CHAPTER 354 Extracranial Vertebral Artery Diseases
Historical Background
As early as 1844, Quain1 described the anatomy and operative surgery of the extracranial vertebral artery in lithographic drawings. Subsequently, in 1853, Maisonneuve successfully ligated the vertebral artery at the transverse foramen of the sixth cervical vertebra for a stab wound to the neck.2 Elective ligation of the vertebral artery was also used to treat aneurysms. In 1888, Matas2 was the first surgeon who fully excised an aneurysm between the occiput and the atlas through a posterior approach. Forty years later, Moniz3 performed the first vertebral angiogram in 1927. Radner4 first reported selective angiography of the vertebral artery in 1947. The role of pathology of the extracranial vessels in relation to cerebral ischemia was emerging,5 and with this revolution in the diagnosis of diseased arteries, revascularization was being performed. In 1958, Crawford and coworkers6 presented their results of surgical treatment of brainstem ischemia by reconstructing the vertebral artery after removing atherosclerotic plaque. The next year, Cate and Scott7 first described the technique of transsubclavian endarterectomy of the subclavian-vertebral artery.
Angiography allowed visualization of other causes of extracranial vertebral artery disease, including extrinsic compression of the vertebral artery by osteophytes,8 constricting bands,9 and rotational obstruction,10 all of which were diagnosed and treated by surgical decompression. Angiography also provided the first extensive cooperative study of the incidence of extracranial arterial stenosis caused by atherosclerotic lesions in patients with cerebrovascular insufficiency. In 1968, stenosis was proposed to be a compromised lumen of more than 50% by the Joint Study of Extracranial Arterial Occlusion.11 Of 4748 patients with cerebral ischemic symptoms, 80% had four-vessel angiograms that were categorized by location of the arterial stenosis. For the first time, this study provided a frequency distribution of sites of stenosis in the extracranial vertebral artery,11 identifying at least some degree of stenosis in 22% and 18% of left and right proximal vertebral arteries, respectively, and 5% to 6% of the distal extracranial vertebral.
Clinical Presentation
The external vertebral arteries provide blood flow to a large distribution through the basilar artery and posterior cerebral arteries. Therefore, symptoms can arise from the occipital or temporal lobes, cerebellum, pons, and brainstem with its cranial nerves, a syndrome referred to as vertebrobasilar insufficiency (VBI) and characterized by intermittent episodes of multiple symptoms that can be sudden or severe (Table 354-1). Eventually, the symptoms either resolve or become permanent. These symptoms are caused not only by embolic or thrombotic sources but also by hemodynamic mechanisms. Changes in the blood flow of the vertebral artery can account for symptoms of VBI,12 even those created in some instances by head turning.13
| The presence of at least two of these symptoms is required to diagnose vertebrobasilar insufficiency: |
In a review of the literature, Ausman and coworkers14 concluded that the symptoms of VBI have multiple causes and that one can seldom localize pathologic lesions in the posterior circulation by clinical examination alone. Most physicians use the presence of any two of the common symptoms to define the syndrome. The most common symptom that physicians have difficulty relating to VBI is vertigo or dizziness, which is often associated with other diseases; many articles have ruled out the occurrence of isolated vertigo or dizziness as evidence of transient ischemic attack (TIA). In general, vertigo or dizziness alone is not considered a presenting symptom of VBI,15 although some authors still assert that these could be exclusive symptoms of VBI.16,17
Anatomy of the Extracranial Vertebral Artery
The vertebral artery varies in diameter from 0.5 to 5.5 mm. The left vertebral artery is larger than the right in about 75% of cases.18 The vertebral artery flow in normal volunteers, as measured by quantitative magnetic resonance angiography (QMRA), ranges from 7 to 199 mL per minute (mean, 90 ± 32 mL per minute; median, 88 mL per minute) on the right, and 21 to 245 mL per minute (mean, 99 ± 40 mL per minute; median, 100 mL per minute) on the left.19
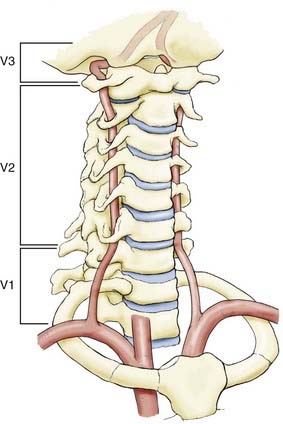
The extracranial vertebral arteries can be divided into three segments—the first (V1), second (V2) and third (V3), which can alternatively be termed the proximal, middle (intraosseous), and distal segments (Fig. 354-1). The designation is helpful because the associated pathology can differ among the segments.
First Vertebral Artery Segment (V1)
The V1 extends from the superior portion of the subclavian artery to enter the transverse foramen of C6. It enters at levels other than C6 10% of the time (C5, 7%; C6, 90%; C7, 3%). Instead of arising from the superior portion of the left subclavian artery, the left vertebral artery can arise from the proximal subclavian trunk. In 5% of cases, it arises separately from the aortic arch. Typically, the right vertebral artery is the first branch of the right subclavian artery, but it can arise from various places: the right common carotid artery (CCA), the right internal carotid artery (ICA), the aortic arch directly, or the right subclavian artery distal to the thyrocervical trunk.20
Pathophysiology of Extracranial Vertebral Artery Disease
Regional hypoperfusion is the predominant stroke mechanism for patients with posterior circulation ischemia. Large or small vessel occlusive disease occurs more commonly in the posterior circulation than thromboembolism.21,22 Stroke secondary to vertebral stenosis can be hemodynamic in the setting of anatomic variations in the other artery, such as a hypoplastic vertebral artery, termination of one artery into the posterior inferior cerebellar artery, or complete occlusion of the contralateral vertebral.20 There also may be associated pathology in the carotid system or an incomplete circle of Willis,18 which affects the collateral circulation. Hemodynamic changes can also result systemically from cardiac insufficiency or postural hypotension.
Atherosclerosis
Atherosclerosis is the most common form of vertebral artery disease. Although it is a potential source of thromboembolic plaque, it can cause significant hypoperfusion by obstructing blood flow. One of the most extensive studies on the incidence of extracranial disease in symptomatic patients was presented by Hass and associates11 from the Joint Study of Extracranial Arterial Occlusion. They found that the most common site of plaque formation in the vertebrobasilar arterial system was the origin of the proximal vertebral artery (right vertebral artery, 18.4%; left vertebral artery, 22.3%). The second most common site was the middle vertebral artery. In this region, it is believed that the blood flow is dampened as it passes through the transverse foramina. Atherosclerosis occurs less frequently intracranially, in the mid basilar artery, and at the entry of the vertebral artery through the dura.
The natural history of extracranial vertebral artery atherosclerotic disease is not well known. Moufarrij and associates reviewed 96 patients with vertebral disease, 89 (93%) of whom had proximal extracranial disease at the vertebral origin.23 Most (75%) of these patients were asymptomatic in relation to their vertebral disease at presentation. Over an average of 4.6 years of follow-up, 19.8% experienced probable VBI symptoms, and another 2% suffered brainstem infarction. Recurrent stroke risks in patients with symptomatic extracranial vertebral atherosclerosis have not been well defined, although estimates of stroke risk in patients with symptomatic intracranial vertebrobasilar disease are 10% to 15% per year.24
Dissection
Spontaneous
The annual incidence of vertebral artery dissection (VAD) is about 1 to 1.5 per 100,25 and VAD is bilateral in 22% of cases.26 Spontaneous VAD is the term used to describe vertebral dissections that do not involve blunt or penetrating trauma as a precipitating factor. However, a history of trivial or minor injury is elicited frequently from patients with so-called spontaneous VAD. The diagnosis of traumatic VAD is reserved for patients with a history of significant trauma, including motor vehicle crashes, falls, or penetrating injuries. Spontaneous dissections are associated with systemic diseases affecting the arterial walls. In both the carotid and vertebral arteries, fibromuscular dysplasia is the most common associated condition in spontaneous dissection. It tends to affect areas where there is significant movement of the cervical spine and therefore occurs in the middle and distal segments of the vertebral artery. The formation of pseudoaneurysms is also quite common, although these lesions are often asymptomatic.18 Other risk factors include spinal manipulation, ceiling painting, nose blowing, oral contraceptive use, hypertension, cystic medial necrosis, migraine headaches, female sex, and recent infection.25 Extracranial vertebral artery dissection is characterized by headache (often occipital) or neck pain and signs of ischemia in the posterior circulation.26,27 Infarcts in the territory of the posterior-inferior cerebral artery (commonly with a lateral medullary syndrome) are seen in 13% of vertebral dissections.28 Intracranial vertebrobasilar dissection may present with symptoms of posterior circulation ischemia (particularly brainstem), subarachnoid hemorrhage (occurs in half of patients), or both.
Traumatic
Trauma is the third most common cause of vertebral artery disease. Both blunt and penetrating trauma can dissect the vertebral artery. Blunt injury occurs from cervical spine fractures and dislocations that may result in occlusion, pseudoaneurysm, or arteriovenous fistula (AVF) of the vertebral artery, especially in the middle portion. This type of injury can also be created iatrogenically from chiropractic manipulation. The most frequent site of thrombosis is at the level of C2 in the distal vertebral artery. This tendency may reflect the posterior placement of the vertebral foramina with respect to the vertebral body. The vertebral artery has an increased vulnerability to compression by subluxation of the cervical apophyseal joints. Blunt injury to the vertebral artery may be more common than has been quoted in the literature because these patients seldom undergo angiography unless they show symptoms of VBI. However, computed tomography (CT) can readily identify the patterns of cervical spine injuries, such as transverse foramen fractures and facet joint dislocations,29,30 which have been most frequently associated with vertebral artery injury, and CT angiography can provide a less invasive method for diagnosis of an underlying vertebral dissection.31 Penetrating trauma to the vertebral artery is less common than blunt trauma.
