11 Extraaxial Brain Tumors
Introduction
Extraaxial brain tumors (EBTs) include an array of tumors that arise from structures and tissues directly adjacent to the brain, including the meninges, nerve sheaths, and the pituitary gland, which give rise to meningiomas, schwannomas, and pituitary adenomas, respectively. As a group, they account for over 50% of all brain tumors diagnosed in the United States and, therefore, a large proportion of brain tumors seen by neurologists and neurosurgeons.1 In fact, meningiomas (which account for 32.1% of all brain tumors) are the most common brain tumor diagnosed in patients greater than 34 years of age and pituitary adenomas (8.4% of all brain tumors) are the most common brain tumor diagnosed in patients between 20 and 34 years of age.1 While these tumors are usually benign, they can be associated with significant morbidity and, rarely, mortality, because of their prevalence and occasionally malignant behavior. In this chapter, we discuss the current management of extraaxial brain tumors, with particular emphasis on the three major types of EBTs, including meningiomas, schwanommas, and pituitary adenomas. General principles of diagnosis, management, and treatment can be extrapolated to other EBTs, such as craniopharygiomas and chordomas. We consider recent advances in the diagnosis and treatment of these tumors that have either impacted or may prospectively impact the management of patients.
Incidence and epidemiology
EBTs account for three out of the four most common primary brain tumors diagnosed in the United States. According to the Central Brain Tumor Registry of the United States (CBTRUS), the incidence of EBTs is 8.3 cases per 100,000 person-years.1 Meningiomas, with an incidence of 5.3 per 100,000 person-years, are the single most common brain tumor histology diagnosed in the United States, occurring in nearly twice as many patients as high-grade gliomas. The incidence of meningiomas is 2.2 times greater in females than males. Despite their classical presentation in middle-aged women, the incidence steadily increases with age (i.e., greatest incidence in the 85+ age group). While there is no reported racial predilection in the United States, there is an increased incidence in the Polynesian population, who more frequently have multiple and larger tumors than other populations.2
Nerve sheath tumors and pituitary tumors are the third and fourth most common brain tumors after meningiomas and glioblastoma (accounting for 9.0% and 8.4% of brain tumors, respectively). Although these tumors are the most common brain tumors diagnosed in patients 20 to 34 years of age, their incidence actually peaks in the 65 to 74-year-old age group. Neither tumor has different gender-specific incidence rates, but pituitary tumors are significantly more likely to be diagnosed in blacks, whereas vestibular schwannomas are significantly more likely to be identified in whites.1
Like gliomas, the incidence of EBTs has increased steadily over the last decade, presumably due to an increased rate of diagnosis rather than a true increase in incidence.3 The increased availability and use of neuroimaging modalities have significantly increased the detection of incidental intracranial pathologies, including meningiomas and vestibular schwannomas.4 The increased incidence is also partially attributed to an increased willingness of the medical community to pursue a diagnosis and treatment in older patients.5
Many risk factors have been suggested for EBTs. Of these, radiation exposure is the only universally accepted factor placing people at risk for meningioma induction.6 This concept gained wide acceptance after Modan and colleagues retrospectively discovered a four-fold increase in the incidence of meningiomas among children treated with the Kienbock-Adamson protocol for tinea capitis, a low dose radiation treatment targeting the scalp.7 Low-dose radiation-induced meningiomas are those associated with exposure to less than 10 Gy, but meningiomas have been induced by as little as 1 to 2 Gy. Higher radiation doses are associated with a decrease in latency of meningioma induction.8
Other risk factors for meningiomas have also been explored. One of the early postulates was that head injury caused meningiomas. Associations between head trauma (especially in young males 10 to 19 years of age) and meningiomas have been reported, with a latency of 15 to 24 years.9,10 The evidence supporting this hypothesis is inconsistent and the seemingly conflicting data leaves no definitive proof of a causal relationship between head injury and the subsequent development of meningiomas. The female predilection of meningiomas suggests that female sex hormones may also be a risk factor for tumorigenesis. This theory was bolstered by the identification of estrogen and progesterone receptors on subsets of meningiomas. Tumors expressing progesterone receptors (PR+) behave in a more benign clinical fashion and are less likely to recur. Those expressing estrogen receptors or lacking progesterone receptors display more frequent genotypic alterations and karyotype abnormalities consistent with more aggressive meningiomas.11 Despite these findings, the evidence that exogenous hormones affect tumor frequency is mixed. Blitshteyn and colleagues retrospectively reviewed records from 355,318 women at the Mayo Clinic between 1993 and 2003 and found that women with either current or past hormone replacement therapy (HRT) had a 2.2-fold risk of developing meningiomas.12 A much smaller population-based case-control study failed to find such an association.13 The fact that receptor status affects gene expression profiles, particularly of those near the NF2 gene locus, which has been implicated in meningioma tumorigenesis, suggests that further examination into the effect of female sex hormones may be warranted.14 Increased body mass index (BMI) has also been associated with a greater incidence of meningiomas.15,16 The association between increased BMI and meningioma incidence may be mediated by aromatase in adipose tissue which increases circulating estrogen levels.
Recently, many studies have studied the impact of mobile phone use on tumor incidence. Hardell and colleagues performed a meta-analysis of such studies and concluded that there is a slight statistically significant increased risk for development of glioma (odds ratio [OR] = 2.0, 95% confidence interval [CI] = 1.2 to 3.4) and vestibular schwanomma (OR = 2.4, 95% CI = 1.1 to 5.3), but not meningiomas, using a greater than or equal to 10 years latency period.17
Presentation
The precise symptomatology with which a tumor presents depends on its location and size. The most common locations of meningiomas in descending order of frequency are: convexity, parasagittal, sphenoid and middle cranial fossa, frontal base and posterior fossa, cerebellar convexity, cerebellopontine angle, intraventricular, and clivus. Schwannomas arise most commonly from the vestibular component of the vestibulocochlear nerve (>90%), sensory division of the trigeminal nerve (1% to 10%), facial nerve (1%), nerves of the jugular foramen (glossopharyngeal, vagus, and spinal accessory nerve), hypoglossal nerve, extraocular nerves, and the olfactory nerve. The various syndromes with which EBTs present are outlined in Tables 11-1 through 11-3.
