the transposed latissimus dorsi will be routed. The insertion of the biceps is then exposed through a bayonet incision over the anterior aspect of the elbow. If the paralyzed biceps is to be resected, the resection is carried out through these two incisions and care is taken to protect the neurovascular bundle of the arm and to preserve a long segment of distal biceps tendon. The latissimus dorsi muscle is then passed under the skin bridge of the axilla, protecting its neurovascular pedicle from any kinking or tension. The insertion of the transposed muscle is passed deep to the pectoralis major tendon and up to the coracoid process while its distal end is passed downward toward the elbow beneath the skin of the arm. It is convenient at this time to close the thoracic incision over several drains. The distal biceps aponeurosis is opened up and spread out so that it can be wrapped around the distal end of the latissimus dorsi. A significant amount of distal latissimus muscle usually has to be excised because it is too long. After the distal anastomosis is completed, the distal skin incision is closed. The proximal end is then fixed at the junction of the conjoined tendon with the coracoid process, the length of the transplant being adjusted so that the elbow remains spontaneously at 90 to 100 degrees of flexion with some trial sutures in place. When the proper length has been determined, the final suturing of the proximal end is carried out and the shoulder wound is closed. A plaster Velpeau bandage is then applied with the elbow flexed and the forearm supinated.
Myocutaneous transplantation of the latissimus dorsi is performed using the same technique, except that an appropriately sized segment of skin is left attached to the muscle (Fig. 71-9).
Unipolar Transfer
This description is based on that of Hovnanian (Fig. 71-10).117 The patient is positioned as for the bipolar transfer. The incision begins in the loin, extends along the lateral margin of the latissimus to the posterior axillary fold, and continues across the axilla and downward along the medial arm to the elbow. The muscle is detached from its origin, preserving part of the aponeurosis with it, and is freed up proximally. Branches from the thoracodorsal artery to the serratus anterior muscle are ligated. The muscle is then transferred to the anterior arm, where it is attached to the biceps tendon and periosteal tissues of the bicipital tuberosity. The arm is bandaged to the thorax for 3 to 4 weeks, at which time active and passive exercises are started.
The posterosuperior muscle fibers are shorter than the anteroinferior muscles; therefore, one must ensure that all muscle fibers are anchored caudally as well as cranially.70,153
Results
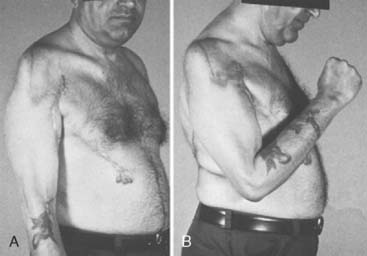
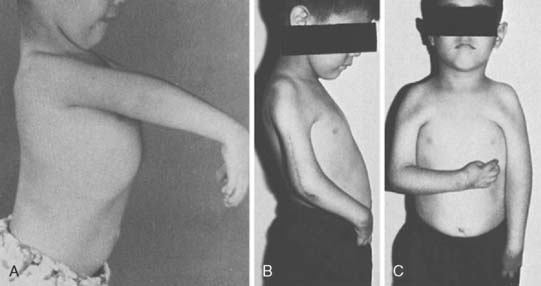
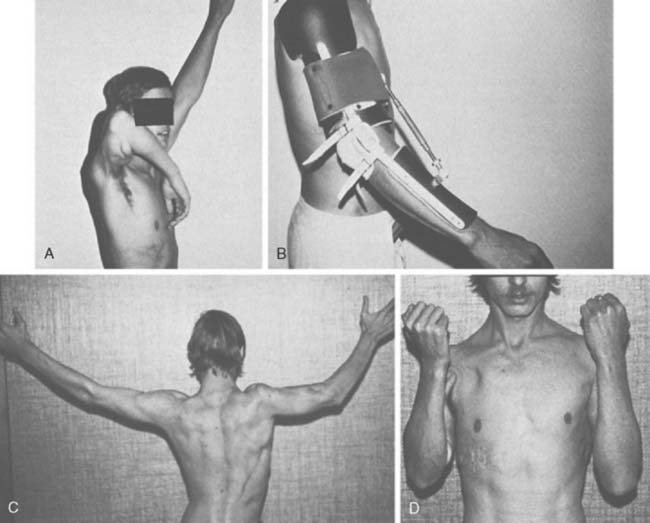
Experience is with small patient samples.11,27,117 Zancolli and Mitre254 observed eight patients who had had bipolar transplantation of the latissimus dorsi for more than 4 years. Active range of flexion was 105 to 140 degrees, and flexion strength varied from 0.7 to 5 kg. Flexion contractures of 10 degrees and 15 degrees occurred in two, and active supination of 20 to 50 degrees was achieved in six. Takami and others230 reported on two patients. One obtained elbow motion from 0 to 125 degrees and supination of 30 degrees and could lift 3 kg; the other had motion from 0 to 130 degrees with 60 degrees of supination and could lift 2 kg. Moneim and Omer171 achieved satisfactory flexion (100 degrees or more) in three of five patients. The two others achieved 65 to 70 degrees of flexion and initially had paralysis of the latissimus dorsi, so they were advised that the procedure should not be done unless the muscle was normal. Preoperative EMG evaluation of the muscle was recommended. Hirayama and associates111 reported four excellent results, three good results, and one failure among eight patients who had transfers of the latissimus dorsi. Botte and Wood28 noted satisfactory function in four of five patients treated with unipolar or bipolar latissimus dorsi transfer, and flexion that averaged 87 degrees (range 35 to 130). Chen48 achieved satisfactory function with average motion from 32 degrees of extension to 126 degrees of flexion in six patients who had bipolar transfers of the latissimus dorsi. They could lift only 1.5 to 2.5 kg, which was sufficient for most activities of daily living but not for heavy manual work. Concurrent skin coverage with restoration of elbow flexion is accomplished nicely with a latissimus dorsi myocutaneous flap.33,112,199,225 Clinical evidence of a functional deficit after transfer or transplantation of the latissimus dorsi is unusual. Russell and coworkers203 did document some minimal changes in shoulder strength and range of motion after transfer or transplantation of the latissimus dorsi in 24 patients.
Patients are able to hold weights between 8 and 15 kg,18 and up to 115 degrees of elbow flexion can be achieved (Fig. 71-11).171 Haninec and Smrcka102 reported M3 flexion up to 120 degrees at 16 months, unchanged from 5 months, in two patients.
Pectoralis Muscle Transfer
History
The first use of the pectoralis major muscle to restore elbow flexion was reported in the European literature in 1917 by Schulze-Berge,209 who transferred its tendon of insertion directly into the belly of the biceps. Subsequent modifications of the method of insertion of the pectoralis major included the use of fascia lata or strands of silk to form tendons of insertion, either into the biceps tendon or directly into the ulna.115,137,138,198 Clark59 reported the first really successful physiologic transfer of the pectoralis major in 1946. In his procedure, the sternocostal origin of the muscle with its separate nerve (medial pectoral) and blood supply was mobilized, passed subcutaneously down the upper arm, and attached to the biceps tendon. Seddon modified Clark’s operation by elevating a segment of rectus abdominis sheath in continuity with the distal end of the transplant to act as a tendon.210
Brooks and Seddon34 described a unipolar transfer of the entire pectoralis major muscle, employing the devascularized long head of the biceps as its tendon of insertion. They recommended this operation instead of a Clark transfer when either the lower part of the pectoralis major was paralyzed but the clavicular head strong, or the whole muscle was weak.213
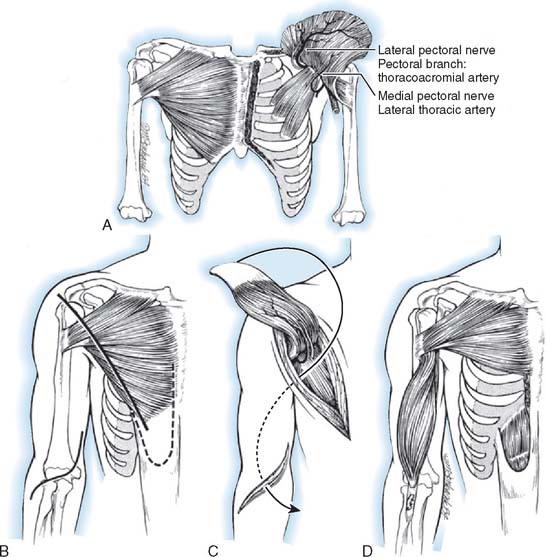
In 1955, Schottstaedt and others first reported bipolar transplantation of the chondrosternal portion (lower two thirds) of the pectoralis major on its neurovascular pedicle to restore elbow flexion.207 The humeral insertion was detached and shifted to the coracoid process, and the origin was transplanted to the biceps tendon. Carroll and Kleinman46 transplanted the entire pectoralis major muscle on both of its neurovascular pedicles. The muscle origin with its attached anterior rectus abdominis sheath was attached to the biceps tendon, and its tendon of insertion was secured to the anterior aspect of the acromion. With bipolar transplantation of the entire muscle, they noted increased shoulder stability, which obviated shoulder arthrodesis and improved mechanical efficiency for elbow flexion as compared with the unipolar transfer. Matory and coworkers157 transferred the lower sternocostal portion of the muscle with a 4-cm portion of rectus sheath mobilized with the medial and lateral pectoral nerves and accompanying vessels. The muscle was “tubularized” and woven through the biceps tendon, and the transverse aponeurosis was repaired to restore a pulley. Separate midline and deltopectoral incisions were used to avoid the undesirable scar from the standard pectoralis transfer approach.
Tsai and associates239 added unipolar transfer of the pectoralis minor muscle to a bipolar pectoralis major transplant, noting excellent strength of elbow flexion without endangering the two muscles’ common neurovascular bundles. The lateral half of the clavicular origin of the pectoralis major was left intact to preserve shoulder adduction.
The first transfer of the pectoralis minor to the paralyzed biceps to restore elbow flexion was done in 1910 by Bradford.30 Spira219 reported on one patient who had complete paralysis of the pectoralis major secondary to poliomyelitis who enjoyed excellent function after the transfer of the origin of the pectoralis minor into the distal biceps. Alnot7 recommended transferring the pectoralis minor to the biceps at the time of plexus exploration and nerve repair.
Concerns such as diminished excursion, the obligatory supination of the forearm, and the oblique scar on the chest have been the focus of several modifications to the technique. Especially in women, there are cosmetic concerns because the procedure may create breast asymmetry.155 Another concern has been that the pectoralis major muscle is not strong enough to substitute for the biceps in cases of complete absence of elbow flexion.218
Anatomy
Phylogenetically, the pectoralis major muscle evolved from three separate ones, and today it has a segmental configuration with an independent neurovascular supply (Fig. 71-12). The pectoralis major muscle has two constant (clavicular and sternocostal) subunits, and in about half of humans, an abdominal subunit.236 The clavicular portion of the muscle originates from the medial third of the clavicle. The sternocostal portion arises from the anterior surface of the manubrium and the body of the sternum and cartilage of the first six ribs. The abdominal portion, when present, arises from the aponeurosis of the external oblique muscle and is found posterior to the axillary border of the sternocostal portion. The lateral pectoral nerve, derived from the lateral cord and containing fibers from nerve roots C5, C6, and C7, supplies the clavicular and upper portions of the sternocostal parts of the muscle. The medial pectoral nerve, derived from the medial cord and containing fibers from C8 and T1, innervates the lower sternocostal and abdominal parts of the pectoralis major after piercing the pectoralis minor or passing around its lateral edge as it also innervates this muscle. The clavicular and upper sternocostal portions of the pectoralis major are supplied by the pectoral branch of the thoracoacromial artery. The lower sternocostal portion and the abdominal portion, when present, receive their blood supply from the lateral thoracic artery.
Unipolar Transfer
The unipolar transfer approach is based on that of Clark59 and Holtmann and associates116 (see Fig. 71-12). The patient is supine with a sandbag behind the shoulder. The arm is draped free and supported on a hand table or Mayo stand. An incision is made parallel to the lateral border of the pectoralis major muscle from the axilla to the seventh rib. The origin of the lower third of the muscle is detached from the sternum and fifth and sixth costal cartilages in continuity with a 6-cm segment of anterior rectus abdominis sheath. The sternocostal segment is carefully freed from the rest of the pectoralis major muscle to protect its nerve (medial pectoral) and vascular (lateral thoracic branches) supplies.
Partial Bipolar Transplantation of Pectoralis Major
This description follows that of Schottstaedt and colleagues.207 The muscle is exposed through an incision extending from 2.5 cm distal to the margin of the axilla along the lateral border of the pectoralis major muscle to within 5 cm of the midline. The lower half of the pectoralis major muscle is detached from its sternocostal origin and separated from the underlying pectoralis minor, care being taken to protect its nerve (medial pectoral) and vascular supply (lateral thoracic arterial branches).
The postoperative position of immobilization and the exercise program are as described earlier.
Complete Bipolar Transplantation of Pectoralis Major
This procedure is based on that of Carroll and Kleinman46 (Fig. 71-13). The patient is placed in the supine position, with a flat bolster under the blade of the scapula and the upper extremity draped free. A long, curvilinear incision is made from the seventh sternocostal joint proximally to two fingerbreadths inferior to the clavicle. The incision continues laterally to the coracoid process, then distally along the anteromedial aspect of the arm to the level of the axilla.
With the acromion and the entire pectoralis major muscle exposed, a second curvilinear incision is made over the antecubital fossa with its transverse limb across the fossa and the longitudinal limb extending medially and distally 6 cm. The entire pectoralis major muscle is then detached from its origin along the medial half of the clavicle and its sternocostal border with a 10- by 4-cm strip of attached rectus abdominis fascia. In the process of freeing the pectoralis major from the chest wall and the underlying pectoralis minor, meticulous care is given to preserving its two neurovascular pedicles. Care must be taken during dissection because the muscle’s neurovascular bundle can easily be avulsed.218
Results
The original description was of only one patient, who had flexion limited by only 15 degrees, extension limited by 5 degrees, and flexion power 40% of normal 16 weeks after a partial bipolar transfer of the pectoralis major.59 Seddon noted excellent results (powerful flexion against gravity and resistance) in seven of 16 Clark’s transfer patients on whom he reported.210 In the remainder, the elbow could be flexed against gravity and slight resistance. Seven patients regained supination against resistance (from 10 to 90 degrees). In four cases in which the pectoralis major power was subnormal, the pectoralis minor was used in addition. D’Aubigne reported excellent active flexion, independent of shoulder adduction, in two patients who had Clark’s transfer.62 Using the unipolar transfer of the pectoralis major into the devascularized long head of the biceps, Brooks and Seddon34 achieved three excellent, three good, and two fair results (less active flexion than passive or flexion against gravity but not against resistance), and two complete failures. Two of the good results, however, required second operations (triceps to biceps) because of simultaneous action of the pectoralis major and the triceps, a phenomenon they ascribed to axonal confusion during regeneration after brachial plexus lesions. Holtmann and associates116 noted useful active elbow flexion through a mean range of 96 degrees, accompanied by supination of the forearm, in all seven of Clark’s transfers on which they reported. Four of the patients had arthrogryposis, and, in all cases, the transfer was extended with a 6-cm segment of anterior rectus sheath. Leffert and Pess141 reported the results of 15 pectoralis transfers: good result in eight, improvement in four, and no improvement in three cases. They recommended that shoulder fusion be carried out several months before the transfer in patients who have a paralyzed shoulder.
Botte and Wood28 recorded satisfactory results in all five patients who had bipolar pectoralis major transfers but cautioned against using it in women because of the cosmetic disfigurement. Matory and associates157 transferred the lower sternocostal segment of the pectoralis major in seven patients and achieved a functional range of elbow flexion (mean 98 degrees) in all. Extension loss was only 15 to 25 degrees, and sustained flexion strength averaged 8 pounds. Tsai and others used modified bipolar transplantation of the pectoralis major (leaving the lateral half of the clavicular origin intact), supplemented with a unipolar transfer of the pectoralis minor, in four patients.239 Three of the four achieved excellent results (full extension with at least 60 degrees of flexion), and the other patient required a secondary Steindler’s flexorplasty.
