Chapter 10 Extraocular Muscles
This chapter begins with a brief review of the microscopic and macroscopic anatomy of striated muscle, then discusses eye movements and describes the characteristics and actions of each extraocular muscle. (The smooth intrinsic muscles are discussed in Chapter 3.)
Microscopic Anatomy of Striated Muscle
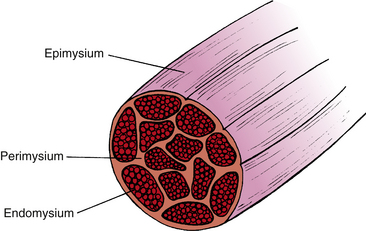
Striated muscle is surrounded by a connective tissue sheath known as the epimysium; continuous with this sheath is a connective tissue network, the perimysium, which infiltrates the muscle and divides it into bundles. The individual muscle fiber within the bundle is surrounded by a delicate connective tissue enclosure, the endomysium (Figure 10-1). The individual muscle fiber is comparable to a cell; however, each fiber is multinucleated, with the nuclei arranged at the periphery of the fiber. The plasma cell membrane surrounding each muscle fiber, the sarcolemma, forms a series of invaginations into the cell, the transverse tubules (T tubules), which allow ions to spread quickly through the cell in response to an action potential. The cell cytoplasm, sarcoplasm, contains normal cellular structures and special muscle fibers, the myofibrils.
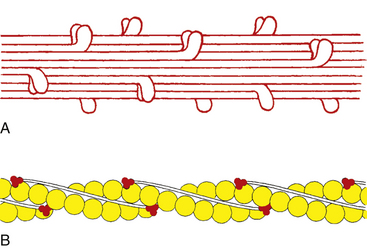
Myofibrils comprise two types, thick and thin. The thick myofibrils are composed of hundreds of myosin subunits. Each subunit is a long, slender filament with two globular heads attached by arms at one end. These filaments lie next to each other and form the “backbone” of the myofibril, with the heads projecting outward in a spiral (Figure 10-2, A). The thin myofibrils are formed by the protein actin arranged in a double-helical filament, with a molecular complex of troponin and tropomyosin lying within the grooves of the double helix (Figure 10-2, B).
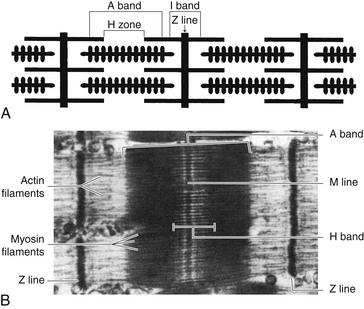
The alternating light and dark bands characteristic of striated muscle are produced by the manner in which these two types of myofibrils are arranged. The light band is the I (isotropic) band, and the dark band is the A (anisotropic) band. These names describe the birefringence to polarized light exhibited by the two areas.1
The I band contains two sets of actin filaments connected to each other at the Z line, a dark stripe bisecting the I band. Only actin myofibrils are found in the I band. The A band contains both myosin and actin; the central lighter zone of the A band—the H zone—contains only myosin. Overlapping actin and myosin filaments form the outer darker edges of the A band (Figure 10-3). The M line bisects the H zone and contains proteins that interconnect the myosin fibrils.
Sliding Ratchet Model of Muscle Contraction
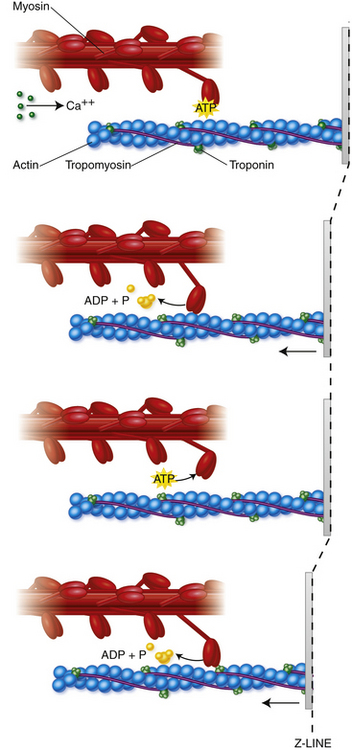
The process of muscle contraction and sarcomere shortening is explained by the sliding ratchet model2–4 (Figure 10-4). The initiation of a muscle contraction occurs when a nerve impulse causes the release of acetylcholine into the neuromuscular junction. The sarcolemma depolarizes and an action potential passes along the surface and is carried into the muscle fiber through the system of T-tubules. Ionic channels are opened and calcium ions are released from the sarcoplasmic reticulum into sarcoplasm. Ca2+ binds to the troponin-tropomyosin complex, resulting in a configurational change, allowing an active site on the actin protein to be available for binding with a myosin head. Coincidentally, adenosine triphosphate (ATP) attached to the myosin head is broken down and released, allowing a cross-bridge to bind with the active actin site. Once this bond is formed, the head tilts toward the shaft of the myosin filament, pulling the actin filament along with it.
Structure of the Extraocular Muscles
The extraocular muscles have a denser blood supply, and their connective tissue sheaths are more delicate and richer in elastic fibers than is skeletal muscle.5 Fewer muscle fibers are included in a motor unit in extraocular muscle than are found in skeletal muscle elsewhere. Striated muscle of the leg can contain several hundred muscle fibers per motor unit6; in the extraocular muscles, each axon innervates 3 to 10 fibers.7 This dense innervation provides for precise fine motor control of the extraocular muscles resulting in high velocity ocular movements, necessary in saccades, (up to 1000 degrees per second) and very accurate pursuits (velocities of 100 degrees per second) and fixations.8 Singly innervated fibers have the classic end plate (en plaque) seen in skeletal muscle; multiply innervated fibers have a neuromuscular junction resembling a bunch of grapes (en grappe).9,10
The extraocular muscles have a range of fiber sizes, with the fibers closer to the surface generally having smaller diameters (5 to 15 μm) and those deeper within the muscle generally having larger diameters (10 to 40 μm).11–14 They can be divided into groups based on characteristics such as location, size, morphology, neuromuscular junction type, or various biochemical properties.5,12,13,15,16 The fibers range from typical twitch fibers at one end of the spectrum to typical slow fibers at the other end, with gradations in between. It would seem that the fast-twitch fibers should produce quick saccadic movements and the slow fibers should produce slower pursuit movements and provide muscle tone. However, all fibers apparently are active at all times and share some level of involvement in all ocular movements.12,14,16,17 Extraocular muscles are among the fastest and most fatigue-resistant of striated muscle.18
Muscle spindles and Golgi tendon organs of typical striated muscle have been identified in human extraocular muscle, although it is unclear whether these structures provide any useful proprioceptive information relative to the extraocular muscles.19,20 Afferent information regarding extraocular muscle proprioception is thought to be mediated by a receptor that is unique to extraocular muscle, the myotendinous cylinder (palisade ending).16,21
Eye Movements
Fick’s Axes
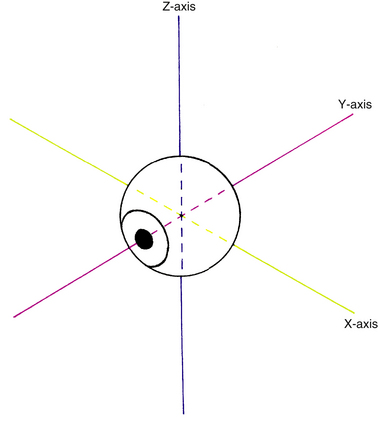
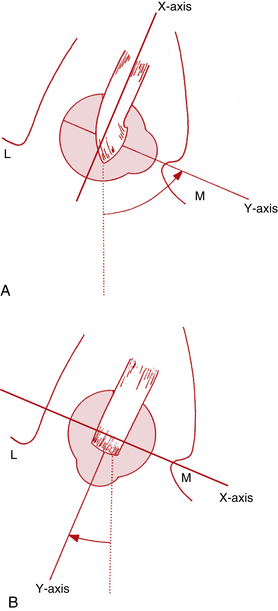
Before a discussion of the individual muscles and the resultant eye movements caused by their contraction, it is necessary to define certain terms. All eye movement can be described as rotations around one or more axes. According to Fick, these axes divide the globe into quadrants and intersect at the center of rotation, a fixed nonmoving point22 and the approximate geometric center of the eye. For convenience, it is assumed that the eye rotates around this fixed point, located 13.5 mm behind the cornea; this point varies in ametropia, is slightly more posterior in myopia, and is slightly more anterior in hyperopia.5 The x-axis is the horizontal or transverse axis and runs from nasal to temporal. The y-axis is the sagittal axis running from the anterior pole to the posterior pole. The z-axis is the vertical axis and runs from superior to inferior (Figure 10-5). When the front of the eye moves up, the back moves down. When the front of the eye moves right, the back of the eye moves left. The anterior pole of the globe is the reference point used in the description of any eye movement. Eye movements are described and based on the movement of the muscle insertion towards its origin.
