Chapter 7 Epilepsy and Sleep
Introduction
Epilepsy and sleep are related in several aspects, from physiology and pathophysiology to clinical appearance and neurophysiological diagnosis to treatment. It has been known since the days of Aristotle that epileptic seizures may occur exclusively during sleep.1 Later, several authors observed that certain epileptic seizures may show a tendency to occur during sleep or waking periods and recognized the relation to the sleep–wake cycle.2–8 Also, epileptiform discharges occur more frequently during non-REM sleep than in REM sleep and waking periods,9–11 and arousals and transitions periods between sleep stages are considered to facilitate epileptiform discharges.12–15 Antiepileptic drugs have effects on sleep, and it has even been speculated that some of their antiepileptic effect is due to their effect on sleep.16
Physiology and Diagnostic Studies of Sleep
Sleep is a complex behavioral trait, in which consciousness and responsiveness to environmental stimuli are reduced. There is an ongoing discussion as to why we sleep, but some physiologic functions, such as memory consolidation,17 have been shown to be dependent on sleep. The pressure on the organism to initiate sleep depends largely on circadian and homeostatic influences.18 Several brain regions are actively involved in the sleep process. Commonly, the cortical activity, and with it the sleep electroencephalography (EEG), is described as “synchronous” in a periodical fashion. The synchronicity refers in particular to the cortical neurons.19 Deeper brain structures such as the midbrain and brainstem involved in this active process do not necessarily reflect the same level of synchronicity as the cortex. There are two general different states of sleep, rapid eye movement sleep (REM sleep) and non–rapid eye movement sleep (NREM sleep), with the latter being divided further into four stages.20 Over the course of a night, NREM and REM sleep alternate with an approximately 90-minute rhythmicity, creating four to six sleep cycles. The NREM stages differ in their amount of slow-wave sleep and special defining patterns like K-complexes and sleep spindles. Electrophysiologically, the dynamic of NREM sleep can be understood as the neurobehavioral attempt to reach a high level of synchrony, expressed as slow-wave sleep and a deafferentiation of sensory input.21 REM sleep is defined by fast eye movements, loss of muscle tone, and an EEG desynchrony. These sleep oscillations are mainly generated by the corticothalamic system, the reticular thalamic and thalamocortical circuit, and thalamically projecting brainstem structures.22 This difference in cellular and systemic neuronal activity is important for the difference in occurrence of seizures and interictal epileptiform discharges during sleep.
During sleep, external (for example, environmental noises) and internal stimuli (for example, sleep apnea episodes) may evoke arousal reactions. In the arousals, an adequate reaction to the stimulus and the attempt to stay asleep are competing impulses. A night with many arousals will be not as restful as a night with few arousals. In the classic sleep evaluation according to the criteria of Rechtschaffen and Kales20 an arousal is an event of fast, desynchronized EEG discharges. Terzano and colleagues23 described a 20- to 40-second rhythmicity of changes in the EEG as the cyclic alternating pattern (CAP), defined by an alternation of phasic EEG events and background activity. Now CAP is considered an expression of different arousal mechanisms that are expressed differentially in the various phases of sleep and in sleep stage transitions.24 During arousals and during stage shifts, interictal epileptic discharges and seizures are more likely to develop, especially in generalized epilepsies.13,25,26
Despite not incorporating all the derivations needed for polysomnography, routine EEG or EEG-video monitoring will already enable the diagnosis of all sleep stages, although with some ambiguity. The prefrontal leads (Fp1 and Fp2) and the lateral temporal leads (FT9 and FT10 or T7 and T8) can be used to interpret eye movements. An epilepsy-focused EEG will allow the evaluation of sleepiness (eye rolling in the frontal leads, temporal theta of drowsiness), falling asleep (change of background, vertex waves, and posterior sharp transients of sleep [POSTS]), and sleep stages 2, 3, and 4 (K-complexes, sleep spindles in stage 2, increase of delta activity in stages 3 and 4). REM sleep can be detected by fast eye movements and the low-amplitude beta and gamma activity in the EEG, but its exact beginning may be obscured without an EMG recording of the chin muscles. The formal scoring of conventional sleep stages requires 30-second epochs starting with the lights-off signal.20
Excessive daytime sleepiness can be assessed by the multiple sleep latency test (MLST), which provides more objective data than questionnaires.27 The MLST is usually performed on a day following a polysomnography to provide accurate documentation of the preceding night’s sleep.
