Evaluation of the Developmentally or Physically Disabled Patient
Perspective
Children with special health care needs are those who have or are at increased risk for a chronic physical, developmental, behavioral, or emotional condition and who also require health and related services of a type or amount beyond that required by children generally.1
The prevalence and characteristics of the CSHCN community depend on the definition used. With use of the federal definition derived by the Maternal and Child Health Bureau of 1998, a study analyzing data from 2006 to 2008 found that approximately one in six children in the United States experienced a special health care need.2 There is a higher prevalence among older children, boys, and non-Hispanic white and black children, and after adjustment for demographic factors, families in poverty are more likely to have CSHCN.
Advances in medical science and technology have been instrumental in allowing children with complex medical problems to survive beyond the neonatal period. Moreover, family-centered care has been the goal for the care of these children, and as a result, many are living at home instead of in institutions.3 CSHCN are frequent users of the emergency medical system. Emergency medical services are typically activated during the time of a crisis. This crisis may occur because of equipment failure or panic by the caregiver who is fatigued or new to caring for the child with special needs. However, a survey of 100 families with CSHCN conducted at a tertiary care urban pediatric center demonstrated that although 97% of the families had sought emergency care in the past, only 23% of the caregivers had ever called 911 before, and 93% had driven their child to the hospital during an emergency.4 Families may transport their children either to the nearest community hospital or to their “home” institution, which is likely to be a tertiary care center. Most emergency medical service jurisdictions transport to the nearest “appropriate” medical facility.
Children (and youth) with special health care needs are usually cared for by adults who have been trained to manage their child’s daily care. In general, families are knowledgeable about their child’s medical conditions and technologic needs. Families may have detailed medical plans that specify such things as the size of tubes, how often to change tubes, dosages of medications, and ventilator settings. One example of a program that provides information to medical care providers is the Emergency Preparedness for Children with Special Health Care Needs program of the American Academy of Pediatrics and the American College of Emergency Physicians.5 This emergency notification program’s purpose is to communicate specific information to medical caregivers about the conditions and medications of a child or adolescent with special needs. An emergency information form is completed by the child’s primary care physician or family and is carried with the child. Although most caregivers are adept at handling many of their children’s emergencies, caregivers seek help for a variety of reasons, including the need for respite, equipment malfunction, an overwhelming medical problem, or help with transport to the child’s home hospital.
In contrast to the pediatric population, there is no universally recognized definition for adults with special health care needs. The Institute of Medicine (IOM) has used several different definitions for disability, including an “inability or limitation in performing socially defined activity and roles expected of individuals within a social and physical environment.”6 The number of adults who meet this broad definition is difficult to calculate. Current estimates indicate that as many as 50 million U.S. residents have some type of disability.7 Estimates come from various national surveys that vary in their definitions, questions, and target populations. Even more importantly, of 18 different national surveys reviewed by the IOM Committee on Disability in America, only four include institutionalized individuals.7 Given the large number of surveillance groups, the IOM has recommended adoption of the World Health Organization’s International Classification of Functioning, Disability and Health, which is currently being used in many countries as a standardized assessment tool for both individuals and populations.
Distinguishing Principles of Disease
General Issues for Children and Youth with Special Health Care Needs
CSHCN differ from other children in a variety of ways. Some CSHCN may be neurologically impaired and therefore have intellectual disabilities. Their physical growth may be limited, resulting in stature smaller than that of other children of the same age. As a result, medical personnel cannot rely on age- and weight-based norms and should either ask caregivers for this information or use length-based tapes to estimate weight, to calculate drug dosages, and to determine fluid management. Children and adolescents with chronic health conditions may have vital signs that differ from those expected for children without chronic conditions of the same age.4 For example, baseline vital signs are altered in children with cardiac conditions or who are on mechanical ventilators. A child with an unrepaired complex congenital heart defect may have a baseline pulse oximetry reading in the low 80s. Some children with lung diseases may have respiratory rates higher than expected, yet these higher respiratory rates are considered their baseline.4 Also, some children may have sensorineural deficits, such as blindness and deafness, that may make history taking and physical assessment challenging. Medical care providers need to be cognizant of these differences.
Specific Disorders
Airway and Pulmonary Conditions
Tracheomalacia
There are three causes of tracheomalacia: congenital or intrinsic tracheal anomaly (occurring within the trachea), which may be associated with a tracheoesophageal fistula (a patent connection between the trachea and esophagus); extrinsic defects (occurring outside the trachea), such as abnormal development of the vasculature around the trachea that may create a ring, which places pressure on the trachea and interferes with airflow; and acquired malacia, which occurs in children with prolonged intubation or chronic tracheal infections.8
Bronchopulmonary Dysplasia
Bronchopulmonary dysplasia (BPD), a chronic lung disease occurring in infants, is characterized by poor lung compliance and chronic exacerbations. BPD is a worldwide problem, with 5000 to 10,000 new cases reported each year. BPD, cystic fibrosis, and asthma are the three most common chronic lung diseases in infants.9
BPD develops primarily in low-birth-weight infants who have respiratory distress syndrome. Babies born before 32 weeks’ gestation may not have enough surfactant to keep their alveoli open.10 However, BPD development is not limited to survivors of respiratory distress syndrome. BPD may result from alveolar damage caused by other lung diseases, exposure to prolonged high oxygen concentrations, use of mechanical ventilation after birth because of conditions such as neonatal pulmonary hypertension and pneumonia or other infections, or trauma to the lungs.10
Apnea
Apnea in infants and young children is defined as a cessation in breathing for more than 20 seconds or associated with a change in color, limpness, altered mental status, or bradycardia. Apnea occurs more frequently in infants born prematurely and generally reflects immature neurologic and respiratory control mechanisms.11 These pauses in respiration may be due to central apnea, which is the result of an absence of a neurologic signal to the respiratory muscles. Underlying causes include encephalitis, brainstem infarction or tumor, neuromuscular disorders such as muscular dystrophy, and thoracic restrictive disorders such as kyphoscoliosis. These children are discharged home from the hospital on an apnea monitor. Apnea monitor alarms often result in ED visits. The apnea monitor should be transported with the child to the hospital. Most monitors contain a computer chip that records information that can be downloaded into a computer at the child’s regular hospital (home hospital) to determine the origin of the monitor alarms (high or low heart rate, apnea, or artifact).
