Chapter 76 Envenomation
Envenomation by species of snakes, spiders, ticks, bees, ants, wasps, jellyfish, octopuses or cone shell snails may threaten life, while envenomation by other creatures may cause serious illness.1 Although this chapter focuses on Australia, the principles of management are widely applicable elsewhere except for stings by scorpions which are not a significant health problem in Australia. Immediate advice on management may be obtained from the Australian Venom Research Unit (AVRU) advisory service on their 24-hour telephone number within Australia on 1300 760 451, from overseas on 61 3 8344 7753 or from their website at http://www.avru.org.
SNAKES
EPIDEMIOLOGY
The mean death rate in Australia from 1981 to 1999 was 2.6 per year1 (∼0.014/100 000), usually occurring because of massive envenomation, snake bite in remote locations, rapid collapse, or due to delayed or inadequate antivenom therapy. However, as many as 2000 people are bitten each year and of these at least 300 require antivenom treatment. This morbidity and mortality is far less than that observed in surrounding countries. Death and critical illness is due to (1) progressive paralysis leading to respiratory failure, (2) bleeding, or (3) renal failure occurring as a complication of rhabdomyolysis, disseminated intravascular coagulation (DIC), haemorrhage, haemolysis or to their combinations. Rapid collapse within minutes after a snake bite is due to anaphylaxis to venom or possibly due to the myocardial effects of DIC causing hypotension.
Snake bite is often ‘accidental’ when a snake is trodden upon or suddenly disturbed. However, many bites occur when humans deliberately interfere with snakes or handle them. The herpetologist or snake collector is at special risk. Not only do they invariably sustain bites in the course of their work2 or hobby, but they are also at risk of developing allergic reactions to venoms and to the antivenoms used in their treatment. Contact with exotic snakes has additional problems.
SNAKE VENOMS
Venoms are complex mixtures of toxins, usually proteins, which kill the snake’s prey and aid its digestion. Many are phospholipases. The main toxins cause paralysis, coagulopathy, rhabdomyolysis and haemolysis (Table 76.1). Coagulopathy may be due to a procoagulant effect by prothrombin activators (Factor Xa-like enzymes), with consumption of clotting factors, or due to a direct anticoagulant effect.
| Neurotoxins |
SNAKE BITE AND ENVENOMATION
SYMPTOMS AND SIGNS OF ENVENOMATION
Not all possible symptoms and signs occur in a particular case: in some cases, one symptom or sign may dominate the clinical picture, and in other cases they may wax and wane (Table 76.2). These phenomena are explained by variations in toxin content of venoms of the same species in different geographical areas, and by variable absorption of different toxins.
Table 76.2 Progressive onset of major systemic symptoms and signs of untreated envenomation (in massive envenomation or in a child, a critical illness may develop in minutes rather than hours)
| < 1 hour after bite |
The cause of transient hypotension soon after envenomation is obscure but it may be related to intravascular coagulation.3,4 Prothrombin activators gain access to the circulation within a number of minutes after subcutaneous injection. There is often tachycardia and relatively minor ECG abnormalities. Other causes of hypotension such as direct cardiac toxicity remain unproven. Hypotension may be secondary to myocardial hypoxaemia.
Tender or even painful regional lymph nodes are moderately common but are not per se an indication for antivenom therapy, since lymphadenitis also occurs with bites by mildly venomous snakes which do not cause serious systemic illness.
IDENTIFICATION OF THE SNAKE
Identification of the snake guides selection of the appropriate antivenom, and provides an insight into the expected syndrome. Administration of the wrong antivenom may endanger the victim’s life because there may be very little neutralisation of venom. A venom detection kit can be used to identify the snake venom. If the snake cannot be identified, a specific antivenom, or a combination of monovalent antivenoms or polyvalent antivenom should be administered on a geographical basis (see Tables 76.3 and 76.4).
