11 Evolution of Perioperative Echocardiography
Many characteristics define a cardiac anesthesiologist, and these have evolved over time.1 Some of these include intimate knowledge of cardiac diseases and the interaction of anesthesia with them, knowledge of the special techniques used by cardiac surgeons including, initially, pure moderate hypothermia and, subsequently, cardiopulmonary bypass (CPB), deep hypothermic circulatory arrest, cardioplegia, off-pump coronary artery bypass grafting, and minimally invasive and robotic surgery. But one of the cardinal characteristics of cardiac anesthesiologists has been their adoption and application of special monitoring techniques. Early on this involved electrocardiographic (ECG), electroencephalographic, arterial, central venous, and left atrial pressure monitoring, and use of arterial blood gases and tests of coagulation. In the 1970s, use of the pulmonary artery catheter (PAC) was introduced. Since about 1990, expertise with use of echocardiography has become one of the defining characteristics of the accomplished cardiac anesthesiologist. Today, few anesthesia or cardiac surgical groups would accept an anesthesiologist who wishes to provide anesthesia for cardiac cases if they have not been trained in perioperative echocardiography. Residents seeking fellowship training in cardiothoracic anesthesia view the quality of experience and teaching of echocardiography as critical criteria in selecting a fellowship. The authors believe that the introduction of echocardiography into cardiac surgery and anesthesia has contributed to the improved success and safety of cardiac surgery since the early 1990s.
As Feigenbaum2 notes, the word evolution usually is reserved for changes of natural phenomena, but it is appropriate to use it in reference to the development of diagnostic ultrasound (e.g., echocardiography) because this represents an attempt to mimic a natural phenomenon. Some mammals (e.g., bats and aquatic mammals) use “diagnostic ultrasound” to visualize their environments, a phenomenon that was first recognized by Lazzaro Spallanzani (1729–1799). A number of previous articles and sections of other texts have reviewed the history of medical diagnostic ultrasound and echocardiography.2–15 The authors have relied heavily on these prior works and frequently quote from them. What becomes immediately apparent in reviewing this history is that it has been an international effort with contributions from engineers, scientists, and clinicians from many different nations. The knowledge and experience of the authors of this chapter result in a particular emphasis on developments in North America, and they acknowledge that they may have unintentionally overlooked important contributions and events that have occurred elsewhere.
Early developments leading to the medical use of ultrasound
The Roman architect Vitruvius coined the word echo (Table 11-1). The French friar Marin Mersenne (1588–1648) frequently is referred to as the “father of acoustics” because he first measured the velocity of sound, whereas the English physicist Robert Boyle (1627–1691) recognized that a medium was necessary for the propagation of sound.13 The Italian Lazzaro Spallanzani (1729–1799) is sometimes referred to as the “father of ultrasound” because he deduced that bats must emit ultrasound waves (inaudible to humans) and listen to the echoes to navigate, based on his observations in the 1790s that bats navigate well when blindfolded but not when he plugged their ears. The Austrian mathematician and physicist Christian Johann Doppler (1803–1853) noted that the pitch of sound varied if the source of sound was moving and derived the mathematical relationship between change in pitch (frequency) and the relative motion (velocity) between the source and the observer. As an indication of the ingenuity of early scientific investigators, Buys Ballot at Utrecht in 1845 confirmed Doppler’s theory and formula experimentally by having trumpeters play notes on railroad cars pulled at different speeds and employed musicians with perfect pitch to identify the frequencies they heard.9 Doppler also predicted (which was later confirmed) that the frequency of light would decrease from a receding object (e.g., stars [“red shift”]) and increase (toward blue) if approaching. Interestingly, manufacturers of color-flow Doppler systems chose to use the opposite convention, using red to color-code flow moving toward the transducer and blue for flow moving away from the transducer (“Red returning, Blue away”).
TABLE 11-1 Early Developments Leading to Medical Use of Ultrasound
| Name | Date* | Development |
|---|---|---|
| Marcus Vitruvius (Roman) | 80–15 BC | Coined word echo |
| Marin Mersenne (French) | 1588–1648 | “Father of acoustics” Measured speed of sound |
| Robert Boyle (English) | 1627–1691 | Recognized a medium was necessary for propagation of sound |
| Lazzaro Spallanzani (Italian) | 1727–1799 | “Father of ultrasound” Demonstrated that bats navigated by echo reflection |
| Christian Doppler (Austrian) | 1803–1853 | In 1842, he described Doppler effect and mathematical relations between change in frequency (of sound and light) and relative motion |
| Jacque and Pierre Curie (French) | 1880–1881 | Discovered piezoelectric effect |
| Lewis F. Richardson (British) | 1912 | British engineer after the Titanic disaster suggested that an echo technique could be used to detect underwater objects |
| Paul Langevin (French) | WWI | Developed SONAR; used the piezoelectric effect to develop transmitters and receivers |
| Sergei Sokolov (Russian) | 1929–1937 | Used reflection of ultrasound to detect flaws in metal |
| Floyd Firestone (American) | 1942 | Flaw detection in metals |
* Lifespan of first five; date of development for last five.
The creation and recording of ultrasound were made possible by the discovery of the piezoelectric effect by the French brothers Jacques and Pierre Curie. In 1880, they discovered that when certain quartz crystals were subjected to mechanical stress (compression), they developed an electrical charge. A year later, in 1881, they observed the converse: When crystals were placed in an alternating electrical field, they would rapidly change shape (vibrate).9
After the Titanic disaster in 1912, the British engineer Lewis F. Richardson suggested that an echo technique could be used to detect underwater objects. In 1917, the Frenchman Paul Langevin (1872–1946) conceived of the idea to use piezoelectric quartz crystal as both transmitter and receiver of ultrasound, which culminated in the development of SONAR (sound navigation and ranging) used to detect enemy submarines. In 1937, the Soviet scientist Sergei Sokolov and, in 1942, the American engineer Floyd Firestone described the use of reflected ultrasound to detect flaws in metals that, as explained later, ultimately led to the medical use of ultrasound in cardiac medicine.11,13,14
Early medical use of ultrasound
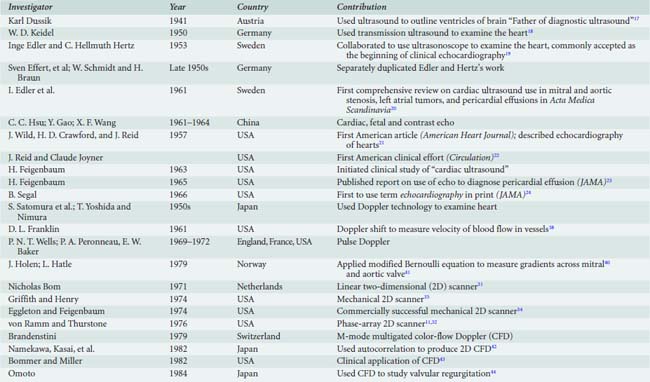
The Austrian neurologist Karl T. Dussik (Figure 11-1), sometimes referred to as the “father of diagnostic ultrasound,”11 is credited with being the first to apply ultrasound for medical diagnosis16 (Table 11-2). In 1941, he reported on his use of transmission ultrasound to outline the ventricles of the brain.17 He considered use of echo reflection but abandoned this approach when his idea was ridiculed.11 In the 1940s, the German physicist W. D. Keidel used transmitted ultrasound through the chest (like X-rays) in an attempt to measure cardiac volumes.11,18 Subsequently, both investigators appear to have abandoned their efforts.
Early history of clinical echocardiography
Edler and Hertz and the Beginning of Clinical Echocardiography (Ultrasound Cardiography) and M-Mode Imaging
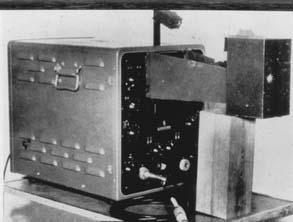
It was the collaboration between cardiologist Inge Edler (1911–2001) and physicist Carl Hellmuth Hertz (1920–1990) (Figure 11-2) at Lund University in Sweden in the early 1950s that is commonly accepted as the beginning of clinical echocardiography.13*

Figure 11-2 I. Edler (right) and C. H. Hertz (left) in 1979.
(From Roelandt JR: Seeing the invisible: A short history of cardiac ultrasound. Eur J Echocardiogr 1:8–11, 2000.)
In a search of a better way to evaluate the mitral valve function before closed mitral commissurotomy, Edler considered the use of something like RADAR (see Table 11-2). He was referred to a young physicist at Lund University, C. Hellmuth Hertz. Hertz was the son of a Nobel Prize winner, and, remarkably, his uncle, Heinrich Hertz, had lent his name to the unit of frequency. Hertz thought that ultrasound (frequencies higher than that audible to the human ear) might be the solution to Edler’s needs.14 Hertz borrowed an ultrasonic reflectoscope used in nondestructive testing of metals at the ship-building yard in Malmo for a weekend, and Elder tested it on Hertz’s chest and detected movement in the heart. The Siemens Corporation provided them with a reflectoscope in October 1953. They first studied isolated hearts in the laboratory with A-mode (static amplitude), but then devised a recording technique to display motion versus time (M-mode) by photographing the image off the oscilloscope (Figures 11-3 and 11-4). Their machine could produce ultrasound frequencies of 0.5, 1.0, 2.5, and 5.0 MHz, and Hertz opted to use 2.5 in humans as the optimal compromise between penetration and resolution. They first used this machine in a patient on October 29, 1953; they termed it “ultrasound cardiography” (UCG).19 At first they attributed the reflectors to the posterior wall of the left ventricle and the anterior wall of the left atrium, but, subsequently, Edler demonstrated that the latter came from the anterior leaflet of the mitral valve. He determined this by passing an ice pick in the comparable direction through the chest at the time of an autopsy on a patient he had echoed shortly before death.2 He subsequently used UCG to evaluate pericardial effusions and, in 1956, detected a left atrial myxoma (but this was not published until 1960).12 However, his principal use of UCG was to evaluate mitral stenosis, and he described the value of the E-F slope in quantifying the severity of the stenosis. In the mid-1950s, Edler and Hertz actually experimented with introducing a transducer into the esophagus to overcome the attenuation in lung tissue, but they abandoned this effort because of difficulties in obtaining acoustic coupling between the transducer and the esophageal wall.12 They published the first review article on UCG in Acta Medica Scandinavia in 1961.20 Hertz left the field of cardiac ultrasound fairly early after he developed ink-jet technology, and Edler made few innovations after 1960.2 In 1977, Edler and Hertz were awarded the Lasker Clinical Medicine Research Prize (often referred to as the American Nobel prize in Medicine).
Others Enter the Field
In the late 1950s, Schmidt and Braun, and Sven Effert in Germany separately began duplicating Elder and Hertz’s work, and in 1959, Effert published the first report identifying an intra-atrial tumor by echocardiography. Between 1961 and 1965, workers in Shanghai and Wuhan, China began to report their use of echocardiography including fetal echocardiography.13
The American Experience
In 1957, engineers John Wild and John Reid at the University of Minnesota described ultrasonic echocardiographic imaging of excised hearts.21 When Reid went to the University of Pennsylvania, he joined forces with cardiologist Claude Joyner, building an ultrasonoscope that they used to study mitral stenosis. Their report, which appeared in the journal Circulation in 1963, was the first American publication on clinical echocardiography.22 Harvey Feigenbaum in Indianapolis, author of one of the first (first published in 1972) and still published textbooks of echocardiography, first became interested in echocardiography in 1963 (Figure 11-5). He published his first article on use of ultrasound to diagnose pericardial effusions in 1965,23 and as mentioned later, he collaborated with Reggie Eggleton to develop a mechanical two-dimensional (2D) scanner. Dr. Feigenbaum also may have been the first to train nonphysicians to do echocardiograms, leading to the development of cardiac sonographers.13 Feigenbaum believes that Bernie Segal of Philadelphia was the first to use the term echocardiography in print in an article published in 1966.24,25 The senior author (E.A.H.) recalls cardiologists presenting their primitive, indistinct, and difficult to interpret M-mode images of the mitral valve at preoperative case conferences in the late 1960s and thinking that echocardiography did not have much future. How wrong he was!
Between the 1950s and the 1970s, M-mode was the only clinically useful format for echocardiography, and many advances and applications were described as the equipment and the skill of the echocardiographers improved, peaking in the late 1970s. But then its role rapidly declined with the development of 2D instrumentation.9
Two-Dimensional Scanners
Work to develop real-time 2D scanners began in the 1960s, made possible by advances in sonar and radar technology and circuitry. Early pioneering work was done by the previously mentioned Americans Wild and Reid,26 and Howry and Bliss27 in the 1950s. In the later 1960s, Ebina28 in Japan and Asberg29 reported producing ultrasound-generated tomographic images of thoracic structures in humans. Their clinical application was limited because of transducer size.
