Chapter 19 Fiberoptic and Flexible Endoscopic-Aided Techniques
I. Introduction and Historical Background
II. Flexible Fiberoptic and Non-fiberoptic Bronchoscope Design and Care
III. Rationale for Fiberoptic Intubation
V. Clinical Techniques of Fiberoptic Intubation
VII. Special Flexible Fiberoptic Bronchoscope Uses
VIII. Rarely Recommended: Oxygen Insufflation via Bronchoscope
IX. Fiberoptic Intubation in Infants and Children
X. Advantages of Flexible Fiberoptic Bronchoscopes
XI. Disadvantages and Complications of Flexible Fiberoptic Bronchoscopes
XII. Other Causes of Failure with Fiberoptic Bronchoscopes
XIII. Learning Fiberoptic Intubation
XV. Introducing Fiberoptic Intubation into One’s Own Practice
I Introduction and Historical Background
The purpose of this chapter is threefold:
1. Incorporate recent airway management developments for integration with flexible endoscopes.
2. Impart knowledge concerning flexible endoscopic techniques and tips for airway use.
3. Increase the likelihood that readers might achieve a level of expertise in flexible endoscopic intubation and airway management.
Historically, a wide variety of devices have been employed to visualize the airway in living human subjects. Manuel Garcia, a singing instructor, ingeniously used mirrors to reflect sunlight in 1854, allowing him to examine his own moving vocal cords. He was considered the “first laryngoscopist” after a presentation to the Royal Society of Medicine.1 Using a blind digital technique, William Macewen completed the first endotracheal intubation under anesthesia as well as the first awake intubation for surgery and anesthesia in 1878.2 On hearing of an accidental intratracheal insertion in 1895, Alfred Kirstein used an esophagoscope that incorporated a proximal light source and spatula-like area to perform the first “direct” laryngoscopy.3
Bronchoscopy history began to develop in 1887, when Gustav Killian extracted a foreign body from a farmer’s respiratory tract with a rigid bronchoscope. Chevalier Jackson modified this rigid bronchoscope by inclusion of a distal tungsten light bulb and suction channel in 1904, resulting in the significantly greater likelihood of successful endotracheal intubation.4
Finally, a remarkable development took place in 1966. Shigeto Ikeda proposed his ideas for construction of a flexible fiberoptic bronchoscope (FOB) to two companies, Machida Endoscope and Olympus.5 Meanwhile, in 1967, Peter Murphy ingeniously used a choledochoscope for endotracheal intubation.6 In 1968, the Machida company at last produced an extremely “bendable,” guided FOB, which featured an eyepiece for seeing images transmitted along 15,000 glass fibers that were 14 µm in diameter. Clinically, Ikeda’s technique was to insert this very flexible instrument through a rigid bronchoscope. Later that year, Olympus produced a more maneuverable FOB with a working channel that allowed the application of cytology brushes. Ikeda’s new FOB pictures of the distal airway, presented at the International Congress on Disease of the Chest in Copenhagen, were instantaneously news-making revelations. He was able to demonstrate a stand-alone FOB insertion method for endotracheal intubation in many countries, and he published his experiences in 1971.7
Initially many clinicians advanced FOB use for diagnostic purposes, for ordinary intubation procedures, and, eventually, for airway management in patients with very challenging airways.8–12 The inherent characteristic of FOB superiority forged its involvement as an instrument of first choice in difficult intubation (DI) cases, cervical spine risk scenarios, and as a diagnostic and therapeutic tool for patients with hypoxemia, high airway pressure, pulmonary tumors, infection, foreign bodies, airway stenosis, obstructive sleep apnea, tracheomalacia, and many more wide-ranging abnormalities.
Raj advocated the role of FOB assistance in double-lumen tube (DLT) placement11 while Ovassapian further delineated its advantages in one-lung isolation in 1987.13 Through FOB use, Benumof compared bronchial insertions of right and left DLTs to explain why the relationship of bronchial anatomy to DLT construction inevitably resulted in less successful right-sided DLT positioning.14
In the 1980s, the Asahi Pentax Company’s integration of the FOB with a preexisting invention, the charge-coupled device (CCD), permitted video monitor viewing of the airway and heightened its educational and clinical benefits.15 FOB construction creativity has continued to evolve, with sizes available for all types of patients, many featuring full-color imaging and a working suction/injection channel. Light-emitting diodes (LED) and micro video complementary metal-oxide semiconductor chips (CMOS) for transmission capability have also been implemented.
Although universal acceptance of FOB use for intubation was somewhat slow for many years, Ovassapian and colleagues were leading and effective pioneers in the educational promotion of its use through workshop training and simulation, eventually popularizing this astonishingly effective technique.16 Currently, the FOB resides worldwide within all algorithms for DI, and there is a clear, growing tendency to use this technique in combination with other recent advances as a multimodal approach to difficult airway (DA) management.
II Flexible Fiberoptic and Non-Fiberoptic Bronchoscope Design and Care
A Fiberoptic Bronchoscope Design
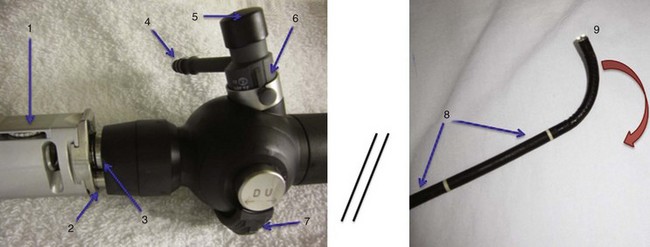
Variations in design may seem endless, but all flexible endoscopes have certain commonalities. Differences are described later in this chapter. There are three main parts: handle, insertion tube, and flexible tip (Fig. 19-1).
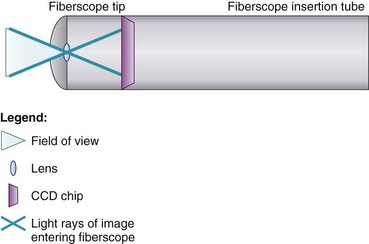
At the end of the handle is either a battery-operated light source, which allows for more portability, or an optical cable connection to an external light source. The proximal “visual section” of the handle is the location of an eyepiece and lens, a video output adaptor, or an actual video screen with an integrated camera (depending on the model). FOBs with a CCD or CMOS camera chip at the tip have a wide-angle image and a higher resolution of the picture transmitted, compared with older models, which have a narrower field of view and less optical detail (Fig. 19-2). This makes video fiberscope deployment useful clinically, particularly in teaching.
Four specific components travel inside the insertion tube along the FOB length (Fig. 19-3). The first allows the transmission of light going toward the tip by way of one or two light guide bundles constructed of noncoherent glass fibers. A high degree of light intensity is focused on the proximal light guide bundle, but heat filters or reflecting mirrors prevent injury to the insertion tube components.
On the back of the handle is a bending or angulation lever. Through an up or down movement of the thumb, it causes pulling on the third insertion tube component located along the sagittal plane, the two angulation wires. This initiates movement of the FOB tip in the opposite direction (i.e., down or up, respectively) by as much as 240 to 350 degrees (see Fig. 19-1). These delicate wires can also be broken with excessive pressure, including lever motion if the FOB tip is still within the ETT lumen.
The FOB tip or bending section is hinged and has an objective lens (approximately 2 mm in diameter) with a fixed focal point and a short field of view (75 to 120 degrees) (see Figs. 19-1 to 19-3).
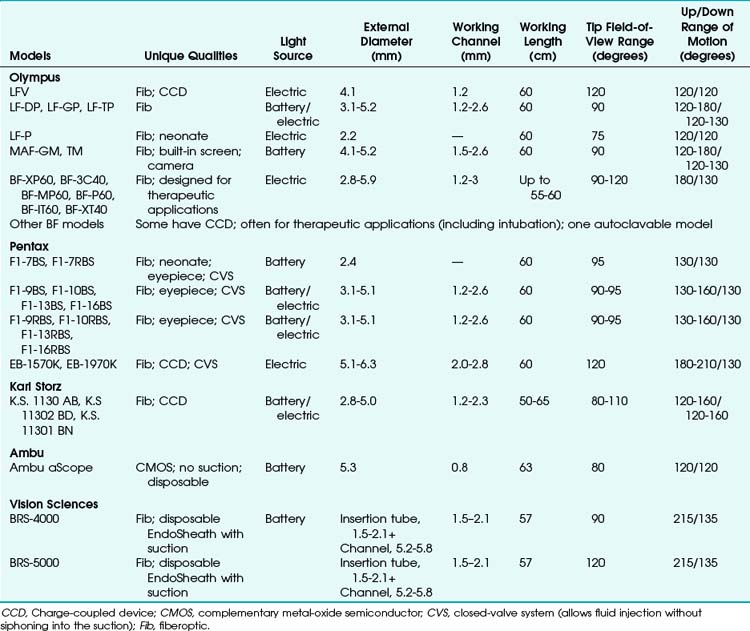
Many models are available in sizes from premature infant to large adult and with varying fields of view, video monitors, closed-valve systems, and other features (Table 19-1).
