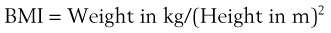Chapter 9 Evaluation and Recognition of the Difficult Airway
I Introduction
The quest to forecast difficult airway (DA) management has spanned more than a half century. In that time, a plethora of scientific investigations, book chapters, editorials, lectures, and workshops have been devoted to this essential issue. In 1956, Cass and colleagues underscored the point and began the search for a simple beside test to determine whose airway will be difficult to manage.1 As of this writing, such a test does not exist.2 Practitioners apply multiple tests, none of which are accurate. Absent reliable data, airway managers depend on case reports and personal experience to formulate critically important airway management plans.3
The medical literature boasts an abundance of articles discussing predictors of difficult intubation (DI) but relatively few explaining predictors of difficult mask ventilation (DMV). Among all of them, practitioners seek to determine which criteria are dependable and which are not.4 Having established a basis on which to predict DMV or DI or both, it becomes possible to select modes of airway management that optimize patients’ safety and comfort.
Generally, failed endotracheal intubation occurs once in every 2230 attempts.5 The average anesthesiologist in the United States has one failed intubation every other year. The incidence is small, but the potential consequences of DA management are of major importance. Failed ventilation accounted for 44% of intraoperative cardiac arrests reported by Keenan and Boyan.6 Thirty-four percent of liability claims identified by Caplan and coauthors were based on adverse respiratory events,7 of which three quarters were judged to be preventable.8
Radiographs and other imaging techniques have been advocated to predict DI but are too expensive and inconvenient to serve as routine screening tests. Highly specialized techniques such as acoustic reflectometry are of dubious reliability.9 More quantitative, noninvasive measurements, such as those with the laryngeal indices caliper,10 bubble inclinometer,11 and goniometer, offer the potential for accurate measurements but have never found their way into clinical practice.
A history of DA is a strong predictor of future problems12; in my opinion, it is the single most reliable predictor of a DA. The contrapositive is not necessarily true, however. A history of problem-free airway management is suggestive of future ease but not a guarantee. Many factors that contribute to difficulty are progressive with time. Examples of such problems include rheumatoid arthritis and obesity. An airway history should be elicited from all patients. Review of prior anesthesia records is frequently helpful; they may describe previously encountered problems, failed therapies, and successful solutions.13
II Problematic Ventilation by Traditional Face Mask
DMV occurs when a practitioner cannot provide sufficient gas exchange because of inadequate mask seal, large volume leaks, or excessive resistance to the ingress or egress of gas.13 The incidence of DMV varies between 0.08% and 5%.14,15 This wide range is probably related to conflicting definitions of DMV.16 Impossible mask ventilation occurs in 0.07% to 0.16% of patients.15,17 Risk factors for DMV include full beard, massive jaw, edentulous state, skin sensitivity (e.g., burns, epidermolysis bullosa, fresh skin grafts), facial dressings, obesity, age older than 55 years, and a history of snoring.15 Other criteria that suggest the possibility of DMV include large tongue, heavy jaw muscles, history of obstructive sleep apnea (OSA), poor atlanto-occipital extension, some types of pharyngeal pathology, facial burns, and facial deformities18 (Box 9-1).
Box 9-1 Risk Factors for Difficult Mask Ventilation
Many types of pharyngeal problems can produce DMV, including lingual tonsil hypertrophy,19 lingual tonsillar abscess, lingual thyroid,20 and thyroglossal cyst.21–24 Many abnormalities cannot be diagnosed by classic airway examination techniques. The presence of any one factor is suggestive of DMV, and as factors increase in number, the likelihood of difficulty increases. A greater than normal mandibulohyoid distance has been associated with OSA, the pathophysiology of which may be related to DMV.25–27 Anesthetic technique could contribute to DMV. El-Orbany and Woehlck suggested that high-dose opioid–induced vocal cord adduction produces DMV.28
Traditional face mask airway management is generally safe and effective. In the unusual instances in which it is not, endotracheal intubation remains one fallback option. Although this scheme works well in most cases, approximately 15% of all DIs are also DMVs.29 Some factors that predispose to DMV also contribute to DI; they include OSA, history of snoring, obese neck, and poor mandibular translation. Some 25% of impossible mask ventilation patients are also difficult to intubate. The overall incidence of impossible mask ventilation and DI is 1 in every 2800 patients.30 Proposed predictors of impossible mask ventilation are listed in Box 9-2.
III Problematic Intubation by Traditional Laryngoscopy
A Sniffing Position
DI occurs when multiple attempts at endotracheal intubation are required.13 The presence or absence of airway pathology does not influence its definition. Traditional laryngoscopy is performed to visualize the laryngeal opening. The observing laryngoscopist is situated outside the airway, above the patient’s head. To see through the airway, light must travel from the glottic opening to the laryngoscopist’s eye. Because light travels in straight lines, the technique requires an uninterrupted linear path between larynx and observer. Most of the manipulations performed attempt to satisfy this criterion.
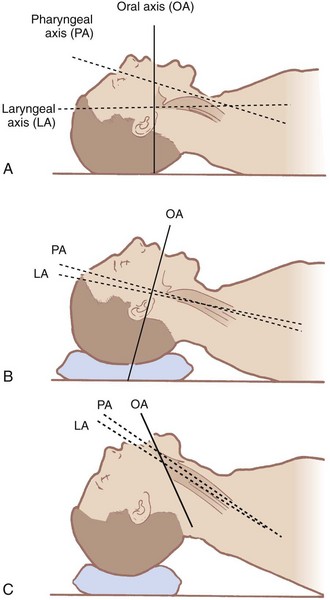
The airway contains three visual axes. They are the long axes of the mouth, oropharynx, and larynx. In the neutral position, these axes form acute and obtuse angles with one another (Fig. 9-1). Light cannot bend around these angles under normal circumstances. To bring all three axes into better alignment, McGill suggested the “sniffing the morning air” position.31 The true sniffing position has two components: cervical flexion and atlanto-occipital extension. Cervical flexion approximates the pharyngeal and laryngeal axes, and atlanto-occipital extension brings the oral axis into better alignment with the other two. Normal atlanto-occipital extension measures 35 degrees.32 With optimal alignment of the airway’s visual axes, it becomes possible to look through the airway into the laryngeal opening. A reduced atlanto-occipital gap or a prominent C1 spinous process impairs laryngoscopy33,34 if vigorous attempts at extension are performed, because the trachea bows and the larynx is forced anteriorly.33
The ability to achieve the sniffing position is easily tested. One simply has the patient flex the lower cervical vertebrae and extend at the atlanto-occipital joint. Pain, tingling, numbness, or inability to achieve these maneuvers predicts DI.35 The benefits of the sniffing position have been dogma for more than 70 years, but Adnet and colleagues and Chou and Wu have questioned its utility.36,37
B Mouth Opening
Mouth opening is important because it determines the available space for placing and manipulating laryngoscopes as well as endotracheal tubes.38,39 A small mouth opening may not accommodate either. Mouth opening also provides room to see through the uppermost part of the airway. Mouth opening relies on the temporomandibular joint (TMJ), which has both a hingelike movement and a gliding motion. The gliding motion is known as translation. The hingelike movement allows the mandible to pivot on the maxilla. The more the mandible swings away from the maxilla, the bigger the mouth opening.
