CHAPTER 70 Epilepsy Surgery
Outcome and Complications
The past 25 years has witnessed the evolution of epilepsy monitoring and surgery into a mature neuroscience subspecialty.1,2 The proliferation of multidisciplinary epilepsy centers has increased our capacity to provide multidisciplinary evaluations of patients with intractable epilepsy, thereby leading to greater numbers of patients being offered surgical treatment (Table 70-1). A robust clinical literature has documented both improvements in surgical approaches and a set of measures of surgical outcomes that has stimulated changes in our approach to these patients. In addition, the emergence of a nosologic context of “surgically remediable syndromes” has been spurred by advances in brain imaging and studies of the neurobiologic underpinnings of epileptogenesis.
TABLE 70-1 Worldwide Surgical Procedures for Temporal Lobe Epilepsy
| PROCEDURE | BEFORE 1986* | 1986-1990† |
|---|---|---|
| ATLX | 2336 | 4862 |
| SAH | — | 568 |
| Neocortical resection | 825 | 1073 |
| Lesionectomy | — | 440 |
ATLX, anterior temporal lobectomy; SAH, selective amygdalohippocampectomy.
From Engel J. Surgery for seizures. N Engl J Med. 1996;334:647-652.
Traditional en bloc resective approaches, which predominated during the first 60 years of epilepsy surgery, have been supplemented by more focused, minimalistic approaches, including selective amygdalohippocampectomy (SAH), multiple subpial transection (MST), keyhole deafferentation hemispherotomy, radiosurgery, and neuromodulatory approaches such as deep brain stimulation (DBS), vagal nerve stimulation (VNS), and the NeuroPace device (Table 70-2).
TABLE 70-2 Surgical Approaches for Epilepsy
Advances in our understanding of the intractable epilepsies have facilitated a discrete taxonomy of “surgically remediable syndromes” (Table 70-3).1 Each syndrome and each new treatment approach engender a new data set with regard to (1) seizure, neurobehavioral, and psychosocial outcomes; (2) complications (surgical, neurological, neuropsychological, and psychobehavioral); (3) “health outcomes” expressed in terms of alterations in quality of life (QOL); and (4) “cost-effectiveness” in comparison to alternative therapeutic interventions.
TABLE 70-3 Surgically Remediable Syndromes
| Temporal lobe epilepsy (TLE) | Idiopathic Mesial temporal sclerosis/mesial temporal lobe epilepsy Lesional (tumor, vascular malformation, developmental, ischemic, traumatic) |
| Extratemporal epilepsy | Idiopathic Lesional (tumor, vascular malformation, developmental, ischemic, traumatic) |
| Catastrophic epilepsy | Lesional Hemimegalencephaly Diffuse cortical dysplasias Sturge-Weber syndrome Rasmussen’s encephalitis Porencephalic cysts |
| Secondarily generalized epilepsies | Lennox-Gastaut syndrome |
Epidemiology and Health Care Costs of Intractable Epilepsy
Epilepsy is a relatively common disorder, with more than 2 million Americans (1.3%) afflicted and approximately 1 in 10 Americans having a minimum of one seizure over the course of a lifetime.3 Up to a third of all individuals with epilepsy are refractory to medical therapy.4 Medically intractable epilepsy is costly. Estimates of the lifetime cost of intractable epilepsy incorporate both direct cost (cost of medical care) and indirect cost (i.e., productivity, lost earnings). In a 2000 study, patients with medically intractable epilepsy in the United States were found to account for 42% of the estimated $1.7 billion in annual direct medical costs for epilepsy and 86% of the $10.8 billion in indirect costs.5 Beyond these estimates, the burden of epilepsy incorporates additional, unmeasurable indirect costs that encompass pain, suffering, and reduction in the QOL of epileptic patients and their caretakers.6,7
Advances in Outcome Assessment of Epilepsy Surgery
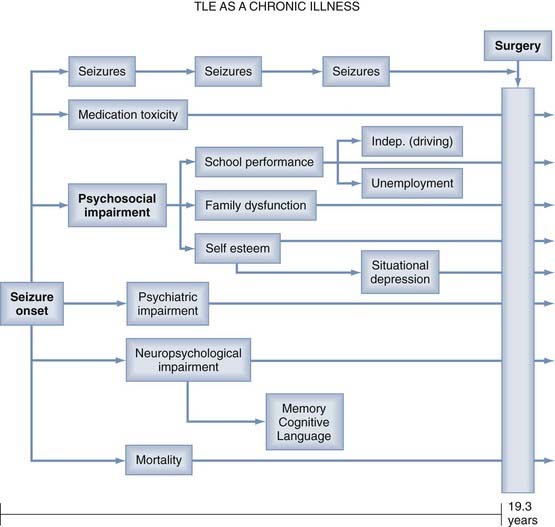
Seizure frequency, previously considered the “gold standard” of outcome measures, is an inadequate measure of surgical outcome for many reasons. Persistent epileptic seizures are often associated with significant psychosocial, psychiatric, and neuropsychological impairments, as well as medication toxicity and excess mortality rates (Fig. 70-1).8 These disabilities may persist despite relief of seizures after “successful” surgery. Therefore, contemporary studies of postoperative outcome emphasize neuropsychological and psychosocial functions and “health outcomes” commensurate with the World Health Organization’s definition of health as “… a state of complete physical, mental and social well-being …”.3,8
Classification of Seizure Outcomes
Studies have demonstrated that “health-related quality of life” (HRQOL) and psychosocial measures may not improve significantly with as much as a 70% reduction in seizure frequency after surgery.9 In recognition of the benefits of postoperative freedom or near freedom from seizures, contemporary seizure outcome classification schemes have emphasized patterns of seizure reduction that are likely to have an impact on QOL.10–12 The Engel classification scheme (Table 70-4) provides four categories of seizure outcome: I, seizure free; II, “rare” seizures (two to three per year): III, “worthwhile improvement” (>90% reduction); and IV, “no worthwhile improvement” (<90% reduction).8,11 That a class IV (“no worthwhile improvement”) outcome may be associated with a 70% reduction in frequency is emphasized by HRQOL studies showing that epilepsy-specific measures are affected even by a few seizures per year.
From Engel J. Surgery for seizures. N Engl J Med. 1996;334:647-652.
Neuropsychological Outcomes
Neuropsychological assessment is useful both during the surgical patient selection process and as a tool to assess outcomes after surgery. The aim of the evaluation is to establish a profile of the patient’s strengths and weaknesses in multiple domains on a variety of standardized tests and questionnaires (Table 70-5) in relation to normative values derived from the general population.13
TABLE 70-5 Neuropsychological Assessment of Epileptic Patients
| FUNCTIONS | TESTS |
|---|---|
| Sensory functions | Halstead-Reitan examination |
| Motor functions: dexterity, coordination, speed, flexibility | Purdue Pegboard, Grooved Pegboard, Thurstone’s Uni- and Bimanual Coordination Test |
| Perceptual-motor functions | Beery Visuo-Motor Integration Test, Block Design, Rey-Osterrieth Complex Figure |
| Psychomotor development and intelligence | Griffith or Bayley Developmental Scales, Wechsler Intelligence Scales for Adults or Children (WISC, WAIS), Stanford-Binet |
| Attention | Concentration Endurance Test, Auditory Continuous Performance Test |
| Memory and Learning | |
| General | Wechsler Memory Scales for Children and Adults |
| Verbal: word lists, story recall | California Verbal Learning Test (CVLT) |
| Visual: faces, patterns | Rey-Osterrieth Complex Figure |
| Expressive: sentence construction | Boston Naming Test, Token Test |
| Receptive: comprehension | Peabody Picture Vocabulary Test (PPVT) |
| Written: reading, spelling | Wide Range Individual Achievement Test (WIAT) |
| Numerical operations | WIAT, Woodcock-Johnson Achievement Battery |
| Executive functions | Tower of London, Wisconsin Card Sorting Test, Fluency Tests |
| Personality | Rorschach, Thematic Apperception Test for Children or Adults, SCL-90R |
| Affective state | Beck Depression Inventory, Hamilton Anxiety Scale |
| Social adjustment | Vineland Adaptive Behavior Scales, Achenbach Child Behavior Check List (CBCL) |
| Quality of life | Quality of Life in Epilepsy questionnaires (e.g., QOLIE-31, QOLIE-AD-48) |
Of particular concern in temporal lobe surgery are losses of memory function, including the rare but disabling syndrome of global amnesia, as well as the more common material-specific memory losses affecting short-term verbal memory in the language-dominant hemisphere and visual-spatial memory in non–dominant-hemisphere, temporal lobe operations. The Wada test, which was originally developed to determine hemispheric lateralization of language function,14 was subsequently adapted by Milner and colleagues15 to provide a measure of the risk for loss of memory function postoperatively. The Wada test has been used for many years to identify patients at risk for global memory loss,16 and in fact, such losses are uncommon since the Wada test was universally adopted. However, reports of favorable memory outcomes in patients who failed the Wada test preoperatively (“false positives”) have called into question the reliability of this procedure in some patients.17 The Wada test has also been useful in identifying lateralized temporal lobe dysfunction, which may correlate with the side of seizure onset,18,19 the likelihood of a favorable seizure outcome, and more recently, prediction of the risk for material-specific memory loss (particularly verbal memory loss) after surgery.20,21 Nonetheless, the Wada test is invasive and requires a degree of patient cooperation, which may be suboptimal in young children and mentally retarded patients. Wada test results may be difficult to interpret in patients with bilateral language representation, excessive agitation, insufficient hemispheric inactivation by amobarbital (Amytal), or other procedural factors.13,22 Functional neuroimaging modalities (i.e., functional magnetic resonance imaging [fMRI], [18F]fluorodeoxyglucose positron emission tomography [FDG-PET], and [99mTc]-hexamethylpropyleneamine oxime single-photon emission computed tomography [HMPAO-SPECT]) are increasingly being used as noninvasive tools for localization of epileptogenic cortex and assessment of focal functional deficits in patients with intractable epilepsy.23–26 [18F]FDG-PET, by imaging the rate of cerebral glucose metabolism, reflects neuronal losses and focal functional deficits in epileptic patients and serves as a predictor of postoperative seizure outcomes. In 70% to 90% of patients with temporal lobe epilepsy (TLE), interictal [18F]FDG-PET detects unilateral temporal hypometabolism or asymmetric bitemporal hypometabolism. This reflects neuronal loss in the damaged temporal lobe, correlates with clinical and neurophysiologic findings,24–26 and has been associated with favorable postsurgical seizure control in several studies.25,27,28 Patients with left mesial temporal lobe epilepsy (MTLE) and regional left hemispheric hypometabolism tend to have impairments in verbal memory and word fluency.29 Although the laterality of hypometabolism as determined by [18F]FDG-PET is related to memory deficits measured by the Wada test,30–33 the location or spatial pattern of the metabolic changes that can predict impairment in Wada memory performance has not been characterized. If future studies can demonstrate a strong correlation between Wada memory scores and metabolism on [18F]FDG-PET, presurgical [18F]FDG-PET could play a role in predicting memory laterality during the presurgical evaluation.
fMRI represents neuronal activity indirectly via hemodynamic changes in the brain. The size of the cortical area activated and the number of involved neurons directly influence the magnitude of the changes in regional cerebral blood flow.34 Studies comparing fMRI and Wada test results in the same patient have shown a high correlation (from 80% to 100%) between the two procedures when the studies were aimed at either investigating language lateralization35–38 or assessing memory asymmetries.39–41
Health-Related Quality of Life
Over the past decade, epilepsy-specific HRQOL instruments have been developed to assess the impact of epilepsy surgery on the “health” and “quality of life” of patients afflicted with intractable epilepsy. These instruments incorporate “generic” measures of health status previously validated in studies of health outcomes in other disease states, as well as “epilepsy-specific” measures of health status, which are more sensitive to the cognitive, memory, and role limitation issues implicit in the disease of intractable epilepsy.8,42,43 Contemporary outcome studies incorporate “health outcomes” measures in which the individual patient’s perspective becomes an integral aspect of the health care outcomes assessment.44 To facilitate HRQOL studies in epilepsy surgery patients, assessment tools such as the Epilepsy Surgery Inventory (ESI-55) have been developed and validated for use in this population.42 This instrument incorporates a “generic core” adapted from the RAND 36-Item Health Survey, along with 12 epilepsy-specific items addressing the domains of cognitive function, role limitations because of memory problems, and health perceptions.45 Other instruments developed to assess HRQOL have included the Quality of Life in Epilepsy—89 (QOLIE-89)46 and a shorter version, the QOLIE-10.44
Cost-Effectiveness
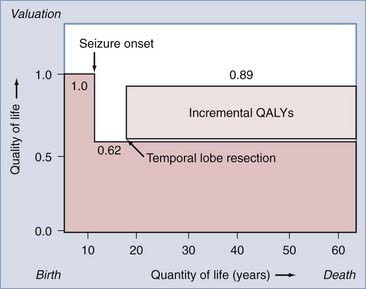
The reduction in the morbidity associated with chronic epilepsy achieved with epilepsy monitoring and surgery appears to be accompanied by parallel reductions in long-term health care costs and unemployment. Recent advances in epidemiologic and “health outcomes” investigations have facilitated cost-effectiveness studies of epilepsy surgery. Cost-effectiveness studies incorporate established patient preferences for being in certain states of health, along with cost estimates of each health state, to assess the cost-effectiveness of surgical intervention. “Health states” are typically quantified on a scale of 0 to 1. For example, preference-based values for epilepsy-related health states include a healthy, disease-free patient (1.0), a patient with intractable complex partial seizures (0.62), and a postoperative, seizure-free patient (0.89).47 This approach permits calculation of “quality-adjusted life years” (QALYs) by multiplying the expected postoperative survival in years by the appropriate quality adjustment factor (from 0 to 1), which reflects the outcome state.47,48 The number of QALYs added to a patient’s life by successful epilepsy surgery can be estimated (Fig. 70-2) and, when combined with the cost of therapy, can be expressed as the cost, in dollars, of providing an incremental QALY to the life of a patient with intractable epilepsy. The “cost-effectiveness” of surgical intervention can then be expressed in various ways to provide a measure of the cost-effectiveness of epilepsy surgery that can be compared with other unrelated health care interventions.
Complications of Diagnostic/Surgical Procedures
Epilepsy surgery is safe and effective. Nevertheless, invasive diagnostic procedures and definitive surgical interventions do carry some risk, which must be considered when recommending surgical intervention to patients with intractable seizures.8
Intracarotid Amytal Procedure (Wada Test)
Complications of the Wada test encompass the complications of transfemoral carotid angiography, including thromboembolism and stroke (0.5% to 1.0%), allergic reactions to contrast agents (1 in 40,000), and local complications of femoral artery puncture.49 Rare mortality has been reported.16
Depth Electrodes
For many years, depth electrodes were used routinely as part of the surgical evaluation of patients with intractable epilepsy.50 In contemporary practice, noninvasive assessments, including structural MRI scans and improved surface electroencephalographic (EEG) monitoring techniques, have reduced the requirement for invasive recordings. An advantage of depth electrodes is that low-voltage, localized discharges emanating from medial structures, including the amygdala and hippocampus, may be detected as evidence of the site of seizure onset. Depth electrodes are invasive and associated with risk for infection (1% to 4%) and intracerebral hemorrhage, which has been noted to occur in 3% of parasagittal placements and 1% of lateral placements.49 Rare mortality from depth electrodes has been reported to be caused by lacerations of large parasagittal draining and bridging veins or the posterior cerebral artery, as noted in one older series of 163 patients.51 The use of modern stereotactic techniques has reduced the morbidity associated with depth electrodes, and recent studies report few complications with no permanent neurological deficits in most cases.52,53 One study has suggested that a postoperative decline in verbal memory may occur with the implantation of hippocampal depth electrodes bilaterally.54
Subdural Strip Electrodes
Subdural strip electrodes have provided a safe alternative to brain penetration by depth electrodes.55 Strip electrodes are usually placed symmetrically over suspected sites of seizure onset and yield excellent recordings from neocortical structures. Although recordings from intracerebral sites are not provided with this modality, these electrodes do not require cortical penetration with its associated risks. The principal risk with subdural electrodes is infection, which may be manifested as superficial infection, meningitis, or brain abscess, as reviewed in an extensive series of 350 patients.55 Other reported adverse events have included fever with temperatures higher than 102°F, migraine, and temporalis muscle fibrosis, as indicated in a prospective study of 55 patients. In another recent multicenter study, only five minor complications occurred in 131 patients, three of which were reported to be small hematomas not requiring evacuation.56
Subdural Grid Electrodes
Subdural grid electrodes provide electrographic tracings from a large expanse of cortex and permit extraoperative brain mapping to localize eloquent functions in relation to the epileptogenic zone. Grid electrode placement requires a large craniotomy and the egress of numerous electrode cables through the scalp for the duration of the monitoring period (usually 1 to 2 weeks). The grid is removed at a second craniotomy, during which the definitive cortical resection is performed. It is not surprising that bone flap infection and meningitis are prominent concerns as complications of this procedure. Infection rates of 22% were identified in an early Cleveland Clinic series but declined to 7% when cables were tunneled to exit percutaneously.57 A contemporary series of 49 patients undergoing grid implantation reported a 4% infection rate, subdural hematoma formation requiring emergency evacuation in 8%, and brain swelling in 2%.58 Other series have reported subdural hematoma formation in 8% and increased intracranial pressure and brain shift requiring premature removal of the grid.49 Meticulous surgical technique with avoidance of injury to or compression of large cortical or bridging veins, tunneling of electrode cables, administration of perioperative antibiotics, and full utilization of mannitol and dexamethasone (Decadron) are likely to improve outcomes and prevent complications.49
Resective Surgery
Temporal Lobe Epilepsy
Intractable epilepsy of temporal lobe origin is the most common syndrome for surgical consideration. It is thus no surprise that the majority of outcome data in the literature have focused on temporal lobe surgery. The syndrome of mesial temporal lobe epilepsy typically incorporates a history of an early insult in infancy or childhood,59,60 hippocampal sclerosis and atrophy on MRI,61 an abnormal creatine–N-acetylaspartate (NAA) ratio on magnetic resonance spectroscopy (MRS),62 temporal hypometabolism on interictal PET,63,64 and a characteristic pattern of hyperperfusion and hypoperfusion on ictal SPECT.59 EEG studies reveal an anteromedial epileptogenic zone, and Wada testing reveals appropriate memory deficits.8,65 Histopathologic analysis of resected hippocampi reveals loss of principal hippocampal neurons, synaptic reorganization, sprouting of mossy fibers, and enhanced expression of glutamate receptors.8,66,67
A smaller population of patients with “cryptogenic” TLE have normal MRI findings preoperatively.60 Patients with “lesional” TLE have temporal lobe neoplasms, vascular malformations, disorders of cortical development, or traumatic/ischemic insults within the temporal lobe. These lesions may variably involve mesial temporal lobe structures or may be associated with hippocampal sclerosis (“dual pathology”)68 and thus lead to distinct surgical approaches and outcomes.
