Erythrocyte Production and Destruction
After completion of this chapter, the reader will be able to:
1. List and describe the erythroid precursors in order of maturity, including the morphologic characteristics, cellular activities, normal location, and length of time in the stage for each.
2. Correlate the erythroblast, normoblast, and rubriblast nomenclatures for red blood cell (RBC) stages.
3. Name the stage of erythroid development when given a written description of the morphology of a cell in a Wright-stained bone marrow smear.
4. List and compare the cellular organelles of immature and mature erythrocytes and describe their specific functions.
5. Name the erythrocyte progenitors and distinguish them from precursors.
6. Explain the nucleus-to-cytoplasm (N : C) ratio. Describe the appearance of a cell when given the N : C ratio or estimate the N : C ratio from the appearance of a cell.
7. Explain how reticulocytes can be recognized in a Wright-stained peripheral blood film.
8. Define and differentiate the terms polychromasia, diffuse basophilia, punctate basophilia, and basophilic stippling.
9. Discuss the differences between the reticulum of reticulocytes and punctate basophilic stippling in composition and conditions for viewing.
10. Define and differentiate erythron and RBC mass.
11. Explain how hypoxia stimulates RBC production.
12. Describe the general chemical composition of erythropoietin (EPO) and name the site of production.
13. Discuss the various mechanisms by which EPO contributes to erythropoiesis.
14. Define and explain apoptosis resulting from Fas/FasL interactions and how this regulatory mechanism applies to erythropoiesis.
15. Explain the effect of bcl-xL and the general mechanism by which it is stimulated in red blood cell progenitors.
16. Describe the features of the bone marrow that contribute to establishing the microenvironment necessary for the proliferation of RBCs, including location and arrangement relative to other cells, with particular emphasis on the role of fibronectin.
17. Discuss the role of macrophages in RBC development.
18. Explain how RBCs enter the bloodstream and how premature entry is prevented and, when appropriate, promoted.
19. Describe the characteristics of senescent RBCs and explain why RBCs age.
20. Explain and differentiate the two normal mechanisms of erythrocyte destruction, including location and process.
Normoblastic Maturation
Terminology
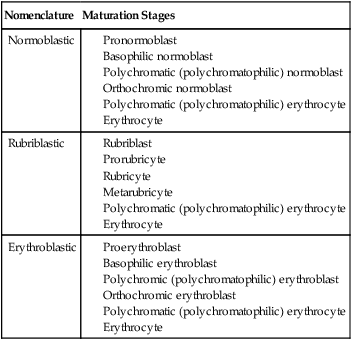
Three nomenclatures are used for naming the erythroid precursors (Table 8-1). The erythroblast terminology is used primarily in Europe. Like the normoblastic terminology used more often in the United States, it has the advantage of being descriptive of the appearance of the cells. Some prefer the rubriblast terminology because it parallels the nomenclature used for white blood cell development. Normoblastic terminology is used in this chapter.
TABLE 8-1
Three Erythroid Precursor Nomenclature Systems
| Nomenclature | Maturation Stages |
| Normoblastic | |
| Rubriblastic | |
| Erythroblastic |
Maturation Process
Erythroid Progenitors
As described in Chapter 7, the morphologically identifiable erythrocyte precursors develop from two functionally identifiable progenitors, burst-forming unit–erythroid (BFU-E) and colony-forming unit–erythroid (CFU-E), committed to the erythroid cell line. Estimates of time spent at each stage suggest that it takes about 1 week for the BFU-E to mature to the CFU-E and another week for the CFU-E to become a pronormoblast,1 which is the first morphologically identifiable RBC precursor. While at the CFU-E stage, the cell completes approximately three to five divisions before maturing further.1 As seen later, it takes approximately another 6 days for the precursors to become mature cells ready to enter the circulation, so approximately 18 to 21 days are required to produce a mature RBC from the BFU-E.
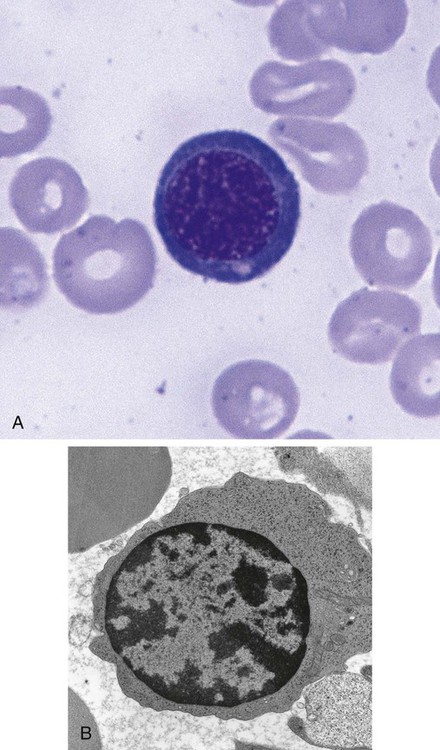
Erythroid Precursors
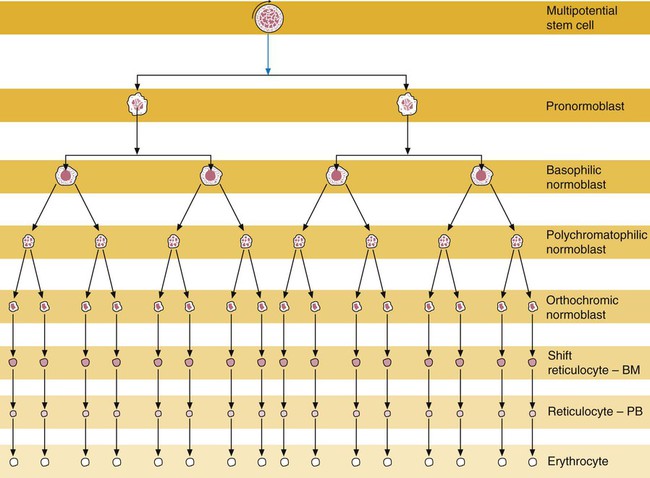
Normoblastic proliferation, similar to the proliferation of other cell lines, is a process encompassing replication (i.e., division) to increase cell numbers and development from immature to mature cell stages (Figure 8-1). The earliest morphologically recognizable erythrocyte precursor, the pronormoblast, is derived via the BFU-E and CFU-E from the multipotential stem cells as discussed in Chapter 7. The pronormoblast is able to divide, with each daughter cell maturing to the next stage of development, the basophilic normoblast. Each of these cells can divide, with each of its daughter cells maturing to the next stage, the polychromatophilic normoblast. Each of these cells also can divide and mature. In the erythrocyte cell line, there are typically three and occasionally as many as five divisions2 with subsequent nuclear and cytoplasmic maturation of the daughter cells, so that from a single pronormoblast, eight mature RBCs usually result. The conditions under which the number of divisions can be increased or reduced are discussed later.
