CHAPTER 34 Epidemiology of gynaecological cancer
General Overview
Globally, breast cancer and cancer of the cervix are the two most common female malignancies. However, both ovarian and endometrial cancer rank in the top 10 female malignancies. Cervical cancer is most common in developing countries, while cancer of the uterine corpus and ovarian cancer have higher incidence in industrialized countries. Indeed, in the presence of cervical screening, these cancers are more common than cervical cancer in most Western countries. Table 34.1 provides an overview of age-standardized incidence and mortality rates of these three gynaecological cancers around the world. Other gynaecological cancers (i.e. cancers of the vulva and vagina) are relatively rare in all parts of the world. The age-standardized rates are less than one per 100,000 in most countries (Curado et al 2007).
Table 34.1 Age-standardized rates (per 100,000 women-years) for cancer of the cervix, corpus uterus and ovary (world standard population)
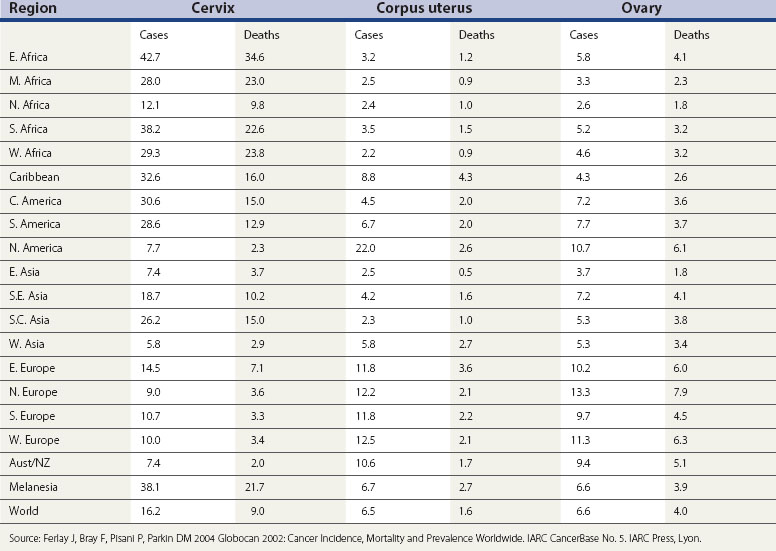
The variation in the rates of gynaecological cancers around the world is enormous (Table 34.1). Cervical cancer rates in East Africa are 12 times greater than in West Asia (the Middle East). The rates for cancer of the uterine corpus are nearly 10 times higher in North America than in West Africa, and ovarian cancer is two and a half times more common in Northern Europe than in Middle Africa. Some, but certainly not all, of these variations can be explained in terms of differences in lifestyle and public health interventions.
Cervical Cancer
The cervical epithelium is composed of two distinct cell types. The ectocervix is covered by non-keratinized squamous cells similar to those of the lining of the vagina. The endocervical canal is covered by columnar cells of the same origin as those of the endometrium. Cervical cancers initiate in the region where these two cell types meet — the squamo-columnar junction. There are three main types of cervical cancer (squamous, adenocarcinoma and adenosquamous carcinoma), with squamous cell carcinoma being the most common. It used to be said that this accounted for approximately 90% of all cases of cervical cancer. However, more recent data show that the proportion of cervical cancer that is adenocarcinoma or adenosquamous carcinoma has doubled, particularly in younger women. Squamous cell carcinoma now only accounts for approximately 75% of all cases of cervical cancer. The reason for the increasing proportion of adenocarcinoma seems to be three-fold: adenocarcinoma really is becoming more common, having been very rare; due to the introduction of mucin staining and greater awareness of adenocarcinoma, it is being reported more often on pathology reports; and cytological screening is more able to detect precancerous squamous lesions than precancerous glandular (adeno) lesions, and thus the relative incidence of the two types of cancer has changed (Sasieni et al 2009).
Descriptive epidemiology
Cervical cancer is the second most commonly diagnosed cancer in women. Worldwide, it is estimated that there are approximately half a million new cases of cervical cancer each year, accounting for approximately 12% of all female cancers (Garcia et al 2007). The cumulative incidence rate up to 74 years of age (assuming no prior death) ranges from 5% in parts of Latin America to approximately 0.5% in parts of the Middle East and Finland. In most European countries, it is under 2% (Curado et al 2007). Cervical cancer is also extremely common in sub-Saharan Africa, but African incidence data are unreliable, particularly for older women.
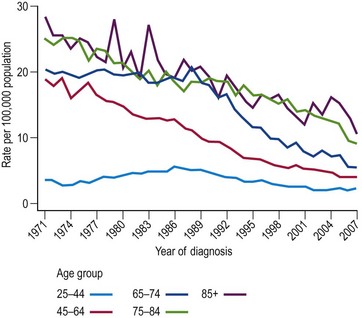
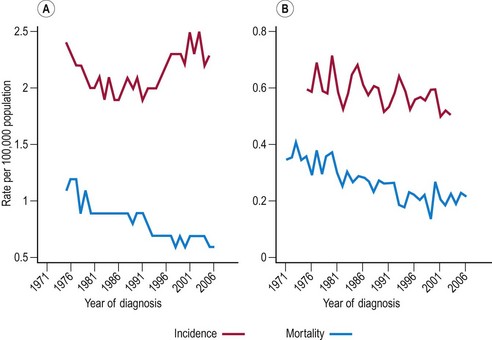
The incidence of cervical cancer in most countries has decreased significantly since the 1960s. In the UK, mortality from cervical cancer has been declining since 1950. The difference between mortality rates in 1950–1952 compared with 2005–2007 varies with age, from an extraordinary 85% reduction in women aged 55–64 years to a more moderate 15% reduction in women aged 25–34 years. Figure 34.1 shows the age-specific mortality rates for cervical cancer in the UK since 1971. It is seen that the greatest decreases have been in older women (aged ≥65 years), and that the increasing rates in younger women between 1970 and 1985 reversed in the 1990s.
The level of environmental exposure will be determined by social norms and will vary between ethnic groups and over time. Modelling shows that the idea that incidence and mortality rates can be modelled by age and cohort effects works well until the 1980s. However, more recent data require the addition of age-specific time trends corresponding to a beneficial effect of screening, particularly in younger women, to provide a satisfactory model (Sasieni and Adams 2000). From a public health perspective, it is important to note that women born in the 1960s are at three- to four-fold higher risk of cervical cancer compared with women born in the 1930s.