Ductions
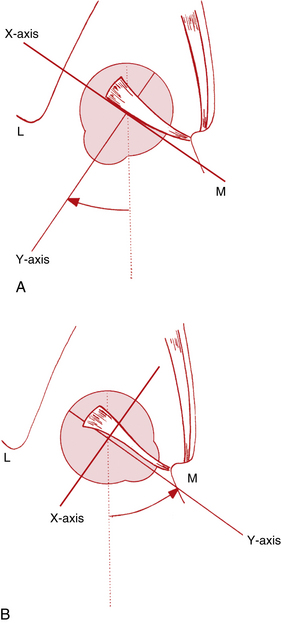
Movements involving just one eye are called ductions (Figure 10-6). Rotations around the vertical axis move the anterior pole of the globe medially—adduction—or laterally—abduction. Rotations around the horizontal axis move the anterior pole of the globe up—elevation (supraduction)—or down—depression (infraduction).
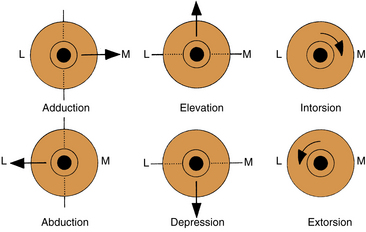
FIGURE 10-6 Movements of the eye in duction. Anterior pole is point of reference. L, Lateral; M, medial.
Torsions or cyclorotations are rotations around the sagittal axis and are described in relation to a point at the 12-o’clock position on the superior limbus. Intorsion (incyclorotation) is the rotation of that point nasally, and extorsion (excyclorotation) is the rotation of that point temporally. Torsional movements may occur in an attempt to keep the horizontal retinal raphe parallel to the horizon.23 With a head tilt of 30 degrees, the ipsilateral eye is intorted approximately 7 degrees, and the contralateral eye is extorted approximately 8 degrees.24
Torsional movements have been questioned by some investigators who believe that true torsion occurs only in pathologic conditions.25 Others have documented torsion in normal eye movements and with head tilt.26–29
Vergences and Versions
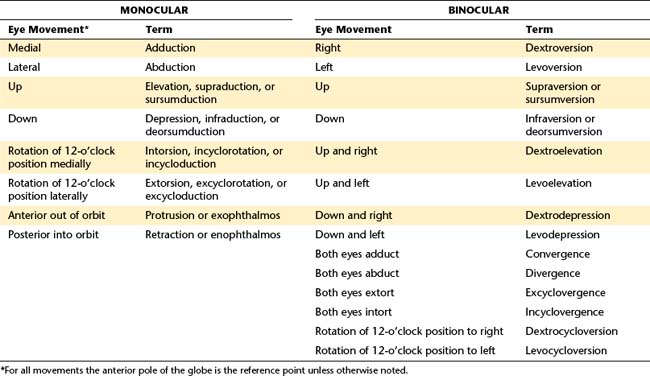
Movements involving both eyes are either vergences or versions, depending on the relative directions of movement. In vergence movements, the eyes move in opposite left-right directions; these are disjunctive movements. In convergence each eye is adducted, and in divergence each eye is abducted. Version movements are conjugate movements and occur when the eyes move in the same direction. Dextroversion is right gaze, and levoversion is left gaze. In supraversion both eyes are elevated, and in infraversion both eyes are depressed. Table 10-1 lists terms for monocular and binocular eye movements and some combination movements.
Positions of Gaze
The primary position of gaze is defined as the position of the eye with the head erect, the eye located at the intersection of the sagittal plane of the head and the horizontal plane passing through the centers of rotation of both eyes, and the eye focused for infinity.17 Secondary positions of gaze are rotations around either the vertical axis or the horizontal axis; tertiary positions are rotations around both the vertical and the horizontal axes.
Macroscopic Anatomy of the Extraocular Muscles
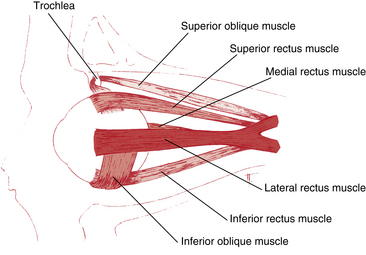
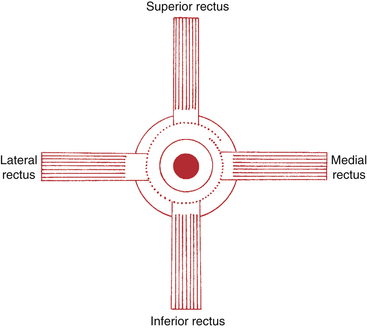
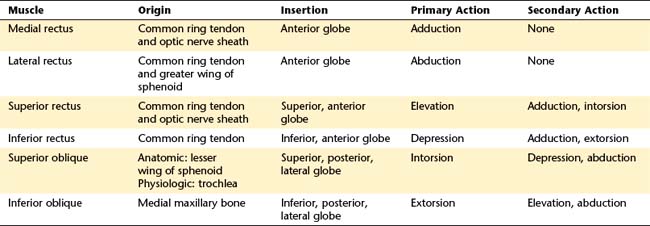
The six extraocular muscles are medial rectus, lateral rectus, superior rectus, inferior rectus, superior oblique, and inferior oblique (Figure 10-7). From longest to shortest, the rectus muscles are the superior, the medial, the lateral, and the inferior.30
Origin of the Rectus Muscles
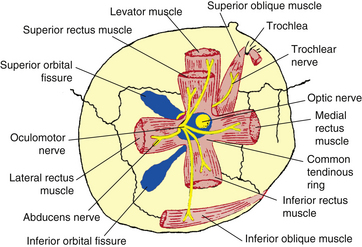
The four rectus muscles have their origin on the common tendinous ring (annulus of Zinn). This oval band of connective tissue is continuous with the periorbita and is located at the apex of the orbit anterior to the optic foramen and the medial part of the superior orbital fissure. The upper and lower areas are thickened bands and sometimes are referred to as the upper and lower tendons or limbs. The medial and lateral rectus muscles take their origin from both parts of the tendinous ring. The superior rectus is attached to the upper limb, and the inferior rectus is joined to the lower (Figure 10-8). The medial rectus and the superior rectus also attach to the dural sheath of the optic nerve.30
Clinical Comment: Retrobulbar Optic Neuritis
RETROBULBAR OPTIC NEURITIS is an inflammation affecting the sheaths of the optic nerve. Generally, there are no observable fundus changes in this condition, but pain with extreme eye movement can be one of the early presenting signs.1,30 The optic nerve sheath is supplied with a dense sensory nerve network and because of the close association of muscle sheath and optic nerve sheath, eye movement can cause stretching of the optic nerve sheath, resulting in a sensation of pain.31
The area enclosed by the tendinous ring is called the oculomotor foramen, and several blood vessels and nerves pass through the foramen, having entered the orbit either through the optic canal or the superior orbital fissure (see Figure 10-8). The optic nerve and ophthalmic artery enter the oculomotor foramen from the optic canal; the superior and inferior divisions of the oculomotor nerve, the abducens nerve, and the nasociliary nerve enter the oculomotor foramen from the superior orbital fissure (see Figure 10-8). These structures lie within the muscle cone, the area enclosed by the four rectus muscles and the connective tissue joining them. Thus the motor nerve to each rectus muscle can enter the surface of the muscle that lies within the muscle cone.
