Fallopian Tube
Anatomy, Histology, and Function of the Fallopian Tube
Approach to Examining Tubal Specimens
Bilateral Tubal Ligation for Sterilization
Salpingectomy for Tubal Ectopic Gestation
Salpingectomy (with or without Oophorectomy and/or Hysterectomy)
Introduction
As the fallopian tube is the intermediary between the ovary and the uterus, it is the seat of various interactions that culminate in a normally implanted pregnancy. Its multiple functions include conditioning of both gametes before fertilization, guiding their journeys before encounter, providing an appropriate chemical environment for fertilization, supplying nutriment to the fertilized ovum for its first few hours of life, and delivering it to the uterine cavity at the proper time for nidation. The exact processes by which these various mechanisms are accomplished are still rather poorly understood, partly because many of them vary significantly from species to species, so that the study of experimental animals does not always help to understand the situation in women. Recently, the fallopian tube has been implicated for its potential role in a more sinister pathologic process. A growing body of evidence strongly suggests that the distal fallopian tube is the site of origin for some proportion of high-grade pelvic serous cancers previously classified as primary ovarian or peritoneal malignancies. For a detailed discussion of this concept, see Chapter 25.
Anatomy, Histology, and Function of the Fallopian Tube
Anatomy
The fallopian tube is divided into four zones, which, extending distally to proximally, are the infundibulum, the ampulla, the isthmus, and the interstitial (or intramural) portions. The infundibulum is the distal end of the tube and forms the funnel-like expansion opening onto the peritoneal cavity, about 1 cm in length and diameter that ends in a variable number of irregular, fringe-like extensions, the fimbriae. Proximal to the infundibulum and making up about half of the length of the tube is the ampullary portion. The ampulla is narrower than the infundibulum and runs a tortuous course. The isthmus, which is 2–3 cm in length, has a narrower lumen and more muscular wall than the ampulla. The interstitial portion of the tube has a lumen with a simple, stellate, or almost circular cross section. In this segment of the tube, the muscle of the tubal wall merges with that of the myometrium.
Histology
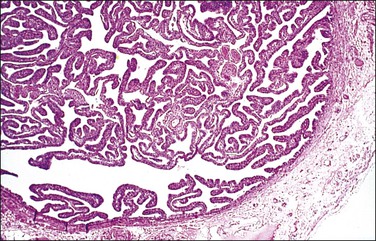
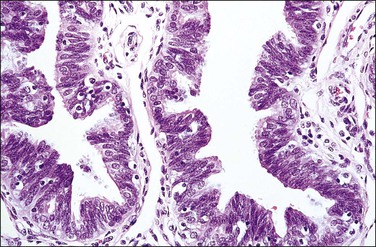
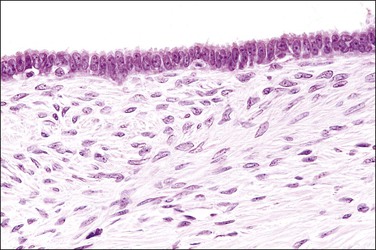
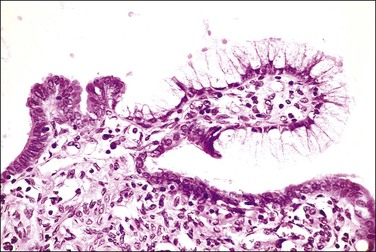
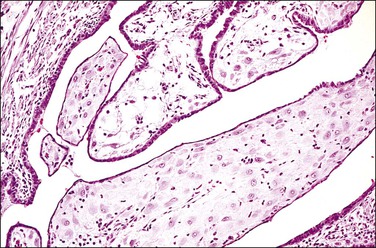
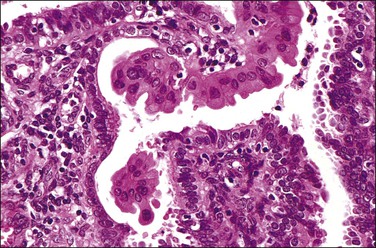
The fallopian tube is composed of a mucosal lining, a muscular layer, and an outer serosa. The mucosa consists of nonstratified epithelium and a sparse underlying fibrovascular lamina propria. The epithelium (Figure 21.1) comprises three cell types: ciliated cells, secretory cells, and intercalated (or peg) cells. The ciliated cells have a centrally placed nucleus with a perinuclear halo, a prominent terminal bar, and definable surface cilia. The nuclei of the secretory cells vary in position, depending on the stage in the menstrual cycle. The intercalated cells are secretory cells that have discharged their secretions with the result that the cell walls are collapsed around the nucleus. The ciliated component is the most prominent near the fimbrial end of the tube and the secretory cells are more numerous in the isthmus than they are in the ampulla.
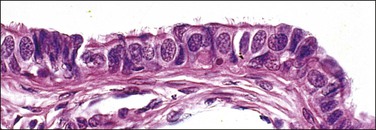
Figure 21.1 Normal tubal epithelium An admixture of secretory and ciliated cells constitute the majority of the cells.
The tubal epithelium shows well-defined histologic alterations in response to cyclic hormonal variations, which affect the height of the epithelial cells, rather than the number of cilia, as happens in other primates. In the proliferative phase of the cycle the estrogen predominance results in epithelial cells with increasing height whereas in the progesterone-dominated secretory phase, the cell height may be as little as half that seen in the first half of the cycle. Similarly, the height of both epithelial cell types is low in pregnancy. Oral contraceptives produce a similar appearance to those of pregnancy; the epithelial cells are relatively flat and show a lack of secretory activity, features that doubtless play some part in the effectiveness of the medication. In the late postmenopausal state, the epithelium becomes thin and atrophic (Figure 21.2).
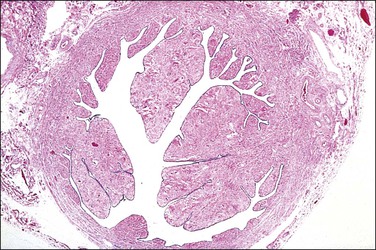
Figure 21.2 Atrophic tubal mucosa. The epithelium is thin and atrophic and the plical lamina propria is fibrotic.
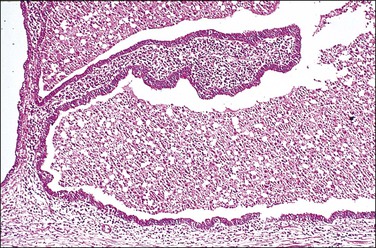
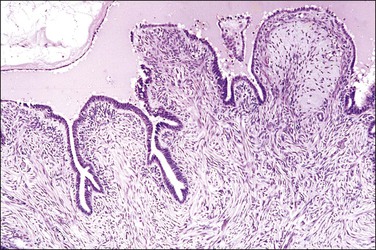
In cross section, at low-power magnification, the mucosa of the tubal ampulla (Figure 21.3) forms a complicated maze-like pattern of folds (the plicae) that branch but do not join. The epithelial surfaces of the plicae are apposed to one another so that even in the widest part of the tube the traversing ovum is not floating in a spacious lumen but is at all times nurtured by the ciliated and secretory epithelium with which it is in contact on all sides. These folds become less complex as the medial end of the tube is approached, becoming stellate in the isthmus (Figure 21.4) and forming an irregular, almost rounded, outline to the lumen in the interstitial part. In postmenopausal women, the plical stroma becomes fibrous and the plicae themselves club shaped, an appearance that may be mistaken for one sequela of infection, and the epithelium flattened.
Mucosal Epithelial Alterations
Secretory Cell Outgrowths and p53 Signatures
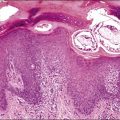
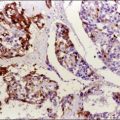
Expansions of morphologically unremarkable secretory cells, uninterrupted by ciliated cells, can occasionally be identified as discrete, linear foci in a background of the usual mixed ciliated and secretory cells populating the fallopian tube epithelium. Such expansions have been designated as secretory cell outgrowths (SCOUTs) when they comprise at least 30 or more secretory cells. The majority are associated with loss of expression of PAX2, a member of the pair box gene family, which is normally expressed in structures derived from the müllerian duct.1 The related p53 signature is also composed of secretory cells, but with the incremental changes of acquired p53 mutation, evidence of DNA damage, and relative predominance in the distal portion of the fallopian tube (Figure 21.5A and B).2 Neither SCOUTs nor p53 signatures exhibit significant proliferative activity (Figure 21.5C). Both are currently interpreted as early clonal expansions short of neoplastic proliferation.
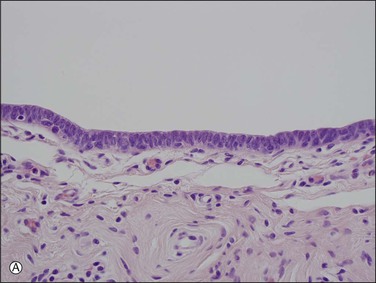
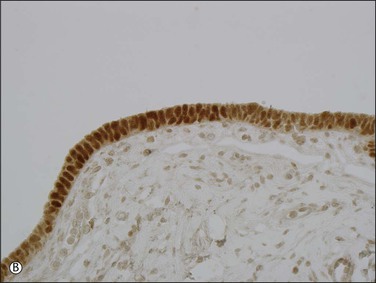

Figure 21.5 (A–C) The p53 signature is a histologically unremarkable stretch of tubal epithelium (A) that is associated with p53 mutations, exhibiting strong nuclear staining for p53 (B) and has a low MIB1 index (C).
Both SCOUTs and p53 signatures are candidate early steps toward high grade serous tubal carcinoma, as discussed in Chapter 25. The reader is cautioned, however, that the prospective cancer risk of women with isolated SCOUTs and/or p53 signature is unknown, so neither is a diagnostic entity that should be invoked in clinical practice. Fallopian tubes from women with high-grade pelvic serous carcinoma harbor a significantly higher frequency of SCOUTs than those from women whose tubes were removed for a non-malignant condition.1 Tubes in which serous tubal intraepithelial carcinoma is present are more likely to contain p53 signatures than those without; in some cases, a p53 signature may be found in direct continuity with intraepithelial carcinoma. In a given case of pelvic serous carcinoma, shared p53 mutations may be identified among p53 signatures, serous tubal intraepithelial carcinoma, and invasive tumor elsewhere in the pelvis.2–5
Other Mucosal Epithelial Proliferations
Another notable pattern of epithelial growth in the fallopian tube, which does not appear to be associated with malignancy, either in the tube or elsewhere, is characterized by an apparent ‘hyperplasia’ of the normal mucosal epithelial cells. Such areas are populated by a mix of ciliated and secretory cells, which appear stratified or are arranged as small tufts. These areas may be focal or more diffuse. The cells themselves may exhibit some nuclear atypia, but not to the degree found in intraepithelial carcinoma. Mitotic activity is generally minimal (Figure 21.6). This mucosal alteration may be seen in the setting of pregnancy, but can be found in the tubes of non-pregnant women as well.