Risk factors
Evidence for an association between cervical cancer and sexual activity dates back to 1842, when Rigorni-Stern published data showing that whereas married women were more likely to die of cancer of the uterus (predominantly cervix) than breast cancer, nuns very rarely died of cancer of the uterus. Since then, the epidemiological evidence suggestive of a sexually transmitted agent causing cervical cancer has grown steadily. Traditional risk factors include the number of sexual partners and age at first sexual intercourse. The behaviour of men is also important, as shown by increasing risk in women with just one partner according to the number of partners of their husband (Buckley et al 1981). More recently, the sexually transmitted agent has been identified as certain types of HPV. The evidence that the relationship between HPV infection and cervical cancer is causal is overwhelming (Bosch et al 2002). Several large studies have been carried out to determine the prevalence of HPV in cervical cancer and precancerous lesions. Over 90% of cervical cancers have been found to include HPV DNA (Smith et al 2007), and when full adjustment for tissue adequacy and a range of polymerase chain reaction primers are used, the estimates rise to almost 100% (Walboomers et al 1999). Several studies have shown that HPV-negative women have an extremely low risk of CIN 3 or cancer (Bulkmans et al 2007, Cuzick et al 2008a, Dillner et al 2008). Furthermore, a recent study (Sankaranarayanan et al 2009) found no cancer deaths among 30,000 HPV-negative women in the subsequent 8-year period.
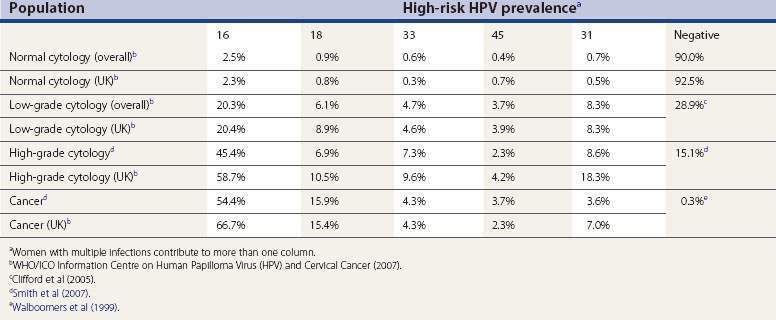
There are over 100 types of HPV, and only some of these infect the anogenital region. These can be split into low-risk types, which cause genital warts, and high-risk types, which can lead to cervical cancer. Types 16 and 18 are strongly associated with cervical cancer; other types including 31, 33, 35, 39, 45, 51, 52, 56, 58 and 66 are associated with a more moderate elevated risk. Table 34.2 details the prevalence of the most common high-risk types of HPV in women with normal cytology, precancerous cervical lesions and invasive cervical cancer in the UK and worldwide.
Table 34.2 High-risk human papilloma virus (HPV) prevalence in women with normal cytology, precancerous cervical lesions and invasive cervical cancer
Other risk factors are less clear cut. Smoking is generally found to be associated with cervical cancer, but it is difficult to disentangle the confounding caused by the sociological link between smoking and increased number of sexual partners. Nevertheless, the body of evidence available suggests that smoking increases the risk of cervical cancer two- to three-fold by reducing the local immune response to HPV (Kapeu et al 2009). The association between oral contraceptive use and cervical cancer is also confounded by sexual behaviour; however, a large pooled analysis including 24 studies found that women who had used oral contraceptives for 5 years or more were at almost double the risk of cervical cancer compared with women who had never used oral contraceptives. This risk declines after the use of oral contraceptives is stopped; within 10 years, the risk is similar to non-users (Appleby et al 2007). High parity and young age at first full-term pregnancy have, independently, been found to increase the risk of invasive cervical carcinoma, and this association remains after adjusting for other reproductive factors. Women with seven or more pregnancies are at approximately 78% greater risk than women with one or two pregnancies. It is estimated that cervical cancer could be reduced by 30% in developing countries if parity and age at first intercourse were the same as in developed countries (International Collaboration of Epidemiological Studies of Cervical Cancer 2006). Previous exposure to sexually transmitted diseases, in particular Chlamydia trachomatis and herpes simplex virus type 2, also increases the risk of cervical cancer, even after adjusting for HPV infection (Bosch and de Sanjose 2007).
Immunosuppression certainly conveys an increased risk of cervical cancer, as shown in studies on renal transplant patients receiving immunosuppressive drugs (Birkeland et al 1995) and on women who are human immunodeficiency virus (HIV) positive (Grulich et al 2007). It seems likely that diet plays a role in the immune response to HPV, but studies on diet and cervical cancer have found little evidence for a strong effect of intake of fruit and vegetables on the risk of cervical cancer (IARC Handbooks of Cancer Prevention 2003). It has been shown recently that cervical neoplasia (including CIS) exhibits familial clustering and that the strength of association increases with increasing genetic relatedness (Magnusson et al 1999). Independently, several groups have found an association between certain human leukocyte antigen class II antigens and cervical neoplasia. However, much more remains to be done in understanding the factors that determine why some women infected with oncogenic HPVs develop cervical cancer but the vast majority do not.
Natural history
There are few data from studies that directly observe the natural history of cervical cancer development because it is generally felt to be unethical not to treat precancerous cervical disease. The situation is further complicated by the possibility that the process of taking a biopsy, required for definitive diagnosis of disease, may affect the natural history by stimulating regression. Therefore, most of what is known of the natural history of cervical precancer is derived from the follow-up of women with cytological abnormalities and the study of the incidence and prevalence of cervical lesions. There is one exception — cervical CIS in Auckland, New Zealand between 1965 and 1974. Women with CIS were not treated. A judicial inquiry in 1988 concluded that this practice was unethical, but allowed the histological and other material to be used for further research. Two studies have been published using this data (McIndoe et al 1984, McCredie et al 2008), with the most recent paper including over 25 years of follow-up (McCredie et al 2008). This study provides the most direct estimates available of the rate of progression from CIS to invasive cancer; the results show that approximately 30% of CIS progress to cancer within 30 years.
For the vast majority (estimated as well over 95%) (Walboomers et al 1999) of cervical cancers, the first step is exposure to one of the oncogenic HPVs. The time from infection to the development of invasive cancer is thought to be many years; typically between 10 and 40. Longitudinal studies on young women show that the majority of HPV infections are transient (Moscicki et al 2004) and that the virus is indeed sexually transmitted (Burk et al 1996). Persistence of infection has been shown to be associated with the development of cervical lesions (Ho et al 1995). It is generally believed that one of the key steps in the development of cancer is integration of the viral DNA in the host genome (Das et al 1992), although some carcinomas only have episomal viral DNA (Cullen et al 1991).
