Chapter 87 Enteral and parenteral nutrition
It is standard practice to provide nutritional support to critically ill patients, in order to
However, despite the universality of this practice, the evidence underlying it is often conflicting and of disappointingly poor quality.1 The failings in the evidence seem to extend to some of the resulting debates, in which certainty appears inversely proportional to justification.2 Inevitably, these difficulties have led many to seek clarity in meta-analysis; perhaps equally inevitably,3 they have usually been disappointed. On so basic a question as the relative merits of enteral and parenteral routes of feeding, the two most recent meta-analyses have produced conflicting results.4,5 Instead of choosing on which trials to base patient care, it seems the clinician must now decide which meta-analysis to believe.
The problem persists with the publication of numerous clinical practice guidelines,6–11 which differ in important areas, although one at least has been used in a cluster randomised trial showing a 10% reduction in mortality that just failed to reach statistical significance in those intensive care units (ICUs) randomised to use the guideline – the ACCEPT study.6 While such validation does not mean that each component of the ACCEPT guideline (Figure 87.1) is optimal, it does at least provide some support.
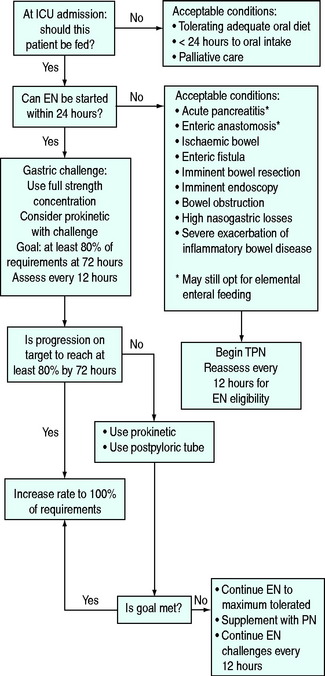
Figure 87.1 Algorithm for nutritional support used in the ACCEPT trial.6 EN, enteral nutrition, PN, parenteral nutrition.
NUTRITIONAL ASSESSMENT
Objective assessment of nutritional status is difficult in ICU, because disease processes confound methods used in the general population. Anthropometric measures such as triceps skin-fold thickness and mid-arm circumference may be obscured by oedema. Voluntary handgrip strength, a test of functional capacity, is impractical in unconscious patients. Laboratory measures, including transferrin, pre-albumin and albumin levels, lymphocyte counts and skin-prick test reactivity, are abnormal in critical illness. Clinical evaluation – the so-called Subjective Global Assessment – is better than objective measurement at predicting morbidity.12 Historical features of malnutrition include weight loss, poor diet, gastrointestinal symptoms, reduced functional capacity and a diagnosis associated with poor intake. Physical signs include loss of subcutaneous fat, muscle wasting, peripheral oedema and ascites.
While laboratory measures are of little value in assessing nutritional status in critically ill patients, intensivists are increasingly involved in preoperative assessment of patients undergoing major surgery. In these patients the serum albumin and operative site were closely associated with the risk of postoperative complications.13 This raises the possibility that outcomes may be improved by treating preoperative malnutrition identified by a simple screening test.
PATIENT SELECTION AND TIMING OF SUPPORT
There are reasonable grounds to believe that it is better to provide nutritional support to critically ill patients than not to do so. This belief is based on the close association between malnutrition, negative nitrogen and calorie balance and poor outcome, and the inevitability of death if starvation continues for long enough. In otherwise healthy humans this takes several weeks to occur. There is also some direct evidence from small studies of parenteral nutrition in patients with severe head injuries14 and of jejunal feeding in those operated on for severe pancreatitis,15 in which the control groups received little or no nutritional support. Both studies showed decreased mortality in the groups receiving adequate nutrition.
Quite good evidence now supports the early institution of nutritional support, and the trend is both to tolerate much shorter periods without nutrition and to begin feeding more rapidly after initial resuscitation.
