CHAPTER 65 Epimacular membranes and vitreomacular traction syndromes
Epidemiologic considerations and terminology
Idiopathic, primary epimacular membranes are a quite common finding, especially in elder people. The prevalence ranges between 2 and 20% in patients aged 70–80 years1. It is hypothesized that this type of epimacular membrane is the result of glial cell migration and proliferation through small breaks of the internal limiting membrane (ILM), which represent a sequelae of posterior vitreous detachment2. Other, secondary, pathomechanisms include retinal breaks, laser coagulation or cryotherapy of the retina, inflammation, and vascular diseases3. An association with macular holes has been described4.
Clinical features, diagnosis, and differential diagnoses
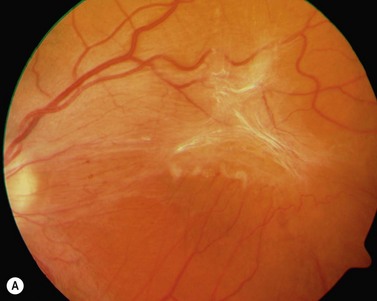
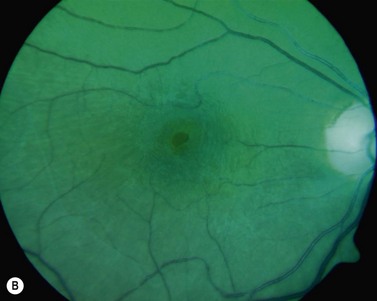
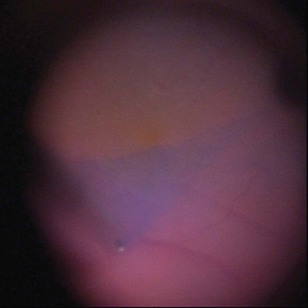
The diagnosis of epimacular membranes can be very well established using clinical examination techniques such as slit-lamp biomicroscopy with a 78 or 90 diopter lens to assess the macula. As mentioned above, the clinical features and findings can vary considerably and are dependent on the extent of the epimacular proliferation. Using biomicroscopy one may see a translucent, glistening reflex over the macula, wrinkles of the retinal surface potentially associated with a distortion of the adjacent vasculature in more pronounced cases of traction, or even an opaque sheet of tissue covering the retinal surface associated with full-thickness retinal folds and localized tractional retinal detachment (Fig. 65.1). The contraction of such membranes forming a macular pseudohole may be misinterpreted as a full-thickness macular hole in some cases. Secondary tissue alterations are the result of tractional forces and include macular edema, which can also be noted during fluorescein angiography, and small retinal hemorrhages.
Anatomical considerations
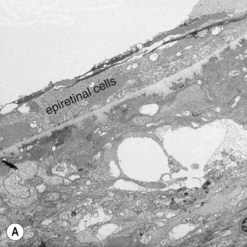
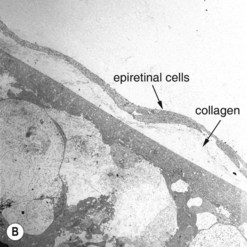
The most common cellular components of epimacular membranes are fibrous astrocytes, retinal pigment epithelial cells, fibrocytes, myofibroblasts, and macrophages. Myofibroblasts were also identified as the predominant cell type in vitreomacular traction syndrome5. The predominance of myofibroblasts may help to explain the high prevalence of cystoid macular edema and progressive vitreomacular traction characteristic of this disorder. Müller cells may also play a relevant role. A very important aspect of the pathogenesis of epimacular membranes is the anomalous PVD or splitting of the vitreous cortex (vitreoschisis) where collagen fibers are left adherent to the inner surface of the retina. These collagen fibers serve as a scaffold for cellular proliferations. Histological studies revealed that the cellular layers, which may vary from single cells to single sheet or multi-sheet layers, may grow either directly on the ILM or on a collagen layer being interspersed between the ILM and the cellular proliferation6 (Fig. 65.2). Similar observations were made in patients with vitreomacular traction syndrome5, where two distinct clinicopathologic features were identified suggesting different forms of epiretinal fibrocellular proliferation, with epiretinal membranes being interposed in native vitreous collagen and single cells or a cellular monolayer proliferating directly on the ILM. These histological findings have implications for surgery, as in one type the vitreoretinal surgeon will remove fragments of the ILM along with the epiretinal proliferation in many cases, whereas in the other type the epiretinal proliferation will be removed without the ILM, as the plane of dissection is within the collagen layer. As a consequence, parts of the collagen fibers and the ILM will remain in place. The surgeon needs to consider that in the first type only one membrane is to be removed, whereas in the second type two are to be removed.
