Chapter 2 Febrile Seizures
What are Febrile Seizures?
Several definitions of febrile seizures exist. The National Institutes of Health (NIH) Consensus Conference1,2 defined febrile seizures as “events in infancy or childhood, usually occurring between 3 months and 5 years of age, associated with fever but without evidence of intracranial infection or defined cause. Seizures with fever in children who have suffered a previous nonfebrile seizure are excluded.” More recently the International League Against Epilepsy (ILAE)3 defined febrile seizures as “seizures occurring in childhood after age one month, associated with a febrile illness not caused by an infection of the central nervous system, without previous neonatal seizures or a previous unprovoked seizure, and not meeting criteria for other acute symptomatic seizures.”
Note that the NIH and ILAE definitions differ in both the minimal and maximal age “permitted” for febrile seizures, and this may cause confusion among parents and physicians. In addition, although both the NIH and ILAE definitions exclude children with prior afebrile seizures and those with seizures due to an intracranial infection or other specific cause, they do not exclude children with preexisting neurological deficits. This may confound studies looking at the cognitive outcome of these seizures. Note also that “fever” is not defined. In general, a temperature higher than 38°C4,5 or 38.4°C (101°F) has been defined as constituting fever.
The majority of febrile seizures occur early in the febrile episode and often are the presenting sign of the fever.6–8 A typical scenario is a child who presents with a seizure, and the presence of fever is discovered later, once the child arrives in an emergency room. Whether the rate of temperature rise is an important determinant of the occurrence of a febrile seizure, rather than the actual temperature achieved, is not well substantiated.9,10
Frequency and Pathophysiology of Febrile Seizures
Several large population-based studies examined the incidence (the number of new cases occurring in a defined population over a specified period of time) and the prevalence (proportion of individuals in a population that has ever had the disorder) of febrile seizures. They found similar rates (2 to 5%) in the Western world and higher rates in Japan and Guam (Figure 2-1). For examples, in Rochester, Minnesota, the annual incidence of febrile seizures was ∼4/1000 children younger than age 5, and ∼2% of children experienced a febrile seizure by the age of 5.11,12 The large Oakland, California,13 and British studies14 also found that by the age of 5 years 2 to 2.3% of children had had a febrile seizure. The National Collaborative Perinatal Project (NCPP) reported a somewhat higher rate (3.5%15,16), that was similar to results from Sweden (cumulative prevalence of 4.1% in children under age 517) and Holland (a rate of 3.9%18).
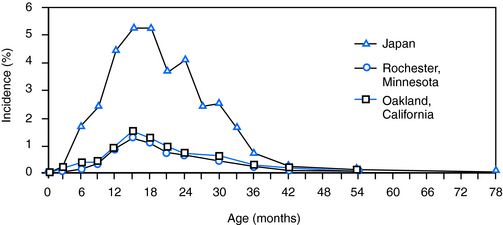
Figure 2–1 Age-specific incidence of febrile seizures in the United States and in Japan.
Reprinted with permission from Hauser [1994], Blackwell Publishing.
In contrast to their frequency in the Western world, febrile seizures occur in ∼8 to 10% of Japanese children4 and of those in Guam.19,20 This increased incidence is likely a result of both environmental factors (e.g., parents and children sleep in close proximity, so that febrile seizures are more likely to be observed), as well as genetic elements.
Why will a child have a febrile seizure? Current evidence supports the notion that in a given child, environmental and genetic factors may interact to determine whether that child will have a febrile seizure. Furthermore, the relative contribution of each varies significantly: febrile seizures occur sporadically as well as run in families, where the contribution of genetic background to their onset is likely higher.21 Looking at risk factors for febrile seizures, Bethune et al.22 found that both attending day care (increasing the chance of fever and infection) and a history of a family member with febrile seizure tripled the risk of having such seizures. Contribution of presumed genetic background (presence of febrile seizures in a relative), as well as environmental factors (e.g., maternal smoking during pregnancy), was reported also by Berg et al.23
How does fever lead to a febrile seizure? Temperature influences neuronal electrophysiological activity.24 For example, the function of ion channels such as the TRPV4 (transient receptor potential vanilloid 4) are markedly dependent on temperature in the physiological and fever ranges, ∼36°C to 42°C.25 Children can also develop seizures on simple hyperthermia and not fever, such as hyperthermia related to anticholinergic overdose26 or hot showers,27 suggesting that increased temperature in itself can be a convulsant in children.