Table 76.3 Antivenom and initial dosages when snake identified
| Snake | Antivenom | Dose (units) |
|---|---|---|
| Common Brown Snake | Brown Snake | 4000 |
| Chappell Island Tiger Snake | Tiger Snake | 12 000 |
| Copperheads | Tiger Snake | 3000–6000 |
| Death Adders | Death Adder | 6000 |
| Dugite | Brown Snake | 4000 |
| Gwardar | Brown Snake | 4000 |
| Mulga (King Brown) Snake | Black Snake | 18 000 |
| Papuan Black Snake | Black Snake | 18 000 |
| Red-bellied Black Snake | Tiger Snake or Black Snake* | 3000 |
| 18 000 | ||
| Rough-scaled (Clarence River) Snake | Tiger Snake | 3000 |
| Sea-Snakes | Sea-Snake or | 1000 |
| Tiger Snake | 3000 | |
| Small-scaled (Fierce) Snake | Taipan | 12 000 |
| Taipans | Taipan | 12 000 |
| Tasmanian Tiger Snake | Tiger Snake | 6000 |
| Tiger Snake | Tiger Snake | 3000 |
* Smaller protein mass Tiger Snake antivenom preferable. Antivenom units per vial: Brown Snake 1000; Tiger Snake 3000; Black Snake 18 000; Taipan 12 000; Death Adder 6000; polyvalent 40 000. Note: (1) If the victim on presentation is critically ill, 2–3 times these amounts should be given initially; (2) additional antivenom may be required in the course of management since absorption of venom may be delayed.
Table 76.4 Antivenom and initial dosages when identity of snake uncertain
| State | Antivenom | Dose (units) |
|---|---|---|
| Tasmania | Tiger Snake | 6000 |
| Victoria | Tiger Snake and | 3000 |
| Brown Snake | 4000 | |
| New South Wales and ACT; Queensland; South Australia; Western Australia; Northern Territory | Polyvalent | 40 000 |
| Papua New Guinea | Polyvalent | 40 000 |
Note: (1) If the victim on presentation is critically ill, 2–3 times these amounts should be given initially; (2) additional antivenom may be required in the course of management since absorption of venom may be delayed.
IDENTIFICATION BY VENOM DETECTION KIT TEST
The venom detection kit (VDK) is an in vitro test for detection and identification of snake venom at the bite site, in urine, blood or other tissue in cases of snake bite in Australia and Papua New Guinea. It is an enzyme immunoassay using rabbit antibodies and chromogen and peroxide solutions. A positive result will indicate the type of antivenom to be administered. It detects venom from a range of snake genera including Tiger, Brown, Black, Death Adder and Taipan. Individual species of snake cannot be identified by the test and several genera may yield a positive result in a specified well. The incidences of false-positive and false-negative tests of the kit remain unknown, but are generally regarded as low. The test isvery sensitive, able to detect venom in concentrations as low as 10 ng/ml, and can yield a visual qualitative result in test wells in approximately 25 minutes. On occasion, a positive test may be present but the patient is asymptomatic. A decision to administer antivenom should be made on clinical grounds. A very high concentration of venom in a sample may overwhelm the test and yield a spuriously negative result (Hook effect). If that possibility exists, a diluted sample should be re-tested.
IDENTIFICATION BY PHYSICAL CHARACTERISTICS
This can be misleading. Non-herpetologists should consult an identification guide1 with reference to scale patterns to identify a specimen correctly if antivenom therapy is to be based on morphological characteristics alone.
