Chapter 50 Extubation and Reintubation of the Difficult Airway
I Introduction
Tracheal extubation has received relatively limited critical scrutiny compared with that accorded to intubation. Textbooks, reviews, and conferences focusing on the airway frequently ignore this aspect of management, despite the observation that airway complications are significantly more likely to be associated with extubation than intubation.1 These complications range from the relatively minor, such as coughing and transient breath holding that have little impact on outcome, to those that are life-threatening. The American Society of Anesthesiologists (ASA) Closed Claims Project analyzed adverse respiratory events and found that 17% of brain injuries and deaths occurred after extubation in the operating room or postanesthesia care unit (PACU).2 Complications associated with extubation in critical care areas are likely to occur more frequently and have more serious consequences.3,4
The ASA Task Force on Management of the Difficult Airway and the Canadian Airway Focus Group recommended a preformulated strategy for extubation of the difficult airway and an airway management plan for dealing with post-extubation hypoventilation.5,6 This chapter classifies the complications associated with routine and more complex tracheal extubation or reintubation, proposes a risk stratification for extubation and reintubation in various clinical settings, and suggests strategies that may prove helpful in reducing serious complications or death.
Low-risk or routine extubations have been reviewed elsewhere and are not the focus of this chapter. Although they are dealt with only briefly, concerns include the adequacy of recovery from neuromuscular blockade, depressant effects of narcotics and residual volatile or intravenous sedatives, level of consciousness, hemodynamic stability, adequate ventilation and oxygenation, normothermia, freedom from noxious stimulation, and airway patency.7–10 The controversy about deep versus awake extubation has been addressed elsewhere,10–12 and most of the present discussion concentrates on extubation of more challenging airways and strategies that may increase the probability of success.
II Extubation Failures and Challenges
In the ICU, the ability to predict readiness for extubation is imprecise despite a host of predictive criteria.13–16 To minimize the risks, discomfort, and expense of prolonged intubation, a trial of extubation is occasionally attempted, but it may be followed sometime thereafter by a need to reintubate. The incidence of required reintubation ranges from 6% to 20%, and it depends on the clinical mix of patients,15,17,18 their critical acuity, critical care resources, and the threshold levels for extubation. Even if reintubation is successful, patients who failed their initial extubation had increased rates of ICU mortality and cost of care, prolonged ICU stay, and hospital length of stay.19 Compared with routine postoperative patients, intensive care patients are more likely to fail extubation because neurologic obtundation may leave them unable to protect their airways. Debilitation and impaired mucociliary clearance may interfere with pulmonary toilet, and diminished strength, altered pulmonary mechanics, increased dead space, and venous admixture may result in hypercapnic or hypoxemic respiratory failure.
Although the complications associated with extubation of postoperative patients may be more common than those associated with intubation, they rarely require reintubation. Studies involving a wide case mix of postoperative patients show a high degree of concordance regarding the incidence of required reintubation. Combining the results of four large studies enrolling more than 150,000 patients, the incidence ranged from 0.09% to 0.19%.20–23 The reintubation rate appears to be significantly higher (1% to 3%) after selected surgical procedures such as panendoscopy 20 and a variety of head and neck procedures.24–28
III Extubation Risk Stratification
Risks related to extubation fall into two broad categories: the risk of extubation failing and the risk of reintubation failing. Within each category, there is a continuum from low to high risk. Like the prediction of a difficult intubation, this is an inexact science; it is probably safest to err on the cautious side. Most extubations are expected and turn out to be uneventful, but even these routine extubations may be associated with complications (Box 50-1).
A Routine Extubations
A retrospective database review from The University of Michigan analyzed 107,317 general anesthetics administered between 1994 and 1999.23 It identified 191 required reintubations in the operating room or PACU, 112 (58.6%) of which were for respiratory reasons. The most common respiratory causes were hypercapnic or hypoxemic respiratory insufficiency (60%), respiratory obstruction (20.5%), and laryngospasm or bronchospasm (19.5%). Failed extubations from a respiratory cause occurred at a rate of 112/107,301 or 0.1%. Unintended extubation accounted for 25 of 191 reintubations, all of which occurred in the operating room. Surgical complications, including neck hematoma, pneumothorax, laryngeal nerve paralysis, and bleeding, accounted for 16 of 191 reintubations. Contrary to their expectations, the investigators found that excessive narcotics (9 cases) and prolonged neuromuscular blockade with pancuronium (2 cases) were responsible for only 11 of 191 reintubations. This self-reported information was retrospective, and sometimes required interpretation of the causes from the therapies applied (e.g., narcotic antagonists, additional doses of anticholinergics).
A prospective study from Thailand found a rate of reintubation occurring within 24 hours of 27 per 10,000 patients.29 This was twice the rate observed in the University of Michigan study.23 The precipitating factor in the Thai study was thought to be residual neuromuscular weakness in almost three quarters of cases requiring reintubation in the operating room or PACU. In a Taiwanese database that surveyed almost 138,000 patients undergoing general anesthesia between 2005 and 2007, 83 reintubations were performed after intended extubation.30 Overall, this represented a rate of 6 reintubations per 10,000 patients. Comparing these patients with a matched cohort not requiring reintubation, the investigators identified the following factors as most predictive of a need for reintubation: chronic obstructive pulmonary disease (odds ratio [OR] = 7.17; 95% confidence interval [CI], 1.98 to 26.00), pneumonia (OR = 7.94; 95% CI, 1.03 to 32.78), ascites (OR = 13.86; 95% CI, 1.08 to 174.74), and systemic inflammatory response syndrome (OR = 11.90; 95% CI, 2.63 to 53.86).
1 Hypoventilation Syndromes
The ASA Closed Claims Project found that 4% of 1175 closed claims resulted from critical respiratory events in the PACU. The highest proportion was attributed to inadequate ventilation, and many of these patients died or suffered brain damage.31
Many clinical conditions may give rise to postoperative ventilatory failure. A multicenter, prospective survey in France that looked at almost 200,000 general anesthetics administered between 1978 and 1982 found that postoperative respiratory depression accounted for 27 of 85 respiratory complications that were life-threatening or had serious sequelae. These complications were responsible for seven deaths and five cases of hypoxic encephalopathy.32 A respiratory rate of less than 8 breaths/min was observed by PACU nurses among 0.2% of 24,000 patients after general anesthesia.22
Hypoventilation may be mediated centrally at the level of the upper motor neuron, anterior horn cell, lower motor neuron, neuromuscular junction, or respiratory muscles. Clinical correlates include central sleep apnea, carotid endarterectomy,33 medullary injuries, demyelinating disorders, direct injury to peripheral nerves, poliomyelitis, Guillain-Barré syndrome, motor neuron disease, myasthenia gravis, and botulism. Hypoventilation may result from the loss of lung or pleural elasticity, diaphragmatic splinting caused by abdominal pain or distention, thoracic deformities such as kyphoscoliosis, or multifactorial entities such as morbid obesity and severe chronic obstructive pulmonary disease. Rarely, hypercapnia results from excess carbon dioxide production or a marked increase in physiologic dead space.
The residual effects of anesthetic drugs contribute to inadequate postoperative ventilation.34–36 It may be aggravated by incomplete reversal of neuromuscular blockers,37 hypocalcemia or hypermagnesemia, or the administration of other drugs, including antibiotics, local anesthetics, diuretics, and calcium channel blockers, which may potentiate neuromuscular blockade.
5 Inadvertent Extubations
Inadvertent extubations may result from movement of or by the patient with an inadequately secured tracheal tube. Intraoperatively, this may occur in the prone position, when the airway is shared with the surgeon, when the head and neck are extended, when draping obscures the view, or when drapes adhere to the endotracheal tube (ETT) or circuit and are carelessly removed. In the ICU, extubation may occur when the patient is repositioned for a radiograph or routine nursing care. Patients insufficiently sedated are at greater risk for deliberate self-extubation,38 but those more heavily sedated are more likely to require reintubation.39 Fastidious attention to securing and supporting the ETT and breathing circuit is essential. Self-extubation may occur during emergence from anesthesia, when the patient is confused, agitated, and distressed, which promotes premature extubation. In the ICU, it may not be possible to know whether a self-extubation is accidental or deliberate, but many of these patients require reintubation,40 are more likely to exhibit post-extubation stridor, and may need multiple intubation attempts, increasing the likelihood of esophageal intubation and death.41–43
6 Entrapment
The tracheal tube may become entrapped due to an inability to deflate the cuff,44,45 or there may be difficulties with the pilot tube.46,47 Difficulties include a crimped pilot tube, a defective pilot valve, and fixation of the tracheal tube by Kirschner wires,48 screws,49 ligatures,50 or entanglement with other devices.51,52 Entrapment can also occur during a percutaneous tracheostomy.53 Mechanical obstruction of an entrapped tube is a life-threatening complication. Partial transection of the tracheal tube by an osteotome during a maxillary osteotomy has resulted in the partially cut tube forming a barb that caught on the posterior aspect of the hard palate.54 One report of tube entrapment with fatal consequences involved a Carlens tube that was inadvertently sutured to the pulmonary artery.55 Lang and colleagues recommended routine intraoperative testing for tracheal tube movement when fixation devices are used in proximity to the airway.49 Uncertainty about tube movement should prompt fiberoptic examination before emergence from general anesthesia.
7 Hypertension and Tachycardia
Transient hemodynamic disturbances accompany extubation in most adults. These responses may be prevented by deep extubation,56 insertion of a laryngeal mask airway (LMA) before emergence, or attenuated by concurrent medication.57–59 Most healthy patients not on antihypertensive agents or other cardioactive drugs exhibit increases in heart rate and systolic blood pressure of more than 20% in association with extubation.60 After coronary artery bypass surgery, these changes tend to be transient, lasting 5 to 10 minutes, and they usually are not associated with electrocardiographic evidence of myocardial ischemia.61 Coronary sinus lactate extraction measurements, however, indicate that among patients with poor cardiac function, extubation may be associated with myocardial ischemia.62 Patients with inadequately controlled hypertension, carcinoid, pheochromocytoma, hypertension associated with pregnancy, or hyperthyroidism may be expected to display even greater increases in blood pressure in response to tracheal extubation. The specific strategies needed to attenuate these usually transient changes are dictated by the clinical context. The strategies, which are not universally effective, include the use of intracuff,63–65 intratracheal,66 or intravenous lidocaine61,67,68; beta-blockers60,69–71; dexmedetomidine72,73; and nitrates.
8 Intracranial Hypertension
Tracheal intubation and suctioning are associated with a rise in intracranial pressure. Extubation probably is associated with comparable or even greater increases in intracranial pressure. There is evidence, albeit contradictory, that intravenous and endotracheal lidocaine attenuate this effect.74,75
9 Intraocular Pressure
Madan and colleagues compared the intraocular pressure changes of tracheal intubation and extubation in children with and without glaucoma.76 They observed significantly greater increases 30 seconds and 2 minutes after deep extubation compared with the corresponding times after uncomplicated intubations. These differences were seen in both groups of children. It is likely that significant increases in intraocular pressure observed after deep extubation would have been even higher had extubation occurred after recovery of consciousness. Lamb and coworkers observed similar effects of extubation on intraocular pressure in adults and commented that this increase could be prevented by using an LMA rather than a tracheal tube.77
10 Coughing
Coughing on emergence from general anesthesia is virtually ubiquitous, particularly when an ETT is used.78 No difference between smokers and nonsmokers is observed. Although coughing is a protective reflex, it can be particularly troublesome in the setting of ophthalmologic, neurologic, oropharyngeal, and neck surgery.
Several strategies have been proposed to minimize coughing, including deep endotracheal extubation, primary use of or conversion to an LMA,57,79,80 use of the sedative dexmedetomidine,73 intravenous or topical application of a local anesthetic to the vocal folds, and use of intracuff lidocaine.63,65,67,81 However, coughing on emergence is common and relatively benign for most patients.
The emergence of severe acute respiratory syndrome (SARS) and the high prevalence of drug-resistant droplet or airborne diseases in some locales make coughing potentially hazardous to the airway manager. In 2003, Toronto was the North American epicenter for SARS, and coughing assumed life-threatening proportions for medical personnel.82–87 Patients with cough, fever, and pulmonary infiltrates were dangerous, and coughing on emergence was not protective, but rather posed a threat by dispersing infectious respiratory droplets on those in the patient’s vicinity. The strategies adopted at that time have been largely relaxed, although they may again become necessary when new risks threaten patients and care providers.
11 Laryngeal Edema
Several of the complications of endotracheal intubation do not become apparent until after extubation occurs.88 Glottic or tracheal injury may occur despite a good laryngeal view or during awake fiberoptic intubation.89–91 Anatomic or functional laryngeal problems are more likely to develop as a consequence of a difficult or prolonged intubation attempts.22 Possible airway injuries include laryngeal edema, laceration, hematoma, granuloma formation, vocal fold immobility, and dislocation of the arytenoid cartilages.92
Glottic edema has been classified as supraglottic, retroarytenoidal, and subglottic.93 Supraglottic edema may result in posterior displacement of the epiglottis, reducing the laryngeal inlet and causing inspiratory obstruction. Retroarytenoidal edema restricts movement of the arytenoid cartilages, limiting vocal cord abduction on inspiration. Subglottic edema, a particular problem in neonates and infants, results in swelling of the loose submucosal connective tissue that is confined by the non-expandable cricoid cartilage. In neonates and small children, this is the narrowest part of the upper airway, and small reductions in diameter result in a significant increase in airway resistance. In children, laryngeal edema is promoted by a tight-fitting ETT, traumatic intubation, intubation longer than 1 hour, coughing on the tracheal tube, and intraoperative alterations of head position.94 Koka and coworkers found an incidence of 1% among children younger than 17 years. Laryngeal edema should be suspected when inspiratory stridor develops within 6 hours of extubation. Management of laryngeal edema depends on its severity. Treatment options include head-up positioning, supplemental humidified oxygen, racemic epinephrine, helium-oxygen administration, reintubation, and tracheostomy.
Clinical studies of children and adults that evaluated the role of prophylactic corticosteroids in the prevention of post-extubation stridor have yielded contradictory findings.95–98 In the setting of adult ICUs, a large, multicenter, prospective, randomized, double-blind trial of methylprednisolone (20 mg administered 12 hours before and every 4 hours until extubation) versus placebo found that steroids significantly reduced post-extubation laryngeal edema (11 of 355 versus 76 of 343, P < 0.0001) and required reintubation due to laryngeal edema (8% versus 54%, P = 0.005). Laryngeal edema was defined by any two of stridor, inspiratory prolongation, and use of accessory muscles. It was classified as severe if reintubation was required within 36 hours. Laryngoscopy was not performed unless reintubation was required. Factors associated with a greater risk of laryngeal edema included female sex, shorter height, larger-diameter ETT, admission for trauma, and shorter duration of intubation. The absence of pretreatment with methylprednisolone was associated with a hazard ratio of more than 8.99 It is possible that the benefits of steroids are restricted to high-risk populations and require administration of multiple doses.100, 101 Contradictory findings may relate to risk factors in the study populations and variability of dosage regimens.
An alternative classification has been proposed for laryngotracheal injury after prolonged intubation.102–104 Immediate post-extubation airway obstruction results from glottic and subglottic granulation tissue, which may swell on removal of the ETT. Posterior glottic and subglottic stenosis due to contracting scar tissue results in increasing obstruction weeks or months after extubation. Benjamin found that fiberoptic evaluation or laryngoscopy with the tube in situ was of limited value.102 An ETT obscures the view of the posterior glottis and subglottis. These lesions were best identified using rigid telescopes with image magnification during general anesthesia. This approach permitted anticipation of problems and development of a management strategy.
12 Laryngospasm
Laryngospasm is believed to be a common cause of post-extubation airway obstruction, particularly in children.105 Even in adults, Rose and colleagues found that it accounted for 23.3% of critical postoperative respiratory events, although the diagnosis was presumptive.106 Olsson and Hallen observed an increased incidence among patients presenting for emergency surgery, those requiring nasogastric tubes, and patients undergoing tonsillectomy, cervical dilation, hypospadias correction, oral endoscopy, or excision of skin lesions.105 A variety of triggers are recognized, including vagal, trigeminal, auditory, phrenic, sciatic, and splanchnic nerve stimulation; cervical flexion or extension with an indwelling ETT; or vocal cord irritation from blood, vomitus, or oral secretions.107 A risk assessment questionnaire was used in a study to prospectively evaluate almost 10,000 children undergoing general anesthesia. A positive history of nocturnal dry cough, exertional wheezing, or more than three wheezing episodes in the prior 12 months was associated with a fourfold increase in the risk of laryngospasm in the PACU and a 2.7-fold increased risk of airway obstruction during surgery or in the PACU.108 Twice as many children were managed with an LMA than an ETT, and an equal number had their devices removed awake and asleep. The depth of anesthesia at the time of device removal did not influence the incidence of laryngospasm.
Laryngospasm involves bilateral adduction of the true vocal folds, vestibular folds, and aryepiglottic folds that outlasts the duration of the stimulus. This is protective to the extent that it prevents aspiration of solids and liquids. It becomes maladaptive when it restricts ventilation and oxygenation. The intrinsic laryngeal muscles are the main mediators of laryngospasm, and they include the cricothyroids, lateral cricoarytenoids, and thyroarytenoid muscles. The cricothyroid muscles are the vocal cord tensors, an action mediated by the SLN. Management of laryngospasm consists of prevention by either extubating at a sufficiently deep plane of anesthesia or awaiting recovery of consciousness.56 Potential airway irritants should be removed and painful stimulation should be discontinued. If laryngospasm occurs, oxygen by sustained positive pressure may be helpful, although this may push the aryepiglottic folds more tightly together.109 Larson described a technique of applying firm digital pressure anteriorly directed to the “laryngospasm notch” between the ascending mandibular ramus and the mastoid process and observed that this technique is rapid and highly effective.110 Very small doses of a short-acting neuromuscular blocker with or without reintubation may be necessary.111,112
13 Macroglossia
Massive tongue swelling may complicate prolonged posterior fossa surgery performed with the patient in the sitting, prone, or park bench position.113–116 It is also seen with very steep or prolonged Trendelenburg positioning, hypothyroidism, acromegaly, lymphangioma, idiopathic hyperplasia, metabolic disorders, amyloidosis, cystic hygroma, neurofibromatosis, rhabdomyosarcoma, sublingual or submandibular infections, and chromosomal abnormalities such as the Beckwith-Wiedemann syndrome.117
The most common and dramatic presentation of macroglossia results from angioedema.118 It can be congenital or acquired. Hereditary angioedema results from a deficiency of C1 esterase inhibitor; acquired C1 esterase deficiency is associated with histamine release, a physical stimulus, or most commonly, a reaction to angiotensin-converting enzyme inhibitors or angiotensin receptor blockers.118,119 Although involvement of the tongue is the most obvious manifestation, the uvula, soft tissues, and larynx may also be involved.
In the ICU setting, macroglossia may be seen as a complication of extreme volume overloading or tongue trauma, particularly when it is further complicated by a coagulopathic state. If this occurs or progresses after extubation, it can lead to partial or complete airway obstruction, making reintubation necessary but difficult or impossible.114 Lam and Vavilala postulate that positioning in most cases results in venous compression leading to arterial insufficiency and subsequent reperfusion injury.115 Alternatively, local compression may cause venous or lymphatic obstruction with resultant immediate and typically milder tongue swelling. The latter form is less severe but more apparent, and extubation is likely to be postponed.
14 Laryngeal or Tracheal Injury
Airway injuries, such as lacerations, edema, arytenoid dislocation, and vocal fold paralysis, may occur from the lips to the distal trachea. The lip or tongue may become entrapped between the laryngoscope blade and the mandibular teeth, resulting in swelling or bleeding, although this is unlikely to be severe enough to complicate extubation. The glottis may be injured as a result of insertion of a round tube through a triangular opening. The trachea can be lacerated or penetrated by the ETT or its introducer or by ischemic compression by the cuff on the tracheal mucosa. Palatopharyngeal injuries have been described as a consequence of blind insertion of an ETT during video laryngoscopy.120–127 Although these injuries are not often apparent at the time of laryngoscopy, they have been managed conservatively and should not complicate extubation. The epiglottis can be downfolded during intubation, but the consequences of downfolding, even if prolonged, are unknown.128
Laryngeal injuries accounted for 33% of all airway injury claims and 6% of all claims in the ASA Closed Claims Project database.129 They range from transient hoarseness to vocal fold paralysis. Even when direct laryngoscopy provides a satisfactory glottic view or intubation is facilitated by fiberoptic instrumentation,89,91 airway injury can occur and go unsuspected until after the ETT is removed. Airway injuries are presumed to be less likely if intubation is easy, but analysis of the ASA Closed Claims Project revealed that 58% of airway trauma and 80% of laryngeal injuries were associated with intubations that were not difficult.129,130 Judging from the findings of the Closed Claims Project, difficult intubations were more likely to result in pharyngeal and esophageal than tracheal injuries.
Vocal fold immobility may result from injury to the recurrent laryngeal nerve or the arytenoid cartilages.92,131–139 Arytenoid immobility has resulted from seemingly uneventful Macintosh and McCoy direct laryngoscopy,137,140 double-lumen tube insertion, and lightwand intubation.134 The mechanism of this injury is uncertain. It may be a consequence of a subluxation or a hemarthrosis with subsequent resolution or fixation. Prolonged or stressful contact between the ETT and the posteromedial aspects of the vocal cords, arytenoids, or posterior commissure may result in ulceration of the perichondrium, which heals with fibrous adhesions that produce an immobile glottis. This complication may be more common than the literature indicates.92,141 Persistent post-extubation hoarseness, a breathy voice, and an ineffective cough should prompt assessment by an otolaryngologist. The diagnosis is confirmed by endoscopic visualization of an immobile vocal cord associated with a rotated arytenoid cartilage.88 If the diagnosis is made early, before the onset of ankylosis, it may be possible to manipulate the arytenoid back into position.