Recently, hyperthermia in an animal model has been shown to induce hyperventilation resulting in alkalosis that in turn provoked seizures.28 This happened 25 to 30 minutes after the onset of the hyperthermia and was not observed in a second febrile seizure model in an immature rat.29 In the latter case, seizures commenced within A: 2.9 ∀ 0.1 minutes of the onset of the hyperthermia procedure (n = 52), and respiratory rates when seizures started B: 167 ∀ 4.9, n = 12 did not differ significantly from those rates prior to C: 161.8 ∀ 2.6 or during the first minute of D: 173 ∀ 3.7 the hyperthermia. Whether alkalosis plays a role in febrile seizures in children remains unknown.
Genetic susceptibility may increase the ability of fever to provoke seizures. Recently, mutations of several ion channels that predispose to febrile seizures have been described,30–33 including mutations of sodium30,31 and chloride (GABAA receptor32,33) channels. In many families, these febrile seizures are associated with epilepsy of diverse phenotypes, a syndrome termed GEFS+ (generalized epilepsy with febrile seizures plus).34 Other single-gene mutations, such as in the interleukin gene promoter (see later discussion) might also render individuals more susceptible to febrile seizures,35,36 and multigene interactions might contribute to the occurrence of these seizures in a more complex and subtle way.
Fever may promote seizures via the function of pyrogenic cytokines including interleukin-1.37,38 This notion is supported by reports of mutations in the interleukin-1 gene promoter that result in increased production of the cytokine in individuals with febrile seizures,36 although the significance of this finding has been debated.39 In summary, the contribution of gene-environment interaction to the generation of febrile seizures, and to their potential causal relationship to epilepsy,40 is a topic of active investigation; increasingly sophisticated experimental tools should rapidly advance our understanding of this question.
Specific infectious etiology of the fever might contribute to the generation of human febrile seizures. A disproportionate number of febrile seizures has been associated with infection with the human herpes virus 6 (HHV6) in some,41 but not all, studies.42 This suggests that mechanisms specific to this virus, including perhaps a unique profile of cytokine induction, might selectively augment neuronal excitability and thus preferentially provoke seizures.
Types of Febrile Seizures
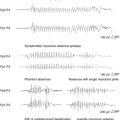
Febrile seizures are divided into: (a) simple (∼70 to 80% of the total5,15,18,43–45), (b) complex, and (c) febrile status epilepticus (FSE).
Simple febrile seizures are short generalized seizures, typically but not necessarily motor, that last less than 108,46 or 1516,45 minutes. Specifically, these seizures do not have focal features and do not recur within the same febrile episode (or within 24 hours from the first seizure). Complex febrile seizures are either longer, lasting >10 minutes8,46 or >15 minutes,16,45 have focal features, or recur within 24 hours of the first episode15,46 or within the same febrile illness.47 Febrile status epilepticus (FSE) is defined as febrile seizures longer than 30 minutes. Note that Scott et al.48,49 define seizures lasting more than 30 minutes in normal children without intracranial infection as prolonged febrile seizures rather than status epilepticus.
Complex febrile seizures, and particularly febrile status epilepticus, are associated with a higher risk of later epilepsy (see later discussion), so recognizing them is of clinical importance. Recent research has found that the duration of a seizure is regulated in each child independent of the probability of having a seizure. Thus, Shinnar et al.50 have found that once a child has had a prolonged seizure, a subsequent seizure is likely to be prolonged as well. This is important for targeting children for abortive therapy to prevent long febrile seizures (see later discussion).