Effects of Epilepsy on Sleep
Epilepsy patients typically report poorer sleep than healthy controls,28 and it seems that sleep disturbance affects their quality of life.29,30 The prevalence is higher in patients with comorbid anxiety and depression.30
SLEEP IN PATIENTS WITH GENERALIZED EPILEPSIES
Janz16 described that patients with awakening epilepsy and those with sleep epilepsy had distinct sleep patterns and sleep habits. According to that study, patients with generalized epilepsy like to stay up late in the evening and often have difficulty falling asleep. Their sleep appeared to be disrupted. In the morning they feel drowsy and unrefreshed, and they prefer to get up late if they can.6,16 Early polygraphic studies seemed to support this concept,31,32 but the sleep EEG recordings were discontinuous in one study,32 which does not allow interpretation of the sleep structure. Other polysomnographic sleep studies in patients with idiopathic generalized epilepsy differentiated more and found normal sleep patterns unless seizures occurred during the night.33–35 These studies were performed either on patients with chronic antiepileptic medication or on patients in whom the drugs (usually phenobarbital and phenytoin) were discontinued a few days prior to the sleep investigations. Thus, chronic drug effects or rebound effects after discontinuation, which may influence the results, cannot be excluded. Only one study36 investigated the night sleep of unmedicated epilepsy patients. Adaptation nights to the sleep lab were also included in this study36 to avoid “first-night” effects.37 Photosensitive patients with generalized epilepsy had significantly more deep sleep (sleep stages 3 and 4) and less light sleep (sleep stages 1 and 2) than the other patients with generalized epilepsy.36 However, this effect was no more significant if the patients were age matched, as the photosensitive patients were younger, and younger patients tend to have more deep sleep. Thus, the systematic polysomnographic evaluation of unmedicated patients with generalized epilepsy36 did not reveal distinct sleep patterns in patients with “awakening” and “sleep” epilepsy, as hypothesized by Janz6 based on unstructured interviews.
SLEEP IN PATIENTS WITH FOCAL EPILEPSIES
Patients with focal epilepsy have poorer sleep as compared to controls in many categories: epilepsy patients sleep less, have a poorer quality of sleep, and report a disturbed sleep.30 This was found not only for patients with catastrophic epilepsy, but also already for patients who are relatively well controlled on 1 or 2 AED.29 Deep sleep is reduced and there are more arousals and more stage shifts,38 especially in nights with seizures, but also in seizure-free nights. REM sleep is decreased if seizures occur during the night as well as during the preceding day. The earlier the seizures appear during the night, the shorter the total REM time,39 but the amount of REM sleep increases with better seizure control.40 Interictal epileptiform discharges (IEDs) alone seem to have little effect on the sleep architecture.41
Effects of Sleep and Sleep Deprivation on Epilepsy
Langdon-Down and Brain5 were the first to subdivide epilepsy patients according to the occurrence of their seizures. They described (1) a “diurnal type,” whose seizure occurred predominantly during the day with a maximum following morning awakening and two smaller peaks in the afternoon; (2) a “nocturnal type,” in whom seizures occurred during the night with maxima shortly after falling asleep and in the early morning hours; and (3) a group without any discernible pattern (“diffuse type”).5 Relations of epileptic seizures to the sleep–wake cycle were evident from the observation that the times at which the seizures occurred changed when the sleep regimen was altered.3,42
The circadian sleep–wake cycle also significantly influences the occurrence of epileptiform discharges.11 During non-REM sleep, generalized epileptiform discharges are more frequent than during waking.9,10,43 For this reason, sleep EEGs are performed on patients in whom the diagnosis of epilepsy is not established or the epilepsy syndrome is unclear and the waking EEG is unrevealing.