Criteria Used in Identification of the Erythroid Precursors
Morphologic identification of blood cells depends on a well-stained peripheral blood film or bone marrow smear (see Chapter 16). In hematology, a modified Romanowsky stain, such as Wright or Wright-Giemsa, is commonly used. The descriptions that follow are based on the use of these types of stains.
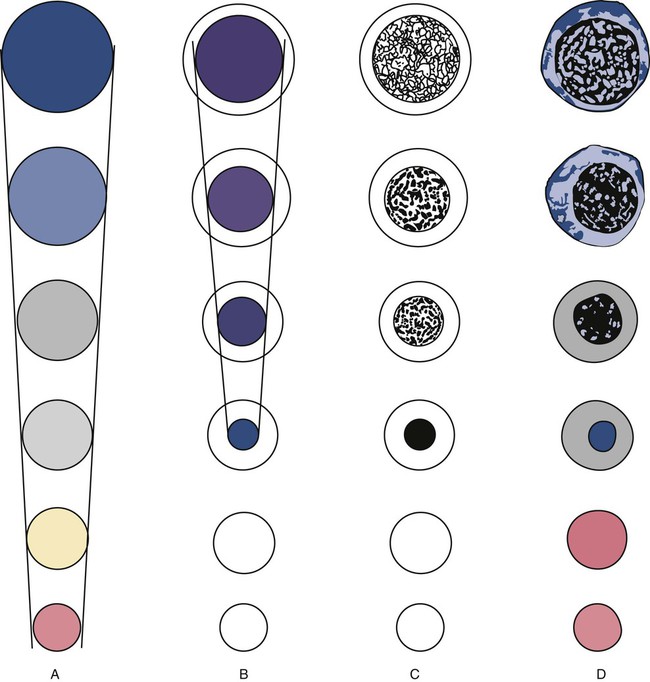
The stage of maturation of any blood cell is determined by careful examination of the nucleus and the cytoplasm. The qualities of greatest importance in identification of RBCs are the nuclear chromatin pattern (texture, density, homogeneity), nuclear diameter, nucleus/cytoplasm (N : C) ratio (Box 8-1), presence or absence of nucleoli, and cytoplasmic color.
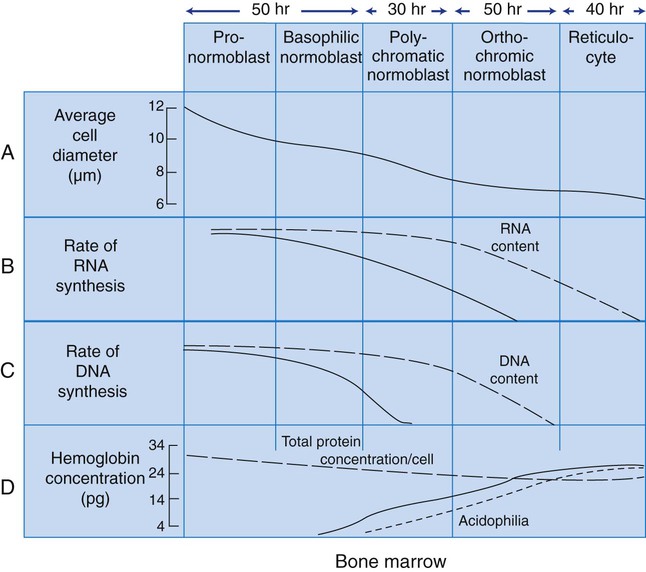
As RBCs mature, several general trends affect their appearance. Figure 8-2 graphically represents these trends.
1. The overall diameter of the cell decreases.
2. The diameter of the nucleus decreases more rapidly than does the size of the cell. As a result, the N : C ratio also decreases.