Compression
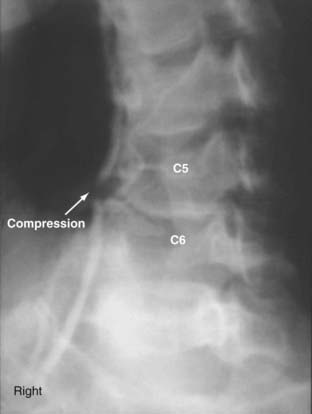
Compression of the vertebral artery can cause VBI. The anterior scalene muscle has been found to compress the vertebral artery at the level of C6. Osteophytes and disk spurs, found between levels C6 and C2, can encroach on and compress the middle vertebral artery, causing vascular symptoms. Usually, rotation or extension of the neck triggers symptoms. Such symptoms have been referred to as bow hunter’s syndrome, an uncommon condition in which the vertebral artery becomes symptomatically occluded during neck rotation.32 Typically, this has been described in relation to compression at the C1-2 level33,34 but can occur at other levels.35 Dynamic angiography has been recommended for patients who show vertebral artery symptoms on flexion, extension, or rotation of the neck.18
Subclavian Steal Syndrome
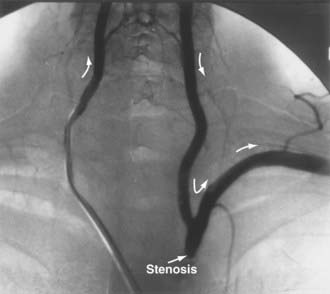
Subclavian steal syndrome was first described by Reivich and colleagues36 in 1961 when they discovered reversed flow in the vertebral artery. It is a disease not affecting the extracranial vertebral artery directly but leading to symptoms of VBI due to hemodynamic alterations in the vertebrobasilar system. It is caused by stenosis or occlusion in the subclavian or innominate artery proximal to the vertebral artery. If the pressure in the subclavian artery distal to the obstruction is low enough, it acts as a “sink” for the flow of blood from the vertebral artery and drains blood from the contralateral vertebral artery and even as far as the circle of Willis (Fig. 354-2). Hence, patients can experience VBI. Most of these symptoms are caused by use of the extremities when the demand for blood flow is increased and the pressure sink becomes more pronounced. In many cases, patients rarely experience symptoms at rest. The symptoms can be provoked by an arm challenge test. This has been further evaluated recently with flow measurements using QMRA before and after the arm challenge test,37 in which a basilar flow index (basilar artery flow to total intracranial flow) was calculated during the challenge test and compared with the baseline. A low basilar flow index correlated with VBI symptoms from steal.
Diagnostic Evaluation
Audiometric and Vestibular Tests
Occasionally, the presentation of vertigo or dizziness with no other findings requires consultation with an otolaryngologist to rule out labyrinthitis or vestibular causes. Noninvasive audiometric and vestibular tests can be performed. Audiometric tests include a pure-tone audiogram and a speech discrimination test to indicate hearing loss. A vestibular test can indicate decruitment and hyperactivity, which can be strong indicators of a centrally located lesion (sensitivity, 92%).17
Noninvasive Anatomic Imaging Techniques
Anatomic imaging of the brain and the posterior fossa is required as part of the evaluation. CT is an excellent imaging technique for ruling out mass lesions or hemorrhages and, in the setting of trauma, for evaluation of cervical spine fractures associated with vertebral injury. Magnetic resonance imaging (MRI) is highly sensitive and can detect demyelinating disease, stroke, and mass lesions. Diffusion-weighted imaging (DWI) is used in the evaluation of acute ischemic stroke38 and will provide early information about the location of acute focal ischemic brain injury.
Magnetic resonance angiography (MRA) or computed tomographic angiography (CTA) is a good noninvasive screening technique for evaluating the intracranial and extracranial arteries. Its ability to accurately and definitively identify stenosis is limited, however. The use of contrast enhancement may increase the utility of MRA.39 Transcranial Doppler (TCD) is another screening modality for vertebral disease but also lacks the sensitivity and specificity provided by other techniques.
Cerebral Angiography
Cerebral angiography is considered the gold standard for evaluating the intracranial and extracranial vessels of the brain. Unlike MRA, it is an invasive procedure and carries a low risk for stroke (1% overall incidence of neurological deficit, and 0.5% incidence of persistent deficit).40
In cases of extracranial vertebral artery disease, the aortic arch must be visualized, as well as the four major intracranial arteries. VBI symptoms can be caused by primary intracranial disease, and good visualization of the intracranial vessels is essential, including collateral supply, such as patent posterior communicating artery into the basilar top. Similarly, subclavian steal would be identified by reversal of flow in the vertebral artery ipsilateral to the subclavian artery stenosis or occlusion. Dynamic angiography can also be used to monitor vascular changes associated with head position, caused by soft tissue (ligament or muscle), neuronal tissue, or bone.18
Hemodynamic Evaluation
After an obstructive lesion has been identified, it is important to determine whether the VBI symptoms are from poor perfusion caused by the obstruction or by emboli. Cerebral angiography can give some sense of the cause, but it is far from reliable. Several methods have been used to evaluate the hemodynamics. Ultrasonography of the vertebral arteries has been used, but insonation is difficult, and its sensitivity is questionable. Variability in equipment, institutions, and technicians makes this method unsatisfactory.41 Intracranial hemodynamic changes have also been monitored with transcranial Doppler ultrasonography, but this technique is difficult to use in the posterior fossa. Imaging techniques used to assess tissue level perfusion, such as positron emission tomography (PET), single-photon emission computed tomography (SPECT), and xenon CT are of limited use in the posterior fossa because of imaging difficulties related to skull base artifacts and imaging resolution.
Since the 1980s, flow quantification using phase-contrast QMRA of the blood vessels has been studied.42–44 Although static MRI or conventional angiography is useful for determining the anatomy of the vessel, QMRA provides actual flow rates of blood in the vessel (in milliliters per minute). Both in vitro and in vivo flow studies have shown that velocities and volumetric flow rates can be estimated accurately for the carotid, vertebral, and major cerebral arteries. Normal values for flow rates in these vessels have been estimated.19 QMRA provides a noninvasive method for analyzing patients with VBI. Patients with VBI in the setting of steno-occlusive vertebrobasilar disease stratified by QMRA to have a normal flow in their distal intracranial vasculature had a recurrent stroke–free survival rate of 100% at 2 years, compared with those with low distal flow, who had a 71% stroke-free survival rate.45 Based on knowledge of the normal range of flow rates in these vessels, it can be determined whether obstructive lesions are significant enough to cause hypoperfusion of the vertebral artery. This knowledge may be helpful in selecting candidates for surgical or endovascular treatment.
Medical Management
Atherosclerotic Disease
Antiplatelet Therapy
Several antiplatelet trials have shown that aspirin reduces the relative risks for stroke, myocardial infarction, and vascular death by about 25%.46 Ticlopidine is more effective than aspirin but has important side effects. Clopidogrel is as effective as ticlopidine, with fewer side effects. In 1996, the European Stroke Prevention Study showed that dipyridamole effectively prevents stroke and, when combined with aspirin, is equivalent to ticlopidine or clopidogrel.47 These results can be applied to the medical treatment of extracranial vertebral artery disease. Recently, the American Heart Association/American Stroke Association Committee published their recommendation for the prevention of stroke in patients with noncardioembolic ischemic stroke or TIA.48 They concluded that aspirin monotherapy, clopidogrel monotherapy, and aspirin combined with extended-release dipyridamole all remain accepted options for initial therapy in patients with noncardioembolic ischemic stroke and TIA.
Two studies have reported outcomes from medical therapy for extracranial vertebral artery disease. Millikan and colleagues49 showed a decline in the mortality rate from 43% to 14% when heparin was used systemically to treat extracranial vertebral artery disease. Twenty years later in a 4-year follow-up, Whisnant and coworkers50 reported that the incidence of brainstem stroke decreased from 35% to 15% when oral anticoagulants were used. In neither study were the patients chosen randomly, nor did most of the patients undergo angiography. Meanwhile, outcome studies on the use of aspirin compared with warfarin for intracranial symptomatic atherosclerosis24 in the posterior circulation disease failed to show any benefit of warfarin over aspirin. The findings can be extrapolated with caution to the extracranial vertebral arteries.
Statin Therapy
The use of 3-hydroxy-3-methyglutaryl coenzyme-A reductase inhibitors (statins) has been evaluated for the prevention of ischemic stroke. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial51 demonstrated an 18% relative reduction in the risk for recurrent stroke in patients randomized to atorvastatin 80 mg per day versus placebo. Based on this clinical trial, the American Heart Association/American Stroke Association Committee recommended statin therapy with intensive lipid-lowering effects for patients with atherosclerotic ischemic stroke or TIA even without known coronary artery disease to reduce the risk for stroke and cardiovascular disease.48 Furthermore, recent data indicate that high-intensity statin therapy might regress plaque burden over time in patients with coronary disease.52 No such studies have looked specifically at extracranial vertebral artery disease.