Spira achieved strong flexion through a range of 135 degrees with virtually full extension in a patient with total paralysis of the pectoralis major and elbow flexors secondary to poliomyelitis whose pectoralis minor origin he transferred to the biceps tendon.219
Arthrogryposis
In arthrogrypotic children, Lloyd-Roberts and Lettin149 observed that preliminary posterior release and triceps lengthening may be necessary to secure passive flexion. They modified Clark’s transfer by obtaining a longer anterior rectus abdominis sheath and inserting the transfer into the ulna, because the biceps tendon frequently is absent. All seven patients who had the modified transfer could get their hands to their mouth against gravity and some resistance. Atkins and coworkers10 transferred the pectoralis major by detaching its clavicular origin to allow its insertion to be tied into the biceps tendon remnant in six children, five of whom were arthrogrypotic. The operations achieved good results in three, three others were fair, and one was poor. Doyle and coworkers74 reported on seven cases of arthrogryposis in which a bipolar transfer of the entire sternal head of the pectoralis major with a generous tongue of anterior rectus abdominis sheath was performed. All seven patients achieved improved elbow motion, and six were able to feed themselves using only one hand. Three of the four patients reported on by Carroll and Kleinman46 who had bipolar transfers of the entire pectoralis major muscle achieved excellent results. Because of the increased shoulder stability achieved with this operation, shoulder arthrodesis was not necessary.
Comparative Studies
Segal and associates, using combined objective and subjective evaluations, compared the results of 13 flexorplasties, three triceps transfers, 17 Clark’s transfers, and eight Brooks-Seddon transfers.213 Fifty-three percent of Clark’s transfers and 75% of Brooks-Seddon transfers either had fair results or failed, whereas only 31% of the flexorplasties had a fair result and there were no failures. The decreased flexion contracture of the elbow, simultaneous contraction of the triceps when the pectoralis transfer flexed the elbow, and undesirable shoulder adduction and internal rotation movements accompanying elbow flexion all contributed to less satisfactory results in the pectoralis transfer group. These authors, and Clark himself, advised against using the pectoralis transfer when some biceps activity was present or could be anticipated.61
Rostoucher200 reported the results in 15 cases of pectoralis minor transfer, with good (M4, <120 degrees flexion) results in 53%. Six failures were salvaged by a secondary Steindler flexorplasty, improving the results to 80% good. The results were good or very good in seven of the nine C5-6 lesions. One good result and three failures were reported in the patients with C5-7 lesions, possibly owing to the lower strength of power of the transferred muscle in these combined lesions.
Complications
Doyle and associates74 reported on two patients who developed transient nerve palsy, one medial and one radial, after pectoralis major transfer. Both problems resolved completely after several weeks. Simultaneous contraction of the triceps when the elbow is flexed by the transfixed pectoralis major was noted in three cases by Segal and coworkers.213 All three had brachial plexus injuries and initially had had paralysis of the triceps and elbow flexors. Axonal confusion after regeneration seems to be the likely explanation, but the complication cannot be predicted, because in six other cases in which the triceps initially was paralyzed and later recovered, no simultaneous flexor-extensor action developed.
Triceps Transfer
History
Biesalski and Mayer23 and later Vulpius and Stoffel246 were the first to describe the transfer of part of the triceps muscle to compensate for the loss of elbow flexion. Since that time, it has been used in the treatment of the flaccid elbow in brachial plexus palsy to a varying degree.7,28,43 Use of the triceps muscle to restore elbow flexion was condemned by Steindler in 1939 because, he felt, “Loss of the normal function of the triceps is too great a sacrifice.”223 Bunnell, however, described a successful triceps transfer.39 In his opinion, “It is more important to flex than to extend the elbow.”40 Some modern authors have again abandoned this technique because the concurrent loss of elbow extension, critical for joint stabilization, is considered too disadvantageous.233 In 1955, Carroll described the technical details43 and, in 1970, reported the results of triceps transfer in 15 patients.45 They advised against performing triceps transfer bilaterally or in a patient who uses crutches. Botte and Wood28 also advised against the procedure in patients who use a cane or a wheelchair and those who have to work with their hands overhead. Several authors have recommended triceps-to-biceps transfer with poorly functioning biceps when there is co-contraction of a reinnervated triceps.7,28,202 This may yield a favorable result even when there is insufficient triceps strength. Otherwise this would be a contraindication. A clear disadvantage is the loss of active extension but when compared with transfers of the hand flexors and extensors, the power of elbow extension is greater, with fewer extension deficits.202
Anatomy
The triceps muscle arises from three heads—one from the scapula (long head) and two from the posterior humerus (lateral and medial heads). The medial head has an extensive origin from the posterior shaft of the humerus, extending from the insertion of the teres major to within 2.5 cm of the trochlea of the humerus. The muscle is supplied by the radial nerve (C7 and C8, with a smaller contribution from C6) through multiple branches that arise above the spiral groove. An exception is the posterior branch to the medial head, which leaves the radial nerve just as it enters the groove.32
Technique
This description is based on that of Carroll (Fig. 71-14). The patient is placed in the distal decubitus position and the extremity is draped free. A posterior midline incision is made that extends over the distal two thirds of the arm and then curves laterally to the olecranon, extending distally over the subcutaneous border of the ulna for 5 cm. The skin flaps are widely undermined so that the ulnar nerve can be exposed medially and the lateral intermuscular septum laterally. A tail of periosteum as long as possible is raised from the ulna in continuity with the triceps insertion, and the medial head is mobilized from the distal third of the shaft of the humerus. The radial motor nerves enter the muscle in the interval between the lateral and medial heads as the radial nerve enters the spiral groove. The raw surface of the stripped medial head is then covered by suturing its two edges together into a tube.
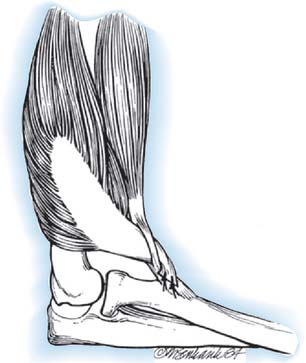
FIGURE 71-14 Transfer of triceps insertion around the lateral side of the elbow to the biceps tendon.
The biceps tendon is exposed through a curvilinear incision in the antecubital fossa, and the tendon is dissected free to its insertion onto the radius. The biceps tendon is split longitudinally. The triceps tendon is passed through a laterally placed subcutaneous tunnel between the two incisions, superficial to the radial nerve. The triceps tendon is then passed through the split biceps tendon and sutured in place under maximum tension with the elbow at 90 degrees of flexion and the forearm in full supination. As an alternative, the triceps tendon can be attached to the radial tuberosity using a pull-out wire technique, as described by Bunnell.40 The elbow is immobilized in a posterior splint at 90 degrees of flexion and full supination for 4 weeks, at which time active exercises are begun.
Results
Carroll and Hill recorded the results of 15 triceps transfers.45 Five successes (flexion against gravity with ability to bring the hand to the mouth), one limited result, and one failure were noted in the seven trauma or paralysis patients. Among the eight patients with arthrogryposis, there were five successes, one limited result, and two failures. The average range of motion was 116 degrees and average flexion contracture 24 degrees in the first group, whereas in the arthrogrypotic group motion averaged only 43 degrees and the average fixed flexion deformity was 59 degrees (Fig. 71-15). Seven of the eight arthrogrypotic patients required adjunctive procedures such as excision of a dislocated radial head or modified elbow arthroplasty to achieve an acceptable passive range of motion before triceps transfer. Williams reported improvement measured by flexion against gravity in all 19 arthrogrypotic elbows submitted to triceps transfer, and he did not hesitate to perform the procedure bilaterally.248 However, extension was usually restricted to approximately a right angle because of the tenodesis effect of the triceps. Other authors have reported variable improvement, if any, after triceps transfer in arthrogrypotic patients.74,96,169 Leffert and Pess141 recorded four good and three improved results in seven patients who underwent triceps-to-biceps transfers. Botte and Wood28 reported mean flexion of 125 degrees in three patients who had triceps-to-biceps transfers. No effort was made to mobilize elbow extension past a 30-degree flexion contracture, because this was thought to be mechanically advantageous for elbow flexion. Alnot7 achieved 10 good and one acceptable result in 11 triceps-to-biceps transfers. Three were done after spontaneous triceps recovery, two in patients with co-contraction, and eight after nerve grafting. The investigators noted that 36% of their patients had a dominant triceps innervation from C8 and T1, making it available for transfer in C5, C6, and C7 lesions in these patients. Rühmann202 reported on a series of three patients, resulting in active elbow function in all patients (mean flexion 113 degrees, range 90 to 30 degrees) with function against resistance (M4-5) and no passive stretching deficit. Loss of active elbow extension was not considered of great importance to these patients. Complications have not been reported for any of these series.
Miscellaneous Transfers for Elbow Flexion
Sternocleidomastoid transfer
In 1951, Bunnell reported on a single patient with a paralyzed upper extremity secondary to poliomyelitis who gained elbow flexion from transfer of the insertion of the sternocleidomastoid muscle, extended with a strip of fascia lata, into the bicipital tuberosity.44 Carroll reported 80% satisfactory results in 15 cases with this technique.40 Kumar et al130 described a modification to Bunnell’s40 original technique, whereby the sternocleidomastoid muscle is sutured to a fascia lata tube under the clavicle to eliminate the bow-stringing and unsightly skin fold that would otherwise occur during flexion.
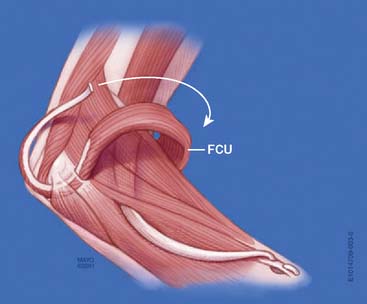
Flexor Carpi Ulnaris Transfer
In 1975, Ahmad published a single case report of a patient with a brachial plexus palsy who achieved 130 degrees of elbow flexion after the transfer of the insertion of the flexor carpi ulnaris, turned back on itself, into the distal humerus.1 No subsequent reports of this transfer have appeared in the literature.
Free-Functioning Muscle Transfers
History
Free muscle transfer for elbow reanimation is indicated when there are no available local donor muscles,59,155 due to trauma, tumor resection, or chronic denervation. Microneurovascular muscle transfer for recovery of motor function in cases of severely injured extremities has grown from successful experimental studies81,129,231,234 into a clinical reality119,120,152,184 and is now a useful reconstructive option in these difficult cases.50,52,69 Despite requiring meticulous technique and microsurgical skills, overall survival and reinnervation of transplanted muscles are consistently successful in large series.14,52,69 Although reconstruction of elbow function alone with a free-functioning muscle transfer in acute cases of brachial plexus palsy is inferior to direct neurotization of the musculocutaneous nerve when performed within 6 months of injury,3,181 it is indicated to restore elbow flexion in chronic cases,69 when there is irreparable injury to the biceps or distal portion of the musculocutaneous nerve, or when used for a dual purpose combining elbow flexion with animation of the wrist or hand.14,19,67,68,71 Important prerequisites for free muscle transfer include adequate skin cover; a normal or at least functional arc of passive elbow motion; a compliant, stable soft tissue bed permitting tendon gliding; and available motor nerves and blood vessels. The presence of vascular bypass grafts at the planned site of vessel repair, for example, is in general a contraindication to functioning free muscle transfer.
In cases of flaccid elbow paralysis resulting from a brachial plexus injury or lesion, innervation may be provided, even when the limb is flail and all nerve roots avulsed, by direct nerve transfer of two to four intercostal motor nerves or spinal accessory nerve, or following regeneration across a banked nerve graft from the contralateral C7 root.3,52,69,70,153,218 In more favorable circumstances, intraplexal nerve transfer using two to three fascicles of the ulnar nerve supplying flexor carpi ulnaris motor branches,186 median nerve fascicles supplying flexor carpi radialis,147,151,157 or direct repair/graft of the musculocutaneous nerve itself will provide superior results and minimal donor morbidity. In chronic cases requiring free functioning muscle transfer, neurotization of the motor nerve can also be performed using an ulnar nerve fascicle.105,229 In any of the above-mentioned nerve procedures, efforts should be made to place the nerve transfer or repair in close proximity to the transferred muscle motor point, thus minimizing the reinnervation time. An accurate epineural repair using microsurgical methods is important. Intercostal nerve transfer is performed with the shoulder in maximal external rotation and 90 degrees of abduction, to allow postoperative shoulder motion up to this positioning limit.66
At surgery, certain principles must be followed to optimize results. It is desirable to place the transferred muscle at its optimal resting tension, with a straight line of pull and anatomic insertion, avoiding crossing of a flail or unstable shoulder when possible, and no bow-stringing.66 Best results are seen when antagonistic (triceps) function is either present or reconstructed through direct reinnervation, tendon transfer, tenodesis, or the addition of another free-functioning muscle transfer. Use of the muscle for a second purpose has been demonstrated to weaken elbow flexion.16
Selection of Donor Muscle
To be useful as a biceps substitute, the free muscle must have adequate strength and excursion, be expendable, and have a neurovascular pedicle permitting microsurgical transfer and nerve repair.66 Strength is roughly proportional to the cross-sectional area and weight of the muscle, whereas excursion is most directly related to fiber length but is also affected by fiber orientation relative to the muscle long axis (pennate vs. strap muscle) and fascial connections. The points of origin and insertion, and distance from the joint center are further factors to be considered.66 Tendon quality (both proximal and distal) affects the ease and quality of muscle insertion through tendinous and neurovascular connections.
Volitional control should be provided through a single motor nerve to allow reinnervation by direct nerve transfer. The pattern of blood supply must maintain muscle perfusion following transfer. Mathes and Nahai156 have classified muscles based upon blood vessel pattern, and have determined that their type I (single vascular pedicle), type II (one dominant and minor pedicles) and type V (one dominant and segmental pedicles) groups may serve as functioning free muscle donors. Examples of each include rectus femoris (type I), gracilis and biceps femoris (type II), and latissimus dorsi (type V).
A comparison of available donor parameters to biceps has led to recommendations including latissimus dorsi or vastus lateralis,218 or rectus femoris3 muscles. The rectus femoris muscle, for example, being bipennate and having a short muscle fascicle length of 6 to 7 cm, has a relatively low contractile capacity.153 Therefore, it is not a good choice to reconstruct two functions and is prone to create an elbow flexion contracture.69 On review, the gracilis muscle would seem ill-suited to biceps replacement because it is too weak. However, gracilis transfers have become the preferred choice of most authors because of its exceptional length, proximal neurovascular pedicle, and excellent distal tendon.24,142 There is a defined but low level of donor-site morbidity, with a nonsubjective loss of hip adduction of 11%, hypesthesia or dysesthesia of the cutaneous territory of the obturator nerve in 50%, and aesthetic changes to the thigh.64 The satisfactory results reported using the gracilis muscle for elbow function belie the anatomic measurements that would seem to render it inferior as a biceps replacement.24
Patient Considerations
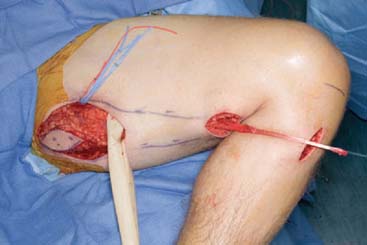
Free muscle transfer is a lengthy procedure, and careful preparation of the patient is important. Adequate positioning and protection of bony prominences as well as maintenance of body core temperature (that facilitates peripheral perfusion) are critical aspects of preparation. The surgical procedure requires meticulous planning. An adequate or reconstructible skin cover and bed for tendon gliding are important considerations before a free-functioning muscle is transferred. When a muscle is used for both soft tissue coverage and functional restoration of elbow function, as is often the case in large post-traumatic reconstructions, the resultant excursion may be insufficient for a functional range of motion.26 An expendable motor nerve must be in the vicinity of the muscle neurovascular pedicle. Preoperative assessments should also include a magnetic resonance or conventional angiogram to prepare for vascular physiologic or post-traumatic variations. The rate of subclavian or axillary artery avulsion, especially associated with first rib and upper extremity fractures, has been reported to be as high as 10% to 25% in brachial plexus injury.100,227 In gracilis transfer, the donor thigh should be the contralateral side, due to the resulting optimal placement of the neurovascular pedicle.
