Epidural anesthesia
Applied anatomy of the epidural space
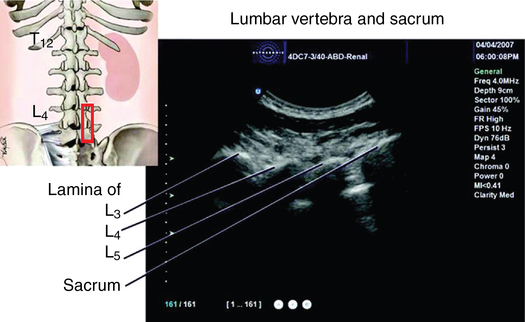
The epidural space, a potential space surrounding the spinal meninges, contains fat, nerve roots, and vascular plexuses. The anatomy of the spine, ligaments, meninges, and blood flow throughout the spinal cord are described in detail in Chapter 57. Knowledge of surface anatomy (Figure 123-1) and key anatomic features of the cervical, thoracic, and lumbar spinal regions (Box 123-1) are critical to the performance of safe and reliable epidural needle placement.
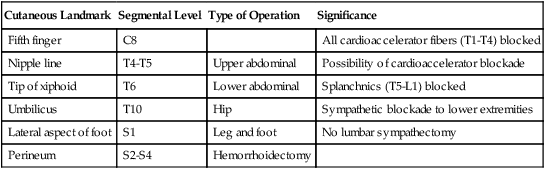
All segments of the spinal canal from the base of the skull to the sacral hiatus are accessible to epidural injection. Epidural anesthesia, provided either alone or in combination with general anesthesia, may be adapted to almost any surgical procedure that takes place below the level of the patient’s chin. Ideally, needle and catheter placement should occur at the level of the surgical incision (e.g., lumbar placement for lower extremity operations and thoracic placement for thoracic/abdominal operations) to allow for block of only the parts of the body that fall within the surgical field. However, a lumbar technique may be used for even upper abdominal procedures, although it would result in a complete sympathectomy, including potentially blocking the cardiac accelerator fibers. Assessment of the dermatomal sensory level enables the anesthesiologist to determine approximate level of sympathectomy and anticipate the resulting hemodynamic effects (Table 123-1).
Table 123-1
Sensory Level of Epidural Blockade Required for Surgical Procedures
| Cutaneous Landmark | Segmental Level | Type of Operation | Significance |
| Fifth finger | C8 | All cardioaccelerator fibers (T1-T4) blocked | |
| Nipple line | T4-T5 | Upper abdominal | Possibility of cardioaccelerator blockade |
| Tip of xiphoid | T6 | Lower abdominal | Splanchnics (T5-L1) blocked |
| Umbilicus | T10 | Hip | Sympathetic blockade to lower extremities |
| Lateral aspect of foot | S1 | Leg and foot | No lumbar sympathectomy |
| Perineum | S2-S4 | Hemorrhoidectomy |
Identification of the epidural space
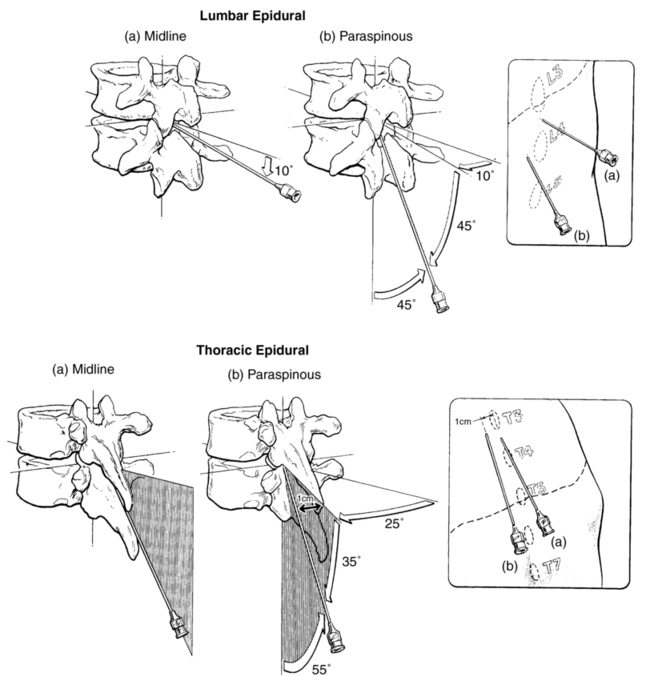
The epidural space may be approached using a midline or a paramedian needle insertion (Figure 123-2). The epidural space is identified by the passage of the needle from an area of high resistance (ligamentum flavum) to an area of low resistance (epidural space). After the needle is positioned in the ligamentum flavum, a syringe with a freely movable plunger is attached, and continuous pressure is applied to the plunger. If the needle is positioned correctly in the ligament, the syringe should not inject when pressure is applied to the plunger. As the needle passes into the epidural space, a sudden loss of resistance in the plunger will be felt, and the air or fluid will easily inject. At this point, a flexible nylon catheter may be advanced 3 to 4 cm through the needle into the epidural space to allow repeated and incremental injections. Preinsertion ultrasound imaging has been demonstrated to accurately identify the level of the vertebrae as well as to estimate the depth of the epidural space (Figure 123-3).