Follow-up studies of women with CIN have found that approximately 60% of CIN I regresses compared with approximately 33% of CIN III; 11% and 22% of CIN I and II, respectively, progressed to CIN III (Östör 1993). Modellers find that regression is more common in younger women, and that three-quarters of CIN in women under 35 years of age will regress (van Ballegooijen et al 1997). They estimate the mean duration of CIN to be 12 years, and that the time from HPV infection to CIN is between 1 and 10 years. Although the details of progression and regression are largely speculative, it is clear that, at most, approximately one-third of high-grade CIN will progress to cancer over approximately 15 years and that the majority of CIN I will regress.
CIN III rates rise rapidly before 30 years of age. Rates then decrease, rather more slowly, being at approximately half their peak by 40 years of age and just 10–20% of their peak by 50 years of age (Office for National Statistics 2006). The extent to which published CIN III rates reflect the prevalence of an untreated condition and the extent to which they mirror incidence is not completely clear.
Prevention and screening
Estimates of the sensitivity of cytology are available from studies in which a large number of women with negative cytology have colposcopy. Ideally, colposcopy should be offered to all women, but this would be expensive and taking a biopsy from all women would be unethical. Many studies comparing two or more screening tests (such as cytology, HPV testing, direct visual inspection) offer colposcopy to all women who are positive on one or more of the screening tests. From such studies, the sensitivity of cytology for high-grade CIN is found to be between 50% and 75% (Nanda et al 2000, Cuzick et al 2006). However, most cases of missed CIN 3 would not become cancerous within 5 years. It is for this reason that screening is recommended, in most countries, to start around the age of 20 years and continue at regular intervals (of between 3 and 5 years) up to the age of 65 years.
There is now sufficient evidence that testing for HPV infections with a primary screening tool can reduce cervical cancer incidence and mortality rates (IARC Monographs 2007). Although HPV testing has high sensitivity (>90%), it is, on average, 6% less specific than cytology (Cuzick et al 2008b). This leads to a higher number of women being referred to colposcopy with no visible lesions. This is especially important in women under 30 years of age, in whom transient HPV infection and cervical lesions are most common. In many high-resource settings, HPV testing is being used in the triage of women over 30 years of age with borderline or mild smears. However, in low-resource settings where organized cytology is not feasible, introduction of HPV testing with subsequent ablative treatment for those women that are positive is an attractive alternative (Sankaranarayanan et al 2009). The introduction of HPV testing to the screening programme would allow the screening interval to be extended (Bulkmans et al 2007, Cuzick et al 2008b, Dillner et al 2008).
Human papilloma virus vaccines
Prophylactic HPV vaccines are L1 virus-like particles (VLPs) in which the surface L1 protein is made to aggregate into particles, mimicking the virus in antigenic respects but not containing the viral DNA. VLPs have been shown to induce a high titre of in-vitro neutralizing antibodies and to protect against experimental challenge with homologous virus in animal models. As VLPs do not contain any viral genes, they are non-infectious and non-oncogenic. To date, two vaccines are commercially available (Cervarix™ and Gardasil™); both are licensed to be applied in three doses within 6 months. Both preparations immunize against HPV 16 and 18, and Gardasil also immunizes against HPV 6 and 11. Results from a number of randomized controlled trials have been published to date. The phase III trials are designed to evaluate the endpoint of CIN 2/3 associated with the HPV types included in the vaccine (Harper et al 2006, Garland et al 2007, Paavonen et al 2007). The smaller phase II trials were powered primarily to evaluate protection from infection by the vaccine types (Villa et al 2005, Mao et al 2006).
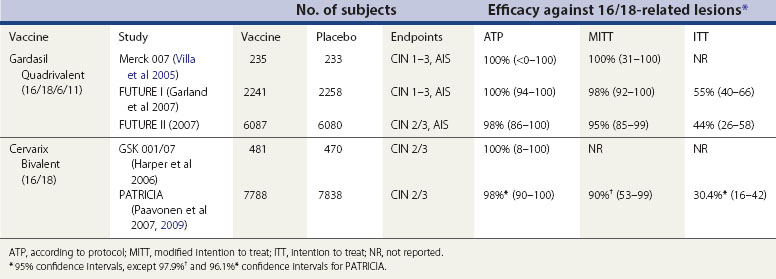
There is no evidence that vaccination is effective against an HPV infection that is already present at the time of vaccination (i.e. vaccines do not induce HPV clearance or reduce progression) (Hildesheim et al 2007). This is important when interpreting the results from clinical trials. There are three main types of analysis for efficacy studies. The according-to-protocol analysis (ATP) only included those women who were HPV negative at enrolment, received all three vaccine doses and remained HPV negative up to the final dose. The modified intention-to-treat (MITT) analysis included study participants who received at least one dose of vaccine, and who were negative for relevant HPV types at enrolment (Cervarix studies) or were randomly assigned to study group irrespective of their baseline HPV status (Gardasil studies). Finally, the intention-to-treat analysis included all randomized subjects.
The vaccine trial subjects differed in age (mostly aged 15–26 years) from the recommended age at vaccination (10–14 years). As younger women are less likely to be infected with HPV at the time of vaccination, the ATP or MITT analysis will more closely reflect the efficacy that one would expect to observe in individuals vaccinated at 10–14 years of age. Vaccine efficacy results by analysis type are detailed in Table 34.3. Overall, HPV vaccines are over 95% effective against vaccine HPV types in HPV-naïve women. Recent results from the PATRICIA trial suggest that this vaccine also confers a high degree of cross-protection against other HPV types (specifically HPV 31, 45 and 33), and that the overall efficacy against CIN 2 or worse associated with any HPV type in the ATP analysis is 70% (Paavonen et al 2009).
Endometrial Cancer
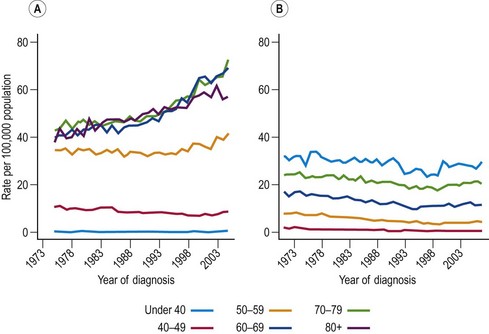
Most cancers of the uterine corpus are adenocarcinomas of the endometrium. Sarcomas of the myometrium (muscle) are rare and are not considered in this chapter. Endometrial cancer comprises approximately 4% of all female cancers worldwide (Bray et al 2005). Incidence rates of endometrial cancer in Western countries are three to eight times as great as mortality rates, reflecting the high cure rate for these cancers (Table 34.1). In fact, 5-year survival for endometrial cancer patients relative to the general population is approximately 70% (Garcia et al 2007). Endometrial cancer is rare in premenopausal women, but becomes relatively common in postmenopausal women; in 2006, the incidence of endometrial cancer in England was 14 per 100,000 in women aged 45–49 years, compared with 29 per 100,000 in women aged 50–54 years. In recent years, endometrial cancer rates have been substantially greater in women over 60 years of age compared with women aged 50–59 years, whereas the rates were similar in the past (Figure 34.2A). Incidence rates are highest in North America; up to 10 times higher than in parts of Africa and almost double those in Northern Europe (Table 34.1). Data from the US Cancer Statistics 2001–2005 show that incidence rates are higher among US Whites (23.5 per 100,000) than Blacks (19.1 per 100,000) (US Cancer Statistics Working Group 2009).
