CHAPTER 10 Equipment
Humidifiers
During normal breathing, the nasal mucosa and upper airway serve as a reservoir for condensed water. This water is evaporated during breathing and reduces the heat expended by the body in warming and humidifying inhaled gases. In typical atmospheric conditions, air in the pulmonary periphery contains 44 mg of water per liter at 37° C (100% relative humidity). Breathing ambient air at a temperature of 22° C, water content of 10 mg/dL would require the addition of 34 mg/L of evaporated water. This evaporation results in a loss of energy with the resultant cooling of the respiratory mucosa. The caloric expenditure of humidification consumes approximately five times the energy required to heat the inspired gases; this may amount to 20% of the basal metabolic rate of an infant (Rashad and Benson, 1967). Nasopharyngeal humidification is bypassed with the placement of common airway devices such as tracheal tubes, tracheostomy tubes, and laryngeal masks. Benefits of heating and humidifying anesthetic gases include prevention of intraoperative hypothermia, decreased atelectasis, and improved mucociliary clearance. Consequences of overhumidification include impaired mucociliary clearance related to reduced mucus viscosity, atelectasis, accumulation of secretions, infection, thermal injury, and surfactant inactivation (Schiffmann, 2006). Partial humidification of gas in the anesthesia breathing circuit takes place within the carbon-dioxide absorber, which uses an exothermic reaction that may raise the water vapor content to as much as 29 mg/L. Further humidification is accomplished by reducing the amount of fresh-gas flow, thereby increasing rebreathing of humidified gases and using a heat and moisture exchanger (HME, or “artificial nose”). The HME uses a fine mesh to cause condensation of exhaled water vapor. The HME may increase the resistance to breathing or the dead space for some infants and children, although these changes are usually tolerable. Low fresh-gas flow alone is often inadequate at maintaining humidity and temperature in infants and neonates, therefore active or passive humidification devices should always be incorporated when ventilating this population (Hunter et al., 2005). HMEs increase airway humidification and preserve temperature in anesthetized children at a lower cost than active humidification systems (Bissonnette and Sessler, 1989) and they require 80 minutes to achieve optimal saturation of the membrane, during which time they are less efficient. HMEs come in a variety of sizes, enabling selection based on size of the patient, so that dead space or resistance can be minimized. Specially designed HMEs filter out infectious pathogens and minimize the risk of cross-infection between patients (Wilkes et al., 2000).
Active humidification is the most efficient means by which to heat and humidify inspired gases (Bissonnette and Sessler, 1989). A servocontrolled, shielded heated wire in the fresh gas line helps to prevent cooling and condensation of the water as it passes through the inspiratory limb. The temperature should be regulated by a probe near the patient connection, because overheating of the inspired gases can produce injury to the airway (Klein and Graves, 1974). Active humidifiers may also increase the compression volume of the breathing circuit; thus, compensatory increases in the tidal volume during controlled ventilation may be necessary except in ventilators with compliance compensation (Coté et al., 1983).
Anesthesia breathing systems
Since Philip Ayre’s (1937) landmark article began the modern era of breathing systems for pediatric anesthesia, this has been a topic of controversy. Using Magill’s technique of tracheal intubation for the repair of cleft lip and palate in infants, Ayre noted adverse results. Breathing through a “closed” high-resistance system, these infants often developed “rapid, ‘sighing’ respirations” and “ashy pallor and sweating.” They exhibited a “dark, congested oozing at the site of operation.” Postoperatively, the infants were “in varying degrees of shock: some…for days” (Ayre, 1937). The contribution of hypotension or hypovolemia to this picture remains unknown, because blood pressure was not measured, and blood loss was difficult to quantify by Ayre’s account.
Ayre noted dramatic clinical improvement when he adopted an open T-piece breathing system. The T piece, an extremely simple device, consists of an inspiratory limb, a connection to the patient, and an expiratory limb. It has no unidirectional or overflow valves, and there is no breathing bag. The expiratory limb serves as a reservoir for fresh gas, a means of monitoring the infant’s respirations, and if the distal end is intermittently occluded, a means of providing positive pressure ventilation. If the volume of the expiratory limb is one third of the tidal volume, rebreathing can be virtually eliminated during spontaneous ventilation with a fresh-gas flow that is twice the minute ventilation (Ayre, 1956). Ayre attributed the salutary effect of the T piece to marked reductions in resistance to gas flow and rebreathing.
Nonrebreathing and Partial Rebreathing Systems
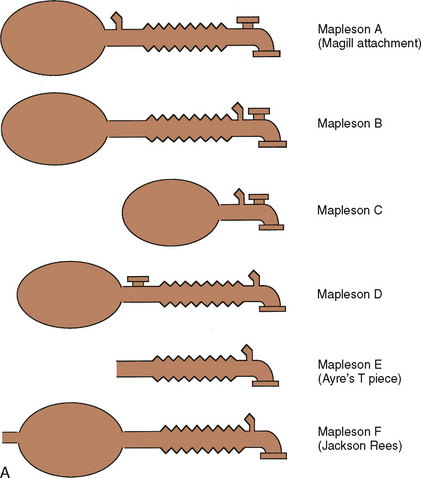
Despite its apparent benefits, the T piece is far from ideal. The major flaws are its release of anesthetic gases into the operating room and its inability to provide assisted or controlled ventilation. A series of modifications occurred. Rees (1950) first proposed the addition of a breathing bag to the expiratory limb. Another system, the Magill attachment, which predated Ayre’s publication, introduced fresh gas distal to a breathing bag and an overflow valve near the patient connection. These and other variations were brought together under a single classification scheme proposed by Mapleson (1954), in which each system was distinguished on the basis of the location of its fresh gas inflow and overflow valves relative to the patient connection.
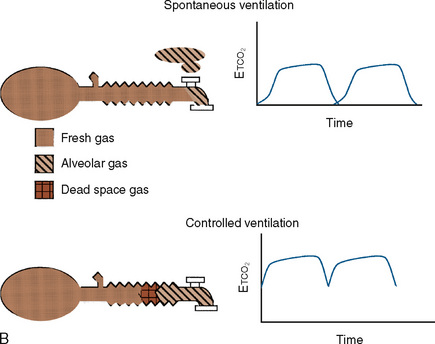
These Mapleson circuits share the benefit of reduced resistance to breathing by virtue of the absence of unidirectional valves and canisters; the elimination of these components results in varying degrees of rebreathing that depend on the fresh-gas flow (Fig. 10-1, A). Rebreathing is not necessarily bad because it serves to conserve heat, humidity, and anesthetic gases. Yet in the absence of a mechanism by which to monitor the accumulation of carbon dioxide, the consequences of hypercarbia and respiratory acidosis probably outweigh these benefits.
Mapleson A System
The Mapleson A system results in no rebreathing during spontaneous ventilation when the fresh-gas flow is more than 75% of the minute ventilation; it requires a larger fresh-gas flow to eliminate rebreathing during controlled ventilation (Fig. 10-1, B) (Waters and Mapleson, 1961; Kain and Nunn, 1967). This design is impractical in the operating room, because the proximal location of the overflow valve makes it cumbersome for scavenging waste gases, difficult to adjust during head-and-neck surgery, and potentially dangerous, because the heavy valve could dislodge a small tracheal tube.
Mapleson D System
The Mapleson D system is characterized by a proximal fresh-gas inflow and a distal overflow valve. It is a modification of the T piece, in which a breathing bag and an overflow valve have been added to the distal expiratory limb. Although it requires slightly more fresh-gas flow to eliminate rebreathing during spontaneous ventilation than the Mapleson A system, it is the most economical during controlled ventilation (Waters and Mapleson, 1961). On balance, considering both spontaneous and controlled ventilation, the Mapleson D requires the lowest fresh-gas flow rates among all Mapleson circuits. This system has become the most widely used of the Mapleson circuits for pediatric anesthesia.
The precise flow dynamics in the Mapleson D system have been a subject of discussion that has resulted in a variety of complex recommendations (Mapleson, 1954; Waters and Mapleson, 1961, Nightingale et al., 1965; Bain and Spoerel, 1973; Rose et al., 1978; Spoerel et al., 1978; Rose and Froese, 1979). To eliminate rebreathing, higher fresh-gas flows are needed during spontaneous ventilation than during controlled ventilation. With spontaneous ventilation, rebreathing is eliminated by provision of fresh-gas flow equal to the mean inspiratory flow rate (Mapleson, 1954; Rose et al., 1978). If an inspiratory/expiratory ratio is 1:1 or 1:2, the mean inspiratory flow rate is 2 to 3 times the minute ventilation. Although Spoerel and others (Bain, 1979) have demonstrated that a normal arterial carbon dioxide tension (Paco2) can be maintained during spontaneous ventilation at fresh-gas flows as low as 100 mL/kg per minute, an increased minute ventilation (and hence more respiratory work) is required to compensate for rebreathed carbon dioxide.
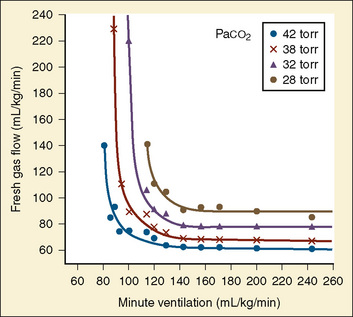
The recommendations for fresh-gas flow during controlled ventilation are complex and varied (Waters and Mapleson, 1961; Nightingale et al., 1965; Bain and Spoerel, 1973). This reflects the importance of several factors that were summarized by Rose and Froese (1979) (Fig. 10-2). When a high fresh-gas flow (greater than 100 mL/kg per minute) is used, the Paco2 is governed by minute ventilation (ventilation limited). At low fresh-gas flow (less than 90 mL/kg per minute), Paco2 is independent of minute ventilation, varying instead as a function of the amount of rebreathing, which is governed by the fresh-gas–flow rate (flow limited).
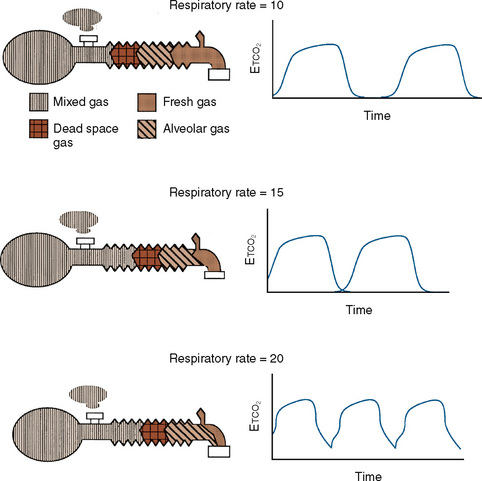
Additional important factors that govern the magnitude of rebreathing include carbon dioxide production, respiratory rate, and respiratory waveform characteristics (e.g., inspiratory flow, inspiratory and expiratory times, and expiratory pause) (Rose and Froese, 1979). Adjustments to the ventilatory pattern that allow the fresh-gas flow to constitute a larger proportion of the inspired gas (e.g., slow inspiratory time or low inspiratory flow) or that enable exhaled gases to be more completely washed out (e.g., long expiratory pause or slow rate) reduce the amount of rebreathing. With a Mapleson D circuit that has controlled ventilation and low fresh-gas flow (flow limited), an attempt to reduce the Paco2 by increasing the respiratory rate would reduce the expiratory pause and thus promote rebreathing (Fig. 10-3). In this situation, the increased ventilation is offset by increased fraction of inspired oxygen (Fico2), resulting in no net change in Paco2. To wash out the exhaled gas at this higher respiratory rate and take advantage of the increased minute ventilation, the fresh-gas flow must be increased. These fresh-gas flow and ventilatory recommendations are predicated for a normal metabolic rate and hence normal carbon dioxide production (Bain and Spoerel, 1977; Nightingale and Lambert, 1978). Conditions that increase carbon dioxide production (e.g., fever, catabolic state, or malignant hyperthermia) must be met with a proportional increase in fresh-gas flow or ventilation.
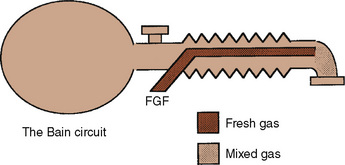
Bain Modification of Mapleson D
The Bain modification of the Mapleson D circuit incorporates the fresh-gas supply within the expiratory limb in a coaxial arrangement (Fig. 10-4) (Bain and Spoerel, 1972). This circuit is light and streamlined with only a single hose to the patient. It also provides some countercurrent warming of the inspired gases and effective scavenging of expired gases. Its major disadvantage lies in the inability to directly inspect the integrity of the inspiratory limb. Pethick (1975) described an indirect test of the Bain inspiratory-limb integrity in which the oxygen flush is passed through the circuit for several seconds. If the inspiratory limb is intact, the rapid flow of gas through it exerts a Venturi effect on the expiratory limb, resulting in a slight negative pressure and collapse of the breathing bag. With a leak from the inspiratory limb into the expiratory limb, the pressure in the latter rises, tending to inflate the reservoir bag. The rebreathing characteristics of the Bain circuit are identical to those of any other Mapleson D. The major reasons that proponents have advocated the use of these Bain circuits for pediatric anesthesia are their relatively lower resistance to breathing offered by an open system, the countercurrent warming of gases, and the low profile of a single hose attached to the patient.
The primary sources of resistance in an anesthesia delivery system are the tracheal tube, the valves, and the carbon dioxide absorber. With modern equipment, the tracheal tube represents a major source of resistance in the neonate (Cave and Fletcher, 1968; Brown and Hustead, 1969). Light-weight, large-diameter, modern disc valves exert resistance in two ways. There is a minimum, flow-independent resistance necessary to displace the valve, usually much less than 1 cm H2O (Hunt, 1955). A much higher resistance may be required when the expiratory valve is wet. At high gas flows (more than 30 L/min), the valves also become a source of turbulent resistance proportional to the flow through them. Carbon dioxide canisters are also a source of turbulence. Their resistance is inversely proportional to the length of the path the gas must take through the resistor. Modern absorbers are short and wide to minimize this path of resistance.
Approximately a half century after Ayre introduced the T piece, the extent to which his work applies remains unclear. Perhaps the infants Ayre studied were subjected to the significant resistance imposed by the valves of that era. Although the neonate has a lower proportion of fatigue-resistant fibers in the diaphragm, infants as young as 2 weeks old have been shown to compensate for increases in resistance of 200% without changes in blood gases, at least for relatively short lengths of time (Muller et al., 1979). The benefits of the Mapleson systems must be weighed against their inherent problems on an individual basis. If a practitioner seldom uses Mapleson circuits and is unfamiliar with their characteristics, or if a practitioner has to make substantial alterations to an anesthesia machine to accommodate them, the additional risks of the situation must be considered. Even if a circle system is used for most children, it is important to understand the flow requirements of these circuits, as they continue to be used for resuscitation outside of the operating room and for transporting critically ill patients.
Circle Systems
The circle system is standard equipment on anesthesia machines that have been designed to meet the standards specified in ASTM 1850-00 (2005). When functioning properly, it enables lower fresh-gas flows than the Mapleson D circuit; a circle system conserves heat, humidity, and anesthetic agent. It also minimizes environmental pollution. Compared with the resistance of an endotracheal tube, the additional resistance imposed by the addition of unidirectional valves and a carbon-dioxide canister is trivial. Because the ratio of the patient’s tidal volume to the volume of the inspiratory limb is small in young children, changes in the anesthetic concentration can take some time to reach equilibrium unless higher fresh-gas flows are used. It is important to be vigilant for the manifestations of stuck (resistance) or floating (rebreathing) unidirectional valves, because both have harmful consequences in small children.