Vocal fold paralysis results from injury to the vagus or one of its branches (i.e., recurrent laryngeal nerve [RLN] or external division of the superior laryngeal nerve [ex-SLN]) and may resemble arytenoid dislocation or ankylosis. Differentiation may require palpation of the cricoarytenoid joints under anesthesia or laryngeal electromyography.88 When vocal fold paralysis occurs as a surgical complication, it is usually associated with neck, thyroid, or thoracic surgery. The left RLN can also be compressed by thoracic tumors, aortic aneurysmal dilatation, left atrial enlargement, or during closure of a patent ductus arteriosus. Occasionally, a surgical cause cannot be implicated. Cavo and coworkers postulated that an overinflated ETT cuff might result in injury to the anterior divisions of the RLN.142
The RLN supplies all of the intrinsic laryngeal muscles except the cricothyroid, the true vocal cord tensor, which is innervated by the ex-SLNs. Unilateral ex-SLN injury results in a shortened, adducted vocal fold with a shift of the epiglottis and the anterior larynx toward the affected side. This produces a breathy voice but no obstruction and usually resolves within days to months. Bilateral ex-SLN injury causes the epiglottis to overhang, making the vocal folds difficult to visualize. If seen, they are bowed. This produces hoarseness with reduction in volume and range but no obstruction. Unilateral RLN injury causes the vocal fold to assume a fixed paramedian position and produces a hoarse voice. There may be a marginal airway with a weak cough. Bilateral RLN injury results in both vocal folds being fixed in the paramedian position and inspiratory stridor, often necessitating a surgical airway.143
Pharyngeal, nasopharyngeal, and esophageal injuries include perforation, lacerations, contusions, and infections. These injuries may be associated with difficult laryngoscopy or intubation, but they may also result from the blind passage of a gum elastic bougie,144 nasogastric tube,145 nasotracheal tube,146 suction catheter, esophageal stethoscope transesophageal echo probe, or temperature probe.147 Penetrating injuries can communicate with the esophagus, resulting in a tracheoesophageal fistula, or with the mediastinum, which may go unrecognized and result in mediastinitis, retropharyngeal abscess, and death.131 After a brief intubation, soft tissue injuries resulting in airway obstruction are more likely to result from edema or hematoma than infection. Most of the described injuries do not significantly complicate extubation. Laryngeal and tracheal stenoses are serious complications, but they are rarely evident at the time of extubation.
15 Airway Injury
Burn patients can have intrinsic and extrinsic airway injuries. Circumferential neck involvement is an example of an extrinsic injury. Smoke inhalation or thermal injuries are examples of intrinsic injuries. Burn patients are at particular risk of requiring reintubation. They can have bronchorrhea, impaired mucociliary clearance and local defenses, laryngeal and supraglottic edema, increased carbon dioxide production, and progressive acute respiratory distress syndrome. Carbon monoxide may also diminish their level of consciousness and the ability to protect their airway. It may be difficult to secure the tracheal tube because of involvement of the adjacent skin, and burn victims may be agitated or uncooperative, increasing the risk of unintended extubation.148 Kemper and coworkers reported their management of 13 burn patients younger than 15 years, 7 of whom exhibited post-extubation stridor. Patients treated with helium-oxygen mixtures had lower stridor scores than patients treated with an air-oxygen mixture.149 They found that 11 of 30 extubated burn victims required treatment for stridor after extubation, consisting of racemic epinephrine, helium-oxygen, reintubation (n = 5), or tracheostomy (n = 1). The absence of a cuff leak was considered to be the best predictor of failure, with a sensitivity of 100% and a positive predictive value of 79%.149
A variety of conditions may lead to airway edema severe enough to result in post-extubation stridor. It occurred in 2% to 37% of ICU patients after “prolonged intubation.”150 A test was sought to predict patients with sufficient airway swelling to compromise safe extubation. The cuff-leak test was initially proposed for children with croup.151 The concept is that marked airway swelling is likely if air does not escape around the deflated ETT cuff when the ETT is occluded as the patient exhales. The cuff-leak test was the best predictor of successful extubation in a pediatric burn and trauma unit, and although sensitive, it was not specific for predicting stridor, necessitating reintubation in 62 adults.152,153
The test has been refined by evaluating cuff-leak volume as the difference between inspiratory and expiratory tidal volumes during assist-control ventilation after cuff deflation.41,154 Two studies found the cuff-leak volume could predict post-extubation stridor. In one study, 8 of 45 patients exhibited stridor, 4 of whom required reintubation.154 In the other study involving 88 adult medical ICU patients, 6 patients exhibited stridor, 3 of whom required reintubation.41 They observed a significantly smaller cuff leak in patients who subsequently developed stridor, concluding that this measurement was the best predictor of the presence or absence of stridor. However, a study of 561 consecutive cardiothoracic patients who were extubated within 24 hours (median, 12 hours) defined a the cuff leak as the difference between the inspired and expired tidal volumes during assist-controlled ventilation. None of the (20) patients with leaks <110 mL developed stridor whereas one patient with a leak of 350 mL did and required reintubation. This led the investigators to conclude that the quantitative cuff-leak test was not reliable in this patient population.155
In another study involving 110 trauma victims, cuff leaks of less than 10% had a 96% specificity for predicting post-extubation stridor or the need for reintubation.156 De Bast and colleagues studied 76 adults in a combined medical-surgical ICU.157 An equal number of patients had been intubated for more or less than 48 hours. Assist-control ventilation was reinstituted after cuff deflation, and the percentage of cuff leak was determined. Receiver operating characteristic (ROC) curves yielded a cutoff value of 15.5% cuff leak to equalize the false positives and false negatives for reintubation due to laryngeal edema, but this study was relatively small. Among patients intubated for more than 48 hours, only 2 of 22 with large-volume leaks and 6 of 16 with small-volume leaks required reintubation for laryngeal edema.
Kriner and colleagues evaluated the cuff-leak test for its ability to predict post-extubation stridor among 462 adult patients intubated for longer than 24 hours.150 They evaluated the two thresholds previously described; a positive test was defined as a cuff-leak volume of 110 mL or less or of 15.5% or less of the exhaled tidal volume. Ten of 82 patients with leak volumes of 110 mL or less developed post-extubation stridor, and 10 of 380 with larger leaks developed post-extubation stridor, giving a positive predictive value for stridor of 0.12 and negative predictive value of 0.97. The sensitivity and specificity of the test under these conditions were 0.50 and 0.84, respectively.150 With leak volumes of 15.5% or less, 7 of 48 patients developed post-extubation stridor, and 13 of 414 patients with volumes greater than 15.5% developed stridor, yielding positive and negative predictive values of 0.15 and 0.97, respectively. The sensitivity and specificity of this test for post-extubation stridor were 0.35 and 0.91, respectively. The prevalence of post-extubation stridor was 20 of 462. Seven of these patients required reintubation; 15 were managed with racemic epinephrine or helium-oxygen mixtures, or both, and 2 of the 15 failed, requiring intubation. The investigators concluded that neither threshold adequately predicted post-extubation stridor or could justify delaying extubation.
The reported variability of post-extubation stridor (2% to 37%) results in part from inconsistent diagnostic criteria,150 but undoubtedly there are many other factors at play. Factors increasing the risk of post-extubation stridor include longer duration of intubation,150 trauma and burns,14,148,154,158 pediatric age group,158 female gender,95,97,150 traumatic or emergent intubations, periods of hypotension, agitation, persistent attempts at phonation, inadequate ETT fixation, aggressive tracheal suctioning, highly positive fluid balance, low plasma oncotic pressure, gastroesophageal reflux, increased ETT diameter, and the presence of a nasogastric tube.95,97,150
In summary, it appears that despite the intuitive appeal of the cuff-leak test, neither the qualitative nor quantitative test adequately predicts adult patients who will develop stridor after extubation.150,155,159 It did not predict where patients were likely to require reintubation. Deem argued that absence of a cuff leak might result in an unnecessary delay of extubation, whereas a large cuff leak might produce false reassurance that there will be no difficulties.159 At least in adults, the value of this test is uncertain.
16 Postobstructive Pulmonary Edema
Severe airway obstruction from any cause may complicate extubation and lead to postobstructive pulmonary edema, also called negative-pressure pulmonary edema.160 This edema occurs when a forceful inspiratory effort is made against an obstructed airway (i.e., Mueller maneuver), often a closed glottis, generating large negative intrapleural pressures that promote venous return. The increase in venous pressure is aggravated by a lowered alveolar pressure, resulting in transudation of fluids into the pulmonary interstitium and alveoli. It may also result in a rightward shift of the interatrial and interventricular septa, raising left atrial and ventricular pressures. Some instances may be complicated by a permeability defect with exudative fluid and inflammatory cells.161–166
Postobstructive pulmonary edema usually occurs in adult patients with upper airway tumors, severe laryngospasm, or rarely, bilateral vocal cord palsy,167 whereas in children, it occurs most commonly as a complication of croup or epiglottitis.168 The onset may be within minutes of the development of airway obstruction. It typically resolves with relief of the obstruction and supportive treatment for pulmonary edema.166
B Higher-Risk Extubations
Although the previously described complications may follow a routine extubation, two additional groups of patients may be affected: those with a higher risk for reintubation and those in whom accomplishing reintubation can be challenging or impossible. Patients at higher risk for reintubation have preexisting medical conditions that reduce their physiologic reserve, ranging from moderate disability to an extremely marginal state. The possibility of higher-risk extubations also exists for a continuum of patients, from those in whom mask ventilation and reintubation should be easily achieved to those in whom both would pose a significant challenge. They may have their jaws wired shut or have a neck that is very poorly suited for emergent surgical access.169,170
Chronic pulmonary or cardiac disease may compromise spontaneous ventilation and necessitate intubation. The patient with an ineffective cough or increased secretions may have a need for pulmonary toilet. An obtunded patient may be unable to protect his airway. A list of higher-risk extubations is provided in Table 50-1.
| Complication | Surgical and Medical Settings |
|---|---|
| Inability to Tolerate Extubation and Required Reintubation | |
| Airway obstruction | |
IV Clinical Settings of Complications
A Operative Conditions
1 Laryngoscopic Surgery
Mathew and colleagues looked at 13,593 consecutive PACU admissions from 1986 through 1989.21 Twenty-six (0.19%) of these patients required reintubation while in the PACU; seven of them had undergone ear, nose, and throat procedures. Of the seven patients, three had laryngeal edema, one was obstructed from a large thyroid, two bled at the operative site, and one developed postobstructive pulmonary edema after a tonsillectomy.
Patients undergoing laryngoscopy and panendoscopy (i.e., laryngoscopy, bronchoscopy, and esophagoscopy) are at an increased postoperative risk for airway obstruction and are approximately 20 times as likely to require reintubation as patients undergoing a wide variety of other surgical procedures.20 Reviewing the records of 324 diagnostic laryngoscopies and 302 panendoscopies, Hill and colleagues found that patients who had undergone laryngeal biopsy were at the greatest postoperative airway risk. Thirteen (5%) of 252 patients required reintubation, most within 1 hour of extubation. Twelve of 13 had undergone laryngeal biopsy. Most of these patients had chronic obstructive pulmonary disease, and their need for reintubation was attributed largely to this.
Robinson prospectively studied 183 patients who had 204 endoscopic laryngeal procedures.171 Seven patients had tracheostomies before or after their surgery because of high-risk airways. Two of the remaining patients developed postoperative stridor; one required reintubation, and the other required a delayed tracheostomy. Indirect laryngoscopy carried out 4 to 6 hours after surgery revealed mucosal hemorrhage or laryngopharyngeal swelling in 32% of cases. Because the patients undergoing tracheostomy were not described, it is possible that the low incidence of reintubation resulted from an aggressive approach to preemptive tracheostomy.
2 Thyroid Surgery
A variety of airway-related injuries can be associated with thyroidectomies, including SLN and RLN injuries, wound hematoma, and tracheomalacia. Lacoste and colleagues retrospectively reviewed the records of 3008 patients who underwent thyroidectomies between 1968 and 1988.27 The RLN had been identified intraoperatively in 2427 of these patients. Indirect laryngoscopy was performed on the third or fifth postoperative day. The RLN was damaged in 0.5% of patients with benign goiters and 10.6% of patients with thyroid cancer. RLN injury produces hoarseness, persistent coughing with phonation, and risk of aspiration. Unilateral RLN palsy was observed in 1.1% of patients. Three patients had bilateral RLN palsy and required tracheostomy. Six of a total of 16 deaths during the first 30 postoperative days were attributed to respiratory complications. One death occurred after failed intubation due to a deviated, constricted trachea. A second death was attributed to difficulties performing a tracheostomy. Two deaths resulted from aspiration or pneumonia, possibly related to RLN dysfunction.
SLN injury is more challenging to diagnose. It produces dysphonia and vocal fatigue, particularly in the higher registers. In a 5-year, multicenter study involving 42 centers and almost 15,000 thyroid operations, the diagnosis was suspected in 3.7% and confirmed in 0.4% of patients.172
Local hemorrhage or hematoma occurs postoperatively in 0.1% to 1.6% of patients undergoing thyroid surgery and in 0.36% of the patients cared for by Lacoste and colleagues.27,172–174 These complications occurred 5 minutes to 3 days postoperatively. Re-exploration within the first day was required only twice. Airway obstruction may result from significant laryngeal and pharyngeal edema, and wound evacuation may be of limited value in the relief of airway obstruction.173,175,176 It may result from or be aggravated by ligature slippage, coughing, vomiting,174 coagulopathies, and reoperation.173 The prophylactic placement of surgical drains likely reduces the incidence of this complication. Wound evacuation may result in significant improvement; however this is not always the case. If time permits or intubation fails, wound evacuation should be considered.176 Laryngeal edema may persist after the wound has been evacuated, necessitating postoperative intubation.
Tracheomalacia is rarely diagnosed after thyroidectomies, even in patients with significant retrosternal tracheal compression, although it may exist subclinically.177–181 Although symptoms, computed tomography (CT), and pulmonary function test results make it easy to recognize airway compression preoperatively, tracheomalacia may be difficult to predict or even detect from the surgical field, and it does not become apparent until after the ETT is removed and spontaneous ventilation has resumed.182
3 Carotid Artery Surgery
Neck swelling or hematoma formation after carotid endarterectomy may be relatively common. The New York Carotid Artery Surgery (NYCAS) study analyzed 9308 procedures performed between 1998 and 1999 at 167 hospitals.183 A hematoma was identified in 5% of patients, substantially increasing the risk of death (OR = 4.30; 95% CI, 2.72 to 5.00) and stroke (OR = 3.89; 95% CI, 2.82 to 5.38). Hematoma occurrence reported in the literature ranges from 1.2% to 12%, depending on the definition used.183 The overall rate of wound hematomas in the North American Symptomatic Carotid Endarterectomy Trial (NASCET), involving 1415 patients was 7.1%, 3.9% of which cases were considered to be mild (i.e., no delay in discharge), 3.0% were moderate (i.e., delay in discharge), and 0.3% were severe (i.e., permanent functional disability or death). The moderate and severe cases required re-exploration or wound evacuation (3.3%). Hematoma contributed to the death of four patients.184 When wound hematomas are identified by a comparison of preoperative and postoperative CT scans, it occurs far more frequently (26%).185 The postoperative reintubation or exploration rate is 1% to 3.3%.184,186
Kunkel and colleagues described 15 patients who developed wound hematomas after carotid endarterectomy.187 Eight of these were evacuated under local anesthesia. In six of seven cases in which general anesthesia was induced before opening the wound, difficulties arose with airway management, resulting in two deaths and one patient with severe neurologic impairment. O’Sullivan and coworkers reported a similar experience for six patients with airway obstruction after carotid endarterectomy.188 Stridor was not relieved by wound evacuation. Administration of muscle relaxants made manual mask ventilation and tracheal intubation virtually impossible due to marked glottic or supraglottic edema. Cyanosis and extreme bradydysrhythmias or asystole occurred in four patients. The providers endorsed Kunkle’s recommendation for wound evacuation but thought that much of the airway compromise was caused by edema from venous or lymphatic congestion. They emphasized that the outward appearance may lead to an underestimation of the situation’s gravity. Voice changes are early signs of danger and may be relatively subtle. Rapid clinical deterioration can occur after stridor develops.189
Studies by Carmichael and colleagues provided additional evidence for the role of swelling and bleeding in a small but elegant study. They compared the CT scans of 19 patients before and after carotid endarterectomy surgery.185 Clinically, 1 patient had severe swelling, 4 had moderate swelling, and 3 had mild swelling, but 10 were deemed normal. However, postoperative CT scans demonstrated significant swelling of the retropharyngeal space and a reduction of the anteroposterior and transverse airway diameter, particularly at the level of the hyoid. Compared with preoperative CT scans, the calculated volume reduction averaged 32% ± 7% for extubated patients. The scans revealed a wound hematoma estimated to be greater than 10 mL (range, 44 to 94 mL) in 5 of 19 patients. Patients who remained intubated postoperatively showed a significantly greater volume reduction of 62% ± 9% (P < 0.025). Those remaining intubated as a result of swelling had contralateral extension of the swelling. These observations may help to explain why opening the wound frequently fails to provide benefit in many patients. Nonetheless, it may be difficult to clinically differentiate bleeding from swelling. After radiologic demonstration of swelling, the same group evaluated the benefits of prophylactic dexamethasone but failed to demonstrate any clinical benefit.190
A 10-year, retrospective review of 3224 carotid endarterectomies performed at The Mayo Clinic revealed that 44 (1.4%) patients required wound exploration within 72 hours of surgery, despite the nonreversal of heparin.191 In two patients, re-exploration occurred before the initial extubation. The decision to re-explore was made in the PACU for 7 patients; the remaining 35 were identified in the ICU or ward. Only one patient required a surgical airway when direct laryngoscopy failed in the ICU. Several techniques were initially employed: awake bronchoscopic intubation (15 of 20 of which were successful), direct laryngoscopy after induction (13 of 15 were successful), and awake direct laryngoscopy (5 of 7 were successful). When awake bronchoscopy failed, direct laryngoscopy was successful whether the patient was awake (3 of 3) or asleep (2 of 2). When direct laryngoscopy initially failed after induction in two patients, it succeeded after opening the incision. When awake direct laryngoscopy failed, one patient required a surgical airway; in the other, direct laryngoscopy succeeded after opening the incision. Despite the size of this series, it is not possible to draw conclusions about which techniques are most successful. Success likely depends on the skill and judgment of the airway manager. It is also possible that this study differed from the other studies in that the decision to re-explore was made earlier and patients had less airway distortion.
Several nerve injuries can result from carotid artery surgery or the anesthetic technique. The range reported in the literature is 3% to 23%, although most of these cases resolve within 4 months of surgery.192 In the NYCAS study, cranial nerve palsies occurred in 514 (5.5%) of 9308 patients and involved, in descending order, the hypoglossal nerve (170), producing tongue deviation to the operative side; a branch of the facial nerve (126), resulting in lip or facial droop; the glossopharyngeal nerve (41); a branch of the vagus, which may involve the RLN and produce vocal cord paresis (31); the trigeminal nerve (19); a branch of the cervical plexus (10); or more than one nerve group (117).183
Bilateral vocal cord and bilateral hypoglossal nerve palsies have been described after staged, bilateral carotid endarterectomies.24,25 In the latter case, the first procedure, performed under regional anesthesia, had been complicated by a wound hematoma, resulting in numbness over the anterior neck and diminished sensation in the C2 and C3 distribution. The subsequent endarterectomy, done 4 weeks later under deep cervical plexus block with subcutaneous infiltration, caused intraoperative airway obstruction and asystole. The airway was secured, but repeated attempts at extubation resulted in persistent obstruction due to bilateral hypoglossal nerve palsy. In another case, performed under cervical plexus block, the patient developed bilateral vocal cord paralysis that required intubation and subsequent tracheotomy. It is suspected that she had a previously unrecognized contralateral vocal cord palsy from a prior thyroidectomy.193 In this case, the vocal cord dysfunction was co-incident with retraction of the carotid sheath—although it can also be induced by a cervical plexus block—but it raises the importance of the preoperative assessment of patients who have had prior head and neck surgery.
4 Cervical Surgery
Cervical spine procedures may be followed by airway-related complications, including vocal cord paralysis and airway obstruction. Vocal cord dysfunction was seen in 5% of 411 patients undergoing anterior cervical discectomy and fusion.194 Stridor was observed in one patient with bilateral vocal cord paralysis who required a tracheostomy. Fifteen of 17 patients had recovered by 12 months. One additional patient had recovered by 15 months, and the remaining patient was lost to follow-up.
Emery and colleagues studied the records of 133 patients who underwent cervical corpectomies with arthrodesis between 1974 and 1989.26 The patients had undergone an anterior approach to achieve a three-level vertebral body and disc resection with bone grafting. This surgical approach requires tracheal and esophageal retraction toward the opposite side to permit exposure. Drains were placed, and all patients were immobilized by a halo vest or a rigid head-cervical-thoracic orthosis. They identified seven patients (5.3%) who required postoperative reintubation, and although they did not compare these patients with those not requiring reintubation, they attempted to identify common features that increased the risk of postoperative airway compromise. Three patients were immediately reintubated in the operating room, and four were reintubated 12 to 91 hours postoperatively. Severe hypopharyngeal swelling was observed at reintubation in four of seven patients and possibly in a fifth. Five of the seven reintubations had no serious sequelae; these patients were extubated within 2 to 8 days. One patient required a cricothyroidotomy, but delay resulted in hypoxic encephalopathy and death. Another patient was reintubated but developed and succumbed to severe adult respiratory distress syndrome. The investigators think that preexisting pulmonary disease, moderate or severe preoperative myelopathy, extensive multilevel decompression with prolonged surgery, and tissue retraction were risk factors for postoperative airway obstruction, but there were no controls.26 They recommended 1 to 3 days of elective intubation postoperatively, a cuff-leak test, and direct laryngoscopy at extubation.
Venna and Rowbottom reviewed the records of 180 patients who had undergone a variety of cervical surgical procedures.28 Based on the Emery study, they had made the decision to keep high-risk patients intubated until they met specified criteria, including a demonstrable cuff leak and the absence of significant airway edema on laryngoscopy. The average time to extubation was 33.5 hours. Despite the delay and the aforementioned criteria, 12 patients (6.6%) demonstrated post-extubation stridor and breathing difficulties, and 5 (2.7%) required reintubation. Two patients required tracheostomy, and two deaths were attributed to airway obstruction and unsuccessful reintubation.
Sagi and coworkers conducted a retrospective chart review of 311 anterior cervical procedures in an effort to identify the factors associated with airway complications.195 In this series, 19 (6.1%) of patients had airway complications, but only 6 (1.9%) required reintubation. Most of these complications were attributed to pharyngeal edema. Risk factors included increased intraoperative bleeding, prolonged surgery (>5 hours), and exposure of more than three vertebral bodies, particularly when they included C2, C3, or C4. Reviewing the literature, these investigators identified an airway complication rate of 2.4% (from 1615 cases), 35 of whom required reintubation or tracheostomy. On average, those requiring reintubation did so at 24 hours.
Epstein and coworkers developed a collaborative protocol involving the neurosurgeon and anesthesiologist. Their objective was avoidance of reintubations.196 Although their study enrolled only 58 patients, they required high-risk, lengthy procedures involving several cervical levels and significant blood loss. All patients remained electively intubated overnight and underwent fiberoptic airway examination before considering extubation. Most patients were extubated the day after surgery, but three remained intubated until day 7. Only one patient required reintubation. This reintubation rate was essentially the same as that observed by Emery and colleagues,26 but Epstein’s cohort appeared to undergo higher-risk surgery.
In an effort to better understand the mechanism of postoperative airway obstruction, Andrew and Sidhu compared the soft tissue changes on the preoperative and postoperative cervical spine radiographs of 32 consecutive patients after a one- or two-level anterior cervical discectomy and fusion.197 They found that the swelling was maximal at the C3-C4 level, corresponding to the area where Emery observed pharyngeal edema. None of their patients experienced dyspnea despite mean differences of 9.4 mm (95% CI, 7.41 to 11.09 mm) to 10.7 mm (95% CI, 7. 8.82 to 12.58 mm) between the preoperative and postoperative radiographs at C3 and C4, respectively. It appears that this area is most vulnerable, regardless of the level operated on. Compared with Sagi’s study, these patients underwent much shorter and more limited procedures.