These findings also indicate the important role of the ILM in the treatment of epimacular membranes. The understanding of the clinical relevance of the ILM for macular surgery emerged in the 1980s, when histopathological examinations of removed epiretinal membranes disclosed fragments of the ILM to be a common feature of these membranes and no functional deficits were noted in these patients7,8. It took years to transfer these findings into clinical consequences. The topic of intentional ILM removal returned to the focus of attention following the presentation of the Gass’ classification of macular holes coinciding with the first report on successful vitrectomy for macular hole closure by Kelly and Wendel in 19919,10. Intentional ILM removal was then first described by Morris et al. in patients with Terson syndrome where ILM peeling led to excellent functional results even in the long term; as a consequence, ILM peeling was then suggested for other ‘traction maculopathies’11.
Fundamental principles and goals of surgery
Obviously, the fundamental principle of surgery is to relieve the tractional force in the area of the macula and prevent a recurrence of the disease. The tractional force can be removed by inducing a PVD in cases of vitreomacular traction syndrome and to remove the visible epimacular membrane. The prevention of recurrences is related to the removal of the ILM during surgery, as clinical trials have shown, with less reproliferations having been seen in patients in whom the ILM had been removed12,13. Therefore, ILM peeling has become a widely accepted and important step for successful surgery for epimacular membranes and vitreomacular traction syndromes, despite single reports on potential negative effects14.
Anesthesia
Vitreoretinal surgery for the treatment of epimacular membranes and vitreomacular traction syndrome may be performed in general or local retrobulbar or parabulbar anesthesia, with the letter being the most frequent form. If available, the addition of hyaluronidase to the local anaesthetic for retrobulbar anesthesia may enhance the safety of the surgical procedure due to more complete akinesia and quicker onset of complete anesthesia15. If the surgery is performed in general anesthesia, an additional preoperative retrobulbar injection of a local anesthetic may significantly reduce postoperative pain16. This concept of ‘pre-emptive analgesia’ is based on the idea that analgesia initiated before a nociceptive event will be more effective than analgesia commenced afterwards.
Operation techniques
Today, small gauge sutureless (23- or 25-gauge) instruments and systems appear to be the most appropriate tools for vitrectomy in epimacular membrane and vitreomacular traction cases, as postoperative discomfort is significantly reduced. If peripheral pathologies need to be approached, less flexible 23- or 20-gauge systems (compared with 25-gauge) may be preferred17. During surgery, there are actually three relevant target structures for a successful surgery in epimacular membrane and vitremacular traction syndrome: the vitreous cortex, the epimacular cellular proliferation, and the ILM.
Induction of PVD
The visualization of the vitreous can be greatly facilitated by the intravitreal injection of triamcinolone acetate (TA), as the crystaline parts of the suspension will become entrapped between the collagen fibers and its gel structure18. Especially in vitreoschisis, TA particles help to determine where the vitreous cortex is still present. It has been recently demonstrated, that the visualization of the vitreous using triamcinolone allowed for a more efficient removal of the posterior hyaloid.
