Chapter 116 Epiretinal Membranes
Prevalence
Evidence regarding the epidemiology of ERMs predominantly comes from two large population studies, the Beaver Dam Eye Study and the Blue Mountains Eye Study.1–3 The overall prevalence of ERM in these populations was 7–11.8%, with a 5-year incidence of 5.3%.1–3 Idiopathic ERMs were bilateral in 19.5–31%, with a 13.5% 5-year incidence of second eye involvement.1–3
The age distribution shows a peak between the ages of 70 and 79 (11.6%), with ERMs being uncommon before the age of 60 (1.9%).3 The increased odds of ERM for persons over the age of 70 compared with those younger than 60 was 7.4.3 ERMs were more commonly diagnosed in women in the Beaver Dam cohort but not in the Blue Mountains Study and this difference may merely reflect the greater survival rate of women in this age group.
The prevalence of ERM appears to vary with ethnicity.4,5 Higher prevalence was noted in Chinese participants in a multiethnic epidemiological study (39% in Chinese versus 27.5% in Caucasians),5 whereas lower rates have been observed in the Japanese (4%).4 In the Blue Mountains Study, the prevalence of ERM was significantly increased following cataract surgery (16.8%) and retinal vein occlusion (12.5%);3 9.1% of patients with no retinal abnormality at baseline developed an ERM following cataract surgery.2
Classification
The majority of ERMs have no associated ocular abnormality and are termed idiopathic. ERMs may be classed as secondary when a pre-existing or coexisting condition has had a significant impact on development, and iatrogenic if they occur following medical or surgical intervention (Table 116.1).6
| Idiopathic
Secondary Retinal vascular disease Vascular occlusion, e.g. BRVO, CRVO Diabetic retinopathy Telangiectasias Macroaneurysm Sickle cell retinopathy Intraocular inflammation Trauma Retinal detachment and retinal tears Intraocular tumors Retinal angiomas Hamartomas Retinal dystrophies Retinitis pigmentosa Iatrogenic Postoperative Cataract Retinal detachment Silicone oil Retinopexy Laser or cryotherapy |
Clinical features
ERM is a spectrum of disease where mild forms are characterized by an abnormal retinal light reflex on fundoscopy with mild retinal thickening and no distortion of the retinal surface. In a proportion of patients, there may be progressive contraction of the ERM resulting in distortion of the retinal surface and, at times, intraretinal fluid accumulation while the membrane may become thicker and more opaque.7
A clinical grading system was proposed by Gass to describe the different stages of the disease.8
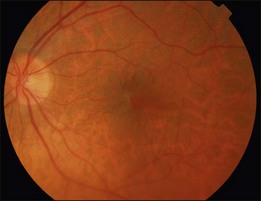
In Grade 0 (also termed cellophane maculopathy), a translucent membrane with no underlying retinal distortion is observed. These membranes are asymptomatic and the diagnosis is often an incidental finding during routine ophthalmic or optometric examination (Fig. 116.1).
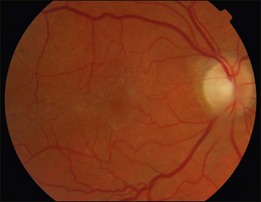
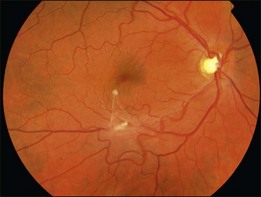
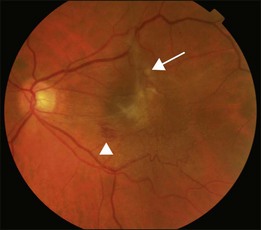
An epiretinal membrane associated with irregular wrinkling of the inner retina is classed as Grade 1 (Fig. 116.2). When this involves the fovea, patients often complain of distorted or blurred vision. These symptoms may only be perceptible when the unaffected eye is covered, particularly if the ERM occurs in the nondominant eye. Other symptoms that patients may report include loss of binocularity, central photopsia, and macropsia. Anecdotally, monocular diplopia may also be a presenting symptom of ERM, however this does not appear to have been described by patients in cohorts published to date.7 Eccentric grade 1 ERMs that do not involve the fovea may be asymptomatic (Fig. 116.3).