3. The nuclear chromatin pattern becomes coarser, clumped, and condensed. The nuclear chromatin of RBCs is inherently coarser than that of myeloid precursors, as if it is made of rope rather than yarn. It becomes even coarser and more clumped as the cell matures, developing a raspberry-like appearance, in which the dark staining of the chromatin is distinct from the almost white appearance of the parachromatin. This chromatin/parachromatin distinction is more dramatic than in myeloid cells. Ultimately, the nucleus becomes quite condensed, with no parachromatin evident at all, and the nucleus is said to be pyknotic.
4. Nucleoli disappear. Nucleoli represent areas where the ribosomes are formed and are seen early as cells begin actively synthesizing proteins. As RBCs mature, the nucleoli disappear, which precedes the ultimate cessation of protein synthesis.
5. The cytoplasm changes from blue to gray to pink. Blueness or basophilia is due to acidic components that attract the basic stain, such as methylene blue. The degree of cytoplasmic basophilia correlates with the amount of ribosomal RNA. These organelles decline over the life of the developing RBC, and the blueness fades. Pinkness or eosinophilia is due to more basic components that attract the acid stain eosin. Eosinophilia of erythrocyte cytoplasm correlates with the accumulation of hemoglobin as the cell matures. Thus the cell starts out being active in protein production on the ribosomes that make the cytoplasm quite basophilic, transitions through a period in which the red of hemoglobin begins to mix with that blue, and ultimately ends with a thoroughly pink-red color when the ribosomes are gone and only hemoglobin remains.
Maturation Sequence
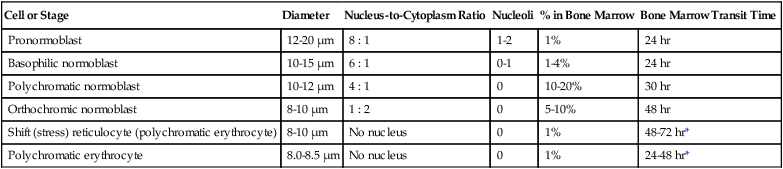
Table 8-2 lists the stages of RBC development in order and provides a convenient comparison. The listing makes it appear that these stages are clearly distinct and easily identifiable. Similar to the maturing of children into adults, however, the process of cell maturation is a gradual process, with changes occurring in a generally predictable sequence but with some variation for each individual. The identification of a given cell’s stage depends on the preponderance of characteristics, although the cell may not possess all the features of the archetypal descriptions that follow. Essential features of each stage are in italics in the following descriptions. The cellular functions described subsequently also are summarized in Figure 8-3.
TABLE 8-2
Normoblastic Series: Summary of Stage Morphology
| Cell or Stage | Diameter | Nucleus-to-Cytoplasm Ratio | Nucleoli | % in Bone Marrow | Bone Marrow Transit Time |
| Pronormoblast | 12-20 µm | 8 : 1 | 1-2 | 1% | 24 hr |
| Basophilic normoblast | 10-15 µm | 6 : 1 | 0-1 | 1-4% | 24 hr |
| Polychromatic normoblast | 10-12 µm | 4 : 1 | 0 | 10-20% | 30 hr |
| Orthochromic normoblast | 8-10 µm | 1 : 2 | 0 | 5-10% | 48 hr |
| Shift (stress) reticulocyte (polychromatic erythrocyte) | 8-10 µm | No nucleus | 0 | 1% | 48-72 hr* |
| Polychromatic erythrocyte | 8.0-8.5 µm | No nucleus | 0 | 1% | 24-48 hr* |
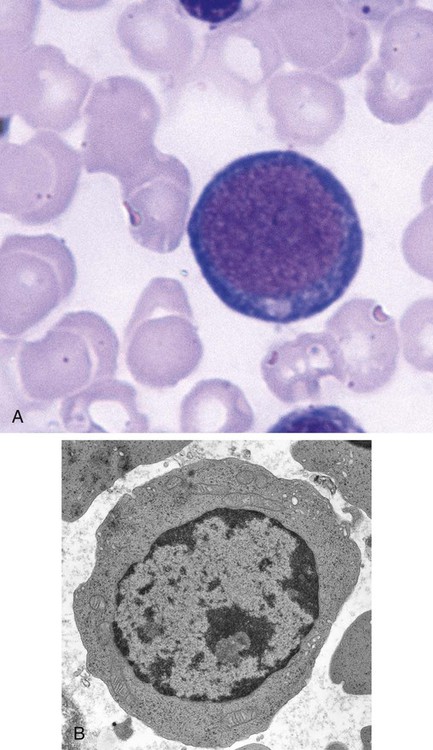
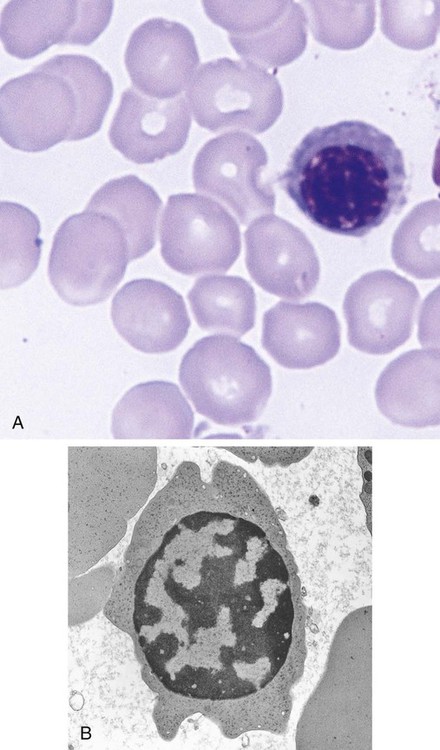
Orthochromic Normoblast (Metarubricyte)
Figure 8-7 shows the orthochromic normoblast.