Function
Ovum Transport
The ciliary action in the tube is toward the uterus and is under the influence of many mediators. Physiologic levels of prostaglandins F2α, E1, and E2 stimulate the ciliary activity as do β-adrenergic agonists; the latter effect is potentiated by estrogen and progesterone. Recent data have shown that progesterone affects the tubal ciliary beat frequency.6 Incubation with progesterone suppresses the beat frequency by 40–50% but estradiol has no effect. Cilia from the tubal ampulla beat significantly faster than those from fimbrial segments.
Approach to Examining Tubal Specimens
Bilateral Tubal Ligation for Sterilization
The most important aspect in the sectioning and preparation of the excised specimen for microscopic examination is that sufficient sections are made to show the complete cross section of the tube (Figure 21.7). An efficient method is to cut the fallopian tube into sections no more than 1–2 mm thick so that a single slide contains up to six sections. In this manner, even if some sections are embedded improperly or cut on a bias, at least a few should show the complete cross section, if in fact it is present. Any case where none of the pieces shows a complete cross section should be reported as incomplete (Figure 21.8). This can occur when the surgeon has removed arteries and fascia or only the tubal fimbriae. In these cases, it commonly happens that the clinician had difficulty definitively identifying the fallopian tube, even though this information was not transmitted to the pathologist. It is most important that an incomplete ligation be clearly reported in part for medicolegal consideration. The pathologist must be careful not to interpret a paratubal cyst as fallopian tube lumen; the latter has a much thicker wall with ample smooth muscle.
Salpingectomy (with or Without Oophorectomy And/or Hysterectomy)
The SEE-FIM Protocol
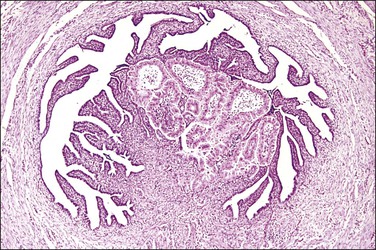
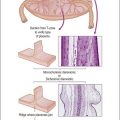
The majority of early serous carcinomas in the fallopian tube are detected microscopically rather than by gross examination, in the distal portion of the fallopian tube, in the fimbriae. Salpingectomy performed in high-risk patients, such as those undertaken prophylactically in BRCA mutant women, are a common setting for clinically and grossly occult disease. In an effort to facilitate a thorough examination of the distal fallopian tube a protocol for sectioning and extensively examining the fimbriated end (SEE-FIM) was developed (see also Chapter 35).6 According to this protocol, the entire fallopian tube is first well fixed to prevent exfoliation of the mucosal epithelial cells. The fimbriae are then amputated from the proximal tube and sectioned longitudinally into multiple sections to permit maximum exposure of the fimbrial mucosa. The remainder of the tube is cross sectioned at 2−3 mm intervals and the entire tube is submitted for histopathologic review (Figure 21.9).
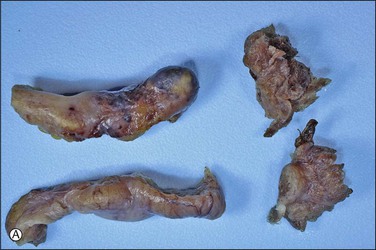
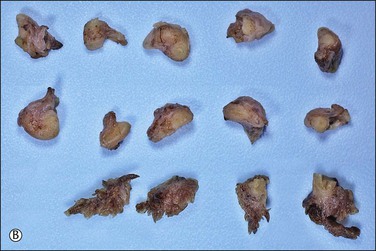
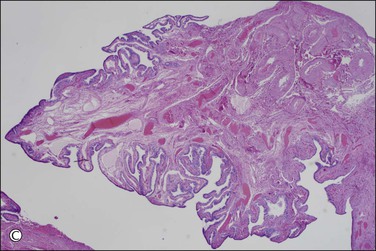
Figure 21.9 (A-C) The protocol for sectioning and extensively examining the fimbriated end (SEE-FIM protocol). Tubes are first fixed. The fimbriae are then amputated from the rest of the tube (A), sectioned longitudinally into multiple sections (B), and then submitted with the remainder of the tube, which has been cross sectioned at 2–3 mm intervals. Longitudinal sectioning permits maximum evaluation of the fimbrial mucosal epithelium (C, low-power magnification).
Indications for extensive tubal sampling are not yet clearly defined by clinical necessity, but should be considered in cases where it is desirable to maximize the likelihood of detecting early tubal cancer. Appropriate specimen types might include risk-reducing (prophylactic) salpingectomies; salpingectomies from women with a history of breast cancer; and salpingectomies in the setting of uterine, ovarian, or peritoneal disease. Some consensus is emerging from within the pathology community, with, for example, the Association of Directors of Anatomic and Surgical Pathology recommending an approach similar to SEE-FIM in prophylactically removed specimens, with an emphasis on longitudinal sectioning of the fimbriae.7 This group also recommends sampling fimbriae as part of processing of routine tubal specimens.
Given that the distal fallopian tube is the site most likely to harbor occult tubal malignancy, the suggestion has been made by some authors to submit the entire fimbriae for histopathologic review even in cases in which tubes are removed for benign disease.8 Currently, the incidence of occult carcinoma in the fallopian tube in the general population is unknown. However, with increasing attention focused on the distal tube, incidental tubal cancers are being discovered in cases otherwise unsuspected of harboring a malignancy.8 A standard clinical response to incidentally discovered occult in situ tubal cancer has not yet been formulated, but there is a possibility for identification of individuals who may benefit from increased surveillance, or even early disease intervention.
Non-Neoplastic Lesions
Inflammation of the Fallopian Tubes
Inflammatory disease resulting from infection of the fallopian tubes and adjacent ovary is an increasing problem. The investigation and treatment of women with the disease is demanding more and more in the way of time and other resources. Identification of the disease early in its natural history is important to enable treatment to be effected before the damage becomes extensive and the consequent surgery destructive.
Non-Granulomatous Salpingitis
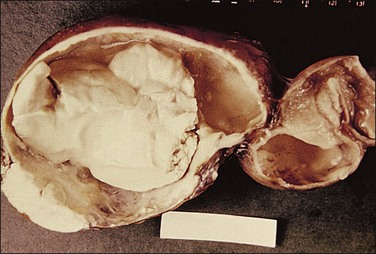
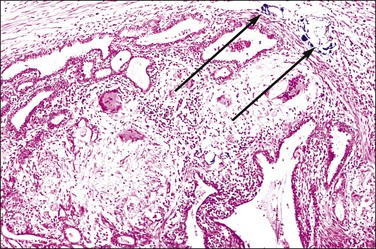
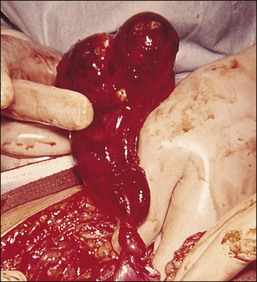
Non-granulomatous salpingitis is predominantly a disease of young, sexually active women, and 70% of those with the disease are under the age of 25. Other factors that have an influence on the development of salpingitis include the method of contraception, induced abortion, and instrumentation of the cervix. Among the infectious organisms responsible for salpingitis, Chlamydia trachomatis and Neisseria gonorrhoeae remain of paramount importance and are responsible for ascending salpingitis.9 Other causative microbial agents are the anaerobic bacteria (bacteroides, clostridia, and streptococci), Mycoplasma hominis, and Ureaplasma urealyticum, and miscellaneous organisms such as Haemophilus influenzae and group A streptococci. Bacterial vaginosis is a common concurrent disorder of women with acute salpingitis, and bacterial vaginosis microorganisms are commonly isolated from the upper genital tracts of patients with pelvic inflammatory disease.9 Salpingitis caused by Actinomyces israelii is associated with the presence of an intrauterine contraceptive device, and may result in the development of a tubo-ovarian abscess (Figure 21.10). Actinomyces-like organisms can be identified in the pus (Figure 21.11). In practice, however, microbiologic investigations often show that the cultured material is already sterile by the time of the investigation, or else there is a combination of organisms.
The spread of etiologic organisms from the lower to the upper genital tract is canalicular, through the cervical canal and endometrial cavity and then into the fallopian tubes. Blockage of this route, either by cornual resection of the fallopian tubes or by sterilization, reduces the risk of salpingitis.10 Salpingitis begins as a mucosal rather than a serosal infection.
Microscopic Features
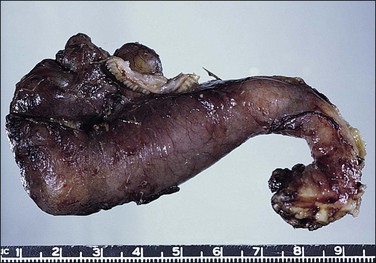
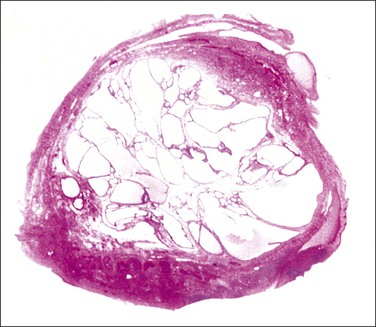
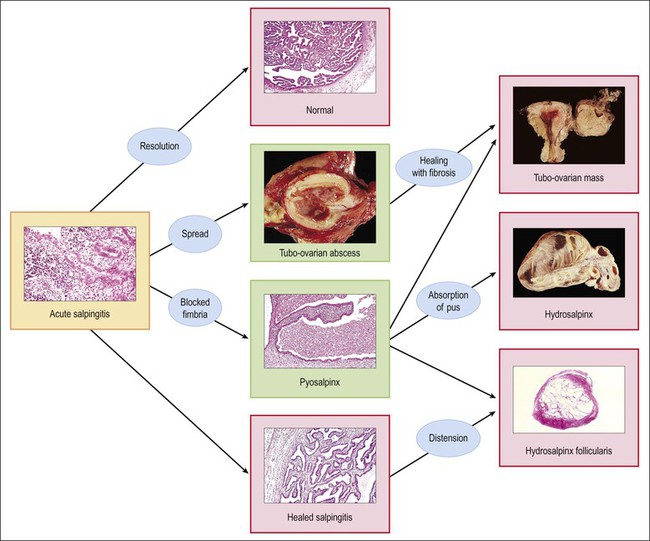
The plicae are greatly swollen and densely infiltrated by neutrophils (Figure 21.12). The epithelial cells soon lose their cilia and the epithelium is shed in severe disease. The lumen contains pus (Figure 21.13). As the disease progresses to chronic salpingitis (Figures 21.14 and 21.15), the inflammatory infiltrate consists predominantly of plasma cells and then lymphocytes. The progression from acute to chronic non-granulomatous salpingitis may take several courses. If the fimbrial end of the tube remains patent, a chronic interstitial salpingitis may ensue, in which case the tube is thickened and the plicae fuse to form epithelial-lined cysts, which are the hallmark of chronic salpingitis. ‘Follicular salpingitis’ (Figure 21.16) rather confusingly refers to the epithelium-lined spaces rather than lymphoid collections. The inflammatory process may eventually become quiescent, leaving only the architectural sequelae of chronic salpingitis (Figure 21.17). Severe inflammation in the tube may spread to the adjacent ovary, resulting in a tubo-ovarian abscess (Figure 21.18). Occlusion of the fimbrial end of the tube prevents release of the tubal contents, so that a pyosalpinx may result (Figure 21.19). In pyosalpinx, the lumen is filled with pus (Figure 21.20) and the wall is thinned, although the attenuation is commonly less marked than in hydrosalpinx. Acute and chronic inflammatory cells infiltrate the plicae and wall. As the exudate is reabsorbed into the tubal wall concurrently with the quiescence of the inflammatory process, clear fluid replaces this pus and a hydrosalpinx results (Figures 21.21 and 21.22). In hydrosalpinx, the epithelium is generally thin and non-ciliated, although a few areas of morphologically normal cells may be seen. The wall of a hydrosalpinx is markedly thinned, with attenuation of smooth muscle and plicae. Usually, by this advanced stage in the disease process, there is little, if any, residual inflammatory infiltrate in the tissues of the wall. From a practical point of view, hydrosalpinx with tubo-ovarian adhesions is commonly misdiagnosed as ovarian ‘serous cystadenoma’ since it commonly presents as an adnexal mass. A careful microscopic examination identifying the muscular layer is diagnostic for hydrosalpinx. A small hydrosalpinx in which there is plical conglutination will have a ‘honeycomb’ cut surface and is termed a ‘hydrosalpinx follicularis’ (Figure 21.23). The relations among these inflammatory changes are shown in Figure 21.24.