In 1887, Motais described a common muscle sheath between the rectus muscles enclosing the space within the muscle cone.5 More recently, dissections by Koornneef32 revealed no definitive, continuous muscle sheath between the rectus muscles in the retrobulbar region.
The lacrimal and frontal nerves and the superior ophthalmic vein lie above the common ring tendon, and the inferior ophthalmic vein lies below. They are outside the muscle cone (see Figure 8-15).
Insertions of the Rectus Muscles: Spiral of Tillaux
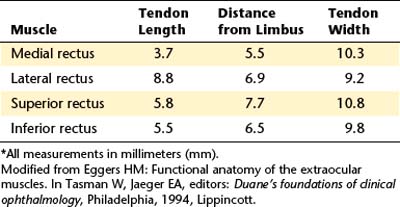
The four rectus muscles insert into the globe anterior to the equator. A line connecting the rectus muscle insertions forms a spiral, as described by Tillaux. This spiral starts at the medial rectus, the insertion that is closest to the limbus, and proceeds to the inferior rectus, the lateral rectus, and finally the superior rectus, the insertion farthest from the limbus5 (Figure 10-9). In a recent study, variations were found from person to person in specific measurements, but the spiral of Tillaux was always observed.33 The tendons of insertion pierce Tenon’s capsule and merge with scleral fibers. A sleeve of the capsule covers the tendon for a short distance, and the muscle can slide freely within this sleeve.5,13 Connective tissue extends from the insertions joining them to each other.
Medial Rectus Muscle
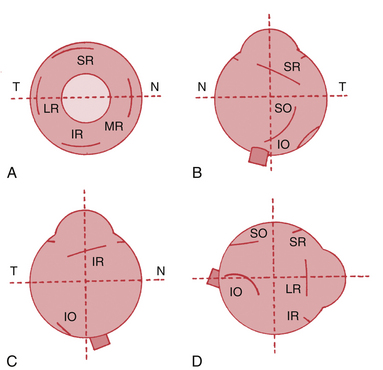
The medial rectus muscle is the largest of the extraocular muscles, with its size probably resulting from the frequency of its use in convergence.30 Its origin is from both the upper and the lower parts of the common ring tendon and from the sheath of the optic nerve. The medial rectus muscle parallels the medial orbital wall until it passes through a connective tissue pulley just posterior to the equator of the globe; at this point it follows the curve of the globe to its insertion.34 The insertion of the medial rectus is about 5.5 mm from the limbus, and the tendon is approximately 3.7 mm long5 (Table 10-2). The insertion line lies vertically such that the horizontal plane of the eye approximately bisects it (Figure 10-10, A). The superior oblique muscle, ophthalmic artery, and nasociliary nerve lie above the medial rectus. Fascial expansions from the sheath of the muscle run to the medial wall of the orbit and form the well-developed medial check ligament (see Figure 8-18). The medial rectus is innervated by the inferior division of cranial nerve III, the oculomotor nerve, which enters the muscle on its lateral surface.
Lateral Rectus Muscle
The lateral rectus muscle has its origin on both limbs of the common tendinous ring and the spina recti lateralis, a prominence on the greater wing of the sphenoid bone. The lateral rectus muscle parallels the lateral orbital wall until it passes through a connective tissue pulley just posterior to the equator of the globe; at this point it follows the curve of the globe to its insertion.34,35 The insertion parallels that of the medial rectus and is approximately 6.9 mm from the limbus, and the length of the tendon is approximately 8.8 mm.5
The lacrimal artery and nerve run along the superior border of the lateral rectus muscle. The ciliary ganglion, the abducens nerve, and the ophthalmic artery lie medial to the lateral rectus between it and the optic nerve. Fascial expansions from the muscle sheath attach to the lateral wall of the orbit and form the lateral check ligament (see Figure 8-18). The lateral rectus is innervated by cranial nerve VI, the abducens nerve, which enters on the medial side of the muscle.
Superior Rectus Muscle
The superior rectus muscle has its origin on the superior part of the common tendinous ring and the sheath of the optic nerve.30 The muscle passes forward beneath the levator muscle; the sheaths enclosing these two muscles are connected to each other, allowing coordination of eye movement with eyelid position and resulting in elevation of the eyelid with upward gaze. An additional band of this tissue connects to the superior conjunctival fornix. The superior rectus muscle parallels the roof of the orbit until it passes through a connective tissue pulley just posterior to the equator of the globe; at this point it follows the curve of the globe to its insertion.34,35
The insertion of the superior rectus is approximately 7.7 mm from the limbus5 and is curved slightly, with the convex side forward. The line of the insertion is oblique, with the nasal side closer to the limbus than the temporal side (see Figure 10-10, B). The tendon length is approximately 5.8 mm.5 A line drawn from the origin to the insertion along the muscle will form an angle of approximately 23 degrees with the sagittal axis.
The frontal nerve runs above the superior rectus and levator muscles, and the nasociliary nerve and the ophthalmic artery lie below. The tendon of insertion for the superior oblique muscle runs below the anterior part of the superior rectus muscle (see Figure 10-7).
Inferior Rectus Muscle
The inferior rectus muscle has its origin on the lower limb of the tendinous ring; its insertion is about 6.5 mm from the limbus in an arc, convex side forward, with the nasal side nearer the limbus; the tendon length is approximately 5.5 mm.5 The inferior rectus approximately parallels the superior rectus, making an angle of 23 degrees with the sagittal axis. The inferior rectus muscle parallels the orbital floor until it passes through a connective tissue pulley just posterior to the equator of the globe; at this point it follows the curve of the globe to its insertion,34 which is parallel to the insertions of the superior rectus (see Figure 10-10, C).
Below the inferior rectus lies the floor of the orbit and above it is the inferior division of the oculomotor nerve. Anteriorly, the inferior oblique muscle comes between the inferior rectus and the orbital floor (see Figure 10-7). The sheaths of these two inferior muscles unite to contribute to the suspensory ligament of Lockwood (see Figure 8-17). The capsulopalpebral fascia, an anterior extension from the sheath of the inferior rectus muscle and the suspensory ligament, inserts into the inferior edge of the tarsal plate, allowing coordination of eye movements with eyelid position and ensuring lowering of the lid on downward gaze.36 The inferior rectus is innervated by the inferior division of cranial nerve III, the oculomotor nerve, which enters the muscle on its superior surface.