Cellular Activity
Hemoglobin production continues on the remaining ribosomes using messenger RNA produced earlier. Late in this stage, the nucleus is ejected from the cell. The cell develops pseudopod-like projections into which the nucleus squeezes. Ultimately, the projection separates from the cell, with the nucleus enveloped by cell membrane.4 The extruded nucleus is then engulfed by splenic macrophages. Often, small fragments of nucleus are left behind if the projection is pinched off before the entire nucleus is enveloped. These fragments are called Howell-Jolly bodies when seen in peripheral blood cells (see Table 18-3) and are typically removed from the circulating cells by the splenic pitting process when the cell enters the circulation.
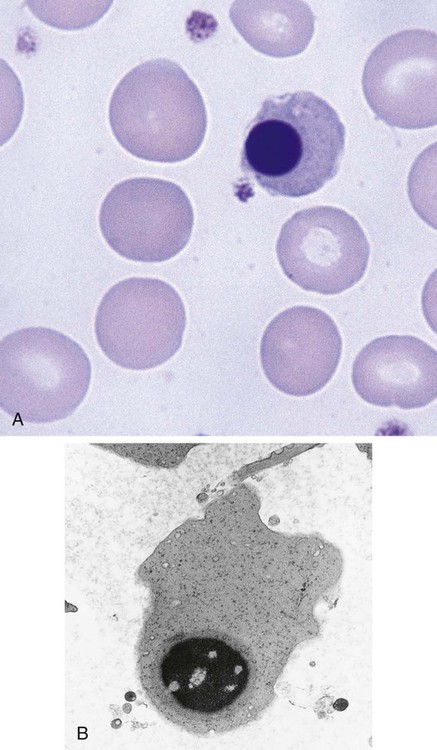
Polychromatic (Polychromatophilic) Erythrocyte
Figure 8-8 shows the polychromatic erythrocyte.
Cytoplasm
The cytoplasm can be compared easily with that of the orthochromic normoblast in that the predominant color is that of hemoglobin. By the end of the polychromatic erythrocyte stage, the cell is the same color as a mature RBC, salmon pink. It remains larger than a mature cell, however. The shape of the cell is not the mature biconcave disc but is irregular in electron micrographs, like a lumpy potato (Figure 8-8, B). It requires the polishing activity of the spleen to assist the RBC into its final discoid shape.
Location
The polychromatic erythrocyte resides in the marrow for 1 day or longer and then moves into the peripheral blood, where it circulates about 1 day. During the first several days after exiting the marrow, the polychromatic erythrocyte is retained in the spleen for pitting and polishing by splenic macrophages, which results in the biconcave discoid mature RBC.5
Cellular Activity
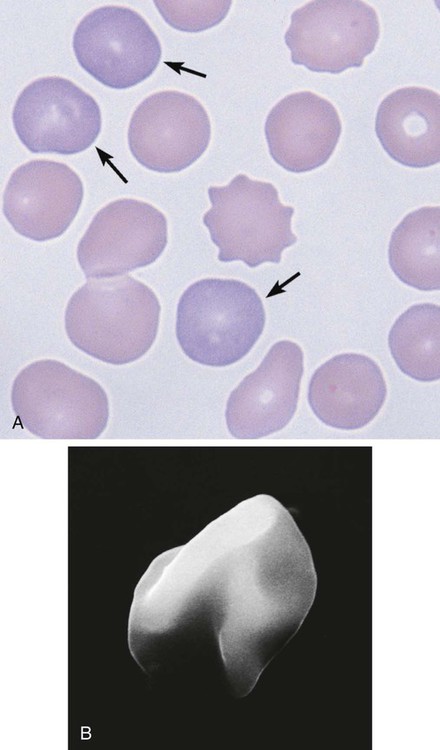
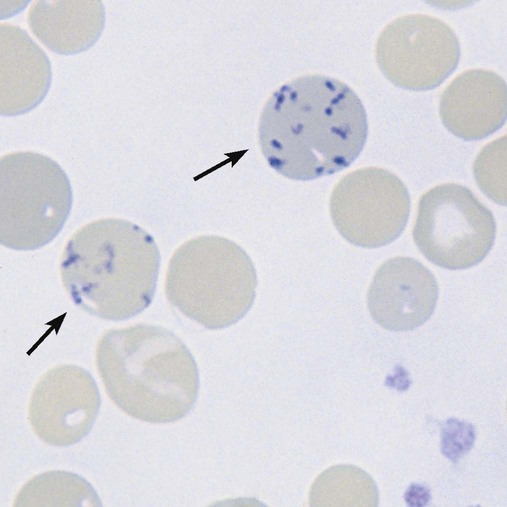
The polychromatic erythrocyte completes production of hemoglobin from residual messenger RNA using the remaining ribosomes. The cytoplasmic protein production machinery is simultaneously being dismantled. Endoribonuclease, in particular, digests the ribosomes. The acidic components that attract the basophilic stain decline over this stage to the point that the polychromatophilia is not readily evident in the polychromatic erythrocytes on a normal peripheral blood smear stained with Wright stain. A small amount of residual ribosomal RNA is present, however, and can be visualized with a vital stain such as new methylene blue, so called because the cells are stained while alive in suspension (i.e., vital), before the smear is made (Box 8-2). The residual ribosomes appear as a mesh of small blue strands, a reticulum, or, when more fully digested, merely blue dots (Figure 8-9). When so stained, the polychromatic erythrocyte is renamed the reticulocyte.
