Chapter 144 Enucleation for Choroidal Melanomas
Introduction
Approximately 5% of all melanomas occur in the eye and surrounding adnexal structures and 85% of these are uveal in origin.1 Uveal melanomas are the most common primary intraocular malignant tumor.2 According to one report, the overall incidence of uveal melanomas is 5.1 per million and 80–90% of uveal melanomas involve the choroid.3
Over 30 years ago, Zimmerman investigated the benefit of enucleation in eyes with choroidal melanoma.4 He observed a peak in mortality 2–3 years after enucleation and suggested that the rise of post-enucleation mortality was a direct result of the enucleation.4 This controversy led to the trend away from enucleation towards vision and eye-sparing treatments. Subsequent studies have demonstrated that the observed post-enucleation rise in mortality was a reflection of natural history of the primary tumor and its metastases rather than direct effects of enucleation causing iatrogenic tumor seeding. Metastasis was independent of the method of treatment.5
For the treatment of the primary tumor, there are many therapeutic options such as observation, transpupillary thermotherapy, plaque radiotherapy, local resection, and enucleation.2 In the USA, the two most frequently used methods of treatment are plaque radiotherapy and enucleation.2 The COMS demonstrated no statistical difference in all-cause mortality between the brachytherapy and enucleation arms in either medium or large choroidal melanomas.6
As mortality data show, no significant difference between enucleation and brachytherapy, quality-of-life (QOL) measurements become important in deciding on a therapeutic plan. The Collaborative Ocular Melanoma Study (COMS) ascertained QOL measures of driving difficulties, near vision activities, activities requiring stereopsis, anxiety levels, and depression levels.7 Patients reported higher levels of function for driving and peripheral vision in the brachytherapy arm compared to the enucleation arm for the first 2 years post-treatment but these differences diminished 3–5 years post-treatment.7 Patients in the brachytherapy arm also had more symptoms of anxiety after treatment compared with the enucleation group.7 Enucleation still remains a viable treatment option for choroidal melanoma in the proper setting.
The COMS group demonstrated a cumulative metastasis rate of 25% at 5 years and 34% at 10 years.8 Once metastasis occurs, the median survival time is 3.6 months, and death is inevitable as there are no effective treatment options at present.8,9
Purpose of enucleation
Implant description
An ideal implant should restore volume, transmit motility, possess a low complication rate, and demonstrate cost-effectiveness.10 To transmit motility, the extraocular muscles should attach to the implant directly or surround the implant indirectly.10 An implant of proper size restores volume.11 Current orbital implant designs fall into two general categories: solid spheres and porous, integrated implants. Solid spheres include silicone and polymethyl methacrylate (PMMA), while porous implants include coralline hydroxyapatite and porous polyethylene.10 Bioceramic and other implants also exist.10 Porous materials allow for fibrovascular ingrowth which may induce epithelialization of a drill hole to accept a prosthesis coupling peg to improve motility. Pegged hydroxyapatite implants offer subjective improved motility over unpegged implants and vascular ingrowth possesses some theoretical advantages, including decreased rates of infection, exposure, and extrusion.10,12
Coralline hydroxyapatite (HA) is a porous, nontoxic implant composed of inorganic, nonreplenishable marine coral.13 High-density porous polyethylene is a porous, synthetic implant that can be molded into various shapes.14 Hydroxyapatite has a brittle nature, which precludes direct suturing to the implant surface.15 Pore sizes and interconnectivity vary, which could affect vascularization.16 Some surgeons drill holes into porous implants to facilitate integration.17 The use of porous materials adds additional costs of the implant, wrapping material, and imaging studies, and possesses higher complication rates.18
Some surgeons wrap porous implants to protect the anterior tissues from hydroxyapatite bone spicules and to facilitate muscle attachment and implantation. However the perceived benefits have not been proven.15 Wrapping materials include donor sclera, autogenous fascia or polyglycolic acid mesh. While some surgeons wrap porous polyethylene implants, the extraocular muscles can be directly sutured to polyethylene.19,20 Wrapping materials may pose infection risk and decrease vascularization rates.17
When surveyed in 1995, the majority (56%) of the American Society of Ophthalmic Plastic and Reconstructive Surgeons (ASOPRS) preferred hydroxyapatite implants and human donor sclera wrapping in enucleations.21 When resurveyed in 2002, the majority (43%) of ASOPRS surgeons preferred porous polyethylene and only 27% preferred hydroxyapatite.18 In the later survey, most implants (60%) were not wrapped and only 25% were wrapped in human donor sclera.18 The majority (92%) of implants were left unpegged.18 In 2007, a survey from the UK supported these trends, demonstrating that 55% of surgeons used porous implants, 57% wrapped the implant (with autogenous sclera being most popular; 20%), and only 7% pegged the implants.22 Custer demonstrated no significant difference in motility between unpegged hydroxyapatite and solid implants.23 The potential advantages of using porous implants and wrapping materials should be weighed against the risks, morbidity, and additional cost.20 If the surgeon does not plan to peg, if the patient has systemic illnesses or factors that may limit fibrovascular ingrowth, or if a porous implant may not improve a patient’s quality of life, the surgeon should give consideration to a solid implant.