Outcome of Febrile Seizures
The outcome of febrile seizures is measured in terms of cognitive function (e.g., school performance, cognitive tests) and the risk for developing epilepsy. Several large studies have suggested that simple febrile seizures, nonfocal and with a duration of less than 10 to 15 minutes, do not lead to long-term sequelae16,43,46,51,52 in either of these realms. However, a smaller study from Taiwan cautioned that infants less than a year of age at the time of their seizure might function less well in certain learning tasks.53,54 By and large, however, the overwhelming evidence regarding simple febrile seizures is that they are benign.
The outcome of complex febrile seizures and of febrile status epilepticus is more controversial.16,45,46,55 In terms of cognitive function, both the American National Collaborative Perinatal Project16,56 and the British cohort51 used general broad measures such as school performance as outcome and evaluated children at age 6 or 10, not later. They concluded that complex febrile seizures do not alter cognition, but their design, as mentioned earlier, limits the confidence of these conclusions. Evaluations of cognitive outcome of prolonged febrile seizures or febrile status are complicated by the fact that many epidemiological studies do not exclude children who are prior neurologically compromised (see definition of febrile seizures in the section “What Are Febrile Seizures?”). Inclusion of children with neurological deficits before the seizures occurred complicates attempts to look at the impact of these seizures on intellect. Notably, British investigators have recently begun to better define the populations they study to exclude children with neurological deficits. The results of these studies should be helpful to sort out the consequences of these seizures on the normal developing brain.57
Whether prolonged febrile seizures and febrile status epilepticus lead to epilepsy has been a subject of debate. Whereas prospective epidemiological evaluations have provided little evidence for epileptogenesis,16,45,46 retrospective analyses have strongly linked a history of complex, and particularly of prolonged, febrile seizures to temporal lobe epilepsy,58–61 suggesting a potential contribution of febrile seizures to epileptogenesis. It is not easy to reconcile these conflicting data, yet the reader should bear in mind that each type of study has inherent limitations. Prospective epidemiological studies benefit from large numbers and from the fact that they are not skewed by clinical populations in referral centers. In contrast, they usually do not have long follow-up and may miss important associations. Retrospective studies involve people who are identified after the fact (or with a known outcome, such as epilepsy) and evaluated retroactively. These studies can identify associations in relatively small populations, but are open to ascertainment and recall biases and may assess selected (e.g., more severely affected) populations that do not reflect a general principle.
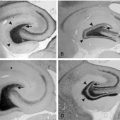
Animal models of long febrile seizures have been used to address the question of the potential epileptogenic consequences of these seizures. These models involved induction of experimental febrile seizures in a normal rodent brain28,29,37,62–71 or in animals that have been subjected to prior insults.72–73 Recently, the use of an experimental model of prolonged febrile seizures in immature rats found that these seizures may promote limbic, temporal lobe epilepsy (TLE) in a minority of animals. The mechanisms by which these seizures contribute to temporal lobe epilepsy in this model are not fully resolved. However, the evidence suggests that the seizures do not lead to “brain damage” (neuronal death).65,74,75 Specifically, transient neuronal injury was provoked by these seizures, but the involved neurons did not die.65 Neuronal counts in several brain regions with established vulnerability to seizures failed to reveal cell loss.65,74,75 Studies for apoptosis did not show increased cell death at any time point after the seizures,65 even when the seizures were carried out for 60 minutes. In addition, neurogenesis that may be induced in other developmental seizures74 did not follow experimental febrile seizures. Other expected structural changes, including mossy fiber sprouting, were also minimal after these seizures74,76,77 and did not explain the conversion of the hippocampal circuit into a hyperexcitable one.