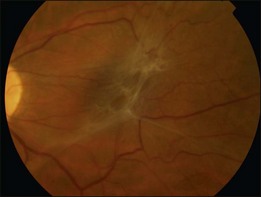
A Grade 2 ERM is characterized by an opaque membrane causing obscuration of underlying vessels and marked full-thickness retinal distortion (Fig. 116.4). Increasing vascular tortuosity and size of vessel involved tend to be a sign of more advanced disease. ERMs with full-thickness retinal distortion may also be associated with cotton-wool spots, exudates, blot hemorrhages and microaneurysms (Fig. 116.5). Cystoid macular edema is present in approximately 20–40% of patients (Fig. 116.6).9–11 Vascularization of the ERM and underlying RPE disturbance are rare but indicate more severe disease. Although symptoms are more commonly associated with increasing severity of ERM, patients may have significant ERMs and remain asymptomatic.6 Approximately 80% of patients with Grade 2 ERM will have symptoms of blurred vision or metamorphopsia.12
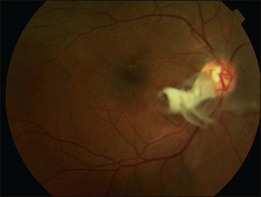
A posterior vitreous detachment (PVD) is present in approximately 60–90% of patients at the time of diagnosis.9–11 Those with partial PVD and persistent vitreomacular adhesion are more likely to develop cystoid macular edema and present with a lower visual acuity.9 In cases where a PVD does not exist, the clinical findings may be very similar to those commonly seen in vitreomacular traction syndrome (VMT) (see Chapter 118, Cystoid macular edema and vitreomacular traction). The subsequent evolution of a PVD can result in avulsion of the ERM and this may be observed as a ‘scroll’ of tissue at the membrane edge or within the vitreous gel (Fig. 116.7). Avulsion of the ERM is usually accompanied by a reduction in or resolution of symptoms.
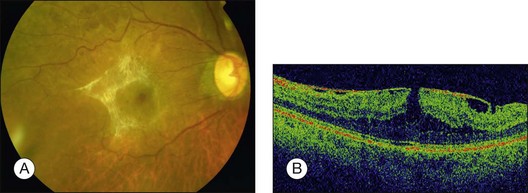
ERMs may also be associated with macular pseudoholes, lamellar holes, and much less commonly, with full-thickness macular holes, presumably as a result of tangential traction. It is possible that lamellar holes form when retinal cysts associated with the ERM rupture forming inner neural defects. When contraction of the ERM distorts the underlying retina to form a steepened foveal contour that clinically resembles a macular hole, the term pseudohole is used (Fig. 116.8). This has been described in up to 20% of patients.13
Although rapid progression of the disease has been described,14 the usual course is one of stability or slow progression over years with the visual acuity rarely deteriorating below 20/200. In a study by Appiah et al., of 324 idiopathic ERMs with a mean follow-up of 33.6 months, 49.5% maintained a visual acuity within 1 line of the initial acuity; 37.4% showed a reduction in vision, and 13.1% stayed the same.9 In the Blue Mountains Study, the area of retina occupied by the ERM remained stable in 39%, regressed in 25.7% and progressed 28.6% in a 5-year period, where change was defined as a change in area of >25%.
Pathogenesis
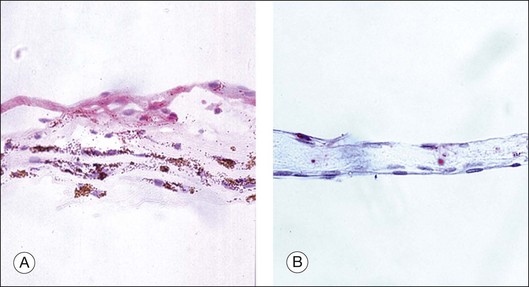
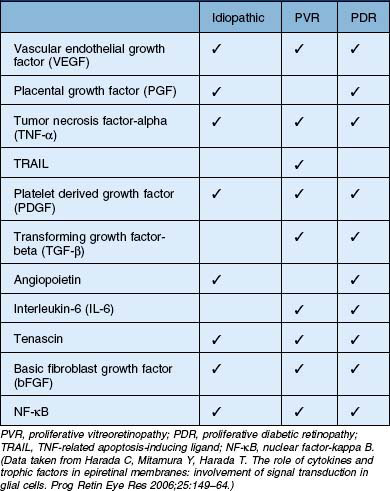
In general terms, ERMs have two main components: an extracellular matrix (consisting of collagen, laminin, tenascin, fibronectin, vitronectin, etc.) and cells of retinal and extra retinal origin (such as glial cells, neurites, retinal pigment epithelium, immune cells and fibrocytes).15,16 The relative abundance of these components within the ERM reflects the underlying etiology, severity of disease or its duration. For example, ERMs secondary to proliferative vitreoretinopathy (PVR) are more heavily pigmented due to a high RPE content when compared with idiopathic ERMs which are composed predominantly of glial elements (Fig. 116.9).17–19 Similarly, ERMs that develop in response to retinal ischemia and neovascular proliferation may have a larger vascular component. Growth factors play an important role in the formation, progression and transformation of membranes with differential expression being observed in membranes according to their etiology.20 A summary of some of the growth factors involved in the development of idiopathic and secondary ERMs is given in Table 116.2.