When studying trends in endometrial cancer, it is important to look at ‘cancer of the uterus, site not specified’ since, in many countries, the majority of those cancers will have originated in the endometrium. In both England (Sasieni et al 1997) and the USA (Ries et al 2001), mortality rates fell steadily and substantially (approximately 60%) between 1950 and 1990. More recent UK trends are shown in Figure 34.2B. Incidence rates in women over 55 years of age have been increasing in the UK and across Northern Europe. In contrast, rates in premenopausal women have been declining (Bray et al 2005). The observed trends in younger women have been attributed to the widespread use of the combined oral contraceptive pill (COCP), while the increasing incidence observed in older women is believed to be linked to the increased prevalence of obesity. Interpretation of trends is complicated by the prevalence of women who have had a hysterectomy, since women without a uterus are obviously not susceptible to uterine cancer.
Risk factors
Obesity is consistently found to be associated with endometrial cancer in both case–control and cohort studies. Due to the high prevalence of obesity in many populations, is likely to be responsible for a significant proportion of cases of endometrial cancer. Typically, studies report a two- to three-fold risk of endometrial cancers associated with obesity (Schouten et al 2004), but some studies have found much stronger effects (Weiderpass et al 2000). Obesity is likely to affect cancer rates by increasing the level of circulating oestrogens, particularly in postmenopausal women. This occurs due to increased conversion of androstenedione to oestrogen, and decreased levels of sex-hormone-binding globulin (Cook et al 2006). There is some evidence that greater upper body fat, as opposed to overall obesity, might be a better indicator of the risk of endometrial cancer, and that waist circumference and waist:hip ratio could be a better predictor of risk than body mass index (BMI; Xu et al 2005). Diabetes has been associated with a two-fold increased risk of endometrial cancer (Friberg et al 2007). Although it is difficult to distinguish between the risks associated with increased body weight and the risks of diabetes per se, there is evidence that hyperinsulinaemia increases the risk of endometrial cancer (Lukanova et al 2004).
Oestrogens are the primary stimulation for endometrial proliferation. In premenopausal women, oestrogens are counteracted by progestagens; however, in postmenopausal women, they go unopposed. Any state that reduces the exposure of uterine tissue to oestrogen or oestrogen-like substances reduces the risk of endometrial cancer, and inversely, any medical condition that results in high levels of endogenous oestrogens increases the risk (i.e. polycystic ovaries, oestrogen-secreting ovarian tumours). Pregnancy and parity reduce the risk of this cancer by 30% for the first birth and by 25% for each successive birth. The use of the COCP reduces the risk by 10% for each year of use, so that there is approximately a 65% reduction with 10 years of use. The protective effect of the COCP lasts for at least 15 years after last use (Pike et al 2004). Oestrogen replacement therapy has been consistently found to be associated with an increase in endometrial cancer. A meta-analysis estimated a relative risk of 2.3 in ever-users compared with never-users, and found that the risk remains elevated for several years after cessation of use (Grady et al 1995). The risk appears to increase both with increasing duration of use and increased dose of oestrogen, with a relative risk of 10 associated with 10 years of use (Grady et al 1995). Newer hormone replacement therapy preparations including a progestogen either as a continuous combined replacement or used sequentially for the last 10 days per month cause minimal, if any, increased risk of endometrial cancer (Beresford et al 1997). The oestrogen receptor modulator hormone used in the treatment and prevention of breast cancer, tamoxifen, has been shown to increase the risk of endometrial cancer two- to three-fold (Cuzick et al 2003). Physical activity and smoking have been shown to decrease the risk of endometrial cancer. Physical activity independent of body weight has been shown to reduce the risk of endometrial cancer by 23% (Voskuil et al 2007). Smoking has consistently been associated with a decreased risk of endometrial cancer; this effect might be linked to the fact that smokers metabolize oestrogens into less harmful metabolites than non-smokers. A recent study (Viswanathan et al 2005) found that smoking reduced the risk of endometrial cancer by 30–40% among current and past smokers, and that the effect was greater in those who had been smoking for longer.
Women with Lynch syndrome (hereditary non-polyposis colorectal cancer) are at very high risk of endometrial cancer from 40 years of age. The risks differ depending on the affected gene, but lifetime risk is thought to be between 40% and 60% (Meyer et al 2009). For this reason, most women with Lynch syndrome will have a prophylactic hysterectomy between 40 and 50 years of age.
Ovarian Cancer
Ovarian cancer is the sixth most common type of cancer and the sixth most common cause of cancer mortality in women in the developed world (Garcia et al 2007). Survival is very poor, except when the tumour is detected early, which is rare in most countries. Overall 5-year survival statistics estimate survival to be 46% in the USA (Horner et al 2008) and 39% in England (Office for National Statistics 2008). However, when the cancer is detected at an early stage, 5-year survival increases to over 70%. Approximately 90% of ovarian cancers are adenocarcinomas of the epithelium, but germ cell tumours are the most common histological type in women under 30 years of age.
Descriptive epidemiology
Variation in the rates of ovarian cancer around the world is less marked for ovarian cancer than for other gynaecological cancers (Table 34.1). The highest rates are found in Northern Europe and in White women in North America and Western Europe. Similarly, rates tend to be lower in most Asian countries, but women of Japanese and Chinese descent in the USA have rates that are higher than in their countries of origin.
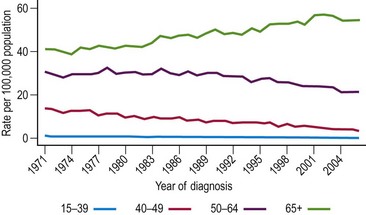
Trends in ovarian cancer in England and Wales differ by age. Mortality rates in premenopausal women have fallen considerably since the 1970s (Figure 34.3). By 1999, the rates in women aged 30–44 years were just half of what they had been in 1970. The picture for older women is dramatically different. Rates in women over 65 years of age have increased, and by 2006, the rates in women over 65 years of age were over 30% greater than they were in 1971. The incidence of ovarian cancer is only slightly greater than the mortality rates, reflecting the poor cure rate for this disease. Consequently, trends in incidence are similar to the trends in mortality. Recently, however, survival has improved substantially due to a combination of earlier detection and the use of platinum-based chemotherapies (Kitchener 2008).
