CHAPTER 310 Evaluation and Management of Spinal Axis Tumors
Benign and Primary Malignant
Although up to 90,000 new cases of metastatic spinal disease are diagnosed each year, primary tumors of the vertebral column are exceedingly rare. Less than 10% of all primary bony tumors arise from the vertebral column, and it is estimated that only approximately 7500 new primary spinal tumors are diagnosed each year in the United States.1 Primary vertebral column tumors can be benign or malignant. Commonly occurring benign and malignant spinal tumors are listed in Table 310-1. In this chapter, the clinical features and surgical management of common primary spinal tumors are discussed.
TABLE 310-1 List of Common Benign and Malignant Primary Vertebral Column Tumors
| Benign Primary Spinal Tumors |
| Malignant Primary Spinal Tumors |
Clinical Features
In general, primary spinal tumors are typically diagnosed at a younger age than metastatic spinal tumors. Several of the primary spinal tumors are related to commonly occurring childhood primary bone tumors seen in other skeletal regions, including Ewing’s sarcoma, osteoblastoma, and giant cell tumors (GCTs). Nevertheless, primary vertebral tumors also occur in adults. The most common benign vertebral column tumor in adults is vertebral hemangioma. Based on autopsy studies, approximately 10% to 20% of the population have vertebral hemangiomas.2,3 In contrast, the most frequently occurring malignant primary vertebral tumor in adults is plasmacytoma or multiple myeloma. Although they are lymphoproliferative tumors, they most commonly arise from bone marrow within the vertebral bodies. Moreover, patients with plasmacytoma or multiple myeloma most often have mechanical back pain, spinal fractures, or symptoms of epidural spinal cord compression.
The most frequent initial symptom in patients with primary spinal tumors is pain. Weinstein found that almost 85% of patients with primary spinal tumors complain of pain.4 Pain related to neoplastic disease may be difficult to differentiate from that caused by other benign conditions; however, it is often worse at night and occurs even at rest. The pain can also be axial or mechanical in nature secondary to erosion of the vertebral column by tumor or spinal instability. Radicular pain can occur if the spinal roots are irritated. Progressive neurological deficits can be associated with either benign or malignant primary spinal neoplasms. However, an aggressive lesion is more likely to be accompanied by pathologic fractures or significant spinal cord compression. Spinal deformity can occur in patients with primary spinal tumors, but it is rare for patients with primary spinal tumors to have gross spinal instability. Nevertheless, one classic finding in patients with osteoid osteoma is painful scoliosis.5–9
Clinical Evaluation
Young patients with protracted spinal pain, pain that is worse at night, pain that occurs at rest, or pain associated with neurological deficits should prompt a medical evaluation for spinal tumors. The initial study to evaluate for spinal tumors is generally plain radiography. Plain radiographs can localize the lesion and are diagnostic in selected cases. However, plain radiographs require approximately 50% loss of mineralization before osteolytic lesions are detected.10 As a result, plain radiographs have low sensitivity for detecting primary spinal tumors, and they often fail to detect small or early tumors.
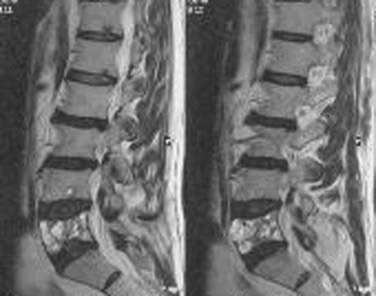
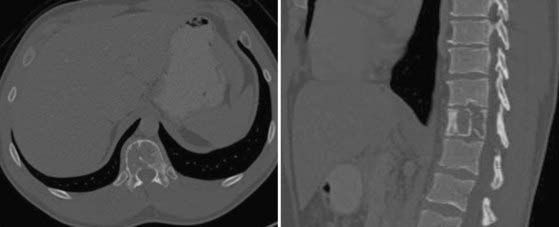
Several primary spinal tumors have characteristic imaging findings on plain radiography, CT, and MRI. Occasionally, congruent findings with these modalities can be diagnostic and obviate the need for further testing or procedures, the primary example of which is an aneurysmal bone cyst (ABC). ABCs typically have cystic structures with fluid-fluid levels from blood degradation products on MRI and CT (Fig. 310-1). In selected cases, identification of the characteristic CT and MRI findings can eliminate the need for tissue biopsy and avoid the potential complication of a hematoma.
Other imaging techniques that are valuable in the evaluation of primary spinal tumors include bone scans, positron emission tomography (PET), and angiography. A bone scan is a nuclear medicine study that can identify new areas of bone growth or breakdown resulting from tumor involvement. The lesion can be considered “cold” or “hot,” depending on the amount of local cell activity and function in the osteocytes. Bone scans generally have high sensitivity but poor specificity. Small lesions that are not well visualized with plain radiography, CT, or MRI, such as osteoid osteomas, can be detected with a bone scan.11,12 PET is another nuclear medicine imaging technique that involves the use of a metabolically active radiotracer. The most commonly used tracer is fludeoxyglucose F 18. After intravenous injection, it accumulates and becomes concentrated in the neoplasm. PET is emerging as one of the most commonly used diagnostic tools in oncology.13–19 It has high sensitivity and provides both anatomic and functional information regarding a tumor.
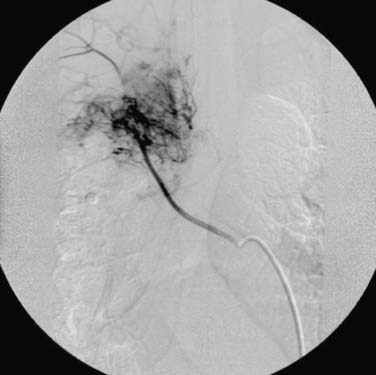
Angiography is used for diagnostic and therapeutic purposes in the management of primary spinal tumors. It can assist in the diagnosis of a vascular lesion, such as an ABC or vertebral hemangioma. In addition, angiography can be used to determine the anatomy and extent of the vascular supply to a primary spinal tumor before surgical intervention (Fig. 310-2). Lesions, including ABCs, hemangiomas, GCTs, and sarcomas, are often associated with increased vascularity. Preoperative embolization in these cases can reduce the intraoperative blood loss associated with surgery and decrease the perioperative morbidity associated with blood loss and transfusion of blood products.20–26
Histopathologic Diagnosis
To make an accurate diagnosis, a CT-guided biopsy is typically performed to obtain tissue specimens for histopathologic analysis. CT-guided biopsy is a fast, economical, and minimally invasive procedure. In addition, it is a safe procedure with a low complication rate. In several studies, percutaneous CT-guided biopsy of spinal lesions has been demonstrated to have a diagnostic accuracy of up to 93%.27–31 Rimondi and colleagues, in their review of 430 patients who underwent percutaneous CT-guided biopsy, found that the success rate for accurate diagnosis is higher with malignant lesions.31 Conversely, the success rate was lower with inflammatory lesions, particularly chronic ones.31 An accurate diagnosis can have a significant impact on treatment and the overall prognosis. In the following section, specific evaluation and management of common benign and malignant primary spinal tumors are discussed.
Benign Primary Spinal Tumors
Aneurysmal Bone Cyst
ABCs were first described by Jaffe and Lichtenstein in 1942.32 ABCs are cystic and osteolytic lesions that typically affect patients younger than 20 years, and they have a slight female preponderance. ABCs usually arise from long bones, but about 12% to 30% of cases involve the spine.33–36 These tumors typically involve the posterior elements, and the thoracic spine is the most frequent site of involvement.36
CT and MRI are most useful for the assessment of ABCs. Expansile, osteolytic, and cystic lesions with thin cortical eggshells are seen on CT. In addition, a fluid-fluid level demonstrating the layering of blood products from previous hemorrhages is seen within the cystic lesions. ABCs have a heterogeneous appearance on both T1- and T2-weighted MRI. Again, a fluid-fluid level is usually seen within the cystic lesions, with heterogeneous signals denoting the various hemoglobin degradation products (see Fig. 310-1). Histologically, ABCs show cystic cavities filled with blood products separated by fibrous septa. They are expansile and associated with significant bony destruction with thin layers of reactive cortical bone surrounding the lesion.
Although the majority of ABCs are primary lesions, it is known that they can be secondary lesions associated with other spinal tumors, including GCT, osteoblastoma, chondroblastoma, and osteogenic sarcoma.35 Generally, symptomatic patients are treated by embolization or surgery. Embolization can be used as first-line and the sole therapy for ABCs.36–45 Several successful cures after embolizations of ABCs have been reported.46–48 In addition, embolization is used preoperatively to decrease intraoperative blood loss and morbidity.33,37,38,42,44 Surgically, complete excision of the tumor is the goal but may be difficult. Incomplete tumor excision may be associated with significant rates of tumor recurrence.33,49–52 Radiotherapy can be considered in patients with residual or recurrent tumor. ABCs are sensitive to radiation, but the recurrence rate remains significant despite adjuvant radiotherapy.33,52–55 In addition, radiation therapy in young patients with these benign tumors is concerning for the development of radiation-induced sarcomas in the future.