TABLE 11-1 Meningioma Location and Associated Typical Clinical Presentations
| Parasagittal and falcine meningiomas | Anterior 1/3 | Headache and mental status changes |
| Middle 1/3 | Jacksonian seizures and progressive hemiparesis | |
| Posterior 1/3 | ||
| Headache, visual symptoms, seizures, or mental status changes | ||
| Sphenoid wing meningiomas | Lateral / pterional | Similar to convexity tumors |
| Middle 1/3 (alar) | Hemiparesis / dysphasia | |
| Medial (clinoidal) | Visual acuity/field disturbance due to optic nerve compression, proptosis, cranial nerve dysfunction (III,IV,V,VI) | |
| Olfactory groove | Foster-Kennedy syndrome (anosmia, ipsilateral optic atrophy with contralateral papilledema), frontal lobe syndromes / mental status changes, urinary incontinence, seizure | |
| Tuberculum sella / suprasellar | Visual acuity/field disturbance, anosmia, hydrocephalus, endocrinologic syndromes | |
| Cavernous sinus | Cranial nerve deficits (III,IV,V,VI) | |
| Cerebellopontine angle | Hearing loss, facial pain / numbness / weakness / spasm, headaches, cerebellar signs | |
| Foramen magnum | Unilateral cervical pain, extremity motor and sensory loss (clockwise involvement), cold and clumsy hands with intrinsic hand atrophy | |
| Petroclival | Hearing loss, vertigo, tinnitus, facial pain, diplopia, cranial nerve deficits (V,VI,VII,VII) |
TABLE 11-2 Intracranial Schwannomas: Typical Clinical Presentation
| Vestibular | Unilateral sensory hearing loss, tinnitus, disequilibrium |
| Trigeminal | Trigeminal nerve dysfunction (numbness, pain), headache, diplopia, hearing loss/tinnitus |
| Facial | Hearing loss, facial paralysis (may be acute), facial pain, hemifacial spasm, tinnitus, vertigo |
| Jugular foramen | Cranial nerve palsies (IX,X,XI) |
| Accessory nerve | Chronic neck and shoulder pain, muscle spasms |
| Hypoglossal | Headache, cranial nerve dysfunction (IX,X,XI), limb weakness |
TABLE 11-3 Pituitary Adenomas: Typical Clinical Presentation
| Nonfunctioning adenoma | Headache, bitemporal hemianopia, diplopia, dysmenorrhea, fatigue |
| Prolactinoma | Amenorrhea, lactation, impotence |
| Cushing disease | Weight gain, hypertension, diabetes mellitus, moon facies, supraclavicular fat pads, central obesity, facial plethora, abdominal striae, muscle weakness, osteoporosis |
| Acromegaly | Increasing hand/foot size, prominent brow/jaw, diabetes mellitus, carpal tunnel syndrome |
| TSH-secreting adenoma | Signs and symptoms consistent with hyperthyroidism |
Diagnosis
MENINGIOMAS
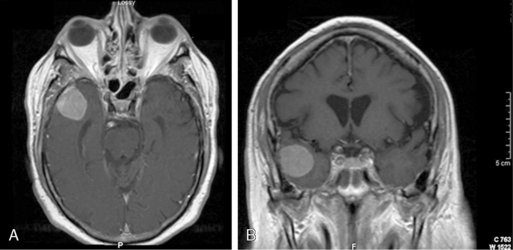
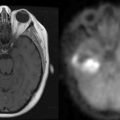
The classic radiographic features of meningiomas include presence of a broad dural base, dural tails, diffuse contrast enhancement, and the presence of an arachnoid plane.18 These criteria can help distinguish between a CP angle meningioma and a vestibular schwannoma (Figure 11-1). Using these classic criteria, MRI has a sensitivity and specificity of 98% and 97%, respectively. However, the sensitivity and positive-predictive value of conventional MRI drops significantly in high-grade (i.e., atypical and malignant) meningiomas.19 MR spectroscopy, which displays distinct peaks for various intratumoral metabolites including choline, creatine, N-acetyl-aspartate (NAA), and lactate, can more definitively identify meningiomas by identifying an alanine peak (unique to meningiomas) and an increased glutamate/creatine ratio.20 Moreover, atypical meningiomas (WHO grade II) are much more likely to have a lactate peak (a marker of proliferation) than WHO grade I tumors.21
SCHWANNOMAS
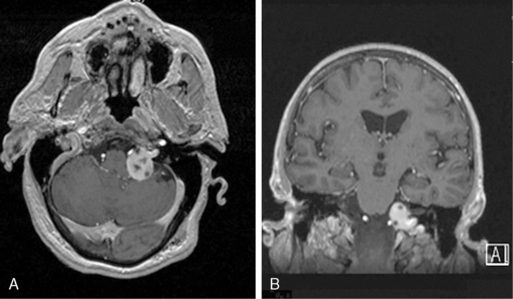
The imaging evaluation of schwannomas includes conventional MR sequences with contrast. Like meningiomas, schwanommas are extraaxial lesions that enhance diffusely. In contrast, schwannomas generally do not have dural tails but rather follow the course of cranial nerves along the skull base, such as into the internal auditory meatus and Meckel’s cave (Figure 11-2). Besides imaging appearance, brainstem auditory evoked responses (BAERs) are a critical diagnostic test for management planning. While for large tumors with brainstem compression it is nearly impossible to preserve hearing, with smaller tumors there is a great interest to preserve functional hearing.
PITUITARY TUMORS
Pituitary tumors are the most common tumor of the sellar region, accounting for over 90% of such lesions. Unlike other EBTs, because of the hypervascularity of the pituitary gland, microadenomas (i.e., pituitary adenomas < 10mm) are often identified by their lack of contrast enhancement while the remainder of the gland enhances briskly (Figure 11-3). Still, in some cases, especially in Cushing disease, an adenoma cannot be identified on MRI despite biochemical evidence of an ACTH-secreting pituitary adenoma. Dynamic pituitary MRI, which uses multiple sequential image acquisition following gadolinium intravenous contrast, significantly increases adenoma detection to nearly 100%, a rate much higher than the 50% to 60% rate reported for nondynamic MRI.22
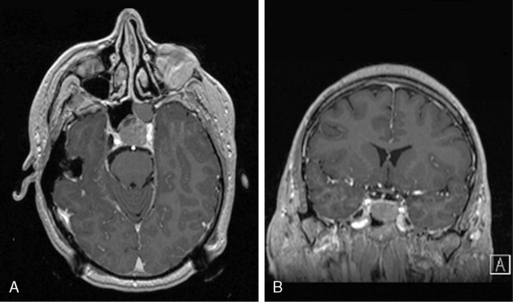
Figure 11-3 (A) and coronal (B) T1-weighted postcontrast MRIs of a patient with a nonfunctioning pituitary adenoma.
In addition to imaging, all patients with sellar or suprasellar lesions need thorough biochemical evaluation of their hypothalamic-pituitary axis, which can provide insight into the nature of an otherwise seemingly nonfunctional pituitary tumor. In addition to biochemical evaluation of circulating hormone levels in the peripheral blood, inferior petrosal sinus sampling (IPSS) has become an integral part of the evaluation of patients with suspected Cushing disease. Comparing central-to-peripheral ratios of ACTH levels at baseline and in response to corticotrophin releasing hormone (CRH) (≥ 2.0 and ≥ 3.0, respectively) is very sensitive for confirming a “central” etiology for Cushing syndrome, or Cushing disease.23
Management
OBSERVATION
Expectant management potentially provides patients with an overall improved quality of life (QOL) for the duration of the disease by not exposing them to the morbidity associated with other treatment paradigms.24 The morbidity of surgery can be quite significant; in one study, among patients older than age 70 years who underwent operation for asymptomatic meningioma, the neurological morbidity rate was 23.3%.25 Expectant management is therefore particularly pertinent to older patients with incidentally discovered and asymptomatic tumors.
The majority of incidental meningiomas show minimal growth. This is particularly true for heavily calcified meninigiomas. Thus, they may be observed without surgical intervention unless specific symptoms appear. Tumor growth is associated with patient age, with tumors in younger patients having a shorter doubling time than in older patients. While radiological features, such as calcification or T2 signal hypointensity or isointensity, may predict decreased growth potential, initial tumor size does not correlate with growth rate.26 Despite minimal growth in most tumors, asymptomatic tumors must be followed: in one study of 40 patients with incidental meningiomas, 33% of tumors grew during a mean follow-up of 32 months, and 36% of patients had symptomatic progression.27
Like incidental meningiomas, there is a place for expectant management of schwannomas. Small and medium-sized vestibular schwannomas that are found incidentally (accounting for approximately 10% of all vestibular schwannomas and found in up to 0.02% of the general population) may have a more benign nature and be less likely to require intervention.28,29 In one series, conservatively managed schwannomas either did not grow or regressed in 42% of patients and had an overall average growth rate of 0.91 mm per year.30 Conservative management can be successful in up to 85% of patients selected for expectant management.31 First-year growth rate is an good predictor of future growth, and therefore must be monitored, if tumors are to be managed conservatively, to determine whether intervention will be necessary.32 The indications for intervention should be based on a combination of rapid tumor growth with the development of symptoms.30 In patients older than 65 years with vestibular schwannomas, these tumors may not require surgical intervention.