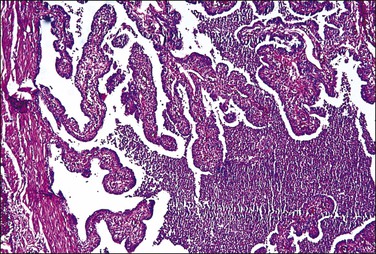
Figure 21.13 Acute salpingitis. The lumen is filled with pus and the plicae are engorged and inflamed.
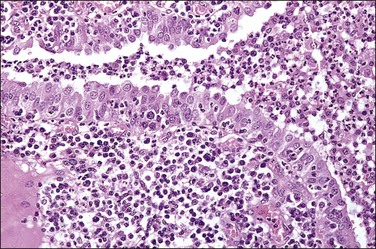
Figure 21.14 Chronic salpingitis. In this early stage of the disease, the plicae are infiltrated by plasma cells and lymphocytes and there is pus in the lumen. The epithelium is intact, although containing inflammatory cells.
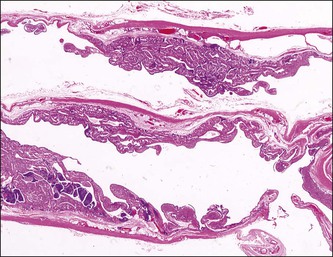
Figure 21.15 Chronic salpingitis. The infiltrate is now predominantly lymphocytic, with germinal centers.
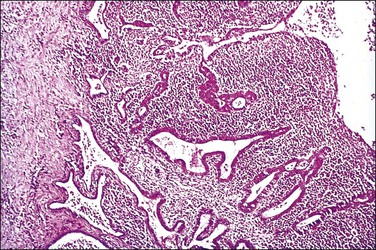
Figure 21.16 Chronic salpingitis. The plicae are partly fused, forming separate epithelium-lined spaces or ‘follicles’ separate from the central lumen (‘follicular salpingitis’). Inflammation is still active.
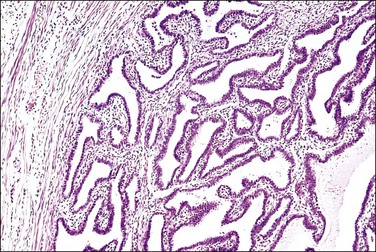
Figure 21.17 Chronic salpingitis, healed. The inflammation has subsided and is near absent, but the plicae remain fused by fibrosis.

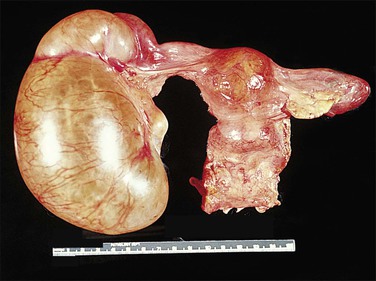
Figure 21.18 Tubo-ovarian abscess. The central mass is the ovary, which contains an abscess. Its cavity communicates with pus in the lumen of the tube (arrow).
Granulomatous Salpingitis
Granulomatous salpingitis is nearly always tuberculous in origin. All age groups may be affected but the pattern of the disease has changed over the last few decades. Until the 1970s, tuberculosis of the female genital tract in the developed world affected mainly women of childbearing age but more recently the majority of cases are in postmenopausal women.11 Whereas previously the main complaint of these women was infertility, tuberculosis accounting for about 40% of all cases of infertility,12 the common symptoms are now pain and bleeding.11 When tuberculosis affects the female genital tract, the tube is affected in nearly all cases and involvement of the endometrium is always secondary to it. The pelvic disease is, in turn, secondary to primary disease in the lungs or bowel, spreading to the tubes by hematogenous and lymphatic routes, respectively.
Gross Features
Tuberculous salpingitis is nearly always bilateral. The tube is thickened and congested and there are serosal adhesions. The fimbrial end of the tube is usually patent and the lumen may contain caseous debris (Figure 21.25). The wall is thickened and foci of caseation may be recognized within the tissue of the wall.
Microscopic Features
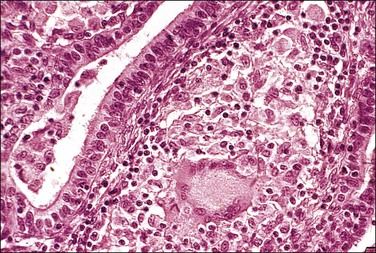
The hallmark of tuberculous salpingitis histologically is the epithelioid cell granuloma that is situated in the lamina propria of the plicae (Figures 21.26 and 21.27) and, rarely, within the muscular wall. Caseation may or may not be present, but is more often seen in older women. A surrounding, dense lymphocytic infiltrate is found, both in the plical lamina propria and in the muscle, the latter is usually more conspicuous. A frequent finding is the presence of striking epithelial proliferation of the endosalpinx, a feature that may cause confusion with carcinoma (see later). Schaumann bodies are occasionally seen in tuberculous salpingitis (Figure 21.26). Although typically associated with sarcoidosis, these rounded, concentrically laminated calcified bodies may be seen in most forms of epithelioid cell granuloma. The histologic suggestion of tubal tuberculosis is confirmed if acid-fast bacilli are found in the sections, but this is achieved in only 1% of cases in which the culture proves positive. On a practical basis, staining is not useful. With the development of molecular pathology, polymerase chain reaction-based tuberculosis-specific bacilli DNA can be detected in 48 hours.13 The differential diagnosis of tuberculous salpingitis in Europe is sarcoidosis, Crohn disease, and foreign-body granuloma. Worldwide, other conditions include schistosomiasis, blastomycosis, coccidioidomycosis, histoplasmosis, and enterobiasis. A positive tissue diagnosis of tuberculosis is often not possible and the decision to treat the patient must rest on the degree of clinical suspicion.
Salpingitis Isthmica Nodosa
Pathology
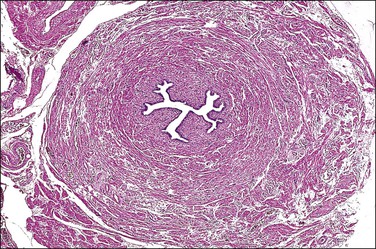
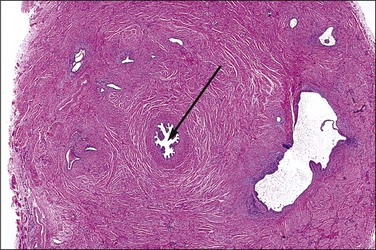
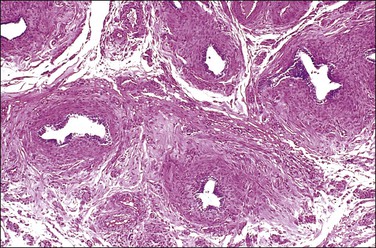
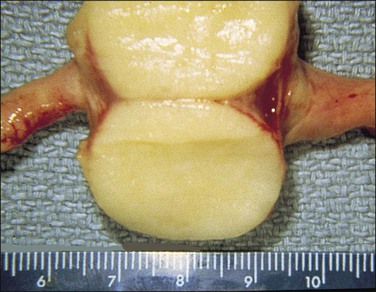
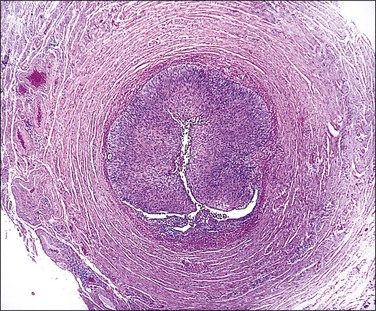
SIN may be recognized grossly as a rounded, firm swelling, up to 2 cm in diameter, at the isthmic end of the tube (Figure 21.28), often merging with the cornual extremity of the uterus. The nodules are often bilateral and, occasionally, there may be more than one swelling on each tube. Most cases, however, cannot be detected macroscopically. The histologic appearances are striking and consist of a thickened wall due to hypertrophied musculature with epithelial-lined channels running between the muscle bundles and reaching close to the serosa (Figures 21.29 and 21.30). The epithelium lining these spaces is of normal, tubal type. The central lumen is always recognizable and the additional channels communicate with it and with each other, but not with the peritoneal cavity.
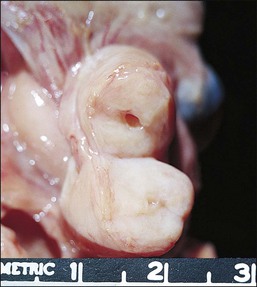
Figure 21.28 Salpingitis isthmica nodosa. The firm, round, isthmic nodule is bisected to demonstrate the central lumen.
Histogenesis
The histogenesis of SIN remains unknown. Three potential mechanisms include:
Tubal Pregnancy
Etiology and Pathogenesis
Any factor that impairs the tube’s ability to transport the fertilized ovum will predispose to tubal implantation of the ovum. Hence, congenital tubal abnormalities, failed tubal ligation, reconstructive tubal surgery, SIN, and, most importantly, postinflammatory tubal damage are all associated with an increased incidence of tubal pregnancies.14
Natural History
Tubal Abortion.
