Chapter 35 Extraspinal Anatomy and Surgical Approaches to the Thoracic Spine
The thoracic spine contains more vertebrae than any other segment of the spinal column. With its 12 vertebrae, the thoracic spine is responsible for the load bearing and flexibility that has allowed Homo sapiens to stand erect. Given its critical role in the biomechanics of movement and its large contribution to the spinal column (almost a third of the total vertebrae), it is not surprising that the thoracic segment is also a frequent site of pathology (Table 35-1). Trauma, primary and metastatic tumors of the column, infections, vascular malformations, congenital disorders, and deformity all affect the thoracic column, making the ability to operate in this region an essential skill set for the competent neurosurgeon.
TABLE 35-1 Indications for Surgery
| Indication | Type |
|---|---|
| Trauma | Vertebral body fracture causing spinal cord compression |
| Infection | Tuberculosis of the vertebral body |
| Deformity | Scoliosis, kyphosis |
| Degeneration | Any type |
| Tumor | Primary and metastatic |
Anatomy
The thoracic vertebrae arise from a mesodermal origin. There are three primary centers of ossification in the cartilaginous template of the vertebra, the centrum and the two neural arches.1,2 These initial three centers of primary ossification mature into five secondary centers at the tips of the transverse processes, the spinous processes, and the annular epiphysial discs.1,2 Development of the spinal column proceeds postnatally and continues into adolescence, whereby the lordotic and kyphotic curves necessary for weight-bearing are established and completed.
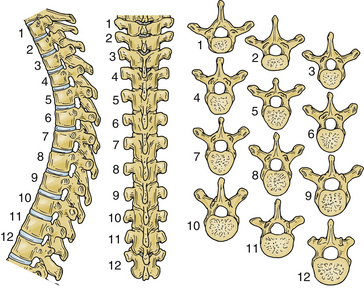
During early development, the intricate connection between the ribs and the thoracic spine begins to contour the posture of humans. The ribs articulate with the vertebral bodies via the costovertebral joints, the transverse processes via the costotransverse joints, and the pedicle of the vertebrae. As is the rule in the spinal column, the size of a vertebra increases from the cervical to the lumbar regions. Therefore the size of the thoracic vertebrae is intermediate compared with their adjacent vertebrae (Fig. 35-1).3 From T1 to T12, the length of the transverse processes decreases. The spinous processes of the thoracic vertebrae are not uniform. At the midthoracic levels, the spinous processes are long and oriented inferiorly compared with their more horizontal orientation at the lower thoracic levels. From T1 to T4, the spinal canal is heart shaped and gradually transitions to a more circular shape from T4 to T8. An imaging study of the thoracic spine frequently shows a vascular groove caused by the impression of the descending aorta. Relative to their cervical counterparts, the thoracic laminae are thicker and deeper, albeit their width is considerably decreased.4 The thoracic pedicles are short, and their height and radius increase from T1 to T12.3,5
Throughout the thoracic spine, the angle between the pedicle and midsagittal plane changes dramatically depending on the level.5 This observation has important clinical implications, such as for the placement of pedicle screws for fixation.6 At T1 the angle between the pedicle and the midsagittal plane is wide, but by T12 the pedicles are parallel to the midsagittal plane. The thoracic pedicles are shorter and thinner than their lumbar counterparts, making them more susceptible to perforation during screw placement.
The relationship between the transverse process and the pedicle is variable in the thoracic spine; this variability makes the use of intraoperative fluoroscopy a necessity for thoracic cases.7 At the upper thoracic levels, the transverse process is located rostral to the pedicle; at the lower levels, the transverse process is caudal to the pedicle with the crossover occurring at T6-7.7
From T1 to T10 the facets are oriented coronally. The orientation becomes oblique between T10 and T12.8 The coronal orientation is important for flexion-extension movements in the lower thoracic spine. The thickness and width of the laminae overlying the facets increase as they progress from rostral to caudal in the thoracic spine. Throughout the thoracic levels, the short and broad laminae in the upper and middle thoracic spine prevent hyperextension.3 The multitude of ligamentous connections, most notably the anterior longitudinal ligament, provides additional stability by increasing the tensile strength of the column. The articular surface of the superior facets is on the ventral aspect, whereas the articular surface of the inferior facets is dorsal. The articular surfaces of the facet joints are flat and slope in an oblique coronal plane, in the same plane as the lamina.