A second functional change in polychromatic erythrocytes is the reduced production of receptors for the adhesive molecules that hold developing RBCs in the marrow (see details later).6–8 As these receptors decline, the cells are freed to leave the marrow.
Erythrocyte
Figure 8-10 shows the erythrocyte.
Cellular Activity
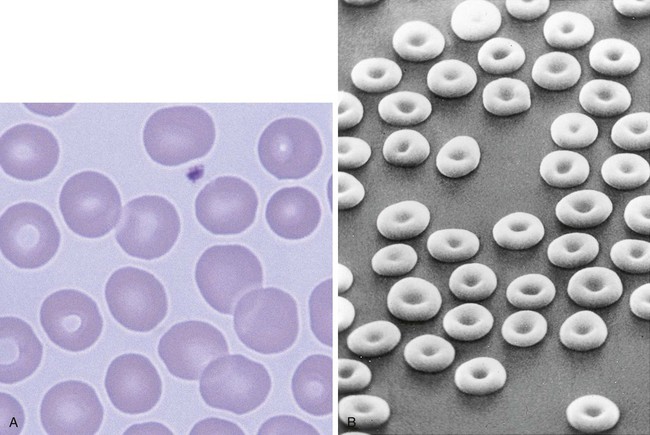
The mature erythrocyte delivers oxygen to tissues, releases it, and returns to the lung to be reoxygenated. The dynamics of this process are discussed in detail in Chapter 10. The interior of the erythrocyte contains mostly hemoglobin, the oxygen-carrying component, and a small proportion of water. It has a surface-to-volume ratio and shape that enable optimal gas exchange to occur. If the cell were to be spherical, it would have hemoglobin at the center of the cell that would be relatively distant from the membrane and would not be readily oxygenated and deoxygenated. With the biconcave shape, even hemoglobin molecules that are toward the center of the cell are not distant from the membrane and are able to exchange oxygen.
The cell’s main function of oxygen delivery throughout the body requires a membrane that is flexible and deformable. The interaction of various membrane components described in Chapter 9 creates these properties. RBCs must squeeze through small spaces such as the basement membrane of the marrow venous sinus. Similarly, when a cell enters the red pulp of the spleen, it must squeeze between epithelial cells to move into the venous outflow. Flexibility is crucial for RBCs to enter and subsequently remain in the circulation.
Erythrokinetics
Hypoxia—the Stimulus to Red Blood Cell Production
The primary oxygen-sensing system of the body is located in peritubular interstitial cells of the kidney.10,11 Hypoxia, too little tissue oxygen, is detected by the peritubular cells, which produce erythropoietin (EPO), the major stimulatory cytokine for RBCs. Under normal circumstances, the amount of EPO produced is fairly consistent, maintaining a level of RBC production that is sufficient to replace the approximately 1% of RBCs that normally die each day (see section on erythrocyte destruction). When there is hemorrhage, increased RBC destruction, or other factors that diminish the oxygen-carrying capacity of the blood (Box 8-3), the production of EPO can be increased.
The mechanism by which hypoxia increases EPO production in peritubular cells is mainly transcriptional regulation. There is a hypoxia-sensitive region of the 3′ regulatory portion of the EPO gene that promotes gene transcription.12 A hypoxia-inducible factor, a transcription factor, is assembled in the cytoplasm when oxygen tension in the cells is decreased.13 It migrates to the nucleus and interacts with the 3′ enhancer for the EPO gene and upregulates the gene expression. With hypoxia, more messenger RNA molecules are produced, which results in more EPO production.
Erythropoietin
Structure
EPO is a thermostable, nondialyzable, glycoprotein hormone with a molecular weight of 34 kD.14 It consists of a carbohydrate unit that reacts specifically with RBC receptors and a terminal sialic acid unit, which is necessary for biologic activity in vivo.15 On desialation, EPO activity ceases.16
Action
EPO is a true hormone, being produced at one location (the kidney) and acting at a distant location (the bone marrow). It is a growth factor (or cytokine) that initiates an intracellular message to the developing RBCs; this process is called signal transduction. EPO must bind to its receptor on the surface of cells to initiate the signal or message. Relatively few receptors require binding to initiate intracellular signaling.17 The EPO-responsive cells vary in their sensitivity to EPO.14 Some are able to respond to low levels of EPO, whereas others require higher levels. In healthy circumstances when RBC production needs to proceed at a modest but regular rate, the cells requiring only low levels of EPO respond. If EPO levels rise secondary to hypoxia, however, a larger population of EPO-sensitive cells are able to respond.
The binding of EPO, the ligand, to its receptor on erythrocyte progenitors initiates a cascade of intracellular events that ultimately leads to more RBCs entering the circulation in a given time. EPO’s effects are mediated by intracellular Janus tyrosine kinase signal transducers that in turn affect gene activities in the RBC nucleus.18 The resulting nuclear activities have two major effects: allowing early release of reticulocytes from the bone marrow and preventing apoptotic cell death. In addition, EPO can reduce the time needed for cells to mature in the bone marrow. These processes are described in detail later. The essence is that EPO puts more RBCs into the circulation at a faster rate than occurs without its stimulation.