Hemangiomas
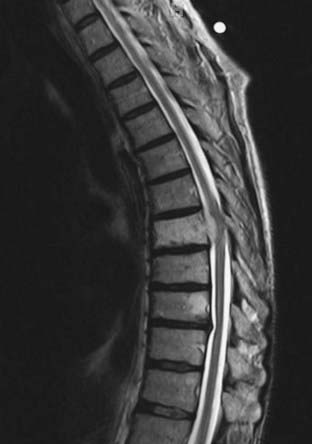
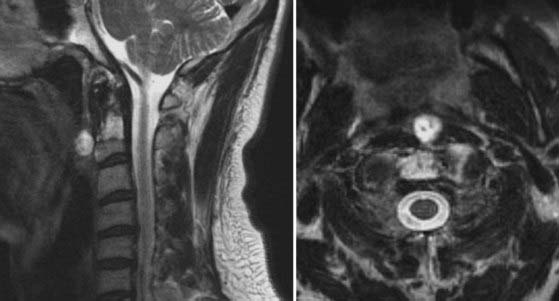
Vertebral hemangiomas are the most commonly occurring benign primary spinal tumors. They affect 10% to 12% of the population and are often incidental findings detected during imaging of the thoracolumbar spine. Vertebral hemangiomas are considered non-neoplastic, and only about 1% of those affected are symptomatic.2,3,56,57 Aggressive hemangiomas can cause local pain from involvement of the vertebral body. In addition, they can cause radiculopathy from nerve root compression or myelopathy with epidural disease and spinal cord compression (Fig. 310-3). Moreover, pathologic fractures can also occur with vertebral hemangiomas.
On CT, vertebral hemangiomas display patterns of coarse vertical striations or bony trabeculation that are often referred to as “honeycomb” or “polka dot” signs, respectively (Fig. 310-4).56 MRI of these lesions demonstrates hyperintense signals on both T1- and T2-weighted sequences. The combination of these characteristic CT and MRI findings is typically sufficient to diagnose vertebral hemangiomas, particularly those that are incidental findings during medical evaluation for other complaints. However, in the event of uncertainty, percutaneous CT-guided biopsy can be performed to obtain a tissue diagnosis. On histopathologic examination, hemangiomas are composed of an aggregate of irregularly shaped sinusoidal channels of thin-walled vessels with erosion of the bony architecture.
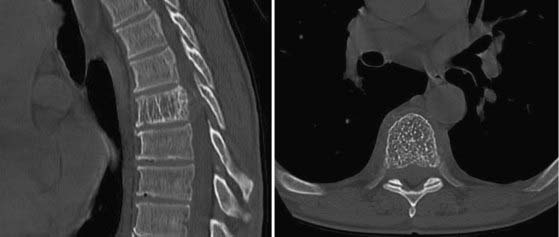
FIGURE 310-4 Sagittal and axial computed tomographic scans of a hemangioma illustrating the “honeycomb” and “polka dot” signs.
Although the prevalence of vertebral hemangiomas is high, they seldom cause clinical symptoms. These lesions most commonly involve the thoracic and lumbar spine.2,57 The lower thoracic spine is the most frequent site of symptomatic vertebral hemangiomas,2,58,59 and they become symptomatic by expansile enlargement of the vertebral body, pedicle, or lamina. Direct invasion of the extradural space can also occur and cause epidural spinal cord compression. In addition, there appears to be an association between pregnancy and the development of symptomatic vertebral hemangiomas.60,61
The majority of vertebral hemangiomas are managed by medical observation. Painful hemangiomas can be treated by vertebroplasty or kyphoplasty. Galibert and associates described the first attempt to treat these lesions by percutaneous injection of acrylic cement.62 Since their report, vertebroplasty or kyphoplasty is now well accepted as primary treatment of painful vertebral hemangiomas without neurological compromise.60,63–69 In symptomatic patients with spinal cord compression or neurological symptoms, surgical excision of the tumor plus spinal cord decompression is the treatment of choice. Preoperative angiography to determine the vascularity of the tumor, followed by tumor embolization, is recommended to decrease intraoperative blood loss (see Fig. 310-2).2,57,60 Postoperative radiotherapy for these lesions is controversial but should be considered in patients with significant residual or recurrent tumors.2,70
Osteoid Osteoma and Osteoblastoma
Osteoid osteomas are benign bone lesions that arise from cancellous bone throughout the musculoskeletal system. Less than 10% of these lesions originate from the spine. By definition, osteomas are smaller than 2 cm. Those larger than 2 cm are considered osteoblastoma. Patients with osteomas are mostly seen in their teenage years initially, and there is a slight male preponderance. Most patients have pain that is typically worse at night and relieved with salicylates. Occasionally, these patients will have painful scoliosis, which is a classic manifestation of this tumor.5–956
Osteoid osteomas most commonly affect the posterior elements of the spine, with the lumbar spine being the most frequently involved site.56,57 A round or oval lesion with a radiolucent center and peripheral sclerosis is the radiographic hallmark of osteomas. Radiographs can help diagnose these lesions but have low sensitivity. CT is the imaging modality of choice and can determine the extent of vertebral involvement. Radionuclide bone scans can also be used to detect osteomas and often demonstrate significant uptake by the tumor. Bone scans have high sensitivity and are particularly useful for detecting small lesions that are otherwise not detected on radiographs or CT.71–73 On histologic analysis, osteomas are composed of well-organized and interconnected trabeculation in a background of vascularized connective tissue with surrounding reactive cortical bone.56
Conservative management with salicylates should be the initial treatment of patients with osteoid osteoma.72–74 Patients who fail conservative management may undergo surgical excision. Curettage or intralesional excision is acceptable, but the goal of surgery is complete removal to prevent recurrence. There is evidence that alcohol, laser, or radiofrequency ablation is an effective alternative treatment of these tumors.75–83
Osteoblastomas are radiographically and histologically similar to osteoid osteomas. However, osteoblastomas are larger (>2 cm) and are associated with more constant pain that is not relieved by salicylates.56,57 Approximately 90% of patients with osteoblastoma are initially seen in their second or third decade of life. Osteoblastomas are associated with a higher rate of neurological compromise because of their larger size and more aggressive biology. Unlike osteomas, complete surgical excision is the primary treatment. However, this can be challenging because of the tumor’s larger size and more diffuse involvement. Wide en bloc excision is preferable to decrease the risk for tumor recurrence. A recurrence rate of up to 50% is associated with intralesional or marginal resection of aggressive osteoblastomas.84–87 Irradiation of these tumors is controversial, but it can be considered for residual or recurrent tumors.88–90
Enchondroma
Enchondroma is a common benign cartilaginous tumor that accounts for 5% of all bone tumors, and it affects the short tubular bones of the hands and feet in more than 50% of cases. It is the most common primary tumor in the hand and is normally found in the diaphysis. On the contrary, it is extremely rare in the vertebral column and accounts for only about 2% of all cases.91–97 Enchondroma can arise either from hyperplasia of immature spinal cartilage trapped within the vertebral bodies or from metaplasia of connective tissues in connection with the spine. Most patients with enchondroma come to medical attention during the second to fourth decades, and there is no gender predilection.
Radiographically, enchondromas show well-circumscribed, round to oval osteolytic lesions that may widen the cortex. On CT, they are homogeneous lesions with or without calcification that may enhance with injection of contrast material. On MRI, enchondromas have intermediate to low signal intensity on T1-weighted sequences and high signal intensity on T2-weighted images (Fig. 310-5).94
Solitary and painless enchondromas can be observed medically. Painful lesions or those associated with neurological symptoms should be treated. In addition, enchondromas with large size, a large unmineralized component, and elevated activity on bone scans are concerning for malignancy.94 Lesions with these features should undergo biopsy, followed by surgical excision. Complete tumor excision is the goal and can be achieved by intralesional resection. Recurrence after surgical excision is rare, and malignancy should be excluded in such cases. Malignant degeneration is possible and is seen in up to 30% of those with conditions involving multiple enchondromatosis, such as Ollier’s disease or Maffucci’s syndrome.98–100
Osteochondroma
Spinal osteochondromas are uncommon primary spinal tumors that account for less than 4% of all primary spine tumors. These benign spinal tumors arise during development when cartilage is trapped outside the physeal plate.56 Radiation is also an unusual cause of this tumor.101–105 Patients with radiation-induced osteochondroma often received radiotherapy at an age younger than 2 years, and there is typically a latent period of 17 months to 9 years.103,104
Patients with osteochondroma are typically seen initially in their third or fourth decades, and there is a male preponderance. Most patients have a solitary lesion, except in cases associated with hereditary multiple exostoses. Osteochondromas have a predilection for the cervical spine, with the axis being the most common site of involvement.56 Patients may complain of pain or myelopathy. Osteochondroma most commonly affects the posterior elements, and patients can have a palpable mass. Radiographically, these lesions are often pedunculated in appearance and are composed of healthy bone with a cartilaginous cap.56 CT is the test of choice to detect the exostosis and the pathognomonic finding of the exostotic lesion in continuity with the cortex and bone marrow of the underlying bone.56,57 MRI can help in evaluating the relationship of the lesion to its surrounding soft tissues and examining the cartilaginous end cap. Thickening of the cartilaginous cap greater than 1 to 2 cm is suggestive of malignant transformation to chondrosarcoma.56
Medical observation is indicated for incidental or dormant lesions that are stable in size, whereas surgical excision is the treatment of choice for symptomatic lesions. Complete excision is the goal, and intralesional resection to achieve complete excision is acceptable, but incomplete excision may result in tumor recurrence.56 In addition, malignant transformation of osteochondroma to chondrosarcoma has been reported.106–109 En bloc excision should be considered for aggressive lesions.110,111 However, en bloc tumor resection is technically demanding in patients with tumor involvement of the axis or subaxial cervical spine. Radiation therapy is ineffective and not generally considered for the treatment of these tumors. Furthermore, radiation-induced transformation of these benign tumors to chondrosarcoma is a concern.107,112
Chondroblastoma
Chondroblastoma is a rare primary bone tumor that accounts for about 1% of all cases.56 Chondroblastoma arises from immature cartilage and is most often seen in the epiphyseal region of long bones. Although only a few cases of spinal involvement have been reported in the literature, patients with chondroblastoma are typically seen in adolescence or young adulthood. There is a slight male preponderance. Most patients complain of pain, but spinal canal invasion is common with chondroblastoma.57 Because its radiographic findings are nonspecific, CT-guided biopsy should be performed for definitive histopathologic diagnosis. On histologic analysis, chondroblastomas consist of round or polygonal chondroblast-like cells and multinucleated giant cells in a background of cartilaginous intercellular matrix and focal calcification.113–115 The limited surgical experience with chondrosarcoma demonstrates significant recurrence rates ranging from 24% to 100% with incomplete resection. Consequently, wide en bloc resection should be highly considered in the management of this rare primary spinal tumor.113–115
Giant Cell Tumor
GCTs account for about 7% to 10% of all cases of primary spinal tumor.56,57 Patients with GCTs are most often seen initially in the third or fourth decades, and the sacrum is the most common site. There is a slight female preponderance associated with GCTs. Common symptoms of GCTs include pain, weakness, sensory deficit, and bladder or bowel dysfunction. Those with sacral GCTs often have large tumors as a result of significant tumor growth before the development of neurological symptoms.