The likely mechanisms for seizure-evoked hippocampal hyperexcitability29,78,79 involve profound and persistent molecular changes in hippocampal neurons that lead to a change of their intrinsic excitability and their responses to input from other neurons. Whereas the repertoire and sequence of molecular changes evoked by experimental prolonged febrile seizures in this model have not yet been fully determined, persistent changes in the expression of specific ion channels likely play a role, as do alterations in endocannabinoid signaling.67
Following experimental prolonged febrile seizures, the properties of Ih, a hyperpolarization-triggered cationic current that contributes to the maintenance of neuronal membrane potential, subthreshold oscillations, and dendritic integration,80–82 in hippocampal neurons were altered.28,79 This led to increased probability of frequency-dependent rebound depolarization in response to hyperpolarizing input.78,79 At the molecular level, these changes were a result of long-lasting altered expression of hyperpolarization-activated cyclic-nucleotide–gated (HCN) channels that conduct this current, particularly a reduction of HCN1 isoforms, and perhaps also of increased formation of HCN1/HCN2 heteromeric channels.83,84 The relevance of the alterations in HCN channels and Ih to human epileptogenesis remains to be fully defined. Altered HCN1 channel expression was found in a subset of resected hippocampi from patients with temporal lobe epilepsy and mesial temporal sclerosis, often with a history of early life seizures.85 This suggests that HCN channels are altered in human epilepsy. However, whether mutations in HCN channel genes that alter Ih contribute to human epilepsy remains unknown.
Altered endocannabinoid signaling also followed prolonged experimental febrile seizures, contributing to hyperexcitability. This resulted from an increase in the number of presynaptic cannabinoid type 1 receptors, which increased retrograde inhibition of GABA release, promoting hyperexcitability.67 It is very likely that many other enduring changes in the expression of key genes that govern neuronal and network excitability may take place after experimental febrile seizures and perhaps human prolonged febrile seizures. These are expected to coordinately alter the probability of the occurrence of spontaneous seizures (i.e., epilepsy).
Evaluation and Management of Febrile Seizures
The evaluation and management of the different types of febrile seizures depend on the age and clinical setting of the child that presents with the seizure, as well as the type of the seizures (simple, complex, or febrile status epilepticus). The first consideration is whether the febrile seizure might be a herald of meningitis or meningoencephalitis, especially in young children (less than 12 months), where clinical signs of CNS infection might be few.86 Because seizures may be the first sign of meningitis in 13 to 16% of children with meningitis, and meningeal sign may be absent or subtle in younger children, the American Academy of Pediatrics recommends a lumbar puncture for all individuals younger than 12 months who present with a febrile seizure and strong consideration of the procedure for older individuals, up to 18 months of age.87 Beyond this consideration, several guidelines recommend minimal evaluation and no treatment of a normal child with a simple febrile seizure.1,87–90 Thus, EEG, imaging, and preventive or abortive therapy are not indicated.87,91,92
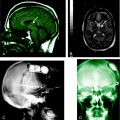
For prolonged febrile seizures (and less clearly for recurrent or focal types of “complex” febrile seizures) the approach of clinicians is in flux. In the proper clinical setting (e.g., with a strong family history) evaluation for GEFS+ (e.g., DNA for SCN1A mutations) might be indicated. With a prolonged seizure, particularly if focal, magnetic resonance imaging (MRI) changes have been reported,48,49,93 and imaging may be indicated, together with follow-up.
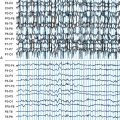
The role of electroencephalography (EEG) is still not clear. An early, large study94 described EEG abnormalities in about a third of children with febrile seizures, mostly consisting of global slowing. However, the presence of an abnormal EEG did not correlate with seizure recurrence,94,95 or with the subsequent development of epilepsy95 and is not a required element of the evaluation of febrile seizures.87 Novel data from the multicenter study of febrile status epilepticus (FEBSTAT) is now emerging, suggesting that focal slowing after these seizures that last more than an hour might predict MRI abnormalities (S. Shinnar, personal communication).