B Fiberoptic Bronchoscope Cleaning
Almost half a million bronchoscopies are performed per year in the United States.17 Whenever any equipment is reused between patients, there is always a concern for avoiding the transmission of communicable diseases. Although there have not been any reports of infection or cross-contamination caused by fiberoptic intubation for elective or emergency purposes, sporadic references testify to instances of infection or contamination after flexible bronchoscopic procedures such as bronchial washings or bronchoscopic lavage. Between the years 2000 and 2007, the American Association for Respiratory Care (AARC), American College of Chest Physicians (AACP), American Association for Bronchology and Interventional Pulmonology (AABIP), and Association for Professionals in Infection Control (APIC) issued guidelines and consensus statements to assist in the prevention of FOB-associated infection or contamination.18–20
Since the 1970s, sources of contamination have included a number of factors: sentinel patients, contaminated water, inadequate sterilization technique (due to insufficient quality or quantity of sterilizing solution), repeated use of cleaning fluid or brushes, automated endoscope reprocessors, and FOB instruments with design errors or damage.21–24 Valves and working channels are usually the most suspect areas for ineffective FOB sterilization. Multiple organisms have been found, including Pseudomonas aeruginosa, non-tuberculosis mycobacteria, Serratia marcescens, Mycobacterium tuberculosis, Stenotrophomonas maltophilia, Legionella pneumophila, Rhodotorula rubra, Klebsiella species, Proteus species, and fungi.21,25–28
Disinfection may take up to 45 min (Fig. 19-4). The assistant should carefully insert a cleaning brush into portals entering the working channel. The brush must be advanced fully along the compete span of this conduit to effect early removal of secretions according to the instructions of the manufacturer and the health care organization.
The suction ports and biopsy ports (if present) need to be disassembled from the rest of the FOB. All nondisposable parts must be gently placed in glutaraldehyde, peracetic acid, orthophthaldehyde, hydrogen peroxide gas plasma, or other manufacturer-indicated sterilization solution, and the working channel must be syringe-flushed with this disinfectant conforming to manufacturer and health care organization instructions.29–31
After completion of the specified sterilization time, all parts must be removed to prevent caustic damage to the instrument. The FOB should be gently washed and thoroughly rinsed with sterile water, including flushing of the working channel to prevent toxicity from residual chemicals.32 It is mandatory to dry the channel by suctioning or purging with 70% alcohol and compressed air for a time period in keeping with manufacturer and health care organization instructions.
Most bacteria, fungi, and viruses are susceptible to these sterilization processes, including human immunodeficiency virus (HIV) and hepatitis. FOBs that have been used in patients with contagious diseases (e.g., tuberculosis) may require a lengthy ethylene oxide sterilization and aeration procedure for up to 24 hours, with the venting cap secured to the venting connector. In the case of suspected prion infection (Creutzfeldt-Jakob or variants), it is best to avoid bronchoscopy if possible, because the sterilization process for most medical instruments would be damaging.18–20,33
Presently, routine surveillance cultures have not been recommended by the AARC, AACP, AABIP, or APIC because of lack of criteria to determine testing frequency, relevance when positive results occur, indeterminate courses of action, and costs of testing procedures.18–20
During all handling and sterilization techniques, keeping the FOB as straight as possible is extremely important to avoid damage. Once the device has been cleaned, it is preferable to store it suspended by its handle in a moderately lighted, climate- and humidity-controlled, safe location. Close proximity to radiation should be avoided because of deleterious effects on the FOB material.34
C Disposable Flexible Bronchoscopes
1 Sheathed Fiberoptic Bronchoscope
Vision Sciences, Inc. (Orangeburg, NY) has produced two adult-sized, channel-less FOBs for use with a clear, durable, snugly fitting, presterilized flexible, thermoplastic, elastomer EndoSheath that incorporates its own 1.5- to 2.1-mm working channel. The disposable sheath potentially prevents transmission of organisms and may increase FOB availability by requiring no down-time for sterilization. It might also reduce costs in centers where economic factors limit the purchase of more than a minimum number of bronchoscopy systems or limit the availability of sterilization equipment or personnel.35,36
2 Non-fiberoptic Flexible Bronchoscope
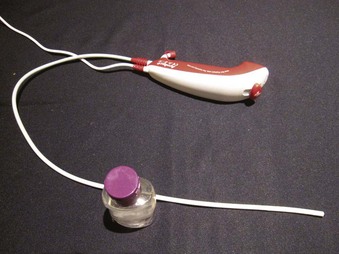
The Ambu aScope (Ambu A/S, Ballerup, Denmark) is a fully disposable, sterile, battery-operated non-FOB with a small (0.8-mm) working channel capable of drug instillation but not suctioning. It has a CMOS chip, a steering button to flex the tip, and a distal LED (Fig. 19-5). Images are transmitted along a cable within the insertion tube and transferred to a small portable monitor by way of a video connection cable at the handle. In its early version, this non-FOB had a timing mechanism allowing it to function for a maximum of 30 minutes during an 8-hour period starting from the initial power startup. A more recent version of this device comes without these time limitations. The monitor can be used for 150 intubations and is connected to an external monitoring system. Apart from routine use of this instrument, a particular advantage may occur in patients with highly contagious disease (e.g., Creutzfeldt-Jakob), in immunosuppressed patients, or in situations in which cheaper devices may be beneficial (e.g., where economic factors limit purchases or where FOB is seldom used).37,38
III Rationale for Fiberoptic Intubation
Airway management failure is a leading cause of patient morbidity and mortality in closed claims analyses.39 During the period 1999 to 2005, failed intubation or DI was the cause of 2.3% of the 2211 anesthesia-related deaths in the United States.40 With rigid laryngoscopes (RLs), DI was reported to have a frequency ranging from 1% to 13%.41–45 Shiga and colleagues performed a meta-analysis of studies on 50,760 patients and estimated the average occurrence of difficult intubation to be 5.8%,46 with a range of 4.5% to 7.5% overall47,48 and an even greater incidence in obstetric or obese patients.49–51 Anesthesia Associates, Inc. (AincA), San Marcos, CA Benumof’s estimate of “cannot intubate, cannot mask-ventilate” scenario was verified by Heidegger and associates at 0.007%,52,53 whereas Kheterpal and coworkers showed the incidence of impossible mask ventilation to be 0.15% in a prospective series of 53,041 patients.54
FOB intubation has always had a place in these scenarios, but this simple technique seems formidable to many. However, despite the increasing availability of less expensive intubating supraglottic airways (SGAs), lighted stylets, video laryngoscopes (VLs), and optical laryngoscopes (OLs), an explosion of improved laryngoscopic views, and greater intubating success rates, there is still a definite need for FOB intubation. A survey of American anesthesiologists by Rosenblatt and colleagues revealed a strong preference for FOB intubation for DA patients.55 Avarguès and associates’ survey of French anesthetists in 1999 revealed that 64% of responders expressed the need for more training in FOB intubation.56 Furthermore, FOB use was mentioned twice in the revised 2003 DA algorithm by a panel of experts who formulated the American Society of Anesthesiologists (ASA) Practice Guidelines for Difficult Airway Management.57 In a 2009 review of the existing algorithms for DA management, Frova and Sorbello affirmed that FOB is universally recognized as the gold standard in the awake, sedated, or anesthetized “difficult to intubate” patient.58
Finally and more practically, there have been numerous cases in which older devices and even the most recently developed airway management devices have proved to be inadequate. FOB employment and assistance steadfastly facilitates the execution of successful intubations, often rescuing these failures, either alone or as part of a multimodal approach to the DA.59,60
The FOB has a number of unique, wide-ranging characteristics:
• Its flexible, gliding attributes allow FOB skimming along the most twisted of DA obstacle courses while still posting the highest success rates for intubation.
• During intubation, the FOB is the device least likely to result in any cervical spine motion.61
• The FOB can be used for intubation in any body position, via oral or nasal routes, in all age groups.
• Under excellent local airway anesthesia, FOB intubation is associated with negligible hemodynamic changes.
• The FOB has multiple diagnostic capabilities for determining abnormal anatomy, assisting with implementation of other airway devices, and trouble-shooting ventilatory problems.
A Indications for Flexible Fiberoptic or Endoscopic Intubation
Although many anesthesiologists routinely carry out FOB intubation on all of their patients, most tend to adhere to specific indications. There is no hard or fast rule for “awake” versus “asleep” fiberoptic intubation. An awake approach is usually chosen to lessen significant patient risk in comparison to other methods, especially rigid laryngoscopy. The awake approach may be more advisable in cases of a very difficult airway or with a somewhat difficult airway in a patient with intolerant comorbidities endangered by the trauma or hypoxemia of non-FOB techniques (e.g., critical coronary artery disease). A list of indications for flexible airway endoscopy is presented in Box 19-1.
Box 19-1 Indications for Use of Flexible Fiberoptic or Endoscopic Intubation
3. Prevention of cervical spine motion in at-risk patients
4. Avoidance of traumatic oral or nasal effects of intubation (e.g., loose teeth, nasal polyps)
FOB, Fiberoptic bronchoscope; SGA, supraglottic airway device.
B Contraindications: Absolute, Moderate, and Relative
The most important contraindication to FOB use may well be lack of skill of the endoscopist (i.e., FOB operator) due to inadequate training or inadequate maintenance of formerly acquired skills. Any lack of a trained assistant or of available, ready to use, proper equipment may also negate FOB plans. There are few other contraindications to FOB use. These may involve situations in which harm to the instrument may occur (e.g., uncooperative patients), cases in which FOB use is extremely likely to be unsuccessful (e.g., patients with known near-total upper airway obstruction [Fig. 19-6]), or rare instances in which another technique may pose less risk for airway management (e.g., patients who have massive facial trauma with excessive bleeding might do better with a tracheostomy). Contraindications for awake and asleep FOB intubation are listed in Boxes 19-2 and 19-3, respectively.
Box 19-2 Contraindications for Awake FOB Techniques
ENT, Ear, nose, and throat; ETT, endotracheal tube; FOB, fiberoptic bronchoscope.
