Establishment of the Basic Embryonic Body Plan
As seen in Chapter 5, despite the relatively featureless appearance of the gastrulating embryo, complex patterns of gene expression set up the basic body plan of the embryo. One of the earliest morphological manifestations of this pattern is the regular segmentation that becomes evident along the craniocaudal axis of the embryo. Such a segmental plan, which is a dominant characteristic of all early embryos, becomes less obvious as development progresses. Nonetheless, even in an adult the regular arrangement of the vertebrae, ribs, and spinal nerves persists as a reminder of humans’ highly segmented phylogenetic and ontogenetic past.
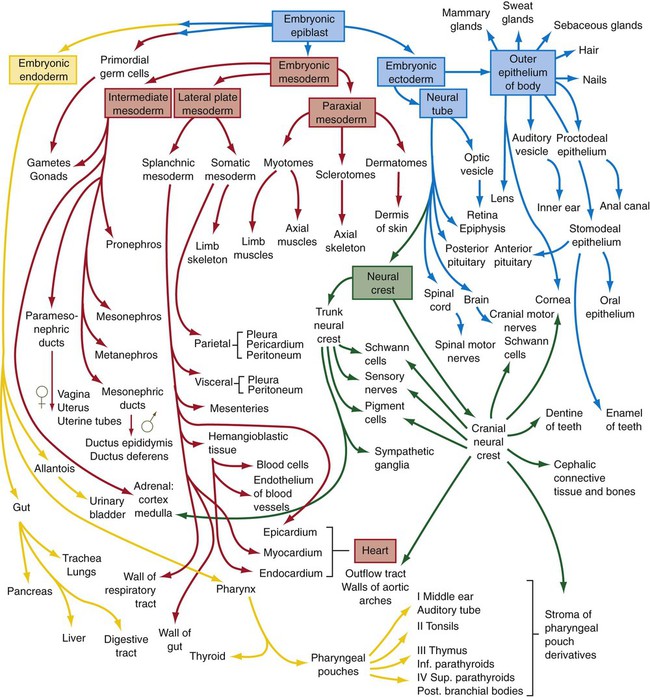
This chapter concentrates on the establishment of the basic overall body plan. In addition, it charts the appearance of the primordia of the major organ systems of the body from the undifferentiated primary germ layers (see Fig. 6.27).
Development of the Ectodermal Germ Layer
Neurulation: Formation of the Neural Tube
The principal early morphological response of the embryonic ectoderm to neural induction is an increase in the height of the cells that are destined to become components of the nervous system. These transformed cells are evident as a thickened neural plate visible on the dorsal surface of the early embryo (Figs. 6.1A and 6.2A). Unseen but also important is the restricted expression of cell adhesion molecules (Ig-CAMs), from N-CAM and E-cadherin in the preinduced ectoderm to N-CAM and N-cadherin in the neural plate.
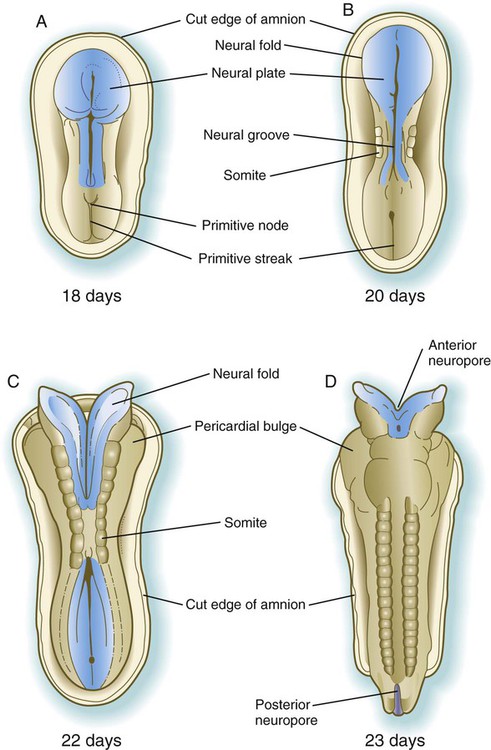
A, At 18 days. B, At 20 days. C, At 22 days. D, At 23 days.
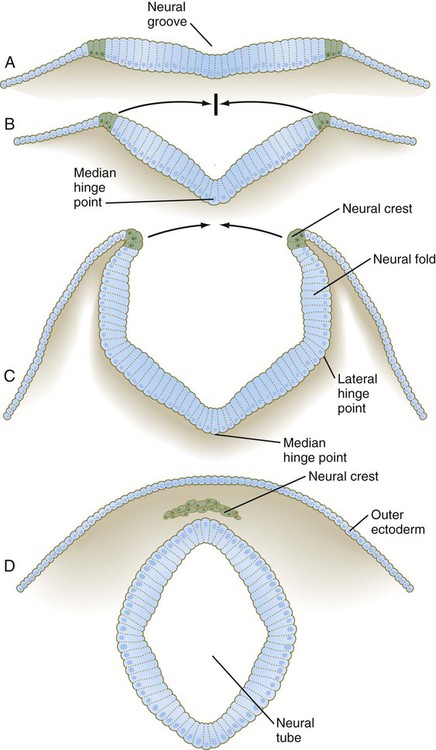
A, Neural plate. B, Neural fold. C, Neural folds apposing. D, Neural tube complete. (Neural crest before and after its exit from the neural epithelium is shown in green.)
The first of four major stages in the formation of the neural tube is transformation of the general embryonic ectoderm into a thickened neural plate. The principal activity of the second stage is further shaping of the overall contours of the neural plate so that it becomes narrower and longer. To a great extent, this is accomplished by convergent extension, during which the ectodermal cells forming the neural plate migrate toward the midline and also become longer along the anteroposterior axis and narrower laterally. This process, which is guided by planar cell polarity (see p. 87), results in the formation of a key-shaped neural plate (see Fig. 6.1A).
The third major stage in the process of neurulation is the lateral folding of the neural plate that results in the elevation of each side of the neural plate along a midline neural groove (see Figs. 6.1B and 6.2B). Many explanations have been proposed for lateral folding of the neural plate and ultimate closure of the neural tube. Most of these explanations have invoked a single or dominant mechanism, but it is now becoming apparent that lateral folding is the result of numerous region-specific mechanisms intrinsic and extrinsic to the neural plate.
The ventral midline of the neural plate, sometimes called the median hinge point, acts like an anchoring point around which the two sides become elevated at a sharp angle from the horizontal. At the median angle, bending can be largely accounted for by notochord-induced changes in the shape of the neuroepithelial cells of the neural plate. These cells become narrower at their apex and broader at their base (see Fig. 6.2B) through a combination of a basal position of the nuclei (thus causing a lateral expansion of the cell in that area) and a purse string–like contraction of a ring of actin-containing microfilaments in the apical cytoplasm. Throughout the lateral folding of the neural plate in the region of the spinal cord, much of the wall area of the neural plate initially remains flat (see Fig. 6.2B), but in the brain region, a lateral hinge point forms as a result of apical constriction of cells in a localized area (see Fig. 6.2C). Elevation of the neural folds seems to be accomplished largely by factors extrinsic to the neural epithelium, in particular, pushing forces generated by the expanding surface epithelium lateral to the neural plate.
The fourth stage in the formation of the neural tube consists of apposition of the two most lateral apical surfaces of the neural folds, their fusion (mediated by cell surface glycoconjugates), and the separation of the completed segment of the neural tube from the overlying ectodermal sheet (see Fig. 6.2C and D). At the same time, cells of the neural crest begin to separate from the neural tube.
Closure of the neural tube begins almost midway along the craniocaudal extent of the nervous system of a 21- to 22-day-old embryo (see Fig. 6.1C). Over the next couple of days, closure extends caudally in a zipperlike fashion, but cranially there are commonly two additional discontinuous sites of closure. The unclosed cephalic and caudal parts of the neural tube are called the anterior (cranial) and posterior (caudal) neuropores. The neuropores also ultimately close off so that the entire future central nervous system resembles an irregular cylinder sealed at both ends. Occasionally, one or both neuropores remain open, and serious birth defects result (see p. 248).
Segmentation in the Neural Tube
Morphological Manifestations of Segmentation
Soon after the neural tube has taken shape, the region of the future brain can be distinguished from the spinal cord. The brain-forming region undergoes a series of subdivisions that constitute the basis for the fundamental gross organization of the adult brain. Segmentation by subdivision of an existing structure (in the case of the neural tube) contrasts with segmentation by adding terminal segments, as is the case in the formation of somites (see p. 99). An early set of subdivisions results in a three-part brain, consisting of a forebrain (prosencephalon), midbrain (mesencephalon), and hindbrain (rhombencephalon). Later, the prosencephalon becomes subdivided into a telencephalon and diencephalon, and the rhombencephalon is subdivided into a metencephalon and myelencephalon (see Fig. 11.2).
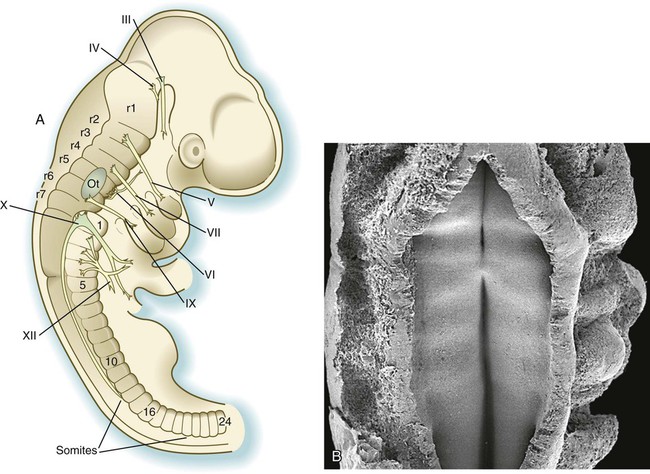
Superimposed on the traditional gross morphological organization of the developing brain is another, more subtle, level of segmentation, which subdivides certain regions of the brain into transiently visible series of regular segments called neuromeres (Fig. 6.3). In the hindbrain, the neuromeres, often called rhombomeres, are visible from early in the fourth to late in the fifth week (Fig. 6.3B). The midbrain does not seem to be segmented, but the prosencephalon contains a less regular series of prosomeres.