Approaches
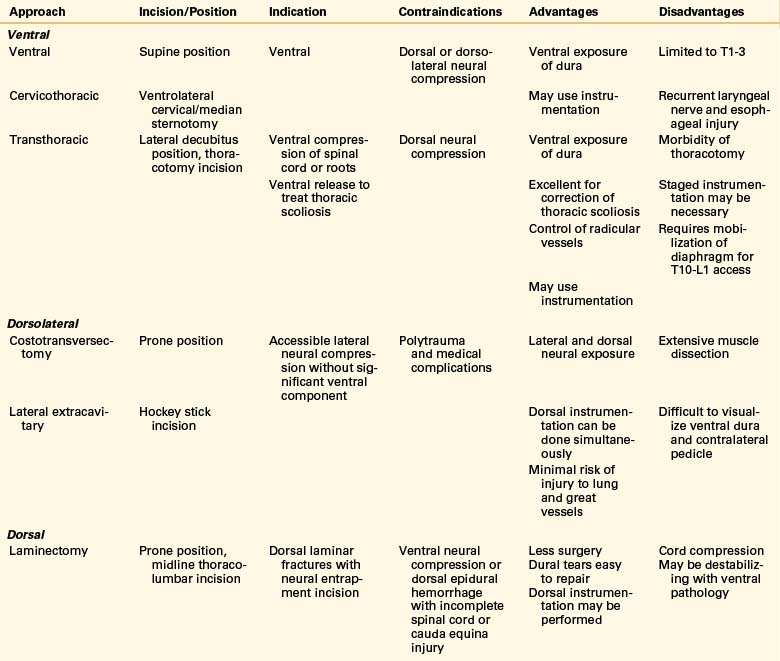
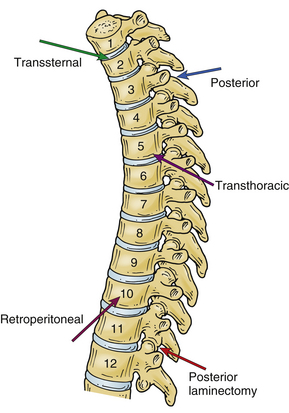
The choice of the approach to the thoracic spine largely depends on the location of the pathology that the surgeon is treating (Tables 35-2 and 35-3; Fig. 35-2). Traditionally, the operative technique of choice for treatment of pathology involving the thoracic spine has been laminectomy. Although this approach is useful for reaching dorsal pathology, it can cause great damage if used to treat ventral lesions.9,10 With the recent advent of novel microsurgical techniques and hardware for ventral stabilization, ventral approaches have become commonplace for the treatment of ventral pathology.