Incidental pituitary tumors, or pituitary “incidentalomas,” can also be managed without surgical intervention. Patients with pituitary incidentalomas usually follow a benign course for at least 6 years, not requiring neurosurgical intervention as long as clinical observation is continued.33 Generally, only those that are greater than 10 mm (macroadenomas) enlarge or cause complications and may require closer clinical observation or upfront surgery. Regardless of size, patients who are treated conservatively should undergo biochemical assessment and ophthalmological examination, since occult endocrine dysfunction or visual field defects may be present at the time a pituitary incidentaloma is detected.33–35
MICROSURGICAL RESECTION
Meningiomas
The importance of complete surgical excision for meningiomas has been well documented for over 50 years. In 1957, Simpson retrospectively reviewed the postoperative course of 265 patients with meningiomas, 55 of whom experienced recurrences (21%). Patients with a gross total resection of tumor, dural attachments, and abnormal bone (grade I excision) had a recurrence rate of 9%, those with gross total excision with coagulation of dural attachments (grade II) had a recurrence rate of 19%, those with gross total excision without coagulation of dural attachments (grade III) had a recurrent rate of 29%, and those with partial resection (grade IV) had a recurrence rate of 44%.36 These recurrence rates most likely are underestimates since this study was conducted in the pre-CT and pre-MRI era. Nonetheless, these findings highlight the fact that surgical excision is the most important factor in the prevention of recurrence. Tumors that cannot be totally excised because of their adjacency to critical structures such as cranial nerves and sinuses (e.g., medial sphenoid wing, petroclival, clinoidal, and tentorial-based tumors, and posterior parasagittal lesions, respectively) therefore are at highest risk for tumor recurrence. The highest recurrence rates are found for patients with sphenoid wing meningiomas(>20%), parasagittal meningiomas (8% to 24%), and suprasellar meningiomas (5% to 10%). In contrast, convexity meningiomas, which are relatively easily excisable, have reported recurrence-free rates at 5,10, and 15 years of 93%, 80%, and 68%, respectively.37 Other risk factors for recurrence include histopathologic findings of increased mitosis/Ki-67 labeling index, focal necrosis, nuclear pleomorphism, prominent nucleoli, syncytial tumors, the presence of brain invasion, and loss of 1p36.1-p34.38,39 Interestingly, “high-risk tumors” occur more frequently at the brain surface than at the cranial base, suggesting that the tendency of cranial base meningiomas to recur depends on surgical rather than biological factors.40
The morbidity of microsurgical excision, like extent of resection, is intimately related to tumor location. Easily accessible tumors, such as convexity, lateral and middle third sphenoid wing meningiomas, and anterior third parasagittal and falcine meningiomas, are amenable to complete resection and associated with low morbidity (10% of patients with neurologic sequelae) and mortality (0 to 3%). Neurologic sequelae associated with these resections typically manifest secondary to compromise of adjacent cerebrovascular structures, immediate postoperative edema, and epilepsy. Tumors of the skull base, tentorium, foramen magnum, and other difficult locations are associated with significantly higher morbidity and mortality due to associations with cranial nerves and proximal cerebral vessels. Permanent neurologic deficit ascribed to cranial nerve dysfunction has been reported in a wide range (18% to 86%).41 The highest of these complication rates are typically associated with petroclival and cavernous sinus meningiomas, especially in cases where a complete resection is performed. Preoperative embolization has led to decreased morbidity in patients in whom the tumor blood supply may be difficult to access at the time of surgery.
Despite improvements in microsurgical techniques, image guidance and perioperative critical/medical care, mortality rates in large series remain at 1% to 14%.42,43 Factors increasing mortality include poor preoperative clinical condition, compressive symptoms from tumor, older age, incomplete tumor removal, pulmonary embolism, and intracranial hemorrhage.44
Vestibular Schwannomas
The introduction of the operating microscope, more sensitive diagnostic imaging, and intraoperative facial and cochlear monitoring have steadily decreased the morbidity and mortality associated with resection of vestibular schwannomas. In a meta-analysis of 16 studies including 5005 patients undergoing microsurgery for sporadic unilateral vestibular schwannomas, tumor resection was complete in 96% of cases, with a mortality rate of 0.63%. The most common nonneurologic complication was cerebrospinal fluid leak, which occurred in 6.0% of patients.45 The challenge of surgical resection lies in preserving facial nerve and auditory function. Detailed evaluation of individual large series shows preservation of facial nerve function is inversely proportional to tumor size. Indeed, when evaluating facial nerve preservation after resection of intracanalicular lesions alone, multiple studies have reported 100% postoperative grade I House-Brackmann function.46,47 Resection of small tumors (<2.0 cm), medium sized tumors (2.0 to 3.9 cm), and large tumors (>4.0 cm) is associated with a 95%−97%, 61%−73%, and 28%−57% preservation of grade I or II House-Brackmann function, respectively.48–50 The suboccipital and translabyrinthine approaches afford comparable and excellent results as compared to the middle fossa approach in which increased manipulation of the superiorly located facial nerve in the internal auditory canal may account for a higher risk to facial nerve function.
The importance of preserving serviceable ipsilateral hearing is also paramount. Risk to serviceable auditory function is directly related to tumor size and operative approach. Functional ipsilateral hearing is retained in 29% to 60% of cases, primarily among tumors less than 3 cm in size, with a precipitous decline in hearing preservation rates in larger tumors.47,51,52 Resection of purely intracanalicular tumors is associated with a 57% to 82% preservation of ipsilateral serviceable auditory function.46,47 The translabyrinthine approach, on the other hand, with its destruction of the otic capsule, is not compatible with hearing preservation but is often necessary to approach large CP angle tumors that otherwise would require excessive retraction and manipulation of the cerebellum.
Pituitary adenomas
Surgical decompression remains the treatment of choice for nonfunctioning pituitary adenomas as well as for symptomatic craniopharyngiomas and Rathke cleft cysts. With the exception of craniopharyngiomas, the most common surgical approach to these tumors is a transsphenoidal procedure. Transsphenoidal surgery yields low morbidity and mortality rates, and leads to improvement in visual symptoms in 87% to 90% of cases.53 The recent development of the endoscopic transsphenoidal approach to the pituitary region offers potential advantages over traditional surgical approaches because of its minimal invasiveness and panoramic visualization. The wider operating field of vision and angled views increase the likelihood of a more thorough and safer tumor removal and preservation of normal gland. Despite its advantages, the endoscopic approach does not allow for three-dimensional visualization and requires the surgeon to operate at an increased working distance. Moreover, the improved visualization requires a larger sellar opening, which makes it more difficult to repair CSF leaks when they occur. For suprasellar tumors that are difficult to resect transsphenoidally, a variety of transcranial approaches (pterional, subfrontal, anterior interhemispheric, and transcallosal) allow adequate visualization and decompression of the optic nerves and chiasm. Variations of the pterional craniotomy include resection of the orbital rim and zygoma to provide a more basal view and better access to suprasellar tumors.
Apart from prolactinomas, surgical resection remains the primary treatment for functional pituitary adenomas. Surgical excision is successful in the majority of patients, with long-term remission rates for Cushing disease ranging from 50% to 98%, for acromegaly ranging from 50% to 85%, and for TSH adenomas ranging from 80% to 91%.54–57 Although pharmacological therapy with a dopamine agonist is the primary and most efficacious treatment for prolactinomas, 10% to 20% of patients fail medical therapy, either because they are intolerant of the drugs (e.g., nausea, headache, fatigue, orthostatic hypotension, and depression) or because their tumors are refractory to pharmacological therapy (despite increasing doses).58,59 In these cases, transsphenoidal surgery can obtain remission in up to 91% of patients with microprolactinomas.60
Following surgery, new endocrine deficits have been reported in up to 40% of patients.61 Immediate postoperative polyuria (diabetes insipidus) may occur in up to 30% of patients, but the majority of cases resolve within the first week following surgery. Delayed hyponatremia, occurring most often 7 to 10 days after surgery, is evident in 1% to 9% of patients.62 Worsening of preoperative visual function is seen in 1% to 4% of patients. Anatomic complications include nasal septal perforations (7%) and chronic sinusitis. Postoperative cerebrospinal fluid leaks and meningitis occur in 0.5% to 3.9% of cases.61,63 Adrenal insufficiency often follows surgery, and patients may require steroid replacement therapy for 6 to 12 months postoperatively.61
In 10% to 20% of pituitary adenoma patients, tumors recur within 10 years following surgical intervention.18,64 Subtotal resection and cavernous sinus invasion are prognostic of recurrence.18 Patients with functional adenomas need to be followed carefully for recurrence, which can occur more than 10 years after surgery. In acromegalics who have undergone transsphenoidal surgery, up to 8% of patients recur within 10 years, of whom up to 80% again achieve remission with repeat transsphenoidal surgery.65 Recurrence rates for Cushing disease generally range between 5% and 15%, with a median time to recurrence of 33 to 59 months.55,66,67
RADIOSURGERY
Stereotactic radiosurgery (SRS) allows relatively safe treatment of those EBTs for which surgical resection is associated with exceedingly high rates of morbidity and mortality or in which surgical resection fails to achieve remission. SRS, which includes Gamma Knife surgery (GKS), modified linear accelerator-based technologies (LINAC), and the proton beam devices, delivers a high dose of radiation in 1 to 5 sessions to a stereotactically-defined target by converging multiple beams of ionizing radiation. By creating a steep radiation dose fall-off around the target, SRS minimizes damage to surrounding structures. Like fractionated radiotherapy, SRS takes advantage of the natural difference in susceptibility of pathological and normal tissue due to differences in mitotic activity. By delivering the radiation dose in less than or equal to 5 sessions, SRS improves the biological effectiveness of the target dose by 2.5 to 3 times that of the same dose delivered in a fractionated manner. Little is known about the pathophysiological mechanisms of SRS-mediated tumor control at the cellular level. Tumor control is mediated, at least in part, by inducing DNA damage and apoptosis in proliferating cells and altering the microvascular supply of tumors. For example, reduced blood flow has been seen over time in meningiomas after GKS.68–70 SRS does not, however, generally cause tumor necrosis (which requires higher radiation doses than are typically used). The radiobiology of SRS is fundamentally different from that of fractionated radiation therapy, and this difference appears in part to be due to vascular changes following radiosurgery.