Box 19-3 Contraindications for Asleep FOB Techniques
ENT, Ear, nose, and throat; ETT, endotracheal tube; FOB, fiberoptic bronchoscope.
IV Equipment
A Fiberoptic and Non-fiberoptic Bronchoscope Model Details
FOB specifications and characteristics have been presented in Table 19-1. Some models have eyepieces or video attachments. Others have varying degrees of portability. Sizes and ranges of view are also variable, from the smallest infant to the largest adult size. In 2010, Olympus developed a unique MAF-GM system; it features a lithium rechargeable battery-operated FOB with a fixed, rotatable video screen by the handle––all totally immersible for sterilization. Its CCD camera has a memory card and an xD chip for still photography and video recording.
B Fiberoptic Bronchoscope Cart
Preparation for highly useful FOB techniques is important to increase the likelihood of success. The incorporation of an FOB cart as a movable asset capable of rapid transport to operating rooms, specialty units, or floors is ideal for routine or emergency use. Contents need to be arranged logically in a readily available design so that the composition and organization will be uniform across the facility in the event that more than one cart is needed for an institution. All airway specialists must be familiar with the contents of the FOB cart and know how to have it transported to required patient sites without delay. Preferably, the cart should have two widely separated tubular structures for hanging a clean FOB and, later, a used one (Fig. 19-7). Typically, the FOB cart will also have a light source, a video monitor (ideally), endoscopy masks, bronchoscopy swivel adapters, oral intubating airways, bite blocks, atomizers, tongue blades, cotton-tipped swabs, gauze, soft nasal airways, and local anesthetics (e.g., 2% and 4% lidocaine, 2% lidocaine gel or paste).
Optionally, an FOB cart setup could also be incorporated as part of a more complete DA cart that includes items needed to follow the DA algorithm. Among other items, a DA cart should contain a VL and screen, SGAs, intubating SGAs, ETT introducer/exchangers,62,63 and percutaneous airway rescue sets.
C Ancillary Equipment
1 Bronchoscopy Swivel Adapters and Endoscopy Masks
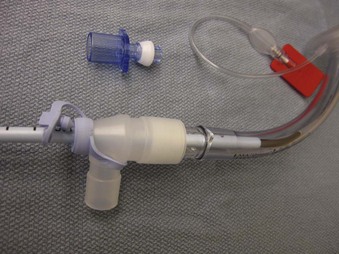
Bronchoscopy swivel adapters have the capacity to rotate when placed between the ventilating system and a mask, SGA, or ETT. They look like elbow adapters but are equipped with a “flip-cap port” covering a diaphragm with a central opening (Fig. 19-8). This permits passage of an FOB with virtually no leak during use of the continuous ventilation system on a patient.
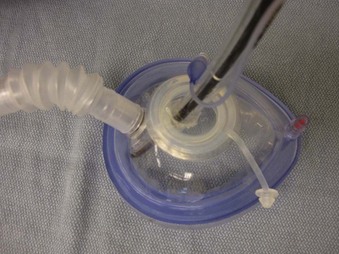
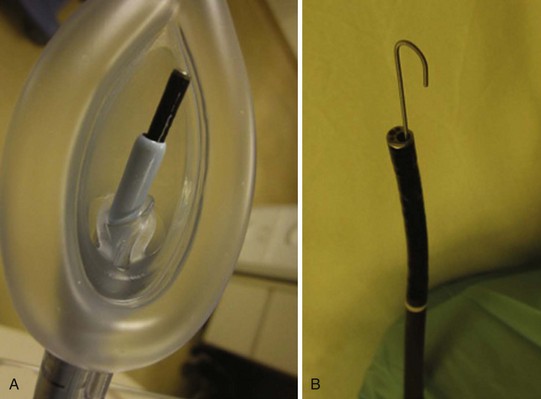
Endoscopy masks differ from regular masks by having at least one larger additional opening whose function is similar to that of a bronchoscopy swivel adapter port (Fig. 19-9). The opening permits both FOB and ETT passage for intubation during continuous mask ventilation. Some masks have recessed one-way, valve-like openings for FOB and ETT entry.
2 Intubating Oral Airways
Intubating oral airways (IOAs) are often used in unconscious patients or in the topically anesthetized oropharynx of awake patients to act as a conduit through which an FOB is inserted for oral intubation (Fig. 19-10). It is extremely useful when an assistant holds the IOA midline throughout the intubation procedure. These airways should not be jammed fully into the patient if they seem too large for smaller mouth openings. If no IOA is used secondary to inadequate mouth opening or endoscopist preference, a bite block should be placed between the molars or incisors to prevent FOB injury.
Most studies comparing characteristics of different IOA involve non-DA.64 No IOA is ideal, and some have disadvantages: Sizes may be too great for patients with small mouth openings; their construction may cause palate or other tissue discomfort or trauma during placement; they may lead the FOB astray (off midline); ETT cuffs may catch and tear on them; and their removal may be slightly intricate. All IOAs are disposable except for the aluminum-made Patil-Syracuse (Anesthesia Associates, Inc. (AincA), San Marcos, CA) oral airway.
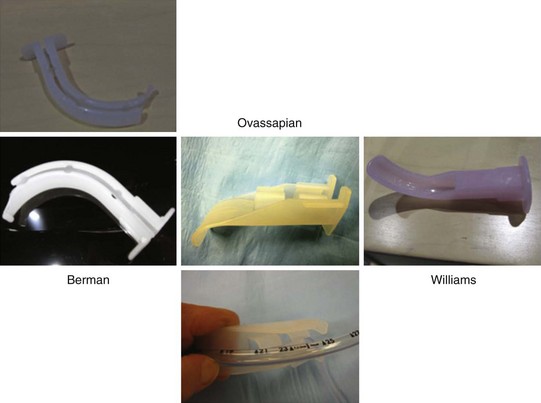
The Berman intubating pharyngeal airway (Teleflex Medical Research, Triangle Park, NC) admits ETTs of 8.5 mm or smaller diameter and comes in various adult, child, and neonatal sizes. Its full-length tubular shape does not permit FOB maneuvering, but it has a wide slit the length of its side for breaking away from the ETT after intubation. If kept midline, it provides a better lead to the glottis, assuming its length is appropriate.65 If the length is too long, its distal end is usually found in the vallecula; pulling it out 1 to 2 cm may rectify that situation. Lateral breaking away of this airway from the ETT can be difficult, particularly if periglottic ETT impingement has occurred.
The proximal half of the Ovassapian fiberoptic intubating airway (Teleflex Medical Research Triangle Park, NC) has a channel that permits passage of an ETT with a diameter of 9 mm or less. This channel has flexible lateral walls and an opening posteriorly for easy ETT removal. The distal half of the airway has a wide, flat, curved area designed to keep the tongue and soft tissues away from the FOB. Its flatness makes it easier to keep it in the midline, and its openness permits free FOB movement throughout its length. Once the FOB is near the carina and the ETT enters between the teeth, this IOA can be partially broken away to avoid tearing the ETT cuff on its corners. FOB orientation during passage through the device can be assisted by drawing a line with a marker lengthwise down the middle of the concave surface of the IOA.66
The Williams airway intubator (Williams Airway Intubator LTD, Calgary, Canada) has two adult sizes that allow passage of an ETT of 8.5 mm diameter or smaller. Proximally, it has a circumferentially closed channel and a distally curved section with a scalloped opening on the lingual surface. The distal area permits free FOB movement. As long as this airway is kept midline and its length is appropriate, it may be the best guide to the larynx.67 If it is too long, visualization can be problematic. There may be a concern for more frequent cuff damage with ETT sizes larger than 7.0 mm when using the smaller size of this IOA. For removal, the ETT has to be disengaged from the 15-mm ETT connector. The enclosed channel part should have been liberally prelubricated to accomplish easier ETT release. Sometimes removal can be difficult.68
3 Short, Soft Nasopharyngeal Airways
Two techniques have described use of nasopharyngeal airways (NPAs) during FOB intubation. A method to simplify nasal FOB intubation by using a lengthwise, laterally cut NPA for breakaway capability was reported by Lu and colleagues (Fig. 19-11).69 The lubricated, modified NPA is gently inserted into one nostril. Afterward, the lubricated FOB is guided through this NPA with the idea that the FOB tip will lead directly toward the glottis. After the FOB has entered just above the carina, the NPA is stripped away, and the ETT is railroaded over the FOB into the trachea.
A second advantage of NPA use is its role during FOB intubation in oxygen administration and as a conduit for inhalation anesthesia administration (most commonly in pediatric patients).70,71 This is particularly advantageous when FOB intubation is preferred to be undertaken unhurriedly in spontaneously breathing patients who need extra anesthesia. An NPA (often with a Murphy eye–type hole cut distally) is placed in one nostril and attached to a breathing circuit by a 15-mm ETT adapter while the FOB intubation procedure is completed orally or nasally through the opposite nostril.72
4 Endotracheal Tubes
Regular polyvinylchloride (PVC) ETTs are used for most FOB intubations. However, the most distal ETT tip area can get caught on the arytenoids, especially the right, causing difficulty for passage into the trachea. Other centrally-curved, soft-tip ETTs have been reported to have greater success rates of passage.73–75 However, Joo and coworkers demonstrated no difference in insertion success rate or number of manipulations required during awake FOB intubation when these two types of ETTs were compared, so long as the correct orientation of the common PVC ETT tip was made. The orientation required during insertion is that in which the Murphy eye of the ETT faces anteriorly with respect to the patient’s body, to avoid arytenoid contact. This represents a 90-degree counterclockwise rotation of the ETT from the normal ETT direction used during rigid laryngoscopy (Fig. 19-12).76
V Clinical Techniques of Fiberoptic Intubation
A Oral Fiberoptic Intubation of the Conscious Patient
During awake FOB intubation, the patient’s state of consciousness may vary from completely awake to arousable with moderate stimulation (level 3 or 4 on the Ramsay scale) (Box 19-4).