MANAGEMENT OF SNAKE ENVENOMATION
The essentials of management are:
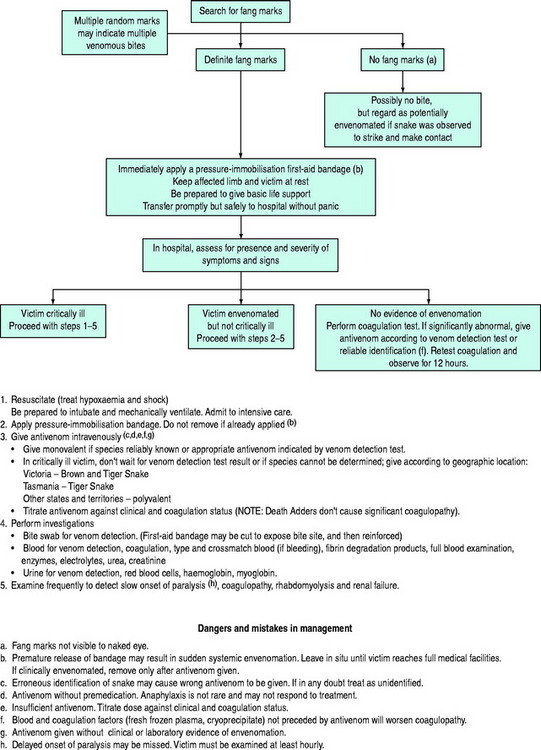
From a practical point of view, one of three clinical situations arises after snake bite. A plan of management for each of these is summarised in Figure 76.1.
When the envenomated victim is not critically ill, more time is available to identify the snake by investigationsand to administer specific monovalent antivenom. A pressure-immobilisation bandage should be applied if not already in place, and not removed until antivenom has been administered.
THE PRESSURE-IMMOBILISATION TECHNIQUE OF FIRST AID
Since at least 95% of snake bites occur on the arms or legs,1 Sutherland’s first-aid pressure-immobilisation technique5 is applicable to the majority of cases. With this technique, a crêpe (or crêpe-like) bandage is applied from the fingers or toes up the limb as far as possible, encompassing the bite site. It should be as firm as required for a sprained ankle. Additional immobilisation is applied to the entire limb by a rigid splint, with the aim of immobilising the joints either side of the bite site.
Venom is usually deposited subcutaneously. The systemic spread of venom is largely dependent on its absorption by way of the lymphatics6 or the small blood vessels. Application of a pressure less than arterial to the bitten area when combined with immobilisation of the limb effectively delays the movement of venom to the central circulation.5 Although it is a first-aid technique designed for use in the field, it should be part of initial management in hospital since it halts further absorption of venom. There is some experimental1 and anecdotal7 evidence with Death Adder bites that the technique inactivates some venom at the bite site but prolonged application has not been subjected to a controlled study.
ANTIVENOM
Antivenom should be administered according to the identity of the snake or, if unknown or doubtful, according to the result of a VDK test (Table 76.3). If neither of these criteria can be fulfilled, and if the situation warrants immediate antivenom therapy, the geographical location may be used a guide, since the distribution of many species is known (Table 76.4). Polyvalent antivenom should not be used when a monovalent antivenom could be used appropriately. For bites by uncommon snakes, when antivenom is indicated, polyvalent antivenom, or a monovalent antivenom as indicated by a VDK test, should be chosen.
Dose
The initial doses of antivenom are given in Tables 76.3 and 76.4. The need for subsequent doses should be guided by the clinical response. After bites by species with coagulopathic effects the victim’s coagulation status is a useful guide.
Administration
The decision to administer antivenom must be based on clinical criteria of envenomation, not restricted to the result of a VDK test. A positive VDK test establishes the diagnosisof envenomation and the choice of antivenom, but does not imply that antivenom should or should not be given.