In 1997, recommendations from a conference sponsored by the US National Institutes of Health, the American Society for Parenteral and Enteral Nutrition and the American Society for Clinical Nutrition suggested that nutritional support be started in any critically ill patient unlikely to regain oral intake within 7–10 days.10 The basis for this was that at a typical nitrogen loss of 20–40 g/day dangerous depletion of lean tissue may occur after 14 days of starvation. Others have suggested a maximum acceptable delay of 7 days. A meta-analysis comparing early (first 48 hours after admission to ICU) with late enteral feeding revealed a reduction in infectious complications.16 Two subsequent meta-analyses comparing early enteral feeding with no artificial nutritional support, and early parenteral feeding with delayed enteral feeding, both found a reduction in mortality with early support.17 Finally, early institution of enteral feeding was an important component of the ACCEPT study guideline.6 Patients in this study received nutritional support if they were thought unlikely to tolerate oral intake in the next 24 hours, and the goal was to start feeding within 24 hours of admission to ICU. The weight of evidence is presently in favour of this more aggressive approach.18
NUTRITIONAL REQUIREMENTS OF THE CRITICALLY ILL
ENERGY
Some muscle wasting and nitrogen loss are unavoidable in critical illness, despite adequate energy and protein provision.19 This fact, coupled with the realisation that caloric requirements had previously been overestimated, has led to downward revision of intake, a process which may still be ongoing. In 1997, the American College of Chest Physicians (ACCP) published guidelines recommending a daily energy intake of 25 kcal/kg,9 and this has remained the standard target energy intake for critically ill patients. More recently, concerns have been raised that this may be excessive. An observational study found lower mortality in those patients who received 9–18 kcal/kg/day than in those with higher and lower intakes.20 However, meaningful benefits of hypocaloric feeding have yet to be demonstrated in prospective trials. It is, moreover, extremely important to realise that enterally fed patients frequently fail to achieve their target intake, and that significant underfeeding is certainly associated with worse outcomes.20–22
Indirect calorimetry is the gold standard, and its use is becoming easier with the availability of devices designed for ICU patients. It permits measurement of the resting energy expenditure (REE). This value excludes the energy cost of physical activity, which increases later in the course of an ICU admission.23 Calorimetry reveals deviations from values predicted by equations, such that two thirds of patients in one study were being either under- or overfed.24 On the other hand, it could not be shown that outcomes are improved by the use of calorimetry.25 Moreover, there are no clear data to relate measured REE to total energy expenditure in the individual patient. As a result, many units do not use calorimetry; in those that do, a target energy provision of 1.3 × measured REE is usual.22
There are a large number of equations claiming to predict basal metabolic rate (BMR) on the basis of weight, sex and age. The best known is the Harris–Benedict equation, which dates back more than 80 years. Schofield’s equations were derived anew in the 1980s.26 Correction factors exist to convert predictions of BMR into estimated energy expenditure by adjusting for such variables as diagnosis, pyrexia and activity. In the past these correction factors have been excessive and may have contributed to overfeeding; a more conservative approach is now advocated. The recommendations of the British Association for Parenteral and Enteral Nutrition are:27
Table 87.1 Basal metabolic rate in kcal/day by age and gender26
| Age | Female | Male |
|---|---|---|
| 15–18 | 13.3 W + 690 | 17.6 W + 656 |
| 18–30 | 14.8 W + 485 | 15.0 W + 690 |
| 30–60 | 8.1 W + 842 | 11.4 W + 870 |
| > 60 | 9.0 W + 656 | 11.7 W + 585 |
W = weight in kg.