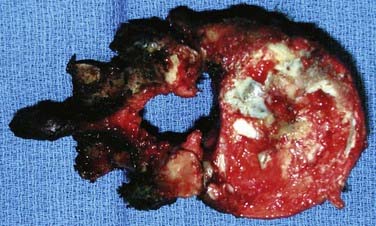
Histologically, GCT is composed of abundant osteoclastic giant cells mixed with spindle cells. Based on this appearance, it is speculated that GCT originates from osteoclasts. Radiographically, GCT demonstrates an expansile mass with destruction of the vertebral bodies. These tumors generally involve the vertebral body but can extend to involve the posterior elements (Fig. 310-6). In the sacrum, GCT can involve the vertebral body bilaterally and can extend across the sacroiliac joints.56
GCTs are locally aggressive and associated with a high recurrence rate after incomplete excision. As a result, the prognosis for patients with GCTs is not as favorable as for those with other benign primary spinal tumors. Before surgery, it is advisable to perform tumor embolization because these tumors are vascular and associated with significant intraoperative blood loss. Complete surgical tumor excision is the treatment of choice for patients with GCTs. However, complete excision is not always possible, given the large size of these lesions and their tendency to invade local surrounding tissues. Wide en bloc excision of these tumors is the preferred surgical treatment and is associated with a lower recurrence rate.116–121 Wide en bloc resection should be attempted when feasible; however, it may require judicious sacrifice of normal surrounding tissues, including nerve roots. Intralesional resection is an alternative treatment when en bloc excision is not possible, but it is associated with significant rates of local recurrence.119
Radiation therapy can be used as adjuvant treatment after incomplete resection or tumor recurrence. In their review of the literature, Leggon and coauthors identified and reviewed 239 lesions treated over the past 50 years.118 Their study demonstrated that there was no statistically significant difference in the recurrence rate after radiotherapy, incomplete surgical resection, or incomplete surgical resection followed by radiotherapy. The recurrence rate in these three groups ranged from 46% to 49%. In contrast, the recurrence rate was 0% in those who underwent wide surgical excision. Therefore, radiation therapy alone appears to be a viable alternative treatment when wide surgical excision is not achievable. Nevertheless, there is concern for radiation-induced sarcoma in long-term survivors. Leggon and colleagues found that radiation-induced sarcoma developed in 11% of patients who received radiotherapy for GCT as primary treatment or after tumor recurrence.118
Malignant Primary Spinal Tumors
Chordoma
Chordoma is an uncommon tumor that accounts for 2% to 4% of all primary malignant bone tumors. The annual incidence rate is low, around 1 case per 100,000 per year.122 However, other than lymphoproliferative tumors, chordoma is the most common primary malignant tumor of the spine in adults. These tumors arise from remnants of the notochord and can be found from the skull base to the coccyx. Most chordomas arise from the clivus or the sacrococcygeal regions. In the spine, the sacrum is the most common site of disease, followed by the lumbar spine and then the cervical spine.122
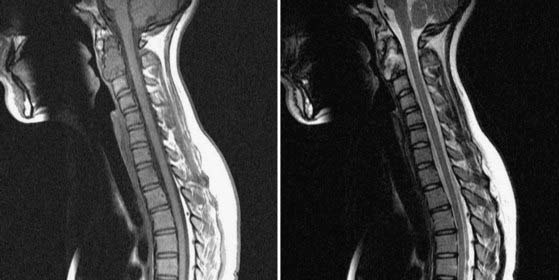
Patients with chordoma are generally seen in middle age, with the peak incidence in the fifth or sixth decades of life. There is a slight male preponderance in patients with spinal chordomas.122 Initial symptoms in these patients include pain, weakness, sensory disturbance, and bladder or bowel dysfunction. Similar to GCTs, chordomas have insidious growth and are often large at the time of detection. On radiography and CT, these tumors have an osteolytic lesion near the center of the vertebral body with surrounding bony expansion and intratumor calcification. MRI of chordoma with T1-weighted sequences typically demonstrates low or intermediate intensity within the lesion. On T2-weighted imaging, chordoma displays its characteristic high intensity signals as a result of the high water content within these tumors (Fig. 310-7).56 Enhancement of the tumor is generally seen with infusion of intravenous contrast material.
On histologic evaluation, chordomas display elongated cords of clear cells known as physaliphorous cells. The cells contain intracytoplasmic vacuoles with a copious amount of mucin in both the intracellular and extracellular compartments. Overall, the histologic appearance is quite bland and benign. Moreover, chordomas are slow-growing neoplasms. Nevertheless, they are highly invasive and are capable of metastasis.123 In addition, some chordomas can have sarcomatous features that include fibrous, osteoid, or chondroid elements.56 Chordomas with these features are referred to as dedifferentiated chordomas.
The ideal treatment of chordomas consists of wide or marginal en bloc resection (Fig. 310-8). En bloc tumor excision is technically challenging but is associated with longer median disease-free survival than is the case with subtotal excision (Fig. 310-9).123–125 Adjuvant radiation therapy can be used for the treatment of residual or recurrent tumors. The reported 5-year local control rate is below 20% after treatment with photon-based radiotherapy at doses of up to 40 to 60 Gy.126–128 The use of highly charged proton beam radiation has increased the local control rate of chordomas to the range of 46% to 63% at 5 years.128–131 Chemotherapy is traditionally ineffective in treating these tumors, but clinical trials with the tyrosine kinase inhibitor imatinib are currently under way.132–134
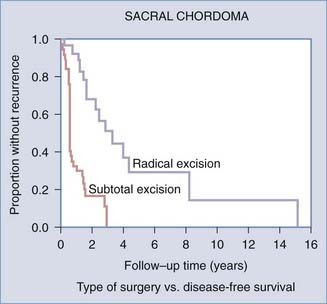
FIGURE 310-9 Kaplan-Meier survival curve demonstrating improved survival of patients after en bloc resection of sacral chordomas.
Chordomas can metastasize, and metastatic disease develops in 5% to 43% of patients.135,136 However, most patients succumb to their disease because of local recurrence and invasion. With wide resection, chordomas are slow-growing neoplasms, and patients generally have a favorable probability of 5-year survival. The 5-year survival rate for chordomas is estimated to be about 67.6%, whereas the 10-year survival rate is around 39.9%.122 Prognostic factors associated with favorable survival include young age, complete resection, and adjuvant radiation treatment in those with residual tumor.56
Chondrosarcoma
Other than lymphoproliferative tumors, chondrosarcoma is the second most common primary vertebral column tumor, and it arises from the spine in up to 12% of all cases.56 Chondrosarcoma is frequently difficult to distinguish from chordoma because these tumors have very similar clinical, radiographic, and histopathologic features. Patients with chondrosarcoma seek medical attention in their fifth or sixth decades, often because of pain and neurological symptoms. Chondrosarcomas have a male preponderance and are 2 to 4 times more likely to occur in men than women.56 Chondrosarcomas are commonly found in the sacrum and the thoracic spine. Radiographically, they resemble an osteolytic lesion with an associated soft tissue mass. These tumors can involve both the vertebral bodies and the posterior elements. CT can demonstrate the degree of bony destruction, and MRI can demonstrate the relationship between the tumor and surrounding soft tissue, including the neural elements. Unlike chordomas, which tend to arise centrally within the vertebral body, chondrosarcomas generally arise in paracentral locations. They typically develop near the petroclival region in the skull base and near the sacroiliac joints in the pelvis.
Standard management of chondrosarcoma is complete surgical excision.56,137 Wide en bloc excision is the ideal treatment and is associated with increased disease-free survival.137 Cure is possible with complete tumor excision, particularly with low-grade lesions.57 The recurrence rate is high without complete tumor excision. Surgery should be performed in patients with tumor recurrence when possible. Although both radiation therapy and chemotherapy are ineffective in treating chondrosarcoma, they can be used for palliation when surgery is not an option.138,139 Nevertheless, long-term survivors are common with chondrosarcoma because they generally have a slow growth rate and low histologic grade.