Membranes appear to develop predominantly where Müller cells have proliferated and migrated onto the inner surface of the retina and where hypertrophied cell processes extend towards the vitreous cavity. In idiopathic ERMs, it is possible that vitreous separation at the time of PVD exerts traction on the retina and induces Müller cell gliosis, a process of cellular hypertrophy, upregulation of cellular proteins such as vimentin and GFAP and transient cellular proliferation. Müller cells are thought to migrate to the epiretinal surface via small defects in the internal limiting membrane (ILM),15 which may occur naturally, such as those commonly seen near retinal vessels, or as a result of larger paravascular breaks observed following PVD.21,22 Activation of Müller cells may continue even once the original stimulus has been withdrawn. In retinal detachment activated Müller cells, that have penetrated the ILM and migrated on to the epiretinal surface, continue to proliferate even once retinal reattachment is achieved.23
Where ERMs form in the absence of PVD, a period of vitreomacular traction may cause chronic irritation of Müller cells inducing gliosis and vascular leakage. Foos noted the presence of vitreous between ERM and ILM in some specimens suggesting that an ERM may form prior to PVD and is a distinct entity from vitreomacular traction.21 In the absence of a PVD, glial cells appear to grow through the posterior hyaloid which then in turn becomes incorporated into the membrane. This may explain the mechanism behind spontaneous avulsion of ERMs following PVD.
More advanced ERMs have contractile components that exert traction on the underlying retina and distort the retinal vasculature with or without a breakdown of blood–retina barrier and fluid accumulation in the macula. In these membranes a higher content of contractile actin or myofibroblasts is observed. This is also described in some secondary ERMs such as those found in PVR and PDR, and in these cases ERMs may lead to detachment of the underlying retina. As the ERM matures the contractile nature of the membrane may change. This is characterized by an alteration in the expression of surface proteins, for example a reduction in GFAP and increase in α-smooth-muscle actin are associated with increasing contractility.24
Clinical assessment and differential diagnosis
Vitreomacular traction (VMT) is a condition characterized by incomplete separation of the posterior vitreous with persistent macular adhesion and traction. Like ERM, it may cause increased retinal thickness, cystoid macular edema and lamellar or full-thickness macular holes.25 ERMs may coexist with VMT in 26–83% and it has been suggested that this is a distinct subgroup of the condition.26–28 VMT is said to differ from ERM by the degree of vitreous separation in the mid-periphery. In VMT with ERM the vitreous in the mid-periphery is detached, whereas in ERM without PVD the vitreous is attached.29 It is also possible that these two conditions form part of a spectrum of disease where the vitreomacular interface is abnormal.
Cystoid macula edema (CME) may also have a similar appearance to an ERM. CME differs from an ERM in that; there is no distortion of the microvasculature, it is always centered on the fovea and may be seen on fluorescein angiography as a ‘star pattern’ in late pictures.7 CME may be the final common pathway of many diseases and may also coexist with ERM, e.g. following a retinal vein occlusion, and management will depend on determining which is the primary condition. ERM surgery is considered if it is thought to be responsible for the CME or if more conservative management has failed.