Box 19-4 Ramsay Sedation Scale
1. Patient is anxious and agitated, restless, or both.
2. Patient is cooperative, oriented, and tranquil.
3. Patient responds to commands only.
4. Patient exhibits brisk response to light glabellar tap or loud auditory stimulus.
5. Patient exhibits a sluggish response to light glabellar tap or loud auditory stimulus.
2 Psychological Preparation of the Patient
Only oxygen is given if airway endangerment is a concern (i.e., no sedative or narcotic). Certainly, those patients for whom no sedation or extremely little sedation is advisable must be prepared to understand why. Explaining safety and how cooperation makes the process easier, faster, and less worrisome is beneficial.77 Hypnosis has been used for FOB use, but special training is required.
C Sedation and Analgesia
Ketamine produces less respiratory depression but is longer acting and has no known reversal therapy in humans. One study on ketamine/xylazine use in primates showed reversal with atipamezole (Antisedan), an α-adrenergic antagonist, but because xylazine is a clonidine analogue, it is difficult to say whether the ketamine alone would be reversed to the same extent.78
Dexmedetomidine (0.7 to 1.0 µg/kg bolus over 10 minutes followed by 0.5 to 1.0 µg/kg/hr infusion) is associated with hypnosis, amnesia, analgesia, and less respiratory depression. It has been used and titrated so that the patient remains responsive to verbal command. In animal studies, dexmedetomidine has been shown to be reversible by atipamezole, but no human studies are available.79
The most appropriate way to administer precisely titrated sedation seems to be with target-controlled infusion. The technique described for nasal FOB intubation has valid principles for oral approaches. Propofol and remifentanil have been studied most extensively.80 Mean target plasma concentrations for propofol and remifentanil were 1.3 µg·mL−1 (standard deviation [SD], 0.2 µg·mL−1) and 3.2 ng·mL−1 (SD, 0.2 ng·mL−1), respectively. Rai and colleagues found no difference in patient satisfaction between the two but reported better FOB intubation conditions and a higher incidence of recall with remifentanil than with propofol.81 Similar results were found by Cafiero and coworkers.82 In patients with cervical trauma, a remifentanil effect site concentration as low as 0.8 ng·mL−1 proved to be effective for awake FOB intubation.83 The very short induction and recovery times with remifentanil may represent a major advantage. Nevertheless, the possibility of increased thoracic wall rigidity and laryngospasm should be kept in mind.84
D Local Anesthesia Purposes and Preparedness
1 Innervation of Orotracheal Airway Structures
Cranial nerve IX, the glossopharyngeal nerve (GPN), supplies sensory innervation to the soft palate, the posterior third of the tongue, the tonsils, and most of the pharyngeal mucosa.85,86 Some fibers also supply the lingual surface of the epiglottis.87 This nerve controls the afferent component of the gag reflex. Cranial nerve V, the trigeminal, gives sensory supply to the anterior two thirds of the tongue but this area rarely needs anesthesia.
2 Topical Orotracheal Anesthesia Techniques
a Glossopharyngeal Anesthesia
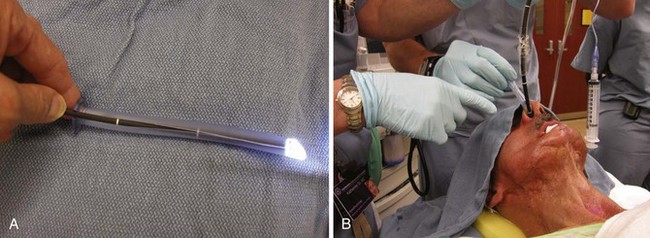
In advance of performing a GPN nerve block, give instructions to the patient that he or she may be requested to take deep breaths, gargle, or swallow. It is helpful to vocally mimic gargling so that the patient understands this directive. While pressure is applied on the lateral tongue surface with a tongue depressor, the operator shifts the tongue medially and sprays the LA during inspiration at the anterior tonsillar pillar (Fig. 19-13). After waiting a number of seconds for patient recovery, the process is repeated on the opposite side, then once more on both sides.
b Alternative Methods of Intraoral Anesthesia
In the “lollipop” method of LA, 3 cm of lidocaine paste is placed on both sides of one end of a tongue depressor. The operator puts this end on the middle of the patient’s tongue and advises the patient to keep the depressor held in place between the teeth. For the “toothpaste” method, the gel is placed directly down the middle of the tongue, while the tongue tip is held with gauze (Fig. 19-14). In these latter two instances, the lidocaine is left to dissolve over 5 to 10 minutes.
c Superior Laryngeal Nerve Block
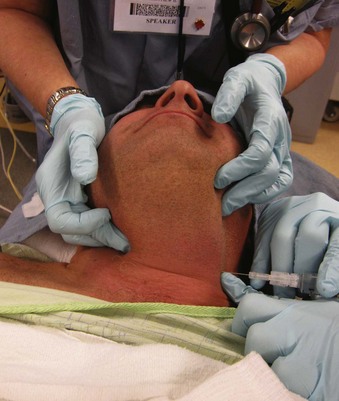
Two techniques are available for the external neck approach to bilateral SLN block. In the simpler technique, attach a 10-mL syringe with 6 mL of 1% lidocaine to a 21- or 22-G needle as an injection system (Video 19-1![]() ). Clean the neck area with antiseptic solution and locate both hyoid cornua (Fig. 19-15). Keep the forefinger of the nondominant hand by one cornu for visual reference, and hold the syringe dart-like near the needle hub while advancing the needle perpendicularly toward that cornu. Once bone is felt, shift the nondominant hand to brace against the neck and hold the system near the hub. Aspirate the syringe gently; if no blood returns, inject 3 mL slowly. Repeat the process on the other side.
). Clean the neck area with antiseptic solution and locate both hyoid cornua (Fig. 19-15). Keep the forefinger of the nondominant hand by one cornu for visual reference, and hold the syringe dart-like near the needle hub while advancing the needle perpendicularly toward that cornu. Once bone is felt, shift the nondominant hand to brace against the neck and hold the system near the hub. Aspirate the syringe gently; if no blood returns, inject 3 mL slowly. Repeat the process on the other side.
The second external approach to SLN block is more complex and takes longer to perform. The same system is used to hit the cornu. Then the operator walks off anteromedially and caudally 0.1 to 0.4 cm and feels the click of going through the thyrohyoid membrane at a depth of 1 to 2 cm.88 The needle is then braced, and the remainder of the technique is identical. The block has to be repeated on the other side.
Trouble-Shooting
1. The cervical collar is in the way: Open the collar on one side of the neck and flip its anterior portion over to the other side. Perform both blocks and close the collar when finished.
2. The hyoid is hard to feel with only one hand: It will be more prominent if an assistant presses gently against the opposite hyoid cornu toward the operator’s finger on the side being blocked.
3. Blood is aspirated: The system has to be withdrawn slightly, redirected, and braced until aspiration is negative. (This is particularly important to prevent intracarotid arterial injection and seizures.)
4. Bone is felt but injection is not possible: The needle tip is probably in the periosteum. Using two hands, brace the system constantly, and imperceptibly withdraw 1 mm at a time until negative aspirations are followed by the ability to inject. Excessive distance during withdrawal can cause failure.
d Transtracheal Block
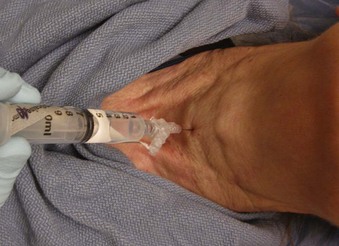
If a collar is in place, locate the cricothyroid area through the anterior collar opening or by flipping the anterior part laterally, as described previously (Video 19-2![]() ). Apply antiseptic, and mark the area before refastening the collar. Straddle the trachea with two digits of the nondominant hand. With the dominant hand, direct a 10-mL syringe containing 4 mL of 4% lidocaine attached to a 21- or 22-G needle as a system, aiming posteriorly in the cricothyroid space while aspirating gently (Fig. 19-16). Shift the other hand to keep the system braced on the neck, if possible, and help guide it until many air bubbles are freely obtained. At this point, brace or encircle the system firmly while the dominant thumb is poised to inject. Instruct the patient to breathe in gently and exhale gently but maximally. When exhalation is at completion, rapidly inject the anesthetic. As the LA enters the trachea, the patient may cough, but because exhalation has taken place, a quick breath must be taken to initiate any more cough, and this will help spread the anesthetic. Usually more coughing ensues. By bracing against the neck (or collar) and encircling the system, one need not worry about excessive needle penetration, removal, or breakage even if the patient’s neck moves quite a bit. The system can be removed when finished.
). Apply antiseptic, and mark the area before refastening the collar. Straddle the trachea with two digits of the nondominant hand. With the dominant hand, direct a 10-mL syringe containing 4 mL of 4% lidocaine attached to a 21- or 22-G needle as a system, aiming posteriorly in the cricothyroid space while aspirating gently (Fig. 19-16). Shift the other hand to keep the system braced on the neck, if possible, and help guide it until many air bubbles are freely obtained. At this point, brace or encircle the system firmly while the dominant thumb is poised to inject. Instruct the patient to breathe in gently and exhale gently but maximally. When exhalation is at completion, rapidly inject the anesthetic. As the LA enters the trachea, the patient may cough, but because exhalation has taken place, a quick breath must be taken to initiate any more cough, and this will help spread the anesthetic. Usually more coughing ensues. By bracing against the neck (or collar) and encircling the system, one need not worry about excessive needle penetration, removal, or breakage even if the patient’s neck moves quite a bit. The system can be removed when finished.