TABLE 35-2 Approaches to the Thoracic Spine as a Function of Level of Pathology
| Approach | Vertebral Level |
|---|---|
| Standard ventral cervical | T1-4 |
| Transsternal | T1-4 |
| Transthoracic | T3-11 |
| Transthoracic, transdiaphragmatic, retroperitoneal | T11-L1 |
Dorsal Approaches
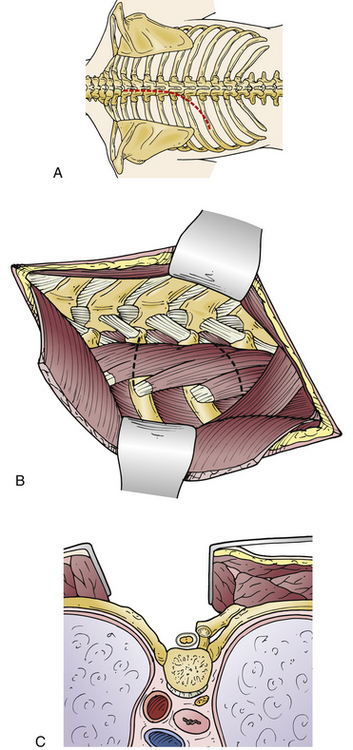
The skin is incised in the midline above the spinous process, and the subcutaneous tissue is dissected (Fig. 35-3A). The thoracolumbar fascia is incised with electrocautery. The paraspinal muscles are detached subperiosteally from the spinous process and lamina (Fig. 35-3B). A sponge may be used to perform the blunt dissection of the paraspinal muscles laterally. The sponge may also be used to control bleeding. During the blunt dissection it is important to ensure that the joint capsule of the facet is not damaged.
Ventral Surgical Approaches
Historically, the impetus for the development of spinal approaches and stabilization was the treatment of trauma, tumors, and Pott disease caused by the tuberculosis pandemics of the late 1800s and early 1900s. Hodgson and Stock described the first ventral approach with an acceptable morbidity rate of 2.9% for the debridement of a tuberculosis abscess.11
The ventral approaches can be divided on the basis of the level on the thoracic column that they reach (see Table 35-2). Generally, the higher thoracic levels (T1-3) are readily accessible using a standard ventral cervical approach, occasionally extended with a median sternotomy or sternal window. This approach is excellent for ventrally located disease with minimal paraspinal involvement. The T2-11 vertebrae can be approached through a dorsolateral thoracotomy, usually from the left side to avoid the liver and azygos vein. However, some surgeons prefer the right-sided approach to avoid the aorta. When the approach is used to correct a deformity, the rule is to use the side of the apex or convexity of the curve to allow application of interbody devices. The lower thoracic region and the thoracolumbar junction can be approached via a left thoracotomy combined with dorsal detachment of the diaphragm via a retroperitoneal approach. Again, the preference of the side depends on the surgeon’s comfort and the location of the pathology.
Cervical Exposure with a Median Sternotomy/Transmanubrial Approach
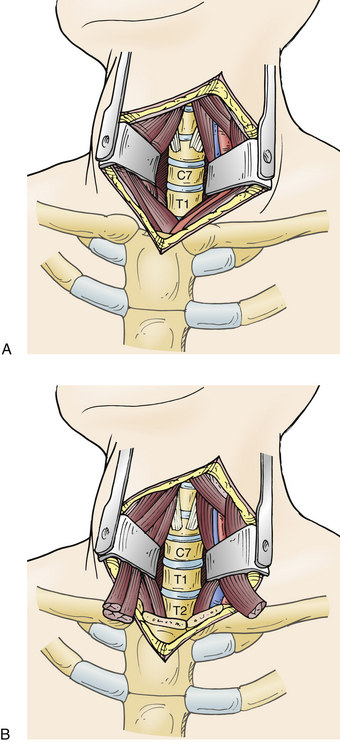
The high thoracic levels can be accessed via a standard cervical approach (Fig. 35-4A). A cervical approach paralleling the medial border of the sternocleidomastoid muscle is usually appropriate for T1 and T2 lesions. A medial sternotomy or sternal osteotomy is occasionally necessary to extend the surgical field to the level of T3 or T4. Most surgeons prefer a left-sided approach, which lowers the risk of injury to the recurrent laryngeal nerve on this side.12 A preoperative CT scan can be helpful in determining the relationship of the clavicle and sternum to the spine and for planning purposes.