Risk factors
One of the first observations linking reproductive history to ovarian cancer risk was that never-married women were at approximately one and a half times the risk of ever-married women. Subsequently, decreasing risk with increasing numbers of full-term pregnancies has been shown in over a dozen case–control studies (Whittemore et al 1992). Typically, having had one or two children is associated with a decrease in risk of approximately 30%, and having had three or more children is associated with a decrease in risk of approximately 60%. In a recent US study of 563 cases and 523 controls, one child was associated with a 40% reduction in risk, two children with a 60% reduction in risk and five or more children with a 80% reduction in risk (Titus-Ernstoff et al 2001). A large cohort study in Sweden including 4128 ovarian cancer cases found that having had one child was associated with a 25% reduction in risk and having had two children was associated with a 43% reduction in risk (Granstrom et al 2008). The effect of parity does not appear to be attributable to confounding by age at first birth, age at last birth, age at menarche or age at menopause, none of which seem to be associated with ovarian cancer risk to any significant extent.
Infertility, however, does have an independent effect on ovarian cancer risk. In a pooled analysis of eight case–control studies (Ness et al 2002), nulliparous women who tried to conceive for more than 5 years had a 2.7-fold risk of ovarian cancer compared with women who had not tried to conceive for more than 1 year. There have been claims that women who received fertility treatment had an increased risk compared with those who had never been treated with fertility drugs, but there is no evidence to support this claim (Kashyap et al 2004).
Although not established conclusively, breast feeding seems to reduce the risk of ovarian cancer, even after adjusting for parity. A recent prospective study found that women who had breast fed for 18 months or more were at significantly lower risk of ovarian cancer compared with women who had not breast fed (Danforth et al 2007).
Exogenous hormones
The protective effect of the COCP on ovarian cancer risk has been demonstrated beyond any reasonable doubt (Collaborative Group on Epidemiological Studies of Ovarian Cancer 2008). Pooled estimates of the protection indicate that the risk is reduced by approximately 50% with 5 years of use, and that protection increases with the duration of use (Whittemore et al 1992). The protective effect of oral contraceptives persists for up to 30 years after cessation of use, and is not confined to any particular formulation of the pill. The benefit in absolute terms can be expected to increase over the next few decades as the cohort of women with extensive use of oral contraceptives reaches the peak age of ovarian cancer incidence. Hormone replacement therapy has been associated with a 19–24% increased risk of ovarian cancer; however, the risk is not increased in women using hormone replacement therapy for less than 5 years, and decreases once a woman stops taking it (Zhou et al 2008).
Family history
It is a well-known clinical observation that ovarian cancer tends to aggregate in families. Rarely, such clustering can be extreme with increased risk of breast cancer (in BRCA1 and BRCA2 gene mutation carriers) and colon and endometrial cancer in cancer family syndromes. The risk of ovarian cancer associated with BRCA1 and BRCA2 mutation carriers is 40% and 18%, respectively (Chen and Parmigiani 2007). Case–control studies generally find a relative risk of approximately 3 associated with one affected family member, and a relative risk of approximately 7 with two affected relatives. More recently, the Swedish Cancer Family Database reported relative risks of 2.54 in women with an affected mother and 2.76 in women with an affected sister (Granstrom et al 2008). Relative risks of such magnitude are typical of most cancers, but the high absolute risk in women with a family history of both early breast cancer and ovarian cancer is such that many such women consider having a prophylactic oophorectomy once they have completed their families, particularly since having an oophorectomy before the natural menopause also reduces the risk of breast cancer.
Other factors
Inconsistent results have been found from epidemiological studies regarding BMI (Vaino and Bianchini 2002). A recent meta-analysis (Schouten et al 2008) concluded that a BMI of 30 or more in premenopausal women increases the risk of ovarian cancer by 30% compared with women with a BMI below 24. No effect was observed in postmenopausal women.
There has been great interest in whether talcum powder applied to the perineal area increases the risk of ovarian cancer. In an attempt to clarify the evidence, a systematic review was carried out in 2003. This found a significantly increased risk associated with talc use (relative risk 1.33, 95% confidence interval 1.16–1.45). However, there was no clear association with dose, and the authors concluded that the association could be confounded or biased (Huncharek et al 2003). A further two case–control studies (Gates et al 2008, Merritt et al 2008) have been published, both of which seem to suggest a significant trend of increasing risk with more frequent use. Despite this finding, the authors advise caution because the majority of studies failed to observe a dose–response relationship and there is the possibility of recall bias differentially affecting cases and controls.
The relationship between smoking and ovarian cancer is unclear. A meta-analysis including 10 studies found that the risk of mucinous ovarian cancer was doubled in current smokers compared with never-smokers; however, a risk reduction for clear cell cancers was also observed (Jordan et al 2006). In 2008, the Norwegian–Swedish Women’s Lifestyle and Health cohort including 103,081 women found a doubling of risk for borderline tumours for former and current smokers compared with non-smokers. They also found a dose–response relationship according to smoking intensity and duration for borderline and serous tumours (Gram et al 2008).
Prevention and screening
No effective screening methods are available for the detection of early ovarian cancer. Recent hopes are centred on multimodal screening involving serum tumour marker CA125 for primary screening followed by ultrasound (Menon et al 2009). Transvaginal ultrasound examination on its own is not justified as a screening technique at population level due to its lack of specificity and predictive value, because it is unable to differentiate benign cysts from malignant disease. The serum tumour marker CA125 is present in approximately 80% of epithelial cancers, but is also raised in the presence of other cancers (i.e. pancreatic, breast, bladder) as well as in some benign diseases (e.g. diverticulitis, endometriosis) and some physiological conditions (e.g. pregnancy, menstruation) (Menon 2004). The best evidence to date regarding multimodal screening comes from a large randomized-controlled trial in the UK; although this trial did not find a difference in deaths from ovarian cancer, it did find a significant difference in the median rate of survival in the screened group compared with the control group (Jacobs et al 1999). Currently, the UK Collaborative Trial of Ovarian Cancer Screening has randomized over 200,000 women to multimodal screening or no screening. The main endpoint is death from ovarian cancer, and the results are expected in 2015. In the multimodal arm, triage is offered based on a substantial increase in CA125 and not simply on the absolute level. There is much interest in other proteomic markers detectable in serum, but it is too early to evaluate these fully (Rifai et al 2006, Gagnon and Ye 2008).