Rhombomeres are arranged as odd and even pairs, and when established, they act like isolated compartments in insect embryos. Because of specific surface properties, cells from adjacent rhombomeres do not intermingle across boundaries between even and odd segments; however, marked cells from two even or two odd rhombomeres placed side by side do intermingle. During their brief existence, rhombomeres provide the basis for the fundamental organization of the hindbrain. In an adult, the segmental organization of the rhombomeres is manifest in the rhombomere-specific origin of many cranial nerves and parts of the reticular formation within the brainstem (see Fig. 11.13).
Mechanisms of Early Segmentation of the Neural Tube
While gastrulation is still taking place, the newly induced neural tube is subjected to vertical inductions from the notochord and head organizing regions (anterior visceral endoderm and prechordal plate), which are important in inducing the forebrain region. These inductions, together with a gradient of Wnt-8 (product of a gene homologous with Wingless, a segment polarity gene in Drosophila [see Fig. 4.1]) signaling, effectively subdivide it into forebrain/midbrain and hindbrain/spinal cord segments. This subdivision is marked by the expression of two transcription factors, Otx-2 (orthodenticle homologue 2) in the forebrain/midbrain region, and in the hindbrain, Gbx-2 (gastrulation brain homeobox 2), whose boundaries sharply define the midbrain-hindbrain border (Fig. 6.4A). Fibroblast growth factors (FGFs), produced in the early primitive streak, are known to exert a posteriorizing effect on the newly forming neural plate.
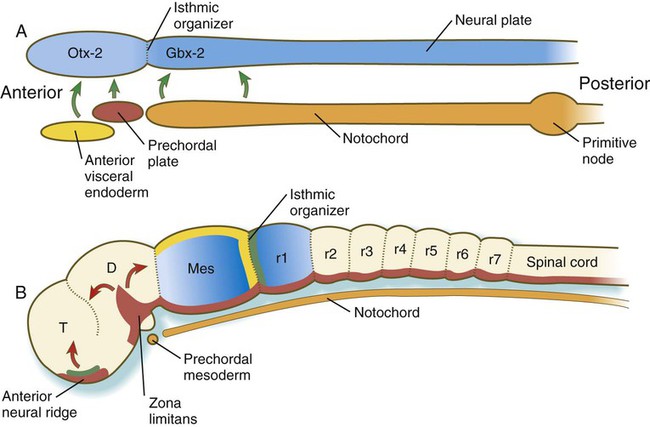
A, In response to signals (green arrows) from the anterior visceral endoderm, the prechordal plate, and the notochord, the neural tube expresses Otx-2 in the future forebrain and midbrain regions and Gbx-2 in the hindbrain and spinal cord. B, Later in development, signals (fibroblast growth factor-8 [FGF-8] [green] and Wnt-1 [yellow]) from the isthmic organizer induce decreasing gradients of En-1 and En-2 (blue) on either side. Another organizer—the anterior neural ridge—secretes sonic hedgehog (red) and FGF-8 (green), and both the zona limitans and the ventral part (floor plate) of the neural tube secrete sonic hedgehog. D, diencephalon; Mes, mesencephalon; r, rhombomere; T, telencephalon. (B, After Lumsden A, Krumlauf R: Science 274:1109-1115, 1996.)
The midbrain-hindbrain border becomes a powerful local signaling center, called the isthmic organizer. Wnt-1 is synthesized in the neural ectoderm anterior, and FGF-8 is formed posterior to the isthmic organizer (Fig. 6.4B). The transcription factors Pax-2 and Pax-5 and engrailed (En-1 and En-2) are expressed on both sides of the isthmic organizer as gradients that are crucial in organizing the development of the midbrain and the cerebellum, a hindbrain derivative.
Two additional organizing or signaling centers are established early in the formation of the forebrain region. One, the anterior neural ridge, is located at the anterior pole of the brain (see Fig. 6.4B). It is a site of sonic hedgehog and FGF-8 signaling activity and is important in organizing the formation of the telencephalon, parts of the diencephalon, the olfactory area, and the pituitary gland. A third signaling center, the zona limitans (see Fig. 6.4B), is a sonic hedgehog–secreting group of cells that organize the border between the future dorsal and ventral thalamus. Chapter 11 presents additional information on the organization and segmentation of the forebrain.
Segmentation in the Hindbrain Region
Segmentation of the hindbrain into seven rhombomeres in humans, and eight in some other animals, is the result of the expression of several categories of genes, which operate in a manner remarkably reminiscent of the way in which the early Drosophila embryo becomes subdivided into segments (see Fig. 4.1). Individual rhombomeres are initially specified through the ordered expression of unique combinations of transcription factors; this patterning is then translated into cellular behavior by the patterned expression of cell surface molecules.
After the Gbx-2–expressing area defines the rough limits of the hindbrain, several segmentation genes are involved in setting up the basic pattern of segmentation that leads to rhombomere formation. Krox 20, a zinc finger transcription factor, is expressed in and guides the formation of rhombomeres 3 and 5 (r3 and r5) (see Fig. 11.12), whereas kreisler, another transcription factor, and Hoxa-1 are also involved in the formation of r5. A decreasing gradient of retinoic acid, produced by the anterior somites, plays an important role in the formation of the posterior rhombomeres (r4 to r7). These molecules are not involved in the specification of r1 to r3, which is regulated by Gbx-2.
The Hox genes are principally involved in specifying segmental identity, but before any molecular marker of morphological segmentation exists, the previously mentioned gradient of retinoic acid stimulates the expression of Hoxa-1 and Hoxb-1. The influence of these two Hox genes and of the segmentation genes, Krox 20 and kreisler, initiates the expression of the various Hox paralogues in a highly specific sequence along the hindbrain and spinal cord (see Fig. 11.12). As seen in Chapters 11 and 14, the pattern of Hox gene expression determines the morphological identity of the cranial nerves and other pharyngeal arch derivatives that arise from specific rhombomeres. At successive times during the formation of the hindbrain, different regulatory networks controlling Hox gene expression come into play, but details of these networks are not presented in this text. The orderly expression of Hox gene paralogues extends anteriorly through r2. Hox proteins are not found in r1 largely because of the antagonistic action of FGF-8, which is produced in response to signals from the isthmic organizer at the anterior end of r1. In the absence of FGF-8, Hox proteins are expressed in r1. Another rhombencephalic protein, sprouty 2, acts as an antagonist of FGF-8, and this protein, in addition to the presence of Hoxa-2 in r2, confines FGF-8 mostly to r1 and contains the primordium of the cerebellum to the anterior part of r1.
Another family of genes, the ephrins and their receptors, determines the behavioral properties of the cells in the rhombomeres. The action of ephrins, which are expressed in even-numbered rhombomeres (2, 4, and 6), and of ephrin receptors, which are expressed in odd-numbered rhombomeres (3 and 5), seems to account for the lack of mixing behavior of cells from adjacent rhombomeres and maintains the separation of the various streams of neural crest cells that emigrate from the rhombomeres (see Fig. 12.8).
Formation and Segmentation of the Spinal Cord
As the body axis is elongating, and somites are forming, the caudalmost part of the newly induced neural plate possesses the properties of a stem cell zone (Fig. 6.5). Under the influence of FGF-8, secreted by the adjacent presomitic paraxial mesoderm, these cells, which go on to form the spinal cord, proliferate without undergoing differentiation. Some of the daughter cells are left behind by the posteriorly advancing stem cell zone. These cells fall under the influence of retinoic acid, produced by the newly formed somites, which are also being formed in a posterior direction (see Fig. 6.8). Retinoic acid stimulates these cells to differentiate into neurons. Elongation of the tail bud region comes to a close when the caudal extent of presomitic mesoderm is reduced, thus allowing the retinoic acid produced in the area to diffuse farther posteriorly and inhibit the action of FGF-8. As a result, proliferation of tail bud mesenchyme is greatly reduced, causing growth to cease.
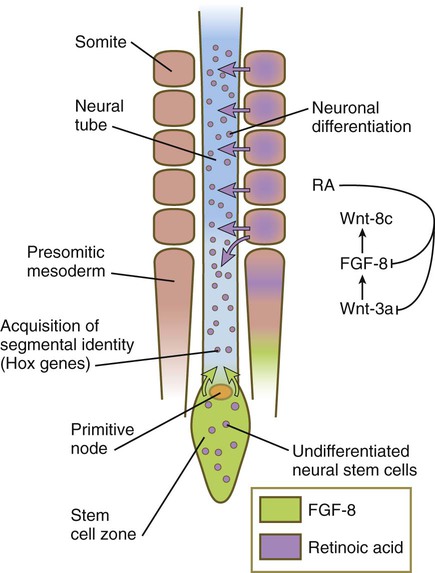
Under the influence of fibroblast growth factor-8 (FGF-8) secreted by presomitic paraxial mesoderm, cells in the most posterior region continue to proliferate, whereas retinoic acid (RA), secreted by newly formed somites, stimulates the neuronal differentiation.
The opposing actions of retinoic acid, which promotes differentiation, and FGF, which fosters proliferation at the expense of differentiation, represent a recurring theme in the development of other structures. For example, the spread of FGF-8 from the isthmic organizer (see Fig. 6.4B) antagonizes the influence of retinoic acid in r1. This permits the exuberant proliferation of the cells in this rhombomere, which is necessary for the formation of the large cerebellum from this structure. Interactions between FGF-8 and retinoic acid in the forming spinal cord and paraxial mesoderm help to set the Hox code that confers anteroposterior identity to regions of the spinal cord and the adjacent somites.
Neural Crest
As the neural tube is closing and separating from the general cutaneous ectoderm, a population of cells called the neural crest leaves the dorsal part of the neural tube and begins to spread throughout the body of the embryo (see Fig. 6.2). The neural crest produces an astonishing array of structures in the embryo (see Table 12.1), and its importance is such that the neural crest is sometimes called the fourth germ layer of the body. (The neural crest is discussed further in Chapter 12.)