Table 87.2 Stress adjustment in the calculation of basal metabolic rate27
| Partial starvation (> 10% weight loss) | Subtract 0–15% |
| Mild infection, inflammatory bowel disease, postoperative | Add 0–13% |
| Moderate infection, multiple long bone fractures | Add 10–30% |
| Severe sepsis, multiple trauma (ventilated) | Add 25–50% |
| Burns 10–90% | Add 10–70% |
PROTEIN
Assessment of nitrogen balance by measuring urinary urea nitrogen is too variable to be useful in estimating protein requirements in ICU.28 As there is an upper limit to the amount of dietary protein that can be used for synthesis,29 there is no benefit from replacing nitrogen lost in excess of this. A daily nitrogen provision of 0.15–0.2 g/kg per day is therefore recommended for the ICU population; this is equivalent to 1–1.25 g protein/kg per day. Severely hypercatabolic individuals, such as those with major burns, are given up to 0.3 g nitrogen/kg per day, or nearly 2 g protein/kg per day.27
MICRONUTRIENTS
Critical illness increases the requirements for vitamins A, E, K, thiamine (B1), B3, B6, vitamin C and pantothenic and folic acids.30 Thiamine, folic acid and vitamin K are particularly vulnerable to deficiency during total parenteral nutrition (TPN). Renal replacement therapy can cause loss of water-soluble vitamins and trace elements. Deficiencies of selenium, zinc, manganese and copper have been described in critical illness, in addition to the more familiar iron-deficient state. Subclinical deficiencies in critically ill patients are thought to cause immune deficiency and reduced resistance to oxidative stress. Suggested requirements for micronutrients in critically ill patients vary between authors and depending on route of administration; the most comprehensive guidance30 is reproduced in Tables 87.3 and 87.4. More recent but broadly similar recommendations for some compounds are also available.31
| Vitamin | Function | Dose |
|---|---|---|
| Vitamin A | Cell growth, night vision | 10000–25000 IU |
| Vitamin D | Calcium metabolism | 400–1000 IU |
| Vitamin E | Membrane antioxidant | 400–1000 IU |
| β-Carotene* | Antioxidant | 50 mg |
| Vitamin K | Activation of clotting factors | 1.5 μg/kg per day |
| Thiamine (vitamin B1) | Oxidative decarboxylation | 10 mg |
| Riboflavin (vitamin B2) | Oxidative phosphorylation | 10 mg |
| Niacin (vitamin B3) | Part of NAD, redox reactions | 200 mg |
| Pantothenic acid | Part of coenzyme A | 100 mg |
| Biotin | Carboxylase activity | 5 mg |
| Pyridoxine (vitamin B6) | Decarboxylase activity | 20 mg |
| Folic acid | Haematopoiesis | 2 mg |
| Vitamin B12 | Haematopoiesis | 20 μg |
| Vitamin C | Antioxidant, collagen synthesis | 2000 mg |
Table 87.4 Trace element requirements in critical illness30
| Element | Function | Dose |
|---|---|---|
| Selenium | Antioxidant, fat metabolism | 100 μg |
| Zinc | Energy metabolism, protein synthesis, epithelial growth | 50 mg |
| Copper | Collagen cross-linking, ceruloplasmin | 2–3 mg |
| Manganese | Neural function, fatty acid synthesis | 25–50 mg |
| Chromium | Insulin activity | 200 mg |
| Cobalt | B12 synthesis | |
| Iodine | Thyroid hormones | |
| Iron | Haematopoiesis, oxidative phosphorylation | 10 mg |
| Molybdenum | Purine and pyridine metabolism | 0.2–0.5 mg |
ROUTE OF NUTRITION
When possible patients should be fed enterally. The advantages over the parenteral route are:
These appear to be the only advantages of the enteral route. Despite the fervour with which some pursue the debate,2 there is little basis for the widespread belief that the enteral route provides a clear benefit in terms of outcome, and that the advantages of enteral feeding are unassailable.
Two hypotheses are commonly advanced in support of the putative superiority of enteral feeding. First, it appears that the lipid contained within TPN is immunosuppressive. Intravenous lipid is known to suppress neutrophil and reticuloendothelial system function, and a comparison of TPN with and without lipid in critically ill trauma patients showed a lower complication rate in those not receiving lipid.32 Second, enteral feeding may protect against infective complications. Absence of complex nutrients from the intestinal lumen is followed in rats by villus atrophy and reduced cell mass of the gut-associated lymphoid tissue (GALT). Starved humans show these changes to a much lesser extent. Lymphocytes produced in the GALT are redistributed to the respiratory tract, and contribute heavily to mucosal immunity. In mice, this contribution is lost during TPN.