Anesthesia machines
Gas and Anesthetic Vapor Delivery
Oxygen
All modern anesthesia machines are designed to ensure continuous oxygen delivery from either pipeline or cylinder supplies and to notify the user if the oxygen-supply pressure fails. Because pressure in the oxygen supply does not guarantee that oxygen is indeed flowing through the pipes, an oxygen analyzer located in the inspiratory limb of the anesthesia circuit is an essential safety monitor for every anesthetic (Dorsch and Dorsch, 2007). Indeed, an inspired oxygen-concentration monitor is considered to be the standard of care for every anesthetic according to the American Society of Anesthesiologists (2005) (Box 10-1).
Box 10-1 Standards for Basic Anesthetic Monitoring Committee of Origin: Standards and Practice Parameters
These standards apply to all anesthesia care although, in emergency circumstances, appropriate life support measures take precedence. These standards may be exceeded at any time based on the judgment of the responsible anesthesiologist. They are intended to encourage quality patient care, but observing them cannot guarantee any specific patient outcome. They are subject to revision from time to time, as warranted by the evolution of technology and practice. They apply to all general anesthetics, regional anesthetics and monitored anesthesia care. This set of standards addresses only the issue of basic anesthetic monitoring, which is one component of anesthesia care. In certain rare or unusual circumstances, 1) some of these methods of monitoring may be clinically impractical, and 2) appropriate use of the described monitoring methods may fail to detect untoward clinical developments. Brief interruptions of continual† monitoring may be unavoidable. These standards are not intended for application to the care of the obstetrical patient in labor or in the conduct of pain management.
Standard II
Oxygenation
Objective
To ensure adequate oxygen concentration in the inspired gas and the blood during all anesthetics.
Methods
Ventilation
Methods
Circulation
Methods
† Note that “continual” is defined as “repeated regularly and frequently in steady rapid succession,” whereas “continuous” means “prolonged without any interruption at any time.”
* Under extenuating circumstances, the responsible anesthesiologist may waive the requirements marked with an asterisk (*); it is recommended that when this is done, it should be so stated (including the reasons) in a note in the patient’s medical record.
(Approved by the ASA House of Delegates on October 21, 1986, and Last Amended on October 25, 2005)
Indications for Careful Control of Fio2
Studies have implicated arterial oxygen tension as one of several variables linked to retinopathy of prematurity (ROP) in preterm infants (weighing less than 1300 g) whose retinas are immature (Flynn et al., 1992). The contribution of brief episodes of intraoperative hyperoxemia to the development of ROP remains unknown, and oxygen concentrations greater than necessary should be avoided in this vulnerable population. It is not known whether there is any real impact on outcome dependent on whether air or nitrous oxide is used as the balance gas in these patients. Given the potential for nitrous oxide to interfere with deoxyribonucleic acid (DNA) synthesis, it may be prudent to avoid this gas in the developing neonate, although there are no definitive data indicating that nitrous oxide is dangerous for these patients.
During airway laser surgery it is desirable to reduce the inspired oxygen concentration to less than 30% to minimize the risk of igniting the tracheal tube or any other combustible materials. Because nitrous oxide also supports combustion, it should not be used in these cases. Air can be used alone or with small amounts of additional oxygen. Helium is also a useful balance gas in these cases, because it does not support combustion and can improve the flow of inspired gases beyond any obstructions in the airway (Pashayan et al., 1988). Specially adapted anesthesia machines capable of delivering inspired carbon dioxide to achieve hypercarbia or increased inspired nitrogen to produce hypoxia may be indicated in the care of the neonate with specific types of congenital heart disease (Tabbutt et al., 2001). The important aspect of caring for these patients is to maintain the balance of pulmonary and systemic blood flow by controlling pulmonary vascular resistance. Both hypoxic oxygen concentrations and carbon dioxide can be used toward this end.
Anesthetic Vapor Delivery
All modern anesthesia machines deliver anesthetic vapor by vaporizing a controlled amount of liquid anesthetic and mixing it with the fresh gas entering the anesthesia circuit. The only exception is the Zeus anesthesia machine (Draeger Medical; Lubeck, Germany), which injects liquid anesthetic directly into the anesthesia circuit under closed-loop control. There are excellent sources of information on the details of vaporizer design; however, they are beyond the scope of this chapter (Dorsch and Dorsch, 2007).
Controlled Ventilation
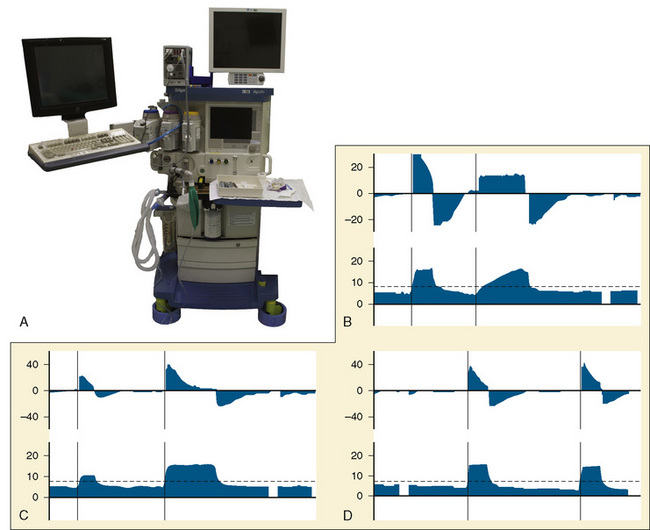
The most important change to date in modern anesthesia machines is the ability to precisely deliver small tidal volumes accurately by compensating for breathing-circuit compliance and changes in fresh-gas flow. Newer anesthesia machines have ventilators that can precisely deliver small volumes at high rates (Stayer et al., 2000). Delivering small tidal volumes requires not only an accurate ventilator, but a means for compensating for the compliance of the breathing system and the interaction between fresh-gas flow and inspired tidal volume. Breathing-system compliance exists in any ventilator-circuit combination and results from the compression of gas within the breathing circuit as pressure builds in the circuit, as well as the elastic expansion of the breathing-circuit tubing. Breathing-system compliance is expressed as milliliters of volume that are taken up per centimeter of water pressure, and it is determined by the internal volume of the breathing system and circuit, as well as the elastic properties of the circuit. Longer breathing circuits have an increased compliance primarily because of the greater internal volume. To better understand the impact of breathing-circuit compliance on delivered tidal volume, consider a case where the compliance value is 1 mL/cm H2O and the inspiratory pressure is 20 cm H2O. In that case, 20 mL of tidal volume delivered by the ventilator will not reach the patient. If the desired tidal volume is small, for example 100 mL or less, the fraction that does not reach the patient because of circuit compliance becomes a significant fraction of the desired tidal volume.
To compensate for the compliance of the breathing system, the preuse checkout must be performed to allow the ventilator to measure the compliance of the breathing system and then compensate for that compliance factor during VCV. To use these ventilators effectively, it is essential that the preuse checkout procedures be followed with the breathing-circuit configuration that is to be used during the procedure (Fig. 10-5, A) (Bachiller et al., 2008). Different anesthesia-machine vendors have different approaches to eliminate the interaction between fresh-gas flow and tidal volume. Some anesthesia machines (e.g., Draeger Medical; Lubeck, Germany) use a decoupling valve between the ventilator and the fresh-gas inflow so that the valve closes during inspiration. Other anesthesia machine designs (e.g., General Electric; Madison, Wisconsin) compensate for the fresh-gas flow by using closed-loop feedback between the inspiratory flow sensor and the ventilator.
Assisted Spontaneous Ventilation
Another advantage of modern anesthesia ventilators for pediatric patients is the advent of pressure-supported modes of ventilation. These modes are specifically designed to allow for spontaneous ventilation when an endotracheal tube or supralaryngeal airway is in place and the associated work of breathing would be an impediment to effective spontaneous ventilation. In pediatric patients the work of breathing is a major problem, because small endotracheal tubes impose a significant resistance to inspiration, the ventilator circuits and valves add to the work of breathing, and the anesthetized patients have reduced inspiratory force. In general, long periods of unassisted spontaneous ventilation during anesthesia are undesirable and can result in progressive atelectasis. Supported ventilation is an excellent method for allowing a patient to breathe spontaneously, and it has been demonstrated to improve gas exchange during anesthesia (von Goedecke et al., 2005).
Three typical options for assisted spontaneous ventilation are listed as follows:
Warming Devices
Heat conservation is critical in the care of the newborn infant. A decrease of 2° C in the environmental temperature is sufficient to double the oxygen consumption of a full-term infant (Hill and Rahimtulla, 1965). To meet this increase in oxygen consumption, the neonate must double its minute ventilation. For a critically ill premature infant who is unable to mount this increase in ventilation, an oxygen debt and progressive lactic acidosis ensue (see Chapter 6, Thermoregulation: Physiology and Perioperative Disturbances). The rate of heat loss in newborn infants is four times that in adults because of a higher surface area-to-body weight ratio, increased curvature of body surfaces, and decreased insulation from skin and subcutaneous fat (Adamson and Towell, 1965). Special efforts must be made to maintain body temperature during anesthesia, especially in small infants undergoing operative procedures accompanied by large insensible heat losses.
Forced Warm-Air Device
The flow of warm air across a child’s skin produced by a forced-air warming blanket prevents heat loss to the environment and may even effectively warm patients via radiant shielding and convection. Of all devices, this is one of the most effective and should be used in all cases where hypothermia is a possibility (Sessler et al., 1991; Camus et al., 1993; Kurz et al., 1993). Forced-air systems inject warm air through a connecting hose into a quiltlike blanket that has small holes on one surface. Warm air escaping through these holes provides conductive and convective warming to the patient. Different sizes of blankets permit maximum coverage of specific body areas over a range of patient sizes. The most effective units direct flow toward areas with major blood vessels, like the chest, axillae, abdomen, and groin, where convective heating is most effective (Giesbrecht et al., 1994). Like overhead radiant warmers and circulating-water blankets, forced-air systems can produce burns and overheating when used inappropriately (Truell et al., 2000). Maximum air temperatures on these devices are designed to minimize the risk of burns. Ensuring that the tube carrying the warm air to the blanket does not touch the patient also decreases the likelihood of thermal injury. Furthermore, directing warm air from the hose without the blanket (“free-hosing”) has been associated with patient burns. Patients with compromised circulations are at a higher risk of burns even when the blanket is used correctly; the temperature should not be set to the highest setting in these patients, especially during prolonged surgery (Siddik-Sayyid et al., 2008). Blankets that are wet are ineffective at maintaining temperature and should be replaced (Lin et al., 2008).
Wrapping and Draping with Plastic Sheets
Because of their large surface area, infants have significant evaporative and radiant heat loss. Wrapping and draping the patient with special blankets is also effective in decreasing heat loss during prolonged surgery, especially when forced-air devices are impractical. Radiant heat loss can be decreased with the use of Webril cotton wrapping (Kendall Corporation; Boston, Massachusetts) covered with plastic bags over exposed extremities. For small infants with relatively large heads, cranial heat loss can be decreased up to 73% by wrapping the head in an insulated hat or plastic bag. This is significant for neonates, because their brains are responsible for 44% of their total heat production (Rowe et al., 1983). The use of reflective blankets, made from the material used in outdoor survival apparel (e.g., space blankets), is also effective in reducing heat loss (Bourke, et al., 1984). Cutaneous heat loss is proportional to the surface area of the patient, making the percentage of skin surface covered far more important than the region of the body covered or the type of material used for passive insulation (Sessler et al., 1991).
Humidification of Inspired Gases
Humidification of inspired gases, as noted previously, is an important technique for conserving heat in anesthetized patients. Because 12% to 14% of body heat is lost through the respiratory tract, the use of warm, humidified gases decreases the potential heat drain in addition to preventing damage to the ciliated cells of the tracheobronchial tree caused by dry gases (Clarke et al., 1954). The humidifier should be servocontrolled, with a temperature sensor close to the patient to automatically shut off the heater when the preset temperature equals the patient’s airway temperature, minimizing the risk of airway burns. Maximum temperature limits, causing automatic shutoff, should also be an integral aspect of the humidifier. Special anesthesia circuits with a heated wire in the expiratory limb minimizes condensation that can occur with humidifiers. HMEs (e.g., Humid-Vent) can provide 80% inspired humidity when 80 minutes of use is allowed to saturate the hygroscopic membrane (Bissonnette et al., 1989a, 1989b). Many HMEs are incorporated into breathing-system filters. These serve to minimize the passage of infectious materials to the breathing circuit and provide warmth and humidification of inhaled gases. The efficacy of various HME brands to perform these functions is variable. The internal volumes of these devices can range from 10 to 60 mL, and small volume filters are preferred in the neonatal population. HME filters may increase the work of breathing and dead space and should be accounted for when ventilating small children. Filtration efficacy is assessed by the penetrance of sodium chloride particles (0.1 to 0.3 mcm); tests in pediatric HME filters show that their filtration ability is less than in adult filters. Based on these findings the Centers for Disease Control (CDC) recommend changing the breathing circuit for each new case. Berry and Nolte (1991) examined the efficacy of the Pall HME filter in preventing bacterial contamination of the circle-breathing system in a laboratory model and found it to be an effective microbial barrier between the patient and the circle system. They concluded that when using the Pall HME filter the components beyond the filter may be reused. However the reuse of single-use equipment is not recommended and use is borne by the individual practitioner or institution. To date there has not been a reported case of infection transmission between patients related to anesthesia-breathing systems with filters in place.
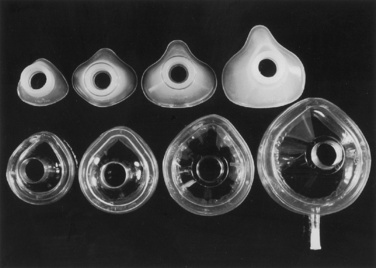
Face Masks
The most common anesthesia face mask in use today is the plastic disposable type that contains an adjustable pneumatic cushion, which when inflated or deflated with air can be altered to conform to the shape of a child’s face. A variety of different manufacturers produce this type of face mask (Fig. 10-6). An alternative variety for use in pediatric patients is the Rendell-Baker-Soucek mask, which remains in use in many centers (Fig. 10-7). This mask is available in malleable rubber or nonlatex silicone and allows an effective seal on a child’s face while minimizing internal dead space. It was originally designed on the basis of anatomic molds taken from a large number of children (Rendell-Baker and Soucek, 1962).
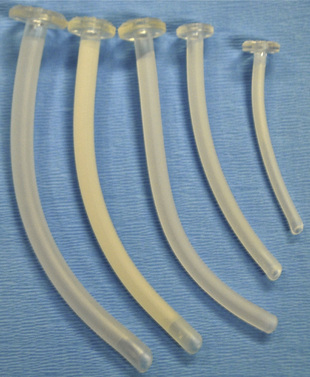
Oral and Nasal Airway Devices
Oral and nasal pharyngeal airway devices are used in pediatric anesthesia to improve patency of the upper airway and to facilitate delivery of oxygen or anesthetic gases to the lungs. Minimum requirements for these devices are noted in ANSI/ISO5364-08 (ANSI/ISO, 2008). The Guedel-type oral airway device is probably most commonly used in pediatric patients. It contains a central lumen for the passage of airflow and for suctioning of the posterior pharynx (Fig. 10-8, A). The oral airway device is primarily used to alleviate upper airway obstruction caused by tonsillar or adenoidal hypertrophy, or normal pharyngeal tissue obstruction, as often occurs in small infants (see Chapter 3, Respiratory Physiology).