A high proportion of tubal pregnancies abort at an early stage and may be expelled from the fimbrial end of the tube. This is invariably the case if implantation has been fimbrial or plical, simply because these sites offer insufficient tissue for adequate placentation, but it is also seen with mural implantation. There is often intramural and intraluminal hemorrhage and subsequent fetal death. Following abortion, degenerating chorionic villi may be retained in the tube as so-called ‘chronic ectopic pregnancy’ or they may be expelled via the uterus or be gradually absorbed. Hyalinized ghost villi may be identified in the tube as an incidental finding many months later.15
Tubal Rupture.
Tubal rupture complicates about 50% of cases of tubal pregnancy and appears to be due partly to the limited distensibility of the tube and partly to transmural trophoblastic invasion with penetration of the serosa. It is particularly likely to occur when the implantation is isthmic, because of this area’s limited distensibility. Rupture is usually acute and is accompanied by intraperitoneal hemorrhage and the clinical features of an acute abdomen. Less commonly, there is a slow leakage of tubal contents and blood from the tube, which results in a gradually enlarging peritubal hematoma with dense adhesions between the tube and surrounding structures such as omentum and intestines. Occasionally, the ureters obstruct as a result of involvement in this peritubal mass. Although tubal rupture usually results in fetal death, the fetus occasionally retains sufficient attachment to its blood supply to maintain its viability. The trophoblast grows out through the rupture site and forms a secondary placental site in the abdomen or broad ligament. A secondary abdominal pregnancy of this type can occasionally proceed to term.
Pathology
Tubal Changes.
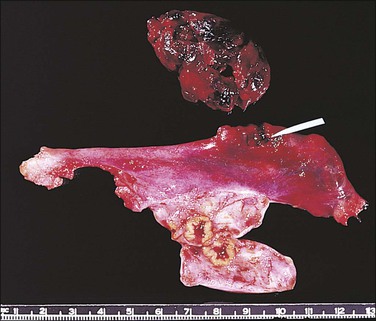
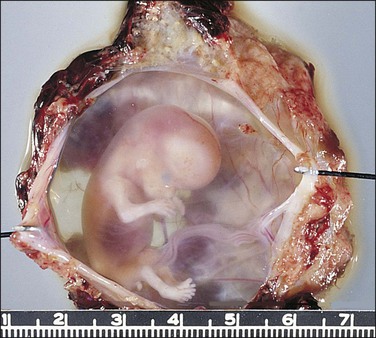
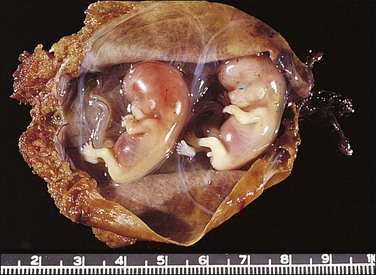
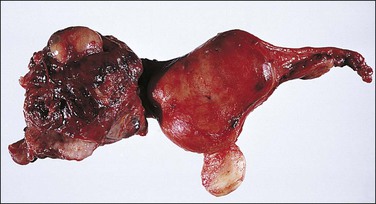
The fallopian tube, as received by the pathologist after salpingectomy, can show a range of appearances that vary with the site of nidation, viability of the fetus, duration of pregnancy, and presence or absence of rupture. In typical cases the tube is focally or generally distended while the peritoneal surface is congested and sometimes inflamed (Figure 21.31). The fimbrial ostium can be occluded by blood clot or blood may be oozing from the ostium. If rupture has occurred, blood clot and placental tissue are sometimes seen protruding through the rupture site (Figure 21.32), and blood clot may envelop the tube. On opening the tube a complete amniotic sac and fetus is occasionally seen (Figures 21.33 and 21.34). More commonly, the lumen contains only fresh and old blood clot (Figure 21.35).
Histologic examination is usually required to confirm the diagnosis of tubal gestation (Figure 21.36). A critical aspect is determination of the tissue to be submitted for microscopic examination. In nearly all cases, tissue within the blood clot will contain chorionic villi. Occasionally, they will be attached to the tubal wall. The villi may appear fully normal, but more often show degenerative change such as fibrosis or hydropic swelling. Dysmorphic changes, associated with chromosomal anomalies or molar change (e.g., triploidy), are only rarely seen as these do not constitute the cause for the pregnancy loss. In some cases many sections of the blood clot have to be examined before placental villi are seen. In the rare case no residual villous tissue will be found, the only detectable abnormalities are the presence of inflammatory debris and nonspecific granulation tissue. Even in such circumstances, however, an implantation site can often be identified by the presence of extravillous trophoblastic cells that have infiltrated the tubal wall and invaded the vascular spaces. Curiously, tubal tissue more than a short distance from the region of the ectopic pregnancy may show no abnormality other than a minor degree of nonspecific inflammation. It is important to take a block from the fallopian tube medial to the implantation site to assess the presence or absence of pre-existing salpingitis, usually manifest as plical adhesions, as this offers not only some pointer as to a likely cause of the present ectopic, but is useful in the further management of the patient. Salpingitis is usually bilateral and a second ectopic in the contralateral tube is more likely in such patients (who frequently end up having some form of assisted conception).
Laparoscopic Salpingostomy.
Although salpingectomy offers almost a 100% cure, laparoscopic methods are widely used that not only prevent maternal hemorrhage but also allow rapid recovery, preserve fertility, and reduce costs.16 Linear salpingostomy is now the standard laparoscopic operation when an ectopic pregnancy is unruptured but measures more than 4 cm by ultrasound. The products of conception are removed after an incision is made along the bulging antimesenteric border of the tube. The specimen the pathologist receives consists mainly of blood clot but frequently contains chorionic villi and trophoblastic fragments, enabling the diagnosis to be confirmed.
Uterine Changes.
In tubal pregnancies the uterine endometrium undergoes decidual change to a degree similar to that found in an intrauterine pregnancy in about 45% of cases.14 The endometrial glands are hypersecretory and an Arias-Stella change is seen focally in 60–70% of cases showing gestational changes. These appearances can, however, occur in any type of pregnancy and are not a specific feature of an ectopic gestation. In contrast to intrauterine pregnancies, curetted material shows little necrosis or inflammation. Indeed, this often is an important clue to the presence of an ectopic pregnancy. Depending on the time interval between fetal demise and curettage, the endometrium may show relatively poorly formed secretory changes and be inactive or even proliferative. In evaluating a curettage or biopsy specimen as part of a clinically suspected ectopic pregnancy, we have never personally encountered the situation where there was a documented pregnancy in the fallopian tube and chorionic villi were simultaneously found in the endometrial cavity. However, the pathologist must always be aware that villi are easily dislodged during tissue processing and may be artifactually introduced as contaminants from another case. Clues as to this possibility include position of the villi relative to other tissue fragments, lack of implantation site in the maternal tissues, and discordance between villous maturity and clinically suspected ectopic gestational age.
Cysts
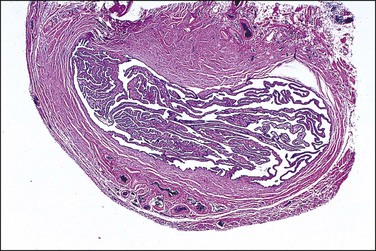
Cysts lying alongside the fallopian tube are referred to as paratubal cysts (Figure 21.37). They can be classified based on their origin as müllerian (paramesonephric), wolffian (mesonephric), or mesothelial. These subtypes are not always diagnostic at the time of presentation, because of either uncertain anatomic relationships or distortion of the wall and lining by dilation.
Paramesonephric Cysts
Paramesonephric cysts, when small, tend to be more laterally situated than those of mesonephric origin. The hydatid of Morgagni, usually seen as a cyst on a pedicle arising from the fimbria, is the most common example of a paramesonephric cyst. Others are found in close proximity to the tube or on its subserosal aspect. Paramesonephric cysts, often also termed ‘broad ligament cysts,’ are lined by a single layer of columnar cells that may be ciliated or non-ciliated (Figures 21.38 and 21.39). Smooth muscle is often present in their wall but is less prominent than in the mesonephric variety. Distinction between the two types of cyst is not always possible. Larger paramesonephric cysts may have papillary excrescences arising from the internal surface (Figure 21.40).
Mesonephric Cysts
The mesonephric remnants are the epoophoron and paroophoron, which continue as vestigial tubular structures between the tube and ovary (rete ovarii), passing medially toward the body of the uterus, to enter it at about the level of the internal cervical os and pass anterolaterally in the cervix as Gartner’s duct. Non-neoplastic, noncystic mesonephric remnants are universally present between the tube and the ovary and are seen as a collection of thick-walled tubular structures lined by cuboidal epithelium with smooth muscle in their walls (Figure 21.41). These remnants may become cystic at any point along their course, so that mesonephric cysts may be found within the mesosalpinx and broad ligament or they may be pedunculated and situated just lateral to the ovary (Kobelt’s cyst). Typically, the mesonephric cyst is lined by a single layer of epithelium that is of low columnar or cuboidal, non-ciliated type. Smooth muscle may be prominent in the wall of these cysts, often together with dense connective tissue and elastic fibers.
Mesothelial Inclusion Cysts
Paratubal cysts lined by mesothelial cells can demonstrate a variety of morphologic appearances and exhibit a range of sizes. The cysts may be multilocular or unilocular, the latter of which are sometimes referred to as simple cysts. Their thin walls are composed of fibrous tissue. The lining cells are generally flattened or cuboidal and ciliated cells are absent. Transitional, or müllerian, metaplasia can also be observed within surface inclusion cysts.
Metaplasias and Rests
Mucinous Metaplasia
This is an uncommon finding in which mucinous epithelium, exhibiting either an endocervical or gastrointestinal phenotype, replaces areas of tubal epithelium (Figure 21.42). Tubal mucinous metaplasia may occur in women with Peutz–Jeghers syndrome and has been reported in women with both ovarian and cervical mucinous tumors; this phenomenon may be associated with a mutation in the tumor suppressor gene STK11.17 Tubal metaplasia in the setting of multifocal mucinous metaplasia and neoplasia of the female genital tract has also been described in women without Peutz–Jeghers syndrome.18 The histogenesis of these multifocal mucinous lesions is unclear.
Endometriosis and Endosalpingiosis
Uterine endometrium is found in the interstitial and isthmic segments of the fallopian tube (Figure 21.43) in up to 25% and 10% of women, respectively. This change is identified both as an incidental finding in hysterectomy specimens and, more significantly, in cornual resections for infertility. The lesion represents a shift of the junction between endometrium and fallopian tube mucosa into the fallopian tube. It may be considered a normal morphologic variation even though it is often called ‘endometriosis’ or ‘endometrial colonization.’ This phenomenon, which may be related to the microenvironment adaptation, is often encountered in histologic examination of the tubal proximal stump, usually 1–4 years after tubal ligation. Endometrial colonization can also be caused by complete occlusion of the fallopian tube. It accounts for 15–20% of cases of infertility and may be associated with tubal pregnancy.19
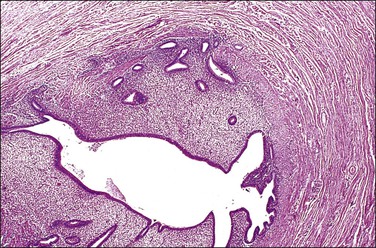
Figure 21.43 Endometrial replacement (‘endometrialization or endometrial colonization’). Endometrial glands and stroma replace the tubal mucosa in the isthmus.