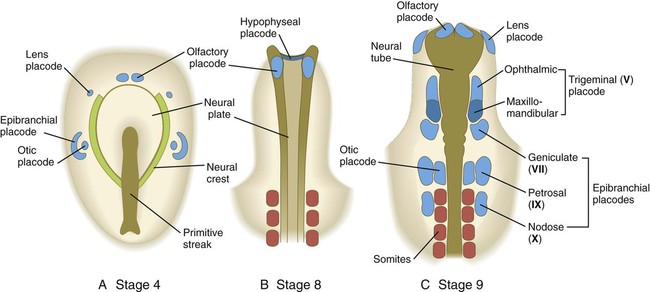
Sensory Placodes and Secondary Inductions in the Cranial Region
As the cranial region begins to take shape, several series of ectodermal placodes (thickenings) appear lateral to the neural tube and neural crest (Fig. 6.6). These placodes arise from a horseshoe-shaped preplacodal domain around the anterior neural plate that is established during the gastrulation and early neurulation periods, and the individual placodes result from a variety of secondary inductive processes between neural or mesenchymal tissues and the overlying ectoderm (see Table 13.1). In several cases, cells from the placodes and neural crest interact closely to form the sensory ganglia of cranial nerves (V, VII, IX, and X). Deficiencies of one of these two components can often be made up by an increased contribution by the other component. Further details of placodes and their developmental fate are given in Chapter 13.
Development of the Mesodermal Germ Layer
Basic Plan of the Mesodermal Layer
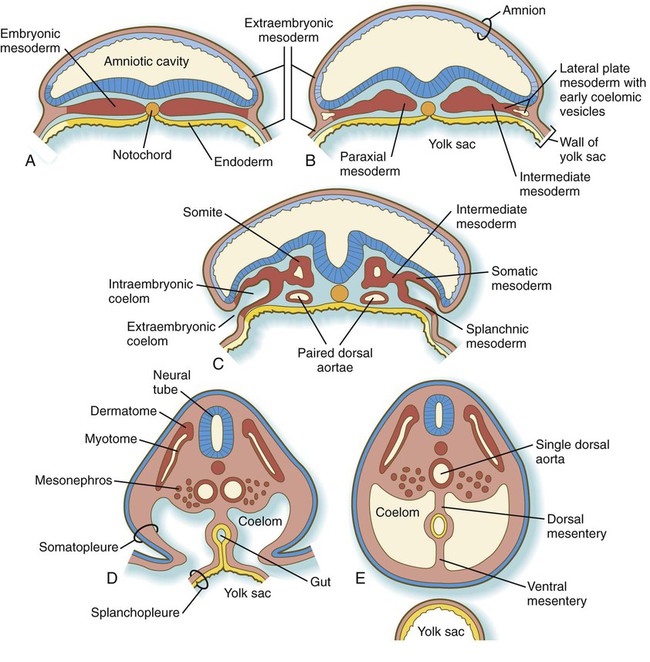
After passing through the primitive streak, the mesodermal cells spread laterally between the ectoderm and endoderm as a continuous layer of mesenchymal cells (see Fig. 5.6). Subsequently, three regions can be recognized in the mesoderm of cross-sectioned embryos (Fig. 6.7B). Nearest the neural tube is a thickened column of mesenchymal cells known as the paraxial mesoderm, or segmental plate. This tissue soon becomes organized into somites. Lateral to the paraxial mesoderm is a compact region of intermediate mesoderm, which ultimately gives rise to the urogenital system. Beyond that, the lateral plate mesoderm ultimately splits into two layers and forms the bulk of the tissues of the body wall, the wall of the digestive tract, and the limbs (see Fig. 6.27).
Paraxial Mesoderm
As the primitive node and the primitive streak regress toward the caudal end of the embryo, they leave behind the notochord and the induced neural plate. Lateral to the neural plate, the paraxial mesoderm appears to be a homogeneous strip of closely packed mesenchymal cells. However, if scanning electron micrographs of this mesoderm are examined with stereoscopic techniques, a series of regular pairs of segments can be discerned. These segments, called somitomeres, have been most studied in avian embryos, but they are also found in mammals. New pairs of somitomeres form along the primitive node as it regresses toward the caudal end of the embryo (Fig. 6.8). Not until almost 20 pairs of somitomeres have formed, and the primitive node has regressed quite far caudally, does the first pair of somites (brick-shaped masses of paraxial mesoderm) form behind the seventh pair of somitomeres.
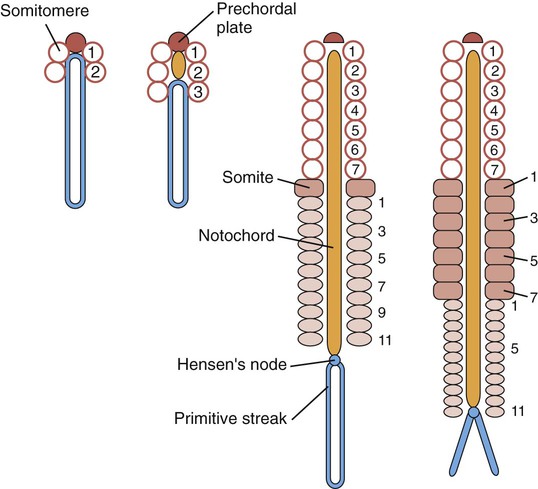
Cranial somitomeres (open circles) take shape along Hensen’s node until 7 pairs have formed. Caudal to the seventh somitomere, somites (rectangles) form from caudal somitomeres (ovals). As the most anterior of the caudal somitomeres transform into somites, additional caudal somitomeres take shape posteriorly. For a while, the equilibrium between transformation into somites anteriorly and new formation posteriorly keeps the number of caudal somitomeres at 11.
After the first pair of somites has been established (approximately 20 days after fertilization), a regular relationship develops between the regression of the primitive streak and the formation of additional somites and somitomeres. The first 7 pairs of somitomeres in the cranial region do not undergo further separation or segmentation. Cells from these somitomeres (cranial mesoderm) will form most of the skeletal musculature of the head, and they have quite different cellular and molecular properties from those derived from somites of the trunk. The first pair of somites forms at the expense of the eighth pair of somitomeres. In the types of embryos studied to date, there is a constant relationship between the caudalmost pair of definitive somites and the number of somitomeres (usually 10 to 11) that can be shown behind them. Every few hours, the pair of somitomeres located caudal to the last-formed somites becomes transformed into a new pair of somites, and a new pair of somitomeres is laid down at the caudal end of the paraxial mesoderm near the primitive node (see Fig. 6.8). As regression of the primitive streak comes to a close, the formation of paraxial mesoderm continues through the cells contributed by the tail bud. The cervical, thoracic, and lumbar vertebrae and associated structures are derived from cells migrating through the primitive streak, whereas the cellular precursors of the sacrum and coccyx come from the tail bud.
Formation of Individual Somites
The formation of individual somites from a seemingly homogeneous strip of paraxial mesoderm is a complex process that involves a variety of levels of molecular control and changes in cellular behavior within the paraxial mesoderm. Our basic understanding of somitogenesis (somite formation) comes from studies on the chick. The first significant step in somitogenesis is segmentation of the paraxial mesoderm. In contrast to segmentation in the hindbrain (see p. 95), somite formation occurs by the sequential addition of new segments in a craniocaudal sequence.
Somitogenesis involves two mechanisms in what is often referred to as a clock and wavefront model. The first step (the wavefront) is associated with the elongation of the caudal end of the body through proliferative activity of mesenchymal cells in the most posterior nonsegmented part of the primitive streak (Fig. 6.9A). Cells in this area divide actively under the influence of a high local concentration of FGF-8. More anteriorly, where the cells are older, the concentration of FGF-8 decreases as the FGF molecules become broken down over time. Conversely, the cells closer to the last-formed somite become exposed to increasing concentrations of retinoic acid, which is produced in the most posterior somites and whose action opposes that of FGF. At some point in their life history, the mesenchymal cells are exposed to a balance of FGF-8 and retinoic acid concentrations that results in their crossing a developmental threshold (the wavefront, or determination front) that prepares them for entering the process of segmentation (somite formation). This is characterized by the expression of a transcription factor, Mesp-2, which prefigures a future somite. With the continued caudal elongation of the embryo and the addition of new somites, the location of the wavefront extends caudally in the growing embryo, but it remains a constant distance from the last-formed somite pair.
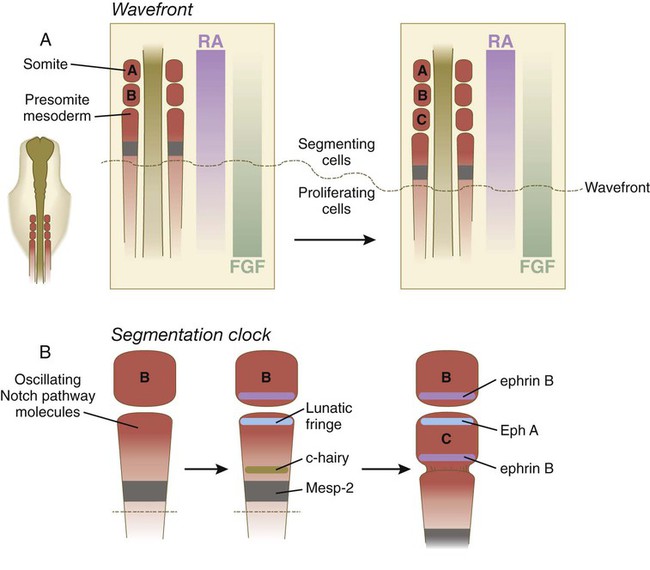
A, The wavefront, consisting of opposing gradients of retinoic acid (RA) and fibroblast growth factor-8 (FGF). B, The segmentation clock, in which oscillating molecules in the Notch pathway stimulate the expression of lunatic fringe at the anterior and c-hairy at the posterior border of a future somite. Later interactions between Eph A and ephrin B maintain the intersomitic space.
Next, the segmentation clock is initiated in those presomitic cells that have passed over the previously mentioned threshold and are expressing Mesp-2. The exact mechanism that starts the clock is still not fully defined, but many molecules in the interacting Notch, Wnt, and FGF pathways are known to be synthesized at regular periodic intervals and become localized at critical locations in the forming somite. In the chick, in which a new somite forms every 90 minutes, lunatic fringe becomes concentrated at the future anterior border of the somite, and c-hairy (a homologue of a segmentation gene in Drosophila) becomes concentrated along the future posterior border (Fig. 6.9B).