Meningiomas
Most groups use a prescription dose of 15 to 20 Gy. In determining dose, one must not only consider tumor dose but also the radiation tolerance of adjacent structures, including cranial nerves and vasculature. Lower doses of 13 or 14 Gy are therefore often used for treating cavernous sinus meningiomas, due to the adjacency of critical structures such as the optic apparatus. Some argue that a lower margin dose of 12 Gy affords the same degree of tumor control with a reduced risk of complications.71 Unacceptably high rates of failure to control tumor growth have been reported with margin doses of 10 Gy or less.72
Outcomes for GKS treatment of skull base meningiomas have been reported extensively. Most reports have a median follow-up of approximately 3 years and report tumor control rates between 91% and 100%, with tumor shrinkage reported in 23% to 73% of treated skull base meningiomas.73–76 Treatment success is associated with tumor volumes less than 10.0 cc, female gender, a high conformality index, and dural tail inclusion in the treatment plan.77 Kondziolka and colleagues additionally note that local tumor progression after radiosurgery is related to a history of prior resection and multiple meningiomas.78
While SRS appears to work very well for typical meningiomas, the results for atypical and malignant meningiomas are less favorable. Harris and colleagues reported 5-year progression-free survival rates of 83% and 72% for atypical and malignant meningiomas, respectively.79 Kreil and colleagues reported less favorable results, reporting 5-year actuarial control rate of 49% in atypical meningiomas and 0% in malignant meningiomas.80 Five-year overall survival rates vary between 59% and 76% in patients with atypical meningiomas and 0% and 59% in patients with malignant meningiomas.79,81,82
In addition to radiographic control, SRS can often improve cranial nerve function after treatment of skull base meningiomas. Pollock and colleagues reported that 12 out of 38 patients who presented with cranial neuropathies associated with cavernous sinus meningiomas had improvement in cranial nerve function on follow-up.83 Roche and colleagues reported similar success in GKS-treated petroclival meningiomas: 13 out of 32 patients treated with GKS for petroclival meningiomas had clinical improvement in cranial nerve dysfunction.75 Kreil and colleagues reported that 96% of patients with skull-based meningiomas treated with GKS had improved or stable neurological status, with improvement noted in a broad range of areas including vision and other cranial nerve functions, hemiparesis, ataxia, vertigo, seizures, and exophthalmus.71
Vestibular schwannomas
Like meningiomas, SRS is used both as a first-line treatment and as a treatment of residual or progressive disease in patients with schwannomas. High rates of tumor control have been reported with a margin dose of 12 Gy, although some suggest a margin dose as low as 10 Gy may be adequate.84 In order to obtain an adequate margin dose and a maximum dose within accepted limits (20 to 25 Gy), only tumors measuring up to 30 mm should be treated with SRS (although treatment of tumors up to 40 mm has been reported).85 Hasegawa and colleagues have reported an 87% to 92% 10-year progression-free survival (PFS).86 Failure of treatment usually occurred within 3 years. Tumor volume thresholds of both 8 ml and 15 ml as well as tumors not compressing the brainstem and deviating the fourth ventricle have been identified as prognostic factors for PFS.86,87 The outcome in terms of postradiosurgical volume reduction in patients who had prior microsurgery is worse than those who were primarily treated with Gamma Knife surgery. This difference is often attributed to increased difficulty with accurate targeting in those who have undergone prior microsurgery. Patients who fail SRS for vestibular schwannomas, either due to tumor enlargement or radiation-related edema, usually require further surgical intervention, including resection or shunting.
As with microsurgery, the safety of SRS must consider hearing preservation and facial nerve palsies. When tumors are treated with a marginal dose of 13 Gy or less, the hearing preservation rate is 58% to 68%, transient facial palsy develops at a rate of 1%, and facial numbness develops at a rate of 2%.86,87 Hearing loss usually occurs within the first 2 years of treatment, but may occur up to 8 years after treatment. The trigeminal nerve is affected in a variety of ways in 33% of patients; a mild hypesthesia is most common. The dose to the brainstem is a more informative predictor of postradiosurgical cranial neuropathy than the length of the nerve that is irradiated; prior resection increases the risk of late cranial neuropathies after radiosurgery.84
Pituitary adenomas
Most series define tumor control as either an unchanged or decreased volume on follow-up radiological imaging studies. A weighted average tumor control rate for all published series detailing such findings and encompassing a total of 1283 patients was 96%, with results of individual studies ranging between 83% and 100%.88
Endocrine outcomes after SRS for pituitary adenomas are reasonable and demonstrate a role for SRS in the management of these tumors. In the largest series of patients treated with GKS for Cushing disease (N=90), endocrine remission was achieved in 54% of patients at an average of 13 months after treatment. Recurrence occurred in 20% of patients after an average of 27 months.89 Remission rates for acromegaly treated with SRS vary between 20% and 96% in studies with at least 10 patients with 2 years of follow-up.88 Results for prolactinomas treated with SRS are just as variable, with remission rates varying between 0% and 84%.88 Some of the variability in outcomes for acromegaly and prolactinomas may be attributable to the use of somatostatin analogues and dopamine agonists, respectively. Three separate reports have found a negative association between endocrine remission and the use of medical therapy at the time of SRS.90–92 These medical therapies for secretory pituitary adenomas likely not only reduce hormone synthesis and secretion but also reduce the metabolism and cell cycling of these tumors, thereby making them less susceptible to radiation effects. Therefore, in preparation for radiosurgery, many centers now recommend a temporary cessation of antisecretory medications in the perioperative time period (starting 2 months before SRS). Stopping antisecretory medication is not without risks, possibly allowing the adenoma to enlarge and thereby increasing the risk of radiosurgery to adjacent structures (e.g., the optic apparatus), necessitating a lower prescription dose, and making effective radiosurgical treatment more difficult.
Complications of SRS
Cranial neuropathies, accounting for approximately 75% of the overall 8.4% rate of complications reported by Pollock and colleagues include, in order of decreasing frequency, trigeminal, abducens, optic, oculomotor, facial, and vestibulocochlear palsies.93 Of all the cranial nerves, the optic and acoustic nerves are the most sensitive to radiation. Recommendations for upper limits of radiation to the optic apparatus range from 8 to 14.1 Gy. The cranial nerves in the cavernous sinus are relatively robust and less susceptible to adverse radiation effects. Neuropathies have not been seen with doses up to 40 Gy. Pollock and Stafford reported that 5 out of 49 patients treated with GKS for cavernous sinus meningiomas had new or worsened trigeminal dysfunction and one patient had new oculomotor palsy; it is unclear whether this is due to radiation effects or tumor progression.83 On the other hand, 12 of 38 patients reported improvements in cranial nerve function. In our series of over 350 pituitary adenomas treated with GKS, eight cases of cranial nerve palsies have been identified.89,92 The cavernous carotid artery is rarely injured by SRS, but many have recommended limiting the dose to the carotid artery, when possible, to <30 Gy.
Symptomatic perilesional edema after GKS occurs in 1% to 25% of patients.71,94 In general, symptoms occur 1 to 6 months after treatment; patients complain of severe headache that can be managed with oral corticosteroids. Edema formation is far less likely in posterior fossa lesions and more likely in parasagittal or superficially-located tumors. Limiting the margin dose to 14 or 18 Gy may reduce the risk of edema.
Symptomatic cyst formation has also been reported after GKS of meningiomas in approximately 1% to 3 % of cases.82,95 All affected patients have undergone prior surgery and some require further surgery for treatment of symptomatic cysts. Postirradiation cysts have been attributed to degenerative and secretory changes, radiation-induced ischemic necrosis, and intratumoral hemorrhage.95
Radiation-induced tumors are a known complication of both fractionated radiotherapy and radiosurgery, although their incidence is greater with the former. Two cases of glioblastoma multiforme have been reported after GKS for meningiomas.96,97 Given the total number of meningiomas treated with GKS, this is an extremely rare event and should not necessarily temper the use of GKS in cases where GKS is indicated. Ironically, patients with radiation therapy-induced meningiomas have been successfully treated with radiosurgery.