Removal of epimacular membranes
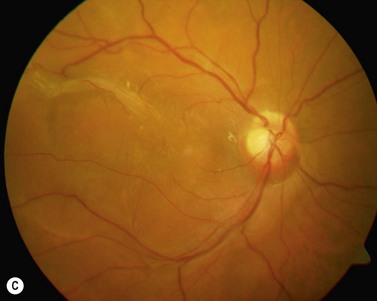
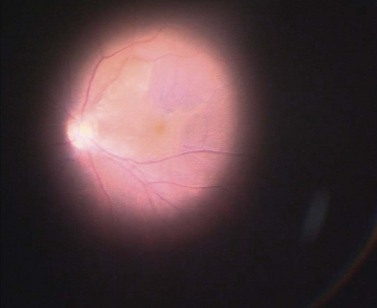
The epimacular membrane could be visualized using trypan blue19,20. Trypan blue is a vital dye with a high biocompatibility21,22 and is commercially available for the application in the posterior segment in a concentration of 0.15%. It is especially helpful to visualize the edges of the membrane, so that the surgeon can easier determine where to start the peeling (Fig. 65.3). The main drawback of trypan blue for vitreoretinal surgery is that a fluid–air exchange is required before injecting the dye in order to achieve high enough concentrations on the retinal surface and prevent a diffusion of the dye though the vitreous cavity. To avoid the need for a fluid–air exchange it was suggested combining the dye with glucose (heavy trypan blue). By eliminating the need for a fluid–air exchange, repeated applications of the dye can easily be carried out23. The removal of the epimacular membrane is often followed by an intentional peeling of the ILM, or of the ILM fragments still present.
Peeling of the internal limiting membrane (ILM)
Indocyanine green (ICG) was the first dye introduced for ILM staining24. ICG is a hydrophilic tricarbocyanine dye, first introduced in 1956. Since then ICG has been used for a variety of diagnostic purposes25,26 including the imaging of choroidal perfusion during angiography27. While the intravenous application of the dye in humans is considered very safe, the ‘off label’ intravitreal application of ICG has become a subject of ongoing discussion as toxic effects of the dye leading to functional impairment of the patients cannot be ruled out28–32. The main advantage of ICG is the good contrast provided at the level of the ILM and its selective staining properties. If applied, ICG should be injected into the fluid filled globe and then washed out immediately. The dye concentration used today varies between 0.05 to 0.5%.
Brilliant blue G (BBG) is a triarylmethane dye introduced in 2006 by Enaida and co-workers for ILM staining33,34. Meanwhile BBG is commercially available in a concentration of 0.025% for use in vitreoretinal surgery. Similar to ICG, BBG selectively stains the ILM but provides a weaker contrast, which is still enough to visualize the ILM and allow for its controlled removal. There is no clinical and experimental evidence so far indicating adverse effects of BBG on functional outcome as seen for ICG35,36. BBG can be injected into either the fluid filled globe or the air filled globe, but should be washed out immediately.
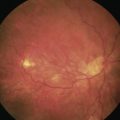
However, in the presence of epimacular membranes, any dye selectively staining the ILM has no access to the ILM and the formation of epimacular membranes may impair sufficient staining and visualization of the ILM. Poor staining may result in an incomplete removal of the ILM associated with residual ILM fragments, with an indefinite amount of cells and collagen remnants at the vitreal side of the ILM. Therefore, different approaches could be considered to achieve the best possible result. First, the ILM-dye is injected prior to the removal of the epimacular membrane. This will allow for a demarcation of the membrane due to the ‘negative’ staining of the epimacular tissue (Fig. 65.4). The removal can be initiated in an area of stained ILM and both tissue sheets may be removed together. Second, the surgeon may remove the unstained epiretinal membrane first, followed by an injection of the ILM dye. This will allow for an identification of ILM remnants after epimacular membrane peeling. Third, trypan blue is injected first, to stain and remove the epimacular membrane, followed by an ILM dye injection (double staining technique)37. However, with respect to potential toxic effects it is recommended to limit the application of any dye, exposure times, and concentrations.
Postoperative complications
Recurrences of the epimacular membrane can be reduced by a meticulous removal of the ILM12,13, as the ILM serves as a scaffold for cellular proliferation. There is a significant difference when comparing recurrences in ILM-peeled and non-ILM-peeled eyes.