The patient is positioned supine with the midline of the head placed on a donut and the neck extended. A roll can be used between the scapulae to augment the manubrium. The incision is made in a T-shaped or cervicosternal fashion parallel to the border of the sternocleidomastoid muscle. During the dissection it may be necessary to sacrifice veins in the surgical path. The sternocleidomastoid muscle can be detached from its origin to increase the field of view. The sternohyoid and sternothyroid muscles are sectioned above the clavicles and sternal notch. The platysma is divided along its fibers in the direction of the incision. If necessary, the medial third of the clavicles can be sectioned and disarticulated from the manubrium. The inferior thyroid vein is ligated and sectioned. The carotid sheath is identified and retracted laterally. Similarly the trachea and esophagus are mobilized medially to create a plane for dissection. Next, the sternohyoid muscle is mobilized from the medial clavicle and sternum (Fig. 35-4B). Finger dissection is used to create a plane beneath the sternum and into the upper mediastinum. The superior thyroid artery should be identified and ligated.
A median sternotomy is performed down to the manubrium, and the mediastinum is opened with a retractor.13 The pleura is opened, and the left innominate vein is divided with ligatures for adequate caudal exposure. A plane is developed beneath the esophagus and above the prevertebral fascia. The longus colli muscles are elevated, and self-retaining retractors are placed. Care must be taken to avoid damage to the recurrent laryngeal nerve. The area of interest is identified using fluoroscopy. At the end of the case, the manubrium is fixed with stainless steel wires and the clavicle with plates and screws.
Thoracotomy
This approach traverses the thorax to gain access to the spinal column, most notably the vertebrae from T3 to T10.14,15 A thoracotomy can be performed from either the right or left side, and the approach largely depends on the location of the pathology. The indications for thoracotomy are correction of deformity, degenerative disease, repair of fractures, resection of tumors, stabilization after trauma, and treatment of infection involving the thoracic column. When the approach is used to correct a deformity, the approach is always from the side of the apex of the curve of the spine. This approach provides excellent visualization of the thoracic vertebrae but is also associated with complications. Among the most avoidable of such complications are procedures performed on the wrong side or the exposure being too high or too low relative to the pathology. These problems can be largely avoided by using fluoroscopy and image-guided systems in the operating room (OR) and the use of standard OR time-outs. Preoperative marking by a radiologist can also be helpful.
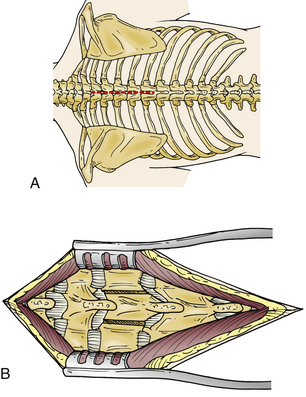
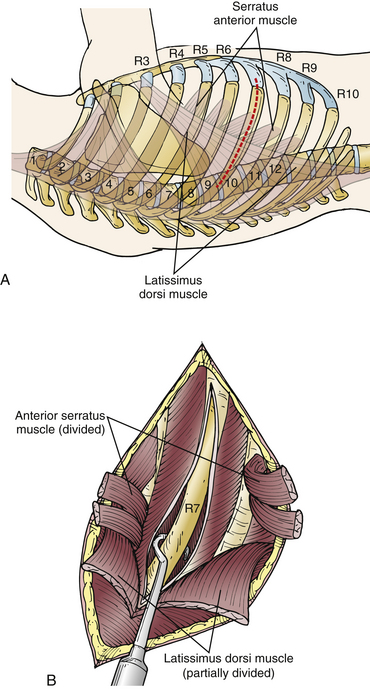
The incision should be placed as close over the pathology as possible. During this approach, the rib resected usually dictates the highest vertebral level that can be accessed and the best exposure for the vertebra two levels below it. The skin incision starts at the lateral border of the paraspinous muscles and extends to the sternocostal junction of the ribs. After the incision is made through the subcutaneous tissue, the latissimus dorsi and serratus anterior muscles are divided. In the authors’ experience it is best to incise the latissimus dorsi only partially and to lift it with a retractor to minimize the risk of damage to underlying tissues (Fig. 35-5). The serratus anterior muscle should be dissected as far distally as possible, especially in the higher thoracic levels, to minimize damage to the long thoracic nerve.