According to Todd, concern about coughing in patients with cervical spine risk is unfounded, because patients cough periodically and those coughing during transtracheal blockade have never been shown to develop secondary neurologic damage.89 Some clinicians prefer using a 20-G angiocatheter in lieu of the needle. They perform the technique in a similar fashion, but after air is aspirated they advance the catheter 0.5 to 1 cm, brace the catheter, remove the needle and syringe, disconnect the syringe, connect the syringe to the catheter, aspirate the catheter for air, and proceed in as previously described. The benefit of the needle technique is that it is faster, it is less likely to provoke more coughing caused by hitting the posterior tracheal wall (particularly when the catheter is threaded in too far), and it is more successful (because the needles are shorter, less flexible, and, even if pulled out of the trachea, can be advanced easily). In contrast, with a catheter coming out of the trachea, the needle would have to be reinserted with it, or the angiocatheter would have to be entirely replaced if the plastic is damaged.
Trouble-Shooting
1. The cricothyroid space is impossible to feel: A cricotracheal injection is acceptable.
2. More than a tiny wisp of blood is aspirated: Remove the system and refill the syringe with fresh local anesthetic to avoid injecting blood into the respiratory system.
3. The hyoid bone seems very close to the cricothyroid space: Reassess anatomy or abandon the technique to prevent vocal cord damage.
4. The patient is at very high risk for aspiration: To maintain the patient’s protective reflexes, avoid transtracheal anesthesia. Instead, use the “spray as you go” technique (discussed later) on the visualized vocal cords. Rapidly insert the FOB, and follow immediately with ETT passage and cuff inflation.
f “spray as You Go” Technique
Patients are more likely to react and cough after these pulses, and the FOB view may be obscured for some seconds, after which the FOB can be advanced until another unanesthetized area is reached.90 An interesting method to avoid the obscured view is to attach a syringe containing local anesthetic to an epidural catheter (0.5 to 1 mm ID, single distal hole) and pass it through a three-way stopcock attached at the FOB injection port through the working channel. On the second stopcock port, attach the suction tubing for easy use by a simple turn. Extend the catheter tip 1 cm beyond the FOB tip so that as LA is injected, the spray goes a farther distance away, causing less visual disturbance to the FOB. Taping the proximal epidural at the working channel port area keeps its tip a fixed distance.
3 Local Anesthetic Drug Choices
LA dosing for various airway blocks is listed in Table 19-2. Toxicities of these injectable drugs have been described elsewhere, but with regard to the airway, some considerations need clarification.
| Drug | Form | Appropriate Blocks |
|---|---|---|
| Lidocaine | Pacey’s paste (slurry): 7 mL 2% solution + 7 mL 2% viscous in two syringes with 3 mL air each + sweetener (mix back and forth via three-way stopcock); should be swished intraorally in 2 aliquots | |
| 2-4% solution | All blocks except as listed below | |
| 1% solution | Preferred for SLN neck blocks | |
| 4% solution | Preferred for transtracheal blocks | |
| 2% viscous lidocaine | Intraoral or nasopharyngeal | |
| 2-5% gel/ointment/paste | Intraoral | |
| Exactacain (benzocaine, butamben, tetracaine HCl) | Metered spray | Intraoral |
| Cetacaine (benzocaine, tetracaine HCl, butamben, benzalkonium chloride) | Spray | Intraoral |
| Hurricaine (benzocaine) | Spray | Intraoral |
| Tessalon Perle (benzonatate) | 200-mg capsule | Intraoral |
SLN, Supralaryngeal nerve.
Nydahl and colleagues demonstrated that 20 mL of 2% lidocaine gel in the nasopharynx was mostly swallowed in the stomach. Only one eighth of the described toxic blood level of an equal amount of injected drug resulted. Doubling the volume to 40 mL was equivalent to producing less than one third of the toxic level.91
Higher dosing and routes are most significant and should depend on a patient’s height and size. Woodall and associates reported symptoms attributable to local anesthetic in 36% of 200 healthy subjects (anesthesiologists attending an FOB course).92 Local anesthetics used included nebulized lidocaine (200 mg or 5 mL 4%), nasopharyngeal lidocaine (100 mg or 2 mL 5%), and “spray as you go” lidocaine (600 mg or the equivalent of 15 mL 4%). The maximum dose in their study, 9 mg/kg lidocaine, was somewhat higher than the average dose of 5 mg/kg cited for performance of the described blocks in this chapter whose total dosage is 370 mg (i.e., GPN, 150 mg; SLN, 60 mg; and transtracheal, 160 mg).
Exactacain spray is a metered anesthetic with a six-spray maximum limit in adults to reduce the risk of toxicity. Each spray delivers 9.3 mg of benzocaine. Onset is within 30 seconds, and the duration is 30 to 60 minutes. Care must be taken to avoid use in patients with ester allergies or if the possibility of methemoglobinemia is likely due to patient comorbidities or concomitant drug therapy. Methemoglobinemia is more often associated with unlimited, continuous spraying of the Cetacaine spray (benzocaine 14.0%, butyl aminobenzoate 2.0%, tetracaine hydrochloride 2.0%) or Hurricaine spray (benzocaine 20%), which deliver 28 mg of benzocaine in a 0.5-second spray.93–95 According to Khorasani and colleagues, dosing can be variable with these agents due to the orientation of the container to the patient during spraying or fluctuations in LA canister volume.94 Between 1950 and 2005, only 26 of 126 total reported methemoglobinemia cases were related to endotracheal intubation. Transesophageal echocardiography was the procedure with the most frequent association, with a documented incidence of 0.115%.95 Some sources consider this to be an underestimation. Because of recent Exactacain straw applicator connection manufacturing changes, care must be taken to use the same straws that come with the local anesthetic canister or to hold them securely while spraying, to prevent “popping off” of straws into a patient.96
A general plan of patient preparation is itemized in Box 19-5. Steps that are inappropriate for any specific patient should be eliminated or changed.
Box 19-5 General Plan of Patient Preparation for Awake FOB Intubation
2. Obtain history and physical examination and discuss the plan with the patient (psychological preparation).
4. Administer sedative and insert an intravenous line if none is present.
5. Administer sodium citrate if aspiration risk is present.
8. Administer oxygen by cannula.
9. Position patient supine (ideally), or sitting for patients who are unable to lie flat, with the intention of facing the patient during endoscopy (note that anatomy will be inverted in the latter case). See obesity concerns discussed in the text.
10. Titrate sedative/narcotic while local airway anesthesia is applied.
E Fiberoptic Orotracheal Technique
1 Three Successive Directions for Fiberoptic Guidance
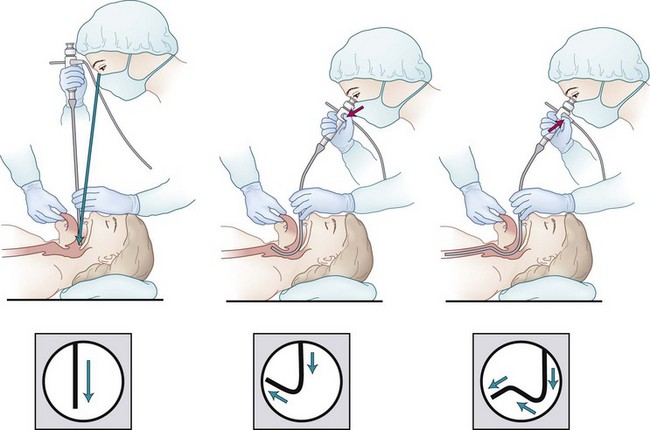
Airway specialists are remarkably familiar with the upper airway configuration as routinely observed during direct laryngoscopy. On the other hand, the different axes successively followed by the structures involved during FOB intubation, and their reciprocal spatial organization, are less a matter of emphasis in the classic teaching programs. Moreover, many sketches or drawings in medical textbooks are inaccurate, showing the upper airways as a regularly curved line joining the oropharynx or nasopharynx to the carina. This is obviously misleading, because three successive directions must be followed in the supine patient in order to reach the trachea through the nasal or the oral route.97 These successive directions are (1) downward to the posterior wall of the oropharynx or nasopharynx, (2) upward to the anterior commissure of the vocal cords, and (3) downward again into the laryngeal and tracheal lumen to the carina.
During the second phase of the fiberscope progression, in the upward direction to the anterior commissure of the vocal cords, the posterior part of the glottic aperture comes into view. In the absence of anatomic distortions, the temptation to push the fiberscope unswervingly in this direction should be resisted. It may be better to continue toward the anterior commissure of the cords, keeping the tip of the device in a sharp anterior path (by pushing down with the thumb on the button of the handle). Only when the anterior commissure is approached should the tip be angled correctly downward, toward the middle of the lumen of the larynx. This maneuver aligns the fiberscope with the anatomic axis of the larynx successively in its supraglottic and infraglottic segments, avoiding contact damage to the mucosa and arytenoid cartilages (Fig. 19-17).