At the level of cellular behavior, cells at the anterior border of the forming somite express the ephrin receptor Eph A. Because the cells on the posterior border of the previously formed somite express the ephrin ligand ephrin B, the cells of the two adjacent somites are prevented from mixing (as is the case with adjacent rhombomeres in the developing hindbrain), and a fissure forms between the two somites. Finally, the action of Wnt-6 from the overlying ectoderm stimulates the expression of the transcription factor paraxis in the newly forming somite. This, along with the downregulation of Snail, results in the transformation of the mesenchymal cells of the anterior part of the somite, and later all the mesenchymal cells, into an epithelial cell type (Fig. 6.10A). While in the earliest stages of its formation, a somite also undergoes an internal subdivision into anterior and posterior halves. Differences in cellular properties stemming from this subdivision are of great significance in the formation of vertebrae and in guiding the migration of neural crest cells and outgrowing axons.
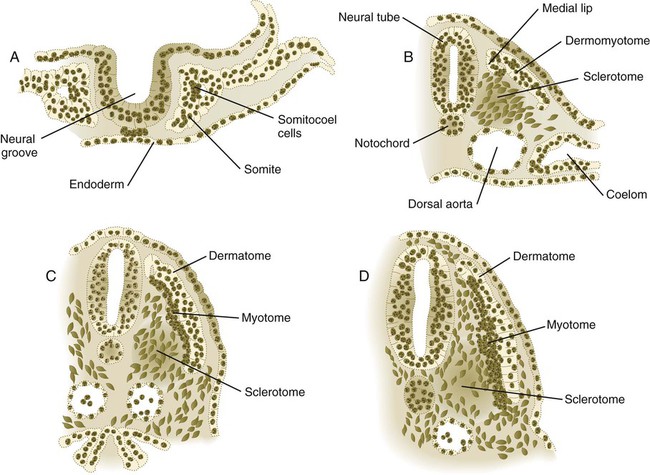
A, Epithelial stage of a somite in the preneural tube stage. B, Epitheliomesenchymal transformation of the ventromedial portion into the sclerotome. C, Appearance of a separate myotome from the original dermomyotome. D, Early stage of breakup of the epithelial dermatome into dermal fibroblasts.
The continued development of a somite involves the complete transformation of the segmented blocks of mesenchymal cells into a sphere of epithelial cells through the continued action of paraxis (see Fig. 6.10A). The cells of the epithelial somite are arranged so that their apical surfaces surround a small central lumen, the somitocoel (which contains a few core cells), and their outer basal surfaces are surrounded by a basal lamina (containing laminin, fibronectin, and other components of the extracellular matrix).
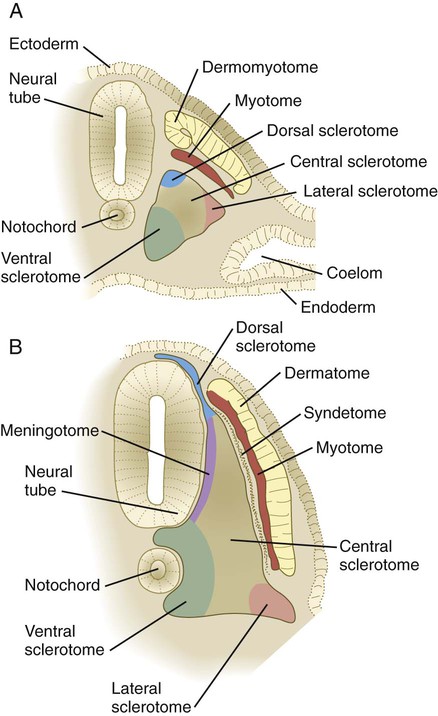
Shortly after the formation of the epithelial somite, the cells of its ventromedial wall are subjected to an inductive stimulus in the form of the signaling molecules sonic hedgehog and noggin, originating from the notochord and the ventral wall of the neural tube. The response is the expression of Pax1 and Pax9 in the ventral half of the somite, which is now called the sclerotome (Fig. 6.11). This leads to a burst of mitosis, the loss of intercellular adhesion molecules (N-cadherin), the dissolution of the basal lamina in that region, and the transformation of the epithelial cells in that region back to a mesenchymal morphology (these cells are called secondary mesenchyme). These secondary mesenchymal cells migrate or are otherwise displaced medially from the remainder of the somite (see Fig. 6.10B) and begin to produce chondroitin sulfate proteoglycans and other molecules characteristic of cartilage matrix as they aggregate around the notochord.
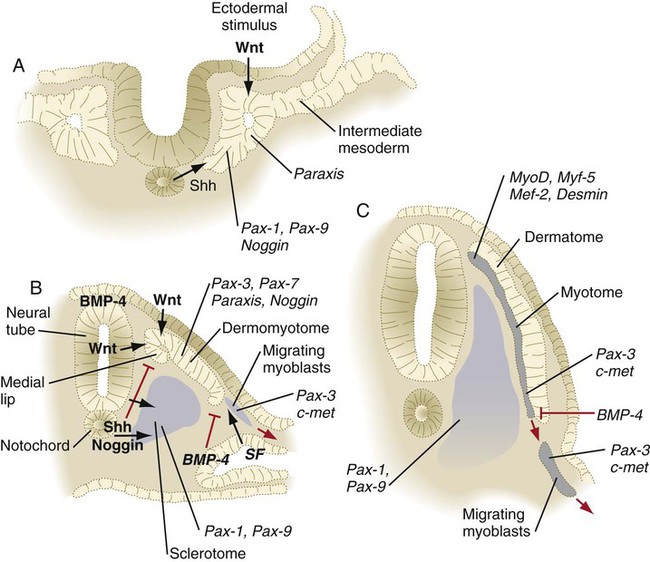
Signaling molecules are represented by black arrows. Inhibitory signals are represented by red lines. Genes expressed in responding tissues are indicated in italics. BMP, bone morphogenetic protein; SF, scatter factor; Shh, sonic hedgehog. (Adapted from Brand-Saberi B and others: Int J Dev Biol 40:411-420, 1996.)
Under the influence of secreted products of Wnt genes produced by the dorsal neural tube and the surface ectoderm, the dorsal half of the epithelial somite becomes transformed into the dermomyotome (see Fig. 6.10B) and expresses its own characteristic genes (Pax3, Pax7, paraxis). Mesenchymal cells arising from the dorsomedial and ventrolateral borders of the dermomyotome form a separate layer, the myotome, beneath the remaining somitic epithelium, which is now called the dermatome (see Fig. 6.10C). As their names imply, cells of the myotome produce muscle, and cells of the dermatome contribute to the dermis.
Organization of the Somite and the Basic Segmental Body Plan
The fates of the cells in the most recently formed somites are not fixed; if such a somite is rotated 180 degrees in a dorsoventral direction, the cells respond to their new environment and form perfectly oriented derivatives. By the time three other new somites have formed behind a somite, however, its cells have received sufficient environmental input that their developmental course is set in place. Even within the early epithelial somite (see Fig. 6.10A), the structures that form from cells of the main epithelial sectors of the somite and from the mesenchymal somitocoel cells in the center of the somite can be mapped out.
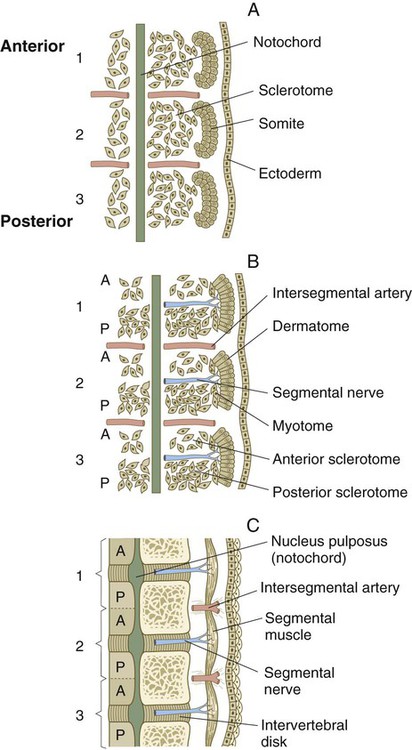
The breakup of the ventral part of the epithelial somite into mesenchyme under the influence of sonic hedgehog and noggin coming from the notochord leads to the formation of the early sclerotome. As the sclerotome develops, it can be subdivided into many compartments, each of which gives rise to specific derivatives (Box 6.1 and Fig. 6.12). Cells from several somitic compartments—the ventral, central, and dorsal—come together to form a vertebra (see Box 6.1), whereas cells from the central and lateral compartments form the ribs. Late in the development of the sclerotome, cells from its medial edge (meningotome) surround the developing spinal cord to form the meninges and their vasculature. Cells of the somitocoel (arthrotome) join with some ventral cells to form the intervertebral disks and the vertebral joint surfaces.
After the Wnt-mediated formation of the dermomyotome, cells in its dorsomedial sector find themselves exposed to a balance of sonic hedgehog signaling from the notochord and Wnt signaling from the dorsal neural tube and overlying surface ectoderm that leads them to becoming committed to the myogenic lineage. Conditions for myogenesis here are set by the inhibition of ectodermally produced bone morphogenetic protein-4 (BMP-4) (which in itself inhibits myogenesis) by noggin. These cells then stop producing Pax-3 and Pax-7 and begin to express myogenic regulatory molecules, such as MyoD and Myf-5 (see p. 184). Ultimately, these cells form the intrinsic back (epaxial) musculature.
In the anterior and posterior borders of the somite, FGF signals from the developed myotome induce a layer of cells along the lateral edge of the sclerotome to produce scleraxis, a transcription factor found in tendons. These cells form a discrete layer, called the syndetome, and they represent the precursors of the tendons that connect the epaxial muscles to their skeletal origins and insertions. Research with cell markers has shown that almost all components of the somites are able to give rise to blood vessels that nourish the various structures derived from the somitic mesoderm. Box 6.2 lists the types of mature cells that are derived from somites.
Within a single somite, cells of the posterior sclerotome multiply at a greater rate than do those of the anterior part, and the result is higher cellular density in the posterior sclerotome (Fig. 6.13B). Properties of these cells and their extracellular matrix (see p. 255) do not permit the passage of either outgrowing nerve fibers or neural crest cells, which instead pass through the anterior sclerotome. Because of the outgrowing neural structures that either pass through or are derived from the anterior sclerotome, it has sometimes been called the neurotome.
