1 Fetal Circulation
II. OVERVIEW OF THE FETAL CIRCULATION
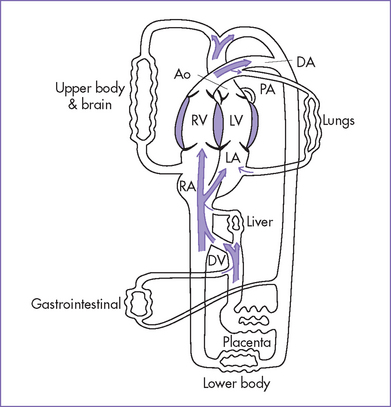
Fetal circulation is shown in Figure 1-1.
A. Oxygenation
1. Blood is oxygenated in the placenta.
2. Oxygenated blood returns to the fetus via the umbilical vein.
3. The umbilical vein courses into the hilum of the liver and joins the portal vein.
4. Umbilical venous blood is directed in part through the ductus venosus, which joins the inferior vena cava (IVC).
5. Oxygen-rich ductus venosus blood is preferentially directed into the left atrium and left heart through the foramen ovale.
6. Venous return of the left heart is composed of both ductus venosus and pulmonary venous blood.
7. The less-oxygenated blood of the liver (largely right lobe) and the superior vena cava (SVC) is directed into the right atrium and right heart.
B. Function
1. The left and right hearts function in parallel rather than in series. The left ventricle (LV) ejects largely to the coronary arteries and upper body with an arterial PaO2 (in fetal lambs) of 25 to 28 mm Hg. The right ventricle (RV) ejects largely to the lungs, the lower body, and the umbilical artery via the ductus arteriosus with an arterial PaO2 of 20 to 23 mm Hg (in fetal lambs).
2. With a high pulmonary vascular resistance, less than 20% of the cardiac output goes through the fetal lungs. This percentage increases in the third trimester.
3. The combined cardiac output of the fetal heart is 450 mL/kg per minute with increasing RV dominance through the second and third trimesters. In contrast, the combined output is 800 mL/kg per minute in the series circulation after birth.
III. THE PLACENTA
B. Function
1. The placenta provides nutrients and respiratory gas and metabolic exchange between the mother and fetus.
2. Uteroplacental flow increases during gestation in three phases as a result of vasodilation.
a. The first phase may occur within days or weeks of pregnancy.
b. The second phase occurs with development of intravillous spaces.
c. The third phase occurs during rapid fetal growth after 30 weeks. This corresponds with a change in uterine blood flow from less than 1% of the maternal cardiac output to 16% to 25% near term.
a. Complete fetoplacental circulation is established around the start of the fifth week after conception.
b. Fetoplacental blood flow, like uteroplacental flow, exponentially increases throughout gestation.
c. Placental blood volume is approximately 30% of the combined fetal cardiac output between 20 weeks and term.
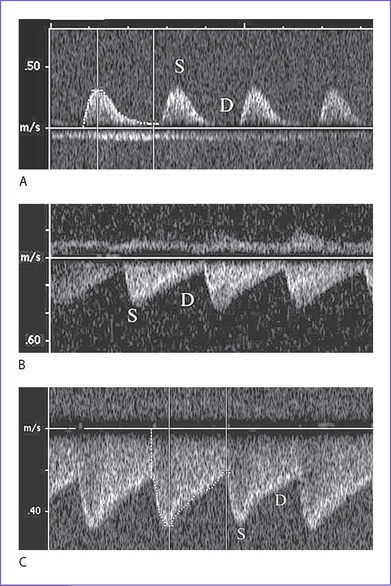
a. In the first trimester, umbilical artery Doppler flow studies have demonstrated a gestational-age-dependent fall in placental resistance, with essentially no forward diastolic flow in the late first trimester.
b. In the second and third trimesters, Doppler flow studies indicate increasing flow velocities in diastole in keeping with the decreasing placental resistance.
IV. FETAL SHUNTS
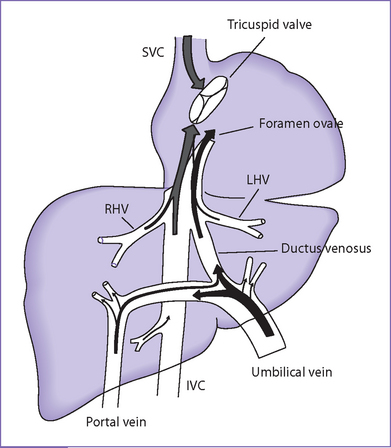
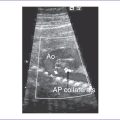
A. Ductus venosus (Fig. 1-3)
1. The ductus venosus provides access for much of the highly oxygenated blood from the umbilical vein to the fetal IVC, which streams preferentially into the left atrium as a consequence of the IVC’s central position and relationship with the foramen ovale, septum primum, and septum secundum.