Apnea in adults is most frequently related to obstructive sleep apnea. This can be due to altered oropharyngeal anatomy but is most commonly secondary to obesity. Decreased muscle tone during sleep leads to obstruction of the pharynx and hypopharynx with redundant tissue. Central sleep apnea is also seen in patients with heart failure. Regardless of etiology, sleep apnea can result in mild to severe sleep disturbances leading to daytime somnolence and irritability. It is also associated with significant morbidity and mortality, including heart failure, acute coronary syndrome, cerebrovascular accidents, and death.12–15 Like pediatric obstructive apnea, obstructive sleep apnea in adults is treated with noninvasive positive-pressure ventilation, such as CPAP, during sleep.
Chronic Respiratory Failure in Adults
Myasthenia gravis is a neuromuscular disorder that can cause respiratory insufficiency and failure. Myasthenia gravis is an autoimmune process that attacks the postsynaptic acetylcholine receptors at the neuromuscular junction. It most commonly affects the bulbar muscles but can also result in generalized weakness.16 Other neuromuscular disorders that can affect the respiratory muscles include muscular dystrophy, multiple sclerosis, and amyotrophic lateral sclerosis. In any patient with respiratory muscle weakness secondary to a neuromuscular disease, pulmonary function tests are an important component of the assessment. One test that can rapidly be obtained in the ED is a negative inspiratory force or maximal inspiratory pressure. A value of less than 20 cm H2O is the generally accepted cutoff for severe impairment and can help determine which patients need ventilatory support. This test is usually performed by a respiratory therapist with use of a pressure gauge connected to a disposable mouthpiece.
Tracheostomy Tubes
Tracheostomy tubes are placed to facilitate mechanical ventilation, to provide a bypass of the upper airway, or to keep the airways clear of secretions. The more common conditions for which tracheostomy tubes are placed include tracheal stenosis, tracheomalacia, certain craniofacial anomalies, BPD, muscular dystrophy, spinal cord injury or disease, traumatic or anoxic brain injury, and cerebrovascular accidents.8
Because the tubes are available from several manufacturers, their sizing and markings vary. Common brands are Shiley, Bivona, Hollinger, Portex, and Bardeen. Typically, the sizes are marked on the packaging and on the flange, or wings, of each tube. They range in size from 00 for newborns to 7.0 for older adolescents and adults. The inner and outer diameter ranges are provided so that comparisons between brands can be made. They range from 2.5 mm for infants to 10.0 mm for adolescents and adults. This information is helpful for emergency personnel in replacing a tracheostomy tube or in choosing an endotracheal tube for oral intubation or use through a stoma. The inner diameter of a tracheostomy tube should be used to choose the endotracheal tube size for insertion through a stoma. Tracheostomy tubes also come in various lengths. Neonatal tubes are shorter than pediatric tubes, although the inner diameters may be the same.8
There are several types of tracheostomy tubes and attachments: single-cannula tubes (Fig. 187-1), double-cannula tubes (Fig. 187-2), cuffed tubes (Fig. 187-3), and fenestrated tubes (Fig. 187-4).8 Neonates, infants, and young children use single-cannula tracheostomy tubes. A double-cannula tube can be used in older children and adults. It contains an outer tube that stays in the stoma and an inner tube that is removable for cleaning and pulmonary toilet. Tracheostomy tubes for older children and adults have an inflatable cuff to keep the tube in place and to prevent air leaks. Fenestrated tubes have a hole in the cephalic portion of the tube that redirects air into the upper airway to allow the individual to speak and to breathe through the nose and mouth. This is facilitated by a decannulation plug that is attached to the opening of the tube.
Figure 187-1 Single-cannula tracheostomy tube. (Modified from Susan Gilbert in Teaching Resource for Instructors in Prehospital Pediatrics [TRIPP]; www.cpem.org.)
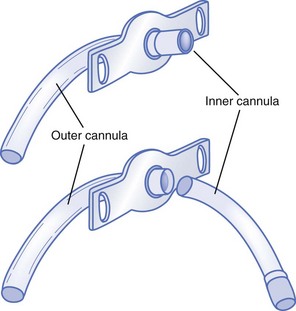
Figure 187-2 Double-cannula tracheostomy tube. (Modified from Susan Gilbert in Teaching Resource for Instructors in Prehospital Pediatrics [TRIPP]; www.cpem.org.)
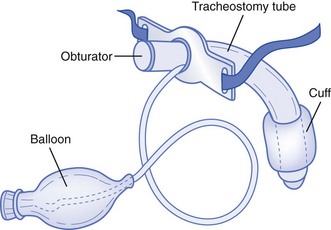
Figure 187-3 Cuffed tracheostomy tube. (Modified from Susan Gilbert in Teaching Resource for Instructors in Prehospital Pediatrics [TRIPP]; www.cpem.org.)
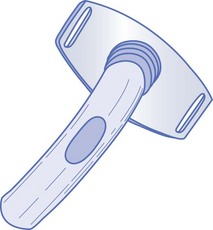
Figure 187-4 Fenestrated tracheostomy tube. (Modified from Susan Gilbert in Teaching Resource for Instructors in Prehospital Pediatrics [TRIPP]; www.cpem.org.)
For sudden onset of respiratory distress, suctioning of the tracheostomy tube may be helpful. Approximately 2 to 3 mL of normal saline should be instilled before suctioning. If suctioning two or three times does not relieve the obstruction or respiratory distress, the medical provider should change the tracheostomy tube because there may be a thick mucous plug obstructing airflow through the tube. An endotracheal tube can be used as a substitute if the appropriate size of a tracheostomy tube is not available. For patients cared for at home, the parents or caregivers often have spare equipment in their “go” bags that can be used (for troubleshooting, see Tables 187-1 and 187-2).
Table 187-1
Medical Devices: Common Problems and Solutions
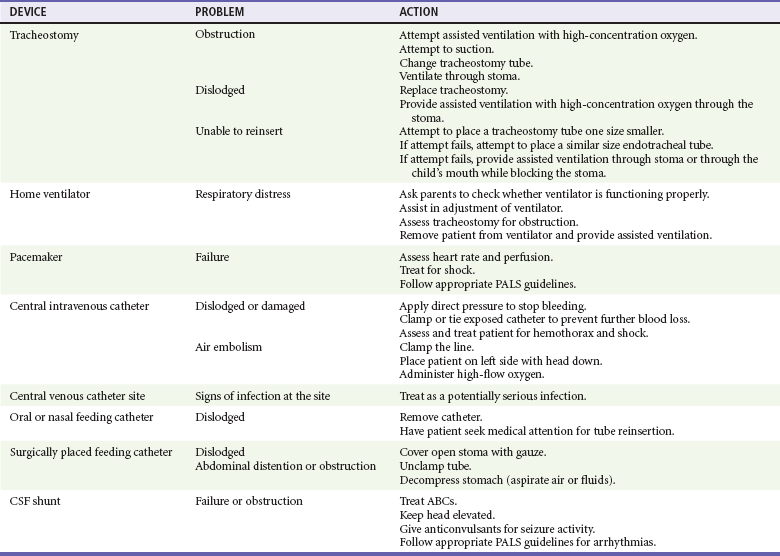
Modified from Adirim T, Smith E: Special Children’s Outreach and Prehospital Education. Sudbury, Mass, Jones and Bartlett, 2006.