The adequacy of mouth opening is assessed by measuring the interincisor distance. An interincisor distance of 3 cm provides sufficient space for intubation, absent other complicating factors. This corresponds approximately to two finger breadths.40 The two finger breadth test is performed by placing the examiner’s second and third digits between the patient’s central incisors. If they fit, there should be adequate room to perform laryngoscopy; if they do not fit, laryngoscopy may be difficult. Factors that interfere with mouth opening include masseter muscle spasm, TMJ dysfunction, and various integumentary aliments. Skin problems that adversely effect mouth opening include burn scar contractures and progressive systemic sclerosis. Masseter muscle spasm can be relieved by induction of anesthesia and administration of muscle relaxants. Mechanical problems at the TMJ remain unaltered by medications. Some patients demonstrate adequate mouth opening when awake but not after anesthesia induction.41 The problem can often be relieved by pulling the mandible forward. A mouth opening that was sufficient for a previous anesthetic may not be so after temporal neurosurgical procedures.42
D Tongue
A normal-size tongue usually fits easily into a normal-size mandibular space. A large tongue fits poorly into a normal-size mandibular space. After filling the space, a large tongue still occupies some of the oropharyngeal airway and obstructs it. For this reason, a large tongue (macroglossia) is a predictor of DI. Similarly, a normal-size tongue fits poorly into a small mandibular space.43 It, too, occupies some of the oropharyngeal airway, thereby obstructing the line of sight. Consequently, a small mandible (micrognathia) is a predictor of DI. In essence, a tongue that is large compared with the size of the mouth (oropharynx) and mandible takes up excessive space in the oropharynx and interferes with visualization.
The base of the tongue is located so close to the larynx that inability to adequately displace it anteriorly creates another problem. Because the base of the tongue hangs down over the larynx, the glottis is hidden from view. The glottic aperture is then anatomically anterior to the base of tongue—hence, the term anterior larynx. Under such circumstances, the larynx is anterior to the base of the tongue and cannot be seen because the tongue hides it. In this manner, glottic and supraglottic masses can create DI if they force the base of the tongue posteriorly. Some of the masses that may be encountered are lingual tonsils,19,44 epiglottic cysts,23 and thyroglossal duct cysts.
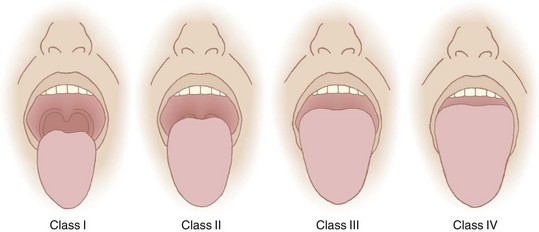
Recognizing the implications of tongue size for successful laryngoscopy, Mallampati and colleagues45 in 1985 and Samsoon and Young5 in 1987 devised classification systems to predict DL utilizing this concept. Mallampati and Samsoon reasoned that a large tongue could be identified on visual inspection of the open mouth. Both classification systems relate the size of the tongue to the oropharyngeal structures identified. A normal-size tongue allows visualization of certain oropharyngeal structures. As the tongue size increases, some structures become hidden from view. Both investigators proposed systems that reasoned backward from this premise.
Application of the Mallampati or the Samsoon classification system is easy and painless. The patient is seated in the neutral position. The mouth is opened as wide as possible, and the tongue is protruded as far as possible. Phonation is discouraged because it raises the soft palate and allows visualization of additional structures.46 The observer looks for specific anatomic landmarks: the fauces, pillars, uvula, and soft palate. The Mallampati classification system uses three groups and the Samsoon system uses four (Fig. 9-2). Both systems suggest that as tongue size increases, fewer structures are visualized and laryngoscopy becomes more difficult. Mallampati class tends to be higher in pregnant patients than in nonpregnant ones.47
Just as the size of tongue can be estimated, so too can the size of the mandible. The patient’s head is extended at the atlanto-occipital joint. The mentum of the mandible and the thyroid cartilage are identified. The “Adam’s apple” (thyroid notch) is the most superficial structure in the neck and serves as a good landmark for the thyroid cartilage. The vocal cords lie just caudad to the thyroid notch. The distance between the thyroid cartilage and the mentum (thyromental distance, or TMD) is measured in one of three ways: with a set of spacers, with a small pocket ruler, or with the observer’s fingers. The normal TMD is 6.5 cm. A TMD greater than 6 cm is predictive of easy intubation, and a TMD of less than 6 cm is suggestive of DI.48 If a ruler is not present at the bedside, practitioners can judge the TMD with their own fingers. The width of one’s middle three fingers frequently approximates 6 cm, and the TMD can be compared with the fingers’ span. In that way, clinically relevant approximations can be taken into account when examining patients for the purpose of predicting DI.
The ability to translate the TMJ is easily assessed before induction. The patient is simply asked to place the mandibular incisors (bottom teeth) in front of the maxillary incisors (upper teeth). Inability to perform this simple task usually results from one of two sources. First, the TMJ may not glide, predicting DI.49 Second, some patients find it difficult to coordinate the maneuver, in which case there is no implication for DI.
The upper lip bite test was proposed as a modification of the TMJ displacement test.50 The upper lip bite test is performed by asking the patient to move the mandibular incisors as high on the upper lip as possible. The maneuver is similar to biting the lip. Contact of the teeth above or on the vermilion border is thought to predict adequate laryngoscopic views. Inability to touch the vermilion boarder with the mandibular teeth is thought to predict poor laryngoscopic views. Both the TMJ translation test and the upper lip bite test assess TMJ glide, which is an important consideration during laryngoscopy. Khan and coworkers confirmed the predictive importance of this maneuver.51 Table 9-1 summarizes a quick, easy, bedside scheme for predicting DI.
TABLE 9-1 Generally Accepted Predictors of Difficult Intubation
| Criterion | Suggestion of Difficult Intubation |
|---|---|
| History of difficult intubation | Positive history |
| Length of upper incisors | Relatively long |
| Interincisor distance | Less than two finger breadths (<3 cm) |
| Overbite | Maxillary incisors override mandibular incisors |
| Temporomandibular joint translation | Inability to extend mandibular incisors anterior to maxillary incisors |
| Mandibular space | Small, indurated, encroached upon by mass |
| Cervical vertebral range of motion | Cannot touch chin to chest or cannot extend neck |
| Thyromental distance | Less than three finger breadths (<6 cm) |
| Mallampati-Samsoon classification | Mallampati III/Samsoon IV—relatively large tongue: uvula not visible |
| Neck | Short, thick |
Adapted from American Society of Anesthesiologists Task Force on Difficult Airway Management: Practice guidelines for management of the difficult airway: An updated report. Anesthesiology 98:1269, 2003.
IV Special Situations
A Morbid Obesity
In patients with MO, adipose tissue deposits in the lateral pharyngeal walls. These deposits are not fixed to bone and are highly mobile. They protrude into the airway, narrowing it, and are drawn farther into the airway during periods of negative airway pressure, such as during inspiration. In these ways, reduced dilator muscle function or pharyngeal adipose depositions predispose to OSA. (See Chapter 43 for further details.)
Although time to oxyhemoglobin desaturation is not a predictor of DI, it is an important consideration. The longer the time available to perform laryngoscopy, the greater the likelihood of success. Rapid hemoglobin desaturation limits that time and thereby reduces the chance of endotracheal intubation. A patient with MO and a BMI of 40 kg/m2, breathing room air, who becomes apneic desaturates to an oxygen saturation in arterial blood (SaO2) of 90% in approximately 1 minute, and to 60% in the next minute. In contrast, if the same patient is breathing 100% O2 before induction of anesthesia, the SaO2 takes approximately 21/2 minutes to fall to 90% and does not reach 60% for an additional 11/2 minutes.51,52 The data show that successful oxygenation before induction of anesthesia extends the period of time until oxyhemoglobin desaturation takes place. Consequently, preoxygenation provides a longer period for laryngoscopy, which should increase the chances of successful intubation.