2 Practical Application
Preparation before the FOB procedure should follow in sequence while adjustments are made depending on individual patient concerns: equipment preparation, patient preparation, antisialogogues, sedatives, narcotics, local anesthetics, monitoring, and nasal cannula oxygen (if permitted; see Box 19-5). If there are not enough people available for assistance, the thumb-control port of the suction may be sealed ahead of time with tape, to make it a one-handed tool, which may help in the midst of a busy FOB procedure. The bed or table is moved to its lowest setting while keeping the patient supine. Use a stepstool to keep the FOB straight, if needed; in the case of a sitting, dyspneic patient approach the FOB procedure by facing the patient.
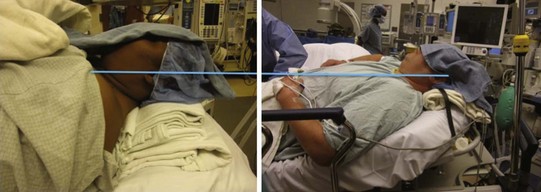
Employ the back-up bed position at whatever height makes the patient’s comfort of breathing better. Traditionally, endoscopists like to “ramp” overweight patients up with many blankets under the upper thorax, neck, and head regions to align the airway when using RL, to decrease soft tissue encroachment on the airway. Rao and colleagues compared ramping with elevating the back of the bed until the patient’s external auditory meatus is at the level of the sternal notch (Fig. 19-18).98 There was no difference between the two methods in demographics, time to intubation, grade of glottic view, or number of RL attempts until intubation was successful. There was a significant difference in the added time and effort needed to position patients with blankets and then to remove blankets (and most likely in laundering cost, at 75¢ per blanket, although this was not mentioned in the study). By extrapolation, elevating the back of the bed or using a reverse Trendelenburg position to align the external auditory meatus with the sternal notch may facilitate FOB intubation in obese patients with less wasted resources.
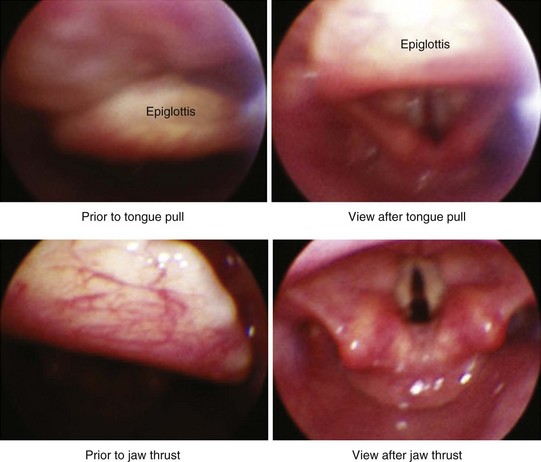
Have an assistant hold the tip of the patient’s tongue with gauze by grasping its anterior and posterior surfaces and extending it carefully so as not to cause tearing of the frenulum linguae. This lifts the tongue off the palate and elevates the epiglottis (Fig. 19-19). Apply 2 cm of 5% lidocaine paste to the distal lingual side of the IOA before gently positioning it, but only if using an Ovassapian or Berman type of airway. The assistant should hold the IOA midline during endoscopy to prevent deviation from the path to the larynx.99 Alternatively, use a bite block (e.g., BiteGard [Gensia Automedics, San Diego, CA]) placed between the premolars to protect the FOB from dental trauma (Fig. 19-20).

Figure 19-20 BiteGard for dental and fiberoptic bronchoscope protection is inserted between the premolars.
Hold the scope immobile and slide the ETT forward with the Murphy eye oriented anteriorly if using a PVC ETT, to prevent it from getting hung up on the arytenoid. Statistically, it gets caught on the right side most often.100 Do not let the FOB go forward as the ETT advances toward the back of the oropharynx. At this point, look at the patient to judge when the ETT may be near the larynx. Ask the patient to take some deep breaths so that the vocal cords open more widely to admit the ETT. Time the breathing, and when ready, quickly push the ETT forward. After judging that it has gone beyond the vocal cords, look at the FOB tracheal view again, and slide the ETT until it ends up two to three rings above the carina and has just passed the FOB tip. Inflate the ETT, stabilize it, and remove the FOB. Put the FOB in the used vertical holder, or hand it in a straight fashion to an assistant. Attach the ventilating system while checking for PETCO2, tape the ETT, and continue whatever patient care is anticipated next.
Trouble-Shooting
1. The tongue extended preoperatively, but now, just before FOB insertion, it cannot be fished out for a tongue pull: Have the assistant firmly apply a large suction tubing to the tongue and slowly draw it out enough so that it can be grasped with the gauze. An alternative, especially for larger tongues, is to snag it with a clamp (e.g., ring forceps) or, rarely, with a large-diameter suture.
2. The patient has excessive oropharyngeal secretions or blood, and visualization with the FOB is almost impossible:
3. The optics are not clear; maybe the FOB is fogged: For an unclear view, try turning the focus dial. For fogging, touch the FOB tip on mucosa to clear it (Video 19-3![]() ). For secretions, suction with the FOB or use a suction catheter in the oropharynx, or both. Otherwise, FOB removal and cleaning of the tip may be the last choice. To clean, do not jab the FOB tip into anything, because delicate fibers can be broken. Hold the tip still but quite tightly near the very end, and gently wipe with alcohol.
). For secretions, suction with the FOB or use a suction catheter in the oropharynx, or both. Otherwise, FOB removal and cleaning of the tip may be the last choice. To clean, do not jab the FOB tip into anything, because delicate fibers can be broken. Hold the tip still but quite tightly near the very end, and gently wipe with alcohol.
4. It is impossible to look to the left or right because the lever only moves the FOB tip up and down: To look left, loosen the grip on the distal FOB insertion section between the thumb and two fingers while simultaneously rotating the handle section counter-clockwise, to avoid torque. To look right, turn the handle section clockwise in a similar fashion. Alternatively, rotate both hands equally in the same direction.
5. The FOB cannot get under the epiglottis because it is stuck on the posterior pharynx: Ask the assistant to pull the tongue out farther and perform a jaw thrust maneuver to lift the epiglottis (see Fig. 19-19).101 Try moving the FOB more caudally or laterally, to get under the epiglottis.
6. The patient keeps gagging or coughing as the FOB advances: Consider repeating the LA blocks or use the “spray as you go” method.
7. After the FOB enters the trachea, the patient gets agitated and desaturates: In a compromised patient with tracheal stenosis or obstructive pathology, this may be the result of inadequate room around the FOB for the patient to breathe. The intubation process should be sped up, and the FOB should be removed as soon as possible. Be especially wary of starting oxygen insufflation through the working channel in this situation, in case not enough tidal volume can be expelled around the FOB. This sequence of events could precipitate the development of a pneumothorax.
8. The FOB is above the carina, but the ETT will not enter the trachea: Try not to jam the ETT, because it is advancing blindly and could damage perilaryngeal structures. Withdraw the ETT 1 to 2 cm, turn it 180 degrees counter-clockwise, and advance it rapidly after asking for a deep breath. If this is unsuccessful, rotate the ETT 180 degrees clockwise and repeat. If it is still not passing, repeat the other way. Try giving jaw thrust or even releasing jaw thrust. Use cricoid pressure, or neck flexion.85 Finally, a rescue may be effected by removing the FOB and trying a smaller ETT; using a centrally curved, soft-tip ETT; inserting an Aintree Intubation Catheter (Cook Medical Inc., Bloomington, IN) on a pediatric FOB; or using a nasopharyngeal approach.
9. While using a pediatric FOB in an adult patient, tracheal rings were seen; the ETT went in, the FOB was removed, and the ETT was attached to the ventilator, but there was no PETCO2, breath sounds, or chest movement: The tip of the FOB must end up within 2 to 3 cm of the carina in an adult, particularly if a pediatric FOB is being used. If it is only partially down the trachea, pressure against the ETT as it pushes forward in the periglottic area against tissue (e.g., arytenoid) may divert it into the esophagus or bend it back into the oropharynx and cause “flippage.” Flippage may occur when a stiff ETT tip indents into the somewhat flexible insertion section of the FOB as the ETT is diverted into the esophagus or pharynx, causing the short entry of the FOB to flip out of the trachea.
10. A centrally curved, soft-tip ETT was partially inserted into the oropharynx of an adult patient; next, the FOB was inserted through the ETT, and the tip of the adult FOB was positioned 2 to 3 cm above the carina; the ETT was then railroaded over the FOB, but in spite of all maneuvers, it will not pass through the glottis: If time and resources are available, look in the oropharynx with another FOB to diagnose the problem. If the FOB crossed through the Murphy eye, the ETT will never advance into the trachea. Trying to force the ETT forward may cause serious FOB damage and injure the patient. Occasionally, when this happens, the FOB might not even be able to be withdrawn easily, particularly if forceful moves were already made. Strong attempts at removing an FOB stuck in the ETT may result in severe damage due to “sharper” Murphy eye edges digging into the outer plastic wrap of the FOB. If any significant resistance occurs in this “ETT insertion first” type of scenario, remove both devices simultaneously.
11. More than just a few drops of blood are in the trachea after a transtracheal injection: Do not give positive-pressure ventilation (PPV). Attach the oxygen system, and suction the ETT. To avoid coughing, give sedative agents and, if desired, muscle relaxants before suctioning.
F Nasal Fiberoptic Intubation of the Conscious Patient
1 Innervation of Nasopharyngeal Airway Structures
Cranial nerve V, the trigeminal, supplies sensation to the anterior half of the nasopharynx by its first branch. The second branch of the trigeminal nerve forms part of the sphenopalatine ganglion, innervating some of the anterior, superior, and central regions.85,86 Cranial nerve IX, the GPN, supplies parasympathetic innervation, while the carotid plexus supplies sympathetics.102 Cranial nerve VIII, the facial nerve, has parasympathetic function and forms part of the sphenopalatine ganglion to assist in control of nasopharyngeal reflexes.