Dissection
Anticoagulation Therapy
The treatment of vertebral artery dissection is based on rather incomplete evidence. Anticoagulation with heparin followed by oral anticoagulation therapy remains popular in most centers and is supported by demonstration of emboli as the most common cause of stroke in these patients.53 Anticoagulation with a target international normalized ratio between 2 and 3 is generally recommended for 3 to 6 months. This practice is supported by several small case series demonstrating good outcome with low complication rates in patients receiving anticoagulation.54,55 After 3 months, a follow-up angiogram or MRI is recommended. If the pathology persists, oral anticoagulation is continued for another 3 months. If reevaluation after 3 months (6 months after dissection) shows persistence of the pathology, oral anticoagulation is stopped, and the patient is kept on antiplatelet therapy for life. The rationales behind this approach are the high recanalization rate within the first 2 to 3 months after the dissection and the observation that, after discontinuation of anticoagulation, recurrence of symptoms occasionally may occur between 3 and 6 months after the onset of dissection but rarely after 6 months. Oral anticoagulation is contraindicated in intracranial dissections complicated by subarachnoid hemorrhage and in presence of a large infarct with associated mass effect or intracranial extension of the dissection.54 Antiplatelet therapy is an alternative to anticoagualtion53,54; however, there are no clinical trials available to determine whether antiplatelet therapy is equal or superior to anticoagulation owing to the low rate of recurrent ischemic events in patients with dissection. Furthermore, in a meta-analysis of 26 studies including 327 patients, Lyrer and Engelter found no significant difference between the two treatment options in the odds of death and in the odds of being alive but disabled.56
Thrombolysis
The role of thrombolysis in patients with acute infarction secondary to dissection has been explored.57,58 Thrombolysis treatment (intravenous and intra-arterial) has been successful in some patients with arterial occlusion caused by dissection if performed within several hours of onset.59,60 The thrombolytic agent acts on clots in both the true lumen and the false lumen of the dissecting vessel, resulting in reduced stenosis. However, in cases with associated subarachnoid hemorrhage, thrombolytic therapy has the potential to aggravate the risk for subarachnoid hemorrhage and should be avoided.61,62
Compression
After a diagnosis of rotational vertebral artery compression is established by dynamic vertebral imaging, surgical treatment is recommended. Conservative medical therapy consists of anticoagulation or neck immobilization, either by instructing the patient to refrain from head turning or by the use of a collar. In one review series of those treated conservatively, however, nearly 50% went on to infarct or had residual neurological deficits.63
Endovascular Management
Atherosclerotic Disease
Angioplasty was first introduced by Dotter and Judkins64 in 1964 using flexible dilators, but it was not until the 1980s that angioplasty was successfully performed in the subclavian and vertebral arteries. In 1980, Bachman and Kim65 first reported dilation of the subclavian artery for the treatment of subclavian steal syndrome. In 1986, Higashida and colleagues66 reported successful percutaneous transluminal angioplasty (PTA) of the vertebral arteries.
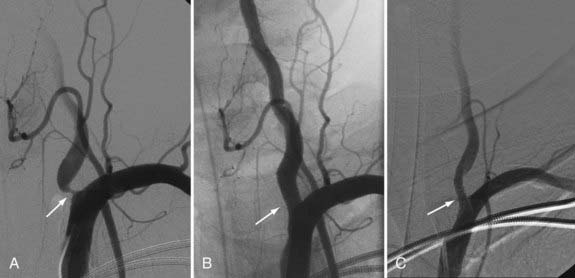
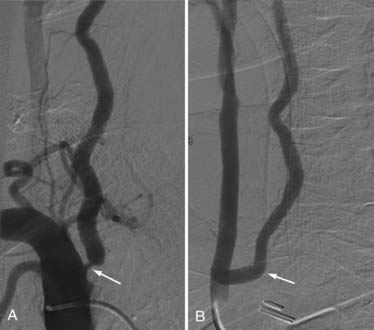
Although PTA has been widely used to treat the cervical carotid artery with or without the placement of stents, vertebral artery PTA is becoming more common. Its main application to the external vertebral artery is for the treatment of atherosclerotic plaque, which most often occurs at the origin of the vertebral artery. The plaque is often fibrous with a smooth surface (ulcerated in <4% of cases), making it ideal for PTA. However, the vertebral artery origin has a well-developed muscularis and, therefore, the potential for a high risk for restenosis. Additionally, the disease causing the stenosis is usually within the lumen of the subclavian artery, with a large burden of plaque. An ideal stent would have a high radial force, spanning the ostium and 2 to 3 mm into the lumen of the subclavian artery. Currently, balloon-mounted coronary platform stents are favored because they tend to have the best combination of high outward radial force, limited foreshortening, low crossing profile, and appropriate diameters.67 The Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA) trial contained a subset of patients (18 of 61 patients) with extracranial vertebral disease treated with angioplasty and stent placement.68 Of this subset, 6 (33%) of 18 patients had vertebral origin stenosis, whereas 12 (67%) of 18 patients had nonostial extracranial vertebral stenosis. Technical success (<50% residual stenosis) was achieved for PTA and stenting for origin stenosis in 94% to 98% of cases in recent series.68–70 However, angiographic follow-up demonstrated ostial in-stent re-stenosis (>50%) ranging up to 67%68–70 at 6 months, compared with 25% of other extracranial vertebral and 32% of intracranial lesions.68 Further analysis in the SSYLVIA trial showed that diabetes, ostial stenosis location, the postprocedure percentage of stenosis, pretreatment vessel diameter, and age contributed significantly to the prediction of stenosis at 6 months, with higher pretreatment vessel diameter and age lessening the likelihood of stenosis.68 Most cases of restenosis at 6 months remained asymptomatic (61%)68 because neointimal proliferation, which typically causes restenosis, likely carries a lower thromboembolic risk than atherosclerosis.71 Nonetheless, these results suggest that endovascular treatment for vertebral origin disease is not the optimal strategy (Fig. 354-3).
Dissection
In rare patients with symptoms refractory to medical management and in those with expanding dissecting aneurysms, endovascular therapy may be indicated. Endovascular therapy is an alternative to surgery as the initial therapy of choice when medical therapy fails or is contraindicated.72,73 Procedures include balloon angioplasty followed by placement of one or more balloon-expandable or self-expanding stents. Associated dissecting aneurysm may require coil embolization or the deployment of a covered stent.74 The published short-term clinical and radiographic results of endovascular therapy of carotid and vertebral artery dissection have been excellent with a low, but not negligible, complication rate. Because the risk for thromboembolic complications from a residual chronic dissecting aneurysm is small, and because extracranial dissecting aneurysms do not rupture, there is no good indication for endovascular treatment of residual aneurysm or luminal irregularity in the absence of symptoms.75,76
Surgical Management
Unlike those for the carotid artery, no clinical trials have shown the beneficial effect of surgery for high-grade stenosis of the vertebral artery. The surgical approach to each anatomic segment of the extracranial vertebral artery is different. The outcome of patients undergoing surgery of the external vertebral artery depends on the cause of the disease, the segment of the vertebral artery affected, and the type of treatment performed. In the hands of an experienced surgeon, surgery is associated with a very low mortality rate, and life expectancy is not substantially different from that of the general population with cerebrovascular and peripheral vascular disease, at about 50% survival at 10 years.77,78
Surgery of the First Vertebral Artery (V1) Segment
Several operations have been devised to treat lesions of the proximal artery.79–82 As microsurgery evolved in the 1970s, various reconstructive techniques also developed. Wylie and Ehrenfeld83 first treated pathology of the proximal vertebral artery by the transposition technique, with anastomosis between the vertebral artery and the CCA. Berguer and associates84 used vein grafts in this region of the vertebral artery to connect the subclavian artery to the proximal vertebral artery. The treatment of penetrating vertebral artery injuries also changed significantly with the advent of the Vietnam War. Surgeons no longer relied solely on ligation of the artery as treatment; in many cases, they actually reconstructed the vertebral artery.18 Transposition of the proximal vertebral artery onto the CCA is the most common procedure performed in this section of the artery, primarily for treatment of atherosclerotic vertebral origin stenosis. Bypasses using vein grafts are also performed from the adjacent subclavian artery or CCA to the proximal vertebral artery. Endarterectomy of the subclavian artery or the proximal vertebral artery can also be performed. Berguer77 detailed the long-term outcome and experience with surgery of the proximal vertebral artery. Of 230 patients, 2 died. Complications included recurrent laryngeal nerve palsy in 2%, Horner’s syndrome in 15%, lymphocele in 4%, chylothorax in 0.5%, and immediate thrombosis in 1%. Symptoms resolved substantially or were cured in 83% of the patients. Patency rates were 95% and 91% at 5 and 10 years, respectively.
Approach to the Proximal Vertebral Artery
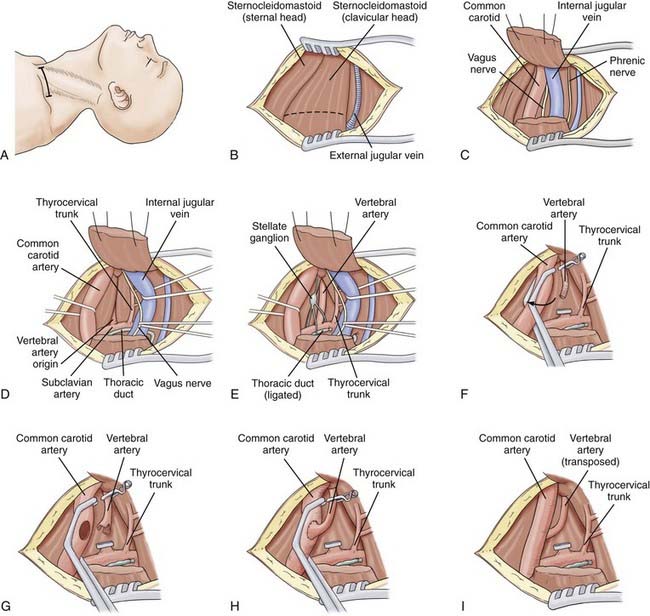
The standard approach to the proximal vertebral artery is a supraclavicular approach (Fig. 354-4A). The patient’s head is placed in a donut headrest. Downward traction of the arm provides better exposure. The head is kept midline for this approach. A supraclavicular incision is made about 2 cm above and parallel to the clavicle and extends from the suprasternal notch to 7 to 8 cm laterally. The skin is retracted superiorly and inferiorly, leaving the platysma, which is divided horizontally. The superficial veins flank the edge of the sternocleidomastoid muscle with the external jugular vein lying at the lateral edge (Fig. 354-4B). The sternocleidomastoid muscle has two origins: the clavicular head from the superior surface of the medial third of the clavicle, and the sternal head from the anterior surface of the manubrium of the sternum. The clavicular head is divided, leaving a cuff on the clavicle, and the muscle is retracted superiorly and laterally (Fig. 354-4C). The omohyoid muscle can also be divided if needed. The dissection is kept medial to expose the carotid sheath. The anterior scalene muscle lies laterally, with the phrenic nerve lying on top of it. This muscle is usually far lateral to the exposure and rarely requires division. The carotid sheath is separated from the overlying fascia and opened. Inside can be found the CCA, the internal jugular vein, and the vagus nerve. The jugular vein and vagus nerve are retracted laterally, and the CCA is retracted medially (Fig. 354-4D). From this point, dissection proceeds below the deep fascia layer caudally.