Gracilis Transfer For Elbow Flexion
Preparation of the Recipient Site
If the surgical plan includes use of spinal accessory or intercostal nerve transfer, these nerves are next prepared. The spinal accessory nerve is identified through the same incision on the deep anterior surface of the trapezius muscle. A nerve stimulator demonstrates its motor function, and dissection is used to spare two branches, those to the sternocleidomastoid muscle and to the upper part of the trapezius. This preserves muscle function that provides scapulohumeral movement. This is especially important if the shoulder is to be arthrodesed.2 In order to avoid needing a nerve graft, a dorsal approach can be used to dissect more distally, as described by Vathana.244 This provides nerve length reaching to the terminal branches of the brachial plexus and certainly to the motor nerve supplying the free functioning muscle. Another option is to harvest the obturator nerve from the level of the obturator foramen, permitting its length to reach 10 cm. The phrenic nerve remains an alternative choice when no plexal nerves are available, and staged reconstruction with contralateral C7 has also been described, although few results have been reported.56 For hand reanimation, contralateral cross-chest C7 donors have been associated with inferior outcome in some hands,235 whereas others report more encouraging preliminary results.50
Should intercostal nerves be required, the deltopectoral incision is extended across the anterior wall of the axilla, curving beneath the nipple to allow elevation of the pectoralis muscles caudally to expose the ribs. Generally, two intercostal nerves will be sufficient to satisfy the obturator branch fascicles. Usually the third and fourth intercostal nerves are used (T3 and T4), easily transferred to lie next to the thoracoacromial trunk. The periosteum is dissected free circumferentially from ribs from the costochondral junction anteriorly to the axilla. By incising the deep (osseous) surface of the reflected interior periosteum, the motor branch is identified at the nipple line and dissected to the costochondral junction. Meticulous dissection is needed to avoid injury to the pleura. Simultaneous biceps reinnervation with the T5 and T6 motor nerves may also be performed. For each transfer, the shoulder is positioned in 90 degrees of abduction and maximal external rotation to ensure adequate nerve length.
Harvest of the Gracilis Muscle
The gracilis muscle is a superficial muscle of the medial aspect of the thigh. It is supplied by the obturator nerve, entering the muscle obliquely, cephalad to the main vascular pedicle, at 6 to 12 cm from its origin. The vascular supply is from a 4 to 6 cm long branch of the profunda femoris artery located 8 to 12 cm from the muscle origin and passing between the adductor longus anteriorly and the adductor magnus posteriorly. The gracilis may be harvested to include a paddle of skin serving as a buoy flap centered at this location (Fig. 71-16).154 Three small incisions are sufficient, beginning with exposure of the distal tendon medial and proximal to the tibial tubercle, then a second posteromedially overlying the tendon in the distal thigh to visualize the distal musculotendinous junction. A more proximal incision is made including the skin paddle at the location of the proximal neuromuscular junction. The position of the skin, centered over the muscle, is facilitated by palpation with traction on the distal tendon previously exposed. The skin paddle base includes fascia overlying both the adductor longus anteriorly and the adductor magnus posteriorly. The dissection then converges on the intermuscular plane between the adductor muscles, at which time the neurovascular pedicle is easily identified. The motor nerve lies cephalad, aligned roughly 45 degrees to the muscle, whereas the vessels pass perpendicularly toward the gracilis muslce. Dissection of the vessels requires careful elevation of the adductor longus and ligation of multiple small muscular branches from the pedicle to it. The nerve is dissected as far cephalad as feasible, often aided with fiberoptic-illuminated retractors, and then divided. The muscle is next released from the pubic symphysis proximally and from the pes anserinus distally. Use of lighted retractors permits tunneling between the three incisions to harvest the muscle. One or more secondary pedicles distally require ligation. Only when the recipient site is fully prepared and the operating microscope positioned is the vascular pedicle divided and the muscle immediately transferred (Fig. 71-17).
Free Muscle Transfer
When all is ready, the gracilis muscle is brought to the arm, and the proximal tendon connections made and the distal tendon tunneled into the antecubital incision, but not yet fixed. The microarterial anastomosis is made next, most frequently performed end to end to an appropriately sized arterial branch of the thoracoacromial trunk or thoracodorsal artery. A single venous connection to the cephalic vein is made. The nerve transfer is next performed, followed by repair of the distal tendon to the biceps tendon (Fig. 71-18). Tension is adjusted to produce a 30-degree flexion contracture at the elbow. We have found this method to provide reliable postoperative arc of motion without use of marking sutures, as described by Manktelow and Zuker, which require more extensive incisions for muscle exposure in the thigh before harvest.153 The distal end can also be sutured to more distal targets, if needed. In our brachial plexus practice, for example, a single gracilis muscle transfer is sometimes used to provide finger flexion in addition to elbow flexion (Fig. 71-19). The distal gracilis tendon is prolonged with a forearm tendon graft (usually the paralyzed flexor carpi radialis) to reach the flexor digitorum profundus and flexor pollicis longus tendons. To avoid bow-stringing, an antecubital pulley is used, which is either the intact lacertus fibrosis or a fabricated antecubital pulley using the flexor carpi ulnaris (Fig. 71-20). In such a procedure, we also reinnervate the biceps with two additional intercostal nerves to improve elbow flexion strength and reconstruct antagonist triceps function with nerve transfer or grafting.
Technique—Two-Stage Reconstruction
Doi et al66,67,71 have described a method of double gracilis transfer combined with neurotization of the triceps and sensory neurotization of the hand to provide patients with four or five brachial plexus root avulsions with shoulder stability and function, as well as active elbow flexion and extension, protective hand sensibility, and rudimentary hand grasp and release. Simple nerve transfers, such as intercostal to musculocutaneous nerve to reconstruct elbow function alone, is not included in this procedure, even in acute cases. In the first stage, the gracilis muscle attached to the clavicle is neurotized with the spinal accessory nerve and an available C5 root, phrenic nerve, or contralateral hemi-C7 transfer is used for shoulder motor reconstruction. The gracilis tendon is sutured to the extensor digitorum communis tendon in the proximal forearm (Fig. 71-21). The applied tension is such that full finger flexion is permitted with the elbow flexed. Using wrist extensors as the distal insertion instead of the finger extensors may improve finger flexion through a tenodesis effect.24 Originally, the brachioradialis muscle was used as a pulley to prevent bow-stringing of the gracilis muscle transfer at the elbow. However, bow-stringing would still sometimes occur, prompting the use of a distal portion of the flexor carpi ulnaris, which is detached and used to create a pulley at the level of the proximal forearm (see Fig. 71-20) (David Khoo, personal communication). Alternatively, the flexor carpi radialis has been used for the same purpose.20
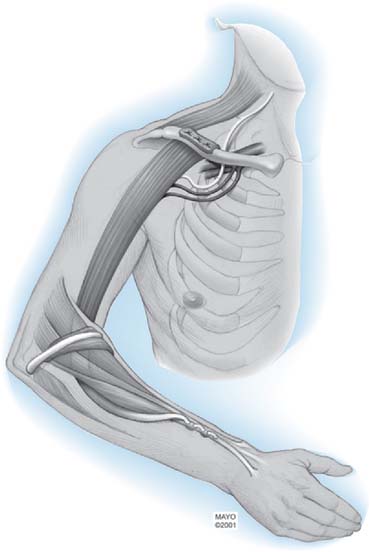
FIGURE 71-21 Doi stage I procedure.71 Free muscle transfer is used for restoration of elbow flexion and wrist extension. This procedure is usually combined with neurotization of shoulder girdle musculature (not shown).
(By permission of Mayo Foundation for Medical Education and Research. All rights reserved.)
The second-stage gracilis transfer, used for finger flexion, is neurotized by two or three intercostal nerves, and is attached to the second rib with multiple drill holes proximally (Fig. 71-22). Vascular connections are to the thoracodorsal vessels. Through a separate incision in the forearm, the flexor digitorum profundus and flexor pollicis longus tendons are sutured in such a way as to create a key pinch and grasp on traction. The gracilis tendon is tunneled under the pronator teres muscle, which serves as the pulley for this transfer, and is secured to the flexor digitorum profundus and flexor pollicis longus tendons using a Pulvertaft weave. Tension is set such that the fingers can extend with elbow flexion and such that the fingers and thumb can close with elbow extension. The second stage is completed by neurotization of the motor branch of the triceps brachii muscle with two intercostal motor nerves and the neurotization of the lateral portion of the median nerve with intercostal nerve sensory branches to restore protective hand sensibility (Figs. 71-23 and 71-24). The supraclavicular nerve sensory rami may also be used for this purpose.
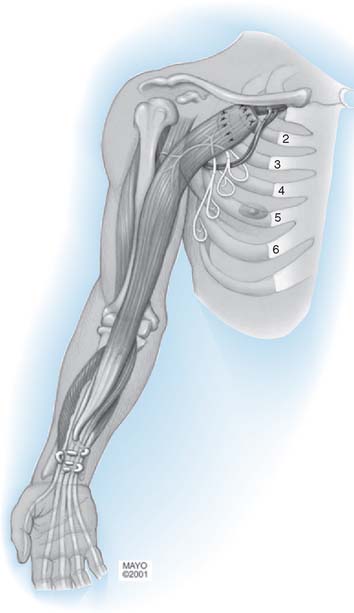
FIGURE 71-22 Doi stage II procedure.71 A second free-functioning gracilis transfer for finger flexion is performed, neurotized with two intercostal motor nerves. Additional nerve transfers for protective hand sensibility and triceps motor function are also performed (see Fig. 71-23).
(By permission of Mayo Foundation for Medical Education and Research. All rights reserved.)
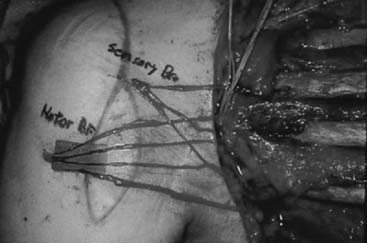
FIGURE 71-24 Intercostal nerves can be divided into sensory and motor branches under the operating microscope.
Shoulder function and stability are important pre-requisites for prehension, and for this reason, Doi has augmented his double free-muscle transfer technique with neurotization of the suprascapular nerve with the C5 or C6 nerve root via a nerve graft. If these are not available, neurotization can be performed from a contralateral C7 nerve donor and a vascularized ulnar nerve graft.65
Latissimus Dorsi Transfer
The latissimus dorsi as a free vascularized muscle flap offers the advantages of a large and long vascular pedicle (diameter 2-3 mm, length 8-12 cm), a single motor nerve, and the relatively large area of skin that can be carried as an island. For this reason, some authors prefer it as a first choice for restoration of elbow flexion.218 Adequate muscle innervation must be determined preoperatively, because C5, C6, and C7 may also be involved in the injury and not provide reliable latissimus function after transfer.
Results
Early results of free-functioning muscle reconstruction for elbow flexion were variable. Gilbert reported an excellent result in a 5-year-old child treated with free transplantation of the gracilis, using the musculocutaneous nerve as the recipient nerve, after traumatic destruction of the biceps muscle.91 Five other patients (two with poliomyelitis and three with brachial plexus palsies), however, achieved no useful flexion after a gracilis transplantation that was innervated through a long nerve graft connected to the sternocleidomastoid nerve. O’Brien and colleagues also recorded a failure in one patient 8 years after a free gracilis transplant that was innervated by the second and third intercostal nerves.184 Hirayama and others111 used a free latissimus dorsi muscle flap innervated by the intercostal nerve in a 35-year-old man with a complete brachial plexus palsy and disuse atrophy of the biceps. Three years later, he had 90 degrees of flexion and a 30-degree flexion contracture. Botte and Wood28 achieved no useful flexion in three patients who had free latissimus dorsi transfers (two had distant neurotization) and recommended against the procedure. Manktelow152 reported on one patient who had a free gracilis transfer that was innervated by the musculocutaneous nerve to replace crushed biceps and brachialis muscles. Eighteen months after surgery, the patient could flex to 120 degrees from the fully extended position with a 15-pound weight.
More recently, Chuang and associates52 reported on the results of 38 cases of brachial plexus injury treated with free-functioning gracilis muscle transfer for elbow flexion. Thirty-one muscle transfers were reinnervated by intercostal nerves; three cases with traumatic loss of the biceps treated at an interval of 6 to 12 months were reinnervated by the original musculocutaneous nerve; the remaining four cases were reinnervated by the spinal accessory nerve. All 38 patients were followed for at least 2 years. All three patients with traumatic biceps loss regained M4 flexion power within 1 year of rehabilitation. Where three intercostal nerves were used for reinnervation, 78% regained M4 strength. Failures (M3 or less) in the intercostal nerve neurotization group were attributed to the site of proximal muscle insertion, with 1 of 5 failures where attachment was to the coracoid process of the scapula (N = 20), and 4 of 5 failures where attachment was to the second rib or the clavicle (N = 11). Transfer for combined elbow flexion and finger flexion yielded only a limited range of motion (0 to 90 degrees) but M4 strength. Finger flexion was attributed to a tenodesis effect rather than to actual muscle excursion. Rehabilitation for patients reinnervated with two instead of three intercostal nerves was longer (more than 2 years) and yielded less power. After a shorter delay to treatment (6-12 months), reinnervation of a free-functioning muscle by two intercostal nerves supplemented by two intercostal nerve transfers to the musculocutaneous nerve yielded M4 power from both the free-functioning muscle and the brachialis muscles. Last, the group reinnervated by the spinal accessory nerve achieved only M2 to M3 elbow flexion strength even after 3 years’ rehabilitation.
Barrie et al14 reported our results at least 1 year after gracilis muscle transfer for elbow flexion and found ≥M4 strength in 79% of single-function transfers, and in 63% of muscles transferred for combined motion (P > 0.05). Comparing results between the two stages of the double gracilis transfer, 75% of stage I transfers (elbow flexion and wrist extension) achieved ≥M4 strength, whereas only one of seven procedures for finger flexion yielded ≥M4 strength. Protective sensation returned in two of seven patients who underwent a two-stage reconstruction.14
After double gracilis transfer, Doi et al68 restored excellent to good elbow flexion in 96%, and 65% achieved more than 30 degrees of total active finger motion with the second stage. Other authors have not been able to consistently restore prehension, although in general, some active flexion is achieved.24 Active motion may be improved by a tenolysis68 or improving the gliding surface of the muscle transfer with silicone sheeting,24 although some authors144 believe that weak muscle excursion is usually due to a lack of muscle recovery as opposed to tendon or muscle adhesion. The number of patients achieving M4 strength is slightly diminished when a single free muscle transfer is used for two functions, and it seems the results are more predictable when a single muscle is used for a single function.24 Shoulder stability and triceps recovery are important added functions that improve outcome after free muscle transfer.65,72 Results in children seem to be superior to those in adults, presumably due to the increased potential for nerve recovery.13,106
In a group of 58 single or double free muscle transfers, Doi et al69 found significantly shorter times to reinnervation by the spinal accessory nerve (mean 3.3 months) as opposed to the intercostal nerves (mean 5.25 months) (P < 0.002), with no difference in motor nerve length. These authors found no difference in time to reinnervation between gracilis, latissimus dorsi, and rectus femoris muscles. The power of elbow flexion was equally excellent in these muscles, and no significant difference in the means was found. The rectus femoris muscle, however, had a significantly lower range of motion (mean 34 degrees) and a flexion contracture at the elbow, compared with the gracilis muscle (mean 74 degrees) and latissimus dorsi (mean 78 degrees). When reinnervated with the spinal accessory nerve, the latissimus dorsi transfer gave the most powerful contraction and the greatest range of joint motion. No statistical relationship could be found in brachial plexus patients younger than 50 years of age between patient age and functional recovery or time to reinnervation. Those patients treated after oncologic resection and requiring postoperative chemotherapy showed no significant difference in time to reinnervation. Patients whose shoulder function was augmented by ipsilateral suprascapular nerve neurotization demonstrated statistically better ranges of motion for shoulder flexion and abduction.65
RESTORATION OF ELBOW EXTENSION
INTRODUCTION
For all persons with tetraplegia, the restoration of elbow extension is uniformly considered essential.¶ In tetraplegia, the deltoid, biceps, brachialis, brachioradialis, and supinator muscles are often spared because of their high-level innervation (C5-C6).127 The triceps muscle (C7) is usually paralyzed, however, and requires reconstruction.80,131,166,190 In large series of tetraplegic patients, the percentages of triceps muscles achieving less that M3 grade strength vary from 59%78 to 73%.167 Active elbow extension is required to reach against gravity, for wheelchair propulsion, pressure relief, independent transfer, and to adequately position the hand for useful ADL function.86 Active elbow extension improves hand positioning by providing an antagonist to elbow flexion, and improves elbow stability after tendon transfer of the brachioradialis and extensor carpi radialis longus muscles.110,131 Because these muscles have a 32-mm and 19-mm excursion on elbow extension (tenodesis effect), respectively, active restoration of elbow extension should be achieved before hand rehabilitation is undertaken.29 Additionally, the presence of an active elbow extensor facilitates prehension movements with the arm in pronation, probably by stabilization of the elbow during the transport of objects.114,133
The posterior deltoid-to-triceps transfer and the biceps-to-triceps transfer are two frequently used transfers for elbow extension. The posterior deltoid-to-triceps transfer has several disadvantages,131 including the need for a free tendon graft,166 long immobilization,159 and decreased strength in time, possibly due to stretching.159,190 Early mobilization is associated with poor results after this transfer.63,134 The biceps-to-triceps transfer can be used when the supinator and brachialis muscles are strong enough to replace the function of the transferred biceps. There are several advantages to this procedure, including the ability to simultaneously correct flexion-supination deformities, no need for a tendon graft, earlier time to mobilization, and less extension power loss over time.