Early release of reticulocytes
EPO promotes early release of developing erythroid precursors from the marrow. Part of that results from EPO-induced changes in the adventitial cell layer of the marrow/sinus membrane.19 Merely increasing the width of the spaces through which cells might move is insufficient for cells to leave the marrow, however. RBCs are held in the marrow because they possess membrane receptors for adhesive molecules. EPO downregulates the production of the receptors on the RBC surface so that cells can exit the marrow earlier than they normally would.6–8 The result is the presence in the circulation of reticulocytes that are still very basophilic, because they have not spent as much time in the marrow as they normally would. These may be called shift reticulocytes because they have been shifted from the bone marrow early (see Figure 8-8, A). Even nucleated RBCs (i.e., normoblasts) can be released early in cases of extreme anemia when the demand for RBCs in the peripheral circulation is great. Releasing cells from the marrow early is a quick fix, so to speak; it is limited in effectiveness because the available precursors in the marrow are depleted within several days and still may not be enough to meet the need in the peripheral blood for more cells. A more sustained response is required in times of increased need for RBCs in the circulation.
Inhibition of apoptosis
A second, and probably more important, mechanism by which EPO increases the number of circulating RBCs is by increasing the number of cells that will be able to mature into circulating erythrocytes. It does this by decreasing apoptosis, the programmed death of RBC progenitors.20,21 To understand this process, an overview of apoptosis in general is helpful.
Apoptosis is a sequential process characterized by, among other things, the degradation of chromatin into large fragments of 5 to 300 kilobases that are degraded further into smaller monomers of 200 bases; protein clustering; and activation of transglutamase. In contrast to necrosis, in which cell injury causes swelling and lysing with release of cytoplasmic contents that stimulate an inflammatory response, apoptosis is not associated with inflammation.22
During the sequential process of apoptosis, the following morphologic changes can be seen: (1) condensation of the nucleus, causing increased basophilic staining of the chromatin; (2) nucleolar disintegration; and (3) shrinkage of cell volume with concomitant increase in cell density and compaction of cytoplasmic organelles while mitochondria remain normal.23 This is followed by a partition of cytoplasm and nucleus into membrane-bound apoptotic bodies that contain varying amounts of ribosomes, organelles, and nuclear material. The last stage of degradation produces nuclear DNA consisting of multimers of 180 base pairs. Characteristic blebbing of the plasma membrane is observed. The apoptotic cell contents remain membrane-bound and are ingested by macrophages, which avoids an inflammatory reaction.
Evasion of apoptosis by erythroid progenitors and precursors
Apoptosis of RBCs is a cellular process that depends on a signal from either the inside or outside of the cell. Among the crucial molecules in the messaging system are the death receptor on the earliest RBC precursors, Fas, and its ligand, FasL, which is expressed by more mature RBCs.23,24
When EPO levels are low, cell production should be at a low rate, because hypoxia is not present. The excess early erythroid precursors should undergo apoptosis. This occurs when the older FasL-bearing erythroid precursors, such as polychromatic normoblasts, cross-link with Fas-marked immature erythroid precursors, such as pronormoblasts, which are then stimulated to undergo apoptosis.23 As long as the more mature cells with FasL are present in the marrow, erythropoiesis is subdued. If the FasL-bearing cells are depleted, as when EPO-stimulated early release occurs, the younger Fas-positive precursors are allowed to develop, which increases the overall output of RBCs from the marrow.
A second mechanism for escaping apoptosis exists for RBC progenitors: EPO rescue. This is the major way in which EPO is able to increase RBC production. When EPO binds to its receptor, one of the effects is to stimulate antiapoptotic molecules, which allows the cell to survive and mature.25 The cell that has the most EPO receptors and is most sensitive to EPO is the CFU-E, although the late BFU-E and early pronormoblast have some receptors.26 Without EPO, the CFU-E would not survive.27
EPO’s effect is mediated by the transcription factor GATA-1.28 The two molecules cooperate to promote the production of the anti-apoptotic molecule bcl-xL.25 EPO-stimulated cells develop this molecule on their mitochondrial membranes and are able to resist the FasL activation of apoptosis.
Reduced marrow transit time
EPO stimulates the synthesis of RBC RNA and effectively increases the rate of many processes, especially hemoglobin production.29 The accumulation of hemoglobin is a factor that may control other cellular processes.30 RBCs will continue to cycle through division and maturation until a critical concentration of hemoglobin is achieved,30 typically after three to five divisions. Under the influence of EPO, the messenger RNA increases and the critical concentration of hemoglobin is achieved in two, rather than three, divisions. The cell is released from the marrow earlier than usual. Such cells are larger, due to a lost mitotic division, and do not have time before entering the circulation to dismantle the protein production machinery that gives the bluish tinge to the cytoplasm. These cells are true shift reticulocytes similar to those in Figure 8-8, A, recognizable in the stained peripheral blood film as especially large, bluish cells lacking central pallor. They also are called stress reticulocytes because they exit the marrow early during conditions of bone marrow stress, such as certain anemias. Conversely, if hemoglobin accumulates slowly, a cell may divide more times before achieving the critical hemoglobin concentration to shut off division (Box 8-4).
The process by which hemoglobin exerts this influence is probably as a transcription factor. The presence of hemoglobin in the nucleus has been recognized for decades,31 and its role as a transcription factor was hypothesized even before the concept of such influential molecules had been fully deduced.30 It seems that hemoglobin is transferred to the nucleus in proportion to its accumulation in the cytoplasm,31 and there it influences other cellular processes.