Implant sizing
Proper implant sizing is essential for cosmesis, volume restoration, and to minimize extrusion, exposure, and sulcus deformities. Most adult patients require at least a 20 mm implant. Traditionally, surgeons use a set of sizing spheres to individualize implant size and use standard volume tables to maximize volume restoration. Alternatively, orbital implant size can be determined using the contralateral eye axial length measurements subtracted by 2 mm, or by 3 mm for hyperopia.11 Another algorithm subtracts the volume of an ideal 24 mm eye by the volume of sclera and 2 mL to determine implant volume.24
Enucleation technique
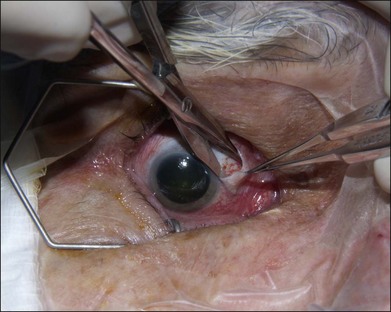
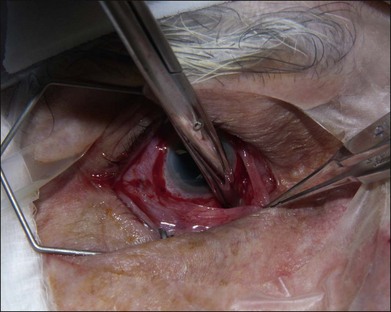
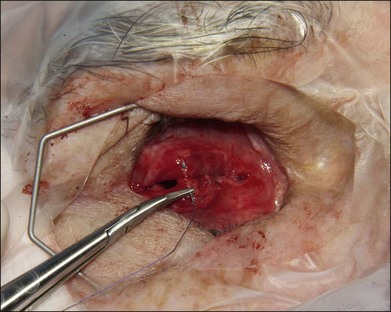
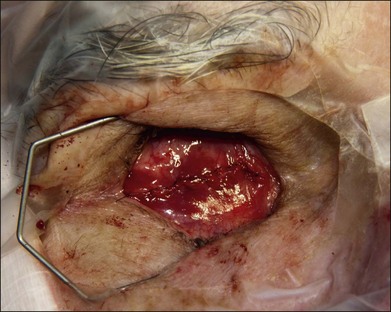
The authors’ technique is as follows: A 360° peritomy is created with blunt Westcott scissors. Conjunctiva and the Tenon layer are dissected from underlying sclera using Stevens scissors (Fig. 144.1). The Stevens scissors are used to bluntly dissect in each oblique quadrant (Fig. 144.2) and each rectus muscle is isolated on a muscle hook. The Tenon layer attachments to the rectus muscles are stripped and the muscle is secured on a double armed 6–0 polyglactin 910 suture through its insertion. Each muscle is disinserted from the globe using blunt Westcott scissors or Abley scissors (Fig. 144.3).
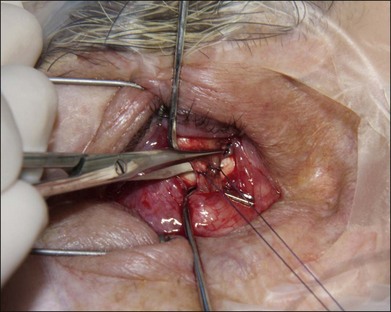
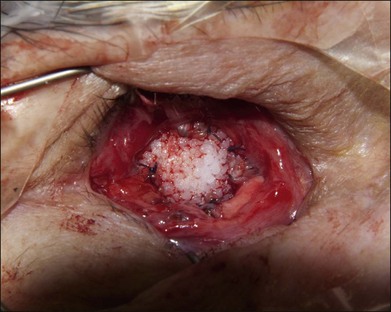
The orbital implant is placed using an injector or periosteal elevators with modest posterior digital pressure. If the implant is wrapped, then four windows can be created just posterior to the rectus muscle attachments. If a wrapping material is not employed, the muscles are sutured directly to or over the implant. Direct attachment of the vertical recti muscles to each other may lead to fornix insufficiency. We suture each rectus muscle to the adjacent rectus muscle suture using a 4–0 polyglactin 910 suture to create a physiologic attachment of the extraocular muscles over the implant with a diameter of approximately 10 mm (Fig. 144.4). The anterior Tenon layer is closed using a running 4–0 polyglactin 910 suture (Fig. 144.5). Finally, the conjunctiva is closed using a running 6–0 plain gut suture (Fig. 144.6).