Mesial Temporal Lobe Epilepsy
Pathologic Substrate
The pathologic features of mesial temporal sclerosis (MTS) include (1) loss of principal neurons (atrophy), (2) glial proliferation (gliosis), and (3) sprouting of dentate granule cells.69,70 When the loss of hippocampal principal neurons exceeds 50%, hippocampal atrophy is visible on MRI69 and gliosis will be manifested as high signal on fluid-attenuated inversion recovery (FLAIR) and T2-weighted images. Not uncommonly, some degree of hippocampal sclerosis is present contralateral to the side of resection and may not be detected by standard MRI assessment. Bilateral MTS is visible on MRI scans less commonly (5% to 10%).71
Seizure Outcomes
Imaging, Neuropsychological, Electrographic, and Clinical Features
A variety of preoperative factors have been recognized over the years as identifiers of more favorable postsurgical outcomes with regard to seizure control. Although seizures were well controlled in 91% of patients with MRI-defined unilateral MTS, the favorable outcome response to surgical intervention declined sharply to 62% in patients found to have bilateral MTS and was notably worse (only 50%) in patients without MTS.72 However, depth electrode recordings in patients with radiographic bilateral MTS have suggested that some may have only a unilateral onset, and in this subset of patients excellent seizure outcomes may still be achieved, as evidenced in a small study of 5 such patients, 4 of whom were completely seizure free after 2 years of follow-up.71 It has also been found in several studies that hippocampal hypometabolism noted on PET studies is strongly correlated with MRI findings of MTS and histologically confirmed neuronal atrophy and gliosis in this region,73,74 thus outlining the prospective utility of PET in preoperative evaluation. In a similar vein, another study evaluating postsurgical patients seen at 1-year follow-up reported an 83% seizure-free rate in those with preoperative unilateral hippocampal hypometabolism,64 in sharp contrast to just a 38% seizure-free rate in patients with either normal PET studies or evidence of multilobar hypometabolism. In a 5-year outcome assessment of 135 operated patients with imaging evidence of lesions, MTS, and normal hippocampi, 80% of patients with lesions, 62% of patients with MTS, and 36% of patients with normal hippocampi were found to be seizure free 2 years postoperatively.75 Multivariate analysis may improve the prediction of outcome.76 MTS, a “known cause” of epilepsy, and the absence of generalized seizures portend a satisfactory outcome. Satisfactory outcomes were achieved in 78% to 83% when both of these features were present, in 53% to 61% when one was present, and in 29% of patients when neither was present.77 Preoperative evaluation with the intracarotid Amytal test (Wada test) has suggested that lateralizing memory deficits are independently predictive of a seizure-free outcome.20,65
Lateralization of seizure onset can be identified with either interictal78,79 or ictal76,80,81 EEG monitoring. In patients with nonlesional TLE, favorable seizure control was noted most often in those with concordance between their interictal EEG and MRI findings.82 A 5-year follow-up study of 28 patients demonstrated that in 26 of them, a 75% reduction in seizures was noted in those who had a unilateral anterior to middle temporal epileptiform focus without discordant findings in other studies, the majority of whom (61%) were reported to be seizure free.79
In a recent review of 126 articles on temporal lobe resection published between 1991 and 2001, the median seizure-free rate was 70%, and of 63 factors analyzed, favorable outcomes were associated with (1) preoperative MTS, (2) anterior temporal localization of interictal epileptiform activity, (3) absence of preoperative generalized seizures, and (4) absence of seizures in the first postoperative week.83 Age at seizure onset, preoperative seizure frequency, and extent of lateral resection had no association with outcome.
Age at the time of surgery (i.e., >45 years old) may be related to seizure outcome, with some studies suggesting less favorable outcomes84 and others suggesting that older age does not predispose to a less favorable outcome.85,86
Surgical Versus Best Medical Management
A 5-year follow-up study compared the seizure outcomes of 148 operated and 94 nonoperated patients with TLE.87 Freedom from seizures during the final year of follow-up was achieved in 62% of operated and 7.5% of nonoperated patients. Complete seizure freedom over the entire study period was achieved in 44.6% of operated and 4.3% of nonoperated patients. None of the nonoperated and 8.8% of the operated patients were free of the need for antiepileptic drugs (AEDs) at follow-up. Other adult studies have documented AED freedom in 21% and 35% of patients at 1- and 2-year follow-up, respectively.88,89 Pediatric studies have documented 30% to 44% AED-free rates at 2 years78,89 and 30%, 35%, and 60% AED-free rates at 2, 5, and 10 years postoperatively, respectively.90 The only prospective, randomized, controlled study of surgical versus best medical management for TLE compared the outcomes in 40 medically managed and 40 surgically managed patients.2 At 1-year follow-up, 58% of the surgically treated and only 8% of the medically treated patients were free of seizures impairing consciousness.
In general, systematic reviews suggest that 66% to 70% of patients are seizure free at short- term (<5 years) follow-up.83,91–94 Long-term (>5 years) follow-up studies show that 41% to 79% of patients remain seizure free after temporal lobe resection87,91,93,95–99 and that 15% to 20% of patients have relapses after initial seizure freedom at 5 to 10 years after surgery.92,93,96,100
Surgical Approaches to Mesial Temporal Lobe Epilepsy
The traditional “en bloc temporal lobectomy” incorporated a 5- to 6-cm lateral resection along with a portion of the amygdala and anterior hippocampus.8,101,102 More centers now use a focused anteromedial resection in which restricted resection of the middle and inferior temporal gyrus is combined with thorough hippocampal removal.8,103–105 Transsylvian SAH permits exclusive resection of medial structures.8,106,107 Awake surgery with intraoperative electrocorticography (ECoG) and functional brain mapping permits tailored resection of both lateral and medial structures.8,108,109
Extent of Cortical and Hippocampal Resection
In the early era of epilepsy surgery, lateral resection alone often yielded disappointing results with regard to freedom from seizures.110 More recent studies in which lateral and mesial structures were resected have suggested that the extent of lateral resection does not correlate with seizure outcome.111,112 One study reported that 53 of 100 patients were seizure free after a standard lateral resection was combined with complete amygdalectomy and minimal hippocampal resection.113 The favorable outcomes after SAH,11,114 the effectiveness of removal of any residual posterior hippocampus in reoperative surgery,115–117 and the identification of posterior hippocampal onsets in depth electrode studies103 all suggest that thorough hippocampal resection may be essential to optimize seizure outcomes.8 Although earlier reports suggested that the extent of mesial resection had no association with seizure outcome, two more recent studies addressed this issue effectively and came to different conclusions.83 In the first study, postoperative MRI was used to confirm the extent of mesial resection in 94 TLE patients and revealed a correlation of the extent of mesiobasal resection with seizure outcome, regardless of the extent of lateral resection.117 In a separate, prospective randomized controlled trial, 70 patients with unilateral ictal onsets confirmed on intracranial recordings underwent temporal lobe resection and were randomized to partial or total hippocampectomy.105 At 1- and 2-year follow-up, the group that underwent complete hippocampectomy experienced superior seizure outcomes (69% versus 38% seizure free at 1 year) without increased neuropsychological or neurological morbidity.
Impact of Surgical Approach
Although a small percentage of TLE patients may harbor epileptogenic zones exclusively in the lateral temporal neocortex,118 the majority of candidates for nonlesional temporal lobe resection have the syndrome of MTLE, for which resection of mesial structures is emphasized.8,59 A long-term follow-up study of 50 patients managed with lateral resection alone revealed 44% to be seizure free at follow-up.119 In Engel’s compilation of seizure outcomes from 107 centers worldwide, outcomes were similar between centers regardless of the surgical approach, provided that the mesial structures were adequately resected.1 In single-center studies, SAH and standard resections produced similar results.72 In Yasargil’s SAH series, 22 of 30 (73%) patients with TLE and depth electrode–confirmed hippocampal onsets were seizure free postoperatively.114 These results after SAH compare favorably with studies of patients with histologically confirmed MTS undergoing standard anteromesial resections.120
A single-center study reported on the seizure outcomes of 321 patients who underwent various temporal lobe resections between 1989 and 1997.121 This series incorporated 96 standard anterior temporal resections, 84 restricted lateral and generous mesial resections, and 91 SAH procedures. The notable finding was the absence of significant differences in seizure outcome between the three operative cohorts in which different resection strategies were used.8
Improvement in Seizure Outcomes Over Time
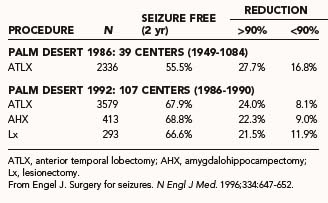
Early investigations of temporal lobe resections documented seizure-free outcomes in 27% to 44% of patients in long-term follow-up.122–125 This was during an era in which lateral resection was emphasized. Engel compared worldwide outcomes of earlier (1949 to 1984) and more recent (1986 to 1990) eras and documented superior outcomes in contemporary series (Table 70-6).1,8,10 This was thought to result from improved methods of patient selection and convergence of surgical resection approaches to emphasize medial resection. Ultimately, in many patients seizure control is not static and may fluctuate over time.
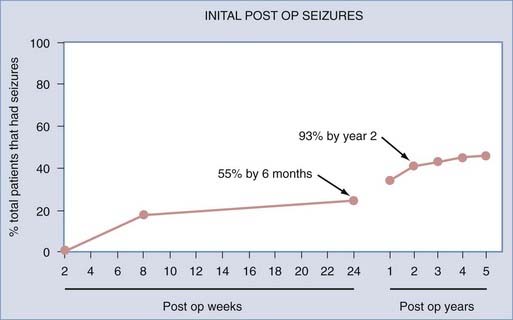
In a study of patients with refractory TLE by Sperling and colleagues, 89 patients who underwent anterior temporal lobe resections between 1986 and 1990 were monitored postoperatively for 5 years.12 Approximately 70% of the patients reported being seizure free over the past year (Engel class I). Only 55% of the patients were seizure free over the entire 5-year period after surgery. In those in whom seizures did develop, 55% experienced them within the first 6 months postoperatively, and almost 93%, relapsed within the first 2 years after surgery (Fig. 70-3). This suggested that in patients who were seizure free for the first 2 years after surgery, recurrent seizures were unlikely to develop thereafter.
A similar outcome study reviewed 148 patients who had an overall 44.6% seizure-free rate after 5 years, 62% of whom were seizure free in the previous year.87 Another 5-year outcome study demonstrated similar results, with an overall 50% seizure-free rate at 5 years and a similar 62% seizure-free rate in the last 2 years of the study.75
Acute Postoperative Seizures and Late Improvement
Traditionally, immediate postoperative seizures were considered incidental to the trauma of surgery itself and not predictive of surgical outcome, analogous to early seizures after stroke or traumatic brain injury, which have not been shown to be reflective of long-term seizure outcomes.10 This “running down” phenomenon was initially characterized by Rasmussen, whose early studies suggested that it occurred in as many as 15% to 20% of patients.126–128 Recent investigations have not confirmed this belief, with only 5% of patients with TLE and immediate postoperative seizures experiencing improved seizure control over time. “Running down” may occur more commonly in patients with unilateral epileptiform discharges preoperatively.129 Other studies, however, suggest that immediate postoperative seizure activity may predict poorer long-term seizure control in both adults130,131 and children.132
Intraoperative Electrocorticography
For many years, temporal lobe resections “tailored” on the basis of intraoperative ECoG provided a standard surgical approach in many centers. Recent experience suggests that standardized anatomic resections may produce similar outcomes, and the appropriate role of intraoperative ECoG is not well defined in current practice. Early studies suggested that intraoperative ECoG may be useful to predict outcome, particularly if epileptiform discharges are present or absent in either preresection or postresection cortex.128,133,134 Ojemann’s recent study used ECoG to define the extent of hippocampal resection in an attempt to both optimize seizure outcomes and minimize postoperative memory deficits though hippocampal sparing.109 In contrast, two studies in which standardized anatomic resections were performed in the context of intraoperative ECoG before and after resection failed to identify any predictive value with regard to ultimate seizure outcomes.135,136 Other studies suggest a limited value of ECoG as a guide to the extent of resection for TLE.111,137,138 Another study in patients with lesional temporal or extratemporal epilepsy in which resection was carried to normal tissue margins found that the extramarginal spike distribution was not associated with seizure outcome.139 In contemporary practice, in which patient selection is guided by advanced imaging and video-EEG data, intraoperative ECoG is not used in some centers in the context of defined surgically remediable syndromes.83
Neuropsychological Outcomes
Cognitive Outcome
Intellectual function is generally preserved in adults12 and children140 after temporal lobe resection, and when seizure control is achieved, improvement in some measures has been reported. In the Graduate Hospital series of 89 consecutive patients undergoing dominant- and non–dominant-hemisphere resections, measures of verbal IQ were unchanged postoperatively and the study demonstrated improvements in performance and full-scale IQ.12 In part, these improvements were believed to be attributable to practice effect. Subsequent studies have reported similar findings, including improvement in verbal IQ after non–dominant-hemisphere resections and performance IQ after dominant-hemisphere resections.141–144
Global Memory Deficits
Although uncommon in modern practice, global amnesia is a disabling complication of temporal lobe surgery. Two patients with global amnesia were described in an early Montreal Neurological Institute series of 90 dominant-hemisphere temporal resections.145 These patients exhibited a syndrome of profound anterograde memory loss with preservation of cognitive performance, personality, early memory, and technical skills.146,147 Earlier reports had described global amnesia after unilateral resection in either the dominant or nondominant hemispheres.143,147,148 Evidence that hippocampal rather than lateral neocortical removal is critical to the production of global amnesia is provided by a report of a patient undergoing a staged resection in whom global amnesia occurred only after the hippocampus was removed.145 This is further supported by reports of global amnesia after SAH.149 Contemporary series report rare postoperative global memory deficits at a frequency of less than 1%,49,143,150,151 whereas a less profound postoperative “severe amnesia” may be more common.152
Material-Specific Memory Deficits
Reported “material-specific” memory deficits include loss of short-term verbal and nonverbal memory postoperatively. In particular, short-term verbal memory loss is common after dominant temporal lobe resections, with significant decrements in verbal memory being reported in 25% to 50% of operated patients.153 Verbal memory loss may accompany resections in the nondominant hemisphere, although at a much lower frequency.153 Nonverbal memory deficits are less commonly identified, even after non–dominant-hemisphere resections,154 although some authors report that these losses may be obscured by “practice effects.”153 In the Graduate Hospital series, evidence of significant short-term verbal memory loss was identified in many patients after dominant-hemisphere temporal lobe resections, with a trend toward improvement after nondominant temporal lobe resections.12 In a recent 10-year series of 321 TLE patients undergoing a variety of surgical approaches for the treatment of nonlesional and lesional TLE, verbal memory declined in 34%, improved in 19%, and remained stable in 46% of patients.121 Weak preoperative performance on measures of verbal memory, young age at surgery, and operations on the nondominant side were associated with stability or improvement in verbal memory. Short-term nonverbal memory measures exhibited similar rates of improvement and deterioration. Weak preoperative performance on measures of nonverbal memory and dominant-side operations were associated with improvement, whereas advanced performance preoperatively and older age were associated with deterioration.121
The high frequency at which verbal memory impairment occurs after dominant-hemisphere temporal lobe surgery has stimulated interest in predicting which patients are at risk for postoperative deficits.8 Recent studies have documented significantly greater risk for verbal memory loss in two categories of patients: (1) those with intact memory function and a normal hippocampus ipsilateral to the seizure focus (“functional adequacy” hypothesis) and (2) those with ipsilateral hippocampal atrophy but impaired memory function, presumably related to poor function within the hemisphere contralateral to the seizure focus to be resected (“functional reserve” hypothesis).8,155 Patients with dominant-hemisphere TLE and a reversed Wada memory asymmetry score (i.e., better memory performance in the epileptogenic temporal lobe, with poor right temporal lobe performance) have been shown to have a greater risk for memory morbidity after left-sided resection, as well as poorer seizure outcome postoperatively.156 Patients with dominant-hemisphere hippocampal atrophy who undergo contralateral, non–dominant-hemisphere resections are also at risk for verbal memory deficits.157 Preoperative MRI studies of hippocampal volumes and left hippocampal MRS profiles (creatine/NAA ratio) also help predict the risk to verbal memory performance after surgery.62
In a recent study, a multivariate risk factor model for predicting postoperative decline in verbal memory was developed in which five risk factors were independently associated with outcome, including (1) dominant-hemisphere resection, (2) MRI findings other than exclusively ipsilateral MTS, (3) intact preoperative delayed recall verbal memory, (4) relatively poorer preoperative immediate recall verbal memory, and (5) intact ipsilateral memory performance on the Wada test.8,158 With this model, individual patients can be assessed with respect to their risk for deficits in verbal memory function after surgery.