If the right side is exposed, several steps are needed. The lymphatic drainage on the right side of the neck is different from that on the left. Delicate lymphatic trunks empty into the right subclavian and jugular veins, which are usually smaller than the lymphatic ducts on the left. Because they do not coagulate completely, it is better to identify and ligate them (Fig. 354-4D and E). The right recurrent laryngeal nerve exits the vagus nerve and loops below the right subclavian artery as it approaches the trachea and larynx. Consequently, medial retraction of the trachea can cause ipsilateral paresis of the vocal cord. If the left side is exposed, the thoracic duct is encountered as it arches from the side of the esophagus laterally to the angle between the internal jugular and subclavian veins. The proximal portion of this duct is ligated twice, and smaller branches are also ligated. The left recurrent laryngeal nerve can be retracted with greater ease because it loops around the aortic arch and approaches the trachea much lower.
Working in the deep fascial layer, the vertebral artery can now be identified (Fig. 354-4E) as the first branch of the subclavian artery exiting from its posterosuperior surface. This feature distinguishes it from the thyrocervical trunk, which has multiple branches and exits from the anterosuperior surface. Alternatively, and more easily, the vertebral artery can be first located superiorly as it exits the transverse foramen of C6. The transverse process of C6 can be identified adjacent to its foramen. The artery arises from the apex of two muscles as they attach to the carotid tubercle: the anterior scalene muscle and the longus colli. The vertebral vein, which overlies the artery, can be divided or retracted. The vertebral vein is formed at the lower end of the canal of the transverse foramina from a venous plexus within the canal around the vertebral artery. The vein is anterior to the artery and often adherent to it. It is important to identify and preserve the sympathetic chain. The vertebral artery is looped and dissected from C6 to the subclavian artery. Care is taken to avoid destroying the sympathetic trunks and stellate or intermediate ganglia that lie on it. The anterior surface is freed.
Transposition of Proximal Vertebral Artery to Common Carotid Artery
This procedure is used because of the ease of exposure. Its limitation, however, is the requirement for simultaneous occlusion of both carotid and vertebral arteries (Fig. 354-4F). Using the standard approach described earlier for isolation of the proximal vertebral artery, the CCA is prepared for the vertebral artery. Adventitia is cleared from the carotid artery. The patient is given a bolus of 3000 to 5000 U of heparin. Five minutes later, the vertebral artery is clamped at the level of C6 with a temporary clip. The proximal vertebral artery just above the stenosis is occluded with a hemoclip and cut. The artery is freed from the surrounding sympathetic trunk and moved medially toward the CCA. If the vertebral artery is not lax enough, it may be necessary to remove it from the C6 transverse process. A double-ended fish-mouth opening is made in the proximal end of the vertebral artery (Fig. 354-4G).
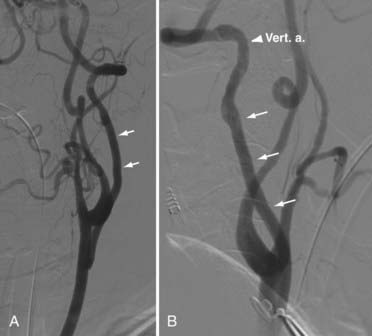
An occluding clamp is placed on the carotid artery at the selected level and used to rotate the vessel laterally (Fig. 354-4F and G). This maneuver allows the anastomosis to be performed on the posterolateral wall of the CCA in line with the trajectory of the vertebral artery. With 7-0 monofilament nylon suture, the superior and inferior ends of the fish-mouth opening are sutured to the corresponding ends of the hole in the carotid artery. One suture is used to form a running anastomosis on the back wall and is tied to the opposite end on completion. The front wall is then sutured. Before the last suture is tied, the lumens of both arteries are flushed with heparinized saline. First the vertebral artery and then the CCA are back-flushed. The final suture is tied, and all clamps are removed (Fig. 354-4H and I). If blood continues to ooze, gentle pressure is placed over the anastomosis with Gelfoam. After copious irrigation and hemostasis, the sternocleidomastoid muscle is reapproximated. A suction drain is placed in the neck for 24 hours, and the neck opening is closed in multilayers. Postoperative imaging using conventional angiography, MRA, or CTA can be used to confirm patency and for follow-up (Fig. 354-5).
Alternative Transpositions and Vein Graft to the Proximal Vertebral Artery
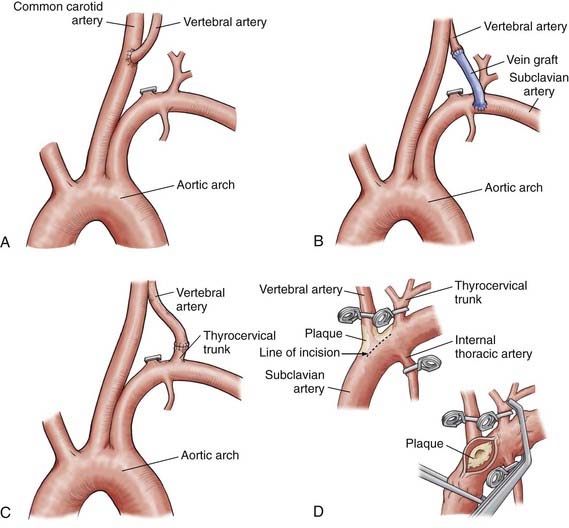
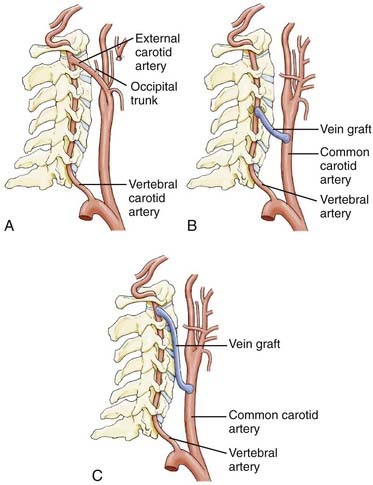
If the carotid artery is stenotic or otherwise compromised, the subclavian artery can be used. The desired segment of the subclavian artery is in the area of the anterior scalene muscle or more distally. Similarly, if transposition to the CCA (Fig. 354-6A) is not feasible because of the length of the proximal vertebral artery, a vein graft bypass can be performed.80,84 Berguer and Feldman80 anastomosed a saphenous vein graft to the subclavian artery distal to the site of origin of the vertebral artery and then attached it by an end-to-end anastomosis to the vertebral artery (Fig. 354-6B). Although this procedure does not interrupt carotid blood flow, it requires two anastomoses and is time consuming. The vein is usually autogenous saphenous vein, although prosthetic materials have been used. The proximal vertebral artery can be also be transposed from the subclavian artery to the thyrocervical trunk (Fig. 354-6C).
Subclavian-Vertebral Endarterectomy
In 1959, Cate and Scott7 described an endarterectomy of the origin of the vertebral artery through a subclavian approach (Fig. 354-6D). Although subclavian-vertebral endarterectomies have been used successfully since the 1960s, they are associated with several technical problems. The endarterectomy is a difficult approach to use with a low-lying subclavian artery. With other safer alternatives now available, the procedure is seldom used today.
Decompression of the Proximal Vertebral Artery
The proximal vertebral artery can be compressed by bands from the tendon of the anterior scalene or the longus colli muscle.79,81 After exposure of the proximal vertebral artery (see Fig. 354-4A to E), the vertebral artery is mobilized from its origin to the transverse foramen of C6. Ligaments, muscles, and bands overlying the artery are excised. In some cases, the sympathetic ganglia or nerve fibers can constrict the artery. If the ganglia are divided, a mild Horner’s syndrome will develop. Segmental resection and end-to-end anastomosis can be used when obstruction is caused by entrapment,82 but the vertebral artery must be long and its diameter adequate.
Surgery of the Second Vertebral Artery (V2) Segment
The V2 segment of the vertebral artery is partially encased in a bony channel as it travels through the transverse foramina of the C1 through C6 cervical segments. Consequently, direct surgical reconstruction of the V2 artery is rarely undertaken.85,86 Anatomically, however, the vertebral artery has more redundancy and is more easily accessible at the distal V2 segment (between C1 and C2) than in the more proximal V2 segment. Most surgeons use angioplasty as the first choice of treatment for intrinsic stenotic lesions in the V2 segment, but extrinsic compression with osteoarthritic spurs is associated with excellent results from surgical decompression, as first reported by Hardin and colleagues8 and then by subsequent reports.87 Single or minor extrinsic lesions can be removed to relieve compression on or kinking of the vertebral artery. In some cases of extensive V1 segment artery disease, or V2 segment disease, it may be necessary to revascularize the V2 segment. Diaz and coworkers86 reported surgical treatment (vertebral endarterectomy, vertebral-to-carotid transposition or grafting) in 11 of 12 patients with disease extending into the middle vertebral artery. Patency was found in 11 of the 12 patients with a follow-up of 4 to 34 months and relief of VBI symptoms after surgery.