Patients undergoing posterior cervical surgery may face the risk of macroglossia and significant retropharyngeal and hypopharyngeal swelling, which may be aggravated by fixation of the cervical spine and make intubation more difficult.198 There is a low probability (1.1% to 1.7%) that reintubation will be required,196,198 but accomplishing this may be very difficult.
5 Maxillofacial Surgery and Trauma
Although these concerns demand special attention, deaths rarely occur. In a review of 461 perioperative deaths reported to the Ontario, Canada, coroner between 1986 and 1995, the investigators found only one death associated with orthognathic surgery, although they were unable to determine how many such cases had been performed (see “In Vivo Studies”).199 They were unable to identify nonlethal complications. Meisami and others performed magnetic resonance imaging (MRI) approximately 24 hours after maxillary or mandibular surgery in 40 patients.200 Despite the significant facial swelling seen in almost all the patients, none exhibited soft tissue swelling from the base of the tongue to the glottis.
Complete airway obstruction after elective orthognathic surgery has been reported. Dark and colleagues described a case involving a young woman who underwent seemingly uneventful mandibular and maxillary osteotomies with submental liposuction.201 Immediately after extubation, she developed airway obstruction requiring reintubation. Repeated fiberoptic examination and CT showed severe and extensive edema from the tongue to the trachea, which was maximal at the level of the hyoid. By the fourth postoperative day, a cuff leak was detected, and the patient was successfully extubated over a tube exchanger. Hogan and Argalious described a patient in whom maxillomandibular advancement was performed for OSA.202 The procedure lasted 9 hours, during which he received 7200 mL of crystalloid and 500 mL hetastarch. He remained intubated overnight, and after demonstrating adequate spontaneous ventilation and a cuff leak, he was extubated (over a 19-F AEC). Extubation was immediately followed by clinical evidence of airway obstruction, and he was reintubated. The obstruction was attributed to fractured hardware and a hematoma in the piriform fossa that caused extrinsic compression. This could easily have resulted from periglottic edema. The investigators concluded that patients undergoing this type of surgery face a high risk of airway complications and recommended nasopharyngolaryngoscopy before extubation.
Clinical assessment of airway edema is unreliable,200 and studies indicate that the cuff-leak test is neither sufficiently sensitive nor specific to determine when to extubate these patients. Endoscopic assessment may help to identify patients who harbor occult clots (i.e., “coroner’s clot”) behind the soft palate or adjacent to the glottis, but it may miss or itself give rise to troublesome bleeding.
Maxillofacial injuries often result from unrestrained occupants of motor vehicles encountering an unyielding dashboard, windshield, or steering wheel. Gunshot wounds or physical altercations also cause maxillofacial injury. Airway obstruction is a primary cause of morbidity and mortality in these patients, and many die before they reach the hospital.203 Those with less life-threatening injuries are likely to present with a full stomach, and many have associated head and neck injuries, lacerations, loose or avulsed teeth, intraoral fractures, and fractures extending into the paranasal sinuses, into the orbit, or through the cribriform plate. They may also have an unstable cervical spine or damage to the neural axis. Injuries to the lower face raise the possibility of a laryngeal fracture. Intermaxillary fixation may be part of the surgical plan, necessitating a nasal intubation or a surgical airway. Timing of tracheal extubation is complex and must take into consideration factors such as the patient’s level of consciousness, ability to maintain satisfactory gas exchange, coagulation status, and integrity of protective airway reflexes. Attention must be paid to the difficulties originally encountered in securing the airway and an evaluation of whether reintubation would be easier or more difficult after surgery and resuscitation. Most of the trauma literature about airway management addresses intubation and offers little help with extubation, making cooperation between the anesthesiologist,204,205 surgeon, and critical care physician essential. Intermaxillary fixation requires wire cutters to be immediately available and personnel to know which wires to cut. A flexible bronchoscope, provisions for an emergency surgical airway, and the required expertise should be immediately available at the time of extubation. Alternatives include prophylactic tracheotomy, submental intubation,206–208 nasal intubation, and bronchoscopic airway evaluation performed before extubation,209 although assessment may be limited to supraglottic structures and exclusion of tube entrapment. Ideally, extubation should be accomplished in a reversible manner, permitting supplemental oxygenation, ventilation, and reintubation if needed (see “Extubation Strategies”).
6 Deep Neck Infections
Infections involving the submandibular, sublingual, submental, prevertebral, parapharyngeal, and retropharyngeal spaces are significant airway management challenges, whether intubation is achieved for surgical drainage or for protection during medical management. In expert hands, bronchoscopy-assisted intubation can often be achieved.210 When this is unsuccessful or constitutes a significant risk of rupturing the abscess, a surgical airway before incision and drainage may be called for.211 Potter and colleagues retrospectively compared the outcomes of 34 patients in whom a tracheotomy was performed with 51 patients who remained intubated after surgical drainage.212 All patients had undergone surgical drainage for impending airway compromise and required airway support postoperatively. It was not always evident to the investigators why a particular strategy was chosen, and these groups were not likely identical. Airway loss occurred more commonly in the intubated patients, but this characteristic was not statistically significant. Two deaths occurred, one resulting from an unintended extubation and the other from post-extubation laryngeal edema and an inability to reestablish the airway. The latter patient had a cuff leak before extubation, and signs of obstruction developed 30 minutes after the ETT was removed. Surgical drainage rarely results in immediate airway improvement, and reintubation or emergent placement of a surgical airway, if required, may be complicated by edema, tissue distortion, and urgency.
7 Posterior Fossa Surgery
Posterior fossa surgery can cause injury to cranial nerves, bilateral vocal cord paralysis, brainstem or respiratory control center injury, and macroglossia.114,115,167,213–216 Because the nerve roots may be very close to the operative site, the resultant injuries may be bilateral, extensive, and transient or permanent. Gorski and coworkers suggested that tolerance of the ETT and the absence of a gag reflex on oral suctioning should arouse suspicion of such an injury.214 Howard and colleagues described a patient with a recurrent choroid plexus papilloma involving the fourth ventricle.215 Preoperatively, the patient displayed bulbar dysfunction. His extubation on the first postoperative day was complicated by complete airway obstruction, hypoxia, and a seizure. Laryngoscopy performed after neuromuscular blockade revealed mildly edematous vocal cords. After reintubation and elective tracheostomy, fiberoptic examination showed the vocal folds in a neutral position. Nocturnal ventilation and tracheostomy were still required at 1 and 3 months, respectively. This patient demonstrated central apnea and bulbar dysfunction with hypoglossal paralysis and unopposed vocal fold adduction.
Artru and colleagues described a patient with a cerebellar mass, severe papilledema, and bulbar signs.213 Despite recovery of consciousness and strength, the patient remained apneic and required ventilatory support for 7 days. The investigators cautioned that the dorsal pons and medulla are the sites of the cardiovascular and respiratory centers that control hemodynamics and ventilation. The area is also host to several cranial nerve nuclei. Damage to these areas can result from edema, disruption, ischemia, or compression and may cause a loss of respiratory drive or airway obstruction.
Dohi and coworkers described a patient who developed bulbar signs, including bilateral vocal cord paralysis after excision of a recurrent cerebellopontine angle tumor.167 Negative-pressure pulmonary edema developed as a consequence of a bilateral, presumably central RLN injury, and a tracheostomy was required until recovery 3 months later. During the initial intubation, the glottis could not be seen by direct laryngoscopy, and blind intubation was performed. The details of three subsequent unsuccessful extubations and reintubations were not described. Trials of extubation in a patient known to be a difficult (direct) laryngoscopy case are life-threatening and cannot be justified. A tracheostomy was performed, and vocal cord function recovered after 3 months.
Early vocal cord evaluation after extubation has been advocated along with the involvement of a neurosurgeon, otolaryngologist, speech therapist, and intensivist to manage patients who have developed laryngeal dysfunction.216 A tracheostomy and an enteral feeding tube may be needed. A more preemptive approach (described later in more detail) involves flexible laryngoscopic assessment through a supraglottic airway (SGA) after removal of the ETT.
8 Stereotactic Surgery and Cervical Immobilization
Stereotactic neurosurgical and neuroradiologic procedures are finding increasing applications. When head frames are used, they may impede access for SGA placement or laryngoscopy. Similarly, patients in cervical immobilization devices for spinal cord protection may undergo high-risk surgical procedures.116 Careful planning for their extubation is critical because reintubation may be difficult, and rapid surgical access may be virtually impossible. Full recovery of strength and consciousness, persistence of respiratory drive, the presence of a cuff leak, preservation of protective reflexes, and absence of significant tongue swelling are the essential prerequisites for extubation. Postoperative seizures, vomiting, elevated intracranial pressure, and neurologic obtundation may make extubation particularly hazardous. Several of the strategies described subsequently should be given serious consideration in managing these patients.
9 Tracheal Resections
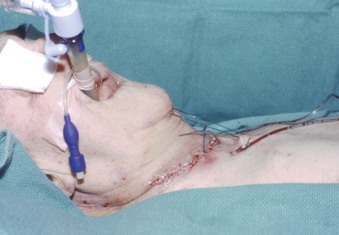
Patients with moderate or severe tracheal stenosis may come for surgical tracheal resection. These patients usually have tracheal stenosis or tracheomalacia, often caused by prolonged intubation or occasionally caused by a retrosternal mass. Some patients may have compromised preoperative respiratory function. After an end-to-end anastomosis, the surgeon may elect to place a “guardian suture” from the chin to the chest, maintaining the head and neck in flexion and thereby minimizing traction on the suture lines (Fig. 50-1).217,218 The preference is for early extubation to avoid positive pressure, coughing, and presence of a foreign body in the airway.217–221 A cough-free extubation is highly desirable, as is avoidance of a need for reintubation, which if required could prove very challenging.
10 Palatoplasty
A variety of surgical procedures have been employed to treat OSA, including uvulopalatopharyngoplasty, midline glossectomy, mandibular advancement, limited mandibular osteotomies with genioglossal advancement, and hyoid bone suspension.222 Pepin and colleagues published a critical analysis of the literature on the risks and benefits of surgical treatment of snoring and OSA.223 They identified “at least five deaths” after uvulopalatopharyngoplasty and found that few studies had adequate numbers to allow conclusions to be drawn regarding their outcomes. Less than one half of the studies commented on the frequency of complications. A retrospective review of 101 uvulopalatopharyngoplasties identified an early postoperative respiratory complication rate of 10%.224 Ten of 11 patients required reintubation, and 1 death resulted from airway obstruction.
Uvulopalatopharyngoplasty was introduced to deal with retropalatine collapse. However, in approximately one half of the adult patients with OSA, obstruction occurs at the retrolingual pharynx. Tongue suspension is one of several approaches introduced to manage the latter group of patients.225 The procedure involves placement of an anchoring screw in the genial tubercle and attachment of a suture through the base of the tongue. Szokol described a morbidly obese patient with OSA in whom this procedure was performed.222 Laryngoscopy and bag-mask ventilation had been difficult. At the conclusion of the procedure, the patient was fully awake, was able to sustain a head lift for 5 seconds, demonstrated a negative inspiratory pressure of 40 cm H2O, and was extubated. Stridor was observed immediately, and bag-mask and laryngeal mask ventilation were ineffective. Attempts to reintubate the patient were unsuccessful, necessitating a cricothyroidotomy. Subsequent direct laryngoscopy showed a markedly swollen epiglottis and grossly edematous laryngeal and hypopharyngeal tissues. The patient developed negative-pressure pulmonary edema, and a tracheostomy was performed 2 days later because of persistent swelling. Tracheal decannulation occurred uneventfully 2 weeks later. The physicians speculated that airway manipulation during the surgery was the cause of this patient’s swelling. They did not consider that the swelling might have resulted from or at least been aggravated by repeated attempts at laryngoscopy.
Palatoplasty, alone or in combination with other procedures, may be performed on patients with cleft palates or other congenital abnormalities. In one study, 14 (5.7%) of 247 patients undergoing palatoplasty had postoperative airway problems, 12 of whom required reintubation. One half of the patients experiencing complications had Pierre Robin sequence; three of the patients required reintubation 24 to 48 hours postoperatively.226
B Preexisting Medical Conditions
1 Paradoxical Vocal Cord Motion
Paradoxical vocal cord motion (PVCM) is the quintessential example of a situation wherein reintubation will be required. Intubation is not more difficult; extubation is the challenge. This uncommon and poorly understood condition is frequently mistaken for refractory asthma or recurrent laryngospasm.227–229 The diagnosis is both overlooked and overused, leading to confusion.230 It also is called vocal cord dysfunction, Munchausen stridor, psychogenic stridor, factitious asthma, pseudoasthma, and irritable larynx syndrome.230,231 Normal vocal cord motion involves inspiratory abduction and 10% to 40% adduction on expiration. With PVCM, adduction of the true vocal cords occurs on inspiration or expiration, or both. The false vocal cords and the posterior laryngeal wall may further contribute to the airway obstruction.231–234 This condition may be associated with psychosocial disorders, stress, exercise, gastroesophageal reflux, irritant exposure, or airway manipulation. Pulmonary function tests show normal expiratory but flattened inspiratory flow loops. It is important to differentiate this condition from asthma, laryngospasm, anaphylaxis, angioedema, gastroesophageal reflux, and vocal cord paralysis. The incidence of PVCM is unknown.
Harbison and coworkers described two patients who had post-extubation stridor after thyroidectomies.235 This is a particularly challenging situation with a complex differential diagnosis, especially because one of the patients had unilateral vocal cord paralysis preoperatively. In that patient, post-extubation stridor developed 24 hours postoperatively and could be observed while awake and asleep. Fiberoptic examination under sedation showed paradoxical motion of the mobile cord. She was managed successfully with speech therapy. They speculated that these cases might have resulted from surgical manipulation of the RLN during the thyroidectomies.
Hammer and colleagues described a 32-year-old woman with recurrent episodes of stridor,236 sometimes associated with cyanosis, despite normal flow-volume loops and pulmonary function tests. The diagnosis of PVCM was made endoscopically and managed with relaxation techniques. After preoperative sedation, topical lidocaine, and bilateral SLN blocks, she underwent an awake fiberoptic intubation. At the conclusion of surgery, extubation was performed after she was fully awake, but sustained inspiratory stridor ensued, resulting in reintubation. A subsequent attempt the next day confirmed inspiratory vocal fold adduction, and a tracheostomy was required for 58 days. In the absence of features predicting a challenging intubation, there seems little justification for awake intubation, and it may contribute unnecessarily to an anxiety disorder.
PVCM imposes no special requirements for intubation. The abnormality is functional rather than anatomic. Appropriate management depends on having the correct diagnosis, which requires clinical suspicion and endoscopic confirmation of inspiratory adduction of the vocal cords. Adequate oxygenation, consideration of CPAP or helium-oxygen administration, positioning, reassurance, and support may suffice, although sedation may be required after the diagnosis is confirmed. Speech therapy, psychotherapy, hypnosis, and reassurance may be helpful in the long-term management,237 but such is not always the case.227 Some reports have recommended electromyographically guided botulinum toxin injection into the thyroarytenoid muscle for recalcitrant cases. The optimal anesthetic management of these patients is unknown. Regional anesthesia avoids airway intervention, but it does not ensure that a condition that may be stress related will not occur. Familiarity with this condition, calm reassurance when there is prior suspicion, and perhaps deep extubation seem prudent.
2 Parkinson’s Disease
Susceptibility to aspiration is common among patients with Parkinson’s disease and is the most common cause of death. Dysphonia, most frequently hypophonia, occurs in approximately 70% to 90% of patients with Parkinson’s disease.238,239 Video stroboscopic findings include laryngeal tremor, vocal fold bowing, and abnormal glottic opening and closing.239 Several neurodegenerative diseases, including multiple system atrophy, have some features in common with Parkinson’s disease, including dysphonia, and these patients may exhibit bilateral abductor vocal fold paresis. Typically, symptoms in patients with Parkinson’s disease progress insidiously, are not recognized by the patient, and may be associated with nocturnal stridor. These features resemble those of OSA identified by polysomnography. Many of these patients may benefit from nocturnal CPAP or bi-level positive airway pressure (BiPAP).238
Patients with multiple system atrophy have daytime hypoxemia associated with abnormal laryngopharyngeal movements, including obstruction at the arytenoids, epiglottis, base of the tongue, and soft palate. The significance of these problems is unclear, but they may contribute to complications after extubation.240
Vincken and colleagues studied 27 patients with extrapyramidal disorders.241 Twenty-four had flow-volume loops, many of which demonstrated saw-toothed oscillations, even in the absence of respiratory symptoms. They observed oscillations with rhythmic (4 to 8 Hz) or irregular movements of the glottis and supraglottic structures. Ten patients exhibited intermittent upper airway obstruction. Four patients had stridor or dyspnea. The investigators believed that the upper airway was the primary site of involvement. In a subsequent report, they observed symptomatic improvement with levodopa despite persistence of the oscillatory pattern on flow-volume loops.242 Inspiratory and expiratory flows after levodopa increased from 1.40 to 3.50 L/sec and 0.95 to 5.05 L/sec, respectively. Bronchodilators provided no additional benefit. This case may have important implications for the perioperative management of patients with Parkinson’s disease.
Easdown and colleagues described a patient with Parkinson’s disease who had a respiratory arrest 60 hours after surgery.243 Before that event, the patient had episodic desaturation, labored breathing, and progressive hypercapnia in the absence of tremor or rigidity. Treatment with bronchodilators produced no benefit, and his condition improved immediately after intubation. With the ETT, compliance and resistance appeared normal. This patient’s levodopa or carbidopa had not been resumed postoperatively, and the investigators speculated that this caused or contributed to upper airway obstruction. Because most patients with Parkinson’s disease are elderly and may have comorbidities that can make the diagnosis uncertain, it is important to consider involvement of the upper airway and the dramatic effect withdrawal and reinstatement of medications can have on their clinical course. This concern is reinforced by a case report describing a patient who developed airway obstruction and acute respiratory acidosis requiring intubation preoperatively because five doses of his antiparkinsonian medications were withheld while he was being fasted.244 Easdown and colleagues emphasized the importance of continuing these medications, and avoidance of dopamine antagonists throughout the perioperative period.243
Backus and colleagues described a patient with long-standing Parkinson’s disease who became aphonic, developed stridor, and suffered respiratory arrest shortly after taking cough medication.245 Complete upper airway obstruction recurred with vocal fold apposition immediately after extubation. Four days later, the patient extubated herself with no further complications. The investigators interpreted this spontaneous laryngospasm as a manifestation of Parkinson’s disease. Others have observed upper airway dysfunction, airflow limitation, and bilateral abductor vocal cord paralysis in association with Parkinson’s disease. The first episode might not have been spontaneous but instead a consequence of aspiration of the cough medicine. Nonetheless, there remains a possibility that these patients are more prone to laryngospasm, whether spontaneous or induced by glottic stimulation.
Liu and coworkers described airway obstruction during induction of anesthesia.246 Despite being unable to visualize the larynx, they attributed the obstruction to laryngospasm. The obstruction resolved with awake, blind nasal intubation but recurred 24 hours later on extubation. At that point, fiberoptic examination showed inspiratory vocal fold adduction, necessitating reintubation. It is unclear whether they were observing manifestations of Parkinson’s disease or PVCM, but extubation was uneventful 24 hours later after increasing the dosage of levodopa or carbidopa.
Parkinson’s disease is a common disorder, but only 13 cases of stridor have been attributed to it.239 The pathogenesis of upper airway obstruction is unknown. It may be mediated by the basal ganglia and nucleus ambiguus. A similar phenomenon involving esophageal spasm has been associated with Parkinson’s disease. One theory invokes laryngeal hypertonicity, which may be triggered by copious secretions.
3 Rheumatoid Arthritis
In patients with rheumatoid arthritis, the airway manager needs to be concerned about three joint areas: the cervical spine, the temporomandibular joint (TMJ), and the cricoarytenoid joint.247 Autopsy studies suggest that 30% to 50% of patients with rheumatoid arthritis have significant cervical spine involvement. Cervical subluxation has been identified clinically in 43% to 86% of these patients and may represent a serious neurologic risk during intubation with flexion or extension.145,247–249 The spectrum of cervical involvement ranges from ligamentous destruction with subluxation and impaction to extreme limitations in the range of motion because of fibrosis and ankylosis. These patients may have a narrowed glottic aperture, limited mouth opening due to involvement of the TMJs, micrognathism, laryngeal deviation, and cricoarytenoid and cricothyroid involvement.250,251 Kohjitani and colleagues retrospectively described four patients undergoing bilateral TMJ replacement; three had glottic erythema and swelling on endoscopy, three had OSA, and three experienced laryngospasm at intubation and after extubation.251 TMJ involvement may result in loss of ramal height and micrognathia with or without ankylosis and associated OSA.
Cricoarytenoid arthritis and its consequences have long been recognized in the anesthesia and general medical literature.252–256 Although rheumatoid arthritis is the most common cause of this condition, it also may be associated with bacterial infections, mumps, diphtheria, syphilis, tuberculosis, Reiter’s syndrome, ankylosing spondylitis, systemic lupus erythematosus, gout, progressive systemic sclerosis, and other conditions.257 The cricoarytenoid joint has a synovial lining and bursa. Its mobility is vital for speech, respiration, and protection from aspiration. Inflammatory changes may include effusion, pannus formation, joint erosion, and ankylosis, any of which may compromise the joint’s functions. Its involvement may be unsuspected or mistaken for asthma until intubation or after extubation and may necessitate a surgical airway.258,259 Dysphonia, dyspnea, or stridor should raise suspicion of this possibility. Complete airway obstruction is a well-described but uncommon complication, despite involvement of the cricoarytenoids in 26% to 86% of patients with rheumatoid arthritis.251,259 Laryngoscopy may reveal a rough and thick mucosa with narrowing of the vocal chink. Although airway obstruction occurs most commonly in patients with long-standing rheumatoid arthritis with polyarticular and systemic involvement, laryngeal stridor has been described as the sole manifestation of this disease.260
Keenan and coworkers described tracheal scoliosis, which consisted of tracheal deviation, laryngeal rotation, anterior angulation, and vocal fold adduction seen fiberoptically and on CT scans.255 It was presumed to result from the loss of vertical height and asymmetrical bony erosions.