Standard Anterior Temporal Lobectomy Versus Selective Amygdalohippocampectomy: Memory Outcome
In patients thought to be at risk for global or material-specific memory deficits postoperatively, various management strategies have been proposed to reduce these losses,8 including memory mapping in the temporal neocortex with restriction of neocortical resection, SAH, or simple denial of surgery to these patients.159 With reports of global amnesia occurring in patients undergoing SAH,149 it was thought that it may be advantageous to perform selective mesial resection from the standpoint of preservation of material-specific memory, particularly short-term verbal memory function. Although some early outcome studies in small series of patients suggested a possible advantage of SAH over standard anterior temporal lobectomy from the standpoint of postoperative memory outcome,88,160 this has not been supported by other studies, and there have been reports to the contrary.161
In a recent review of 140 patients undergoing either right or left SAH, a decline in verbal learning and memory occurred after 32% of the right-sided and 51% of the left-sided resections.54 The left SAH patients were particularly at risk when preoperative testing revealed intact verbal memory function, late onset of epilepsy, and the absence of MTS on MRI. Collateral damage to adjacent temporolateral tissue during the transsylvian dissection may exacerbate the deficits caused by hippocampal resection.54,162 The role of deafferentation of the temporal circuitry during resection of the parahippocampal gyrus, amygdala, and hippocampus also needs to be considered. This is supported by PET evidence of worsening hypometabolism of the remaining temporal lobe neocortex after SAH.163
Postoperative Language Dysfunction
After dominant-hemisphere temporal lobe resection, a syndrome of transitory postoperative dysnomia or even aphasia is observed in as many as 30% of operated patients.164 In most cases, the dysnomia or aphasia gradually disappears over a period of a few weeks. This occurs even when resections are guided by intraoperative or extraoperative language mapping.126,127 The cause of this transitory phenomenon is unclear, but it is more common when resections are carried to within 1 to 2 cm of essential language sites as determined by mapping procedures.8,159,165 Other explanations for this phenomenon include resection of inferior temporal lobe “inessential” language sites,166 brain retraction and associated “neuroparalytic edema,”167,168 or deafferentation of white matter pathways. Some authors have suggested that such word-finding deficits represent an acute postoperative exacerbation of the preoperative deficits common in patients with TLE and that they last no longer than 1 year.169
Although some investigations of naming have not revealed enduring deficits at 6 and 18 months postoperatively,170–172 others have suggested that significant, persistent word-finding difficulties do occur commonly after standard or anteromesial temporal lobe resection.164,173,174 Such deficits have been reported to be associated with early risk factors for the development of seizures173 and with the pathologic state of the resected hippocampus.175 In one study, 7% of patients undergoing standard dominant-hemisphere resections exhibited persistent postoperative dysnomia.174 Ojemann described enduring language deficits after resections within 1 to 2 cm of identified language sites.176
The aforementioned findings stimulated interest in the value of intraoperative mapping and tailoring of the lateral neocortical resection. Ojemann and colleagues suggested that up to 17% of patients undergoing left temporal resections 4.0 to 4.5 cm from the temporal tip (a “standard” temporal lobe resection) without mapping would experience postoperative deficits.8,177 Some centers now restrict cortical resections to 3 cm of the middle and inferior gyrus without mapping and have reported minimal postoperative language deficits.103 A general trend toward restricted lateral cortical resection in the temporal lobe has resulted in language mapping being less commonly used. It has not been studied whether such restricted resection may engender deficits not seen in patients undergoing mapping.
Persistent, severe dysphasia has been reported in 1% to 2% of patients undergoing dominant-hemisphere temporal resections, even with language mapping.116,143,178,179 Such adverse postoperative outcomes occur as a result of resection of essential language cortex or manipulation or thrombosis of the middle cerebral or anterior choroidal artery.180
Neurobehavioral and Psychosocial Outcomes
Psychiatric Outcome
Psychiatric morbidity has been reported to occur in 15% to 50% of patients with epilepsy in the literature.181,182 There is a high prevalence of psychopathology, including depression, in candidates for temporal lobe resection both preoperatively and postoperatively.183,184 One study reported postoperative improvement or resolution of long-standing depressive symptoms in 47% of patients undergoing temporal lobe resections, thus suggesting that preoperative depression is not a contraindication to surgery.185 In the same study, depression occurred de novo in 10% of operated patients. Improvement in depression postoperatively is more likely in patients who are rendered seizure free.186,187 Preoperative assessment of the risk for chronic depressive symptoms postoperatively may be achieved by using measures of emotional adjustment, such as the Washington Psychosocial Seizure Inventory.187 The early postoperative period is characterized by the dynamic expression of varying psychopathologic conditions. In one study, half of the patients with no psychopathology preoperatively exhibited symptoms of anxiety, depression, and emotional lability 6 weeks postoperatively.188 Other reports have documented new psychiatric problems in 31% of patients and resolution of psychiatric diagnoses in 15% of patients in the 6 months after surgery.183 One study reported that 10% of 121 patients with TLE who underwent epilepsy surgery required postoperative psychiatric hospitalization.189 The de novo appearance of hypomania requiring psychiatric hospitalization,190 psychogenic seizures (particularly in females undergoing nondominant temporal lobe surgery),183,191 and neurotic or psychotic symptoms192 postoperatively demonstrates the necessity for comprehensive psychosocial and psychiatric assessments both preoperatively and postoperatively. In the context of a thorough preoperative evaluation, a history of psychotic symptoms does not represent an absolute contraindication to surgical intervention, although an exacerbation of symptoms may occur postoperatively.8
Psychosocial Outcome
It is increasingly being recognized that the syndrome of intractable TLE embraces comorbid conditions beyond the encumbrance of frequent, intractable seizures. Such comorbidity includes psychosocial, psychiatric, and neuropsychological impairment, medication toxicity, and excess mortality rates.8 These impairments develop as a result of frequent, disabling seizures during critical stages of personal development and may not resolve immediately after surgery.
Patients are aware of epilepsy-associated disabilities and hope for their resolution after surgery. In a study of 69 preoperative patients, their aims for epilepsy surgery beyond freedom from seizures included desire for work, ability to drive, independence, socializing, and freedom from AEDs.9 The psychosocial outcomes of successful surgery were assessed in a 5-year follow-up study of the long-term changes in 61 surgical and 23 medically managed TLE patients.193 In this study, 68% of the surgery group exhibited improved psychosocial status, as opposed to 5% of the medically managed group. Individuals who underwent surgery were found to be more likely to drive, live independently, work full-time, and be financially independent. Remaining seizure free was not a prerequisite for improvement in psychosocial measures in this study, although other investigations have documented diminished psychosocial adjustment in patients with recurrent seizures.194
Health-Related Quality of Life
A clear relationship has been documented between psychosocial status and quality of seizure control in medically managed epileptics.195 Similarly, in postoperative patients, seizure-free patients score more favorably than those with either auras or recurrent seizures on a variety of measures.45 In the only randomized, controlled trial of epilepsy surgery versus best medical management to date, patients who underwent surgery were documented to have improvement in both seizure outcomes and HRQOL.2 The surgical group consistently scored higher than the medical group as early as 3 months after surgery and continuing to 12 months on both the QOLIE-89 and measures of school or job performance. Another study showed better HRQOL in postoperative seizure-free patients than in those with persistent auras and persistent seizures.42 In another study of patients 2 years postoperatively, both seizure-free patients and those with a 90% reduction in frequency experienced significant improvement in HRQOL.89 When compared with the health status of patients with other chronic diseases, postoperative patients with persistent seizures scored worse than did those with heart disease, hypertension, or diabetes. When patients were seizure free postoperatively, they scored better than patients with these non-neurological illnesses.196 In a study reviewing nonsurgical and surgical patients evaluated preoperatively and 1 and 2 years postoperatively, significant improvement was identified on 10 of the 17 scales of the QOLIE-89 in patients who were entirely seizure free.197 Significantly more improvement was noted at 2-year follow-up than at 1-year follow-up. In addition, patients with persistent auras were not significantly improved when compared with patients with persistent seizures.197
In a large prospective surgical series of 396 patients in which the QOLIE-89 was administered before surgery and up to 5 years after surgery, the most substantial improvement in HRQOL occurred immediately after surgery in all patients, but additional improvements over time were seen in the seizure-free group.198 The effect, in this study, seemed to stabilize at 2 years after surgery and was related to the duration of freedom from seizures. Another method of determining well-being is to assess patients’ perceived effect of surgery or their satisfaction with the results. A recent study found that of 396 patients, 80% would make the same decision (to have surgery) if given the choice again, and 91% to 92% reported a strong or very strong positive impact of surgery (influenced by freedom from seizures and gainful employment).199
Cost-Effectiveness of Surgical Treatment
Wiebe and coworkers used decision-analysis modeling and an intention-to-treat approach to compare medical and surgical treatment of intractable TLE in a Canadian population of 200 patients treated either surgically or medically over a 35-year period.48 In their model, surgery required a larger initial expenditure; however, by 8 years after surgery, the cost savings engendered by the 57 seizure-free patients made surgical management less expensive than medical management across the entire cohort of surgical patients. Thus, surgical therapy was more cost-effective than medical management in this population.
In a decision-analysis model of surgical versus best medical management of intractable TLE, Langfitt used Rochester, New York, cost data to address the relative cost-effectiveness of different treatments.8,47 This investigation used public health clinical research methods that express the cost-effectiveness of treatment as a “marginal cost-effectiveness ratio” (MCER), which represents the dollar cost per QALY added to treated patients’ lives postoperatively. Each postoperative outcome state was assigned a quality adjustment on the basis of the ESI-55 scores achieved by 42 patients undergoing evaluation for surgery. With a state of total health adjusted to 1.0, patients with intractable seizures preoperatively were adjusted to 0.62; postoperative states were adjusted as follows: no seizures, 0.89; auras only, 0.80; and recurrent complex partial seizures, 0.72. In this model, a patient rendered seizure free after surgery would improve from 0.62 to 0.89 on the adjustment scale, and if this patient lived for 40 years in this state of health, the patient would accrue an additional 10 QALYs. The calculated MCER was $15,581 per QALY, which compares quite favorably with the cost of other health care interventions. Another study reported a cost-effectiveness ratio of $27,200 per QALY.200 By comparison, the calculated MCER for lifetime tuberculosis screening for a 20-year-old African American was $324,537 per QALY.47 The MCER calculated for stenting versus balloon angioplasty for symptomatic, single-vessel coronary artery disease was $29,893 per QALY.47 In addition, the calculated MCER for asymptomatic intracranial aneurysm repair was $28,441 per QALY.47
A recent study sought to determine whether health care costs change when seizures become controlled after surgery.201 Total costs for seizure-free patients had declined 32% by 2 years after surgery because of less use of AEDs and inpatient care. Costs did not change in patients with persisting seizures, regardless of whether they underwent surgery. In the 18 to 24 months after evaluation, epilepsy-related costs were $2068 to $2094 in patients with persisting seizures versus $582 in seizure-free patients. They concluded that costs remain stable for more than 2 years after evaluation in patients with TLE whose seizures persist but that patients who become seizure free after surgery use substantially less health care than before surgery. Further cost reductions in seizure-free patients can be expected as AEDs are successfully eliminated.
Complications of Temporal Lobe Resection
In a review of the accumulated worldwide experience on temporal lobe resective surgery before 1993,49 significant/impairing complications were uncommon and included death,51,124,143,202–204 infection,49,143,167 hemiparesis from manipulation or thrombosis of the middle cerebral artery or anterior choroidal vasculature or from direct brainstem injury or resection,203,205–209 visual field deficits from resection of Meyer’s loop fibers in the roof of the temporal horn,209–211 hemianopia179,211,212 as a result of excessive tissue resection or infarction, postoperative hematoma formation,124,143,202 and rare third cranial nerve203,206 and seventh cranial nerve213 palsies. There was a trend toward reduced mortality and morbidity over time in a large, single-center series and in a worldwide survey of 2282 operations performed between 1928 and 1973.124
In a contemporary, single-center study of 329 temporal lobe neurosurgical interventions (in 321 consecutive patients), 28 complications were reported (8.5%), including no mortalities, meningitis (1.5%), subdural hematoma (0.6%), deep venous thrombosis (1.2%) and neurological complications (5.2%).121 In another single-center study of 215 patients undergoing temporal lobe surgery between 1984 and 1999, complications included mild hemiparesis, hemianopia, transient cranial nerve palsies, and transient language difficulties.214
In a multicenter study at six different centers in Sweden, the complications in 449 operated patients were reviewed.56 In 247 temporal lobe resections, one mortality occurred in a 62-year-old woman who experienced a postoperative hematoma. Hemiparesis occurred in 5 patients, in 1 patient after neocortical resection and in 4 patients after resections involving the hippocampus. These complications were thought to be due to anterior choroidal artery infarction and manipulation of “perforating vessels.” Other complications included hemianopia (0.4%) and cranial nerve injury (0.9%). A clear correlation between age and severity of complications was noted. Few complications occurred in those younger than 35 years. “Manipulation hemiplegia” was originally described by Penfield and colleagues208 and may be caused by manipulation/injury to the anterior choroidal artery or the middle cerebral artery in the sylvian fissure. The resultant hemiparesis was thought to be more likely in older patients with atherosclerosis and hypertension and was one of the main complications of temporal lobe surgery in those older than 35 years. In a Norwegian epilepsy surgery series,215,216 “large” complications occurred in 1 of 64 patients younger than 19 years and in 7 of 61 adult patients, thus confirming an increased risk for postoperative complications in older patients. Additional support is provided by another study of 215 operations performed between 1983 and 1999 in which permanent complications occurred in only 3 of 215 patients, and these patients were older than 30 years.214
Recent reports of unusual complications after temporal lobe resection include four cases of cerebellar hemorrhage believed to be related to postoperative epidural suction drains217 and diplopia associated with transient trochlear nerve palsy in three patients.218
Mortality After Temporal Lobe Resection
The annual death rate attributable to epilepsy, which reflects accidents, suicide, and sudden unexpected death due to epilepsy (SUDEP), is higher in patients with chronic epilepsy than in the general population.8 Studies of the impact of postoperative freedom from seizures have revealed that successful temporal lobe surgery lowers but does not normalize the overall mortality associated with chronic epilepsy.219 In the Graduate Hospital series, all late mortalities (four) occurred in patients with recurrent seizures, including three with SUDEP and one suicide.12 In another study, late mortality was studied and occurred in 2% of seizure-free patients and in 11.9% of patients with recurrent seizures.214
Lesional Temporal Lobe Epilepsy
Lesions of various types are identified in 15% to 30% of patients with intractable TLE.8,220,221 These lesions may be neoplastic (astrocytoma, ganglioglioma, pleomorphic xanthoastrocytoma, dysembryoplastic neuroepithelial tumor), vascular (cavernous hemangioma, arteriovenous malformation [AVM], angioma), dysgenetic (microdysgenesis, focal or diffuse dysplasia, Sturge-Weber syndrome, tuberous sclerosis), or traumatic/ischemic. In a review of 167 patients with temporal or extratemporal lesions, 15% had hippocampal sclerosis or “dual pathology.”68 In further investigations of dual pathology, significant hippocampal neuron loss was identified in patients with lesions located adjacent to the hippocampus and in those with a history of “early injury.”222,223
In patients with mesial temporal lobe lesions and intractable epilepsy, studies of lesional resection alone, without resection of mesial structures (“lesionectomy”), have produced disappointing results, with 22%,224 19%,225 and 43%226 of patients rendered seizure free in small series.8 In those with laterally located lesions, seizure outcome is improved when complete lesion resection is achieved.117 When lesional removal is performed along with standard mesial resection, seizure outcomes were improved, with 85%,226 91%,227 and 92%228 of patients being rendered seizure free in various series. Other authors have recommended gross total resection of the lesion along with an additional 5 to 10 mm of adjacent epileptogenic tissue (“lesionectomy plus”) and sparing of mesial structures in the case of lateral lesions without dual pathology (i.e., normal hippocampus) and have reported favorable seizure outcomes with this approach.8,121,229 In patients with temporal lobe lesions and dual pathology, resection of mesial structures along with the lesion has been recommended.230
The value of ECoG in guiding decisions regarding the extent of extralesional tissue resection is controversial231 and has been addressed in reports of patients with TLE and various tumors57,111,225,228 and AVMs227; these reports suggest an advantage conferred by resection of epileptogenic tissue, including mesial structures, along with lesional resection. Despite a possible advantage from the standpoint of seizure control, hippocampal resection in lesional cases may cause significant neuropsychological morbidity when hippocampal sclerosis is absent on MRI, particularly in dominant-hemisphere resections. In cases in which the hippocampus is not invaded by tumor, the approach of “lesionectomy plus” may confer less morbidity in dominant-hemisphere resections while maintaining favorable seizure outcomes.229 Excision of a presumed epileptogenic region without lesional resection tends to result in poor outcomes.231
Extratemporal Epilepsy
The protean clinical manifestations of the extratemporal epilepsies result from the varied pathogenetic features of these disorders and the eloquent brain regions that are affected by seizures arising in the broad expanse of the frontal, parietal, and occipital lobes.8 Extratemporal epilepsy patients undergo surgery less commonly than do patients with TLE.1 The epileptogenic regions are often large and ill defined, thus mandating larger resections, and surgical approaches include lobar and multilobar, central and tailored resections, topectomy, and MST. Extratemporal resections are often combined with callosotomy or MST to improve efficacy. In a series of 2177 patients older than 51 years at the Montreal Neurological Institute, operations included temporal (56%), frontal (18%), central/rolandic (7%), parietal (6%), occipital (1%) and multilobar resections, as well as hemispherectomy (11%).49,232
Seizure Outcomes