Endosalpingiosis involving the tube and paratubal tissue is morphologically identical to that found elsewhere in the peritoneum. The glandular deposits of benign tubal epithelium can be found on the serosal surface of the tube and in the mesosalpinx. Not infrequently, psammoma bodies are present in association with the epithelium. Occasionally it is identified in women who also have endometriosis, suggesting a common etiology in some cases. A thorough discussion of both endometriosis and endosalpingiosis can be found in Chapter 22.
Transitional Metaplasia
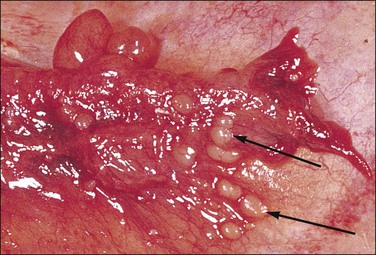
Walthard rests are extremely common, small collections of transitional cells, rarely more than 1 mm in diameter, situated immediately beneath the tubal serosa (Figures 21.44 and 21.45). Grossly, Walthard rests are clear to tan-white soft nodules, usually less than 1 mm in diameter (Figure 21.46). The cells are of a rather nondescript type but some show longitudinal grooves in the nuclear membrane, resembling the appearance seen in Brenner tumor of the ovary. There is speculation that the cells of the Walthard rest and Brenner tumor arise in the same way, by transitional cell metaplasia of the serosal mesothelium. They may be solid or cystic. Walthard rests are of no significance whatsoever, apart from the importance of being recognized grossly for what they are and not mistaken clinically for pelvic tuberculosis, endometriosis, or disseminated tumor.
Compared with the very common transitional cell metaplasia of the serosa that Walthard rests represent, transitional cell metaplasia of the mucosa is extremely rare. It is likely that this is the same change that is described as ‘reserve cell metaplasia’ and may serve as a possible source of tubal transitional cell carcinomas.20
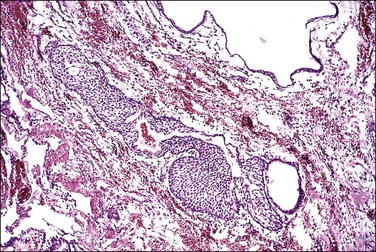
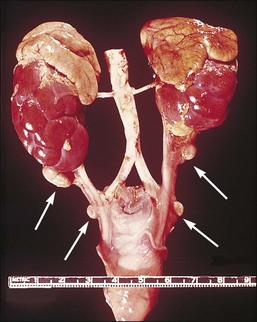
Adrenal Rest
An encapsulated collection of adrenal cortical cells is encountered as 1–3 mm yellow nodules in the broad ligament or mesosalpinx in as many as one-fourth of women (Figure 21.47). Also termed ‘Marchand rest,’ the heterotopic adrenal cells are identical to those of the adrenal cortex and are arranged in cords mimicking the zona fascicularis of the latter (Figure 21.48). Benign and malignant tumors may arise from them.
Pseudodecidual Change (Ectopic Decidua)
The stromal cells of the fallopian tube lamina propria and subserosa readily undergo pseudodecidual change (Figure 21.49). It is seen in about one-third of salpingectomy specimens containing ectopic pregnancies, and in 5–8% of tubal segments excised for sterilization performed during cesarean section or in the immediate postpartum period.19
Torsion of the Fallopian Tube
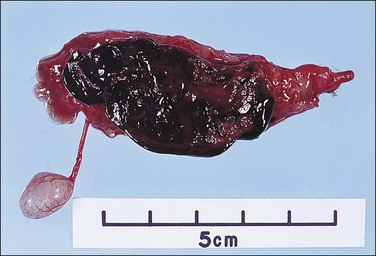
The fallopian tube usually undergoes torsion with the ovary. Both become twisted together, often because the ovary is enlarged. However, torsion may affect either organ independently and the tube is particularly at risk if it is diseased, as with a hydrosalpinx. The torsed tube is swollen and dark red-blue (Figure 21.50). Microscopy shows marked congestion initially (Figure 21.51), followed by infarction.
Prolapse of the Fallopian Tube
Tubal prolapse occurs occasionally after a hysterectomy, especially with vaginal hysterectomy. On clinical examination, a lesion simulating granulation tissue is seen at the vaginal apex. A misdiagnosis of papillary adenocarcinoma may happen if the tubal plicae and their lining of bland epithelium are not recognized (see Chapter 5).
Epithelial Proliferation Associated with Salpingitis
Definition
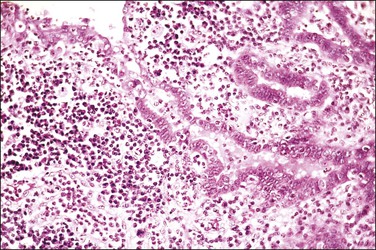
Reactive hyperplasia of tubal epithelium is associated with salpingitis. It may be mistaken for carcinoma since the salpingitis may present as pseudocarcinomatous hyperplasia.21
Microscopic Features
This change results in the formation of multiple small glandular structures, often arranged in a highly complex pattern, amid inflamed, often edematous, tubal plicae (Figures 21.52–21.54). The complexity of the architectural pattern is compounded by the fusion of adjacent plicae, resulting in a striking back-to-back pseudoglandular pattern or a sieve-like pattern.21 Epithelial stratification is often present and there may be loss of nuclear polarity. Nuclear atypia is of a mild to moderate degree only. Nucleoli are prominent in only half of the cases. Mitotic figures are rarely observed and these are normal. Moderate to marked chronic inflammatory changes are, of course, always present.
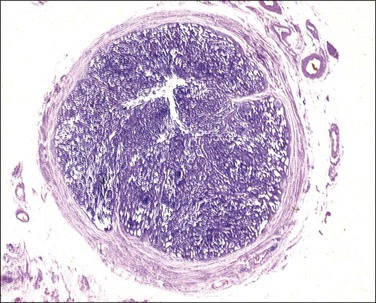
Figure 21.52 Epithelial proliferation associated with salpingitis. The diameter of the tube is greatly increased by what appears to be solid tissue.
Tumors of the Fallopian Tube
Primary tubal neoplasms, most of which are malignant, are uncommonly diagnosed preoperatively. A modification of the World Health Organization (WHO) classification of fallopian tube tumors is shown in Table 21.1.19
Benign Tumors
Adenomatoid Tumor
Pathology
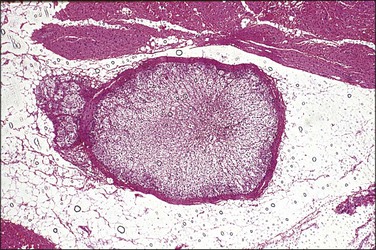
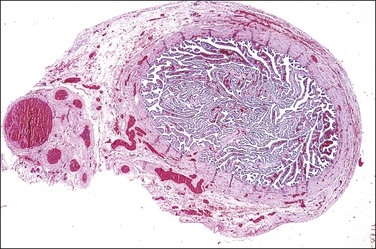
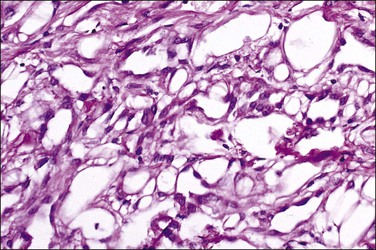
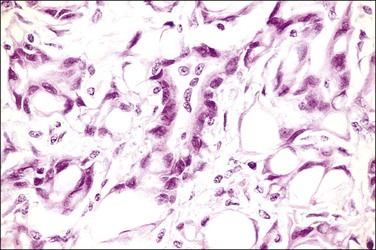
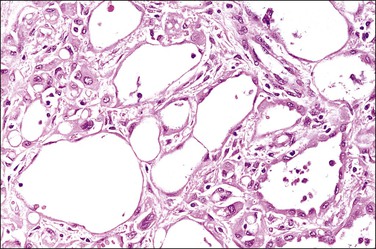
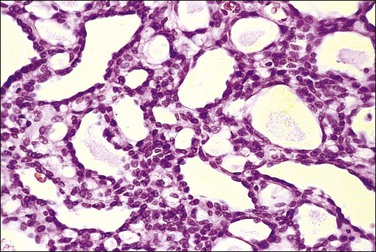
The adenomatoid tumor appears as a round or oval nodule, usually 1–3 cm in diameter, distending the tube (Figure 21.55). It is usually subserosal and in the outer wall, although it may be confined to the endosalpinx or spread throughout the wall. The neoplasm shows numerous slit-like, ovoid, and round spaces of various sizes, separated by bands of connective tissue (Figure 21.56). The spaces are lined by a single layer of low cuboidal or flattened cells with eosinophilic cytoplasm and oval nuclei (Figures 21.57–21.59). The tumor is not encapsulated and infiltrates the muscle of the tubal wall at its margins.
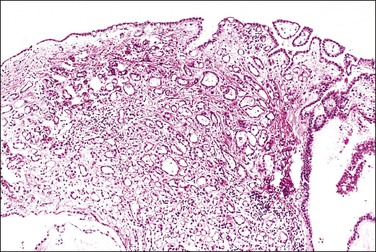
Figure 21.56 Adenomatoid tumor, which at low-power magnification discloses numerous slit-like, ovoid, and round spaces of various sizes, separated by bands of connective tissue.
The main interest in these clinically unimportant tumors is in their histogenesis. Suggestions of their origin have included mesonephric, vascular, lymphatic, müllerian, and mesothelial derivation. Electron microscopic and, more recently, immunohistochemical studies indicate that it is of mesothelial origin.
Adenofibroma
Once considered to be extremely rare, adenofibromas involving the fallopian tube have been recognized with greater frequency recently, due largely to the implementation of protocols that extensively sample the fimbriated end of the fallopian tube, where the overwhelming majority of tubal adenofibromas are located.22 Most are present as microscopic foci; those forming larger masses are uncommon. Microscopically, they share features with ovarian adenofibromas, both morphologically and immunohistochemically, with the stromal component exhibiting positive staining for inhibin and CD10.22
Other Benign Tumors
Of the rare benign tumors, about 50 mature cystic teratomas (dermoid cysts) have been reported, nearly all as incidental findings.24 They resemble grossly and histologically the ovarian mature cystic teratoma and are thought to arise from misplaced germ cells, in this case extragonadal.
Rare examples of placental site nodule25,26 and placental site trophoblastic tumor27 have also been reported in the fallopian tube. Rarely, papillomas, ranging up to 3 cm in diameter, may occur in the tubal lumen.
