CHAPTER 24 Ethical and Legal Issues in Medical Genetics
Ethics is the branch of knowledge that deals with moral principles, which in turn relate to principles of right, wrong, justice, and standards of behavior. Traditionally, the reference points are based on a synthesis of the philosophical and religious views of well-informed, respected, thinking members of society. In this way, a code of practice evolves that is seen as reasonable and acceptable by a majority, which often forms the basis for professional guidelines or regulations. It might be argued that there are no ‘absolutes’ in ethical and moral debates. In complex scenarios, in which there may be competing and conflicting claims to an ethical principle, practical decisions and actions often have to be based on a balancing of duties, responsibilities, and rights. Ethics, like science, is not static but moves on, and in fact the development of the two disciplines is closely intertwined.
Ethical issues arise in all branches of medicine, but human genetics poses particular challenges because genetic identity impinges not just on an individual, but also on close relatives and the extended family, as well as society in general. In the minds of the general public, clinical genetics and genetic counseling can easily be confused with eugenics—defined as the science of ‘improving’ a species through breeding. It is important to stress that the modern specialty of clinical genetics has absolutely nothing in common with the appalling eugenic philosophies that were practiced in Nazi Germany and, to a much lesser extent, elsewhere in Europe and the United States between the two world wars. Emphasis has already been placed on the fundamental principle that genetic counseling is a non-directive and non-judgmental communication process whereby factual knowledge is imparted to facilitate informed personal choice (see Chapter 17). Indeed, clinical geneticists have been pioneers in recent times in practicing and promoting non-paternalism in medicine, and 5% of the original budget for the Human Genome Project was set aside for funding studies into the ethical and social implications of the knowledge gained from the project. Coercion and eugenics certainly have no place in modern medical genetics.
General Principles
The time-honored four principles of medical ethics that command wide consensus are listed in Box 24.1. Developed and championed by the American ethicists Tom Beauchamp and James Childress, these principles provide an acceptable framework, although close scrutiny of many difficult dilemmas highlights limitations in these principles and apparent conflicts between them. Everyone involved in clinical genetics will sooner or later be confronted by complex and challenging ethical situations, some of which pose particularly difficult problems with no obvious solution, and certainly no perfect one. Just as patients need to balance risks when making a decision about a treatment option, so the clinician/counselor may need to balance these principles one against the other. A particular difficulty in medical genetics can be the principle of autonomy, given that we all share our genes with our biological relatives. Individual autonomy needs sometimes to be weighed against the principle of doing good, and doing no harm, to close family members.
Box 24.1
Fundamental Ethical Principles
The Beauchamp and Childress framework of ethical principles is, unsurprisingly, not the only one in use and others have developed them into practical approaches. These include the Jonsen framework (Box 24.2) and the more detailed scheme developed by Mike Parker of Oxford’s Ethox Centre (Box 24.3), which builds on previous proposals. Taken together, these provide a practical approach to clinical ethics, which is an expanding discipline in health care.
Box 24.2
The Jonson Framework
a Practical Approach to Clinical Ethics
Box 24.3
The Ethox Centre Clinical Ethics Framework (Mike Parker)
Informed Consent
A patient is entitled to an honest and full explanation before any procedure or test is undertaken. Information should include details of the risks, limitations, implications, and possible outcomes of each procedure. In the current climate, with respect to full information and the doctor-patient contract, some form of signed consent is increasingly being obtained for every action that exposes the patient—access to medical records, clinical photography, genetic testing, and storage of DNA. In fact, there is no legal requirement to obtain signed consent for taking a blood test from which DNA is extracted and stored. The issue was addressed by the UK Human Tissue Act 2004. According to the act, DNA does not constitute ‘human tissue’ in the same way as biopsy samples or cellular material, for which formal consent is required, whether the tissue is from the living or the dead. The act does require that consent is formally obtained where cellular material is used to obtain genetic information for another person. In a clinical setting, this must be clearly discussed and documented.