The outcomes of extratemporal resections have historically been less favorable than those achieved with temporal lobe surgery, with approximately 45% being seizure free after surgery.1 Improved imaging, patient selection, mapping, and surgical methods have resulted in improved outcomes in contemporary reports. In a study of 60 patients with extratemporal epilepsy, structural abnormalities were present in 83% of the patients.233 Surgical resection of the frontal, parietal, and occipital lobes was performed. Preoperative mapping with grids and strips was performed in 50%, and the remainder underwent intraoperative mapping with ECoG. At 4 years’ follow-up, 61% of the patients with focal lesions were seizure free versus 20% of the patients without histopathologic abnormalities. In a review of patients undergoing frontal lobe resections with adjunctive MST and callosotomy when appropriate, 72% were Engel class I or II postoperatively.234 In another study of patients undergoing frontal lobe surgery, 24 underwent intracranial monitoring and 80% were Engel class I or II postoperatively (64% seizure free).235 In this series, patients without lesions had better outcomes than those with lesions. In a review of frontal lobe epilepsies, 72% of lesional and 40% of nonlesional patients had an excellent outcome (Engel class I, II) after frontal lobe resection, with seizure-free rates of 44% and 24%, respectively.236 In a report of seizure outcomes in 37 patients with intractable frontal tumoral epilepsy, 67% of patients were Engel class I or II with 35% being seizure free.237
Complications of Extratemporal Resection
Complications of extratemporal resections include those related to invasive monitoring with grids, surgical complications of resection or MST, and neurological sequelae of intentional resection of or inadvertent injury to regions of eloquent cortex.8 The complications in one study included three wound infections and three neurological deficits that resolved slowly.233 In frontal lobe resections in Broca’s area, within the posterior 2.5 cm of the opercula, the inferior frontal gyrus is usually spared,232 and language sites identified by stimulation mapping techniques within the middle frontal gyrus may contribute, if resected, to transient or long-standing expressive aphasias.177 Resection of the supplementary motor cortex may produce a transient syndrome consisting of postoperative mutism, contralateral neglect or hemiparesis, and diminished spontaneous movement, which usually resolves spontaneously over a period of weeks.238,239 The cognitive effects of frontal resections are usually well tolerated.240 Preservation of draining veins and arterial supply to the central area is a key consideration.49 Partial resection of the non–dominant-hemisphere facial motor cortex is usually well tolerated; however, complete removal may produce long-standing perioral weakness.115,241,242 The superior resection margin should be located no closer than 2 to 3 mm below the lowest elicited thumb response. Rasmussen described successful removal of the dominant-hemisphere facial motor cortex, provided that the vascular supply to the central area is meticulously preserved.8,241,242 Large parietal resections behind the rolandic cortex can be accomplished with reported hemiparesis rates as low as 0.5%.242 When resections are extended into the parietal operculum, visual field defects may occur if the resections are carried deep into the white matter.203,242 A non–dominant-hemisphere parietal syndrome develops in some patients after large parietal resections, and in the dominant hemisphere, care must be taken to preserve Wernicke’s area. In the occipital lobe, complete resection produces the expected contralateral hemianopia, and excision in the dominant hemisphere to within 2 cm of Wernicke’s area may result in dyslexia.242
Extratemporal Lesional Epilepsy
Lesional resection alone has provided favorable results in extratemporal sites, with 9 of 14 patients (64%) being seizure free in one study224 and 17 of 18 patients (94%) being seizure free in another study.243 A meta-analysis of lesional epilepsy in all sites showed that 44% of the patients were seizure free after simple excision and 67% were seizure free after “seizure surgery.”244 Lesionectomy with removal of hemosiderin-stained brain resulted in freedom from seizures in 73% of patients with occult vascular malformations.245
Cortical dysplasias are associated with a unique pattern of intrinsic epileptogenicity, and intraoperative ECoG is thought by some authors to provide useful information for guiding resection and ensuring optimal seizure outcomes.246,247 In a large series of patients undergoing surgery for focal epilepsy secondary to cortical dysplasia, 49% were seizure free.248 Fifty-eight percent of those undergoing complete resection and 27% of those with incomplete resection were seizure free. Other reports suggest universal freedom from seizures in 100% of patients with Taylor’s balloon cell–type cortical dysplasia after complete lesionectomy without ECoG mapping.249 Other neuronal migration abnormalities, such as “double cortex,” do not benefit from resective surgery.250
Hypothalamic Hamartomas
“Intrahypothalamic” hypothalamic hamartomas (HH) may be associated with intractable partial, gelastic, and generalized seizures,251 as well as retardation and behavioral disorders, whereas precocious puberty predominates in the “parahypothalamic” subset.252 Although several reports have documented successful surgical removal of these lesions and relief of seizures with transcallosal or modified subfrontal approaches,253 such intrahypothalamic surgery raises concern regarding complications of the approach, as opposed to direct intrahypothalamic resection of these lesions. A recent study reported the results of transcallosal surgical resection of HH in 26 patients with refractory epilepsy in a prospective outcome study.254 Fourteen (54%) patients were completely seizure free, and 9 (35%) had at least a 90% improvement in total seizure frequency. They also reported postoperative improvement in behavior and cognition. The likelihood of a seizure-free outcome seemed to correlate with younger age, shorter lifetime duration of epilepsy, smaller preoperative HH volume, and 100% HH resection. Another recent study looked at 37 patients with HH and symptomatic epilepsy who underwent transcortical transventricular endoscopic resection.255 Eighteen patients (48.6%) were seizure free. Seizures were reduced more than 90% in 26 patients (70.3%) and by 50% to 90% in 8 patients (21.6%). Additionally, the mean postoperative hospital stay may be shorter in endoscopic patients than in patients who undergo transcallosal resection.
Cerebellar Seizures
The classic teaching that epileptic seizures do not arise from the cerebellar cortex has been challenged by several reports of focal motor seizures with secondary generalization in which the seizure focus appeared to be within the cerebellum.8,256 Five of eight patients achieved freedom from seizures after resection of their cerebellar lesions.
Catastrophic Epilepsy
“Catastrophic epilepsies” are those in which panhemispheric syndromes are associated with intractable seizures. Such syndromes include Rasmussen’s encephalitis, developmental syndromes (i.e., hemimegalencephaly, tuberous sclerosis, hamartomas, Sturge-Weber syndrome), and congenital hemiplegia/porencephaly.257
Hemispherectomy
Although the original surgical approach of anatomic, complete, en bloc hemispherectomy with sparing of the basal ganglia, hypothalamus, and diencephalon114,242,258 was successful from the standpoint of seizure control, the immediate and delayed complications were daunting.49 In particular, these procedures created a large area of denuded, unsupported subcortical tissue and significant volumes of intracranial dead space that led to repeated microhemorrhage and subdural membrane formation, referred to as “superficial cerebral hemosiderosis.”8 Late complications in the postoperative course occur in as many as 38% of patients259 and include hydrocephalus,260 increased intracranial pressure, neurological demise, or even death.261 An alternative approach to hemispheric decortication (i.e., removal lobe by lobe) was reviewed in a large pediatric series.257 The study reported that 26 of 48 patients were seizure free with a reduced rate of delayed complications.257 Nevertheless, perioperative mortality occurred in 3 patients, and intraoperative blood loss and coagulopathy complicated the clinical course, particularly in children without brain atrophy or with hemimegalencephaly. In another version of “cerebral hemicorticectomy,” the entire cortical surface is “degloved” to the level of the white matter. In one study this resulted in 8 of 11 patients being seizure free, 1 patient with hydrocephalus, and no mortalities or delayed complications.262
With the introduction by Rasmussen of the technique of modified or “functional hemispherectomy,” in which a generous central and temporal resection is juxtaposed with deafferentation of the frontal and occipital lobes, postoperative complications were significantly reduced.115,242 With deafferentation rather than removal of the frontal and occipital lobes, the volume of intracranial dead space is reduced.8 In a 7-year follow-up study of 14 patients, no hemosiderosis or hydrocephalus occurred, and 10 of the 14 patients were seizure free.
Over the last 15 years, the approach of hemispheric deafferentation as a preferred alternative to resection has been advanced by several authors, all of whom perform increasingly limited resections in concert with hemispheric deafferentation. During this period, “peri-insular hemispherotomy” was introduced, in which a smaller craniotomy and a much reduced peri-insular (opercular frontal, parietal, temporal) resection are combined with deafferentation of the frontal, parietal, occipital, and temporal lobes.263 This study reported favorable seizure outcomes (9 of 11 patients seizure free and 1 of 11 improved 95%). In addition, the study documented reduced operative time, as well as a decrease in perioperative and delayed complications. Hemispherectomy in children has been found in recent studies to result in freedom from seizures in 43% to 79% of patients.264–273
The “transsylvian keyhole functional hemispherectomy” advanced by Schramm and colleagues274,275 represents a true “minimalist” approach to hemispheric deafferentation.8 A linear scalp incision and a 4- by 4-cm craniotomy provide the limited exposure required for a transsylvian approach to the circular sulcus, through which access to the entire ventricular system is gained.8 Transventricular hemispheric deafferentation and amygdalohippocampectomy resulted in significantly decreased blood loss and a reduced mean operating time when compared with a Rasmussen-type functional hemispherectomy. Of the 20 patients reviewed, 88% were seizure free, and 6% had improvement in their seizures. This approach is facilitated in patients with hemispheric atrophy and not recommended in those with hemimegalencephaly.8 In a modification of this technique, another study evaluated 34 patients undergoing “transopercular hemispherotomy,” after which 67% of patients were seizure free.276
Disconnection Surgery
Multiple Subpial Transection
MST was developed by Morrell and colleagues to permit the treatment of partial epilepsies in which the seizure focus resides exclusively or partially within eloquent cortical regions.277 In a review of the Rush Presbyterian experience with 100 patients, seizure outcomes were stratified according to MST performed alone (32 patients) or in conjunction with cortical resection (68 patients).277 Class I and II outcomes were achieved, respectively, in 38% and 25% of patients with partial seizures undergoing MST alone, in 58% and 13% of patients with Landau-Kleffner syndrome treated by MST alone, and in 49% and 10% of patients when MST was performed in conjunction with resection procedures. In another review of 20 MST procedures performed without resection, less favorable outcomes were reported.278 Yet another study of 12 patients undergoing MST with or without resection revealed less favorable outcomes, including Engel class II (1), III (2), and IV (9).279 A study of long-term outcome reported a late increase in seizure frequency in 19% of patients treated by MST with or without resection.280 A meta-analysis of an aggregate international experience consisting of 211 patients at six centers revealed that 53 underwent MST alone and 158 underwent MST plus resection.281 An “excellent outcome,” defined as greater than a 95% reduction in seizures, was achieved with MST plus resection in 87% of patients with generalized seizures and in 68% with complex partial seizures; with MST alone, 71% of patients with generalized seizures and 62% of patients with complex partial seizures had excellent outcomes.
As an increasing number of patients have undergone MST, we have been able to evaluate the neurological deficits resulting from this procedure. MST has been associated with a reduction in verbal fluency but preservation of spoken and written language abilities.277 In this same study, 41 of 45 patients undergoing MST in Wernicke’s area had preserved receptive function, including comprehension of spoken and written words. One patient suffered a deep hemorrhage causing a speech deficit. In 44 transections in the hand motor cortex, strength was preserved, and activities of daily living could be performed with the affected hand.277 Of 7 transections in the leg area, which were described as technically difficult, 2 patients suffered footdrop because of subcortical venous hemorrhage. Overall, neurological complications were observed in 17% and permanent deficits were identified in 7%. No mortalities occurred. Two cases of “remarkable” intraoperative brain swelling and edema have been described, with a large intracerebral hematoma discovered in 1 patient.278
Corpus Callosotomy
The procedure of subtotal or staged total corpus callosotomy has been recommended less frequently since widespread introduction of the vagal nerve stimulator.8 Nevertheless, an abundant literature attests to the utility of callosotomy as palliative treatment in patients with multiple or poorly lateralized (and unresectable) epileptogenic foci, secondarily generalized tonic-clonic seizures, and injurious drop attacks because of tonic or atonic seizures with resultant falls and injury.282–286 Early studies revealed increased focal seizures in 25% of patients undergoing callosotomy.285 It has been reported that 70% of patients will achieve elimination of seizures or at least a greater than 80% reduction in their frequency.284,285,287
In a recent series of 23 patients with intractable generalized seizures, patients underwent partial division (17) or total division (6) of the corpus callosum.288 Forty-one percent of the patients were completely seizure free or nearly free of the seizure types targeted for treatment. Forty-five percent of the patients experienced a greater than 50% reduction in seizure frequency. Simple partial motor seizures developed in 4 patients postoperatively. In addition, mentally retarded patients tended to have poorer outcomes. Fifty-seven percent of patients experienced a transient disconnection syndrome that resolved. One patient suffered a clinically silent right frontal infarction related to venous thrombosis. The average hospital stay was 7.7 days.
Callosotomy is particularly effective for drop attacks. In a study of 52 patients with drop attacks (tonic or atonic seizures), 42 (81%) exhibited complete cessation of drop attacks, with greater success occurring in those undergoing total callosal section.289 Two adult patients suffered a marked disconnection syndrome that gradually remitted, and 14 patients experienced transient akinetic states that resolved in several weeks. In another study of 20 patients monitored for 3 years, 10 exhibited a marked improvement in QOL, and 10 had greater than a 50% reduction in their seizures.290 In another cohort of 17 patients, 9 had greater than an 80% reduction in targeted seizures, and overall, 88% of the parents reported satisfaction with the surgical outcome because of improved alertness and responsiveness.291
The surgical and functional complications attributable to corpus callosotomy are well described in the literature, with a larger number of complications noted in earlier series.49,228 The main complications reported are acute disconnection syndromes, more common with total callosotomy, and the rare “split brain” syndrome. Subtotal (70% to 80%) callosotomy has been recommended as an initial procedure to minimize this complication. Surgical complications such as hemorrhage and infarction are related more to obtaining access to the interhemispheric fissure. With modern advances in microsurgical approaches and careful patient selection, corpus callosotomy is a safe procedure and a technique that is currently underused.
Neuromodulatory Surgery
Vagus Nerve Stimulation
Since Food and Drug Administration approval in 1997 of VNS as palliative treatment of patients older than 12 years with intractable partial seizures, tens of thousands of patients have undergone implantation of a left vagus nerve stimulator.292,293 In prospective clinical trials, a median partial seizure reduction of 34% after 3 months and 45% at 12 months was achieved in patient groups both younger and older than 50 years. Twenty percent of patients at 12 months had 75% or greater reductions in seizures, thus demonstrating improved seizure control over time.292,293 At 3 months, generalized seizures were reduced by 46%. Improvements in mood have also been reported.294 Although it has been observed that figural memory worsens when VNS is active during memory tasks,295 no change in cognitive functions has been noted.296 In patients with greater than 50% improvement in seizure frequency, QOL measures were improved.296,297 In a long-term study looking at the effectiveness of VNS in epilepsy patients, seizure frequency was reduced by 26% 1 year after implantation, by 30% 5 years after surgery, and by 52% 12 years after implantation.298 A more recent study suggested that VNS could be a safe and effective alternative therapy in children with drug-resistant epilepsy who are not candidates for epilepsy surgery.299 In this study, after a mean follow-up of 31 months, 38% of the patients had a reduction in seizure frequency of greater than 90%.
Reported side effects include voice alteration, hoarseness, throat or neck pain, headache, cough, and dyspnea.300 Adverse events in adults include infection requiring antibiotics or removal of the device (or both) and transient paralysis of the left vocal cord with hoarseness and aspiration.301 There is an extraordinary report of self-inflicted vocal cord paralysis in 2 developmentally disabled patients by manipulation and rotation of the pulse generator within the subclavicular pocket.302 Such a report mandates that patients be observed for manipulation of the device. In a review of adverse events in 24 children implanted with the vagal nerve stimulator, 15 events occurred in 11 patients, including lead fractures, wound erythema, requested removal of the device, abscess, malfunction, gastrostomy, recurrent psychosis, and diminished speech volume.300 Removal of electrodes from the vagus nerve can be difficult. One paper reported resolution of a deep wound infection with antibiotics alone, thus suggesting that removal of the device might not always be necessary.303 No increase in sudden unexpected, unexplained death with the vagal nerve stimulator was identified when implanted patients were compared with appropriate cohort populations.304
Deep Brain Stimulation
In the past, brain stimulation in the cerebellum, the caudate nucleus, and the anterior, centromedian, and ventralis intermedius thalamic nuclei has been performed in an attempt to modulate cortical excitability.305,306 Small controlled trials in 14 patients who underwent cerebellar stimulation showed that 2 were improved and 12 were unchanged.307 In another study, 4- to 6-Hz stimulation of the ventral caudate nucleus led to a reduction in neocortical and mesial temporal epileptic discharges and electrical spread of seizures, but clinical seizure data were not assessed. Caudate nucleus stimulation for epilepsy has not yet been tested in controlled studies. A small placebo-controlled study of stimulation of the centromedian nucleus showed no significant benefit.308 An initial report of patients undergoing DBS in the subthalamic nucleus described a greater than 80% reduction in daytime seizures.305 In another study, 5 patients with various seizure types underwent stimulation through bilateral electrodes in the anterior thalamus.309 They reported a significant decrease in seizure frequency, with a mean 54% reduction (mean follow-up of 15 months). Two of the patients had a 75% or greater reduction in seizures. The observed benefits, however, did not differ between stimulation-on and stimulation-off periods, thus suggesting that either a placebo or carryover effect was present. Currently, a multicenter prospective randomized trial of scheduled chronic anterior thalamic stimulation in patients with medically intractable localization-related epilepsy is under way. Electrical stimulation of the hippocampus has also been reported in an attempt to block temporal lobe seizures.310 In another small series, 3 patients with complex partial seizures had DBS electrodes implanted in the amygdala-hippocampal region.311 Over a mean follow-up of 5 months, all patients had a greater than 50% reduction in seizure frequency. In 2 patients, AEDs could be reduced. Complications with DBS for epilepsy, such as hemorrhage and infection, have been reported in about 5% of patients,312 although the hemorrhage is often not clinically significant.