The possibility that multiple organ failure may be driven by translocation of bacteria or endotoxin across an impaired mucosal barrier has been extensively investigated in animals. While it is known that TPN is associated with increased gut permeability to macromolecules in humans, this does not seem to result in translocation.33 Although translocation does occur following surgery, and seems to be associated with sepsis;34 a causal relation with multiple organ failure is unproven. In fact, a reduction in septic morbidity has only been found in certain groups, primarily abdominal trauma victims,35,36 in whom parenteral nutrition was associated with a higher incidence of abdominal abscess and pneumonia. A third study found no difference.37 In head-injured patients there is one trial showing no effect and one each supporting either route; however in the study favouring TPN the enteral nutrition group were grossly underfed.14,38,39
None of these studies is less than 15 years old, and the techniques of both enteral and parenteral feeding have changed a great deal in that time. Reductions in septic complications have also been found using enteral feeding in pancreatitis.40 In contrast, no benefit was found in sepsis, though enteral feeding was instituted late.41 A review of 31 clinical trials comparing enteral with parenteral feeding found no consistent difference.42
More recent systematic analyses have, as mentioned earlier, produced conflicting results. One found a reduction in infectious complications with enteral feeding, but no difference in mortality.5 The most recent and most robust meta-analysis considered only high-quality trials using an intention-to-treat principle. It showed a clear reduction in mortality in patients fed parenterally.4
The same authors performed a prospectively defined subgroup analysis comparing early enteral with parenteral feeding, which showed no difference in mortality. Another recent meta-analysis of this question reached the same conclusion.43 On this basis, as in the ACCEPT study, the authors recommended early use of the enteral route, with rapid recourse to parenteral nutrition if this was not possible.6,17 At present it appears that timing of nutritional support matters as much as the route used.
A well-powered randomised study comparing early parenteral with enteral feeding will soon start.18 However, in view of the practical and financial advantages of enteral feeding, it will probably need to find a significant mortality difference in favour of the parenteral route if it is to change the present preference for enteral feeding.
ENTERAL NUTRITION
ACCESS
Nasojejunal tubes are beneficial if impaired gastric emptying is refractory to prokinetic agents6 (see below) or in pancreatitis. Spontaneous passage through the pylorus following blind placement is not reliable, but may be increased by the administration of single doses of 200 mg erythromycin or 20 mg metoclopramide.44 However endoscopic or fluoroscopic assistance is needed for truly reliable transpyloric tube placement, although new developments in tube technology may soon obviate the logistic difficulties these methods entail. Unsurprisingly, two meta-analyses of various inconclusive studies have produced conflicting results on the question of whether nasojejunal feeding reduces the risk of aspiration or ventilator-associated pneumonia.7,45 At present, the uncertain quality of the evidence concerning their benefits, coupled with the cost and logistic difficulty of placing them, precludes the routine use of nasojejunal tubes for all patients.
REGIMEN
Slowly building up the rate of feeding is not proven to avoid diarrhoea or high gastric residual volumes. Head-injured patients fed with target intake from the outset have fewer infective complications.46 Nonetheless, it is presently common practice to start delivering around 30 ml/hour and build up to the target intake depending on tolerance, as judged by gastric residual volumes. These are assessed by aspiration of the tube every 4 hours. Gastric residual volumes over 150 ml on two successive occasions have been associated with an increased incidence of ventilator-associated pneumonia;47 contrastingly, others have found no link between high residual volumes and the risk of aspiration.48 Nevertheless, if the residual volume is consistently greater than 200 ml, treatment with prokinetic agents (metoclopramide 10 mg every 8 hours or erythromycin 250 mg every 12 hours intravenously) appears to increase tolerance of feeding, though there is no discernible effect on mortality or morbidity.44 In refractory cases a nasojejunal tube often permits successful enteral feeding, because small bowel function is resumed quicker than gastric emptying. A nasogastric tube is still needed to drain the stomach. Diarrhoea, abdominal distension, nausea and vomiting may suggest intolerance, despite low gastric volumes. Absence of bowel sounds is common in ventilated patients and should not be taken to indicate ileus.