Investigations
Optical coherence tomography
Although fluorescein angiography was once the first-line investigation for epiretinal membranes, this has now been largely replaced with optical coherence tomography (OCT), which allows clinicians to assess the anatomical relationship between the vitreous face and the retinal surface. Initial studies evaluating the application of OCT to the diagnosis of ERM reported variable success, however development of more sensitive machines has significantly increased diagnostic capabilities with some studies detecting ERM in up to 90% cases.13,30–32
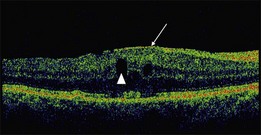
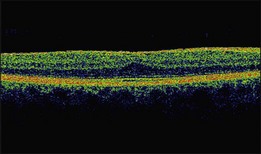
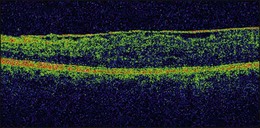
On OCT, an epiretinal membrane can be seen as a hyper-reflective layer on the surface of the retina. It may be associated with an underlying corrugation of the retinal surface, blunting of the foveal contour (Fig. 116.10), increased retinal thickness and intraretinal cysts. Idiopathic ERMs tend to be adherent globally to the underlying retina, whereas secondary ERMs are more likely to be characterized by focal retinal adhesion (Fig. 116.11).33 OCT may be a useful tool to monitor the clinical course of ERMs both prior to and following surgery. More recently, OCT has also been used at the time of surgery with some success.34 Following successful ERM surgery macular thickness and foveal contour tend to improve on OCT, although neither returns to normal. This does not, however, preclude a gradual improvement in visual function.13
Fluorescein angiography
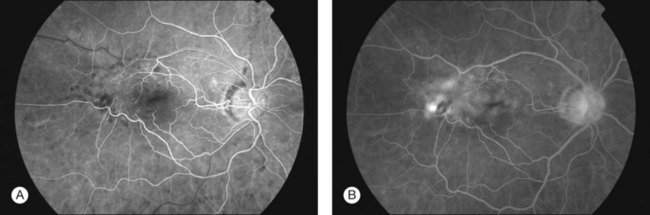
Despite the advantages of OCT, fluorescein angiography (FFA) still remains a useful tool, particularly in cases where an underlying vascular event or choroidal neovascular membrane is suspected. FFA can highlight the extent of retinal wrinkling, degree of retinal vascular tortuosity and presence of macular edema (Fig. 116.12). Wise demonstrated diffuse leakage from retinal capillaries or small veins in the late venous phase, however some ERMs showed no leakage despite having advanced disease with deep retinal edema.7 Extensive leakage from retinal capillaries or veins was associated with more rapidly progressing lesions.7 Displacement or distortion of the foveal capillary network may indicate foveal ectopia.
When to offer surgery and prognostic indicators
The principal indications for ERM surgery are patient-reported symptoms of reduced visual acuity with or without metamorphopsia. Although surgery is usually reserved for patients with a reduction in acuity to ≤20/60, improvements in surgical techniques have led to patients undergoing surgery with better visual acuity if disturbed by symptoms of metamorphopsia or diplopia and for occupational reasons. Although patients with lower presenting visual acuity make the most significant gains in acuity following surgery, their final acuity is still less than those who present with good acuity.35,36
Following surgery there is often a period of several weeks without any noticeable visual improvement. Patients can expect a visual improvement of two or more lines in 60–85% of cases 6–12 months after surgery, with 44–55% achieving a visual acuity of 20/50 or better.12,13,37,38 Those with a worse visual acuity preoperatively are more likely to have a greater visual improvement following surgery, in one series a mean improvement of 4.1 lines was achieved in those presenting with an acuity of 20/200 or worse.37 However, patients with a higher preoperative visual acuity tend to have a higher final acuity even though the percentage improvement may be less. Both idiopathic and secondary ERMs appear to benefit to an equal extent from surgery, however in some cases a pre-existing macular pathology may limit the visual recovery.37
The variability in visual results in the presence of anatomically successful surgery has led to a search for favorable prognostic factors. The available studies are largely retrospective and must therefore be interpreted with caution. Preoperative characteristics include visual acuity, central foveal thickness, presence of a macular pseudohole, cystoid macular edema, and the location, thickness and degree of opacification of the membrane.32 Poor preoperative visual acuity and long duration of symptoms are most commonly associated with worse visual outcomes.12,35,37,39,40 Preoperative foveal thickness on OCT was found to have some association with the visual improvement attained at 6 months but does not appear to be a significant factor in determining the final visual acuity.13,41 Some studies have found the presence of CME to be significantly associated with a poor prognosis but others have failed to demonstrate an association.12,35,39,40,42,43
The introduction of ultra-high-resolution OCT led to the identification of a distinct, highly reflective line just vitread to the retinal pigment epithelium (RPE). This line represents the junction of photoreceptor inner and outer segments, and when intact, it is strongly suggestive of viable photoreceptors. Some studies have demonstrated an association between preoperative disruption of the inner segment/outer segment junction (IS/OS) and poor visual outcomes following surgery,39,41,44 however others have failed to find a significant association.45–47 More recently, Ooto et al. used adaptive optics scanning laser ophthalmoscopy to describe an association between structural abnormalities of photoreceptors termed “microfolds” and symptoms of metamorphopsia.47
Focal macular ERG (FmERG) has also been used in an attempt to predict postoperative outcomes. Oscillatory potentials, “b” wave and “a” wave are all reduced in patients with ERM. A reduced b : a wave ratio appears to be a sign of early disease with a reduction in the a-wave amplitude being correlated with poor postoperative visual acuity.48
Surgery
Use of vital dyes to assist in ERM peeling
Vital dyes are a useful adjunct to ERM surgery and there is some evidence that they may improve visual outcomes.49–51 A number of dyes are in use with different affinities for intraocular collagen and cellular elements. Commonly used dyes in clinical practice include indocyanine green (ICG), trypan blue (TB), and brilliant blue.