In clinical genetics, many patients who are candidates for clinical examination and genetic testing are children or individuals with learning difficulties who may lack capacity to grant informed consent. Furthermore, the result of any examination or test may have only a small chance of directly benefiting the patient but is potentially very important for family members. Here the law is important. In England and Wales, the Mental Capacity Act of 2005 came into effect in 2007 and applies to adults aged 16 and older. It replaced case law for health (and social) care and there is a legal duty to use the legislation and apply the ‘Test for Capacity’ (Box 24.4) for any relevant decision for people who lack capacity. Decisions must take into account the ‘best interests’ of the patient, but can also embrace the wider interests that relate to the family. In England and Wales, the law allows for an appropriate person appointed by the Court of Protection to act on their behalf, whereas in Scotland it is legally permitted for certain designated adults, including family members, to give consent (or refuse) on behalf of a person lacking capacity.
Box 24.4
Mental Capacity Act, 2005, England and Wales (Outline)—Principles, Definition, and Test for Capacity
Ethical Dilemmas in the Genetic Clinic
Prenatal Diagnosis
Many methods are now widely available for diagnosing structural abnormalities and genetic disorders during the first and second trimesters (see Chapter 21). The past 35 to 40 years have seen the first real availability of choice in the context of pregnancy in human history. Not surprisingly, the issue of prenatal diagnosis and subsequent offer of termination of pregnancy raises many difficult issues for individuals and families, and raises serious questions about the way in which society views and cares for both children and adults with disability. In the United Kingdom, termination of pregnancy is permitted up to and beyond 24 weeks’ gestation if the fetus has a lethal condition such as anencephaly, or if there is a serious risk of major physical or mental handicap. For good reason, terms such as ‘serious’ are not defined in the relevant legislation, but this can inevitably lead to controversy over interpretation.
The difficulties surrounding prenatal diagnosis can be illustrated by considering some of the general principles that have already been discussed. At the top of the list comes informed consent. In the United Kingdom, approximately 70% of all pregnancies are monitored for the presence of a neural tube defect by measurement of α-fetoprotein in maternal serum at approximately 16 weeks’ gestation (p. 328). In theory, all women undergoing this test should have a full understanding of its potential implications. This also applies to every woman who is offered a detailed ultrasonographic scan to assess fetal anatomy at around 18 to 20 weeks’ gestation (p. 326). For fully informed consent to be obtained in these situations, it is essential that pregnant women should have access to detailed counseling by unhurried staff members who are knowledgeable, experienced, and sympathetic. In practice this may not always be so; indeed, there is evidence that the quality of information provided varies widely.
The results of public consultation exercises conducted by the Advisory Committee on Genetic Testing (subsumed into the Human Genetics Commission which was abolished in 2010) and the HFEA are reasonably reassuring. The views expressed support the applications of genetics in prenatal testing for serious disorders but concern over wider applications of the techniques. Similarly, research published by the British Social Attitudes survey, in the context of genetics research and gene manipulation for the detection of disease, suggested that the public supports these activities in general but expressed deep reservations for application of the technologies for genetic enhancement. Genetic enhancement, through manipulation of embryos or gametes, strikes at the very heart of what it means to have one’s own identity through natural laws of chance. This, it seems, is a powerful undercurrent in the understanding of who we are as individuals and as a species.
Predictive Testing In Childhood
The situation is very different if predictive testing could directly benefit the child by identifying the need for a medical or surgical intervention in childhood. This applies to conditions such as familial hypercholesterolemia (p. 175), for which early dietary management can be introduced, and also to some of the familial cancer-predisposing syndromes (p. 225) for which early screening, and sometimes prophylactic surgery, is indicated. Generally, it is thought that in these situations genetic testing is acceptable at around the time when other screening tests or preventive measures would be initiated.