COMPOSITION
Electrolyte composition varies widely, with sodium- and potassium-restricted formulations available. Vitamins and trace elements are usually added by the manufacturers so that daily requirements are present in a volume containing roughly 2000 kcal. The possible benefits of providing additional doses of some of these substances to critically ill patients are considered below.
COMPLICATIONS
Enteral feeding is an independent risk factor for ventilator-associated pneumonia.49 Sinusitis due to nasogastric intubation may necessitate changing to an orogastric tube. Fine-bore tubes are vulnerable to misplacement in the trachea or to perforation of the pharynx, oesophagus, stomach or bowel. Percutaneous endoscopic gastrostomy is associated with a high 30-day all-cause mortality in acutely ill patients, in whom it may be best avoided.50 Other complications include insertion site infection, serious abdominal wall infection and peritonitis. Surgically placed jejunostomies can cause similar problems, and may also obstruct the bowel.
PARENTERAL NUTRITION
In ICU patients the daily requirements are infused continuously over 24 hours. Careful biochemical and clinical monitoring is important, particularly at the outset (Table 87.6).
Table 87.6 Minimum monitoring during total parenteral nutrition. Less stable patients may require more intensive surveillance
| Nursing | Temperature |
| Pulse | |
| Blood pressure | |
| Respiratory rate | |
| Fluid balance | |
| Blood sugar (4 hourly when commencing feed) | |
| Daily (at least) | Review of fluid balance |
| Review of nutrient intake | |
| Blood sugar | |
| Urea, electrolytes and creatinine | |
| Weekly (at least) | Full blood picture |
| Coagulation screen | |
| Liver function tests | |
| Magnesium, calcium and phosphate | |
| Weight | |
| As indicated | Zinc |
| Uric acid |
ACCESS
The major concern with central venous access for TPN is prevention of infection. The following considerations apply:51
COMPLICATIONS
Parenteral nutrition has the potential for severe complications.
NUTRITION AND SPECIFIC DISEASES
LIVER DISEASE10,52
Energy requirements in ICU patients are not altered by the presence of chronic liver disease. Lipolysis is increased, so lipid must be used with caution to avoid hypertriglyceridaemia (not more than 1 g/kg per day). Protein restriction may be required in chronic hepatic encephalopathy; starting with 0.5 g/kg per day, the dose may be cautiously increased towards a normal intake. Hepatic encephalopathy may in part be due to depletion of branched-chain amino acids (BCAAs) permitting increased cerebral uptake of aromatic amino acids, which produce inhibitory neurotransmitters. In protein-intolerant patients the use of feeds enriched with BCAAs may permit greater protein intake without worsening encephalopathy. Their routine use is not indicated.17 Thiamine and fat-soluble vitamin deficiencies are common in patients with chronic liver disease.
ACUTE PANCREATITIS
Until recently, TPN was a cornerstone of the management of severe acute pancreatitis to minimise pancreatic stimulation. This has changed with the publication of studies showing jejunal feeding to be safe, effective and associated with reductions in infective complications compared with TPN.40 Elemental feeds and pancreatic enzyme supplements are logical if malabsorption is a problem. Despite the shift towards enteral feeding of patients with pancreatitis, some can only be fed intravenously.
OBESITY
The possibility that obese patients specifically may benefit from hypocaloric feeding was assessed by comparing 40 obese patients fed target intakes of < 20 or 25–30 kcal/kg per day.53 Other than a reduction in ICU stay, no benefit was shown. At present there is insufficient evidence to justify feeding obese patients differently from others.
