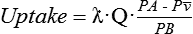Factors affecting anesthetic gas uptake
Solubility
The solubility of an anesthetic agent is defined by its blood-gas partition coefficient. It describes the relative affinity of an inhaled anesthetic agent for the blood. For example, isoflurane has a blood-gas partition coefficient of 1.4. This means that, at equilibrium, the isoflurane concentration in the blood would be 1.4 times the concentration in the gas (alveolar) phase. By definition, the partial pressures of the agent in blood and gas are identical at equilibrium, but the blood would contain more isoflurane. The blood-gas partition coefficients of commonly used inhalation anesthetic agents are listed in Table 61-1.
Table 61-1
Partition Coefficients at 37°C
| Anesthetic Agent | Blood-Gas Partition Coefficient |
| Desflurane | 0.45 |
| Nitrous oxide | 0.47 |
| Sevoflurane | 0.65 |
| Isoflurane | 1.4 |
| Enflurane | 1.8 |
| Halothane | 2.5 |
| Diethyl ether | 12.0 |
| Methoxyflurane | 15.0 |
Modified from Eger EI II. Effect of inspired anesthetic concentration on the rate of rise of alveolar concentration. Anesthesiology. 1963;24:153-157.
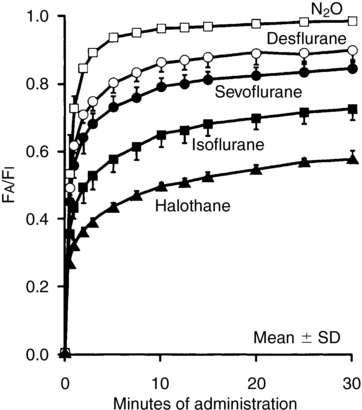
The higher the blood-gas partition coefficient, the greater the amount of anesthetic agent dissolved in blood at equilibrium, and onset of anesthesia is delayed because it is not the total amount of drug in the blood, but the partial pressure of inhalation agent in the blood and, therefore, in the brain that induces anesthesia. For agents with a high coefficient, it takes a relatively long time to “fill the tank” before the partial pressure begins to rise high enough to induce anesthesia. Gases with a high coefficient, because the gas diffuses so quickly into blood, have a relatively low alveolar/inspired gas ratio (FA/FI) (Figure 61-1), which also delays onset. Uptake of the more soluble inhalation agents can be increased by anesthetic overpressuring, that is, delivering a concentration of inspired gas two to four times the MAC (minimum alveolar concentration).
Cardiac output
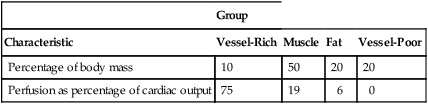
The anesthetic agent in the blood is initially distributed to the vessel-rich tissues (Table 61-2). Soon after blood returns to the lungs, depending on its blood-gas partition coefficient, it has the same partial pressure that it had on leaving the lungs. As the gradient for the uptake of anesthetic approaches 0, less agent is taken up, and alveolar concentration rises (PI ∼ PA ∼ PBLOOD). Because children have greater perfusion of the vessel-rich group than do adults, FA/FI rises more rapidly in children, so anesthesia is achieved more rapidly in these patients.
Table 61-2
| Group | ||||
| Characteristic | Vessel-Rich | Muscle | Fat | Vessel-Poor |
| Percentage of body mass | 10 | 50 | 20 | 20 |
| Perfusion as percentage of cardiac output | 75 | 19 | 6 | 0 |
From Eger EI II. Effect of inspired anesthetic concentration on the rate of rise of alveolar concentration. Anesthesiology. 1963;24:153-157.
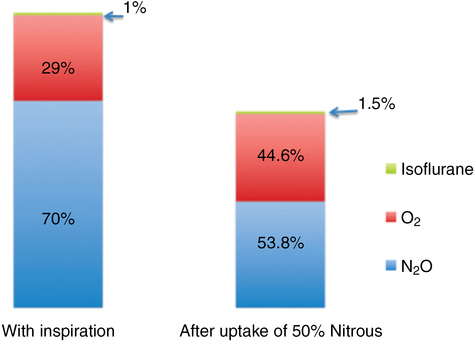
Second-gas effect
The phenomenon known as the second-gas effect results from large volumes of a first gas (usually N2O) being taken up from alveoli, as described in the concentrating effect, increasing the rate of increase in alveolar concentration of the second gas given concomitantly. Factors responsible for the concentration effect also govern the second-gas effect. The effective increase in alveolar ventilation should increase the alveolar concentration of all concomitantly inspired gases regardless of their inspired concentration. Moreover, uptake of the first gas reduces the total gas volume, thereby increasing the concentration of the second gas (Figure 61-2).