Wattenmaker and colleagues studied patients with rheumatoid arthritis undergoing posterior cervical spine procedures.250 Their primary objective was to compare the perioperative airway complications seen in rheumatoid arthritis patients when intubation was performed by direct laryngoscopic or flexible bronchoscopy. Retrospectively reviewing 128 consecutive posterior cervical procedures, upper airway obstruction characterized by stridor occurred in 9 of 128 patients, 1 of 70 patients intubated with bronchoscopic guidance, and 8 of 58 patients intubated otherwise (i.e., direct laryngoscopy or blind nasotracheal technique). Five patients (all in the nonbronchoscopic group) required emergency reintubation that proved to be very difficult, with two near fatalities and one death. Although the two groups were similar with regard to age, gender, American Rheumatology Association classification, ASA physical status, duration of surgery and anesthesia, fluid balance, and postoperative immobilization, there were significant differences in time to extubation. Seven of the patients could not be intubated by flexible bronchoscopy and were therefore intubated by a nonfiberoptic technique. The patients were not randomized to different methods; criteria for the method of intubation and techniques were not described; all patients were intubated awake; and the study was carried out over an 11-year period.261 Although it is not possible to draw firm conclusions from this study, there was a high incidence (7%) of post-extubation stridor and difficult or failed reintubations, regardless of the intubation technique.
Patients with rheumatoid arthritis qualify as higher-risk extubation cases because they may have a fixed or unstable cervical spine, TMJ ankylosis, difficult intubations by direct or flexible laryngoscopy, and increased risk of post-extubation airway obstruction. Several investigators have recommended postponing extubation until the patient is wide awake. Unfortunately, this provides increased protection against nothing other than laryngospasm and aspiration. The prevailing wisdom is that patients with limited mouth opening and a potentially unstable cervical spine should be intubated with a flexible bronchoscope.250 This method involves blind passage of the ETT through the cords, which may be traumatic,91,261 particularly in the face of preexisting cricoarytenoid arthritis. Regional anesthesia should be considered as an alternative to general anesthesia when appropriate. When intubation cannot be avoided, proposed extubation strategies include a preemptive tracheostomy or a method that increases the reversibility of extubation. Neither strategy has been prospectively evaluated in this population.
4 Tracheomalacia
Tracheomalacia is a dynamic airway obstruction resulting from loss of the cartilaginous tracheal support. This results in the posterior membranous wall bulging anteriorly when the intratracheal pressure is reduced or the intrathoracic pressure is increased.141 Although rare, it should be considered when the patient has dyspnea on exertion with difficulty clearing secretions and a seal-like, incessant cough.141,262
Patients frequently are misdiagnosed with asthma and fail to respond to escalating therapy. Pulmonary function tests (i.e., forced expiratory volume at 1 second, forced vital capacity, and peak expiratory flow) show severely diminished expiratory flow with relative preservation of the inspiratory flow. The diagnosis may be confirmed fiberoptically during spontaneous breathing. Tracheomalacia may be congenital263 or result from vascular compression,264 an intrathoracic goiter,265 chronic obstructive pulmonary disease, or prolonged intubation. The latter may be caused by ETT cuff-induced erosion of the tracheal cartilage with or without extension to the membranous trachea.
The severity of the dynamic obstruction is proportional to the expiratory force. It may be unapparent during quiet breathing but disabling in a distressed patient. Positive pressure or bypassing the lesion with a tracheal tube provides temporary relief while further management options are considered. They may include medical management, surgical resection, or placement of a stent.262 Additional suggestions for the extubation of a patient with suspected tracheomalacia are described later.
Relapsing polychondritis is an example of extensive tracheobronchomalacia. It is a rare, multisystem disease characterized by episodic inflammation of cartilaginous structures resulting in tissue destruction.262,266 Laryngeal and tracheal tract involvement occurs in approximately one half of patients. It usually occurs early in the course of the disease and may manifest as hoarseness, nonproductive cough, shortness of breath, and stridor. Upper airway obstruction is usually diffuse and may progress to involve the glottis, subglottic area, trachea, and bronchial cartilages. Histologically, there is evidence of perichondral inflammation and replacement of cartilage by fibrous tissue that manifests as inflammatory swelling and progressive destruction of cartilage. The clinical manifestations range from bronchorrhea and recurrent pneumonia to airway collapse. Medical management consists of steroids, nonsteroidal anti-inflammatory drugs, and immunosuppressant agents, but their benefit varies. Surgical management consists of external airway splinting or self-expanding metallic stents. These patients may present for bronchoscopy, tracheostomy, tracheal or nasal reconstruction, aortic valve replacement, or stent placement.264,267–271 Airway collapse after extubation should be anticipated and may be temporarily dealt with by CPAP.272,273
5 Obstructive Sleep Apnea Syndrome
In the ASA Closed Claims Project analysis of adverse respiratory events, 65 of the 156 perioperative events involved obese patients; for the claims specifically related to extubation, 12 of the 18 were obese, and 5 of these patients had been diagnosed with OSA.2 OSA correlates positively with age and obesity, both of which are becoming increasingly prevalent. The pathophysiology and perioperative airway management of OSA in obese patients has been comprehensively reviewed.274–276 Many surgical patients have undiagnosed or untreated OSA. OSA syndrome is associated with an increased risk of gastroesophageal reflux, difficult mask ventilation276–278 and laryngoscopic intubation,279–282 and accelerated arterial oxygen desaturation.276,283 The risk of airway obstruction after surgery is increased for patients with OSA; life-threatening post-extubation obstruction occurred in 7 (5%) of 135 patients.279,284 Rapid desaturation, difficult mask ventilation, and difficult direct laryngoscopy make this a particularly high-risk setting.285 The ASA practice guidelines for the management of patients with OSA provided limited guidance beyond a strong recommendation that they be fully awake and that the airway manager verify that neuromuscular blockade is completely reversed before extubation. If possible, extubation and recovery should be carried out in the lateral or semi-upright position,286 nasal CPAP should be available or routinely implemented, and consideration should be given to extubation over a tube exchanger.274,276,280,284,287 These strategies have been associated with better outcomes, and anecdotal comparisons are compelling, but they have not been subjected to controlled, randomized trials, and they were not addressed by the ASA Task Force.
6 Laryngeal Incompetence
Laryngeal function may be disturbed for at least 4 hours after tracheal extubation.288 Immediately after extubation, 8 (33%) of 24 patients aspirated swallowed radiopaque dye; 5 showed radiologic evidence of massive aspiration. Four hours after extubation, 4 (20%) of 20 patients aspirated dye; 3 had massive aspirations. At 24 hours, the rate was reduced to 5%. In this study, patients had been intubated for 8 to 28 hours during and after cardiac surgery. Although the investigators did not observe a relationship between duration of intubation (8 to 28 hours) and aspiration, it is unclear whether the presumed laryngeal incompetence occurs after brief intubation or is more common and severe with prolonged intubation. The mechanism of laryngeal incompetence was postulated to be primarily sensory because patients who aspirated dye did not cough.
Residual neuromuscular paralysis is a common problem in postoperative patients and may result in hypoventilation, hypoxemia, pharyngeal and laryngeal dysfunction, or increased pulmonary aspiration.264,289 Pharyngeal function was impaired in conscious volunteers receiving a continuous infusion of vecuronium and resulted in laryngeal penetration of contrast medium proportional to the degree of blockade.290 Relaxation of the upper esophageal sphincter was also observed. None of the volunteers coughed or had respiratory symptoms. Berg and colleagues found a higher incidence of postoperative pulmonary complications (i.e., pulmonary infiltrate or atelectasis associated with cough, sputum, or shortness of breath) among patients randomly assigned to receive a long-acting or intermediate-acting neuromuscular blocker.291 It is intriguing to speculate on how residual neuromuscular blockade may contribute to laryngeal incompetence.
7 Pulmonary Aspiration of Gastric Contents
Although more patients are being diagnosed with gastroesophageal reflux, the diagnosis of perioperative pulmonary aspiration has not increased.292,293 Aspiration is estimated to complicate 1 of 2000 to 3000 general anesthetics and was responsible for only 3 of 156 perioperative events in the ASA Closed Claims Project review.2,293,294 Nonetheless, it is the leading cause of pneumonia in the ICU and a common cause of acute respiratory distress syndrome.295 Many of these cases are ventilator-associated pneumonia and occur with the ETT in situ. Factors predisposing a surgical patient to aspiration include emergency surgery, pain, obesity, narcotics, nausea, ileus, bowel obstruction, pregnancy, some surgical positions, depressed level of consciousness, inadequate depth of anesthesia, postoperative drowsiness, and residual neuromuscular blockade. Despite the ubiquity of these conditions, perioperative aspiration is not commonly identified. Before intubation, difficult bag-mask ventilation may result in gastric distention, which may be further complicated if laryngoscopy proves difficult because it may delay securing the airway. Repeated laryngoscopic attempts may cause edema, thereby increasing glottic resistance. Aspiration may also result from obtundation or conditions that impair vocal cord apposition (e.g., vocal cord paralysis, laryngeal incompetence, residual neuromuscular blockade, granulomas). Aspiration can cause serious morbidity and death.292–295
Although most incidents of aspiration seem to occur at induction, many occur during maintenance and recovery from anesthesia.296 Numerous strategies have been described to reduce the risk at induction, but relatively little information is available on how best to prevent this later. Premature extubation, postoperative nausea, delayed gastric emptying, residual neuromuscular blockade, relaxation of the esophageal sphincters, decreased level of consciousness, gagging on an ETT, supine recovery, and impaired laryngeal competence may make emergence from anesthesia and tracheal extubation as problematic as induction. A kinked or clamped nasogastric tube may promote regurgitation and aspiration. Evidence-based recommendations on an extubation strategy to reduce aspiration are not available. It would seem logical to minimize the contributing factors: postoperative nausea and vomiting, residual neuromuscular blockade, decreased level of consciousness and associated diminished protective airway reflexes, and gastric evacuation. We do not know whether gastric decompression reduces aspiration; a well-seated i-gel SGA297 or ProSeal LMA may or may not offer some protection from aspiration.298–300 With the current information, it is not appropriate to recommend the elective use of these devices in a patient at increased risk for aspiration.
V Factors Affecting Intubation and Extubation
A Previously Encountered Airway Difficulties
Multiple attempts at laryngoscopy by experienced personnel, a need for alternative airway management techniques due to failure of direct laryngoscopy, and prior difficulty prompting the primary use of alternative techniques are settings in which reintubation may be problematic. In urgent or emergent circumstances, methods that had previously been successful may not be available or appropriate. The required equipment, necessary skills, or time required to perform alternative techniques may not be available. Uncertainty regarding the ease of ventilation or intubation may correctly lead to disinclination to administer paralytic and sedating drugs. Although they may facilitate ventilation and intubation, failure will result in an apneic patient who can neither be ventilated nor intubated. Knowledge of prior difficulties may result in intubation conditions that are less favorable to success. Repeated attempts at laryngoscopy are associated with a significant increase in the risk of hypoxemia, esophageal intubation, regurgitation, aspiration, bradycardia, and cardiac arrest.2,301 To avoid this risk, flexible bronchoscopic intubation may be considered, but in an agitated, hypoxic patient with secretions or blood in the airway, it may be difficult to achieve adequate topical anesthesia, and the procedure may be difficult or impossible.
B Limited Access
Limited access to the airway is exemplified by intermaxillary fixation, severe cervical restriction, instability, or immobilization, and the chin-to-chest guardian suture (see Fig. 50-1) to prevent traction tracheal resection. More commonly, this situation may arise in the confining space of a PACU, ICU, or patient room where access is limited. In each case, there may be additional risks related to oxygenation, ventilation, airway obstruction, or pulmonary toilet. For example, after cervical fixation or orthognathic surgery, the patient may have macroglossia or supraglottic edema. A patient requiring tracheal resection may be unable to clear blood or secretions from the airway.
C High-Risk Cases
The clinical playing field may not be level at all hours of the day. The immediate availability of highly trained primary and support personnel, equipment, and the necessary clinical information may be problematic at night or during periods of intense activity. The ASA Task Force on Management of the Difficult Airway and the Canadian Airway Focus Group recommended a preformulated strategy for extubation of the difficult airway.5,6 The ASA Closed Claims Project supported the need for such a strategy.2 Patients at risk for hypoventilation, hypoxemia, and loss of airway patency have been discussed. The remainder of this chapter addresses specific extubation strategies.
VI Extubation Strategies
If any of the higher-risk extubation conditions exists or is anticipated, the clinician should consider a strategy that does not cut off access to the airway. Ideally, the strategy should permit continued administration of oxygen or ventilation of a failing patient even while the airway is being reestablished. These objectives are consistent with the ASA Task Force and Canadian Airway Focus Group recommendations.5,6
A Deep versus Awake Extubation
Extubations may be performed before or after recovery of consciousness. Deep extubation ordinarily occurs after full recovery of neuromuscular function and the resumption of spontaneous ventilation. Its purported advantage is avoidance of the adverse reflexes associated with extubation, such as hypertension, dysrhythmias, coughing, laryngospasm, and increased intraocular or intracranial pressures. The fundamental disadvantage of deep extubation is the patient’s inability to protect his airway against obstruction and aspiration. When deep extubation is improperly executed, laryngospasm and its attendant complications are more likely to occur. Although not having to wait for the recovery of consciousness may accelerate operating room turnover, this approach is more difficult to justify when anesthetic agents having a faster elimination time are available. Delays in recovery usually are brief. Unscavenged volatile anesthetic agents may also represent an occupational health hazard. A significant proportion of American anesthesiologists practice the technique, at least some of the time, but there are few data for adults that compare the safety of deep extubation with that of awake extubation.12 Koga and colleagues compared three small groups of adult patients who underwent deep extubation, awake extubation, or deep extubation after the insertion of an LMA.80 Straining occurred in a high (but comparable) proportion of patients whether the ETT was removed before or after recovery of consciousness.
B Extubation with a Laryngeal Mask or Other Supraglottic Airway
On emergence from general anesthesia, most patients tolerate an LMA with less coughing and changes in intraocular, intracranial, and arterial pressures (see Fig. 50-1).57,77,80,302,303 Silva and Brimacombe substituted an LMA for the ETT in a small series of patients while still asleep and paralyzed after completion of neurosurgical procedures.304 Muscle relaxation was then reversed, and the anesthetic was discontinued. The LMAs were removed when the patients resumed spontaneous ventilation and obeyed commands. None of the 10 patients coughed, and changes in the rate-pressure product (indicating cardiac oxygen requirements) were minimal. The investigators concluded that the technique might prove useful in patients undergoing other types of surgical procedures. They stressed that this substitution should be performed only by those skilled in LMA insertion. Patients must be at a sufficient depth of anesthesia or coughing, breath-holding, laryngospasm, and the very pressor responses this substitution is intended to avoid may occur. Bailey and others recommended that the LMA be inserted before removal of the ETT to prevent losing the airway after tracheal extubation.80,305 Compared with deep tracheal extubation followed by Guedel airway insertion, there was a lower incidence of coughing and requirement for airway manipulation.305 Koga and coworkers compared this technique with deep and awake tracheal extubation.80 They observed no difference in recovery conditions between patients in whom the ETT was removed by deep or awake methods; however, they noticed a significant improvement in recovery conditions when the LMA substitution was performed. This technique is useful but can jeopardize a secure airway if not properly executed. It should be practiced on routine airways before use in higher-risk extubations.306 Brimacombe suggested (personal communication, December 2010) that a ProSeal LMA or LMA Supreme with a gum elastic bougie inserted through the drainage tube could produce a more secure substitution for an ETT.307
Sometimes, it is desirable to perform the exchange of an ETT for an SGA in reverse. Several types of tube exchanges have been described. Asai wanted to replace a damaged ETT in a patient who had been a difficult intubation.308 He inserted an LMA Classic behind the existing ETT. A fiberoptic bronchoscope (FOB) with a replacement 7-mm ETT was introduced through the LMA; the FOB was advanced through the vocal cords, and the original ETT was removed. The new ETT was then advanced over the FOB, which was removed. To extend the length of the ETT to enable its removal from the LMA, another ETT was inserted into the proximal end of the replacement ETT.308 This technique was complicated and could easily have failed.
Matioc and Arndt wished to substitute an ETT for a ProSeal LMA.309 Using an Arndt Airway Exchange Catheter Set (Cook Critical Care, Bloomington, IN) (see Fig. 50-6), they introduced an FOB through the ProSeal LMA into the trachea. The set comes with a 144-cm extrastiff Amplatz guidewire, which was passed through the FOB, and the latter was removed. An 11-F, 70-cm, Cook airway exchange catheter (CAEC) was introduced over the guidewire and the ProSeal LMA was removed. The replacement ETT was then advanced over the exchange catheter.
A simpler approach involving the Aintree intubation catheter (Cook Critical Care) has been described with a variety of SGAs, including the cuffed oropharyngeal airway (COPA),310 the LMA Classic,311–313 the LMA ProSeal,314,315 and the LMA Supreme.316 The Aintree intubation catheter is 56 cm long and has an internal diameter (ID) of 4.7 mm; its outer diameter (OD) is 6.3 mm (19 F). A bronchoscope (<4 mm) can be inserted through the device, leaving 3 or 4 cm protruding beyond its tip. A 7-mm or larger ETT can be advanced over it. Only the distal 3 to 4 cm of the protruding bronchoscope is flexible, but it is usually sufficient to allow successful maneuvering into the trachea, after which the SGA is removed and a replacement ETT is advanced over the Aintree catheter. A Rapi-Fit adapter is provided to enable positive-pressure ventilation while the substitution is performed. The Aintree intubation catheter and FOB are inserted together through the SGA. There are several advantages of this technique.315,317 It can be used to facilitate conversion from an unmodified LMA to an oral ETT of adequate size. Sufficient length allows the LMA to be removed with minimal risk of losing the airway. The Aintree intubation catheter fits tightly to the insertion cord of the FOB and to the ETT, thereby reducing the size discrepancy that often results in difficult glottic passage. The catheter can be used as a conduit for manual or jet ventilation during an exchange. An LMA Classic, inserted as a rescue device (for “cannot intubate, cannot ventilate” cases) can facilitate safe tube exchange without the need for an intubating LMA.
C Extubation or Reintubation over a Fiberoptic Bronchoscope or Laryngoscope
When tube entrapment is a possibility, extubation over an FOB can avert a disastrous outcome. For a spontaneously breathing patient, extubation over an FOB provides the opportunity of visually assessing the trachea and laryngeal anatomy and function. This can help in the patient suspected of having tracheomalacia, vocal cord paresis, or PVCM. It also permits assessment of supraglottic structures.318 These opportunities can be maximized by reassuring the patient and by providing judicious sedation, an antisialagogue, and the use of a Yankauer sucker for oral secretions. The oropharynx is suctioned, taking care to avoid inducing a gag reflex. The FOB is placed above the carina, and the cuff is slowly deflated to minimize coughing. The ETT is slowly withdrawn into the oropharynx, followed by very gradual withdrawal of the FOB to the supraglottic region. After the patient is comfortable, the FOB is further withdrawn to a position just above the vocal cords. Even with this deliberate technique, the exercise is frequently frustrated by excessive secretions, coughing, swallowing, or poor tolerance with insufficient opportunity of visualizing the structures of interest.
If the technique is successful, it may identify problems and anticipate complications. When significant abnormalities are identified, a decision must be made about whether to immediately reinsert the ETT or withdraw the FOB and manage the patient with agents such as corticosteroids, racemic epinephrine, and helium-oxygen.149,152 This technique is not practical for performing a trial of extubation, in part because such a trial lasts only seconds or minutes.
Watson endorsed the use of an FOB to exchange ETTs, citing the advantages of minimal sedation, risk of aspiration, hemodynamic embarrassment, and uncertainty about tube placement.319 His technique involved passing the loaded FOB alongside the existing ETT. He had used this technique successfully in 13 of 15 attempts. Dellinger suggested that use of an FOB to perform tube exchanges offered the greatest likelihood of reintubation success and recommended techniques for conversion from nasal to oral, oral to nasal, and oral to oral ETT exchange in addition to extubation.318 We have found this method of extubation to be unreliable and one that demands immediate and often incorrect clinical judgment.
D Extubation with a Supraglottic Airway with or Without a Bronchoscope
This technique is useful in patients with recurrent post-extubation stridor or those at risk for static or dynamic tracheal stenosis. We frequently employ this technique in patients undergoing thyroidectomy when tracheomalacia or vocal fold paralysis is suspected.181
E Use of a Gum Elastic Bougie or Mizus Endotracheal Tube Replacement Obturator
Finucane and Kupshik described an awake, blind nasal intubation in a patient with cervical instability, but the cuff became damaged, requiring a tube exchange.320 They used the 63-cm-long, 4-mm-OD plastic sleeve from a brachial central venous catheter as a tube exchanger. Others have used a gum elastic bougie to achieve similar objectives.321–324
Cook Critical Care designed the Mizus Endotracheal Tube Replacement Obturator (METTRO) for the replacement of endotracheal and tracheostomy tubes (Fig. 50-2). It is available in two sizes: 70 cm long (7.0 F, 2.3 mm) for replacement of ETTs as small as 3 mm and 80 cm long (19 F, 6.3 mm) for passing through tubes 7 mm or larger. The METTRO is a single-use, flexible, radiopaque, solid device with a tapered tip and distance markings. The package insert instructs the user to advance this device until resistance is encountered, but this recommendation can result in coughing, discomfort, hypertension, and tachycardia. Tracheal perforation has been reported using different devices but following similar recommendations.325,326
The smaller airway obturator has been used to maintain airway access during tracheostomies in 22 patients and for “tentative extubations” in 7 patients.327 The smaller-caliber device was preferred by Audenaert and colleagues because patient discomfort was minimal during tube exchanges and the device was unobtrusive during surgical tracheostomies. The obturator was removed when it was apparent that the patient was unlikely to require reintubation. The 19-F obturator was not conducive to spontaneous breathing. Chipley and coworkers used a METTRO in an obese patient with a fractured occipital condyle recovering from respiratory failure.328 They left this in place for 48 hours, removing it when extubation appeared to be successful. They also described the use of the obturator to stimulate coughing, although this might have been ill advised given the possibility of tracheal perforation. The METTRO obturator is being phased out and will soon be unavailable.
In a modification of the Eschmann gum elastic bougie (Intubation Guide, Smiths Medical, Keene, NH), both ends of the bougie were amputated, exposing a hollow core. A cannula, syringe, and ETT connector enabled this assembly to be connected to an oxygen source.329
F Use of Jet Stylets
The ubiquitous nasogastric tube has been used as an exchange catheter,330 but these devices are specifically formulated to become softer as they are warmed. This thermolability is not a desirable characteristic for a tube exchanger.
Bedger and Chang coined the term jet stylet to refer to a self-fashioned, 65-cm-long, plastic catheter with a removable 15-mm adapter at the proximal end. It could be connected to an anesthesia machine or jet injector.331 They created three side ports cut into the distal 5 cm to minimize catheter whip during jet ventilation. They used the stylet for extubation or reintubation of 59 patients. It also functioned adequately in patients when it was used for jet ventilation and oxygen insufflation. Although no complications were described in this series, in an earlier report, Bedger and Chang described tension pneumothoraces in 3 of 600 patients ventilated through a 3.5-mm-OD pediatric chest tube at 15 psi.332 This stylet had been used to provide airway access and ventilation during direct laryngoscopy. They speculated that the pneumothoraces might have resulted from endobronchial migration of the catheter. They did not consider the possibility that barotrauma occurred as a result of jet ventilation against apposed vocal cords as the patients were recovering from neuromuscular blockade.