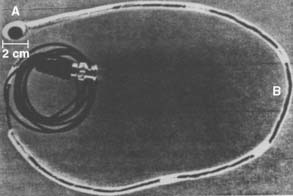
In 1968, Somer30 constructed the first electronic phased-array scanner based on the wavefront theory formulated in the 17th century by Huygens.11 In 1971, this was followed by the description by Nicholas Bom31 of Rotterdam of an electronic linear scanner (Figure 11-6) that generated a rectangular image and, in 1974, of an electronic phased-array scanner by F. L. Thurstone and O.T. von Ramm at Duke University.11,32 At the same time, J. Griffith and W. Henry, at the National Institutes of Health, introduced a mechanical sector scanner.11,33 This was said to be cumbersome to manipulate, and hence shortly thereafter Reggie Eggleton, working with Feigenbaum in Indianapolis, developed a handheld real-time mechanical 2D scanner34 that was more “user friendly” and commercially successful13 (Figure 11-7). In 1976 and 1977, Kisslo and colleagues35,36 at Duke University reported on the clinical use of the von Ramm and Thurston phased-array scanner for 2D echocardiography (2DE).
Development of Vascular and Cardiac Doppler
In 1956, Satomura reported the application of the Doppler principle in the use of ultrasound to measure blood flow velocity.11,37 However, in 1961, Franklin, at the University of Washington, was the first to measure Doppler shift of frequency, and hence velocity of flow of blood in vessels using a continuous-wave ultrasonic device.38 Pulse-wave Doppler (in which flow at a defined depth could be analyzed) was nearly simultaneously introduced in 1969 and 1970 by P. N. T. Wells of England, P. A. Peronneau of France, and D. W. Baker in the United States.11 In 1974, F. E. Barber, D. W. Baker, and colleagues at the University of Washington introduced the combination of pulse-wave Doppler with 2D scanning to produce the subsequently widely used “Duplex Scanner.”11,39 Subsequently, in Norway, but at different institutions, J. Holen40 and L. Hatle41 applied the Bernoulli principle to estimate the pressure decline across stenotic mitral and aortic valves, respectively.
Color-Flow (Doppler) Echocardiography
In 1978, the Swiss-born M. A. Brandestini, working at the University of Washington, described a method (based on pulse-wave Doppler) of color encoding flow velocity data and superimposing it on M-mode images.13 His initial observations were extended by Namekawa et al42 in Japan who described, in 1982, color Doppler flow imaging (CFI) utilizing autocorrelation to produce real-time images of flow in 2D sector scans. Bommer and Miller in the United States43 and Omoto working with the Namekawa group44 described clinical use of 2D color Doppler flow imaging.9,11
Prelude to Perioperative Transesophageal Echocardiography
The first reported use of intraoperative echocardiography (IOE) was in 1972 when Johnson and his colleagues45 at the University of Colorado reported on the use of intraoperative epicardial M-mode echocardiography to evaluate the results after open mitral commissurotomy. In 1976, Wexler and Pohost, of Massachusetts General Hospital, wrote a review article in the journal Anesthesiology on noninvasive techniques for hemodynamic monitoring, in which they described the basic principles and the potential application to anesthesia of the relatively new technique of “echocardiography.”46 At that time, this was limited to M-mode technology, but they anticipated that in the future 2D sector scanning would overcome some of the limitations of M-mode scanning. In that same year, at the annual meeting of the American Society of Anesthesiologists (ASA) in October in San Francisco, Rathod and colleagues,47 at Cook County Hospital and Loyola University in Chicago, and Paul Barash and colleagues,48 at Yale University, described use of transthoracic M-mode echocardiography to monitor the effects of anesthetics on cardiac function during anesthesia in 20 adults and 13 children, respectively. They concluded that this technique was a useful method to assess cardiac function, and Barash et al48 predicted that it “has the potential to be a useful tool for clinical anesthesiology that may supplant the invasive monitors currently available.”
In 1978, Strom and colleagues at Albert Einstein College of Medicine in Bronx, New York, published their experience with use of intraoperative epicardial M-Mode scanning during cardiac surgery,49 which led to their investigations of transesophageal echocardiography (TEE; see later).
When superior handheld 2D transducers became available, they began to be used epicardially during cardiac surgery after gas sterilization or wrapping them in sterile sheaths. In the early 1980s, Spotnitz and colleagues,51 at Columbia University College of Medicine in New York City (NYC), reported use of intraoperative epicardial 2D scanning after CPB to detect intracardiac air50 and to assess left ventricular ejection fraction before and after cardiac surgery. In 1984, Goldman and colleagues, at Mount Sinai Medical Center in NYC, reported the use of intraoperative epicardial contrast 2DE to evaluate regurgitation after mitral valve surgery52 and to assess myocardial perfusion during cardiac surgery.53
Transesophageal echocardiography
TEE has had a profound effect on the practice of cardiac anesthesiology starting in the mid 1980s, and conversely, at least in North America, anesthesiologists played a key role in the introduction of this modality into the practice of cardiac anesthesia and cardiology8 (Table 11-3).
TABLE 11-3 Notable Developments in Transesophageal Echocardiography
| Investigator (Reference) | Year | Contribution |
|---|---|---|
| Side and Gosling60 | 1971 | Continuous-wave Doppler of the thoracic aorta mounted on a standard gastroscope |
| Duck61 | 1974 | Pulse-wave Doppler of thoracic aortic blood flow with an esophageal probe |
| Daigle62 | 1975 | Pulse-wave Doppler of thoracic aortic blood flow with an esophageal probe |
| Frazin63 | 1976 | M-mode of heart via a cable-mounted transducer |
| Matsumoto, Oka, et al64,65 | 1979–1980 | Transesophageal M-mode monitoring during cardiac surgery |
| Hisanaga66 | 1977–1980 | Mechanical 2D scanner mounted on gastroscope |
| DiMagna67 | 1980 | Electronic linear phased-array 2D scanner mounted on an endoscope mainly used to examine the gastrointestinal tract |
| Souquet68 | 1980–1982 | Electronic phased-array 2D scanner mounted on gastroscope |
| Schulter, Hanrath, Sorquet69 | 1980–1982 | Clinical evaluation of Sorquet’s TEE probe |
| Goldman et al93 | 1976 | TEE with color-flow Doppler |
| Takamoto et al94 | 1987 | TEE with color-flow Doppler |
| deBruijn et al95 | 1987 | TEE with color-flow Doppler |
| Omoto98; and others | ~1989 | Biplane probe |
| Roelandt100; and others | ~1992 | Multiplane probe |
2D, two-dimensional; TEE, transesophageal echocardiography.
Intravascular Ultrasound
The development of TEE had its roots in the search for alternative ultrasound windows because of difficulties encountered in obtaining good ultrasound signals through the chest using the early insensitive transthoracic transducers.7 In the early 1960s, this led to the investigation of intravascular probes. In 1960, Cieszynski54 inserted a single-element transducer on a catheter into the jugular vein of dogs. In 1963, Omoto et al55 reported obtaining static cross-sectional images in patients by slowly rotating a single-element transducer inserted into the right atrium,7 and a year later, Kimoto et al56 reported obtaining C-scans of the atrial septum in humans from an intravascular catheter.9 In 1968, Carleton and Clark57 reported similar studies. 1970, Reggie Eggleton58 mounted four elements on a catheter and created the first cross-sectional images of intracardiac structures by computer reconstruction of images obtained by slow rotation and ECG triggering,5 and in 1972, Nicholas Bom,59 in Rotterdam, described a real-time intra- cardiac scanner using a 32-element circular electronically phased-array transducer placed at the tip of a 9 F-catheter. Thereafter, interest faded as sophisticated TEE probes became available, only to re-emerge in the late 1980s (see later).
Transesophageal Ultrasound (Doppler and M-Mode)
Side and Gosling60 were the first to use transesophageal (continuous-wave) Doppler (transducer mounted on a steerable gastroscope) to assess flow in the heart and aorta in 1971. This was followed by similar reports by Olson and Shelton in 1972. In 1974 and 1975, Duck et al61 and Daigle et al62 used transesophageal pulsed Doppler to measure flow in the thoracic aorta.7 However, true TEE is said to have its embryonic beginnings in 1976 when Lee Frazin (a Chicago cardiologist) et al63 reported recordings of M-mode echocardiograms of the aortic root and valve, mitral valve, and left atrium from the esophagus by attaching a nonfocused 3.5-mHz transducer attached to a 3-mm coaxial cable (Figure 11-8). They reported superior recordings compared with the transthoracic approach in 38 patients.63 Following this lead, Matsumoto and Oka,64 at Albert Einstein College of Medicine, fabricated a stiff transesophageal probe supporting an M-mode transducer (Figure 11-9). They first used this in 1979 to measure ventricular dimensions and volume during mitral valve surgery in a 65-year-old woman.64 They subsequently reported intraoperative transesophageal M-mode monitoring in 21 patients undergoing cardiac surgery.65 At that time, M-mode TEE was difficult to interpret except by the extremely sophisticated clinician and hence was not widely adopted. However, the development of a new generation of gastroscopes with steerable tips in the late 1960s, onto which echo transducers could be mounted, had a significant positive impact on the development of TEE by facilitating direct contact with the wall of the esophagus,7 overcoming the problem encountered by Edler and Hertz a decade earlier.
Transesophageal Two-Dimensional Imaging
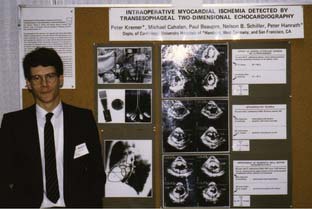
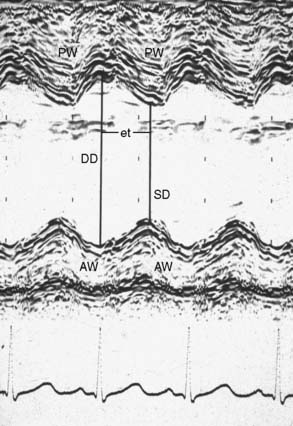
Although some primitive 2D TEE mechanical scanners and linear were described between 1977 and 1980 (e.g., Hisanaga et al66 and DiMagno et al67), it was not until Jacques Souquet68 (Figure 11-10), working in conjunction with the Varian Corporation, developed their phased-array transducer (Figure 11-11) mounted on the end of a gastroscope for their 2D sector scanner system (Figures 11-12 and 11-13) that TEE became a practical reality. These new TEE probes were evaluated clinically by cardiologists Michael Schluter and Peter Hanrath in Hamburg, Germany,69 and their preliminary results were highlighted at an international conference held in Hamburg in 1981.8 The early subsequent use of TEE took different directions in the United States and Europe. Cardiologists in Germany and the Netherlands rapidly began using TEE in awake patients to aid in the diagnosis of a variety of cardiac pathologies. In the United States, cardiologists seemed reluctant to adopt this new technology.6 In the early 1980s, Peter Hanrath sent prototypes (first M-mode transducers and then with the 2D transducers mounted on gastroscopes) to cardiologists James Seward at Mayo Clinic and Nelson Schiller (Figure 11-14) at the University of California in San Francisco (UCSF), who passed them on to anesthesiologists. Schiller70 spoke with William Hamilton, Chair of the Department of Anesthesiology at UCSF, who put him in contact with two young faculty members, Michael Cahalan and Michael Roizen. At that time (1981–1983), a cardiology fellow from Hanrath’s group in Germany, Peter Kremer (Figure 11-15), came to UCSF and collaborated with Cahalan (Figure 11-16) and Roizen to investigate these new TEE instruments. At the 1982 annual meeting of the ASA, they started the American TEE “revolution” when they presented their results in monitoring cardiac and vascular surgery patients with this new TEE probe, displaying the high-quality recordings and images obtained (Figures 11-17 and 11-18), and describing its usefulness in assessing filling and function of the left ventricle, and in detecting myocardial ischemia and intracardiac air.71,72 Subsequently, they reported its superiority to PAC in assessing filling in the operating room73 and usefulness in evaluating the hemodynamic changes during anaphylaxis74 and during surgery for pheochromocytoma.75 Topol and colleagues at Johns Hopkins University demonstrated its usefulness in determining the cause of hypotension immediately after CPB76 and documented improvement in myocardial dysfunction immediately after coronary revascularization.77 Cucchiara et al,78 at the Mayo Clinic, described its usefulness in detecting air embolism during neurosurgery, whereas the UCSF group described its value in monitoring during vascular surgery79,80 and its superiority over ECG in detecting myocardial ischemia.81 In 1984, the Hamburg group and Cahalan published an anatomic analysis of six standard TEE views,82 which was expanded on a few years later by the Mayo Clinic group.83 Meanwhile, the group at Mount Sinai Medical Center in NYC (Martin Goldman, Joel Kaplan, Daniel Thys, Zaharia Hillel, and Steven Konstadt) was quick to adopt and evaluate perioperative TEE, following the experience of Kaplan working with the early Diasonic TEE probes in the early 1980s.84–88 In 1987, Cahalan et al89 and Clements and deBruijn90 at Duke University wrote review articles on the use of TEE in anesthesiology, whereas the group at Mayo Clinic reported on the reproducibility of measurements obtained during intraoperative TEE.91 In that same year (1987), the second edition of this text edited by Kaplan on Cardiac Anesthesia contained for the first time a 63-page thoroughly referenced chapter on Intraoperative Echocardiography.92

(From Image: A Conversation with…Jacques Souquet, PhD. Available at: http://www.rt-image.com/A_Conversation_with_Jacques_Souquet_PhD_The_diagnostic_capabilities_of_ShearWave/content=8504J05E48BE588440B6967644A0B0441.)