Although ICG has been associated with toxicity in macular hole surgery, a study comparing ILM peeling with or without ICG in idiopathic ERM found no difference in outcomes.52–54 ICG has greater affinity for ILM than ERM and may be more useful when viewed as a negative stain, i.e. the ERM will be seen as an area free of stain while the surrounding ILM will be clearly visible.55,56 A number of studies reporting ICG toxicity have been published with varying incubation times, osmolarity and concentrations. In general, RPE toxicity has been more commonly demonstrated with a solution that has an osmolarity <270 mOsm, a concentration above 0.5% and incubation time >30 seconds.57 Additional factors that may be associated with RPE toxicity include application technique and duration of light exposure.57 A similar approach of negative staining can be employed using Brilliant blue (0.25 mg/mL). Brilliant blue stains ILM but does not appear to have the concerns regarding toxicity of ICG and may be a good alternative.57
Trypan blue highlights ERMs due to its strong affinity for glial cells, allowing good visualization of the extent of the membrane and thus aiding peeling. The facilitation of peeling with dyes does not appear to correlate with improvement in visual outcomes although there is a trend favoring the use of trypan blue.49–51 Providing that the dye does not enter the subretinal space, there has been no evidence of RPE toxicity.58–60
Benefit of peeling internal limiting membrane in ERM surgery
Although patches of ILM are often removed at the time of ERM surgery,61 there is debate as to the potential benefit of completing an ILM peel following the removal of ERM. It has been proposed that removal of ILM at the time of surgery removes the scaffold for myofibroblast proliferation and any residual microscopic ERM, thus reducing the risk of recurrence as well as improving visual outcomes.61,62 Conversely, there are concerns that loss of retinal tissue and damage to Müller cell footplates may adversely affect visual function and that rates of recurrence are not affected.38,63 There is currently no high-level evidence to inform this debate with published series being either small in number, retrospective or with variable staining and peeling techniques.
Intraoperative and postoperative complications
Intraoperative
Small petechial hemorrhages from the perifoveal capillary bed have been reported in 19% of cases but these do not appear to be associated with reduced visual outcome.36 Preretinal hemorrhages are usually self-limiting and do not require any treatment. Raising the infusion bottle height may reduce the clotting time particularly in patients using anticoagulant medications.
Peripheral retinal breaks are observed intraoperatively in 4–9% of patients following 20-gauge vitrectomy.37,43 More recent data suggest that the incidence of entry site breaks is reduced further with 23-gauge systems to as low as 1%.64,65 If present, such retinal breaks are treated at the time of surgery with cryotherapy or laser, according to their position, followed by gas or air tamponade.
Lens touch may rarely occur during surgery. In most cases, this will not prevent successful removal of the ERM, however if the view is significantly impaired a lensectomy may be required. Patients with a lens touch cataract will show rapid progression postoperatively and may exhibit more postoperative inflammation. These patients will require early cataract surgery and are at increased risk of peroperative complications.66
Postoperative complications
Cataract
The most common complication following ERM surgery is the formation or progression of cataract. This is true of all vitrectomy surgery and has been reported to occur with an incidence of 6–100% depending on the underlying condition, duration of follow-up and method of measuring cataract formation.37,67 The incidence of cataract within the first year ranges from 30% to 65% and increases with increasing length of follow-up.43
The cause of cataract progression following vitrectomy is still unclear but hypotheses include: alterations in oxygen tension and glucose concentrations, disruption of the anterior vitreous in the retrolental area and orientation of the infusion cannula at the time of surgery.43,67,68 Interestingly, a reduced incidence of cataract formation has been reported in series of patients who had no infusion line inserted and did not undergo a vitrectomy at the time of ERM removal, instead the ERM was peeled and left floating in the vitreous.69
Retinal detachment
Retinal detachment following ERM surgery occurs in 2–14% of eyes.35–37,42 The most common source of detachment is unidentified entry site breaks at the time of surgery. This emphasizes the importance of careful search for retinal tears using scleral indentation at the end of surgery.