NEUROTIZATION
History
Neurotization of both triceps and biceps muscles with intercostals in adults will result in crippling co-contraction and should be avoided.233 If biceps neurotization has been carried out by intraplexus donors, the triceps may be neurotized by three intercostal nerves. However, because some authors prefer to sacrifice the triceps and use it for biceps function, many do not attempt neurotization. Many patients suffering from co-contraction between the biceps and triceps have co-contraction between the deltoid and the biceps, giving musculocutaneous nerve neurotization cases the advantage of separate motion of the shoulder and elbow.123
Neurotizations for elbow extension in brachial plexus injuries with root avulsions have been performed with the suprascapular nerve, phrenic nerve, contralateral C7 root, partial median or ulnar nerves, and intercostal nerves.94,97,140,149
Early series, especially those using intercalated grafts, reported poor results.212 Later series report satisfactory restoration of elbow extension after intercostal nerve transfer to the long head of the triceps branch without intercalated grafts.72,93
Technique
Intercostal nerve to the nerve of the long head of the triceps branch was described by Goubier and Teboul in 2007.93 The technique involves exposing the third to fifth intercostal nerves and mobilizing the motor branches after their isolation by electrical stimulation. The radial nerve is accessed through a brachial and axillary approach and usually found behind the brachial vein. Dissection is facilitated through partial or total division of the pectoralis major tendon. The long head of the triceps branch is found at the level of the latissimus dorsi and teres major tendons, and the nerve is followed into the radial nerve and split to increase its length before being sutured to the intercostal nerve endings using microsurgical technique. The three intercostal nerves can be glued together with fibrin before microneural suture. The pectoralis muscle tendon is repaired before skin closure.
Results
At an average follow-up of 23 months, intercostal nerve transfer to long head of the triceps branch performed in 10 patients yielded M4 strength in seven patients, M3 in one patient, and M1 and M2 strength in two patients. The average time to recover extension against resistance was 15 months.93 Doi et al. described the results of third and fourth intercostal nerve transfer to the triceps motor branch during the second stage of their double free muscle technique to restore elbow and finger flexion and extension in 32 patients with complete brachial plexus avulsion.68 They found elbow extension postoperatively to be limited by contracture. Positioning of the hand in space was possible in 50% of cases, of which two patients were able to stabilize the elbow while moving the fingers. Recovery of even weak triceps function proved useful in his patients, permitting the double free muscle transfers to provide grasp function independent of elbow position in many cases. On failure of all triceps recovery from intercostal nerve neurotization, some elbow stabilization could be achieved from transfer of the reinnervated infraspinatus muscle, at least with the aid of gravity and its tenodesis effect.72
MUSCLE TENDON TRANSFERS
Latissimus Dorsi Transfer or Transplant
History
Several authors have proposed unipolar transfer of the latissimus dorsi insertion into the extensor mechanism to restore elbow extension.103,115,137 Harmon103 added the teres major to the latissimus dorsi transfer in one case report. Hovnanian118 devised a unipolar transfer of the latissimus dorsi in which its origin was transferred to the triceps tendon. Myocutaneous unipolar transfer of the latissimus dorsi was reported by Landra to provide skin coverage and elbow extension at the same time.136 Tobin and associates performed a similar procedure but used only the lateral segment of the latissimus dorsi with an attached island of skin.237
Bipolar transplantation of the latissimus dorsi was first reported by Schottstaedt and coworkers in 1955.207 The origin was sutured to the triceps tendon, and the insertion was moved to the acromion. DuToit and Levy75 emphasized the necessity for discarding the distal 3 inches of the latissimus dorsi to get the transplant tight enough.
Technique
Unipolar Transfer
This description is based on that of Hovnanian (Fig. 71-25). The patient is placed on the side, with the arm draped free. The technique of exposing, detaching, and mobilizing the latissimus dorsi on its neurovascular pedicle is the same as that described for flexor replacement. The incision on the arm, however, is carried over from the posterior axillary fold onto the posteromedial aspect without crossing the neurovascular bundle. Distally, the incision is carried over the medial epicondyle onto the posterior aspect of the proximal ulna. The aponeurotic fascia at the free end of the latissimus dorsi muscle is then sutured under tension to the triceps tendon, the periosteum over the olecranon, and the connective tissue and muscle septa on the extensor surface of the forearm, keeping the elbow in full extension. A considerable amount of muscle usually has to be excised to make the transfer as tight as possible.
Bipolar Transplantation
This description is based on the technique of Schottstaedt and coworkers (Fig. 71-26). The latissimus dorsi muscle is exposed, freed, and completely detached on its neurovascular pedicle through an incision extending from the posterior axillary fold along the muscle’s lateral margin to within 5 to 7.5 cm of the iliac crest. The lower triceps and olecranon are exposed through a posterior incision. A subcutaneous tunnel is then created between this incision and the dorsolateral incision, and the origin of the latissimus dorsi is drawn through it. This portion of the muscle is then attached to the triceps tendon and adjacent soft tissue around the olecranon. The acromion is exposed through a 5-cm transverse incision over its posterior edge. The insertion of the latissimus dorsi is then drawn up over the deltoid and sutured securely through drill holes to the acromion and the adjacent soft tissues under maximal tension with the elbow fully extended. The elbow is maintained in full extension for 3 to 4 weeks before flexion is allowed.
Results
Unipolar
Hovnanian117 reported satisfactory results in two patients who had a unipolar latissimus transfer. Tobin and colleagues237 used a unipolar transfer of the lateral portion of the latissimus dorsi with a large island of attached skin to restore extension and to cover a large ulcer involving the elbow joint in a 58-year-old man with syringomyelia. Active elbow extension was achieved. The wound was covered, and latissimus dorsi muscle function in the donor site was said to have been preserved by the innervated medial muscle branch retained in situ. Prudzansky and others193 reported on a 48-year-old man with a recurrent extra-abdominal desmoid tumor that required excision of the entire deltoid-end three fourths of the triceps with the overlying skin. Reconstruction using a large latissimus dorsi myocutaneous flap restored normal elbow extension power with a range from full extension to 95 degrees of flexion.
Bipolar
Schottstaedt and associates207 achieved full extension of the elbow against gravity after bipolar transplantation of the latissimus dorsi in a 5-year-old child with poliomyelitis. DuToit and Levy75 reported on a 44-year-old man who was able to do push-ups after bipolar transplantation of the latissimus dorsi. They noted that the distal 3 inches of muscle had to be discarded to secure the proper tension in the transplant, and they made the proximal acromial attachment before the distal attachment. No complications have been reported. An illustrative case is shown in Figure 71-27.
Posterior Deltoid Transfer
History
In 1949, d’Aubigne62 mentioned the possibility of transferring the posterior part of the deltoid into the triceps as a method of restoring active extension of the elbow. The first procedure was carried out in a tetraplegic patient in 1975 by Moberg, who mobilized the separately innervated posterior part of the deltoid, extending it with multiple toe extensor tendon grafts inserted into the triceps aponeurosis.166 Converting the transferred deltoid from a one-joint to a two-joint muscle achieved elbow extension without creating any detectable shoulder dysfunction. Since this communication, there have been several other reports of tetraplegic patients in whom posterior deltoid transfer has been used to achieve elbow extension.37,107 Hentz and colleagues’ communication108 indicates that the deltoid transfer is now being attached directly to the triceps aponeurosis without any intervening graft material. Castro-Sierra and Lopez-Pita47 used two opposing periosteal flaps, one from the deltoid insertion and one turned backward from the triceps insertion into the olecranon, to join the deltoid and triceps. Freehafer and associates82 advised using the anterior tibial tendon as an interposition graft. If the patient can walk or might later be able to, they recommended using fascia lata to connect the deltoid to the triceps insertion.
The deltoid-to-triceps transfer is the most frequently used method to restore elbow extension173 and has become the standard method of treatment over the past 10 years when the deltoid has a minimum of M4 strength.78 The varying techniques differ in the type of material used to attach the deltoid to the triceps.84 Most surgeons prefer lower extremity tendon graft or fascia lata, although a consensus has not been reached on the optimum interpositional graft.127 A new technique described by Mennen and Boonzaier162 involves the use of the bony insertion of the deltoid, which is attached by steel wires to the bony insertion of the triceps into the olecranon reflected cranially with a 10-mm wide central section of the triceps tendon and aponeurosis. No subsequent use of this technique has been described in the literature. A number of investigators have found adequate strength and excursion for elbow extension from this transfer.83,134,167 This procedure is indicated in tetraplegic patients with a good passive range of motion (to within 30 degrees of full extension) at the elbow and with sufficient strength in the posterior deltoid. Inadequate strength on manual muscle testing of the deltoid is an absolute contraindication, and fixed contracture greater than 30 to 45 degrees is a relative contraindication to this procedure.78,110
Anatomy
The deltoid muscle is supplied by two terminal branches of the circumflex nerve, which divides about 6 cm below the level of the acromion. The posterior branch is short and runs almost horizontally to supply the portion of the deltoid arising from the spine of the scapula. The anterior branch is longer and runs horizontally to supply the acromial and clavicular portions of the deltoid.32
The architectural properties of the posterior deltoid and triceps muscle were studied by Fridén et al86 to determine whether triceps function is appropriately matched by that of the posterior deltoid. It was found that the cross-sectional area of the posterior deltoid was significantly less than that of the three heads of the triceps and that only 20% of the maximum isometric tension could be provided. Despite this factor, the posterior deltoid muscle fiber length was significantly greater, allowing its force to be generated over a much wider range of muscle length, which would explain the relative independence of perioperative posterior deltoid tension on the functional results after transfer.86 The large fiber excursion approaches 25 mm and can potentially offset the potential tendon stretch.118
Technique
This procedure is based on that of Moberg (Fig. 71-28). Through a slightly curved incision along the posterior border of the deltoid, the posterior half of the muscle is exposed to its insertion. There is usually a natural cleavage between the two parts of the deltoid where the separation can be accomplished by blunt dissection. Because the deltoid muscle lacks a large tendon of insertion, the tendon of insertion and the surrounding periosteum and a rectangular strip of fascia from the adjacent brachialis muscle should remain attached to the deltoid muscle. The posterior deltoid is mobilized proximally, attaining 3 cm of amplitude, the amount necessary for elbow extension. Care is taken to avoid injury to the axillary nerve branches.
The triceps aponeurosis is exposed through separate, slightly curved, longitudinal incisions proximal to the olecranon. The conjoined extensor tendons to the second, third, and fourth toes (nonfunctioning in tetraplegic patients) are removed with the aid of a Brand tendon stripper. The graft is attached to the deltoid muscle by at least twice looping the end of the graft through the deltoid tendon and adjacent fascia and periosteum and suturing the graft to the adjacent structures. The graft is then passed distally through a subcutaneous tunnel created between the two incisions. The tendon graft is looped twice through and sutured to the triceps aponeurosis, passed again subcutaneously in a proximal direction and again attached to the deltoid muscle. Suturing of the graft should be done with the elbow extended fully and the shoulder abducted 30 degrees. Fascia lata is an alternative of comparable effectiveness. This graft is applied and attached as shown in Figure 71-28B. The posterior deltoid muscle is infiltrated with 20 mL of 1% lidocaine with epinephrine 1:200,000 to prevent disruption of the anastomosis during recovery from anesthesia.
The central third portion of the triceps tendon is used as the interposition graft.173 The procedure can be performed simultaneously with other tendon transfers for hand reanimation, such as a brachioradialis-to-flexor pollicis longus transfer for key pinch.190 Also, the procedure is possible with the patient in supine position under axillary block and a block of the axillary nerve with or without local anesthesia.78
Aftercare
A long-arm cast, applied from the upper humerus to just proximal to the wrist with the elbow in 10 degrees of flexion, is worn for 6 weeks. Then the patient begins active extension exercises. To prevent stretching of the newly transferred muscle-tendon unit, however, elbow flexion is increased only 10 degrees per week. A polycentric adjustable elbow splint that allows full extension but blocks flexion at whatever position is desired is useful.107 During the rehabilitation period, elbow extension power must be closely monitored. If a temporary decrease in extension power is observed, reimmobilization in the extended position is carried out until active full extension is regained. Bryan postulated that late stretching originates in the central portion of the grafts secondary to revascularization and advised protecting the arm from flexion past 90 degrees for 3 months after surgery.37
Some authors, after seeing unpredictable results and the considerable elongation that takes place in the two junctions with immediate wheelchair use, have altered their aftercare regimen to include an electric wheelchair and initial casting (4 weeks) and treatment with hinged splints permitting increasing levels of flexion (6 weeks).78
Results
Only one failure is reported by Moberg’s experience with posterior deltoid transfers.167 In general, elbow extension power was noted to be several times stronger with the extremity at the side in the position for lifting the body than with the limb extended over the head. Bryan37 reported satisfactory results in 10 of 14 deltoid transfers performed in seven tetraplegic patients. In all but one, bilateral deltoid-to-triceps transfers were performed in single operations. De Bendetti63 evaluated 13 tetraplegic patients with 14 deltoid-to-triceps transfers. He noted a mean function of 5, as compared with a preoperative mean of 0.5. Castro-Sierra and Lopez-Pita47 noted 10 satisfactory results in 13 tetraplegic patients using Moberg’s technique but were able to shorten the postoperative immobilization period to 35 days. A proximally based flap of triceps tendon with an attached flap of olecranon periosteum is turned back on itself and sutured to the deltoid insertion with the attached periosteum. Lamb and Chan134 reported satisfactory results after 16 Moberg transfers in 10 tetraplegic patients. Hentz and others used a tubed fascia lata strip inserted through drill holes in the olecranon and allowed up to 30 degrees of immediate flexion postoperatively,107 but no follow-up results were given.107,108 Significant preoperative flexion contracture was believed to compromise the result of deltoid-to-triceps transfer. Under these circumstances, they recommend biceps-to-triceps transfer.
Freehafer and others82 reported successful results in all 15 cases of triceps paralysis treated by deltoid-to-triceps transfer using the anterior tibial tendon as an interposition graft. All of the patients could reach above their heads and noted significant improvement in performing depressor push-ups and transfers from bed to chair. Lacey and associates132 performed a similar procedure in 16 elbows. At surgery, they documented excursion of the posterior deltoid of 7.31 cm. They believed that optimal tension was achieved by suturing the graft with the deltoid pulled to its original insertion length and with the elbow at 90 degrees of flexion. All but one transfer achieved muscle strength of 3 or better. Ejeskar77 recorded the results of deltoid-to-triceps transfer in 40 elbows of 32 patients. Ten of the 30 procedures performed using Moberg’s technique and 1 of the 10 procedures using the method of Castro-Sierra and Lopez-Pita produced full extension of the elbow. Twenty elbows showed no extension beyond 60 degrees, but most had enough extension to maintain elbow control in daily activities. Most of the patients did not have significant use of the elbow extensor when moving between bed and chair, and stretching of some of the transfers was documented.159 Vanden Berghe and colleagues242 initially used the Moberg technique for deltoid-to-triceps transfer. After the first two cases, they changed to the Castro-Sierra and Lopez-Pita technique for the next six cases because of the shorter immobilization time. All eight elbows gained extensor power of 3 or better. One patient ruptured a transfer in a fall.