Figure 11-16 Michael K. Cahalan, Professor Anesthesiology, University of Utah.
(Photograph courtesy M. K. Cahalan.)
In 1986, Goldman and colleagues,93 at Mount Sinai Medical Center in NYC, reported their experience with a new 2D TEE probe that also provided Doppler color-flow imaging (Aloka and Irex), as did a Japanese group.94 That same year, the Hewlett-Packard Corporation also introduced a color-flow Doppler TEE probe; and in 1987, deBruijn and Clements, together with pioneering echocardiographer, Joseph Kisslo at Duke University, reviewed their early experience with this new technology.95 In that same year, pulse-wave Doppler was added to 2D TEE probes,96 although several years earlier the Hamburg group had described results of use of a TEE probe that combined pulse-wave Doppler with M-mode TEE.97 In 1989, biplane probes (Figure 11-19) first became available,98 although prototypes had been described earlier,99 and soon thereafter, multiplane single-transducer TEE probes100 and smaller diameter probes suitable for pediatric use101 appeared. In 1992, Pandian and colleagues, at Tufts-New England Medical Center, reviewed their early clinical experience with a 5-MHz phased-array multiplane TEE probe (“OmniPlane”).102
A survey conducted of Society of Cardiovascular Anesthesiologists (SCA) members in early 1988 provides a “snap-shot” of the state of practice of IOE at that time103; 20% of the responders reported that IOE studies were being performed at their institutions. The majority of responders were practicing in large teaching hospitals. Anesthesiologists were involved 41% of the time and cardiologists in 33%, although in one third of the former group, cardiologists always assisted. TEE technique was used in 51%, epicardial in 37%, and transthoracic in 22% of cases. At the time of the survey, six manufactures made 2D TEE transducers—Hewlett-Packard, Aloka Toshiba, Diasonics, General Electric, Acusonics, and Hoffrel, but only the first three provide color-flow TEE. Most responders reported using either Diasonics (46%) or Hewlett-Packard (42%); 90% used the 2D mode, 75% M-mode, 60% pulsed-Doppler, 58% color-flow Doppler, and only 23% continuous-wave Doppler, whereas 97% believed that IOE had been or could have been helpful. The authors of the survey noted that few academic medical centers offered formal instruction and certification in IOE, and that a definite need for training had been identified.
Along this same line, in an editorial published in 1989, Kaplan104 urged caution and restraint in the adoption of this new and complex technology that had the potential to cause numerous complications, and emphasized the need for anesthesiologists who aspire to be echocardiographers to obtain a high level of training.
In the late 1980s, the group at Duke University demonstrated the utility of routine epicardial echocardiography during surgery for congenital heart disease.105–107 As soon as smaller probes that could be used in infants and children became available, these began to be used. In 1989, Kyo et al,101 in Japan, were among the first to describe the use of TEE in pediatric cardiac surgery, a practice strongly supported by the work of the Seattle group about which they started publishing in 1993.107–110 By the end of that decade, 98% of pediatric cardiac surgery centers used intraoperative TEE.111
Into the Mainstream
The 1990s witnessed the expansion of intraoperative TEE into the mainstream of clinical practice of cardiac surgery and anesthesia,112 as well as into critical care and noncardiac surgery. From the beginning of perioperative TEE, its value to detect myocardial ischemia based on the appearance or regional wall motion abnormalities was recognized.81 When only single-plane TEE transducers were available, the midpapillary transgastric short-axis view often was advocated for this purpose. With the appearance of biplane and multiplane probes in 1996, Cahalan’s group113 documented the limitations of the transgastric short-axis view and the need to use multiple views. In that same year, the group at UCSF also documented the value of TEE in assessing ischemia during CABG surgery114; whereas in 1997, the group at Cleveland Clinic documented the benefit of intraoperative TEE in high-risk CABG.115 In 2000, Aronson and colleagues116 reported on the use of intraoperative low-dose dobutamine echocardiography. Evidence of the increased importance of echocardiography to the practice of cardiac anesthesia is that with its first issue in 2001 (volume 15, number 1, February), Journal of Cardiothoracic and Vascular Anesthesia changed the logo on its cover from a PAC to echocardiographic images, and three TEE images graced the cover of Daniel Thys’ Textbook of Cardiothoracic Anesthesiology published in 2001.117
Other developments
Contrast Echocardiography and Myocardial Contrast Echocardiography
Contrast enhancement of blood represents an important adjunct to echocardiography. It has been used to identify cardiac structures, enhance identification of the endocardial boarder, detect shunts and valvular regurgitation, and assess myocardial perfusion. Feinstein,118 of Rush University in Chicago, and his international colleagues have reviewed the development of what they refer to as “contrast enhanced ultrasound” imaging and have identified important contributors over the years. Both the radiologist R. Gramiak, of University of Rochester,13* and the cardiologist C. R. Joyner, Jr., of the University of Pennsylvania,9 have been credited as being the first to incidentally note dense echoes associated with the injection of normal saline or indocyanine green during M-mode echocardiography. Both of them subsequently described using saline contrast to help identify cardiac structures during M-mode echocardiography.119 In 1975, Seward et al,120 at the Mayo Clinic, described their initial experience with use of indocyanine green as an echocardiographic contrast agent in more than 300 cases, emphasizing its role in analyzing shunts. Bommer et al showed that when echocontrast agents were injected into coronary arteries during echocardiography, they produced myocardial opacification,9 which led to the development of myocardial contrast echocardiography. The echo contrast of these agents was attributed to microbubbles. In these early media, the microbubbles were too large to pass through the capillaries including the lung, and hence direct injection into the left heart was necessary to image the structures in the left heart including the myocardium. In 1984, Feinstein et al described the use of ultrasonic energy (“sonication”) to produce contrast agents consisting of relatively uniform, stable, and small gaseous microbubbles that could pass through capillary beds and allow imaging of the left heart chambers and myocardium via right-sided injection.121 Over time, superior commercial contrast agents have been developed (e.g., Levovist, Albunex), and newer, more sophisticated agents are under development.118
In 1984, Goldman and colleagues52 described the use of intraoperative contrast echocardiography to evaluate mitral regurgitation after mitral valve surgery by injecting saline into the left ventricle and monitoring for any regurgitation of contrast into the left atrium with epicardial 2D imaging. In that same year, this group also was the first to describe intraoperative myocardial contrast epicardial echocardiography (myocardial contrast produced by administration of cardioplegia into the aortic root) to predict the presence of coronary artery disease (CAD).53 In the late 1980s and early 1990s, Aronson and colleagues at the University of Chicago, Kabas and Kisslo at Duke, and Spotnitz and Kaul at University of Virginia used intraoperative contrast echocardiography to assess coronary artery surgery.122–125 In 2000, Aronson126 summarized the progress in measurement of myocardial perfusion by contrast echocardiography in the operating room, and in 2006, Dijkmans and his international colleagues127 summarized the state of the development of myocardial contrast echocardiography. In 2007, the U.S. Food and Drug Administration called attention to concern about the safety of some of these agents, and in 2008, the American Society of Echocardiography (ASE) issued a consensus statement on “the clinical applications of ultrasonic contrast agents in echocardiography.”128
Epicardial and Epiaortic Imaging
As noted earlier, Edler and Hertz first made epicardial ultrasound recordings of cadaver hearts in 1953, as did Wild and Reid in 1957.21 Also, as mentioned earlier, in 1972, Johnson et al45 reported on the use of intraoperative epicardial M-mode imaging to evaluate the results after mitral commissurotomy. In the early 1980s, the groups at Columbia University College of Medicine and the Mount Sinai Medical Center in NYC used intraoperative epicardial 2DE to detect intracardiac air,50 assess left ventricular ejection fraction,51 evaluate regurgitation after mitral valve surgery (after injection of contrast into the left ventricle),52 and assess myocardial perfusion during cardiac surgery.53 Because suitable small transesophageal probes were not available in the late 1980s and early 1990s, epicardial 2D scanning became the standard for echocardiographic surveillance during pediatric cardiac surgery in that era.106
With the introduction of TEE, epicardial imaging largely was discarded, but recently, both epicardial and epiaortic imaging have regained attention and are being utilized more frequently in situations in which TEE is not possible or adequate, and to assess the thoracic aorta, especially in the “blind spots” of the TEE.129 The ASE/SCA have developed guidelines for epicardial130 and epiaortic131 applications.
Intracardiac Echocardiography
As reviewed earlier, intracardiac ultrasound was explored as an alternate method for examination of the heart during the era of limited transthoracic transducers. This approach largely was discarded with improved transthoracic instruments and, especially, the arrival of TEE. Recently, with improved miniaturization and technology of intravascular probes, and the increase in percutaneous intracardiac procedures (e.g., closure devices, percutaneous mitral valve surgery, electrophysiologic procedures), use of intracardiac ultrasound to monitor and aid in the safer performance of these procedures has become more popular.132
Intravascular Ultrasound
The circular-array catheter-mounted transducers ultimately were miniaturized (1 to 3 mm in diameter) and provided with sophisticated electronics so they could be placed into coronary arteries over a guidewire. Utilizing ultra-high frequencies (40 MHz), they permit a resolution of 150 μm to a depth of 2 to 3 cm. In the late 1980s, Nissen and colleagues,133 at the University of Kentucky, were among the first to exploit these new intravascular ultrasound catheters to study CAD. Such technology is now used routinely to study CAD, and larger intravascular ultrasound probes are used to assist with placement of intracardiac devices, as well as to assess larger vessels.13
Echocardiography in Critical Care Medicine: In the Noncardiac Operating Room and Intensive Care Units
In Intensive Care Units and the Care of Critically Ill Patients
The value of transthoracic echocardiography (TTE) in the evaluation and management of critically ill patients was recognized as soon as 2D scanning became available. However, its common use was limited because of difficulty in obtaining adequate views by the transthoracic route in this patient population. As soon as TEE 2D probes arrived, their usefulness and advantages over TTE in critical care situations became obvious. In 1986, groups at Erasmus University in the Netherlands and at the University of California in San Francisco collaborated in illustrating the role of 2D TEE in solving clinical problems.134 In 1987, Erbel et al136 in Mainz, Germany reported the superiority of TEE over TTE in detection of aortic dissection in 21 patients (100% vs. 29%),135 which was followed by a report of a multicenter study comparing echocardiography (TTE and TEE) with CT scanning and angiography in 164 cases of aortic dissection (82 proved). Echocardiography was found to have a sensitivity and specificity, and positive and negative predictive values of 98% or greater, and was at least as good as the other diagnostic modalities. In 1988, Chan,137 at the Ottawa Heart Institute, reported its usefulness in assessing the cause of hypotension after cardiac surgery in seven patients in whom TTE had failed to explain the problem (tamponade in three, ventricular septal rupture in one, and global LV failure in three). Reichert et al138 of the Netherlands followed this with a report of use of TEE in 60 hypotensive patients after cardiac surgery and found it changed the clinical diagnosis in 60% of cases.