As the cells of the sclerotome disperse around the notochord, cells of the anterior half of one somite aggregate with cells of the posterior half of the more cranial somite. Ultimately, this aggregate forms a single vertebra. Such an arrangement, which depends on interactions with the neural tube, places the bony vertebrae out of phase with the myotomally derived segmental muscles of the trunk (Fig. 6.13C). This structure allows the contracting segmental muscles to move the vertebral column laterally. The relationship between the anterior half of one somite and the adjoining posterior half of its neighboring somite is reminiscent of the parasegments of Drosophila (similarly arranged subdivisions of the segments into two parts), but whether they are functionally similar in terms of genetic control is undetermined.
Intermediate Mesoderm
Connecting the paraxial mesoderm and the lateral plate mesoderm in the early embryo is a small cord of cells called the intermediate mesoderm, which runs along the entire length of the trunk (see Fig. 6.7C). How the intermediate mesoderm forms remains unclear. It appears to arise as a response of early mesoderm to BMP, secreted by lateral ectoderm, and activin and other signals emanating from the paraxial mesoderm. The response to these signals is the expression of Pax-2 within what becomes the intermediate mesoderm. The cranial and caudal extent of the intermediate mesoderm is defined by expression of members of the Hox-4 paralogue cranially and Hox-11 caudally. In experiments involving a cranial shift of expression of Hox-4, the cranial border of the intermediate mesoderm is correspondingly shifted toward the head. The intermediate mesoderm is the precursor of the urogenital system. The earliest signs of differentiation of the intermediate mesoderm are in the most cranial regions, where vestiges of the earliest form of the kidney, the pronephros, briefly appear. In the lateral region of the intermediate mesoderm, a longitudinal pronephric duct appears on each side of the embryo. The pronephric duct is important in organizing the development of much of the adult urogenital system, which forms largely from cells of the caudal portions of the intermediate mesoderm (see Chapter 16).
Lateral Plate Mesoderm
The lateral plate mesoderm soon divides into two layers as the result of the formation and coalescence of coelomic (body cavity) spaces within it (see Fig. 6.7B and C). The dorsal layer, which is closely associated with the ectoderm, is called somatic mesoderm, and the combination of somatic mesoderm and ectoderm is called the somatopleure (see Fig. 6.7D). The ventral layer, called splanchnic mesoderm, is closely associated with the endoderm and is specified by the transcription factor Foxf-1. The combined endoderm and splanchnic mesoderm is called the splanchnopleure. The intraembryonic somatic and splanchnic mesodermal layers are continuous with the layers of extraembryonic mesoderm that line the amnion and yolk sac.
Formation of the Coelom
As the embryo undergoes lateral folding, the small coelomic vesicles that formed within the lateral plate mesoderm coalesce into the coelomic cavity (see Fig. 6.7). Initially, the intraembryonic coelom is continuous with the extraembryonic coelom, but as folding is completed in a given segment of the embryo, the two coelomic spaces are separated. The last region of the embryo to undergo complete lateral folding is the area occupied by the yolk sac. In this area, small channels connecting the intraembryonic and extraembryonic coeloms persist until the ventral body wall is completely sealed.
In the cylindrical embryo, the somatic mesoderm constitutes the lateral and ventral body wall, and the splanchnic mesoderm forms the mesentery and the wall of the digestive tract. The somatic mesoderm of the lateral plate also forms the mesenchyme of the limb buds, which begin to appear late in the fourth week of pregnancy (see Fig. 10.1).
Extraembryonic Mesoderm and the Body Stalk
The thin layers of extraembryonic mesoderm that line the ectodermal lining of the amnion and the endodermal lining of the yolk sac are continuous with the intraembryonic somatic and splanchnic mesoderm (see Fig. 6.7A and B). The posterior end of the embryo is connected with the trophoblastic tissues (future placenta) by the mesodermal body stalk (see Fig. 7.1). As the embryo grows and a circulatory system becomes functional, blood vessels from the embryo grow through the body stalk to supply the placenta, and the body stalk itself becomes better defined as the umbilical cord. The extraembryonic mesoderm that lines the inner surface of the cytotrophoblast ultimately becomes the mesenchymal component of the placenta.
Early Stages in the Formation of the Circulatory System
Heart and Great Vessels
The earliest development of the circulatory system consists of the migration of heart-forming cells arising in the epiblast through the primitive streak in a well-defined anteroposterior order. According to a commonly accepted model of heart development, the cells passing through the streak closest to the primitive node form the outflow tract, the cells passing through the midstreak form the ventricles, and the cells that form the atria enter the streak most posteriorly (Fig. 6.14A). After leaving the primitive streak, the precardiac cells, which are associated with endodermal cells as a splanchnic mesoderm, become arranged in the same anteroposterior order in a U-shaped region of cardiogenic mesoderm, called the cardiac crescent (Fig. 6.14B). Following an inductive influence (involving members of the BMP and FGF families) by the endoderm (likely the anterior visceral endoderm, which also serves as the head organizer in mammals), the cells in this field are committed to the heart-forming pathway. In response, these cells express genes for several sets of transcription factors (Nkx2-5, MEF2, and GATA4) that are important in early heart development. From the cardiogenic mesoderm, the heart and great vessels form from bilaterally paired tubes that fuse in the midline beneath the foregut to produce a single tube (Fig. 6.15; see Fig. 6.14C).
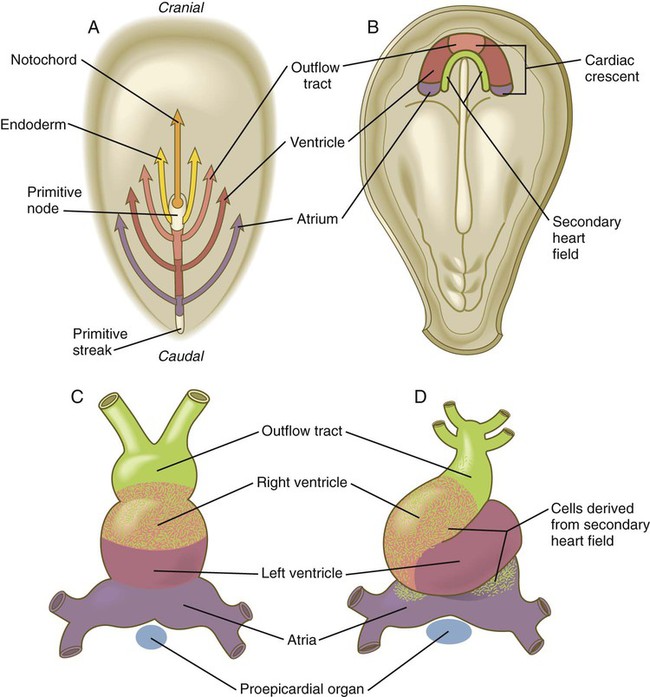
A, Topographically precise movements of cardiogenic cells through the primitive streak. B, Horseshoe-shaped distribution of cardiogenic cells after their migration through the primitive streak. At this stage, the cardiogenic area is anterior to the rostralmost extent of the neural plate. C, The straight tubular heart. D, Ventral view of the S-shaped heart.
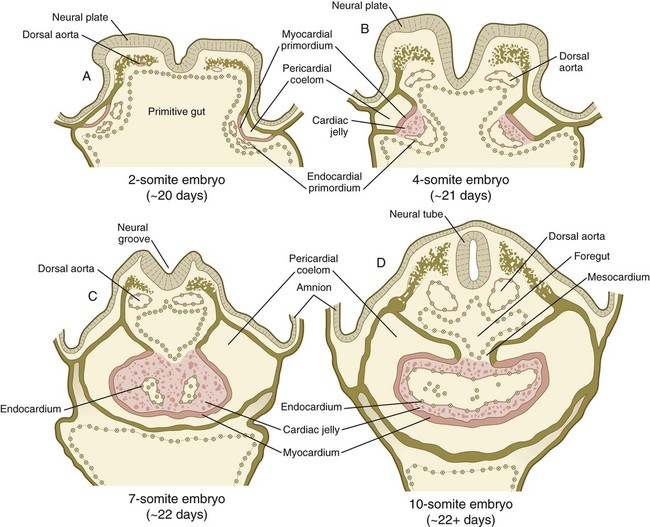
A, Two-somite embryo. B, Four-somite embryo. C, Seven-somite embryo. D, Ten-somite embryo.
A secondary (anterior) heart field has been described in chick and mouse embryos. Located in the splanchnic mesoderm on the posteromedial side of the cardiac crescent (see Fig. 6.14B), cells from the anterior part of the second heart field form most of the outflow tract and the right ventricle, and those from the posterior part of this field contribute to the formation of the atria (see Fig. 6.14D). In contrast, cells derived from the cardiac crescent form the left ventricle and most of the atria and make minor contributions to the outflow tract and right ventricle.
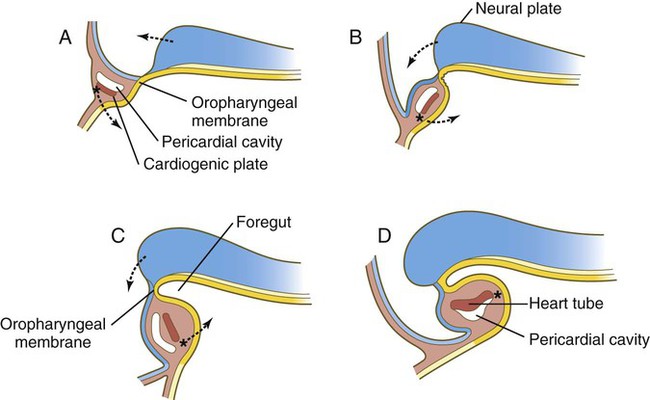
In human embryos, the earliest recognizable precardiac mesoderm is a crescent-shaped zone of thickened mesoderm rostral to the embryonic disk of the gastrulating embryo early in the third week (see Fig. 6.14B). As the mesoderm begins to split into the splanchnic and somatic layers, a cardiogenic plate is recognizable in the splanchnic mesoderm rostral to the oropharyngeal membrane (Fig. 6.16A). In this area, the space between the two layers of mesoderm is the forerunner of the pericardial cavity. The main layer of splanchnic mesoderm in the precardiac region thickens to become the myocardial primordium. Between this structure and the endoderm of the primitive gut, isolated mesodermal vesicles appear, which soon fuse to form the tubular endocardial primordia (see Fig. 6.15A and B). The endocardial primordia ultimately fuse and become the inner lining of the heart.