Approach to the Proximal V2 Segment (C6)
The approach to the proximal V2 segment essentially parallels the approach described for exposure of the proximal (V1) vertebral artery. This would be undertaken to decompress constriction of the artery at the level of the C6 foramen transversarium related to osteophytic bony compression or of fascial or ligamentous bands related to the insertion of the scalene muscles onto the transverse process of C6. The exposure of the vertebral artery as demonstrated in Figure 354-4B to E can be undertaken with the exception that a longitudinal incision medial to the sternocleidomastoid and extending toward the sternal notch would be used rather than a horizontal supraclavicular incision, and the sternocleidomastoid could be retracted laterally rather than divided from its clavicular attachment. The omohyoid is transected as needed. The dissection would focus more superiorly in the deep fascial layer after the carotid has been dissected and retracted medially and the jugular laterally. When the tubercle of C6 is palpated, the insertions of the scalene and longus colli muscles are dissected subperiosteally, and the transverse process of C6 can be resected as needed to decompress the vertebral artery. Dissection can be carried out more superiorly toward C5 as needed.
Approach to the Mid V2 Segment (C2-5)
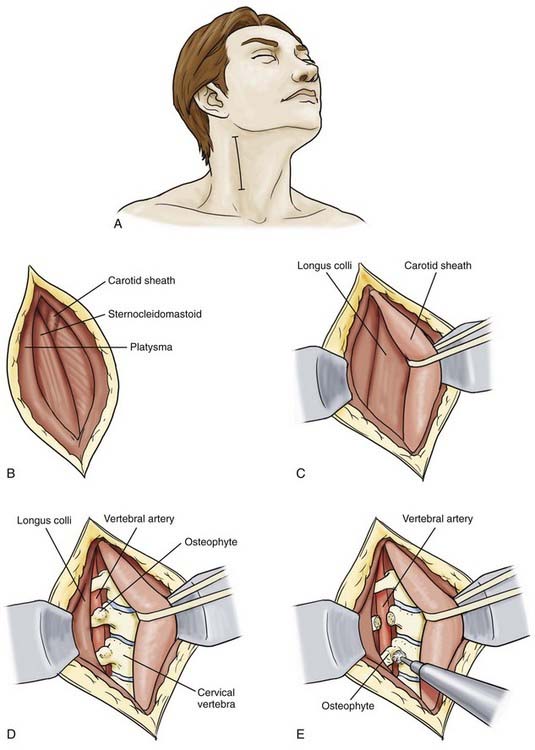
The mid V2 segment (C2-5) can be accessed most readily from an anterior approach.82 The incision can traverse a skin crease or be made longitudinally along the anterior border of the sternocleidomastoid muscle (Fig. 354-7A), depending on whether the pathology involves one or two levels. Initially, a cervical radiograph is used to define the level of interest and should be repeated intraoperatively before the transverse process is drilled. The skin is retracted, and the platysma is left intact until it is completely exposed. An incision is then made longitudinally through the platysma along the anterior border of the sternocleidomastoid muscle (Fig. 354-7B).
By blunt dissection, a plane is developed between the strap muscles and the carotid sheath, which contains the carotid, internal jugular vein, and vagus nerve. The carotid sheath, trachea, and esophagus are bluntly dissected from the prevertebral fascia below, and retracted medially, whereas the sternocleidomastoid muscle is retracted laterally (Fig. 354-7C). The prevertebral fascia is opened to expose the anterior longitudinal ligament and the longus colli muscles. The ligament is incised on its lateral border to allow a periosteal elevator to be used to mobilize the prevertebral musculature (the longus colli and longus capitis). Periosteal dissection is carried out to free the attachment of these muscles from the vertebral body and transverse processes laterally up to the anterior tubercle (Fig. 354-7D). Further, dissection posterolaterally beyond the anterior tubercle must be avoided because this may lead to injury of the cervical nerve roots. The sympathetic ganglia laying on the lateral aspect of the longus colli need to be preserved. The bony canal of the vertebral artery lays just deep and medial to the anterior tubercle. After the muscles have been reflected laterally, the anterior tubercle of the transverse process can be removed using a high-speed drill, rongeur, or curet (Fig. 354-7E) to unroof the bony canal and expose the vertebral artery. Immediately below the transverse process, anterior to the vertebral artery, is the venous plexus, which is coagulated meticulously. Care is taken to preserve radiculomedullary arteries that exit from the vertebral artery between C1 and C5 and supply the spinal cord.
Approach to the Distal V2 Segment (C1-2)
![]() The approach to the distal V2 segment (C1-2) depends on the revascularization technique used. The most common is the anterolateral approach88–90 to access the distal V2 segment for bypass, either through direct anastomosis to donors, such as the external carotid artery (ECA), or through an interposition graft from a vein or the radial artery used to connect the arteries (Video 354-1).
The approach to the distal V2 segment (C1-2) depends on the revascularization technique used. The most common is the anterolateral approach88–90 to access the distal V2 segment for bypass, either through direct anastomosis to donors, such as the external carotid artery (ECA), or through an interposition graft from a vein or the radial artery used to connect the arteries (Video 354-1).
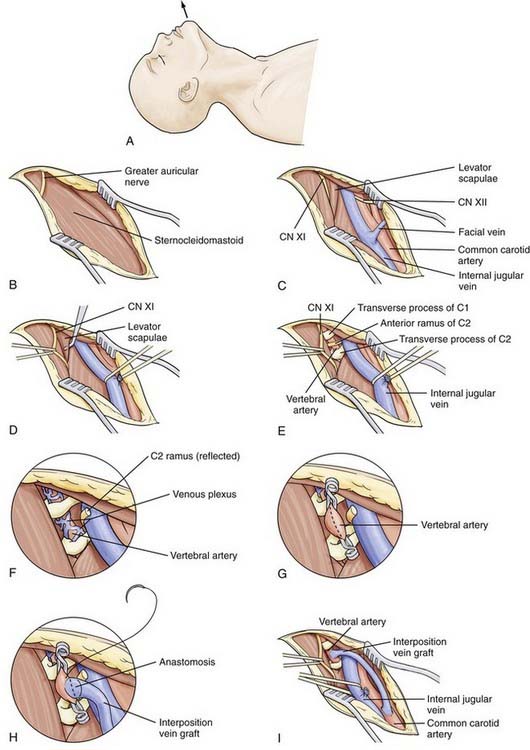
The exposure is made high in the anterior triangle. Subluxation of the jaw is a useful maneuver to provide additional exposure. The incision is made on the medial edge of the sternocleidomastoid muscle and extends in a curvilinear fashion over the mastoid bone (Fig. 354-8A). The auricular nerve may be encountered posterosuperiorly and may need to be transected (Fig. 354-8B). The incision is brought down to the platysma muscle, which is separated from the subcutaneous tissue. Along the medial border of the sternocleidomastoid muscle, the carotid sheath is entered, and the internal jugular vein is identified. The parotid gland is freed from the sternocleidomastoid muscle and reflected anteriorly. A self-retaining retractor is placed to retract the internal jugular vein medially and the sternocleidomastoid muscle laterally (Fig. 354-8C). Below the sternocleidomastoid muscle, the accessory nerve is visible. This nerve is protected by placing a loop around it and retracting it laterally. The belly of the digastric muscle is retracted superiorly, or it can be divided. Below the digastric muscle, the C1 tubercle is palpated. The fascia is cleared, and the fibers of the levator scapulae and splenius cervicis become visible (Fig. 354-8D). The location of the C1 tubercle can be confirmed by placing a clamp at this level and obtaining a lateral cervical radiograph.
The C2 tubercle is also palpated, and the levator scapulae is cut to reveal the anterior ramus of the C2 nerve root, which passes anteriorly to the vertebral artery from medial to lateral (Fig. 354-8E). Cutting this nerve exposes the vertebral artery; the nerve is cut laterally and retracted anteriorly to avoid traction on the proximal root and spinal cord (Fig. 354-8F). Dissection of the overlying tissue reveals a venous plexus surrounding the vertebral artery. Careful coagulation or the use of Gelfoam prevents injury to the artery. About 2 cm of vertebral artery can be exposed in the C1-2 intertransverse interspace. Further exposure can be obtained by removing the lateral wall of the transverse foramen of C1.
Decompression of the V2 Segment
Several series have reported surgery in this segment of the vertebral artery to treat external compressive lesions.10,85–87 Such compressive lesions can be associated with bow hunter’s syndrome, in which symptoms occur during head rotation.35 When the transverse process is reached, the level of the compression can be identified and decompressed. Plain anteroposterior cervical radiographs identify the level. Osteophytes are drilled off or removed with curets. The periosteum must be removed; otherwise, adhesions to the artery can persist. The artery is dissected circumferentially and displaced laterally. In some cases, degenerative changes of the zygapophyseal joint can result in protrusion and compression of the artery and must be removed (see Fig. 354-7E). At the end of the procedure, the artery should be free of all restrictions (Fig. 354-9).