Between 1990 and 1994, the groups at St Louis University,139 Mayo Clinic,140 Cleveland Clinic,141 UCSF,142 National Taiwan University,143 and Baylor College of Medicine144 reported their initial results with use of TEE in more than 450 critically ill patients. In most of these series, TEE was performed because of unsatisfactory or suboptimal TTE, and the authors reported TEE to be invariably informative. Two groups directly compared use of TEE with TTE in intensive care units or the emergency department and found TEE to be vastly superior.141,143 Many of these studies were done by cardiologists on patients with cardiologic issues. In 1998, the group at Mount Sinai Medical Center in NYC demonstrated the superiority of a goal-directed limited-scope TEE examination performed by surgical intensivists who had received limited goal-directed training in TEE, compared with conventional hemodynamic monitoring including use of the PAC.145 Recent articles have reviewed the progress made in the use of TEE in the general intensive care unit.146–148
Intraoperative Transesophageal Echocardiography for Noncardiac Surgery
Although intraoperative TEE initially was adopted by cardiac anesthesiologists for use during cardiac surgery, early use during noncardiac surgery (vascular surgery, pheochromocytoma, and anaphylaxis) was reported by the group in UCSF,72,74,75,79–81 The group at Duke emphasized the potential role of the anesthesiologist as a cardiac diagnostician.149 Beginning in 1988, in a series of annual refresher course lectures given at the ASA annual meeting, Cahalan reviewed the benefits of intraoperative TEE for hemodynamic monitoring and advocated its adoption by all anesthesiologists caring for patients at risk for hemodynamic problems. The adoption of such practice had been limited by lack of educational opportunities (which is now being addressed; see later and Chapter 41) and maintenance of skills. A comprehensive review of the experience with use of TEE outside of the cardiac surgical operating room recently has been published by Mahmood et al,148 whereas Goldstein150 and Green et al151 have debated whether the general anesthesiologist should be trained and certified in basic TEE.
Recent developments
Since the early 2000s, many advances in digital technology and miniaturization, probe technology (including fully sampled matrix-array probes that have replaced the mechanically rotating multiplane probes), image-compression algorithms, high-density digital storage, and broadband communication networks have revolutionized storage and processing of TEE images (see Chapter 12). Secure digital servers have replaced videotape libraries of clinical studies, resulting in vastly faster and more convenient access to high-fidelity stored images even from remote locations.152–155 Other areas that have seen considerable advancement include 3D and 4D, tissue Doppler, speckle tracking, regional wall motion detection, and handheld/hand-carried echocardiography (the “Echo-Stethoscope”).
Three- and Four-Dimensional Echocardiography
Transthoracic Three-Dimensional Echocardiography
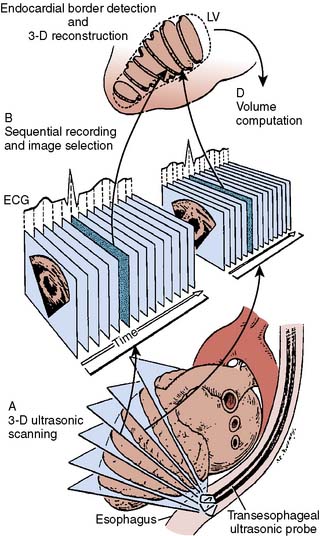
History of 3D imaging in medicine starts in 1961 when Baum and Greenwood156 used 3D ultrasonography for localization of orbital lesions. All early work in cardiac 3DE focused on methods of acquiring multiple 2D images by moving a standard 2D transducer in space and reconstructing the multiple 2D images into a 3D image. The first such publication came in 1974 from Stanford scientists Dekker, Piziali, and Dong157 on a system for ultrasound imaging of the human heart in three dimensions. Dekker’s group accomplished this by mounting the transducer onto a movable mechanical arm, which allowed alignment of multiple 2D images and creation of a 3D image (Figure 11-20).
In 1976, the spark-gap technique of 3D reconstruction of the heart was developed by Moritz and Shreve.158 This technique involved measuring the transit times of spark-generated shock waves to obtain the spatial coordinates of the echocardiographic planes. The microprocessor controlled the operation of the system and performed all the computations required to determine the location and orientation of the ultrasound beam with respect to a known coordinate system. About this same time, Japanese engineers and clinicians with Matsumoto et al,159 from Osaka University, described technology they used to process 3D images of left ventricle, atrium, and aorta: “We applied our newly developed computerized image processing system for 3-D echocardiographic display with binocular parallax shift and for constructing 2-D echocardiographic images in desired planes from sequential recordings of 2-D echocardiograms recorded with anteroposterior emission of ultrasound beams. This system mainly consisted of a flying spot scanner, a minicomputer and a display cathode ray tube.…The frontal plane 2-D echocardiograms provided useful data.”159 In 1979, Raab et al160 developed a magnetic locator that they attached to the transducer and to a central computer that located the transducer in space and subsequently followed its position.
Improvements on these methods led to the freehand imaging, allowing free movement of the transducer at a single or multiple acoustic windows and resulting in a 3D wire-frame image. Images were acquired over short periods during held end-expiration with combination of angulated or rotated scans from parasternal or apical windows. Image quality depended on the patient’s respiration. A magnetic field system was used to track the ultrasound scanhead. Images were digitized and registered with position data and image depth, as well as coordinated with independently acquired ECG. The borders of the left ventricle and associated anatomic structures were manually traced in the selected images.160
The 3D reconstruction of real-time transthoracic 2D images of the left ventricle, using an apical rotation method, was first described in 1982 by Ghosh and colleagues.161 In their method, the transducer was placed on the patient’s chest wall in the region of the apex to obtain the standard four-chamber view to record 2D left ventricular end-diastolic and end-systolic images. The transducer was then rotated in 30-degree increments from 0 to 180 degrees to obtain various planes passing through the apex. Using spatial coordinate data, 3D perspective images were plotted by the computer in any desired view. Three-dimensional reconstructed volumes closely correlated with those obtained by left ventricular angiography. In that same year, Geiser et al162 described their technique of dynamic 3D echocardiographic reconstruction of the intact human left ventricle in patients.
Olaf von Ramm, professor of biomedical engineering at Duke University, with his colleague, Stephen Smith, pioneered the development of clinical 3D ultrasound scanners in the late 1980s. In 1987, they patented the first real-time high-speed 3D ultrasound system, capable of acquiring pyramidal volumetric data at frame rates (about 8 per second) sufficient to depict cardiac movement. Their transthoracic transducer, transmitting at frequency 2.5 to 3.5 MHz, included 256 elements configured in a “sparse array” pattern (not all the transducer head was used and connected to individual elements).163 Later, this technique was developed commercially by Volumetrics Medical Imaging (Chapel Hill, NC) and existed for several years; however, it never made an impact on clinical practice because of poor image quality.
Transesophageal Three-Dimensional Echocardiography
In the 1980s, when rapid development of TEE began, first attempts at 3D reconstruction from TEE were begun. The improved image resolution afforded by TEE was important for quality 3D image reconstruction. In 1986, Martin et al,164 of the Department of Anesthesiology and Center for Bioengineering at the University of Washington, in Seattle, used a precision micromanipulator of a TEE-mounted transversely oriented 32-element 3.5-MHz ultrasonic array transducer to generate a 3D echocardiogram. This allowed them to obtain multiple multiplanar short-axis 2D images of the heart with known angular relations between them over a series of cardiac cycles. An off-line computer analysis of the images was used to form 3D reconstruction of the left ventricular cavity at end-diastole and end-systole from which stroke volume was determined.164 In a canine study in 1989, Martin and Bashein165 were able to demonstrate that the stroke volumes generated from their 3D reconstructed volumes were comparable with radionuclide and thermodilution measurements (Figure 11-21).
The next step in development of 3D TEE was when Wollschläger166 et al reported on their system for TEE computer tomography. Their development was commercialized by TomTec Technology Company (Munich, Germany) and has found application in research and clinical practice. This technology was partially based on the rotational device used by Hewlett-Packard Corporation for their multiplane TEE probe that they introduced in 1992.
In 1992, Pandian et al,167 at Tufts-New England Medical Center, were among the first to report clinical results with 3D TEE. They used a 5-MHz, 64-element, and phased-array TEE probe connected to a computer that directed transducer movement at 1-mm increments. A complete cardiac cycle was recorded at each tomographic level with ECG and respiration gating. These were then processed using dedicated 4D software and displayed as a dynamic 3D tissue image of the heart. In 1997, Chen et al,168 at the Thorax Center in Rotterdam, Netherlands, published a study on the measurements of mitral valve orifice area with 3DE in patients with mitral stenosis. They had developed their own system for TEE acquisition, using TomTec software for off-line reconstruction. Transesophageal acquisition was performed in six patients with a custom-built transducer assembly; the transducer was rotated by a step motor that was commanded by computer algorithm that controlled the acquisition of cross sections within preset ranges of heart cycle length by ECG gating and respiratory phase.
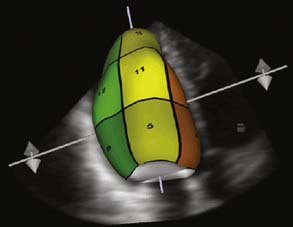
In 2007, Philips introduced its new “Live 3D TEE” probe with a matrix-array transducer (x7-2t, 2500 elements). This probe featured the combination of two new novel technologies: xMATRIX (3D power) technology and “Pure Wave” crystal technology that resulted in improved image clarity. “Live 3DTEE” offers not only conventional imaging modes such as 2D multiplane imaging, M-mode, continuous- and pulse-wave Doppler, and color Doppler, but also real-time 3D imaging: Live 2D, 3D Zoom, Full Volume, and 3D Color Full Volume (Figure 11-22). Live 3D displays a fixed pyramidal data set determined by the depth of the initial 2D images and can be used to visualize any cardiac structures located in the near field. Movement of the probe results in real-time change of the 3D image. 3D Zoom displays a truncated but magnified pyramidal data set of variable size. Placement of the probe over the region of interest with minimized sector width improved temporal resolution and optimized image quality. 3D zoom is used to view the left atrial appendage, interatrial septum, and mitral and tricuspid valves. Full volume provides a pyramidal data set that allows the inclusion of the larger cardiac volume at frame rate greater than 30 Hz. It combines a series of subvolumes acquired with ECG gating to create a final, larger, reconstructed full-volume image. This is the only one of these 3D modes that requires ECG-gated reconstruction. 3D color full volume combines grayscale of full-volume data with color Doppler.
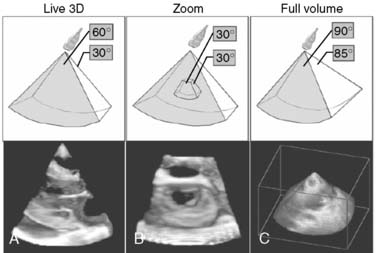
Figure 11-22 Three real-time three-dimensional (3D) imaging modes.
(From Hung J, et al: ASE Position Paper. 3D echocardiography: A review of current status and future directions. J Am Soc Echocardiogr 20:213–233, 2007, Figure 3.)
3DE has found particular application in measuring ventricular volumes (together with stroke volume, cardiac output, and ejection fraction), especially of the right ventricle (which is a particular problem with 2DE because of its complex geometric shape), to evaluate mitral valve, aortic valve, and aortic root pathoanatomy, to evaluate complex congenital heart disease, and as a guide for percutaneous interventional procedures including placement of closure devices, transeptal puncture, ablation procedures, and placement of left atrial closure devices. In 2007, the ASE published a position article on the current status and future directions of 3DE169 and, in 2008, published a practical guide for its use.170
Four-Dimensional Echocardiography
The next decade brought improvement in technology and new clinical studies. In 2005, Corsi et al171 developed a volumetric analysis technique for quantification of global and regional LV function and studied patients with CAD, cardiomyopathy, and valvular disease. They concluded that volumetric analysis of real-time 3DE data was clinically feasible and allows fast, semiautomated, dynamic measurement of left ventricle volume and automated precise detection of regional wall motion abnormalities. More recent studies have used speckle tracking combined with 4DE to more accurately quantify regional LV strain for strain analysis.172
Because the left ventricle is a 3D structure with a complex pattern of wall motion, 4D imaging offers great potential for identification of dyssynchrony. It also could help to identify the optimum pacing site for resynchronization therapy. A recently published study described a novel approach for morphologic and functional quantification of the mitral valve based on a 4D model estimated from ultrasound data.173 The 4D model’s parameters are estimated for each patient using the latest discriminative learning and incremental searching techniques (Figure 11-23).
Tissue Doppler and Speckle Tracking
In 1989, Isaaz et al174 described the application of Doppler echocardiography to evaluate myocardial motion, and tissue Doppler imaging was born.175 By appropriate filtering, they examined the high-amplitude low-velocity signals reflected back from the myocardium instead of the blood with pulsed-wave Doppler. This information has been used to evaluate ventricular function (rate and pattern of filling, and emptying of the ventricle, strain, and strain rate) and to estimate filling pressures. In 1997, Nagueh et al176 at Baylor University suggested that the ratio of early transmitral blood flow (E) to early movement of the basal myocardium as determined by tissue Doppler (Ea or E′), E/E′, could give a clue to left atrial filling pressure. Color coding (mapping) of tissue Doppler velocities in various parts of the myocardium was introduced by the group at the Royal Infirmary of Edinburgh in 1994177 and has been used to detect dyssynchrony.