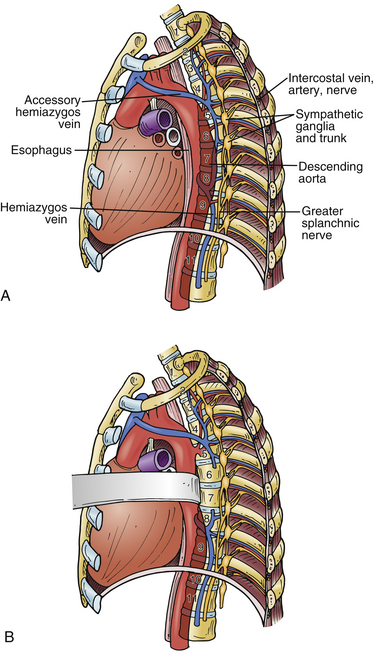
Once the segmental vessels are released, the aorta can be mobilized to the right side and the prevertebral area exposed for surgery (Fig. 35-6). A sponge stick can be used to further expose the vertebral bodies. For approaches to T11-L1, monopolar cauterization is used to divide the diaphragm about 1 cm from the costal margin. The retroperitoneal space is then entered.
The technique for closure is important because several critical structures, including the lung, azygos vein, and aorta, are in the surgeon’s path. One or two chest tubes are placed, and the operative site is irrigated with antibiotic solution. The chest tubes are set to suction and remain in place until drainage decreases to less than 100 mL/day. Chest tubes are placed to water seal in cases where the dura has opened or cerebrospinal fluid has been encountered. The parietal pleura should be closed whenever possible. At our institution we use thoracic drains. The skin incision for the thoracic drains should be placed one level below the targeted intercostal level. A rib approximator may be used to narrow the cavity between the ribs created by the retractor. The ribs may be reapproximated with a suture. The surgeon must ensure that the neurovascular bundle is excluded. At this point the anesthesiologist can test the patency of the lung by reinflating it. Reinflation of the lung is critical to avoid unwanted atelectasis. The soft tissues are closed sequentially. Other potential sources of complication include the risk of injury to the lung, segmental vessels, azygos vein, and aorta or entry into the intervertebral foramen.
Thoracoabdominal Approach
This approach is excellent for the thoracolumbar junction, most notably from T9 to L5.16,17 Although this approach is feasible from both the right and left sides, a left-sided approach is preferred because the liver and vena cava are not in the trajectory of the surgeon’s approach. For a left-sided approach, the patient is placed on the right side. The table can be bent above the pelvis to increase the distance between the pelvis and ribcage, adding exposure to this region. Depending on the target level, it is recommended to resect the ninth or tenth rib. After the skin and subcutaneous tissues are incised at the thoracolumbar junction, the muscle is split in the direction of its fibers to open the superficial muscular layer of the rectus anterior, latissimus dorsi, and external oblique muscles.