Recurrence
Recurrence of ERM occurs in less than 20% of patients and is rarely visually significant. Rates of recurrence appear to be higher in younger patients (25%).6 Other rarer complications include endophthalmitis, retinal toxicity from the use of vital dyes, phototoxicity, visual field defects and subretinal neovascularization.
1 Klein R, Klein BE, Wang Q, et al. The epidemiology of epiretinal membranes. Trans Am Ophthalmol Soc. 1994;92:403–430.
2 Fraser-Bell S, Guzowski M, Rochtchina E, et al. Five-year cumulative incidence and progression of epiretinal membranes: the Blue Mountains Eye Study. Ophthalmology. 2003;110:34–40.
3 Mitchell P, Smith W, Chey T, et al. Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology. 1997;104:1033–1040.
4 Miyazaki M, Nakamura H, Kubo M, et al. Prevalence and risk factors for epiretinal membranes in a Japanese population: the Hisayma study. Graefes Arch Clin Exp Ophthalmol. 2003;241:642–646.
5 Ng CH, Cheung N, Wang JJ, et al. Prevalence and risk factors for epiretinal membranes in a multi-ethnic United States population. Ophthalmology. 2011;118:694–699.
6 McLeod D, Hiscott PS, Grierson I. Age-related cellular proliferation at the vitreoretinal juncture. Eye. 1987:1263–1281.
7 Wise G. Clinical features of idiopathic preretinal macular fibrosis. Am J Ophthalmol. 1975;79:349–357.
8 Gass JD. Stereoscopic atlas of macular diseases: diagnosis and treatment, 3rd edn. St Louis: Mosby; 1987.
9 Appiah AP, Hirose T, Kado M. A review of 324 cases of idiopathic premacular gliosis. Am J Ophthalmol. 1988;106:533–535.
10 Hirokawa H, Jalkh AE, Takahashi M, et al. Role of the vitreous in idiopathic preretinal macular fibrosis. Am J Ophthalmol. 1986;101:166–169.
11 Appiah AP, Hirose T. Secondary causes of premacular fibrosis. Ophthalmology. 1989;96:389–392.
12 Pesin SR, Olk RJ, Grand MG, et al. Vitrectomy for premacular fibroplasia. Prognostic factors, long-term follow-up, and time course of visual improvement. Ophthalmology. 1991;98:1109–1114.
13 Massin P, Allouch C, Haouchine B, et al. Optical coherence tomography of idiopathic macular epiretinal membranes before and after surgery. Am J Ophthalmol. 2000;130:732–739.
14 Sheard RM, Sethi C, Gregor Z. Acute macular pucker. Ophthalmology. 2003;110:1178–1184.
15 Bringmann A, Wiedemann P. Involvement of Muller glial cells in epiretinal membrane formation. Graefes Arch Clin Exp Ophthalmol. 2009;247:865–883.
16 Lesnik Oberstein SY, Lewis GP, Dutra T, et al. Evidence that neurites in human epiretinal membranes express melanopsin, calretinin, rod opsin and neurofilament protein. Br J Ophthalmol. 2011;95:266–272.
17 Wickham LJ, Asaria RH, Alexander R, et al. Immunopathology of intraocular silicone oil: retina and epiretinal membranes. Br J Ophthalmol. 2007;91:258–262.
18 Morino I, Hiscott P, McKechnie N, et al. Variation in epiretinal membrane components with clinical duration of the proliferative tissue. Br J Ophthalmol. 1990;74:393–399.
19 Hiscott P, Morino I, Alexander R, et al. Cellular components of subretinal membranes in proliferative vitreoretinopathy. Eye. 1989:3606–3610.
20 Harada C, Mitamura Y, Harada T. The role of cytokines and trophic factors in epiretinal membranes: involvement of signal transduction in glial cells. Prog Retin Eye Res. 2006;25:149–164.
21 Foos RY. Vitreoretinal juncture; epiretinal membranes and vitreous. Invest Ophthalmol Vis Sci. 1977;16:416–422.
22 Gandorfer A, Schumann R, Scheler R, et al. Pores of the inner limiting membrane in flat-mounted surgical specimens. Retina. 2011;31:977–981.
23 Lewis GP, Charteris DG, Sethi CS, et al. The ability of rapid retinal reattachment to stop or reverse the cellular and molecular events initiated by detachment. Invest Ophthalmol Vis Sci. 2002;43:2412–2420.