Osteosarcoma
Osteosarcoma is the most common primary bony tumor in young patients and typically arises in the long bones. Most patients are initially seen during the first to third decades of life. Primary osteosarcoma of the spine is rare and accounts for roughly 1% to 2% of osteosarcomas.56 Spinal osteosarcoma is detected at a later age, with most patients typically being middle-aged. Some osteosarcomas are in fact secondary lesions from previous radiation treatment or Paget’s disease.140 More than 90% of spinal osteosarcomas involve the vertebral body.57
Historically speaking, the prognosis of patients with osteosarcoma is dismal. Osteosarcomas have a propensity for early and widespread metastasis. In addition, they have a poorer prognosis than their appendicular counterparts. However, in recent years, neoadjuvant and adjuvant chemotherapy in combination with radiotherapy has been used for the treatment of osteosarcoma with success.23,141–147 Wide en bloc resection is the surgical treatment of choice to achieve the most optimal oncologic outcome. Long-term survival and cure are now possible with aggressive multimodality treatment consisting of surgery, chemotherapy, and radiotherapy.141,142,146
Ewing’s Sarcoma
Ewing’s sarcoma is the second most common primary malignant bone tumor and is the most common malignant primary spinal tumor of childhood.148 Ewing’s sarcoma is a poorly differentiated tumor that can arise from bone or soft tissues. It is a tumor with a characteristic histologic appearance and chromosomal alteration. Ewing’s sarcoma consists of small round blue cells on hematoxylin-eosin staining. On cytogenetic analysis, it has a characteristic chromosomal translocation t(11;22)(q24;q12). Other pathologically distinct tumors that are related to Ewing’s sarcoma include primitive neuroectodermal tumors and extraskeletal Ewing’s tumors.
The peak incidence of Ewing’s sarcoma occurs between 10 and 20 years of age, and more than 80% of cases occur before the age of 20.148 Patients with spinal Ewing’s sarcoma have pain, swelling, a mass, or neurological symptoms. The most common site of Ewing’s sarcoma in the spine is the sacrococcygeal region, followed by the lumbar and thoracic spine.149 Ewing’s sarcoma in the mobile spine accounts for only about 5% of cases.56
Ewing’s sarcoma is exquisitely sensitive to radiation therapy and chemotherapy. Nevertheless, before modern combination treatment consisting of surgery, chemotherapy, or radiation therapy, survival rates from Ewing’s sarcoma were dismal. Neoadjuvant radiation therapy and chemotherapy should be initiated in patients with large tumors, extensive extraspinal extension, or metastatic disease. Wide en bloc excision is the ideal surgical treatment when possible. In patients in whom en bloc excision is not possible, intralesional excision to debulk the majority of the tumor is acceptable. Adjuvant radiation therapy and chemotherapy are administered postoperatively and are required to achieve the most optimal oncologic control. With a contemporary multidisciplinary approach, long-term survival rates have improved from 5% to 20% to 50% to 80%.56,150 However, negative prognostic factors include sacrococcygeal location, large size, and metastasis. Patients with metastasis generally have a 5-year survival rate of just 10% to 15%.148
Plasmacytoma/Multiple Myeloma
The most common primary malignant tumors that arise from the vertebral column are plasmacytoma and multiple myeloma. Although they are lymphoproliferative tumors and are considered systemic tumors, plasmacytoma and multiple myeloma generally arise within the bone marrow of the vertebral column.151 They are the most common malignant vertebral column tumors in the elderly.56,57 Plasmacytoma by definition is a solitary lesion, whereas multiple myeloma is truly a systemic neoplasm. The estimated incidence of multiple myeloma is 5 to 7 new cases per 100,000 persons per year, and 19,920 new cases are expected to be diagnosed in the United States in 2008.152,153
Multiple myeloma is a malignancy that arises from plasma cells and is characterized by abnormal production of immunoglobulins. The abnormal proliferation of plasma cells within the spine causes destruction of the bony architecture and results in secondary osteoporosis. Multiple myeloma occurs more frequently in men than in women, and its peak incidence is in the sixth or seventh decade of life.152 The majority of patients complain of back pain. Radiography, CT, and MRI are usually performed during the initial evaluation. Radiographs often demonstrate “punched-out” or osteolytic lesions and areas of decreased mineralization. Other than diffuse abnormal marrow signals of the vertebral column, there is no characteristic MRI finding. However, pathologic fractures are seen in up to 50% of patients.154 CT-guided biopsy of the lesion should be performed, as well as iliac bone marrow aspiration biopsy. In addition, serum and urine electrophoresis should be performed to evaluate for the presence of an abnormal or excessive amount of immunoglobulins.
In cases of plasmacytoma, en bloc spondylectomy followed by radiotherapy can be considered, but such treatment is highly controversial. These tumors are very radiosensitive, and good local control and long-term survival can be achieved with radiotherapy alone.155 In the absence of spinal instability or neurological compromise, the standard initial treatment of plasmacytoma and multiple myeloma is radiotherapy. In a study of 206 patients, Knobel and coworkers found that the 5-year probability of progression from solitary plasmacytoma to multiple myeloma was 51% and the median time to progression was 21 months.155 Therefore, progression from plasmacytoma to multiple myeloma with systemic involvement is a significant problem, and en bloc resection may not provide the desired oncologic outcomes.
The mainstay treatment of multiple myeloma is radiation therapy. Chemotherapy and bone marrow transplantation are used in patients with systemic and advanced disease. In addition, these lymphoproliferative tumors are highly responsive to corticosteroids. Corticosteroids are administered to acutely symptomatic patients and are effective in improving pain and even neurological symptoms. Surgical intervention is reserved for patients with spinal instability, severe pain refractory to conservative treatment, and neurological deficits.151 In recent years, percutaneous vertebroplasty and kyphoplasty have emerged to become very successful treatments in patients with pathologic fractures and spinal pain from multiple myeloma.156–162 For those with spinal canal compromise and neurological deficits or severe spinal instability, surgical decompression and instrumented fusions are indicated. Similar to the management of metastatic spine disease, circumferential decompression with stabilization is the ideal for patients with spinal canal compromise and neurological deficits or severe spinal instability.
Chemotherapy and Radiation Therapy
In general, chemotherapy has a limited role in the management of primary spinal neoplasms. Chemotherapy is typically administered as an adjunct to surgical resection of malignant spinal tumors or to patients with systemic disease. Neoadjuvant chemotherapy has been shown to have favorable results in the treatment of Ewing’s sarcoma and osteogenic sarcoma.23,141–146,148,163–167 Patients with certain primary spinal tumors can benefit from neoadjuvant or postoperative radiation therapy. Adjuvant radiation therapy has been shown to benefit patients with GCT, hemangioma, chordoma, Ewing’s sarcoma, and osteogenic sarcoma.143,146,165,167–171 However, many of the primary spinal tumors have a poor response to radiotherapy. In general, the response rate is worse with low-grade tumors than with high-grade tumors.
The most common form of spinal radiation therapy is external-beam ionizing radiation. However, radiation toxicity to the spinal cord is of great concern because of the proximity of the cord and the high radiation doses required to achieve adequate local tumor control. In addition, exposure of surrounding tissues leading to radiation-induced sarcoma is a concern for young patients with benign tumors.172 They may be long-term survivors from their benign neoplasms, only to succumb to secondary sarcomas. Recent advances in radiation therapy have improved the ability to deliver high-dose radiation therapy to tumors while limiting radiation exposure to the spinal cord and surrounding non-neoplastic tissues. Such advances include proton beam therapy and conformal radiation therapy such as intensity-modulated radiation therapy and other stereotactic radiosurgery techniques. Currently, data are still limited on the long-term efficacy of these advanced radiotherapy techniques for the management of primary spinal tumors, but current and future clinical studies incorporating these therapeutic modalities will be extremely valuable.
Surgical Management
Treatment of primary spinal tumors is often dictated by the histology of the tumor, its location, and the extent of tumor invasion.121,173 For benign lesions, appropriate surgical resection can result in cure, but not every benign lesion warrants surgical resection. Malignant primary spinal tumors usually require surgical intervention. However, treatment strategies may be significantly influenced by knowledge of the tumor’s histology. For selected malignant tumors, neoadjuvant or adjuvant radiation therapy and chemotherapy are required to achieve the best oncologic outcome. Neoadjuvant therapy can significantly reduce the bulk of the tumor to decrease the magnitude of surgery and improve the prospect of achieving wide en bloc resection.
In primary sarcomas from extraspinal sites, long-term tumor control, progression-free survival, and the potential for cure have been shown to correlate with the ability to perform marginal, wide, or radical en bloc tumor resection.174–179 Accordingly, the goal of modern surgical treatment of primary vertebral column tumors is to achieve wide or marginal en bloc vertebral body resection (i.e., total en bloc spondylectomy [TES]). Over the recent decades, evidence is accumulating that en bloc spondylectomy for primary spinal tumors can impart a higher local tumor control rate, longer disease-free survival, and possible cure of chordomas and chondrosarcomas specifically.180–185
The term en bloc resection signifies an attempt to remove the lesion in a single piece. In en bloc spondylectomy, the vertebral body is typically removed in a single piece, and the posterior arch is removed separately as a single piece. Spondylectomy was first described by Stener in 1971,182 and since then, various reports have supported improved local tumor control rates and disease-free survival with spondylectomy.180–185 In the 1990s, Tomita and colleagues further modified and popularized the TES technique originally described by Roy-Camille. In this procedure, en bloc spondylectomy with anterior and posterior spinal reconstruction is performed through an all-posterior approach.121,180 En bloc spondylectomy can be performed in a single level or up to three consecutive levels with this approach. Based on cadaveric study, Kawahara and colleagues found that the single-stage posterior TES approach can be used for lesions located from T1 to L2 and possibly L3 and L4 if the inferior vena cava and iliac vessels have been safely mobilized anteriorly.186 To achieve en bloc spondylectomy with wide margins of resection, the tumor should be contained within the vertebral body with minimal to no paraspinal extension.121,173 In addition, there should be no epidural disease, and at least one pedicle must be free of tumor.