Oral airway devices are usually manufactured from plastic or polyethylene and are latex-free. They are sized according to the total length of the device (50 to 80 mm, flange to tip, for most children) or based on an arbitrary scale designated by the manufacturer. The appropriate size is determined by placing the airway device adjacent to the child’s face to approximate its position in the oral cavity. The appropriately-sized oropharyngeal airway device should extend from the corner of the mouth to the angle of the mandible (Fig. 10-8, B). When appropriately placed, its distal end snugly curves around the base of the tongue, without the proximal end protruding out of the mouth. Too small a device pushes the posterior portion of the tongue against the posterior pharyngeal wall, and too large a device may cause upper airway obstruction at the laryngeal inlet by compressing or distorting the epiglottis.
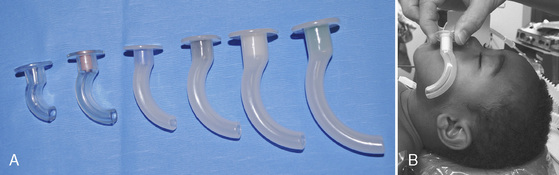
The nasal airway device is made from soft, latex-free rubber to allow easy insertion through the nasal passage and into the nasal or oropharynx. It can be bathed in warm or cold water to decrease or increase its stiffness, respectively. Some nasal airway devices contain an enlarged flange at the proximal end to prevent unintentional advancement into the nasal cavity. Others contain an adjustable ring that can be secured against the outside of the nasal opening. Nasal airways are available in sizes 12 to 36F (outer diameter, or OD) (Fig. 10-9). If required, a stiffer nasal airway can be fashioned out of a standard tracheal tube by cutting it off at the appropriate length. An anesthesia breathing circuit can be connected to any type of nasal airway device using an appropriately sized tracheal tube adaptor (Fig. 10-10). This technique may facilitate intubation in patients with difficult airways by allowing the administration of anesthesia and oxygen during airway manipulation via a modified nasal airway connected to the anesthesia breathing circuit (Holm-Knudsen et al., 2005). The use of an adaptor also permits delivery of continuous positive airway pressure (CPAP).
To avoid trauma and bleeding of the delicate nasal mucosa, the nasal airway device should be lubricated and gently inserted in a posterocaudad direction along the floor of the nasal cavity. A topical vasoconstrictor, such as 0.05% oxymetazoline, can be applied to the nasal mucosa before inserting the nasal airway device to shrink nasal mucosal tissue and reduce bleeding. Vasoconstrictors should be used in recommended doses, particularly because topical phenylephrine use has been associated with cardiac arrest and death in children. The circumstances of these arrests usually involve the topical administration of an unmeasured dose of phenylephrine with resultant hypertension. The anesthesiologists in these cases administered a β-blocking or calcium-channel blocking agent in response to the hypertension, with the consequence of pulmonary edema and cardiac arrest (Groudine et al., 2000). Hypertension secondary to α-agonists should be treated with a α-antagonists and direct vasodilating agents. In children weighing up to 25 kg, the initial dose of phenylephrine should not exceed 20 mcg/kg (Groudine et al., 2000). Oxymetazoline can also cause hypertension, tachycardia, and peripheral vasoconstriction by stimulation of peripheral α2-receptors; some patients exhibit hypotension, bradycardia, and neurologic and respiratory depression related to stimulation of central α2-receptors. Current dosing guidelines recommend oxymetazoline use in children at 6 years of age, although it is safely utilized in children of all ages. A 0.025% solution is recommended for ophthalmic use (1 to 2 drops to affected eye every 6 hours) and a 0.05% solution for nasal use (2 to 3 sprays in each nostril twice a day). The proper diameter of the nasal airway device is determined by approximating the circular diameter of the nasal opening. The proper length of the nasal airway device is estimated by measuring the distance from the nares to the tragus of the ear. When appropriately placed, its distal tip should lie at the level of the angle of the mandible, between the posterior aspect of the tongue and above the tip of the epiglottis.
The most common complication resulting from inserting a nasal airway device is trauma to the nasal or pharyngeal mucosa that results in minor bleeding. Adenoidal tissue may be disrupted and may bleed into the oropharynx. Occasionally, a friable vessel is encountered in the nasal mucosa and bleeding is brisk. Sequential dilation of the nasal passage with nasal airway devices of increasing size is sometimes used in an attempt to minimize trauma during nasal intubation. Adamson et al. (1988) investigated the influence of dilation vs. no dilation on bleeding and found an increased incidence of hemorrhage with dilation and no difference in resistance to passage of the tracheal tube. There are two pathways that devices can take when placed into the nasal passage: a lower pathway below the inferior turbinate and an upper pathway between the middle and inferior turbinates. The middle turbinate is attached anteriorly to the cribriform plate and posteriorly to the ethmoid bone by a thin lamella, making it vulnerable to trauma and avulsion when an airway device is forcibly passed through the upper pathway. The lower pathway is therefore preferred, and airway devices should be inserted perpendicular to the patient’s nare and directed caudally to increase the likelihood of passage into the lower pathway (Hall and Shutt, 2003; Ahmed-Nusrath et al., 2008). Nasal airways should not be inserted in children with a coagulopathy, neutropenia, or suspicion of a traumatic basilar skull fracture.
Tracheal Tubes
The most commonly used tracheal tubes are made of nonreactive polyvinylchloride (PVC). Tracheal tubes should bear a designation that they comply with ANSI/ISO Standard 5361 (1999), which confirms that they are inert, as well as meet dimension, material, and product-marking standards. Biologically inert materials reduce the risk of airway inflammatory reactions.
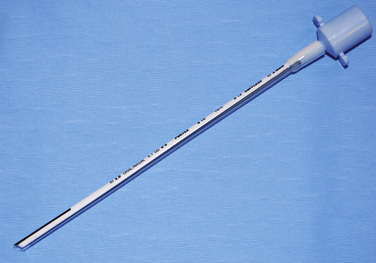
PVC tubes are flammable and cannot be used in laser surgery performed on airways. A tube that is either nonflammable (e.g., metal) or laser-resistant (e.g., red rubber tube wrapped with aluminum foil or manufactured with special surface treatments) must be substituted (see Chapter 24, Anesthesia for Pediatric Otorhinolaryngologic Surgery). These special tubes should have a label indicating that they are intended for use in laser surgery. Patients with difficult airways or tracheal abnormalities often require a spiral wire-embedded silicone, or “anode,” tube (Fig. 10-11). This tube has the flexibility to assume virtually any position, while the wire coil embedded in its wall preserves the lumen. Extreme caution must be taken with anode tubes, because their flexibility makes accidental extubation more likely.
In evaluating tracheal tubes for use in infants and children, their influence on dead space, resistance to breathing, and tracheal or laryngeal injury must be considered. Tracheal intubation reduces the dead space of the natural extrathoracic airway but can have a dramatic negative impact on the resistance to breathing (Glauser et al., 1961). Resistance to laminar flow through a tube is governed by the Hagen-Poiseuille Law, which dictates that resistance is proportional to the length of the tube and inversely proportional to the fourth power of the radius. Thus, small changes in the lumen of a tube can dramatically increase the resistance to flow through it. Assuming that flow is laminar and that all other variables are constant, one can predict that a reduction in internal diameter (ID) from 7.5 to 7.0 mm increases resistance 24%, whereas a reduction from 3.5 to 3.0 mm increases resistance by nearly 50%. Because luminal changes between the tracheal tube and the adapter promote turbulent flow, measured differences are even more exaggerated. The resistance to breathing through the natural airway of a neonate is greater than that through a 3.5-mm ID tracheal tube but significantly less than that through a 2.5-mm ID tracheal tube (Polgar and Kong, 1965). A small amount of secretions or debris can increase resistance substantially, yet these accumulations are difficult to avoid in small-lumen tubes. The use of 2.5-mm ID tracheal tubes should be restricted to situations in which no other tube fits.
Resistance to breathing is governed by the ID of a tube, but the potential for laryngeal or tracheal mucosal injury is related to the OD. Mild trauma to the airway, producing as little as 1-mm mucosal edema, can result in significant narrowing of the infant’s airway. As little as 25 mm Hg pressure on the lateral wall of the trachea causes local ischemia and mucosal injury in adults and presumably in children as well (Nordin et al., 1977; Seegobin and van Hasselt, 1984).
Historically, uncuffed tracheal tubes have been used for children under 8 years. Based on the interpretation of statements by Eckenhoff (1951), the child’s larynx was thought to be conical with a circular cricoid ring at its most narrow dimension and only assumed a more cylindrical shape as the child matured into an adult (Motoyama, 2009). A circular, uncuffed tracheal tube would then conform well to this narrow, circular portion of the airway. More recent data suggest that the cricoid is slightly elliptical with the rima glottidis (the vocal cords) as the narrowest point in the pediatric airway (Litman et al., 2003; Dalal et al., 2009). A snug-fitting tracheal tube would exert more pressure on the lateral mucosa of the trachea than in the anterior-posterior direction. (Motoyama, 2009). This conclusion might suggest the use of smaller, uncuffed tracheal tubes. However, smaller tracheal tubes would increase resistance to spontaneous breathing, make controlled ventilation more difficult caused by a larger leak around the tube, increase environmental pollution, and possibly risk increased rates of pulmonary aspiration.
Yet with the reduction in tube size necessary to pass the glottic opening and the fear of laryngeal and tracheal damage, cuffed tracheal tubes have not been recommended in young children in the past. With the regular use of mechanical ventilation and the advent of pressure-support anesthesia ventilators, increased tracheal tube resistance may be less of an issue for most children undergoing anesthesia. Tissue damage may be the result of overinflated cuffs or prolonged intubation in intensive care settings (Nordin, 1977; Holski, 1997). Several series, however, have demonstrated that cuffed, tracheal tubes perform as well or better than uncuffed tubes in children undergoing anesthesia with muscle relaxant, noting fewer required intubation attempts and no difference in the incidence of postoperative croup (Khine et al., 1997; Murat, 2001; Weiss et al., 2009).
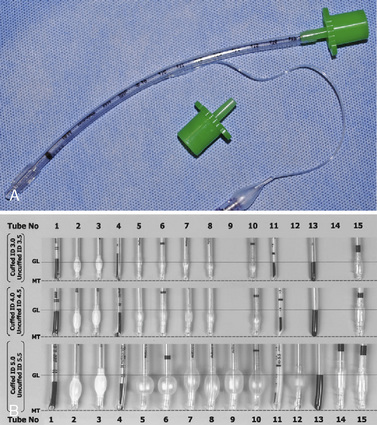
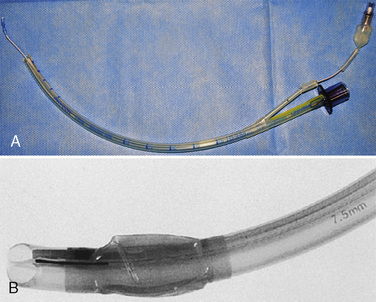
The development of a new, microcuff tracheal tube (Kimberly-Clark Worldwide; Roswell, Georgia) may add additional advantages to management of the pediatric airway (Weiss and Dullenkopf, 2007). These tubes have an anatomically-based intubation depth mark for placement at the level of the cords, providing a margin of safety for tube-tip displacement with head flexion and extension (Weiss et al., 2006). Additionally, these tubes have a distally placed cuff with a smooth, thin membrane that allows the cuff to avoid the cricoid and larynx and facilitates lower inflation pressures to seal the trachea (Fig. 10-12, A).
Whether cuffed tracheal tubes should replace uncuffed tubes in pediatrics is unclear. In a large, randomized, controlled, multicenter trial comparing cuffed with uncuffed endotracheal tubes, Weiss and others (2009) reported that in children from birth to 5 years of age undergoing general anesthesia, the incidence of postextubation stridor was similar for the cuffed tube group (4.4%) vs. the uncuffed group (4.7%). However, the rate of tube exchange was significantly greater in the uncuffed group (30.8%) compared with the cuffed group (2.1%). Unfortunately, some of the major shortcomings of cuffed tracheal tubes are the relative inconsistency of the distance of the distal tube tip to the lower and upper borders of the cuff, the ODs of the tubes, and the maximal cross-sectional area of the inflated cuff and depth markers for positioning of the tube at the appropriate depth of the trachea (Fig. 10-12, B; see Chapter 1, Special Characteristics of Pediatric Anesthesia, Table 1-1) (Weiss et al., 2004a).
A variety of tracheal tubes are available for special needs. A Ring, Adaire, Elwyn (RAE) tube has a preformed contour that facilitates access to the surgical field (Fig. 10-13, A). Oral RAE tubes were initially developed for cleft-palate surgery. The proximal end of the oral RAE tube rests over the middle of the mandible, whereas the proximal end of a nasal RAE tube rests on the forehead (Fig. 10-13, B; see Chapter 25, Anesthesia for Plastic Surgery, Fig. 25-12). These preformed tracheal tubes can be used for any type of head or neck surgery in which the operating room table is turned away from the anesthesiologist and the head is maintained in a neutral position. The greatest disadvantage of preformed tubes is that the flexion point is fixed. The length may be inappropriate, especially in patients whose cricoid diameter is unusually large or small, increasing the risk of endobronchial intubation or accidental extubation, respectively. Similarly, in a patient with a laryngeal or proximal tracheal stenosis, a standard tracheal tube of a caliber small enough to be admitted to the airway may be too short. Tubes of small caliber with extra length are commercially available for this purpose.
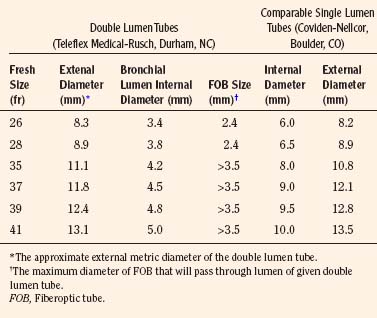
Double-Lumen Tracheal Tubes
A double-lumen tracheal tube is commonly used to attain lung separation in adults. Its advantages include rapid and easy separation of the lungs, access to both lungs to facilitate suctioning, the ability to rapidly switch to two-lung ventilation if needed, and the ability to administer CPAP or oxygen insufflation to the operative lung, when necessary. The smallest commercially available double-lumen tracheal tube is size 26F, which precludes its placement in children weighing less than 30 to 35 kg or younger than 8 to 10 years old (Table 10-1; see Chapter 23, Anesthesia for General Abdominal, Thoracic, Urologic, and Bariatric Surgery, Table 23-9). With this small, double-lumen tracheal tube, bronchoscopic confirmation of its appropriate location within the trachea and bronchus requires use of an ultrathin flexible bronchoscope (Wood, 1985).
Tracheal Tubes for One-Lung Ventilation
Because of mechanical difficulties of one-lung ventilation in small children, pediatric surgeons historically have used retractors and surgical packs to improve surgical exposure during thoracic surgery. With the increasing popularity of thoracoscopic surgical techniques in children, there is an increasing need to provide one-lung ventilation to facilitate surgical exposure (Rowe et al., 1994; Tobias, 1999; Hammer, 2001). The pediatric anesthesiologist has a number of choices for the provision of one-lung ventilation.