As the head of the embryo takes shape by lateral and ventral folding, the bilateral cardiac primordia come together in the midline ventral to the gut and fuse to form a primitive single tubular heart. This structure consists of an inner endocardial lining surrounded by a loose layer of specialized extracellular matrix that has historically been called cardiac jelly (see Fig. 6.15C). Outside the cardiac jelly is the myocardium, which ultimately forms the muscular part of the heart. The outer lining of the heart, called the epicardium, and fibroblasts within the heart muscles are derived from the proepicardial primordium, which is located near the dorsal mesocardium (see Figs. 6.14C and D and 6.18). Cells migrating from the proepicardium cover the surface of the tubular heart. The entire tubular heart is located in the space known as the pericardial coelom. Shortly after the single tubular heart is formed, it begins to form a characteristic S-shaped loop that presages its eventual organization into the configuration of the adult heart (Fig. 6.17). (Additional cellular and molecular aspects of early cardiogenesis are discussed in Chapter 17.)
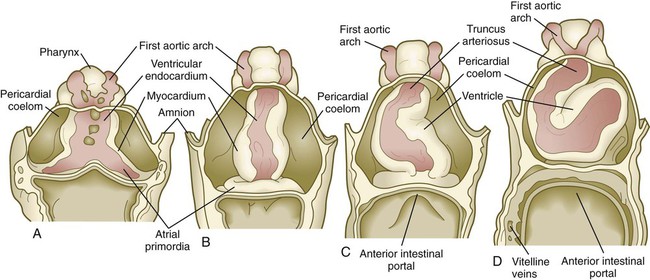
A, Four-somite embryo. B, Eight-somite embryo. C, Ten- to 11-somite embryo. D, Twelve-somite embryo.
The heart is formed from a variety of cell lineages. Within the cardiogenic mesoderm are cells that express N-cadherin and cells that do not (Fig. 6.18A). Depending on their location within the cardiogenic mesoderm, N-cadherin–positive cells go on to form either atrial or ventricular myocytes, whereas N-cadherin–negative cells form the endocardial lining and, later, cells of the endocardial cushions (see p. 428). Cells of the cardiac conduction system are derived from modified atrial and ventricular cardiac myocytes.
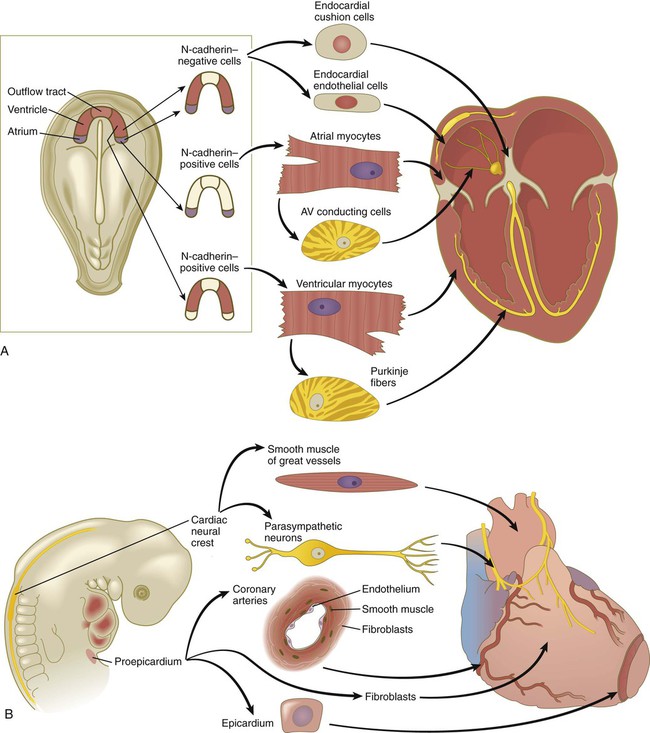
A, Derivatives of cardiogenic mesodermal cells. B, Cellular contributions of the cardiac neural crest and proepicardium to the heart. AV, atrioventricular. (After Mikawa T: In Harvey RP, Rosenthal N, eds: Heart development, San Diego, 1999, Academic Press.)
The early heart does not form in isolation. At its caudal end, the endocardial tubes do not fuse, but rather extend toward the posterior part of the body as the venous inflow tract of the heart (see Fig. 6.17A). Similarly, the endothelial tube leading out from the heart at its cranial end produces vascular arches that loop around the pharynx. Migrating neural crest cells form much of the walls of these vessels. By 22 or 23 days after fertilization, differentiation of cardiac muscle cells in the myocardium is sufficiently advanced to allow the heart to begin beating.
Blood and Blood Vessels
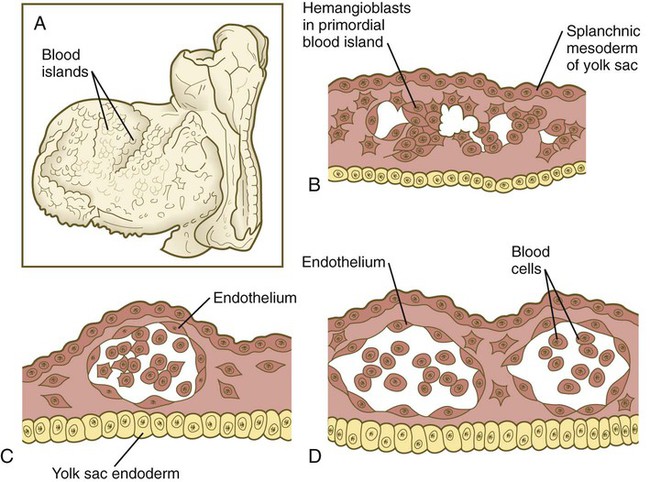
The formation of blood and blood vessels begins in the mesodermal wall of the yolk sac and in the wall of the chorion outside the embryo proper. Stimulated by an inductive interaction with the endoderm of the yolk sac and possibly also with the visceral endoderm, many small blood islands, consisting of stem cells called hemangioblasts, appear in the extraembryonic splanchnic mesoderm of the yolk sac (Fig. 6.19). Experimental evidence suggests that the inductive signal from the yolk sac endoderm is the signaling molecule Indian hedgehog. The yolk sac mesoderm responds to this signal by producing BMP-4, which feeds back onto itself. In a yet undefined manner, this interaction triggers the formation of blood islands within the mesoderm of the yolk sac. Within the blood islands, the central cells are blood-forming cells (hemocytoblasts), whereas the cells on the outside acquire the characteristics of endothelial lining cells, which form the inner walls of blood vessels. As the vesicular blood islands in the wall of the yolk sac fuse, they form primitive vascular channels that extend toward the body of the embryo. Connections are made with the endothelial tubes associated with the tubular heart and major vessels, and the primitive plan of the circulatory system begins to take shape.
Development of the Endodermal Germ Layer
Starting at the time of gastrulation, the developing gut becomes specified into successively more discrete anteroposterior regions. The formation of endoderm as a germ layer depends on nodal signaling during gastrulation. In the high nodal environment close to the primitive node, the endodermal cells assume an anterior fate, whereas more posteriorly, the newly formed endodermal cells, which are exposed to lower levels of nodal and the presence of FGF-4, are specified to become more posterior structures as development proceeds. The posterior gut responds by expressing the transcription factor Cdx-2, which both promotes hindgut identity and suppresses the foregut differentiation program. Within the anterior domain, the gut expresses Hex, Sox-2, and Foxa-2. These early subdivisions of the gut set the stage for the more finely graded partitioning of the gut by the Hox genes (see Fig. 15.2) and the later induction of gut derivatives, such as the liver, pancreas, and lungs.
Development of the endodermal germ layer continues with the transformation of the flat intraembryonic endodermal sheet into a tubular gut as a result of the lateral folding of the embryonic body and the ventral bending of the cranial and caudal ends of the embryo into a roughly C-shaped structure (Fig. 6.20; see Fig. 6.7). A major morphological consequence of these folding processes is the sharp delineation of the yolk sac from the digestive tube.
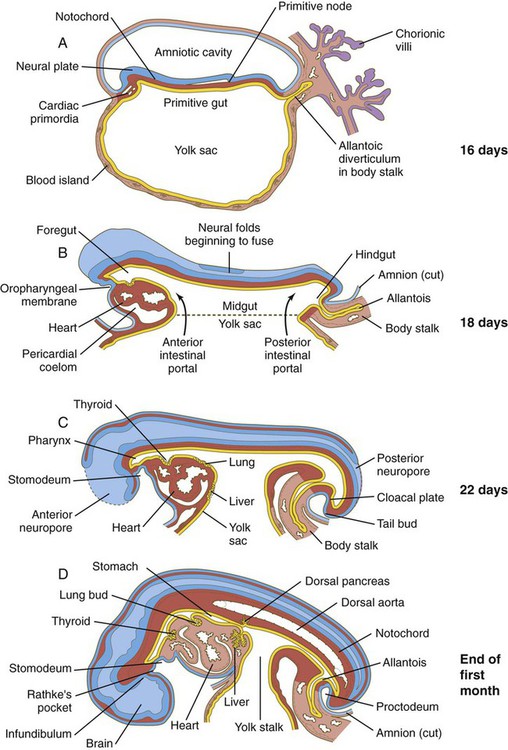
A, At 16 days. B, At 18 days. C, At 22 days. D, At the end of the first month. (After Patten. From Carlson BM: Patten’s foundations of embryology, ed 6, New York, 1996, McGraw-Hill.)
Early in the third week, when the three embryonic germ layers are first laid down, the intraembryonic endoderm constitutes the roof of the roughly spherical yolk sac (see Fig. 6.20). Expansion of either end of the neural plate, particularly the tremendous growth of the future brain region, results in the formation of the head fold and tail fold along the sagittal plane of the embryo. This process, along with concomitant lateral folding, results in the formation of the beginnings of the tubular foregut and hindgut. This process also begins to delineate the yolk sac from the gut proper.
