32 Fetal Intervention
II. RATIONALE FOR FETAL CARDIAC INTERVENTION
A. The cardiac defect
1. The cardiac defect can have a negative impact on postnatal survival (e.g., hypoplastic left heart syndrome [HLHS] with severely restrictive or intact atrial septum).
2. The defect significantly affects quality of life.
a. Single ventricle with Fontan circulation (aortic stenosis that evolves to HLHS with a single right ventricle [RV] as the systemic ventricle).
b. Pulmonary atresia with intact ventricular septum that can lead to hypoplastic right heart syndrome and single-ventricle circulation with a single morphologic left ventricle (LV) as the systemic ventricle.
3. It can lead to fetal demise, as could occur with the evolution of severe tricuspid or mitral insufficiency with progressive severe pulmonary or aortic outflow tract obstruction, respectively.
III. TREATMENT
A. Fetal aortic stenosis
1. Fetal intervention is currently only justifiable for a severe form of aortic stenosis that has a high likelihood of evolving into HLHS or in which heart failure has evolved as a result of the severe LV dysfunction and/or mitral insufficiency.
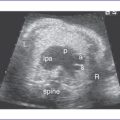
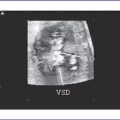
2. Mild or even moderate forms of fetal aortic stenosis do not usually result in LV hypoplasia or fetal heart failure. The fetus can undergo neonatal balloon aortic valvuloplasty (Figs. 32-1 and 32-2) with satisfactory results and long-term outcomes.
3. The postnatal outcomes for HLHS vary among institutions.
a. The best reported survival ranges from 80% to 90% for the first-stage Norwood surgery and 70% to 80% through the Fontan stage.
b. The long-term survival beyond 20 years of age is unknown.
c. In the context of progressive heart failure or fetal hydrops, the likelihood of survival if the fetus is not at a truly viable age and size for intervention is extremely low.
4. Fetal intervention can be justified if the intent is to prevent progression of severe aortic stenosis to HLHS or fetal hydrops.
a. We select patients we believe will have HLHS at birth but whose LV is still within the normal range at the time of diagnosis.
b. Fetal echocardiography shows features of fetal aortic stenosis with evolving HLHS.
d. Contraindications to fetal intervention.
e. Fetal cardiac intervention team.
f. Outcomes and follow-up after fetal intervention for aortic stenosis.
B. HLHS with intact or restrictive atrial septum
1. Rationale for fetal intervention and postnatal outcomes.
a. These cardiac defects have a very high mortality (<50% survival in published reports).
b. The defect might have an additional negative impact on long-term survival on those who survive neonatal surgery.
c. The defect can require urgent interventional catheterization or surgery immediately after birth.
d. The lesion has a significant negative impact on the lungs and pulmonary vasculature and likely has a progressive prenatal component.
2. Indications for fetal intervention.
a. These indications are evolving and will change as we obtain more knowledge about this variant of HLHS.
b. Patient selection requires integration of the following features:
a. To create an atrial defect that persists.
b. To decompress pulmonary venous flow in utero through the remainder of gestation in utero.
c. To enable a more stable baby postnatally.
d. To reduce the need for an emergency procedure immediately after birth.
b. Increased morbidity and mortality.
c. Procedures performed using a purely percutaneous technique.
C. Pulmonary atresia with intact ventricular septum
1. Fetal intervention for this defect is somewhat more controversial.
a. A significant percentage of these patients ultimately achieve a biventricular repair after initial palliative interventional catheterization and surgery.
b. Currently, there are no reliable prenatal criteria to accurately predict which patients will ultimately require single-ventricle palliation postnatally.
c. Occasionally, fetuses with severe pulmonary stenosis or pulmonary atresia develop heart failure, probably largely due to the evolution of significant tricuspid insufficiency.
a. This patient will probably ultimately have a single-ventricle palliation.
b. There is severe tricuspid regurgitation with hydrops and potential fetal demise.
c. A postnatal tricuspid valve z score of less than −3 has been shown to predict which patients ultimately have single-ventricle palliation.
a. Fibromuscular outflow tract atresia.
b. Ebstein-type cardiac defect with functional pulmonary atresia.