Tissue Doppler recording has the same requirement of close alignment of the ultrasound beam with the movement being interrogated. This limitation has been overcome by the identification of small bright spots in the myocardium on the grayscale image because of backscattering from small structures within the myocardium (less than one wavelength) termed Speckles. This was first described by the innovative engineering group at Duke University in the late 1980s and early 1990s.178 These speckles can be identified and tracked (i.e., Speckle Tracking) to generate similar information provided by tissue Doppler but without the alignment constraint. These techniques initially were introduced for TTE and are technically demanding but are being used for investigational purposes with clinical TEE, the clinical importance of which are being investigated.154
Regional Wall Motion and Endocardial Border Detection
The value of echocardiography in detecting regional wall motion abnormalities and especially for early detection of myocardial ischemia has been recognized for many years. This usually was accomplished by leisurely off-line analysis. With the development of perioperative applications (as well as echocardiographic monitoring of stress tests), more accurate online assessment was needed. One of the keys to accurate assessment of wall motion is accurate delineation of the endocardial border. Various techniques have been introduced to facilitate this. Initially, this included bloodstream and myocardial contrast (see earlier). In the early 1990s, Hewlett-Packard Corporation introduced a method of transesophageal real-time automatic (endocardial) border detection using backscatter imaging with lateral gain compensation, which they termed “acoustic quantification.”179 This subsequently was improved by adding color coding, termed “Color Kinesis” (CK) by the manufacturer.180 Its use in perioperative TEE was assessed by the group at the University Hospital in Vienna.181 More recently, tissue Doppler imaging enhanced by color coding,182,183 and Speckle Tracking has been introduced to facilitate detection of abnormal regional myocardial function. Recently, dos Reis et al,184 from University of Brasilia, briefly reviewed the history of attempts at semiautomatic border detection and proposed a new algorithm combining classic mathematical morphology for binary images, high-boost filtering, image segmentation, and motion estimation.
Handheld or Hand-Carried Echocardiography: The “Ultrasound-Stethoscope”
The concept and reality of a portable lightweight battery-operated echocardiograph machine was introduced by Roelandt et al in 1978,185 who referred to this as the “Ultrasound-Stethoscope.” Since then, industry has introduced more miniaturized but also more capable portable high-resolution devices that are now even the subject of television advertising. Their use is advocated to complement and expand the history and physical examination, especially in the age of decreased skills in the latter.186 Mondillo et al187 have classified these devices, based on their size, capabilities, and cost, into four categories. The top-level devices are now capable of providing 2D, M-mode, color-flow, pulse-wave, and continuous-wave and tissue Doppler. Mondillo et al187 and Kobal et al188 have reviewed the capability and limitations of the use of these devices. A key limiting factor is the capabilities of the person conducting and interpreting the examination,189 and there are controversies about how much training and whether certification is required. Cost implications (vs. benefits) of acquiring the equipment and providing appropriate education are other issues. In 2002, the ASE, the American College of Cardiology, and the American Heart Association addressed the use of hand- carried echocardiography190 and recommended that users should have a minimum of Level 1 training (75 supervised studies and 150 supervised interpretations), but strongly recommended that they have Level 2 training (150 supervised studies and 300 supervised interpretations), which are far beyond what other proponents of the use of this technology have suggested is necessary.191
Organizations, training, guidelines, and examinations
A unique feature of the development of the practice of perioperative echocardiography has been the close cooperation and collaboration between cardiologists and anesthesiologists. The former have welcomed and embraced the latter into their specialty, and the two groups have collaborated in training and educational activities, establishing standards for its use and certification, and the development of guidelines and examinations. The ASA and the SCA have cooperated and played key roles in the development of perioperative echocardiography. In 1974, Feigenbaum192 addressed the educational problems in echocardiography, and these have largely been resolved in recent years. In 2001, Aronson and Thys193 gave a historical review of the more recent developments in training and certification in perioperative echocardiography.
The first meeting dedicated to cardiac ultrasound was sponsored by the American College of Cardiology and held at Indiana University in Indianapolis on January 11 and 12, 1968. Among the approximately 50 who attended included Elder, Joyner, Reid, and Feigenbaum. The ASE was created in Indianapolis in 1975. The Journal of the American Society of Echocardiography began in 1988, and the first annual meeting of the ASE was held in Washington, DC, in 1990.13
The SCA has played a key role in the development of perioperative TEE. The SCA offered its first workshop on echocardiography in 1987, initiated the Annual Comprehensive Review on Perioperative Echocardiography in 1998, and in 1991 published a monograph on the Intraoperative Use of Echocardiography.15,194 In 1994, the ASA and SCA embarked on jointly developing practice guidelines for perioperative TEE. The task force was led by Daniel Thys (Figure 11-24) and included representatives from both the American College of Cardiology (William Stewart) and the ASE (Alan Pearlman). These guidelines were published in 1996195 and were updated in the fall of 2009.15,200 This marked the beginning of collaborations with various organizations, especially the ASE, in producing a number of guidelines related to perioperative echocardiography (Table 11-4; see Chapter 41).

Figure 11-24 Daniel M. Thys, Professor (Emeritus) Anesthesiology Columbia University College of Physicians and Surgeons, New York City, President of the Society of Cardiovascular Anesthesiologists (SCA; 1999–2001), chaired the ad hoc Task Force of the ASA/SCA, which developed the Practice Guidelines for Perioperative TEE (1967, 2010). He was chair of the Council on Intraoperative Echocardiography of the American Society of Echocardiography (ASE; 1999–2001), and was on the editorial board of the Journal of the American Society of Echocardiography. He was founding member and president of the National Board of Echocardiography (NBE; 2005–2007). Gave the 2nd Annual Weyman Lecture on the history of cardiac anesthesiology emphasizing importance of echocardiography.15
(Courtesy D. M. Thys.)
In 1993, the ASE launched an examination to test knowledge in echocardiography (ASeXAM). Although it contained questions on perioperative echocardiography, that was not its particular focus. Later that decade, the SCA developed an examination confined to perioperative TEE that was first administered on April 24, 1998. As a result of discussion with the ASeXAM, Inc., the National Board of Echocardiography was established on November 1, 1999, to develop and administer examinations in the field of clinical echocardiography.15 Currently, three examinations are administered, one in adult echocardiography (ASCeXAM), another in advanced perioperative TEE (Advanced PTEeXAM), and, most recently, in basic perioperative TEE (Basic PTEeXAM). In 2002, Aronson and colleagues201 reviewed the development and analyzed the results of the PTE examination. Physicians who successfully pass these examinations are recognized as “testamurs.” As of 2009, 4091 have taken and 2966 have passed the advanced PTE examination.15
In 2003, the National Board of Echocardiography began issuing board certification in Advanced Perioperative TEE to candidates who are testamurs and have met the training and clinical experience requirements. As of 2009, 1111 physicians have become Diplomates in advanced perioperative TEE.15 In 2010, the National Board of Echocardiography initiated a process for certification in Basic PTE.
In 2000, Morewood et al surveyed members of the SCA; 42% had completed fellowship training. The survey revealed that although 94% of those surveyed practiced in institutions that used intraoperative TEE, and 72% personally performed TEE, less than 30% had received formalized training, and only 19% had passed certifying examinations. Their use of TEE increased with the percentage of their practice devoted to cardiac anesthesia (56% utilization if 25% of practice was cardiac and 91% if more than 75% cardiac). They reported that TEE was performed most of the time or always in 90% of valve surgery, 41% of CABG, and only 1% of noncardiac surgery. Notably, less than 50% said their institutions had specific credentialing criteria.202 It is interesting to compare these data with the results of the previously mentioned survey of the members of the SCA conducted 12 years earlier,203 and it is unknown how contemporary practice differs from that reflected in Morewood’s 2000 survey.
A cautionary note
In 1970, Drs. Swan and Ganz introduced their balloon-tipped PAC,204 which rapidly was adopted by cardiac anesthesiologists, critical care physicians, and all anesthesiologists because it provided hemodynamic information that they were sure was improving patient care. It was widely discussed at meetings of the SCA and the subject of an ASA practice guideline. Now, it has largely fallen into disrepute and its use has greatly diminished,205 partly because of limitations of some of the data it provides (especially use of filling pressures to assess volume status), risks associated with its use (although with careful application these have found to be uncommon), but most importantly, because of lack of high-quality evidence that its use improves patient outcome. In fact, several recent randomized, controlled trials have failed to find evidence of benefit.206,207 In an editorial entitled “The Pulmonary Artery Catheter, 1967-2007: Rest in Peace?” Rubenfeld and colleagues208 conclude: “The 40-year story of the PA catheter is nearing its end. It is a cautionary tale of rapid adoption and slow evaluation of a monitoring device that when used correctly, provides exquisitely detailed physiological data that, regrettably, does not appear to benefit patients. Older clinicians will look back wistfully on the hours spent placing, troubleshooting, and debating the data from the PA catheter. Younger colleagues will just wonder what all the fuss was about.” The authors of this chapter hope that the same will not be said about TEE 20 years from now. There is danger of being mesmerized by the new technology that has been developed and applied, without documenting its real clinical benefit and cost-effectiveness. Although clinicians are reasonably confident of the value and apparent clinical benefit that use of perioperative TEE has provided their patients, they recognize that much of the evidence supporting its use and showing its benefit is of a low level. Furthermore, having learned that filling pressures (e.g., central venous pressure [CVP] and pulmonary artery occlusion pressure [PAOP]) are poor predictors of volume status and fluid responsiveness, it is disheartening to encounter a study that also found no correlation between objective measures of right and left ventricular end-diastolic volume (per echocardiography and radionuclide cineangiography) and fluid responsiveness.209 Thus, the reader is urged to look critically at the data. The clinical scientific community is urged to design and conduct valid studies to prove the benefit of these new technologies and modalities so that clinicians learn from history and do not repeat the errors of the past.
1 Hessel E.A.II. Evolution of cardiac anesthesia and surgery. In: Kaplan J.A., Reich D.L., Lake C.L., Konstadt S.N., editors. Kaplan’s cardiac anesthesia. ed 5. Philadelphia: Saunders/Elsevier; 2006:3-32.
2 Feigenbaum H. Evolution of echocardiography. Circulation. 1996;93:1321-1327.
3 Oka Y. Preface. In: Yoka Y., Goldiner P.L., editors. Transesophageal echocardiography. Philadelphia: JP Lippincott Company; 1992:xi-xvi.
4 Goldberg B.B., Gramiak R., Freimanis A.K. Early history of diagnostic ultrasound: The role of American radiologists. AJR Am J Roentgenol. 1993;160:189-194.
5 Wells P.N. Milestones in cardiac ultrasound: Echoes from the past. History of cardiac ultrasound. Int J Card Imaging. 1993;9(Suppl 2):3-9.
6 Labovitz A.J. Pearson: Historical perspectives and technical considerations. In: Transesophageal echocardiography: Basic principles and clinical implications. Philadelphia: Lea and Febiger; 1993:1-11.
7 Roelandt J., Souquet J. The development of TEE: A historical review. In: Stumper O., Sutherland G.R., editors. Transesophageal echocardiography in congenital heart disease. London: Edward Arnold; 1994:1-9.
8 Seward J.B. Tranesophageal echocardiography. Past, present, future. In: Freeman W.K., Seward J.B., Khandheria A.J., Tajak. Tranesophageal echocardiography. Boston: Little Brown and Co; 1994:1-8.
9 Weyman A.E. Principles and practice of echocardiography, ed 2. Philadelphia: Lea & Febiger, 1994.
10 Newman P.G., Rozycki G.S. The history of ultrasound. Surg Clin North Am. 1998;78:179-195.
11 Roelandt J.R. Seeing the invisible: A short history of cardiac ultrasound. Eur J Echocardiogr. 2000;1:8-11.
12 Edler I., Lindström K. The history of echocardiography. Ultrasound Med Biol. 2004;30:1565-1644.
13 Feigenbaum H. History of echocardiography. In: Feigenbaum H., Armstrong W.F., Ryan T., editors. Feigenbaum’s echocardiography. ed 6. Philadelphia: Lippincott Williams & Wilkins; 2005:1-10.
14 Singh S., Goyal A. The origin of echocardiography: A tribute to Inge Edler. Tex Heart Inst J. 2007;34:431-438.
15 Thys D.M. Cardiac anesthesia: Thirty years later—the second annual Arthur, E. Weyman lecture. Anesth Analg. 2009;109:1782-1790.