Cataract formation is the most common complication in the postoperative course and is not related to epimacular membrane or ILM removal but to vitrectomy itself. The incidence of the development of nuclear sclerosis is correlated with the age of the patient and the use of an intraocular tamponade. Rates as high as 50–75% have been reported38. The development of cataract is the most frequent cause for a deterioration of visual acuity following vitreoretinal surgery. With this background many surgeons try to avoid tamponades in these surgeries unless required for other reasons such as retinal breaks.
Other postoperative complications, such as asymptomatic paracentral scotomata, may be related to a mechanical trauma of the nerve fiber layer following ILM peeling39.
The use of adjuvants such as vital dyes, especially ICG, may result in visual field defects or peculiar alterations of the retinal pigment epithelium28,29, which may be associated with less favourable functional outcome.
1 Mitchell P, Smith W, Chey T, et al. Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology. 1997;104:1033-1040.
2 Roth AM, Foos RY. Surface wrinkling retinopathy in eyes enucleated at autopsy. Trans Am Acad Ophthalmol Otolaryngol. 1971;75:1047.
3 Wise GN. Clinical features of idiopathic preretinal macular fibrosis. Am J Ophthalmol. 1975;79:349.
4 Messmer EM, Heidenkummer HP, Kampik A. Ultrastructure of epiretinal membranes associated with macular holes. Graefes Arch Clin Exp Ophthalmol. 1998;236:248-254.
5 Gandorfer A, Rohleder M, Kampik A. Epiretinal pathology of vitreomacular traction syndrome. Br J Ophthalmol. 2002;86(8):902-909.
6 Haritoglou C, Schumann R, Reiniger I, et al. Evaluation of the internal limiting membrane after conventional peeling during macular hole surgery. Retina. 2006;26(1):21-24.
7 Kampik A, Green WR, Michels RG, et al. Ultrastructural features of progressive idiopathic epiretinal membrane removed by vitreous surgery. Am J Ophthalmol. 1980;90:797-809.
8 Kampik A, Kenyon KR, Michels RG, et al. Epiretinal and vitreous membranes. Comparative study of 56 cases. Arch Ophthalmol. 1981;99:1445-1454.
9 Gass JD. Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol. 1988;106:629-639.
10 Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109:654-659.
11 Morris R, Kuhn F, Witherspoon CD, et al. Hemorrhagic macular cysts in Terson’s syndrome and its implications for macular surgery. Dev Ophthalmol. 1997;29:44-54.
12 Park DW, Dugel PU, Garda J, et al. Macular pucker removal with and without internal limiting membrane peeling: pilot study. Ophthalmology. 2003;110:62-64.
13 Haritoglou C, Gass CA, Schaumberger M, et al. Macular changes after peeling of the internal limiting membrane in macular hole surgery. Am J Ophthalmol. 2001;132:363-369.
14 Sivalingam A, Eagle RCJr, Duker JS, et al. Visual prognosis correlated with the presence of internal-limiting membrane in histopathologic specimens obtained from epiretinal membrane surgery. Ophthalmology. 1990;97:1549-1552.
15 Remy M, Pinter F, Nentwich MM, et al. Efficacy and safety of hyaluronidase 75 IU as an adjuvant to mepivacaine for retrobulbar anesthesia in cataract surgery. J Cataract Refract Surg. 2008;34(11):1966-1969.
16 Kristin N, Schönfeld CL, Bechmann M, et al. Vitreoretinal surgery: pre-emptive analgesia. Br J Ophthalmol. 2001;85(11):1328-1331.
17 Rizzo S, Genovesi-Ebert F, Murri S, et al. 25-gauge, sutureless vitrectomy and standard 20-gauge pars plana vitrectomy in idiopathic epiretinal membrane surgery: a comparative pilot study. Graefes Arch Clin Exp Ophthalmol. 2006;244(4):472-479.
18 Sakamoto T, Ishibashi T. Visualizing vitreous in vitrectomy by triamcinolone. Graefes Arch Clin Exp Ophthalmol. 2009;247(9):1153-1163.