IV. FETAL CARDIAC SURGERY
B. Rationale for fetal cardiac surgery intervention
a. Prenatal: It is apparent that prenatal diagnosis of congenital heart defect is much poorer than postnatal diagnosis.
b. Postnatal: It has been found that neonates with cardiac malformation amenable to biventricular repair have a better survival (96%) than a similar cohort with lesions not diagnosed prenatally (76%).
a. Improving gross cardiac development.
b. Improving ultrastructural cardiac development (allowing normal histological and ultrastructural development of the heart).
3. Reducing damage to surrounding organs and development of surrounding intrathoracic structures depends on adequate physical space within the chest for their growth. Ebstein’s anomaly with severe tricuspid regurgitation causes severe enlargement of the RA, and lung development on the right side is compromised, resulting in hypoplasia.
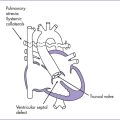
BOX 32-1 PROPOSED THEORY OF FLOW-RELATED FETAL CARDIAC DEVELOPMENT
Simple anatomic lesion during cardiac morphogenesis such as aortic stenosis
Alterations of in utero pressure and flow patterns, such as decreased inflow to left ventricle
Complex postnatal cardiac malformation, such as hypoplastic left heart syndrome
C. Lesions that are likely candidates for fetal intervention
The most likely candidates for fetal cardiac surgical intervention are those whose pathogenesis is related to abnormal intracardiac flow patterns (Box 32-2).
D. What is needed for a successful fetal cardiac intervention
1. The lesion for which the intervention is needed must have a poor outlook with conventional postnatal surgical approaches so that the increased risk of in utero intervention is justified (e.g., single-ventricle physiology).
2. The lesion must be a simple primary cardiac defect that can be readily dealt with.
3. The proposed interventional procedure must be simple, reliable, and quick.
E. Practical issues in a potential cardiac intervention
1. Size of the bypass circuits and oxygenators available to allow the smallest weight of a fetus (infant).
2. Tissue structure: The ultrastructure of fetal tissues is different from that in a neonate, allowing scarless healing in extremely friable tissue.
a. Tissues in the fetus have a higher water content.
b. Ratio of type I to type III collagen gradually rises with fetal maturation, thus increasing the degree of cross-linking.
F. Fetoplacental unit response to bypass
1. The placenta is a natural oxygenator, so there is less priming volume.
2. There is a lower magnitude of inflammatory response because of placental oxygenation.
3. The blood flow depends on the impedance in the parallel circulations (placental and systemic circulation), so any elevation in pulmonary vascular resistance eventually decreases fetal cardiac output.
4. The fetoplacental unit response to bypass remains the major obstacle to the successful clinical application of fetal cardiac surgery.
H. Fetal cardiac pacing
1. About one in 20,000 fetuses develops congenital complete heart block (CCHB) (see Chapter 23).
2. The majority (58%-80%) have structural heart disease.
a. Left atrial isomerism with atrioventricular septal defect.
b. Discordant atrioventricular connection.
c. Endocardial fibroelastosis.
3. When CCHB is associated with severe structural defects, the in utero mortality rate is about 86%. In contrast, fetuses with CCHB with a structurally normal heart (majority due to maternal antibodies to SS-A/Ro or SS-B/La) have a lower mortality rate (11%-25%).
4. Fetuses cannot usually tolerate a ventricular rate less than 55 bpm. Bradycardia results in AV valve regurgitation, low aortic flow velocity (decreased cardiac output), and hydrops.
5. This reaction to bradycardia suggests that there is no apparent inherent myocardial dysfunction in these fetuses and that the cardiac failure is purely secondary to the slow heart rate.
6. Aims of the fetal cardiac pacing.
a. Fetal epicardial pacing (accelerating the heart rate) might reverse the heart failure.
b. Fetal pacing might allow safe progression of pregnancy to a safe gestational age (accepted beyond 32 weeks’ gestation).
c. Pacing could avoid the need for maternal therapy or other modalities of fetal therapy, such as umbilical vein infusions.