ADJUNCTIVE NUTRITION
GLUTAMINE
The evidence on glutamine supplementation in critical illness is as contradictory as in other areas of nutritional support. Reductions in infectious complications and length of ICU stay were shown in small early studies of enterally fed trauma and burns patients, but a much larger study in unselected ICU patients found no effect on any outcome.54 TPN solutions have historically contained no glutamine because of problems with stability and solubility. These are now being overcome by use of dipeptides, but clinical studies of intravenous glutamine supplementation during TPN have also been conflicting. One early trial in ICU patients requiring TPN showed a reduction in late mortality which only became apparent after 20 days, and which persisted at 6 months.55 There are suggestions from meta-analysts that there is now a demonstrable mortality benefit from higher doses of parenteral glutamine;56 this awaits confirmation in a prospective trial. In the meantime, it seems that the patients most likely to benefit are those requiring TPN for more than 10 days.55
SELENIUM
Selenium is crucial to the regulation of glutathione peroxidase, the major scavenging system for oxygen free radicals. Low plasma selenium levels are common in ICU patients, and a number of small studies have shown potential benefits, again mostly when larger doses are given intravenously. A very recent large randomised trial of selenium 1000 μg intravenously for 14 days has shown a reduction in mortality when patients breaching trial protocols were excluded, but the intention-to-treat analysis did not reach statistical significance.57 Parenteral selenium supplementation may well soon be proven to be beneficial in unselected ICU patients.
ANTIOXIDANT VITAMINS
Vitamins A, C and E are also involved in systemic defence against oxidant stress, and have been studied in various combinations and doses, with and without selenium. Of the trials not using selenium, only one has shown a reduction in deaths using large enteral doses of vitamins C and E; the control group, however, had a very high mortality rate.58 It is hard to recommend routine supplementation with antioxidant vitamins on present evidence.
ARGININE AND IMMUNONUTRITION
Arginine is a non-essential amino acid which acts as a precursor of nitric oxide, polyamines (important in lymphocyte maturation) and nucleotides. Animal studies suggest enhanced cell-mediated immunity and survival when arginine is supplemented. Several commercially available enteral feeding solutions combine omega-3 fatty acids, arginine, nucleotides and in one case glutamine to produce so-called immune-enhancing diets. They have been assessed in a number of trials, few of them in patient groups likely to be admitted to ICU outside North America. Meta-analysis has suggested reductions in hospital stay and infections, but not in mortality.59,60 Subgroup analysis revealed an increase in mortality when arginine supplementation was given to septic patients;60 interim safety assessment of a randomised controlled trial61 led to its early cessation when this finding was replicated in the subgroup of patients with sepsis, though this may have been a chance effect.17
1 Doig GS, Simpson F, Delaney A. A review of the true methodological quality of nutritional support trials conducted in the critically ill: time for improvement. Anesth Analg. 2005;100:527-533.
2 Marik PE, Pinsky M. Death by parenteral nutrition. Intensive Care Med. 2003;29:867-869.
3 LeLorier J, Gregoire G, Benhaddad A, et al. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med. 1997;337:536-542.
4 Simpson F, Doig GS. Parenteral vs. enteral nutrition in the critically ill patient: a meta-analysis of trials using the intention to treat principle. Intensive Care Med. 2005;31:12-23.
5 Gramlich L, Kichian K, Pinilla J, et al. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature. Nutrition. 2004;20:843-848.
6 Martin CM, Doig GS, Heyland DK, et al. Multicentre, cluster-randomized clinical trial of algorithms for critical-care enteral and parenteral therapy (ACCEPT). CMAJ. 2004;170:197-204.
7 Heyland DK, Dhaliwal R, Drover JW, et al. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. J Parenter Enteral Nutr. 2003;27:355-373.
8 Jacobs DG, Jacobs DO, Kudsk KA, et al. Practice management guidelines for nutritional support of the trauma patient. J Trauma. 2004;57:660-678.
9 Cerra FB, Benitez MR, Blackburn GL, et al. Applied nutrition in ICU patients. A consensus statement of the American College of Chest Physicians. Chest. 1997;111:769-778.
10 Klein S, Kinney J, Jeejeebhoy K, et al. Nutrition support in clinical practice: review of published data and recommendations for future research directions. Summary of a conference sponsored by the National Institutes of Health, American Society for Parenteral and Enteral Nutrition, and American Society for Clinical Nutrition. Am J Clin Nutr. 1997;66:683-706.
11 Kreymann KG, Berger MM, Deutz NE, et al. ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr. 2006;25:210-223.
12 Baker JP, Detsky AS, Wesson DE, et al. Nutritional assessment: a comparison of clinical judgement and objective measurements. N Engl J Med. 1982;306:969-972.