Twenty-four transfers were evaluated at an average follow-up of 37 months.170 These authors report M4 strength in 42% and M3 in 29%. Even M2 strength provided some useful increase in hand function, allowing hand positioning at eye level or above. Lamb and Chan reported M5 power in 50% of their posterior deltoid-to-triceps transfers,134 but the discrepancy may be explained by the difference in patient positioning during evaluation. When the patient is supine, the contribution from the pectoral girdle muscles is eliminated in raising the arm above the head, thereby giving true elbow extension strength evaluation.170
The short-term versus long-term comparative results after 11 deltoid-to-triceps procedures in six patients were evaluated with a mean follow-up of 3 years and 24 years after surgery by Vastamäki.243 The study group originally consisted of 27 patients with tetraplegia, but many were lost due to death before they could be evaluated, but the functional results showed an average decrease in extension strength of approximately 1% per year, which may be due mostly to patient aging. This study showed the permanency of results obtained with this procedure. A study using motion analysis methods found increased speed and stability of elbow flexion and extension, and a parallel increase in the speed of shoulder flexion and extension.195 Another biomechanically oriented study found that the posterior deltoid was predicted to operate exclusively on the ascending limb of the length-tension curve, that it generated only 65% of its predicted force, and that the elbow extension moment did not change significantly after rehabilitation.143 Other biomechanical studies have shown that the strength of extension depends on the position of the upper arm relative to the shoulder.125 When the shoulder is weak, as is often the case in C5 or C6 tetraplegia, the upper arm is elevated to shoulder level, which unfortunately corresponds to the position in which the posterior deltoid-to-triceps transfer is the weakest.
Complications
Stretching and disruption of the proximal or distal anastomosis may require reoperation. In these instances, Moberg has advanced the triceps tendon by osteotomizing the olecranon, advancing the bone, and reattaching the tendon distally with a screw.159 Spasticity of the elbow flexors may compromise the result.
Complications adversely affecting results have included elongation of the tendon transfer caused by rapid and early mobilization and attenuation over time,173 as well as graft failure, elbow flexion contractures,168 biceps spacicity,82 and the difficulties associated with the tolerance of a long post-operative course of immobilization.109,168–170 There are multiple reasons why a tendon transfer can fail, including incorrect tension, scar formation, disruption or attenuation of the interposition graft or tendon weave, and progressive weakness of the donor muscle.127,131,190
Biceps-to-Triceps Transfer
History
In 1954, Friedenberg reported a patient with poliomyelitis who had difficulty arising from a chair and walking with crutches because of bilateral absence of triceps function.87 After lateral subcutaneous transfer of the biceps tendon into the triceps aponeurosis, the patient was able to support 7.5 pounds in extension on the right and 8.5 pounds on the left. He was able to transfer independently and to use crutches without difficulty. Friedenberg,87 Hentz and associates,107 and Lamb and Chan134 recorded cases of tetraplegic patients for whom this procedure was unsuccessful.
Zancolli255 believes that elbow extension is the most important function one could add for tetraplegic patients. He uses Friedenberg’s method87 after establishing that the supinator is functioning. If there is no supinator muscle function, he transfers the posterior deltoid. Zancolli noted only 24% reduction in elbow flexion power after biceps transfer. Falconer80 has described Zancolli’s technique of biceps transfer. The biceps is exposed through a Z incision along its lateral border, placing the horizontal limb of the Z in the flexion crease. The incision extends 10 cm proximally to the flexion crease and 5 cm distally. The musculocutaneous nerve is identified and mobilized from the undersurface of the biceps muscle 5 cm proximally. The muscle is detached at the radial tubercle and mobilized 10 cm proximally to the elbow, care being taken to protect the radial nerve. A second incision is made over the olecranon, and a flap of triceps aponeurosis is developed. The biceps is passed subcutaneously into the posterior wound and sutured under tension to the aponeurosis flap. The elbow is immobilized in extension for 4 weeks. Ejeskar,77 noting that biceps-to-triceps transfer was the procedure of choice for restoring elbow extension when there is a flexion contracture, reported his results in six cases. One patient died of other causes, two regained full extension, one lacked 80 degrees of extension, and two achieved no extension. One patient sustained a radial nerve palsy that was recovering at 5 months.
Some authors have recently reported better results with biceps-to-triceps than with posterior deltoid-to-triceps transfer.16,173 Before transfer, an elbow contracture of more than 30 degrees is corrected by some authors, because this degree of biceps contracture would prevent the rerouted tendon from reaching the olecranon.16 However, this technique is better suited to alleviate a mild elbow flexion contracture than the posterior deltoid transfer,118 and as such, an elbow flexion contracture of 30 degrees or more is seen by some authors as an indication for biceps-to-triceps transfer over deltoid-to-triceps transfer.78 Also, the need for tendon augmentation is obviated because a direct transfer is possible, and the operation generally takes less time. Active supinator and brachialis muscles must be present (on electromyography) to maintain forearm supination and elbow flexion. If there is doubt about this, the musculocutaneous nerve can be blocked to temporarily paralyze the biceps.110 Some authors4,159,196 argue that paralysis of the pectoralis muscle is an indication for the biceps-to-triceps procedure to avoid the potential for further weakening of the shoulder, although others disagree that this should be taken into account.78 The biceps tendon can be routed around the lateral or medial side of the humerus. Lateral routing techniques have been complicated by radial nerve injuries and consequent diminished function of the brachioradialis and extensor carpi radialis longus.77,131,196 The medial approach, in contrast, has yielded excellent results.77,107,127,131,196 There remains the potential for ulnar nerve injury, but in tetraplegic patients with injury at level C6 or above, the ulnar nerve is nonfunctional. Also, the medial route is a more direct one, with less potential risk of adhesions.131 Even under maximal tension, no median nerve injuries or vascular compression have been observed.197 This procedure is generally associated with a 4078 to 47%196 loss in elbow flexion force, but most authors report that their patients to not register any significant complaints related to this loss110,196 even in contrast to the 24% reduction noted with the lateral route.159,255
Technique
Preoperative clinical examination is essential to ensure the presence of an active supinator and brachialis muscle. Ulnar innervated muscles must be assessed to determine if there is any remaining function that could be reduced. This procedure can also be performed with the patient in supine position under axillary block.79
The medial approach is clearly established.131,196 This requires a longitudinal incision along the medial intermuscular septum, through which the ulnar nerve is identified. The biceps tendon is released from the radial tuberosity anteriorly through an incision in the antecubital fossa, passed over the ulnar nerve, and attached to the olecranon via a bony tunnel posteriorly with the elbow in full extension. Fixation is performed by drilling a unicortical hole from the tip of the olecranon to the posterior cortex with a small drill bit. This hole is enlarged sequentially by drilling until it can accommodate the biceps tendon. Two small drill holes are placed through the opposite posterior cortex through which nonabsorbable braided suture, also placed within the tendon, is passed. The sutures are used to pull the tendon into the unicortical hole, and then tied over the bone with the elbow in extension.
General protocols have been established for the postoperative care.109,159,173 The arm is usually immobilized for 3 weeks, after which a brace is fitted with the elbow in extension. Active flexion and extension develops at 15 degrees per week, and movement is integrated into daily activities in a graded fashion. More rigorous precaution can be afforded through added protection as described by Fridén and Lieber88 and Kuz et al.131
Results
Results of the medial approach have been encouraging, with consistent restoration of elbow extension. Kuz et al131 reported on the outcome in four transfers, and Revol et al196 in 13 cases, with all patients achieving M4 elbow extension strength 18 months postoperatively. Loss of elbow flexion strength was significant (47% on average), but subclinical in all cases. The lateral routing technique has been associated with a 24% reduction in elbow flexion power.159 Mulcahey et al,173 in a prospective study comparing eight deltoid-to-triceps and eight biceps-to-triceps transfers, found M3 or better strength in 7 of 8 biceps transfers compared with 1 of 8 deltoid transfers. Impressive loss in elbow flexion torque was seen (52%), which persisted through the 24-month follow-up period despite some clinical improvement. Kozin and Schloth127 reported a case in which bilateral biceps-to-triceps transfer was successfully used to salvage a failed bilateral deltoid-to-triceps transfer. Furthermore, the technique has been shown to increase the pinch strength from a previous brachioradialis transfer.38 The learning time to activate the biceps as an elbow extensor takes from 3 to 6 months.196
Miscellaneous Transfers for Elbow Extension
Brachioradialis Transfer
In 1938, Ober and Barr185 described the posterior transfer of the freed anterior margin of the brachioradialis into the proximal ulna, olecranon, and triceps tendon to restore active elbow extension. The addition of the extensor carpi radialis to strengthen the brachioradialis was advised if its power was insufficient. Immobilization of the elbow in full extension and supination was advised, and exercises were started at 10 days. Extension of the elbow against gravity was possible in all six cases. No subsequent reports of the procedure have appeared in the literature.
The technique was modified in 2005 by Ozkan et al189 to include a bipolar transfer of the brachioradialis muscle. The short-term results showed slight improvement postoperatively, depending on shoulder abduction. The relative weakness of the brachioradialis muscle was cited as a possible reason for this finding. It is a radial nerve innervated muscle but may be available for transfer due to possible mixed lesions or the higher likelihood of cross-innervation from multiple roots.
Transfers for Pronation
History
Paralytic supination contracture of the forearm can occur as a result of brachial plexus palsy and poliomyelitis,41,188,253 or in tetraplegia levels below C5.89 Secondary partial or complete paralysis of the flexor-pronator muscles in the presence of unopposed biceps and supinator muscles is the underlying common denominator. The deformity not only is cosmetically displeasing but also seriously limits the function of the hand for grasping and two-handed activities. Patients with this deformity cannot perform tasks that require pronation, such as controlling a wheelchair joystick, as well as most personal hygiene functions. In children, this is especially cumbersome because it prevents the use of a computer keyboard.192
Patients presenting with supination contracture concurrent with an anterior dislocation of the radial head can be treated by transfer of the biceps insertion to the ulna. Nondynamic correction of the supination deformity by closed osteoclasis26,41 or open osteotomy of the forearm bones256 was advised by earlier authors. Schottstaedt and colleagues208 suggested changing the biceps from a supinator to a pronator by transferring its tendon to the side of the radial tuberosity opposite its normal insertion, but they did not report their results. Zancolli performed a long Z lengthening of the tendon, rerouting the distal strip of the tendon around the neck of the radius.253 All 14 of his patients also required release of the contracted soft tissues, particularly the interosseous membrane, to obtain passive correction of the supination deformity before the biceps rerouting. Manske and coworkers believed that soft tissue releases could be avoided by performing the biceps rerouting earlier; if correction was unsatisfactory, a secondary percutane-ous osteoclasis of the radius and ulna was recommended.154
The goal for any surgery is active elbow flexion, with the forearm positioned in neutral rotation or slight pronation.113,191 If the radial head is not dislocated, the forearm can be pronated passively, and there is active supination (on electromyography), then a Zancolli-type biceps rerouting can be carried out, with or without release of the contracture.253 Here the goal is to achieve active pronation, and this is the most frequently used corrective procedure. Finally, Hoyen and colleagues119 prefer to perform a radius pronation osteotomy, which allows full correction past that offered by biceps-pronator plasty and interosseous membrane and distal radioulnar joint release. The osteotomy is made just distal to the pronator insertion, and fixation is performed after the radius is rotated a desired amount to position the forearm more optimally. This allows more rapid rehabilitation, and offers reliable results.110 Although biceps rerouting has been found to give the best forearm motion, afterward it will not be available for tendon transfer, leaving elbow extension to be achieved by posterior deltoid-to-triceps transfer in most cases. Elbow extension strength may be improved in patients who undergo forearm osteotomy and a biceps-to-triceps transfer, but at this time, the choice of procedure seems to depend in some degree on surgeon preference.
Biceps Rerouting: Technique
This description is based on those of Zancolli253 and Manske and coworkers154 (Fig. 71-29). A high tourniquet is employed, and the biceps tendon is exposed through an incision starting on the medial aspect of the distal arm and extending across the flexion crease of the elbow and distally on the lateral aspect of the forearm. The lacertus fibrosus is incised, and the median nerve and brachial artery are retracted medially. The biceps tendon is exposed to its insertion into the bicipital tuberosity, and the radial recurrent leash of vessels is divided. The tendon is divided by a long Z-plasty to its insertion, and the distal segment is passed posteriorly around the radial neck at the level of the tuberosity using a ligature carrier. The tendons are reattached by side-to-side suture, effectively lengthening the tendon by about 1.5 cm. A long-arm cast is applied with the forearm in the neutral position and the elbow at a right angle for 4 to 6 weeks. If the deformity is long standing and the patient is older, soft tissue releases often are required to obtain passive forearm rotation. The interosseous membrane and supinator muscle can be released through a long dorsal incision overlying the ulna. The extensor muscles are retracted toward the radius, and this protects the interosseous nerve. Owings and colleagues188 suggested releasing the supinator from the volar lateral surfaces of the radius through the anterior incision.
Results
Zancolli performed soft tissue releases and biceps tendon rerouting in 14 patients with paralytic supination contracture of the forearm (four had poliomyelitis, eight birth palsies, and two tetraplegia).253 Correction to the neutral position or some pronation was maintained in all patients, and eight had 10 to 60 degrees of active pronation. Owings and others reported nine good results, 11 satisfactory results, and two poor results in 26 patients who had biceps tendon rerouting using the Z-plasty technique.188 The two poor results were related to problems with stability of the proximal radius. Manske and associates154 reported the results of Zancolli’s biceps tendon rerouting in 11 patients with supination deformity secondary to obstetric paralysis. The neutral position was obtained in nine, and six had active supination-pronation movement averaging 42 degrees (range 15 to 65 degrees). Two patients, neither of whom had preoperative passive pronation to neutral, obtained satisfactory results after a secondary percutaneous osteoclasis of the radius and ulna. The authors recommended that surgical correction of paralytic supination deformity of the forearm be done between 3 and 6 years of age. Leffert and Pess141 reported four good and two improved results among six patients using Zancolli’s method of biceps rerouting.
More than 2 years’ follow-up was reported on five patients with C5, C6 obstetric brachial plexus palsy, and biceps-to-ulna transfers for correction of supination contractures concurrent with dislocation of the radial head.113 No pronation past neutral was possible preoperatively in each case. Postoperatively, there was full active flexion with no rotation and muscle grades from M3 to M5, with passive pro- and supination from 60 to 75 degrees, respectively. The transfer did not affect active rotation. These authors also reported on eight children with supination contracture and located the radial head where biceps rerouting was performed and evaluated after more than 2 years. In all cases, no passive pronation was possible. Postoperatively, more effective flexion was possible in neutral rotation, with active pronation against gravity possible in all, ranging from 30 to 90 degrees.113 Eleven of 14 flexion-supination contractures in Freehafer’s group were corrected by cast treatment alone, but five elbows in three patients went on to undergo biceps tendon tenotomy. There were no complications, one patient required six tenotomies due to bilateral recurrence, and all elbows achieved acceptable ADL function and regained M4 flexion and supination.86 However, biceps tendon tenotomy in tetraplegia is not found in other current literature.
Complications
Zancolli253 reported on two patients who had overcorrection, presumably because the biceps tendon suture was under excessive tension. Owings and colleagues188 reported on one patient who had persistent weakness of thumb extension, two who had proximal radial instability, and one who developed excessive pronation.
Transfers for Supination
History
Because it is infrequently indicated, muscle transfer to regain supination in the paralyzed upper extremity has received scant attention in the literature. Steindler223 described transferring the flexor carpi ulnaris tendon into the dorsal aspect of the distal radius. Tubby240 described transferring the flexor carpi radialis and pronator teres through the interosseous space to the back of the radius to achieve supination in the spastic upper extremity. Schottstaedt and associates208 noted that providing active supination using the flexor carpi ulnaris or palmaris longus redirected dorsally into the radial shaft was often necessary after Steindler’s flexorplasty.
Technique—Flexor Carpi Ulnaris Transposition to the Radius
This technique is based on Steindler’s method (Fig. 71-30).223 The flexor carpi ulnaris tendon is detached, and its distal half is mobilized through a 12-cm incision along the volar-ulnar aspect of the forearm. The dorsolateral surface of the distal radius is exposed between the extensor pollicis brevis and the extensor carpi radialis longus tendons using a 5-cm longitudinal incision. A subcutaneous tunnel is created between this incision and the proximal end of the first incision, and the flexor carpi ulnaris tendon is pulled through. A hole is drilled through the distal radius, and the tendon is fed through the hole from the dorsal to the volar aspect. Its end is reflected back and sutured to itself under tension with the forearm supinated and the elbow flexed. A long-arm cast, with the wrist in slight dorsiflexion, the forearm in full supination, and the elbow at a right angle, is worn for 3 weeks. Part-time splinting is continued for 2 months.