2. On Doppler flow interrogation in the fetus, the ductus venosus can be readily identified by the flow acceleration that occurs within its lumen.
a. It has the highest flow velocities observed in the systemic venous system.
b. Its pattern of flow is phasic but continuously antegrade, with the lowest velocity during atrial contraction.
3. Absence (or agenesis) of the ductus venosus.
a. The umbilical vein may connect with the portal venous system, another systemic vein such as the IVC, or the right atrium itself.
b. At least in the latter two conditions, a high cardiac output state often develops in the fetus, suggesting that the ductus venosus in some way regulates the umbilical venous return.
4. Ductus venosus patterns change in the context of evolving fetal heart failure. These suggest a protective mechanism that prevents modestly elevated central venous pressures from significantly influencing the critical umbilical venous return.
a. Flow patterns show gradual reduction in forward velocities during atrial systole in the ductus venosus.
b. Flow patterns show ultimate development of a wave reversal that usually occurs before the development of umbilical venous notching.
B. Foramen ovale
1. The foramen ovale is a communication between the left and right atrium.
a. The septum primum is a flap-valve in the foramen ovale that allows the oxygen-rich blood to stream through the ductus venosus from the umbilical vein to enter into the left atrium.
b. The septum primum prevents blood from moving from the left atrium to the right atrium in the normal heart.
2. In humans, Doppler studies suggest the fraction of combined cardiac output that crosses the foramen ovale decreases from 34% at 20 weeks to 18% at term.
C. Ductus arteriosus
1. The ductus arteriosus is a vessel that permits the majority of the RV output to be ejected into the descending aorta.
2. Early in gestation, its size is very similar to that of the aortic arch.
3. Intimal cushions form later in gestation.
a. Likely in preparation for postnatal closure, intimal cushions form within the lumen of the ductus arteriosus as a result of vascular smooth muscle cell proliferation and migration into the subendothelium.
b. The formation of intimal cushions could correspond with the very high velocities observed in the ductus arteriosus in late third trimester human fetuses.
4. Circulating prostaglandin E2 (PGE2) probably controls the patency of the ductus arteriosus in utero.
V. FETAL MYOCARDIUM
A. Developmental changes in structure and growth
a. In the fetus, particularly early in gestation, growth is largely through cardiac myocyte and nonmyocyte proliferation.
b. Myocardial growth and general somatic growth are rapid in fetal life, particularly in the first half of gestation after embryogenesis.
c. In late gestation, cardiac myocytes begin to lose their ability to divide, and subsequent myocardial growth is through cardiac myocyte hypertrophy and nonmyocyte proliferation. The factors that determine when a cardiac myocyte withdraws from the cell cycle, and even the exact timing in humans, are largely unknown.
d. Fetal cardiac and noncardiac myocytes express matrix.
e. The metabolic energy source of the fetal myocardium is largely glucose.
2. Differences between fetal and postnatal myocardium help to explain differences in myocardial function.
a. Noncontractile proteins make up about 60% of fetal versus 30% of the adult myocardium.
b. With increasing gestational age, the number of sarcomeres and the myofibril component increase.
c. The myofibrils are disorganized in early gestation. They gradually become more organized with increasing gestational and postnatal age.
d. With maturation, the transverse tubular system is acquired and the sarcoplasmic reticulum develops.
e. With gestational age and postnatal maturation, the sympathetic nervous system matures and the number of expressed β-adrenergic receptors in the myocardium increases.
f. There are developmental differences in isoforms of myosin heavy chain and troponin expressed.
B. Developmental changes in myocardial function
a. Single muscle strip studies suggest the fetal myocardium has less active tension, which could suggest that its contractility is less robust than in the more mature heart.
b. Echocardiographic parameters of systolic performance in the fetus are not very different from those in the postnatal heart.
c. Cardiac output changes from 450 mL/kg per minute in utero to 800 mL/kg per minute for the combined ventricular output in the neonate.
d. In utero, the RV output is approximately 1.2-1.4 that of the LV.
a. Single muscle strip preparations suggest greater passive tension of the fetal myocardium than in the postnatal myocardium.
b. Doppler ventricular filling patterns demonstrate increasing e wave (early ventricular diastole) with gestation and no significant change in the a wave velocity (during atrial systole, at least from the middle trimester), which suggests that the ability of the ventricles to relax changes with gestational age.
c. Isovolumic relaxation time does not significantly change between the second and third trimesters.