Univent Tracheal Tubes
The Univent Tracheal Tube (Fuji Systems Corporation; Tokyo, Japan) is a single-lumen tracheal tube with a moveable bronchial blocker built into its side wall (Fig. 10-14) (Kamaya and Krishna, 1985; Hammer et al., 1998; MacGillivray, 1988). The bronchial blocker contains a low-pressure high-volume cuff and has a central channel used to suction the blocked lung and to insufflate oxygen. The Univent Tracheal Tube is inserted into the trachea like a standard tracheal tube with the bronchial blocker withdrawn into the main tube. The bronchial blocker can then be advanced into the main-stem bronchus of the operative lung under bronchoscopic guidance. Rotation of the tracheal tube determines the direction that the blocker takes as it is advanced. Inflation of the endobronchial cuff on the balloon-tip catheter enables isolation of the operative lung. At the end of the procedure, the blocker tube can be withdrawn into the main tube, permitting postoperative ventilation without the need to change to a separate single-lumen tube.
Selective Endobronchial Intubation
Bronchial-Blocker Devices
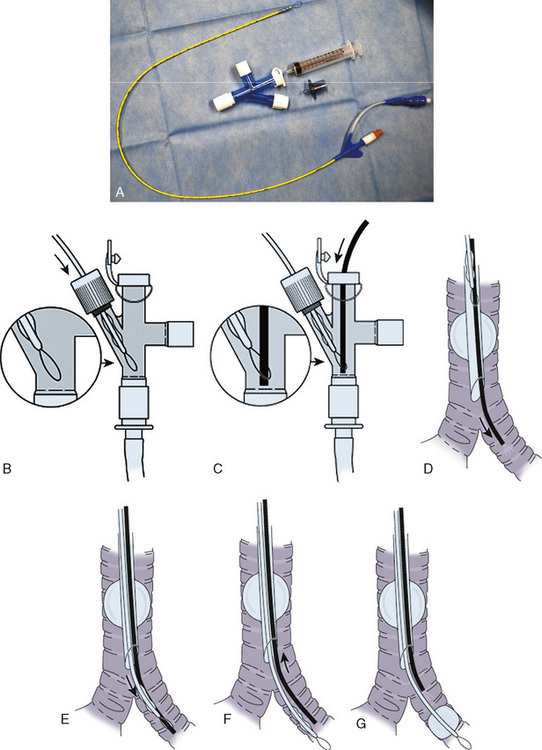
A bronchial-blocker device consists of a small balloon that is purposefully inflated within the proximal portion of the main bronchus to isolate one of the lungs under bronchoscopic guidance. Several different devices can be used as bronchial blockers, including a Fogarty embolectomy catheter and the Arndt endobronchial blocker (Cook Critical Care; Bloomington, Indiana) (Fig. 10-15). The latter device contains a central channel that allows suctioning (for lung deflation) and the application of oxygen and CPAP. It is too large for use in neonates and infants.
The Arndt endobronchial blocker is a bronchial blocker with an inflatable cuff and a central lumen, through which a wire with a looped end has been passed (Arndt et al., 1999; Hammer, 2002). The bronchial blocker is passed through a specialized adapter that is placed at the proximal end of the tracheal tube. This adapter contains the following four ports:
Tracheostomy Tubes
The three most common indications for tracheostomy in children are the need for prolonged mechanical ventilation (more than 50% of cases), upper airway obstruction (40%), and pulmonary toilet (10%) (Wetmore et al., 1999). Within each category are congenital, traumatic, metabolic, infectious, and neoplastic conditions that require tracheostomy. Although the underlying medical conditions may be numerous, the most common diagnoses in pediatric tracheostomy patients are bronchopulmonary dysplasia and neurologic disorders.
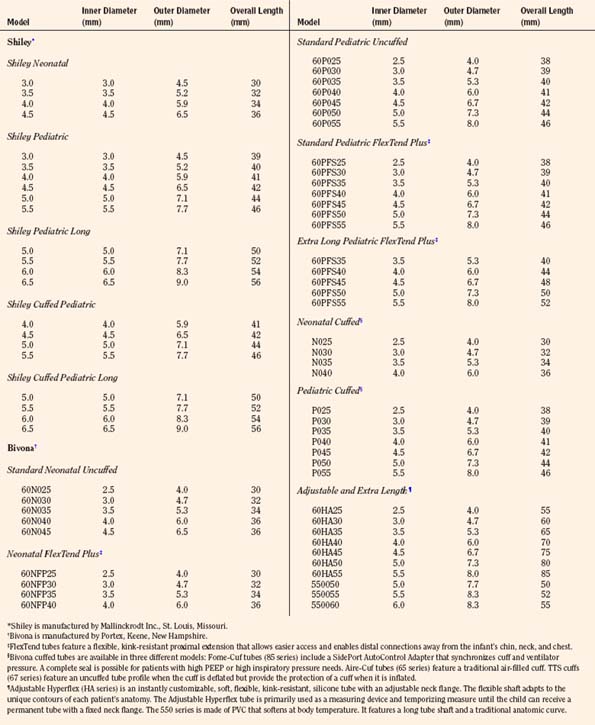
The ideal tracheostomy tube should be made of a material that causes minimal tissue reactivity, can be easily cleaned and maintained, and is available in a variety of shapes, diameters, and lengths (Fig. 10-16). The tube needs to be rigid enough to prevent kinking or collapse, yet soft enough to be comfortable for the patient. Early tracheostomy tubes were made of stainless steel or silver (Downes and Schreiner, 1985). These tubes had the advantages of causing minimal tissue reaction and averting tracheal collapse. Their rigidity caused significant discomfort to the patient because of injury to the tracheal mucosa. Most manufacturers use silicone tubes that have minimal tissue reactivity and conform to the structure of the airway. The ideal tube also contains an inner cannula that can be removed and cleaned. Modern tracheostomy tubes have a 15-mm male connector for the attachment of standard respiratory equipment. To improve the patient’s comfort and ease of care, a low-profile swivel is commonly added to the tracheostomy tube. This allows unrestricted neck movement and easy care through a suction port. Tracheostomy tubes for infants are uncuffed; in larger children and adolescents, cuffed tracheostomy tubes are preferred. Attachments are available to promote verbal communication.
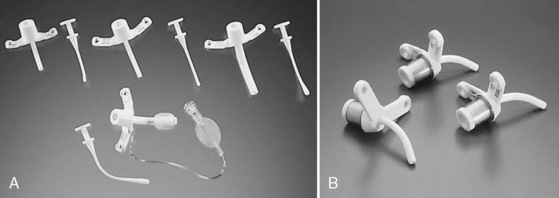
FIGURE 10-16 Representative models of pediatric tracheostomy tubes include the Shiley (A) and the Bivona (B).
The appropriate tracheostomy tube is selected on the basis of ID, OD, and length (Table 10-2). The OD determines the size of the tube that may be inserted, whereas the ID determines the actual airway size. The diameter of the tube should be large enough to allow adequate air exchange, easy suctioning, and clearance of secretions. If the indication for tracheostomy is assisted ventilation, the size of the tube should be adjusted to prevent excessive air leak. Predictors of the appropriate tube size include the child’s age and the size of a preexisting tracheal tube. A tube that is too large compromises the capillary blood flow in the tracheal wall, which may result in mucosal ischemia, ulceration, and development of fibrous stenosis. Overinflation of a cuffed tracheostomy tube for a prolonged period of time may produce similar injuries. This complication may be avoided by selecting the proper size of tracheostomy tube and adjusting the cuff pressure to less than 20 cm H2O (Table 10-2). The choice of the tube size is also influenced by visualization of the size of the tracheal lumen.
Supralaryngeal Airways
Laryngeal Masks
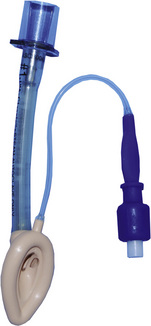
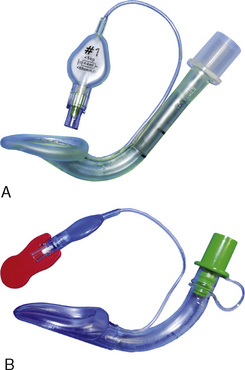
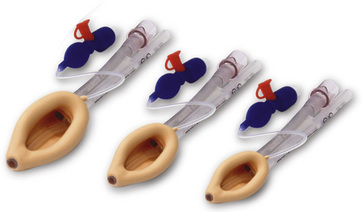
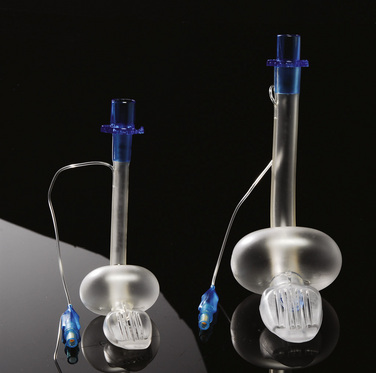
Since the invention of the original laryngeal mask, the Laryngeal Mask Airway (LMA; LMA North America; San Diego, California) (Brain, 1983), several manufacturers have developed laryngeal masks of various designs for use in children (Fig. 10-17, Table 10-3). Examples include the Ambu AuraOnce (Ambu A/S; Ballerup, Denmark), air-Q (Cookgas; St. Louis, Missouri), and the Portex Soft Seal Laryngeal Mask (SS-LM; Portex; Kent, United Kingdom) (Fig. 10-18). Most of these masks share common features, such as a bowl-shaped inflatable cuff attached to an airway tube and a detachable or fixed 15-mm adaptor at the proximal end of the airway tube. Some designs have curved airway tubes, whereas others maintain the original straight configuration. Some of the new designs incorporate a separate gastric access channel to facilitate correct placement and to allow gastric decompression; however, only the Proseal LMA is available in all pediatric sizes (Fig. 10-19). Despite variations in design, one simple principle applies to the use of these devices in children. The incidence of problems with positioning laryngeal masks is inversely proportional to the age of the child; consequently, the use of laryngeal masks in infants and neonates requires an experienced provider with a high level of vigilance. Fiberoptic bronchoscopy and magnetic resonance imaging (MRI) studies in pediatric patients demonstrate a high incidence of malpositioning of the LMA in children (Keidan et al., 2000; Monclus et al., 2007). When placed properly, the distal cuff overlies the laryngeal inlet, and the aperture bars, when present, prevent the epiglottis from obstructing the lumen (Fig. 10-20). Once inflated through a pilot tube, the cuff creates a seal in the pharynx that permits both spontaneous and controlled ventilation without a large gas leak, provided the peak pressure is below 15 cm H2O (Epstein and Halmi, 1994; Lardner et al., 2008). A variety of methods of LMA placement in children is possible. The original “Brain” (1990) technique requires that the mask be advanced across the hard palate with the cuff fully deflated, the distal aperture facing anteriorly, and the head in the classic “sniffing” position. The index finger of the right hand helps guide the LMA over the surface of the tongue while pressing the mask against the palate, thereby simulating the action of the tongue on a bolus of food. A water-based lubricant applied to the posterior surface of the LMA helps to decrease the resistance to insertion. On meeting the characteristic resistance of the upper esophageal sphincter, the cuff is inflated through the pilot tube. With cuff inflation, the tube usually moves outward a short distance as the tube centers itself over the laryngeal inlet. Inflation of the cuff to recommended maximum volumes and the use of clinical endpoints such as outward movement of the LMA may result in cuff pressures that are higher than the maximum recommended pressure of 60 cm H2O (Maino et al., 2006). High cuff pressures are associated with increased incidence of leak around the LMA, excess mucosal pressures, and an increased incidence of sore throat (Wong et al., 2009). Only a fraction of the recommended volume is required to obtain an effective seal, and using a manometer to guide inflation has been shown to decrease leakage around the LMA by promoting more effective molding of the device to the airway (Licina et al., 2008).
| Weight (kg) | Maximum Endotracheal Tube Accepted* | |
| Air-Q Size | ||
| 1 | <7 | 5.0 Cuffed |
| 1.5 | 7-15 | 6.0 Cuffed |
| 2.0 | 15-30 | 6.0 Cuffed |
| 2.5 | 20-50 | 6.5 Cuffed |
| 3.5 | 50-70 | 7.5 Cuffed |
| 4.5 | 70-100 | 8.5 Cuffed |
| Ambu AuraOnce Size | ||
| 1 | <5 | 3.5 Uncuffed |
| 1.5 | 5-10 | 4.5 Uncuffed |
| 2 | 10-20 | 4.5 Cuffed |
| 2.5 | 20-30 | 5.0 Cuffed |
| 3 | 30-50 | 6.0 Cuffed |
| 4 | 50-70 | 6.0 Cuffed |
| 5 | 70-100 | 7.0 Cuffed |
| 6 | >100 | 7.0 Cuffed |
| LMA Classic | ||
| 1 | <5 | 3.5 Uncuffed |
| 1.5 | 5-10 | 4.0 Uncuffed |
| 2 | 10-20 | 4.5 Uncuffed |
| 2.5 | 20-30 | 5.0 Uncuffed |
| 3 | 30-50 | 6.0 Cuffed |
| 4 | 50-70 | 6.0 Cuffed |
| 5 | 70-100 | 7.0 Cuffed |
| 6 | >100 | 7.0 Cuffed |
| LMA Proseal | ||
| 1 | <5 | |
| 1.5 | 5-10 | 4.5 Uncuffed |
| 2 | 10-20 | 4.5 Uncuffed |
| 2.5 | 20-30 | 4.5 Uncuffed |
| 3 | 30-50 | 5.0 Uncuffed |
| 4 | 50-70 | 5.0 Uncuffed |
| 5 | 70-100 | 6.0 Uncuffed |
* Based on Mallinckrodt tracheal tubes.
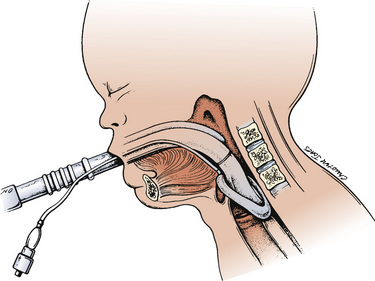
FIGURE 10-20 When properly inserted, the distal outlet of the LMA is situated over the laryngeal inlet.
The LMA can also be inserted with the cuff partially inflated or with the aperture facing posteriorly and then turned 180 degrees once it is in the larynx (Chow et al., 1991; O’Neill et al., 1994; Nakayama et al., 2002). Clinical evaluations of the different insertion techniques suggest that the rotational technique is an acceptable alternative to the standard approach and may improve placement success in children (Tsujimura, 2001; Nakayama et al., 2002; Ghai et al., 2008).
Additional maneuvers that may facilitate ease of insertion include increasing head extension, performing a jaw-thrust maneuver, inserting the LMA slightly laterally in the pharynx to avoid the uvula, or using a laryngoscope to lift the tongue anteriorly (Brain, 1989; van Heerden and Kirrage, 1989; Cass, 1991). Nevertheless, in some children, insertion is difficult and associated with pharyngeal bleeding (Marjot, 1991). The LMA is usually placed in an anesthetized child; insertion in a conscious or sedated neonate is occasionally necessary in situations in which traditional mask ventilation and endoscopy is problematic (Denny et al., 1990; Markakis et al., 1992; Stricker et al., 2008). The LMA can be placed successfully in the prone pediatric patient in the case of accidental extubation and allows for rapid airway control without having to reposition the patient supine. (Dingeman et al., 2005).