24 Sramek SJ, Wallow IH, Stevens TS, et al. Immunostaining of preretinal membranes for actin, fibronectin, and glial fibrillary acidic protein. Ophthalmology. 1989;96:835–841.
25 Jaffe NS. Vitreous traction at the posterior pole of the fundus due to alterations in the vitreous posterior. Trans Am Acad Ophthalmol Otolaryngol. 1967;71:642–652.
26 Odrobina D, Michalewska Z, Michalewski J, et al. Long-term evaluation of vitreomacular traction disorder in spectral-domain optical coherence tomography. Retina. 2011;31:324–331.
27 Koizumi H, Spaide RF, Fisher YL, et al. Yannuzzi LA. Three-dimensional evaluation of vitreomacular traction and epiretinal membrane using spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;145:509–517.
28 Gandorfer A, Rohleder M, Kampik A. Epiretinal pathology of vitreomacular traction syndrome. Br J Ophthalmol. 2002;86:902–909.
29 Heilskov TW, Massicotte SJ, Folk JC. Epiretinal macular membranes in eyes with attached posterior cortical vitreous. Retina. 1996;16:279–284.
30 Nigam N, Bartsch DU, Cheng L, et al. Spectral domain optical coherence tomography for imaging ERM, retinal edema, and vitreomacular interface. Retina. 2010;30:246–253.
31 Witkin AJ, Ko TH, Fujimoto JG, et al. Redefining lamellar holes and the vitreomacular interface: an ultrahigh-resolution optical coherence tomography study. Ophthalmology. 2006;113:388–397.
32 Wilkins JR, Puliafito CA, Hee MR, et al. Characterization of epiretinal membranes using optical coherence tomography. Ophthalmology. 1996;103:2142–2151.
33 Mori K, Gehlbach PL, Sano A, et al. Comparison of epiretinal membranes of differing pathogenesis using optical coherence tomography. Retina. 2004;24:57–62.
34 Falkner-Radler CI, Glittenberg C, Hagen S, et al. Spectral-domain optical coherence tomography for monitoring epiretinal membrane surgery. Ophthalmology. 2010;117:798–805.
35 Rice TA, De Bustros S, Michels RG, et al. Prognostic factors in vitrectomy for epiretinal membranes of the macula. Ophthalmology. 1986;93:602–610.
36 Donati G, Kapetanios AD, Pournaras CJ. Complications of surgery for epiretinal membranes. Graefes Arch Clin Exp Ophthalmol. 1998;236:739–746.
37 Wong JG, Sachdev N, Beaumont PE, et al. Visual outcomes following vitrectomy and peeling of epiretinal membrane. Clin Experiment Ophthalmol. 2005;33:373–378.
38 Lim JW, Cho JH, Kim HK. Assessment of macular function by multifocal electroretinography following epiretinal membrane surgery with internal limiting membrane peeling. Clin Ophthalmol. 2010;4:689–694.
39 Michalewski J, Michalewska Z, Cisiecki S, et al. Morphologically functional correlations of macular pathology connected with epiretinal membrane formation in spectral optical coherence tomography (SOCT). Graefes Arch Clin Exp Ophthalmol. 2007;245:1623–1631.
40 Trese MT, Chandler DB, Machemer R. Macular pucker. I. Prognostic criteria. Graefes Arch Clin Exp Ophthalmol. 1983;221:12–15.
41 Mitamura Y, Hirano K, Baba T, et al. Correlation of visual recovery with presence of photoreceptor inner/outer segment junction in optical coherence images after epiretinal membrane surgery. Br J Ophthalmol. 2009;93:171–175.
42 Poliner LS, Olk RJ, Grand MG, et al. Surgical management of premacular fibroplasia. Arch Ophthalmol. 1988;106:761–764.
43 Pournaras CJ, Donati G, Brazitikos PD, et al. Macular epiretinal membranes. Semin Ophthalmol. 2000;15:100–107.
44 Inoue M, Morita S, Watanabe Y, et al. Preoperative inner segment/outer segment junction in spectral-domain optical coherence tomography as a prognostic factor in epiretinal membrane surgery. Retina. 2011;31:1366–1372.
45 Suh MH, Seo JM, Park KH, et al. Associations between macular findings by optical coherence tomography and visual outcomes after epiretinal membrane removal. Am J Ophthalmol. 147, 2009. 473.e3–80.e3
46 Arichika S, Hangai M, Yoshimura N. Correlation between thickening of the inner and outer retina and visual acuity in patients with epiretinal membrane. Retina. 2010;30:503–508.