a. Fetal myocardium does follow Starling’s law, as is most simply demonstrated by the increased ventricular output following a premature beat.
b. LV preload is determined by the IVC flow, pulmonary venous return, size of the foramen ovale, right heart filling pressures, and the diastolic or filling function of the LV.
c. RV preload is determined by the SVC and IVC flow, left heart filling pressure, foramen ovale size, and RV diastolic function.
a. LV afterload is largely from the upper body.
b. RV afterload is largely determined by the vascular bed of the lower body (including the placenta) and patency of the ductus arteriosus.
c. Afterload has a significant impact on cardiac output.
d. The low vascular impedance of a normal placenta results in a large fraction of the combined ventricular output going through the umbilical circulation.
b. Over the normal range of heart rates, the cardiac output does not significantly change, but the stroke volume changes to maintain a stable cardiac output.
VI. RESPONSE OF THE FETAL CIRCULATION TO STRESS
A. Decreased oxygen delivery to the fetus
1. Decreased oxygen delivery to the fetus is the most common cause of fetal stress.
2. Causes of decreased oxygen delivery include:
a. Decreased maternal arterial oxygen content.
b. Decreased uterine blood flow.
c. Uterine vascular pathology.
e. Fetal factors that alter the oxygen-carrying capacity or change the umbilical blood flow.
B. Cardiac output in response to hypoxia
1. Cardiac output does not significantly increase in response to hypoxia in the fetus.
2. The fetus compensates in other ways.
c. Decreased O2 consumption: The fetus can decrease oxygen consumption by one third without developing metabolic acidosis.
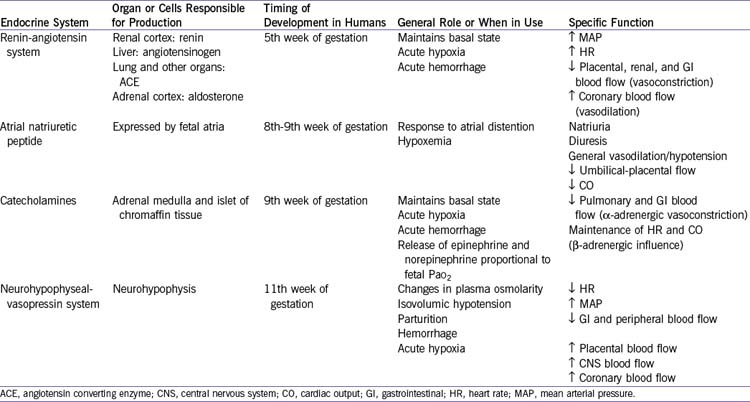
e. Endocrine response (Table 1-1).
VII. TAKE-HOME MESSAGE
A. Normal fetal heart function
1. The fetal left heart and right heart function in parallel rather than in series. This is a result of the unique fetal shunts and placental circulation.
2. As a consequence of the fetal circulation and streaming of blood, the left heart in utero sees more highly oxygenated blood which goes to the fetal myocardium and brain.
3. Normal fetal heart rates are 110 to 150 bpm at less than 10 weeks, 150 to 180 bpm at 10 to 16 weeks, and 120 to 160 bpm from 20 weeks to term.
4. The fetal response to stress includes:
a. Increase in the mean arterial pressure.
b. Decrease in the fetal heart rate.
c. Decrease in the combined cardiac output and redistribution of the cardiac output to critical organs (brain, heart, adrenal glands) late in the course.
B. Differences in fetal and mature hearts
1. There are key developmental differences among the fetal, neonatal, and adult myocardium that result in differences in diastolic and systolic function.
2. The fetal myocardium does follow Starling’s law, as is most simply demonstrated by the increased ventricular output following a premature beat.
Fouron J-C. Fetal cardiovascular physiology. In: Allan L, Hornberger L, Sharland G, editors. Textbook of Fetal Cardiology. London: Greenwich Medical Media; 2000:31-45.
Kiserud T, Acharya G. The fetal circulation. Prenat Diagn. 2004;24:1049-1059.
Morin FC, Weiss K. Response of the fetal circulation to stress. In: Polin RA, Fox WW, editors. Fetal and Neonatal Physiology. Philadelphia: WB Saunders; 1992:598-609.
Rudolph AM. The fetal circulation and postnatal adaptation. In: Rudolph AM, editor. Congenital Diseases of the Heart: Clinical-Physiological Considerations. Armonk, NY: Futura; 2001:3-44.
St John Sutton M, Gill T, Plappert T. Functional anatomic development of the fetal heart. In: Polin RA, Fox WW, editors. Fetal and Neonatal Physiology. Philadelphia: WB Saunders; 1992:598-609.