Revascularization to the V2 Segment Using Transpositions
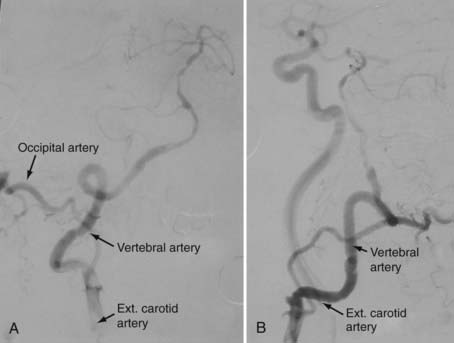
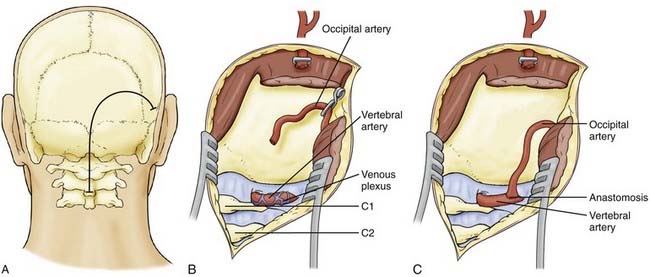
Pritz and associates91 reported transposing the trunk of the ECA to the middle vertebral artery in order to ligate the vertebral artery distal to an aneurysm at C4. In a large series of 100 distal vertebral reconstructions, Berguer and colleagues performed 23 procedures for transposition of the ECA or its occipital branch to the distal vertebral artery at C1-2 for revascularization in the setting of ischemic symptoms.78
This technique requires a carotid bifurcation free of disease and a long ECA trunk. Both the vertebral artery and the ECA are isolated during the approach. The major drawback of this procedure is the need to mobilize the ECA to reach the vertebral artery. The ECA is skeletonized, and all its branches are divided and ligated before the appropriate length is selected (Fig. 354-10A). It is advisable to leave the occipital branch intact primarily because musculoskeletal branches from the occipital artery often feed the more distal vertebral artery. The ECA is mobilized laterally either below or above the ICA and connected to the distal segment of the vertebral artery by an end-to-end (Fig. 354-11) or end-to-side anastomosis. The proximal vertebral artery is permanently occluded with a clip. Use of the occipital artery is considered if the ECA is too short to reach the vertebral artery, although, given that collateral blood flow to the distal vertebral artery often comes from the occipital artery, transposing the occipital artery directly to the distal vertebral artery may not have a significantly greater effect.
Koskas and associates92 have also reported revascularization of the distal vertebral artery segment at the C1-2 level in 91 patients primarily for severe VBI. The authors performed a direct transposition of the vertebral artery, by exposing the vertebral artery at the level of C2, ligating the vessel proximally, and transposing the vessel to the posterolateral aspect of the ICA. There were no deaths or strokes in the series. VBI symptoms were alleviated in 57.5%, 2.3% were unchanged, and the rest improved; the transposed vertebral artery was occluded in early follow-up in 8 patients (8.7%), with a patency rate of 89.1% at 5 years.
Revascularization to the V2 Segment Using Vein Grafts
Vein grafts can be used to bypass disease at a more proximal aspect of the vertebral artery by connecting the V2 segment to the CCA (see Fig. 354-10B), ECA, or ICA. In 1966, Clark and Perry93 used a saphenous vein graft to connect the ECA to the vertebral artery at C2-3. The advantage of the ECA as the donor supply is that the proximal anastomosis does not interfere with the cerebral circulation. In 1977, Carney and Anderson94 performed the first vein bypass from the CCA to the distal vertebral artery at the level of C1 and C2 (Fig. 354-10C). Berguer and colleagues78 published a large series of 100 distal vertebral artery reconstructions over a 14-year period, performed ostensibly for ischemic symptoms. These included 68 vein grafts to the C1-2 segment of the vertebral from the CCA, ICA, or ECA. In the overall series, 16% of grafts failed in the early postoperative period, two symptomatically, and the patency rates were 75% and 70% at 5 and 10 years, respectively.
Although vein grafts to the mid V2 segment can be performed successfully95 using the anterior approach to expose both the vertebral artery and the carotid artery, the C1-2 vertebral artery segment, accessed through the anterolateral approach, is particularly amenable to bypass. In the C1-2 interspace, a 2-cm segment of vessel can be exposed without the need for bony removal and unroofing of the vertebral canal necessary in the mid V2 segment. The donor site can be the CCA (see Fig. 354-10B and C), ICA, or ECA. A saphenous vein of selected length is removed from the leg. The patient is given heparin (5000 U). After 5 minutes, the vertebral artery is gently pulled up to isolate a 2-cm section (see Fig. 354-8G). Temporary clips are used to isolate the vertebral artery, and an incision is made sized to the fish-mouthed end of the vein graft. The vein is connected to the vertebral artery with an end-to-side anastomosis using 8-0 polypropylene (Fig. 354-8H). The clips are removed. If backflow through the graft is good, a temporary clip is used to occlude the vein. The proximal end of the graft is passed to the CCA, ICA, or ECA. The donor vessel is cleared of any surrounding tissue, and a cross-clamp is applied to its proximal and distal portions. Using an aortic punch, a 4- or 5-mm elliptical arteriostomy is made in the wall of the donor. With 6-0 polypropylene, the vein graft is anastomosed end to side. Again, backflow is allowed from the vein and distal CCA before the final suture is placed. The clamp on the proximal carotid artery is then removed (Fig. 354-8I). If the ECA is used, the vein graft can be anastomosed end-to-end to the proximal portion of the artery, and the distal portion of the ECA is tied off. Postoperative imaging using conventional angiography, MRA, or CTA can be used to confirm patency and for follow-up (Fig. 354-12).
Surgery of the Third Vertebral Artery (V3) Segment
The V3 segment is vulnerable to blunt trauma, and injury to the intima can result in thrombosis, embolization, and dissection.18 This region has a high incidence of arteriovenous fistula formation and aneurysmal degeneration. Patency of the distal segment is often maintained through cervical collaterals. In many cases, if angiography reveals collaterals from the occipital artery, this artery is left intact. Before planning any type of procedure, surgeons must consider the biomechanics of the spine at this level. Rotation occurs with the axis line posterior to the neck. Therefore, it is best to place grafts posteriorly to avoid undue torsion. An appropriate length must be used to avoid kinking or torsion.
Approach to the V3 Segment
The V3 segment of the vertebral artery is best exposed through a posterior approach. This approach is used to gain greater exposure of the vertebral artery for an occipital artery–to–distal vertebral artery bypass or to decompress an obstructed vertebral artery. The approach is more familiar to neurosurgeons because the patient is in a full prone or three-quarter prone position. The incision is made from the C2-3 spinous process to the inion in a hockey-stick fashion to the mastoid bone (Fig. 354-13A).79,89 The scalp and posterior musculature are dissected from the occiput and retracted laterally. Similarly, the paraspinous muscles are dissected, exposing the lamina of C1 and C2 ipsilaterally, and the muscles retracted laterally. The segment of the vertebral artery within the sulcus arteriosus is exposed (Fig. 354-13B). The artery is surrounded by a venous plexus, which must be dissected, maintaining hemostasis with gentle packing and bipolar. The artery can be isolated over a longer segment by drilling the transverse foramen of C1 laterally to release and mobilize the vertebral artery. Frequently, muscular branches entering the vessel at the C1 level are present and should be preserved if possible.
Decompression of the V3 Segment
An occasional patient may have severe local obstruction of the distal vertebral artery caused by compression from arterial branches or neighboring nerves. This can easily be treated by using the posterolateral approach with division of the obstruction. Bow hunter’s syndrome due to constriction of the distal segment of the vertebral artery, with occlusion following lateral head rotation, can also occur.32 Traditionally, these patients were simply fused between the skull, C1, and C2. In younger patients, however, it is best to explore the anatomy and remove the compressive pathology.
Revascularization to the V3 Segment
The posterior approach for an occipital artery–to–distal vertebral artery bypass has been advocated79 as a method for revascularization. In the setting of more proximal vertebral occlusion, the occipital often provides collateral to the distal vertebral, as previously noted. In such cases, careful thought must be given to performing this type of bypass because the additional flow from direct occipital to vertebral anastomosis may not be significantly greater than existing collateral. The added challenge is that the occipital artery tends to be more tortuous as it emerges from the trapezius muscle, and large lengths of it can be difficult to isolate and mobilize to perform the bypass. However, the distal vertebral artery is easily accessed with a large exposure from the dura to C1 and by removing the C1 foramen as inferiorly as the exit of C2. Temporary clips are placed on the atlantal segment of the vertebral artery following systemic heparinization. The vertebral is incised, and the dissected occipital artery is fish-mouthed and sutured with 8-0 nylon in an end-to-side fashion (Fig. 354-13C). Attention must be given to allowing adequate redundancy to the occipital to avoid tension on the graft during head rotation, but at the same time avoiding kinking.
Conclusion
The literature reports many series using different approaches to the extracranial vertebral artery with great success. Table 354-2 summarizes the endovascular and surgical approaches to the more commonly encountered extracranial vertebral artery diseases. We must emphasize, however, the need to analyze the clinical and laboratory data of each patient methodically and to define the cause of the symptoms. If surgical intervention is indicated, the choice of procedure must optimize blood flow while minimizing the treatment risk for death or morbidity to the patient. There will always be unique cases that require special approaches to the three segments of the extracranial vertebral artery. In general, however, as more sophisticated endovascular techniques are developed, the use of angioplasty and stent placement to treat occlusive disease will likely become more common.