13 Kudsk KA, Tolley EA, DeWitt RC, et al. Preoperative albumin and surgical site identify surgical risk for major postoperative complications. J Parenter Enteral Nutr. 2003;27:1-9.
14 Rapp RP, Young B, Twyman D, et al. The favorable effect of early parenteral feeding on survival in head-injured patients. J Neurosurg. 1983;58:906-912.
15 Pupelis G, Selga G, Austrums E, et al. Jejunal feeding, even when instituted late, improves outcomes in patients with severe pancreatitis and peritonitis. Nutrition. 2001;17:91-94.
16 Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med. 2001;29:2264-2270.
17 Doig G, Simpson F. Evidence-based guidelines for nutritional support of the critically ill: results of a bi-national guideline development conference. Sydney: EvidenceBased.net; 2005.
18 Doig GS, Simpson F. Early enteral nutrition in the critically ill: do we need more evidence or better evidence? Curr Opin Crit Care. 2006;12:126-130.
19 Streat SJ, Beddoe AH, Hill GL. Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients. J Trauma. 1987;27:262-266.
20 Krishnan JA, Parce PB, Martinez A, et al. Caloric intake in medical ICU patients: consistency of care with guidelines and relationship to clinical outcomes. Chest. 2003;124:297-305.
21 Rubinson L, Diette GB, Song X, et al. Low caloric intake is associated with nosocomial bloodstream infections in patients in the medical intensive care unit. Crit Care Med. 2004;32:350-357.
22 Villet S, Chiolero RL, Bollmann MD, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. 2005;24:502-509.
23 Plank LD, Hill GL. Sequential metabolic changes following induction of systemic inflammatory response in patients with severe sepsis or major blunt trauma. World J Surg. 2000;24:630-638.
24 Makk LJ, McClave SA, Creech PW, et al. Clinical application of the metabolic cart to the delivery of total parenteral nutrition. Crit Care Med. 1990;18:1320-1327.
25 Saffle JR, Larson CM, Sullivan J. A randomized trial of indirect calorimetry-based feedings in thermal injury. J Trauma. 1990;30:776-782.
26 Schofield W. Predicting basal metabolic rate; new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39C:5-41.
27 Working Party of the British Association for Parenteral and Enteral Nutrition. Current Perspectives on Enteral Nutrition in Adults. Maidenhead: BAPEN, 1999.
28 Konstantinides FN, Konstantinides NN, Li JC, et al. Urinary urea nitrogen: too insensitive for calculating nitrogen balance studies in surgical clinical nutrition. J Parenter Enteral Nutr. 1991;15:189-193.
29 Larsson J, Lennmarken C, Martensson J, et al. Nitrogen requirements in severely injured patients. Br J Surg. 1990;77:413-416.
30 Demling RH, DeBiasse MA. Micronutrients in critical illness. Crit Care Clin. 1995;11:651-673.
31 Shenkin A. Micronutrients in the severely-injured patient. Proc Nutr Soc. 2000;59:451-456.
32 Battistella FD, Widergren JT, Anderson JT, et al. A prospective, randomized trial of intravenous fat emulsion administration in trauma victims requiring total parenteral nutrition. J Trauma. 1997;43:52-58.
33 Sedman PC, MacFie J, Palmer MD, et al. Preoperative total parenteral nutrition is not associated with mucosal atrophy or bacterial translocation in humans. Br J Surg. 1995;82:1663-1667.
34 MacFie J, O’Boyle C, Mitchell CJ, et al. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45:223-228.
35 Moore FA, Moore EE, Jones TN, et al. TEN versus TPN following major abdominal trauma – reduced septic morbidity. J Trauma. 1989;29:916-922.
36 Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503-511.
37 Adams S, Dellinger EP, Wertz MJ, et al. Enteral versus parenteral nutritional support following laparotomy for trauma: a randomized prospective trial. J Trauma. 1986;26:882-891.
38 Grahm TW, Zadrozny DB, Harrington T. The benefits of early jejunal hyperalimentation in the head-injured patient. Neurosurgery. 1989;25:729-735.