G Use of Commercial Tube Exchangers
Several commercial products incorporate many of the features described by Bedger and Chang.331 They are long, hollow catheters that may include connectors for jet or manual ventilation. Most have distance and radiopaque markers. They also have end and distal side holes. They can be introduced through an existing ETT, permitting its withdrawal. Oxygen insufflation or jet ventilation can be provided through the tube exchanger. Respiratory monitoring can be achieved by connection to a capnograph. Spontaneous breathing may take place around the device. In most reports, these catheters have been tolerated well enough that they can be left in situ until it is unlikely that reintubation will be required. They must be properly secured to ensure that they do not come out prematurely (Fig. 50-3). Even with the catheter in place, most patients will be able to talk or cough.
These devices are consistent with practice guidelines from the ASA Task Force and Canadian Airway Focus Group recommendations regarding the extubation of the difficult airway.5,6 They increase the probability that reintubation will succeed; if difficulty is encountered, the device can provide a conduit for oxygen insufflation. Jet ventilation, if necessary, can be accomplished while alternative techniques are explored. This may be thought of as a reversible or staged extubation. With the device in place, other options can be pursued, including evaluation of the benefits of helium-oxygen mixtures or inhalation of racemic epinephrine. Knowing that the patient is satisfactorily oxygenated and ventilated, the airway manager can recruit additional information, equipment, and expertise.
1 Tracheal Tube Exchangers
The most basic commercial tube exchangers are the Sheridan T.T.X. (Hudson Respiratory Care Inc. [RCI], Temecula, CA) (Fig. 50-4) and the JEM Endotracheal Tube Changer (Instrumentation Industries Inc., Bethel Park, PA). The T.T.X. devices are available in four ODs (2.0-, 3.3-, 4.8-, and 5.8 mm) and two lengths (56 and 81 cm). The smallest can be inserted into tracheal tubes with IDs as small as 2.5 mm. A longer, 100-cm endobronchial exchanger (Sheridan E.T.X.) is available for exchanging double-lumen tubes. They are firm—Shore hardness of 85, the same durometer measurement as the CAEC and Cardiomed International’s Endotracheal Ventilation Catheter (ETVC)—although thermolabile and therefore subject to softening with heat. They are frosted to minimize drag and have a radiopaque stripe and distance markings. They have no side holes or connectors. An alternative product, the Sheridan JETTX Exchanger, is a longer device (100 cm), but it is available in only a single OD that is suitable for ETTs greater than 6.5-mm ID. It incorporates a proximal slip-fit connector that can be connected by a Luer-Lok to a jet ventilator. As with the T.T.X., there is only a single, distal end hole.
2 Cook Airway Exchange Catheters
Cook Critical Care has developed a family of hollow stylets known as CAECs (Fig. 50-5A). They are available in 8.0-, 11-, 14-, and 19-F sizes, corresponding to 2.7-, 3.7-, 4.7-, and 6.3-mm ODs, respectively. They can be used to exchange ETTs with IDs of 3, 4, 5, and 7 mm, respectively. The 8-F CAEC is 45 cm long, and the others are 83 cm long. The devices are radiopaque, have distance markings between 15 and 30 cm from the distal end, and have two distal side holes and an end hole. Proximally, two types of connectors are secured and released by a patented Rapi-Fit adapter (see Fig. 50-5B). They provide a 15-mm connection or a Luer-Lok jet ventilation attachment and were designed for rapid removal and reattachment while the tracheal tube is being off-loaded and replaced. The length and IDs (1.6 to 3.4 mm) make manual ventilation with a resuscitation bag possible but useful only for short periods because resistance is very high. The Luer-Lok jet Rapi-Fit connector allows jet ventilation, but the paucity of distal side holes potentially increases catheter whip and the risk of barotrauma.333
Atlas and Mort examined the relationship between the diameter of the two larger CAECs and tolerance, as well as the ability to phonate and cough.334 It is unclear whether their patients were randomly assigned to specific sizes of catheters. Phonation and discomfort were similar in both groups, with only 3 of 101 patients experiencing significant discomfort. Cough effort tended to be reduced with the larger CAEC, but this did not achieve significance. Atlas and colleagues also looked at a larger tube exchanger (JEM 400 Endotracheal Tube Changer, Instrumentation Industries Inc.), which they reasoned would have a higher degree of success as a tube exchanger. This device has an OD of 6.35 mm and is said to be stiffer. They adapted the JEM 400 using the Rapi-Fit connector from the CAEC 19-83 to enable jet ventilation.335 This device, like the Sheridan JETTX, has only a single end-hole and is not recommended for jet ventilation.
Mort evaluated the concept of reversible extubation in patients with difficult airways.336 From an institutional database, he identified patients who were extubated in the operating room, PACU, or ICU with a CAEC. The tube exchanger was left in place until the need for reintubation was considered unlikely. Over a 9-year period, 354 patients qualified. Two groups emerged: those who required reintubation while the AEC was still in place and another group who required reintubation within 7 days but after the AEC had been removed. Airway-related complications were compared for the two groups. The AEC dwell time was a mean of 3.9 hours (range, 5 minutes to 72 hours). Of 354 patients, 288 were extubated in the ICU and had previously required three or more laryngoscopic attempts or alternative devices to achieve intubation. Comparing the overall success rate in the two groups, 47 of 51 patients in the AEC group were successfully reintubated, 87% on the first attempt. The four failures in this group resulted from inadvertent removal of the AEC in three; one patient was rescued with an intubating LMA and flexible bronchoscope. Mild (SpO2 < 90%) and severe hypoxia (<70%) were experienced by three and four patients, respectively. Of the 37 patients requiring reintubation without an AEC, the first pass success rate was only 14%, with mild and severe hypoxia in 50% and 19%, respectively; three or more laryngoscopy attempts in 28 of 36 patients; and the need for a rescue device in 32 of 36 patients.336 In all cases, reintubation was attempted by a member of an anesthesia airway team: an attending anesthesiologist or a resident under direct supervision. Although reintubation over an AEC does not guarantee first-pass success, this strategy was strikingly more effective (87% versus 14% first-pass success) and had far fewer life-threatening complications.
3 Arndt Airway Exchange Catheter
The Arndt Airway Exchange Catheter (Cook Critical Care), a radiopaque exchange catheter, consists of an extrastiff Amplatz guidewire with positioning marks, a Rapi-Fit adapter, a bronchoscopic port, and a distally tapered 14-F (4.7-mm-OD), 70-cm-long AEC. It was designed for the exchange of LMAs, endobronchial tubes, and ETTs (Fig. 50-6). Bronchoscopy is performed through the existing airway device. The flexible end of the Amplatz guidewire is introduced through the working channel of the bronchoscope and under visual control and is advanced to the level of the carina. The bronchoscope is removed over the wire, taking care that it is neither advanced nor withdrawn. The Arndt AEC is advanced over the guidewire to the appropriate depth, determined by aligning the distance markings on the airway device with that of the AEC. The original airway is carefully removed, and its replacement is advanced over the AEC.309
4 Endotracheal Ventilation Catheter
Cardiomed’s Endotracheal Ventilation Catheter (Lindsay, Ontario, Canada) is made of a hybrid plastic (Fig. 50-7). It is 85 cm long and has a 4-mm OD (12 F) and 3-mm ID. It has a radiopaque stripe along its entire length and distance markings at 4-cm intervals. Proximally, it has a male hose barb with a threaded adapter welded into the catheter. These attachments have been constructed to prevent restriction of the catheter’s ID. The threaded adapter connects to an easily removed Luer-Lok adapter. Distally, it is blunt ended with one end hole and eight helically arranged side holes to minimize catheter whip and jet ventilation pressures. Unpublished studies by the manufacturer found no significant softening over time at body temperature. This is desirable for a product that may remain in situ and be required to serve as a stylet. A metal guidewire is available to provide additional stiffness, but we have not found this to be necessary.
The ETVC was designed to facilitate reversible extubation.337 We have used it in at least 600 patients, the first 202 of whom has been reported.338 Although the ETVC had been used to facilitate reintubation, this was not required in most cases. In the original series, reintubation or tube exchange was performed in 32 (16%) of 202 cases, a rate that was very similar to that reported by others.339 In both series, the ETVC338 and the CAEC339 were used primarily to maintain airway access. In the original publication, the ETVC was used for oxygen insufflation (31 patients), jet ventilation (45 patients), and post-extubation capnography (54 patients).338
Reintubation was successful in 20 (91%) of 22 attempts. One failure occurred with a softer prototype. The second failure resulted when an inexperienced and unsupervised operator attempted a tube exchange. Difficulty was occasionally encountered advancing the ETT through the glottis, similar to that experienced when using an FOB to intubate.340 Tongue retraction with a laryngoscope blade should always be attempted when possible. ETT rotation or the use of an ETT such as the Parker Flex-Tip (Parker Medical, Boulder, CO) may prove useful. Because the ETVC has a relatively small OD, use of a smaller-diameter ETT is recommended.
Complications included barotrauma, intolerance, unintended dislodgment, and tracheal perforation. Intolerance occurred in 2 of 202 patients (typically because of carinal irritation) and in 1 patient recently recovered from status asthmaticus. Intolerance should prompt reassessment of the depth of insertion. If the depth is clinically or radiographically appropriate, and the ETVC (or other AEC) continues to be required, tolerance usually can be achieved by instilling lidocaine through the device. Most patients, including those with reactive airways, have tolerated the ETVC without difficulty. Dislodgement occurred when the ETVC was inadequately secured or the patient “tongued” the catheter out. Proper fixation is essential (see Fig. 50-3). Tracheal or bronchial perforation with different instrumentation has been described.325,326 In our case, it occurred in a patient with obstructing, proliferative tracheal papillomatosis and a chronic tracheostomy. A rigid prototype catheter was inserted alongside the tracheostomy, penetrating the posterior tracheal wall. Jet ventilation resulted in fatal barotrauma. Aspiration and laryngospasm have not been observed.
H Exchange of Double-Lumen Tubes
Double-lumen tubes are selected for procedures requiring lung isolation. Although the resistance through a larger double-lumen tube does not preclude postoperative ventilation or weaning, it may be desirable to replace it with a single-lumen tube, particularly if care is to be transferred to an area where familiarity is lacking or ventilatory support may be prolonged. The double-lumen tube may have to be changed because of damage to a cuff or because the initial tube was an inappropriate size. Substitution can often be achieved by direct or indirect (e.g., video) laryngoscopy. While the larynx is in view, the double-lumen tube is withdrawn and immediately replaced with a single-lumen tube or replacement double-lumen tube. Occasionally, this cannot be accomplished.341 Whether the substitution is a single- to double-, double- to single-, or double- to double-lumen tube, the requirements are similar, and the previously described tube exchangers may not be sufficiently long or firm.342,343
1 Visually Assisted Tube Exchange
The recently discontinued WuScope (Achi Corp., Fremont, CA, and Asahi Optical, Tokyo, Japan) has been used to exchange double-lumen tubes and conventional ETTs in patients with difficult airways.344–346 The WuScope provided a good glottic view in a morbidly obese patient with adult respiratory distress syndrome and permitted insertion of a suction catheter anterior to the existing nasotracheal tube. The nasotracheal tube was withdrawn, and the replacement oral ETT was easily advanced over the suction catheter with minimal interruption of mechanical ventilation. We think that a visually direct tube exchange with a WuScope, Bullard Scope (Circon, Santa Barbara, CA), Upsher UltraScope (Mercury Medical, Clearwater, FL), or video laryngoscope is preferable to blind passage of an ETT over a flexible bronchoscope or tube exchanger. A hollow tube exchanger, however, would have permitted oxygen insufflation or jet ventilation had desaturation or difficulties with tube advancement occurred. A supraglottic view (in contrast to the infraglottic view provided by a flexible bronchoscope) of the exchange enables identification and correction of an ETT impingement or the outward migration of the exchange catheter.347
The concept of visually direct tube exchange was assessed retrospectively in a population in whom no view of the larynx could be obtained by direct laryngoscopy.348 Using one of three indirect laryngoscopes—the Glidescope (Verathon Medical, Bothell WA), Airtraq (Prodol Meditec, Vizcaya, Spain), or McGrath Series 5 (Aircraft Medical, Edinburgh, Scotland)—in conjunction with an AEC, 51 exchanges were performed. Four of the cases involved a double-lumen tube converted to a conventional ETT or a nasal to oral conversion. Thirty-seven patients had previously difficult intubations requiring multiple attempts or a rescue technique. Most of the patients were obese or morbidly obese and had a positive fluid balance of more than 10 L over the previous 4 days. The intention of the study was not to compare the specific devices but rather to evaluate the advantages of visually directing the tube exchange. Mort reported that this was achieved on the first attempt in 47 of 49 patients.348 In the two failures, use of the Glidescope video laryngoscope afforded an excellent view of the AEC migrating cephalad, allowing rapid reintubation with a styletted ETT. He pointed out that had the tube exchange been performed blindly, the replacement would have failed, with potentially serious consequences. Others have made similar observations.349,350 These findings and our personal experiences are consistent with Mort’s recommendations.
2 Conversion from Nasal to Oral Intubation
Blind or bronchoscopically assisted nasal intubation is sometimes performed when oral approaches are difficult or unsuccessful. The nasal tube may have to be converted to an oral one because of complications or the intended surgery. Whenever possible, this should be done under visual control, assisted by a flexible bronchoscope,351 rigid fiberoptic device, or video or optical laryngoscope.344,348 The more anatomically or physiologically challenging the patient, the more compelling the case for including a tube exchanger.
Gabriel and Azocar described a patient in halo fixation in whom the connector was detached and the nasotracheal tube was advanced deeper into the trachea.352 The tube was then grasped close to the uvula with forceps and digitally extracted through the mouth. Novella used a Sheridan T.T.X. to convert a nasal to an oral tube in a patient with Klippel-Feil syndrome who first underwent orthognathic surgery and subsequent septorhinoplasty.353 After completion of orthognathic surgery, the T.T.X. was inserted into the nasal tracheal tube, and the latter was withdrawn. The T.T.X. was then grasped with two Magill forceps; the caudal one was used to stabilize the catheter, and the cephalad one was used to withdraw the proximal end out of the mouth. An oral tube was then railroaded over the T.T.X. Cooper described a similar maneuver in a patient in whom oral fiberoptic intubation could not be accomplished, but fiberoptic nasal intubation was achieved.354 He passed an ETVC through the existing nasal tube and removed the latter. The ETVC was then stabilized with caudal Magill forceps and withdrawn through the mouth with the cephalad forceps. Oxygen insufflation was provided through the ETVC, which was then used to thread an oral tube into the trachea. In this case, oxygen desaturation was avoided, although the procedure was easily and quickly accomplished.
3 Conversion from Oral to Nasal Intubation
During efforts to convert from an oral to a nasal tracheal tube, Sumiyoshi and coworkers used negative-pressure ventilation during the tube exchange.355 Their patient was in a halo and chest cast due to a cervical injury, and laryngoscopy had been unsuccessful. An attempt to introduce a 4.8-mm FOB adjacent to the existing tube (with a tube exchanger through it) was unsuccessful. A subsequent effort involved a 3.5-mm FOB and a 7-F METTRO using negative pressure to achieve ventilation. A smaller, hollow tube exchanger might have been successful and could have avoided the risk of negative-pressure pulmonary edema resulting from an ETT and FOB occupying a small glottic opening.356 Smith and Fenner performed an oral to nasal conversion using a 4.0-mm-OD, flexible bronchoscope, which they inserted through the glottis and anterior to an oral tube.357 The oral tube was withdrawn, and a nasal tube was advanced over the FOB. Many of the difficulties in performing a tube exchange can be avoided with the use of indirect (fiberoptic or video) laryngoscopes, ideally in combination with AECs.
4 Conversion from Supraglottic Airways to Endotracheal Tubes
A discussion of the conversion of various SGAs to ETTs is beyond the scope of this chapter, but the process is addressed elsewhere. Whenever possible, this conversion should be facilitated by visual guidance, using direct laryngoscopy or an indirect technique. These techniques include the use of a flexible bronchoscope and Aintree catheter or a video laryngoscope.358,359
5 Changing Tracheostomy Tubes
A tracheostomy tube may need to be replaced because of tube damage, such as cuff rupture, occlusion with secretions, or conversion to another type or size of tracheostomy tube. If the tracheostomy was recently performed, the tissue is friable. In patients in whom a false passage has been created during the original procedure, in those with poor tissue integrity, or those highly dependent on supplemental oxygen, exchanging the tracheostomy tube can be difficult and fraught with complications. The Weinmann Tracheostomy Exchange Set (Cook Critical Care) (Fig. 50-8), consists of a Ciaglia Blue Rhino tracheostomy dilator, a CAEC, Rapi-Fit adapter, and two loading dilators (26 and 28 F). The 45-cm-long exchange catheter is passed through the tracheostomy tube to be replaced; its cuff is deflated, and the tube is removed over the exchange catheter. The replacement tracheostomy tube and its loading dilator are placed over the AEC, which is connected to the Rapi-Fit adapter, permitting ventilation as required. The loading catheter and tracheostomy catheter are advanced using the AEC as a guide. If stomal dilatation is required, the set is equipped with Ciglia Blue Rhino dilators (32 to 38 F).360 The only description of this device advocates jet ventilation and high-flow oxygen insufflation. AEC insertion must be atraumatic, confirmed with a carbon dioxide (CO2) tracing, and the depth verified to ensure it is not beyond the carina. Only the amount of oxygen (O2) required to prevent significant oxygen desaturation should be supplied.
VII Jet Ventilation Through Stylets
The preceding sections stressed the importance of being able to supplement oxygenation during a tube exchange. In most circumstances, a patient’s oxygen content can be adequately sustained with insufflation, obviating the need for high-pressure jet ventilation. If oxygen requirements are high before a tube exchange, the equipment should be immediately available to provide jet ventilation. This equipment consists of a manually cycled, Venturi-type jet ventilator with a Luer-Lok adapter and an in-line pressure-reducing valve (see Fig. 50-8).361 The objective of jet ventilation is to correct life-threatening hypoxemia—not to normalize arterial blood gases. Although the achievement of normal PaCO2 may be attainable, the risks quite likely exceed the benefits.343 Barotrauma has occurred through such misguided objectives, and it has been fatal in some cases.
A In Vitro Studies
Transtracheal jet ventilation by means of an intravenous catheter or intratracheal ventilation using a stylet or tube exchanger has been advocated in the management of the “cannot intubate, cannot ventilate” patient.5,362 The inspiratory volume depends on the driving pressure, injection time, respiratory compliance and resistance, and resistance of the tube exchanger. The latter is determined by the catheter’s ID and length. The expiratory volume depends on exhalation time, elastic recoil of the lungs, and airways resistance.361,363 Mismatch between inspiratory and expiratory volumes can have serious consequences.
In vitro studies using jet stylets have been conducted to determine flow, pressure, and entrainment characteristics. Using an in vitro model, with three sizes of Sheridan T.T.X. catheters, Dworkin and colleagues measured the inspiratory and expiratory flows resulting from a 50-psi injection as the simulated upper airway resistance, lung compliance, gas flow rate, and injection times were varied.363 The upper airway resistance was determined by the effective tracheal diameter, which they defined as a computed difference between the OD of the T.T.X. and the tracheal diameter. They simulated upper airway obstruction by using various sizes of ETT adapters (11- to 3.5-mm ID) in the proximal airway. The gas flows through the large, medium, and small tube exchangers, when connected to a pressure source of 50 psi, were 63, 33, and 12 L/min, respectively. In their model, if the difference between the tracheal and T.T.X. diameters resulted in an effective tracheal diameter that was greater than 4 to 4.5 mm, air trapping did not occur. Because increased upper airway resistance and reduced effective tracheal diameter resulted in larger tidal volumes, they concluded that jet ventilation through a long catheter that was positioned close to the carina caused little Venturi effect or air entrainment. Placement of the catheter close to the carina may ensure a higher oxygen concentration by reducing room air entrainment, but it also increases the risk of distal catheter migration and barotrauma.
In another in vitro model, calculations based on oxygen dilution and direct measurement using a pneumotachograph revealed that air entrainment accounted for 0% to 31% of the inspired volume.364 The largest T.T.X. and lung compliance resulted in the greatest entrainment. Gaughan and colleagues used a high driving pressure (50 psi), long inspiratory time (1 second), and brief expiratory time (1 second).364 Even within a low-compliance system, the large T.T.X. was associated with excessive tidal volumes.
Prolonging expiratory time reduces the minute ventilation by reducing the respiratory rate. This technique still exposes the lungs to potentially injurious tidal volumes. An alternative approach is to reduce the driving pressure. Gaughan and coworkers assessed the tidal volumes and air entrainment in a model lung with a range of compliance sets ventilated by high- and low-flow regulators through 14- and 16-gauge intravenous catheters.365 Their high-flow regulator at steady state produced flow rates of 320 L/min at 100 psi, whereas the low-flow regulator produced flows up to 15 L/min at 9 to 5 psi. Intravenous catheters, because of their short length, offer considerably less resistance to flow. Their proximity to the upper airway also results in greater air entrainment (15% to 74%). Both high- and low-flow regulators allowed adequate minute ventilation in the setting of normal tracheal and bronchial diameters and normal compliance. The investigators recommended that during transtracheal jet ventilation, when low-flow regulators were used, an inspiratory-to-expiratory (I : E) ratio of 1 : 1 should be used because it yields the greatest minute ventilation. Although this observation is undoubtedly true, it remains to be determined whether such high minute volumes are clinically necessary or safe.343,361
B In Vivo Studies
Chang and colleagues provided intraoperative jet ventilation using a 3.5-mm chest tube as a jet catheter.332 They ventilated with 15 psi at 10 to 16 breaths/min and continued until spontaneous ventilation was deemed adequate. The patient recovered but had a left pneumothorax that was attributed to catheter migration and unilateral ventilation. The investigators mentioned that they had encountered three cases of pneumothoraces and one pneumoperitoneum in approximately 600 similar procedures. They drew attention to the importance of catheter placement and advised that even brief airway obstruction can result in barotrauma. However, they failed to mention that vocal fold apposition occurring during recovery might promote this complication. In a subsequent paper, the same investigators stated that the jet stylet had been used for the ventilation of six patients, resulting in normocarbia and adequate ventilation.331 Baraka described a patient with a poor laryngeal view in whom an ETT exchange was facilitated using a CAEC.366 This was advanced until resistance was encountered. Jet ventilation at 50 psi was commenced before withdrawal of the ETT or deflation of its cuff. The result was right-chest expansion but incomplete deflation, but within three breaths, asystole was observed. A needle thoracotomy confirmed the diagnosis of tension pneumothorax, and sinus rhythm was restored.366
Egol and colleagues described pneumothoraces and a pneumoperitoneum in three patients using a variety of delivery devices and driving pressures.333 They included an 18-F suction catheter at 50 psi, a nasogastric sump tube at 20 psi (inspiratory time at 30%), and a fiberoptic laryngoscope at 40 psi. They attributed the barotrauma observed to incorrect catheter placement, ventilation during phonation, and possible direct mucosal penetration from jet injection. They examined the relationship between the number of distal side holes in the tube exchanger and the pressure at the catheter tip. At any given driving pressure, the more side holes, the lower the pressure at the catheter tip. They recommended vigilance regarding the location of the catheter tip, including avoidance of direct mucosal contact and insertion into orifices where exhalation might be restricted. They advocated securing the catheter to minimize migration, use of catheters with multiple side holes, use of small-diameter jet catheters to minimize the resistance to exhalation, and use of the minimal effective driving pressure. They encouraged the development and use of an effective pressure sensor and pressure-cutoff device.