RELATION OF EPILEPTIFORM DISCHARGES TO THE SLEEP-WAKE CYCLE AND SLEEP STAGES IN GENERALIZED EPILEPSY
Generalized epileptiform discharges gradually increase with deepening of non-REM sleep34,44 In patients with absence epilepsy, the lowest discharge rates were found during REM sleep.34 Because deep sleep stages are most pronounced during the first sleep cycle, it is not surprising that the highest rate of interictal epileptiform discharges were found during the first sleep cycle.34 The morphology of generalized spike-wave complexes is more irregular during non-REM sleep and is similar to waking and REM sleep.34,35,44
The early hypothesis8 that transitional states between wake and sleep and vice versa may be epileptogenic in selected patients was supported by polygraphic studies. Several authors demonstrated the facilitating effects of transitional states of sleep such as sleep onset, changes between sleep stages, and arousals on the occurrence of epileptiform discharges in patients with absence epilepsy.12,33,35 Niedermeyer45 emphasized that an abnormal paroxysmal response to arousal and the influx of upward traveling stimuli seem to be the most important epileptogenic mechanism in primary generalized epilepsy. The concept of CAP, which is based on these observations, provides a new approach and supports the idea that transitional cyclic sleep patterns activate epileptiform discharges.13
RELATIONS OF EPILEPTIC SEIZURES AND SLEEP IN GENERALIZED EPILEPSIES
Generalized tonic-clonic seizures show a clearer relation to the sleep–wake cycle than “minor seizures.”46 However, myoclonic seizures tend to occur predominantly in the early morning hours shortly after awakening from night sleep in juvenile myoclonic epilepsy.14,47 Based on the patient’s recollection of seizures and the patient’s history, Janz46 classified epilepsies according to the time of occurrence of the “grand mal” seizures. He coined the term awakening epilepsy for patients whose generalized tonic-clonic seizures predominantly occur in the first 2 hours after awakening, with a second peak in the afternoon.46 Janz6 reported earlier that in 45% of his 2110 patients with grand mal epilepsy, seizures occurred predominantly during sleep, in 34% during the first 2 hours after awakening from night sleep, and in 21% no relation to the sleep–wake cycle was found. During the course of the epilepsy, the pattern may change as less patients have an awakening predominance (31%) and more patients show a diffuse pattern (26%).16 The awakening type seemed to be associated with idiopathic generalized epilepsy.46 Half of Janz’s patients with hereditary idiopathic epilepsy belonged to the awakening group, whereas most patients with focal epilepsy secondary to brain tumors (53%) had a diffuse pattern of seizure occurrence, and only 8% showed an awakening pattern.46 The minor seizures in patients with epilepsy on awaking comprised predominantly absence seizures (94%) and myoclonic seizures (96%) and less frequently psychomotor seizures (16%) or Jacksonian seizures (9%).46 A major bias of the study of is the observation that the recollection of epileptic seizures by the patients as well as caregivers is unreliable.48 Also, the etiological diagnoses are subject to uncertainties in the study of Janz46 because at that time no modern imaging studies were available. Few recent studies dealt with the relation of the occurrence of generalized tonic-clonic seizures to the sleep–wake cycle.49,50 The distribution of generalized tonic-clonic seizures was as follows in 77 of 141 patients with generalized epilepsy, in whom generalized tonic-clonic were observed:49 (1) 16.8% had seizures after awakening, (2) 36.3% had seizures during waking hours of the day, (3) 28.5% had seizures during night sleep, and (4) 18.1% had seizures both during night sleep and during waking periods of the day. Morning and nocturnal awakenings accounted for 36 of 51 seizures in 33 patients with juvenile myoclonic epilepsy, whereas the evening relaxation period (n = 6), sleep onset (n = 3), and sleep (n = 6) were less frequently associated with seizures.50
RELATIONS OF FOCAL EPILEPSY AND SLEEP
In focal epilepsies, the rate of IED is increased in sleep, with the highest increase in deep NREM sleep (sleep stages 3 and 4) and a decrease in REM sleep.51–53 The localization of IED is more widespread in NREM sleep and has the best localizing value if occurring in REM sleep.52 An important mechanism in activating IED is the activity changes associated with arousals.15
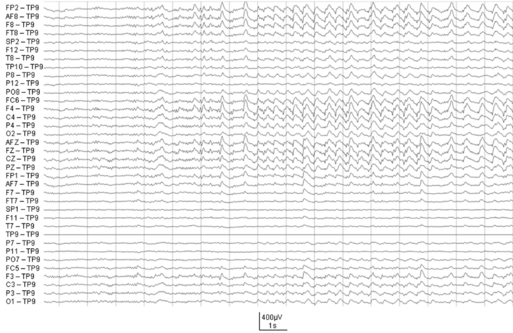
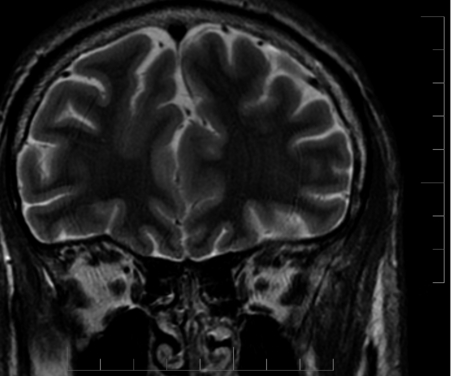
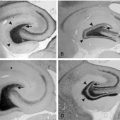
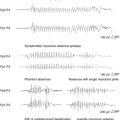
In frontal lobe epilepsy, seizures have a tendency to occur more commonly during night sleep than in other focal epilepsies such as temporal lobe epilepsy54 (Figure 7-1). During sleep, seizures arising from frontal lobes evolve less often into generalized tonic-clonic seizures (22% of the seizures) than do seizures arising from the temporal lobes (45%), whereas during wakefulness, the percentage of generalization was not statistically different (20% vs. 19%).55 Also, the seizures mostly arose from NREM stages 1 and 2, rarely from deep sleep, and almost never from stage REM. Figure 7-1 shows the onset of a right frontal EEG seizure pattern following a sleep spindle. The patient’s MRI (Figure 7-2) depicts a right frontal focal cortical dysplasia. Subclinical seizures were especially prevalent during the night. In sleep, a higher level of synchronization is reached, which explains the smaller number of seizures during deep sleep but is also compatible with the increased portion of secondary generalization.55 An important point for differential diagnosis is that psychogenic nonepileptic seizures do not arise directly from sleep.55 A period of wakefulness after awakening always precedes the psychogenic nonepileptic seizures if they occur during sleep.55
SLEEP DEPRIVATION
Sleep deprivation is one of the most potent precipitators of epileptic seizures and epileptiform discharges, especially in patients with generalized epilepsy. Janz6 reported that sleep deprivation and/or excessive alcohol consumption precipitated the first seizure in 28 of 47 patients with juvenile myoclonic epilepsy. Based on these observations, most neurologists counsel epilepsy patients to avoid sleep deprivation. However, there was no seizure-precipitating effect observed in a study of temporal lobe epilepsy patients who underwent sleep deprivation during EEG-video monitoring for epilepsy surgery evaluation.56 Thus, it is likely that generalized epilepsies and focal epilepsies are differently sensitive to sleep deprivation.