Diagnostic and interventional flexible bronchoscopy is facilitated with LMA use. After LMA placement, the airway is anesthetized with topical lidocaine, and the patient is allowed to breathe spontaneously. Adequate anesthetic depth is maintained with volatile or intravenous anesthetic (Nussbaum and Zagnoev, 2001; Yazbeck-Karam et al., 2003).
Laryngeal masks are critical tools in the management of the airways in children. They have been used successfully to rescue ventilation in patients who have difficult airways and can serve as conduits to intubation (Yang and Son, 2003; Batra, et al., 2006). Attaching a swivel connector (Swivel Connector, Ref. 60-60.305; VBM; Sulz, Germany) to the 15-mm end of an LMA facilitates ventilation during fiberoptic intubation through the LMA and subsequent LMA removal (Weiss et al., 2004b). The use of this adaptor significantly shortens apnea time and allows uninterrupted ventilation of the patient during tracheal tube placement and LMA removal. Despite its many benefits, the LMA has several limitations in children. Cuffed tracheal tubes do not pass through small LMAs, because the pilot balloon becomes lodged in the airway tube (Weiss and Goldmann, 2004). In order to place a cuffed tracheal tube, an uncuffed tube is first inserted and the LMA is removed; a tube exchange is then performed, leaving a cuffed tube. This added step can cause additional morbidity or mortality if the exchange should fail.
Standard tracheal tubes are similar in length to their corresponding LMA sizes. This similarity results in the tracheal tube disappearing into the airway tube before a significant length of tube can be slid over a fiberoptic scope into the trachea. This presents challenges with advancing the tube into the trachea and removing the LMA. Several techniques have been described to address this problem, including using a second tracheal tube or laryngeal forceps as a stabilizer, cutting the airway tube of the LMA to shorten its length, using an extra-long tracheal tube, or leaving the LMA in place after intubation (Benumof, 1992; Yamashita, 1997; Selim et al., 1999; Muraika et al., 2003; Osborn and Soper, 2003; Yang and Son, 2003; Machotta and Hoeve, 2008).
The air-Q is uniquely designed to facilitate tracheal tube placement in children of all ages (Fig. 10-18, B). This laryngeal mask is a curved device with a uniquely designed airway tube. The airway tube readily accommodates the pilot balloon of cuffed tracheal tubes in all its sizes, and the length is shortened, thereby ensuring that the tracheal tube is in the trachea when the proximal end disappears into the device (Table 10-3).
A limitation of the classic LMA is its inability to protect the airway against regurgitation of gastric contents into the trachea. In a 1992 study by Barker et al., in adults without significant risk for reflux or aspiration there was a 25% incidence of regurgitation of methylene blue into the laryngeal mask, though no tracheal soiling was noted. Pulmonary aspiration during LMA use remains an uncommon event, with the incidence reported to be approximately 2 in 10,000 (Brimacombe and Berry, 1995; Keller et al., 2004a).
In general, the smaller the size of the LMA, the higher the incidence of malpositioning, usually with the epiglottis contained within the grill of the LMA (Rowbottom et al., 1991; Mizushima et al., 1992; Dubreuil et al., 1993). Even in the presence of this type of malpositioning, ventilation is not usually impaired, although ventilation is more likely to become problematic with small movements of the child or LMA when the initial position is not optimal. (Mason and Bingham, 1990; Rowbottom et al., 1991).
The Proseal LMA is designed to separate the alimentary and respiratory tracts. It integrates a drain tube placed lateral to the airway tube of the LMA and ending at the tip of the mask (Fig. 10-19) (Brain et al., 2000). When the Proseal LMA is placed correctly, the second tube is contiguous with the alimentary tract and is sealed against the upper esophageal sphincter, allowing the passage of a nasogastric tube and confirmation of adequate positioning of the device. The Proseal LMA forms a more effective airway seal and is associated with higher oropharyngeal leak pressures (11 to 18 cm H2O higher) and less gastric insufflation than the classic LMA in children (Goldmann and Jakob, 2005; Lopez-Gil and Brimacombe, 2005; Wheeler, 2006; Lardner et al., 2008). The first-attempt success rate of placement is reported to be about 90% in children (Wheeler, 2006). The Proseal LMA may effectively separate the gastrointestinal system from the airway; however, much of the evidence for this comes from case reports and requires the assumption that the device position does not change after it has been assessed to be correctly placed (Keller et al., 2004b).
The Proseal LMA is available for use in all infants. The incidence of complications with the Proseal LMA is highest in the youngest patients (size 1.5), most of these relating to poor positioning of the LMA, obstruction, or laryngospasm (Bagshaw, 2002).
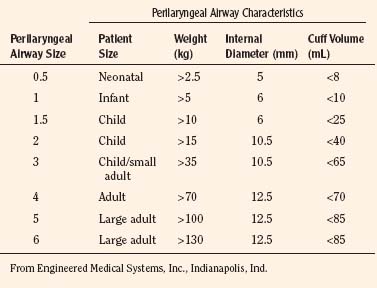
Cobra Perilaryngeal Airway
The Cobra Perilaryngeal Airway (Cobra PLA; Engineered Medical Systems, Indianapolis, Indiana) is another supraglottic device that aids in airway management. The Cobra PLA consists of a softened distal tip that has slotted openings for ventilation and is designed to be positioned in the hypopharynx overlying the laryngeal inlet (Fig. 10-21). It is secured in place by a more proximal cuff and has a proximal 15-mm connector that attaches to the anesthesia breathing circuit. The Cobra PLA is available in eight different sizes, five of which are suitable for pediatric age and weight ranges (Table 10-4). It is suitable for use during spontaneous or controlled ventilation, and the larger sizes can accommodate an appropriately sized fiberoptic bronchoscope and tracheal tube for difficult intubations.
The Cobra PLA is inserted in a similar manner to the LMA. A preliminary study in adults demonstrated that the Cobra PLA often requires readjustment up or down to affect adequate ventilation once inserted (Agro et al., 2003). A comparison examining the anatomic positions of the LMA Unique, Softseal LMA, and the Cobra PLA found that herniation of the arytenoids through the mask aperture bars was more common with the Cobra PLA than with the other devices (van Zundert et al., 2006). Polaner et al. (2006) assessed the orientation of the Cobra PLA using flexible bronchoscopy in infants and children. They found the airway to be acceptable in all children; however, the laryngeal view was nearly or completely obstructed in 76.9% of the patients who weighed 10 kg or less. A comparison of the Cobra PLA to the LMA Unique in children revealed higher seal pressures for the Cobra PLA, with similar insertion ease and times and less gastric insufflation. The fiberoptic view was found to be better based on the comparison of fiberoptic views immediately after induction to views before emergence (Szmuk et al., 2008). Airway obstruction secondary to epiglottic incarceration into the aperture bars has been reported and may be more likely if the device moves upward from the hypopharynx after insertion (Yamaguchi et al., 2006).
Laryngeal Tube
The Laryngeal Tube (LT; VBM Medical; Noblesville, Indiana) is a single-lumen tube consisting of an oval ventilation aperture placed between two distal low-pressure cuffs and is inserted in a similar manner as the LMA (Fig. 10-22). The distal (esophageal) balloon is designed to seal the airway distally and protect against regurgitation. The proximal (oropharyngeal) balloon is designed to seal off the pharynx above the ventilation port. The two balloons are inflated sequentially via a unique connector at a pressure of 60 cm H2O by using a manometer. There are seven sizes that encompass all age ranges. The LT is available in four designs: the standard LT reusable version, a disposable LT-D version, a reusable version with a gastric access channel (LTS-II), and the LTS-D, a disposable single use version with a gastric access channel (Table 10-5). Like the LMA and Cobra PLA, the LT is designed as an alternative to the anesthesia face mask and as a potential tool for providing ventilation in patients with difficult airways. It can be used during spontaneous or controlled ventilation. In adult studies, the rate of successful placement exceeds 90% (Cook et al., 2003). A prospective comparison of the LT with the LMA in children demonstrated similar insertion success rates and higher airway leak pressures with the LT (Genzwuerker et al., 2006). The LT allows higher positive inspiratory pressure than the classic LMA; like other supralaryngeal airway devices it is associated with more ventilation failures in children who weigh less than 10 kg (Genzwuerker et al., 2005). Removal of the LT in an anesthetized state reduces cough, hypersalivation, hypoxia, and tube displacement (Lee et al., 2007).
| Size | Patient | Weight/Height |
| 0 | Newborn | <5 kg |
| 1 | Baby | 5-12 kg |
| 2 | Child | 12-25 kg |
| 2.5 | Adult | 125-150 cm |
| 3 | Adult | <155 cm |
| 4 | Adult | 155-180 cm |
| 5 | Adult | >180 cm |
Flexible Fiberoptic Bronchoscopy
Fiberoptic intubation remains the gold standard for the management of difficult pediatric intubation. Miniaturization of fiberoptic and digital image technology has allowed the design of ultrathin bronchoscopes that facilitate intubation with tracheal tube sizes down to 2.5 mm ID (Fan et al., 1986; de Blic et al., 1991; Roth et al., 1994). In addition, the optical aspects of the equipment have improved with these smaller fiberscopes to allow better screen resolution. With the child sedated or anesthetized and breathing spontaneously, a fiberoptic bronchoscope that has been passed through a tracheal tube is inserted through the mouth or nose to visualize the larynx. The tracheal tube is advanced off the bronchoscope after its entrance into the trachea.
Some ultrathin bronchoscopes contain a working channel, but it is often too narrow to allow effective suctioning of secretions, so secretions and blood are more likely to obscure the view in smaller children. Furthermore, oxygen insufflation should be judiciously applied through this channel in small children because of the possibilities of generating dangerously high intrabronchial pressures and development of a tension pneumothorax (Iannoli and Litman, 2002).
Lighted Stylets
The lighted stylet (“light wand”) consists of a semirigid stylet with a bright light at the distal-most end (Fiberoptic Intubation Stylet, Anesthesia Medical Specialties; Santa Fe Springs, California; and Trachlight, Laerdal Medical, Armonk, New Jersey) (Fig. 10-23). The lighted stylet is prepared by inserting it inside a standard tracheal tube, the tip is then bent at a 60- to 120-degree angle, depending on the anatomy of the patient. The length of the bend may influence intubation success with the lighted stylet and should ideally be approximately equal to the distance from the thyroid prominence to the mandibular angle (Chen et al., 2003). After dimming of the operating room lights, a jaw thrust is performed with the nondominant hand, and the lighted stylet is placed in the midline of the pharynx and advanced slowly. A characteristic glow is noted with transillumination at the cricothyroid membrane, and the tracheal tube is advanced off the stylet into the trachea. Difficulty with placement of the lighted stylet may be caused by entrapment in the vallecula or a nonmidline insertion. In this event, the stylet should be withdrawn and reoriented in the midline to reduce the risk of airway trauma before subsequent insertion attempts (Fisher and Tunkel, 1997). A transient disappearance of the transilluminated light, followed by a reappearance more distally suggests passage behind the larynx into the esophagus.
The lighted stylet is useful when a child has an anatomically normal larynx that is difficult to visualize with direct methods. This may occur with micrognathia, temporomandibular joint disorders (or any condition that limits mandibular mobility), cervical spine instability, or facial trauma (Krucylak and Schreiner, 1992). It is particularly suited to children with limited neck and mandible mobility, but it is not useful in cases of fixed upper or lower airway obstructive pathology or in the presence of a foreign body.
Commercially made lighted stylets can be used in tracheal tubes as small as 4.5 mm ID. For small infants and neonates, a standard tracheal tube stylet can be combined with a 20-gauge fiberoptic illuminating light pipe (Storz Ophthalmics Inc., St. Louis, Missouri) attached to any standard fiberoptic light source (Davis et al., 2000). Few complications have been reported with lighted stylet use. However, arytenoid dislocation and dislodgement of the lighted stylet bulb have been described. The latter complication is less likely with newer stylet designs, and potential for arytenoid dislocation may be decreased by avoiding rotation of the stylet in the pharynx when it slides off the midline. The skill required for lighted-stylet guided intubation is easily acquired, and it remains a very useful adjunct in the management of children who present with difficult intubation.
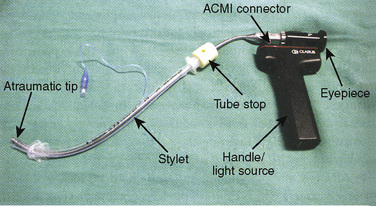
Optical Stylets
The Shikani Optical Stylet (SOS) is a modification of the lighted stylet, which has a fiberoptic core that allows transmission of the image from the distal tip to an eyepiece (Fig. 10-24). It integrates a battery-operated light source and is manufactured in pediatric and adult versions. The pediatric version can accommodate tubes down to 2.5 mm ID. The SOS has an integrated oxygen insufflation port that allows the insufflation of oxygen. Similar to the lighted stylet, a jaw thrust with the nondominant hand facilitates the elevation of the epiglottis off the posterior pharyngeal wall and allows the SOS to be placed in the midline along the tongue base until the epiglottis and glottic opening are visualized. The device can be placed just past the vocal cords, and the tube can then be advanced into the trachea.
The SOS combines the benefits of fiberoptics with a light wand and has been used successfully in patients who are difficult to intubate (Shukry et al., 2005). The SOS is limited by blood, secretions, and fogging, although fogging can be reduced by the application of an antifog solution or warming the device before use.
Bonfils Retromolar Scope
With mechanics of use similar to the SOS, The Bonfils Retromolar Scope is a rigid J-shaped optical stylet with a fixed anterior curve of 40 degrees. The manufacturer suggests a retromolar approach to the glottic opening to shorten the distance travelled to the glottis. Limited by blood, secretions, and fogging, the first-attempt success rate is reported to be about 70% in children (Bein et al., 2008).
Laryngoscopes
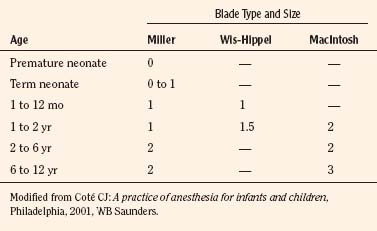
A variety of pediatric-sized laryngoscope blades are available (Table 10-6). The laryngoscope has three basic parts: a handle, a blade, and a light. The blade consists of a spatula, a flange, and a tip. Blades differ in length, width, and curvature. Laryngoscopes can be purchased with incandescent or fiberoptic light sources. Those with fiberoptic light sources provide extremely bright, highly focused light that occasionally can be obscured by the tongue and soft tissue. Selection of a particular laryngoscope is usually based on personal preference and experience, the size of the child, and the peculiarities of a specific airway problem.