The ETVC has an end hole and eight distal side holes (see Fig. 50-7). Its use to provide jet ventilation during general anesthesia with muscle relaxation on 45 occasions was described.338 Its attachment to a handheld jet ventilator with a pressure-reducing valve is shown in Figure 50-9. Between 1991 and 1993, Irish and coworkers used this device with a driving pressure of 50 psi in 25 anesthetized and paralyzed patients undergoing percutaneous dilatational tracheostomies.367 They observed barotrauma in one patient. Arterial blood gases in 12 consecutive, critically ill patients revealed a pH of 7.37 ± 0.09, PaCO2 of 45.5 ± 10.8, and PaO2 of 256 ± 126 (mean ± SD). In a subsequent report, a patient ventilated for 90 minutes at only 20 psi developed a pneumothorax.343 Chan and Manninen also described use of the ETVC to provide jet ventilation.368 After performing a fiberoptic intubation in a patient with an unstable cervical spine, they discovered that the cuff of the ETT had been damaged. They inserted a flexible bronchoscope through the other nostril and advanced it through the vocal cords anterior to the original ETT. They then passed an ETVC through the original ETT and provided three breaths of jet ventilation at 50 psi. The patient developed a pneumothorax. Unfortunately, they used a high driving pressure through an exchange catheter that might have been too deeply inserted in the setting of near-complete glottic occlusion (i.e., partial cuff deflation, 6-mm ETT, and an FOB passing through the vocal cords).
These cases reinforce the general principles previously stated.343,369 The need for jet ventilation should always be weighed against its possible risks. It should be immediately available and used when there is evidence of deteriorating oxygenation. If a 15-mm connector is available, capnography should be used to confirm intratracheal placement. An in-line pressure-reducing valve should be used, and ventilation should begin with the lowest pressure capable of producing adequate chest expansion. The duration of inspiration should be minimized while the duration of exhalation is determined by observing the return of the thoracic diameter to its position before inspiratory. The depth of catheter insertion should be far enough from the carina that distal migration does not occur but not so proximal that jet ventilation results in the catheter’s ejection from the glottis. Multiple distal side holes can reduce catheter whip and the distal catheter pressure during jet ventilation. Every effort must be taken to minimize expiratory resistance.
IX Clinical Pearls
• Careful planning of tracheal extubation or tube exchange is as vital as the planning required for intubation. Airway complications are as common after tube removal as during insertion.
• Extubation carries the risk of reintubation and the risk of failed reintubation.
• Reintubation may be required because of an airway obstruction or because of failure of oxygenation, ventilation, clearance of secretions, or airway protection.
• Anticipating a successful extubation is an inexact science. Any emergent reintubation is likely to be more complex due to urgent conditions and physiologic instability.
• Reintubation may fail because of inadequate access to the airway (e.g., halo fixation, maxillomandibular fixation), preexisting anatomic features (e.g., retrognathism, prominent incisors), inadequate preparation, lack of expertise or insufficient information (e.g., emergencies), a rapidly deteriorating clinical state, or blood, secretions, or swelling obscuring the visual field.
• Many threatening circumstances can be anticipated and managed preemptively with an extubation strategy.
• Extubation strategies include deep extubation, bronchoscopic examination under anesthesia through a supraglottic airway (SGA), substitution of an endotracheal tube with an SGA, and extubation over a tube exchanger.
• The safest extubation strategy may be a surgical airway.
• A supraglottic device or tube exchanger should be left in place until it is likely that reintubation will not be required. Premature withdrawal is a common mistake.
• A reintubation strategy may include the judicious administration of oxygen by insufflation or jet ventilation; advancement of an endotracheal tube over the tube exchanger, preferably with tongue retraction; or indirect laryngoscopy.
All references can be found online at expertconsult.com.
8 Miller KA, Harkin CP, Bailey PL. Postoperative tracheal extubation. Anesth Analg. 1995;80:149–172.
23 Lee PJ, MacLennan A, Naughton NN, O’Reilly M. An analysis of reintubations from a quality assurance database of 152,000 cases. J Clin Anesth. 2003;15:575–581.
99 Francois B, Bellissant E, Gissot V, et al. 12-h pretreatment with methylprednisolone versus placebo for prevention of postextubation laryngeal oedema: A randomised double-blind trial. Lancet. 2007;369:1083–1089.
103 Benjamin B, Cummings CW, Fredrickson JM, et al. Laryngeal trauma from Intubation: Endoscopic evaluation and classification. In: Otolaryngology: Head and neck surgery. St. Louis: Mosby–Year Book; 1998:2018–2033.
150 Kriner EJ, Shafazand S, Colice GL. The endotracheal tube cuff-leak test as a predictor for postextubation stridor. Respir Care. 2005;50:1632–1638.
172 Rosato L, Avenia N, Bernante P, et al. Complications of thyroid surgery: Analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J Surg. 2004;28:271–276.
270 Biro P, Rohling R, Schmid S, et al. Anesthesia in a patient with acute respiratory insufficiency due to relapsing polychondritis. J Clin Anesth. 1994;6:59–62.
330 Steinberg MJ, Chmiel RA. Use of a nasogastric tube as a guide for endotracheal reintubation. J Oral Maxillofac Surg. 1989;47:1232–1233.
342 Hannallah M. Evaluation of tracheal tube exchangers for replacement of double-lumen endobronchial tubes. Anesthesiology. 1992;77:609–610.
363 Dworkin R, Benumof JL, Benumof R, Karagianes TG. The effective tracheal diameter that causes air trapping during jet ventilation. J Cardiothorac Anesth. 1990;4:731–736.
1 Asai T, Koga K, Vaughan RS. Respiratory complications associated with tracheal intubation and extubation. Br J Anaesth. 1998;80:767–775.
2 Peterson GN, Domino KB, Caplan RA, et al. Management of the difficult airway: A closed claims analysis. Anesthesiology. 2005;103:33–39.
3 Schwartz DE, Matthay MA, Cohen NH. Death and other complications of emergency airway management in critically ill adults. A prospective investigation of 297 tracheal intubations. Anesthesiology. 1995;82:367–376.
4 Mort TC. The incidence and risk factors for cardiac arrest during emergency tracheal intubation: A justification for incorporating the ASA Guidelines in the remote location. J Clin Anesth. 2004;16:508–516.
5 Practice guidelines for management of the difficult airway: An updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003;98:1269–1277.
6 Crosby ET, Cooper RM, Douglas MJ, et al. The unanticipated difficult airway with recommendations for management. Can J Anaesth. 1998;45:757–776.
7 Hartley M, Vaughan RS. Problems associated with tracheal extubation. Br J Anaesth. 1993;71:561–568.
8 Miller KA, Harkin CP, Bailey PL. Postoperative tracheal extubation. Anesth Analg. 1995;80:149–172.
9 Rassam S, Sandbythomas M, Vaughan RS, Hall JE. Airway management before, during and after extubation: A survey of practice in the United Kingdom and Ireland. Anaesthesia. 2005;60:995–1001.
10 Jubb A, Ford P. Extubation after anaesthesia: A systematic review. Update Anaesth. 2009;2009:30–36.
11 Karmarkar S, Varshney S. Tracheal extubation. Contin Educ Anaesth Crit Care Pain. 2008;8:214–220.
12 Daley MD, Norman PH, Coveler LA. Tracheal extubation of adult surgical patients while deeply anesthetized: A survey of United States anesthesiologists. J Clin Anesth. 1999;11:445–452.
13 Epstein SK. Extubation failure: An outcome to be avoided. Crit Care. 2004;8:310–312.
14 Demling RH, Read T, Lind LJ, Flanagan HL. Incidence and morbidity of extubation failure in surgical intensive care patients. Crit Care Med. 1988;16:573–577.
15 Marini JJ, Wheeler AP. Weaning from mechanical ventilation. ed 2. Marini JJ, Wheeler AP, eds. Critical care medicine: The essentials. Baltimore: Lippincott Williams & Wilkins; 1997;vol 1:173–195.
16 Gandia F, Blanco J. Evaluation of indexes predicting the outcome of ventilator weaning and value of adding supplemental inspiratory load. Intensive Care Med. 1992;18:327–333.
17 Epstein SK. Putting it all together to predict extubation outcome. Intensive Care Med. 2004;30:1255–1257.
18 Lavery GG, McCloskey BV. The difficult airway in adult critical care. Crit Care Med. 2008;36:2163–2173.
19 Seymour CW, Martinez A, Christie JD, Fuchs BD. The outcome of extubation failure in a community hospital intensive care unit: A cohort study. Crit Care. 2004;8:R322–R327.
20 Hill RS, Koltai PJ, Parnes SM. Airway complications from laryngoscopy and panendoscopy. Ann Otol Rhinol Laryngol. 1987;96:691–694.
21 Mathew JP, Rosenbaum SH, O’Connor T, Barash PG. Emergency tracheal intubation in the postanesthesia care unit: Physician error or patient disease? Anesth Analg. 1990;71:691–697.
22 Rose DK, Cohen MM. The airway: Problems and predictions in 18,500 patients. Can J Anaesth. 1994;41(Pt 1):372–383.
23 Lee PJ, MacLennan A, Naughton NN, O’Reilly M. An analysis of reintubations from a quality assurance database of 152,000 cases. J Clin Anesth. 2003;15:575–581.
24 Levelle JP, Martinez OA. Airway obstruction after bilateral carotid endarterectomy. Anesthesiology. 1985;63:220–222.
25 Tyers MR, Cronin K. Airway obstruction following second operation for carotid endarterectomy. Anaesth Intensive Care. 1986;14:314–316.
26 Emery SE, Smith MD, Bohlman HH. Upper-airway obstruction after multilevel cervical corpectomy for myelopathy. J Bone Joint Surg Am. 1991;73:544–551.
27 Lacoste L, Gineste D, Karayan J, et al. Airway complications in thyroid surgery. Ann Otol Rhinol Laryngol. 1993;102:441–446.
28 Venna RP, Rowbottom JR. A nine year retrospective review of post operative airway related problems in patients following multilevel anterior cervical corpectomy. Anesthesiology. 2002;95:A1171.
29 Chinachoti T, Chau-in W, Suraseranivongse S, et al. Postoperative reintubation after planned extubation in Thai Anesthesia Incidents Study (THAI Study). J Med Assoc Thai. 2005;88(Suppl 7):S84–S94.
30 Ting PC, Chou AH, Yang MW, et al. Postoperative reintubation after planned extubation: A review of 137,866 general anesthetics from 2005 to 2007 in a Medical Center of Taiwan. Acta Anaesthesiol Taiwan. 2010;48:167–171.
31 Zeitlin GA. Recovery room mishaps in the ASA Closed Claims Study. ASA Newslett. 1989;53:28–30.
32 Tiret L, Desmonts JM, Hatton F, Vourc’h G. Complications associated with anaesthesia—A prospective survey in France. Can Anaesth Soc J. 1986;33(Pt 1):336–344.
33 Wade JG, Larson CP, Jr., Hickey RF, et al. Effect of carotid endarterectomy on carotid chemoreceptor and baroreceptor function in man. N Engl J Med. 1970;282:823–829.
34 Knill RL, Gelb AW. Ventilatory responses to hypoxia and hypercapnia during halothane sedation and anesthesia in man. Anesthesiology. 1978;49:244–251.
35 Goodman NW. Volatile agents and the ventilatory response to hypoxia. Br J Anaesth. 1994;72:503–505.
36 Nagyova B, Dorrington KL, Robbins PA. Effect of low-dose enflurane on the ventilatory response to hypoxia in humans. Br J Anaesth. 1994;72:509–514.
37 Eriksson LI. Evidence-based practice and neuromuscular monitoring: It’s time for routine quantitative assessment. Anesthesiology. 2003;98:1037–1039.
38 Moons P, Sels K, De Becker W, et al. Development of a risk assessment tool for deliberate self-extubation in intensive care patients. Intensive Care Med. 2004;30:1348–1355.
39 Curry K, Cobb S, Kutash M, Diggs C. Characteristics associated with unplanned extubations in a surgical intensive care unit. Am J Crit Care. 2008;17:45–52.
40 Betbese AJ, Perez M, Bak E, Rialp G, Mancebo J. A prospective study of unplanned endotracheal extubation in intensive care unit patients. Crit Care Med. 1998;26:1180–1186.
41 Miller RL, Cole RP. Association between reduced cuff leak volume and postextubation stridor. Chest. 1996;110:1035–1040.
42 Mort TC. Unplanned tracheal extubation outside the operating room: A quality improvement audit of hemodynamic and tracheal airway complications associated with emergency tracheal reintubation. Anesth Analg. 1998;86:1171–1176.
43 Vassal T, Anh NG, Gabillet JM, et al. Prospective evaluation of self-extubations in a medical intensive care unit. Intensive Care Med. 1993;19:340–342.
44 Lall NG. Difficult extubation: A fold in the endotracheal cuff. Anaesthesia. 1980;35:500–501.
45 Tanski J, James RH. Difficult extubation due to a kinked pilot tube. Anaesthesia. 1986;41:1060.
46 Sprung J, Conley SF, Brown M. Unusual cause of difficult extubation. Anesthesiology. 1991;74:796–797.
47 Heyman DM, Greenfeld AL, Rogers JS, et al. Inability to deflate the distal cuff of the laser-flex tracheal tube preventing extubation after laser surgery of the larynx. Anesthesiology. 1994;80:236–238.
48 Lee C, Schwartz S, Mok MS. Difficult extubation due to transfixation of a nasotracheal tube by a Kirschner wire. Anesthesiology. 1977;46:427.
49 Lang S, Johnson DH, Lanigan DT, Ha H. Difficult tracheal extubation. Can J Anaesth. 1989;36(Pt 1):340–342.
50 Akers JA, Riley RH. Failed extubation due to ‘sutured’ double-lumen tube. Anaesth Intensive Care. 1990;18:577.
51 Fagraeus L. Difficult extubation following nasotracheal intubation. Anesthesiology. 1978;49:43–44.
52 Hilley MD, Henderson RB, Giesecke AH. Difficult extubation of the trachea. Anesthesiology. 1983;59:149–150.
53 Ciaglia P, Firsching R, Syniec C. Elective percutaneous dilatational tracheostomy. A new simple bedside procedure; preliminary report. Chest. 1985;87:715–719.
54 Schwartz LB, Sordill WC, Liebers RM. Difficulty in removal of accidentally cut endotracheal tube. J Oral Maxillofac Surg. 1982;40:518–519.
55 Dryden GE. Circulatory collapse after pneumonectomy (an unusual complication from the use of a Carlens catheter): Case report. Anesth Analg. 1977;56:451–452.
56 Kempen P. Extubation in adult patients: Who, what, when, where, how, and why? J Clin Anesth. 1999;11:441–444.
57 Nair I, Bailey PM. Use of the laryngeal mask for airway maintenance following tracheal extubation. Anaesthesia. 1995;50:174–175.
58 Asai T, Shingu K. Use of the laryngeal mask during emergence from anesthesia in a patient with an unstable neck. Anesth Analg. 1999;88:469–470.
59 Lee B, Lee JR, Na S. Targeting smooth emergence: The effect site concentration of remifentanil for preventing cough during emergence during propofol-remifentanil anaesthesia for thyroid surgery. Br J Anaesth. 2009;102:775–778.
60 Dyson A, Isaac PA, Pennant JH, et al. Esmolol attenuates cardiovascular responses to extubation. Anesth Analg. 1990;71:675–678.
61 Paulissian R, Salem MR, Joseph NJ, et al. Hemodynamic responses to endotracheal extubation after coronary artery bypass grafting. Anesth Analg. 1991;73:10–15.
62 Wellwood M, Aylmer A, Teasdale S, et al. Extubation and myocardial ischemia. Anesthesiology. 1984;61(3A):A132.
63 Fagan C, Frizelle HP, Laffey J, et al. The effects of intracuff lidocaine on endotracheal-tube-induced emergence phenomena after general anesthesia. Anesth Analg. 2000;91:201–205.
64 Estebe JP, Delahaye S, Le CP, et al. Alkalinization of intra-cuff lidocaine and use of gel lubrication protect against tracheal tube-induced emergence phenomena. Br J Anaesth. 2004;92:361–366.
65 Estebe JP, Gentili M, Le CP, et al. Alkalinization of intracuff lidocaine: Efficacy and safety. Anesth Analg. 2005;101:1536–1541.
66 Takita K, Morimoto Y, Kemmotsu O. Tracheal lidocaine attenuates the cardiovascular response to endotracheal intubation. Can J Anesth. 2001;48:732–736.
67 Staffel JG, Weissler MC, Tyler EP, Drake AF. The prevention of postoperative stridor and laryngospasm with topical lidocaine. Arch Otolaryngol Head Neck Surg. 1991;117:1123–1128.
68 Hung O. Understanding hemodynamic responses to tracheal intubation. Can J Anaesth. 2001;48:723–726.
69 Muzzi DA, Black S, Losasso TJ, Cucchiara RF. Labetalol and esmolol in the control of hypertension after intracranial surgery. Anesth Analg. 1990;70:68–71.
70 O’Dwyer JP, Yorukoglu D, Harris MN. The use of esmolol to attenuate the haemodynamic response when extubating patients following cardiac surgery—A double-blind controlled study. Eur Heart J. 1993;14:701–704.
71 Inoue S, Tanaka Y, Kawaguchi M, Furuya H. The efficacy of landiolol for suppressing the hyperdynamic response following laryngoscopy and tracheal intubation: A systematic review. Anaesth Intensive Care. 2009;37:893–902.
72 Turan G, Ozgultekin A, Turan C, et al. Advantageous effects of dexmedetomidine on haemodynamic and recovery responses during extubation for intracranial surgery. Eur J Anaesthesiol. 2008;25:816–820.
73 Guler G, Akin A, Tosun Z, et al. Single-dose dexmedetomidine attenuates airway and circulatory reflexes during extubation. Acta Anaesthesiol Scand. 2005;49:1088–1091.
74 Bedford RF, Persing JA, Pobereskin L, Butler A. Lidocaine or thiopental for rapid control of intracranial hypertension. Anesth Analg. 1980;59:435–437.
75 Brucia JJ, Owen DC, Rudy EB. The effects of lidocaine on intracranial hypertension. J Neursci Nurs. 1992;24:205–214.
76 Madan R, Tamilselvan P, Sadhasivam S, et al. Intra-ocular pressure and haemodynamic changes after tracheal intubation and extubation: A comparative study in glaucomatous and nonglaucomatous children. Anaesthesia. 2000;55:380–384.
77 Lamb K, James MF, Janicki PK. The laryngeal mask airway for intraocular surgery: Effects on intraocular pressure and stress responses. Br J Anaesth. 1992;69:143–147.
78 Kim ES, Bishop MJ. Cough during emergence from isoflurane anesthesia. Anesth Analg. 1998;87:1170–1174.
79 Asai T. Use of the laryngeal mask after tracheal extubation. Can J Anaesth. 1999;46:997–998.
80 Koga K, Asai T, Vaughan RS, Latto IP. Respiratory complications associated with tracheal extubation. Timing of tracheal extubation and use of the laryngeal mask during emergence from anaesthesia. Anaesthesia. 1998;53:540–544.
81 Estebe JP, Dollo G, Le Corre P, et al. Alkalinization of intracuff lidocaine improves endotracheal tube-induced emergence phenomena. Anesth Analg. 2002;94:227–230.
82 Bevan D. SARS 3: Are we ready? Clin Invest Med. 2003;26:273–274.
83 Peng PWH, Wong DT, Bevan D, Gardam M. Infection control and anesthesia: Lessons learned from the Toronto SARS outbreak [in French]. Can J Anesth. 2003;50:989–997.
84 Bevan JC, Upshur REG. Anesthesia, ethics, and severe acute respiratory syndrome. Can J Anesth. 2003;50:977–982.
85 Loeb M, McGeer A, Henry B, et al. SARS among critical care nurses, Toronto. Emerg Infect Dis. 2004;10:251–255.
86 Varia M, Wilson S, Sarwal S, et al. Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. Can Med Assoc J. 2003;169:285–292.
87 Kamming D, Gardam M, Chung F. I. Anaesthesia and SARS. Br J Anaesth. 2003;90:715–718.
88 Loh KS, Irish JC. Traumatic complications of intubation and other airway management procedures. Anesthesiol Clin North Am. 2002;20:953–969.
89 Mencke T, Echternach M, Kleinschmidt S, et al. Laryngeal morbidity and quality of tracheal intubation: A randomized controlled trial. Anesthesiology. 2003;98:1049–1056.
90 Maktabi MA, Smith RB, Todd MM. Is routine endotracheal intubation as safe as we think or wish? Anesthesiology. 2003;99:247–248.
91 Maktabi MA, Hoffman H, Funk G, From RP. Laryngeal trauma during awake fiberoptic intubation. Anesth Analg. 2002;95:1112–1114.
92 Tolley NS, Cheesman TD, Morgan D, Brookes GB. Dislocated arytenoid: An intubation-induced injury. Ann R Coll Surg Engl. 1990;72:353–356.
93 Blanc VF, Tremblay NA. The complications of tracheal intubation: A new classification with a review of the literature. Anesth Analg. 1974;53:202–213.
94 Koka BV, Jeon IS, Andre JM, et al. Postintubation croup in children. Anesth Analg. 1977;56:501–505.
95 Darmon JY, Rauss A, Dreyfuss D, et al. Evaluation of risk factors for laryngeal edema after tracheal extubation in adults and its prevention by dexamethasone. A placebo-controlled, double-blind, multicenter study. Anesthesiology. 1992;77:245–251.
96 Gaussorgues P, Boyer F, Piperno D, et al. Laryngeal edema after extubation. Do corticosteroids play a role in its prevention? [in French]. Presse Med. 1987;16:1531–1532.
97 Ho LI, Harn HJ, Lien TC, et al. Postextubation laryngeal edema in adults. Risk factor evaluation and prevention by hydrocortisone. Intensive Care Med. 1996;22:933–936.
98 Anene O, Meert KL, Uy H, et al. Dexamethasone for the prevention of postextubation airway obstruction: A prospective, randomized, double-blind, placebo-controlled trial. Crit Care Med. 1996;24:1666–1669.