Superior Oblique Muscle
The superior oblique muscle has its origin on the lesser wing of the sphenoid bone, medial to the optic canal near the frontoethmoid suture.17 The muscle courses forward and passes through the trochlea, a U-shaped piece of cartilage attached to the orbital plate of the frontal bone (see Figure 10-7). The tendon of insertion actually begins approximately 1 cm posterior to the trochlea. Normally, no connective adhesions exist between these two structures, allowing the tendon to slide easily through the trochlea.1
The superior oblique muscle is the longest and thinnest of the extraocular muscles because of its long (2.5 cm) tendon of insertion.30 The tendon of insertion changes direction as it passes through the trochlea to run in a posterior direction and lies inferior to the superior rectus muscle. The insertion of the superior oblique muscle attaches in the superoposterior lateral aspect of the globe37 and is fan shaped, concave forward, and oblique (see Figure 10-10, B).
The trochlea is considered the physiologic or effective origin of the superior oblique muscle in determining muscle action because it acts as a pulley and changes the direction of muscle pull. In considering the action of the superior oblique, a line is drawn from the trochlea to the insertion rather than from the anatomic origin to the insertion. A line drawn from the physiologic origin to the insertion makes an angle of approximately 55 degrees with the sagittal axis.37 The superior oblique muscle lies above the medial rectus, with the nasociliary nerve and the ophthalmic artery lying between them. Innervation is by the trochlear nerve, cranial nerve IV, which enters the posterior area of the muscle.
Inferior Oblique Muscle
The inferior oblique muscle has its origin on the maxillary bone just posterior to the inferior medial orbital rim and lateral to the nasolacrimal canal.38 The inferior oblique is the only extraocular muscle to have its anatomic origin in the anterior orbit. The muscle runs from the medial corner of the orbit to the lateral aspect of the globe, its length approximately paralleling the tendon of insertion of the superior oblique muscle.
The insertion of the inferior oblique is on the posterior portion of the globe on the lateral side, mostly inferior, lying just outer to the macular area (see Figure 10-10, D).1,30 The insertion is curved concave downward, the tendon of insertion is quite short, just 1 mm in length. The muscle makes an angle of approximately 51 degrees with the sagittal axis.34 Above the inferior oblique are the inferior rectus and globe, and below it lies the floor of the orbit. The inferior oblique is innervated by the inferior division of the oculomotor nerve, which enters the muscle on its upper surface.
Table 10-3 lists the motor innervation of the extraocular muscles.
| Muscle | Nerve |
|---|---|
| Medial rectus | Inferior division of oculomotor (CN III) |
| Lateral rectus | Abducens (CN VI) |
| Superior rectus | Superior division of oculomotor (CN III) |
| Inferior rectus | Inferior division of oculomotor (CN III) |
| Superior oblique | Trochlear (CN IV) |
| Inferior oblique | Inferior division of oculomotor (CN III) |
CN, Cranial nerve.
Fibers of the Extraocular Muscles
The fibers of the extraocular muscles have a layered organization. The global layer is adjacent to the globe and consists of fibers of various diameters.39 This group of fibers extends the full length of the muscle and is attached at the origin and insertion through well-defined tendons.18,40 The global layer inserts into the sclera and causes movement of the globe.41 The outer orbital layer is adjacent to orbital bone, consists of smaller-diameter fibers, and is more vascularized than the global layer.39,42 These fibers end before the muscle tendon and have insertions into the muscle sheath. The orbital layer of the oblique muscles may encircle the global layer.18,39,40 The orbital layer inserts into connective tissue muscle pulleys that can influence the rotational axis of the muscle.18,41
The orbital layer fibers make up 40% to 60% of the fibers within an extraocular muscle.40
Fibers of extraocular muscles can be divided into types having some of the usual characteristics of striated muscle. All of these types are involved in all muscle contractions. Orbital fibers with a high number of mitochondria and that are singly innervated have small myofibrils, allowing for rapid access of Ca2+ to contractile fibers. These are generally fast twitch and fatigue resistant fibers resulting in rapid contraction.18 Orbital fibers that are multiply innervated have several nerve terminals along the length of a single fiber, and they include both fast twitch and slowly contracting fibers.18
Among the global fibers that are singly innervated, the red fibers (having high amount of myoglobulin) may be fast twitch and fatigue resistant; the white fibers (lesser amount of myoglobulin) are fast twitch and may be fatigable.18 Global fibers that are multiply innervated are associated with the myotendinous cylinder or palisade endings; they are large myofibrils and appear to be slow and tonic.18
Orbital Connective Tissue Structures
Connective tissue sleeves or pulleys can be identified using detailed magnetic resonance imaging (MRI); the pulleys that couple the rectus muscles to the orbital walls and Tenon’s capsule were the first to be visualized.34,35,43–45 Pulleys along the inferior oblique and superior oblique muscle paths have subsequently been identified.18,40 Although not as prominent as the pulley of the superior oblique muscle, and only consisting of soft tissue,40 the pulleys encircle each extraocular muscle like a sleeve and can affect the mechanisms of muscle positioning. Smooth muscle-connective tissue struts attach the pulleys to the periorbita of the orbital wall and may help to refine coordination of binocular eye movements.35,41,44,46 The smooth muscle of the pulley is richly innervated by sympathetic and parasympathetic nerves, suggesting both excitatory and inhibitory capabilities.18,46 The smooth muscle either regulates the stiffness of the connective tissue or moves the pulleys to alter the pulling direction.41 The pulleys reduce sideslip of the extraocular muscles during globe rotation and help to determine the effective direction of pull.43 Pulley displacement can clinically mimic muscle dysfunction, and orbital imaging may be needed to distinguish it accurately from a palsy. The pulley for the medial rectus is the most fully developed.47
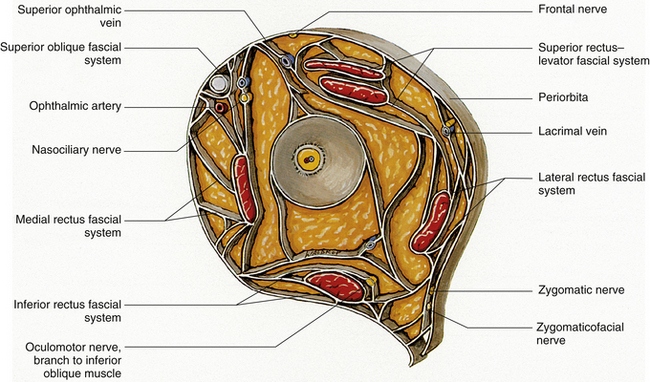
Dense connective tissue septa between the extraocular muscle sheaths and between the sheaths and the orbital bones form a highly organized network that contributes to the framework supporting the globe within the orbit. The horizontal rectus muscles are anchored to the periorbita at the anterior orbital walls through the medial and lateral check ligaments. The medial check ligament is attached to the bones of the medial orbital wall, and the lateral check ligament is attached to the lateral tubercle on the zygomatic bone of the lateral wall; both ligaments are posterior to the orbital septum. The medial check ligament is better developed than the lateral.32 Traditionally, these ligaments were described as “brakes” that limit the extent of movement of the globe; that is, in abduction the medial check ligament stops lateral movement of the globe when extension of the medial rectus muscle starts to exert pull on the relatively inelastic ligament.
The connective tissue septa that connect muscle to muscle and periodically connect individual muscles to the orbital walls along a significant portion of the muscle length have been identified in dissection studies.45,48,49 These intermuscular septa include those joining (1) lateral rectus, inferior rectus, and medial rectus; (2) medial rectus and superior rectus; (3) lateral rectus and superior rectus; (4) medial rectus to superior oblique and to orbital roof and floor; (5) medial rectus to periorbita of ethmoid; (6) superior oblique to frontoethmoid angle; (7) inferior rectus to orbital floor; (8) lateral rectus to lateral wall; (9) levator to adjacent periorbita32; and (10) superior oblique to orbital roof45 (Figure 10-11).