Sleep deprivation also facilitates epileptiform discharges in patients with generalized epilepsy.6,57,58 However, there is some debate on whether sleep deprivation has a genuine activating effect on epileptiform discharges or whether it acts through sleep induction.59 It has been shown that the IED yield in patients with clinically highly probable epilepsy but negative previous routine EEGs (that contained both wake and sleep) could be increased by 52% after sleep deprivation.60 IED were recorded in some of these patients in wake or sleep stage only.60 Thus, it is important that the EEG after sleep deprivation includes both sleep and wake phases. The effect of sleep deprivation on the occurrence of IED in the EEG was also shown in another study that directly compared drug-induced sleep EEG and EEG after sleep deprivation in patients with normal or borderline waking EEG. This study revealed epileptic discharge activation in 44% after sleep deprivation versus 14% during drug-induced sleep.61 Another study compared the rate of epileptiform discharges of waking EEGs after sleep deprivation with sleep EEG after sleep deprivation and concluded that sleep deprivation had an independent activating effect because after sleep deprivation, the waking EEGs showed more epileptiform discharges than the sleep EEGs.62 An increased cortical excitability following sleep deprivation, which could be a reason for increased epileptic discharges, has also been demonstrated by transcranial magnetic stimulation in patients with focal and generalized epilepsies.63
Effects of Epilepsy Treatment on Sleep
The mainstay of treatment in epilepsy is by antiepileptic drugs (AEDs). Many AEDs exert some effects on sleep, most obviously those that have hypnotic properties.64 One has to be careful, though, when trying to interpolate AED effects on sleep from the effect on vigilance. Side effects like drowsiness do not translate into quicker and better sleep. Furthermore, a normal macrostructure with 4 to 6 macrocycles of non-REM and REM sleep does not necessarily imply a good quality of sleep, as sleep quality also depends on arousals and the microstructure of sleep.65 It was hypothesized that some of the antiepileptic effects may even be mediated through an influence on sleep.16 Most studies evaluated short-term effects of the drugs (often with healthy volunteers) on patients (mostly with psychiatric disorders) on chronic treatment, who were then compared with what was considered normal sleep.36
The older, established AEDs have been studied the most and also have more long-term data available. Phenobarbital (PHB) is still a widely used AED, especially in developing countries. Its potency and low cost are its great advantages, but its use is limited in more affluent societies due to enzyme induction and mostly to increased tiredness, its main subjective side effect. Phenobarbital changes the sleep macrostructure by reducing sleep onset latency and accentuating the distribution of deep sleep to the first half of the night and REM sleep to the second part of the night. It also reduces the number of periods of waking and movement time after sleep onset.66 PHB reduces the number of REM interruptions only in patients with generalized epilepsy (including patients with “awakening” epilepsy), which is an interesting finding with respect to the observation that transitions between sleep stages seem to provoke seizure discharges in these patients.12,33,49 The effect of PHB differs in patients with generalized and focal epilepsies, whereas in patients with generalized epilepsy, sleep stages 1 to 3 were decreased, and stages 2 to 4 in patients with focal epilepsy were increased.66
Phenytoin (PHT) is another classical AED. After starting PHT treatment, patients fell asleep more rapidly, deep sleep was increased, and light sleep was decreased when compared to the unmedicated baseline. These effects were more pronounced in the second part of the night. In contrast to the effects of PHB, the amount and the interruptions of REM-sleep remained unchanged with PHT.66 The comparison of patients with generalized and focal epilepsy revealed only minor insignificant differences. A long-term study (at least 6 months) on PHT showed that the aforementioned increase of deep sleep and decrease of light sleep returned to baseline values, and only the reduced sleep latency was lasting.36