Treatment of febrile seizures is rarely indicated. Prophylactic treatment of a child with febrile seizures using barbiturates or valproate may reduce the number of further seizures, but there is no evidence that this approach alters the probability of developing epilepsy,52 and the side effects of daily medication are not justified.21,46,52,96 Attempts at initiating prophylactic diazepam at the onset of fever failed97–99 because children became drowsy and irritable, thus reducing compliance. In addition, most febrile seizures occur at the onset of the febrile illness—before diazepam can be initiated.100
An emerging consensus is the notion that prolonged febrile seizures should be prevented in anyone who has had a previous prolonged febrile seizure. This can be achieved via abortive therapy using a benzodiazepine. The administration of rectal diazepam99,101,102 or buccal or nasal midazolam103,104 is rapidly effective, preventing a short febrile seizure from transforming to a prolonged one or to febrile status epilepticus and often preventing stressful emergency room visits.
1. National Institutes of Health. Febrile seizures: consensus development conference summary. Bethesda, National Institutes of Health 3:2, 1980.
2. Nelson KB, Ellenberg JH. Febrile Seizures. New York: Raven Press, 1981.
3. Commission on Epidemiology and Prognosis, International League Against Epilepsy. Guidelines for epidemiologic studies on epilepsy. Epilepsia. 1993;34:592-596.
4. Tsuboi T. Epidemiology of febrile and afebrile convulsions in children in Japan. Neurology. 1984;34:175-181.
5. Al-Eissa Y A. Febrile seizures: rate and risk factors of recurrence. J. Child Neurol. 1995;0:315-319.
6. Wolf SM, Carr A, Davis D. The value of phenobarbital in the child who has had a single febrile seizure: a controlled prospective study. Pediatrics. 1977;59:378-385.
7. Berg AT, Shinnar S, Hauser WA. Predictors of recurrent febrile seizures: a prospective study of the circumstances surrounding the initial febrile seizure. N Engl J Med. 1992;327:1122-1127.
8. Berg AT, Shinnar S, Doufsky AS. Predictors of recurrent febrile seizures: a prospective cohort study. Arch Pediatr Adolesc Med. 151:371-378.
9. Michon PE, Wallace SJ. Febrile convulsions: electroencephalographic changes related to rectal temperature. Arch Dis Child. 1984;59:371-373.
10. Berg AT. Are febrile seizures provoked by a rapid rise in temperature? Am J Dis Child. 1993;147:1101-1103.
11. Hauser W, Hersdorffer D. Epilepsy: Frequency, Causes and Consequences. New York: Demos, 1990.
12. Hauser WA, Kurland LT. The epidemiology of epilepsy in Rochester, Minnesota, 1935 through 1967. Epilepsia. 1975;16:1-166.
13. van den Berg BJ, Yerushalmy J. Studies on convulsive disorders in young children. I. Incidence of febrile and nonfebrile convulsions by age and other factors. Pediatr Res. 1969;3:298-304.
14. Ross EM, Peckham CS, West PB, Butler NR. Epilepsy in childhood: findings from the National Child Development Study. Brit Med J. 1980;280:207-210.
15. Nelson KB, Ellenberg JH. Predictors of epilepsy in children who have experienced febrile seizures. N Engl J Med. 1976;295:1029-1033.
16. Nelson KB, Ellenberg JH. Prognosis in children with febrile seizures. Pediatrics. 1978;61:720-727.
17. Forsgren L, Sidenvall R, Blomquist HK, Heijbel J. A prospective incidence study of febrile convulsions. Acta Paediatr Scand. 1990;79:550-557.
18. Offringa M, et al. Prevalence of febrile seizures in Dutch schoolchildren. Paediatr Perinat Epidemiol. 1991;5:181-188.
19. Stanhope JM, et al. Convulsions among the Chamorro people of Guam, Mariana Islands. Am J Epidemiol. 1972;95:299-304.
20. Pal DK. Methodologic issues in assessing risk factors for epilepsy in an epidemiologic study in India. Neurology. 1999;53:2058-2063.