V. TAKE-HOME MESSAGE
A. Diagnosis
1. Congenital heart disease diagnosed in utero has a poorer prognosis than the same defect diagnosed postnatally.
2. Determining which is the primary and which is the secondary cardiac defect remains difficult. If the valve pathology is primary, then earlier relief of obstruction can produce a successful outcome. On the other hand, underdevelopment of the valve may be secondary to inadequate cardiac output, crossing it due to poor ventricular function.
B. Treatment
1. Clinical experience has shown that fetal balloon valvuloplasty is possible. Longer-term follow-up is needed to determine the impact on the immediate and intermediate postnatal outcome.
2. The fetoplacental unit response to cardiac bypass remains the major obstacle to fetal cardiac surgery.
3. Fetal cardiac pacing might prove helpful in fetuses with CCHB. It has been suggested that by accelerating the heart rate in the absence of myocardial damage, hydrops could be reversed.
Donofrio MT, Bremer YA, Moskowitz WB. Diagnosis and management of restricted or closed foramen ovale in fetuses with congenital heart disease. Am J Cardiol. 2004;94(10):1348-1351.
Hanley FL, Sade RM, Blackstone EH, et al. Outcomes in neonatal pulmonary atresia with intact ventricular septum. A multi-institutional study. J Thorac Cardiovasc Surg. 1993;105(3):406-423. 424-427, discussion 423-424.
Hanley FL. Fetal cardiac surgery. Adv Card Surg. 1994;5:47-74.
Hedrick HL, Flake AW, Crombleholme TM, et al. Sacrococcygeal teratoma: Prenatal assessment, fetal intervention, and outcome. J Pediatr Surg. 2004;39(3):430-438.
Huhta J, Quintero RA, Suh E, Bader R. Advances in fetal cardiac intervention. Curr Opin Pediatr. 2004;16(5):487-493.
Jouannic JM, Boudjemline Y, Benifla JL, Bonnet D. Transhepatic ultrasound-guided cardiac catheterization in the fetal lamb: A new approach for cardiac interventions in fetuses. Circulation. 2005;111(6):736-741.
Marshall AC, van der Velde ME, Tworetzky W, et al. Creation of an atrial septal defect in utero for fetuses with hypoplastic left heart syndrome and intact or highly restrictive atrial septum. Circulation. 2004;110(3):253-258.
McElhinney DB, Salvin JW, Colan SD, et al. Improving outcomes in fetuses and neonates with congenital displacement (Ebstein’s malformation) or dysplasia of the tricuspid valve. Am J Cardiol. 2005;96(4):582-586.
Michaelsson M, Engle MA. Congenital complete heart block: An international study of the natural history. Cardiovasc Clin. 1972;4(3):85-101.
Reddy VM, Hendricks-Munoz KD, Rajasinghe HA, et al. Post–cardiopulmonary bypass pulmonary hypertension in lambs with increased pulmonary blood flow. A role for endothelin 1. Circulation. 1997;95(4):1054-1061.
Simpson JM, Sharland GK. Natural history and outcome of aortic stenosis diagnosed prenatally. Heart. 1997;77(3):205-210.
Tulzer G, Arzt W, Franklin RC, et al. Fetal pulmonary valvuloplasty for critical pulmonary stenosis or atresia with intact septum. Lancet. 2002;360(9345):1567-1568.
Tworetzky W, Marshall AC. Balloon valvuloplasty for congenital heart disease in the fetus. Clin Perinatol. 2003;30(3):541-550.
Tworetzky W, Marshall AC. Fetal interventions for cardiac defects. Pediatr Clin North Am. 2004;51(6):1503-1513.
Tworetzky W, Wilkins-Haug L, Jennings RW, et al. Balloon dilation of severe aortic stenosis in the fetus: Potential for prevention of hypoplastic left heart syndrome: Candidate selection, technique, and results of successful intervention. Circulation. 2004;110(15):2125-2131.
Vlahos AP, Lock JE, McElhinney DB, van der Velde ME. Hypoplastic left heart syndrome with intact or highly restrictive atrial septum: Outcome after neonatal transcatheter atrial septostomy. Circulation. 2004;109(19):2326-2330.