Premedication
Antivenom should be preceded by premedication with subcutaneous adrenaline (epinephrine), approximately 0.25 mg for an adult and 0.005–0.01 mg/kg for a child, at least 5–10 minutes before commencement of infusion. In the moribund or critically ill victim, when it is essential to administer antivenom quickly, the epinephrine may be given intramuscularly or even intravenously in smaller doses. However, in general, epinephrine is not recommended by either of those routes because of the risk of intracerebral haemorrhage due to the combination of possible hypertension and coagulopathy. Although intracerebral haemorrhage has been recorded in the past in association with premedication, all such cases were accompanied by intravenous epinephrine, none with subcutaneous epinephrine. On the other hand, the incidence of adverse reactions (8–13%) to antivenom is sufficient to warrant premedication with epinephrine, which is the only medication proven effective in reducing the incidence of antivenom-induced reactions and their severity.8
It is not prudent to forgo premedication and elect to treat anaphylaxis if it occurs. Iatrogenic anaphylaxis has a high mortality despite vigorous and expert resuscitation.9 If an adverse reaction to the first vial of antivenom has not occurred, subsequent vials do not need to be preceded by epinephrine. The reaction rate to polyvalent antivenom is higher than to monovalent antivenoms and should not be used when a monovalent antivenom or combinations will suffice.
The antihistamine, promethazine, is ineffective in this setting10 and may cause obtundation and hypotension, both of which may exacerbate and confound a state of envenomation. It is not recommended. Other drugs such as steroids and aminophylline are also not useful in preventing anaphylaxis because their actions, apart from being unproven, are too slow in onset, but steroids are useful for preventing serum sickness.
Adverse reactions
Antivenom infusion should always be administered in a location equipped and staffed by personnel capable of managing anaphylaxis. Management of anaphylaxis is discussed in detail in Chapter 58. Intramuscular epinephrine is the key treatment in a dose of approximately 0.25–1.00 mg for adults and 10 μg/kg for children. Antivenom therapy should be discontinued temporarily and restarted when the victim’s condition is stable.
INVESTIGATIONS AND MONITORING
URINE
Test the urine for venom that may be present when venom in blood has been bound by antivenom and is therefore undetectable. Urine should also be tested for blood and protein. If the urine is pigmented a distinctionshould be made between haemoglobinuria and myoglobinuria, which is impossible with simple ward tests. Urine output should be recorded.
SECONDARY MANAGEMENT
HEPARIN
Although this anticoagulant has prevented the action of prothrombin activators in animal models of envenomation, it does not improve established disseminated intravascular coagulation.11 It is not recommended. Emphasis instead should be on treating the cause by neutralising venom with antivenom.
UNCOMMON AND EXOTIC SNAKE BITE
Zoo personnel, herpetologists and amateur collectors who catch, maintain or breed species of uncommon Australian snakes or who import or breed exotic (overseas) snakes are at risk, as are personnel in the Australian Quarantine and Inspection Service (AQIS). There are no specific antivenoms to the venoms of uncommon Australian snakes, but neutralisation is provided by polyvalent antivenom or by monovalent antivenom, as indicated by the VDK. Exotic snake antivenoms are maintained by Venom Supplies Ltd, Tanunda, South Australia (Tel: 08 8563 0001), Royal Melbourne Hospital (Tel: 03 9342 7000), Royal Adelaide Hospital (Tel: 08 8223 4000), Australian Reptile Park (Tel: 02 4340 1022) and by Taronga Zoo (Mosman, Tel: 02 9969 2777). The locations of antivenoms in Australia for treatment of bites by specific exotic snakes are maintained by AVRU at http://www.avru.org/reference/reference_avhold.html.
LONG-TERM EFFECTS OF SNAKE BITE
After appropriate treatment, recovery is expected but it may be slow, taking many weeks or months, particularlyfrom a critical illness or after delayed presentation with neurotoxicity and rhabdomyolysis. Isolated neurological or ophthalmic signs may persist. Long-term loss of taste or smell occurs occasionally.
SPIDERS
Although several thousand species of spiders exist in Australia, only Funnel-web Spiders (genera Atrax and Hadronyche) and the Red-back Spider (Latrodectus hasselti) have caused death or significant systemic illness. All spiders have venom and a few, particularly the White-tailed Spider (Lampona cylindrata) and the Common Black House Spider (Badumna insignis), have caused severe local injury, although this occurs rarely.1,12,13 Causes for ulcerated skin lesions other than spider bites should be sought.