Table 187-2
Troubleshooting Gastrostomy Tubes and Buttons
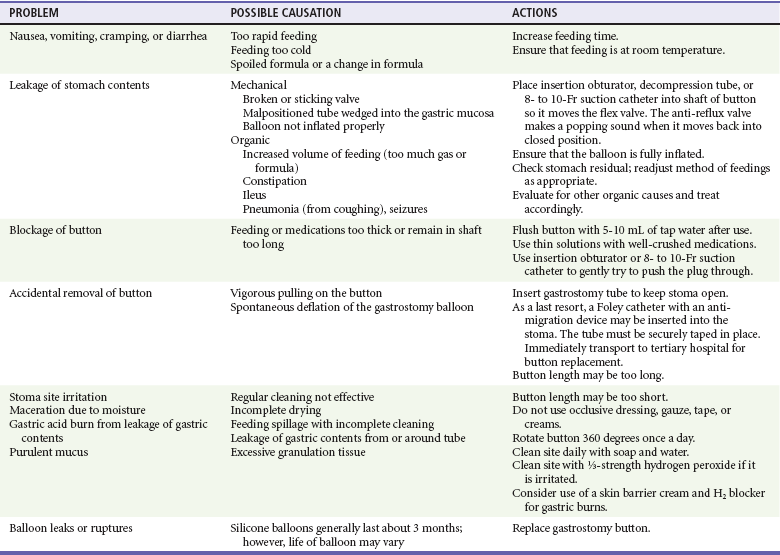
From Adirim TA, Smith E, Singh T: Special Children’s Outreach and Prehospital Education. Sudbury, Mass, Jones and Bartlett, 2006.
Home Mechanical Ventilation
Many individuals with tracheostomy tubes are also mechanically ventilated. Indications for mechanical ventilation include severe lung disease and abnormal respiratory drive either from central causes or secondary to weakness or paralysis. Some people are dependent on ventilators continuously; some depend on ventilators while they are asleep, others for part of the day.11
There are two types of ventilators: pressure-cycled ventilators and volume ventilators. Pressure-cycled ventilators tend to be used in infants. They are set to deliver a given pressure with each breath. Volume ventilators give a tidal volume with each breath. There are two common modes of ventilations: intermittent mandatory ventilation and controlled mechanical ventilation. Intermittent mandatory ventilation mode delivers a mechanical breath between the patient’s own respirations to ensure that the patient achieves a certain number of breaths per minute. In this mode, the ventilator can synchronize its breaths with the patient’s breaths. On the other hand, with controlled mechanical ventilation, the ventilator is set to deliver a certain number of breaths per minute whether or not the patient can breathe without assistance. Some ventilators have an assist-control mode in which the ventilator gives a breath at the same time the patient takes a breath to augment the patient’s effort.8
There are five types of home ventilator alarms: low pressure/apnea, low power, high pressure, setting error, and power switchover. The causes of a low-pressure alarm include a loose or disconnected circuit, a leak in the circuit, and a leak around the tracheostomy site. The low-power alarm indicates that the internal battery is near completion and the ventilator will need to be plugged into an electrical outlet. A high-pressure alarm indicates that there is an obstructed circuit or increased airway pressures. Increased airway pressure can result from any type of lung disease, including bronchospasm, pneumothorax, infection, and pulmonary edema. A setting-error alarm indicates that the setting has been improperly adjusted. Under these circumstances, the patient should be manually ventilated until the ventilator can be set properly. The power-switchover alarm sounds whenever the ventilator switches from AC power to battery power. When this happens, check to ensure that the battery is powering the unit before pressing the “alarm silent” button.8
Neurologic Conditions
Cerebral palsy (CP) is the leading cause of childhood disability,17 and population studies have shown that more than 80% of children with CP will survive into adulthood.18 CP is a group of disorders affecting the development of movement attributed to nonprogressive disturbances that occur in the developing fetal or infant brain. The motor disorders are often accompanied by one or more of the following: disturbances of sensation, cognition, communication, perception, or behavior or a seizure disorder.19 Premature birth is the most common associated historical factor, and prenatal maternal infection and multiple pregnancies have also been associated with CP.17
CP is classified on the basis of the type of movement disorder and the area of the body involved: spastic, athetoid, ataxic, or mixed and hemiplegia, diplegia, or quadriplegia. The most common form, spasticity, is defined as a velocity-dependent resistance to stretch. When the extremities of an individual with the spastic form of CP are stretched beyond their range of motion, the muscles contract and increase resistance. Clonus and persistent primitive reflexes are characteristic. Because CP is a movement disorder, gross motor skills are delayed. There is a lack of coordination and differential strength and tone in the musculature of the individual. Treatment is focused on management of spasticity. Some of the treatments include daily range-of-motion exercises, casting, and splinting. Some people may take oral medications (baclofen, diazepam, and clonidine) to improve muscle spasticity or to decrease the drooling that is evident in 10% of children with CP, caused by poor oromotor control combined with decreased sensation and poor head control.18
Some individuals with CP are now treated with baclofen pumps inserted directly into the thecal sac surrounding the spinal cord. Baclofen is directly infused into this space by a small pump through continuous infusion. Baclofen acts by binding to receptors in the spinal cord, thereby inhibiting spinal reflexes. Complications include overdose when the pump is filled incorrectly or programming is inaccurate. Overdose can lead to respiratory depression and death. Some less serious side effects of the medication are drowsiness, dizziness, hypotension, headache, and nausea. The physician presented with a comatose patient with CP on a baclofen pump must maintain the patient’s airway, breathing, and circulation until the pump is urgently stopped with a specialized device. If this device is not readily available, emptying of the reservoir with a 22-gauge needle is an alternative treatment. This can be done by locating the pump in the abdominal wall and inserting the needle in the center of it. Another complication is medication withdrawal due to pump failure, which is usually the result of tube obstruction or faulty pump programming. Symptoms of withdrawal include increased spasticity, tachycardia, blood pressure changes, severe hyperthermia, and rhabdomyolosis.19,20
Intellectual Disability
Intellectual disability, previously known as mental retardation, is commonly found in both children and adults. Approximately 30 to 50% of individuals with CP have significant intellectual disabilities. Most have milder cognitive deficits and learning disabilities, and some individuals with CP have normal intelligence. Other causes of intellectual disability include prenatal and perinatal exposures, traumatic or anoxic brain injuries, and genetic syndromes. The term developmental delay is often used interchangeably with mental retardation and intellectual disability. Mental retardation has a specific definition, however. Mental retardation is a fixed disability characterized by significant limitations both in intellectual functioning and in adaptive behavior as expressed in conceptual, social, and practical adaptive skills.21 Developmental delay is a failure to achieve age-appropriate milestones; these children have the potential to “catch up” and eventually to progress normally with their growth and development. In all situations, it is important to assess the developmental level of the patient as well as associated problems, such as hearing or visual impairment.