TABLE 354-2 Common Indications and Procedures for Treatment of Extracranial Vertebral Artery Disease
| PROXIMAL VERTEBRAL ARTERY (V1 SEGMENT) | MIDDLE VERTEBRAL ARTERY (V2 SEGMENT) | DISTAL VERTEBRAL ARTERY (V3 SEGMENT) |
|---|---|---|
| Surgical | ||
| Vertebral-carotid transposition for vertebral origin stenosis | Decompression for osteophytic compression Vein graft to mid or distal V2 segment from carotid for proximal occlusive disease |
Occipital artery-vertebral bypass for proximal occlusive disease |
| Endovascular | ||
| Vertebral origin angioplasty and stent for atherosclerotic disease | Vertebral angioplasty and stent for atherosclerotic disease | Vertebral angioplasty and stent for atherosclerotic disease |
Albuquerque FC, Fiorella D, Han P, et al. A reappraisal of angioplasty and stenting for the treatment of vertebral origin stenosis. Neurosurgery. 2003;53:607-614.
Amin-Hanjani S, Du X, Zhao M, et al. Use of quantitative magnetic resonance angiography to stratify stroke risk in symptomatic vertebrobasilar disease. Stroke. 2005;36:1140-1145.
Anson JA, Spetzler RF. Surgery for vertebral basilar insufficiency: Extracranial. In: Carter LP, Spetzler RF, Hamilton MG, editors. Neurovascular Surgery. New York: McGraw-Hill; 1995:383-403.
Ausman JI, Shrontz CE, Pearce JE, et al. Vertebrobasilar insufficiency. A review. Arch Neurol. 1985;42:803-808.
Bauer A, Amin-Hanjani S, Alaraj A, et al. Quantitative magnetic resonance angiography in the evaluation of the subclavian steal syndrome: report of 5 patients. J Neuroimaging. 2009;19:250-252.
Berguer R, Morasch MD, Kline RA. A review of 100 consecutive reconstructions of the distal vertebral artery for embolic and hemodynamic disease. J Vasc Surg. 1998;27:852-859.
Brink B. Approach to the second segment of the vertebral arteries. In: Berguer R, Bauer RB, editors. Vertebrobasilar Arterial Occlusive Disease: Medical and Surgical Management. New York: Raven; 1984:257-264.
Caplan LR. Vertebrobasilar disease. Adv Neurol. 2003;92:131-140.
Caplan LR, Wityk RJ, Glass TA, et al. New England Medical Center Posterior Circulation registry. Ann Neurol. 2004;56:389-398.
Carney AL. Vertebral artery surgery: Pathology, hemodynamics, and technique. In: Robicsek F, editor. Extracranial Cerebrovascular Disease: Diagnosis and Management. New York: Macmillan; 1986:395-424.
Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305-1316.
Diaz FG, Ausman JI, de los Reyes RA, et al. Surgical reconstruction of the proximal vertebral artery. J Neurosurg. 1984;61:874-881.
Diaz FG, Ausman JI, Shrontz C, et al. Surgical correction of lesions affecting the second portion of the vertebral artery. Neurosurgery. 1986;19:93-100.
Engelter ST, Brandt T, Debette S, et al. Antiplatelets versus anticoagulation in cervical artery dissection. Stroke. 2007;38:2605-2611.
Hass WK, Fields WS, North RR, et al. Joint study of extracranial arterial occlusion. II. Arteriography, techniques, sites, and complications. JAMA. 1968;203:961-968.
Horowitz M, Jovin T, Balzar J, et al. Bow hunter’s syndrome in the setting of contralateral vertebral artery stenosis: evaluation and treatment options. Spine. 2002;27:E495-E498.
Phatouros CC, Higashida RT, Malek AM, et al. Endovascular treatment of noncarotid extracranial cerebrovascular disease. Neurosurg Clin N Am. 2000;11:331-350.
Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344:898-906.
Sorensen BF. Bow hunter’s stroke. Neurosurgery. 1978;2:259-261.
Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA). Study results. Stroke. 2004;35:1388-1392.
1 Quain R. The Anatomy of the Arteries of the Human Body and Its Application to Pathology and Operative Surgery, with a Series of Lithographic Drawings. London: Taylor & Watson; 1844.
2 Matas R. Aneurysms and wounds of the vertebral arteries. Ann Surg. 1893;18:477-516.
3 Moniz E. L’encephalographie arterielle son importance dans la localisation des tumeurs cerebrales. Rev Neurol (Paris). 1927;2:72-90.
4 Radner S. Intracranial angiography via the vertebral artery: preliminary report of a new technique. Acta Radiol (Stockh). 1947;28:838-842.
5 Hutchinson EC, Yates PO. The cervical portion of the vertebral artery: a clinico-pathological study. Brain. 1956;79:319-331.
6 Crawford ES, De Bakey ME, Fields WS. Roentgenographic diagnosis and surgical treatment of basilar artery insufficiency. JAMA. 1958;168:509-514.
7 Cate WRJr, Scott HWJr. Cerebral ischemia of central origin: relief by subclavian-vertebral artery thromboendarterectomy. Surgery. 1959;45:19-31.
8 Hardin CA, Williamson WP, Steegmann AT. Vertebral artery insufficiency produced by cervical osteoarthritic spurs. Neurology. 1960;10:855-858.
9 Husni EA, Bell HS, Storer J. Mechanical occlusion of the vertebral artery. A new clinical concept. JAMA. 1966;196:475-478.
10 Hardin CA, Poser CM. Rotational obstruction of the vertebral artery due to redundancy and extraluminal cervical fascial bands. Ann Surg. 1963;158:133-137.
11 Hass WK, Fields WS, North RR, et al. Joint study of extracranial arterial occlusion. II. Arteriography, techniques, sites, and complications. JAMA. 1968;203:961-968.
12 Fields WS, Lemak NA. Joint study of extracranial arterial occlusion. VII. Subclavian steal—a review of 168 cases. JAMA. 1972;222:1139-1143.
13 Toole JF, Tucker SH. Influence of head position upon cerebral circulation. Studies on blood flow in cadavers. Arch Neurol. 1960;2:616-623.
14 Ausman JI, Shrontz CE, Pearce JE, et al. Vertebrobasilar insufficiency. A review. Arch Neurol. 1985;42:803-808.
15 Special report from the National Institute of Neurological Disorders and Stroke. Classification of cerebrovascular diseases III. Stroke. 1990;21:637-676.
16 Grad A, Baloh RW. Vertigo of vascular origin. Clinical and electronystagmographic features in 84 cases. Arch Neurol. 1989;46:281-284.
17 Kumar A, Mafee M, Dobben G, et al. Diagnosis of vertebrobasilar insufficiency: time to rethink established dogma? Ear Nose Throat J. 1998;77:966-969. 972-964
18 Carney AL. Vertebral artery surgery: historical development, basic concepts of brain hemodynamics, and clinical experience of 102 cases. Adv Neurol. 1981;30:249-282.
19 Amin-Hanjani S, Du X, Zhao M, et al. Blood flow in major cerebral vessels: effect of age, blood pressure and vascular anatomy. In: AANS/CNS Cerebrovascular Section and the American Society of Interventional & Therapeutic Neuroradiology. 9th Joint Annual Meeting. Orlando, FL, 2006.
20 Osborne AG. Diagnostic Cerebral Angiography, 2nd ed. New York: Lippincott Williams & Wilkins; 1999.
21 Caplan LR. Vertebrobasilar disease. Adv Neurol. 2003;92:131-140.
22 Caplan LR, Wityk RJ, Glass TA, et al. New England Medical Center Posterior Circulation registry. Ann Neurol. 2004;56:389-398.
23 Moufarrij NA, Little JR, Furlan AJ, et al. Vertebral artery stenosis: long-term follow-up. Stroke. 1984;15:260-263.
24 Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305-1316.
25 Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344:898-906.
26 Dziewas R, Konrad C, Drager B, et al. Cervical artery dissection—clinical features, risk factors, therapy and outcome in 126 patients. J Neurol. 2003;250:1179-1184.
27 Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45:1517-1522.
28 Beletsky V, Nadareishvili Z, Lynch J, et al. Cervical arterial dissection: time for a therapeutic trial? Stroke. 2003;34:2856-2860.
29 Hoit DA, Schirmer CM, Weller SJ, et al. Angiographic detection of carotid and vertebral arterial injury in the high-energy blunt trauma patient. J Spinal Disord Tech. 2008;21:259-266.
30 Inamasu J, Guiot BH. Vertebral artery injury after blunt cervical trauma: an update. Surg Neurol. 2006;65:238-245.
31 Fassett DR, Dailey AT, Vaccaro AR, et al. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech. 2008;21:252-258.
32 Sorensen BF. Bow hunter’s stroke. Neurosurgery. 1978;2:259-261.
33 Horowitz M, Jovin T, Balzar J, et al. Bow hunter’s syndrome in the setting of contralateral vertebral artery stenosis: evaluation and treatment options. Spine. 2002;27:E495-E498.
34 Seki T, Hida K, Akino M, et al. Anterior decompression of the atlantoaxial vertebral artery to treat bow hunter’s stroke: technical case report. Neurosurgery. 2001;49:1474-1476.
35 Vates GE, Wang KC, Bonovich D, et al. Bow hunter stroke caused by cervical disc herniation. Case report. J Neurosurg. 2002;96:90-93.
36 Reivich M, Holling HE, Roberts B, et al. Reversal of blood flow through the vertebral artery and its effect on cerebral circulation. N Engl J Med. 1961;265:878-885.
37 Bauer A, Amin-Hanjani S, Alaraj A, et al. Quantitative magnetic resonance angiography in the evaluation of the subclavian steal syndrome: report of 5 patients. J Neuroimaging. 2009;19:250-252.
38 Karonen JO, Vanninen RL, Liu Y, et al. Combined diffusion and perfusion MRI with correlation to single-photon emission CT in acute ischemic stroke. Ischemic penumbra predicts infarct growth. Stroke. 1999;30:1583-1590.
39 Parker DL, Tsuruda JS, Goodrich KC, et al. Contrast-enhanced magnetic resonance angiography of cerebral arteries. A review. Invest Radiol. 1998;33:560-572.