Metaplastic papillary tumors are rare lesions that have been identified as incidental findings in tubal segments excised postpartum for sterilization.19 They are composed of papillary nests of budding, proliferating cells with abundant eosinophilic cytoplasm (Figures 21.60 and 21.61). Rare mitotic figures may be seen. Whether these are genuine neoplastic lesions or the result of metaplasia and proliferation occurring during pregnancy is not known.
Very rare neoplasms include neurilemmoma, lipoma, chondroma, lymphangioma, ganglioneuroma, and hemangioma.19
Borderline Tumors
Compared with their ovarian counterparts, borderline tumors of the fallopian tube are uncommon, with limited experience about their clinical behavior. Most of the very limited number are serous and a very rare case is endometrioid.28–30 Mucinous borderline tumors have been reported; when such cases are encountered, secondary spread to the tube from an undetected appendiceal mucinous tumor should always be excluded first.
The serous borderline tumor of the fallopian tube shows formation of papillary projections with focally prominent epithelial stratification and atypia. None has recurred, suggesting that these extremely uncommon tumors can be managed conservatively.30
Malignant Tumors
Primary Carcinoma of the Fallopian Tube
General Features
Primary fallopian tube carcinoma has historically been considered to be a relatively uncommon neoplasm, with an estimated incidence of 0.41 per 100,000 (approximately 1/150, as common as ovarian cancer).31 This assumption has been due, in part, to the stringent criteria classically used for ascribing a site of origin to the fallopian tube, including all of the following: the bulk of the tumor must be present in the tube; any tumor present in the ovary, peritoneum, or endometrium must be of smaller quantity than that in the tube; and early cancer should be identified in the tube. There is compelling and mounting evidence, however, to suggest that a significant proportion of cases of high-grade serous carcinoma classified as either ovarian or peritoneal in origin using conventional classification schemes have an origin in the distal fallopian tube. See Chapter 25 for a detailed discussion of this concept.
Clinical Features
The mean age of patients with fallopian tube carcinoma is about 60 years (age range, 26–86). Women who develop tubal carcinomas in the setting of a BRCA mutation present at a slightly younger mean age than those without.32 Tubal carcinomas are uncommon in women under 40 years old.33,34 Nulliparity, previous PID, and hormone replacement therapy may be associated with increased risk of developing tubal carcinoma, while oral contraceptive use may reduce the risk.35,36 Early tubal carcinomas may be discovered in risk-reducing, prophylactic salpingectomies from women with BRCA-1 or BRCA-2 mutations, in specimens with coexisting high-grade serous carcinomas in other pelvic sites (ovary, peritoneum, uterus), and, very rarely, as incidental findings in specimens removed for non-malignant indications.
The correct diagnosis is rarely made preoperatively. The most common presenting signs and symptoms, including serosanguineous vaginal discharge, pain, and a palpable pelvic mass, are nonspecific and are seen in only a minority of women with the disease. ‘Hydrops tubae profluens,’ the classically described finding of colicky lower abdominal pain relieved by an intermittent serous, watery, vaginal discharge, occurs in fewer than 10% of patients.32 Tumor may be identified in cervical cytology specimens or endometrial biopsies or curettages, but the findings are nonspecific for a tubal origin.
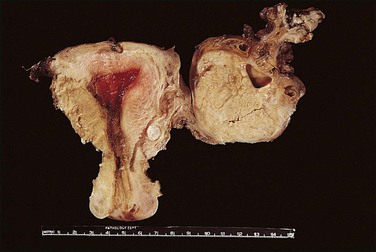
Gross Features
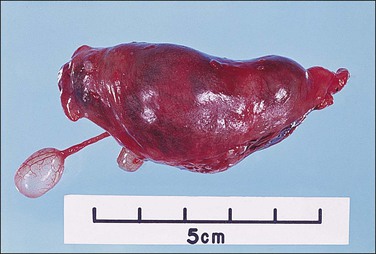
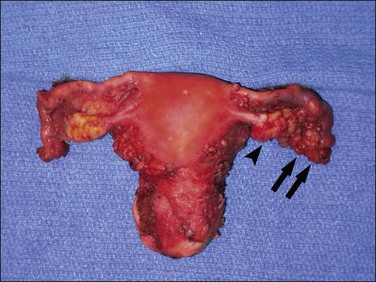
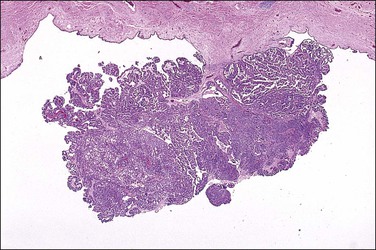
Tubal carcinoma can exhibit a variable gross appearance. The classic description is that of an enlarged, distended, tumor-filled tube with fused fimbriae, resembling a sausage or hydrosalpinx (Figure 21.62). The wall may or may not be thickened. In some cases, the tube is of overall normal size and shape, with grossly evident tumor present as friable nodules studding the fimbriae (Figure 21.63). Cases harboring only intraepithelial carcinoma will appear grossly unremarkable. About 20% of tubal carcinomas are bilateral.37
Microscopic Features
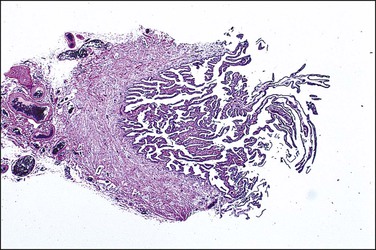
Serous Tubal Intraepithelial Carcinomas.
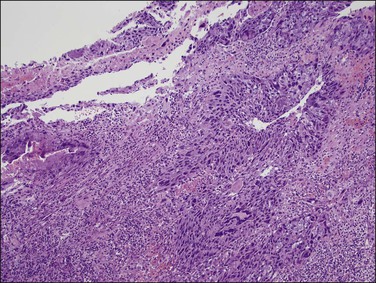
1. A discrete population of epithelial cells replacing the normal mucosa. Some STICs exhibit marked disorganized stratification with irregular fractures separating the cells (Figure 21.64A), while others are minimally stratified and ‘thin’ appearing (Figure 21.64B). The stratified STIC can generally be easily identified at low-power magnification, by virtue of its thickened, somewhat ‘velvety’ appearance relative to the adjacent non-neoplastic epithelium. Additionally, STICs often have an exfoliative appearance, with shedding of tumor cells from the surface, which may also be notable at low power (Figure 21.64A).
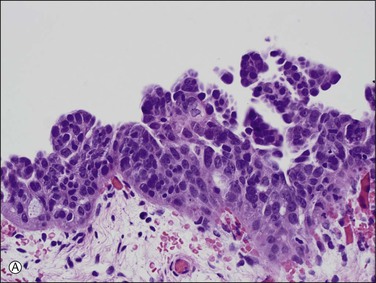
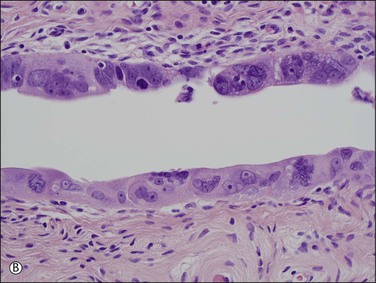
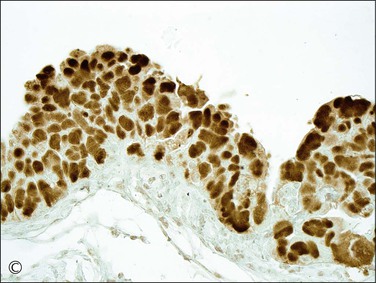
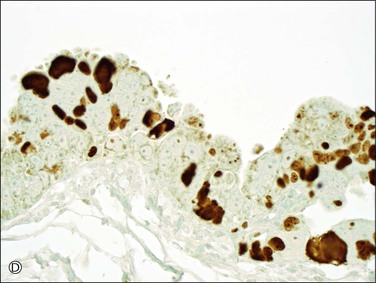
Figure 21.64 Serous tubal intraepithelial carcinoma. Some STICs are markedly stratified with fracture lines between cells; exfoliation of tumor cells from the surface is common (A). Other STICs may be more thin, but still exhibit loss of cell polarity and atypical nuclei (B). Most STICs stain strongly positive for p53 (C), and have a high proliferative (MIB1-positive) index (D).
2. Loss of cell polarity. Even in the absence of stratification, the cells of a STIC almost always exhibit some loss of polarity.
3. Increased nuclear to cytoplasmic ratio.
4. Often rounded nuclei with prominent nucleoli.
6. Usually stain positive for p53. STICs are p53 mutation-associated lesions; the majority exhibit strong, diffuse nuclear staining for p53 (Figure 21.64C). In a minority of STICs, staining for p53 is completely absent, which, in some cases, is due to a p53 mutation with a resultant stop codon, yielding a truncated p53 protein, which is not detectable by immunohistochemical staining.38
7. Increased rate of proliferation. STICs have a high MIB1 index (approximately 70%) by immunohistochemical staining (Figure 21.64D).5
It is important to note that, while immunohistochemistry can be a useful diagnostic adjunct in supporting a morphologic impression of STIC, it should never be used as a substitute for the appropriate morphologic features. Additionally, when considering a diagnosis of STIC, they must be distinguished from lesser lesions of unknown clinical significance and diagnostic reproducibility. For example, the term ‘tubal intraepithelial lesion in transition’ (TILT) has been proposed for atypical foci of mitotically active p53-positive secretory cells, which exhibit features intermediate between p53 signatures and STICs.5
Serous Carcinoma.