Autism and Other Neurodevelopmental Disorders
The 2007 National Survey of Child Health estimates that there are 673,000 children in the United States with autism spectrum disorders, and data from the 2006 Autism and Developmental Disabilities Monitoring Network estimate the prevalence of autism to be 1% of all children in the United States.22,23 Autism spectrum disorders are a group of neurodevelopmental disorders characterized by impaired development in socialization, communication, and behavior. These disorders include autism, Asperger’s syndrome, and pervasive developmental disorder, not otherwise specified. Studies have shown that these children have a high level of functional limitations and poor health status, an accompanying high level of health care use and unmet health needs, and increased parenting stress and family burden.24–26 In fact, a Pennsylvania Autism Needs Assessment from September 2011 reported that both the untreated or undertreated symptoms of autism and co-occurring disorders can result in unwanted outcomes, including police contact, ED visits, and inpatient psychiatric hospital care, and that the incidence of these outcomes increases markedly with age from less than 5% of children to more than 10% for ED visits to almost 25% for police contacts in adults with autism. The most common reasons for emergency and inpatient hospital-based care are aggression and defiant behaviors, followed by self-injury, anxiety, and depression.
More than 40% of children and adolescents with autism and other neurodevelopmental disorders have multiple conditions. For children with autism, more than 95% have a co-occurring developmental disorder, such as attention deficit disorder, attention-deficit/hyperactivity disorder, learning disabilities, mental retardation, stuttering, and other developmental delays.24 Also, children with autism have a higher incidence of seizure disorders. Therefore, children with autism are more likely to be taking prescription medications and to access health care services at higher rates than normal children do.
Strategies typically used to treat aggression and other behavioral disorders are often less effective in people with autism. Consultation with specialists with knowledge of the care of people with autism is recommended (e.g., specialists at regional tertiary care centers). Children with autism spectrum disorders with aggressive behaviors not controlled by behavioral therapy are increasingly being treated with atypical antipsychotic agents, such as risperidone.27 Management of uncontrolled behavior may necessitate medication, especially to protect patients from harming themselves or others.
Seizures
Seizure disorders affect approximately 10% of children who are disabled, compared with only 0.5% of healthy children. The frequency of seizure disorders in adults is more difficult to estimate as adults have less consistent health care and are more likely than children to have seizures secondary to other medical or toxicologic issues. In a 2004 nationwide survey, 2.9% of participants reported a diagnosis of epilepsy or seizure disorder.21 Patients with seizure activity often have normal intelligence, and their seizures can be well controlled by anticonvulsant medication.28 With age comes an increasing frequency of secondary seizure syndromes, such as post-traumatic seizures, adverse reactions to medications, and seizures related to structural lesions such as masses, or encephalomalacia. In someone receiving antiepileptic therapy with stable symptoms, a “breakthrough” seizure may occur as a result of toxic or subtherapeutic blood levels of seizure medication. This may occur for a variety of reasons. These include but are not limited to poor absorption of the medication secondary to a gastrointestinal illness, missing of one or more doses of anticonvulsant medication, medication dosages not being appropriately adjusted for a patient’s growth or weight gain, and lowered seizure threshold from an illness or accidental multidosing of a patient’s medication (Table 187-3). For patients with multiple abnormalities, seizure activity is generally more severe and is more difficult to control with anticonvulsant medications.
Table 187-3
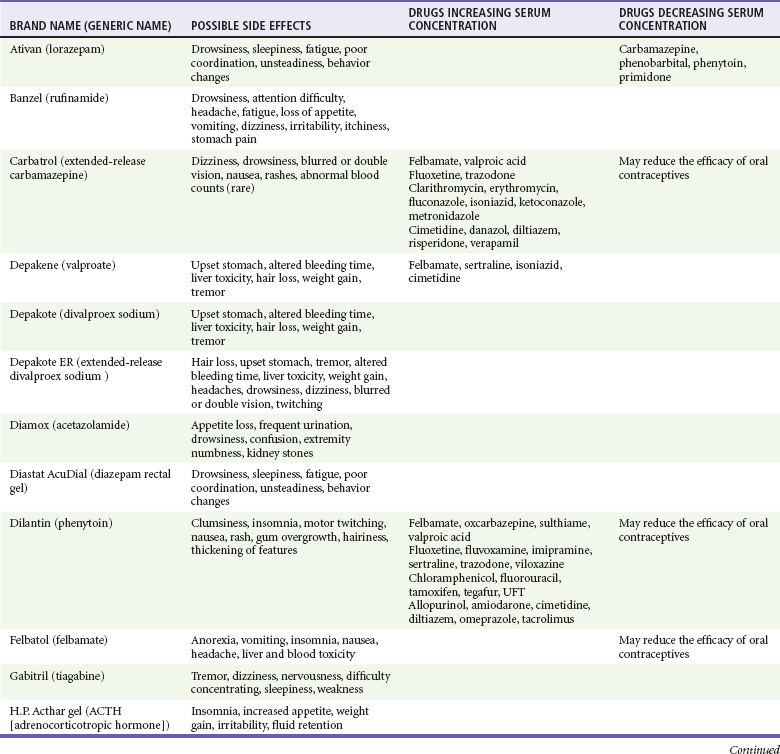
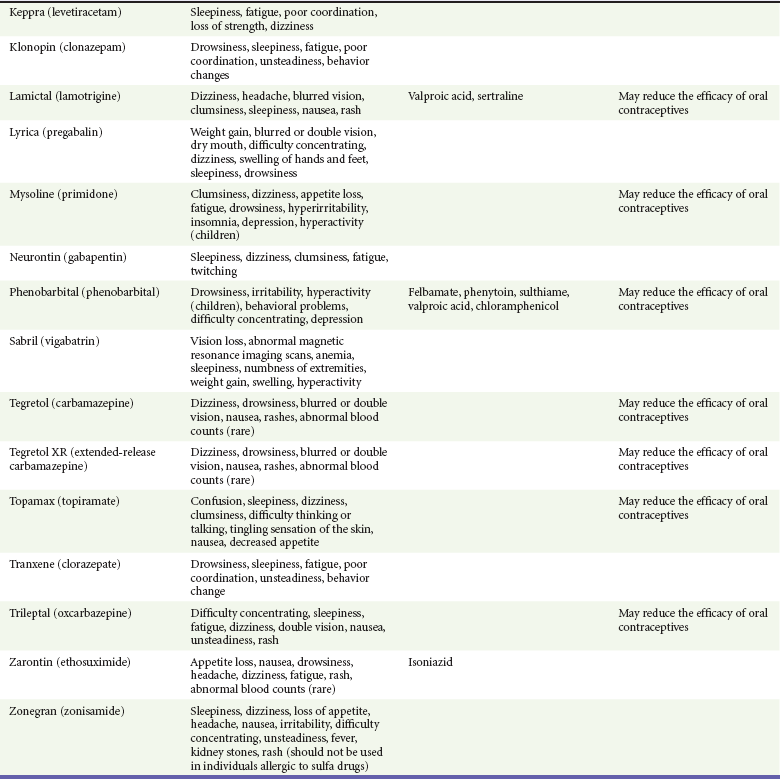
Modified from the Epilepsy Foundation, Western/Central Pennsylvania: Anti-seizure medication and their side effects. Available at www.efwp.org/programs/side_effects.shtml. Accessed November 25, 2011; and Perucca E: Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol 61:246-255, 2006.