21. Berg AT, et al. Childhood-onset epilepsy with and without preceding febrile seizures. Neurology. 1999;53:1742-1748.
22. Bethune P, et al. Which child will have a febrile seizure? Am J Dis Child. 1993;147:35-39.
23. Berg AT, et al. Risk factors for a first febrile seizure: a matched case-control study. Epilepsia. 1995;36:334-341.
24. Hodgkin AL, Katz B. The effect of temperature on the electrical activity of the giant axon of the squid. J Physiol. 1949;109:240-249.
25. Shibasaki K, et al. Effects of body temperature on neural activity in the hippocampus: regulation of resting membrane potentials by transient receptor potential vanilloid 4. J Neurosci. 2007;27:1566-1575.
26. Olson KR, et al. Seizures associated with poisoning and drug overdose. Am J Emerg Med. 1994;12:392-395.
27. Fukuda M, et al. Clinical study of epilepsy with severe febrile seizures and seizures induced by hot water bath. Brain Dev. 1997;19:212-216.
28. Schuchmann S, et al. Experimental febrile seizures are precipitated by a hyperthermia-induced respiratory alkalosis. Nat Med. 2006;12:817-823.
29. Dubé C, et al. Prolonged febrile seizures in the immature rat model enhance hippocampal excitability long-term. Ann Neurol. 2000;47:336-344.
30. Wallace RH, et al. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta1 subunit gene SCN1B. Nat Genet. 1998;19:366-370.
31. Escayg A, et al. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet. 2000;24:343-345.
32. Harkin LA, et al. Truncation of the GABA(A)-receptor gamma2 subunit in a family with generalized epilepsy with febrile seizures plus. Am J Hum Genet. 2002;70:530-536.
33. Kang JQ, et al. Why does fever trigger febrile seizures? GABAA receptor gamma2 subunit mutations associated with idiopathic generalized epilepsies have temperature-dependent trafficking deficiencies. J Neurosci. 2006;26:2590-2597.
34. Berkovic S, Scheffer I. Febrile seizures: genetics and relationship to other epilepsy syndromes. Curr Opin Neurol. 1998;11:129-134.
35. Kanemoto K, et al. Interleukin (IL)1beta, IL-1alpha, and IL-1 receptor antagonist gene polymorphisms in patients with temporal lobe epilepsy. Ann Neurol. 2000;47:571-574.
36. Virta M, et al. Increased frequency of interleukin-1beta (-511) allele 2 in febrile seizures. Pediatr Neurol. 2002;26:192-195.
37. Dubé C, et al. Interleukin-1 beta contributes to the generation of experimental febrile seizures. Ann Neurol. 2005;57:152-155.
38. Vezzani A, Baram TZ. New roles for interleukin-1 beta in the mechanisms of epilepsy. Epilepsy Curr. 2007;7:45-50.
39. Tan NC, et al. Genetic association studies in epilepsy: “the truth is out there.”. Epilepsia. 2004;45:1429-1442.
40. Duncan JS, et al. Adult epilepsy. Lancet. 2006;367:1087-1100.
41. Barone SR, et al. Human herpesvirus-6 infection in children with first febrile seizures. J Pediatr. 1995;127:95-97.
42. Zerr DM, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768-776.
43. Verity CM, et al. Febrile convulsions in a national cohort followed up from birth. I. Prevalence and recurrence in the first five years of life. Brit Med J. 1985;290:1307-1310.
44. Annegers JF, et al. Recurrence risk of febrile convulsions in a population-based cohort. Epilepsy Res. 1990;5:209-216.
45. Berg AT, Shinnar S. Complex febrile seizures. Epilepsia. 1996;37:126-133.
46. Annegers JF, et al. Factors prognostic of unprovoked seizures after febrile convulsions. N Engl J Med. 1987;316:493-498.
47. Camfield P, Camfield C. Febrile seizures. In: Shinnar S, Amir N, Branski D, editors. Childhood Seizures. Basel: Karger; 1995:32-38.
48. Scott RC, et al. Magnetic resonance imaging findings within 5 days of status epilepticus in childhood. Brain. 2002;125:1951-1959.
49. Scott RC, et al. Hippocampal abnormalities after prolonged febrile convulsion: a longitudinal MRI study. Brain. 2003;126:2551-2557.