2 Topical Nasopharyngeal Local Anesthetic Techniques
Besides anesthetization, the nose requires the addition of a vasoconstrictor to decrease the likelihood of epistaxis (see Table 19-2). Dip four Q-tip swabs into solutions of cocaine or a vasoconstrictor mixed with local anesthetic. Swab insertion gives the FOB operator an idea of the caliber of each nasal passage. Apply a single swab to the outermost part within the nostril. Push it inward with a forefinger, perpendicularly to the plane of the face, until slight resistance is met (Video 19-4![]() ). Put the next swab in the opposite nostril, and continue alternating insertion of swabs parallel to previous ones. By the time the last swab is placed, the initial insertions will have caused some vasoconstriction, and slight swab pressure will push each one in further. If desired, midway through the process, spray 0.2 mL of solution with a 20-G angiocatheter (needle removed) in three different directions within each nostril. Continue this process until all swabs are at the back of the nasopharynx. This method uses a total of 2 mL (80 mg) of cocaine. If the possibility of tachycardia is a concern, apply a mixture of lidocaine and a vasoconstrictor instead of cocaine, with swabs positioned as described. Some FOB operators recommend another technique, using lidocaine gel and phenylephrine as a coating on short nasal airways to gauge nasal passage size. Any excess drug that is not absorbed usually drips back to anesthetize the posterior pharyngeal wall.
). Put the next swab in the opposite nostril, and continue alternating insertion of swabs parallel to previous ones. By the time the last swab is placed, the initial insertions will have caused some vasoconstriction, and slight swab pressure will push each one in further. If desired, midway through the process, spray 0.2 mL of solution with a 20-G angiocatheter (needle removed) in three different directions within each nostril. Continue this process until all swabs are at the back of the nasopharynx. This method uses a total of 2 mL (80 mg) of cocaine. If the possibility of tachycardia is a concern, apply a mixture of lidocaine and a vasoconstrictor instead of cocaine, with swabs positioned as described. Some FOB operators recommend another technique, using lidocaine gel and phenylephrine as a coating on short nasal airways to gauge nasal passage size. Any excess drug that is not absorbed usually drips back to anesthetize the posterior pharyngeal wall.
As an alternative, spray the mixture twice in each nostril, but only in an upright patient. Spraying in supine patients can result in overdoses if the spray becomes a stream. Several case reports have been published in which application of topical phenylephrine or a potent vasoconstrictor resulted in dangerous hypertension. Beta-blocking agents administered in this circumstance may promote the occurrence of pulmonary edema and should be avoided, according to the New York State guidelines on the topical use of phenylephrine in the operating room.103
For nasotracheal intubation under GA, vasoconstrictors are very useful to prevent epistaxis.
G Fiberoptic Nasotracheal Technique
Because of the relative narrowness of the nasal passage, an ETT at least one-half size smaller than would be chosen for the oral route should be selected, depending on the patient’s history or size. Some endoscopists erroneously think that inserting progressively larger nasal airways or “trumpets” can mechanically “dilate the passage.” However, Adamson and coworkers reported that use of this method caused increased trauma, hemorrhage, and delay of intubation.104
Trouble-Shooting
1. The plan was to partially insert the ETT first, but it does not want to pass: Be gentle and do not exert force. Try the other side. If that does not work, try alternative methods listed previously.
2. Partially inserting the ETT caused epistaxis (no FOB yet): Inflate the ETT cuff to tamponade the area, or externally pinch the nose for 5 to 10 minutes. If blood is welling out of the nostril but no blood trickles into the back of the pharynx (i.e., there is no choking, swallowing, or continual need for suctioning), try pulling the deflated ETT a little and reinflating the balloon at what may be the site of injury. To prevent additional trauma, try not to remove the ETT unless blood keeps entering into the back of the pharynx regardless of the number of times these maneuvers were engaged.
H Oral or Nasal Fiberoptic Intubation of the Unconscious Patient
1 Under Routine General Anesthesia
Eyes should always be protected in unconscious patients before any airway manipulation. If mask ventilation is expected to be easy under GA before surgery, a purely elective FOB procedure can always be used to intubate patients if consciousness is not required. This form of achieving intubation is very acceptable. It is considered ethical not to inform the patient of this plan because it is one normally used by many patient caregivers.105 In some circumstances, “asleep” FOB intubation may have specific indications (e.g., a patient with extensive anterior dental work).
Trouble-Shooting
1. The technique is not succeeding: Careful monitoring and awareness of 2 to 3 minutes’ passage of time, even in the case of technical difficulty, should keep the situation under control. If unsuccessful, always reinstitute face mask ventilation and decide whether the problem is caused by anatomic difficulty, being off-midline, insufficient assistance, or a lack of equipment. Think about giving more anesthetic before another attempt, or use an alternative intubation plan (e.g., intubation with another device). Frequently, combination FOB techniques should be considered (see “Combination Techniques”).
2 After Rapid-Sequence Induction
If the decision is made to proceed, have two operable suctions attached, one on the FOB and the other on a Yankauer or soft suction tubing in case of regurgitation. Choose a smaller-sized ETT. If not contraindicated, preoperative preparation is identical to that used for rapid-sequence GA, with inclusion of an antisialogogue, clear oral antacid, histamine H2-blocker, and/or gastric motility–inducing agent. After induction and cricoid pressure applied as originally explained by Sellick,106 use the FOB intubation technique with good assistance. Once the FOB nears the carina, the ETT should be inserted quickly and the cuff inflated immediately.
Trouble-Shooting
1. The epiglottis and periglottic areas are easily seen, but the glottis is very small with closed vocal cords, and the FOB cannot enter: Check the nerve stimulator for degree of muscle relaxation. If this is insufficient and laryngospasm is suspected, spray down the FOB with 4% lidocaine. If the patient is relaxed, ask the assistant to slowly decrease the cricoid pressure to see if things improve, because this pressure can be associated with impaired glottic visualization. If regurgitation occurs, another assistant should suction the oropharynx, and pressure may need to be reinstituted, with a tentative release tried later.
VI Combination Techniques
Elective use of an FOB combination with other airway management equipment has a very high rate of success with the added luxury of planning. This may be extraordinarily beneficial in the most difficult airway predicaments. As an illustration, a Fastrach intubating laryngeal mask airway (LMA North America) may be desirable to seal off the laryngeal area from blood and accomplish ventilation in a patient who is difficult to mask ventilate, difficult to intubate, and has significant intraoral bleeding. This intubating airway has a moderately high success rate for blind insertion of its ETT (90% to 96.2% with ≤3 attempts or adjusting maneuvers).107–109 Its use in combination with an FOB, however, can change a blind technique to a more controlled visual one, with a greater chance of success.110
Finally, combining multiple approaches to manage the DA is just another illustration of the general concept of multimodal therapy, in contrast to monotherapy. Multimodal therapy is used in the everyday practice of health caregivers, such as when different classes of drugs are used to provide an overall “tailored” anesthetic, or when combinations of various analgesics are used for chronic pain control. The multimodal approach to the airway, unduly disregarded for a long time, seems to be increasingly accepted as the drawbacks of individual airway devices or techniques become more obvious and the failures of each, when used alone, are more frequently reported.111
A Superficial Airway Devices and Flexible Fiberoptic Bronchoscopes
1 Combination with Endoscopy Masks
Insert the ETT-loaded FOB sequentially through the port, IOA, and trachea for subsequent intubation (Fig. 19-21). After ETT intubation, remove the FOB and mask and join the ETT to the ventilation system. If patient conditions warrant urgent ventilation, institute it through the ETT after removal of the FOB. Retire the mask at any time thereafter. One advantage of using this technique for an elective case is that it affords more time for endoscopy, particularly if surgery will not be delayed because the incision site is at the other end of the body.
2 Combination with Swivel Adapters
For intubation purposes, the combined use of an FOB and a swivel adapter can be undertaken under GA or oxygen-enrichment conditions similar to those for FOB-endoscopy mask use. With the patient under GA, insert a pediatric FOB within an Aintree intubation catheter (ID 4.7 mm, OD 6.3 mm) through the swivel adapter port while the patient is breathing spontaneously or being mask ventilated (Fig. 19-22). Once the FOB enters the trachea and nears the carina, slide the Aintree over it until it is two to three rings above the bifurcation. Hold the Aintree securely while noting the depth of insertion, and take out the FOB. Remove the Aintree connector to extract the mask, SGA, or old ETT. Then, slide a lubricated ETT-loaded FOB through the Aintree and down the trachea, stopping again near the carina. Pass the ETT farther down over the Aintree until the ETT tip lies 2 to 3 cm above the bifurcation. While firmly holding the ETT, remove the FOB and the Aintree. Reconnect the ETT to the ventilation circuit.
B Supraglottic Airways and Flexible Fiberoptic Bronchoscopes
The original LMA for ventilation had obvious airway management benefits from its inception, particularly for difficult mask ventilation and for DI. This device has saved countless lives and is rightfully prominently embedded in the ASA DA algorithm. It can be used in patients who are unconscious, awake with local airway anesthesia, or under GA. Since its development, dozens of SGA and intubating SGA devices have been invented. The idea of performing FOB intubation through the LMA was spawned by the knowledge that, when seated properly, this SGA had to be situated around the glottis and could provide a pathway for an ETT. The combination of FOB-SGA for ETT intubation has been successfully tried with numerous SGA brands (Fig. 19-23).