40 Heiserman JE, Dean BL, Hodak JA, et al. Neurologic complications of cerebral angiography. AJNR Am J Neuroradiol. 1994;15:1401-1407.
41 Srinivasan J, Mayberg MR, Weiss DG, et al. Duplex accuracy compared with angiography in the Veterans Affairs Cooperative Studies Trial for Symptomatic Carotid Stenosis. Neurosurgery. 1995;36:648-653.
42 Enzmann DR, Ross MR, Marks MP, et al. Blood flow in major cerebral arteries measured by phase-contrast cine MR. AJNR Am J Neuroradiol. 1994;15:123-129.
43 Hofman MB, Visser FC, van Rossum AC, et al. In vivo validation of magnetic resonance blood volume flow measurements with limited spatial resolution in small vessels. Magn Reson Med. 1995;33:778-784.
44 Zhao M, Charbel FT, Alperin N, et al. Improved phase-contrast flow quantification by three-dimensional vessel localization. Magn Reson Imaging. 2000;18:697-706.
45 Amin-Hanjani S, Du X, Zhao M, et al. Use of quantitative magnetic resonance angiography to stratify stroke risk in symptomatic vertebrobasilar disease. Stroke. 2005;36:1140-1145.
46 Weksler BB, Lewin M. Anticoagulation in cerebral ischemia. Stroke. 1983;14:658-663.
47 Easton JD. What have we learned from recent antiplatelet trials? Neurology. 1998;51:S36-S38.
48 Adams RJ, Albers G, Alberts MJ, et al. Update to the AHA/ASA recommendations for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke. 2008;39:1647-1652.
49 Millikan CH, Siekert RG, Shick RM. Studies in cerebrovascular disease. III. The use of anticoagulant drugs in the treatment of insufficiency or thrombosis within the basilar arterial system. Proc Staff Meet Mayo Clin. 1955;30:116-126.
50 Whisnant JP, Cartlidge NE, Elveback LR. Carotid and vertebral-basilar transient ischemic attacks: effect of anticoagulants, hypertension, and cardiac disorders on survival and stroke occurrence—a population study. Ann Neurol. 1978;3:107-115.
51 Amarenco P, Bogousslavsky J, Callahan A3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559.
52 Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295:1556-1565.
53 Engelter ST, Brandt T, Debette S, et al. Antiplatelets versus anticoagulation in cervical artery dissection. Stroke. 2007;38:2605-2611.
54 Schievink WI. The treatment of spontaneous carotid and vertebral artery dissections. Curr Opin Cardiol. 2000;15:316-321.
55 Lucas C, Moulin T, Deplanque D, et al. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. 1998;29:2646-2648.
56 Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev. 2003:CD000255.
57 Arnold M, Nedeltchev K, Sturzenegger M, et al. Thrombolysis in patients with acute stroke caused by cervical artery dissection: analysis of 9 patients and review of the literature. Arch Neurol. 2002;59:549-553.
58 Price RF, Sellar R, Leung C, et al. Traumatic vertebral arterial dissection and vertebrobasilar arterial thrombosis successfully treated with endovascular thrombolysis and stenting. AJNR Am J Neuroradiol. 1998;19:1677-1680.
59 Leistner S, Hartmann A, Marx P, et al. Successful thrombolytic treatment of intracranial carotid occlusion due to dissection. Eur Neurol. 2001;45:284-285.
60 Sampognaro G, Turgut T, Conners JJ3rd, et al. Intra-arterial thrombolysis in a patient presenting with an ischemic stroke due to spontaneous internal carotid artery dissection. Catheter Cardiovasc Interv. 1999;48:312-315.
61 Grosman H, Fornasier VL, Bonder D, et al. Dissecting aneurysm of the cerebral arteries. Case report. J Neurosurg. 1980;53:693-697.
62 Hochberg FH, Bean C, Fisher CM, et al. Stroke in a 15-year-old girl secondary to terminal carotid dissection. Neurology. 1975;25:725-729.
63 Kuether TA, Nesbit GM, Clark WM, et al. Rotational vertebral artery occlusion: a mechanism of vertebrobasilar insufficiency. Neurosurgery. 1997;41:427-432.
64 Dotter CT, Judkins MP. Transluminal treatment of arteriosclerotic obstruction: description of a new technic and a preliminary report of its application. Circulation. 1964;30:654-670.
65 Bachman DM, Kim RM. Transluminal dilation for subclavian steal syndrome. AJR Am J Roentgenol. 1980;135:995-996.
66 Higashida RT, Hieshima GB, Tsai FY, et al. Percutaneous transluminal angioplasty of the subclavian and vertebral arteries. Acta Radiol Suppl. 1986;369:124-126.
67 Wehman JC, Hanel RA, Guidot CA, et al. Atherosclerotic occlusive extracranial vertebral artery disease: indications for intervention, endovascular techniques, short-term and long-term results. J Interv Cardiol. 2004;17:219-232.
68 Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA). Study results. Stroke. 2004;35:1388-1392.
69 Albuquerque FC, Fiorella D, Han P, et al. A reappraisal of angioplasty and stenting for the treatment of vertebral origin stenosis. Neurosurgery. 2003;53:607-614.
70 Chastain HD2nd, Campbell MS, Iyer S, et al. Extracranial vertebral artery stent placement: in-hospital and follow-up results. J Neurosurg. 1999;91:547-552.
71 Schillinger M, Exner M, Mlekusch W, et al. Acute-phase response after stent implantation in the carotid artery: association with 6-month in-stent restenosis. Radiology. 2003;227:516-521.
72 DeOcampo J, Brillman J, Levy DI. Stenting: a new approach to carotid dissection. J Neuroimaging. 1997;7:187-190.
73 Phatouros CC, Higashida RT, Malek AM, et al. Endovascular treatment of noncarotid extracranial cerebrovascular disease. Neurosurg Clin N Am. 2000;11:331-350.
74 Biggs KL, Chiou AC, Hagino RT, et al. Endovascular repair of a spontaneous carotid artery dissection with carotid stent and coils. J Vasc Surg. 2004;40:170-173.
75 Schievink WI, Piepgras DG, McCaffrey TV, et al. Surgical treatment of extracranial internal carotid artery dissecting aneurysms. Neurosurgery. 1994;35:809-815.
76 Mokri B. Traumatic and spontaneous extracranial internal carotid artery dissections. J Neurol. 1990;237:356-361.
77 Berguer R. Long-term results of vertebral artery reconstruction. In: Yao JST, Pearce WH, editors. Long-Term Results in Vascular Surgery. Norwalk: Appleton & Lange; 1993:69-80.
78 Berguer R, Morasch MD, Kline RA. A review of 100 consecutive reconstructions of the distal vertebral artery for embolic and hemodynamic disease. J Vasc Surg. 1998;27:852-859.
79 Anson JA, Spetzler RF. Surgery for vertebral basilar insufficiency: Extracranial. In: Carter LP, Spetzler RF, Hamilton MG, editors. Neurovascular Surgery. New York: McGraw-Hill; 1995:383-403.
80 Berguer R, Feldman AJ. Surgical reconstruction of the vertebral artery. Surgery. 1983;93:670-675.
81 Carney AL. Vertebral artery surgery: Pathology, hemodynamics, and technique. In: Robicsek F, editor. Extracranial Cerebrovascular Disease: Diagnosis and Management. New York: Macmillan; 1986:395-424.
82 Diaz FG, Ausman JI, de los Reyes RA, et al. Surgical reconstruction of the proximal vertebral artery. J Neurosurg. 1984;61:874-881.
83 Wylie EJ, Ehrenfeld WK. Extracranial Cerebrovascular Disease. Philadelphia: WB Saunders; 1970.
84 Berguer R, Andaya LV, Bauer RB. Vertebral artery bypass. Arch Surg. 1976;111:976-979.
85 Brink B. Approach to the second segment of the vertebral arteries. In: Berguer R, Bauer RB, editors. Vertebrobasilar Arterial Occlusive Disease: Medical and Surgical Management. New York: Raven; 1984:257-264.
86 Diaz FG, Ausman JI, Shrontz C, et al. Surgical correction of lesions affecting the second portion of the vertebral artery. Neurosurgery. 1986;19:93-100.
87 Nagashima C. Surgical treatment of vertebral artery insufficiency caused by cervical spondylosis. J Neurosurg. 1970;32:512-521.
88 Henry AK. Extensile Exposures. Baltimore: William & Wilkins; 1957.
89 Verbiest H. A lateral approach to the cervical spine: techniques and indications. J Neurosurg. 1968;28:191-203.
90 Verbiest H. Anterolateral operations for fractures and dislocations in the middle and lower parts of the cervical spine. J Bone Joint Surg Am. 1969;51:1489-1530.
91 Pritz MB, Chandler WF, Kindt GW. Vertebral artery disease: radiological evaluation medical management, and microsurgical treatment. Neurosurgery. 1981;9:524-530.
92 Koskas F, Kieffer E, Rancurel G, et al. Direct transposition of the distal cervical vertebral artery into the internal carotid artery. Ann Vasc Surg. 1995;9:515-524.
93 Clark K, Perry MO. Carotid vertebral anastomosis: an alternative technic for repair of the subclavian steal syndrome. Ann Surg. 1966;163:414-416.
94 Carney AL, Anderson EM. Carotid distal vertebral bypass for carotid occlusion: case report and technique. Clin Electroencephalogr. 1978;9:105-109.
95 Kakino S, Ogasawara K, Kubo Y, et al. Symptomatic occlusion at the origin of the vertebral artery treated using external carotid artery-cervical vertebral artery bypass with interposed saphenous vein graft. Surg Neurol. 2008;69:164-168.