Special considerations
Optic nerve invasion and limited extrascleral extension
Overall, choroidal melanoma rarely invades the optic nerve.25 The COMS demonstrated extrascleral extension in only 8.2% of 1527 enucleated eyes.26 Limited extrascleral extension does not contraindicate enucleation. However, the approach to the optic nerve transaction may have to be modified if there is evidence of limited extrascleral extension. Limited extrascleral extension may be suspected based on echographic or radiologic imaging.27 It is recommended that the quadrant opposite to the extrascleral extension be used to introduce the enucleation scissors so as to avoid transecting the extrascleral extension. In some cases, greater access to the posterior orbit may be obtained via lateral orbitotomy approach to transect the optic nerve and for en bloc removal of the extrascleral extension.28,29
Complications
Complications of enucleation are rare and include pyogenic granuloma, conjunctival inclusion cyst formation, conjunctival wound dehiscence causing exposure or extrusion, implant displacement or migration, infection, orbital hemorrhage, socket contracture, and ptosis.18,30 In the COMS large tumor trial, patients who received pre-enucleation radiation had an overall complication rate of 8% and patients who had not received radiation had a complication rate of 4%.30
Additional complications of pegged implants include peg malposition, peg cracking, and an audible peg click.18 Reports on overall complication rates vary widely for porous and nonporous implants and for pegged and unpegged implants. However, general trends among the various implant choices exist.
Nunery et al. demonstrated a higher exposure rate in HA versus silicone implants.31,32 Exposed porous implants may not extrude as often as solid implants because of fibrovascular ingrowth, but nearly all exposures ultimately require repair.31 Exposure rates range from 0% to 33.3% for porous implants.13,33–39 The American Society of Ophthalmic Plastic and Reconstructive Surgery (ASOPRS) survey revealed an exposure rate of only 3% with the majority (81 of 82) of events occurring with porous implants rather than solid implants.18 A meta-analysis of exposure rates of all implants demonstrated a 1.3% (2/153 cases) rate with silicone, a 4.9% rate (96/1959 cases) with coralline hydroxyapatite, and an 8.1% rate (41/507 events) with porous polyethylene.34 For pegged implants, all complication rates are greater, including pyogenic granuloma formation (14%), exposure (6%), infection (5%), and peg malposition (5%).18
1 Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664–1678.
2 Singh AD, Bergman L, Seregrard S, et al. Clin Ophthal Oncol. 2007:198–204.
3 Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118:1881–1885.
4 Zimmerman LE, McLean IW, Foster WD. Does enucleation of the eye containing a malignant melanoma prevent or accelerate the dissemination of tumour cells. Br J Ophthalmol. 1978;62:420–425.
5 Singh AD, Rennie IG, Kivela T, et al. The Zimmerman-McLean-Foster hypothesis: 25 years later. Br J Ophthalmol. 2004;88:962–967.
6 Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch Ophthalmol. 2001;119:670–676.
7 Melia M, Moy CS, Reynolds SM, et al. Quality of life after iodine 125 brachytherapy vs enucleation for choroidal melanoma: 5-year results from the Collaborative Ocular Melanoma Study: COMS QOLS Report No. 3. Arch Ophthalmol. 2006;124:226–238.
8 Diener-West M, Reynolds SM, Agugliaro DJ, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005;123:1639–1643.
9 Augsburger JJ, Correa ZM, Shaikh AH. Effectiveness of treatments for metastatic uveal melanoma. Am J Ophthalmol. 2009;148:119–127.
10 Chalasani R, Poole-Warren L, Conway RM, et al. Porous orbital implants in enucleation: a systematic review. Surv Ophthalmol. 2007;52:145–155.
11 Kaltreider SA, Lucarelli MJ. A simple algorithm for selection of implant size for enucleation and evisceration: a prospective study. Ophthal Plast Reconstr Surg. 2002;18:336–341.
12 Shields CL, Shields JA, De Potter P. Hydroxyapatite orbital implant after enucleation. Experience with initial 100 consecutive cases. Arch Ophthalmol. 1992;110:333–338.
13 Shields JA, Shields CL, De Potter P. Hydroxyapatite orbital implant after enucleation–experience with 200 cases. Mayo Clin Proc. 1993;68:1191–1195.
14 Bilyk JR, Rubin PA, Shore JW. Correction of enophthalmos with porous polyethylene implants. Int Ophthalmol Clin. 1992;32:151–156.