Although the assumption is that en bloc resection of a spinal tumor removes the lesion in its entirety, it does not equate with the classic en bloc radical resection of extremity tumors because of the presence of the neural elements and spinal cord within the lesion, which may often be spared. Therefore, the extent of spinal tumor resection should instead be designated “marginal,” “wide,” or “radical” en bloc resection.173 Any resection would be considered an “intralesional” resection when the tumor has been entered during the resection. In contrast, marginal en bloc resection involves removal of the tumor with dissection along the pseudocapsule but no entrance into the tumor. In wide en bloc resection, a continuous layer of surrounding healthy tissue is removed along with the tumor. Radical en bloc resection requires removal of the tumor along with the entire anatomic compartment of the tumor origin. It is not possible in the spine, given that it would require removal of the entire spine compartment from the skull base to the coccyx.
The ability to accomplish marginal or wide en bloc resection of primary spinal tumors is largely based on tumor location and extension. Weinstein, Boriani, and Biagini devised a classification system (WBB staging system) for primary spinal tumors based on concepts and terms accepted by most oncologists for other musculoskeletal tumors.173 In this classification, the vertebra is divided into 12 sectors numbered from 1 to 12 in clockwise order, with the spinal canal being the center. In addition, the vertebra is divided into five layers ranging from the paravertebral extraosseous region to intradural involvement. Finally, the longitudinal extent of the tumor is defined by the number of spinal segments involved. We recommend that surgical planning for primary spinal tumors be based on this WBB staging system. Accordingly, neoplasms that are confined within the vertebral body or the posterior arch can be excised via marginal or wide en bloc resection. Moreover, tumors located eccentrically with unilateral pedicle or transverse process involvement (or both) and small paraspinal extension can be excised via marginal or wide en bloc resection with the sagittal resection technique.173 Conversely, tumors with extensive epidural involvement are not candidates for en bloc resection because the risk for neurological injury is high.
Despite reports of good clinical results with TES for spinal neoplasms, it is a technically highly demanding procedure that is associated with significant perioperative risks,121,180,186–188 including neurological and dural injury during pedicle resection and vascular injury to the aorta or vena cava during blunt dissection around the anterior vertebral body, which can be fatal. There is also risk for spinal cord ischemia with manipulation and sacrifice of segmental vessels. Excessive bleeding from the epidural plexus or vertebral body can occur. Finally, after completion of the spondylectomy, there is complete disconnection of the spine with spinal instability, which requires a well-planned reconstruction. Moreover, despite meticulous effort to remain extralesional, there is still the inherent risk of tumor contamination of the field during pediculotomy. However, the use of a fine-threaded T-saw has been shown to decrease the risk for tumor contamination during pediculotomy in an animal model.189
At times, en bloc resection cannot be achieved in the management of primary vertebral column tumors because of extension into surrounding anatomic structures. Such structures include the dura, neural elements, major vessels, paraspinal musculature, and visceral organs. Tumor involvement in these structures may limit the ability to achieve wide or marginal excision of the tumor without significant risk. In these cases, intralesional or piecemeal resection can be performed for subtotal tumor removal.190 Intralesional or piecemeal tumor resection has been widely performed with good success, but the limitations of subtotal resection include difficulty identifying the tumor margins and a high risk of tumor contamination in the surrounding tissues.180,190
Boriani S, Bandiera S, Biagini R, et al. Chordoma of the mobile spine: fifty years of experience. Spine. 2006;31:493.
Boriani S, Biagini R, De Iure F, et al. Lumbar vertebrectomy for the treatment of bone tumors: surgical technique. Chir Organi Mov. 1994;79:163.
Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine. 1997;22:1036.
Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res. 1986;204:9.
Ferguson WS, Goorin AM. Current treatment of osteosarcoma. Cancer Invest. 2001;19:292.
Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98:21.
Hitchon PW, Bilsky MH, Ebersold MJ. Primary Bony Spinal Lesions, 2nd ed. Philadelphia: Elsevier; 2005.
Kawahara N, Tomita K, Matsumoto T, et al. Total en bloc spondylectomy for primary malignant vertebral tumors. Chir Organi Mov. 1998;83:73.
Leggon RE, Zlotecki R, Reith J, et al. Giant cell tumor of the pelvis and sacrum: 17 cases and analysis of the literature. Clin Orthop Relat Res. 2004;423:196.
Luther N, Bilsky MH, Hartl R. Giant cell tumor of the spine. Neurosurg Clin N Am. 2008;19:49.
McLoughlin GS, Sciubba DM, Wolinsky JP. Chondroma/chondrosarcoma of the spine. Neurosurg Clin N Am. 2008;19:57.
McMaster ML, Goldstein AM, Bromley CM, et al. Chordoma: incidence and survival patterns in the United States, 1973-1995. Cancer Causes Control. 2001;12:1.
Murphey MD, Andrews CL, Flemming DJ, et al. From the archives of the AFIP. Primary tumors of the spine: radiologic pathologic correlation. Radiographics. 1996;16:1131.
Rimondi E, Staals EL, Errani C, et al. Percutaneous CT-guided biopsy of the spine: results of 430 biopsies. Eur Spine J. 2008;17:975.
Roy-Camille R, Saillant G, Bisserie M, et al. [Total excision of thoracic vertebrae (author’s transl).]. Rev Chir Orthop Reparatrice Appar Mot. 1981;67:421.
Sciubba DM, Chi JH, Rhines LD, et al. Chordoma of the spinal column. Neurosurg Clin N Am. 2008;19:5.
Stener B. Complete removal of vertebrae for extirpation of tumors. A 20-year experience. Clin Orthop Relat Res. 1989;245:72.
Sundaresan N, Rosen G, Huvos AG, et al. Combined treatment of osteosarcoma of the spine. Neurosurgery. 1988;23:714.
Tomita K, Kawahara N, Baba H, et al. Total en bloc spondylectomy. A new surgical technique for primary malignant vertebral tumors. Spine. 1997;22:324.
Tomita K, Kawahara N, Murakami H, et al. Total en bloc spondylectomy for spinal tumors: improvement of the technique and its associated basic background. J Orthop Sci. 2006;11:3.
Weber KL. Current concepts in the treatment of Ewing’s sarcoma. Expert Rev Anticancer Ther. 2002;2:687.
Weinstein JN. Surgical approach to spine tumors. Orthopedics. 1989;12:897.
Yao KC, Boriani S, Gokaslan ZL, et al. En bloc spondylectomy for spinal metastases: a review of techniques. Neurosurg Focus. 2003;15(5):E6.
York JE, Berk RH, Fuller GN, et al. Chondrosarcoma of the spine: 1954 to 1997. J Neurosurg. 1999;90:73.
York JE, Kaczaraj A, Abi-Said D, et al. Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery. 1999;44:74.
1 Chi JH, Bydon A, Hsieh P, et al. Epidemiology and demographics for primary vertebral tumors. Neurosurg Clin N Am. 2008;19:1.
2 Fox MW, Onofrio BM. The natural history and management of symptomatic and asymptomatic vertebral hemangiomas. J Neurosurg. 1993;78:36.
3 Lang EFJr, Peserico L. Neurologic and surgical aspects of vertebral hemangiomas. Surg Clin North Am. 1960;40:817.
4 Weinstein JN. Surgical approach to spine tumors. Orthopedics. 1989;12:897.
5 Haibach H, Farrell C, Gaines RW. Osteoid osteoma of the spine: surgically correctable cause of painful scoliosis. CMAJ. 1986;135:895.
6 Keim HA, Reina EG. Osteoid-osteoma as a cause of scoliosis. J Bone Joint Surg Am. 1975;57:159.
7 Saifuddin A, White J, Sherazi Z, et al. Osteoid osteoma and osteoblastoma of the spine. Factors associated with the presence of scoliosis. Spine. 1998;23:47.
8 Schulze KJ, Fiedler K. [Scolioses in spinal tumors.]. Beitr Orthop Traumatol. 1988;35:65.
9 Taylor LJ. Painful scoliosis: a need for further investigation. Br Med J (Clin Res Ed). 1986;292:120.
10 O’Mara RE. Bone scanning in osseous metastatic disease. JAMA. 1974;229:1915.
11 Schirrmeister H, Glatting G, Hetzel J, et al. Prospective evaluation of the clinical value of planar bone scans, SPECT, and (18)F-labeled NaF PET in newly diagnosed lung cancer. J Nucl Med. 2001;42:1800.
12 Schirrmeister H, Guhlmann A, Elsner K, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40:1623.
13 Buck AK, Schirrmeister H, Mattfeldt T, et al. Biological characterisation of breast cancer by means of PET. Eur J Nucl Med Mol Imaging. 2004;31(suppl 1):S80.
14 Hetzel M, Arslandemir C, Konig HH, et al. F-18 NaF PET for detection of bone metastases in lung cancer: accuracy, cost-effectiveness, and impact on patient management. J Bone Miner Res. 2003;18:2206.
15 Schirrmeister H. Detection of bone metastases in breast cancer by positron emission tomography. Radiol Clin North Am. 2007;45:669.
16 Schirrmeister H, Bommer M, Buck AK, et al. Initial results in the assessment of multiple myeloma using 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2002;29:361.
17 Schirrmeister H, Buck A, Guhlmann A, et al. Anatomical distribution and sclerotic activity of bone metastases from thyroid cancer assessed with F-18 sodium fluoride positron emission tomography. Thyroid. 2001;11:677.
18 Schirrmeister H, Buck AK, Bergmann L, et al. Positron emission tomography (PET) for staging of solitary plasmacytoma. Cancer Biother Radiopharm. 2003;18:841.
19 Schirrmeister H, Guhlmann A, Kotzerke J, et al. Early detection and accurate description of extent of metastatic bone disease in breast cancer with fluoride ion and positron emission tomography. J Clin Oncol. 1999;17:2381.