Implications for the Extended Family
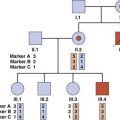
It is widely agreed that the identification of a condition that could have implications for other family members should lead to the offer of tests for the extended family. This applies particularly to balanced translocations and serious X-linked recessive disorders. In the case of translocations, this is sometimes referred to as translocation chasing. For an autosomal recessive disorder such as cystic fibrosis, the term ‘cascade screening’ is applied (p. 304).
The main ethical problem that arises here is that of confidentiality. A carrier of a translocation or serious X-linked recessive disorder is usually urged to alert close family relatives to the possibility that they could also be carriers and therefore at risk of having affected children. Alternatively, permission can be sought for members of the genetics team to make these approaches. Occasionally a patient, for whatever reason, will refuse to allow this information to be disseminated.
Informed Consent in Genetic Research
The issues relating to informed consent when participating in genetic research are just as complex. Many people are perfectly willing to hold out their arm for a blood test which might ‘help others’, particularly if they have personal experience of a serious disorder in their own family. However, few will have given any serious thought to the possible ramifications of their simple act of altruism. For example, it is unlikely that they will ever have considered whether their sample will be tested anonymously, who will be informed of the result, or whether other tests will be carried out on stored DNA in the future as new techniques are developed. These concerns, among others (Box 24.5), have prompted the US National Institutes of Health Office of Protection from Research Risks to draw up proposals on the steps that should be taken to try to ensure that all aspects of informed consent are addressed when samples are collected for genetic research. Just as signed consent for genetic testing and storage of DNA has become routine in the service setting (although not a legal requirement under the UK Human Tissue Act 2004), similar procedures should be adhered to in a research setting.
Ethical Dilemmas and the Public Interest
Genetics and Insurance
The life insurance industry is competitive and profit driven. Private insurance is based on ‘mutuality’, whereby risks are pooled for individuals in similar circumstances. In contrast, public health services are based on the principle of ‘solidarity’, whereby health provision for everyone is funded from general taxation. It is understandable that the life insurance industry is concerned that individuals who receive a positive predictive test result will take out large policies without revealing their true risk status. This is sometimes referred to as ‘antiselection’ or ‘adverse selection’. On the other hand, the genetics community is concerned that individuals who test positive will become victims of discrimination, and perhaps uninsurable. This concern extends to those with a family history of a late-onset disorder, who might be refused insurance unless they undergo predictive testing.
Inevitably, the Association of British Insurers (ABI) had a view. In the 1999 revision of its Code of Practice, the ABI reiterated its view that applicants should not be asked to undergo genetic testing and that existing genetic test results need not be disclosed in applications for mortgage-related life assurance up to a total of £100,000. In 2005 the UK government negotiated an agreement with the ABI to extend restriction on the use of predictive genetic tests by insurers to November 2011. The document, entitled ‘Concordat and Moratorium on Genetics and Insurance’ (Box 24.6), stated that no one will be required to disclose the result of a predictive genetic test unless first approved by the government’s Genetics and Insurance Committee (GAIC). The GAIC approved only one application—for HD for amounts greater than £500,000—and the body was abolished in 2009.
Box 24.6
Key Points in the ‘Concordat and Moratorium on Genetics and Insurance’ Negotiated between the UK Government and the Association of British Insurers (Abi), 2005
Gene Patenting and the Human Genome Project
The legal issues are complex and, not surprisingly, the international community has struggled to identify satisfactory solutions. With the exception of the United States, most national regulatory bodies prohibit payment for the procurement of human genetic material, but their views on patenting are much less well defined. In recent years many important human genes have been patented, and companies such as Myriad Genetics in the United States sought to impose their exclusive licence for genetic testing for BRCA1 and BRCA2 (p. 224). In fact, in 2004 the European Patent Office revoked the patent, denying Myriad a license fee from every BRCA test undertaken in Europe, and thereby setting a precedent for other contentious cases. Gene patents may concern single-gene disorders, such as the BRCA genes, but increasingly commercial companies are offering a range of ‘direct to consumer’ tests, consisting of the analysis of a range of polymorphisms, with the promise of predicting the future risk of ill health from common disorders. These companies are often less than candid about the precise tests offered, and their validation, all of which runs counter to the spirit of scientific enquiry and evidence-based medicine.