15 Custer PL. Enucleation: past, present, and future. Ophthal Plast Reconstr Surg. 2000;16:316–321.
16 Mawn LA, Jordan DR, Gilberg S. Scanning electron microscopic examination of porous orbital implants. Can J Ophthalmol. 1998;33:203–209.
17 Gayre GS, Lipham W, Dutton JJ. A comparison of rates of fibrovascular ingrowth in wrapped versus unwrapped hydroxyapatite spheres in a rabbit model. Ophthal Plast Reconstr Surg. 2002;18:275–280.
18 Su GW, Yen MT. Current trends in managing the anophthalmic socket after primary enucleation and evisceration. Ophthal Plast Reconstr Surg. 2004;20:274–280.
19 Moshfeghi DM, Moshfeghi AA, Finger PT. Enucleation. Surv Ophthalmol. 2000;44:277–301.
20 Perry JD, Tam RC. Safety of unwrapped spherical orbital implants. Ophthal Plast Reconstr Surg. 2004;20:281–284.
21 Hornblass A, Biesman BS, Eviatar JA. Current techniques of enucleation: a survey of 5,439 intraorbital implants and a review of the literature. Ophthal Plast Reconstr Surg. 1995;11:77–88.
22 Viswanathan P, Sagoo MS, Olver JM. UK national survey of enucleation, evisceration and orbital implant trends. Br J Ophthalmol. 2007;91:616–619.
23 Custer PL, Trinkaus KM, Fornoff J. Comparative motility of hydroxyapatite and alloplastic enucleation implants. Ophthalmology. 1999;106:513–516.
24 Custer PL, Trinkaus KM. Volumetric determination of enucleation implant size. Am J Ophthalmol. 1999;128:489–494.
25 Shields CL, Santos MC, Shields JA, et al. Extraocular extension of unrecognized choroidal melanoma simulating a primary optic nerve tumor: report of two cases. Ophthalmology. 1999;106:1349–1352.
26 Histopathologic characteristics of uveal melanomas in eyes enucleated from the Collaborative Ocular Melanoma Study. COMS report no. 6. Am J Ophthalmol. 1998;125:745–766.
27 Boldt HC, Byrne SF, Gilson MM, et al. Baseline echographic characteristics of tumors in eyes of patients enrolled in the Collaborative Ocular Melanoma Study: COMS report no. 29. Ophthalmology. 2008;115:1390–1397.
28 De Potter P, Shields JA, Shields CL, et al. Modified enucleation via lateral orbitotomy for choroidal melanoma with orbital extension: a report of two cases. Ophthal Plast Reconstr Surg. 1992;8:109–113.
29 Singh AD, Jacques R, Rundle PA, et al. Combined enucleation and orbitotomy for choroidal melanoma with orbital extension. Eye (Lond). 2006;20:615–617.
30 The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma III: local complications and observations following enucleation COMS report no. 11. Am J Ophthalmol. 1998;126:362–372.
31 Nunery WR, Heinz GW, Bonnin JM, et al. Exposure rate of hydroxyapatite spheres in the anophthalmic socket: histopathologic correlation and comparison with silicone sphere implants. Ophthal Plast Reconstr Surg. 1993;9:96–104.
32 Nunery WR, Cepela MA, Heinz GW, et al. Extrusion rate of silicone spherical anophthalmic socket implants. Ophthal Plast Reconstr Surg. 1993;9:90–95.
33 Dutton JJ. Coralline hydroxyapatite as an ocular implant. Ophthalmology. 1991;98:370–377.
34 Custer PL, Trinkaus KM. Porous implant exposure: Incidence, management, and morbidity. Ophthal Plast Reconstr Surg. 2007;23:1–7.
35 Jordan DR, Gilberg S, Bawazeer A. Coralline hydroxyapatite orbital implant (bio-eye): experience with 158 patients. Ophthal Plast Reconstr Surg. 2004;20:69–74.
36 Buettner H, Bartley GB. Tissue breakdown and exposure associated with orbital hydroxyapatite implants. Am J Ophthalmol. 1992;113:669–673.
37 Karesh JW, Dresner SC. High-density porous polyethylene (Medpor) as a successful anophthalmic socket implant. Ophthalmology. 1994;101:1688–1696.
38 Blaydon SM, Shepler TR, Neuhaus RW, et al. The porous polyethylene (Medpor) spherical orbital implant: a retrospective study of 136 cases. Ophthal Plast Reconstr Surg. 2003;19:364–371.
39 Kim JH, Khwarg SI, Choung HK, et al. Management of porous polyethylene implant exposure in patients with retinoblastoma following enucleation. Ophthalmic Surg Lasers Imaging. 2004;35:446–452.