Individuals with structural brain abnormalities are more likely to experience status epilepticus. Status epilepticus is a condition of generalized convulsions in adults and older children (older than 5 years) for 5 minutes continuously or two or more discrete seizures without full recovery of consciousness.28–30 Treatment initially includes benzodiazepines, which are often effective; if two doses of a benzodiazepine are not effective, subsequent doses are not likely to work, and phenytoin (Dilantin) or fosphenytoin (Cerebyx) is considered the next line of medication.28 It is important to rapidly assess for and to treat underlying conditions that may have triggered the seizure or make it refractory to standard therapies, even in patients with known seizure disorders. This includes testing for hypoglycemia and electrolyte abnormalities, such as hyponatremia, as well as a focused history and physical examination looking for signs of trauma, infection, or changes in home medications. (For complete discussions of the management of adult and childhood seizures, see Chapters 102 and 175, respectively.) Intractable epilepsy affects approximately 20 to 30% of patients with seizures. Newer antiepileptic drugs and surgical procedures decrease seizure frequency in a significant number of patients. Even among this group, up to 10% continue to have disabling seizures. For these patients, the vagal nerve stimulator (VNS) provides hope for better seizure control.31
The VNS is an implantable device that looks like a pacemaker. It is implanted by a neurosurgeon just under the skin in the chest. In the small number of children who have had the VNS placed, most have had a reduction in seizure frequency of 50%.31 How the VNS works on the brain is not known. The VNS is programmed by the neurologist with a programming wand and a laptop computer to provide baseline intermittent stimulation of the left vagus nerve. Although the VNS system delivers stimulation automatically in regular pulses all the time, a special magnet can be used to deliver extra electronic stimulation in between cycles. The patient or caregiver activates the device by placing the hand-held magnet over the device implanted in the chest.32 Patients who can sense that they are about to experience a seizure can activate the VNS themselves by passing the magnet over the device. Out-of-hospital providers who encounter a seizing patient with the VNS should assist the caregiver with its activation or seek advice from medical control. Individuals with VNS devices should otherwise be treated like other seizing patients, with careful attention to airway, breathing, and circulation. The special magnet can also be used to stop the stimulation by holding the magnet over the device. While the magnet is held directly over the generator, the switch inside the generator remains closed and stimulation is not delivered. When the magnet is removed, the switch opens and the device is able to deliver stimulation.33,34 Sometimes the patient may want to shut the stimulation off. Some reasons for this include when the patient is eating if the stimulation causes problems with swallowing, when the patient plans to speak or to sing in public as the stimulation can change one’s voice, and if the stimulation causes discomfort.33
Myelomeningocele and Hydrocephalus
Clinical presentation depends on the level of the defect. The condition results in partial or complete paralysis and loss of sensory function (not always symmetrical). In addition, the child may present with loss of bladder or bowel control, cognitive impairments, visual deficits, and seizure disorders. Bladder and bowel dysfunction is common among children with spina bifida. In such cases, there is incomplete emptying of the bladder, predisposing the child to urinary tract infections. Management of such cases requires emptying of the bladder by urinary catheterization several times a day. Hydrocephalus associated with Chiari malformation (displacement of the brainstem and part of the cerebellum downward through the skull) occurs in more than 68% of children with spina bifida.34 Because of frequent exposure to latex products early in life, children with spina bifida should be assumed to be allergic to latex.35,36
Spinal Cord Disease
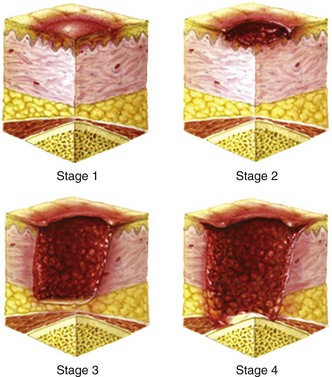
Impaired sensation and strength place individuals with spinal cord disease at increased risk for pressure ulcers. The sacral region is the most commonly recognized site, although any region overlying a bone prominence is at risk for tissue injury. Examples include the occiput, elbows, heels, and posterior aspect of the pinna in the supine patient. Other risk factors for pressure ulcers include age, diabetes, chronic steroid use, tobacco exposure, and malnutrition. Pressure ulcers are categorized as stage 1 to stage 4 (Fig. 187-5). Stage 1 describes the often subtle color, temperature, and tactile changes that occur before actual ulceration. Stage 2 ulcers are shallow, with involvement limited to the epidermis and dermis. Stage 3 lesions penetrate into the subcutaneous fat, and stage 4 lesions penetrate through the deep fascia and may involve underlying muscle and bone. Prevention and early recognition are paramount as severe lesions can be refractory and often require prolonged treatment and surgical intervention. Preventive measures include frequent position changes, padded support devices, daily skin examinations, meticulous hygiene, and avoidance of temperature extremes. In some situations, hospitalization may be necessary for care of extensive wounds or treatment of superimposed infection, which may lead to bacteremia and sepsis. In one prospective cohort, pressure sores accounted for nearly 18% of hospitalizations in patients with spinal cord injury.37 In the case of infection, blood and wound culture specimens should be obtained and antibiotics should be initiated with specific concern for skin flora.