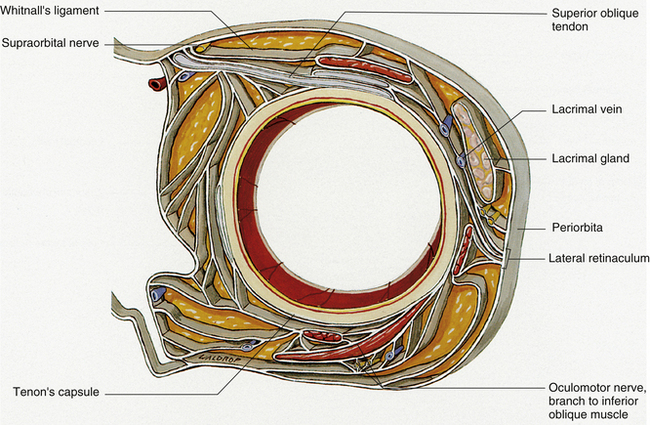
FIGURE 10-11 Connective tissue system in cross section through anterior orbit at level of Whitnall’s ligament.
(From Dutton JJ: Atlas of clinical and surgical orbital anatomy, Philadelphia, 1994, Saunders.)
The presence and orientation of these septa vary from front to back. Figure 10-12 shows a representation of the septa at midorbit. The considerable amount of attachment between muscle and bone helps to stabilize the muscle path and can limit eye movement.18,45
Isolated Agonist Model
One of the earliest models developed to explain eye movement is the isolated agonist model described by Duane.50 This straightforward model has been used widely in the clinical evaluation of extraocular muscles and can be used to describe the movement around the axes that occurs with contraction of each muscle. However, it is important to remember that during eye movements, all six extraocular muscles are in some state of contraction or relaxation, and it is strictly hypothetical to discuss the movement of the eye as if only one muscle contracts. In each of these descriptions the eye begins in primary position.
Movements From Primary Position
Horizontal Rectus Muscles
The medial rectus lies parallel to the sagittal axis and perpendicular to the vertical axis; therefore it has only one action, which is rotation around the vertical axis in a nasal direction–adduction. The lateral rectus also lies parallel to the sagittal axis and perpendicular to the vertical axis; contraction causes rotation in a temporal direction–abduction (Figure 10-13).
Vertical Rectus Muscles
The action of the superior rectus is more complex than that of the medial and lateral rectus muscles because it lies at an angle to each of the axes; with the insertion above the origin and on the anterior globe, movement around the horizontal axis causes elevation. The muscle insertion is lateral to the origin, so movement around the vertical axis causes adduction; the oblique insertion on the superior surface of the globe causes intorsion on contraction (Figure 10-14, A). The primary action of the superior rectus is said to be elevation; adduction and intorsion are secondary actions.
The primary action of the inferior rectus is depression because the insertion is below the origin and on the anterior of the globe. Secondary actions are adduction, because the insertion is lateral to the origin, and extorsion, which results from the oblique insertion on the inferior surface of the globe (Figure 10-14, B).
Oblique Muscles
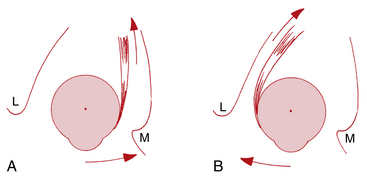
The primary action of the superior oblique muscle is intorsion.5,17,51–53 This action results from the oblique insertion on the posterosuperior lateral aspect of the globe (Table 10-4); contraction rotates the eye around the sagittal axis, causing intorsion. The secondary actions are depression and abduction.5,17,51–53 Depression occurs because the insertion is posterior and inferior to the physiologic origin; contraction of the muscle pulls the back of the eye up, and the anterior pole moves down. Because the insertion is lateral to the trochlea, contraction of the superior oblique pulls the back of the globe medially, thus moving the anterior pole laterally (Figure 10-15, A, page 194).
The primary action of the inferior oblique—extorsion—occurs because the muscle wraps around the lower portion of the globe and the insertion is superior and lateral to the origin. Secondary actions are elevation and abduction.5,17,51–53 Because the insertion is on the posterior eye and above the origin, contraction pulls the back of the eye down, elevating the front. Abduction occurs because the insertion on the back of the eye is pulled toward the medial side; thus the anterior pole is moved laterally in abduction (Figure 10-15, B).
Some authors offer the contrasting view that the primary action of the superior oblique is depression, that of the inferior oblique is elevation, and the torsional actions are secondary.54
Movements from Secondary Positions
As the position of the globe changes, the relationship between the muscle origin and insertion changes relative to the axes, and contraction of a muscle has a different effect than when the eye is in primary position. If the eye is elevated, contraction of the horizontal rectus muscles no longer causes strictly adduction or abduction, but also causes a slight elevation; if the eye is depressed, contraction causes further depression.55
Vertical Rectus Muscles
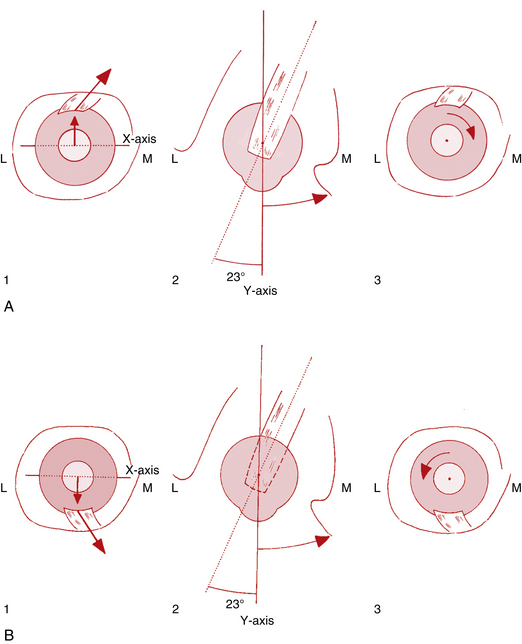
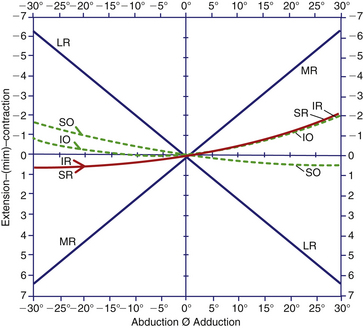
With the eye abducted approximately 23 degrees from primary position, the vertical rectus muscles parallel the sagittal axis, and lie perpendicular to the horizontal axis; thus only vertical movement will occur. In this position, contraction of the superior rectus will cause only elevation, and contraction of the inferior rectus will cause only depression50 (Figure 10-16, B, page 195).
As the eye adducts, it approaches a position where the plane of the vertical rectus muscles is at a right angle to the sagittal axis; this occurs at approximately 67 degrees of adduction (which may be physically impossible because of the connective tissue constraints of the orbit). If the muscle plane of the vertical rectus muscles is at a right angle to the sagittal axis, and thus parallel to the horizontal axis, contraction of the superior or inferior rectus muscle will not cause vertical movement50 (Figure 10-16, A).