FUNNEL-WEB SPIDERS
Many species of the Funnel-web genera Atrax and Hadronyche inhabit Queensland, New South Wales, Victoria, Tasmania and South Australia but only spiders from New South Wales and southern Queensland have caused significant illness and death. These are large dark-coloured aggressive spiders. A systematic review involving 138 cases identified 77 cases of severe envenomation with 13 deaths, but none occurred after introduction of antivenom in 1981 and the vast majority (97%) responded to antivenom therapy.14 All deaths were attributed to the Sydney Funnel-web Spider (A. robustus)1 which inhabits an area within an approximate 160 km radius of Sydney and is inclined to roam after rainfall, enter houses and seek shelter among clothes or bedding, giving a painful bite when disturbed.
RED-BACK SPIDER
If the effects of a bite are trivial and confined to the bite site, antivenom may be withheld; otherwise, antivenom should be given intramuscularly. The antivenom may be given intravenously in cases of refractory pain but the risk of anaphylaxis may be higher than by the intramuscular route which is very low (< 0.5%). A premedication with promethazine is recommended and epinephrine should be at hand. In contrast to a bite from a snake or Funnel-web Spider, a bite from a Red-back Spider is not immediately life threatening. There is no proven effective first aid, but application of a cold pack or iced water may help relieve pain. Bites by Steatoda spp. (cupboard spiders) may cause a similar syndrome and can be treated effectively with Red-back Spider antivenom.15
BOX JELLYFISH
This creature, Chironex fleckeri, is probably the most venomous in the world. It has caused at least 63 deaths in the waters off northern Australia.16 It has a cuboid bell up to 30 cm in diameter. Numerous tentacles arise from the corners of the bell and trail several metres. It is semitransparent and difficult to see by anyone wading or swimming in shallow water. The tentacles are lined with millions of nematocysts which, on contact with skin, dischargea threaded barb which pierces subcutaneous tissue, including small blood vessels. Contact with the tentacles causes severe pain and envenomation, from which death may occur within minutes. Death is probably due to both neurotoxic effects causing apnoea and direct cardiotoxicity, although the precise mode of action of the venom is unknown. In mechanically ventilated animals, fatal hypotension occurs rapidly on envenomation.17 The skin which sustains the injury may heal with disfiguring scars.
IRUKANDJI
Its sting is mild and marked only by a small area of erythema. However, severe general illness may follow with abdominal cramps, hypertension, back pain, nausea and vomiting, limb cramps, chest tightness and marked distress.18 Occasionally, cardiogenic pulmonary oedema has necessitated mechanical ventilation and inotropic therapy, while hypertension has caused two deaths by stroke. The mechanism is uncertain, but experimental studies in animals have shown that Irukandji extracts cause a hyperadrenergic syndrome secondary to massive release of catecholamines19 which may explain at least in part the cause of heart failure. Pain relief is the most important feature of management in mild to moderate cases. In a series of 10 victims with ‘Irukandji syndrome’, intravenous magnesium salts provided pain relief and a reduction in blood pressure.20 Antihypertensive therapy with phentolamine or a ‘titratable’ nitrate may be required in the initial phase of management.
BEES, WASPS AND ANTS
Anaphylactic reactions to bee and wasp stings cause approximately the same number of deaths in Australia each year as do snake bites at an average of 2.3 per annum.20 The common European Honey Bee (Apis mellifera) is largely responsible. Jumper and Bull Ants (Myrmecia spp.) may also cause anaphylaxis. Persons who develop reactions to bites should seek immunotherapy and carry injectable epinephrine.