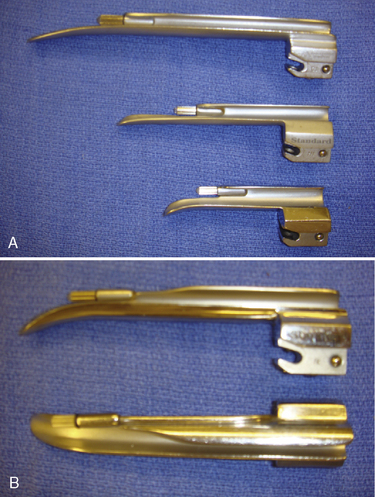
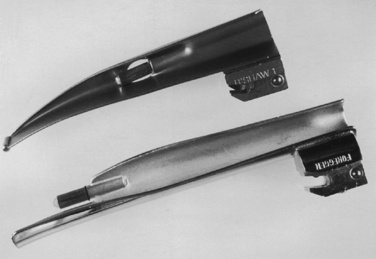
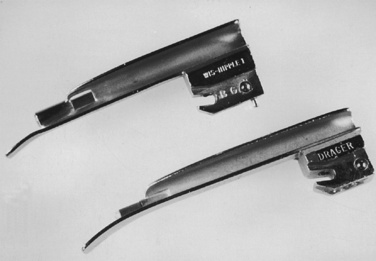
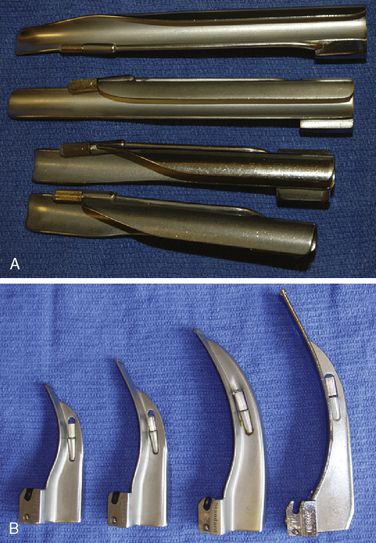
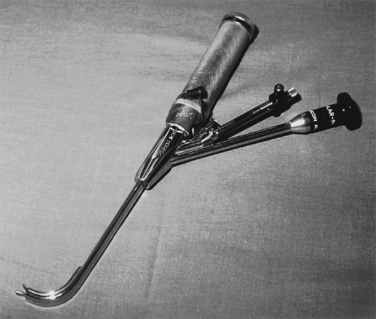
Generally, the straight Miller or Phillips blade is used in children (Fig. 10-25). This blade allows the cephalad aspect of the larynx to be exposed more easily, because the base of the tongue can be lifted out of the line of sight, and the protruding epiglottis can be retracted with the tip. Wide blades and large-flange blades, like the Robertshaw, the Flagg, and the Wis-Hipple blades, allow the wide tongue of the small child to be flattened during laryngoscopy (Fig. 10-26) (Robertshaw, 1962). The length of a 1.5 Wis-Hipple blade is especially useful in toddlers (Fig. 10-27). For older children and young adults, longer straight blades (e.g., the Miller 2) or curved blades (e.g., the MacIntosh) allow the anesthesiologist to achieve good exposure and avoid prominent dentition (Fig. 10-28).
During laryngoscopy, neonates may rapidly develop hypoxemia secondary to apnea, decreased functional residual capacity, and increased oxygen consumption. The Oxyscope is a modified Miller blade that allows insufflation of oxygen into the pharynx and decreases the rapidity with which hypoxemia occurs during laryngoscopy (Fig. 10-29) (Todres and Crone, 1981).
Video Laryngoscopes
Video laryngoscopes of various designs are being manufactured for use in children. Much of the data regarding the efficacy of these devices comes from case reports and case series with very few data from prospective trials. The Bullard laryngoscope represents one of the early designs of an optically enhanced laryngoscope for pediatric use (Fig. 10-30). It incorporates fiberoptics and mirrors to provide an indirect glottic view and has been used successfully in children of all ages (Borland and Casselbrant, 1990).Video laryngoscopes can play an important role in the successful management of the child who is difficult to intubate by direct laryngoscopy. One issue that seems to apply to many of these devices is that even with full view of the glottic opening, guiding the tracheal tube into the trachea may sometimes be difficult.
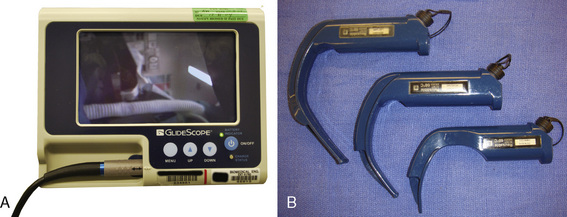
Glidescope
The Glidescope (Verathon Medical, Bothwell, Washington, U.S.) is a plastic laryngoscope with a MacIntosh-style curved blade. The blade has an integrated digital video camera that transmits the image from the tip to a portable video monitor. The Glidescope Cobalt consists of a video baton with a disposable plastic blade; the camera is positioned at the inflection point of the blade, providing a wide-angled view on the monitor (Fig. 10-31) (Cooper et al., 2005). After sweeping the tongue to the left, the Glidescope is placed in the midline of the pharynx or slightly to the left to create space for the passage of the tracheal tube. Because the view of the larynx is not directly in the line of sight as in conventional laryngoscopy, a styleted tracheal tube with similar angulation as the blade or a hockey stick configuration is required for intubation and should be prepared before laryngoscopy. The manufacturer also provides a preformed rigid stylet (Gliderite Rigid Stylet) to facilitate intubation with the Glidescope. During passage of the tracheal tube, the laryngoscopist’s attention should be directed to the passage of the styleted tube to minimize pharyngeal injury caused by blind advancement (Leong et al., 2008).
Storz Video Laryngoscope
The Storz Video Laryngoscope consists of a camera system that couples to several video blades. A Miller-type video blade is available for intubation in neonates and infants and has been used successfully after failed laryngoscopy in children (Hackell et al., 2009). A study in an infant mannequin compared the grade of view of the Miller-1 video blade to the standard Miller blade and demonstrated a one-grade improvement in view with the video blade (Wald et al., 2008; Xue et al., 2008; Fiadjoe et al., 2009). Because of the similarity in design of the Storz Video blades to standard blades, they allow documentation of the traditional direct laryngoscopy view while providing the option for visualization with video if the direct view is poor.
The Airtraq (Prodol Meditec SA; Vizcaya, Spain) is an indirect laryngoscope that uses a series of mirrors and prisms to provide a wide-angled view of the airway during intubation. The image is transmitted from a lens at the tip to a proximal viewfinder. The Airtraq incorporates a channel to house the tracheal tube during laryngoscopy (Fig. 10-32). The device is placed in the midline of the oropharynx, and the tip is advanced to the vallecula; the epiglottis may need to be elevated to obtain optimal exposure. Once adequate positioning has been obtained, the incorporated tracheal tube is advanced into the trachea. The Airtraq is currently manufactured in 4 sizes. The Airtraq has been reported to facilitate difficult intubation in a child; however, further study in a large cohort of pediatric patients is warranted to evaluate its efficacy (Lejus et al., 2009).
Intravenous Equipment
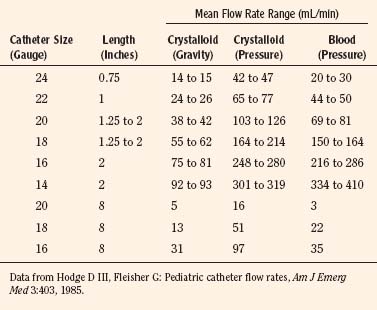
Catheters
In accordance with Poiseuille’s relationship, the resistance to flow through an intravenous catheter is related to the radius of the lumen, length of the catheter, and the viscosity of the fluid (Table 10-7). Although small differences exist between comparable catheters of various manufacturers, major flow reductions occur when they are lengthened to enable central venous cannulation (Hodge et al., 1986; Rosen and Rosen, 1986). Viscosity of the infused fluid (e.g., blood vs. crystalloid) imposes significant resistance changes only when small (smaller than 20-gauge) or long (more than 3 inches) catheters are used (Hodge and Fleisher, 1985; Rothen et al., 1992). For example, an 18-gauge catheter lengthened to 8 inches to enable central venous placement exhibits the flow characteristics of a short 24-gauge catheter.
Central venous catheters are manufactured in various sizes lengths and materials for pediatric use. Catheters are constructed from Silicone, PVC, polyethylene or polyurethane. Each of these materials has unique advantages and disadvantages; however, the most common adverse events related to catheter use are infection and thrombosis. Polyurethane catheters have been shown to have greater pressure tolerance than silicone catheters in experimental models and are less likely to rupture (Smirk et al., 2009). Polyurethane and polyethylene catheters may increase the risk of vessel perforation as compared with silicone and PVC catheters. Silicone catheters are soft and pliable and may be more difficult to insert percutaneously; PVC catheters are stiff on insertion and soften after insertion, making them easier to place (Welch et al., 1997; Macdonald and Ramasethu, 2007). Peripherally inserted central catheters (PICCs) catheters are typically single lumen, made from silicone or polyurethane, and available in sizes as small as 1.2 French for neonatal use. Central venous catheters are available in sizes as small as 2.5 French and are typically single or double lumen for neonatal use—triple-lumen catheters are available for older children (Macdonald and Ramasethu, 2007). Central venous access can be obtained in children using several vessels; they can be peripherally inserted into central veins (PICC lines) or can be inserted using the femoral, subclavian, or jugular veins. The central circulation can also be accessed in neonates through the umbilical vessels. The risk of complications with subclavian access is increased when compared with cannulation of the internal jugular vein. Internal jugular access is associated with less pneumothorax, less accidental arterial puncture, and fewer attempts to puncture the vein (Iovino et al., 2001). Ultrasound facilitates catheter placement in children and lessens the number of attempts for successful cannulation even in experienced operators (Verghese et al., 1999). In young children, the combination of Valsalva’s maneuver, liver compression, and Trendelenburg’s position produces the largest increase in jugular venous size, with Valsalva’s maneuver being the most effective single maneuver to achieve this increase. The optimal positioning of central venous catheters remains a subject of debate; however, avoiding intracardiac placement decreases the risk of cardiac perforation and tamponade. Central venous placement is not without risks, including pleural effusion, pneumothorax and hydrothorax, pneumomediastinum, and hemothorax. A risk factor for perforation is catheter stiffness; the more perpendicular the catheter is with the vessel wall the higher the chance of perforation, particularly in catheters placed on the left side of the head and neck. Ideally, the catheter tip should be placed at the superior vena cava-right atrial (SVC-RA) junction outside of the heart, and the catheter axis should parallel the axis of the vein into which it is inserted (Fletcher and Bodenham, 2000). Central catheters should be placed 1 cm outside the cardiac silhouette in premature infants and 2 cm away in full-term infants, and x-ray technology should be routinely used to diagnose catheter migration into the heart (Nowlen et al., 2002). The carina may provide an estimate of the SVC-RA junction in older children, but this may not be the case in neonates and small children (Yoon et al., 2005; Inagawa et al., 2007).
Intraosseous Access
Intraosseous access should be considered in children who need acute resuscitation and have challenging peripheral or central vascular access (ILCOR, 2005) (see Chapter 30, Anesthesia for the Pediatric Trauma Patient). The intraosseous route provides noncollapsible access to the central venous circulation, making it an ideal route to administer drugs and fluid to patients with cardiovascular compromise or shock. Onset times of drugs administered via this route are similar to those given intravenously. The preferred access site in children and infants is the anteromedial surface of the tibia, approximately 1 to 2 cm below the tibial tuberosity. Other sites that have been successfully used include the sternum, the iliac crest, the distal radius, and several others. Several companies manufacture intraosseous needles; however, access may be obtained with any styleted needle. Spinal needles of 18 or 20 gauge have been successfully used for access. After sterile preparation of the chosen site, the needle is inserted perpendicular to the skin and advanced using a rotating motion until a loss of resistance is felt. Once seated, the stylet may be removed, and successful positioning is confirmed with aspiration of marrow and the ability to flush without extravasation. Risks of intraosseous access placement include infection, extravasation of fluid and medication, injury to the growth plate, and fracture (Tobias and Ross, 2010).
A number of studies have determined that needleless intravenous catheters reduce the rate of needlestick injuries by up to 60% (Orenstein et al., 1995; Needlestick, 2000). In 2001, the U.S. Occupational Safety and Health Administration (OSHA) authored the Needlestick Safety and Prevention Act (HR 5178), which provides a legislative mandate that health-care facility employers provide employees with safety-engineered sharp devices (Federal Register on January 18, 2001).
Infusion Sets
In the face of significant hypovolemia that results from brisk blood loss or other causes of severe intravascular volume depletion, the rapid infusion of blood or crystalloid may prevent an impending disaster. The speed of infusion is directly related to the driving pressure of the infusate and the resistance of the infusion. Unless small-bore infusion tubing is used to minimize infusion dead space, resistance of the infusion circuit is mainly related to the length and diameter of the intravenous catheter. Short catheters with large, IDs have less resistance than longer catheters with smaller diameters, irrespective of peripheral or central placement (Hodge and Fleisher, 1985). The driving pressure of the intravenous solution depends on the height of the fluid bag, gravity, or the mechanical force used to push the solution. A simple method to increase the fluid administration rate in neonates and small children uses the push-pull technique, as noted above. By drawing blood or fluid into a syringe, the fluid can be “pushed” into the patient with significant speed, especially in children weighing less than 40 kg (Stoner et al., 2007). Drawbacks of this technique include the need to manually perform the task and risk of infection that may increase as the same syringe is used repeatedly. Advantages include the ability to accurately deliver fluid at a brisk rate while manually sensing changes in system resistance that may indicate an intravenous catheter infiltration.
Pressurizing or mechanically pumping an intravenous solution or blood also dramatically increases the rate of infusion. By placing an inflatable sleeve over the infusion solution bag, the driving pressure of the solution can be markedly increased. By using a hand pump (C-Fusor, Smith Medical North America, Dublin; Ohio; Infu-Surg, Cardinal Health; Dublin, Ohio), the sleeve can be inflated incrementally to 300 mm Hg to drive blood or intravenous solution into the patient. Disadvantages with this system include the need to hand-inflate the pressurization device, the inability to accurately deliver small volumes of infusate, infiltration of infusate outside of the vein, and the risk of unintended infusion of air causing an air embolism (Linden et al., 1997). Special attention to removing residual air from the blood or infusion bags before spiking the bag is necessary. Before using a rapid-infuser system, the intravenous line should be checked for free flow and patency of the vein. Accuracy of infusion volumes can be increased by using the pressure system to fill a push-pull syringe that has been set up instead of having to manually fill the syringe. Any residual air can be removed using the syringe system. Automated pressure infusers (Level 1 H-1200, Smith Medical North America, Dublin, Ohio; Ranger A1400, Arizant Healthcare; Prairie, Minnesota) eliminate the need for manual inflation through the use of high pressure air or electrical power to create the driving pressure. These devices have fluid warmers and air detectors as integral parts or as accessories and can deliver large volumes of warmed fluids rapidly through special, disposable infusion sets. Roller pump technology has also been used to rapidly infuse blood or intravenous solution. Integrated with an electromagnetic heater, air detector, and electronic flow control, high volumes of warmed fluids can be accurately delivered using this technology (Belmont Rapid Infuser; Billerica, Massachusetts). Because the risk of air is greater with high-pressure infusers, it is crucial that any automated apparatus used stops delivery of fluid if air is detected in the line. Hemolysis and hyperkalemia may also be a concern if rapid infusers are used to deliver blood through 24-gauge intravenous catheters (Miller and Schlueter, 2004).
Adamson S.J., Towell M. Thermal homestasis in the fetus and newborn. Anesthesiology. 1965;26:531-548.
Adamson D.N., Theisen F.C., Barret K.C. Effect of mechanical dilation on nasotracheal intubation. J Oral Maxillofac Surg. 1988;46:372-375.