20 Gokaslan ZL. Spine surgery for cancer. Curr Opin Oncol. 1996;8:178.
21 Heary RF, Bono CM. Metastatic spinal tumors. Neurosurg Focus. 2001;11(6):e1.
22 Hess T, Kramann B, Schmidt E, et al. Use of preoperative vascular embolisation in spinal metastasis resection. Arch Orthop Trauma Surg. 1997;116:279.
23 Papagelopoulos PJ, Galanis EC, Vlastou C, et al. Current concepts in the evaluation and treatment of osteosarcoma. Orthopedics. 2000;23:858.
24 Prabhu VC, Bilsky MH, Jambhekar K, et al. Results of preoperative embolization for metastatic spinal neoplasms. J Neurosurg. 2003;98:156.
25 Schirmer CM, Malek AM, Kwan ES, et al. Preoperative embolization of hypervascular spinal metastases using percutaneous direct injection with n-butyl cyanoacrylate: technical case report. Neurosurgery. 2006;59:E431.
26 Trubenbach J, Nagele T, Bauer T, et al. Preoperative embolization of cervical spine osteoblastomas: report of three cases. AJNR Am J Neuroradiol. 2006;27:1910.
27 Babu NV, Titus VT, Chittaranjan S, et al. Computed tomographically guided biopsy of the spine. Spine. 1994;19:2436.
28 Ghelman B, Lospinuso MF, Levine DB, et al. Percutaneous computed-tomography–guided biopsy of the thoracic and lumbar spine. Spine. 1991;16:736.
29 Lis E, Bilsky MH, Pisinski L, et al. Percutaneous CT-guided biopsy of osseous lesion of the spine in patients with known or suspected malignancy. AJNR Am J Neuroradiol. 2004;25:1583.
30 Monti C, Rimondi E, Rollo G, et al. [Percutaneous computed tomography–guided biopsy in spinal diseases.]. Radiol Med (Torino). 1994;87:299.
31 Rimondi E, Staals EL, Errani C, et al. Percutaneous CT-guided biopsy of the spine: results of 430 biopsies. Eur Spine J. 2008;17:975.
32 Jaffe H, Lichtenstein L. Solitary unicameral bone cyst with emphasis on the roentgen picture, the pathologic appearance, and the pathogenesis. Arch Surg. 1942;44:1004.
33 Boriani S, De Iure F, Campanacci L, et al. Aneurysmal bone cyst of the mobile spine: report on 41 cases. Spine. 2001;26:27.
34 Cottalorda J, Kohler R, Sales de Gauzy J, et al. Epidemiology of aneurysmal bone cyst in children: a multicenter study and literature review. J Pediatr Orthop B. 2004;13:389.
35 Kransdorf MJ, Sweet DE. Aneurysmal bone cyst: concept, controversy, clinical presentation, and imaging. AJR Am J Roentgenol. 1995;164:573.
36 Koci TM, Mehringer CM, Yamagata N, et al. Aneurysmal bone cyst of the thoracic spine: evolution after particulate embolization. AJNR Am J Neuroradiol. 1995;16:857.
37 Burch S, Hu S, Berven S. Aneurysmal bone cysts of the spine. Neurosurg Clin N Am. 2008;19:41.
38 Chuang VP, Soo CS, Wallace S, et al. Arterial occlusion: management of giant cell tumor and aneurysmal bone cyst. AJR Am J Roentgenol. 1981;136:1127.
39 Cottalorda J, Bourelle S. Current treatments of primary aneurysmal bone cysts. J Pediatr Orthop B. 2006;15:155.
40 De Cristofaro R, Biagini R, Boriani S, et al. Selective arterial embolization in the treatment of aneurysmal bone cyst and angioma of bone. Skeletal Radiol. 1992;21:523.
41 DeRosa GP, Graziano GP, Scott J. Arterial embolization of aneurysmal bone cyst of the lumbar spine. A report of two cases. J Bone Joint Surg Am. 1990;72:777.
42 Dysart SH, Swengel RM, van Dam BE. Aneurysmal bone cyst of a thoracic vertebra. Treatment by selective arterial embolization and excision. Spine. 1992;17:846.
43 Konya A, Szendroi M. Aneurysmal bone cysts treated by superselective embolization. Skeletal Radiol. 1992;21:167.
44 Liu JK, Brockmeyer DL, Dailey AT, et al. Surgical management of aneurysmal bone cysts of the spine. Neurosurg Focus. 2003;15(5):E4.
45 Meyer S, Reinhard H, Graf N, et al. Arterial embolization of a secondary aneurysmatic bone cyst of the thoracic spine prior to surgical excision in a 15-year-old girl. Eur J Radiol. 2002;43:79.
46 Dubois J, Chigot V, Grimard G, et al. Sclerotherapy in aneurysmal bone cysts in children: a review of 17 cases. Pediatr Radiol. 2003;33:365.
47 Guibaud L, Herbreteau D, Dubois J, et al. Aneurysmal bone cysts: percutaneous embolization with an alcoholic solution of Zein—series of 18 cases. Radiology. 1998;208:369.
48 Mohit AA, Eskridge J, Ellenbogen R, et al. Aneurysmal bone cyst of the atlas: successful treatment through selective arterial embolization: case report. Neurosurgery. 2004;55:982.
49 de Kleuver M, van der Heul RO, Veraart BE. Aneurysmal bone cyst of the spine: 31 cases and the importance of the surgical approach. J Pediatr Orthop B. 1998;7:286.
50 Mankin HJ, Hornicek FJ, Ortiz-Cruz E, et al. Aneurysmal bone cyst: a review of 150 patients. J Clin Oncol. 2005;23:6756.
51 Ozaki T, Halm H, Hillmann A, et al. Aneurysmal bone cysts of the spine. Arch Orthop Trauma Surg. 1999;119:159.
52 Papagelopoulos PJ, Currier BL, Shaughnessy WJ, et al. Aneurysmal bone cyst of the spine. Management and outcome. Spine. 1998;23:621.
53 Feigenberg SJ, Marcus RBJr, Zlotecki RA, et al. Megavoltage radiotherapy for aneurysmal bone cysts. Int J Radiat Oncol Biol Phys. 2001;49:1243.
54 Papagelopoulos PJ, Choudhury SN, Frassica FJ, et al. Treatment of aneurysmal bone cysts of the pelvis and sacrum. J Bone Joint Surg Am. 2001;83:1674.
55 Yousri B, Aboumaarouf M, El Andaloussi M. [Aneurismal bone cyst in children: 17 cases.]. Rev Chir Orthop Reparatrice Appar Mot. 2003;89:338.
56 Murphey MD, Andrews CL, Flemming DJ, et al. From the archives of the AFIP. Primary tumors of the spine: radiologic pathologic correlation. Radiographics. 1996;16:1131.
57 Sansur CA, Pouratian N, Dumont AS, et al. Part II: spinal-cord neoplasms—primary tumours of the bony spine and adjacent soft tissues. Lancet Oncol. 2007;8:137.
58 Zapalowicz K, Skora P, Myslinski R, et al. Balloon kyphoplasty for painful C-7 vertebral hemangioma. J Neurosurg Spine. 2008;8:458.
59 Laredo JD, Reizine D, Bard M, et al. Vertebral hemangiomas: radiologic evaluation. Radiology. 1986;161:183.
60 Acosta FLJr, Sanai N, Chi JH, et al. Comprehensive management of symptomatic and aggressive vertebral hemangiomas. Neurosurg Clin N Am. 2008;19:17.
61 Chi JH, Manley GT, Chou D. Pregnancy-related vertebral hemangioma. Case report, review of the literature, and management algorithm. Neurosurg Focus. 2005;19(3):E7.
62 Galibert P, Deramond H, Rosat P, et al. [Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty.]. Neurochirurgie. 1987;33:166.
63 Bas T, Aparisi F, Bas JL. Efficacy and safety of ethanol injections in 18 cases of vertebral hemangioma: a mean follow-up of 2 years. Spine. 2001;26:1577.
64 Deramond H, Depriester C, Galibert P, et al. Percutaneous vertebroplasty with polymethylmethacrylate. Technique, indications, and results. Radiol Clin North Am. 1998;36:533.
65 Feydy A, Cognard C, Miaux Y, et al. Acrylic vertebroplasty in symptomatic cervical vertebral haemangiomas: report of 2 cases. Neuroradiology. 1996;38:389.
66 Hamza S, Meddeb N, Elleuch M, et al. [Vertebroplasty success in a case of aggressive hemangioma.]. Tunis Med. 2003;81:824.
67 Noez S, Collignon L, Bex V, et al. [Kyphoplasty for the treatment of painful vertebral hemangioma.]. Rev Med Liege. 2006;61:91.
68 Purkayastha S, Gupta AK, Kapilamoorthy TR, et al. Percutaneous vertebroplasty in the management of vertebral lesions. Neurol India. 2005;53:167.
69 Rawat S, Nangia S, Ezhilalan RB, et al. Variance in the treatment of vertebral haemangiomas. J Indian Med Assoc. 2007;105:42.
70 Faria SL, Schlupp WR, Chiminazzo HJr. Radiotherapy in the treatment of vertebral hemangiomas. Int J Radiat Oncol Biol Phys. 1985;11:387.
71 Enomoto K, Nishimura H, Hamada K, et al. Nuclear imaging of osteoma. Clin Nucl Med. 2008;33:135.
72 Munk PL, Huk ME. Medical management of osteoid osteoma. Can J Surg. 2003;46:60.
73 Kneisl JS, Simon MA. Medical management compared with operative treatment for osteoid-osteoma. J Bone Joint Surg Am. 1992;74:179.