RADIATION THERAPY
Meningiomas
Results for XRT and FSRT of meningiomas in patients who are not appropriate candidates for SRS are comparable to those reported for SRS. In one series of 317 patients with a mean tumor volume of 33.6 ml treated with FSRT (using 57.6 Gy in 1.8 Gy fractions), tumor control was achieved in 97% of patients (including patients treated for recurrent disease) with a mean follow-up of 5.7 years.98 Earlier time to progression was associated with larger tumor volumes (>60 ml) and atypical histology. The side effect profile is similar to that reported for SRS. Grade 3 toxicity was reported in only 2.2% of patients (affecting the optic apparatus and trigeminal nerve). Other side effects may include other cranial neuropathies, brainstem injury, encephalomalacia, or radionecrosis. Nonetheless, the current rate and profile of complications represents a substantial improvement over the side effect profile reported in earlier series using less precise conventional radiation therapy.
Vestibular Schwannomas
FSRT for vestibular schwannomas has gained in popularity because it carries the advantages of both conventional radiotherapy and SRS, sparing adjacent normal tissues (brainstem, cerebellum, cranial nerves) while killing tumor cells. Five-year PFS after FSRT (usually 54 Gy in 1.8 Gy fractions) has been reported to be 93% to 98%, on par with that reported for SRS.87,99,100 Large tumor size at the time of treatment is predictive of a need for future neurosurgical intervention.87 After 2.5 years, 25% of patients had objective hearing loss.99,100 Incidentally, neurofibromatosis patients are more susceptible to hearing loss after FSRT for vestibular schwannomas than patients with sporadic tumors. Facial nerve function is preserved in 91% to 97% of patients 2.5 to 3 years after FSRT.87,99
Pituitary adenomas
XRT and FSRT remains an important tool in the armamentarium for treatment of sellar lesions because these tumors often abut the optic apparatus, excluding the use of SRS. Enthusiasm has waned due to a slow rate of hormone normalization and an increased incidence of complications. Like SRS, XRT is used to treat both postoperative residual disease as well as recurrent or progressive disease. Long-term tumor control rates (in a study of 411 patients) have been reported as high as 94% and 88% at 10 and 20 years, respectively, after 45 to 50 Gy in 25 to 30 fractions.101 Recently, differences in 10-year control rates have been reported between nonfunctional and functional adenomas (98% and 73%, respectively, p=0.0083) and depending on whether the cavernous sinus is involved.101,102 Moreover, improved survival is noted in patients treated with stereotactic RT compared to those in whom two-field or three-field techniques were used. Clinical improvement (e.g., of headache and visual disturbance) has been reported in up to 72% of patients, related to tumor shrinkage, with greatest size reduction seen after 3 years.103
In addition to radiographic control, one must also consider the rate of hormone normalization. In one series, hormone hypersecretion improved in 67% of patients after conventional RT (regardless of the type of functional adenoma), with growth hormone-secreting adenomas being the most responsive.103 In Cushing disease, XRT has been reported to result in remission in up to 83% of patients. There is nonetheless a wide range of endocrine remission rates reported following XRT and FSRT for secretory adenomas. Hormone normalization, when it occurs, generally is seen later than with radiosurgery.
The most common side effect or complication of RT, as with SRS, is hypopituitarism, which occurs in approximately 22% to 50% of patients.101,102 Other complications noted include seizures, visual loss, strokes, and induction of glioblastoma 12 years after RT.101,102 These rates of serious complications appear higher than with SRS.
CHEMOTHERAPY
Meningiomas
Hormone receptor antagonists have been explored as possible chemomodulatory agents for the treatment of meningiomas. Trials of mifepristone, a progesterone receptor antagonist, have been ineffective or only partially successful in producing tumor regression.104,105 Likewise, blockage of estrogen receptors with tamoxifen has shown little benefit.106 Despite these failed studies, recent confirmation that receptor status affects gene expression profiles may stimulate new interest in this avenue of chemotherapy for meningiomas.14
Unlike hormone receptor anatagonists, hydroxyurea remains a chemotherapeutic option for the treatment of meningiomas, especially in patients for whom other options (i.e., repeat surgery or who have already been radiated) may not exist. Hydroxyurea can arrest progression of unresectable or recurrent benign meningiomas, with 93% PFS in benign meningiomas.107
Investigations into the genetic and molecular underpinning of these tumors have led the way to many theoretical opportunities for chemotherapeutic intervention. Studies of meningiomas have found a chromosomal aberration on the long arm of chromosome 22 in 50% to 72% of cases, which involved the tumor suppressor NF-2 gene loci in up to 60% of sporadic meningiomas.108 These mutations/deletions are not consistent across all meningiomas, being identified in as few as 26% of meningiomas in one series, underscoring the notion that non-NF2 mechanisms are also important,109,110 and making these genes less attractive targets for chemotherapy. Other targets of interest that are critical in cellular signaling in meningiomas have been identified as potential targets, including epidermal growth factors receptor (EGFR), transforming growth factor alpha (TGF-alpha), ras protein cascades, Ras-Raf-1-MEK-1-MAPK pathway, and PKC pathways.111 Inhibitors of many of these pathways are being explored primarily in glioblastoma models as well as in meningiomas (e.g., imatinib, sunitinib). There has also been considerable interest in the role of cyclooxygenase inhibitors on meningioma proliferation, largely due to the availability of these drugs. Although there is no evidence of COX-2 amplification in meningiomas, COX-2 inhibitors have been shown to inhibit cell growth and induce apoptosis in meningiomas and have been shown to reduce mean tumor growth rate by 66% in mouse xenograft models.112,113 Further investigation into the therapeutic benefits of COX inhibitors may be warranted.
Pituitary adenomas
In the absence of complications necessitating immediate surgery, such as apoplexy, hydrocephalus, or a cerebrospinal fluid leak, pharmacotherapy with dopamine agonists is considered the first-line treatment approach for prolactin-secreting adenomas. Dopamine agonists (bromocriptine, cabergoline) effectively normalize prolactin levels in as many as 89% of patients.114 These medications decrease tumor volume by at least 50% in more than two-thirds of patients within the first several months of therapy, resulting in visual field improvements in all but 10% of patients. Quinagolide and cabergoline, both selective DA receptor subtype-2−selective agonists, have also been effective in reducing prolactin secretion and tumor size in adult patients with prolactinomas, even in those with a previous poor responsive or intolerance to bromocriptine. Cabergoline, with a longer half-life and weekly dosing, is noted for its tolerability and high compliance rates. For women who wish to maintain fertility, bromocriptine and cabergoline appear safe, without a significant increase in birth defects.
Although surgery is the primary treatment for all other pituitary adenomas, recent reports highlight impressive advancements in the pharmacologic treatment of growth hormone-secreting, TSH-secreting, and ACTH-secreting adenomas. Traditionally, the two options for medical therapy of growth hormone-secreting tumors have been dopamine agonists and somatostatin analogues. Dopamine agonists provide symptomatic relief in the majority of patients but normalize IGF-1 levels in only about 20% to 40% of cases. Somatostatin analogues (octreotide, sandostatin-LAR, lanreotide, lanreotide-SR) can normalize IGF-1 levels in up to 60% of patients and have a more favorable side-effect profile compared to dopamine agonists.115 The recently introduced GH receptor antagonist, pegvisomant, has normalized IGF-1 levels in 75% to 85% of patients with refractory disease, although reported experience with its administration for greater than 2 years remains limited.116
Medical therapy for Cushing disease, in those who fail surgical and/or radiation therapy, has largely focused on inhibiting peripheral steroid production with ketoconazole. Ketoconazole, an antifungal agent, normalizes urinary free cortisol in approximately 50% of patients.117 More recently, however, the somatostatin analogue pasireotide and the dopamine agonist cabergoline (and their combination) have shown promise in the medical therapy of Cushing disease. TSH-secreting adenomas can also be responsive to dopaminergic agonists and somatostatin analogues. Cytotoxic chemotherapy has occasionally provided modest benefit in pituitary carcinoma.118
1. CBTRUS. Statistical Report: Primary Brain Tumors in the United States, 2000–2004. Published by the Central Brain Tumor Registry of the United States, 2008.
2. S. Olson, A. Law. Meningiomas and the Polynesian population. ANZ J Surg. 2005;75:705-709.
3. S. Hoffman, J.M. Propp, B.J. McCarthy. Temporal trends in incidence of primary brain tumors in the United States, 1985–1999. Neuro Oncol. 2006;8:27-37.
4. M.W. Vernooij, M.A. Ikram, H.L. Tanghe, A.J. Vincent, A. Hofman, G.P. Krestin, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357:1821-1828.