Although OSA pathology and pathophysiology predispose to DMV and DI, the true incidence of problems resulting from MO is undefined. The popularity of bariatric surgery has brought numerous patients with MO to the operating room. Because these patients receive face mask ventilation infrequently, there is little practical experience to refute classic teachings about such ventilation. It is reasonable to expect fat cheeks, a short immobile neck, a large tongue, and pharyngeal adipose deposits to complicate face mask ventilation.53 Nevertheless, Brodsky and colleagues reported on a morbidly obese patient with a BMI of 43 kg/m2 and OSA who was ventilated easily by face mask.54 This experience serves to document the clinical findings of many practitioners. Along the same lines, considerable experience with laryngoscopy in patients with MO has developed. Absent findings to the contrary, most of these patients are easy to intubate. In other words, MO does not appear to be a strong independent predictor of DI.54–57 The presence of other DI predictors implies potential problems.58 Gonzalez and colleagues suggested that a neck circumference greater than 43 cm (19 inches) is such a predictor.59 Pretracheal fat accumulation has been investigated as a predictor of DI, but the results have been conflicting.60,61
B Pregnancy
Airway management is one of the most important factors contributing to maternal mortality.62,63 (See Chapter 37 for further details.) Airway difficulties pose a risk of pulmonary aspiration and hypoxic cardiopulmonary arrest. Rocke and associates reported some degree of difficulty during intubation in almost 8% of full-term pregnant patients undergoing cesarean section.55
Pregnant patients suffer from generalized soft tissue swelling. They all gain weight and often reach MO proportions. Short, fat necks tend to prevent achievement of the proper sniffing position, which impairs aligning the visual axes of the mouth, pharynx, and larynx. Redundant pharyngeal tissues may fall into the airway during traditional laryngoscopy, obstructing the line of sight. Tissue edema can produce so much mucosal swelling that landmarks, such as epiglottis and arytenoids, are hard to distinguish. The breasts may be so engorged that they interfere with placement of a laryngoscope handle.64 As the space between mandible and chest diminishes, less room is available for the assistant’s hand to provide cricoid pressure. In fact, the assistant’s hand may take up space needed to open the mouth, thereby preventing adequate mouth opening. Sufficient mouth opening is required to see through the airway as well as to insert and manipulate equipment.
Tongue swelling produces macroglossia. Macroglossia prevents sufficient displacement into the mandibular space, so the line of sight is obstructed. It has been suggested that straining can exacerbate the Mallampati-Samsoon classification.65
Overzealous left lateral uterine displacement can tilt the torso and neck, altering the airway position in relation to the cervical spine. Cricoid pressure may exacerbate this problem by displacing the airway laterally, occluding it,66 or angulating the larynx.
Swelling of the supraglottic airway interferes with face mask ventilation and complicates laryngoscopy and intubation. As the upper airway becomes edematous, it also becomes more friable. Instrumentation of such airways frequently leads to bleeding, which further complicates face mask ventilation and endotracheal intubation.67
Parturients, especially obese parturients, have reduced FRC.68,69 All pregnant women experience an increase in O2 consumption of approximately 20%. Consequently, periods of apnea, such as during laryngoscopy under general anesthesia, predispose to desaturation in these patients much more quickly than in their nonpregnant and nonobese counterparts.70,71 Rapid oxyhemoglobin desaturation reduces the time available for laryngoscopy and detracts from the successful completion of intubation.
Other factors tend to impair successful laryngoscopy. Anxiety on the part of inexperienced physicians has led to intubation attempts before adequate conditions are achieved. Laryngoscopy under general anesthesia requires sufficient depth of anesthesia and profound muscle relaxation.72,73 Absent one or both of these conditions, the chances of success are markedly reduced. The result is poor mouth opening, reduced interincisor distance, retching, vomiting, and aspiration. Well-intentioned, novice assistants degrade intubating conditions or fail to enhance them.73 The Report on Confidential Enquiries into Maternal Deaths in England and Wales pointed to failure to provide cricoid pressure, poorly applied cricoid pressure, or release of cricoid pressure during a vomiting episode as major contributors to maternal morbidity and mortality.62
The application of cricoid pressure during active vomiting has been controversial. Sellick’s original description of cricoid pressure called for release of the maneuver during vomiting episodes.74 A single case report described an elderly woman who vomited during application of cricoid pressure and suffered an esophageal rupture.75 Although other factors could have contributed to or caused the rupture in this patient, some have recommended relinquishing cricoid pressure during vomiting episodes on the basis of this occurrence. Sellick retracted his initial recommendation, and others have supported the maintenance of cricoid pressure during active vomiting,76–78 believing that the risk of aspiration pneumonia is greater than the risk of esophageal rupture.77,78 Furthermore, cricoid pressure sometimes pushes the entire neck posteriorly, resulting in forward flexion of the head on the neck. As a result, the advantages of the sniffing position are lost and laryngoscopy becomes more difficult. To correct this problem, bimanual cricoid pressure was suggested by Crowley and Giesecke.79 To accomplish this maneuver, the assistant’s left hand supports the patient’s neck from underneath as the assistant’s right hand places pressure on the cricoid cartilage.
C Lingual Tonsil Hypertrophy
Over 111/2 years, Ovassapian and colleagues analyzed 33 cases of unanticipated DI. Before induction of anesthesia,19 none of these patients was thought to pose a significant likelihood of difficulty on the basis of careful preanesthetic airway examinations. Of the 33 patients, 15 had normal airway examinations; 3 of those patients had BMIs of 31 to 40 kg/m2. The remaining 18 patients presented with varying predictors of DI but were judged to be at low risk for poor laryngoscopic views. After induction, intubation turned out to be difficult in the hands of experienced attending anesthesiologists. All 33 patients subsequently underwent flexible fiberoptic pharyngoscopy for pharyngeal assessments, and all demonstrated lingual tonsil hypertrophy.
Lingual tonsils are lymph tissues located at the base of the tongue. They usually exhibit hypertrophy bilaterally but may do so unilaterally. Enlarged lingual tonsils can push the epiglottis posteriorly, obstructing the line of sight and preventing anterior displacement of the base of the tongue. They sometimes encroach on the vallecula, preventing optimal location of curved laryngoscope blades. They can distort the epiglottis so that it covers the glottic opening or is difficult to recognize. Not only do hypertrophied lingual tonsils prevent elevation of the base of the tongue from the line of sight, but they also have a tendency to bleed, complicating intubation even further.80,81
Lingual tonsil hypertrophy cannot be identified by classic airway examinations to predict DI. It is often asymptomatic, and its suggestive symptoms are nonspecific. They include sore throat, dysphagia, snoring, OSA, and sensations of fullness or lumps in the throat. Most patients have a history of palatine tonsillectomy or adenoidectomy.44 Lingual tonsil hypertrophy has been associated with airway obstruction and OSA.82–85 Other pharyngeal problems that can complicate laryngoscopy include acute lingual tonsillitis,82 lingual tonsillar abscesses, lingual thyroids,20 and thyroglossal cysts.21–23 These structures reside at the base of the tongue, a location that is hidden from view during routine physical examination.
D Burns
Thermal burns of the head and neck complicate airway management in several ways. (See Chapter 44 for further details.) Burn patients with coexisting airway problems experience approximately 50% greater mortality than burn patients without airway issues. Thermal damage to the upper airway results in massive swelling within 2 to 24 hours. Edematous mucosa encroaches on the airway lumen to create severe narrowing or even occlusion. A narrowed upper airway reduces the amount of air drawn into the lungs. An occluded upper airway prevents any O2 from reaching the alveoli. Both effects result in hypoxia. As the mucosa swells, traditional laryngoscopy becomes difficult or impossible. So much edema can collect in the mucosa that landmarks may be unrecognizable. For these reasons, it is sometimes best to perform endotracheal intubation early in the care of burn patients, before swelling creates hypoxia and DI.86
E Acromegaly
The incidence of DI in acromegaly is four to five times higher than in the general population.87 Acromegaly results from excess growth hormone, which is frequently produced by pituitary tumors. One screening test for acromegaly involves administration of 75 to 100 g of glucose. If plasma growth hormone concentrations do not fall after 1 to 2 hours, acromegaly is suspected. High plasma growth hormone levels may indicate acromegaly. Skull radiographs and computed tomographic scans showing an enlarged sella turcica suggest anterior pituitary adenoma.
Typical acromegalic features include enlarged nose, big tongue, thick mandible, full lips, elevated nasolabial folds, and prominent frontal sinuses. These patients appear to experience overgrowth of mucosa and soft tissues of the pharynx, larynx, and vocal cords.88,89 Many experience OSA.90,91 Early in the disease process, joint spaces may be widened, but later on arthritis develops and limits range of motion. This frequently occurs at the TMJ, resulting in a small mouth opening, and it may occur in cricoarytenoid joints. Overgrowth of tissues can produce vocal cord abnormalities, resulting in hoarseness or recurrent laryngeal nerve paralysis.