Cancers of the Vulva and the Vagina
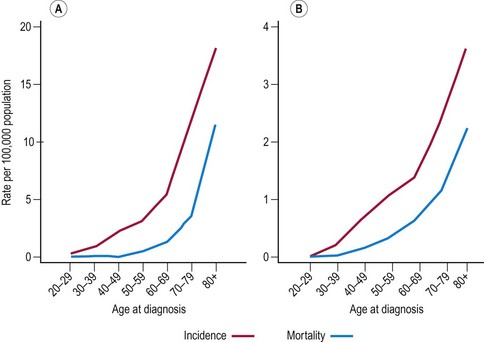
Cancers of the vulva and vagina account for 7% and less than 2% of all gynaecological cancers in the UK, respectively. Over 80% are squamous in origin, with 10% of vulva cancers being melanomas. Incidence and mortality of both cancers is highest in women over 60 years of age (Figure 34.4). The incidence of vaginal cancer in women over 60 years of age is three per 100,000 in England (compared with less than one per 100,000 in women under 60 years of age). The risk in women over 60 years of age is much more pronounced for vulval cancer; incidence increases from approximately three per 100,000 in women aged 40–59 years to 11 per 100,000 in women aged over 60 years. The incidence of vaginal cancer in the UK has remained at approximately 0.6 per 100,000 for the past 15 years. The incidence of vulval cancer fell during the 1970s and 1980s; however, it has increased in recent years and the incidence rate is now comparable to that observed in 1975 (Figure 34.5). This increase reflects the sharp rise of vulval cases in women under 50 years of age, which in turn is thought to be caused by the high prevalence of HPV infection in younger women (Joura et al 2000). The increase in HPV infection of the vulva has been attributed to changing attitudes towards oral sex. Mortality rates from both cancers have fallen considerably since the 1970s. For vaginal cancer, the rates have fallen by 38% since the 1970s (from 0.4 per 100,000 to 0.2 per 100,000), and for vulva cancer, the corresponding figure is 53% (from 1.3 per 100,000 in 1971 to 0.6 per 100,000 in 2006).
Risk factors for these cancers appear to be similar to those for cervical cancer. A well-defined precancerous stage has been identified for both these cancers: vaginal intraepithelial neoplasia and vulval intraepithelial neoplasia. It is estimated that 65–90% of vaginal cancers and 30–35% of vulval cancers can be attributed to HPV infection [WHO/ICO Information Centre on Human Papilloma Virus (HPV) and Cervical Cancer 2007]. HPV 16 has been found to be the most prevalent HPV type in both these cancers; it compromised approximately 60% of the HPV found in vaginal cancer (Hampl et al 2006) and 20–60% of the HPV found in vulval cancer. HPV-positive vulvar cancer is much more common in women under 50 years of age, where high-risk HPV types can be detected in up to 60% of cancers (Hampl et al 2008). It has been hypothesized that vulval cancer arises from two distinct diseases (Trimble et al 1996, Basta et al 1999). The first type develops from in-situ vulval neoplasia, in which a much higher proportion of high-risk HPV types are found (70–90%) (Madeleine et al 1997) and which is more prevalent in young women. The second type, which more often afflicts older women, develops from non-cancerous vulvar epithelial disorders as a result of chronic inflammation (the itch–scratch–lichen sclerosus hypothesis) (Canavan and Cohen 2002). Antibodies to herpes simplex virus type 2 have been associated with increased risk of both vaginal and vulval cancer, even after controlling for HPV infection (Hildesheim et al 1997, Madeleine et al 1997). Smoking increases the risk of vulval cancer six-fold, and this increases to 25-fold when heavy smoking (>20 cigarettes a day) and HPV infection interact (Hildesheim et al 1997, Madeleine et al 1997). The association between smoking and vaginal cancer still remains uncertain.
The one risk factor that is very strongly associated with cancer of the vulva is immunosuppression; the risk of invasive vulval cancer is increased 100-fold in women with renal transplants (Penn 1986). It has also been documented that the incidence of vulval intraepithelial neoplasia is increased in women who are HIV positive (Frisch et al 2000).
Vaginal cancer in adolescents has been attributed to in-utero exposure to diethylstilbestrol (DES), a synthetic oestrogen used to prevent spontaneous abortions between 1940 and 1970 (Greenwald et al 1971). Vaginal cancer is almost never diagnosed in cohorts who could not have been exposed to DES in utero (either because they were born prior to 1945 or because DES was never prescribed in the country). The current estimates suggest a 40-fold increase in the risk of vaginal cancer in the daughters of exposed women (Hatch et al 1998).
KEY POINTS
Appleby P, Beral V, Berrington de Gonzalez A, et al. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. The Lancet. 2007;370:1609-1621.
Basta A, Adamek K, Pitynski K. Intraepithelial neoplasia and early stage vulvar cancer. Epidemiological, clinical and virological observations. European Journal of Gynaecological Oncology. 1999;20:111-114.
Beresford SA, Weiss NS, Voigt LF, McKnight B. Risk of endometrial cancer in relation to use of oestrogen combined with cyclic progestagen therapy in postmenopausal women. The Lancet. 1997;349:458-461.
Birkeland SA, Storm HH, Lamm LU, et al. Cancer risk after renal transplantation in the Nordic countries 1964–1986. International Journal of Cancer. 1995;60:183-189.
Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. Journal of Clinical Pathology. 2002;55:244-265.
Bosch FX, de Sanjose S. The epidemiology of human papillomavirus infection and cervical cancer. Disease Markers. 2007;23:213-227.
Bray F, dos Santos Silva I, Moller H, Weiderpass E. Endometrial cancer incidence trends in Europe: underlying determinants and prospects for prevention. Cancer Epidemiology, Biomarkers and Prevention. 2005;14:1132-1142.
Buckley JD, Harris RW, Doll R, Vessey MP, Williams PT. Case–control study of the husbands of women with dysplasia or carcinoma of the cervix uteri. The Lancet. 1981;2:1010-1015.
Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. The Lancet. 2007;370:1764-1772.
Burk RD, Ho GY, Beardsley L, Lempa M, Peters M, Bierman R. Sexual behavior and partner characteristics are the predominant risk factors for genital human papillomavirus infection in young women. Journal of Infectious Diseases. 1996;174:679-689.
Canavan TP, Cohen D. Vulvar cancer. American Family Physician. 2002;66:1269-1274.
Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. Journal of Clinical Oncology. 2007;25:1329-1333.
Clifford GM, Rana RK, Franceschi S, et al. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiology, Biomarkers & Prevention. 2005;14:1157-1164.
Collaborative Group on Epidemiological Studies of Ovarian CancerBeral V, Doll R, Hermon C, Peto R, Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. The Lancet. 2008;371:303-314.