50. Shinnar S, et al. How long do new-onset seizures in children last? Ann Neurol. 2001;49:659-664.
51. Verity CM, et al. Long-term intellectual and behavioral outcomes of children with febrile convulsions. N Engl J Med. 1998;338:1723-1728.
52. Berg AT, Shinnar S. Do seizures beget seizures? An assessment of the clinical evidence in humans. J Clin Neurophysiol. 1997;14:102-110.
53. Chang YC, et al. Working memory of school-aged children with a history of febrile convulsions: a population study. Neurology. 2001;57:37-42.
54. Baram TZ, Shinnar S. Do febrile seizures improve memory? Neurology. 2001;57:7-8.
55. Raol YS, et al. Epilepsy after early-life seizures can be independent of hippocampal injury. Ann Neurol. 2003;53:503-511.
56. Hirtz DG, et al. Febrile convulsions. In: Engel JJr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook, 3. Philadelphia: Lippincott-Raven; 1997:2483-2488.
57. Raspall-Chaure M, et al. Outcome of paediatric convulsive status epilepticus: a systematic review. Lancet Neurol. 2006;5:769-779.
58. Cendes F, et al. Atrophy of mesial temporal lobe structures in patients with temporal lobe epilepsy: cause or consequence of repeated seizures? Ann Neurol. 1993;34:795-801.
59. French JA, et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774-780.
60. Hamati-Haddad A, Abou-Khalil B. Epilepsy diagnosis and localization in patients with antecedent childhood febrile convulsions. Neurology. 1998;50:917-922.
61. Theodore WH, et al. Hippocampal atrophy, epilepsy duration, and febrile seizures in patients with partial seizures. Neurology. 1999;52:132-136.
62. Holtzman D, et al. Hyperthermia-induced seizures in the rat pup: a model for febrile convulsions in children. Science. 1981;213:1034-1036.
63. Morimoto T, et al. Electroencephalographic study of rat hyperthermic seizures. Epilepsia. 1991;32:289-293.
64. Baram TZ, et al. Febrile seizures: an appropriate-aged model suitable for long-term studies. Dev Brain Res. 1997;98:265-270.
65. Toth Z, et al. Seizure-induced neuronal injury: vulnerability to febrile seizures in an immature rat model. J Neurosci. 1998;18:4285-4294.
66. Chang YC, et al. Febrile seizures impair memory and cAMP-response element binding protein activation. Ann Neurol. 2003;4:706-718.
67. Chen K, et al. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron. 2003;39:599-611.
68. Heida JG, et al. Lipopolysaccharide-induced febrile convulsions in the rat: short-term sequelae. Epilepsia. 2004;45:1317-1329.
69. Heida JG, Pittman QJ. Causal links between brain cytokines and experimental febrile convulsions in the rat. Epilepsia. 2005;46:1906-1913.
70. Lemmens EM, et al. Gender differences in febrile seizure-induced proliferation and survival in the rat dentate gyrus. Epilepsia. 2005;46:1603-1612.
71. Kamal A, et al. Persistent changes in action potential broadening and the slow afterhyperpolarization in rat CA1 pyramidal cells after febrile seizures. Eur J Neurosci. 2006;23:2230-2234.
72. Germano IM, et al. Neuronal migration disorders increase susceptibility to hyperthermia-induced seizures in developing rats. Epilepsia. 1996;37:902-910.
73. Scantlebury MH, et al. Febrile seizures in the predisposed brain: a new model of temporal lobe epilepsy. Ann Neurol. 2005;58:41-49.
74. Bender RA, et al. Mossy fiber plasticity and enhanced hippocampal excitability, without hippocampal cell loss or altered neurogenesis, in an animal model of prolonged febrile seizures. Hippocampus. 2003;13:357-370.
75. Dubé C, et al. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129:911-922.
76. Jiang W, et al. The neuropathology of hyperthermic seizures in the rat. Epilepsia. 1999;40:5-19.
77. Baram TZ, et al. Is neuronal death required for seizure-induced epileptogenesis in the immature brain? Prog Brain Res. 2002;135:365-375.