Indwelling Catheters
Ventriculoperitoneal (VP) shunts are catheters that are inserted into the ventricles within the brain and then threaded under the skin from the skull to the peritoneum, where excess cerebrospinal fluid (CSF) is drained (Fig. 187-6). Less commonly the shunt may drain into the pleural space (ventriculopleural shunt) or right atrium (ventriculoatrial shunt). Hydrocephalus occurs with an obstruction somewhere in the CSF circulation system. If the obstruction is beyond the lateral ventricles, they enlarge with CSF, increasing intracranial pressure. Hydrocephalus can be seen in formerly premature infants who sustained an intracranial hemorrhage during the neonatal period and in adults with a traumatic or spontaneous subarachnoid or intraventricular hemorrhage. Both children and adults can have hydrocephalus secondary to brain tumors or other mass lesions causing obstruction of normal CSF flow. Finally, children with spina bifida (myelomeningocele) often have hydrocephalus if they have an Arnold-Chiari malformation that obstructs CSF flow.
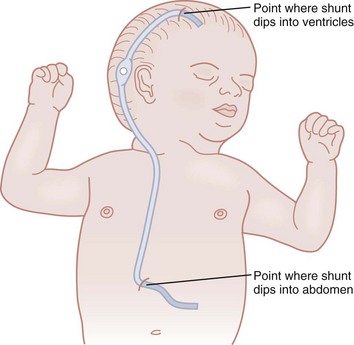
Figure 187-6 Ventriculoperitoneal shunt. (Modified from Susan Gilbert in Teaching Resource for Instructors in Prehospital Pediatrics [TRIPP]; www.cpem.org.)
A person with a VP shunt can rarely have an infection of the shunt track during the first 3 months postoperatively. Symptoms include fever, ill appearance, erythema or tenderness over the shunt site or tubing, abdominal pain and tenderness, vomiting, and altered mental status. Infection can also occur outside of the immediate postoperative setting in patients with bacteremia or primary central nervous system infections leading to seeding of the shunt. A VP shunt infection should be considered in anyone presenting with a fever; if a shunt infection is suggested clinically, CSF should be drawn from the shunt, preferably by a neurosurgeon, and sent for cell count and culture. Broad-spectrum antibiotics should be administered pending cell count and culture results; likely organisms include Staphylococcus epidermidis, Staphylococcus aureus, and rarely Haemophilus influenzae. Neurosurgeons should manage all shunt infections.38,39
The more common complication of a VP shunt is shunt obstruction or malfunction. This can occur with an increase in protein in the CSF that causes a blockage in the tubing or as a result of a mechanical disruption in the shunt tubing. This can cause a buildup of CSF in the ventricles and an increase in intracranial pressure. Signs and symptoms include headache, nausea, vomiting, irritability, altered mental status, ataxia, and change in vital signs. A bulging fontanel may also be seen in infants. Initial management should include elevation of the patient’s head and management of the patient’s airway, breathing, and circulation. Stable patients should have head computed tomography performed, and a neurosurgeon should be consulted as soon as possible. The definitive treatment is surgical shunt revision.40
Gastrostomy Tubes
Gastrostomy tubes are placed in individuals who need long-term nutritional supplementation or cannot take food by mouth. Many conditions necessitate placement of an artificial feeding tube, including severe developmental delay, coma, short bowel syndrome, swallowing difficulties and aspiration burns to the mouth and esophagus, failure to thrive, dysphagia secondary to neurologic disorders, oropharyngeal and esophageal malignant neoplasms, and chronic diseases that affect nutrition (e.g., cystic fibrosis).41
Gastrostomy tubes are surgically or endoscopically placed feeding catheters. These tubes include gastrostomy tubes (G tubes), jejunostomy tubes (J tubes), and percutaneous endoscopic gastrostomy tubes (PEGs or buttons). Gastric feeding is generally preferred, but a J tube may be more appropriate for patients with gastroesophageal reflux disease, delayed or impaired gastric emptying, aspiration, or pancreatitis. The tube is placed in the stomach and then passed through the junction between the stomach and jejunum, bypassing the stomach. PEGs are placed by gastroenterologists and surgeons.42
There are three potential gastrostomy tube emergencies. The tube can leak gastric contents, become obstructed, or come completely out. When a patient presents with any of these problems, assessment of hydration status is important, especially in anyone who is dependent on the feeding tube for hydration and nutrition. One should inquire about prescribed medications and whether any doses were missed.43
If there is leaking around the catheter, management focuses on delineating and fixing the cause of the leak. Possible causes of leakage include balloon deflation, coughing, constipation, bowel obstruction, stoma dilation, and seizure. Addressing the cause may solve the problem; otherwise, consultation with the subspecialty service that manages the tube may be necessary (Table 187-4).
Table 187-4
Potential Central Line Emergencies and Physician Management or Considerations
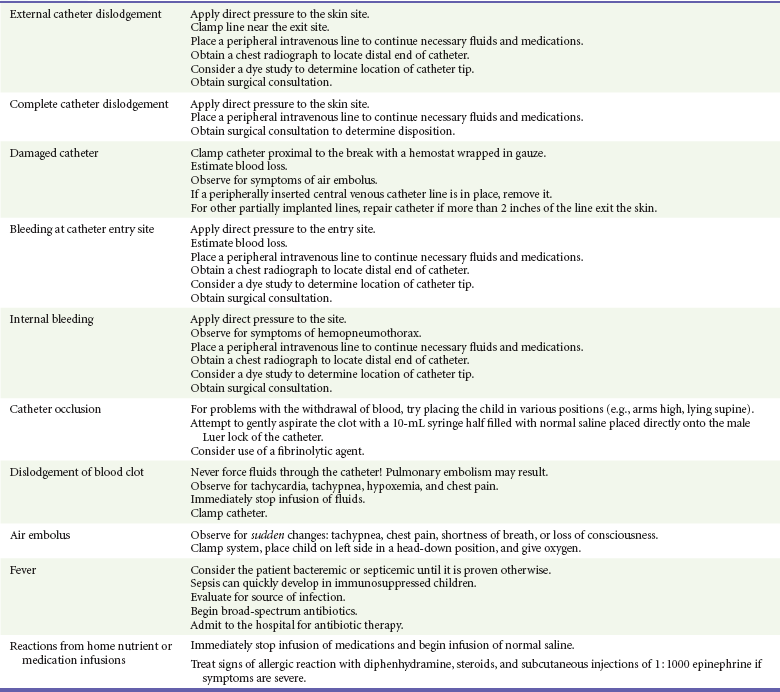
From Adirim TA, Smith E, Singh T: Special Children’s Outreach and Prehospital Education. Sudbury, Mass, Jones and Bartlett, 2006.