The use of ethosuximide brought increased light sleep (stage 1), decreased deep sleep (stages 3 and 4), and prolonged REM sleep in the first sleep cycle as compared with unmedicated baseline in patients with generalized epilepsy.67 Valproic acid use led to an increase of light sleep stage 1, but no deep sleep changes occurred. As with ethosuximide, the first sleep cycle was prolonged.67 The effects of valproic acid and ethosuximide on the first sleep cycle are maybe related to the fact that these drugs were evaluated in patients with generalized epilepsy, and these patients showed similar influences of PHT and PHB on the first sleep cycle.67
Carbamazepine is one of the most widely used AEDs, and its effects on sleep are well documented. The effects differ in acute and long-term treatment. In the first days of treatment, deep sleep and sleep efficiency are increased, but REM sleep is decreased, an effect that is most pronounced after the first doses. In long-term therapy, the sleep macrostructure of patients treated with carbamazepine was closer to the normal macrostructure than before treatment.68,69
For the newer AEDs, there are several studies on sleep, but in most, long-term data is missing. A single dose of levetiracetam increased sleep stage 2 but decreased stage 4 NREM sleep in epileptic patients.70 Gabapentin increases deep sleep and REM and decreases sleep stage 1, but did not change the subjective daytime sleepiness in healthy volunteers.71,72 For some of the newer AEDs, among them tiagabine, vigabatrin, and topiramate, a GABAergic mechanism of action is hypothesized, and so these drugs can be expected to have effects on sleep. For topiramate, though, no effect on sleep could be demonstrated.73 Tiagabine, on the other hand, improved sleep efficiency and increased deep sleep.74
Sleep Disorders and Epilepsy
Sleep myoclonus—or hypnic jerks—occurs in the transition phase between wake and sleep and can be so strong that the sleeper and/or the bed partner awake.75 Sleep starts can more rarely appear as visual, auditory, or other sensory experiences and are then closely related to hypnagogic hallucinations, which are in turn probably only abnormal if occurring in connection with other sleep disorders, such as narcolepsy.76,77
Nightmares are frightening dreams that lead to awakening and are usually remembered by the dreamer. They have to be carefully weighted against epileptic seizures with fearful associations.78 Sleepwalking and pavor nocturnus are NREM parasomnias that lead to complex behavior and are often not remembered by the sleeper. Pavor nocturnus, which is a disorder of children, is also called night terrors because the children experience strong fear during an attack. The differential diagnosis between epileptic seizures associated with fear and sleep terrors may be difficult.79 Both disorders are fairly common.80
REM sleep behavior disorder (RBD) is a REM sleep parasomnia that is characterized by the loss of the REM muscle atonia81 and may be confused with frontal lobe seizures presenting with complex motor seizures. Patients act out their dreams in complex behavior; they often experience violent dreams and are mostly amnesic for these episodes. RBD precedes neurodegenerative disorders, especially multiple system atrophy and Parkinson’s disease.82
Patients with narcolepsy have excessive daytime sleepiness and abnormal REM sleep, which is symptomatic by cataplexy (sudden loss of muscle tone, often after emotional triggers), sleep paralysis (inability to move for a short period of time on waking), or sleep-associated hallucinations.83
Primary sleep disorders are not only important differential diagnoses, but can also be more common in epilepsy patients. Obstructive sleep apnea (OSA) syndromes, for example, are more common in epilepsy patients.84 OSA in these patients was possibly related to medical treatment or AED-associated weight gain.85,86 OSA treatment was able to improve seizure control.87
1. Passouant P. Historical aspects of sleep and epilepsy. Epilepsy Res Suppl. 1991;2:19-22.
2. Hopkins H. The time of appearance of epileptic seizures in relation to age, duration and type of the syndrome. J Nerv Ment Dis. 1993;77:153-162.
3. Gowers WR. Epilepsy and Other Chronic Convulsive Diseases. New York: William Wood, 1885.
4. Griffiths GN, Fox IT. Rhythm in epilepsy. Lancet. 1938;234:409-416.
5. Langdon-Down M, Brain WR. Time of day in relation to convulsions in epilepsy. Lancet. 1929;II:1029-1032.
6. Janz D. “Aufwach”-Epilepsien. Arch Psychiat Nervenkr. 1953;191:53-98.
7. Magnussen G. 18 cases of epilepsy with fits in relation to sleep. Acta Psychiat Scand. 1936;11:28-3219.
8. Patry FL. The relation of time of day, sleep and other factors to the incidence of epileptic seizures. Am J Psychiat. 1931;10:789-813.