These features predispose to DMV and DL. Large tongues and epiglottides produce upper airway obstruction and make laryngoscopy difficult. Big mandibles increase the distance between teeth and vocal cords, necessitating longer laryngoscope blades. Thickened vocal cords and subglottic narrowing may require smaller tracheal tubes than would otherwise be selected. Nasal turbinate enlargement may obstruct the nasal airway and prevent passage of tubes. Dyspnea on exertion, stridor, or hoarseness may suggest laryngeal abnormalities that can complicate intubation. In addition to thick mandibles, acromegalics frequently present with long mandibles. Nonetheless, the resulting increased TMD has not been found to be associated with DL.87
F Epiglottitis
Epiglottitis (supraglottitis) is caused by a potentially life-threatening infection that produces upper airway swelling and obstruction. Formerly, the most common etiology was Haemophilus influenza type B (HIB), and the disease usually occurred in children 2 to 8 years of age. Since the widespread use of HIB vaccine, epiglottitis is an unusual finding in the pediatric population, but it still occurs in immunosuppressed adults. Common presenting signs are sore throat and dysphagia. Other signs and symptoms include drooling, inspiratory stridor, high fever, rapid deterioration to respiratory distress, pharyngitis, tachypnea, cyanosis, lethargy, and tripod positioning. These patients are most comfortable sitting up, extending the neck, and leaning forward. The “muffled voice” is an infrequent finding.92 Lateral radiographs of the neck classically demonstrate a swollen epiglottis. Mild adult forms may be treated with observation for exacerbation of respiratory function.93–95 Severe cases with respiratory compromise require airway management, which usually means tracheal intubation and antibiotics.
G Acute Submandibular Space Cellulitis
Acute cellulitis of the submandibular space (Ludwig’s angina) can progress rapidly to a life-threatening situation. It generally begins as a mixed-flora infection around the mandibular molars. Aerobic and anaerobic pathogens are involved. The floor of the mouth swells and forces the tongue into the airway, creating upper airway obstruction. Extension to sublingual and hypopharyngeal areas produces tongue and pharyngeal swelling.96,97 This mechanism is exacerbated in the supine position, and patients often cannot tolerate lying flat. Inability to assume the supine position precludes computed tomography, but the diagnosis can be confirmed with lateral neck soft tissue radiographs. Potential exists for infection to track down the airway and into the mediastinum, creating significant edema and swelling throughout the airway.
H Rheumatoid Arthritis
Cricoarytenoid arthritis may arise with hoarseness, pain on swallowing, dyspnea, stridor, and tenderness over the larynx. The arytenoids are edematous and fixed in adduction. Swelling may be so severe that the glottis is obscured.98 The vocal cords move poorly,99 which makes the larynx much more difficult to identify.98
V Reliability of Prediction Criteria
Although intuitively it makes sense to perform and is consistent with best medical practices, airway evaluation frequently falls short of its intended goal.13 Numerous rating systems based on recognized prediction criteria have been investigated. Most suffer from recurrent problems.100
The first problem is nomenclature. A standardized definition of the DA did not exist until 1993.101 At that time, it was explained as a situation in which a conventionally trained anesthesiologist experienced difficulty with mask ventilation, difficulty with endotracheal intubation, or both. For years, individual investigators needed to establish their own definition of DI each time studies were conducted. Consequently, the end points of their work were not necessarily comparable to those of other investigations in the field. Multiple meanings of the term DI prevented comparative analysis of studies.
In 1993, the American Society of Anesthesiologists (ASA) Committee on Practice Guidelines for Management of the Difficult Airway offered a generally acceptable definition.101 Ten years later, the definition was altered slightly, and DI now refers to any intubation that requires multiple attempts.13 This is a good clinical definition, but it lacks the precision required for scientific investigation. For example, some practitioners may perform a single laryngoscopy and, on the basis of the view obtained, elect to forgo further attempts at laryngoscopy. Such cases may be handled with supraglottic ventilatory devices, regional anesthesia, or other techniques. This situation does not meet the definition of DI even though it would have if one more attempt at intubation had been made. Failed intubation may be an easier term to understand. A failed intubation exists when laryngoscopists give up and admit that traditional intubation will not be successful. The end point is clear and occurs with an incidence of 1 in 280 obstetric patients and 1 in 2230 among the general surgical population.5
The second problem is identifying features that predict DI. This is frequently accomplished by attempting to recognize characteristics found in patients who have proved to have DI. The problem with such an approach is the lack of information about the same characteristics in patients who have easy intubations. As Turkan and colleagues pointed out, we do not even know the normal values for many prediction criteria.102 A better method is to apply multivariate analysis to populations of patients in a prospective manner. In that way, a single factor can be compared in difficult and easy intubations. Various rating systems have attempted to combine multiple predictors into a formula, but to date, none have been satisfactory.
The fourth problem is experimental methods. Individual practitioners differ in many ways. Any given patient, on any given day, may be difficult to intubate for one laryngoscopist and not for another. Clinical practice has shown that a particular patient who is difficult to intubate in the hands of one laryngoscopist may be successfully intubated by another laryngoscopist. For this reason, experimental designs involving more than one laryngoscopist introduce a source of variation, which detracts from attempts to control experimental conditions. Relying on a single laryngoscopist obviates this problem but limits the number of patients who can be enrolled into a single study. Another source of experimental error is observer variation. Just as laryngoscopists differ, so do observers.103,104 Observations performed by different experimenters are subject to variations and introduce another source of erroneous data. The best way to prevent this problem is to have all observations performed by a single experimenter. This, too, may limit the number of patients enrolled in a single study.
Statistical tests for assessing the usefulness of criteria involve sensitivity and positive predictive value. Sensitivity is the ratio of DI patients correctly identified compared with all the DI patients within the entire population of patients. For example, consider a population of patients in which five people are difficult to intubate. If a particular predictor of DI correctly identifies all five patients, its sensitivity is 100%. A sensitivity of 100% means that all the DI patients are identified by the test. If the test correctly identifies only two of the five patients, its sensitivity is 2/5, or 40%. Positive predictive value is the probability that DI patients identified by the test are, in fact, difficult to intubate. If the test predicts that five patients will be difficult to intubate and all five of those patients are difficult to intubate, the positive predictive value of the test is 100%. A positive predictive value of 100% means that if the test says that any particular patient will be difficult to intubate, that patient will be difficult to intubate. If the test predicts that 10 patients will be difficult to intubate but only 5 of them are in fact difficult to intubate, its predictive value is 5/10, 50%. Unfortunately, statistical tests such as sensitivity and positive predictive value, when applied to classic prediction criteria, have yielded disappointing results.14,100
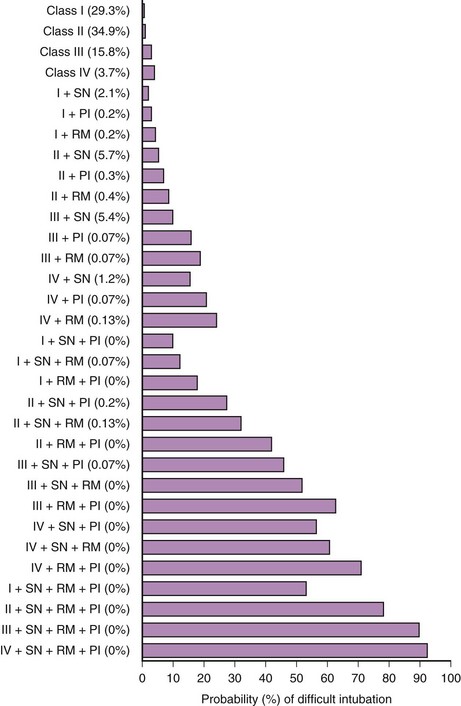
In 1984, Cormack and Lehane described a grading system for comparing laryngoscopic views (Fig. 9-3).105 Cormack-Lehane grade 1 views demonstrate the entire glottic opening. Grade 2 views show the posterior laryngeal aperture but not the anterior portion. Grade 3 views allow visualization of the epiglottis but not any part of the larynx. Grade 4 views allow the laryngoscopist to see the soft palate but not the epiglottis. Early evidence indicated a good correlation between Mallampati-Samsoon classes and laryngoscopic grades5,45 (i.e., a higher Mallampati-Samsoon class predicted a correspondingly higher laryngoscopic grade for any given patient). This concept formed the basis for using Mallampati-Samsoon classes to predict DI. In 1992, Rocke and coworkers disproved that relationship.55 They investigated several classic predictors of DI and demonstrated that none of them was a reliable predictor of DI (Fig. 9-4).