Cook LS, Weiss NS, Doherty JA, Chen C. Endometrial Cancer. Oxford: Oxford University Press; 2006.
Cullen AP, Reid R, Campion M, Lorincz AT. Analysis of the physical state of different human papillomavirus DNAs in intraepithelial and invasive cervical neoplasm. Journal of Virology. 1991;65:606-612.
Curado MP, Edwards B, Shin HR, et al, editors. Cancer Incidence in Five Continents, Vol. IX. Lyon: IARC, 2007. IARC Scientific Publications No. 160
Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. The Lancet. 2003;361:296-300.
Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. International Journal of Cancer. 2006;119:1095-1101.
Cuzick J, Szarewski A, Mesher D, et al. Long-term follow-up of cervical abnormalities among women screened by HPV testing and cytology — results from the Hammersmith study. International Journal of Cancer. 2008;122:2294-2300.
Cuzick J, Arbyn M, Sankaranarayanan R, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;26(Suppl 10):K29-K41.
Danforth KN, Tworoger SS, Hecht JL, Rosner BA, Colditz GA, Hankinson SE. Breastfeeding and risk of ovarian cancer in two prospective cohorts. Cancer Causes and Control. 2007;18:517-523.
Das BC, Sharma JK, Gopalakrishna V, Luthra UK. Analysis by polymerase chain reaction of the physical state of human papillomavirus type 16 DNA in cervical preneoplastic and neoplastic lesions. Journal of General Virology. 1992;73:2327-2336.
Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ (Clinical Research Ed.). 2008;337:a1754.
Ferlay J, Bray F, Pisani P, Parkin DM. Globocan 2002: Cancer Incidence, Mortality and Prevalence Worldwide. IARC CancerBase No. 5. Lyon: IARC Press; 2004.
Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007;50:1365-1374.
Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. Journal of the National Cancer Institute. 2000;92:1500-1510.
FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. New England Journal of Medicine. 2007;356(19):1915-1927.
Gagnon A, Ye B. Discovery and application of protein biomarkers for ovarian cancer. Current Opinions in Obstetrics and Gynecology. 2008;20:9-13.
Garcia M, Jemal A, Ward EM, et al. Global Cancer Facts & Figures 2007. Atlanta, GA: American Cancer Society; 2007.
Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. New England Journal of Medicine. 2007;356:1928-1943.
Gates MA, Tworoger SS, Terry KL, et al. Talc use, variants of the GSTM1, GSTT1, and NAT2 genes, and risk of epithelial ovarian cancer. Cancer Epidemiology, Biomarkers and Prevention. 2008;17:2436-2444.
Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstetrics and Gynecology. 1995;85:304-313.
Gram IT, Braaten T, Adami HO, Lund E, Weiderpass E. Cigarette smoking and risk of borderline and invasive epithelial ovarian cancer. International Journal of Cancer. 2008;122:647-652.
Granstrom C, Sundquist J, Hemminki K. Population attributable fractions for ovarian cancer in Swedish women by morphological type. British Journal of Cancer. 2008;98:199-205.
Greenwald P, Barlow JJ, Nasca PC, Burnett WS. Vaginal cancer after maternal treatment with synthetic estrogens. New England Journal of Medicine. 1971;285:390-392.
Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. The Lancet. 2007;370:59-67.
Hampl M, Sarajuuri H, Wentzensen N, Bender HG, Kueppers V. Effect of human papillomavirus vaccines on vulvar, vaginal, and anal intraepithelial lesions and vulvar cancer. Obstetrics and Gynecology. 2006;108:1361-1368.
Hampl M, Deckers-Figiel S, Hampl JA, Rein D, Bender HG. New aspects of vulvar cancer: changes in localization and age of onset. Gynecological Oncology. 2008;109:340-345.
Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247-1255.
Hatch EE, Palmer JR, Titus-Ernstoff L, et al. Cancer risk in women exposed to diethylstilbestrol in utero. JAMA: the Journal of the American Medical Association. 1998;280:630-634.
Hildesheim A, Han CL, Brinton LA, Kurman RJ, Schiller JT. Human papillomavirus type 16 and risk of preinvasive and invasive vulvar cancer: results from a seroepidemiological case–control study. Obstetrics and Gynecology. 1997;90:748-754.
Hildesheim A, Herrero R, Wacholder S, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA: the Journal of the American Medical Association. 2007;298:743-753.
Ho GY, Burk RD, Klein S, et al. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. Journal of the National Cancer Institute. 1995;87:1365-1371.
Horner MJ, Ries LAG, Krapcho M, et al, editors. SEER Cancer Statistics Review 1975–2006. Bethesda, MD: National Cancer Institute, 2008.
Huncharek M, Geschwind JF, Kupelnick B. Perineal application of cosmetic talc and risk of invasive epithelial ovarian cancer: a meta-analysis of 11,933 subjects from sixteen observational studies. Anticancer Research. 2003;23:1955-1960.
IARC Handbooks of Cancer Prevention. Fruit and Vegetables. Lyon: IARC Press; 2003.
IARC Monographs. Human Papillomaviruses. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon: IARC Press; 2007.
International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical carcinoma and reproductive factors: collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies. International Journal of Cancer. 2006;119:1108-1124.
Jacobs IJ, Skates SJ, MacDonald N, et al. Screening for ovarian cancer: a pilot randomised controlled trial. The Lancet. 1999;353:1207-1210.
Jordan SJ, Whiteman DC, Purdie DM, Green AC, Webb PM. Does smoking increase risk of ovarian cancer? A systematic review. Gynecological Oncology. 2006;103:1122-1129.
Joura EA, Losch A, Haider-Angeler MG, Breitenecker G, Leodolter S. Trends in vulvar neoplasia. Increasing incidence of vulvar intraepithelial neoplasia and squamous cell carcinoma of the vulva in young women. Journal of Reproductive Medicine. 2000;45:613-615.
Kapeu AS, Luostarinen T, Jellum E, et al. Is smoking an independent risk factor for invasive cervical cancer? A nested case–control study within Nordic biobanks. American Journal of Epidemiology. 2009;169:480-488.
Kashyap S, Moher D, Fung MF, Rosenwaks Z. Assisted reproductive technology and the incidence of ovarian cancer: a meta-analysis. Obstetrics and Gynecology. 2004;103:785-794.
Kitchener HC. Survival from cancer of the ovary in England and Wales up to 2001. British Journal of Cancer. 2008;99(Suppl 1):S73-S74.
Lukanova A, Zeleniuch-Jacquotte A, Lundin E, et al. Prediagnostic levels of C-peptide, IGF-I, IGFBP -1, -2 and -3 and risk of endometrial cancer. International Journal of Cancer. 2004;108:262-268.