Oblique Muscles
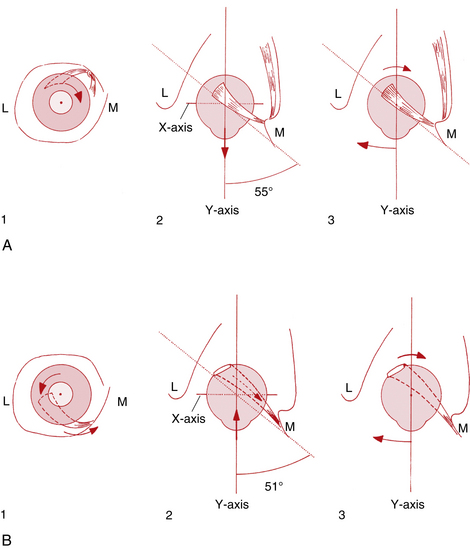
As the eye adducts 51 to 55 degrees, the plane of the oblique muscles becomes parallel to the sagittal axis and perpendicular to the horizontal axis. In this position the superior oblique will cause only depression, and the inferior oblique will cause only elevation. When the eye is abducted 35 to 39 degrees, the plane of the oblique muscles makes a right angle with the sagittal axis and parallels the horizontal axis, and the obliques cannot cause vertical movement50 (Figure 10-17).
This analysis is used in the clinical assessment of extraocular muscle function. As the eye increases in abduction, the elevating and depressing abilities of the vertical rectus muscles increase as the elevating and depressing abilities of the oblique muscles decrease. As the eye increasingly moves into adduction, the elevating and depressing abilities of the oblique muscles increase as the elevating and depressing abilities of the vertical rectus muscles decrease.
Agonist and Antagonist Muscles
In any position of gaze, innervation of all extraocular muscles is controlled carefully by the central nervous system, and each muscle is in some stage of contraction or relaxation. No single muscle acts alone; muscles work together as agonists, antagonists, or synergists. In all these movements, fine motor control should provide for smooth, continuous movements. According to Sherrington’s law of reciprocal innervation, contraction of a muscle is accompanied by a simultaneous and proportional relaxation of the antagonist.56 In adduction the increased contraction of the medial rectus muscle is accompanied by the increased relaxation of the antagonist, the lateral rectus muscle.
When the superior rectus muscle and the inferior oblique muscle contract at the same time, the adduction action of the superior rectus and the abduction action of the inferior oblique, as well as the intorsion of the superior rectus and the extorsion of the inferior oblique, will counteract each other. The resultant eye movement is elevation; the muscles are synergists in elevation. Jampel demonstrated this by stimulating the superior rectus and inferior oblique muscles simultaneously and noting that the eye moved directly up.57
When the superior oblique and inferior rectus muscles were stimulated simultaneously, the eye moved directly downward.57 The superior oblique and the inferior rectus are synergists in depression. The superior oblique is the antagonist for the inferior oblique in vertical movements and torsional movements but is synergistic for abduction.
Clinical Comment: Extraocular Muscle Assessment
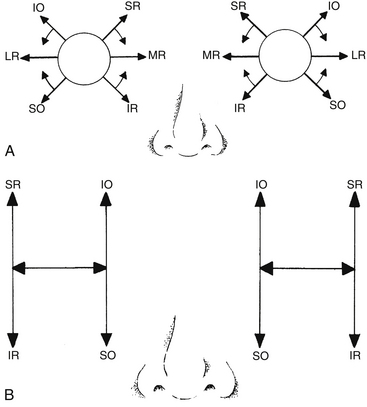
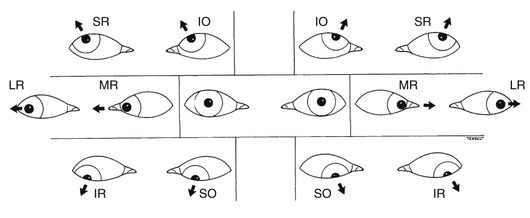
Assessment of eye position and movements can be an important tool in determining the integrity of the extraocular muscles and associated nerves. The practitioner first notes the position of each eye while directing the patient to fixate on a target straight ahead. An eye that is deviated toward the nose would indicate an underactive lateral rectus muscle; the medial rectus is unopposed by the lateral rectus. Figure 10-18, A, shows the direction of pull of each muscle when the eye is in primary position.
With the more complex movements of the other muscles, the most reliable way to determine a dysfunctional muscle is to put the eye into a position in which one muscle is the primary actor. In the adducted position the oblique muscles are the primary elevator and depressor; in the abducted position the vertical rectus muscles are the primary elevator and depressor. This arrangement can be represented by the “H” diagrams in Figure 10-18, B. Thus when doing ocular motility testing, it is important to move the eyes to such a position as to isolate the vertical abilities of these muscles. The usual manner of performing ocular motility testing follows:
The extensive network of connective tissue septa and fibroelastic pulley system associated with the extraocular muscle may affect eye movement, and MRI may be an additional tool to determine the extent of muscle involvement once a strabismic condition is diagnosed.
Clinical Comment: Strabismus Surgery
Surgical correction for strabismus can be complicated because of the extensive connective tissue network linking extraocular muscles to each other and to the orbital bones. This may be one of the reasons why a patient reverts to presurgery strabismic posture.58 The realization that there is connection between the muscle sheath and the connective tissue sheath of the globe, not just at the point of the tendon insertion, should be a consideration in muscle resection surgery.58
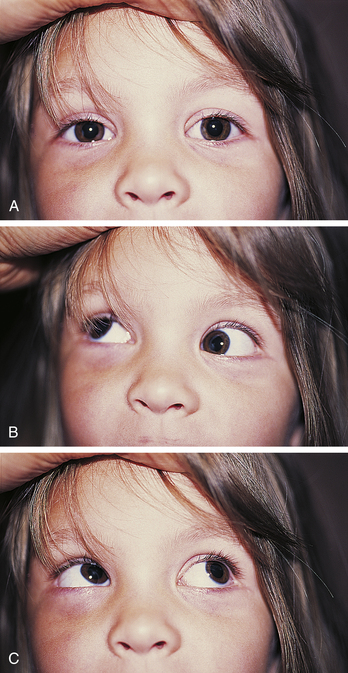
Clinical Comment: Brown Superior Oblique Sheath Syndrome
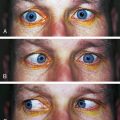
Inability to elevate the eye in the adducted position is usually caused by a dysfunctional inferior oblique muscle. However, such a limitation could also be caused by an immobile superior oblique muscle (Figure 10-19). Using electromyography, Brown59 determined that a patient with an inability to elevate the eye in adduction had a functional inferior oblique muscle, but that the movement of the superior oblique through the trochlea was restricted. The superior oblique could not lengthen when the inferior oblique contracted.59 In congenital Brown’s syndrome, the cause could be a short or anchored tendon; in acquired Brown’s syndrome the cause could be an accumulation of fluid or tissue between the trochlea and the tendon.51,60
Clinical Comment: Hyperthyroidism (Graves’ Disease)
Enlargement of the extraocular muscles produced by Graves’ disease is caused by chronic inflammatory infiltration of the muscles with glycoprotein and mucopolysaccharide deposition, resulting in proptosis.1 In addition, restricted ocular motility is evident. However, evaluation of the restricted movement may not depict the correct dysfunctional muscle because fibrosis of the muscles can occur, limiting muscle activity. For example, if the medial rectus is fibrotic, eye movement may be restricted in the lateral direction because the medial rectus is unable to elongate and acts as a check on lateral movement. Restriction may appear to be impairment of the lateral rectus but may actually be caused by the fibrotic medial rectus muscle.