5. E.B. Claus, P.M. Black. Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973–2001. Cancer. 2006;106:1358-1363.
6. F. Umansky, Y. Shoshan, G. Rosenthal, S. Fraifeld, S. Spektor. Radiation-induced meningioma. Neurosurg Focus. 2008;24:E7.
7. B. Modan, D. Baidatz, H. Mart, R. Steinitz, S.G. Levin. Radiation-induced head and neck tumours. Lancet. 1974;1:277-279.
8. E.E. Mack, C.B. Wilson. Meningiomas induced by high-dose cranial irradiation. J Neurosurg. 1993;79:28-31.
9. L.E. Phillips, T.D. Koepsell, G. van Belle, W.A. Kukull, J.A. Gehrels, W.T. LongstrethJr. History of head trauma and risk of intracranial meningioma: population-based case-control study. Neurology. 2002;58:1849-1852.
10. S. Preston-Martin, J.M. Pogoda, B. Schlehofer, M. Blettner, G.R. Howe, P. Ryan, et al. An international case-control study of adult glioma and meningioma: the role of head trauma. Int J Epidemiol. 1998;27:579-586.
11. S. Pravdenkova, O. Al-Mefty, J. Sawyer, M. Husain. Progesterone and estrogen receptors: opposing prognostic indicators in meningiomas. J Neurosurg. 2006;105:163-173.
12. S. Blitshteyn, J.E. Crook, K.A. Jaeckle. Is there an association between meningioma and hormone replacement therapy? J Clin Oncol. 2008;26:279-282.
13. B. Custer, W.T. LongstrethJr, L.E. Phillips, T.D. Koepsell, G. Van Belle. Hormonal exposures and the risk of intracranial meningioma in women: a population-based case-control study. BMC Cancer. 2006;6:152.
14. E.B. Claus, P.J. Park, R. Carroll, J. Chan, P.M. Black. Specific genes expressed in association with progesterone receptors in meningioma. Cancer Res. 2008;68:314-322.
15. M.K. Aghi, E.N. Eskandar, B.S. Carter, W.T. CurryJr, F.G. Barker2nd. Increased prevalence of obesity and obesity-related postoperative complications in male meningioma patients. Clin Neurosurg. 2007;54:236-240.
16. V.S. Benson, K. Pirie, J. Green, D. Casabonne, V. Beral. Lifestyle factors and primary glioma and meningioma tumours in the Million Women Study cohort. Br J Cancer. 2008;99:185-190.
17. L. Hardell, M. Carlberg, F. Soderqvist, K. Hansson Mild. Meta-analysis of long-term mobile phone use and the association with brain tumours. Int J Oncol. 2008;32:1097-1103.
18. E.F. Chang, G. Zada, S. Kim, K.R. Lamborn, A. Quinones-Hinojosa, J.B. Tyrrell, et al. Long-term recurrence and mortality after surgery and adjuvant radiotherapy for nonfunctional pituitary adenomas. J Neurosurg. 2008;108:736-745.
19. M. Julia-Sape, D. Acosta, C. Majos, A. Moreno-Torres, P. Wesseling, J.J. Acebes, et al. Comparison between neuroimaging classifications and histopathological diagnoses using an international multicenter brain tumor magnetic resonance imaging database. J Neurosurg. 2006;105:6-14.
20. S. Hazany, J.R. Hesselink, J.F. Healy, S.G. Imbesi. Utilization of glutamate/creatine ratios for proton spectroscopic diagnosis of meningiomas. Neuroradiology. 2007;49:121-127.
21. R. Buhl, A. Nabavi, S. Wolff, H.H. Hugo, K. Alfke, O. Jansen, et al. MR spectroscopy in patients with intracranial meningiomas. Neurol Res. 2007;29:43-46.
22. T.C. Friedman, E. Zuckerbraun, M.L. Lee, M.S. Kabil, H. Shahinian. Dynamic pituitary MRI has high sensitivity and specificity for the diagnosis of mild Cushing’s syndrome and should be part of the initial workup. Horm Metab Res. 2007;39:451-456.
23. E.H. Oldfield, J.L. Doppman, L.K. Nieman, G.P. Chrousos, D.L. Miller, D.A. Katz, et al. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing’s syndrome. N Engl J Med. 1991;325:897-905.
24. S. Yano, J. Hamada, Y. Kai, T. Todaka, T. Hara, T. Mizuno, et al. Surgical indications to maintain quality of life in elderly patients with ruptured intracranial aneurysms. Neurosurgery. 2003;52:1010-1015. discussion 1015–1016
25. J. Kuratsu, M. Kochi, Y. Ushio. Incidence and clinical features of asymptomatic meningiomas. J Neurosurg. 2000;92:766-770.
26. M. Nakamura, F. Roser, J. Michel, C. Jacobs, M. Samii. The natural history of incidental meningiomas. Neurosurgery. 2003;53:62-70. discussion; 70–61
27. M. Niiro, K. Yatsushiro, K. Nakamura, Y. Kawahara, J. Kuratsu. Natural history of elderly patients with asymptomatic meningiomas. J Neurol Neurosurg Psychiatry. 2000;68:25-28.
28. A. Jeyakumar, R. Seth, T.M. Brickman, P. Dutcher. The prevalence and clinical course of patients with ‘incidental’ acoustic neuromas. Acta Otolaryngol. 2007;127:1051-1057.
29. D. Lin, J.L. Hegarty, N.J. Fischbein, R.K. Jackler. The prevalence of “incidental” acoustic neuroma. Arch Otolaryngol Head Neck Surg. 2005;131:241-244.
30. S.I. Rosenberg. Natural history of acoustic neuromas. Laryngoscope. 2000;110:497-508.
31. H.G. Deen, M.J. Ebersold, S.G. Harner, C.W. Beatty, M.S. Marion, R.E. Wharen, et al. Conservative management of acoustic neuroma: an outcome study. Neurosurgery. 1996;39:260-264. discussion 264–266
32. D.C. Tschudi, T.E. Linder, U. Fisch. Conservative management of unilateral acoustic neuromas. Am J Otol. 2000;21:722-728.
33. L.E. Donovan, B. Corenblum. The natural history of the pituitary incidentaloma. Arch Intern Med. 1995;155:181-183.
34. J. Feldkamp, R. Santen, E. Harms, A. Aulich, U. Modder, W.A. Scherbaum. Incidentally discovered pituitary lesions: high frequency of macroadenomas and hormone-secreting adenomas—results of a prospective study. Clin Endocrinol (Oxf). 1999;51:109-113.
35. K. Oyama, N. Sanno, S. Tahara, A. Teramoto. Management of pituitary incidentalomas: according to a survey of pituitary incidentalomas in Japan. Semin Ultrasound CT MR. 2005;26:47-50.
36. D. Simpson. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22-39.
37. T. Morimura, J. Takeuchi, Y. Maeda, E. Tani. Preoperative embolization of meningiomas: its efficacy and histopathological findings. Noshuyo Byori. 1994;11:123-129.
38. Y.J. Kim, R. Ketter, W. Henn, K.D. Zang, W.I. Steudel, W. Feiden. Histopathologic indicators of recurrence in meningiomas: correlation with clinical and genetic parameters. Virchows Arch. 2006;449:529-538.
39. D.K. Boker, H. Meurer, F. Gullotta. Recurring intracranial meningiomas. Evaluation of some factors predisposing for tumor recurrence. J Neurosurg Sci. 1985;29:11-17.
40. R. Ketter, J. Rahnenfuhrer, W. Henn, Y.J. Kim, W. Feiden, W.I. Steudel, et al. Correspondence of tumor localization with tumor recurrence and cytogenetic progression in meningiomas. Neurosurgery. 2008;62:61-69. discussion 69–70
41. F. DeMonte, H.K. Smith, O. al-Mefty. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg. 1994;81:245-251.
42. M. Kallio, R. Sankila, T. Hakulinen, J. Jaaskelainen. Factors affecting operative and excess long-term mortality in 935 patients with intracranial meningioma. Neurosurgery. 1992;31:2-12.
43. B. Pertuiset, S. Farah, L. Clayes, J. Goutorbe, J. Metzger, M. Kujas. Operability of intracranial meningiomas. Personal series of 353 cases. Acta Neurochir (Wien). 1985;76:2-11.
44. J. Jaaskelainen. Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol. 1986;26:461-469.
45. I. Yamakami, Y. Uchino, E. Kobayashi, A. Yamaura. Conservative management, gamma-knife radiosurgery, and microsurgery for acoustic neurinomas: a systematic review of outcome and risk of three therapeutic options. Neurol Res. 2003;25:682-690.