Madeleine MM, Daling JR, Carter JJ, et al. Cofactors with human papillomavirus in a population-based study of vulvar cancer. Journal of the National Cancer Institute. 1997;89:1516-1523.
Magnusson PK, Sparen P, Gyllensten UB. Genetic link to cervical tumours. Nature. 1999;400:29-30.
Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstetrics and Gynecology. 2006;107:18-27.
McCredie MR, Sharples KJ, Paul C, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. The Lancet Oncology. 2008;9:425-434.
McIndoe WA, McLean MR, Jones RW, Mullins PR. The invasive potential of carcinoma in situ of the cervix. Obstetrics and Gynecology. 1984;64:451-458.
Menon U. Ovarian cancer screening. CMAJ: Canadian Medical Association Journal. 2004;171:323-324.
Menon U, Gentry-Maharaj A, Hallett R, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). The Lancet Oncology. 2009;10:327-340.
Merritt MA, Green AC, Nagle CM, Webb PM. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. International Journal of Cancer. 2008;122:170-176.
Meyer LA, Broaddus RR, Lu KH. Endometrial cancer and Lynch syndrome: clinical and pathologic considerations. Cancer Control. 2009;16:14-22.
Moscicki AB, Shiboski S, Hills NK, et al. Regression of low-grade squamous intra-epithelial lesions in young women. The Lancet. 2004;364:1678-1683.
Nanda K, Mccrory DC, Myers ER, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Annals of Internal Medicine. 2000;132:810-819.
Ness RB, Cramer DW, Goodman MT, et al. Infertility, fertility drugs, and ovarian cancer: a pooled analysis of case–control studies. American Journal of Epidemiology. 2002;155:217-224.
Office for National Statistics. Cancer Statistics Registrations. Series MB1 No. 34. London: Office for National Statistics; 2006.
Office for National Statistics. Cancer survival, England, patients diagnosed 2001–2006 and followed up to 2007: one year and five-year survival for 21 common cancers, by sex and age. Cancer Survival Trends. SMPS No. 61. London: Office for National Statistics; 2008.
Östör AG. Natural history of cervical intraepithelial neoplasia: a critical review. International Journal of Gynecological Pathology. 1993;12:186-192.
Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161-2170.
Paavonen J, Naud P, Salmeron J et al 2009 Final phase III efficacy analysis of cervarix in young women. Abstract O-29.06. 25th International Papilloma Virus Conference, Malmo.
Penn I. Cancers of the anogenital region in renal transplant recipients. Analysis of 65 cases. Cancer. 1986;58:611-616.
Pike MC, Pearce CL, Wu AH. Prevention of cancers of the breast, endometrium and ovary. Oncogene. 2004;23:6379-6391.
Ries LAG, Eisner MP, Kosary CL, et al, editors. SEER Cancer Statistics Review, 1973–1998. Bethesda, MD: National Cancer Intitute, 2001.
Rifai N, Gillette MA, Carr SA. Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nature Biotechnology. 2006;24:971-983.
Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. New England Journal of Medicine. 2009;360:1385-1394.
Sasieni PD, Adams J, Cuzick J. Trends in gynaecological cancers in England and Wales. Journal of Epidemiology and Biostatistics. 1997;2:187-195.
Sasieni PD, Adams J. Analysis of cervical cancer mortality and incidence data from England and Wales: evidence of a beneficial effect of screening. Journal of the Royal Statistics Society Series A. 2000;Issue 163:191-209.
Sasieni P, Castanon A, Cuzick J. Screening and adenocarcinoma of the cervix. International Journal of Cancer. 2009;125:525-529.
Schouten LJ, Goldbohm RA, van den Brandt PA. Anthropometry, physical activity, and endometrial cancer risk: results from the Netherlands Cohort Study. Journal of the National Cancer Institute. 2004;96:1635-1638.
Schouten LJ, Rivera C, Hunter DJ, et al. Height, body mass index, and ovarian cancer: a pooled analysis of 12 cohort studies. Cancer Epidemiology, Biomarkers and Prevention. 2008;17:902-912.
Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. International Journal of Cancer. 2007;121:621-632.
Titus-Ernstoff L, Perez K, Cramer DW, Harlow BL, Baron JA, Greenberg ER. Menstrual and reproductive factors in relation to ovarian cancer risk. British Journal of Cancer. 2001;84:714-721.
Trimble CL, Hildesheim A, Brinton LA, Shah KV, Kurman RJ. Heterogeneous etiology of squamous carcinoma of the vulva. Obstetrics and Gynecology. 1996;87:59-64.
US Cancer Statistics Working Group. United States Cancer Statistics: 1999–2005 Incidence and Mortality Web-based Report. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2009.
Vaino F, Bianchini F, editors. IARC Handbooks of Cancer Prevention: Weight Control and Physical Activity. Lyon: IARC Press, 2002.
van Ballegooijen M, van den Akker-van Marle ME, Warmerdam PG, Meijer CJ, Walboomers JM, Habbema JD. Present evidence on the value of HPV testing for cervical cancer screening: a model-based exploration of the (cost-)effectiveness. British Journal of Cancer. 1997;76:651-657.
Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18 L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. The Lancet Oncology. 2005;6:271-278.
Viswanathan AN, Feskanich D, de Vivo I, et al. Smoking and the risk of endometrial cancer: results from the Nurses’ Health Study. International Journal of Cancer. 2005;114:996-1001.
Voskuil DW, Monninkhof EM, Elias SG, Vlems FA, van Leeuwen FE. Physical activity and endometrial cancer risk, a systematic review of current evidence. Cancer Epidemiology, Biomarkers and Prevention. 2007;16:639-648.
Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. Journal of Pathology. 1999;189:12-19.
Weiderpass E, Persson I, Adami HO, Magnusson C, Lindgren A, Baron JA. Body size in different periods of life, diabetes mellitus, hypertension, and risk of postmenopausal endometrial cancer (Sweden). Cancer Causes and Control. 2000;11:185-192.
Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case–control studies. IV. The pathogenesis of epithelial ovarian cancer. Collaborative Ovarian Cancer Group. American Journal of Epidemiology. 1992;136:1212-1220.
. WHO/ICO Information Centre on Human Papilloma Virus (HPV) and Cervical Cancer 2007 Human Papillomavirus and Cervical Cancer. [cited 17 April 2009]; available from http://www.who.int/hpvcentre/en
Xu WH, Matthews CE, Xiang YB, et al. Effect of adiposity and fat distribution on endometrial cancer risk in Shanghai women. American Journal of Epidemiology. 2005;161:939-947.
Zhou B, Sun Q, Cong R, et al. Hormone replacement therapy and ovarian cancer risk: a meta-analysis. Gynecologic Oncology. 2008;108:641-651.