47 Ooto S, Hangai M, Takayama K, et al. High-resolution imaging of the photoreceptor layer in epiretinal membrane using adaptive optics scanning laser ophthalmoscopy. Ophthalmology. 2011;118:873–881.
48 Niwa T, Terasaki H, Kondo M, et al. Function and morphology of macula before and after removal of idiopathic epiretinal membrane. Invest Ophthalmol Vis Sci. 2003;44:1652–1656.
49 Haritoglou C, Eibl K, Schaumberger M, et al. Functional outcome after trypan blue-assisted vitrectomy for macular pucker: a prospective, randomized, comparative trial. Am J Ophthalmol. 2004;138:1–5.
50 Haritoglou C, Gandorfer A, Schaumberger M, et al. Trypan blue in macular pucker surgery: an evaluation of histology and functional outcome. Retina. 2004;24:582–590.
51 Kwok AK, Lai TY, Li WW, et al. Trypan blue- and indocyanine green-assisted epiretinal membrane surgery: clinical and histopathological studies. Eye. 2004;18:882–888.
52 Hillenkamp J, Saikia P, Gora F, et al. Macular function and morphology after peeling of idiopathic epiretinal membrane with and without the assistance of indocyanine green. Br J Ophthalmol. 2005;89:437–443.
53 Hillenkamp J, Saikia P, Herrmann WA, et al. Surgical removal of idiopathic epiretinal membrane with or without the assistance of indocyanine green: a randomised controlled clinical trial. Graefes Arch Clin Exp Ophthalmol. 2007;245:973–979.
54 Koestinger A, Bovey EH. Visual acuity after vitrectomy and epiretinal membrane peeling with or without premacular indocyanine green injection. Eur J Ophthalmol. 2005;15:795–799.
55 Foster RE, Petersen MR, Da Mata AP, et al. Riemann CD. Negative indocyanine green staining of epiretinal membranes. Retina. 2002;22:106–108.
56 Sakamoto H, Yamanaka I, Kubota T, et al. Indocyanine green-assisted peeling of the epiretinal membrane in proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol. 2003;241:204–207.
57 Rodrigues EB, Costa EF, Penha FM, et al. The use of vital dyes in ocular surgery. Surv Ophthalmol. 2009;54:576–617.
58 Balayre S, Boissonnot M, Paquereau J, et al. [Evaluation of trypan blue toxicity in idiopathic epiretinal membrane surgery with macular function test using multifocal electroretinography: seven prospective case studies]. J Fr Ophtalmol. 2005;28:169–176.
59 Uno F, Malerbi F, Maia M, et al. Subretinal trypan blue migration during epiretinal membrane peeling. Retina. 2006;26:237–239.
60 Balayre S, Boissonnot M, Fernandez B, et al. [Ultrastructural study of epiretinal membrane stained by trypan blue: 15 case reports]. J Fr Ophtalmol. 2005;28:159–167.
61 Bovey EH, Uffer S, Achache F. Surgery for epimacular membrane: impact of retinal internal limiting membrane removal on functional outcome. Retina. 2004;24:728–735.
62 Park DW, Dugel PU, Garda J, et al. Macular pucker removal with and without internal limiting membrane peeling: pilot study. Ophthalmology. 2003;110:62–64.
63 Kifuku K, Hata Y, Kohno RI, et al. Residual internal limiting membrane in epiretinal membrane surgery. Br J Ophthalmol. 2009;93:1016–1019.
64 Issa SA, Connor A, Habib M, et al. Comparison of retinal breaks observed during 23 gauge transconjunctival vitrectomy versus conventional 20 gauge surgery for proliferative diabetic retinopathy. Clin Ophthalmol. 2011;5:109–114.
65 Nakano T, Uemura A, Sakamoto T. Incidence of iatrogenic peripheral retinal breaks in 23-Gauge vitrectomy for macular diseases. Retina. 2011;31:1997–2001.
66 Narendran N, Jaycock P, Johnston RL, et al. The Cataract National Dataset electronic multicentre audit of 55,567 operations: risk stratification for posterior capsule rupture and vitreous loss. Eye. 2009;23:31–37.
67 Do DV, Hawkins B, Gichuhi S, et al. Surgery for post-vitrectomy cataract. Cochrane Database Syst Rev. 3, 2008. CD006366
68 Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139:302–310.
69 Saito Y, Lewis JM, Park I, et al. Nonvitrectomizing vitreous surgery: a strategy to prevent postoperative nuclear sclerosis. Ophthalmology. 1999;106:1541–1545.