EPO also can reduce the time it takes for cells to mature in the marrow by reducing individual cell cycle time, specifically the length of time that cells spend between mitoses.32 This effect is only about a 20% reduction, however, so that the normal transit time in the marrow of approximately 6 days from pronormoblast to erythrocyte can be shortened by only about 1 day by this effect.
Measurement of Erythropoietin
Quantitative measurements of EPO are performed on plasma and other body fluids. EPO can be measured by immunoassays of various types, including radioimmunoassay and chemiluminescence. The reference range is 5 to 30 mUnits/mL.1 Increased amounts of EPO are expected in the urine of most patients with anemia, with the exception of patients with anemia caused by renal disease.
Other Stimuli to Erythropoiesis
In addition to tissue hypoxia, other factors influence RBC production to a modest extent. It is well documented that testosterone directly stimulates erythropoiesis, which partially explains the higher hemoglobin concentration in men than in women.33 Also, pituitary34 and thyroid35 hormones have been shown to affect the production of EPO and so have indirect effects on erythropoiesis.
Microenvironment of the Bone Marrow
The microenvironment of the bone marrow is described in Chapter 7, and the cytokines essential to hematopoiesis are discussed there. Here, the details pertinent to erythropoiesis (i.e., the erythropoietic inductive microenvironment) are emphasized, including the locale and arrangement of erythroid cells and the anchoring molecules involved.
Hematopoiesis occurs in marrow cords, essentially a loose arrangement of cells in a dilated sinus area between the arterioles that feed the bone and the venous sinus that returns blood to efferent veins. Erythropoiesis typically occurs in what are called erythroid islands (see Figures 7-5 and 7-6). These are macrophages surrounded by erythroid precursors in various stages of development. It was previously believed that these macrophages provided iron directly to the normoblasts for the synthesis of hemoglobin. This was termed the suckling pig phenomenon. Although there is some evidence that macrophages may secrete some ferritin that normoblasts acquire,36 developing RBCs probably obtain most iron via transferrin (see Chapter 11), and no direct contact with macrophages is needed for this. Because macrophages do release ferritin, it is logical to assume that this could account for the intimate arrangement. However, a more credible explanation of the cellular arrangement has been established. Macrophages are now known to elaborate cytokines that are vital to the maturation process of the RBCs.37,38 RBC precursors would not survive without macrophage support via such stimulation.
The major cellular anchor for the RBCs is the macrophage. Several systems of adhesive molecules and RBC receptors tie the developing RBCs to the macrophages.36 At the same time, RBCs are anchored to the extracellular matrix of the bone marrow, chiefly by fibronectin.7
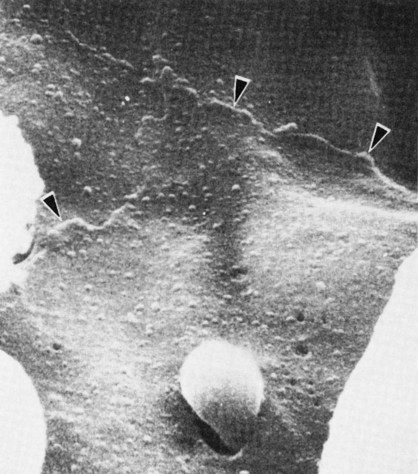
When it comes time for the RBCs to leave the marrow, they cease production of the receptors for the adhesive molecules.7 Without the receptor, the cells are free to move from the marrow into the venous sinus. Fibronectin appears to play an important role in the directional migration of red blood cells toward the sinus.8 Entering the venous sinus requires the RBC to traverse the barrier created by the adventitial cells on the cord side, the basement membrane, and the epithelial cells lining the sinus. Egress through this barrier occurs between adventitial cells, through holes (fenestrations) in the basement membrane, and through pores in the endothelial cells19 (Figure 8-11).39,40
Erythrocyte Destruction
Macrophage-Mediated Hemolysis (Extravascular Hemolysis)
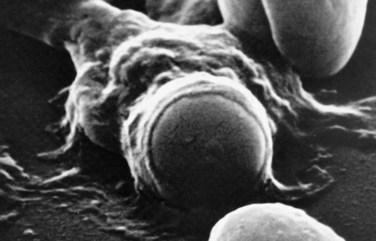
RBCs must remain highly flexible to exit the spleen by squeezing through the so-called splenic sieve formed by the endothelial cells lining the venous sinuses. Spherical RBCs are rigid and are not able to squeeze through the narrow spaces; they become trapped against the endothelial cells and basement membrane that form the sieve. In this situation, the RBC is like a fleeing thief who is trapped against a fence at the end of an alley. There, it is readily ingested by macrophages that patrol along the sinusoidal lining (Figure 8-12).
The mechanisms that signal macrophages to ingest senescent cells are currently under investigation. It is highly likely that there is no single mechanism, but rather that macrophages recognize several signals. Whether a single signal is sufficient to elicit ingestion or whether a cell must express more than one signal is uncertain. Those mechanisms receiving the greatest amount of research interest include binding of autologous immunoglobulin G (IgG) to band 3 clusters,41,42 exposure of phosphatidylserine on the exterior (plasma side) of the membrane,43 and inability to maintain cation balance.44 The macrophage is able to recognize these three and other characteristics of senescent cells that differentiate them from younger cells and thus targets the older cells for ingestion and lysis.