AUTHOR’S PREFERRED METHOD OF TREATMENT
The primary goal in treatment of the flail upper limb should be the restoration of elbow flexion. The second is to restore shoulder external rotation and abduction, or at least glenohumeral stability, followed by grasp function and finally intrinsic motor function. Grasp itself is a complex function requiring sensibility, finger flexion and extension, and stability or active control of the wrist for tenodesis effect. Restoration of elbow extension is important in grasp function at times as well, particularly when powered by free muscle transfers crossing the elbow that would otherwise produce an elbow flexion moment. It is in fact certainly true that the major function of the shoulder and elbow in normal extremities is to position the hand in space for manipulation. More limited use of the proximal extremity, to function as a paper weight, perform arm-to-trunk prehension, or support a tray become important more limited goals in flaccid paralysis. For restoration of elbow flexion, understanding the importance of elapsed time from injury on treatment of elbow paralysis is critical. Recovery of elbow flexion by reanimation of paralyzed muscle requires intervention by 6 months from injury, preferably, with steadily decreasing results over the ensuing 6 months. No recovery is to be expected when nerve surgery is performed after 1 year.
It is also true, as in general experience, that more is better. That is, two muscles are often better than one for this critical function. This is certainly the case for the Steindler flexorplasty, which works best to augment a weak biceps rather than serve as a replacement.18,52,53 Chuang found that although Steindler’s flexorplasty is effective in increasing elbow flexion function from M2 to M3 or M4, it is less useful in upgrading from M0 or M1 to M3. Similarly, free muscle transplantation alone is less effective than free muscle transfer combined with direct biceps neurotization, particularly when elapsed time from injury does not favor full biceps recovery (6-12 months after trauma). Double nerve transfers to both biceps and brachialis produce reliable M4 recovery of elbow flexion, for patients with isolated upper trunk, or upper and middle trunk injury in whom both ulnar and median nerve fascicle transfers are possible.147,151,187
Late reconstruction should be performed with a free-functioning muscle supplied by two or three intercostal nerves. Flexion strength can be increased by a stable shoulder, either resulting from nerve recovery, tendon transfers or shoulder arthrodesis. In plexus injury, shoulder joint stability is usually obtained by reinnervation of the suprascapular nerve and the axillary nerve with nerve graft or transfer. Targeting either nerve alone resulted in 30 degrees of shoulder abduction in one study.57 Transfers to both suprascapular and axillary nerves yielded 70 to 115 degrees of shoulder abduction.57,140
In our experience and that of others,235 in global avulsions, delayed cases, and for hand reanimation, free muscle transfer and other secondary procedures are necessary for satisfactory outcome. Early and aggressive nerve reconstruction usually yields more satisfactory function than the various muscle transfers, and this early surgery should be combined with secondary procedures such as pedicled and free muscle transfers to reach the best possible functional outcome for the upper extremity.
1 Ahmad I. Restoration of elbow flexion by a new operative method. Clin. Orthop. Relat. Res. 1975;106:186.
2 Akasaka Y., Hara T., Takahashi M. Restoration of elbow flexion and wrist extension in brachial plexus paralyses by means of free muscle transplantation innervated by intercostal nerve. Ann. Chir. Main. Memb. Super. 1990;9:341.
3 Akasaka Y., Hara T., Takahashi M. Free muscle transplantation combined with intercostal nerve crossing for reconstruction of elbow flexion and wrist extension in brachial plexus injuries. Microsurgery. 1991;12:346.
4 Allieu Y., Benichou M., Ohanna F., Bousquet P., Chammas M. [Surgical classification of the upper limb in tetraplegic patients]. Ann. Chir. Plast. Esthet. 1993;38:180.
5 Allieu Y., Cenac P. Neurotization via the spinal accessory nerve in complete paralysis due to multiple avulsion injuries of the brachial plexus. Clin. Orthop. Relat. Res. 1988;237:67.
6 Allieu Y., Privat J.M., Bonnel F. [Neurotization with the spinal nerve (nervus accessorius) in avulsions of roots of the brachial plexus]. Neurochirurgie. 1982;28:115.
7 Alnot J.Y. Elbow flexion palsy after traumatic lesions of the brachial plexus in adults. Hand Clin. 1989;5:15.
8 Alnot J.Y., Abols Y. [Restoration of elbow flexion by tendon transfer in traumatic paralysis of the brachial plexus in adults. Apropos of 44 injured patients]. Rev. Chir. Orthop. Reparatrice Appar. Mot. 1984;70:313.
9 Andrisano A., Porcellini G., Stilli S., Libri R. The Steindler method in the treatment of paralytic elbow flexion. Ital. J. Orthop. Traumatol. 1990;16:235.
10 Atkins R.M., Bell M.F., Sharrard W.J.W. Pectoralis major transfer for paralysis of elbow flexion in children. J. Bone Joint Surg. 1985;67B:640.
11 Axer A., Segal D., Elkon A. Partial transposition of the latissimus dorsi. J. Bone Joint Surg. 1973;55A:1259.
12 Bahm J., Noaman H., Becker M. The dorsal approach to the suprascapular nerve in neuromuscular reanimation for obstetric brachial plexus lesions. Plast. Reconstr. Surg. 2005;115:240.
13 Baliarsing A.S., Doi K., Hattori Y. Bilateral elbow flexion reconstruction with functioning free muscle transfer for obstetric brachial plexus palsy. J. Hand Surg. [Br.]. 2002;27:484.
14 Barrie K.A., Steinmann S.P., Shin A.Y., Spinner R.J., Bishop A.T. Gracilis free muscle transfer for restoration of function after complete brachial plexus avulsion. Neurosurg. Focus. 2004;16:E8.
15 Bartlett S.P., May J.W., Yaremchuk M.J. The latissimus dorsi muscle: a fresh cadaver study of the primary neurovascular pedicle. Plast. Reconstr. Surg. 1981;67:631.
16 Barus D., Kozin S.H. The evaluation and treatment of elbow dysfunction secondary to spasticity and paralysis. J. Hand Ther. 2006;19:192.
17 Bentolila V., Nizard R., Bizot P., Sedel L. Complete traumatic brachial plexus palsy. Treatment and outcome after repair. J. Bone Joint Surg. Am. 1999;81:20.
18 Berger A., Brenner P. Secondary surgery following brachial plexus injuries. Microsurgery. 1995;16:43.
19 Berger A., Flory P.J., Schaller E. Muscle transfers in brachial plexus lesions. J. Reconstr. Microsurg. 1990;6:113.
20 Berger A., Schaller E., Becker M.H. [Pulley for strengthening a muscle replacement operation across two joints in brachial plexus lesion: description of the surgical technique]. Handchir. Mikrochir. Plast. Chir. 1994;26:51.
21 Bertelli J.A., Guizoni M.F., Dos Santos A.R., Calixto J.B., Duarte H.E. Cross-chest radial nerve transfer in brachial plexus injuries. Experimental and anatomical basis. Chir. Main. 1999;18:122. discussion 31
22 Bertelli J.A. Platysma motor branch transfer in brachial plexus repair: report of the first case. J. Brachial Plex. Peripher. Nerve Inj. 2007;2:12.
23 Biesalski K., Mayer L. Die Sehnenverpflanzung am Ellenbogen. Operation 19. Ersatz des M. biceps brachii durch den M. triceps brachii. In: Biesalski K., Mayer L., editors. Die physiologische Sehnenverpflanzung. Berlin: Springer; 1916:284.
24 Bishop A.T. Functioning free-muscle transfer for brachial plexus injury. Hand Clin. 2005;21:91.
25 Blaauw G., Slooff A.C. Transfer of pectoral nerves to the musculocutaneous nerve in obstetric upper brachial plexus palsy. Neurosurgery. 2003;53:338. discussion 41
26 Blount W.P. Osteoclasis for supination deformities in children. J. Bone Joint Surg. 1940;22:300.
27 Bostwick J., Nahai F., Wallace J.G., Vasconex L.O. Sixty latissimus dorsi flaps. Plast. Reconstr. Surg. 1979;63:31.
28 Botte M.J., Wood M.D. Flexorplasty of the elbow. Clin. Orthop. Rel. Res. 1989;245:110.
29 Bottero L., Revol M., Cormerais A., Servant J.M. [Effect of brachial-radial and extensor carpi radialis longus tenodesis on elbow flexion-extension movements. Application to tendon transfers in tetraplegia]. Ann. Chir. Plast. Esthet. 2000;45:511.
30 Bradford E.H. The operative treatment of paralysis of the shoulder following anterior poliomyelitis. Am. J. Orthop. Surg. 1910;8:21.
31 Brandt K.E., Mackinnon S.E. A technique for maximizing biceps recovery in brachial plexus reconstruction. J. Hand Surg. [Am.]. 1993;18:726.
32 Brash J.C. Neuro-vascular Hila of Limb Muscles. Edinburgh: E & S Livingstone Ltd., 1955.
33 Brones M.F., Wheeler E.S., Lesavoy M.A. Restoration of elbow flexion and arm contour with the latissimus dorsi myocutaneous flap. Plast. Recontr. Surg. 1982;69:329.
34 Brooks D.M., Seddon H.J. Pectoral transplantation for paralysis of the flexors of the elbow: a new technique. J. Bone Joint Surg. 1959;41B:36.
35 Brunnelli G., Monini L. Neurotization of avulsed roots of brachial plexus by means of anterior nerves of cervical plexus. Clin. Plast. Surg. 1984;11:149.
36 Brunelli G.A., Vigasio A., Brunelli G.R. Modified Steindler procedure for elbow flexion restoration. J. Hand Surg. [Am.]. 1995;20:743.
37 Bryan R.S. The Moberg deltoid-triceps replacement and key pinch operations in quadriplegia: preliminary experiences. Hand. 1977;9:207.
38 Brys D., Waters R.L. Effect of triceps function on the brachioradialis transfer in quadriplegia. J. Hand Surg. [Am.]. 1987;12:237.
39 Bunnell S. Surgery of the Hand. 2nd ed. Philadelphia: J. B. Lippincott Co., 1948.
40 Bunnell S. Restoring flexion to the paralytic elbow. J. Bone Joint Surg. 1951;33A:566.
41 Burman M. Paralytic supination contracture of the forearm. J. Bone Joint Surg. Am. 1956;38A:303.
42 Carroll R.E., Gartland J.J. Flexorplasty of the elbow. J. Bone Joint Surg. 1953;35A:706.
43 Carroll R.E. Restoration of flexor power to the flail elbow by transplantation of the triceps tendon. Surg. Gynecol. Obstet. 1955;95:685.
44 Carroll R.E. Restoration of elbow flexion by transplantation of sternocleidomastoid muscle. J. Bone Joint Surg. 1962;44A:1039.
45 Carroll R.E., Hill N.A. Triceps transfer to restore elbow flexion. J. Bone Joint Surg. 1970;52A:23.
46 Carroll R.E., Kleinman W.B. Pectoralis major transplantation to restore elbow flexion to the paralytic limb. J. Hand Surg. 1979;4:501.
47 Castro-Sierra A., Lopez-Pita A. A new surgical technique to correct triceps paralysis. Hand. 1983;15:42.
48 Chen W.S. Restoration of elbow flexion by latissimus dorsi myocutaneous or muscle flap. Arch. Orthop. Trauma Surg. 1990;109:117.
49 Chen W.S. Restoration of elbow flexion by modified Steindler flexorplasty. Int. Orthop. 2000;24:43.
50 Chuang D.C. Functioning free muscle transplantation for brachial plexus injury. Clin. Orthop. Relat Res. 1995;314:104.
51 Chuang D.C. Neurotization procedures for brachial plexus injuries. Hand Clin. 1995;11:633.
52 Chuang D.C., Carver N., Wei F.C. Results of functioning free muscle transplantation for elbow flexion. J. Hand Surg. [Am.]. 1996;21:1071.
53 Chuang D.C., Epstein M.D., Yeh M.C., Wei F.C. Functional restoration of elbow flexion in brachial plexus injuries: results in 167 patients (excluding obstetric brachial plexus injury). J. Hand Surg. [Am.]. 1993;18:285.
54 Chuang D.C., Hattori Y., Ma H.S., Chen H.C. The reconstructive strategy for improving elbow function in late obstetric brachial plexus palsy. Plast. Reconstr. Surg. 2002;109:116. discussion 27
55 Chuang D.C., Ma H.S., Borud L.J., Chen H.C. Surgical strategy for improving forearm and hand function in late obstetric brachial plexus palsy. Plast. Reconstr. Surg. 2002;109:1934.
56 Chuang D.C., Wei F.C., Noordhoff M.S. Cross-chest C7 nerve grafting followed by free muscle transplantations for the treatment of total avulsed brachial plexus injuries: a preliminary report. Plast. Reconstr. Surg. 1993;92:717. discussion 26
57 Chuang D.C., Yeh M.C., Wei F.C. Intercostal nerve transfer of the musculocutaneous nerve in avulsed brachial plexus injuries: evaluation of 66 patients. J. Hand Surg. [Am.]. 1992;17:822.
58 Clark J.M.P. Reconstruction of the biceps brachii by pectoral muscle transplantation. Br. J. Surg. 1946;34:180.
59 Clark J.M.P. Reconstructive surgery in paralysis of the elbow. Physiotherapy. 1970;56:295.
60 Clarke H.M., Curtis C.G. An approach to obstetrical brachial plexus injuries. Hand Clin. 1995;11:563. discussion 80
61 Colson J.H.C. Physical treatment of pectoral transfer and flexorplasty. Physiotherapy. 1970;56:300.
62 D’Aubigne R.M. Treatment of residual paralysis after injuries of the main nerves (superior extremity). Proc. R. Soc. Med. 1949;42:831.
63 De Bendetti M. Restoration of elbow extension power in the tetraplegic patient using the Moberg technique. J. Hand Surg. 1979;4:86.
64 Deutinger M., Kuzbari R., Paternostro T., Todoroff B., Becker M.H. [Functional and esthetic assessment of donor site defects following transfer of the gracilis muscle]. Handchir. Mikrochir. Plast. Chir. 1995;27:90.
65 Doi K., Hattori Y., Ikeda K., Dhawan V. Significance of shoulder function in the reconstruction of prehension with double free-muscle transfer after complete paralysis of the brachial plexus. Plast. Reconstr. Surg. 2003;112:1596.
66 Doi K., Hattori Y., Tan S.H., Dhawan V. Basic science behind functioning free muscle transplantation. Clin. Plast. Surg. 2002;29:483. v
67 Doi K., Kuwata N., Muramatsu K., Hottori Y., Kawai S. Double muscle transfer for upper extremity reconstruction following complete avulsion of the brachial plexus. Hand Clin. 1999;15:757.
68 Doi K., Muramatsu K., Hattori Y., Otsuka K., Tan S.H., Nanda V., Watanabe M. Restoration of prehension with the double free muscle technique following complete avulsion of the brachial plexus. Indications and long-term results. J. Bone Joint Surg. Am. 2000;82:652.
69 Doi K., Sakai K., Ihara K., Abe Y., Kawai S., Kurafuji Y. Reinnervated free muscle transplantation for extremity reconstruction. Plast. Reconstr. Surg. 1993;91:872.
70 Doi K., Sakai K., Kuwata N., Ihara K., Kawai S. Reconstruction of finger and elbow function after complete avulsion of the brachial plexus. J. Hand Surg. [Am.]. 1991;16:796.
71 Doi K., Sakai K., Kuwata N., Ihara K., Kawai S. Double free-muscle transfer to restore prehension following complete brachial plexus avulsion. J. Hand Surg. [Am.]. 1995;20:408.
72 Doi K., Shigetomi M., Kaneko K., Soo-Heong T., Hiura Y., Hattori Y., Kawakami F. Significance of elbow extension in reconstruction of prehension with reinnervated free-muscle transfer following complete brachial plexus avulsion. Plast. Reconstr. Surg. 1997;100:364. discussion 73
73 Dolenc V.V. Intercostal neurotization of the peripheral nerves in avulsion plexus injuries. Clin. Plast. Surg. 1984;11:143.
74 Doyle J.R., James P.M., Larson L.J., Ashley R.K. Restoration of elbow flexion in arthrogryposis multiplex congenita. J. Hand Surg. 1980;5:149.
75 Du Toit G.T., Levy S.J. Transposition of latissimus dorsi for paralysis of triceps brachii: report of a case. J. Bone Joint Surg. 1967;49B:135.
