Evoked potential monitoring
The evoked potential waveform
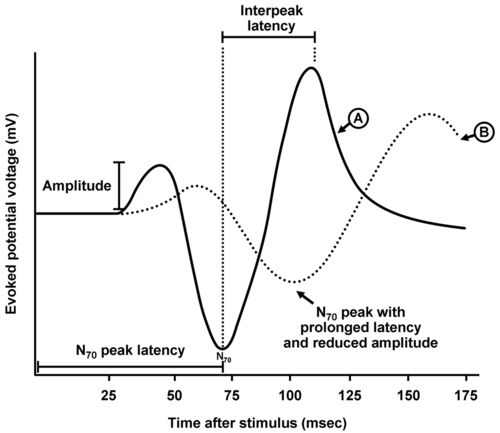
All four EP techniques involve the application of a stimulus that generates a neuronal response with measurement of that response. Typical recordings are expressed as a graph of time (in milliseconds) on the abscissa (i.e., x-axis) and voltage (mV) as the ordinate (i.e., y-axis) (Figure 20-1). The responses are very low voltage and require signal averaging to enhance their quality, that is, recorded waveforms are a composite of 50 to 100 or more measurements following multiple stimulation measurement cycles that serve to “subtract out” higher-voltage interference (e.g., electrocardiogram, electroencephalogram, and electrical noise within the operative suite). Peak voltages in the measured waveform refer to positive or negative deflections, designated by a P or N, respectively.
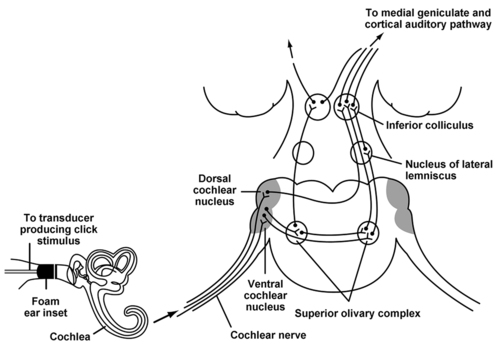
Brainstem auditory evoked responses
BAERs allow monitoring of the integrity of the auditory pathway both peripherally and centrally. Stimuli are loud, repetitive clicks produced by a device placed over or in the auditory canal or canals. Measurement of the response is from electrodes placed on the scalp or external ears to record contralateral and ipsilateral signals that have and have not decussated, respectively. BAER monitoring allows assessment of the acoustic transduction system of the middle and inner ear, the cochlear nerve (i.e., cranial nerve VIII), and the entire central auditory pathway rostrally to the primary auditory cortex located in the temporal lobe of the brain (Figure 20-2). Some anesthetic agents may cause minor changes in amplitude or latency of recorded waveforms; however, these changes are usually very small, even with large changes in anesthetic dose. Therefore, significant intraoperative BAER changes are usually indicative of a surgical trespass.
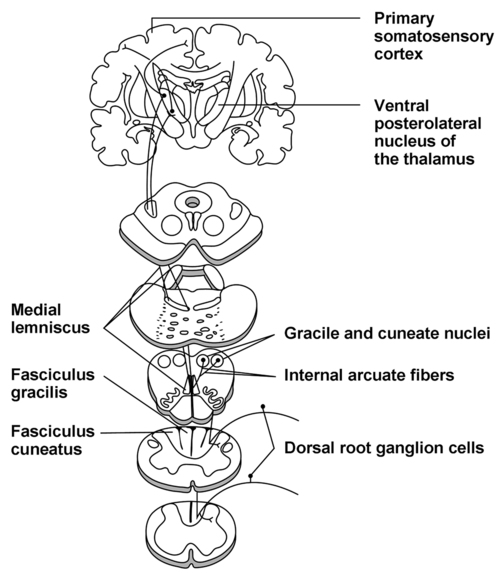
Somatosensory evoked potentials
Monitoring of SSEPs permit assessment of major sensory pathways (within the dorsal column medial lemniscus) responsible for transmission of touch, vibration, and proprioception (Figure 20-3). These sensory pathways consist of first-order neurons originating at peripheral sensory receptors and entering the spinal cord to travel rostrally within the ipsilateral posterior column to synapse on the second-order neurons located within the gracile and cuneate nuclei at the cervicomedullary junction. These neurons then decussate and proceed rostrally through the brainstem as the medial lemniscus to reach the ventral posterolateral nucleus of the thalamus. The second-order neurons synapse here with third-order neurons that then travel rostrally to the primary somatosensory cortex located on the postcentral gyrus.
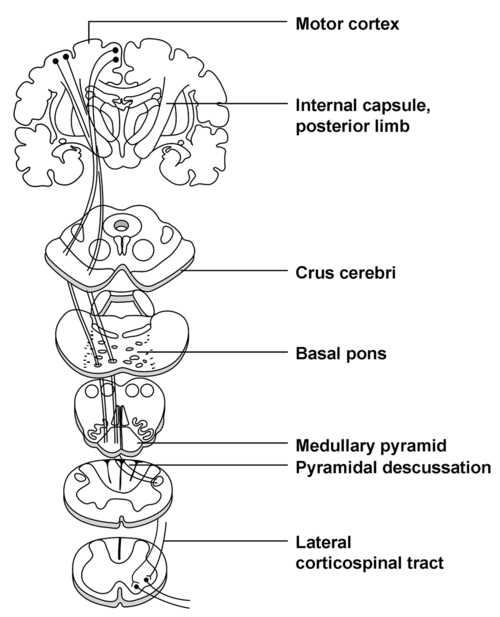
Motor evoked potentials
Unlike SSEP techniques that assess the integrity of afferent neural pathways, MEPs assess the efferent motor pathway: the corticospinal tract (Figure 20-4). Cell bodies of first-order motor neurons exist in the precentral gyrus of the frontal lobe. Axons extend via the internal capsule into the crux cerebri of the midbrain, basal pons, and pyramidal tracts of the medulla. In the caudal region of the medulla, these axons decussate and travel in the lateral region of the spinal cord (i.e., the lateral corticospinal tract) to synapse on secondary motor neurons. These secondary motor neurons then leave the ventral spinal cord, combine to form nerves and plexuses, and proceed to innervate muscles. Blood to the primary motor pathway comes from branches of the middle and anterior cerebral arteries in the cerebrum, the vertebrobasilar system in the brainstem, and the anterior spinal artery in the spinal cord.
Intraoperative changes in evoked potential signals
• Was a drug recently administered or was the dose of a drug recently changed?
• Was an NMBA given during MEP monitoring?
• Is there a physiologic reason for neural ischemia or hypoxia (e.g., hypotension, hypoxia, anemia)? An increase in blood pressure, the O2 content of blood, and hemoglobin concentration may all be helpful in improving O2 delivery to compromised neural tissue even in the event of a surgical cause of signal decrease or loss.
• Is there significant hypothermia?
• Is there a positioning problem that may result in peripheral nerve compression?
• Is there a problem with the monitoring equipment (e.g., displacement of a monitoring lead)?