Clinical Comment: Forced Duction Test
A FORCED DUCTION TEST can be performed if a fibrotic muscle is suspected. With the patient under topical anesthesia, the practitioner grasps the conjunctiva near the limbus and attempts to move the eye in a direction opposite from the suspected restriction. Resistance will be met if the cause is fibrosis, but if the muscle is paralyzed, the eye can be moved. For instance, if the lateral rectus is suspect, the practitioner would attempt to move the eye medially. If the lateral rectus is fibrotic, resistance to movement occurs; if it is paralyzed, the eye can be moved with the forceps.52
Yoke Muscles
Yoke muscles are those muscles of the two eyes acting together to cause binocular movements (Figure 10-20). Hering’s law of equal innervation states that the innervation to the muscles of the two eyes is equal and simultaneous. Thus the movements of the two eyes are normally symmetric.61 In dextroversion, equal and simultaneous innervation is supplied to the yoke muscles—the right lateral rectus and left medial rectus; in convergence, equal and simultaneous innervation is supplied to the yoke muscles—the right medial rectus and left medial rectus.
Paired Antagonist Model
A model by Boeder55 analyzes the actions of the extraocular muscles as antagonist pairs. Figure 10-21 shows the path that the anterior pole of the eye traces with contraction of these pairs. The vertical pair of muscles have primary actions of elevation and depression, and secondary actions of adduction and torsion. Adduction increases with medial movement, as do the torsional effects. In abduction, both muscles must relax.55
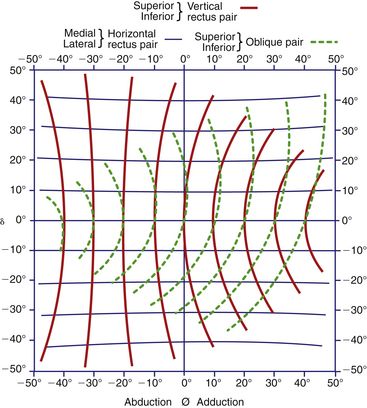
FIGURE 10-21 Traces of line of fixation with activity of each of three muscle pairs in various positions of gaze.
(From Boeder P: The cooperation of extraocular muscles, Am J Ophthalmol 51:469, 1961.)
The primary actions of the paired obliques are intorsion and extorsion. The oblique muscle tendons insert obliquely into the globe, and the torsional effects do not diminish with horizontal movement because the insertion does not act as a unit. In adduction the medial fibers of the superior oblique tendon exert greater contractile force, and in abduction the lateral fibers are shortened.61 In adduction the lateral fibers of the inferior oblique tendon are shortened, and in abduction the medial fibers contract.57
The contraction of one muscle is associated with lengthening of its antagonist. This state of relaxation or extension is considered an activity equivalent to contraction. In all positions of gaze, all muscles are in some state of activity. An analysis of the change of length of each muscle during a simple horizontal excursion shows that as the medial rectus shortens, the lateral rectus lengthens, and vice versa. The vertical rectus muscles behave as one, both shortening in adduction and lengthening in abduction. The obliques cocontract in abduction, but in adduction the superior oblique lengthens and the inferior oblique shortens55 (Figure 10-22).
Complexity of the Oblique Muscles
Some controversy exists over the horizontal abilities of the inferior oblique muscle. The relationship of the muscle plane of the inferior oblique with the vertical axis determines whether the inferior oblique is an adductor or an abductor. If the muscle plane lies in front of the vertical axis, the inferior oblique will aid in adduction. With increasing lateral movement of the eye, however, a point will be reached at which the inferior oblique plane is put behind the vertical axis, causing the inferior oblique then to aid in abduction.37 Animal studies in which the muscles are stimulated directly either singularly or collectively seem to support this view.57,62 When the superior oblique and inferior oblique were stimulated simultaneously, no ocular movement occurred; in some positions, these two muscles appeared to be complete antagonists, and abduction did not occur. These observations do not change the model used clinically. In adduction the obliques are responsible for elevation and depression, and in abduction the vertical recti are responsible for elevation and depression.
Innervation and Blood Supply
Innervation
The medial rectus, inferior rectus, and inferior oblique muscles are innervated by the inferior division of the oculomotor nerve. The superior rectus muscle is innervated by the superior division of the oculomotor nerve. The lateral rectus muscle is supplied by the abducens nerve. The superior oblique muscle is innervated by the trochlear nerve (see Table 10-3 and Figure 10-8).
Blood Supply
The extraocular muscles are supplied by two muscular branches from the ophthalmic artery: The superior (lateral) branch supplies the superior and lateral rectus and the superior oblique muscles, and the inferior (medial) branch supplies the inferior and medial rectus and the inferior oblique muscles.1,63 Other arteries make various contributions to the extraocular muscle blood supply, including the lacrimal, supraorbital, and infraorbital arteries. These vessels and the muscles they supply are described in Chapter 11 (see Table 11-2).
Aging Changes in the Extraocular Muscles
Both horizontal rectus muscles are displaced inferiorly with age, with the medial rectus displaced more than the lateral rectus. This may be the cause of a constant partial depression and may contribute to the impaired ability to elevate the eyes often observed in elderly persons, predisposing them to an incomitant (nonconcomitant) strabismus.47 The superior rectus and inferior rectus muscles do not change locations.47
Other age-related changes in extraocular muscles include a greater variety in fiber sizes, increased connective tissue in the muscle, increased adipose tissue in the bundles, deposits of lipofuscin, and degenerative changes.64
1. Doxanas M.T., Anderson R.L. Clinical orbital anatomy. Baltimore: Williams & Wilkins; 1984. p 116
2. Honda H., Asakura S. Calcium-triggered movement of regulated actin in vitro. A fluorescence microscopy study. J Mol Biol. 1989;205(4):677.
3. Bagni M.A., Cecchi G., Colomo F., et al. Tension and stiffness of frog muscle fibres at full filament overlap. J Muscle Res Cell Motil. 1990;11:371.
4. Smith D.A. The theory for sliding filament models for muscle contraction. IIIDynamics of the five-state model. J Theor Biol. 1990;146(4):433.
5. Eggers H.M. Functional anatomy of the extraocular muscles. Tasman W., Jaeger E.A., editors. Duane’s foundations of clinical ophthalmology, vol 1. Philadelphia: Lippincott, 1994.
6. Guyton A.C. Textbook of medical physiology, ed 8. Philadelphia: Saunders; 1991. p 76
7. Wirtschafter J.D. Neuroanatomy of the ocular muscles. In: Reeh M.J., Wobig J.L., Wirtschafter J.D., editors. Ophthalmic anatomy. San Francisco: American Academy of Ophthalmology, 1981. p 267
8. Karatas M. Internuclear and supranuclear disorders of eye movements: clinical features and causes. Eur J Neurol. 2009;16:1265-1277.
9. Namba T., Nakamura T., Grob D. Motor nerve endings in human extraocular muscle. Neurology. 1968;18:403.
10. Hess A. Further morphological observations of “en plaque” and “en grappe” nerve endings on mammalian extrafusal muscle fibers with the cholinesterase technique. Rev Can Biol. 1962;21:241.
11. Brandt D.E., Leeson C.R. Structural differences of fast and slow fibers in human extraocular muscle. Am J Ophthalmol. 1966;62:478.
12. Breinin G.M. The structure and function of extraocular muscle: an appraisal of the duality concept. Am J Ophthalmol. 1971;71:1.
13. Peachy L. The structure of the extraocular muscle fibers of mammals. In: Bach-y-Rita P., Collins C.C., Hyde J.E., editors. The control of eye movements. New York: Academic Press, 1971. p 47
14. Scott A.B., Collins C.C. Division of labor in human extraocular muscle. Arch Ophthalmol. 1973;90:319.
15. Montagnani S., De Rosa P. Morphofunctional features of human extrinsic ocular muscles. Doc Ophthalmol. 1989;72(2):119.