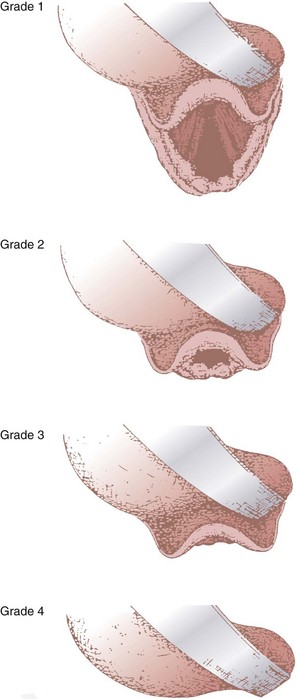
(From Cormack RS, Lehane J: Difficult tracheal intubation in obstetrics. Anaesthesia 39:1105, 1984.)
Classic prediction criteria essentially deal with surface anatomy. They screen for some factors that are associated with DI but fail to address others. Moreover, some potential problems are hidden from surface anatomy examinations. Glottic and supraglottic abnormalities such as lingual tonsil hypertrophy or epiglottic prolapse into the glottic opening cannot be diagnosed by standard physical examinations for predicting DI.19,106,107 Pathophysiologic factors such as mobile TMJ discs or disc fragments can produce severely limited mouth opening after induction of anesthesia when none existed before.106 Precise measurements of atlantoaxial motion sometimes fail to predict DI.108 Supraglottic, glottic, or subglottic pathology may be unrecognized by standard tests but complicate intubation nonetheless.109 As of this writing, no single factor reliably predicts DI. As more and more predictors of DI are found in the same patient at the same time, the likelihood of DI increases.35,104,110–112
VI Nontraditional Considerations in Difficult Airway Prediction
A Imaging
Attempts at predicting DA management by imaging are not new. Standard radiographic films have been used to assess bony structures in an attempt to predict airway problems. However, they have been only minimally helpful. Charters took this concept to the next level; he advocated mathematical modeling, looking at relationships between bony structures.113 One example is the minimal residual volume114: Insufficient volume between the rami of the mandible (i.e., the mandibular space) prevents sufficient anterior tongue displacement and forces the tongue inferiorly, where it drives the epiglottis against the posterior pharyngeal wall and prevents visualization of the larynx. Another example is lateral neck radiographs from which geometric calculations are made.115 Radiography requires the use of large machines, specialty consultation by radiologists, moving patients to radiology suites, and considerable expense. These constraints are certainly not adaptable to screening large numbers of patients.
Other types of mathematical modeling include airway indices. Numerous indices have been promulgated. Despite the appeal of attaching numbers to criteria and performing calculations with these numbers, airway indices, such as those postulated by Wilson and Arne, have failed to accurately predict DI.116 Newly created risk scores, such as the one offered by Eberhart and collegues,117 await independent validation. Interest in airway indices continues, in the hope that reliable predictions can be formulated based on bedside observations.
Among the most interesting of all mathematical models is computerized facial analysis.118,119 Connor and Segal119 are developing programs to assess facial images and predict difficulty of intubation. At present, they require high speed computers, substantial computing power, and network access. As impressive as this program appears, there are likely to be predictors of DI that are not included in their analysis.
B Supraglottic and Glottic Tumors
Various types of imaging can diagnose supraglottic and glottic tumors. However, relatively few patients benefit from such studies. Most patients present for airway management without imaging. Airway assessment relies on the history and examination of surface anatomy. A history of voice changes, respiratory distress, snoring, and swallowing issues is nondescript and frequently absent when airway tumors are present. Traditionally, a high index of suspicion has prompted otolaryngologic consultation, but otherwise many of these tumors go undiagnosed. These masses can directly obstruct visualization of the larynx. They can occupy space in the vallecula, preventing optimal placement of Macintosh blades, and can interfere with displacement of the epiglottis by Miller blades.23 Many supraglottic and glottic tumors remain undiagnosed and interfere with mask ventilation and intubation. The classic example is lingual tonsil hypertrophy. Enlarged lingual tonsils push the epiglottis posteriorly, preventing visualization of the glottis and obstructing mask ventilation.120
D Arthritis
Arthritis is usually associated with joints in the extremities. It also affects laryngeal joints. Cricoarytenoid arthritis can impair Cormack-Lehane views. A related issue is ligament calcification. Stylohyoid ligament calcification can limit forward movement of the hyoid bone, thereby interfering with epiglottic displacement.114
E Cancerous Goiter
In modern practice, thyroid goiters are usually removed before they grow very large. Goiters that bow the trachea generally leave the larynx in the midline. Even tracheomalacia rarely interferes with airway management. Nevertheless, cancerous goiter increases the risk of DI. It is postulated that laryngeal fibrosis extends from the cancer, diminishing soft tissue mobility.121
F Offsetting Features
Individual predictors of DA management carry varying importance in different patients. In some circumstances, a given predictor may produce substantial difficulty, whereas in other situations, it may contribute little. Practitioners have observed this repeatedly. One reason may be offsetting features. One or more factors that contribute to DA management can be offset by others. Perhaps the best recognized example of this point is the edentulous maxilla.122 Absence of upper teeth allows for such an advantageous line of sight that coexisting confounding issues may well be rendered clinically unimportant.
G Interobserver Variation
In order to investigate factors that predict DA management and to apply that information clinically, it becomes important to agree on nomenclature and to standardize tests. If different investigators use varying definitions of terms, their findings cannot be compared. Once agreement has been reached among investigators, they must use the same end points in order to apply their findings. The wide range of reported sensitivity and specificity values among tests could relate to this fundamental issue. Interobserver variability confounds establishing specific tests as worthwhile and adversely affects their clinical use. Interobserver variability is especially well recognized for Mallampatti-Samsoon classification systems,123 and it adversely affects other tests as well.
H Anesthetic Technique
During mask ventilation, partial or complete vocal cord adduction produces upper airway obstruction and interferes with ventilation as well as oxygenation. During laryngoscopy, passage of a tracheal tube between partially or completely adducted vocal cords may lead to problems, including vocal cord hematoma and laceration. These conditions can lead to hoarseness and altered voice quality on a temporary basis, and occasionally the changes can be permanent. For most patients, temporary voice changes are a nuisance, but for those who use their voice professionally, this complication becomes a major problem. For patients who experience permanent aphonia, these complications are major setbacks. More acutely, eliminating neuromuscular blockers from anesthetic induction and laryngoscopy increases the risk of DI or failed endotracheal intubation.124
VII ConclusionS
New tests are sorely needed. They should be painless to perform; patients do not tolerate discomfort for the sake of DA screening. The tests should be quick, should be simple to apply, and should require little or no equipment. Practitioners need to perform these tests on each patient. Complex procedures, cumbersome equipment, and difficult calculations will dissuade many physicians and nurses from using tests. They should be bedside procedures; it is impractical to send ambulatory surgery patients, day-of-admission surgery patients, or emergency patients to distant places for high-technology, expensive imaging studies. The tests should be objective, with little interexaminer variation,16,103 and reproducible. They should be reliable.14,112 High degrees of sensitivity and positive predictive value are crucial.