There is growing interest in methods of neurostimulation that are modulated by input from sensing devices. A small pilot study reported that responsive stimulation controlled with an external computer system terminated some spontaneous seizures in eight patients, four with bilateral anterior thalamic stimulation and four with focal cortical stimulation.313 In this study, analysis of electrographic seizure severity in stimulated versus nonstimulated events was used to rule out non–stimulation-associated effects and suggested both an immediate effect and a possible cumulative antiepileptic effect of high-frequency stimulation. A multi-institutional clinical study is also under way in patients with medically intractable partial onset seizures treated with a cranially implanted responsive neurostimulator (RNS). The RNS pulse generator continuously analyzes the patient’s ECoG tracings and automatically triggers electrical stimulation when specific ECoG characteristics programmed by the clinician are detected. An initial single-center experience in a feasibility study of this device described a 45% decrease in seizures in seven of eight patients with a mean follow-up of 9 months.314
Stereotactic Radiosurgery
Radiosurgery for Hypothalamic Hamartomas
HH may be associated with an epileptic encephalopathy marked by medically intractable gelastic and other seizures and behavioral and cognitive decline. Recent reports of successful treatment of HH with Gamma Knife surgery (GKS) have offered an attractive alternative to open surgery.315–318 In a multicenter study of 10 patients undergoing GKS in seven centers, 4 patients were seizure free (Engel class I), 1 had rare nocturnal seizures, 1 had rare partial seizures, and 2 were improved.316 A European, multicenter, prospective trial of GKS for HH has enrolled 60 patients, 27 of whom have exceeded 3 years of follow-up.319 Ten of the 27 patients (37%) were seizure free (Engel class I). This study emphasized a temporal evolution of changes in seizure frequency during the postradiation period: a slight improvement in seizure frequency within the first 2 months, followed by transient worsening, with a subsequent reduction and ultimate remission in favorable cases. Behavioral improvements, in addition to EEG normalization, occurred in a more linear fashion. Minimal side effects were reported. Patients treated with doses exceeding 17 Gy to the margins of the HH seemed to have greater rates of seizure remission than did those receiving less than 13 Gy.
Radiosurgery for Supratentorial Tumors
Given the diverse pathologic features and locations of central nervous system tumors associated with intractable epilepsy, the effects of GKS on tumor progression and seizure outcome are not well studied. One study divided 24 patients into two groups distinguished by the amount of radiation directed to surrounding tissue.320 Outcome was assessed at a mean of approximately 2 years after GKS as “excellent” (Engel class I or II) or not. Patients in the high-dose group achieved a 66% improvement rate as compared with 42% in the low-dose group, with all patients exhibiting adequate tumor control. This report suggested that higher GKS doses to the epileptogenic region surrounding the tumor may improve seizure outcomes.
Radiosurgery for Arteriovenous Malformations
The potential efficacy of GKS in the treatment of symptomatic localization-related epilepsies has best been demonstrated in the treatment of AVMs. One large series reported that seizures remitted after GKS in 69% of patients with AVM and epilepsy.321 Subsequent studies of both proton beam treatment and GKS showed a combined rate of seizure remission of 73% to 80%.322–324 A recent large case series emphasized that the incidence of seizure remission is better with smaller AVMs.324 However, another study noted that seizures remitted independent of radiologic remission of the AVM, thus suggesting that the effects of irradiation near the lesion, rather than improvement of the AVM itself, may be important in control of seizures after GKS.321
Radiosurgery for Cavernous Malformations
It is difficult to draw conclusions about seizure outcome after radiosurgery for cavernomas given the limited studies in the literature. In general, seizure remission appears to be lower than that encountered in patients after treatment of AVMs. The effect of dose to adjacent brain tissue around the margin of the cavernous malformation, thought to be important in the case of tumors and possibly AVMs, has not been systematically studied with regard to seizure control for patients with cavernomas. Excess morbidity in terms of postoperative hemorrhage and edema remains a concern. An early study suggested that GKS did not appreciably alter the natural course of cavernous malformations while exposing patients to radiation-induced complications that exceeded by seven times those expected with the same dose for AVMs.325 A more recent retrospective comparison concluded that traditional open resection resulted in better seizure control and a lower risk for hemorrhage than GKS did.326 A retrospective multicenter trial reported on 49 patients with cavernomas treated by GKS.327 All patients had epilepsy that was medically intractable and were monitored for more than 12 months after treatment. The mean marginal dose was 19.2 Gy. Twenty-six patients (53%) became seizure free (Engel class I), 10 patients (20%) had a substantial decrease in the number of seizures (Engle class II), and 13 patients (26%) had little or no improvement. The average time to seizure remission was 4 months, and severe radiation-induced edema developed in 7 patients, but they recovered fully.
Radiosurgery for Mesial Temporal Lobe Epilepsy
The rationale for treatment of MTLE with GKS is less compelling than in the disorders discussed previously because MTLE is amenable to open surgery.328 All of the studies in the literature differ in their treatment protocols and results, with most failing to achieve complete remission from seizures.329–334 An earlier study had demonstrated that 6 of 7 patients (86%) studied over a 2-year follow-up period were seizure free after radiosurgery.329 A more recent trial reported that 13 of 21 patients (62%) were seizure free (Engel class I) after radiosurgery for MTLE.334 The variability in outcome of GKS therapy for MTLE may reflect differing approaches to the dose and target volume more than the anatomic target of the mesial temporal lobe structures.328 Taken together, these studies suggest that low-dose protocols are less successful than higher-dose protocols. No significant clinical or neuroimaging changes occur until approximately 9 to 12 months after treatment, and the most dramatic drop in the seizure rate occurs between 12 and 18 months, coincident with the development and resolution of maximal MRI changes. Reported morbidities include visual field deficits, headache, nausea, vomiting, and depression.
Beyond seizure control, studies have begun to evaluate secondary outcome measures such as cognition and QOL. Three reports of neuropsychological outcome after GKS for MTLE are available.332–334 One prospective, multicenter trial reported no mean neurocognitive changes through a 2-year follow-up period.334 Similarly, one small series reported no group mean changes at 6 months of follow-up, although some individuals showed a decline in at least one cognitive domain.332 Another small series reported on three participants with a 27-month follow-up who underwent dominant-hemisphere low-dose GKS.333 No long-term consistent changes in neurocognitive measures were found, although each patient showed a decline in a measure of verbal memory. They concluded that the neurocognitive changes after GKS appear to be similar to those after anterior temporal lobectomy.
Overall, although GKS for MTLE is promising, the optimal treatment protocol has not yet been determined and the relative benefits in terms of seizure resolution and avoidance of complications have yet to be clearly demarcated from those of open surgery. A National Institutes of Health–sponsored multicenter pilot study on the safety of GKS for MTLE has recently been completed.328 In this study, patients who would normally qualify for anterior temporal lobectomy for unilateral MTLE were randomized to either a 20- or 24-Gy dose. A total of 30 subjects were enrolled and monitored for 3 years; the preliminary results are promising, with safety well within that expected after routine GKS and favorable efficacy and neuropsychological profiles.
Bien CG, Kurthen M, Baron K, et al. Long-term seizure outcome and antiepileptic drug treatment in surgically treated temporal lobe epilepsy patients: a controlled study. Epilepsia. 2001;42:1416-1421.
Clusmann H, Schramm J, Kral T, et al. Prognostic factors and outcome after different types of resection for temporal lobe epilepsy. J Neurosurg. 2002;97:1131-1141.
Engel J. Surgery for seizures. N Engl J Med. 1996;334:647-652.
Feindel A, Rasmussen T. Temporal lobectomy with amygdalectomy and minimal hippocampal resection: review of 100 cases. Can J Neurol Sci. 1991;18:603-605.
Fontaine D, Capelle L, Duffau H. Somatotopy of the supplementary motor area: evidence from correlation of the extent of surgical resection with the clinical patterns of deficit. Neurosurgery. 2002;50:297-305.
Gleissner U, Helmstaedter C, Schramm J, et al. Memory outcome after selective amygdalohippocampectomy: a study in 140 patients with temporal lobe epilepsy. Epilepsia. 2002;43:87-95.
Langfitt JT. Cost-effectiveness of anterotemporal lobectomy for medically-intractable complex partial epilepsy. Epilepsia. 1997;38:154-163.
Langfitt JT, Holloway RG, McDermott MP, et al. Health care costs decline after successful epilepsy surgery. Neurology. 2007;68:1290-1298.
Milner B. Psychological defects produced by temporal lobe excision. Res Publ Assoc Res Nerv Ment Dis. 1958;36:244-257.
Ng Y, Rekate HL, Prenger EC, et al. Transcallosal resection of hypothalamic hamartoma for intractable epilepsy. Epilepsia. 2006;47:1192-1202.
Ojemann G, Ojemann J, Lettich E, et al. Cortical language localization in left dominant hemisphere. J Neurosurg. 1989;71:316-326.
Ojemann G. Surgical therapy for medically intractable epilepsy. J Neurosurg. 1987;66:489-499.
Olivier A. Extratemporal resections in the surgical treatment of epilepsy. In: Spencer SS, Spencer DD, editors. Surgery for Epilepsy. Boston: Blackwell Scientific, 1991.
Quigg M, Barbaro NM. Stereotactic radiosurgery for treatment of epilepsy. Arch Neurol. 2008;65:177-183.
Salanova V, Markand O, Worth R. Temporal lobe epilepsy surgery: outcome, complications and late mortality rate in 215 patients. Epilepsia. 2002;43:170-174.
Schachter SC, Wheless JW. The evolving place of vagus nerve stimulation therapy. Neurology. 2002;59(6 suppl 4):S1-S2.
Schramm J, Aliashkevich A, Grunwald T. Multiple subpial transections: outcome and complications in 20 patients who did not undergo resection. J Neurosurg. 2002;97:39-47.
Schramm J, Kral T, Clusmann H. Transsylvian keyhole functional hemispherectomy. Neurosurgery. 2001;49:891-901.
Schwartz TH, Bazil CW, Walczak TS, et al. The predictive value of intraoperative electrocorticography in resections for limbic epilepsy associated with mesial temporal sclerosis. Neurosurgery. 1997;40:302-311.
Spencer SS, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7:525-537.
Sperling M, O’Connor M, Saykin A, et al. Temporal lobectomy for refractory epilepsy. JAMA. 1996;276:470-475.
Stroup E, Langfitt J, Berg M, et al. Predicting verbal memory decline following anterior temporal lobectomy. Neurology. 2003;60:1266-1273.
Wiebe A, Blume W, Girvin J, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311-318.
Wieser HG, Siegel G, Yasargil G. The Zurich Amygdalohoppocampectomy series: a cohort up-date. Acta Neurochir Suppl. 1990;50:122-127.
Wyler AR, Hermann BP, Somes G. Extent of medial temporal resection on outcome from anterior temporal lobectomy: a randomized prospective study. Neurosurgery. 1995;37:982-991.
1 Engel J. Surgery for seizures. N Engl J Med. 1996;334:647-652.
2 Wiebe A, Blume W, Girvin J, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311-318.
3 Rowland LP. National Institutes of Health consensus development conference statement: surgery for epilepsy, 1990 March; 19-21.
4 Schuele SU, Lüders HO. Intractable epilepsy: management and therapeutic alternatives. Lancet Neurol. 2008;7:514-524.
5 Begley CE, Famulari M, Annegers JF, et al. The cost of epilepsy in the United States: an estimate from population-based clinical and survey data. Epilepsia. 2000;41:342-351.
6 Taylor DC, McMackin D, Staunton H, et al. Patients’ aims for epilepsy surgery: desires beyond seizure freedom. Epilepsia. 2001;42:629-633.
7 Begley CE, Annegers JF, Lairson DR, et al. Cost of epilepsy in the United States: a model based on incidence and prognosis. Epilepsia. 1994;35:1230-1243.
8 Pilcher WH. Epilepsy surgery: outcome and complications. In: Winn HR, editor. Youmans Neurological Surgery. Philadelphia: Saunders; 2003:2565-2585.
9 Hermann BP, Wyler AR, Ackerman B, et al. Short-term psychological outcome of anterior temporal lobectomy. J Neurosurg. 1989;71:327-334.
10 Engel JJr. Outcome with respect to epileptic seizures. In: Engel JJr, editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1987:553-571.
11 Engel JJr, Van Ness PC, Rasmussen TB, et al. Outcome with respect to epileptic seizures. In: Engel JJr, editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993:609-621.
12 Sperling M, O’Connor M, Saykin A, et al. Temporal lobectomy for refractory epilepsy. JAMA. 1996;276:470-475.
13 Lassonde M, Sauerwein HC, Gallagher A, et al. Neuropsychology: traditional and new methods of investigation. Epilepsia. 2006;47(suppl 2):9-13.
14 Wada J, Rasmussen T. Intracarotid injection of sodium Amytal for the lateralization of cerebral speech dominance: experimental and clinical observations. J Neurosurg. 1960;17:266-282.
15 Milner B, Branch C, Rasmussen T. Study of short-term memory after intracarotid injection of sodium Amytal. Trans Am Neurol Assoc. 1962;87:224-226.
16 Jones-Gotman MJ. Commentary: psychological evaluation; testing hippocampal function. In: Engel JJr, editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1987:203-211.
17 Kubu CS, Girvin JP, McLachlan RS, et al. Does the intracarotid amobarbital procedure predict global amnesia after temporal lobectomy? Epilepsia. 2000;41:1321-1329.
18 Wyllie E, Naugle R, Chelune G, et al. Intracarotid amobarbital procedure: II. Lateralizing value in evaluation for temporal lobectomy. Epilepsia. 1991;32:865-869.
19 Perrine K, Westerveld M, Sass KJ, et al. Wada memory disparities predict seizure laterality and postoperative seizure control. Epilepsia. 1995;36:851-856.
20 Loring DW, Meador KJ, Lee GP, et al. Wada memory asymmetries predict verbal memory decline after anterior temporal lobectomy. Neurology. 1995;45:1329-1333.
21 Sabsevitz DS, Swanson SJ, Morris GL, et al. Memory outcome after left anterior temporal lobectomy in patients with expected and reversed Wada memory asymmetry scores. Epilepsia. 2001;42:1408-1415.
22 Loring DW, Meador KJ, Lee GP. Criteria and validity issues in Wada assessment. In: Benett T, editor. The Neuropsychology of Epilepsy. New York: Plenum Press; 1992:233.
23 Trenerry MR, Jack CR, Ivnik RJ, et al. MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology. 1993;43:1800-1805.
24 Koutroumanidis M, Hennessy MJ, Seed PT, et al. Significance of interictal bilateral temporal hypometabolism in temporal lobe epilepsy. Neurology. 2000;54:1811-1821.
25 Akanuma N, Koutroumanidis M, Adachi N, et al. Presurgical assessment of memory-related brain structures: the Wada test and functional neuroimaging. Seizure. 2003;12:346.
26 Koutroumanidis M, Binnie CD, Elwes RD, et al. Interictal regional slow activity in temporal lobe epilepsy correlates with lateral temporal hypometabolism as imaged with 18FDG PET: neurophysiological and metabolic implications. J Neurol Neurosurg Psychiatry. 1998;65:170-176.
27 Van Bogaert P, Massager N, Tugendhaft P, et al. Statistical parametric mapping of regional glucose metabolism in mesial temporal lobe epilepsy. Neuroimage. 2000;12:129-138.
28 Theodore WH, Sato S, Kufta C, et al. Temporal lobectomy for uncontrolled seizures: the role of positron emission tomography. Ann Neurol. 1992;32:789-794.
29 Arnold S, Schlaug G, Niemann H, et al. Topography of interictal glucose hypometabolism in unilateral mesiotemporal epilepsy. Neurology. 1996;46:1422-1430.
30 Pelaez JM, Geller EB, Wong CO, et al. Relationship of quantitative FDG-PET temporal lobe metabolism and lateralized memory function on Wada testing. Epilepsia. 1998;39:246.
31 Salanova V, Morris HHIII, Rehm P, et al. Comparison of the intracarotid amobarbital procedure and interictal cerebral 18-fluorodeoxyglucose positron emission tomography scans in refractory temporal lobe epilepsy. Epilepsia. 1992;33:635-638.