39 Young B, Ott L, Twyman D, et al. The effect of nutritional support on outcome from severe head injury. J Neurosurg. 1987;67:668-676.
40 Marik PE, Zaloga GP. Meta-analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis. BMJ. 2004;328:1407.
41 Cerra FB, McPherson JP, Konstantinides FN, et al. Enteral nutrition does not prevent multiple organ failure syndrome (MOFS) after sepsis. Surgery. 1988;104:727-733.
42 Lipman TO. Grains or veins: is enteral nutrition really better than parenteral nutrition? A look at the evidence. J Parenter Enteral Nutr. 1998;22:167-182.
43 Peter JV, Moran JL, Phillips-Hughes J. A metaanalysis of treatment outcomes of early enteral versus early parenteral nutrition in hospitalized patients. Crit Care Med. 2005;33:213-220.
44 Booth CM, Heyland DK, Paterson WG. Gastrointestinal promotility drugs in the critical care setting: a systematic review of the evidence. Crit Care Med. 2002;30:1429-1435.
45 Marik PE, Zaloga GP. Gastric versus post-pyloric feeding: a systematic review. Crit Care. 2003;7:R46-51.
46 Taylor SJ, Fettes SB, Jewkes C, et al. Prospective, randomized, controlled trial to determine the effect of early enhanced enteral nutrition on clinical outcome in mechanically ventilated patients suffering head injury. Crit Care Med. 1999;27:2525-2531.
47 Mentec H, Dupont H, Bocchetti M, et al. Upper digestive intolerance during enteral nutrition in critically ill patients: frequency, risk factors, and complications. Crit Care Med. 2001;29:1955-1961.
48 McClave SA, Lukan JK, Stefater JA, et al. Poor validity of residual volumes as a marker for risk of aspiration in critically ill patients. Crit Care Med. 2005;33:324-330.
49 Drakulovic MB, Torres A, Bauer TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354:1851-1858.
50 Abuksis G, Mor M, Plaut S, et al. Outcome of percutaneous endoscopic gastrostomy (PEG): comparison of two policies in a 4-year experience. Clin Nutr. 2004;23:341-346.
51 Fraenkel DJ, Rickard C, Lipman J. Can we achieve consensus on central venous catheter-related infections? Anaesth Intensive Care. 2000;28:475-490.
52 Mizock BA. Nutritional support in hepatic encephalopathy. Nutrition. 1999;15:220-228.
53 Dickerson RN, Boschert KJ, Kudsk KA, et al. Hypocaloric enteral tube feeding in critically ill obese patients. Nutrition. 2002;18:241-246.
54 Hall JC, Dobb G, Hall J, et al. A prospective randomized trial of enteral glutamine in critical illness. Intensive Care Med. 2003;29:1710-1716.
55 Griffiths RD, Jones C, Palmer TE. Six-month outcome of critically ill patients given glutamine-supplemented parenteral nutrition. Nutrition. 1997;13:295-302.
56 Heyland DK. Critical care nutrition. Critical Care Nutrition, Kingston, Ontario, 2006. http://www.criticalcarenutrition.com.
57 Angstwurm MW, Engelmann L, Zimmermann T, et al. Selenium in Intensive Care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med. 2007;35:118-126.
58 Crimi E, Liguori A, Condorelli M, et al. The beneficial effects of antioxidant supplementation in enteral feeding in critically ill patients: a prospective, randomized, double-blind, placebo-controlled trial. Anesth Analg. 2004;99:857-863.
59 Heys SD, Walker LG, Smith I, et al. Enteral nutritional supplementation with key nutrients in patients with critical illness and cancer: a meta-analysis of randomized controlled clinical trials. Ann Surg. 1999;229:467-477.
60 Heyland DK, Novak F, Drover JW, et al. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA. 2001;286:944-953.
61 Bertolini G, Iapichino G, Radrizzani D, et al. Early enteral immunonutrition in patients with severe sepsis: results of an interim analysis of a randomized multicentre clinical trial. Intensive Care Med. 2003;29:834-840.