Classic prediction criteria deal primarily with surface anatomy and fail to address issues such as lingual tonsils, epiglottic cysts, and other soft tissue pathology. For this reason, despite outward appearances, a history of DI may be the most reliable predictor of future DIs. A history of prior DI is typically elicited in one of two ways—orally or in writing. Frequently, patients discuss prior intubation difficulties during the preanesthetic interview. Alternatively, this information may be discovered in old records of patients, in written letters, or through the Medic Alert National Registry (see Chapter 54). The Medic Alert Registry provides patients with bracelets and cards that identify their basic medical problem; more information is available through an emergency phone number provided to each registrant.
VIII Clinical Pearls
• No single test reliably predicts DMV or DL.
• Generally accepted predictors of DA management tend to have poor sensitivity and low positive predictive value.
• Proposed predictors of impossible facemask ventilation include neck radiation changes, beard, male gender, OSA, and Mallampati-Samsoon class III or IV.
• Recommended tests to predict DI include history of DI, protruding upper incisors, interincisor distance <3 cm, failed TMJ translation, small indurated mandibular space, limited cervical vertebral range of motion, TMD <6 cm, Mallampati-Samsoon Class III or IV, and short thick neck.
• The Cormack-Lehane views are as follows: grade I, entire glottic aperture visualized; grade II, posterior glottis visualized, but not the anterior portion; grade III, epiglottis visualized, but no part of the glottis; grade IV, soft palate visualized, but not the epiglottis.
• High-dose opioids sometimes produce vocal cord adduction leading to DMV and DI.
All references can be found online at expertconsult.com.
2 Shiga T, Wajma Z, Inoue T, et al. Predicting difficult intubation in apparently normal patients. Anesthesiology. 2005;103:429–437.
5 Samsoon GLT, Young JRB. Difficult tracheal intubation: A retrospective study. Anaesthesia. 1987;42:487–490.
7 Caplan RA, Posner KL, Ward RJ, et al. Adverse respiratory events in anesthesia: A closed claims analysis. Anesthesiology. 1990;72:828–833.
13 Practice guidelines for management of the difficult airway. An updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003;98:1269–1277. Erratum 10:565, 2004
15 Langeron O, Masso E, Huraux C, et al. Prediction of difficult mask ventilation. Anesthesiology. 2000;92:1229–1236.
17 Kheterpal S, Han R, Tremper KK, et al. Incidence and predictors of difficult and impossible mask ventilation. Anesthesiology. 2006;105:885–891.
19 Ovassapian A, Glassenberg R, Randel GI, et al. The unexpected difficult airway and lingual tonsil hyperplasia: A case series and a review of the literature. Anesthesiology. 2002;97:124–132.
30 Kheterpal S, Martin L, Shanks AM, et al. Prediction of outcomes of impossible mask ventilation: A review of 50,000 anesthetics. Anesthesiology. 2009;110:891–897.
52 Benumof JL, Dagg R, Benumof R. Critical hemoglobin desaturation will occur before return to an unparalyzed state following 1 mg/kg of intravenous succinylcholine. Anesthesiology. 1997;87:979–982.
55 Rocke DA, Murray WB, Rout CC, et al. Relative risk analysis of factors associated with difficult intubation in obstetric anesthesia. Anesthesiology. 1992;47:67–73.
124 Lunstrom LH, Moller AM, Rosenstock C, et al. Avoidance of neuromuscular blocking agents may increase the risk of difficult tracheal intubation: A cohort study of 103,812 consecutive patients recorded in the Danish anaesthesia database. Br J Anaesth. 2009;103:283–290.
1 Cass NM, James NR, Lines V. Difficult direct laryngoscopy complicating intubation for anaesthesia. Br Med J. 1956;1:488–489.
2 Shiga T, Wajma Z, Inoue T, et al. Predicting difficult intubation in apparently normal patients. Anesthesiology. 2005;103:429–437.
3 Bannister FB, MacBeth RG. Direct laryngoscopy and tracheal intubation. Lancet. 1944;244:651–654.
4 Lee A, Fan LTY, Gin T, et al. A systematic review (meta-analysis) of the accuracy of the Mallampati test to predict the difficult airway. Anesth Analg. 2006;102:1867–1868.
5 Samsoon GLT, Young JRB. Difficult tracheal intubation: A retrospective study. Anaesthesia. 1987;42:487–490.
6 Keenan RL, Boyan CP. Cardiac arrest due to anesthesia: A study of incidence and cause. JAMA. 1985;253:2373–2377.
7 Caplan RA, Posner KL, Ward RJ, et al. Adverse respiratory events in anesthesia: A closed claims analysis. Anesthesiology. 1990;72:828–833.
8 Cheney FW, Posner KL, Caplan RA. Adverse respiratory events infrequently leading to malpractice suits: A closed claims analysis. Anesthesiology. 1991;75:932–939.
9 Ochroch EA, Eckmann DM. Clinical application of acoustic reflectometry in predicting the difficult airway. Anesth Analg. 2002;95:645–649.
10 Roberts JT, Ali HH, Shorten GD. Using the laryngeal indices caliper to predict difficulty of intubation with a Macintosh #3 laryngoscope. J Clin Anesth. 1993;5:302–305.
11 Roberts JT, Ali HH, Shorten GD. Using the bubble inclinometer to measure laryngeal tilt and predict difficulty of laryngoscopy. J Clin Anesth. 1993;5:306–309.
12 Lundstrom LH, Moller AM, Rosenstock C, et al. A documented previous difficult tracheal intubation as a prognostic test for a subsequent difficult tracheal intubation in adults. Anaesthesia. 2009;64:1081–1088.
13 American Society of Anesthesiologists Task Force on Difficult Airway Management. Practice guidelines for management of the difficult airway: An updated report. Anesthesiology. 2003;98:1269–1277. Erratum 10:565, 2004
14 El-Ganzouri AR, McCarthy RJ, Tuman KI, et al. Preoperative airway assessment: Predictive value of a multivariate risk index. Anesth Analg. 1996;82:1197–1204.
15 Langeron O, Masso E, Huraux C, et al. Prediction of difficult mask ventilation. Anesthesiology. 2000;92:1229–1236.
16 Rose DK, Cohen MM. The incidence of airway problems depends on the definition used. Can J Anaesth. 1996;43:30–34.
17 Kheterpal S, Han R, Tremper KK, et al. Incidence and predictors of difficult and impossible mask ventilation. Anesthesiology. 2006;105:885–891. 2006
18 Hagberg C, Boin MH. Management of the airway: Complications. In: Benumof JL, Saidman LJ. Anesthesia and perioperative complications. ed 2. St. Louis: Mosby; 1999:3–24.
19 Ovassapian A, Glassenberg R, Randel GI, et al. The unexpected difficult airway and lingual tonsil hyperplasia: A case series and a review of the literature. Anesthesiology. 2002;97:124–132.
20 Buckland RW, Pedley J. Lingual thyroid: A threat to the airway. Anaesthesia. 2000;55:1103–1105.
21 Henderson LT, Denneny JC, Teichgraeber J. Airway-obstructing epiglottic cyst. Ann Otol Rhinol Laryngol. 1985;94:475–476.
22 Kamble VA, Lilly RB, Gross JB. Unanticipated difficult intubation as a result of an asymptomatic vallecular cyst. Anesthesiology. 1999;91:872–873.
23 Mason DG, Wark KJ. Unexpected difficult intubation: Asymptomatic epiglottic cysts as a cause of upper airway obstruction during anaesthesia. Anaesthesia. 1987;42:407–410.
24 Rashid J, Warltier B. Awake fibreoptic intubation for a rare cause of upper airway obstruction: An infected laryngocoele. Anaesthesia. 1989;44:834–836.
25 Chou HC, Wu TL. Large hypopharyngeal tongue: A shared anatomic abnormality for difficult mask ventilation, difficult intubation, and obstructive sleep apnea. Anesthesiology. 2001;94:936–937.
26 Chou HC, Wu TL. Mandibulohyoid distance in difficult laryngoscopy. Br J Anaesth. 1993;71:335–339.
27 Riley R, Guilleminault C, Herran J, et al. Cephalometric analyses and flow-volume loops in obstructive sleep apnea patients. Sleep. 1983;6:303–311.