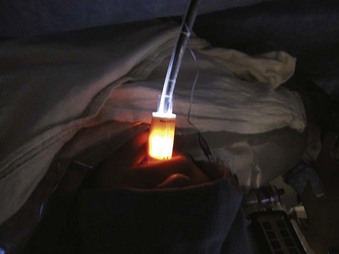
Figure 19-23 Intubating with the combination of a fiberoptic bronchoscope within an Ambu Aura-i intraoperatively.
1 Combination with SGA and Multiple Endotracheal Tube Techniques
Trouble-Shooting
1. The only ETT that could pass is small and too short, prompting concern that its cuff will lodge between the vocal cords:
2 Combination with SGA and Aintree Catheters
As detailed earlier, intubate with an Aintree-loaded FOB via an SGA. Fix the Aintree, and remove the FOB and SGA. Complete the intubation with the ETT-loaded FOB via the Aintree (Fig. 19-24A).
3 Combination with SGA and Guidewires
Similarly, thread a long guidewire (110 to 145 cm long, 0.38 to 0.97 mm diameter) through the working channel of the FOB far into the trachea by way of the SGA (see Fig. 19-24B). While holding the wire in place, extract both FOB and SGA. Subsequently, thread the near end of the wire through the FOB tip, up the working channel of an ETT-loaded FOB. Use the FOB to visually follow the wire into the trachea for completion of ETT intubation.
4 Concerns
There are some limitations to FOB and SGA combinations:
• Correct SGA placement is not successful in 100% of cases.
• The ETT may not fit or pass through the SGA, so the combination must be prechecked.
• Not all lubricants are equally adept at lubricating the ETT. Viscous lidocaine, Exactacain, and Neosporin (polymyxin B sulfate, neomycin sulfate, bacitracin zinc) ointment achieve a far greater degree of “slickness” than common surgical lubricants.
• Compare the length of the ETT with the length of the SGA, because the distance from the periglottic opening of the SGA to the vocal cords may be 3.5 cm or more. Inattention to lengths may produce a “too short” ETT picture. Trying to cut a 4-cm section of a SGA tube to avert this problem (as mentioned earlier) may lead to problems: the SGA connector may not separate easily from its original seat within the SGA tube, or it may have a poor fit when plugged into the cut-off SGA section.
C Intubating Supraglottic Airways and Flexible Fiberoptic Bronchoscopes
Intubating SGAs were originally designed to act as conduits with high success rates for blind passage of an ETT into the trachea. They are more aptly designed for this purpose than most SGA devices, because larger ETTs can be deployed. When used with an FOB in a similar fashion to FOB-SGA combinations, their intubation success rate soars (Fig. 19-25). In a study by Erlacher and associates, 180 patients were intubated blindly through CobraPLUS (Pulmodyne, Indianapolis, IN), air-Q (Trudell Medical Marketing Limited, London, Ontario, Canada), and Fastrach devices with success rates of 47%, 57%, and 95%, respectively.112 Of the approximately 60 patients for whom blind intubation failed when these intubating SGAs were used alone, FOB insertion for intubation was attempted; the overall success rate for the FOB-intubating SGA combination was greater than 98%.
D Combination with Rigid Laryngoscopes
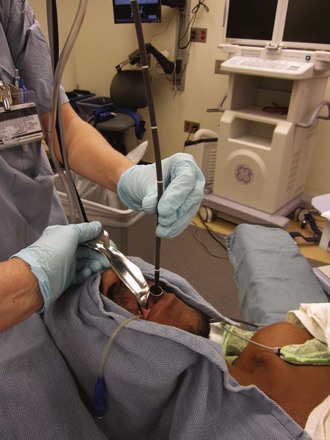
Occasionally, when an FOB is used as the sole airway device, impediments may prevent entry into the glottis; examples include a nonmobile or floppy, posteriorly directed epiglottis and upper airway edema. An RL can assist in lifting the mandible and moving obstructing tissues out of the way to improve the route for the FOB (Fig. 19-26). The FOB-RL technique requires at least two people: two endoscopists, or one endoscopist and a very knowledgeable assistant. The assistant must be able to hold the RL immobile after it has been placed optimally or to manage the FOB controls.110,113 The clinical situation, together with the knowledge and technical abilities of the personnel involved, dictate who handles the RL and who directs the FOB tip or completes glottic insertion of the FOB or both. Successful FOB-aided controlled tracheal extubation and reintubation using the RL-FOB combination has been reported in intensive care unit patients.114
E Combination with Video Laryngoscopes or Optical Laryngoscopes
VLs and OLs are amazing devices that have a firmly rooted place in airway management and definitely should be part of the DA algorithm. Many can give stunning images due to options such as wide-angle camera capability, exceptionally clear optics, and video monitoring. Their learning curves are very fast. In most patients, the 60-degree angulation design with video or optical mirror capability improves Cormack-Lehane views of the larynx by one to two grades over those seen with the RL.115–117 VL devices will most heavily impact what is now obviously an inferior device for airway management—the RL—and will result in continual declining use of that instrument.118
Despite superior visual capabilities, these devices can have failures in diverse airway cases.117–119 It is unlikely that VL will replace FOB use because of the very nature of FOB—the flexibility to conform to intricate airway pathology. The VL can optimize laryngoscopy but does not share the FOB’s second most desirable property, the ability to mechanically guide the direction of an ETT into the trachea to a specific end point. Clinicians may be delighted by the improved images from the VL, but they also are disappointed periodically when it remains impossible to intubate a patient despite “a perfect laryngeal view.” Many such failures have been reported with all types of VLs and OLs.59,119 In addition, pharyngeal perforation (palate or tonsillar pillars) may rarely result from the ETT tip during VL use. The cause is simple to understand. Whereas the ETT is initially advanced under direct vision, at some point it is pushed ahead blindly until it is finally seen again on the video screen.120–122 Care taken to directly observe the ETT hugging the tongue and curving it centrally and caudally within the airway greatly lessens this possibility. This type of trauma is unlikely with an FOB system in which the ETT slides over its insertion section.
Even the FOB has limitations compared to VL devices. FOBs have a more narrow-angled field of view, a shorter focal distance, a greater possibility of obscured optics if the airway is soiled, an inability to open the airway, and a degree of inability to maintain a midline position in the pharynx while advancing toward the glottis.123
VLs can also help diagnose FOB problems. In many cases, the FOB-VL technique provides visualization of the passage of the ETT over the fiberscope into the glottic area, aiding in the resolution of ETT “hang ups” at the arytenoid and other passage difficulties. The combined technique does have a drawback in that it requires both an endoscopist and a very knowledgeable assistant, but management of DI may necessitate two operators in any event. Indeed, use of the combination of FOB and VL has been shown to be advantageous and has been reported in DA patients.124
Another virtue of the FOB-VL combination is that it permits the use of a VL to fully observe FOB manipulation by trainees. This can enhance instruction and maximize learning experiences.125
1 Non–Channel-Loading Devices and Fiberoptic Bronchoscopes
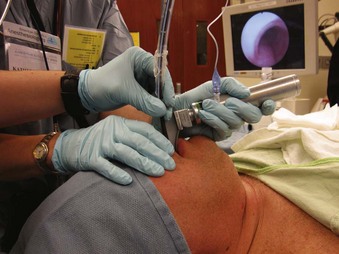
The type and number of personnel and the technique involved with combination of an FOB and a non–channel-loading device are similar to those of the combined FOB-RL method. Situate FOB and VL video screens, if available, near one another. After GA induction, attempt laryngoscopy with the VL until the best view is achieved. While holding the VL in place, direct the tip of the FOB under the epiglottis and toward the glottis (Fig. 19-27). Instruct the assistant to control the FOB tip. When the tip is beyond the view of the VL, use the FOB view. Continue to advance the insertion section, and after locating the tip near the carina, ask the assistant to railroad the ETT as described previously. Alternatively, after the VL attempt, have the assistant fix the VL in place. Take over total control of the FOB to locate the glottis, trachea, and carina and complete ETT passage.
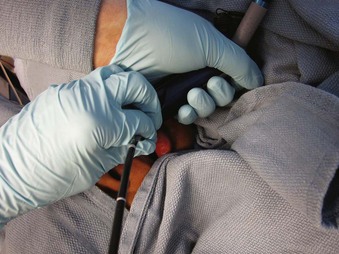
Figure 19-27 Intubating with a combination of a fiberoptic bronchoscope and a GlideScope video laryngoscope.
Trouble-Shooting
1. The brightness of the VL is making the FOB view excessively “whited out”: Dim the brightness of the VL model, if it possesses this feature, to prevent excessive glare when viewing through the FOB (Fig. 19-28). If the VL model does not have this capability, power it off after the FOB insertion if this problem arises. Power it back on intermittently to monitor FOB and ETT progress.
2. It is impossible to get the FOB tip under the epiglottis, even when directly looking into the mouth to position it: The FOB tip may be too flimsy to execute this maneuver. Observe the VL view of the FOB, gently advance the ETT just past the FOB tip, and snake the ETT under the epiglottis. Request a tongue pull or jaw thrust, or both. If successful, advance the FOB and complete the intubation.
2 Channel-Loading Devices and Fiberoptic Bronchoscopes
Visualization of the larynx may be perfect with the channel-loading type of VL or OL and yet result in failure of ETT intubation. In this scenario or even if insufficient visualization occurs, the VL may be used to open an airway path. Insert the FOB within an ETT either in the channel or next to the device. Direct it toward the glottis if it seems easy to do so, or use the FOB-VL techniques detailed earlier (Fig. 19-29).