When an RBC lyses within a macrophage, the major components are catabolized. The iron is removed from the heme. It can be stored in the macrophage as ferritin until transported out. The globin of hemoglobin is degraded and returned to the metabolic amino acid pool. The protoporphyrin component of heme is degraded through several intermediaries to bilirubin, which is released into the plasma and ultimately excreted by the liver in bile. The details of bilirubin metabolism are discussed in Chapter 22.
Mechanical Hemolysis (Intravascular Hemolysis)
When the membrane of the RBC has been breached, regardless of where the cell is located when it happens, the cell contents enter the surrounding plasma. Although mechanical lysis is a relatively small contributor to RBC demise under normal circumstances, the body still has a system of plasma proteins, including haptoglobin and hemopexin, to salvage the released hemoglobin so that its iron is not lost. Hemolysis and the functions of haptoglobin and hemopexin are discussed in Chapter 22.
Summary
• RBCs develop from committed erythroid progenitor cells in the bone marrow, the BFU-E and CFU-E.
• The morphologically identifiable precursors of mature RBCs, in order from youngest to oldest, are the pronormoblast, basophilic normoblast, polychromatic normoblast, orthochromic normoblast, and reticulocyte.
• As erythroid precursors age, the nucleus becomes condensed and ultimately is ejected from the cell, which produces the reticulocyte stage. The cytoplasm changes color from blue, reflecting numerous ribosomes, to pink as hemoglobin accumulates and the ribosomes are degraded. Each stage can be identified by the extent of these nuclear and cytoplasmic changes.
• It takes approximately 18 days for the BFU-E to mature to an RBC, of which about 6 days are spent as identifiable precursors in the bone marrow. The mature erythrocyte has a life span of 120 days in the circulation.
• Hypoxia of peripheral blood is detected by the peritubular cells of the kidney, which then produce EPO.
• EPO, the primary hormone that stimulates the production of erythrocytes, is able to (1) rescue the CFU-E from apoptosis, (2) shorten the time between mitoses of precursors, (3) release reticulocytes from the marrow early, and (4) reduce the number of mitoses of precursors.
• Apoptosis is the mechanism by which excessive production of cells is controlled. Fas, the death receptor, is expressed by young normoblasts, and FasL, the ligand, is expressed by older normoblasts. As long as older cells mature slowly in the marrow, they induce the death of younger cells.
• EPO rescues cells from apoptosis by stimulating the production of antiapoptotic molecules that counteract the effects of Fas and FasL.
• Survival of RBC precursors in the bone marrow depends on cytokines and adhesive molecules, such as fibronectin, which are elaborated by macrophages. RBCs are found in erythroid islands, where erythroblasts at various stages of maturation surround a macrophage.
• As RBC precursors mature, they lose fibronectin receptors and can leave the bone marrow. Egress occurs between adventitial cells but through pores in the endothelial cells of the venous sinus.
• Aged RBCs, or senescent cells, cannot regenerate catabolized enzymes because they lack a nucleus. The semipermeable membrane becomes more permeable to water, so the cell swells and becomes spherocytic and rigid. It becomes trapped in the splenic sieve.
• Extravascular or macrophage-mediated hemolysis accounts for most normal RBC death. The signals to macrophages that initiate RBC ingestion may include binding of autologous IgG, membrane lipid changes, and cation balance changes.
• Intravascular hemolysis results when mechanical factors breach the cell membrane while the cell is in the peripheral circulation. This pathway accounts for a minor component of normal destruction of RBCs.
Review Questions
1. Which of the following is an erythrocyte progenitor?
2. Which of the following is the most mature normoblast?
3. What erythroid precursor can be described as follows: The cell is of medium size compared with other normoblasts, with an N : C ratio of nearly 1 : 1. The nuclear chromatin is condensed and chunky throughout the nucleus. No nucleoli are seen. The cytoplasm is a muddy, blue-pink color.
4. Which of the following is not related to the effects of erythropoietin?
a. The number of divisions of a normoblast
b. The formation of pores in sinusoidal endothelial cells for marrow egress
c. The time between mitoses of normoblasts
d. The production of antiapoptotic molecules by erythroid progenitors
5. Hypoxia stimulates RBC production by:
a. Inducing more pluripotent stem cells into the erythroid lineage
b. Stimulating EPO production by the kidney
c. Increasing the number of RBC mitoses
d. Stimulating the production of fibronectin by macrophages of the bone marrow
6. In the bone marrow, RBC precursors are located:
a. In the center of the hematopoietic cords
b. Adjacent to megakaryocytes along the adventitial cell lining
7. Which of the following determines the timing of egress of RBCs from the bone marrow?
a. Maturing normoblasts slowly lose receptors for adhesive molecules that bind them to stromal cells.
b. Stromal cells decrease production of adhesive molecules over time as RBCs mature.
c. Endothelial cells of the venous sinus form pores at specified intervals of time, allowing egress of free cells.
d. Periodic apoptosis of pronormoblasts in the marrow cords occurs.
8. What single feature of normal RBCs is most responsible for limiting their life span?
9. Intravascular hemolysis is the result of trauma to RBCs while in the circulation.
10. Extravascular hemolysis occurs when:
a. RBCs are mechanically ruptured.
b. RBCs extravasate from the blood vessels into the tissues.
c. Splenic macrophages ingest senescent cells.
d. Erythrocytes are trapped in blood clots outside the blood vessels.
11. A pronormoblast belongs to the RBC mass of the body, but not to the erythron.
12. A cell has an N : C ratio of 4 : 1. Which of the following statements would describe it?