Sometimes a patient’s G tube becomes clogged with medication or food. Obstructed tubes can be cleared with either Coca-Cola or a proteolytic enzyme solution; otherwise, they need to be replaced.8
G tubes that have fallen completely out of the patient need to be replaced as soon as possible so that the stoma does not constrict and make replacement difficult. Gastrostomy-jejunostomy tube placement is more challenging and is usually done under fluoroscopy by a radiologist familiar with this procedure.42 For G tube replacement, the physician should ask when the tube was first inserted. Maturation of a tube site generally occurs within a few weeks but may be prolonged in certain populations. For tubes that are less than 3 months old, consultation with the person or service that performed the procedure is necessary because the track may not yet be fully formed and insertion by a practitioner not adept at replacement of these tubes may create a false track.
When the reinsertion procedure is performed, a similar size gastrostomy tube should be used. Families often have an extra tube with them. When a G tube is not available, a Foley catheter can be used to temporarily keep the stoma open until definitive placement can occur.11
Central Venous Catheters
Centrally inserted central venous catheters include single- and multiple-lumen catheters and can be tunneled or nontunneled. Common insertion sites are the jugular, subclavian, and cephalic veins. Nontunneled catheters are used for temporary access and are generally limited to inpatient use. Tunneled catheters, however, are frequently used in the outpatient setting. These include the Broviac, Hickman, and Groshong catheters. These are generally placed by surgeons or radiologists. In addition to medication administration and blood draws, these catheters are frequently used in dialysis-dependent patients. They are sometimes used temporarily while waiting for more definitive access, such as a maturing arteriovenous fistula, but may also be considered permanent access for those patients with prior arteriovenous fistula complications or failures. Implanted vascular access ports (Port-A-Cath, PAS Port, and Med-A-Ports) are also common. The insertion sites and method are the same as for the tunneled central venous catheters. The distal end of the catheter consists of a reservoir that is covered with a self-healing rubber septum. This reservoir rests subcutaneously, and a special needle is needed to access this line. The advantages of the implanted vascular ports are that they need to be flushed with heparin only once a month and after each access, as opposed to daily heparin flushes, and they are hidden and so may be more acceptable to older children, for whom body image is more of an issue. They have also been shown to have lower infection rates than catheters with external ports.44
Bladder Catheters
Individuals with neurogenic bladder secondary to spinal cord or brainstem disease may use a variety of different approaches to bladder management. These approaches include pharmaceutical agents, intermittent catheterization, and chronic indwelling catheters. Spontaneous voiding and intermittent catheterization are associated with a lower rate of urinary tract infections; however, a chronic indwelling catheter may be necessary, depending on the location of the spinal cord lesion and the individual’s level of function. Indwelling catheters may also be used in patients with altered genitourinary anatomy secondary to traumatic or postsurgical changes. Catheters may be either urethral or suprapubic in placement. Suprapubic catheters are often preferred by patients and may be associated with lower rates of infection.45–47 Regardless of site, indwelling urinary catheters are subject to bacterial colonization. Pyuria and bacteriuria are common findings, and patients are often asymptomatic. Whereas Escherichia coli is still the most common pathogen, patients with spinal cord disease have higher rates of Pseudomonas, Proteus, and Serratia colonization than in the general population.47 Candiduria is also a common finding in asymptomatic patients with long-term catheterization.48 Determination of when to initiate antimicrobial therapy is controversial. The National Institute on Disability and Rehabilitation Research defines significant bacteriuria as ≥104 CFU/mL for clean-void specimens, whereas any degree of bacterial growth is considered significant in patients with indwelling catheters.49 Despite these sensitive definitions, this consensus report recommends against treatment of asymptomatic bacteriuria unless a urease-producing organism is isolated.49 This recommendation is based on the association between urea-producing organisms, such as Proteus, and bladder stone formation. If a patient is febrile without another obvious source, critically ill, or symptomatic with abdominal or flank pain, vomiting, chills, or rigors, it is appropriate to treat a presumed urinary tract infection. When possible, the catheter should be replaced or temporarily removed before urine is sent for microscopy and culture. In immunocompetent patients who have minor or vague symptoms and who have appropriate follow-up care, it may be appropriate to wait for culture results before antibiotics are started.
References
1. McPherson, M, Arango, P, Fox, H, et al. A new definition of children with special health care needs. Pediatrics. 1998;102:137.
2. Boyle, CA, et al. Trends in the prevalence of developmental disabilities in US children, 1997-2008. Pediatrics. 2011;127:1034–1042.
3. Turchi, RM, Berhane, Z. Care coordination for CSHCH: Associations with family-provider relations and family/child outcomes. Pediatrics. 2009;124(Suppl 4):S428–S434.
4. Singh, T, Adirim, TA. Vital sign ranges for children with special health care needs [abstract]. Pediatr Res. 2004:49.
5. American Academy of Pediatrics, Committee on Pediatric Emergency Medicine. Policy statement—emergency information forms and emergency preparedness for children with special health care needs. Pediatrics. 2010;125:829–837.
6. Pope AM, Tarkiv AR, eds. Disability in America: Toward a National Agenda for Prevention. Washington, DC: National Academy Press, 1991.
7. Field MJ, Jette AM, eds. The Future of Disability in America. Washington, DC: National Academy Press, 2007.
8. Adirim, TA, Smith, E, Singh, T. Special Children’s Outreach and Prehospital Education. Sudbury, Mass: Jones and Bartlett; 2006.
9. National Heart, Lung, and Blood Institute, National Institutes of Health. Bronchopulmonary dysplasia. www.nhlbi.nih.gov/health/dci/Diseases/Bpd/Bpd_WhatIs.html.