16 Dussik K.T. Uber die Moglichkeit Hochfrequente Mechanische Schwingungen als Diagnostisches Hilfsmitel zu Verwerten. Z Neurol. 1941;174:153.
17 Shampo M.A., Kyle R.A. Karl Theodore Dussik—pioneer in ultrasound. Mayo Clin Proc. 1995;70(12):1136.
18 Keidel W.D. [New method of recording changes in volume of the human heart.]. Z Kreislaufforsch. 1950;39(9–10):257-271.
19 Edler I., Hertz C.H. The use of the ultrasonic reflectoscope for the continuous recordings of the movements of heart walls. Kungl Fysiografiska Sallskapets I Lund Forhandlingar. 1954;24:1-19.
20 Edler I., Gustafson A., Karlefors T., Christensson B. Ultrasoundcardiography. Acta Med Scand Suppl. 1961;370:5-124.
21 Wild J.J., Crawford H.D., Reid J.M. Visualization of the excised human heart by means of reflected ultrasound of echography; preliminary report. Am Heart J. 1957;54:903-906.
22 Joyner C.R.Jr, Reid J.M., Bond J.P. Reflected ultrasound in the assessment of mitral valve disease. Circulation. 1963;27(4 Pt 1):503-511.
23 Feigenbaum H., Waldhausen J.A., Hyde L.P. Ultrasound diagnosis of pericardial effusion. JAMA. 1965;191:711-734.
24 Segal B.L., Likoff W., Kingsley B. Echocardiography. Clinical application in mitral stenosis. JAMA. 1966;195:161-166.
25 Feigenbaum H. The origin of echocardiography? (Letter). Tex Heart Inst J. 2008;35:87-88.
26 Wild J.J., Reid J.M. Application of echo-ranging techniques to the determination of structure of biological tissues. Science. 1952;115(2983):226-230.
27 Howry D.H., Bliss W.R. Ultrasonic visualization of soft tissue structures of the body. J Lab Clin Med. 1952;40:579-592.
28 Ebina T., Oka S., Tanaka M., et al. The ultrasono-tomography for the heart and great vessels in living human subjects by means of the ultrasonic reflection technique. Jpn Heart J. 1967;8:331-353.
29 Asberg A. Ultrasonic cinematography of the living heart. Ultrasonics. 1967;5:113-117.
30 Somer J.C. Electronic sector scanning for ultrasonic diagnosis. Ultrasonics. 1968;6:153-159.
31 Bom N., Lancée C.T., Honkoop J., Hugenholtz P.G. Ultrasonic viewer for cross-sectional analyses of moving cardiac structures. Biomed Eng. 1971;6:500-503. 5
32 vonRamm O.T. Cardiac imaging using a phased array ultrasound system. I. System design. Circulation. 1976;53:258-262.
33 Griffith J.M., Henry W.L. A sector scanner for real time two-dimensional echocardiography. Circulation. 1974;49:1147-1152.
34 Eggleton R.E., Fiegenbaum H., Johnson K.W., et al. Visualization of cardiac dynamics with real time B-mode ultrasonic scanner (Abstract). Circulation. 1974;49–50(Suppl 3):26.
35 Kisslo J., von Ramm O.T., Thurstone F.L. Cardiac imaging using a phased array ultrasound system. II. Clinical technique and application. Circulation. 1976;53:262-267.
36 Kisslo J.A., von Ramm O.T., Thurstone F.L. Dynamic cardiac imaging using a focused, phased-array ultrasound system. Am J Med. 1977;63:61-68.
37 Satomura S. A study on examining the heart with ultrasonics. Jap Circ J. 1956;20:227.
38 Franklin D.L., Schlegel W., Rushmer R.F. Blood flow measured by Doppler frequency shift of back-scattered ultrasound. Science. 1961;134:564-565.
39 Barber F.E., Baker D.W., Nation A.W., et al. Ultrasonic duplex echo-Doppler scanner. IEEE Trans Biomed Eng. 1974;21:109-113.
40 Holen J., Aaslid R., Landmark K., Simonsen S. Determination of pressure gradient in mitral stenosis with a non-invasive ultrasound Doppler technique. Acta Med Scand. 1976;199:455-460.
41 Hatle L., Angelsen B.A., Tromsdal A. Non-invasive assessment of aortic stenosis by Doppler ultrasound. Br Heart J. 1980;43:284-292.
42 Namekawa K., Kasai C., Tsukamoto M., Koyano A., Realtime bloodflow imaging system utilizing auto-correlation techniques, Ultrasound Med Biol, Suppl 2; 1983:203-208
43 Bommer W., Miller L. Real-time two-dimensional color flow Doppler-enhanced imaging in the diagnosis of cardiovascular disease. Am J Cardiol. 1982;49:944.
44 Omoto R., Yokote Y., Takamoto S., et al. The development of real-time two-dimensional Doppler echocardiography and its clinical significance in acquired valvular diseases. With special reference to the evaluation of valvular regurgitation. Jpn Heart J. 1984;25:325-340.
45 Johnson M.L., Holmes J.H., Spangler R.D., Paton B.C. Usefulness of echocardiography in patients undergoing mitral valve surgery. J Thorac Cardiovasc Surg. 1972;64:922-934.
46 Wexler L.F., Pohost G.M. Hemodynamic monitoring: noninvasive techniques. Anesthesiology. 1976;45:156-183.
47 Rathod R., Jacobs H.K., Kramer N.E., et al. Echocardiographic assessment of ventricular performance following induction with two anesthetics. Anesthesiology. 1978;49:86-90.
48 Barash P.G., Glanz S., Katz J.D., et al. Ventricular function in children during halothane anesthesia: An echocardiographic evaluation. Anesthesiology. 1978;49:79-85.
49 Strom J., Becker R.M., Frishman W., et al. Effects of hypothermic hyperkalemic cardioplegic arrest on ventricular performance during cardiac surgery: Assessment by intraoperative echocardiography. N Y State J Med. 1978;78:2210-2213.
50 Rodigas P.C., Meyer F.J., Haasler G.B., et al. Intraoperative 2-dimensional echocardiography: Ejection of microbubbles from the left ventricle after cardiac surgery. Am J Cardiol. 1982;50:1130-1132.
51 Dubroff J.M., Wong C.Y., et al. Left ventricular ejection fraction during cardiac surgery: A two-dimensional echocardiographic study. Circulation. 1983;68:95-103.
52 Goldman M.E., Mindich B.P., Teichholz L.E., et al. Intraoperative contrast echocardiography to evaluate mitral valve operations. J Am Coll Cardiol. 1984;4:1035-1040.
53 Goldman M.E., Mindich B.P. Intraoperative cardioplegic contrast echocardiography for assessing myocardial perfusion during open heart surgery. J Am Coll Cardiol. 1984;4:1029-1034.
54 Cieszynski T. [Intracardiac method for the investigation of structure of the heart with the aid of ultrasonics.]. Arch Immunol Ther Exp (Warsz). 1960;8:551-557. Polish
55 Omoto R. Ultrasonic tomography of the heart: An intracardiac scan method. Ultrasonics. 1967;5:80-83.
56 Kimoto S., et al. Ultrasonic tomography of the liver and detection of heart atrial septal defect with the aid of ultrasonic intravenous probes. Ultrasonics. 1964;2:82.
57 Carleton R.A., Clark J.G. Measurement of left ventricular diameter in the dog by cardiac catheterization. Validation and physiologic meaningfulness of an ultrasonic technique. Circ Res. 1968;22:545-558.
58 Eggleton R.C., Townsend C., Herrick J., et al. Ultrasonic visualization of left ventricular dynamics. Ultrasonics. 1970;17:142-153.
59 Bom N., Lancée C.T., Van Egmond F.C. An ultrasonic intracardiac scanner. Ultrasonics. 1972;10:72-76.
60 Side C.D., Gosling R.G. Non-surgical assessment of cardiac function. Nature. 1971;232:335-336.
61 Duck F.A., Hodson C.J., Tomlin P.J. An esophageal Doppler probe for aortic flow velocity monitoring. Ultrasound Med Biol. 1974;1:233-241.
62 Daigle R.E., Miller C.W., Histand M.B., et al. Nontraumatic aortic blood flow sensing by use of an ultrasonic esophageal probe. J Appl Physiol. 1975;38:1153-1160.
63 Frazin L., Talano J.V., Stephanides L., et al. Esophageal echocardiography. Circulation. 1976;54:102-108.
64 Matsumoto M., Oka Y., Lin Y.T., et al. Transesophageal echocardiography; for assessing ventricular performance. N Y State J Med. 1979;79:19-21.
65 Matsumoto M., Oka Y., Strom J., et al. Application of transesophageal echocardiography to continuous intraoperative monitoring of left ventricular performance. Am J Cardiol. 1980;46:95-105.
66 Hisanaga K., Hisanaga A., Hibi N., et al. High speed rotating scanner for transesophageal cross-sectional echocardiography. Am J Cardiol. 1980;46:837-842.
67 DiMagno E.P., Buxton J.L., Regan P.T., et al. Ultrasonic endoscope. Lancet. 1980;1:629-631.
68 Souquet J., Hanrath P., Zitelli L., et al. Transesophageal phased array for imaging the heart. IEEE Trans Biomed Eng. 1982;29:707-712.
69 Schluter M., Langenstein B.A., Polster J., et al. Transesophageal cross-sectional echocardiography with a phased array transducer system. Technique and initial clinical results. Br Heart J. 1982;48:67-72.
70 Schiller N.B., Intraoperative TEE: Its inception, development and future [Personal notes of the author (E.A.H.)]. Chicago, Abbott Anesthesia Lecture 21st Annual Meeting of the Society of Cardiovascular Anesthesiologists, April 28; 1999
71 Cahalan M.K., Kremer P., Schiller N.B., et al. Intraoperative monitoring with two-dimensional transesophageal echocardiography (Abstract). Anesthesiology. 1982;57:A-153.
72 Roizen M.F., Kremer P., Cahalan M., et al. Monitoring with transesophageal echocardiography: Patients undergoing supraceliac aortic occlusion (Abstract). Anesthesiology. 1982;57:A-152.
73 Beaupre P.N., Cahalan M.K., Kremer P.F., et al. Does pulmonary artery catheter occlusion pressure adequately reflect left ventricular filling during anesthesia and surgery? (Abstract). Anesthesiology. 1983;59:A3.
74 Beaupre P.N., Roizen M.F., Cahalan M.K., et al. Hemodynamic and two-dimensional transesophageal echocardiographic analysis of an anaphylactic reaction in a human. Anesthesiology. 1984;60:482-484.
75 Roizen M.F., Hunt T.K., Beaupre P.N., et al. The effect of alpha-adrenergic blockade on cardiac performance and tissue oxygen delivery during excision of pheochromocytoma. Surgery. 1983;94:941-945.
76 Topol E.J., Humphrey L.A., Blanck T.J.J., et al. Characterization of post-cardiopulmonary bypass hypotension with intraoperative transesophageal echocardiography. Anesthesiology. 1983;59:A2.
77 Topol E.J., Weiss J.L., Guzman P.A., et al. Immediate improvement of dysfunctional myocardial segments after coronary revascularization: Detection by intraoperative transesophageal echocardiography. J Am Coll Cardiol. 1984;4:1123-1134.
78 Cucchiara R.F., Nugent M., Seward J.B., Messick J.M. Air embolism in upright neuro-surgical patients: Detection and localization by two-dimensional transesophageal echocardiography. Anesthesiology. 1984;60:353-355.
79 Roizen M.F., Beaupre P.N., Alpert R.A., et al. Monitoring with two-dimensional transesophageal echocardiography. Comparison of myocardial function in patients undergoing supraceliac, suprarenal-infraceliac, or infrarenal aortic occlusion. J Vasc Surg. 1984;1:300-305.
80 Gewertz B.L., Kremser P.C., Zarins C.K., et al. Transesophageal echocardiographic monitoring of myocardial ischemia during vascular surgery. J Vasc Surg. 1987;5:607-613.
81 Smith J.S., Cahalan M.F., Benfiel D.J., et al. Intraoperative detection of myocardial ischemia in high-risk patients: Electrocardiography versus two-dimensional transesophageal echocardiography. Circ. 1985;72:1015-1021.
82 Schlüter M., Hinrichs A., Thier W., et al. Transesophageal two-dimensional echocardiography: Comparison of ultrasonic and anatomic sections. Am J Cardiol. 1984;53:1173-1178.
83 Seward J.B., Khandheria B.K., Oh J.K., et al. Transesophageal echocardiography: Technique, anatomic correlations, implementation, and clinical applications. Mayo Clin Proc. 1988;63:649-680.