19 Haritoglou C, Gandorfer A, Schaumberger M, et al. Trypan blue in macular pucker surgery: an evaluation of histology and functional outcome. Retina. 2004;24:582-590.
20 Perrier M, Sebag M. Epiretinal membrane surgery assisted by trypan blue. Am J Ophthalmol. 2003;135:909-911.
21 Schumann R, Gandorfer A, Priglinger SG, et al. Vital dyes for macular surgery: a comparative electron microscopy study of the internal limiting membrane. Retina. 2009;29(5):669-676.
22 Figueroa MS, Rebolleda G, Noval S, et al. Evaluation of trypan-blue toxicity in macular hole surgery with electroretinography. Arch Soc Esp Oftalmol. 2008;83(11):659-664.
23 Lesnik Oberstein SY, Mura M, Tan SH, et al. Heavy trypan blue staining of epiretinal membranes: an alternative to infracyanine green. Br J Ophthalmol. 2007;91(7):955-957.
24 Kadonosono K, Itoh N, Uchio E, et al. Staining of the internal limiting membrane in macular hole surgery. Arch Ophthalmol. 2000;118:1116-1118.
25 Bradley EC, Barr JW. Determination of blood volume using indocyanine green (cardio-green) dye. Life Sci. 1968;7:1001-1007.
26 Lund-Johansen P. The dye dilution method for measurement of cardiac output. Eur Heart J. 1990;11(suppl):6-12.
27 Craandijk A, van Beek CA. Indocyanine green fluorescence angiography of the choroid. Br J Ophthalmol. 1976;60:377-385.
28 Haritoglou C, Gandorfer A, Gass CA, et al. Indocyanine green-assisted peeling of the internal limiting membrane in macular hole surgery affects visual outcome: A clinicopathologic correlation. Am J Ophthalmol. 2002;134:836.
29 Engelbrecht NE, Freeman J, Sternberg PJr, et al. Retinal pigment epithelial changes after macular hole surgery with indocyanine green-assisted internal limiting membrane peeling. Am J Ophthalmol. 2002;133:89-94.
30 Haritoglou C, Gandorfer A, Gass CA, et al. The effect of Indocyanine-green on functional outcome of macular pucker surgery. Am J Ophthalmol. 2003;135:328-337.
31 Gandorfer A, Haritoglou C, Kampik A. Toxicity of indocyanine green in vitreoretinal surgery. Dev Ophthalmol. 2008;42:69-81.
32 Haritoglou C, Langhals H, Kampik A. Indocyanine green should not be used to facilitate removal of the internal limiting membrane in macular hole surgery. Surv Ophthalmol. 2009;54(1):138-141.
33 Enaida H, Hisatomi T, Hata Y, et al. Brilliant blue G selectively stains the internal limiting membrane/brilliant blue G-assisted membrane peeling. Retina. 2006;26:631-636.
34 Enaida H, Hisatomi T, Goto Y, et al. Preclinical investigation of internal limiting membrane staining and peeling using intravitreal brilliant blue G. Retina. 2006;26:623-630.
35 Remy M, Thaler S, Schumann RG, et al. An in-vivo evaluation of Brilliant Blue G in animals and humans. Br J Ophthalmol. 2008;92:1142-1147.
36 Lüke M, Januschowski K, Beutel J, et al. Electrophysiological effects of Brilliant Blue G in the model of the isolated perfused vertebrate retina. Graefes Arch Clin Exp Ophthalmol. 2008;246(6):817-822.
37 Stalmans P, Freon EJ, Parys-Van Ginderdeuren R, et al. Double vital staining using trypan blue and infracyanine green in macular pucker surgery. Br J Ophthalmol. 2003;87:713-716.
38 Crafoord S, Jemt M, Carlsson JO, et al. Long-term results of macular pucker surgery. Acta Ophthalmol Scand. 1997;75(1):85-88.
39 Haritoglou C, Ehrt O, Gass CA, et al. Paracentral scotomata: a new finding after vitrectomy for idiopathic macular hole. Br J Ophthalmol. 2001;85:231-233.