28 El-Orbany M, Woehlck HJ. Difficult mask ventilation. Anesth Analg. 2009;109:1870–1880.
29 Williamson JA, Webb RK, Szekely S, et al. The Australian Incident Monitoring Study. Difficult intubation: An analysis of 2000 incident reports. Anaesth Intensive Care. 1993;21:602–607.
30 Kheterpal S, Martin L, Shanks AM, et al. Prediction of outcomes of impossible mask ventilation: A review of 50,000 anesthetics. Anesthesiology. 2009;110:891–897.
31 McGill IW. Technique in endotracheal anaesthesia. Br Med J. 1930;2:817–819.
32 Nichol HC, Zuck D. Difficult laryngoscopy: The “anterior” larynx and the atlanto-occipital gap. Br J Anaesth. 1983;55:141–144.
33 Brechner VL. Unusual problems in the management of airways: I. Flexion-extension mobility of the cervical vertebrae. Anesth Analg. 1968;47:362–373.
34 White A, Kander PL. Anatomic factors in difficult direct laryngoscopy. Br J Anaesth. 1975;47:468–474.
35 Wilson ME, Spiegelhalter D, Roberston JA, et al. Predicting difficult intubation. Br J Anaesth. 1988;61:211–216.
36 Adnet F, Borron SW, Dumas JL, et al. Study of the “sniffing position” by magnetic resonance imaging. Anesthesiology. 2001;94:83–86.
37 Chou HC, Wu TL. Rethinking the three axes alignment theory for direct laryngoscopy. Acta Anaesthesiol Scand. 2001;45:261–262.
38 Block C, Brechner VL. Unusual problems in airway management: II. The influence of the temporomandibular joint, the mandible, and associated structures on tracheal intubation. Anesth Analg. 1971;50:114–123.
39 De Beer DA, Williams DG, Mackersie A. An unexpected difficult laryngoscopy. Paediatr Anaesth. 2002;12:645–648.
40 Aiello G, Metcalf I. Anesthetic implications of temporomandibular joint disease. Can J Anaesth. 1992;39:610–616.
41 Redick LF. The temporomandibular joint and tracheal intubation. Anesth Analg. 1987;66:675–676.
42 Kawaguchi M, Sakamoto T, Furuya H, et al. Pseudoankylosis of the mandible after supratentorial craniotomy. Anesth Analg. 1996;83:731–734.
43 Woods GM. Mandibular tori as a cause of inability to visualize the larynx. Anesth Analg. 1995;81:870–871.
44 Golding-Wood DG, Whittet HB. The lingual tonsil: A neglected symptomatic structure? J Laryngol Otol. 1989;103:922–925.
45 Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: A prospective study. Can Anaesth Soc J. 1985;32:424–434.
46 Tham EJ, Gildersleve CD, Sanders LD, et al. Effects of posture, phonation, and observer on Mallampati classification. Br J Anaesth. 1992;68:32–38.
47 Pilkington S, Carli F, Dakin MJ, et al. Increase in Mallampati score during pregnancy. Br J Anaesth. 1995;74:638–642.
48 Patil VU, Stehling LC, Zauder HL. Predicting the difficulty of intubation utilizing an intubation guide. Anesthesiol Rev. 1983;10:32–33.
49 Calder I, Calder J, Crockard HA. Difficult direct laryngoscopy in patients with cervical spine disease. Anaesthesia. 1995;50:756–763.
50 Khan ZH, Kashfi A, Ebrahimkhani E. A comparison of the upper lip bite test (a simple new technique) with modified Mallampati classification in predicting difficulty in endotracheal intubation: A prospective blinded study. Anesth Analg. 2003;96:595–599.
51 Khan ZH, Mohammadi M, Rasouli MR, et al. The diagnostic value of the upper lip bite test combined with sternomental distance for prediction of easy laryngoscopy and intubation: A prospective study. Anesth Analg. 2009;109:822–824.
52 Benumof JL, Dagg R, Benumof R. Critical hemoglobin desaturation will occur before return to an unparalyzed state following 1 mg/kg of intravenous succinylcholine. Anesthesiology. 1997;87:979–982.
53 Farmery AD, Roe PG. A model to describe the rate of oxyhaemoglobin desaturation during apnoea. Br J Anaesth. 1996;76:284–291. Erratum 76:890, 1996
54 Brodsky JB, Lemmens HJ, Brock-Utne JG, et al. Morbid obesity and tracheal intubation. Anesth Analg. 2002;94:732–736.
55 Rocke DA, Murray WB, Rout CC, et al. Relative risk analysis of factors associated with difficult intubation in obstetric anesthesia. Anesthesiology. 1992;47:67–73.
56 Lundstrom LH, Moller A, Rosenstock C, et al. High body mass index is a weak predictor of difficult and failed tracheal intubation. Anesthesiology. 2009;110:266–274.
57 Mashour GA, Kheterpal S, Vanaharam V, et al. The extended Mallampati score and a diagnosis of diabetes mellitus are predictors of difficult laryngoscopy in the morbidly obese. Anesth Analg. 2008;107:1919–1923.
58 Voyagis GS, Kyriakis KP, Dimitriou V, et al. Value of oropharyngeal Mallampati classification in predicting difficult laryngoscopy among obese patients. Eur J Anaesthesiol. 1998;15:330–334.
59 Gonzalez H, Minville V, Delanoue K, et al. The importance of increased neck circumference to intubation difficulties in obese patients. Anesth Analg. 2008;106:1132–1136.
60 Ezri T, Gewurtz G, Sessler DJ, et al. Prediction of difficult laryngoscopy in obese patients by ultrasound quantification of anterior soft tissue. Anaesthesia. 2003;58:1111–1114.
61 Komatsu R, Sengupta P, Wadwa A, et al. Ultrasound quantification of anterior soft tissue thickness fails to predict difficult laryngoscopy in obese patients. Anaesth Intensive Care. 2007;35:32–37.
62 Report on Confidential Enquiries into Maternal Deaths in England and Wales, 1982–1984 and 1985–1987. London: Her Majesty’s Stationery Office, 1989. and 1991
63 Rochat RW, Koonin LM, Atrash HK, et al. Maternal mortality in the United States: Report from the Maternal Mortality Collaborative. Obstet Gynecol. 1988;42:91–97.
64 Kay NH. Mammomegaly and intubation. Anaesthesia. 1982;37:221.
65 Farcon EL, Kim MH, Marx GF. Changing Mallampati score during labour. Can J Anaesth. 1994;41:50–51.
66 Lawes EG, Duncan PW, Bland B, et al. The cricoid yoke: A device for providing consistent and reproducible cricoid pressure. Br J Anaesth. 1986;58:925–931.
67 Crowhurst JA. Failed intubation management at caesarean section. Anaesth Intensive Care. 1991;19:305.
68 Awe RJ, Nicotra MB, Newsom TD, et al. Arterial oxygenation and alveolar-arterial gradients in term pregnancy. Obstet Gynecol. 1979;53:182–186.
69 Bevan DR, Holdcroft A, Loh L, et al. Closing volume and pregnancy. Br Med J. 1974;1:13–15.
70 Archer GW, Marx GF. Arterial oxygen tension during apnoea in parturient women. Br J Anaesth. 1974;46:358–360.
71 Baraka AS, Hanna MT, Jabbour SI, et al. Preoxygenation of pregnant and nonpregnant women in the head-up versus supine position. Anesth Analg. 1992;75:757–759.
72 Carnie JC, Street MK, Kumar B. Emergency intubation of the trachea facilitated by suxamethonium: Observations in obstetric and general surgical patients. Br J Anaesth. 1986;58:498–501.
73 Morgan M. The confidential enquiry into maternal deaths. Anaesthesia. 1986;41:689–691.
74 Sellick BA. Cricoid pressure to control regurgitation of stomach contents during induction of anaesthesia. Lancet. 1961;2:404–406.