Agro F., Barzoi G., Carassiti M., et al. Getting the tube in the oesophagus and oxygen in the trachea: preliminary results with the new supraglottic device (cobra) in 28 anaesthetised patients. Anaesthesia. 2003;58(9):920.
Ahmed-Nusrath A., Tong J.L., Smith J.E. Pathways through the nose for nasal intubation: a comparison of three endotracheal tubes. Br J Anaesth. 2008;100(2):269.
American Society of Aneshesiologists, Standards and Pratice Parameters Committee (SaPP). Standards for basic aneshetic monitoring, October 2005. Available at www.asahq.org/publicationsAndServices/standards/02.pdf.
ANSI/ISO. Anaesthetic and respiratory equipment: oropharyngeal airways. 2008.
Ans/Iso. Anaesthetic and respiratory equipment: tracheal tubes and connectors. West Conshohocken, PA: ASTM International, 1999;5361.
ASTM 1850-00. Standard specification for particular requirements for anesthesia workstations and their components, ASTM International. West Conshohoken, Pa, 2005.
Arndt G.A., Delessio S.T., Kranner P.W., et al. One-lung ventilation when intubation is difficult: presentation of a new endobronchial blocker. Acta Anaesthesiol Scand. 1999;43(3):356.
Ayre P. Endotracheal anesthesia for babies: with special reference to hare-lip and cleft palate operations. Anesth Analg. 1937;16:330.
Ayre P. The t-piece technique. Br J Anaesth. 1956;28(11):520.
Bachiller P.R., Mcdonough J.M., Feldman J.M. Do new anesthesia ventilators deliver small tidal volumes accurately during volume-controlled ventilation? Anesth Analg. 2008;106(5):1392.
Bagshaw O. The size 1.5 laryngeal mask airway (LMA) in paediatric anaesthetic practice. Paediatr Anaesth. 2002;12(5):420.
Bain J.A., Spoerel W.E. A streamlined anaesthetic system. Can Anaesth Soc J. 1972;19(4):426.
Bain J.A., Spoerel W.E. Flow requirements for a modified Mapleson d system during controlled ventilation. Can Anaesth Soc J. 1973;20(5):629.
Bain J.A., Spoerel W.E. Carbon dioxide output and elimination in children under anaesthesia. Can Anaesth Soc J. 1977;24(5):533.
Bain J.A., Spoerel W.E. Circuit. Can Anaesh Soc J. 1979;26(1):65-66.
Barker P., Langton J.A., Murphy P.J., et al. Regurgitation of gastric contents during general anaesthesia using the laryngeal mask airway. Br J Anaesth. 1992;69(3):314.
Batra Y.K., Panda N.B., Rajeev S. Airway rescue with laryngeal mask airway during sclerotherapy of a large arteriovenous malformation in the oral and maxillofacial region. Paediatric Anaesthesia. 2006;16:894-895.
Bein B., Wortmann F., Meybohm P., et al. Evaluation of the pediatric Bonfils fiberscope for elective endotracheal intubation. Paediatr Anaesth. 2008;18(11):1040.
Benumof J.L. Use of the laryngeal mask airway to facilitate fiberscope-aided tracheal intubation. Anesth Analg. 1992;74(2):313.
Berry A., Nolte F. An alternative strategy for infection control of anesthesia breathing circuits: a laboratary assessment of the Pall HME Filter. Anesth Analg. 1991;72(5):651-655.
Bissonnette B., Sessler D.I. Passive or active inspired gas humidification increases thermal steady-state temperatures in anesthetized infants. Anesth Analg. 1989;69:783-787.
Bissonnette B., Sessler D.I., LaFlamme P. Intraoperative temperature monitoring sites in infants and children and the effect of inspired gas warming on esophageal temperature. Anesth Analg. 1989;69:192-196.
Bissonnette B., Sessler D.I., LaFlamme P. Passive and active inspired gas humidification in infants and children. Anesthesiology. 1989;71:350-354.
Borland L.M., Casselbrant M. The bullard laryngoscope: a new indirect oral laryngoscope (pediatric version). Anesth Analg. 1990;70(1):105.
Bourke D.L., Wurm H., Rosenberg M., et al. Intraoperative heat conservation using a reflective blanket. Anesthesiology. 1984;60(2):151.
Brain A.I. The laryngeal mask: a new concept in airway management. Br J Anaesth. 1983;55(8):801.
Brain A.I. Further developments of the laryngeal mask. Anaesthesia. 1989;44(6):530.
Brain A.I., Verghese C., Strube P.J. The LMA ‘proseal’: a laryngeal mask with an oesophageal vent. Br J Anaesth. 2000;84:650-654.
Brain A.L. Proper technique for insertion of the laryngeal mask. Anesthesiology. 1990;73(5):1053-1054.
Brimacombe J.R., Berry A. The incidence of aspiration associated with the laryngeal mask airway: a meta-analysis of published literature. J Clin Anesth. 1995;7:297-305.
Brown E.S., Hustead R.F. Resistance of pediatric breathing systems. Anesth Analg. 1969;48(5):842.
Camus Y., Delva E., Just B., et al. Leg warming minimizes core hypothermia during abdominal surgery. Anesth Analg. 1993;77(5):995.
Cass L. Inserting the laryngeal mask. Anaesth Intensive Care. 1991;19(4):615.
Cave P., Fletcher G. Resistance of nasotracheal tubes used in infants. Anesthesiology. 1968;29(3):588.
Chen T.H., Tsai S.K., Lin C.J., et al. Does the suggested lightwand bent length fit every patient? The relation between bent length and patient’s thyroid prominence-to-mandibular angle distance. Anesthesiology. 2003;98:1070-1076.
Chow B.F., Lewis M., Jones S.E. Laryngeal mask airway in children: insertion technique. Anaesthesia. 1991;46(7):590.
Clarke R., Orkin L.R., et al. Body temperature studies in anesthetized man: effect of environmental temperature, humidity, and anesthesia systems. JAMA. 154(311), 1954.
Cook T.M., McKinstry C., Hardy R., et al. Randomized comparison of laryngeal tube with classic laryngeal mask airway for anaesthesia with controlled ventilation. Br J Anaesth. 2003;91(3):373.
Cooper R.M., Pacey J.A., Bishop M.J., et al. Early clinical experience with a new videolaryngoscope (glidescope) in 728 patients. Can J Anaesth. 2005;52:191-198.
Coté C.J., Hartnick C.J. Pediatric transtracheal and cricothyrotomy airway devices for emergency use: which are appropriate for infants and children? Paediatr Anaesth. 2009;19(Suppl 1):66-76.
Coté C.J., Petkau A.J., Ryan J.F., et al. Wasted ventilation measured in vitro with eight anesthetic circuits with and without inline humidification. Anesthesiology. 1983;59:442-446.
Dalal P.G., Murray D., Messner A.H., et al. Pediatric laryngeal dimensions: an age-based analysis. Anesth Analg. 2009;108(5):1475.
Davis L., Cook-Sather S., Schreiner M. Lighted stylet tracheal intubation: a review. Anesht Analg. 2000;90(3):745-756.
de Blic J., Delacourt C., Scheinmann P. Ultrathin flexible bronchoscopy in neonatal intensive care units. Arch Dis Child. 1991;66(12):1383.
Denny N.M., Desilva K.D., Webber P.A. Laryngeal mask airway for emergency tracheostomy in a neonate. Anaesthesia. 1990;45(10):895.
Dingeman R.S., Goumnerova L.C., Goobie S.M. The use of a laryngeal mask airway for emergent airway management in a prone child. Anesth Analg. 2005;100:670-671. table of contents
Dorsch J., Dorsch S. Understanding anesthesia equipment. Lippincott Williams & Wilkins, 2007.
Downes J.J., Schreiner M.S. Tracheostomy tubes and attachments in infants and children. Int Anesthesiol Clin. 1985;23(4):37.
Dubreuil M., Laffon M., Plaud B., et al. Complications and fiberoptic assessment of size 1 laryngeal mask airway. Anesth Analg. 1993;76(3):527.
Eckenhoff J.E. Some anatomic considerations of the infant larynx influencing endotracheal anesthesia. Anesthesiology. 1951;12(4):401.
Epstein R.h., Halmi B.H. Oxygen leakage around the laryngeal mask airway during laser treatment of port-wine stains in children. Anesth Analg. 1994;78(3):486.
Fan L.L., Sparks L.M., Dulinski J.P. Applications of an ultrathin flexible bronchoscope for neonatal and pediatric airway problems. Chest. 1986;89(5):673.
Fiadjoe J.E., Stricker P.A., Hackell R.S., et al. The efficacy of the storz miller 1 video laryngoscope in a simulated infant difficult intubation. Anesth Analg. 2009;108(6):1783.
Fisher Q.A., Tunkel D.E. Lightwand intubation of infants and children. J Clin Anesth. 1997;9(4):275.
Fletcher S.J., Bodenham A.R. Safe placement of central venous catheters: where should the tip of the catheter lie? Br J Anaesth. 2000;85(2):188.
Flynn J.T., Bancalari E., Snyder E.S., et al. A cohort study of transcutaneous oxygen tension and the incidence and severity of retinopathy of prematurity. N Engl J Med. 1992;326(16):1050.
Flynn P.E., Black A.E., Mitchell V. The use of cuffed tracheal tubes for paediatric tracheal intubation: a survey of specialist practice in the United Kingdom. Eur J Anaesthesiol. 2008;25(8):685.
Genzwuerker H.V., Fritz A., Hinkelbein J., et al. Prospective, randomized comparison of laryngeal tube and laryngeal mask airway in pediatric patients. Paediatr Anaesth. 2006;16:1251-1256.
Genzwuerker H.V., Hohl E.C.h., Rapp H.J. Ventilation with the laryngeal tube in pediatric patients undergoing elective ambulatory surgery. Paediatr Anaesth. 2005;15:385-390.
Ghai B., Makkar J.K., Bhardwaj N., et al. Laryngeal mask airway insertion in children: comparison between rotational, lateral and standard technique. Paediatr Anaesth. 2008;18:308-312.
Giesbrecht G.G., Ducharme M.B., McGuire J.P. Comparison of forced-air patient warming systems for perioperative use. Anesthesiology. 1994;80(3):671.
Glauser E.M., Cook C.D., Bougas T.P. Pressure-flow characteristics and dead spaces of endotracheal tubes used in infants. Anesthesiology. 1961;22:339.
Goldmann K., Jakob C. A randomized crossover comparison of the size 2 1/2 laryngeal mask airway proseal versus laryngeal mask airway-classic in pediatric patients. Anesth Analg. 2005;100:1605-1610.
Groudine S.B., Hollinger I., Jones J., et al. New York State guidelines on the topical use of phenylephrine in the operating room: the phenylephrine advisory committee. Anesthesiology. 2000;92:859-864.
Hackell R., Held L., Stricker P., et al. Management of the difficult infant airway with the Storz Video Laryngoscope: a case series. Anesth Analg. 2009;109(3):763-766.
Hall C.E., Shutt L.E. Nasotracheal intubation for head and neck surgery. Anaesthesia. 2003;58(3):249.
Hammer G.B. Pediatric thoracic anesthesia. Anesth Analg. 2001;92(6):1449.
Hammer G.B. Pediatric thoracic anesthesia. Anesthesiol Clin North Am. 2002;20(1):153.
Hammer G.B., Brodsky J.B., Redpath J.H., et al. The univent tube for single-lung ventilation in paediatric patients. Paediatr Anaesth. 1998;8(1):55.
Hill J., Rahimtulla K. Heat balance and the metabolic rate of new-born babies in relation to environmental temperature and the effect of age and of weight on basal metabolic rate. J Physiol. 1965;180(2):239-265.
Hodge D.3rd, Delgado-Paredes C., Fleisher G. Central and peripheral catheter flow rates in “pediatric” dogs. Ann Emerg Med. 1986;15(10):1151.
Hodge D.3rd, Fleisher G. Pediatric catheter flow rates. Am J Emerg Med. 1985;3(5):403.
Holm-Knudsen R., Eriksen K., Rasmussen L.S. Using a nasopharyngeal airway during fiberoptic intubation in small children with a difficult airway. Paediatr Anaesth. 2005;15(10):839.
Holski J. Laryngeal damage from tracheal intubation. Paediatr Anaesth. 1997;7(6):435.
Hunt K.H. Resistance in respiratory valves and canisters. Anesthesiology. 1955;16(2):190.
Hunter T., Lerman J., Bissonnette B. The temperature and humidity of inspired gases in infants using a pediatric circle system: effects of high and low-flow anesthesia. Paediatr Anaesth. 2005;15:750-754.
Iannoli E.D., Litman R.S. Tension pneumothorax during flexible fiberoptic bronchoscopy in a newborn. Anesth Analg. 2002;94(3):512.
Inagawa G., Ka K., Tanaka Y., et al. The carina is not a landmark for central venous catheter placement in neonates. Paediatr Anaesth. 2007;17(10):968.
International Liaison Committee on Resuscitation (ILCOR). Resuscitation, advanced life support. 2005;67:213-247.
Iovino F., Pittiruti M., Buononato M., et al. Central venous catheterization: Complications of different placements. Ann Chir. 2001;126(10):1001.
James I. Cuffed tubes in children. Paediatr Anaesth. 2001:259.
Kain M., Nunn J. Fresh gas flow and rebreathing in the Magill circuit with spontaneous respiration. Proc R Soc Med. 1967;60(8):749-750.
Kamaya H., Krishna P.R. New endotracheal tube (univent tube) for selective blockade of one lung. Anesthesiology. 1985;63(3):342.
Keidan I., Fine G.F., Kagawa T. Work of breathing during spontaneous ventilation in anesthetized children: a comparative study among the face mask, laryngeal mask airway and endotracheal tube. Anesth Analg. 2000;91(6):1381.
Keller C., Brimacombe J., Bittersohl J., et al. Aspiration and the laryngeal mask airway: three cases and a review of the literature. Br J Anaesth. 2004;93:579-582.
Keller C., Brimacombe J., von Goedecke A., et al. Airway protection with the proseal laryngeal mask airway in a child. Paediatr Anaesth. 2004;14:1021-1022.
Khine H.H., Corddry D.H., Kettrick R.G., et al. Comparison of cuffed and uncuffed endotracheal tubes in young children during general anesthesia. Anesthesiology. 1997;86(3):627.
Klein E.F., Graves S.A. “Hot pot” tracheitis. Chest. 1974;65:225-226.
Krucylak C.P., Schreiner M.S. Orotracheal intubation of an infant with hemifacial microsomia using a modified lighted stylet. Anesthesiology. 1992;77(4):826.
Kurz A., Kurz M., Poeschl G., et al. Forced-air warming maintains intraoperative normothermia better than circulating-water mattresses. Anesth Analg. 1993;77(1):89.
Lardner D.R., Cox R.G., Ewen A., et al. Comparison of laryngeal mask airway (LMA)-Proseal and the LMA-classic in ventilated children receiving neuromuscular blockade. Can J Anaesth. 2008;55:29-35.
Lawrence L.W., Delclos G.L., Felknor S.A., et al. The effectiveness of a needleless intravenous connection system: an assessment by injury rate and user satisfaction. Infect Control Hosp Epidemiol. 1997;18(3):175.
Lee J., Kim J., Kim S., et al. Removal of the laryngeal tube in children: anaesthetized compared with awake. Br J Anaesth. 2007;98(6):802.