99 Francois B, Bellissant E, Gissot V, et al. 12-h pretreatment with methylprednisolone versus placebo for prevention of postextubation laryngeal oedema: A randomised double-blind trial. Lancet. 2007;369:1083–1089.
100 Shemie S. Steroids for anything that swells: Dexamethasone and postextubation airway obstruction. Crit Care Med. 1996;24:1613–1614.
101 Bagshaw SM, Delaney A, Farrell C, et al. Best evidence in critical care medicine. Steroids to prevent post-extubation airway obstruction in adult critically ill patients. Can J Anaesth. 2008;55:382–385.
102 Benjamin B. Prolonged intubation injuries of the larynx: Endoscopic diagnosis, classification, and treatment. Ann Otol Rhinol Laryngol Suppl. 1993;160:1–15.
103 Benjamin B, Cummings CW, Fredrickson JM, et al. Laryngeal trauma from intubation: Endoscopic evaluation and classification. In: Cummings CW, Frederickson JM, eds. Otolaryngology: Head and neck surgery, vol 3. St. Louis: Mosby–Year Book; 1998:2018–2033.
104 Benjamin BF, Holinger LM. Laryngeal complications of endotracheal intubation. Ann Otol Rhinol Laryngol. 2008;117:2–20.
105 Olsson GL, Hallen B. Laryngospasm during anaesthesia. A computer-aided incidence study in 136,929 patients. Acta Anaesthesiol Scand. 1984;28:567–575.
106 Rose DK, Cohen MM, Wigglesworth DF, DeBoer DP. Critical respiratory events in the postanesthesia care unit. Patient, surgical, and anesthetic factors. Anesthesiology. 1994;81:410–418.
107 Rex MAE. A review of the structural and functional basis of laryngospasm and a discussion of nerve pathways involved in reflexes and its clinical significance in man and animals. Br J Anaesth. 1970;42:891–899.
108 von Ungern-Sternberg BS, Boda K, Chambers NA, et al. Risk assessment for respiratory complications in paediatric anaesthesia: A prospective cohort study. Lancet. 2010;376:773–783.
109 Sasaki CT, Isaacson G. Functional anatomy of the larynx. Otolaryngol Clin North Am. 1988;21:595–612.
110 Larson PCJ. Laryngospasm—The best treatment. Anesthesiology. 1998;89:1293–1294.
111 Chung DC, Rowbottom SJ. A very small dose of suxamethonium relieves laryngospasm. Anaesthesia. 1993;48:229–230.
112 Landsman IS. Mechanisms and treatment of laryngospasm. Int Anesthesiol Clin. 1997;35:67–73.
113 Drummond JC. Macroglossia, deja vu. Anesth Analg. 1999;89:534–535.
114 Kuhnert SM, Faust RJ, Berge KH, Piepgras DG. Postoperative macroglossia: Report of a case with rapid resolution after extubation of the trachea. Anesth Analg. 1999;88:220–223.
115 Lam AM, Vavilala MS. Macroglossia: Compartment syndrome of the tongue? Anesthesiology. 2000;92:1832–1835.
116 Spiekermann BF, Stone DJ, Bogdonoff DL, Yemen TA. Airway management in neuroanaesthesia. Can J Anaesth. 1996;43:820–834.
117 Murthy P, Laing MR. Macroglossia. BMJ. 1994;309:1386–1387.
118 Kyrmizakis DE, Papadakis CE, Liolios AD, et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists. Arch Otolaryngol Head Neck Surg. 2004;130:1416–1419.
119 Kalra A, Cooley C, Palaniswamy C, Zanotti-Cavazonni SL. Valsartan-induced angioedema in a patient on angiotensin-converting enzyme inhibitor for years: Case report and literature review. Am J Ther. 2011 Sep 20. [Epub ahead of print]
120 Cooper RM. Complications associated with the use of the GlideScope videolaryngoscope. Can J Anesth. 2007;54:54–57.
121 Choo MK, Yeo VS, See JJ. Another complication associated with videolaryngoscopy. Can J Anesth. 2007;54:322–324.
122 Vincent RD, Jr., Wimberly MP, Brockwell RC, Magnuson JS. Soft palate perforation during orotracheal intubation facilitated by the GlideScope videolaryngoscope. J Clin Anesth. 2007;19:619–621.
123 Cross P, Cytryn J, Cheng KK. Perforation of the soft palate using the GlideScope(R) videolaryngoscope. Can J Anesth. 2007;54:588–589.
124 Malik AM, Frogel JK. Anterior tonsillar pillar perforation during GlideScope videolaryngoscopy. Anesth Analg. 2007;104:1610–1611.
125 Hsu WT, Hsu SC, Lee YL, et al. Penetrating injury of the soft palate during GlideScope intubation. Anesth Analg. 2007;104:1609–1610.
126 Williams D, Ball DR. Palatal perforation associated with McGrath videolaryngoscope. Anaesthesia. 2009;64:1144–1145.
127 Holst B, Hodzovic I, Francis V. Airway trauma caused by the Airtraq laryngoscope. Anaesthesia. 2008;63:889–890.
128 van Zundert A, van Zundert T, Brimacombe J. Downfolding of the epiglottis during intubation. Anesth Analg. 2010;110:1246–1247.
129 Domino KB, Posner KL, Caplan RA, Cheney FW. Airway injury during anesthesia: A closed claims analysis. Anesthesiology. 1999;91:1703–1711.
130 Cheney FW, Posner KL, Caplan RA. Adverse respiratory events infrequently leading to malpractice suits. A closed claims analysis. Anesthesiology. 1991;75:932–939.
131 Weber S. Traumatic complications of airway management. Anesthesiol Clin North Am. 2002;20:265–274.
132 Hoffman HT, Brunberg JA, Winter P, et al. Arytenoid subluxation: diagnosis and treatment. Ann Otol Rhinol Laryngol. 1991;100:1–9.
133 Appukutty J. Post-intubation cricoarytenoid joint dysfunction. Br J Anaesth. 2008;100:141.
134 Debo RF, Colonna D, Dewerd G, Gonzalez C. Cricoarytenoid subluxation: Complication of blind intubation with a lighted stylet. Ear Nose Throat J. 1989;68:517–520.
135 Close LG, Merkel M, Watson B, Schaefer SD. Cricoarytenoid subluxation, computed tomography, and electromyography findings. Head Neck Surg. 1987;9:341–348.
136 Dudley JP, Mancuso AA, Fonkalsrud EW. Arytenoid dislocation and computed tomography. Arch Otolaryngol. 1984;110:483–484.
137 Frink EJ, Pattison BD. Posterior arytenoid dislocation following uneventful endotracheal intubation and anesthesia. Anesthesiology. 1989;70:358–360.
138 Tan V, Seevanayagam S. Arytenoid subluxation after a difficult intubation treated successfully with voice therapy. Anaesth Intensive Care. 2009;37:843–846.
139 Paulsen FP, Rudert HH, Tillmann BN. New insights into the pathomechanism of postintubation arytenoid subluxation. Anesthesiology. 1999;91:659–666.
140 Usui T, Saito S, Goto F. Arytenoid dislocation while using a McCoy laryngoscope. Anesth Analg. 2001;92:1347–1348.
141 Gaissert HA, Burns J. The compromised airway: Tumors, strictures, and tracheomalacia. Surg Clin North Am. 2010;90:1065–1089.
142 Cavo JW, Jr. True vocal cord paralysis following intubation. Laryngoscope. 1985;95:1352–1359.
143 Ludlow CL, Gracco C, Sasaki CT, et al. Neurogenic and functional disorders of the larynx. In: Ballenger JJ, Snow JB, Jr., eds. Otorhinolaryngology: Head and neck surgery, vol 15. Philadelphia: Williams & Wilkins; 1996:556–584.
144 Arndt GA, Cambray AJ, Tomasson J. Intubation bougie dissection of tracheal mucosa and intratracheal airway obstruction. Anesth Analg. 2008;107:603–604.
145 Gruen R, Cade R, Vellar D. Perforation during nasogastric and orogastric tube insertion. Aust N Z J Surg. 1998;68:809–811.
146 Seaman M, Ballinger P, Sturgill TD, Maertins M. Mediastinitis following nasal intubation in the emergency department. Am J Emerg Med. 1991;9:37–39.
147 Kharasch ED, Sivarajan M. Gastroesophageal perforation after intraoperative transesophageal echocardiography. Anesthesiology. 1996;85:426–428.
148 Mlcak RP, Suman OE, Herndon DN. Respiratory management of inhalation injury. Burns. 2007;33:2–13.
149 Kemper KJ, Ritz RH, Benson MS, Bishop MS. Helium-oxygen mixture in the treatment of postextubation stridor in pediatric trauma patients. Crit Care Med. 1991;19:356–359.
150 Kriner EJ, Shafazand S, Colice GL. The endotracheal tube cuff leak test as a predictor for postextubation stridor. Respir Care. 2005;50:1632–1638.
151 Adderley RJ, Mullins GC. When to extubate the croup patient: The “leak” test. Can J Anaesth. 1987;34(Pt 1):304–306.
152 Kemper KJ, Izenberg S, Marvin JA, Heimbach DM. Treatment of postextubation stridor in a pediatric patient with burns: The role of heliox. J Burn Care Rehabil. 1990;11:337–339.
153 Fisher MM, Raper RF. The ‘cuff-leak’ test for extubation. Anaesthesia. 1992;47:10–12.
154 Efferen LS, Elsakr A. Post-extubation stridor: Risk factors and outcome. J Assoc Acad Minor Phys. 1998;9:65–68.
155 Engoren M. Evaluation of the cuff-leak test in a cardiac surgery population. Chest. 1999;116:1029–1031.
156 Sandhu RS, Pasquale MD, Miller K, Wasser TE. Measurement of endotracheal tube cuff leak to predict postextubation stridor and need for reintubation. J Am Coll Surg. 2000;190:682–687.
157 De Bast Y, De Backer D, Moraine JJ, et al. The cuff leak test to predict failure of tracheal extubation for laryngeal edema. Intensive Care Med. 2002;28:1267–1272.
158 Kemper KJ, Benson MS, Bishop MJ. Predictors of postextubation stridor in pediatric trauma patients. Crit Care Med. 1991;19:352–355.
159 Deem S. Limited value of the cuff-leak test. Respir Care. 2005;50:1617–1618.
160 Oswalt CE, Gates GA, Holmstrom MG. Pulmonary edema as a complication of acute airway obstruction. JAMA. 1977;238:1833–1835.
161 Halow KD, Ford EG. Pulmonary edema following post-operative laryngospasm: A case report and review of the literature. Am Surg. 1993;59:443–447.
162 Frank LP, Schreiber GC. Pulmonary edema following acute upper airway obstruction [letter]. Anesthesiology. 1986;65:106.
163 Holmes JR, Hensinger RN, Wojtys EW. Postoperative pulmonary edema in young, athletic adults. Am J Sports Med. 1991;19:365–371.
164 Lathan SR, Silverman ME, Thomas BL, Waters WC. Postoperative pulmonary edema. South Med J. 1999;92:313–315.
165 Dolinski SY, MacGregor DA, Scuderi PE. Pulmonary hemorrhage associated with negative-pressure pulmonary edema. Anesthesiology. 2000;93:888–890.
166 Krodel DJ, Bittner EA, Abdulnour R, et al. Case scenario: Acute postoperative negative pressure pulmonary edema. Anesthesiology. 2010;113:200–207.
167 Dohi S, Okubo N, Kondo Y. Pulmonary oedema after airway obstruction due to bilateral vocal cord paralysis. Can J Anaesth. 1991;38:492–495.
168 Lang SA, Duncan PG, Shephard DA, Ha HC. Pulmonary oedema associated with airway obstructon. Can J Anaesth. 1990;37:210–218.
169 Ovassapian A, Land P, Schafer MF, et al. Anesthetic management for surgical corrections of severe flexion deformity of the cervical spine. Anesthesiology. 1983;58:370–372.
170 Doyle DJ, Zura A, Ramachandran M, et al. Airway management in a 980-lb patient: Use of the Aintree intubation catheter. J Clin Anesth. 2007;19:367–369.
171 Robinson PM. Prospective study of the complications of endoscopic laryngeal surgery. J Laryngol Otol. 1991;105:356–358.
172 Rosato L, Avenia N, Bernante P, et al. Complications of thyroid surgery: Analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J Surg. 2004;28:271–276.
173 Harding J, Sebag F, Sierra M, et al. Thyroid surgery: Postoperative hematoma—Prevention and treatment. Langenbecks Arch Surg. 2006;391:169–173.
174 Bononi M, Bonapasta SA, Scarpini M, et al. Incidence and circumstances of cervical hematoma complicating thyroidectomy and its relationship to postoperative vomiting. Head Neck. 2010;32:1173–1177.
175 Bexton MD, Radford R. An unusual cause of respiratory obstruction after thyroidectomy. Anaesthesia. 1982;37:596.
176 Bukht D, Langford RM. Airway obstruction after surgery in the neck. Anaesthesia. 1983;38:389–390.
177 Ayabe H, Kawahara K, Tagawa Y, Tomita M. Upper airway obstruction from a benign goiter. Surg Today. 1992;22:88–90.
178 Shen WT, Kebebew E, Duh QY, Clark OH. Predictors of airway complications after thyroidectomy for substernal goiter. Arch Surg. 2004;139:656–659. discussion 659–660
179 Abraham D, Singh N, Lang B, et al. Benign nodular goitre presenting as acute airway obstruction. ANZ J Surg. 2007;77:364–367.
180 Abrams JT, Horrow JC, Bennett JA, et al. Upper airway closure: A primary source of difficult ventilation with sufentanil induction of anesthesia. Anesth Analg. 1996;83:629–632.
181 Palazzo FF, Allen JG, Greatorex RA. Laryngeal mask airway and fibre-optic tracheal inspection in thyroid surgery: A method for timely identification of tracheomalacia requiring tracheostomy. Ann R Coll Surg Engl. 2000;82:141–142.
182 Palazzo FF, Allen JG, Greatorex RA. Respiratory complication after thyroidectomy and the need for tracheostomy in patients with a large goitre. Br J Surg. 1999;86:967–968.
183 Greenstein AJ, Chassin MR, Wang J, et al. Association between minor and major surgical complications after carotid endarterectomy: Results of the New York Carotid Artery Surgery study. J Vasc Surg. 2007;46:1138–1144. discussion 1145–1136
184 Ferguson GG, Eliasziw M, Barr HWK, et al. The North American Symptomatic Carotid Endarterectomy Trial: Surgical results in 1415 patients. Stroke. 1999;30:1751–1758.
185 Carmichael FJ, McGuire GP, Wong DT, et al. Computed tomographic analysis of airway dimensions after carotid endarterectomy. Anesth Analg. 1996;83:12–17.
186 Self DD, Bryson GL, Sullivan PJ. Risk factors for post-carotid endarterectomy hematoma formation. Can J Anaesth. 1999;46:635–640.
187 Kunkel JM, Gomez ER, Spebar MJ, et al. Wound hematomas after carotid endarterectomy. Am J Surg. 1984;148:844–847.
188 O’Sullivan JC, Wells DG, Wells GR. Difficult airway management with neck swelling after carotid endarterectomy. Anaesth Intensive Care. 1986;14:460–464.
189 Munro FJ, Makin AP, Reid J. Airway problems after carotid endarterectomy. Br J Anaesth. 1996;76:156–159.
190 Hughes R, McGuire G, Montanera W, et al. Upper airway edema after carotid endarterectomy: The effect of steroid administration. Anesth Analg. 1997;84:475–478.
191 Shakespeare WA, Lanier WL, Perkins WJ, Pasternak JJ. Airway management in patients who develop neck hematomas after carotid endarterectomy. Anesth Analg. 2010;110:588–593.
192 Cunningham EJ, Bond R, Mayberg MR, et al. Risk of persistent cranial nerve injury after carotid endarterectomy. J Neurosurg. 2004;101:445–448.
193 Kwok AO, Silbert BS, Allen KJ, et al. Bilateral vocal cord palsy during carotid endarterectomy under cervical plexus block. Anesth Analg. 2006;102:376–377.
194 Morpeth J, Williams M. Vocal fold paralysis after anterior cervical diskectomy and fusion. Laryngoscope. 2000;110:43–46.
195 Sagi HC, Beutler W, Carroll E, Connolly PJ. Airway complications associated with surgery on the anterior cervical spine. Spine. 2002;27:949–953.
196 Epstein NE, Hollingsworth R, Nardi D, Singer J. Can airway complications following multilevel anterior cervical surgery be avoided? J Neurosurg. 2001;94(Suppl):185–188.
197 Andrew SA, Sidhu KS. Airway changes after anterior cervical discectomy and fusion. J Spinal Disord Tech. 2007;20:577–581.
198 Lee YH, Hsieh PF, Huang HH, Chan KC. Upper airway obstruction after cervical spine fusion surgery: Role of cervical fixation angle. Acta Anaesthesiol Taiwan. 2008;46:134–137.
199 Beed SD, Devitt JH. Mortality associated with orthognathic surgery. Can J Anaesth. 1996;43:A40.
200 Meisami T, Musa M, Keller MA, et al. Magnetic resonance imaging assessment of airway status after orthognathic surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:458–463.
201 Dark A, Armstrong T. Severe postoperative laryngeal oedema causing total airway obstruction immediately on extubation. Br J Anaesth. 1999;82:644–646.
202 Hogan PW, Argalious M. Total airway obstruction after maxillomandibular advancement surgery for obstructive sleep apnea. Anesthesia Analgesia. 2006;103:1267–1269.
203 Phero JC, Weaver JM, Peskin RM. Anesthesia for maxillofacial/mandibular trauma. In: Benumof J, ed. Anesthesiology clinics of North America. Philadelphia: WB Saunders; 1993:509–523.
204 Krausz AA, El-Naaj IA, Barak M. Maxillofacial trauma patient: Coping with the difficult airway. World J Emerg Surg. 2009;4:21.
205 Dupanovic M, Fox H, Kovac A. Management of the airway in multitrauma. Curr Opin Anaesthesiol. 2010;23:276–282.
206 Caron G, Paquin R, Lessard MR, et al. Submental endotracheal intubation: An alternative to tracheotomy in patients with midfacial and panfacial fractures. J Trauma. 2000;48:235–240.
207 Sharma RK, Tuli P, Cyriac C, et al. Submental tracheal intubation in oromaxillofacial surgery. Indian J Plast Surg. 2008;41:15–19.
208 Mohan R, Iyer R, Thaller S. Airway management in patients with facial trauma. J Craniofac Surg. 2009;20:21–23.
209 Bahr W, Stoll P. Nasal intubation in the presence of frontobasal fractures: A retrospective study. J Oral Maxillofac Surg. 2009;50:445–447.
210 Ovassapian A, Tuncbilek M, Weitzel EK. Airway management in adult patients with deep neck infections: A case series and review of the literature. Anesth Analg. 2005;100:585–589.
211 Wolfe MM, Davis JW, Parks SN. Is surgical airway necessary for airway management in deep neck infections and Ludwig angina? J Crit Care. 2011;26:11–14.
212 Potter JK, Herford AS, Ellis E, III. Tracheotomy versus endotracheal intubation for airway management in deep neck space infections. J Oral Maxillofac Surg. 2002;60:349–354.
213 Artru AA, Cucchiara RF, Messick JM. Cardiorespiratory and cranial-nerve sequelae of surgical procedures involving the posterior fossa. Anesthesiology. 1980;52:83–86.
214 Gorski DW, Rao TL, Scarff TB. Airway obstruction following surgical manipulation of the posterior cranial fossa, an unusual complication. Anesthesiology. 1981;54:80–81.
215 Howard R, Mahoney A, Thurlow AC. Respiratory obstruction after posterior fossa surgery. Anaesthesia. 1990;45:222–224.
216 Thompson JW, Newman L, Boop FA, Sanford RA. Management of postoperative swallowing dysfunction after ependymoma surgery. Childs Nerv Syst. 2009;25:1249–1252.
217 Pearson FG, Gullane P. Subglottic resection with primary tracheal anastomosis: Including synchronous laryngotracheal reconstructions. Semin Thorac Cardiovasc Surg. 1996;8:381–391.
218 Pinsonneault C, Fortier J, Donati F. Tracheal resection and reconstruction. Can J Anaesth. 1999;46(Pt 1):439–455.
219 Gaissert HA, Honings J, Grillo HC, et al. Segmental laryngotracheal and tracheal resection for invasive thyroid carcinoma. Ann Thorac Surg. 2007;83:1952–1959.
220 Sandberg W. Anesthesia and airway management for tracheal resection and reconstruction. Int Anesthesiol Clin. 2000;38:55–75.
221 Saravanan P, Marnane C, Morris EA. Extubation of the surgically resected airway—A role for remifentanil and propofol infusions. Can J Anesth. 2006;53:507–511.
222 Szokol JW, Wenig BL, Murphy GS, Drezek E. Life-threatening upper airway obstruction after tongue base surgery. Anesthesiology. 2001;94:532–534.
223 Pepin JL, Veale D, Mayer P, et al. Critical anaylsis of the result of surgery in the treatment of snoring, upper airway resistance syndrome (UARS), and obstructive sleep apnea (OSA). Sleep. 1996;19(Suppl):S90–S100.
224 Haavisto L, Suonpaa J. Complications of uvulopalatopharyngoplasty. Clin Otolaryngol. 1994;19:243–247.
225 Coleman J, Bick PA. Suspension sutures for the treatment of obstructive sleep apnea and snoring. Otolaryngol Clin North Am. 1999;32:277–285.
226 Antony AK, Sloan GM. Airway obstruction following palatoplasty: Analysis of 247 consecutive operations. Cleft Palate Craniofac J. 2002;39:145–148.
227 Hayes JP, Nolan MT, Brennan N, FitzGerald MX. Three cases of paradoxical vocal cord adduction followed up over a 10-year period. Chest. 1993;104:678–680.
228 Golden SE. The management and treatment of recurrent postoperative laryngospasm. Anesth Analg. 1997;84:1392.
229 Mevorach DL. The management and treatment of recurrent postoperative laryngospasm. Anesth Analg. 1996;83:1110–1111.
230 Christopher KL. Understanding vocal cord dysfunction: A step in the right direction with a long road ahead. Chest. 2006;129:842–843.
231 Christopher KL, Wood RP, Eckert RC, et al. Vocal-cord dysfunction presenting as asthma. N Engl J Med. 1983;308:1566–1570.
232 Tousignant G, Kleiman SJ. Functional stridor diagnosed by the anaesthetist. Can J Anaesth. 1992;39:286–289.
233 Sukhani R, Barclay J, Chow J. Paradoxical vocal cord motion: An unusual cause of stridor in the recovery room. Anesthesiology. 1993;79:177–180.
234 Arndt GA, Voth BR. Paradoxical vocal cord motion in the recovery room: A masquerader of pulmonary dysfunction. Can J Anaesth. 1996;43:1249–1251.
235 Harbison J, Dodd J, McNicholas WT. Paradoxical vocal cord motion causing stridor after thyroidectomy. Thorax. 2000;55:533–534.