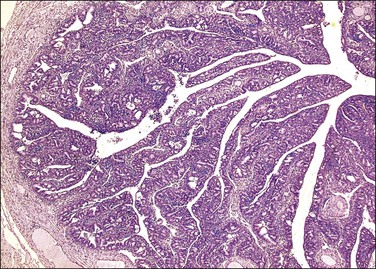
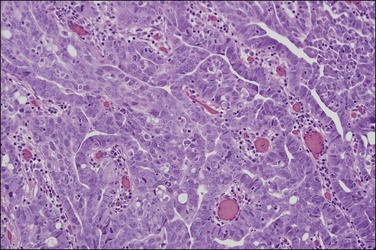
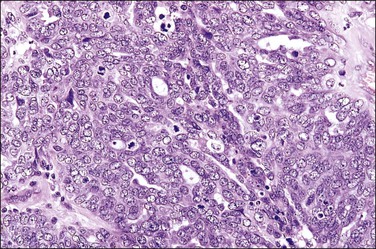
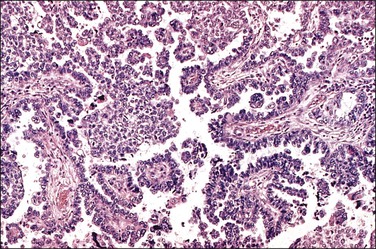
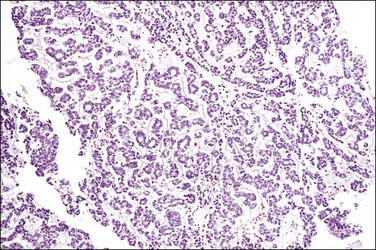
Serous carcinomas are the most common histologic type of epithelial tubal malignancy. Using classical criteria of a dominant tumor mass within the fallopian tube to assign a primary site, these are over half of all tubal carcinomas. This underestimates the true incidence, as new evidence suggests that grossly occult tubal serous carcinomas frequently present with a dominant metastatic ovarian mass, and thus have been considered primary ovarian tumors (see Chapter 25). As is true elsewhere in the pelvis, the majority of serous carcinomas involving the fallopian tube are high grade and demonstrate defects in the p53 gene.37 These poorly differentiated tumors are predominantly solid with usually at least focal areas of poorly formed papillae. Characteristic slit-like spaces are seen through solid sheets of tumor cells (Figure 21.65). The cells themselves have marked nuclear pleomorphism and a high mitotic rate with occasional structurally abnormal mitotic figures (Figures 21.66 and 21.67). Necrosis is common. STICs are frequently identified in association with high-grade serous carcinoma, and the presumed precursor lesion.3 Low-grade serous tumors are rare in the fallopian tube. These are extensively papillary tumors with areas of stromal invasion. Papillae are lined by columnar to cuboidal cells, some of which may be ciliated, exhibiting generally mild cytologic atypia. Mitotic figures are typically infrequent (Figures 21.68 and 21.69). A transition from areas of borderline malignancy to carcinoma is common.
Endometrioid Carcinoma.
About 12–25% of fallopian tube carcinomas are of endometrioid type.37,39,40 Their pattern resembles that of endometrioid carcinomas in the uterus and ovary and is predominantly more glandular and less papillary than serous carcinomas. They are less likely to be bilateral than serous carcinomas.40 A subtype of endometrioid carcinoma may resemble the female adnexal tumor of probable wolffian origin, and shows a mostly solid proliferation of small, oval to spindle cells punctured by small to cystic glands, many with luminal periodic acid–Schiff (PAS)-positive colloid-like secretions. Foci of typical endometrioid carcinoma are usually present but may be minor. These tumors usually are noninvasive.
Transitional Cell Carcinoma.
Transitional cell carcinoma is the third most common histologic type of carcinoma found in the fallopian tube. One recent report found that 12% of tubal carcinomas were entirely of transitional cell type37 while another found that as many as 57% of tubal carcinomas contained a transitional cell element.41 These tumors are arranged as solid nests that show stratification. The cells have clear cytoplasm that may be slightly eosinophilic and some nuclei show nuclear grooves, the so-called ‘coffee bean’ appearance.37 In our view, many such cases are more likely to be poorly differentiated serous carcinomas with a broad papillary or pseudopapillary growth pattern than a truly transitional cell type.
Clear Cell Carcinoma.
Clear cell carcinoma of the fallopian tube is rare, constituting about 2% of tubal carcinomas.37 The tumor has the same appearances as seen in the uterus and ovary. Solid zones of clear cells and papillary areas are present, together with a tubulocystic pattern with prominent hobnail cells.
Spread, Treatment, and Prognosis of Tubal Carcinoma
The current clinical staging system for carcinoma of the fallopian tube is shown in an abbreviated fashion in Appendix A. The spread of tubal carcinoma is generally similar to that of ovarian carcinoma. Transluminal spread into the peritoneal cavity with subsequent implantation on peritoneal surfaces is the predominant mode of spread, but direct involvement of adjacent organs, particularly ovary and uterus, is also important. Occasionally, spread may occur transluminally to involve endometrium and endocervix. Lymphatic spread from the fimbria is principally to the pelvic nodes, and to the para-aortic nodes from that portion of the tube near the uterus. In one small series, two of six patients with disease limited to the fallopian tube already had nodal metastases.43,44
Survival depends upon the extent of the disease at the time of diagnosis. The overall 5 year survival for stages I and II is about 50–60% and for stages III and IV is about 15–20%.45,46 The overall 5 year survival for all stages is under 40%. Histologically high-grade lesions have the worst outcome but a better prognosis is associated with endometrioid type37 and an inflammatory reaction around the tumor. The most important adverse prognostic factor remains advanced stage. Other adverse factors have included increasing age, vascular space invasion, and a high volume of residual tumor.
Carcinosarcoma
Definition
A rare mixed tumor composed of malignant glands and malignant mesenchyme, with an annual incidence of about 0.25 cases per million women.47 Carcinosarcoma may be found anywhere along the female genital tract and, although much more common in the endometrium and ovary, about 70 cases of this tumor have been reported in the fallopian tube.
Pathology
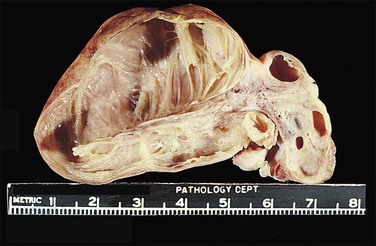
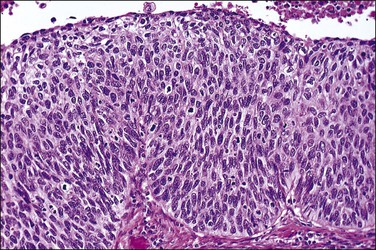
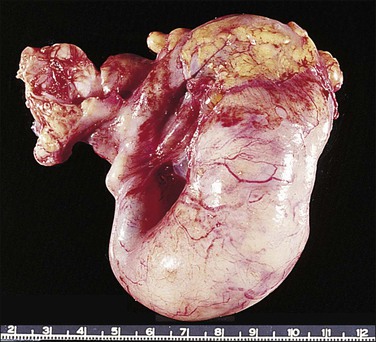
Carcinosarcomas of the tube are often relatively small when diagnosed. Larger tumors are difficult to distinguish from ovarian primaries. They appear grossly as polypoid growths filling the lumen of the tube, often with areas of hemorrhage and necrosis (Figure 21.72).
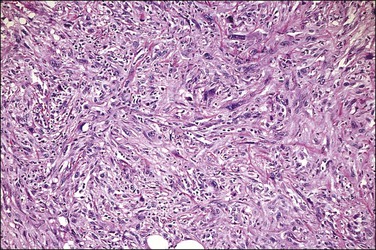
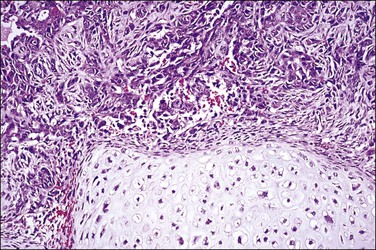
Microscopically, carcinosarcoma discloses a malignant epithelial element that may or may not have the pattern of an endometrioid adenocarcinoma, together with a mesenchymal element. As in the endometrium and ovary, the mesenchymal element may be homologous, containing elements indigenous to the endometrium, or heterologous, containing elements foreign to the endometrium, such a cartilage (Figure 21.73) or striated muscle.
Treatment and Prognosis
Recommended treatment is by surgery, followed by radiotherapy and/or chemotherapy. The prognosis is poor, the 5 year survival rate being about 15% and the mean survival only 16–20 months. Early stage disease is associated with a better prognosis,48 and several long-term survivals are recorded.47
Other Malignant Tumors
Other rare malignant tumors that have been reported arising in the tube include leiomyosarcoma,49 embryonal rhabdomyosarcoma,50 immature teratoma,51 and choriocarcinoma (Figure 21.74).52
Tumors Metastatic to the Fallopian Tube
Metastatic tumors from other gynecologic sites can spread to the fallopian tube, including rare transgenital spread of cervical SILs as described above (Figures 21.70 and 21.71).42 Tumors from non-gynecologic sites can spread to the fallopian tube, typically evident as deposits on the serosal surface of the tube or as tumor within lymphovascular spaces within the tube stroma.
Female Adnexal Tumor of Wolffian Origin
Definition
A tumor of the broad ligament for which there is very strong evidence of a wolffian origin. About 40 cases have been reported.19
Gross Features
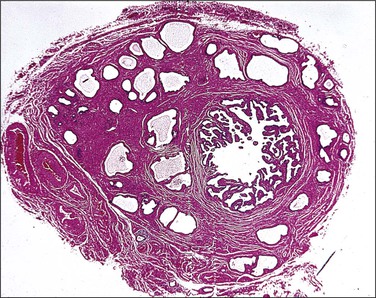
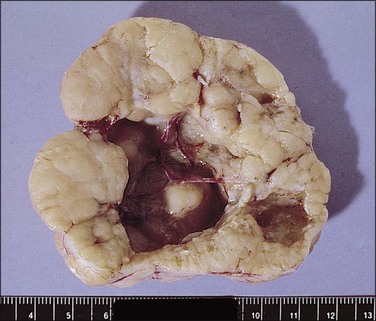
The tumors are unilateral, ranging from 0.5 to 18 cm in diameter, and are situated within the leaves of the broad ligament or pedunculated from it. Virtually all have bosselated, smooth outer surfaces. The cut surfaces (Figure 21.75) are gray-white to tan in color and rubbery to firm in consistency. Some are gritty, with focal areas of calcification that are sometimes sufficiently extensive to be seen on pelvic X-ray. Cystic areas are sometimes present.
Microscopic Features
Three main histologic patterns are described in these tumors:
• A sieve-like pattern (Figure 21.76), in which there are hollow tubules of varying size and shape, sometimes with cyst formation.
• Closely packed tubules, giving a dense, solid appearance (Figure 21.77). The tubules are winding, branching, and anastomosing, and are lined by cuboidal or columnar epithelial cells.
The nuclei are round or oval and pale, with evenly dispersed chromatin. Most tumors have a low mitotic count. Some of those that behave in a malignant fashion contain pleomorphic nuclei and numerous mitotic figures, but not all clinically malignant tumors display these features.19
Origin
The tumors found in the broad ligament and mesosalpinx are considered by convention to be of wolffian origin because of their occurrence in the broad ligament, which is where wolffian remnants are located. Also, they do not resemble müllerian tumors by light microscopy, electron microscopy, or immunohistochemistry.19 However, as the majority of female adnexal tumors of wolffian origin (FATWOs) share histologic features of sex cord–stromal tumors, particularly of Sertoli cell tumors or Sertoli–Leydig cell tumors, a possible extragonadal sex cord–stromal origin has been proposed.53 It is difficult to study the tumor origin because of low incidence.
Differential Diagnosis
The differential diagnosis is sex cord–stromal tumors, particularly those containing Sertoli cells. No stromal cells of Leydig type have been demonstrated, nor has any patient shown hormonal manifestations. Inhibin reactivity is not helpful to differentiate FATWOs, Sertoli cell, or Sertoli–Leydig cell tumors as all of these tumors are consistently reactive.53–55 In addition, although some areas of the tumors have a passing similarity to granulosa cell tumors, endometrioid adenocarcinomas, and clear cell carcinomas, these resemblances are only superficial and do not stand up to close scrutiny, although occasional confusion may occur.56
References
1. Chen, EY, Mehra, K, Mehrad, M, et al. Secretory cell outgrowth, PAX2 and serous carcinogenesis in the fallopian tube. J Pathol. 2010; 222:110–116.