46. S.J. Haines, S.C. Levine. Intracanalicular acoustic neuroma: early surgery for preservation of hearing. J Neurosurg. 1993;79:515-520.
47. M. Samii, C. Matthies, M. Tatagiba. Intracanalicular acoustic neurinomas. Neurosurgery. 1991;29:189-198. discussion 198–189
48. M.J. Ebersold, S.G. Harner, C.W. Beatty, C.M. HarperJr, L.M. Quast. Current results of the retrosigmoid approach to acoustic neurinoma. J Neurosurg. 1992;76:901-909.
49. R.G. Ojemann. Management of acoustic neuromas (vestibular schwannomas) (honored guest presentation). Clin Neurosurg. 1993;40:498-535.
50. L.N. Sekhar, W.B. Gormley, D.C. Wright. The best treatment for vestibular schwannoma (acoustic neuroma): microsurgery or radiosurgery? Am J Otol. 1996;17:676-682. discussion 683–679
51. J.B. NadolJr, C.M. Chiong, R.G. Ojemann, M.J. McKenna, R.L. Martuza, W.W. Montgomery, et al. Preservation of hearing and facial nerve function in resection of acoustic neuroma. Laryngoscope. 1992;102:1153-1158.
52. D.L. Baldwin, T.T. King, A.W. Morrison. Hearing conservation in acoustic neuroma surgery via the posterior fossa. J Laryngol Otol. 1990;104:463-467.
53. J.A. JaneJr, E.R. LawsJr. The surgical management of pituitary adenomas in a series of 3,093 patients. J Am Coll Surg. 2001;193:651-659.
54. M. Losa, P. Mortini, A. Franzin, R. Barzaghi, C. Mandelli, M. Giovanelli. Surgical management of thyrotropin-secreting pituitary adenomas. Pituitary. 1999;2:127-131.
55. G.D. Hammer, J.B. Tyrrell, K.R. Lamborn, C.B. Applebury, E.T. Hannegan, S. Bell, et al. Transsphenoidal microsurgery for Cushing’s disease: initial outcome and long-term results. J Clin Endocrinol Metab. 2004;89:6348-6357.
56. N. Sonino, M. Zielezny, G.A. Fava, F. Fallo, M. Boscaro. Risk factors and long-term outcome in pituitary-dependent Cushing’s disease. J Clin Endocrinol Metab. 1996;81:2647-2652.
57. E.R. Laws. Surgery for acromegaly: evolution of the techniques and outcomes. Rev Endocr Metab Disord. 2008;9:67-70.
58. A. Colao, G. Vitale, P. Cappabianca, F. Briganti, A. Ciccarelli, M. De Rosa, et al. Outcome of cabergoline treatment in men with prolactinoma: effects of a 24-month treatment on prolactin levels, tumor mass, recovery of pituitary function, and semen analysis. J Clin Endocrinol Metab. 2004;89:1704-1711.
59. J. Verhelst, R. Abs, D. Maiter, A. van den Bruel, M. Vandeweghe, B. Velkeniers, et al. Cabergoline in the treatment of hyperprolactinemia: a study in 455 patients. J Clin Endocrinol Metab. 1999;84:2518-2522.
60. J. Kreutzer, R. Buslei, H. Wallaschofski, B. Hofmann, C. Nimsky, R. Fahlbusch, et al. Operative treatment of prolactinomas: indications and results in a current consecutive series of 212 patients. Eur J Endocrinol. 2008;158:11-18.
61. I. Ciric, A. Ragin, C. Baumgartner, D. Pierce. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40:225-236. discussion 236–227
62. D.F. Kelly, E.R. LawsJr, D. Fossett. Delayed hyponatremia after transsphenoidal surgery for pituitary adenoma. Report of nine cases. J Neurosurg. 1995;83:363-367.
63. J.R. Davis, W.E. Farrell, R.N. Clayton. Pituitary tumours. Reproduction. 2001;121:363-371.
64. E.R. LawsJr, N.C. Fode, M.J. Redmond. Transsphenoidal surgery following unsuccessful prior therapy. An assessment of benefits and risks in 158 patients. J Neurosurg. 1985;63:823-829.
65. M. Kurosaki, D.K. Luedecke, T. Abe. Effectiveness of secondary transnasal surgery in GH-secreting pituitary macroadenomas. Endocr J. 2003;50:635-642.
66. D. Bochicchio, M. Losa, M. Buchfelder. Factors influencing the immediate and late outcome of Cushing’s disease treated by transsphenoidal surgery: a retrospective study by the European Cushing’s Disease Survey Group. J Clin Endocrinol Metab. 1995;80:3114-3120.
67. A.L. Utz, B. Swearingen, B.M. Biller. Pituitary surgery and postoperative management in Cushing’s disease. Endocrinol Metab Clin North Am. 2005;34:459-478. xi
68. M. Marekova, J. Vavrova, D. Vokurkova, J. Psutka. Modulation of ionizing radiation-induced apoptosis and cell cycle arrest by all-trans retinoic acid in promyelocytic leukemia cells (HL-60). Physiol Res. 2003;52:599-606.
69. T. Tsuzuki, S. Tsunoda, T. Sakaki, N. Konishi, Y. Hiasa, M. Nakamura, et al. Tumor cell proliferation and apoptosis associated with the Gamma Knife effect. Stereotact Funct Neurosurg. 1996;66(Suppl. 1):39-48.
70. H. Hawighorst, R. Engenhart, M.V. Knopp, G. Brix, M. Grandy, M. Essig, et al. Intracranial meningeomas: time- and dose-dependent effects of irradiation on tumor microcirculation monitored by dynamic MR imaging. Magn Reson Imaging. 1997;15:423-432.
71. W. Kreil, J. Luggin, I. Fuchs, V. Weigl, S. Eustacchio, G. Papaefthymiou. Long term experience of gamma knife radiosurgery for benign skull base meningiomas. J Neurol Neurosurg Psychiatry. 2005;76:1425-1430.
72. J.C. Ganz, E.O. Backlund, F.A. Thorsen. The results of Gamma Knife surgery of meningiomas, related to size of tumor and dose. Stereotact Funct Neurosurg. 1993;61(Suppl. 1):23-29.
73. G. Pendl, O. Schrottner, S. Eustacchio, K. Feichtinger, J. Ganz. Stereotactic radiosurgery of skull base meningiomas. Minim Invasive Neurosurg. 1997;40:87-90.
74. B.R. Subach, L.D. Lunsford, D. Kondziolka, A.H. Maitz, J.C. Flickinger. Management of petroclival meningiomas by stereotactic radiosurgery. Neurosurgery. 1998;42:437-443. discussion 443–435
75. P.H. Roche, W. Pellet, S. Fuentes, J.M. Thomassin, J. Regis. Gamma knife radiosurgical management of petroclival meningiomas results and indications. Acta Neurochir (Wien). 2003;145:883-888. discussion 888
76. R. Liscak, A. Kollova, V. Vladyka, G. Simonova, J. NovotnyJr. Gamma knife radiosurgery of skull base meningiomas. Acta Neurochir Suppl. 2004;91:65-74.
77. S.J. DiBiase, Y. Kwok, S. Yovino, C. Arena, S. Naqvi, R. Temple, et al. Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys. 2004;60:1515-1519.
78. D. Kondziolka, E.I. Levy, A. Niranjan, J.C. Flickinger, L.D. Lunsford. Long-term outcomes after meningioma radiosurgery: physician and patient perspectives. J Neurosurg. 1999;91:44-50.
79. A.E. Harris, J.Y. Lee, B. Omalu, J.C. Flickinger, D. Kondziolka, L.D. Lunsford. The effect of radiosurgery during management of aggressive meningiomas. Surg Neurol. 2003;60:298-305. discussion 305
80. I. Malik, J.G. Rowe, L. Walton, M.W. Radatz, A.A. Kemeny. The use of stereotactic radiosurgery in the management of meningiomas. Br J Neurosurg. 2005;19:13-20.
81. S.G. Ojemann, P.K. Sneed, D.A. Larson, P.H. Gutin, M.S. Berger, L. Verhey, et al. Radiosurgery for malignant meningioma: results in 22 patients. J Neurosurg. 2000;93(Suppl. 3):62-67.