The sequence of steps in the formation of the tubular gut can be likened to a purse string constricting the ventral region of the embryo, although the actual mechanism is more related to the overall growth of the embryo than to a real constriction. The region of the imaginary purse string becomes the yolk stalk (also called the omphalomesenteric or vitelline duct), with the embryonic gut above and the yolk sac below it (see Figs. 6.7D and 6.20D). The portion of the gut that still opens into the yolk sac is called the midgut, and the points of transition between the open-floored midgut and the tubular anterior and posterior regions of the gut are called the anterior and posterior intestinal portals (see Fig. 6.20B).
The endodermal edges of the anterior and posterior intestinal portals are also sites of expression of the signaling molecule sonic hedgehog. In the posterior intestinal portal, the appearance of sonic hedgehog in the endoderm is followed shortly by the expression of another signaling molecule, BMP-4. This is followed by the appearance of a gradient of mesodermal expression of paralogous groups 9 through 13 of the Hox genes (see Fig. 4.5 for an illustration of paralogous groups), with Hoxa-d9 being expressed most cranially and Hoxa-d13 being expressed most caudally, near the cloaca. This distribution of Hox gene expression associated with hindgut formation is reminiscent of that already described for the early hindbrain region (see p. 96).
In some cases, normal development of the gut and its related structures can proceed only when sonic hedgehog signaling is repressed. As discussed further on page 355, the dorsal pancreatic bud (see Fig. 6.20D) is induced by the notochord. A direct result of this induction is repression of sonic hedgehog signaling within the gut endoderm in the area of the dorsal pancreas. This repression allows the expression of the genes associated with the formation of the pancreas. At roughly the same anteroposterior level, but on the ventral side of the gut where the liver will form, hepatic endoderm expresses albumin in response to signals from the adjacent precardiac mesoderm.
The anterior end of the foregut remains temporarily sealed off by an ectodermal-endodermal bilayer called the oropharyngeal membrane (see Fig. 6.20B). This membrane separates the future mouth (stomodeum), which is lined by ectoderm, from the pharynx, the endodermally lined anterior part of the foregut. Without an intervening layer of mesoderm, this bilayer of two epithelial sheets is inherently unstable and eventually breaks down. As discussed in Chapter 14, the endoderm of the anterior foregut acts as a powerful signaling center. Pharyngeal gut-derived molecular signals guide the formation and specific morphology of the pharyngeal arches.
The rapid bulging of the cephalic region, in conjunction with the constriction of the ventral region, has a major topographic effect on the rapidly developing cardiac region. In the early embryo, the cardiac primordia are located cephalic to the primitive gut. The forces that shape the tubular foregut cause the bilateral cardiac primordia to turn 180 degrees in the craniocaudal direction while the paired cardiac tubes are moving toward one another in the ventral midline (see Fig. 6.16).
In the region of the hindgut, the expansion of the embryo’s body is not as prominent as it is in the cranial end, but nevertheless a less exaggerated ventral folding also occurs in that region. Even as the earliest signs of the tail fold are taking shape, a tubular evagination of the hindgut extends into the mesoderm of the body stalk. This evagination is called the allantois (see Fig. 6.20B). In most mammals and birds, the allantois represents a major structural adaptation for the exchange of gases and the removal of urinary wastes. Because of the efficiency of the placenta, however, the allantois never becomes a prominent structure in the human embryo. Nevertheless, because of the blood vessels that become associated with it, the allantois remains a vital part of the link between the embryo and the mother (see Chapter 7).
Caudal to the allantois is another ectodermal-endodermal bilayer called the cloacal plate, or proctodeal membrane (see Fig. 6.20C). This membrane, which ultimately breaks down, covers the cloaca, which in the early embryo represents a common outlet for the digestive and the urogenital systems. The shallow depression outside the proctodeal membrane is called the proctodeum.
As the gut becomes increasingly tubular, a series of local inductive interactions between the epithelium of the digestive tract and the surrounding mesenchyme initiates the formation of most of the major digestive and endocrine glands (e.g., thyroid gland, salivary glands, pancreas), the respiratory system, and the liver. In the region of the stomodeum, an induction between forebrain and stomodeal ectoderm initiates the formation of the anterior pituitary gland. (Further development of these organs is discussed in Chapters 14 and 15.)
Basic Structure of a 4-Week-Old Embryo
Gross Appearance
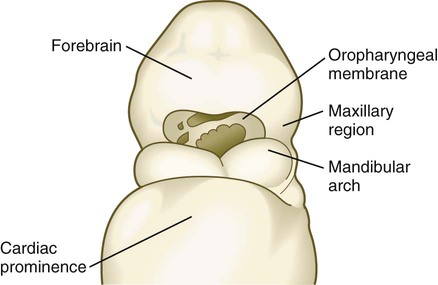
By the end of the fourth week of pregnancy, the embryo, which is still only about 4 mm long, has established the rudiments of most of the major organ systems except for the limbs (which are still absent) and the urogenital system (which has developed only the earliest traces of the embryonic kidneys). Externally, the embryo is C-shaped, with a prominent row of somites situated along either side of the neural tube (Figs. 6.21 and 6.22). Except for the rudiments of the eyes and ears and the oropharyngeal membrane, which is beginning to break down (Fig. 6.23), the head is relatively featureless. In the cervical region, pharyngeal arches are prominent (Fig. 6.24; see Fig. 6.21B and C). The body stalk still occupies a significant part of the ventral body wall, and cephalic to the body stalk, the heart and liver make prominent bulges in the contours of the ventral body wall. Posterior to the body stalk, the body tapers to a spiraled tail, which is prominent in embryos of this age.
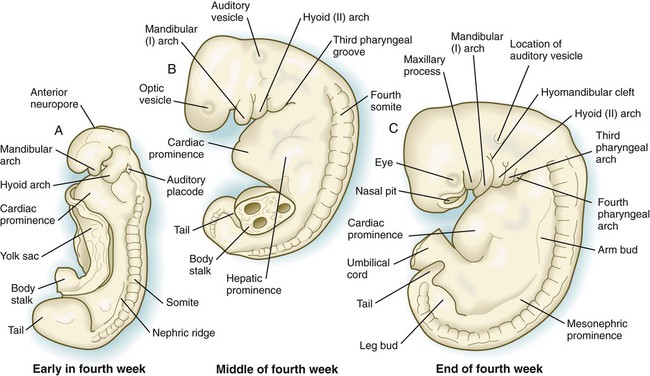
A, Early in the fourth week. B, Middle of the fourth week. C, End of the fourth week.
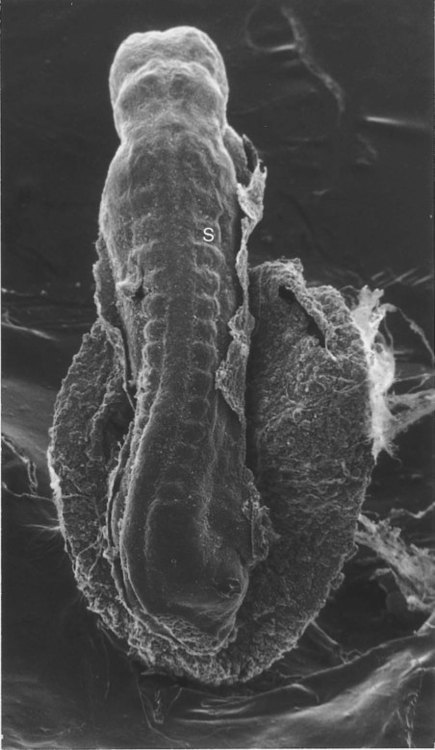
S, Somite. (From Jirásek JE: Atlas of human prenatal morphogenesis, Amsterdam, 1983, Martinus Nijhoff.)
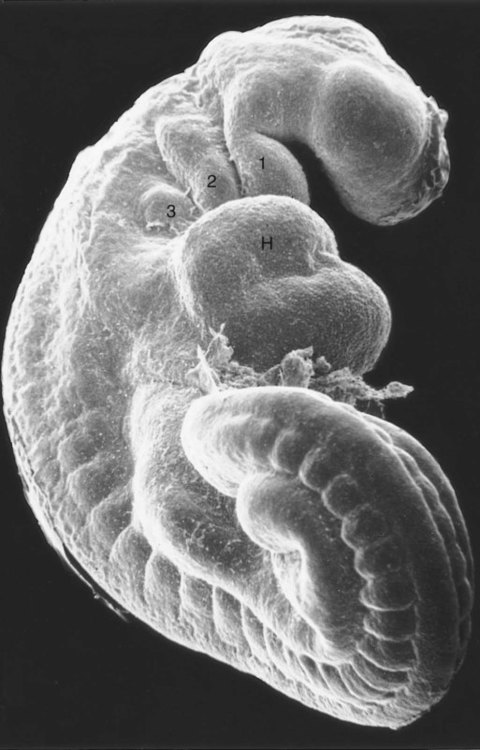
H, heart. Numbers 1 to 3 indicate pharyngeal arches. (From Jirásek JE: Atlas of human prenatal morphogenesis, Amsterdam, 1983, Martinus Nijhoff.)
Another prominent but little understood feature of embryos of this age is a ring of thickened ectoderm, called the wolffian ridge, which encircles the lateral aspect of the body (Fig. 6.25). Its function is not well understood, but it spans the primordia of many structures (e.g., nose, eye, inner ear, pharyngeal arches, limbs) that require tissue interactions for their early development. The wolffian ridge is marked molecularly by the expression of members of the Wnt signaling pathway. What role the thickened ectoderm plays in early organogenesis remains to be determined.
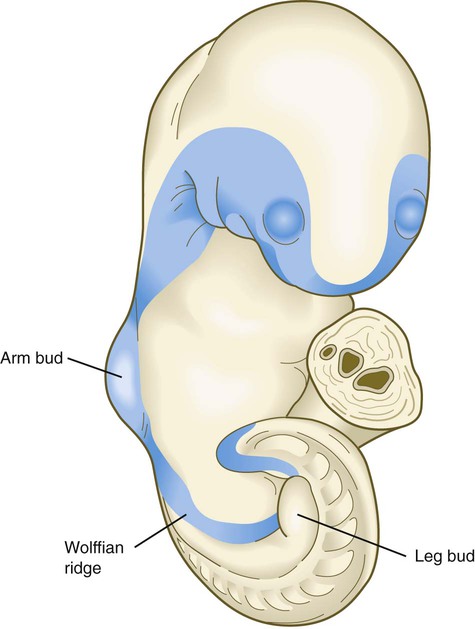
The portion of the ring between the upper and lower limb buds is the wolffian ridge. (Based by O’Rahilly R, Gardner E: Anat Embryol 148:1-23, 1975.)