1 Sutherland SK, Tibballs J. Australian Animal Toxins. Melbourne: Oxford University Press, 2001.
2 Pearn JH, Covacevich J, Charles N, et al. Snakebite in herpetologists. Med J Aust. 1994;161:706-708.
3 Tibballs J, Sutherland S, Kerr S. Studies on Australian snake venoms. Part I: the haemodynamic effects of Brown Snake (Pseudonaja) species in the dog. Anaesth Intensive Care. 1989;17:466-469.
4 Tibballs J. The cardiovascular, coagulation and haematological effects of Tiger Snake (Notechis scutatus) venom. Anaesth Intensive Care. 1998;26:529-535.
5 Sutherland SK, Coulter AR, Harris RD. Rationalization of first-aid measures for elapid snakebite. Lancet. 1979;1:183-186.
6 Howarth DM, Southee AE, Whyte IM. Lymphatic flow rates and first-aid in simulated peripheral snake or spider envenomation. Med J Aust. 1994;161:695-700.
7 Oakley J. Managing death adder bite with prolonged pressure bandaging. 6th Asia–Pacific Congress on Animal, Plant and Microbial Toxins and 11th Annual Scientific Meeting of the Australasian College of Tropical Medicine, 8–12 July 2002. Cairns Colonial Club, Queensland, Australia, 2002;29.
8 Premawardhena AP, de Silva CE, Fonseka M, et al. Low dose subcutaneous adrenaline to prevent acute adverse reactions to antivenom serum in people bitten by snakes: randomised, placebo controlled trial. BMJ. 1999;318:1041-1043.
9 Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 2000;30:1144-1150.
10 Fan HW, Marcopito LF, Cardoso JL, et al. A sequential randomised and double blind trial of promethazine prophylaxis against early anaphylactic reactions to antivenom for Bothrops snake bites. BMJ. 1999;318:1451-1453.
11 Tibballs J, Sutherland SK. The efficacy of heparin in the treatment of Common Brown Snake (Pseudonaja textilis) envenomation. Anaesth Intensive Care. 1992;20:33-37.
12 Isbister GK, Gray MR. White-tail spider bite: a prospective study of 130 definite bites by Lampona species. Med J Aust. 2003;179:199-202.
13 Pincus SJ, Winkel KD, Hawdon GM, et al. Acute and recurrent skin ulceration after spider bite. Med J Aust. 1999;171:99-102.
14 Isbister GK, Gray MR, Balit CR, et al. Funnel-web spider bite: a systematic review of recorded clinical cases. Med J Aust. 2005;182:407-411.
15 Isbister GK, Gray MR. Effects of envenoming by comb-footed spiders of the genera Steatoda and Achaearanea (family Theridiidae: Araneae) in Australia. J Toxicol Clin Toxicol. 2003;41:809-819.
16 Williamson JA, Fenner PJ, Burnett JW, et al. Venomous and Poisonous Marine Animals. Sydney: University of New South Wales Press, 1996.
17 Tibballs J, Williams D, Sutherland SK. The effects of antivenom and verapamil on the haemodynamic actions of Chironex fleckeri (Box jellyfish) venom. Anaesth Intensive Care. 1998;26:40-45.
18 Little M, Mulcahy RF. A year’s experience of Irukandji envenomation in far north Queensland. Med J Aust. 1998;169:638-641.
19 Winkel KD, Tibballs J, Molenaar P, et al. Cardiovascular actions of the venom from the Irukandji (Carukia barnesi) jellyfish: effects in human, rat and guinea-pig tissues in vitro and in pigs in vivo. Clin Exp Pharmacol Physiol. 2005;32:777-788.
20 Corkeron M, Pereira P, Makrocanis C. Early experience with magnesium administration in Irukandji syndrome. Anaesth Intensive Care. 2004;32:666-669.
21 Levick NR, Schmidt JO, Harrison J, et al. Review of bee and wasp sting injuries in Australia and the USA. In: Austin AD, Dowton M, editors. Hymenoptera. Melbourne: CSIRO Publishing, 2000.