32 Salanova V, Markand O, Worth R. Focal functional deficits in temporal lobe epilepsy on PET scans and the intracarotid amobarbital procedure: comparison of patients with unitemporal epilepsy with those requiring intracranial recordings. Epilepsia. 2001;42:198-203.
33 Hong SB, Roh SY, Kim SE, et al. Correlation of temporal lobe glucose metabolism with the Wada memory test. Epilepsia. 2000;41:1554-1559.
34 Belliveau JW, Kennedy DNJr, McKinstry RC, et al. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991;254:716-719.
35 Carpentier A, Pugh KR, Westervelt M, et al. Functional MRI of language processing: dependence on input modality and temporal lobe epilepsy. Epilepsia. 2001;42:1241-1254.
36 Desmond JE, Sum JM, Wagner AD, et al. Functional MRI measurement of language lateralization in Wada-tested patients. Brain. 1995;118:1411-1419.
37 Gaillard WD, Balsamo L, Xu B, et al. fMRI language task panel improves determination of language dominance. Neurology. 2004;63:1403-1408.
38 Hert-Pannier L, Gaillard WD, Mott SH, et al. Noninvasive assessment of language dominance in children and adolescents with functional MRI: a preliminary study. Neurology. 1997;48:1003-1012.
39 Golby AJ, Poldrack RA, Illes J, et al. Memory lateralization in medial temporal lobe epilepsy assessed by functional MRI. Epilepsia. 2002;43:855-863.
40 Killgore WDS, Glosser G, Casasanto DJ, et al. Functional MRI and the Wada test provide complementary information for predicting post-operative seizure control. Seizure. 1999;8:450-455.
41 Rabin ML, Narayan VM, Kimberg DY, et al. How close is fMRI to providing the memory component of the Wada test? Curr Lit Clin Sci. 2005;5:184-186.
42 Vickrey BG, Hays RD, Graber J, et al. A health-related quality of life instrument for patients evaluated for epilepsy surgery. Med Care. 1992;30:299-319.
43 Privitera M, Ficker DM, Privitera M, et al. Assessment of adverse events and quality of life in epilepsy: design of a new community-based trial. Epilepsy Behav. 2004;5:841-846.
44 Cramer JA, Perrine K, Devinsky O, et al. Development and cross-cultural translations of a 31-item quality of life in epilepsy inventory. Epilepsia. 1998;39:81-88.
45 Vickrey BG. A procedure for developing a quality of life measure for epilepsy surgery patients. Epilepsia. 1993;34(suppl 4):S22-S27.
46 Devinsky O, Perrine K, Hirsch J, et al. Relation of cortical language distribution and cognitive function in surgical epilepsy patients. Epilepsia. 2000;41:400-404.
47 Langfitt JT. Cost-effectiveness of anterotemporal lobectomy for medically-intractable complex partial epilepsy. Epilepsia. 1997;38:154-163.
48 Wiebe S, Gafni A, Blume WT, et al. An economic evaluation of surgery for temporal lobe epilepsy. J Epilepsy. 1995;8:227-235.
49 Pilcher WH, Ojemann GA. Presurgical evaluation and epilepsy surgery. In: Apuzzo MLJ, editor. Brain Surgery: Complication Avoidance and Management. New York: Churchill Livingstone, 1993.
50 Diehl B, Luders HO. Temporal lobe epilepsy: when are invasive recordings needed? Epilepsia. 2000;41(suppl 3):S61-S74.
51 Engel JJr, Crandall PH, Rausch R. The partial epilepsies. In: Rosenberg RN, editor. The Clinical Neurosciences, Vol 2. New York: Churchill Livingstone; 1983:1249.
52 Blatt DR, Roper SM. Invasive monitoring of limbic epilepsy using stereotactic depth and subdural strip electrodes: surgical technique. Surg Neurol. 1997;48:74-79.
53 Fernandez GA, Hufnagel A. Safety of intrahippocampal depth electrodes for presurgical evaluation of patients with intractable epilepsy. Epilepsia. 1997;38:922-929.
54 Gleissner U, Helmstaedter C, Schramm J, et al. Memory outcome after selective amygdalohippocampectomy: a study in 140 patients with temporal lobe epilepsy. Epilepsia. 2002;43:87-95.
55 Wyler AR, Ojemann GA, Lettich E, et al. Subdural strip electrodes for localizing epileptogenic foci. J Neurosurg. 1984;60:1195-1200.
56 Rydenhag B, Silander HC. Complications of epilepsy surgery after 654 procedures in Sweden, September 1990-1995: a multicenter study based on the Swedish national epilepsy surgery register. Neurosurgery. 2001;49:51-57.
57 Wyllie E, Luders H, Morris H, et al. Clinical outcome after complete or partial cortical resection for intractable epilepsy. Neurology. 1987;37:1634-1641.
58 Lee WS, Lee JK. Complications and results of subdural grid electrode implantation in epilepsy surgery. Surg Neurol. 2000;54:346-351.
59 Engel J. Epilepsy surgery. Curr Opin Neurol. 1994;7:140-147.
60 Wieser H, Engel J, Williamson P, et al. Surgically remediable temporal lobe syndromes. In: Engel JJr, editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993:49-63.
61 Jack CR, Sharbrough FW, Twomey CK, et al. Temporal lobe seizures: lateralization with MR volume measurements of the hippocampal formation. Radiology. 1990;175:423-429.
62 Sawrie SM, Martin RC, Knowlton R, et al. Relationships among hippocampal volumetry, proton magnetic resonance spectroscopy and verbal memory in temporal lobe epilepsy. Epilepsia. 2001;42:1403-1407.
63 Salanova V, Markand O, Worth R. Focal functional deficits in temporal lobe epilepsy of PET scans and the intracarotid amobarbital procedure: comparison of patients with unitemporal epilepsy with those requiring intracranial recordings. Epilepsia. 2001;42:198-203.
64 Manno EM, Sperling MR, Ding X, et al. Predictors of outcome after anterior temporal lobectomy: positron emission tomography. Neurology. 1994;44:2331-2336.
65 Sperling MR, Saykin AJ, Glosser G, et al. Predictors of outcome after anterior temporal lobectomy: the intracarotid amobarbital test. Neurology. 1994;44:2325-2330.
66 Nakasato N, Levesque MF, Babb TL. Seizure outcome following standard temporal lobectomy: correlation with hippocampal neuron loss and extrahippocampal pathology. J Neurosurg. 1992;77:194-2000.
67 Lynd-Balta E, Pilcher WH, Joseph SA. Distribution of AMPA receptor subunits in the hippocampal formation of temporal lobe epilepsy patients. Neuroscience. 1996;72:15-29.
68 Cendes F, Andermann F, Gloor P, et al. Atrophy of mesial structures in patients with temporal lobe epilepsy: cause or consequence of repeated seizures? Ann Neurol. 1993;34:795-801.
69 De Lanerolle NC, Kim JH, Brines ML. Cellular and molecular alterations in partial epilepsy. Clin Neurosci. 1994;2:64-81.
70 Babb TL, Kupfer WR, Pretorius JK, et al. Synaptic reorganization by mossy fibers in human epileptic fascia dentate. Neuroscience. 1991;42:351-363.
71 King D, Spencer SS, McCarthy G, et al. Bilateral hippocampal atrophy in medial temporal lobe epilepsy. Epilepsia. 1995;36:905-910.
72 Arruda F, Cendes F, Andermann F, et al. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol. 1996;40:446-450.
73 Semah F, Baulac M, Hasboun D, et al. Is interictal temporal hypometabolism related to mesial temporal sclerosis? A positron emission tomography/magnetic resonance imaging confrontation. Epilepsia. 1995;36:447-456.
74 Engel JJr, Brown WJ, Kuhl DE, et al. Pathological findings underlying focal temporal lobe hypometabolism in partial epilepsy. Ann Neurol. 1982;12:518-528.
75 Berkovic SF, McIntosh AM, Kalnins RM, et al. Preoperative MRI predicts outcome of temporal lobectomy. Neurology. 1995;45:1358-1363.
76 Spencer SS, McCarthy G, Spencer DD. Diagnosis of medial temporal lobe seizure onset: relative specificity and sensitivity of quantitative MRI. Neurology. 1993;43:2117-2124.
77 Spencer SS. Long term outcome after epilepsy surgery. Epilepsia. 1996;37:807-813.
78 Gilliam F, Wyllie E, Kashden J, et al. Epilepsy surgery outcome: comprehensive assessment in children. Neurology. 1997;48:1368-1374.
79 Holmes MD, Dodrill CB, Ojemann LM, et al. Five-year outcome after epilepsy surgery in nonmonitored and monitored surgical candidates. Epilepsia. 1996;37:748-752.
80 Risinger M, Engel J, Van Ness P, et al. Ictal localization of temporal lobe seizures with scalp/sphenoidal recordings. Neurology. 1989;39:1288-1293.
81 Murro A, Park Y, King D, et al. Seizure localization in temporal lobe epilepsy: a comparison of scalp-sphenoidal and volumetric MRI. Neurology. 1993;43:2531-2533.
82 Gilliam F, Bowling S, Bilir E, et al. Association of combined MRI, interictal EEG and ictal EEG results with outcome and pathology after temporal lobectomy. Epilepsia. 1997;38:1315-1320.
83 McIntosh AM, Wilson SJ. Seizure outcome after temporal lobectomy: current research practice and findings. Epilepsia. 2001;42:1288-1307.
84 Sirven JI, Malamut BL. Temporal lobectomy outcome in older versus younger adults. Neurology. 2000;54:2166-2170.
85 Boling W, Andermann F, Reutens D, et al. Surgery for temporal lobe epilepsy in older patients. J Neurosurg. 2001;95:242-248.
86 McLachlan RS, Chovaz CJ, Blume WT, et al. Temporal lobectomy for intractable epilepsy in patients over age 45 years. Neurology. 1992;42:662-665.
87 Bien CG, Kurthen M, Baron K, et al. Long-term seizure outcome and antiepileptic drug treatment in surgically treated temporal lobe epilepsy patients: a controlled study. Epilepsia. 2001;42:1416-1421.
88 Wieser HG. Selective amygdalo-hippocampectomy for temporal lobe epilepsy. Epilepsia. 1988;29:S100-S113.
89 McLachlan RS, Rose KJ, Derry PA, et al. Health-related quality of life and seizure control in temporal lobe epilepsy. Ann Neurol. 1997;41:482-489.
90 Mathern GW, Giza CC, Yudovin S, et al. Postoperative seizure control and antiepileptic drug use in pediatric epilepsy surgery patients: the UCLA experience, 1986-1997. Epilepsia. 1999;40:1740-1749.
91 Engel J, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resection for epilepsy. Epilepsia. 2003;44:741-751.
92 McIntosh AM, Kalnins RM, Mitchell LA, et al. Temporal lobectomy: long term seizure outcome, late recurrence, and risks for seizure recurrence. Brain. 2004;127:2018-2030.
93 Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188-1198.
94 Tonini C, Beghi E, Berg AT, et al. Predictors of epilepsy surgery outcome: a meta-analysis. Epilepsy Res. 2004;62:75-87.
95 Zaatreh MM, Firlik KS, Spencer DD, et al. Temporal lobe tumoral epilepsy: characteristics and predictors of surgical outcome. Neurology. 2003;61:636-641.
96 Yoon HH, Kwon HL, Mattson RH, et al. Long-term seizure outcome in patients initially seizure-free after resective epilepsy surgery. Neurology. 2003;61:445-450.
97 So EL, Radhakrishnan K, Silbert PL, et al. Assessing changes over time in temporal lobectomy outcome by scoring seizure frequency. Epilepsy Res. 1997;27:119-125.
98 Al-kaylani M, Konrad P, Lazenby B, et al. Seizure freedom off antiepileptic drugs after temporal lobe epilepsy surgery. Seizure. 2007;16:95-98.
99 Asztely F, Ekstedt G, Rydenhag B, et al. Longterm followup of the first 70 operated adults in the Gotenborg epilepsy surgery series with respect to seizures, psychosocial outcome and use of antiepileptic drugs. J Neurol Neurosurg Psychiatry. 2007;78:605-609.
100 Spencer SS, Berg AT, Vickrey BG, et al. For the multicenter study of epilepsy surgery: predicting long-term seizure outcome after resective epilepsy surgery. Neurology. 2005;65:912-1018.
101 Walker AE. Surgery for epilepsy. In: Magnus O, Lorentz de Hass AM, editors. Handbook of Clinical Neurology. 15: The Epilepsies. Amsterdam: North Holland; 1974:739-757.
102 Crandall PH. Standard en bloc anterior temporal lobectomy. In: Spencer SS, Spencer DD, editors. Surgery for Epilepsy. Boston: Blackwell Scientific, 1991.
103 Spencer DD, Spencer SS, Mattson RH, et al. Access to the posterior temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurgery. 1984;15:667-671.
104 Spencer DD, Inserni J. Temporal lobectomy. In: Luders H, editor. Epilepsy Surgery. New York: Raven Press; 1992:533-545.
105 Wyler AR, Hermann BP, Somes G. Extent of medial temporal resection on outcome from anterior temporal lobectomy: a randomized prospective study. Neurosurgery. 1995;37:982-991.
106 Wieser HG, Yasargil G. Selective amygdalo-hippocampectomy as a surgical treatment of mediobasal limbic epilepsy. Surg Neurol. 1984;17:445-457.
107 Yasargil MG, Teddy P, Roth P. Selective amygdalohippocampectomy: operative anatomy and surgical technique. Adv Tech Stand Neurosurg. 1985;12:93.
108 Ojemann GA. Intraoperative tailoring of temporal lobe resections. In: Engel JJr, editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993:481-488.
109 McKhann GMII, Schoenfeld-McNeill JS, Born DE, et al. Intraoperative hippocampal electrocorticography to predict the extent of hippocampal resection in temporal lobe epilepsy surgery. J Neurosurg. 2000;93:44-52.
110 Penfield W, Jasper H. Epilepsy and the Functional Anatomy of the Human Brain. Boston: Little Brown; 1954.
111 Cascino GD, Trenerry MR, Jack CRJr, et al. Electrocorticography and temporal lobe epilepsy: relationship to quantitative MRI and operative outcome. Epilepsia. 1995;36:692-696.
112 Hermann B, Davies K, Foley K, et al. Visual confrontation naming outcome after standard left anterior temporal lobectomy with sparing versus resection of the superior temporal gyrus: a randomized prospective clinical trial. Epilepsia. 1999;40:1070-1076.
113 Feindel A, Rasmussen T. Temporal lobectomy with amygdalectomy and minimal hippocampal resection: review of 100 cases. Can J Neurol Sci. 1991;18:603-605.
114 Wieser HG, Siegel G, Yasargil G. The Zurich Amygdalohoppocampectomy series: a cohort up-date. Acta Neurochir Suppl. 1990;50:122-127.
115 Rasmussen T. Surgery for epilepsy arising in regions other than the temporal and frontal lobes. In: Advances in Neurology: Neurosurgical Management of the Epilepsies. New York: Raven Press; 1975:207-226.
116 Rasmussen T. Surgical treatment of patients with complex partial seizures. Adv Neurol. 1975;11:415-449.
117 Nayel MH, Awad IA, Luders H. Extent of mesiobasal resection determines outcome after temporal lobectomy for intractable complex partial seizures. Neurosurgery. 1991;29:55-61.
118 Walczak T. Neocortical temporal lobe epilepsy: characterizing the syndrome. Epilepsia. 1995;36:633-635.
119 Keogan M, McMackin D, Peng S, et al. Temporal neocorticectomy in management of intractable epilepsy: long term outcome and predictive factors. Epilepsia. 1989;33:852-861.
120 Hennessy MJ, Elwes RD, Rabe-Hesketh S, et al. Prognostic factors in the surgical treatment of medically intractable epilepsy associated with mesial temporal sclerosis. Acta Neurologica Scand. 2001;103:344-350.
121 Clusmann H, Schramm J, Kral T, et al. Prognostic factors and outcome after different types of resection for temporal lobe epilepsy. J Neurosurgery. 2002;97:1131-1141.
122 Penfield W, Flanigan H. Surgical therapy of temporal lobe seizures. Arch Neurol Psychiatry. 1950;64:491-500.
123 Bailey P. Surgical treatment of psychomotor epilepsy: five year followup. South Med J. 1961;54:299-301.
124 Jensen I. Temporal lobe surgery around the world. Acta Neurol Scand. 1975;52:354-373.
125 Rasmussen TB. Surgical treatment of the complex partial seizures: results, lessons and problems. Epilepsia. 1983;24(suppl 1):65-76.
126 Bladin PF. Post-temporal lobectomy seizures. Clin Exp Neurol. 1987;24:77-83.
127 Rasmussen T. Cortical resection for medically refractory focal epilepsy: results, lessons and questions. In: Rasmussen T, Marino R, editors. Functional Neurosurgery. New York: Raven Press; 1979:253-269.
128 Salanova V, Andermann F, Rasmussen T, et al. The running down phenomena in temporal lobe epilepsy. Brain. 1996;119:989-996.
129 Ficker DM, So EL, Mosewich RK, et al. Improvement and deterioration of seizure control during the postsurgical course of epilepsy surgery patients. Epilepsia. 1999;40:62-67.
130 Garcia PA, Barbaro NM, Laxer KD. The prognostic value of postoperative seizures following epilepsy surgery. Neurology. 1991;41:1511-1512.
131 Luders H, Murphy D, Awad I, et al. Quantitative analysis of seizure frequency 1 week and 6, 12 and 24 months after surgery of epilepsy. Epilepsia. 1994;35:1174-1178.