76 Dutton R.O., Dawson E.G. Elbow flexorplasty: an analysis of long-term results. J. Bone Joint Surg. 1981;63A:1064.
77 Ejeskar A. Upper limb surgical rehabilitation in high-level tetraplegia. Hand Clin. 1988;4:585.
78 Ejeskar A. Elbow extension. Hand Clin. 2002;18:449.
79 Eyler, D. L.: Modification of Steindler flexorplasty. Georgia Warm Springs Foundation, Warm Springs, GA. Unpublished circular letter. Oct. 19, 1950.
80 Falconer D.P. Tendon transfers about the shoulder and elbow in the spinal cord-injured patient. Hand Clin. 1988;4:211.
81 Free muscle transplantation by microsurgical neurovascular anastomoses. Report of a case. Chin. Med. J. (Engl.). 1976;2:47.
82 Freehafer A.A., Kelly C.M., Peckham P.H. Tendon transfer for the restoration of upper limb function after a cervical spinal cord injury. J. Hand Surg. 1984;9A:887.
83 Freehafer A.A. Tendon transfers in patients with cervical spinal cord injury. J. Hand Surg. [Am.]. 1991;16:804.
84 Freehafer A.A. Tendon transfers in tetraplegic patients: the Cleveland experience. Spinal Cord. 1998;36:315.
85 Freehafer A.A. Elbow extension and flexion-supination deformities in tetraplegia. J. Hand Surg. [Br.]. 2000;25:366.
86 Fridén J., Lieber R.L. Quantitative evaluation of the posterior deltoid to triceps tendon transfer based on muscle architectural properties. J. Hand Surg. [Am.]. 2001;26:147.
87 Friedenberg Z.B. Transposition of the biceps brachii for triceps weakness. J. Bone Joint Surg. 1954;36A:656.
88 Friedman A.H., Nunley J.A.2nd, Goldner R.D., Oakes W.J., Goldner J.L., Urbaniak J.R. Nerve transposition for the restoration of elbow flexion following brachial plexus avulsion injuries. J. Neurosurg. 1990;72:59.
89 Gellman H., Kan D., Waters R.L., Nicosa A. Rerouting of the biceps brachii for paralytic supination contracture of the forearm in tetraplegia due to trauma. J. Bone Joint Surg. Am. 1994;76:398.
90 Ghahremani S., Nejad A.A. Restoring elbow flexion by pectoralis major transplantation in war-injured patients. Microsurgery. 1996;17:97.
91 Gilbert A. Free muscle transfer. Int. Surg. 1981;66:33.
92 Gilbert A., Tassin J.L. [Surgical repair of the brachial plexus in obstetric paralysis]. Chirurgie. 1984;110:70.
93 Goubier J.N., Teboul F. Transfer of the intercostal nerves to the nerve of the long head of the triceps to recover elbow extension in brachial plexus palsy. Tech. Hand Up. Extrem. Surg. 2007;11:139.
94 Goubier J.N., Teboul F. Technique of the double nerve transfer to recover elbow flexion in C5, C6, or C5 to C7 brachial plexus palsy. Tech. Hand Up. Extrem. Surg. 2007;11:15.
95 Green D.P., McCoy H. Turnbuckle orthotic correction of elbow-flexion contracture after acute injuries. J. Bone Joint Surg. 1979;61A:1092.
96 Greene M.H. Cryptic problems of arthrogryposis multiplex congenita. J. Bone Joint Surg. 1963;45A:885.
97 Gu Y.D., Chen D.S., Zhang G.M., Cheng X.M., Xu J.G., Zhang L.Y., Cai P.Q., Chen L. Long-term functional results of contralateral C7 transfer. J. Reconstr Microsurg. 1998;14:57.
98 Gu Y.D., Wu M.M., Zhen Y.L., Zhao J.A., Zhang G.M., Chen D.S., Yan J.G., Cheng X.M. Phrenic nerve transfer for brachial plexus motor neurotization. Microsurgery. 1989;10:287.
99 Guan S.B., Hou C.L., Chen D.S., Gu Y.D. Restoration of shoulder abduction by transfer of the spinal accessory nerve to suprascapular nerve through dorsal approach: a clinical study. Chin. Med. J. (Engl.). 2006;119:707.
100 Gupta A., Jamshidi M., Rubin J.R. Traumatic first rib fracture: is angiography necessary? A review of 730 cases. Cardiovasc. Surg. 1997;5:48.
101 Gutowski K.A., Orenstein H.H. Restoration of elbow flexion after brachial plexus injury: the role of nerve and muscle transfers. Plast. Reconstr. Surg. 2000;106:1348. quiz 58; discussion 59
102 Haninec P., Smrcka V. Reconstruction of paralyzed biceps brachii muscle by transposition of pedicled latissimus dorsi muscle: report of two cases. Acta Chir. Plast. 1998;40:41.
103 Harmon P.H. Muscle transplantation for triceps palsy: the technique of utilizing the latissimus dorsi. J. Bone Joint Surg. 1949;31A:409.
104 Harris W., Low V.W. On the importance of accurate muscular analysis in lesions of the brachial plexus and the treatment of Erb’s palsy and infantile paralysis of the upper extremity by cross-union of nerve roots. Br. Med. J. 1903;2:1035.
105 Hattori Y., Doi K., Baliarsing A.S. A part of the ulnar nerve as an alternative donor nerve for functioning free muscle transfer: a case report. J. Hand Surg. [Am.]. 2002;27:150.
106 Hattori Y., Doi K., Ikeda K., Pagsaligan J.M., Watanabem M. Restoration of prehension using double free muscle technique after complete avulsion of brachial plexus in children: a report of three cases. J. Hand Surg. [Am.]. 2005;30:812.
107 Hentz V.R., Brown M., Keoshrian L.A. Upper limb reconstruction in quadriplegia: functional assessment and proposed treatment modifications. J. Hand Surg. 1983;8:119.
108 Hentz V.R., Hamlin C., Keoshian L.A. Surgical reconstruction in tetraplegia. Hand Clin. 1988;4:601.
109 Hentz V.R., House J., McDowell C., Moberg E. Rehabilitation and surgical reconstruction of the upper limb in tetraplegia: an update. J. Hand Surg. [Am.]. 1992;17:964.
110 Hentz V.R. Surgical strategy: matching the patient with the procedure. Hand Clin. 2002;18:503.
111 Hirayama T., Takemitsu Y., Atsuta Y., Ozawa K. Restoration of elbow flexion by complete latissimus dorsi muscle transposition. J. Hand Surg. 1987;12B:194.
112 Hochberg J., Borges Fortes da Silva F. Latissimus dorsi myocutaneous flap to restore elbow flexion and axillary burn contracture: a report on two pediatric patients. J. Pediatr. Orthop. 1982;2:565.
113 Hoffer M.M., Phipps G.J. Surgery about the elbow for brachial palsy. J. Pediatr. Orthop. 2000;20:781.
114 Hoffmann G., Laffont I., Hanneton S., Roby-Brami A. How to extend the elbow with a weak or paralyzed triceps: control of arm kinematics for aiming in C6-C7 quadriplegic patients. Neuroscience. 2006;139:749.
115 Hohmann G. Ersatz des gelahmten Bizeps Brachii durch den Pectoralis Major. Munch. Med. Wochenschr. 1918;65:1240.
116 Holtmann B., Wray R.C., Lowrey R., Weeks P. Restoration of elbow flexion. Hand. 1975;7:256.
117 Hovnanian A.P. Latissimus dorsi transplantation of the elbow. Ann. Surg. 1956;143:493.
118 Hoyen H., Gonzalez E., Williams P., Keith M. Management of the paralyzed elbow in tetraplegia. Hand Clin. 2002;18:113.
119 Ikuta Y., Kubo T., Tsuge K. Free muscle trans-plantation by microsurgical technique to treat severe Volkmann’s contracture. Plast. Reconstr. Surg. 1976;58:407.
120 Ikuta Y., Yoshioka K., Tsuge K. Free muscle graft as applied to brachial plexus injury—case report and experimental study. Ann. Acad. Med. Singapore. 1979;8:454.
121 Kanaya F., Gonzalez M., Park C., Kutz J.E., Kleinert H.E., Tsai T.M. Improvement in motor function after brachial plexus surgery. J. Hand Surg. 1990;15A:30.
122 Kawai H., Kawabata H., Masada K., Ono K., Yamamoto K., Tsuyuguchi Y., Tada K. Nerve repairs for traumatic brachial plexus palsy with root avulsion. Clin. Orthop. Relat. Res. 1988;237:75.
123 Kawano K., Nagano A., Ochiai N., Kondo T., Mikami Y., Tajiri Y. Restoration of elbow function by intercostal nerve transfer for obstetrical paralysis with co-contraction of the biceps and the triceps. J. Hand Surg. Eur. Vol. 2007;32:421.
124 Kettelkamp D.B., Larson C.B. Evaluation of the Steindler flexorplasty. J. Bone Joint Surg. 1963;45A:513.
125 Kirsch R.F., Acosta A.M., Perreault E.J., Keith M.W. Measurement of isometric elbow and shoulder moments: position-dependent strength of posterior deltoid-to-triceps muscle tendon transfer in tetraplegia. I. E. E. E. Trans. Rehabil. Eng. 1996;4:403.
126 Koman L.A., Gelberman R.H., Toby E.B., Poehling G.G. Cerebral palsy. Management of the upper extremity. Clin. Orthop. Relat. Res. 1990;253:62.
127 Kozin S.H., Schloth C. Bilateral biceps-to-triceps transfer to salvage failed bilateral deltoid-to-triceps transfer: a case report. J. Hand Surg. [Am.]. 2002;27:666.
128 Krakauer J.D., Wood M.B. Intercostal nerve transfer for brachial plexopathy. J. Hand Surg. [Am.]. 1994;19:829.
129 Kubo T., Ikuta Y., Tsuge K. Free muscle transplantation in dogs by microneurovascular anastomoses. Plast. Reconstr. Surg. 1976;57:495.
130 Kumar V.P., Satku K., Pho R.W. Modified technique of sternomastoid transfer for elbow flexion. J. Hand Surg. [Am.]. 1992;17:812.
131 Kuz J.E., Van Heest A.E., House J.H. Biceps-to-triceps transfer in tetraplegic patients: report of the medial routing technique and follow-up of three cases. J. Hand Surg. [Am.]. 1999;24:161.
132 Lacey S.H., Wilber R.G., Peckham P.H., Freehafer A.A. The posterior deltoid to triceps transfer: a clinical and biomechanical assessment. J. Hand Surg. 1986;11A:542.
133 Laffont I., Hoffmann G., Dizien O., Revol M., Roby-Brami A. How do C6/C7 tetraplegic patients grasp balls of different sizes and weights? Impact of surgical musculo-tendinous transfers. Spinal Cord. 2007;45:502.
134 Lamb D.W., Chan K.M. Surgical reconstruction of the upper limb in traumatic tetraplegia. A review of. 41 patients. J. Bone Joint Surg.. 1983;65B:291.
135 Landi A., Cavazza S., Caserta G., Leti Acciaro A., Sartini S., Gagliano M.C., Manca M. The upper limb in cerebral palsy: surgical management of shoulder and elbow deformities. Hand Clin. 2003;19:631. vii
136 Landra A.P. The latissimus dorsi musculocutaneous flap used to resurface defect on the upper arm and restore extension to the elbow. Br. J. Plast. Surg. 1979;32:275.
137 Lange F. Die Epidemische Kinderlahmung. Munich: J. F. Lehmann, 1930.
138 Lange M. Orthopaedisch-Chirurgische Operationslehre. Munich: J. F. Bergmann, 1951.
139 Leechavengvongs S., Witoonchart K., Uerpairojkit C., Thuvasethakul P., Ketmalasiri W. Nerve transfer to biceps muscle using a part of the ulnar nerve in brachial plexus injury (upper arm type): a report of 32 cases. J. Hand Surg. [Am.]. 1998;23:711.
140 Leechavengvongs S., Witoonchart K., Uerpairojkit C., Thuvasethakul P., Malungpaishrope K. Combined nerve transfers for C5 and C6 brachial plexus avulsion injury. J. Hand Surg. [Am.]. 2006;31:183.
141 Leffert R.D., Pess G.M. Tendon transfers for brachial plexus injury. Hand Clin. 1988;4:273.
142 Liao H.T., Chuang D.C., Ulusal A.E., Schrag C. Surgical strategies for brachial plexus polio-like paralysis. Plast. Reconstr. Surg. 2007;120:482.
143 Lieber R.L., Friden J., Hobbs T., Rothwell A.G. Analysis of posterior deltoid function one year after surgical restoration of elbow extension. J. Hand Surg. [Am.]. 2003;28:288.
144 Lin S.H., Chuang D.C., Hattori Y., Chen H.C. Traumatic major muscle loss in the upper extremity: reconstruction using functioning free muscle transplantation. J. Reconstr. Microsurg. 2004;20:227.
145 Lindholm T.S., Einola S. Flexorplasty of paralytic elbows: analysis of late functional results. Acta Orthop. Scand. 1973;44:1.
146 Liu T.K., Yang R.S., Sun J.S. Long-term results of the Steindler flexorplasty. Clin. Orthop. Relat. Res. 1993;296:104.
147 Liverneaux P.A., Diaz L.C., Beaulieu J.Y., Durand S., Oberlin C. Preliminary results of double nerve transfer to restore elbow flexion in upper type brachial plexus palsies. Plast. Reconstr. Surg. 2006;117:915.
148 Lloyd-Roberts G.C., Lettin A.W.F. Arthrogryposis multiplex congenita. J. Bone Joint Surg. 1970;52B:494.
149 Luedemann W., Hamm M., Blömer U., Samii M., Tatagiba M. Brachial plexus neurotization with donor phrenic nerves and its effect on pulmonary function. J. Neurosurg. 2002;96:523.
150 Mackinnon S.E. Preliminary results of double nerve transfer to restore elbow flexion in upper type brachial plexus palsies. Plast. Reconstr. Surg. 2006;118:1273. author reply 4
151 Mackinnon S.E., Novak C.B., Myckatyn T.M., Tung T.H. Results of reinnervation of the biceps and brachialis muscles with a double fascicular transfer for elbow flexion. J. Hand Surg. [Am.]. 2005;30:978.
152 Manktelow R.T. Functioning microsurgical muscle transfer. Hand Clin. 1988;4:289.
153 Manktelow R.T., Zuker R.M. The principles of functioning muscle transplantation: applications to the upper arm. Ann. Plast. Surg. 1989;22:275.
154 Manske P.R., McCarroll H.R., Hale R. Biceps tendon rerouting and percutaneous osteoclasis in the treatment of supination deformity in obstetrical palsy. J. Hand Surg. 1980;5:153.
155 Marshall R.W., Williams D.H., Birch R., Bonney G. Operations to restore elbow flexion after brachial plexus injuries. J. Bone Joint Surg. Br. 1988;70:577.
156 Mathes S.J., Nahai F. Classification of the vascular anatomy of muscles: experimental and clinical correlation. Plast. Reconstr. Surg. 1981;67:177.
157 Matory W.E., Morgan W.J., Breen T. Technical considerations in pectoralis major transfer for treatment of the paralytic elbow. J. Hand Surg. 1991;16A:12.
158 Mayer L., Green W. Experiences with Steindler flexorplasty of the elbow. J. Bone Joint Surg. 1954;36A:775.
159 McDowell C.L., Moberg E.A., House J.H. Proceedings of the Second International Conference on Surgical Rehabilitation of the Upper Limb in Tetraplegia (Quadriplegia). J. Hand Surg. 1986;11A:604.
160 Meade N.G., Lithgow W.C., Sweeney H.J. Arthrogryposis multiplex congenita. J. Bone Joint Surg. 1958;40A:1285.
161 Medical Research Council. Aids to the Investigation of Peripheral Nerve Injuries. War Memorandum No 7, 2nd ed.. His Majesty’s Stationery Office, London, 1943.
162 Mennen U., Boonzaier A.C. An improved technique of posterior deltoid to triceps transfer in tetraplegia. J. Hand Surg. [Br.]. 1991;16:197.
163 Merrell G.A., Barrie K.A., Katz D.L., Wolfe S.W. Results of nerve transfer techniques for restoration of shoulder and elbow function in the context of a meta-analysis of the English literature. J. Hand Surg. [Am.]. 2001;26:303.
164 Millesi H. Surgical management of brachial plexus injuries. J. Hand Surg. 1977;5:367.
165 Millesi H. Brachial plexus injuries management and results. Clin. Plast. Surg. 1984;11:115.
166 Moberg E. Surgical treatment for absent single-hand grip and elbow extension in quadriplegia: principles and preliminary experience. J. Bone Joint Surg. 1975;57A:196.