236 Hammer G, Schwinn D, Wollman H. Postoperative complications due to paradoxical vocal cord motion. Anesthesiology. 1987;66:686–687.
237 Larsen B, Caruso LJ, Villariet DB. Paradoxical vocal cord motion: An often misdiagnosed cause of postoperative stridor. J Clin Anesth. 2004;16:230–234.
238 Blumin JH, Berke GS. Bilateral vocal fold paresis and multiple system atrophy. Arch Otolaryngol Head Neck Surg. 2002;128:1404–1407.
239 Gan EC, Lau DP, Cheah KL. Stridor in Parkinson’s disease: A case of ‘dry drowning’? J Laryngol Otol. 2010;124:668–673.
240 Shimohata T, Shinoda H, Nakayama H, et al. Daytime hypoxemia, sleep-disordered breathing, and laryngopharyngeal findings in multiple system atrophy. Arch Neurol. 2007;64:856–861.
241 Vincken WG, Gauthier SG, Dollfuss RE, et al. Involvement of upper-airway muscles in extrapyramidal disorders. A cause of airflow limitation. N Engl J Med. 1984;311:438–442.
242 Vincken WG, Darauay CM, Cosio MG. Reversibility of upper airway obstruction after levodopa therapy in Parkinson’s disease. Chest. 1989;96:210–212.
243 Easdown LJ, Tessler MJ, Minuk J. Upper airway involvement in Parkinson’s disease resulting in postoperative respiratory failure. Can J Anaesth. 1995;42:344–347.
244 Fitzpatrick AJ. Upper airway obstruction in Parkinson’s disease. Anaesth Intensive Care. 1995;23:367–369.
245 Backus WW, Ward RR, Vitkun SA, et al. Postextubation laryngeal spasm in an unanesthetized patient with Parkinson’s disease. J Clin Anesth. 1991;3:314–316.
246 Liu EH, Choy J, Dhara SS. Persistent perioperative laryngospasm in a patient with Parkinson’s disease. Can J Anesth. 1998;45:495.
247 Matti MV, Sharrock NE. Anesthesia on the rheumatoid patient. Rheum Dis Clin North Am. 1998;24:19–34.
248 Gurley JP, Bell GR. The surgical management of patients with rheumatoid cervical spine disease. Rheum Dis Clin North Am. 1997;23:317–332.
249 Norton ML, Ghanma MA. Atlantoaxial instability revisited. An alert for endoscopists. Ann Otol Rhinol Laryngol. 1982;91(Pt 1):567–570.
250 Wattenmaker I, Concepcion M, Hibberd P, Lipson S. Upper-airway obstruction and perioperative management of the airway in patients managed with posterior operations on the cervical spine for rheumatoid arthritis. J Bone Joint Surg Am. 1994;76:360–365.
251 Kohjitani A, Miyawaki T, Kasuya K, et al. Anesthetic management for advanced rheumatoid arthritis patients with acquired micrognathia undergoing temporomandibular joint replacement. J Oral Maxillofac Surg. 2002;60:559–566.
252 Lofgren RH, Montgomery WW. Incidence of laryngeal involvement in rheumatoid arthritis. N Engl J Med. 1962;267:193–195.
253 Funk D, Raymon F. Rheumatoid arthritis of the cricoarytenoid joints: An airway hazard. Anesth Analg. 1975;54:742–745.
254 Chalmers A, Traynor JA. Cricoarytenoid arthritis as a cause of acute upper airway obstruction. J Rheumatol. 1979;6:541–542.
255 Keenan MA, Stiles CM, Kaufman RL. Acquired laryngeal deviation associated with cervical spine disease in erosive polyarticular arthritis. Use of the fiberoptic bronchoscope in rheumatoid disease. Anesthesiology. 1983;58:441–449.
256 Skues MA, Welchew EA. Anaesthesia and rheumatoid arthritis. Anaesthesia. 1993;48:989–997.
257 Leicht MJ, Harrington TM, Davis DE. Cricoarytenoid arthritis: A cause of laryngeal obstruction. Ann Emerg Med. 1987;16:885–888.
258 Bamshad M, Rosa U, Padda G, Luce M. Acute upper airway obstruction in rheumatoid arthritis of the cricoarytenoid joints. South Med J. 1989;82:507–511.
259 Kolman J, Morris I. Cricoarytenoid arthritis: A cause of acute upper airway obstruction in rheumatoid arthritis. Can J Anaesth. 2002;49:729–732.
260 Guerra LG, Lau KY, Marwah R. Upper airway obstruction as the sole manifestation of rheumatoid arthritis. J Rheumatol. 1992;19:974–976.
261 Cooper RM. Rheumatoid arthritis is a common disease with clinically important implications for the airway. J Bone Joint Surg Am. 1995;77:1463–1465.
262 Wright CD. Tracheomalacia. Chest Surg Clin North Am. 2003;13:349–357. viii
263 Masters IB, Chang AB, Patterson L, et al. Series of laryngomalacia, tracheomalacia, and bronchomalacia disorders and their associations with other conditions in children. Pediatr Pulmonol. 2002;34:189–195.
264 Triglia JM, Nicollas R, Roman S, Kreitman B. Tracheomalacia associated with compressive cardiovascular anomalies in children. Pediatr Pulmonol. 2001;23(Suppl):8–9.
265 Bailey B. Laryngoscopy and laryngoscopes—Who’s first? The forefathers/four fathers of laryngology. Laryngoscope. 1996;106:939–943.
266 Hochberg MC, Ruddy S, Harris ED, Sledge CB. Relapsing polychondritis. ed 5. Kelley WN, Ruddy S, Harris ED, Jr., Sledge CB, eds. Kelley’s textbook of rheumatology. Philadelphia: WB Saunders; 2001;vol 6:1463–1468.
267 Sarodia BD, Dasgupta A, Mehta AC. Management of airway manifestations of relapsing polychondritis: Case reports and review of literature. Chest. 1999;116:1669–1675.
268 Hayward AW, al Shaikh B. Relapsing polychondritis and the anaesthetist. Anaesthesia. 1988;43:573–577.
269 Burgess FW, Whitlock W, Davis MJ, Patane PS. Anesthetic implications of relapsing polychondritis: A case report. Anesthesiology. 1990;73:570–572.
270 Biro P, Rohling R, Schmid S, et al. Anesthesia in a patient with acute respiratory insufficiency due to relapsing polychondritis. J Clin Anesth. 1994;6:59–62.
271 Fitzmaurice BG, Brodsky JB, Kee ST, et al. Anesthetic management of a patient with relapsing polychondritis. J Cardiothorac Vasc Anesth. 1999;13:309–311.
272 Tso AS, Chung HS, Wu CY, et al. Anesthetic management of a patient with relapsing polychondritis—A case report. Acta Anaesthesiol Sin. 2001;39:189–194.
273 Adliff M, Ngato D, Keshavjee S, et al. Treatment of diffuse tracheomalacia secondary to relapsing polychondritis with continuous positive airway pressure. Chest. 1997;112:1701–1704.
274 Benumof JL. Obstructive sleep apnea in the adult obese patient: Implications for airway management. Anesthesiol Clin North Am. 2002;20:789–811.
275 Chung SA, Yuan H, Chung F. A systemic review of obstructive sleep apnea and its implications for anesthesiologists. Anesth Analg. 2008;107:1543–1563.
276 Isono S. Obstructive sleep apnea of obese adults: Pathophysiology and perioperative airway management. Anesthesiology. 2009;110:908–921.
277 Langeron O, Masso E, Huraux C, et al. Prediction of difficult mask ventilation. Anesthesiology. 2000;92:1229–1236.
278 Kheterpal S, Han R, Tremper KK, et al. Incidence and predictors of difficult and impossible mask ventilation. Anesthesiology. 2006;105:885–891.
279 Esclamado RM, Glenn MG, McCulloch TM, Cummings CW. Perioperative complications and risk factors in the surgical treatment of obstructive sleep apnea syndrome. Laryngoscope. 1989;99:1125–1129.
280 Riley RW, Powell NB, Guilleminault C, et al. Obstructive sleep apnea surgery: Risk management and complications. Otolaryngol Head Neck Surg. 1997;117:648–652.
281 Chou HC, Wu TL. Large hypopharyngeal tongue: A shared anatomic abnormality for difficult mask ventilation, difficult intubation, and obstructive sleep apnea? Anesthesiology. 2001;94:936–937.
282 Aziz MF, Healy D, Kheterpal S, et al. Routine clinical practice effectiveness of the Glidescope in difficult airway management: An analysis of 2,004 Glidescope intubations, complications, and failures from two institutions. Anesthesiology. 2011;114:34–41.
283 Benumof JL, Dagg R, Benumof R. Critical hemoglobin desaturation will occur before return to an unparalyzed state following 1 mg/kg intravenous succinylcholine. Anesthesiology. 1997;87:979–982.
284 Rennotte MT, Baele P, Aubert G, Rodenstein DO. Nasal continuous positive airway pressure in the perioperative management of patients with obstructive sleep apnea submitted to surgery. Chest. 1995;107:367–374.
285 Juvin P, Lavaut E, Dupont H, et al. Difficult tracheal intubation is more common in obese than in lean patients. Anesth Analg. 2003;97:595–600.
286 Practice guidelines for the perioperative management of patients with obstructive sleep apnea: A report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Anesthesiology. 2006;104:1081–1093.
287 Johnson JT, Braun TW. Preoperative, intraoperative, and postoperative management of patients with obstructive sleep apnea syndrome. Otolaryngol Clin North Am. 1998;31:1025–1030.
288 Burgess GE, III., Cooper JR, Jr., Marino RJ, et al. Laryngeal competence after tracheal extubation. Anesthesiology. 1979;51:73–77.
289 Debaene B, Plaud B, Dilly MP, Donati F. Residual paralysis in the PACU after a single intubating dose of nondepolarizing muscle relaxant with an intermediate duration of action. Anesthesiology. 2003;98:1042–1048.
290 Eriksson LI, Sundman E, Olsson R, et al. Functional assessment of the pharynx at rest and during swallowing in partially paralyzed humans: Simultaneous videomanometry and mechanomyography of awake human volunteers. Anesthesiology. 1997;87:1035–1043.
291 Berg H, Roed J, Viby-Mogensen J, et al. Residual neuromuscular block is a risk factor for postoperative pulmonary complications. A prospective, randomised, and blinded study of postoperative pulmonary complications after atracurium, vecuronium and pancuronium. Acta Anaesthesiol Scand. 1997;41:1095–1103.
292 Ng A, Smith G. Gastroesophageal reflux and aspiration of gastric contents in anesthetic practice. Anesth Analg. 2001;93:494–513.
293 Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78:56–62.
294 Olsson GL, Hallen B, Hambraeus-Jonzon K. Aspiration during anaesthesia: A computer-aided study of 185,358 anaesthetics. Acta Anaesthesiol Scand. 1986;30:84–92.
295 Raghavendran K, Nemzek J, Napolitano LM, Knight PR. Aspiration-induced lung injury. Crit Care Med. 2011;39:818–826.
296 Kluger MT, Short TG. Aspiration during anaesthesia: A review of 133 cases from the Australian Anaesthetic Incident Monitoring Study (AIMS). Anaesthesia. 1999;54:19–26.
297 Gibbison B, Cook TM, Seller C. Case series: Protection from aspiration and failure of protection from aspiration with the i-gel airway. Br J Anaesth. 2008;100:415–417.
298 Mark DA. Protection from aspiration with the LMA-ProSeal after vomiting: A case report. Can J Anesth. 2003;50:78–80.
299 Cooper RM. The LMA, laparoscopic surgery and the obese patient—Can vs should. Can J Anesth. 2003;50:5–10.
300 Cooper RM. Use of a new videolaryngoscope (GlideScope®) in the management of a difficult airway. Can J Anesth. 2003;50:611–613.
301 Mort TC. Emergency tracheal intubation: Complications associated with repeated laryngoscopic attempts. Anesth Analg. 2004;99:607–613.
302 Brimacombe J. The advantages of the LMA over the tracheal tube or facemask: A meta-analysis. Can J Anaesth. 1995;42:1017–1023.
303 Fujii Y, Toyooka H, Tanaka H. Cardiovascular responses to tracheal extubation or LMA removal in normotensive and hypertensive patients. Can J Anaesth. 1997;44:1082–1086.
304 Silva LCE, Brimacombe JR. Tracheal tube/laryngeal mask exchange for emergence. Anesthesiology. 1996;85:218.
305 Dob DP, Shannon CN, Bailey PM. Efficacy and safety of the laryngeal mask airway vs Guedel airway following tracheal extubation. Can J Anaesth. 1999;46:179–181.
306 Stix MS, Borromeo CJ, Sciortino GJ, Teague PD. Learning to exchange an endotracheal tube for a laryngeal mask prior to emergence. Can J Anaesth. 2001;48:795–799.
307 Gasteiger L, Brimacombe J, Perkhofer D, et al. Comparison of guided insertion of the LMA ProSeal vs the i-gel. Anaesthesia. 2010;65:913–916.
308 Asai T. Use of the laryngeal mask for exchange of orotracheal tubes. Anesthesiology. 1999;91:1167–1168.
309 Matioc A, Arndt GA. Intubation using the ProSeal laryngeal mask airway and a Cook airway exchange catheter set. Can J Anesth. 2001;48:932.
310 Hawkins M, Roberts EA. Use of a cuffed oropharyngeal airway and Aintree catheter in a difficult airway. Anaesthesia. 1999;54:909–910.
311 Zura A, Doyle DJ, Orlandi M. Use of the Aintree intubation catheter in a patient with an unexpected difficult airway. Can J Anaesth. 2005;52:646–649.
312 Higgs A, Clark E, Premraj K. Low-skill fibreoptic intubation: Use of the Aintree Catheter with the classic LMA. Anaesthesia. 2005;60:915–920.
313 Farag E, Bhandary S, Deungria M, et al. Successful emergent reintubation using the Aintree intubation catheter and a laryngeal mask airway. Minerva Anestesiol. 2010;76:148–150.
314 Cook TM, Brooks TS, Van der Westhuizen J, Clarke M. The Proseal LMA is a useful rescue device during failed rapid sequence intubation: Two additional cases. Can J Anesth. 2005;52:630–633.
315 Cook TM, Seller C, Gupta K, et al. Non-conventional uses of the Aintree intubating catheter in management of the difficult airway. Anaesthesia. 2007;62:169–174.
316 Greenland KB, Tan H, Edwards M. Intubation via a laryngeal mask airway with an Aintree catheter—Not all laryngeal masks are the same. Anaesthesia. 2007;62:966–967.
317 Zura A, Doyle DJ, Avitsian R, DeUngria M. More on intubation using the Aintree catheter. Anesth Analg. 2006;103:785.
318 Dellinger RP. Fiberoptic bronchoscopy in adult airway management. Crit Care Med. 1990;18:882–887.
319 Watson CB. Use of fiberoptic bronchoscope to change endotracheal tube endorsed. Anesthesiology. 1981;55:476–477.
320 Finucane BT, Kupshik HL. A flexible stilette for replacing damaged tracheal tubes. Can Anaesth Soc J. 1978;25:153–154.
321 Desai SP, Fencl V. A safe technique for changing endotracheal tubes [letter]. Anesthesiology. 1980;53:267.
322 Tomlinson AA. Difficult tracheal intubation. Anaesthesia. 1985;40:496–497.
323 Baraka A, Louis F, Sibai AN, Usta N. A simple manoeuvre for changing the tracheal tube. Intensive Care Med. 1987;13:216–217.
324 Robles B, Hester J, Brock-Utne JG. Remember the gum-elastic bougie at extubation. J Clin Anesth. 1993;5:329–331.
325 DeLima LG, Bishop MJ. Lung laceration after tracheal extubation over a plastic tube changer. Anesth Analg. 1991;73:350–351.
326 Seitz PA, Gravenstein N. Endobronchial rupture from endotracheal reintubation with an endotracheal tube guide. J Clin Anesth. 1989;1:214–217.
327 Audenaert SM, Montgomery CL, Slayton D, Berger R. Application of the Mizus endotracheal obturator in tracheostomy and tentative extubation. J Clin Anesth. 1991;3:418–421.
328 Chipley PS, Castresana M, Bridges MT, Catchings TT. Prolonged use of an endotracheal tube changer in a pediatric patient with a potentially compromised airway. Chest. 1994;105:961–962.
329 Arndt GA, Ghani GA. A modification of an Eschmann endotracheal tube changer for insufflation. Anesthesiology. 1988;69:282–283.
330 Steinberg MJ, Chmiel RA. Use of a nasogastric tube as a guide for endotracheal reintubation. J Oral Maxillofac Surg. 1989;47:1232–1233.
331 Bedger RC, Jr., Chang JL. A jet-stylet endotracheal catheter for difficult airway management. Anesthesiology. 1987;66:221–223.
332 Chang JL, Bleyaert A, Bedger R. Unilateral pneumothorax following jet ventilation during general anesthesia. Anesthesiology. 1980;53:244–246.
333 Egol A, Culpepper JA, Snyder JV. Barotrauma and hypotension resulting from jet ventilation in critically ill patients. Chest. 1985;88:98–102.
334 Atlas G, Mort TC. Extubation of the difficult airway over an airway exchange catheter: Relationship of catheter size and patient tolerance. Crit Care Med. 1999;27:A57.
335 Atlas G. A high-risk endotracheal tube exchanger. Anesth Analg. 2002;95:785.
336 Mort TC. Continuous airway access for the difficult extubation: The efficacy of the airway exchange catheter. Anesth Analg. 2007;105:1357–1362.
337 Cooper RM, Levytam S. Use of an endotracheal ventilation catheter for difficult extubations. Anesthesiology. 1992;77:A1110.
338 Cooper RM. The use of an endotracheal ventilation catheter in the management of difficult extubations. Can J Anaesth. 1996;43:90–93.
339 Loudermilk EP, Hartmannsgruber M, Stoltzfus DP, Langevin PB. A prospective study of the safety of tracheal extubation using a pediatric airway exchange catheter for patients with a known difficult airway. Chest. 1997;111:1660–1665.
340 Katsnelson T, Frost EA, Farcon E, Goldiner PL. When the endotracheal tube will not pass over the flexible fiberoptic bronchoscope. Anesthesiology. 1992;76:151–152.
341 Benumof JL. Difficult tubes and difficult airways. J Cardiothorac Vasc Anesth. 1998;12:131–132.
342 Hannallah M. Evaluation of Tracheal Tube Exchangers for replacement of double-lumen endobronchial tubes. Anesthesiology. 1992;77:609–610.
343 Cooper RM, Cohen DR. The use of an endotracheal ventilation catheter for jet ventilation during a difficult intubation. Can J Anaesth. 1994;41:1196–1199.
344 Andrews SR, Norcross SD, Mabey MF, Siegel JB. The WuScope technique for endotracheal tube exchange. Anesthesiology. 1999;90:929–930.
345 Andrews SR, Mabey MF. Tubular fiberoptic laryngoscope (WuScope) and lingual tonsil airway obstruction. Anesthesiology. 2000;93:904–905.
346 Sprung J, Wright LC, Dilger J. Use of WuScope for exchange of endotracheal tube in a patient with difficult airway. Laryngoscope. 2003;113:1082–1084.
347 Asai T, Shingu K. Difficulty in advancing a tracheal tube over a fibreoptic bronchoscope: Incidence, causes and solutions. Br J Anaesth. 2004;92:870–881.
348 Mort TC. Tracheal tube exchange: Feasibility of continuous glottic viewing with advanced laryngoscopy assistance. Anesth Analg. 2009;108:1228–1231.
349 Hirabayashi Y. GlideScope-assisted endotracheal tube exchange. J Cardiothorac Vasc Anesth. 2007;21:777.
350 Matioc AA, Lopukhin O. The Airtraq to facilitate endotracheal tube exchange in a critically ill, difficult-to-intubate patient. J Clin Anesth. 2007;19:485–486.
351 Tapnio RU, Viegas OJ. An alternative method for conversion of a nasal to an orotracheal intubation. Anesthesiology. 1998;88:1683–1684.
352 Gabriel DM, Azocar RJ. A novel technique for conversion of nasotracheal tube to orotracheal [letter]. Anesthesiology. 2000;93:911.
353 Novella J. Intraoperative nasotracheal to orotracheal tube change in a patient with Klippel-Feil syndrome. Anaesth Intensive Care. 1995;23:402–403.
354 Cooper RM. Conversion of a nasal to an orotracheal intubation using an endotracheal tube exchanger. Anesthesiology. 1997;87:717–718.
355 Sumiyoshi R, Kai T, Takahashi S. Application of negative-pressure ventilation when changing endotracheal tubes. Anesthesiology. 1994;81:1551–1552.
356 Cooper RM. Negative pressure ventilation during tracheal tube exchange. Anesthesiology. 1995;82:1533–1534.
357 Smith JE, Fenner SG. Conversion of orotracheal to nasotracheal intubation with the aid of the fibreoptic laryngoscope. Anaesthesia. 1993;48:1016.
358 Avitsian R, Doyle DJ, Helfand R, et al. Successful reintubation after cervical spine exposure using an Aintree intubation catheter and a laryngeal mask airway. J Clin Anesth. 2006;18:224–225.
359 Lam NC, Hagberg CA, Bassili LM. Use of the video laryngoscopy for Combitube exchange in a difficult airway. J Clin Anesth. 2009;21:294–296.
360 Weinmann M, Bander JJ. Introduction of a new tracheostomy exchange device after percutaneous tracheostomy in a patient with coagulopathy. J Trauma. 1996;40:317–319.
361 Benumof JL, Gaughan SD. Concerns regarding barotrauma during jet ventilation. Anesthesiology. 1992;76:1072–1073.
362 Benumof JL, Scheller MS. The importance of transtracheal jet ventilation in the management of the difficult airway. Anesthesiology. 1989;71:769–778.
363 Dworkin R, Benumof JL, Benumof R, Karagianes TG. The effective tracheal diameter that causes air trapping during jet ventilation. J Cardiothorac Anesth. 1990;4:731–736.
364 Gaughan SD, Benumof JL, Ozaki GT. Quantification of the jet function of a jet stylet. Anesth Analg. 1992;74:580–585.
365 Gaughan SD, Ozaki GT, Benumof JL. A comparison in a lung model of low- and high-flow regulators for transtracheal jet ventilation. Anesthesiology. 1992;77:189–199.
366 Baraka AS. Tension pneumothorax complicating jet ventilation via a Cook airway exchange catheter. Anesthesiology. 1999;91:557–558.
367 Irish JC, Brown DH, Cooper RM. Airway control during percutaneous tracheotomy. Laryngoscope. 1994;104:1178–1180.
368 Chan AS, Manninen PH. Bronchoscopic findings of a tension pneumothorax. Anesth Analg. 1995;80:628–629.
369 Benumof JL. Airway exchange catheters: Simple concept, potentially great danger. Anesthesiology. 1999;91:342–344.

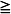 3 for laryngeal view
3 for laryngeal view