84 Kaplan J.A. Transesophageal echocardiography. Mt Sinai J Med. 1984;51:592-594.
85 Konstadt S., Goldman M., Thys D., et al. Intraoperative diagnosis of myocardial ischemia. Mt Sinai J Med. 1985;52:521-525.
86 Konstadt S.N., Thys D., Mindich B.P., et al. Validation of quantitative intraoperative transesophageal echocardiography. Anesthesiology. 1986;65:418-421.
87 Thys D.M., Hillel Z., Goldman M.E., et al. A comparison of hemodynamic indices derived by invasive monitoring and two-dimensional echocardiography. Anesthesiology. 1987;67:630-634.
88 Konstadt S.N., Kaplan J.A., Tannenbaum M.A., et al. Case 5—1987. 45-year-old woman develops acute left ventricular ischemia and dysfunction after subxiphoid drainage of a pericardial tamponade. J Cardiothorac Anesth. 1987;1:469-478.
89 Cahalan M.K., Litt L., Botvinick E.H., Schiller N.B. Advances in noninvasive cardiovascular imaging: Implication for the anesthesiologist. Anesthesiology. 1987;66:356-372.
90 Clements F.M., deBruijn N.P. Perioperative evaluation of regional wall motion by transesophageal two-dimensional echocardiography. Anesth Analg. 1987;66:249-261.
91 Abel M.D., Nishimura R.A., Callahan M.J., et al. Evaluation of intraoperative transesophageal two-dimensional echocardiography. Anesthesiology. 1987;66:64-68.
92 Thys D.M., Hillel Z., Konstadt S.N., Goldman M.E. Intraoperative echocardiography. In: Kaplan J.A., editor. Clinical anesthesia. ed 2. Orlando: Grune & Stratton; 1987:255-318.
93 Goldman M.E., Thys D., Ritter S., et al. Transesophageal real time Doppler flow imaging: A new method for intraoperative cardiac evaluation. Journal of the American College of Cardiology. 1986;7:1A.
94 Takamoto S., Omoto R. Visualization of thoracic dissecting aortic aneurysm by transesophageal Doppler color flow mapping. Herz. 1987;12:187-193.
95 deBruijn N.P., Clements F.M., Kisslo J.A. Intraoperative color flow mapping: Initial experience. Anesth Analg. 1987;66:386-390.
96 Roewer N., Bednarz F., Schulte am Esch J. Continuous measurement of intracardiac and pulmonary blood flow velocities with transesophageal pulsed Doppler echocardiography: Technique and initial clinical experience. J Cardiothorac Anesth. 1987;1:418-428.
97 Schlüter M., Langenstein B.A., Hanrath P., et al. Assessment of transesophageal pulsed Doppler echocardiography in the detection of mitral regurgitation. Circulation. 1982;66:784-799.
98 Omoto R., Kyo S., Matsumura M., et al. Bi-plane color transesophageal Doppler echocardiography (color TEE): Its advantages and limitations. Int J Card Imaging. 1989;4:57-58.
99 Curling P.E., Newsom L.R., Rogers A., et al. 2D transesophageal echocardiography: A bidirectional phased array probe with temperature monitoring. Anesthesiology. 1984;61:A159.
100 Roelandt J.R., Thomson I.R., Vletter W.B., et al. Multiplane transesophageal echocardiography: Latest evolution in an imaging revolution. J Am Soc Echocardiogr. 1992;5:361-367.
101 Kyo S., Koike K., Takanawa E., et al. Impact of transesophageal Doppler echocardiography on pediatric cardiac surgery. Int J Card Imaging. 1989;4:41-42.
102 Pandian N.G., Hsu T.L., Schwartz S.L., et al. Multiplane transesophageal echocardiography. Imaging planes, echocardiographic anatomy, and clinical experience with a prototype phased array OmniPlane probe. Echocardiography. 1992;9:649-666.
103 Hillel Z., Mikula S., Thys D. The current state of intraoperative echocardiography in North America: Results of a survey. J Cardiothorac Anesth. 1988;2:803-811.
104 Kaplan J.A. Monitoring technology: Advances and restraints. J Cardiothorac Anesth. 1989;3:257-259.
105 Ungerleider R.M., Kisslo J.A., Greeley W.J., et al. Intraoperative prebypass and postbypass epicardial color flow imaging in the repair of atrioventricular septal defects. J Thorac Cardiovasc Surg. 1989;98:90-99. discussion 99–100
106 Ungerleider R.M., Greeley W.J., Sheikh K.H., et al. Routine use of intraoperative epicardial echocardiography and Doppler color flow imaging to guide and evaluate repair of congenital heart lesions. A prospective study. J Thorac Cardiovasc Surg. 1990;100:297-309.
107 Ungerleider R.M., Kisslo J.A., Greeley W.J., et al. Intraoperative echocardiography during congenital heart operations: Experience from 1,000 cases. Ann Thorac Surg. 1995;60(6 Suppl):S539-S542.
108 Stevenson J.G., Sorensen G.K., Gartman D.M., et al. Transesophageal echocardiography during repair of congenital cardiac defects: Identification of residual problems necessitating reoperation. J Am Soc Echocardiogr. 1993;6:356-365.
109 Stevenson J.G., Sorensen G.K. Proper probe size for pediatric transesophageal echocardiography. Am J Cardiol. 1993;72:491-492.
110 Stevenson J.G. Role of intraoperative transesophageal echocardiography during repair of congenital cardiac defects. Acta Paediatr Suppl. 1995;410:23-33.
111 Stevenson J.G. Utilization of intraoperative transesophageal echocardiography during repair of congenital cardiac defects: A survey of North American centers. Clin Cardiol. 2003;26:132-134.
112 Savage R.M. Preface. In: Savage R.M., Aronson S., editors. Comprehensive textbook of intraoperative transesophageal echocardiography. Philadelphia: Lipppincott Williams & Wilkins; 2005:xiii.
113 Rouine-Rapp K., Ionescu P., Balea M. Detection of intraoperative segmental wall-motion abnormalities by transesophageal echocardiography: The incremental value of additional cross sections in the transverse and longitudinal planes. Anesth Analg. 1996;83:1141-1148.
114 Bergquist B.D., Bellows W.H., Leung J.M. Transesophageal echocardiography in myocardial revascularization: II. Influence on intraoperative decision making. Anesth Analg. 1996;82:1139-1145.
115 Savage R.M., Lytle B.W., Aronson S., et al. Intraoperative echocardiography is indicated in high-risk coronary artery bypass grafting. Ann Thorac Surg. 1997;64:368-373. discussion 373–374
116 Aronson S., Dupont F., Savage R., et al. Changes in regional myocardial function after coronary artery bypass graft surgery are predicted by intraoperative low-dose dobutamine echocardiography. Anesthesiology. 2000;93:685-692.
117 Thys D.M., Hillel Z., Schwartz A.J. Textbook of cardiothoracic anesthesiology. New York: McGraw-Hill Companies, 2001.
118 Feinstein S.B., Coll B., Staub D., et al. Contrast enhanced ultrasound imaging. J Nucl Cardiol. 2010;17:106-115.
119 Gramiak R., Shah P.M. Echocardiography of the aortic root. Invest Radiol. 1968;3:356-366.
120 Seward J.B., Tajik A.J., Spangler J.G., Ritter D.G. Echocardiographic contrast studies: Initial experience. Mayo Clin Proc. 1975;50:163-192.
121 Feinstein S.B., Ten Cate F.J., Zwehl W., et al. Two-dimensional contrast echocardiography. I. In vitro development and quantitative analysis of echo contrast agents. J Am Coll Cardiol. 1984;3:14-20.
122 Aronson S., Savage R., Toledano A., et al. Identifying the cause of left ventricular systolic dysfunction after coronary artery bypass surgery: The role of myocardial contrast echocardiography. J Cardiothorac Vasc Anesth. 1998;12:512-518.
123 Kabas J.S., Kisslo J., Flick C.L., et al. Intraoperative perfusion contrast echocardiography. Initial experience during coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1990;99:536-542.
124 Spotnitz W.D., Kaul S. Intraoperative assessment of myocardial perfusion using contrast echocardiography. Echocardiography. 1990;7:209-228.
125 Aronson S., Lee B.K., Wiencek J.G., et al. Assessment of myocardial perfusion during CABG surgery with two-dimensional transesophageal contrast echocardiography. Anesthesiology. 1991;75:433-440.
126 Aronson S. Measurement of myocardial perfusion by contrast echocardiography: Application in the operating room. Coron Artery Dis. 2000;11:227-234.
127 Dijkmans P.A., Senior R., Becher H., et al. Myocardial contrast echocardiography evolving as a clinically feasible technique for accurate, rapid, and safe assessment of myocardial perfusion: The evidence so far. J Am Coll Cardiol. 2006;48:2168-2177.
128 Mulvagh S.L., Rakowski H., Vannan M.A., et al. American Society of Echocardiography: American Society of Echocardiography Consensus 174. Statement on the Clinical Applications of Ultrasonic Contrast Agents in Echocardiography. J Am Soc Echocardiogr. 2008;21:1179-1201.
129 Whitley W.S., Glas K.E. An argument for routine ultrasound screening of the thoracic aorta in the cardiac surgery population. Semin Cardiothorac Vasc Anesth. 2008;12:290-297.
130 Reeves S.T., Glas K.E., Eltzschig H., et al. Council for Intraoperative Echocardiography of the American Society of Echocardiography; Society of Cardiovascular Anesthesiologists: Guidelines for performing a comprehensive epicardial echocardiography examination: Recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg. 2007;105:22-28.
131 Glas K.E., Swaminathan M., Reeves S.T., et al. Council for Intraoperative Echocardiography of the American Society of Echocardiography; Society of Cardiovascular Anesthesiologists; Society of Thoracic Surgeons: Guidelines for the performance of a comprehensive intraoperative epiaortic ultrasonographic examination: Recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists; endorsed by the Society of Thoracic Surgeons. Anesth Analg. 2008;106:1376-1384.
132 Foster G.P., Picard M.H. Intracardiac echocardiography: Current uses and future directions. Echocardiography. 2001;18:43-48.
133 Nissen S.E., Grines C.L., Gurley J.C., et al. Application of a new phased-array ultrasound imaging catheter in the assessment of vascular dimensions. In vivo comparison to cineangiography. Circulation. 1990;81:660-666.
134 Gussenhoven E.J., Taams M.A., Roelandt J.R., et al. Transesophageal two-dimensional echocardiography: Its role in solving clinical problems. J Am Coll Cardiol. 1986;8:975-979.
135 Erbel R., Börner N., Steller D., et al. Detection of aortic dissection by transoesophageal echocardiography. Br Heart J. 1987;58:45-51.
136 Erbel R., Engberding R., Daniel W., et al. Echocardiography in diagnosis of aortic dissection. Lancet. 1989;1:457-461.
137 Chan K.-L. Transesophageal echocardiography for assessing cause of hypotension after cardiac surgery. Am J Cardiol. 1988;62:1142-1143.
138 Reichert C.L., Visser C.A., Koolen J.J., et al. Transesophageal echocardiography in hypotensive patients after cardiac operations. Comparison with hemodynamic parameters. J Thorac Cardiovasc Surg. 1992;104:321-326.
139 Pearson A.C., Castello R., Labovitz A.J. Safety and utility of transesophageal echocardiography in the critically ill patient. Am Heart J. 1990;119:1083-1089.
140 Oh J.K., Seward J.B., Khandheria B.K., et al. Transesophageal echocardiography in critically ill patients. Am J Cardiol. 1990;66:1492-1495.
141 Foster E. The role of transesophageal echocardiography in critical care: UCSF experience. J Am Soc Echocardiogr. 1992;5:368-374.
142 Font V.E., Obarski T.P., Klein A.L., et al. Transesophageal echocardiography in the critical care unit. Cleve Clin J Med. 1991;58:315-322.
143 Hwang J.J., Shyu K.G., Chen J.J., et al. Usefulness of transesophageal echocardiography in the treatment of critically ill patients. Chest. 1993;104:861-866.
144 Khoury A.F., Afridi I., Quiñones M.A., Zoghbi W.A. Transesophageal echocardiography in critically ill patients: Feasibility, safety, and impact on management. Am Heart J. 1994;127:1363-1371.
145 Benjamin E., Griffin K., Leibowitz A.B., et al. Goal-directed transesophageal echocardiography performed by intensivists to assess left ventricular function: Comparison with pulmonary artery catheterization. J Cardiothorac Vasc Anesth. 1998;12:10-15.
146 Hüttemann E., Schelenz C., Kara F., et al. The use and safety of transoesophageal echocardiography in the general ICU—a minireview. Acta Anaesthesiol Scand. 2004;48:827-836.