9. Gibbs EL, Gibbs FA. Diagnostic and localizing value of electroencephalic studies in sleep. Res Publ Ass Nerv Ment Dis. 1947;26:366-376.
10. Gloor P, Tsai C, Haddad F. An assessment of the value of sleep-electroencephalography for the diagnosis of temporal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1958;10:633-648.
11. Shouse MN, Martins da Silva A, Sammaritano M. Circadian rhythm, sleep, and epilepsy. J Clin Neurophysiol. 1996;13:32-50.
12. Halasz P. Sleep, arousal and electroclinical manifestations of generalized epilepsy with spike and wave pattern. Epilepsy Res Suppl. 1991;2:43-48.
13. Terzano MG, Parrino L, Anelli S, Halasz P. Modulation of generalized spike-and-wave discharges during sleep by cyclic alternating pattern. Epilepsia. 1989;30:772-781.
14. Gigli GL, Calia E, Marciani MG, et al. Sleep microstructure and EEG epileptiform activity in patients with juvenile myoclonic epilepsy. Epilepsia. 1992;33:799-804.
15. Parrino L, Halasz P, Tassinari CA, Terzano MG. CAP, epilepsy and motor events during sleep: the unifying role of arousal. Sleep Med Rev. 2006;10:267-285.
16. Janz D. Epilepsy and the sleeping-waking cycle. In: Vincken PJ, Bruyn GW, editors. Handbook of Clinical Neurology. Amsterdam: North Holland; 1974:457-490.
17. Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272-1278.
18. Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195-204.
19. Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679-685.
20. Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles: Brain Information Service/Brain Research Institute, 1968.
21. Steriade M, Contreras D, Curro Dossi R, Nunez A. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993;13:3284-3299.
22. Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087-1106.
23. Terzano MG, Mancia D, Salati MR, Costani G, Decembrino A, Parrino L. The cyclic alternating pattern as a physiologic component of normal NREM sleep. Sleep. 1985;8:137-145.
24. Ferri R, Bruni O, Miano S, et al. The time structure of the cyclic alternating pattern during sleep. Sleep. 2006;29:693-699.
25. Shouse MN, Langer J, King A, et al. Paroxysmal microarousals in amygdala-kindled kittens: could they be subclinical seizures? Epilepsia. 1995;36:290-300.
26. Eisensehr I, Parrino L, Noachtar S, Smerieri A, Terzano MG. Sleep in Lennox-Gastaut syndrome: the role of the cyclic alternating pattern (CAP) in the gate control of clinical seizures and generalized polyspikes. Epilepsy Res. 2001;46:241-250.
27. Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519-524.
28. Alanis-Guevara I, Pena E, Corona T, Lopez-Ayala T, Lopez-Meza E, Lopez-Gomez M. Sleep disturbances, socioeconomic status, and seizure control as main predictors of quality of life in epilepsy. Epilepsy Behav. 2005;7:481-485.
29. de Weerd A, de Haas S, Otte A, et al. Subjective sleep disturbance in patients with partial epilepsy: a questionnaire-based study on prevalence and impact on quality of life. Epilepsia. 2004;45:1397-1404.
30. Xu X, Brandenburg NA, McDermott AM, Bazil CW. Sleep disturbances reported by refractory partial-onset epilepsy patients receiving polytherapy. Epilepsia. 2006;47:1176-1183.
31. Jovanovic UJ. Das Schlafverhalten der Epileptiker. I. Schlafdauer, Schlaftiefe und Besonderheiten der Schlafperiodik. Dtsch Z Nervenheilk. 1967;190:159-198.
32. Christian W. Schlaf-Wach-Periodik bei Schlaf- und Aufwachepilepsien. Nervenarzt. 1961;32:266-275.
33. Passouant P, Besset A, Carriere A, Billiard M. Night sleep and generalized epilepsy. In: Koella WP, Levin P, editors. Sleep 1974. Basel: Karger; 1975:185-196.
34. Sato S, Dreifuss FE, Penry JK. The effect of sleep on spike-wave discharges in absence seizures. Neurology. 1973;23:1335-1345.
35. Tassinari CA, Bureau-Paillas M, Dalla Bernadina B, et al. Generalized epilepsies and sleep. A polygraphic study. In: Van Praag HM, Meinardi H, editors. Brain and Sleep. Amsterdam: De Erven Bohn; 1974:154-166.
36. Röder-Wanner UU, Wolf P, Danninger T. Are sleep patterns in epileptic patients correlated with their type of epilepsy? In: Martins da Silva A, Binnie C, Meinradi H, editors. Biorhythms and Epilepsy. New York: Raven Press; 1985:109-121.