2. Lee, Y, Miron, A, Drapkin, R, et al. A candidate precursor to serous carcinoma that originates in the distal tube. J Pathol. 2007; 211:26–35.
3. Kindelberger, DW, Lee, Y, Miron, A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007; 31:161–169.
4. Carlson, JW, Miron, A, Jarboe, EA, et al. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J Clin Oncol. 2008; 26:4160–4165.
5. Jarboe, E, Folkins, A, Nucci, MR, et al. Serous carcinogenesis in the fallopian tube: a descriptive classification. Int J Gynecol Pathol. 2008; 27:1–9.
6. Medeiros, F, Muto, MG, Lee, Y, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006; 30:230–236.
7. Longacre, TA, Oliva, E, Soslow, RA. Recommendations for the reporting of fallopian tube neoplasms. Virchows Arch. 2007; 450:25–29.
8. Semmel, DR, Folkins, AK, Hirsch, MS, et al. Intercepting early pelvic serous carcinoma by routine pathological examination of the fimbria. Mod Pathol. 2009; 22:985–988.
9. Crossman, SH. The challenge of pelvic inflammatory disease. Am Fam Physician. 2006; 73:859–864.
10. Abbuhl, SB, Muskin, EB, Shofer, FS. Pelvic inflammatory disease in patients with bilateral tubal ligation. Am J Emerg Med. 1997; 15:271–274.
11. Honore, LH. Pathology of the fallopian tube and broad ligament. In: Fox H, Wells M, eds. Haines and Taylor obstetrical and gynaecological pathology. 4th ed. New York: Churchill Livingstone; 1995:623–671.
12. Parikh, FR, Nadkarni, SG, Kamat, SA, et al. Genital tuberculosis—a major pelvic factor causing infertility in Indian women. Fertil Steril. 1997; 67:497–500.
13. Ortu, S, Molicotti, P, Sechi, LA, et al. Rapid detection and identification of Mycobacterium tuberculosis by real time PCR and Bactec 960 MIGT. New Microbiol. 2006; 29:75–80.
14. Ramirez, NC, Lawrence, WD, Ginsburg, KA. Ectopic pregnancy. A recent five-year study and review of the last 50 years’ literature. J Reprod Med. 1996; 41:733–740.
15. Jacques, SM, Qureshi, F, Ramirez, NC, et al. Retained trophoblastic tissue in Fallopian tubes: a consequence of unsuspected ectopic pregnancies. Int J Gynecol Pathol. 1997; 16:219–224.
16. Carson, SA, Buster, JE. Current concepts—ectopic pregnancy. N Engl J Med. 1993; 329:1174–1181.
17. Kuragaki, C, Enomoto, T, Ueno, Y, et al. Mutations in the STK11 gene characterize minimal deviation adenocarcinoma of the uterine cervix. Lab Invest. 2003; 83:35–45.
18. Mikami, Y, Kiyokawa, T, Sasajima, Y, et al. Reappraisal of synchronous and multifocal mucinous lesions of the female genital tract: a close association with gastric metaplasia. Histopathology. 2009; 54:184–191.
19. Scully, RE, Young, RH, Clement, RB. Tumors of the broad ligament and other uterine ligaments. Washington, DC: Armed Forces Institute of Pathology; 1998.
20. Egan, AJM, Russell, P. Transitional (urothelial) cell metaplasia of the fallopian tube mucosa: morphological assessment of three cases. Int J Gynecol Pathol. 1996; 15:72–76.
21. Cheung, AN, Young, RH, Scully, RE. Pseudocarcinomatous hyperplasia of the fallopian tube associated with salpingitis. A report of 14 cases. Am J Surg Pathol. 1994; 18:1125–1130.
22. Bossuyt, V, Medeiros, F, Drapkin, R, et al. Adenofibroma of the fimbria: a common entity that is indistinguishable from ovarian adenofibroma. Int J Gynecol Pathol. 2008; 27:390–397.
23. Schust, D, Stovall, DW. Leiomyomas of the fallopian tube. A case report. J Reprod Med. 1993; 38:741–742.
24. Kutteh, WH, Albert, T. Mature cystic teratoma of the fallopian tube associated with an ectopic pregnancy. Obstet Gynecol. 1991; 78:984–986.
25. Campello, TR, Fittipaldi, H, O’Valle, F, et al. Extrauterine (tubal) placental site nodule. Histopathology. 1998; 32:562–565.
26. Nayar, R, Snell, J, Silverberg, SG, et al. Placental site nodule occurring in a fallopian tube. Hum Pathol. 1996; 27:1243–1245.
27. Su, YN, Cheng, WF, Chen, CA, et al. Pregnancy with primary tubal placental site trophoblastic tumor: a case report and literature review. Gynecol Oncol. 1999; 73:322–325.
28. Alvarado-Cabrero, I, Navani, SS, Young, RH, et al. Tumors of the fimbriated end of the fallopian tube: a clinicopathologic analysis of 20 cases, including nine carcinomas. Int J Gynecol Pathol. 1997; 16:189–196.
29. Krasevic, M, Stankovic, T, Petrovic, O, et al. Serous borderline tumor of the fallopian tube presented as hematosalpinx: a case report. BMC Cancer. 2005; 5:129.
30. Zheng, W, Wolf, S, Kramer, EE, et al. Borderline papillary serous tumor of the fallopian tube. Am J Surg Pathol. 1996; 20:30–35.
31. Stewart, SL, Wike, JM, Foster, SL, et al. The incidence of primary fallopian tube cancer in the United States. Gynecol Oncol. 2007; 107:392–397.
32. Nordin, AJ. Primary carcinoma of the fallopian tube: a 20-year literature review. Obstet Gynecol Surv. 1994; 49:349–361.
33. Brescia, RJ, Cardoso de Almeida, PC, Fuller, AF, Jr., et al. Female adnexal tumor of probable Wolffian origin with multiple recurrences over 16 years. Cancer. 1985; 56:1456–1461.
34. Daya, D. Malignant female adnexal tumor of probable Wolffian origin with review of the literature. Arch Pathol Lab Med. 1994; 118:310–312.
35. Hellstrom, AC, Silfversward, C, Nilsson, B, et al. Carcinoma of the fallopian tube. A clinical and histopathologic review: the Radiumhemmet series. Int J Gynecol Cancer. 1994; 4:395–400.
36. Vicus, D, Finch, A, Rosen, B, et al. Risk factors for carcinoma of the fallopian tube in women with and without a germline BRCA mutation. Gynecol Oncol. 2010; 118:155–159.
37. Alvarado-Cabrero, I, Young, RH, Vamvakas, EC, et al. Carcinoma of the fallopian tube: a clinicopathological study of 105 cases with observations on staging and prognostic factors. Gynecol Oncol. 1999; 72:367–379.
38. Jarboe, EA, Miron, A, Carlson, JW, et al. Coexisting intraepithelial serous carcinomas of the endometrium and fallopian tube: frequency and potential significance. Int J Gynecol Pathol. 2009; 28:308–315.
39. Fujiwaki, R, Takahashi, K, Ryuko, K, et al. Primary endometrioid carcinoma of the fallopian tube. Acta Obstet Gynecol Scand. 1996; 75:508–510.
40. Navani, SS, Alvarado-Cabrero, I, Young, RH, et al. Endometrioid carcinoma of the fallopian tube: a clinicopathologic analysis of 26 cases. Gynecol Oncol. 1996; 63:371–378.
41. Uehira, K, Hashimoto, H, Tsuneyoshi, M, et al. Transitional cell carcinoma pattern in primary carcinoma of the fallopian tube. Cancer. 1993; 72:2447–2456.
42. Cheung, ANY, So, KF, Ngan, HYS, et al. Primary squamous cell carcinoma of fallopian tube. Int J Gynecol Pathol. 1994; 13:92–95.
43. Cormio, G, Lissoni, A, Maneo, A, et al. Lymph node involvement in primary carcinoma of the fallopian tube. Int J Gynecol Cancer. 1996; 6:405–409.
44. di Re, E, Grosso, G, Raspagliesi, F, et al. Fallopian tube cancer. Incidence and role of lymphatic spread. Gynecol Oncol. 1996; 62:199–202.
45. Rauthe, G, Vahrson, HW, Burkhardt, E. Primary cancer of the fallopian tube. Treatment and results of 37 cases. Eur J Gynaecol Oncol. 1998; 19:356–362.
46. Rosen, AC, Ausch, C, Hafner, E, et al. A 15-year overview of management and prognosis in primary fallopian tube carcinoma. Austrian Cooperative Study Group for Fallopian Tube Carcinoma. Eur J Cancer. 1998; 34:1725–1729.
47. Hellstrom, AC, Auer, G, Silfversward, C, et al. Prognostic factors in malignant mixed müllerian tumor of the fallopian tube. Int J Gynecol Cancer. 1996; 6:467–472.
48. Ebert, AD, Perez-Canto, A, Schaller, G, et al. Stage I primary malignant mixed müllerian tumor of the fallopian tube: report of a case with five-year survival after minimal surgery without adjuvant treatment. J Reprod Med. 1998; 43:598–600.
49. Ebert, A, Goetze, B, Herbst, H, et al. Primary leiomyosarcoma of the fallopian tube. Ann Oncol. 1995; 6:618–619.
50. Buchwalter, CL, Jenison, EL, Fromm, M, et al. Pure embryonal rhabdomyosarcoma of the fallopian tube. Gynecol Oncol. 1997; 67:95–101.
51. Li, S, Zimmerman, RL, LiVolsi, VA. Mixed malignant germ cell tumor of the fallopian tube. Int J Gynecol Pathol. 1999; 18:183–185.
52. Muto, MG, Lage, JM, Berkowitz, RS, et al. Gestational trophoblastic disease of the fallopian tube. J Reprod Med. 1991; 36:57–60.
53. Zheng, W, Senturk, BZ, Parkash, V. Inhibin immunohistochemical staining: a practical approach for the surgical pathologist in the diagnoses of ovarian sex cord-stromal tumors. Adv Anat Pathol. 2003; 10:27–38.
54. Kommoss, F, Oliva, E, Bhan, AK, et al. Inhibin expression in ovarian tumors and tumor-like lesions: an immunohistochemical study. Mod Pathol. 1998; 11:656–664.
55. McCluggage, WG. Value of inhibin staining in gynecological pathology. Int J Gynecol Pathol. 2001; 20:79–85.
56. Fukunaga, M, Bisceglia, M, Dimitri, L. Endometrioid carcinoma of the fallopian tube resembling a female adnexal tumor of probable wolffian origin. Adv Anat Pathol. 2004; 11:269–272.