147 Salem R., Vallee F., Rusca M., Mebazaa A. Hemodynamic monitoring by echocardiography in the ICU: The role of the new echo techniques. Curr Opin Crit Care. 2008;14:561-568.
148 Mahmood F., Christie A., Matyal R. Transesophageal echocardiography and noncardiac surgery. Semin Cardiothorac Vasc Anesth. 2008;12:265-289.
149 Hodgins L., Kisslo J.A., Mark J.B. Perioperative transesophageal echocardiography: The anesthesiologist as cardiac diagnostician. Anesth Analg. 1995;80:4-6.
150 Goldstein S. Pro: The general anesthesiologist should be trained and certified in transesophageal echocardiography. J Cardiothorac Vasc Anesth. 2010;24:183-188.
151 Green M. Con: General anesthesiologists should not be trained and certified in basic transesophageal echocardiography. J Cardiothorac Vasc Anesth. 2010;24:189-190.
152 Vezina D.P., Johnson K.B., Cahalan M.K., Transesophageal echocardiography. ed 7, Miller R.E., Eriksson L.A., Fleisher, et al., Miller’s anesthesia; 2010. Churchill Livingstone/Elsevier. Philadelphia
153 Yeates T.M., Zimmerman J.M., Cahalan M.K. Perioperative echocardiography: Two-dimensional and three-dimensional applications. Anesthesiol Clin. 2008;26:419-435.
154 Marcucci C., Lauer R., Mahajan A. New echocardiographic techniques for evaluating left ventricular myocardial function. Semin Cardiothorac Vasc Anesth. 2008;12:228-247.
155 Weyman A.E. Future directions in echocardiography. Rev Cardiovasc Med. 2009;10:4-13.
156 Baum G. Orbital lesion localization by three dimensional ultrasonography. N Y State J Med. 1961;61:4149-4157.
157 Dekker D.L., Piziali R.L., Dong E. A system for ultrasonically imaging the human heart in three dimensions. Comput Biomed Res. 1974;7:544-553.
158 Moritz W.E. A microprocessor-based spatial locating system for use with diagnostic ultrasound. IEEE Trans Biomed Eng. 1976;64:966-974.
159 Matsumoto M., Matsuo H., Kitabatake A., et al. Three-dimensional echocardiograms and two-dimensional echocardiographic images at desired planes by a computerized system. Ultrasound Med Biol. 1977;3:163.
160 Raab F.H., Blood E.B., Steiner T.O., et al. Magnetic position and orientation tracking system. IEEE Trans Aerospace Elec Sys. 1979;AES-15:709-718.
161 Ghosh A., Maurer G. Three-dimensional reconstruction of echo-cardiographic images using the rotation method. Ultrasound Med Biol. 1982;8:655-661.
162 Geiser E.A., Ariet M., Conetta D.A., et al. Dynamic three-dimensional echocardiographic reconstruction of the intact human left ventricle: Technique and initial observations in patients. Am Heart J. 1982;103:1056-1065.
163 vonRamm O.T., Smith S.W. Real time volumetric ultrasound imaging system. J Digit Imaging. 1990;3:261-266.
164 Martin R.W., Bashein G., Zimmer R., Sutherland J. An endoscopic micromanipulator for multiplanar transesophageal imaging. Ultrasound Med Biol. 1986;12:965-975.
165 Martin R.W., Graham M.M., Kao R., Bashein G. Measurement of left ventricular ejection fraction and volumes with three-dimensional reconstructed transesophageal ultrasound scans: Comparison to radionuclide and thermal dilution measurements. J Cardiothorac Anesth. 1989;3:260-268.
166 Wollschläger H., Zeiher A.M., Klein H.P., et al. [Transesophageal echo computer tomography (ECHO-CT): a new method of dynamic 3-D reconstruction of the heart]. Biomed Tech (Berl). 1989;34(Suppl):10-11.
167 Pandian N.G., Schwartz S.L., et al. Three-dimensional and four-dimensional transesophageal echocardiographic imaging of the heart and aorta in humans using a computed tomographic imaging probe. Echocardiography. 1992;9:677-687.
168 Chen Q., Nosir Y.F., Vletter W.B., et al. Accurate assessment of mitral valve area in patients with mitral stenosis by three-dimensional echocardiography. J Am Soc Echocardiogr. 1997;10:133-140.
169 Hung J., Lang R., Flachskampf F., et al. ASE Position Paper: 3D echocardiography: A review of the current status and future directions. J Am Soc Echocardiogr. 2007;20:213-233.
170 Yang H.S., Bansal R.C., Mookadam F., et al. American Society of Echocardiography. Practical guide for three-dimensional transthoracic echocardiography using a fully sampled matrix array transducer. J Am Soc Echocardiogr. 2008;21:979-989.
171 Corsi C., Lang R.M., Veronesi F., et al. Volumetric quantification of global and regional left ventricular function from real-time three-dimensional echocardiographic images. Circulation. 2005;112:1161-1170.
172 Nesser HJ., Mor-Avi V., Gorissen W., et al. Quantification of left ventricular volumes using three-dimensional echocardiographic speckle tracking: Comparison with MRI. Eur Heart J. 2009;30:1565-1573.
173 Voigt I., Ionasec R.I., Georgescu B., Houle H., et al, Model-driven physiological assessment of the mitral valve from 4D TEE,. 72610R.11. Progress in biomedical optics and imaging; 10. 1; 2009:72610R.1. no 37
174 Isaaz K., Thompson A., Ethevenot G., et al. Doppler echocardiographic measurement of low velocity motion of the left ventricular posterior wall. Am J Cardiol. 1989;64:66-75.
175 Marcucci C., Lauer R., Mahajan A. New echocardiographic techniques for evaluating left ventricular myocardial function. Semin Cardiothorac Vasc Anesth. 2008;12(4):228-247.
176 Nagueh S.F., Middleton K.J., Kopelen H.A., et al. Doppler tissue imaging: A noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527-1533.
177 Fleming A.D., Xia X., McDicken W.N., et al. Myocardial velocity gradients detected by Doppler imaging. Br J Radiol. 1994;67:679-688.
178 Bohs L.N., Trahey G.E. A novel method for angle independent ultrasonic imaging of blood flow and tissue motion. IEEE Trans Biomed Eng. 1991;38:280-286.
179 Pérez J.E., Prater D.M., et al. Automated, on-line quantification of left ventricular dimensions and function by echocardiography with backscatter imaging and lateral gain compensation. Am J Cardiol. 1992;70:1200-1205.
180 Lang R.M., Vignon P., Weinert L., et al. Echocardiographic quantification of regional left ventricular wall motion with color kinesis. Circulation. 1996;93:1877-1885.
181 Hartmann T., Kolev N., Blaicher A., et al. Validity of acoustic quantification colour kinesis for detection of left ventricular regional wall motion abnormalities: A transoesophageal echocardiographic study. Br J Anaesth. 1997;79:482-487.
182 Gorcsan I.3rd, Gulati V.K., Mandarino W.A., Katz W.E. Color-coded measures of myocardial velocity throughout the cardiac cycle by tissue Doppler imaging to quantify regional left ventricular function. Am Heart J. 1996;131:1203-1213.
183 Katz W.E., Gulati V.K., Mahler C.M., Gorcsan J. Quantitative evaluation of the segmental left ventricular response to dobutamine stress by tissue Doppler echocardiography. Am J Cardiol. 1997;79:1036-1042.
184 dos Reis M.d.o.C., da Rocha A.F., Vasconcelos D.F., et al. Semi-automatic detection of the left ventricular border. Conf Proc IEEE Eng Med Biol Soc. 2008:218-221.
185 Roelandt J., Ten Cate F.J., Hugenholtz P. The ultrasonic stethoscope: A miniature hand-held device for real time cardiac imaging. Circulation. 1978;58(II):II-79–II-81.
186 Roelandt J.R. Ultrasound stethoscopy: A renaissance of the physical examination? Heart. 2003;89:971-973.
187 Mondillo S., Giannotti G., Innelli P., et al. Hand-held echocardiography: Its use and usefulness. Int J Cardiol. 2006;111:1-5.
188 Kobal S.L., Atar S., Siegel R.J. Hand-carried ultrasound improves the bedside cardiovascular examination. Chest. 2004;126:693-701.
189 Alexander J.H., Peterson E.D., Chen A.Y., et al. Feasibility of point-of-care echocardiography by internal medicine house staff. Am Heart J. 2004;147:476-481.
190 Seward J.B., Douglas P.S., Erbel R., et al. Hand-carried cardiac ultrasound (HCU) device: Recommendations regarding new technology. A report from the Echocardiography Task Force on New Technology of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:369-373.
191 Vignon P., Dugard A., Abraham J., et al. Focused training for goal-oriented hand-held echocardiography performed by noncardiologist residents in the intensive care unit. Intensive Care Med. 2007;33:1795-1799.
192 Feigenbaum H. Educational problems in echocardiography (Editorial). Am J Cardiol. 1974;34:741-742.
193 Aronson S., Thys D.M. Training and certification in perioperative transesophageal echocardiography: A historical perspective. Anesth Analg. 2001;93:1422-1427.
194 deBruijn N.P., Clements F.M., editors. Intraoperative use of echocardiography. A Society of Cardiovascular Anesthesiologists Monograph. Philadelphia: JB Lippincott, 1991.
195 Thys D.M., Abel M., Bollen B.A., et al. Practice guidelines for perioperative transesophageal echocardiography. A report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Anesthesiology. 1996;84:986-1006.
196 Shanewise J.S., Cheung A.T., Aronson S., et al. ASE/SCA guidelines for performing a comprehensive intraoperative multiplane transesophageal echocardiography examination: Recommendations of the American Society of Echocardiography Council for Intraoperative Echocardiography and the Society of Cardiovascular Anesthesiologists Task Force for Certification in Perioperative Transesophageal Echocardiography. Anesth Analg. 1999;89:870-884.
197 Cahalan M.K., Stewart W., Pearlman A., et al. Society of Cardiovascular Anesthesiologists; American Society of Echocardiography Task Force. American Society of Echocardiography and Society of Cardiovascular Anesthesiologists task force guidelines for training in perioperative echocardiography. J Am Soc Echocardiogr. 2002;15:647-652.
198 Quiñones M.A., Douglas P.S., Foster E., et al. American Society of Echocardiography; Society of Cardiovascular Anesthesiologists; Society of Pediatric Echocardiography: ACC/AHA clinical competence statement on echocardiography: A report of the American College of Cardiology/American Heart Association/American College of Physicians-American Society of Internal Medicine Task Force on clinical competence. J Am Soc Echocardiogr. 2003;16:379-402.
199 Mathew J.P., Glas K., Troianos C.A., et al. Council for Intraoperative Echocardiography of the American Society of Echocardiography: ASE/SCA recommendations and guidelines for continuous quality improvement in perioperative echocardiography. Anesth Analg. 2006;103:1416-1425.
200 American Society of Anesthesiologists and Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Practice guidelines for perioperative transesophageal echocardiography. An updated report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Anesthesiology. 2010;112(5):1084-1096.
201 Aronson S., Subhiyah R., et al. Development and analysis of a new certifying examination in perioperative transesophageal echocardiography. Anesth Analg. 2002;95:1476-1482.
202 Morewood G.H., Gallgher M.E., Conlay L.A. Current practice patterns for perioperative transesophageal echocardiography in the United States (Abstract). Anesth Analg. 2001;92:SCA 94.
203 Hillel Z., Mikula S., Thys D. The current state of intraoperative echocardiography in North America: Results of a survey. J Cardiothorac Anesth. 1988;2(6):803-811.
204 Swan H.J., Ganz W., Forrester J., et al. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med. 1970;283:447-451.
205 Wiener R.S., Welch H.G. Trends in the use of the pulmonary artery catheter in the United States, 1993-2004. JAMA. 2007;298:423-429.
206 Shah M.R., Hasselblad V., Stevenson L.W., et al. Impact of the pulmonary artery catheter in critically ill patients: Meta-analysis of randomized clinical trials. JAMA. 2005;294:1664-1670.
207 Wheeler A.P., Bernard G.R., Thompson B.T., et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213-2224.
208 Rubenfeld G.D., McNamara-Aslin E., Rubinson L. The pulmonary artery catheter, 1967-2007: Rest in peace? JAMA. 2007;298:458-461.
209 Kumar A., Anel R., Bunnell E., et al. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit Care Med. 2004;32:691-699.
* The interested reader is referred to the tribute written by Singh and Goyal14 and the historic review written by Edler himself and published posthumously.12
* Goldberg, Gramiak, and Freimanis4 have written a history of the contribution of American radiologists to the early development of diagnostic ultrasound.