Selection and dose of local anesthetic agent
The major sites of action of epidurally injected local anesthetics are the spinal nerve roots, where the dura is relatively thin. Only a small amount of local anesthetic agent actually diffuses across the dura into the subarachnoid space. A local anesthetic agent should be selected on the basis of speed of onset, degree of motor blockade required, and duration of the surgical procedure (Table 123-2).
Table 123-2
Clinical Effects of Local Anesthetic Solutions Commonly Used for Epidural Blockade
| Drug | Time Spread to ± 4 Segments ± 1 SD (min) | Approximate Time to 2-Segment Regression ± 2 SD* (min) | Recommended Top-up Time from Initial Dose* (min) |
| Lidocaine, 2% | 25 ± 5 | 100 ± 40 | 60 |
| Prilocaine, 2%-3% | 15 ± 4 | 100 ± 40 | 60 |
| Chloroprocaine, 2%-3% | 12 ± 5 | 60 ± 15 | 45 |
| Mepivacaine, 2% | 15 ± 5 | 120 ± 150 | 60 |
| Bupivacaine, 0.5%-0.75% | 18 ± 10 | 200 ± 80 | 120 |
| Ropivicaine, 0.75%-1% | 20.5 ± 7.9 | 177 ± 49 | 120 |
| Levobupivacaine, 0.5%-0.75% | 20 ± 9 | 200 ± 80 | 120 |
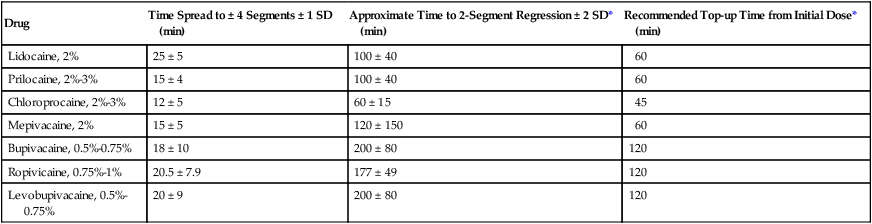
*Note that top-up time is based on duration +/− 2 SD, which encompasses the likely duration in 95% of the population. In a conscious cooperative patient, an alternative is to use frequent checks of segmental level to indicate the need to top-up. All solutions contain 1:200,000 epinephrine.
Reprinted, with permission, from Veering BT, Cousins MJ. Epidural neural blockade. In: Cousins MJ, Carr DB, Horlocker TT, Bridenbaugh PO, eds. Neural Blockade in Clinical Anesthesia and Management of Pain, 4th ed. Philadelphia: Lippincott Williams & Williams; 2009:241-295.
Local anesthetic dose may be calculated by the following formula: dose equals 1 to 1.5 mL of local anesthetic agent per segment blocked. The dose may need to be significantly reduced in parturients and in obese and elderly patients. In these situations, incremental doses are advised. A second dose of approximately 50% of the initial dose will maintain the original level of anesthesia if injected when the blockade has regressed 1 or 2 dermatomes (see Table 123-2).