10. Stanley, FJ. Cerebral palsy trends: Implications for perinatal care. Acta Obstet Gynecol Scand. 2004;73:5.
11. Adirim, TA. Children with special health care needs: The technology dependent child. In Cooper A, et al, eds.: APLS: The Pediatric Emergency Resource, 4th ed, Sudbury, Mass: Jones and Bartlett, 2006.
12. Yaggi, HK, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041.
13. Marshall, NS, et al. Sleep apnea as an independent risk factor for all-cause mortality: The Busselton Health Study. Sleep. 2008;31:1079–1085.
14. Young, JS, et al. Sleep in hospitalized medical patients, part 1: Factors affecting sleep. J Hosp Med. 2008;3:473–482.
15. Gottlieb, DJ, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The Sleep Heart Health Study. Circulation. 2010;122:352–360.
16. National Center for Biotechnology Information, U.S. National Library of Medicine. Myasthenia gravis. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001731, 2011.
17. Bax, M, et al. Proposed definition and classification of cerebral palsy. Dev Med Child Neurol. 2005;47:571.
18. Green, LB, Hurvitz, EA. Cerebral palsy. Phys Med Rehabil Clin North Am. 2007;18:859.
19. Hutton, JL, Cooke, T. Life expectancy in children with cerebral palsy. BMJ. 1994;309:431–435.
20. Shirley, K, Kothare, S, Piatt, J, Adirim, TA. Baclofen overdose by intrathecal pump. Pediatr Emerg Care. 2006;22:258.
21. American Association of Intellectual and Developmental Disabilities. FAQ on intellectual disability. www.aaidd.org/content_104.cfm.
22. Centers for Disease Control and Prevention, Prevalence of autism spectrum disorders—Autism and developmental disabilities monitoring network, United States, 2006. MMWR Surveill Summ 2009;58:1. www.cdc.gov/mmwr/preview/mmwrhtml/ss5810a1.htm.
23. Kogan, MD, et al, Prevalence of parent reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics 2009;124:1395–1403. www.pediatrics.org/cgi/doi/10.1542/peds.2009-1522.
24. Boulet, SL, Boyle, CA, Schieve, LA. Health care use and health and functional impact of developmental disabilities among US children. Arch Pediatr Adolesc Med. 2009;163:19–26.
25. Kogan, MD, et al, A national profile of the health care experiences and family impact of autism spectrum disorder among children in the United States, 2005-2006. Pediatrics 2008;122:e1149–e1158. www.pediatrics.org/cgi/content/full/122/6/e1149.
26. Schieve, LA, Blumberg, SJ, Rice, C, Visser, SN, Boyle, C. The relationship between autism and parenting stress. Pediatrics. 2007;119(Suppl 1):S114–S121.
27. McCracken, JT, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314–321.
28. Weinstein, SL, Gaillard, WD. Epilepsy. In: Batshaw M, ed. Children with Disabilities. 6th ed. Baltimore, Md: Paul H. Brookes; 2007:439–460.
29. Lowenstein, DH, Bleck, T, Macdonald, RL. It’s time to revise the definition of status epilepticus. Epilepsia. 1999;40:120.
30. Abend, NS, Dlugos, DJ. Treatment of refractory status epilepticus: Literature review and a proposed protocol. Pediatr Neurol. 2008;38:377–390.
31. Pellock, JM. Overview: Definitions and classifications of seizure emergencies. J Child Neurol. 2007;22(Suppl):9S.
32. McLachlan, RS. Vagus nerve stimulation for intractable epilepsy: A review. J Clin Neurophysiol. 2007;14:358.
33. Al-Otaibi, FA, Hamani, C, Lozano, AM. Neuromodulation in epilepsy. Neurosurgery. 2011;69:957–979.
34. Epilepsy Foundation. Treatment options: Vagus nerve stimulation. www.epilepsyfoundation.org/about/treatment/vns/.
35. Stevenson, KL. Chiari Type II malformation: Past, present, and future. Neurosurg Focus. 2004;16:5.
36. Paker, N, Soy, D. Reasons for rehospitalization in patients with spinal cord injury: 5 years’ experience. Int J Rehabil Res. 2006;29:71–76.
37. Korinek, AM, Fulla-Oller, L. Morbidity of ventricular cerebrospinal fluid shunt surgery in adults: An 8-year study. Neurosurgery. 2011;68:985–994.
38. Bokhary, MA, Kamal, H. Ventriculo-peritoneal shunt infections in infants and children. Libyan J Med. 2008;3:20–22.
39. Khan, AA, Jabbar, A. Cerebrospinal shunt malfunction: Recognition and emergency management. Br J Hosp Med (Lond). 2007;68:651–655.
40. Gottrand, F, Sullivan, PB. Gastrostomy tube feeding: When to start, what to feed and how to stop. Eur J Clin Nutr. 2010;64(Suppl 1):S17–S21.
41. Shah, M, Klooster, M. Frequency and methods of gastrojejunal tube replacement in children. Curr Gastroenterol Rep. 2010;12:223–227.
42. El-Matary, W. Percutaneous endoscopic gastrostomy in children. Can J Gastroenterol. 2008;22:993–998.
43. Pinon, M, Bezzio, S. A prospective 7-year survey on central venous catheter–related complications at a single pediatric hospital. Eur J Pediatr. 2009;168:1505–1512.
44. Mitsui, T, Minami, K. Is suprapubic cystostomy an optimal urinary management in high quadriplegics? A comparative study of suprapubic cystostomy and clean intermittent catheterization. Eur Urol. 2000;38:434–438.
45. MacDiarmid, SA, Arnold, EP. Management of spinal cord injured patients by indwelling suprapubic catheterization. J Urol. 1995;154(Pt 1):492–494.
46. Esclarín De Ruz, A, García Leoni, E. Epidemiology and risk factors for urinary tract infection in patients with spinal cord injury. J Urol. 2000;164:1285–1289.
47. Newman, E, Price, M. External catheters: Hazards and benefits of their use by men with spinal cord lesions. Arch Phys Med Rehabil. 1985;66:310–313.
48. Goetz, LL, Howard, M. Occurrence of candiduria in a population of chronically catheterized patients with spinal cord injury. Spinal Cord. 2010;48:51–54.
49. National Institute on Disability and Rehabilitation Research Consensus Statement. The prevention and management of urinary tract infections among people with spinal cord injuries. January 27-29, 1992. J Am Paraplegia Soc. 1992;15:194–204.