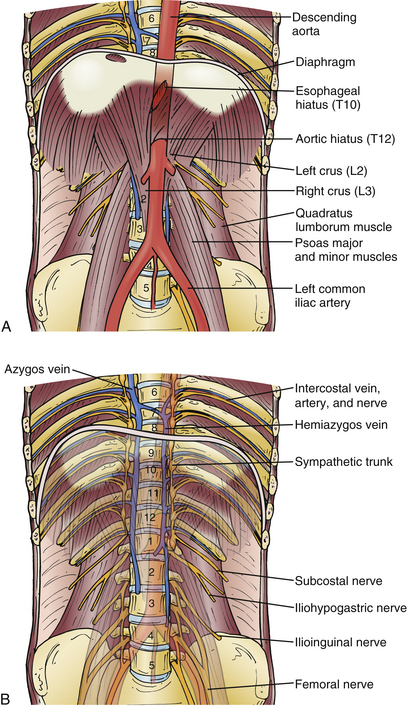
Starting with a retroperitoneal approach, the external oblique, internal oblique, and transversus muscles are split. The peritoneum is mobilized to the midline and freed with a sponge stick from the diaphragm. The ninth or tenth rib is resected as in a thoracotomy. This rib can be used later as a structural bone graft or morcellized and placed in a cage. The ventral resection is performed as near the cartilage-bone junction of the rib as possible. The costal cartilage is split, and the diaphragm is transected about 2 cm medial to its insertion into the thoracic wall. The transected diaphragm should be mobilized by using holding sutures, which are used during closure to approximate the tissues. The mobilized diaphragm is transected about 2 cm above the medial and lateral arcuate ligaments. The parietal pleura is incised at the thoracic level of the pathology. The psoas muscle should be mobilized dorsally to augment the field of view. The segmental vessels may be ligated at the level of the pathology to minimize bleeding during the approach and operation (Fig. 35-7).
Complications associated with this procedure include entry into the peritoneal space and damage to the greater splanchnic nerve, ascending lumbar vein, sympathetic trunk, thoracic duct, or the great vessels.
Dorsolateral Surgical Approaches
Costotransversectomy
The patient is typically placed in the prone position; some surgeons also use the lateral decubitus position.18,19 A roll may be placed under the scapula to augment the exposure of the chest. The choice of incision depends on the location and extent of the pathology and may include either a longitudinal paraspinal incision or a midline or transverse paraspinal incision. The paraspinal incision is made in a curvilinear fashion almost 2 inches from the midline of the vertebra of choice. Cautery is used to cut the paraspinal muscle and thoracolumbar fascia, and a Cobb elevator is used to separate the muscles at the level of interest (Figs. 35-8A and B).
The pleura lies immediately deep to the ribs; therefore the surgeon must use caution during the resection. Using sharp periosteal dissection, the surgeon separates the periosteum from the rib (Fig. 35-8C). The costotransverse joint is incised, and the periosteum of the rib is elevated circumferentially. During the dissection, the neurovascular bundle should be identified and care exerted to preserve it. The rib is cut at its angle and disarticulated from the costovertebral joint to enable dissection of the parietal pleura and endothoracic fascia. A self-retaining retractor or a rib spreader can be used to augment the exposure.
Summary
The thoracic spine may be approached from the front or back and from either side. The complex anatomy of the mediastinum and the abdomen means that a multidisciplinary team of thoracic surgeons, general surgeons, and neurosurgeons is required to approach, treat, and stabilize the spinal column. Given the diversity of pathology that involves the thoracic spine, comfort with approaching the thoracic levels is requisite for a spinal surgeon.
Chaynes P., Sol J.C., Vaysse P., et al. Vertebral pedicle anatomy in relation to pedicle screw fixation: a cadaver study. Surg Radiol Anat. 2001;23(2):85-90.
Dias M.S. Normal and abnormal development of the spine. Neurosurg Clin North Am. 2007;18(3):415-429.
Ebraheim N.A., Xu R., Ahmad M., Yeasting R.A. The quantitative anatomy of the thoracic facet and the posterior projection of its inferior facet. Spine (Phila Pa 1976). 1997;22(16):1811-1817.
Lesoin F., Thomas C.E.III, Autricque A., et al. A transsternal biclavicular approach to the upper anterior thoracic spine. Surg Neurol. 1986;26(3):253-256.
Maiman D.J., Pintar F.A. Anatomy and clinical biomechanics of the thoracic spine. Clin Neurosurg. 1992;38:296-324.
McCormack B.M., Benzel E.C., Adams M.S., et al. Anatomy of the thoracic pedicle. Neurosurgery. 1995;37(2):303-308.