Circulatory System
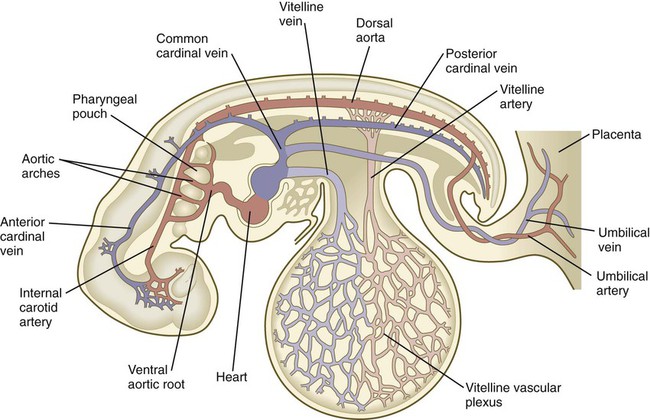
At 4 weeks of age, the embryo has a functioning two-chamber heart and a blood vascular system that consists of three separate circulatory arcs (Fig. 6.26). The first, the intraembryonic circulatory arc, is organized in a manner similar to that of a fish. A ventral aortic outflow tract from the heart splits into a series of aortic arches passing around the pharynx through the pharyngeal arches and then collecting into a cephalically paired dorsal aorta that distributes blood throughout the body. A system of cardinal veins collects the blood and returns it to the heart via a common inflow tract.
The second arc, commonly called the vitelline or omphalomesenteric arc, is principally an extraembryonic circulatory loop that supplies the yolk sac (see Fig. 6.26). The third circulatory arc, also extraembryonic, consists of the vessels associated with the allantois. In humans, this third arc consists of the umbilical vessels, which course through the body stalk and spread in an elaborate network in the placenta and chorionic tissues. This set of vessels represents the real lifeline between the embryo and mother. Although the two extraembryonic circulatory loops do not persist as such after birth, the intraembryonic portions of these arcs are retained as vessels or ligaments in the adult body.
Derivatives of the Embryonic Germ Layers
By the end of the fourth week of development, primordia of most of the major structures and organs in the body have been laid down, many of them the result of local inductive interactions. Each of the embryonic germ layers contributes to the formation of many of these structures. Figure 6.27 summarizes the germ layer origins of most of the major structures in the embryonic body. This figure is designed to be a guide that allows specific structures that are being studied to be viewed in the context of the whole body, rather than something that should be memorized at this stage. Students have found that a flow chart such as this is useful for review at the end of an embryology course.
Summary
 The response of dorsal ectodermal cells to primary induction is to thicken, thus forming a neural plate. Neurulation consists of lateral folding of the neural plate at hinge points to form a neural groove. Opposing sides of the thickened epithelium of the neural groove join to form a neural tube. The temporarily unclosed cranial and caudal ends of the neural tube are the anterior and posterior neuropores.
The response of dorsal ectodermal cells to primary induction is to thicken, thus forming a neural plate. Neurulation consists of lateral folding of the neural plate at hinge points to form a neural groove. Opposing sides of the thickened epithelium of the neural groove join to form a neural tube. The temporarily unclosed cranial and caudal ends of the neural tube are the anterior and posterior neuropores.
 Cranially, the neural tube subdivides into a primitive three-part brain consisting of the prosencephalon, mesencephalon, and rhombencephalon. The caudal part of the early brain also becomes subdivided into segments called neuromeres, of which the rhombomeres are most prominent. Specific homeobox genes are expressed in a regular order in the rhombomeres. A signaling center, the isthmic organizer, located at the midbrain and hindbrain junction acts via the production of Wnt-1 anteriorly and FGF-8 posteriorly.
Cranially, the neural tube subdivides into a primitive three-part brain consisting of the prosencephalon, mesencephalon, and rhombencephalon. The caudal part of the early brain also becomes subdivided into segments called neuromeres, of which the rhombomeres are most prominent. Specific homeobox genes are expressed in a regular order in the rhombomeres. A signaling center, the isthmic organizer, located at the midbrain and hindbrain junction acts via the production of Wnt-1 anteriorly and FGF-8 posteriorly.
 As the neural tube closes, neural crest cells emigrate from the neural epithelium and spread through the body along well-defined paths. Secondary inductions acting on ectoderm in the cranial region result in the formation of several series of ectodermal placodes, which are the precursors of sense organs and sensory ganglia of cranial nerves.
As the neural tube closes, neural crest cells emigrate from the neural epithelium and spread through the body along well-defined paths. Secondary inductions acting on ectoderm in the cranial region result in the formation of several series of ectodermal placodes, which are the precursors of sense organs and sensory ganglia of cranial nerves.
 The embryonic mesoderm is subdivided into three craniocaudal columns: the paraxial, intermediate, and lateral plate mesoderm. Paraxial mesoderm is the precursor tissue to the paired somites and somitomeres. Segmentation of the paraxial mesoderm into somites occurs through the action of a clock mechanism that leads to the periodic expression of c-hairy and a variety of downstream molecules. As the result of a complex series of inductive interactions involving numerous signaling molecules, the epithelial somites become subdivided into sclerotomes (precursors of vertebral bodies) and dermomyotomes, which form dermatomes (dermal precursors) and myotomes (precursors of axial muscles). In further subdivisions, precursor cells of limb muscles are found in the lateral halves of the somites, and precursor cells of axial muscles are found in the medial halves. The posterior half of one sclerotome joins with the anterior half of the next caudal somite to form a single vertebral body.
The embryonic mesoderm is subdivided into three craniocaudal columns: the paraxial, intermediate, and lateral plate mesoderm. Paraxial mesoderm is the precursor tissue to the paired somites and somitomeres. Segmentation of the paraxial mesoderm into somites occurs through the action of a clock mechanism that leads to the periodic expression of c-hairy and a variety of downstream molecules. As the result of a complex series of inductive interactions involving numerous signaling molecules, the epithelial somites become subdivided into sclerotomes (precursors of vertebral bodies) and dermomyotomes, which form dermatomes (dermal precursors) and myotomes (precursors of axial muscles). In further subdivisions, precursor cells of limb muscles are found in the lateral halves of the somites, and precursor cells of axial muscles are found in the medial halves. The posterior half of one sclerotome joins with the anterior half of the next caudal somite to form a single vertebral body.
 Intermediate mesoderm forms the organs of the urogenital system. The lateral plate mesoderm splits to form somatic mesoderm (associated with ectoderm) and splanchnic mesoderm (associated with endoderm). The space between becomes the coelom. The limb bud arises from lateral plate mesoderm, and extraembryonic mesoderm forms the body stalk.
Intermediate mesoderm forms the organs of the urogenital system. The lateral plate mesoderm splits to form somatic mesoderm (associated with ectoderm) and splanchnic mesoderm (associated with endoderm). The space between becomes the coelom. The limb bud arises from lateral plate mesoderm, and extraembryonic mesoderm forms the body stalk.
 Blood cells and blood vessels form initially from blood islands located in the mesodermal wall of the yolk sac. The heart, originating from a horseshoe-shaped region of splanchnic mesoderm anterior to the oropharyngeal membrane, forms two tubes on either side of the foregut. As the foregut takes shape, the two cardiac tubes come together to form a single tubular heart, which begins to beat around 22 days after fertilization.
Blood cells and blood vessels form initially from blood islands located in the mesodermal wall of the yolk sac. The heart, originating from a horseshoe-shaped region of splanchnic mesoderm anterior to the oropharyngeal membrane, forms two tubes on either side of the foregut. As the foregut takes shape, the two cardiac tubes come together to form a single tubular heart, which begins to beat around 22 days after fertilization.
 The embryonic endoderm initially consists of the roof of the yolk sac. As the embryo undergoes lateral folding, the endodermal gut forms cranial and caudal tubes (foregut and hindgut), but the middle region (midgut) remains open to the yolk sac ventrally. Regional specification of the gut begins with sonic hedgehog signals from the endoderm of the intestinal portals, which are translated to gradients of Hox gene expression in the neighboring mesoderm. As the tubular gut continues to take shape, the connection to the yolk sac becomes attenuated to form the yolk stalk. The future mouth (stomodeum) is separated from the foregut by an oropharyngeal membrane, and the hindgut is separated from the proctodeum by the cloacal plate. A ventral evagination from the hindgut is the allantois, which in many animals is an adaptation for removing urinary and respiratory wastes.
The embryonic endoderm initially consists of the roof of the yolk sac. As the embryo undergoes lateral folding, the endodermal gut forms cranial and caudal tubes (foregut and hindgut), but the middle region (midgut) remains open to the yolk sac ventrally. Regional specification of the gut begins with sonic hedgehog signals from the endoderm of the intestinal portals, which are translated to gradients of Hox gene expression in the neighboring mesoderm. As the tubular gut continues to take shape, the connection to the yolk sac becomes attenuated to form the yolk stalk. The future mouth (stomodeum) is separated from the foregut by an oropharyngeal membrane, and the hindgut is separated from the proctodeum by the cloacal plate. A ventral evagination from the hindgut is the allantois, which in many animals is an adaptation for removing urinary and respiratory wastes.
 In a 4-week-old embryo, the circulatory system includes a functioning two-chamber heart and a blood vascular system that consists of three circulatory arcs. In addition to the intraembryonic circulation, the extraembryonic vitelline circulatory arc, which supplies the yolk sac, and the umbilical circulation, which is associated with the allantois and supplies the placenta, are present.
In a 4-week-old embryo, the circulatory system includes a functioning two-chamber heart and a blood vascular system that consists of three circulatory arcs. In addition to the intraembryonic circulation, the extraembryonic vitelline circulatory arc, which supplies the yolk sac, and the umbilical circulation, which is associated with the allantois and supplies the placenta, are present.