37. Agnew HW, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2:263-266.
38. Touchon J, Baldy-Moulinier M, Billiard M. Sleep organization and epilepsy. In: Degen R, Rodin EA, editors. Epilepsy, Sleep and Sleep Deprivation. 2nd. Amsterdam: Elsevier; 1991:73-81.
39. Bazil CW, Kothari M, Luciano D, et al. Provocation of nonepileptic seizures by suggestion in a general seizure population. Epilepsia. 1994;35:768-770.
40. Hrachovy RA, Frost JDJr, Kellaway P. Sleep characteristics in infantile spasms. Neurology. 1981;31:688-793.
41. Malow BA, Lin X, Kushwaha R, Aldrich MS. Interictal spiking increases with sleep depth in temporal lobe epilepsy. Epilepsia. 1998;39:1309-1316.
42. Marchand L. Des influences cosmiques sur les accidents epileptiques. L’Encéphale. 1931;26(Suppl. 10):237.
43. Billiard M, Besset A, Zachariev Z, Touchon J, Baldy-Moulinier M, Cadilhac J. Relation of seizures and seizure discharges to sleep stages. In: Wolf P, Dam M, Janz D, Dreifuss FE, editors. Advances in Epileptology. New York: Raven Press; 1987:665-670.
44. Ross JJ, Johnson LC, Walter RD. Spike and wave discharges during stages of sleep. Arch Neurol. 1966;14:399-407.
45. Niedermeyer E. Petit mal, primary generalized epilepsy and sleep. In: Sterman MB, Passouant P, Shouse MN, editors. Sleep and Epilepsy. New York: Academic Press; 1982:191-207.
46. Janz D. The grand mal epilepsies and the sleeping-waking cycle. Epilepsia. 1962;3:69-109.
47. Janz D, Christian W. Impulsiv-Petit mal. Dtsch Z Nervenheilk. 1957;176:346-356.
48. Blum DE, Eskola J, Bortz JJ, Fisher RS. Patient awareness of seizures. Neurology. 1996;47:260-264.
49. Billiard M. Epilepsies and the sleep wake cycle. In: Sterman MB, Passouant P, Shouse MN, editors. Sleep and Epilepsy. New York: Academic Press; 1982:269-286.
50. Touchon J, Baldy-Moulinier M, Billiard M, Besset A, Cadilhac J. Effect of awakening on epileptic activity in primary generalized myoclonic epilepsy. In: Sterman MB, Shouse MN, Passouant P, editors. Sleep and Epilepsy. New York: Academic Press; 1982:239-248.
51. Malow BA, Selwa LM, Ross D, Aldrich MS. Lateralizing value of interictal spikes on overnight sleep-EEG studies in temporal lobe epilepsy. Epilepsia. 1999;40:1587-1592.
52. Sammaritano M, Gigli GL, Gotman J. Interictal spiking during wakefulness and sleep and the localization of foci in temporal lobe epilepsy. Neurology. 1991;41:290-297.
53. Rossi GF, Colicchio G, Pola P. Interictal epileptic activity during sleep: a stereo-EEG study in patients with partial epilepsy. Electroencephalogr Clin Neurophysiol. 1984;58:97-106.
54. Crespel A, Baldy-Moulinier M, Coubes P. The relationship between sleep and epilepsy in frontal and temporal lobe epilepsies: practical and physiopathologic considerations. Epilepsia. 1998;39:150-157.
55. Bazil CW, Walczak TS. Effects of sleep and sleep stage on epileptic and nonepileptic seizures. Epilepsia. 1997;38:56-62.
56. Malow BA, Passaro E, Milling C, Minecan DN, Levy K. Sleep deprivation does not affect seizure frequency during inpatient video-EEG monitoring. Neurology. 2002;59:1371-1374.
57. Bechinger D, Kriebel J, Schlager M. Das Schlafentzugs-EEG, ein wichtiges diagnostisches Hilfsmittel bei cerebralen Anfällen. Z Neurol. 1973;205:193-206.
58. Pratt KL, Mattson RH, Weikers NJ, Williams R. EEG activation of epileptics following sleep deprivation: a prospective study of 114 cases. Electroencephalogr Clin Neurophysiol. 1968;24:11-15.
59. Degen R, Degen HE. The diagnostic value of the sleep EEG with and without sleep deprivation in patients with atypical absences. Epilepsia. 1983;24:557-566.
60. Fountain NB, Kim JS, Lee SI. Sleep deprivation activates epileptiform discharges independent of the activating effects of sleep. J Clin Neurophysiol. 1998;15:69-75.
61. Rowan AJ, Veldhuisen RJ, Nagelkerke NJ. Comparative evaluation of sleep deprivation and sedated sleep EEGs as diagnostic aids in epilepsy. Electroencephalogr Clin Neurophysiol. 1982;54:357-364.