74 Kan P, Schmidt MH. Osteoid osteoma and osteoblastoma of the spine. Neurosurg Clin N Am. 2008;19:65.
75 Gangi A, Basile A, Buy X, et al. Radiofrequency and laser ablation of spinal lesions. Semin Ultrasound CT MR. 2005;26:89.
76 Migues A, Velan O, Solari G, et al. Osteoid osteoma of the calcaneus: percutaneous radiofrequency ablation. J Foot Ankle Surg. 2005;44:469.
77 Rosenthal DI. Percutaneious radiofrequency treatment of osteoid osteomas. Semin Musculoskelet Radiol. 1997;1:265.
78 Rosenthal DI. Radiofrequency treatment. Orthop Clin North Am. 2006;37:475.
79 Gangi A, Alizadeh H, Wong L, et al. Osteoid osteoma: percutaneous laser ablation and follow-up in 114 patients. Radiology. 2007;242:293.
80 Adam G, Neuerburg J, Vorwerk D, et al. Percutaneous treatment of osteoid osteomas: combonation of drill biopsy and subsequent ethanol injection. Semin Musculoskelet Radiol. 1997;1:281.
81 Akhlaghpoor S, Tomasian A, Arjmand Shabestari A, et al. Percutaneous osteoid osteoma treatment with combination of radiofrequency and alcohol ablation. Clin Radiol. 2007;62:268.
82 Duda SH, Schnatterbeck P, Harer T, et al. [Treatment of osteoid osteoma with CT-guided drilling and ethanol instillation.]. Dtsch Med Wochenschr. 1997;122:507.
83 Sanhaji L, Gharbaoui IS, Hassani RE, et al. [A new treatment of osteoid osteoma: percutaneous sclerosis with ethanol under scanner guidance.]. J Radiol. 1996;77:37.
84 Berry M, Mankin H, Gebhardt M, et al. Osteoblastoma: a 30-year study of 99 cases. J Surg Oncol. 2008;98:179.
85 Healey JH, Ghelman B. Osteoid osteoma and osteoblastoma. Current concepts and recent advances. Clin Orthop Relat Res. 1986;204:76.
86 Jackson RP, Reckling FW, Mants FA. Osteoid osteoma and osteoblastoma. Similar histologic lesions with different natural histories. Clin Orthop Relat Res. 1977 Oct;128:303-313.
87 Saglik Y, Atalar H, Yildiz Y, et al. Surgical treatment of osteoblastoma: a report of 20 cases. Acta Orthop Belg. 2007;73:747.
88 Berberoglu S, Oguz A, Aribal E, et al. Osteoblastoma response to radiotherapy and chemotherapy. Med Pediatr Oncol. 1997;28:305.
89 Rajkumar A, Basu R, Datta NR, et al. Radiation therapy for sacral osteoblastoma. Clin Oncol (R Coll Radiol). 2003;15:85.
90 Uhl V, Castro JR, Knopf K, et al. Preliminary results in heavy charged particle irradiation of bone sarcoma. Int J Radiat Oncol Biol Phys. 1992;24:755.
91 Antic B, Roganovic Z, Tadic R, et al. Chondroma of the cervical spinal canal. Case report. J Neurosurg Sci. 1992;36:239.
92 Gabibov GA, Gasparina SS, Taniashin SV. [Chondroma of the spine. Diagnosis and surgical treatment.]. Zh Vopr Neirokhir Im N N Burdenko. 1986:48.
93 Lozes G, Fawaz A, Perper H, et al. Chondroma of the cervical spine. Case report. J Neurosurg. 1987;66:128.
94 McLoughlin GS, Sciubba DM, Wolinsky JP. Chondroma/chondrosarcoma of the spine. Neurosurg Clin N Am. 2008;19:57.
95 Morard M, De Tribolet N, Janzer RC. Chondromas of the spine: report of two cases and review of the literature. Br J Neurosurg. 1993;7:551.
96 Palaoglu S, Akkas O, Sav A. Chondroma of the cervical spine. Clin Neurol Neurosurg. 1988;90:253.
97 Willis BK, Heilbrun MP. Enchondroma of the cervical spine. Neurosurgery. 1986;19:437.
98 Flemming DJ, Murphey MD. Enchondroma and chondrosarcoma. Semin Musculoskelet Radiol. 2000;4:59.
99 Kosaki N, Yabe H, Anazawa U, et al. Bilateral multiple malignant transformation of Ollier’s disease. Skeletal Radiol. 2005;34:477.
100 Ramina R, Coelho Neto M, et al. Maffucci’s syndrome associated with a cranial base chondrosarcoma: case report and literature review. Neurosurgery. 1997;41:269.
101 Cree AK, Hadlow AT, Taylor TK, et al. Radiation-induced osteochondroma in the lumbar spine. Spine. 1994;19:376.
102 Gorospe L, Madrid-Muniz C, Royo A, et al. Radiation-induced osteochondroma of the T4 vertebra causing spinal cord compression. Eur Radiol. 2002;12:844.
103 Jaffe N, Ried HL, Cohen M. Radiation induced osteochondroma in long-term survivors of childhood cancer. and associates. Int J Radiat Oncol Biol Phys. 1983;9;:665.
104 Paulino AC, Fowler BZ. Secondary neoplasms after radiotherapy for a childhood solid tumor. Pediatr Hematol Oncol. 2005;22:89.
105 Taitz J, Cohn RJ, White L, et al. Osteochondroma after total body irradiation: an age-related complication. Pediatr Blood Cancer. 2004;42:225.
106 Ahmed AR, Tan TS, Unni KK, et al. Secondary chondrosarcoma in osteochondroma: report of 107 patients. Clin Orthop Relat Res. 2003;411:193.
107 Altay M, Bayrakci K, Yildiz Y, et al. Secondary chondrosarcoma in cartilage bone tumors: report of 32 patients. J Orthop Sci. 2007;12:415.
108 Du YK, Shih HN, Wang JM, et al. Dedifferentiated chondrosarcoma arising from osteochondromatosis. A case report. Chang Gung Yi Xue Za Zhi. 1991;14:130.
109 Garrison RC, Unni KK, McLeod RA, et al. Chondrosarcoma arising in osteochondroma. Cancer. 1982;49:1890.
110 O’Connor MI, Bancroft LW. Benign and malignant cartilage tumors of the hand. Hand Clin. 2004;20:317.
111 Samartzis D, Marco RA. Osteochondroma of the sacrum: a case report and review of the literature. Spine. 2006;31:E425.
112 Merchan EC, Sanchez-Herrera S, Gonzalez JM. Secondary chondrosarcoma. Four cases and review of the literature. Acta Orthop Belg. 1993;59:76.
113 Hoeffel JC, Brasse F, Schmitt M, et al. About one case of vertebral chondroblastoma. Pediatr Radiol. 1987;17:392.
114 Leung LY, Shu SJ, Chan MK, et al. Chondroblastoma of the lumbar vertebra. Skeletal Radiol. 2001;30:710.
115 Wisniewski M, Toker C, Anderson PJ, et al. Chondroblastoma of the cervical spine. Case report. J Neurosurg. 1973;38:763.
116 Doita M, Harada T, Iguchi T, et al. Total sacrectomy and reconstruction for sacral tumors. Spine. 2003;28:E296.
117 Junming M, Cheng Y, Dong C, et al. Giant cell tumor of the cervical spine: a series of 22 cases and outcomes. Spine. 2008;33:280.
118 Leggon RE, Zlotecki R, Reith J, et al. Giant cell tumor of the pelvis and sacrum: 17 cases and analysis of the literature. Clin Orthop Relat Res. 2004;423:196.
119 Luther N, Bilsky MH, Hartl R. Giant cell tumor of the spine. Neurosurg Clin N Am. 2008;19:49.
120 Shikata J, Yamamuro T, Shimizu K, et al. Surgical treatment of giant-cell tumors of the spine. Clin Orthop Relat Res. 1992;278:29.
121 Tomita K, Kawahara N, Baba H, et al. Total en bloc spondylectomy. A new surgical technique for primary malignant vertebral tumors. Spine. 1997;22:324.
122 McMaster ML, Goldstein AM, Bromley CM, et al. Chordoma: incidence and survival patterns in the United States, 1973-1995. Cancer Causes Control. 2001;12:1.
123 York JE, Kaczaraj A, Abi-Said D, et al. Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery. 1999;44:74.
124 Sciubba DM, Chi JH, Rhines LD, et al. Chordoma of the spinal column. Neurosurg Clin N Am. 2008;19:5.
125 Boriani S, Bandiera S, Biagini R, et al. Chordoma of the mobile spine: fifty years of experience. Spine. 2006;31:493.
126 Fuller DB, Bloom JG. Radiotherapy for chordoma. Int J Radiat Oncol Biol Phys. 1988;15:331.
127 Catton C, O’Sullivan B, Bell R, et al. Chordoma: long-term follow-up after radical photon irradiation. Radiother Oncol. 1996;41:67.
128 Park L, Delaney TF, Liebsch NJ, et al. Sacral chordomas: impact of high-dose proton/photon-beam radiation therapy combined with or without surgery for primary versus recurrent tumor. Int J Radiat Oncol Biol Phys. 2006;65:1514.
129 Hug EB. Review of skull base chordomas: prognostic factors and long-term results of proton-beam radiotherapy. Neurosurg Focus. 2001;10(3):E11.
130 Suit HD, Goitein M, Munzenrider J, et al. Definitive radiation therapy for chordoma and chondrosarcoma of base of skull and cervical spine. J Neurosurg. 1982;56:377.