75 Ralph SJ, Wareham CA. Rupture of the oesophagus during cricoid pressure. Anaesthesia. 1991;46:40–41.
76 Vanner RG. Mechanisms of regurgitation and its prevention with cricoid pressure. Int J Obstet Anesth. 1993;2:207–215.
77 Sellick BA. Rupture of the oesophagus following cricoid pressure? Anaesthesia. 1982;37:23–24.
78 Robinson JS, Thompson JM. Fatal aspiration (Mendelson’s) syndrome despite antacids and cricoid pressure. Lancet. 1979;2:228–230.
79 Crowley DS, Giesecke AH. Bimanual cricoid pressure. Anaesthesia. 1990;45:588–589.
80 Henderson K, Abernathy S, Bays T. Lingual tonsillar hypertrophy: The anesthesiologist’s view. Anesth Analg. 1994;79:814–815.
81 Tokumine J, Sugahara K, Ura M, et al. Lingual tonsil hypertrophy with difficult airway and uncontrollable bleeding. Anaesthesia. 2003;58:390–391.
82 Bourne RA, Cameron PA, Dziukas L. Respiratory obstruction with lingual tonsillitis. Anaesth Intensive Care. 1992;20:367–369.
83 Dindzans LJ, Irvine BW, Hayden RE. An unusual cause of airway obstruction. J Otolaryngol. 1984;13:252–254.
84 Kashyap A, Farid A, Aldridge R, et al. Lingual tonsil causing airway obstruction. Ear Nose Throat J. 1994;73:830–831. 834
85 Olsen KD, Suh KW, Staats BA. Surgically correctable causes of sleep apnea syndrome. Otolaryngol Head Neck Surg. 1981;89:726–731.
86 Thompson PB, Herndon DN, Traber DL, et al. Effect on mortality of inhalation injury. J Trauma. 1986;26:163–165.
87 Ali Z, Bithal PK, Prabhaker H, et al. An assessment of the predictors of difficult intubation in patients with acromegaly. J Clin Neurosci. 2009;16:1043–1045.
88 Bhatia ML, Misra SC, Prakash J. Laryngeal manifestations in acromegaly. J Laryngol Otol. 1966;80:412–417.
89 Kitahata LM. Airway difficulties associated with anaesthesia in acromegaly: Three case reports. Br J Anaesth. 1971;43:1187–1190.
90 Grunstein RR, Ho KY, Berthon-Jones M, et al. Central sleep apnea is associated with increased ventilatory response to carbon dioxide and hypersecretion of growth hormone in patients with acromegaly. Am J Respir Crit Care Med. 1994;150:496–502.
91 Piper JG, Dirks BA, Traynelis VC, et al. Perioperative management and surgical outcome of the acromegalic patient with sleep apnea. Neurosurgery. 1995;63:70–75.
92 Park KW, Darvish A, Lowenstein E. Airway management for adult patients with acute epiglottitis: A 12 year experience at an academic medical center (1984-1995). Anesthesiology. 1998;88:254–261.
93 Crosby E, Reid D. Acute epiglottitis in the adult: Is intubation mandatory? Can J Anaesth. 1991;38:914–918.
94 MayoSmith MF, Hirsch PJ, Wodzinski SF, et al. Acute epiglottitis in adults: An eight-year experience in the state of Rhode Island. N Engl J Med. 1986;314:1133–1139.
95 Muller BJ, Fliegel JE. Acute epiglottitis in a 79-year-old man. Can Anaesth Soc J. 1985;32:415–417.
96 Barkin RM, Bonis SL, Elghammer RM, et al. Ludwig angina in children. J Pediatr. 1975;87:563–565.
97 Sethi DS, Stanley RE. Deep neck abscesses: Changing trends. J Laryngol Otol. 1994;108:138–143.
98 Vetter TR. Acute airway obstruction due to arytenoiditis in a child with juvenile rheumatoid arthritis. Anesth Analg. 1994;79:1198–1200.
99 Futran ND, Sherris D, Norante JD. Cricoarytenoid arthritis in children. Otolaryngol Head Neck Surg. 1991;104:366–370.
100 Yentis SM. Predicting difficult intubation: Worthwhile exercise or pointless ritual? Anaesthesia. 2002;57:105–109.
101 Practice guidelines for management of the difficult airway: A report by the American Society of Anesthesiologists’ Task Force on Management of the Difficult Airway. Anesthesiology. 1993;78:597–602.
102 Turkan S, Ates Y, Cuhruk H, et al. Should we reevaluate the variables for predicting the difficult airway in anesthesiology? Anesth Analg. 2002;94:1340–1344.
103 Karkouti K, Rose DK, Ferris LE, et al. Inter-observer reliability of ten tests used for predicting difficult tracheal intubation. Can J Anaesth. 1996;43:554–559.
104 Lewis M, Keramati S, Benumof JL, et al. What is the best way to determine oropharyngeal classification and mandibular space length to predict difficult laryngoscopy? Anesthesiology. 1994;81:69–75.
105 Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39:1105–1111.
106 Lim BS, Andrews R. Unexpected difficult intubation in a patient with normal airway on assessment. Anaesth Intensive Care. 2001;29:642–643.
107 Ramachandran K, Bhishma R. Unexpected difficult airway. Anaesthesia. 2003;58:392–393.
108 Urakami Y, Takenaka I, Nakamura M, et al. The reliability of the Bellhouse test for evaluating extension capacity of the occipitoatlantoaxial complex. Anesth Analg. 2002;95:1437–1441.
109 Carroll CD, Saunders NC. Respiratory papillomatosis: A rare cause of collapse in a young adult presenting to the emergency department. Emerg Med J. 2002;19:362–365.
110 Bellhouse CP, Dore C. Criteria for estimating likelihood of difficulty of endotracheal intubation with the Macintosh laryngoscope. Anaesth Intensive Care. 1988;16:329–337.
111 Frerk CM. Predicting difficult intubation. Anaesthesia. 1991;46:1005–1008.
112 Tse JC, Rimm EB, Hussain A. Predicting difficult tracheal intubation in surgical patients scheduled for general anesthesia: A prospective blind study. Anesth Analg. 1995;81:254–258.
113 Charters P. Analysis of mathematical model for osseous factor in difficult intubation. Can J Anaesth. 1994;41:594–602.
114 Horton WA, Fahy L, Charters P. Factor analysis in difficult tracheal intubation: Laryngoscopy-induced airway obstruction. Br J Anaesth. 1990;65:801–805.
115 Kamalipour H, Bagheri M, Kamlai K, et al. Lateral neck radiographs for prediction of difficult orotracheal intubation. Eur J Anaesthesiol. 2005;22:687–693.
116 Drolet P. Management of the unanticipated difficult airway: A systemic approach. Continuing professional development. Can J Anaesth. 2009;56:683–701.
117 Eberhart LHJ, Ardnt C, Aust HJ, et al. A simplified risk score to predict difficult intubation: Development and prospective evaluation of 3763 patients. Eur J Anaesthesiol. 2010;27:935–940.
118 Suzuki N, Isono S, Ishikawa T, et al. Submandible angle in nonobese patients with difficult tracheal intubation. Anesthesiology. 2007;106:916–923.
119 Connor CW, Segal S. Accurate classification of difficult intubation by computerized facial analysis. Anesth Analg. 2011;112:84–93.
120 Asbjornsen H, Kuwelker M, Softeland E. A case of unexpected difficult airway due to lingual tonsil hypertrophy. Acta Anaesthesiol Scand. 2008;53:310–312.
121 Bouaggad A, Nejmi SE, Bouderka MA, et al. Prediction of difficult tracheal intubation in thyroid surgery. Anesth Analg. 2004;99:603–606.
122 King TA, Adams AP. Predicting difficult intubation: What factors influence the thyromental distance? [Letter]. Br J Anaesth. 1992;47:623.
123 Bindra A, Prabhakar H, Singh GP, et al. Is the modified Mallampati test performed in supine position a reliable predictor of difficult tracheal intubation? J Anesth. 2010;24:482–485.
124 Lunstrom LH, Moller AM, Rosenstock C, et al. Avoidance of neuromuscular blocking agents may increase the risk of difficult tracheal intubation: A cohort study of 103,812 consecutive patients recorded in the Danish anaesthesia database. Br J Anaesth. 2009;103:283–290.