132 Park K, Buchhalter J, McClelland R, et al. Frequency and significance of acute postoperative seizures following epilepsy surgery in children and adolescents. Epilepsia. 2002;43:874-881.
133 Fiol ME, Gates JR, Torres F, et al. The prognostic value of residual spikes in the postexcision electrocorticogram after temporal lobectomy. Neurology. 1991;41:512-516.
134 McBride MC, Binnie CD, Janota I, et al. Predictive value of intraoperative electrocorticograms in resective epilepsy surgery. Ann Neurol. 1991;30:526-532.
135 Schwartz TH, Bazil CW, Walczak TS, et al. The predictive value of intraoperative electrocorticography in resections for limbic epilepsy associated with mesial temporal sclerosis. Neurosurgery. 1997;40:302-311.
136 Tran TA, Spencer SS, Marks D, et al. Significance of spikes recorded on electrocorticography in nonlesional medial temporal lobe epilepsy. Ann Neurol. 1995;38:763-770.
137 Kanazawa O, Blume WT, Girvin JP. Significance of spikes at temporal lobe electrocorticography. Epilepsia. 1996;37:50-55.
138 Tuunainen A, Nousiainen U, Mervaala E, et al. Postoperative EEG and electrocorticography: relation to clinical outcome in patients with temporal lobe surgery. Epilepsia. 1994;35:1165-1173.
139 Tran TA, Spencer SS, Javidan M, et al. Significance of spikes recorded on intraoperative electrocorticography in patients with brain tumor and epilepsy. Epilepsia. 1997;38:1132-1139.
140 Westerveld M, Sass KJ, Chelune CJ, et al. Temporal lobectomy in children: cognitive outcome. J Neurosurg. 2000;92:24-30.
141 Milner B. Visual recognition and recall after right temporal lobe excision in man. Neuropsychologia. 1968;6:191-209.
142 Milner B. Psychological aspects of focal epilepsy and its neurosurgical management. Adv Neurol. 1975;8:299-321.
143 Olivier A. Risk and benefit in the surgery of epilepsy: complications and positive results on seizure tendency and intellectual function. Acta Neurol Scand Suppl. 1988;78:114-121.
144 Rausch R, Crandall P. Psychological status related to surgical control of temporal lobe seizures. Epilepsia. 1982;23:191-201.
145 Milner B. Psychological defects produced by temporal lobe excision. Res Publ Assoc Res Nerv Ment Dis. 1958;36:244-257.
146 Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. 1957. J Neuropsychiatry Clin Neurosci. 2000;12:103-113.
147 Dimsdale H, Logue V, Piercy M. A case of persisting impairment of recent memory following right temporal lobectomy. Neuropsychologia. 1964;1:287-298.
148 Walker E. Recent memory loss in unilateral temporal lesions. Arch Neurol Psychiatry. 1957;78:543-552.
149 Rausch R. Psychological evaluation. In: Engel JJr, editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1987:181-195.
150 King D, Flanigan H, Gallagher B, et al. Temporal lobectomy for partial complex seizures: evaluation, results and 1 year follow up. Neurology. 1986;36:334-339.
151 Walczak T, Radtke R, McNamara J, et al. Anterior temporal lobectomy for complex partial seizures: evaluation, results and long-term follow up in 100 cases. Neurology. 1990;40:413-418.
152 McClone J, Black SE. Criterion-based validity of an intracarotid amobarbital recognition-memory protocol. Epilepsia. 1999;40:430-438.
153 Martin RC, Sawrie SM, Roth DL, et al. Individual memory change after anterior temporal lobectomy: a base rate analysis using regression-based outcome methodology. Epilepsia. 1998;39:1075-1082.
154 Trenerry MR. Neuropsychologic assessment in surgical treatment of epilepsy. Mayo Clin Proc. 1996;71:1196-1200.
155 Chelune GJ. Hippocampal adequacy versus functional reserve: predicting memory functions following temporal lobectomy. Arch Clin Neuropsychol. 1995;10:413-432.
156 Davies KG, Hermann BP, Foley KT. Relation between intracarotid amobarbital memory asymmetry scores and hippocampal sclerosis in patients undergoing anterior temporal lobe resections. Epilepsia. 1996;37:522-525.
157 Trenerry MR, Jack CR, Ivnik RJ, et al. MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology. 1993;43:1800-1805.
158 Stroup E, Langfitt J, Berg M, et al. Predicting verbal memory decline following anterior temporal lobectomy. Neurology. 2003;60:1266-1273.
159 Ojemann GA, Dodrill CS. Intraoperative techniques for reducing language and memory deficits with left temporal lobectomy. In: Advances in Epileptology. New York: Raven Press; 1987:327-330.
160 Pauli E, Pickel S, Schulemann H, et al. Neuropsychologic findings depending on the type of the resection in temporal lobe epilepsy. Adv Neurol. 1999;81:373-377.
161 Goldstein LH, Polkey CE. Short-term cognitive changes after unilateral temporal lobectomy or unilateral amygdalo-hippocampectomy for the relief of temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 1993;56:135-140.
162 Van Roost D, Clusmann H, Urbach H, et al. Transcortical keyhole approach versus transsylvian approach for selective amygdalohippocampectomy: which procedure is better. Acta Neurochir (Wien). 2000;142:1191.
163 Dupont S, Croize A, Semah F, et al. Is amygdalohippocampectomy really selective in medial temporal lobe epilepsy? A study using positron emission tomography with (18) fluorodeoxyglucose. Epilepsia. 2001;42:731-740.
164 Langfitt JT, Rausch R. Word-finding deficits persist after left anterotemporal lobectomy. Arch Neurol. 1996;53:72-76.
165 Ojemann G. Surgical therapy for medically intractable epilepsy. J Neurosurg. 1987;66:489-499.
166 Luders H, Lesser R, Dinner D, et al. Language deficits elicited by electrical stimulation of the fusiform gyrus. In: Engel JJr, editor. Fundamental Mechanisms of Human Brain Function. New York: Raven Press; 1987:83-90.
167 Penfield W. Pitfalls and success in surgical treatment of focal epilepsy. BMJ. 1958;1:669-672.
168 Penfield W, Milner B. Memory deficit produced by bilateral lesions in the hippocampal zone. Arch Neurol Psychiatry. 1958;79:475-497.
169 Chelune GJ. Using neuropsychological data to predict postsurgical cognitive outcome. In: Luders HO, editor. Epilepsy Surgery. New York, NY: Raven Press; 1992:477-486.
170 Stafiniak P, Saykin AJ, Sperling MR, et al. Acute naming deficits following dominant temporal lobectomy: prediction by age at first risk for seizures. Neurology. 1990;40:1509-1512.
171 Davies KG, Maxwell RE, Beniak TE, et al. Language function after temporal lobectomy without stimulation mapping of cortical function. Epilepsia. 1995;36:130-136.
172 Hermann BP, Wyler AR, Somes G. Language function following anterior temporal lobectomy. J Neurosurg. 1991;74:560-566.
173 Saykin AJ, Stafiniak P, Robinson LJ, et al. Language before and after temporal lobectomy: specificity of acute changes and relation to early risk factors. Epilepsia. 1995;36:1071-1077.
174 Hermann BP, Wyler AR, Somes G. Dysnomia after left anterior temporal lobectomy without functional mapping: frequency and correlates. Neurosurgery. 1994;35:52-57.
175 Davies KG, Bell BD. Naming decline after left anterior temporal lobectomy correlates with pathological status of resected hippocampus. Epilepsia. 1998;39:407-419.
176 Ojemann G. Brain organization of language from the perspective of electrical stimulation mapping. Behav Brain Sci. 1983;6:189-230.
177 Ojemann G, Ojemann J, Lettich E, et al. Cortical language localization in left dominant hemisphere. J Neurosurg. 1989;71:316-326.
178 Rasmussen T. Cortical resection in the treatment of focal epilepsy. Adv Neurol. 1975;8:139-154.
179 Katz A, Awad I, Kong A, et al. Extent of resection in temporal lobectomy for epilepsy: II. Memory changes and neurologic complications. Epilepsia. 1989;30:763-771.
180 Helgason C, Bergen D, Bleck T, et al. Infarction after surgery for focal epilepsy: manipulation hemiplegia revisited. Epilepsia. 1987;28:340-345.
181 Fenwick P. Psychiatric assessment and temporal lobectomy. Acta Neurol Scand Suppl. 1988;78:96-101.
182 Fenwick PB, Blumer D, Caplan R, et al. Presurgical psychiatric assessment. In: Engel JJr, editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993:273-290.
183 Glosser G, Zwil AS, Glosser DS, et al. Psychiatric aspects of temporal lobe epilepsy before and after anterior temporal lobectomy. J Neurol Neurosurg Psychiatry. 2000;68:53-58.
184 Matsuura M. Indication for anterior temporal lobectomy in patients with temporal lobe epilepsy and psychopathology. Epilepsia. 2000;41:39-42.
185 Altshuler L, Rausch R, Delrahim S, et al. Temporal lobe epilepsy, temporal lobectomy and major depression. J Neuropsychiatry Clin Neurosci. 1999;11:436-443.
186 Herman BP, Wyler AR. Depression, locus of control and the effects of epilepsy surgery. Epilepsia. 1989;30:332-338.
187 Derry P, Rose K, McLachlan RS. Moderators of the effect of preoperative emotional adjustment on postoperative depression after surgery for temporal lobe epilepsy. Epilepsia. 2000;41:177-185.
188 Ring HA, Moriarty J. A prospective study of the early postsurgical psychiatric associations of epilepsy surgery. J Neurol Neurosurg Psychiatry. 1998;64:601-604.
189 Anhoury S, Brown RJ, Krishnamoorthy ES, et al. Psychiatric outcome after temporal lobectomy: a predictive study. Epilepsia. 2000;41:1608-1615.
190 Kanemoto K. Hypomania after temporal lobectomy: a sequel to the increased excitability of the residual temporal lobe? J Neurol Neurosurg Psychiatry. 1995;59:448-454.
191 Montenegro MA, Guerreiro MM. De novo psychogenic seizures after epilepsy surgery: a case report. Arq Neuropsiquiatr. 2000;58:535-537.
192 Mayanagi Y, Watanabe E. Psychiatric and neuropsychological problems in epilepsy surgery: analysis of 100 cases that underwent surgery. Epilepsia. 2001;42:19-23.
193 Jones JE, Berven NL, Ramirez L, et al. Long-term psychosocial outcomes of anterior temporal lobectomy. Epilepsia. 2002;43:896-903.
194 Wheelock I, Peterson C, Buchtel HA. Presurgery expectations, postsurgery satisfaction and psychosocial adjustment after epilepsy surgery. Epilepsia. 1998;39:487-494.
195 Jacoby A, Baker G, Steen N, et al. The clinical course of epilepsy and its psychosocial correlates: findings from a U.K. community study. Epilepsia. 1996;37:148-161.
196 Vickrey BG, Hays RD, Rausch R, et al. Quality of life of epilepsy surgery patients as compared with outpatients with hypertension, diabetes, heart disease and/or depressive symptoms. Epilepsia. 1994;35:597-607.
197 Markand O, Salanova V. Health-related quality of life outcome in medically refractory epilepsy treated with anterior temporal lobectomy. Epilepsia. 2000;41:749-759.
198 Spencer SS, Berg AT, Vickrey BG, et al. Health related quality of life over time since resective epilepsy surgery. Ann Neurol. 2007;62:327-334.
199 Chin PS, Berg AT, Spencer SS, et al. Patient-perceived impact of resective epilepsy surgery. Neurology. 2006;66:1882-1887.
200 King JT, Sperling MR. A cost-effectiveness analysis of anterior temporal lobectomy for intractable temporal lobe epilepsy. J Neurosurg. 1997;87:20-28.
201 Langfitt JT, Holloway RG, McDermott MP, et al. Health care costs decline after successful epilepsy surgery. Neurology. 2007;68:1290-1298.
202 Rasmussen T. The role of surgery in the treatment of focal epilepsy. Clin Neurosurg. 1968;16:288-314.
203 Van Buren JM. Complications of surgical procedures in the diagnosis and treatment of epilepsy. In: Engel JJr, editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1987:465-475.
204 Ojemann G. Temporal lobectomy tailored to electrocorticography and functional mapping. In: Spencer S, Spencer D, editors. Surgery for Epilepsy. Boston: Blackwell Scientific; 1991:137-145.
205 Silfvenius H, Gloor P, Rasmussen T. Evaluation of insular ablation in surgical treatment of temporal lobe epilepsy. Epilepsia. 1964;5:307-320.
206 Crandall PH. Postoperative management and criteria for evaluation. In: Purpura DP, Penry JK, Walter RD, editors. Advances in Neurology. Neurosurgical Management of the Epilepsies, Vol 8. New York: Raven Press; 1975:265-279.
207 Ojemann GA. Neurosurgical management of epilepsy: a personal perspective in 1983. Appl Neurophysiol. 1985;46:11-18.
208 Penfield W, Lende R, Rasmussen T. Manipulation hemiplegia. J Neurosurg. 1961;18:760-776.
209 Babb T, Wilson CL, Crandall P. Asymmetry and ventral course of the human geniculostriate pathway as determined by hippocampal visual evoked potentials and subsequent visual field defects after temporal lobectomy. Exp Brain Res. 1982;47:317-328.
210 Falconer M, Wilson J. Visual field changes following anterior temporal lobectomy: their significance in relation to Myer’s loop of the optic radiation. Brain. 1958;81:1-14.
211 Marino R, Rasmussen T. Visual field changes after temporal lobectomy in man. Neurology. 1968;18:825-835.
212 Van Buren JM, Baldwin M. The architecture of the optic radiation in the temporal lobe of man. Brain. 1958;81:15-40.
213 Anderson J, Awad I, Hahn J. Delayed facial nerve palsy after temporal lobectomy for epilepsy: report of four cases and discussion of possible mechanisms. Neurosurgery. 1991;28:453-456.
214 Salanova V, Markand O, Worth R. Temporal lobe epilepsy surgery: outcome, complications and late mortality rate in 215 patients. Epilepsia. 2002;43:170-174.
215 Guldvog B, Løyning Y, Hauglie-Hanssen E, et al. Surgical treatment for partial epilepsy among Norwegian adults. Epilepsia. 1994;35:540-553.
216 Guldvog B, Løyning Y, Hauglie-Hanssen E, et al. Surgical treatment for partial epilepsy among Norwegian children and adolescents. Epilepsia. 1994;35:554-565.
217 Toczek MT, Morrell MJ, Silverberg GA. Cerebellar hemorrhage complicating temporal lobectomy. J Neurosurg. 1996;85:718-722.
218 Jacobson DM, Warner JJ, Ruggles KH. Transient trochlear nerve palsy following anterior temporal lobectomy for epilepsy. Neurology. 1995;45:1465-1468.
219 Hennessy MJ, Langan Y. A study of mortality after temporal lobe epilepsy surgery. Neurology. 1999;53:1276-1283.
220 Babb TL, Brown WJ. Pathological findings in epilepsy. In: Engel JJr, editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1987:511-540.
221 Spencer DD, Spencer SS, Mattson RH, et al. Temporal lobe masses in patients with intractable partial epilepsy. Neurology. 1984;34:432-436.
222 Fried I, Kim J, Spencer D. Hippocampal pathology in patients with intractable seizures and temporal lobe masses. J Neurosurg. 1992;76:735-740.
223 Mathern GW, Babb TL, Pretorius JK, et al. The pathophysiologic relationships between lesion pathology, intracranial ictal EEG onsets, and hippocampal neuron losses in temporal lobe epilepsy. Epilepsy Res. 1995;21:133-147.
224 Cascino GD, Kelly PJ, Sharbrough F, et al. Long-term follow-up of stereotactic lesionectomy in partial epilepsy: predictive factors and electroencephalographic results. Epilepsia. 1992;33:639-644.
225 Jooma R, Yeh H, Privitera M, et al. Lesionectomy versus electrophysiologically guided resection for temporal lobe tumors manifesting with complex partial seizures. J Neurosurg. 1995;83:231-236.
226 Moore J, Cascino G, Trenerry M, et al. A comparative study of lesionectomy versus corticectomy in patients with temporal lobe lesional epilepsy. J Epilepsy. 1999;6:239-242.
227 Yeh HS, Kashwagi S, Tew J, et al. Surgical management of epilepsy associated with cerebral arteriovenous malformations. J Neurosurgery. 1990;72:216-223.
228 Pilcher WH, Silbergeld DL, Berger MS, et al. Intraoperative electrocorticography during tumor resection: impact on seizure outcome in patients with gangliogliomas. J Neurosurg. 1993;78:891-902.
229 Schramm J, Kral T. Surgical treatment for neocortical temporal lobe epilepsy: clinical and surgical aspects and seizure outcome. J Neurosurg. 2001;94:33-42.
230 Cascino GD, Jack CR, Parisi JE, et al. Operative strategy in patients with MRI-identified dual pathology and temporal lobe epilepsy. Epilepsy Res. 1993;14:175-182.
231 Fried I, Cascino G. Lesional surgery. In: Engel J Jr, ed. Surgical Treatment of the Epilepsies, 2nd ed. New York: Raven Press.; 1993.
232 Olivier A. Extratemporal resections in the surgical treatment of epilepsy. In: Spencer SS, Spencer DD, editors. Surgery for Epilepsy. Boston: Blackwell Scientific, 1991.
233 Zentner J, Hufnagel A, Ostertun B, et al. Surgical treatment of extratemporal epilepsy: clinical, radiologic and histopathologic findings in 60 patients. Epilepsia. 1996;37:1072-1080.