Overby M.C., Rothman A.S. Anterolateral decompression for metastatic epidural spinal cord tumors. Results of a modified costotransversectomy approach. J Neurosurg. 1985;62(3):344-348.
1. Dias M.S. Normal and abnormal development of the spine. Neurosurg Clin North Am. 2007;18(3):415-429.
2. Oskouian R.J.Jr., Sansur C.A., Shaffrey C.I. Congenital abnormalities of the thoracic and lumbar spine. Neurosurg Clin North Am. 2007;18(3):479-498.
3. Maiman D.J., Pintar F.A. Anatomy and clinical biomechanics of the thoracic spine. Clin Neurosurg. 1992;38:296-324.
4. Xu R., Burgar A., Ebraheim N.A., Yeasting R.A. The quantitative anatomy of the laminas of the spine. Spine (Phila Pa 1976). 1999;24(2):107-113.
5. Chaynes P., Sol J.C., Vaysse P., et al. Vertebral pedicle anatomy in relation to pedicle screw fixation: a cadaver study. Surg Radiol Anat. 2001;23(2):85-90.
6. Attar A., Ugur H.C., Uz A., et al. Lumbar pedicle: surgical anatomic evaluation and relationships. Eur Spine J. 2001;10(1):10-15.
7. McCormack B.M., Benzel E.C., Adams M.S., et al. Anatomy of the thoracic pedicle. Neurosurgery. 1995;37(2):303-308.
8. Ebraheim N.A., Xu R., Ahmad M., Yeasting R.A. The quantitative anatomy of the thoracic facet and the posterior projection of its inferior facet. Spine (Phila Pa 1976). 1997;22(16):1811-1817.
9. Standefer M., Hardy R.W.Jr., Marks K., Cosgrove D.M. Chondromyxoid fibroma of the cervical spine—a case report with a review of the literature and a description of an operative approach to the lower anterior cervical spine. Neurosurgery. 1982;11(2):288-292.
10. Smith T.K., Stallone R.J., Yee J.M. The thoracic surgeon and anterior spinal surgery. J Thorac Cardiovasc Surg. 1979;77(6):925-928.
11. Hodgson A.R., Stock F.E. Anterior spinal fusion: a preliminary communication on the radical treatment of Pott’s disease and Pott’s paraplegia. Br J Surg. 1956;44(185):266-275.
12. Micheli L.J., Hood R.W. Anterior exposure of the cervicothoracic spine using a combined cervical and thoracic approach. J Bone Joint Surg [Am]. 1983;65(7):992-997.
13. Lesoin F., Thomas C.E.III, Autricque A., et al. A transsternal biclavicular approach to the upper anterior thoracic spine. Surg Neurol. 1986;26(3):253-256.
14. Anderson T.M., Mansour K.A., Miller J.I.Jr. Thoracic approaches to anterior spinal operations: anterior thoracic approaches. Ann Thorac Surg. 1993;55(6):1447-1451.
15. McElvein R.B., Nasca R.J., Dunham W.K., Zorn G.L.Jr. Transthoracic exposure for anterior spinal surgery. Ann Thorac Surg. 1988;45(3):278-283.
16. Burrington J.D., Brown C., Wayne E.R., Odom J. Anterior approach to the thoracolumbar spine: technical considerations. Arch Surg. 1976;111(4):456-463.
17. Heitmiller R.F. The left thoracoabdominal incision. Ann Thorac Surg. 1988;46(2):250-253.
18. Garrido E. Modified costotransversectomy: a surgical approach to ventrally placed lesions in the thoracic spinal canal. Surg Neurol. 1980;13(2):109-113.
19. Overby M.C., Rothman A.S. Anterolateral decompression for metastatic epidural spinal cord tumors. Results of a modified costotransversectomy approach. J Neurosurg. 1985;62(3):344-348.
20. Larson S.J., Holst R.A., Hemmy D.C., Sances A.Jr. Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. J Neurosurg. 1976;45(6):628-637.