62. Klingler D, Trägner H, Deisenhammer E. The nature of the influence of sleep deprivation on the EEG. In: Degen R, Rodin EA, editors. Epilepsy, Sleep and Sleep Deprivation. Amsterdam: Elsevier; 1991:231-234.
63. Badawy RA, Curatolo JM, Newton M, Berkovic SF, Macdonell RA. Changes in cortical excitability differentiate generalized and focal epilepsy. Ann Neurol. 2007;61:324-331.
64. Sammaritano M, Sherwin A. Effect of anticonvulsants on sleep. Neurology. 2000;54:S16-S24.
65. Terzano MG, Parrino L, Spaggiari MC, Palomba V, Rossi M, Smerieri A. CAP variables and arousals as sleep electroencephalogram markers for primary insomnia. Clin Neurophysiol. 2003;114:1715-1723.
66. Wolf P, Röder-Wanner UU, Brede M. Influence of therapeutic phenobarbital and phenytoin medication on the polygraphic sleep of patients with epilepsy. Epilepsia. 1984;25:467-475.
67. Wolf P, Röder-Wanner UU, Brede M, Noachtar S, Sengoku A. Influences of antiepileptic drugs on sleep. In: Martins da Silva A, Binnie C, Meinardi H, editors. Biorhythms and Epilepsy. New York: Raven Press; 1985:137-153.
68. Yang JD, Elphick M, Sharpley AL, Cowen PJ. Effects of carbamazepine on sleep in healthy volunteers. Biol Psychiatry. 1989;26:324-328.
69. Gigli GL, Placidi F, Diomedi M, et al. Nocturnal sleep and daytime somnolence in untreated patients with temporal lobe epilepsy: changes after treatment with controlled-release carbamazepine. Epilepsia. 1997;38:696-701.
70. Bell C, Vanderlinden H, Hiersemenzel R, Otoul C, Nutt D, Wilson S. The effects of levetiracetam on objective and subjective sleep parameters in healthy volunteers and patients with partial epilepsy. J Sleep Res. 2002;11:255-263.
71. Foldvary-Schaefer N, De Leon Sanchez I, Karafa M, Mascha E, Dinner D, Morris HH. Gabapentin increases slow-wave sleep in normal adults. Epilepsia. 2002;43:1493-1497.
72. Placidi F, Mattia D, Romigi A, Bassetti MA, Spanedda F, Marciani MG. Gabapentin-induced modulation of interictal epileptiform activity related to different vigilance levels. Clin Neurophysiol. 2000;111:1637-1642.
73. Bonanni E, Galli R, Maestri M, et al. Daytime sleepiness in epilepsy patients receiving topiramate monotherapy. Epilepsia. 2004;45:333-337.
74. Mathias S, Wetter TC, Steiger A, Lancel M. The GABA uptake inhibitor tiagabine promotes slow wave sleep in normal elderly subjects. Neurobiol Aging. 2001;22:247-253.
75. Oswald I. Sudden bodily jerks on falling asleep. Brain. 1959;82:92-103.
76. Dagnino N, Loeb C, Massazza G, Sacco G. Hypnic physiological myoclonias in man: an EEG-EMG study in normals and neurological patients. Eur Neurol. 1969;2:47-58.
77. Ohayon MM, Priest RG, Caulet M, Guilleminault C. Hypnagogic and hypnopompic hallucinations: pathological phenomena? Br J Psychiatry. 1996;169:459-467.
78. Epstein AW. Recurrent dreams; their relationship to temporal lobe seizures. Arch Gen Psychiatry. 1964;10:25-30.
79. Lombroso CT. Pavor nocturnus of proven epileptic origin. Epilepsia. 2000;41:1221-1226.
80. Hublin C, Kaprio J, Partinen M, Heikkila K, Koskenvuo M. Prevalence and genetics of sleepwalking: a population-based twin study. Neurology. 1997;48:177-181.
81. Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293-308.
82. Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology. 1996;46:388-393.
83. Overeem S, Mignot E, van Dijk JG, Lammers GJ. Narcolepsy: clinical features, new pathophysiologic insights, and future perspectives. J Clin Neurophysiol. 2001;18:78-105.
84. Malow BA, Levy K, Maturen K, Bowes R. Obstructive sleep apnea is common in medically refractory epilepsy patients. Neurology. 2000;55:1002-1007.
85. Takhar J, Bishop J. Influence of chronic barbiturate administration on sleep apnea after hypersomnia presentation: case study. J Psychiatry Neurosci. 2000;25:321-324.
86. Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015-3021.
87. Malow BA, Weatherwax KJ, Chervin RD, et al. Identification and treatment of obstructive sleep apnea in adults and children with epilepsy: a prospective pilot study. Sleep Med. 2003;4:509-515.