234 Shimizu H, Maehara T. Neuronal disconnection for the surgical treatment of pediatric epilepsy. Epilepsia. 2000;41:28-30.
235 Jobst BC, Siefel AM, Thadani VM, et al. Intractable seizures of frontal lobe origin: clinical characteristics, localizing signs and results of surgery. Epilepsia. 2000;41:1139-1152.
236 Mosewich RK, So EL, O’Brien TJ, et al. Factors predictive of the outcome of frontal lobe epilepsy surgery. Epilepsia. 2000;41:843-849.
237 Zaatreh MM, Spencer DD, Thompson JL, et al. Frontal lobe tumoral epilepsy: clinical, neurophysiologic features and predictors of surgical outcome. Epilepsia. 2002;43:727-733.
238 Zentner J, Hufnagel A, Pechstein U, et al. Functional results after resective procedures involving the supplementary motor area. J Neurosurg. 1996;85:542-549.
239 Fontaine D, Capelle L, Duffau H. Somatotopy of the supplementary motor area: evidence from correlation of the extent of surgical resection with the clinical patterns of deficit. Neurosurgery. 2002;50:297-305.
240 Milner B. Visually-guided maze learning in man: effects of bilateral hippocampal, bilateral frontal and unilateral cerebral lesions. Neuropsychologia. 1965;3:317-338.
241 Rasmussen T. Surgery of frontal lobe epilepsy. Adv Neurol. 1975;8:197-205.
242 Rasmussen T. Commentary: extratemporal cortical excisions and hemispherectomy. In: Engel JJr, editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1987:417-424.
243 Awad I, Rosenfeld J, Ahl J, et al. Intractable epilepsy and structural lesions of the brain: mapping, resection strategies and seizure outcome. Epilepsia. 1991;32:179-186.
244 Weber J, Silbergeld D, Winn HR. Surgical resection of epileptogenic cortex associated with structural lesions. Neurosurg Clin N Am. 1993;4:327-336.
245 Kraemer DL, Griebel ML, Lee N, et al. Surgical outcome in patients with epilepsy with occult vascular malformations treated with lesionectomy. Epilepsia. 1998;39:600-607.
246 Palmini A, Gambardella A, Andermann F, et al. Intrinsic epileptogenicity of human dysplastic cortex as suggested by corticography and surgical results. Ann Neurol. 1995;37:476-487.
247 Hong S, Kang K, Seo D, et al. Surgical treatment of intractable epilepsy accompanying cortical dysplasia. J Neurosurg. 2000;93:766-773.
248 Edwards J, Wyllie E, Ruggeri P, et al. Seizure outcome after surgery for epilepsy due to malformation of cortical development. Neurology. 2000;55:1110-1114.
249 Urbach H, Scheffler B, Heinrichsmeier, et al. Focal cortical dysplasia of Taylor’s balloon cell type: a clinicopathological entity with characteristic neuroimaging and histopathological features and favorable postsurgical outcome. Epilepsia. 2002;43:33-40.
250 Bernasconi A, Martinez V, Rosa-Neto P, et al. Surgical resection for intractable epilepsy in “double cortex” syndrome yields inadequate results. Epilepsia. 2001;42:1124-1129.
251 Kuzniecky R, Guthrie B. Intrinsic epileptogenesis of hypothalamic hamartomas in gelastic epilepsy. Ann Neurol. 1997;42:60-67.
252 Arita K, Ikawa F, Kurisu K, et al. The relationship between magnetic resonance imaging findings and clinical manifestations of hypothalamic hamartoma. J Neurosurg. 1999;91:212-220.
253 Rosenfeld JV, Harvey AS. Transcallosal resection of hypothalamic hamartomas, with control of seizures, in children with gelastic epilepsy. Neurosurgery. 2001;48:108-118.
254 Ng Y, Rekate HL, Prenger EC, et al. Transcallosal resection of hypothalamic hamartoma for intractable epilepsy. Epilepsia. 2006;47:1192-1202.
255 Ng Y, Rekate HL, Prenger EC, et al. Endoscopic resection of hypothalamic hamartomas for refractory symptomatic epilepsy. Neurology. 2008;70:1543-1548.
256 Mesiwala AH, Kuratani JD, Avellino AM, et al. Focal motor seizures with secondary generalization arising in the cerebellum. J Neurosurg. 2002;97:190-196.
257 Carson B, Javedan S, Freeman J, et al. Hemispherectomy: a hemidecortication approach and review of 52 cases. J Neurosurg. 1996;84:903-911.
258 Krynauw R. Infantile hemiplegia treated by removing one cerebral hemisphere. J Neurol Neurosurg Psychiatry. 1950;13:243-267.
259 Wilson P. Cerebral hemispherectomy for infantile hemiplegia. A report of 50 cases. Brain. 1970;93:147-180.
260 Kalkanis S, Blumenfeld H, Sherman JC, et al. Delayed complications thirty-six years after hemispherectomy: a case report. Epilepsia. 1996;37:758-762.
261 White H. Cerebral hemispherectomy in the treatment of infantile hemiplegia. Confin Neurol. 1961;21:1-50.
262 Winston K. Cerebral hemicorticectomy for epilepsy. J Neurosurg. 1992;77:889-895.
263 Villemure JG, Mascott CR. Peri-insular hemispherotomy: surgical principles and anatomy. Neurosurgery. 1995;37:975-981.
264 Wyllie E, Comair YG, Kotogal P, et al. Seizure outcome after epilepsy surgery in children and adolescents. Ann Neurol. 1998;44:740-748.
265 Terra-Bustamante VC, Inuzuca LM, Fernandes RM, et al. Temporal lobe surgery in children and adolescents: clinical characteristics and clinical outcome. Seizure. 2005;14:274-281.
266 Maehara T, Shimizu H, Kawai K, et al. Postoperative development of children after hemispherotomy. Brain Dev. 2002;24:155-160.
267 Devlin AM, Cross JH, Harkness W, et al. Clinical outcomes of hemispherectomy for epilepsy in childhood and adolescence. Brain. 2003;126:556-566.
268 Gonzales-Martinez J, Gupta A, Kotagal P, et al. Hemispherectomy for catastrophic epilepsy in infants. Epilepsia. 2005;46:1518-1525.
269 Shimizu H. Our experience with pediatric epilepsy surgery focusing on corpus callosotomy and hemispherotomy. Epilepsia. 2005;46(suppl 1):30-31.
270 Delalande O, Bulteau C, Dellatolas G, et al. Vertical parasagittal hemispherotomy: surgical procedures and clinical long-term outcomes in a population of 83 children. Neurosurgery. 2007;60(suppl 1):19-32.
271 Basheer SN, Connolly MB, Lautzenhiser A, et al. Hemispheric surgery in children with refractory epilepsy: seizure outcome, complications, and adaptive function. Epilepsia. 2007;48:133-140.
272 Lettori D, Battaglia D, Sacco A, et al. Early hemispherectomy in catastrophic epilepsy a neurocognitive and epileptic long-term follow-up. Seizure. 2008;17:49-63.
273 Terra-Bustamante VC, Inuzuka LM, Fernandes RM, et al. Outcome of hemispheric surgeries for refractory epilepsy in pediatric patients. Childs Nerv Syst. 2007;23:321-326.
274 Schramm J, Behrens E, Entzian W. Hemispherical deafferentation: an alternative to functional hemispherectomy. Neurosurgery. 1995;36:509-516.
275 Schramm J, Kral T, Clusmann H. Transsylvian keyhole functional hemispherectomy. Neurosurgery. 2001;49:891-901.
276 Shimizu H, Taketoshi M. Modification of peri-insular hemispherotomy and surgical results. Neurosurgery. 2000;47:367-373.
277 Smith MC. Multiple subpial transections in patients with extratemporal epilepsy. Epilepsia. 1998;39:S81-S89.
278 Schramm J, Aliashkevich A, Grunwald T. Multiple subpial transections: outcome and complications in 20 patients who did not undergo resection. J Neurosurg. 2002;97:39-47.
279 Mulligan LP, Spencer DD, Spencer SS. Multiple subpial transactions: the Yale experience. Epilepsia. 2001;42:226-229.
280 Orbach D, Romanelli P, Devinsky O, et al. Late seizure recurrence after multiple subpial transections. Epilepsia. 2001;42:1130-1133.
281 Spencer SS, Schramm J, Wyler A, et al. Multiple subpial transection for intractable partial epilepsy: an international meta-analysis. Epilepsia. 2002;43:141-145.
282 Shimizu H, Maehara T. Neuronal disconnection for the surgical treatment of pediatric epilepsy. Epilepsia. 2000;41:28-30.
283 Purves SJ. Selection of patients for corpus callosum section. In: Spencer SS, Spencer DD, editors. Surgery for Epilepsy. Boston: Blackwell Scientific; 1991:69.
284 Spencer SS, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7:525-537.
285 Spencer SS, Spencer DD, Williamson PD, et al. Corpus callosotomy for epilepsy. I. Seizure effects. Neurology. 1988;38:19.
286 Wilson DH, Reeves A, Gazzaniga M, et al. Cerebral commissurotomy for control of intractable seizures. Neurology. 1977;27:708-715.
287 Purves SJ, Wada JA, Woodhurst WB, et al. Results of anterior corpus callosum section in 24 patients with medically intractable seizures. Neurology. 1988;38:1194-1201.
288 Sorenson JM, Wheless JW, Baumgartner JE, et al. Corpus callosotomy for medically intractable seizures. Pediatr Neurosurg. 1997;27:260-267.
289 Maehara T, Shimizu H. Surgical outcome of corpus callosotomy in patients with drop attacks. Epilepsia. 2001;42:67-71.
290 Andersen B, Rogvi-Hansen B, Kruse-Larsen C, et al. Corpus callosotomy: seizure and psychosocial outcome, a 39 month follow-up of 20 patients. Epilepsy Res. 1996;23:77-85.
291 Gilliam F, Wyllie E, Kotagal P, et al. Parental assessment of functional outcome after corpus callosotomy. Epilepsia. 1996;37:753-757.
292 Schachter SC, Wheless JW. The evolving place of vagus nerve stimulation therapy. Neurology. 2002;59(6 suppl 4):S1-S2.
293 Schacter SC. Vagus nerve stimulation therapy summary, five years after FDA approval. Neurology. 2002;59(suppl 4):S15-S20.
294 Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. 2000;42:203-210.
295 Helmstaedter C, Hope C, Elger CE. Memory alterations during acute high intensity vagus nerve stimulation. Epilepsy Res. 2001;47:37-42.
296 Dodrill CB, Morris GL. Effects of vagal nerve stimulation on cognition and quality of life in epilepsy. Epilepsy Behav. 2001;2:46-53.
297 Morrow JI, Bingham E, Crain JJ, et al. Vagal nerve stimulation in patients with refractory epilepsy: effect on seizure frequency, severity and quality of life. Seizure. 2000;9:442-445.
298 Uthman BM, Reichl AM, Dean JC, et al. Effectiveness of vagus nerve stimulation in epilepsy patients: a 12-year observation. Neurology. 2004;63:1124-1126.
299 Benifla M, Rutka JT, Logan W, et al. Vagal nerve stimulation for refractory epilepsy in children: indications and experience at The Hospital for Sick Children. Childs Nerv Syst. 2006;22:1018-1026.
300 Murphy JV. Left vagal nerve stimulation in children with medically refractory epilepsy. J Pediatr. 1999;134:563-566.
301 Ben-Menachem E, Hellstrom K, Verstappen D. Analysis of direct hospital costs before and 18 months after treatment with vagus nerve stimulation therapy in 43 patients. Neurology. 2002;50(suppl 4):S44-S47.
302 Kalkanis JG, Krisha P, Espinose JA, et al. Self-inflicted vocal cord paralysis in patients with vagus nerve stimulators. J Neurosurg. 2002;96:949-951.
303 Ortler M, Luef G, Kofler A, et al. Deep wound infection after vagus nerve stimulator implantation: treatment without removal of the device. Epilepsia. 2001;42:133-135.
304 Annegers JF, Coan SP, Hauser WA, et al. Epilepsy, vagal nerve stimulation by the NCP system, mortality and sudden, unexpected, unexplained death. Epilepsia. 1998;39:206-212.
305 Benabid AL, Minotti L, Koudsie A, et al. Antiepileptic effect of high-frequency stimulation of the subthalamic nucleus (corpus luysi) in a case of medically intractable epilepsy caused by focal dysplasia: a 30 month follow-up: technical case report. Neurosurgery. 2002;50:1385-1392.
306 Velasco F, Velasco M, Velasco AL, et al. Electrical stimulation of the centromedian thalamic nucleus in control of seizures: long-term studies. Epilepsia. 1995;36:63-71.
307 Theodore WH, Fisher R, Theodore WH, et al. Brain stimulation for epilepsy. Acta Neurochir Suppl. 2007;97:261.
308 Fisher RS, Uematsu S, Krauss GL, et al. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia. 1992;33:841-851.
309 Hodaie M, Wennberg RA, Dostrovsky JO, et al. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia. 2002;43:603-608.
310 Velasco M, Velasco F, Velasco AL, et al. Subacute electrical stimulation of the hippocampus blocks intractable temporal lobe seizures and paroxysmal EEG activities. Epilepsia. 2000;41:158-169.
311 Vonck K, Boon P, Achten E, et al. Long-term amygdalohippocampal stimulation for refractory temporal lobe epilepsy. Ann Neurol. 2002;52:556-565.
312 Fisher R. Anterior thalamic nucleus stimulation: issues in study design. In: Lüders H, editor. Deep Brain Stimulation and Epilepsy. London: Martin Dunitz; 2003:307-322.
313 Osorio I, Frei MG, Sunderam S, et al. Automated seizure abatement in humans using electrical stimulation. Ann Neurol. 2005;57:258-268.
314 Fountas KN, Smith JR, Murro AM, et al. Implantation of a closed-loop stimulation in the management of medically refractory focal epilepsy: a technical note. Stereotact Funct Neurosurg. 2005;83:153-158.
315 Unger F, Schrottiner O, Haselberger K, et al. Gamma knife radiosurgery for hypothalamic hamartomas in patients with medically intractable epilepsy and precocious puberty. J Neurosurg. 2000;92:726-731.
316 Regis J, Fabrice B, de Toffol B, et al. Gamma knife surgery for epilepsy related to hypothalamic hamartomas. Neurosurgery. 2000;47:1343-1352.
317 Parrent AG. Stereotactic radiofrequency ablation for the treatment of gelastic seizures associated with hypothalamic hamartoma. Case report. J Neurosurg. 1999;91:881-884.
318 Dunoyer C, Ragheb J, Resnick T, et al. The use of stereotactic radiosurgery to treat intractable childhood partial epilepsy. Epilepsia. 2002;43:292-300.
319 Regis J, Scavarda D, Tamura M, et al. Epilepsy related to hypothalamic hamartomas: surgical management with special reference to gamma knife surgery. Childs Nerv Syst. 2006;22:881-895.
320 Schrottner O, Eder HG, Unger F, et al. Radiosurgery in lesional epilepsy: brain tumors. Stereotact Funct Neurosurg. 1998;70(suppl 1):50-56.
321 Steiner L, Lindquist C, Adler JR, et al. Clinical outcome of radiosurgery for cerebral arteriovenous malformations. J Neurosurg. 1992;77:1-8.
322 Gerszten PC, Adelson PD, Kondziolka D, et al. Seizure outcome in children treated for arteriovenous malformations using gamma knife radiosurgery. Pediatr Neurosurg. 1996;24:139-144.
323 Kurita H, Kawamoto S, Suzuki I, et al. Control of epilepsy associated with cerebral arteriovenous malformations after radiosurgery. J Neurol Neurosurg Psychiatry. 1998;65:648-655.
324 Schauble B, Cascino GD, Pollock BE, et al. Seizure outcomes after stereotactic radiosurgery for cerebral arteriovenous malformations. Neurology. 2004;63:683-687.
325 Karlsson B, Kihlstrom L, Lindquist C, et al. Radiosurgery for cavernous malformations. J Neurosurg. 1998;88:293-297.
326 Shih YH, Pan DH. Management of supratentorial cavernous malformations: craniotomy versus gamma knife radiosurgery. Clin Neurol Neurosurg. 2005;107:108-112.
327 Regis J, Bartolomei F, Kida Y, et al. Radiosurgery for epilepsy associated with cavernous malformation: retrospective study in 49 patients. Neurosurgery. 2000;47:1091-1097.
328 Quigg M, Barbaro NM. Stereotactic radiosurgery for treatment of epilepsy. Arch Neurol. 2008;65:177.
329 Regis J, Bartolomei F, Rey M, et al. Gamma knife surgery for mesial temporal lobe epilepsy. Epilepsia. 1999;40:1551-1556.
330 Cmelak AJ, Abou-Khalil B, Konrad PE, et al. Low-dose stereotactic radiosurgery is inadequate for medically intractable mesial temporal lobe epilepsy: a case report. Seizure. 2001;10:442-446.
331 Kawai K, Suzuki I, Kurita H, et al. Failure of low-dose radiosurgery to control temporal lobe epilepsy. J Neurosurg. 2001;95:883-887.
332 Srikijvilaikul T, Najm I, Foldvary-Schaefer N, et al. Failure of gamma knife radiosurgery for mesial temporal lobe epilepsy: report of five cases. Neurosurgery. 2004;54:1395-1402.
333 McDonald CR, Norman MA, Tecoma E, et al. Neuropsychological change following gamma knife surgery in patients with left temporal lobe epilepsy: a review of three cases. Epilepsy Behav. 2004;5:949-957.
334 Regis J, Rey M, Bartolomei F, et al. Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia. 2004;45:504-515.