Gene Therapy
One of the most exciting aspects of recent progress in molecular biology is the prospect of successful gene therapy (p. 350), although it is obviously disappointing that this potential has not yet been realized. It is understandable that both the general public and the health care professions should be concerned about the possible side effects and abuse of gene therapy. Frequent reference has been made to the ‘slippery slope’ argument, whereby to take the first step leads incrementally and inevitably to uncontrolled experimentation. To address these anxieties, advisory or regulatory committees have been established in several countries to assess the practical and ethical aspects of gene therapy research programs.
Population Screening
Population screening programs offering carrier detection for common autosomal recessive disorders, have been in operation for many years (p. 318), and in many cases well received (e.g., thalassemia and Tay-Sachs disease). This was not so with respect to neonatal screening for α1 antitrypsin deficiency in Scandinavia, which was abandoned because it proved stressful. Such is the progress in comparative genome analysis that it will be technically possible to compare the genome of the unborn baby with that of its parents; de novo differences may represent mutations that would lead to serious disease and it has been proposed that the technique is offered to couples undergoing PGD. Chorionic villus tissue could also be analyzed in this way, and even free fetal DNA from maternal blood eventually. The ethical problem relates to the range of diseases that might be tested this way, and the choices couples might face.
With respect to screening programs that detect carrier status for disease, the issues are slightly different. Early efforts to introduce sickle-cell carrier detection in North America were largely unsuccessful because of misinformation, discrimination, and stigmatization. Also, pilot studies assessing the responses to cystic fibrosis (CF) carrier screening in white populations yielded conflicting results (p. 321). These experiences illustrate the importance of informed consent and the difficulties of ensuring both autonomy and informed choice. For example, CF screening in the United Kingdom has been implemented as part of neonatal screening. Although aimed at identifying babies with CF, the screening detects a proportion who are simply carriers, but obviously newborn infants cannot make an informed choice. Consequently, some pilot studies have focused on adults and their responses to the offer of carrier testing, either from their general practitioner or at the antenatal clinic. This has raised the vexed question of whether an offer from a respected family doctor could be interpreted as an implicit recommendation to participate, and such an approach yields a higher acceptance rate than a casual written invitation to attend for screening at a future date. This begs the question as to whether, depending on the approach, individuals feel pressured to undergo a test that they do not necessarily want.
In population screening, confidentiality is also important. Many will not wish their carrier status to be known by classmates or colleagues at work. The issue of confidentiality will be particularly difficult for individuals found by genetic testing to be susceptible to a medical problem through environmental industrial hazards, which could lead to employment discrimination (p. 367). These anxieties led in 1995 to the Equal Employment Opportunity Commission in the United States issuing a guideline that allows for anyone denied employment because of disease susceptibility to claim protection under the Americans with Disabilities Act.
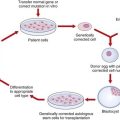
Cloning and Stem Cell Research
The main ethical difficulty arises in relation to the source of stem cells. No one voices serious ethical difficulties in relation to stem cells harvested from the fully formed person, whether taken from the umbilical cord or the mature adult, and there have been significant advances using these sources. But a strong school of scientific opinion maintains that there is no substitute for studying embryonic stem cells to understand how cells differentiate from primitive into more complex types. In 2005 the UK Parliament moved swiftly to approve an extension to research on early human embryos for this purpose. Research on human embryos up to 14 days of age was already permitted under the Human Fertilization and Embryology Act 1990. The United Kingdom therefore became one of the most attractive places to work in stem-cell research because, although regulated, it is legal. Publicly funded research of this kind was not permitted in the United States until a change of political direction in 2009. Progress has been painfully slow for those engaged in this work, and the focus shifted to the creation of animal-human (‘human-admixed’) hybrids and chimeras because of the poor supply and quality of human oocytes (usually ‘leftovers’ from infertility treatment) for use in nuclear cell transfer. In the United Kingdom, Newcastle was granted a license to collect fresh eggs for stem-cell research from egg donors in return for a reduction in the cost of IVF treatment, a decision greeted with alarm in some quarters. This group was also the first, in 2005, to create a human blastocyst after nuclear transfer.