Lejus C., Pichenot V., Péan D., et al. Intubation with an Airtraq of a 7-year-old child with severe cervical burned sequels. Ann Fr Anesth Reanim. 2009;28(4):399.
Leong W.L., Lim Y., Sia A.T., et al. Palatopharyngeal wall perforation during Glidescope intubation. Anaesth Intensive Care. 2008;36(6):870.
Licina A., Chambers N.A., Hullett B., et al. Lower cuff pressures improve the seal of pediatric laryngeal mask airways. Paediatr Anaesth. 2008;18(10):952.
Lin E.P., Smith K., Valley R.D. Wet forced-air warming blankets are ineffective at maintaining normothermia. Paediatr Anaesth. 2008;18(7):642.
Linden J.V., Kaplan H.S., Murphy M.T. Fatal air embolism due to perioperative blood recovery. Anesth Analg. 1997;84(2):422.
Litman R.S., Weissend E.E., Shibata D., et al. Developmental changes of laryngeal dimensions in unparalyzed, sedated children. Anesthesiology. 2003;98:41.
Lopez-Gil M., Brimacombe J. The Proseal laryngeal mask airway in children. Paediatr Anaesth. 2005;15(3):229.
MacGillivray R.G. Evaluation of a new tracheal tube with a movable bronchus blocker. Anaesthesia. 1988;43(8):687.
Machotta A., Hoeve H. Airway management and fiberoptic tracheal intubation via the laryngeal mask in a child with Marshall-Smith syndrome. Paediatr Anaesth. 2008;18:341-342.
Maino P., Dullenkopf A., Keller C., et al. Cuff filling volumes and pressures in pediatric laryngeal mask airways. Paediatr Anaesth. 2006;16:25-30.
Mapleson W.W. The elimination of rebreathing in various semi-closed anaesthetic systems. Br J Anaesth. 1954;26(5):323.
Marjot R. Trauma to the posterior pharyngeal wall caused by a laryngeal mask airway. Anaesthesia. 1991;46(7):589.
Markakis D.A., Sayson S.C., Schreiner M.S. Insertion of the laryngeal mask airway in awake infants with the Robin sequence. Anesth Analg. 1992;75(5):822.
Mason D.G., Bingham R.M. The laryngeal mask airway in children. Anaesthesia. 1990;45(9):760.
Macdonald M.G., Ramasethu J. Atlas of procedures in neonatology. Lippincott Williams & Wilkins, 2007.
Miller M.A., Schlueter A.J. Transfusions via hand-held syringes and small-gauge needles as risk factors for hyperkalemia. Transfusion (Paris). 2004;44(3):373.
Mizushima A., Wardall G.J., Simpson D.L. The laryngeal mask airway in infants. Anaesthesia. 1992;47(10):849.
Monclus E., Garcés A., De Jose Maria B. Study of the adjustment of the Ambu laryngeal mask under magnetic resonance imaging. Paediatr Anaesth. 2007;17:1182-1186.
Motoyama E.K. The shape of the pediatric larynx: cylindrical or funnel shaped? [editorial]. Anesth Analg. 2009;108(5):1379.
Muller N., Gulston G., Cade D., et al. Diaphragmatic muscle fatigue in the newborn. J Appl Physiol. 1979;46(4):688.
Muraika L., Heyman J.S., Shevchenko Y. Fiberoptic tracheal intubation through a laryngeal mask airway in a child with Treacher Collins syndrome. Anesth Analg. 2003;97:1298-1299.
Murat I. Cuffed tubes in children: a 3-year experience in a single institution. Paediatr Anaesth. 2001;11(6):748.
Nakayama S., Osaka Y., Yamashita M. The rotational technique with a partially inflated laryngeal mask airway improves the ease of insertion in children. Paediatr Anaesth. 2002;12:416-419.
Needlestick Prevention and Safety Act: 106th Congress. Public Law; 2000:106-430.
Nightingale D.A., Lambert T.F. Carbon dioxide output in anaesthetised children. Anaesthesia. 1978;33(7):594.
Nightingale D.A., Richards C.C., Glass A. An evaluation of rebreathing in a modified t-piece system during controlled ventilation of anaesthetized children. Br J Anaesth. 1965;37(10):762.
Nordin U. The trachea and cuff-induced tracheal injury: an experimental study on causative factors and prevention. Acta Otolaryngol Suppl. 1977;345:1.
Nordin U., Lindholm C.E., Wolgast M. Blood flow in the rabbit tracheal mucosa under normal conditions and under the influence of tracheal intubation. Acta Anaesthesiol Scand. 1977;21(2):81.
Nowlen T.T., Rosenthal G.L., Johnson G.L., et al. Pericardial effusion and tamponade in infants with central catheters. Pediatrics. 2002;110(1 Pt 1):137.
Nussbaum E., Zagnoev M. Pediatric fiberoptic bronchoscopy with a laryngeal mask airway. Chest. 2001;120:614-616.
O’Neill B., Templeton J.J., Caramico L., et al. The laryngeal mask airway in pediatric patients: factors affecting ease of use during insertion and emergence. Anesth Analg. 1994;78(4):659.
Orenstein R., Reynolds L., Karabaic M., et al. Do protective devices prevent needlestick injuries among health care workers? Am J Infect Control. 1995;23(6):344.
Osborn I.P., Soper R. It’s a disposable LMA, just cut it shorter: for fiberoptic intubation. Anesth Analg. 2003;97(1):299.
Pashayan A.G., Gravenstein J.S., Cassisi N.J., et al. The helium protocol for laryngotracheal operations with CO2 laser: a retrospective review of 523 cases. Anesthesiology. 1988;68(5):801.
Pethick S.L. Letter to the editor. Can Anaesth Soc J. 1975;22:115.
Polaner D.M., Ahuja D., Zuk J., et al. Video assessment of supraglottic airway orientation through the perilaryngeal airway in pediatric patients. Anesth Analg. 2006;102:1685-1688.
Polgar G., Kong G.P. The nasal resistance of newborn infants. J Pediatr. 1965;67(4):557.
Rashad K.F., Benson D.W. Role of humidity in prevention of hypothermia in infants and children. Anesth Analg. 1967;46:712-718.
Rees G.J. Anaesthesia in the newborn. Br Med J. 1950;2(4694):1419.
Rendell-Baker L., Soucek D. New pediatric face masks and anaesthetic equipment. Br Med J. 5293(1690), 1962.
Robertshaw F. A new laryngoscope for infants and children. Lancet. 1962;2(7264):1034.
Rose D.K., Byrick R.J., Froese A.B. Carbon dioxide elimination during spontaneous ventilation with a modified Mapleson d system: studies in a lung model. Can Anaesth Soc J. 1978;25(5):353.
Rose D.K., Froese A.B. The regulation of Paco2 during controlled ventilation of children with a t-piece. Can Anaesth Soc J. 1979;26(2):104.
Rosen K.R., Rosen D.A. Comparative flow rates for small bore peripheral intravenous catheters. Pediatr Emerg Care. 1986;2(3):153.
Roth A.G., Wheeler M., Stevenson G.W., et al. Comparison of a rigid laryngoscope with the ultrathin fibreoptic laryngoscope for tracheal intubation in infants. Can J Anaesth. 1994;41(11):1069.
Rothen H.U., Lauber R., Mosimann M. An evaluation of the rapid infusion system. Anaesthesia. 1992;47(7):597.
Rowbottom S.J., Simpson D.L., Grubb D. The laryngeal mask airway in children: a fibreoptic assessment of positioning. Anaesthesia. 1991;46(6):489.
Rowe M.I., Weinberg G., Andrews W. Reduction of neonatal heat loss by an insulated head cover. J Pediatr Surg. 1983;18(6):909.
Rowe R., Andropoulos D., Heard M., et al. Anesthetic management of pediatric patients undergoing thoracoscopy. J Cardiothorac Vasc Anesth. 1994;8(5):563.
Schiffmann H. Humidification of respired gases in neonates and infants. Respir Care Clin North Am. 2006;12:321-336.
Seegobin R.D., Van Hasselt G.L. Endotracheal cuff pressure and tracheal mucosal blood flow: endoscopic study of effects of four large volume cuffs. Br Med J. 1984;288(6422):965.
Selim M., Mowafi H., Al-Ghamdi A., et al. Intubation via LMA in pediatric patients with difficult airways. Can J Anaesth. 1999;46:891-893.
Sessler D.I., McGuire J., Sessler A.M. Perioperative thermal insulation. Anesthesiology. 1991;74(5):875.
Shukry M., Hanson R.D., Koveleskie J.R., et al. Management of the difficult pediatric airway with Shikani optical stylet. Paediatr Anaesth. 2005;15(4):342.
Siddik-Sayyid S.M., Abdallah F.W., Dahrouj G.B. Thermal burns in three neonates associated with intraoperative use of bair hugger warming devices. Paediatr Anaesth. 2005;18:337-339.
Smirk C., Soosay Raj T., Smith A.L., et al. Neonatal percutaneous central venous lines: fit to burst. Arch Dis Child Fetal Neonatal Ed. 2009;94(4):F298.
Spoerel W.E., Aitken R.R., Bain J.A. Spontaneous respiration with the Bain breathing circuit. Can Anaesth Soc J. 1978;25(1):30.
Stayer S.A., Bent S.T., Campos C.J., et al. Comparison of nad 6000 and servo 900c ventilators in an infant lung model. Anesth Analg. 2000;90(2):315.
Stoner M.J., Goodman D.G., Cohen D.M., et al. Rapid fluid resuscitation in pediatrics: testing the American College of Critical Care Medicine Guideline. Ann Emerg Med. 2007;50(5):601.
Stricker P.A., Budac S., Fiadjoe J.E., Rehman M.A. Awake laryngeal mask insertion followed by induction of anesthesia in infants with the Pierre Robin sequence. Acta Anaesthesiol Scand. 2008;52(9):1307.
Szmuk P., Ghelber O., Matuszczak M., et al. A prospective, randomized comparison of cobra perilaryngeal airway and laryngeal mask airway unique in pediatric patients. Anesth Analg. 2008;107:1523-1530.
Tabbutt S., Ramamoorthy C., Montenegro L.M., et al. Impact of inspired gas mixtures on preoperative infants with hypoplastic left heart syndrome during controlled ventilation. Circulation. 2001;104(12 Suppl 1):1159.
Tobias J.D. Anaesthetic implications of thoracoscopic surgery in children. Paediatr Anaesth. 1999;9(2):103.
Tobias J.D., Ross A.K. Intraosseous infusions: a review for the anesthesiologist with a focus on pediatric use. Anesth Analg. 2010;110(2):391.
Todres I.D., Crone R.K. Experience with a modified laryngoscope in sick infants. Crit Care Med. 1981;9(7):544.
Truell K.D., Bakerman P.R., Teodori M.F., et al. Third-degree burns due to intraoperative use of a Bair hugger warming device. Ann Thorac Surg. 2000;69(6):1933.
Tsujimura Y. Downfolding of the epiglottis induced by the laryngeal mask airway in children: a comparison between two insertion techniques. Paediatr Anaesth. 2001;11:651-655.
Van Heerden P.V, Kirrage D. Large tonsils and the laryngeal mask airway. Anaesthesia. 1989;44(8):703.
van Zundert A., Brimacombe J., Kamphuis R., et al. The anatomical position of three extraglottic airway devices in patients with clear airways. Anaesthesia. 2006;61(9):891.
Verghese S.T., McGill W.A., Patel R.I., et al. Ultrasound-guided internal jugular venous cannulation in infants: a prospective comparison with the traditional palpation method. Anesthesiology. 1999;91(1):71.
von Goedecke A., Brimacombe J., Hörmann C., et al. Pressure support ventilation versus continuous positive airway pressure ventilation with the proseal laryngeal mask airway: a randomized crossover study of anesthetized pediatric patients. Anesth Analg. 2005;100(2):357.
Wald S.H., Keyes M., Brown A. Pediatric video laryngoscope rescue for a difficult neonatal intubation. Paediatr Anaesth. 2008;18(12):1251-1252.
Waters D.J., Mapleson W.W. Rebreathing during controlled respiration with various semiclosed anesthetic systems. Br J Anaesth. 1961;33:374-381.
Weiss M., Dullenkopf A. Cuffed tracheal tubes in children: Past, present and future. Exp Rev Med Device. 2007;4(1):73.
Weiss M., Dullenkopf A., Fischer J.E., et al. Prospective randomized controlled multicentre trial of cuffed or uncuffed endotracheal tubes in small children. Br J Anaesth. 2009;103(6):867-873.
Weiss M., Dullenkopf A., Gysin C., et al. Shortcomings of cuffed paediatric tracheal tubes. Br J Anaesth. 2004;92(1):78-88.
Weiss M., Gerber A.C., Schmitz A. Continuous ventilation technique for laryngeal mask airway (LMA) removal after fiberoptic intubation in children. Paediatr Anaesth. 2004;14:936-940.
Weiss M., Goldmann K. Caution when using cuffed tracheal tubes for fibreoptic intubation through paediatric-sized laryngeal mask airways. Acta Anaesth Scand. 2004;48:523.
Weiss M., Knirsch W., Kretschmar O., et al. Tracheal tube-tip displacement in children during head-neck movement: a radiological assessment. Br J Anaesth. 2006;96(4):486.
Welch R.H., Gravenstein N., Blackshear R.H. Multilumen central venous catheters in children: relative potential to perforate vessels. An in vitro study. J Clin Monit. 1997;13(2):75.
Wetmore R.F., Marsh R.R., Thompson M.E., et al. Pediatric tracheostomy: a changing procedure? Ann Otol Rhinol Laryngol. 1999;108(7 Pt 1):695.
Wheeler M. Proseal laryngeal mask airway in 120 pediatric surgical patients: a prospective evaluation of characteristics and performance. Paediatr Anaesth. 2006;16:297-301.
Wilkes A.R., Benbough J.E., Speight S.E., et al. The bacterial and viral filtration performance of breathing system filters. Anaesthesia. 2000;55:458-465.
Wong J.G., Heaney M., Chambers N.A. Impact of laryngeal mask airway cuff pressures on the incidence of sore throat in children. Paediatr Anaesth. 2009;19(5):464.
Wood R.E. Clinical applications of ultrathin flexible bronchoscopes. Pediatr Pulmonol. 1985;1(5):244.
Xue F.S., Tian M., Liao X., et al. Safe and successful intubation using a Storz video laryngoscope in management of pediatric difficult airways. Paediatr Anaesth. 2008;18(8):790-792.
Yamaguchi S., Urabe K., Ikeda T., et al. Airway obstruction due to incarceration of the epiglottis into the epiglottic bars during general anesthesia with a new perilaryngeal airway (Cobra PLA). Anesth Analg. 2006;102:973.
Yamashita M. Longer tube length eases endotracheal intubation via the laryngeal mask airway in infants and children. J Clin Anesth. 1997;9(5):432.
Yang S.Y., Son S.c. Laryngeal mask airway guided fibreoptic tracheal intubation in a child with a lingual thyroglossal duct cyst. Paediatr Anaesth. 2003;13:829-831.
Yazbeck-Karam V.G., Aouad M.T., Baraka A.S. Laryngeal mask airway for ventilation during diagnostic and interventional fibreoptic bronchoscopy in children. Paediatr Anaesth. 2003;13(8):691.
Yoon S.Z., Shin J.H., Hahn S., et al. Usefulness of the carina as a radiographic landmark for central venous catheter placement in paediatric patients. Br J Anaesth. 2005;95(4):514.