16. Porter J.D. Extraocular muscle: cellular adaptations for a diverse functional repertoire. Ann N Y Acad Sci. 2002;956:7.
17. Burde R.M., Feldon S.E. The extraocular muscles. In: Hart W.M.Jr., editor. Adler’s physiology of the eye. ed 9. St Louis: Mosby; 1992:101.
18. Porter J.D., Andrade F.H., Baker R.S. The extraocular muscles. In: Kaufman P.L., Alm A., editors. Adler’s physiology of the eye. ed 10. St Louis: Mosby; 2003:787.
19. Ruskell G.L. Extraocular muscle proprioceptors and proprioception. Prog Retin Eye Res. 1999;18(3):269.
20. Weir C.R., Knox P.C., Dutton G.N. Does extraocular proprioception influence oculomotor control? Br J Ophthalmol. 2000;84:1071-1074.
21. Richmond F.J.R., Johnston W.S.W., Baker R.S., et al. Palisade endings in human extraocular muscles. Invest Ophthalmol Vis Sci. 1984;25:471-476.
22. Alpern M. Movements of the eyes. In: Dawson H., editor. The eye. New York: Academic Press, 1962.
23. Walls G.L. The evolutionary history of eye movements. Vis Res. 1962;2:69.
24. Linwong M., Herman S.J. Cycloduction of the eyes with head tilt. Arch Ophthalmol. 1971;85:570.
25. Jampel R.S. Ocular torsion and the function of the vertical extraocular muscles. Am J Ophthalmol. 1975;77:292.
26. Duke-Elder S. Textbook of ophthalmologyvol, 4. St Louis: Mosby; 1949.
27. Diamond S.G., Markham C.H. Ocular counterrolling as an indicator of vestibular otolith function. Neurology. 1983;33:1460.
28. Collewijin H., Van der Steer J., Ferman L., et al. Human ocular counterroll: assessment of static and dynamic properties from electromagnetic scleral coil recordings. Exp Brain Res. 1985;59:185.
29. Ott D., Eckmiller R. Ocular torsion measured by TV and scanning laser ophthalmoscopy during horizontal pursuit in humans and monkeys. Invest Ophthalmol Vis Sci. 1989;30(12):2512.
30. Warwick R. Eugene Wolff’s anatomy of the eye and orbit, ed 7. Philadelphia: Saunders; 1976. p 248
31. Burton H. Somatic sensations from the eye. In: Hart W.M.Jr., editor. Adler’s physiology of the eye. ed 9. St Louis: Mosby; 1992:185.
32. Koornneef L. Orbital connective tissue. In: Jakobiec F.A., editor. Ocular anatomy, embryology, and teratology. Philadelphia: Harper & Row; 1982:835.
33. DeGottrau P., Gajisin S. Anatomic, histologic, and morphometric studies of the ocular rectus muscles and their relation to the eye globe and Tenon’s capsule. Klin Monatsbl Augenheilkd. 1992;200(5):515. (abstract)
34. Porter J.D., Poukens V., Baker R.S., et al. Structure-function correlations in the human medial rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 1996;37:468.
35. Demer J.L., Miller J.M., Poukens V. Surgical implications of the rectus extraocular muscle pulleys. J Pediatr Ophthalmol Strabismus. 1996;33(4):208.
36. Wobig J.L. The eyelids. In: Reeh M.J., Wobig J.L., Wirtschafter J.D., editors. Ophthalmic anatomy. San Francisco: American Academy of Ophthalmology; 1981:38.
37. Krewson W.E. Comparison of the oblique extraocular muscles. Arch Ophthalmol. 1944;32:204.
38. Wobig J.L. The extrinsic ocular muscles. In: Reeh M.J., Wobig J.L., Wirtschafter J.D., editors. Ophthalmic anatomy. San Francisco: American Academy of Ophthalmology; 1981:33.
39. Wasicky R., Ziya-Ghazvini F., Blumer R., et al. Muscle fiber types of human extraocular muscles: a histochemical and immunohistochemical study. Invest Ophthalmol Vis Sci. 2000;41(5):980.
40. Kono R., Poukens V., Demer J.L. Superior oblique muscle layers in monkeys and humans. Invest Ophthalmol Vis Sci. 2005;46:2790-2799.
41. Demer J.L., Oh S.Y., Poukens V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 2000;41:1280.
42. Oh S.Y., Poukens V., Cohen M.S., et al. Structure-function correlation of laminar vascularity in human rectus extraocular muscles. Invest Ophthalmol Vis Sci. 2001;42:17.
43. Clark R.A., Miller J.M., Demer J.L. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787.
44. Clark R.A., Miller J.M., Demer J.L. Location and stability of rectus muscle pulleysMuscle paths as a function of gaze. Invest Ophthalmol Vis Sci. 1997;38:227.
45. Ettl A., Kramer J., Daxer A., et al. High-resolution magnetic resonance imaging of the normal extraocular musculature. Eye. 1997;11:793.
46. Demer J.L., Poukens V., Miller J.M., et al. Innervation of extraocular pulley smooth muscle in monkeys and humans. Invest Ophthalmol Vis Sci. 1997;38(9):1774.
47. Clark R.A., Demer J.L. Effect of aging on human rectus extraocular muscle paths demonstrated by magnetic resonance imaging. Am J Ophthalmol. 2002;134:872.
48. Miller J.M. Functional anatomy of normal human rectus muscles. Vis Res. 1989;29(2):223.
49. Miller J.M., Demer J.L., Rosenbaum A.L. Effects of transposition surgery on rectus muscle paths by magnetic resonance imaging. Ophthalmology. 1993;100(4):475.
50. Duane A. The monocular movements. Arch Ophthalmol. 1936;8:531.
51. Leigh R.J., Zee D.S. The neurology of eye movements. Philadelphia: Davis; 1983. pp 145, 170
52. Von Noorden G.K., Maumenee A.E. Atlas of strabismus, ed 2. St Louis: Mosby; 1973. pp 6, 112
53. Kanski J.J. Clinical ophthalmology, ed 3. Oxford, England: Butterworth-Heinemann; 1994. p 428
54. Bron A.J., Tripathi R.C., Tripathi B.J. Wolff’s anatomy of the eye and orbit, ed 8. London: Chapman & Hall; 1997.
55. Boeder P. The cooperation of extraocular muscles. Am J Ophthalmol. 1969;51:469.
56. Sherrington C.S. Experimental note on two movements of the eyes. J Physiol (Lond). 1984;17:27.
57. Jampel R.S. The fundamental principle of the action of the oblique ocular muscles. Am J Ophthalmol. 1970;69:623.
58. Hakim O.M., Gruber El-Hag Y., Maher H. Persistence of eye movement following disinsertion of extraocular muscle. J AAPOS. 2008;12:62-65.
59. Brown H.W. Congenital structural muscle anomalies. In: Allen E.D., editor. Strabismus ophthalmic symposium. St Louis: Mosby; 1950:250.
60. Helveston E.M., Merriam W.W., Ellis F.D., et al. The trochlea. A study of the anatomy and physiology. Ophthalmology. 1982;89:124.
61. Hering E. Theory of binocular vision. New York: Plenum; 1977.
62. Jampel R.S. The action of the superior oblique muscleAn experimental study in the monkey. Arch Ophthalmol. 1966;75:535.
63. Hayreh S.S. The ophthalmic artery: III. Branches. Br J Ophthalmol. 1962;46:212.
64. McKelvie P., Friling R., Davey K., et al. Changes as the result of ageing in extraocular muscles: a post-mortem study, Aust N Z J Ophthalmol. 1999;27:420.