Those who object to the use of embryonic stem cells believe it is not only treating the human embryo with disrespect and tampering with the sanctity of life, but also could lead eventually to reproductive cloning. In fact, the Human Fertilization and Embryology Act of 1990 permits the creation of human embryos for research, but very few have been created since the HFEA began granting licenses. This Act of Parliament has been reviewed and updated to accommodate new developments, and came into effect in 2009. The main provisions are listed in Box 24.7 and will continue to generate contentious ethical debate.
Box 24.7
The Key 2008 Amendments to the Human Fertilization and Embryology Act (HFEA) 1990
American Society of Human Genetics Report. Statement on informed consent for genetic research. Am J Hum Genet. 1996;59:471-474.
Association of British Insurers. Genetic testing. ABI Code of Practice. London, UK: ABI, London; 1999.
Baily MA, Murray TH, editors. Ethics and newborn genetic screening: new technologies, new challenges. Baltimore, MD: Johns Hopkins University Press, Baltimore, 2009.
British Medical Association. Human genetics. Choice and responsibility. Oxford, UK: Oxford University Press; 1998.
Bryant J, Baggott la Velle L, Searle J, editors. Bioethics for scientists. Chichester, UK: John Wiley, 2002.
A multiauthor text of wide scope with many contributions relevant to medical genetics.
Buchanan A, Daniels N, Wikler D, Brock DW. From chance to choice: genetics and justice. Cambridge, UK: Cambridge University Press; 2000.
Clarke A, editor. The genetic testing of children. Oxford, UK: Bios Scientific, 1997.
A comprehensive multiauthor text dealing with this important subject.
Clothier Committee. Report of the Committee on the Ethics of Gene Therapy. London, UK: HMSO; 1992.
Collins FS. Shattuck lecture—medical and societal consequences of the human genome project. N Engl J Med. 1999;341:28-37.
Harper PS, Clarke AJ. Genetics society and clinical practice. Oxford, UK: Bios Scientific; 1997.
Human Genetics Commission. Inside information: balancing interests in the use of personal genetic data. London, UK: Department of Health; 2002.
Jonsen AR, Siegler M, Winslade WJ. Clinical ethics: a practical approach to ethical decisions in clinical medicine, 3rd edn. New York: McGraw-Hill; 1992.
The key reference that outlines the Jonsen framework for decision-making in clinical ethics.
Knoppers BM. Status, sale and patenting of human genetic material: an international survey. Nat Genet. 1999;22:23-26.
Knoppers BM, Chadwick R. Human genetic research: emerging trends in ethics. Nat Rev Genet. 2005;6:75-79.
An overview of current international policies on gene patenting.
McInnis MG. The assent of a nation: genetics and Iceland. Clin Genet. 1999;55:234-239.
Nuffield Council on Bioethics. Genetic screening: ethical issues. London, UK: Nuffield Council on Bioethics; 1993.
A very helpful document for professional guidance.
Nuffield Council on Bioethics. Mental disorders and genetics: the ethical context. London, UK: Nuffield Council on Bioethics; 1998.
A further detailed document dealing with genetic issues in the context of mental health.
Pokorski RJ. Insurance underwriting in the genetic era. Am J Hum Genet. 1997;60:205-216.
A detailed account of the issues surrounding the use of genetic tests by the insurance industry.
Royal College of Physicians, Royal College of Pathologists, British Society of Human Genetics: Consent and Confidentiality in Genetic Practice. Guidance on genetic testing and sharing genetic information. Report of the Joint Committee on Medical Genetics. London, UK: RCP, RCPath, BSHG; 2006.
Elements