131 Verhey LJ, Munzenrider JE. Proton beam therapy. Annu Rev Biophys Bioeng. 1982;11:331.
132 Casali PG, Messina A, Stacchiotti S, et al. Imatinib mesylate in chordoma. Cancer. 2004;101:2086.
133 Casali PG, Stacchiotti S, Sangalli C, et al. Chordoma. Curr Opin Oncol. 2007;19:367.
134 Orzan F, Terreni MR, Longoni M, et al. Expression study of the target receptor tyrosine kinase of imatinib mesylate in skull base chordomas. Oncol Rep. 2007;18:249.
135 Bjornsson J, Wold LE, Ebersold MJ, et al. Chordoma of the mobile spine. A clinicopathologic analysis of 40 patients. Cancer. 1993;71:735.
136 McPherson CM, Suki D, McCutcheon IE, et al. Metastatic disease from spinal chordoma: a 10-year experience. J Neurosurg Spine. 2006;5:277.
137 York JE, Berk RH, Fuller GN, et al. Chondrosarcoma of the spine: 1954 to 1997. J Neurosurg. 1999;90:73.
138 Foweraker KL, Burton KE, Jena R, et al. High dose photon radiotherapy in the management of chordoma and chondrosarcoma of the skull base and cervical spine. Clin Oncol (R Coll Radiol). 2007;19:S28.
139 Foweraker KL, Burton KE, Maynard SE, et al. High-dose radiotherapy in the management of chordoma and chondrosarcoma of the skull base and cervical spine. Part 1—Clinical outcomes. Clin Oncol (R Coll Radiol). 2007;19:509.
140 Barwick KW, Huvos AG, Smith J. Primary osteogenic sarcoma of the vertebral column: a clinicopathologic correlation of ten patients. Cancer. 1980;46:595.
141 Eilber F, Giuliano A, Eckardt J, et al. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol. 1987;5:21.
142 Link MP, Goorin AM, Horowitz M, et al. Adjuvant chemotherapy of high-grade osteosarcoma of the extremity. Updated results of the Multi-Institutional Osteosarcoma Study. Clin Orthop Relat Res. 1991;270:8.
143 Eilber FR, Grant T, Morton DL. Adjuvant therapy for osteosarcoma: preoperative and postoperative treatment. Cancer Treat Rep. 1978;62:213.
144 Link MP. Adjuvant therapy in the treatment of osteosarcoma. Important Adv Oncol. 1986:193.
145 Eilber FR. Adjuvant treatment of osteosarcoma. Surg Clin North Am. 1981;61:1371.
146 Ferguson WS, Goorin AM. Current treatment of osteosarcoma. Cancer Invest. 2001;19:292.
147 Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600.
148 Weber KL. Current concepts in the treatment of Ewing’s sarcoma. Expert Rev Anticancer Ther. 2002;2:687.
149 Pilepich MV, Vietti TJ, Nesbit ME, et al. Ewing’s sarcoma of the vertebral column. Int J Radiat Oncol Biol Phys. 1981;7:27.
150 Sharafuddin MJ, Haddad FS, Hitchon PW, et al. Treatment options in primary Ewing’s sarcoma of the spine: report of seven cases and review of the literature. Neurosurgery. 1992;30:610.
151 Bilsky MH, Azeem S. Multiple myeloma: primary bone tumor with systemic manifestations. Neurosurg Clin N Am. 2008;19:31.
152 Kyle RA, Rajkumar SV. Epidemiology of the plasma-cell disorders. Best Pract Res Clin Haematol. 2007;20:637.
153 The Multiple Myeloma Research Foundation. About Myeloma. Available at www.multiplemyeloma.org, 2008.
154 Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21.
155 Knobel D, Zouhair A, Tsang RW, et al. Prognostic factors in solitary plasmacytoma of the bone: a multicenter Rare Cancer Network study. BMC Cancer. 2006;6:118.
156 Yeh HS, Berenson JR. Myeloma bone disease and treatment options. Eur J Cancer. 2006;42:1554.
157 McDonald RJ, Trout AT, Gray LA, et al. Vertebroplasty in multiple myeloma: outcomes in a large patient series. AJNR Am J Neuroradiol. 2008;29:642.
158 Latif T, Hussein MA. Advances in multiple myeloma and spine disease. Clin Lymphoma Myeloma. 2005;6:228.
159 Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98:21.
160 Cortet B, Cotten A, Boutry N, et al. Percutaneous vertebroplasty in patients with osteolytic metastases or multiple myeloma. Rev Rhum Engl Ed. 1997;64:177.
161 Chakrabarti I, Burton AW, Rao G, et al. Percutaneous vertebroplasty of a myelomatous compression fracture in the presence of previous posterior instrumentation. Report of two cases. J Neurosurg Spine. 2006;5:168.
162 Bartolozzi B, Nozzoli C, Pandolfo C, et al. Percutaneous vertebroplasty and kyphoplasty in patients with multiple myeloma. Eur J Haematol. 2006;76:180.
163 Bacci G, Balladelli A, Forni C, et al. Adjuvant and neoadjuvant chemotherapy for Ewing sarcoma family tumors in patients aged between 40 and 60: report of 35 cases and comparison of results with 586 younger patients treated with the same protocols in the same years. Cancer. 2007;109:780.
164 Rosen G, Caparros B, Nirenberg A, et al. Ewing’s sarcoma: ten-year experience with adjuvant chemotherapy. Cancer. 1981;47:2204.
165 Brown AP, Fixsen JA, Plowman PN. Local control of Ewing’s sarcoma: an analysis of 67 patients. Br J Radiol. 1987;60:261.
166 Bacci G, Forni C, Longhi A, et al. Long-term outcome for patients with non-metastatic Ewing’s sarcoma treated with adjuvant and neoadjuvant chemotherapies. 402 patients treated at Rizzoli between 1972 and 1992. Eur J Cancer. 2004;40:73.
167 Scully SP, Temple HT, O’Keefe RJ, et al. Role of surgical resection in pelvic Ewing’s sarcoma. J Clin Oncol. 1995;13:2336.
168 Suit HD. Radiotherapy in osteosarcoma. Clin Orthop Relat Res. 1975;111:71.
169 Hristov B, Shokek O, Frassica DA. The role of radiation treatment in the contemporary management of bone tumors. J Natl Compr Canc Netw. 2007;5:456.
170 Hershey A, Bos GD, Stevens K. Successful treatment of spinal osteosarcoma with radiation and chemotherapy. Orthopedics. 1996;19:617.
171 DeLaney TF, Park L, Goldberg SI, et al. Radiotherapy for local control of osteosarcoma. Int J Radiat Oncol Biol Phys. 2005;61:492.
172 Hitchon PW, Bilsky MH, Ebersold MJ. Primary Bony Spinal Lesions, 2nd ed. Philadelphia: Elsevier; 2005.
173 Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine. 1997;22:1036.
174 Springfield DS, Schmidt R, Graham-Pole J, et al. Surgical treatment for osteosarcoma. J Bone Joint Surg Am. 1988;70:1124.
175 Heslin MJ, Lewis JJ, Nadler E, et al. Prognostic factors associated with long-term survival for retroperitoneal sarcoma: implications for management. J Clin Oncol. 1997;15:2832.
176 Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res. 1986;204:9.
177 Dirix LY, Vermeulen P, De Wever I, et al. Soft tissue sarcoma in adults. Curr Opin Oncol. 1997;9:348.
178 Dirix LY, Somville J, van Oosterom AT. Diagnosis and treatment of soft tissue sarcomas in adults. Curr Opin Oncol. 1996;8:289.
179 Brady MS, Gaynor JJ, Brennan MF. Radiation-associated sarcoma of bone and soft tissue. Arch Surg. 1992;127:1379.
180 Tomita K, Kawahara N, Murakami H, et al. Total en bloc spondylectomy for spinal tumors: improvement of the technique and its associated basic background. J Orthop Sci. 2006;11:3.
181 Sundaresan N, Rosen G, Huvos AG, et al. Combined treatment of osteosarcoma of the spine. Neurosurgery. 1988;23:714.
182 Stener B. Total spondylectomy in chondrosarcoma arising from the seventh thoracic vertebra. J Bone Joint Surg Br. 1971;53:288.
183 Stener B. Complete removal of vertebrae for extirpation of tumors. A 20-year experience. Clin Orthop Relat Res. 1989;245:72.
184 Roy-Camille R, Saillant G, Bisserie M, et al. [Total excision of thoracic vertebrae (author’s transl).]. Rev Chir Orthop Reparatrice Appar Mot. 1981;67:421.
185 Boriani S, Biagini R, De Iure F, et al. Lumbar vertebrectomy for the treatment of bone tumors: surgical technique. Chir Organi Mov. 1994;79:163.
186 Kawahara N, Tomita K, Matsumoto T, et al. Total en bloc spondylectomy for primary malignant vertebral tumors. Chir Organi Mov. 1998;83:73.
187 Marmor E, Rhines LD, Weinberg JS, et al. Total en bloc lumbar spondylectomy. Case report. J Neurosurg. 2001;95:264.
188 Yao KC, Boriani S, Gokaslan ZL, et al. En bloc spondylectomy for spinal metastases: a review of techniques. Neurosurg Focus. 2003;15(5):E6.
189 Abdel-Wanis Mel S, Tsuchiya H, Kawahara N, et al. Tumor growth potential after tumoral and instrumental contamination: an in-vivo comparative study of T-saw, Gigli saw, and scalpel. J Orthop Sci. 2001;6:424.
190 Talac R, Yaszemski MJ, Currier BL, et al. Relationship between surgical margins and local recurrence in sarcomas of the spine. Clin Orthop Relat Res. 2002;397:127.