167 Moberg E. The Upper Limb in Tetraplegia: A New Approach to Surgical Rehabilitation. Stuttgart: Thieme, 1978.
168 Moberg E. The new surgical rehabilitation of arm-hand function in the tetraplegic patient. Scand. J. Rehabil. Med. Suppl. 1988;17:131.
169 Moberg E. Surgical rehabilitation of the upper limb in tetraplegia. Paraplegia. 1990;28:330.
170 Mohammed K.D., Rothwell A.G., Sinclair S.W., Willems S.M., Bean A.R. Upper-limb surgery for tetraplegia. J. Bone Joint Surg. Br. 1992;74:873.
171 Moneim M.S., Omer G.E. Latissimus dorsi muscle transfer for restoration of elbow flexion after brachial plexus disrruption. J. Hand Surg. 1986;11A:135.
172 Monreal R. Steindler flexorplasty to restore elbow flexion in C5-C6-C7 brachial plexus palsy type. J. Brachial Plex. Peripher. Nerve Inj. 2007;2:15.
173 Mulcahey M.J., Lutz C., Kozin S.H., Betz R.R. Prospective evaluation of biceps to triceps and deltoid to triceps for elbow extension in tetraplegia. J. Hand Surg. [Am.]. 2003;28:964.
174 Nagano A. Intercostal nerve transfer for elbow flexion. Tech. Hand Up. Extrem. Surg. 2001;5:136.
175 Nagano A., Ochiai N., Okinaga S. Restoration of elbow flexion in root lesions of brachial plexus injuries. J. Hand Surg. [Am.]. 1992;17:815.
176 Narakas A. Surgical treatment of traction injuries of the brachial plexus. Clin. Orthop. 1978;133:71.
177 Narakas A. Neurotization in the treatment of brachial plexus injuries. In: Gelberman R.H., editor. Operative Nerve Repair and Reconstruction. Philadelphia: JB Lippincott; 1991:1329.
178 Narakas A.O. The surgical treatment of traumatic brachial plexus lesions. Int. Surg. 1980;65:522.
179 Narakas A.O. Thoughts on neurotization or nerve transfers in irreparable nerve lesions. Clin. Plast. Surg. 1984;11:153.
180 Narakas A.O. The treatment of brachial plexus injuries. Int. Orthop. 1986;9:29.
181 Narakas A.O. Neurotization in brachial plexus injuries: indications and results. Clin. Orthop. Relat. Res. 1988;237:43.
182 Narakas A.O. Muscle transpositions in the shoulder and upper arm for sequelae of brachial plexus palsy. Clin. Neurol. Neurosurg. 1993;95(suppl):S89.
183 Nyholm K. Elbow flexorplasty in tendon transposition (an analysis of the functional results in 26 patients). Acta Orthop. Scand. 1963;33:32.
184 O’Brien B., Morrison W.A., MacLeod A.M., Weinglein O. Free microneurovascular muscle transfer in limbs to provide motor power. Ann. Plast. Surg. 1982;9:381.
185 Ober F.R., Barr J.S. Brachioradialis muscle transposition for triceps weakness. Surg. Gynecol. Obstet. 1938;67:105.
186 Oberlin C. [Brachial plexus palsy in adults with radicular lesions, general concepts, diagnostic approach and results]. Chir. Main. 2003;22:273.
187 Oberlin C., Béal D., Leechavengvongs S., Salon A., Dauge M.C., Sarcy J.J. Nerve transfer to biceps muscle using a part of ulnar nerve for C5-C6 avulsion of the brachial plexus: anatomical study and report of four cases. J. Hand Surg. [Am.]. 1994;19:232.
188 Owings R., Wickstrom J., Perry J., Nickel V.L. Biceps brachii rerouting in treatment of paralytic supination contracture of the forearm. J. Bone Joint Surg. 1971;53A:137.
189 Ozkan T., Okumus A., Aydin A., Ozkan S., Tuncer S. Brachioradialis transposition for elbow extension in obstetrical brachial plexus palsy. Tech. Hand Up. Extrem. Surg. 2005;9:60.
190 Paul S.D., Gellman H., Waters R., Willstein G., Tognella M. Single-stage reconstruction of key pinch and extension of the elbow in tetraplegic patients. J. Bone Joint Surg. Am. 1994;76:1451.
191 Peterson C.2nd, Maki S., Wood M.B. Clinical results of the one-bone forearm. J. Hand Surg. Am. 1995;20:609.
192 Price A., Tidwell M., Grossman J.A. Improving shoulder and elbow function in children with Erb’s palsy. Semin. Pediatr. Neurol. 2000;7:44.
193 Prudzansky M., Kelly M., Weinberg H. Latissimus dorsi musculocutaneous flap for elbow extension. J. Surg. Oncol. 1991;47:62.
194 Pruksakorn D., Sananpanich K., Khunamornpong S., Phudhichareonrat S., Chalidapong P. Posterior approach technique for accessory-suprascapular nerve transfer: a cadaveric study of the anatomical landmarks and number of myelinated axons. Clin. Anat. 2007;20:140.
195 Remy-Neris O., Milcamps J., Chikhi-Keromest R., Thevenon A., Bouttens D., Bouilland S. Improved kinematics of unrestrained arm raising in C5-C6 tetraplegic subjects after deltoid-to-triceps transfer. Spinal Cord. 2003;41:435.
196 Revol M., Briand E., Servant J.M. Biceps-to-triceps transfer in tetraplegia. The medial route. J. Hand Surg. [Br.]. 1999;24:235.
197 Revol M., Cormerais A., Laffont I., Pedelucq J.P., Dizien O., Servant J.M. Tendon transfers as applied to tetraplegia. Hand Clin. 2002;18:423.
198 Rivarola R.A. Tratamiento de las paralisis definitivas del miembro superior. Bul. Trabajos Soc. Cirug. Buenos Aires. 1928;12:688. (reported in Sernana Med. Buenos Aires 2:1294, 1928)
199 Rivet D., Boileau R., Saiveau M., Baudet J. Restoration of elbow flexion using the latissimus dorsi musculo-cutaneous flap. Ann. Chir. Main. 1989;8:110.
200 Rostoucher P., Alnot J.Y., Touam C., Oberlin C. Tendon transfers to restore elbow flexion after traumatic paralysis of the brachial plexus in adults. Int. Orthop. 1998;22:255.
201 Ruch D.S., Friedman A., Nunley J.A. The restoration of elbow flexion with intercostal nerve transfers. Clin. Orthop. Relat. Res. 1995;314:95.
202 Rühmann O., Wirth C.J., Gosse F. Triceps to biceps transfer to restore elbow flexion in three patients with brachial plexus palsy. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2000;34:355.
203 Russell R.C., Pribaz J., Zook E.G., Leighton W.D., Eriksson E., Smith C.J. Functional evaluation of latissimus dorsi donor site. Plast. Reconstr. Surg. 1986;78:336.
204 Samardzic M., Grujicic D., Antunovic V. Nerve transfer in brachial plexus traction injuries. J. Neurosurg. 1992;76:191.
205 Samardzic M., Grujicic D., Rasulic L., Bacetic D. Transfer of the medial pectoral nerve: myth or reality? Neurosurgery. 2002;50:1277.
206 Samardzic M., Rasulic L., Grujicic D., Milicic B. Results of nerve transfers to the musculocutaneous and axillary nerves. Neurosurgery. 2000;46:93. discussion, 3
207 Schottstaedt E.R., Larsen L.J., Bost F.C. Complete muscle transposition. J. Bone Joint Surg. 1955;37A:897.
208 Schottstaedt E.R., Larsen L.J., Bost F.C. The surgical reconstruction of the upper extremity paralyzed by poliomyelitis. J. Bone Joint Surg. 1958;40A:633.
209 Schulze-Berge R. Ersatz der Benger des Voderarames (Bizeps und Brachialis) durch den Pectoralis major. Dtsch. Med. Wochenschr. 1917;43:433.
210 Seddon H.J. Transplantation of pectoralis major for paralysis of the flexors of the elbow. Proc. R. Soc. Med. 1949;43:837.
211 Seddon H.J. Nerve grafting. J. Bone Joint Surg. 1963;45B:447.
212 Sedel L. The results of surgical repair of brachial plexus injuries. J. Bone Joint Surg. 1982;64B:54.
213 Segal A., Seddon H.J., Brooks D.M. Treatment of paralysis of the flexors of the elbow. J. Bone Joint Surg. 1959;41B:44.
214 Shin A.Y., Spinner R.J., Steinmann S.P., Bishop A.T. Adult traumatic brachial plexus injuries. J. Am. Acad. Orthop. Surg. 2005;13:382.
215 Simesen K., Haase J. Microsurgery in brachial plexus lesions. Acta Orthop. Scand. 1985;56:238.
216 Solonen K.A., Vastamaki M., Strom B. Surgery of the brachial plexus. Acta Orthop. Scand. 1984;55:436.
217 Songcharoen P., Mahaisavariya B., Chotigavanich C. Spinal accessory neurotization for restoration of elbow flexion in avulsion injuries of the brachial plexus. J. Hand Surg. [Am.]. 1996;21:387.
218 Soucacos P.N., Vekris M.D., Zoubos A.B., Johnson E.O. Secondary reanimation procedures in late obstetrical brachial plexus palsy patients. Microsurgery. 2006;26:343.
219 Spira E. Replacement of biceps brachii by pectoralis minor transplant. J. Bone Joint Surg. 1957;39B:126.
220 Steindler A. Orthopaedic reconstruction work on hand and forearm. N. Y. Med. J. 1918;108:1117.
221 Steindler A. A muscle plasty for the relief of flail elbow in infantile paralysis. Interstate Med. J. 1918;25:235.
222 Steindler A. Operative treatment of paralytic conditions of the upper extremity. J. Orthop. Surg. 1919;1:608.
223 Steindler A. Tendon transplantation in the upper extremity. Am. J. Surg. 1939;44:260.
224 Steindler A. Muscle and tendon transplantation at the elbow. In: AAOS: Instructional Course Lectures on Reconstruction Surgery. Ann Arbor: JW Edwards; 1944:276.
225 Stern P.J., Neale H.W., Gregory R.O., Kreilein J.G. Latissimus dorsi musculocutaneous flap for elbow flexion. J. Hand Surg. 1982;7:25.
226 Stern P.J., Caudle R.J. Tendon transfers for elbow flexion. Hand Clin. 1988;4:297.
227 Sturm J.T., Perry J.F.Jr. Brachial plexus injuries from blunt trauma—a harbinger of vascular and thoracic injury. Ann. Emerg. Med. 1987;16:404.
228 Sunderlund S. Repair of the brachial plexus directed to restoring elbow flexion. Bull. Hosp. Joint Dis. Orthop. Inst. 1984;44:485.
229 Sungpet A., Suphachatwong C., Kawinwonggowit V. Transfer of one fascicle of ulnar nerve to functioning free gracilis muscle transplantation for elbow flexion. A. N. Z. J. Surg. 2003;73:133.
230 Takami H., Takahashi S., Ando M. Latissimus dorsi transplantation to restore elbow flexion to the paralysed limb. J. Hand Surg. 1984;9B:61.
231 Tamai S., Komatsu S., Sakamoto H., Sano S., Sasauchi N. Free muscle transplants in dogs, with microsurgical neurovascular anastomoses. Plast. Reconstr. Surg. 1970;46:219.
232 Teboul F., Kakkar R., Ameur N., Beaulieu J.Y., Oberlin C. Transfer of fascicles from the ulnar nerve to the nerve to the biceps in the treatment of upper brachial plexus palsy. J. Bone Joint Surg. Am. 2004;86-A:1485.
233 Terzis J.K., Kostopoulos V.K. The surgical treatment of brachial plexus injuries in adults. Plast. Reconstr. Surg. 2007;119:73e.
234 Terzis J.K., Sweet R.C., Dykes R.W., Williams H.B. Recovery of function in free muscle transplants using microneurovascular anastomoses. J. Hand Surg. [Am.]. 1978;3:37.
235 Terzis J.K., Vekris M.D., Soucacos P.N. Outcomes of brachial plexus reconstruction in 204 patients with devastating paralysis. Plast. Reconstr. Surg. 1999;104:1221.
236 Tobin G.R., Bland K.I., Adcock R. Surgical anatomy of the musculus pectoralis major and neurovascular supply. Am. Coll. Surg. Forum. 1981;32:574.
237 Tobin G.R., Shusterman B.A., Peterson G.H., Nichols G., Bland K.I. The intramuscular neurovascular anatomy of the latissimus dorsi muscle: the basis for splitting the flap. Plast. Reconstr. Surg. 1981;67:637.
238 Tomita Y., Tsai T., Burns J.T., Karaoguz A., Ogden L.L. Intercostal nerve transfers in brachial plexus injuries: an experimental study. Microsurgery. 1983;4:95.
239 Tsai T., Kalisman M., Burns J., Kleinert H.E. Restoration of elbow flexion by pectoralis major and pectoralis minor transfer. J. Hand Surg. 1983;8:186.
240 Tubby A.H. Deformities Including Diseases of the Bones and Joints. London: MacMillan, 1912.
241 Tuttle H. Exposure of the brachial plexus with nerve transplantation. J. A. M. A. 1913;61:15.
242 Vanden Berghe A., Van Laere M., Hellings S., Vercauteren M. Reconstruction of the upper extremity in tetraplegia: functional assessment, surgical procedures and rehabilitation. Paraplegia. 1991;29:103.
243 Vastamaki M. Short-term versus long-term com-parative results after reconstructive upper-limb surgery in tetraplegic patients. J. Hand Surg. [Am.]. 2006;31:1490.
244 Vathana T., Larsen M., de Ruiter G.C., Bishop A.T., Spinner R.J., Shin A.Y. An anatomic study of the spinal accessory nerve: extended harvest permits direct nerve transfer to distal plexus targets. Clin. Anat. 2007;20:899.
245 VonLanz T., Wachsmuth W. Praktische Anatomie. 2nd ed. Berlin: Springer-Verlag, 1959.
246 Vulpius O., Stoffel A. Muskel- und Sehnentransplantation. Überpflanzungen an der oberen Extremität. In: Vulpius O., Stoffel A., editors. Orthopädische Operationslehre. 2nd ed. Stuttgart: Ferdinand Enke; 1920:266.
247 Waikakul S., Wongtragul S., Vanadurongwan V. Restoration of elbow flexion in brachial plexus avulsion injury: comparing spinal accessory nerve transfer with intercostal nerve transfer. J. Hand Surg. [Am.]. 1999;24:571.
248 Williams P.F. The elbow in arthrogryposis. J. Bone Joint Surg. 1973;55B:834.
249 Wynn Parry C.B. Brachial plexus injuries. Br. J. Hosp. Med. 1984;32:130.
250 Xu J., Cheng X., Gu Y. Different methods and results in the treatment of obstetrical brachial plexus palsy. J. Reconstr. Microsurg. 2000;16:417. discussion 20
251 Xu W.D., Gu Y.D., Xu J.G., Tan L.J. Full-length phrenic nerve transfer by means of video-assisted thoracic surgery in treating brachial plexus avulsion injury. Plast. Reconstr. Surg. 2002;110:104. discussion 10
252 Yamada S., Peterson G.W., Soloniuk D.S., Will A.D. Coaptation of the anterior rami of C-3 and C-4 to the upper trunk of the brachial plexus for cervical nerve root avulsion. J. Neurosurg. 1991;74:171.
253 Zancolli E.A. Paralytic supination contracture of the forearm. J. Bone Joint Surg. 1967;49A:1275.
254 Zancolli E.A., Mitre H. Latissimus dorsi transfer to restore elbow flexion. J. Bone Joint Surg. 1973;55A:1265.
255 Zancolli E.A. Structural and Dynamic Bases of Hand Surgery. 2nd ed. Philadelphia: J. B. Lippincott Co., 1979.
256 Zaoussis A.L. Osteotomy of the proximal end of the radius for paralytic supination deformity in children. J. Bone Joint Surg. 1963;45B:523.
* See references 25, 31, 53, 58, 128, 174, 175, 181, 201, 204–206, 212, and 233.
† See references 34, 46, 58, 90, 101, 207, 209, 218, and 219.
‡ See references 3, 51–53, 55, 69, 70, 152, 155, and 218.
§ See references 73, 88, 121, 122, 164, 165, 176, 178–181, 212, 215, 216, 228, and 238.
¶ See references 37, 63, 82, 107–109, 135, 159, 170, and 173.