78. Chen K, et al. Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits. Nat Med. 1999;5:888-894.
79. Chen K, et al. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat Med. 2001;7:331-337.
80. Magee JC. Dendritic lh normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci. 1999;2:508-514.
81. Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453-480.
82. Santoro B, Baram TZ. The multiple personalities of h-channels. Trends Neurosci. 2003;26:550-554.
83. Brewster AL, et al. Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform- and cell-specific manner. J Neurosci. 2002;22:4591-4599.
84. Brewster AL, et al. Formation of heteromeric hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in the hippocampus is regulated by developmental seizures. Neurobiol Dis. 2005;19:200-207.
85. Bender RA, et al. Enhanced expression of a specific hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epileptic hippocampus. J Neurosci. 2003;23:6826-6836.
86. Rosman PN. Evaluation of the child with febrile seizures. In: Baram TZ, Shinnar S, editors. Febrile Seizures. Academic Press; 2002:266-271.
87. American Academy of Pediatrics Provisional Committee on Quality Improvement, Subcommittee on Febrile Seizures. Practice parameter: the neurodiagnostic evaluation of the child with a first simple febrile seizure. Pediatrics. 1996;97:769-775.
88. Knudsen FU. Febrile seizures—treatment and outcome. Brain Dev. 1996;18:438-449.
89. Knudsen FU. Febrile seizures: treatment and prognosis. Epilepsia. 2000;41:2-9.
90. Baumann RJ, Duffner PK. Treatment of children with simple febrile seizures: the AAP practice parameter. American Academy of Pediatrics. Pediatr Neurol. 2000;23:11-17.
91. Berg AT, et al. An EEG should not be obtained routinely after first unprovoked seizure in childhood. Neurology. 2000;55:898-899.
92. Hirtz D, Berg A, Bettis D, et alfor the Quality Standards Subcommittee of the American Academy of Neurology; Practice Committee of the Child Neurology Society. Practice parameter: treatment of the child with a first unprovoked seizure. Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2003;60:166-175.
93. VanLandingham KE, et al. Magnetic resonance imaging evidence of hippocampal injury after prolonged focal febrile convulsions. Ann Neurol. 1998;43:413-426.
94. Frantzen E, et al. Longitudinal EEG and clinical study of children with febrile convulsions. Electroencephalogr Clin Neurophysiol. 1968;24:197-212.
95. Stores G. When does an EEG contribute to the management of febrile seizures? Arch Dis Child. 1991;66:554-557.
96. American Academy of Pediatrics: Committee on Quality Improvement, Subcommittee on Febrile Seizures. Practice parameter. Long-term treatment of the child with simple febrile seizures. Pediatrics. 1999;103:1307-1309.
97. Autret E, et al. Double-blind, randomized trial of diazepam versus placebo for prevention of recurrence of febrile seizures. J Pediatr. 1990;117:490-494.
98. Rosman NP, et al. A controlled trial of diazepam administered during febrile illnesses to prevent recurrence of febrile seizures. N Engl J Med. 1993;329:79-84.
99. Uhari M, et al. Effect of acetaminophen and of low intermittent doses of diazepam on prevention of recurrences of febrile seizures. J Pediatr. 1995;126:991-995.
100. Berg AT. Diazepam to prevent febrile seizures. N Engl J Med. 1993;329:2033-2034.
101. Rossi LN, et al. Short-term prophylaxis of febrile convulsions by oral diazepam. Acta Paediatr. 1993;82:99.
102. O’Dell C, et al. Rectal diazepam gel in the home management of seizures in children. Pediatr Neurol. 2005;33:166-172.
103. Lahat E, et al. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. BMJ. 2000;321:83-86.
104. Scott RC, et al. Intranasal midazolam for treating febrile seizures in children. Buccal midazolam should be preferred to nasal midazolam. BMJ. 2001;322:107.