82. S.L. Stafford, B.E. Pollock, R.L. Foote, M.J. Link, D.A. Gorman, P.J. Schomberg, et al. Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery. 2001;49:1029-1037. discussion 1037–1028
83. B.E. Pollock, S.L. Stafford. Results of stereotactic radiosurgery for patients with imaging defined cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys. 2005;62:1427-1431.
84. K.D. Foote, W.A. Friedman, J.M. Buatti, S.L. Meeks, F.J. Bova, P.S. Kubilis. Analysis of risk factors associated with radiosurgery for vestibular schwannoma. J Neurosurg. 2001;95:440-449.
85. H.K. Inoue. Low-dose radiosurgery for large vestibular schwannomas: long-term results of functional preservation. J Neurosurg. 2005;102(Suppl.):111-113.
86. T. Hasegawa, S. Fujitani, S. Katsumata, Y. Kida, M. Yoshimoto, J. Koike. Stereotactic radiosurgery for vestibular schwannomas: analysis of 317 patients followed more than 5 years. Neurosurgery. 2005;57:257-265. discussion 257–265
87. A.W. Chan, P. Black, R.G. Ojemann, F.G. Barker2nd, H.M. Kooy, V.V. Lopes, et al. Stereotactic radiotherapy for vestibular schwannomas: favorable outcome with minimal toxicity. Neurosurgery. 2005;57:60-70. discussion 60–70
88. J.P. Sheehan, J. Jagannathan, N. Pouratian, L. Steiner. Stereotactic radiosurgery for pituitary adenomas: a review of the literature and our experience. Front Horm Res. 2006;34:185-205.
89. J. Jagannathan, J.P. Sheehan, N. Pouratian, E.R. Laws, L. Steiner, M.L. Vance. Gamma Knife surgery for Cushing’s disease. J Neurosurg. 2007;106:980-987.
90. A.M. Landolt, D. Haller, N. Lomax, S. Scheib, O. Schubiger, J. Siegfried, et al. Octreotide may act as a radioprotective agent in acromegaly. J Clin Endocrinol Metab. 2000;85:1287-1289.
91. A.M. Landolt, N. Lomax. Gamma knife radiosurgery for prolactinomas. J Neurosurg. 2000;93(Suppl. 3):14-18.
92. N. Pouratian, J. Sheehan, J. Jagannathan, E.R. LawsJr, L. Steiner, M.L. Vance. Gamma knife radiosurgery for medically and surgically refractory prolactinomas. Neurosurgery. 2006;59:255-266. discussion 255–266
93. B.E. Pollock. Stereotactic radiosurgery for intracranial meningiomas: indications and results. Neurosurg Focus. 2003;14:e4.
94. V.P. Singh, S. Kansai, S. Vaishya, P.K. Julka, V.S. Mehta. Early complications following gamma knife radiosurgery for intracranial meningiomas. J Neurosurg. 2000;93(Suppl. 3):57-61.
95. T. Shuto, S. Inomori, H. Fujino, H. Nagano, N. Hasegawa, Y. Kakuta. Cyst formation following gamma knife surgery for intracranial meningioma. J Neurosurg. 2005;102(Suppl.):134-139.
96. J.S. Loeffler, A. Niemierko, P.H. Chapman. Second tumors after radiosurgery: tip of the iceberg or a bump in the road? Neurosurgery. 2003;52:1436-1440. discussion 1440–1432
97. J.S. Yu, W.H. Yong, D. Wilson, K.L. Black. Glioblastoma induction after radiosurgery for meningioma. Lancet. 2000;356:1576-1577.
98. S. Milker-Zabel, A. Zabel, D. Schulz-Ertner, W. Schlegel, M. Wannenmacher, J. Debus. Fractionated stereotactic radiotherapy in patients with benign or atypical intracranial meningioma: long-term experience and prognostic factors. Int J Radiat Oncol Biol Phys. 2005;61:809-816.
99. G. Horan, G.A. Whitfield, K.E. Burton, N.G. Burnet, S.J. Jefferies. Fractionated conformal radiotherapy in vestibular schwannoma: early results from a single centre. Clin Oncol (R Coll Radiol). 2007;19:517-522.
100. M. Fuss, J. Debus, F. Lohr, P. Huber, B. Rhein, R. Engenhart-Cabillic, et al. Conventionally fractionated stereotactic radiotherapy (FSRT) for acoustic neuromas. Int J Radiat Oncol Biol Phys. 2000;48:1381-1387.
101. M. Brada, B. Rajan, D. Traish, S. Ashley, P.J. Holmes-Sellors, S. Nussey, et al. The long-term efficacy of conservative surgery and radiotherapy in the control of pituitary adenomas. Clin Endocrinol (Oxf). 1993;38:571-578.
102. F.E. Snead, R.J. Amdur, C.G. Morris, W.M. Mendenhall. Long-term outcomes of radiotherapy for pituitary adenomas. Int J Radiat Oncol Biol Phys. 2008;71:994-998.
103. R. Sasaki, M. Murakami, Y. Okamoto, K. Kono, E. Yoden, T. Nakajima, et al. The efficacy of conventional radiation therapy in the management of pituitary adenoma. Int J Radiat Oncol Biol Phys. 2000;47:1337-1345.
104. S.W. Lamberts, H.L. Tanghe, C.J. Avezaat, R. Braakman, R. Wijngaarde, J.W. Koper, et al. Mifepristone (RU 486) treatment of meningiomas. J Neurol Neurosurg Psychiatry. 1992;55:486-490.
105. S.M. Grunberg, M.H. Weiss, I.M. Spitz, J. Ahmadi, A. Sadun, C.A. Russell, et al. Treatment of unresectable meningiomas with the antiprogesterone agent mifepristone. J Neurosurg. 1991;74:861-866.
106. J.W. Goodwin, J. Crowley, H.J. Eyre, B. Stafford, K.A. Jaeckle, J.J. Townsend. A phase II evaluation of tamoxifen in unresectable or refractory meningiomas: a Southwest Oncology Group study. J Neurooncol. 1993;15:75-77.
107. B.M. Hahn, U.M. Schrell, R. Sauer, R. Fahlbusch, O. Ganslandt, G.G. Grabenbauer. Prolonged oral hydroxyurea and concurrent 3d-conformal radiation in patients with progressive or recurrent meningioma: results of a pilot study. J Neurooncol. 2005;74:157-165.
108. J.P. Dumanski, G.A. Rouleau, M. Nordenskjold, V.P. Collins. Molecular genetic analysis of chromosome 22 in 81 cases of meningioma. Cancer Res. 1990;50:5863-5867.
109. K.J. Drummond, J.J. Zhu, P.M. Black. Meningiomas: updating basic science, management, and outcome. Neurologist. 2004;10:113-130.
110. A. Perry, D.X. Cai, B.W. Scheithauer, P.E. Swanson, C.M. Lohse, I.F. Newsham, et al. Merlin, DAL-1, and progesterone receptor expression in clinicopathologic subsets of meningioma: a correlative immunohistochemical study of 175 cases. J Neuropathol Exp Neurol. 2000;59:872-879.
111. M.D. Johnson, B. Sade, M.T. Milano, J.H. Lee, S.A. Toms. New prospects for management and treatment of inoperable and recurrent skull base meningiomas. J Neurooncol. 2008;86:109-122.
112. B.T. Ragel, R.L. Jensen, D.L. Gillespie, S.M. Prescott, W.T. Couldwell. Celecoxib inhibits meningioma tumor growth in a mouse xenograft model. Cancer. 2007;109:588-597.
113. B.T. Ragel, A.L. Asher, N. Selden, J.D. MacDonald. Self-assessment in neurological surgery: the SANS wired white paper. Neurosurgery. 2006;59:759-765. discussion 765–756
114. J. Webster. Dopamine agonist therapy in hyperprolactinemia. J Reprod Med. 1999;44:1105-1110.
115. F. Castinetti, I. Morange, P. Jaquet, B. Conte-Devolx, T. Brue. Ketoconazole revisited: a preoperative or postoperative treatment in Cushing’s disease. Eur J Endocrinol. 2008;158:91-99.
116. K.I. Alexandraki, A.B. Grossman. Pituitary-targeted medical therapy of Cushing’s disease. Expert Opin Investig Drugs. 2008;17:669-677.
117. T. Kienitz, M. Quinkler, C.J. Strasburger, M. Ventz. Long-term management in five cases of TSH-secreting pituitary adenomas: a single center study and review of the literature. Eur J Endocrinol. 2007;157:39-46.
118. M.B. Lopes, B.W. Scheithauer, D. Schiff. Pituitary carcinoma: diagnosis and treatment. Endocrine. 2005;28:115-121.