Chapter 84 Endovascular Management of Intracranial Aneurysms
Microsurgical clipping of intracranial aneurysms has been the historical definitive standard for treatment of intracranial aneurysms.1 Today’s surgical techniques routinely achieve complete exclusion of the aneurysm from the circulation without compromise of the parent vessel or arterial perforators in a large number of patients. However, several risk factors may put a patient at increased risk for morbidity and mortality. These factors include the aneurysm’s size, its location, the patient’s age, and the medical condition of the patient.2 In addition, according to the International Subarachnoid Aneurysm Trial (ISAT), patients with subarachnoid hemorrhage fared better with endovascular coiling than with surgical clipping.3 To overcome some of the limitations of surgical clipping, endovascular treatments were developed. They have grown considerably over the last 15 years, since U.S. Food and Drug Administration (FDA) approval of the Guglielmi detachable coil (GDC) in 1995.4,5 This chapter discusses the basic techniques utilized in coiling of ruptured and nonruptured saccular intracranial aneurysms. After a brief discussion of each technique, we give a short review of the results of each form of treatment, concentrating on the large reported case series. Finally, we discuss specific complications related to endovascular treatment of saccular intracranial aneurysms.6,7
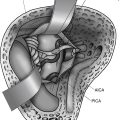
Conventional Coiling of a Simple Saccular Aneurysm
Technique
At our institution, all intracranial aneurysm coiling procedures are performed under general anesthesia with neurologic monitoring. An arterial line is placed in the radial artery to closely monitor the patient’s blood pressure. The anesthesiologist is aware of the need to avoid transient blood pressure spikes, especially when intubating or extubating the patient. This is particularly important in patients who have a ruptured aneurysm. The case can sometimes take a few hours; therefore, anesthesia is necessary to maintain immobility, because three-dimensional (3D) imaging and a fluoroscopic road map are very motion sensitive. A 6-French (Fr) sheath is inserted into the right common femoral artery. If balloon remodeling or additional microcatheters are needed, puncture of the left common femoral artery may be necessary. Just after the sheath is inserted in the groin, a baseline activated clotting time (ACT) is drawn. Then, 6 to 10 international units (IUs) of heparin per kilogram of body weight are given intravenously as a bolus. For patients with unruptured aneurysms, the ACT is checked every 30 minutes throughout the procedure and heparin is given intermittently to keep the ACT between 250 to 300 seconds.8 For patients with a ruptured aneurysm, 3000 IU of heparin are given after placement of framing coils; the patient is given additional heparin to maintain an ACT range of 250 to 300 seconds. A syringe of protamine is prepared in advance and readily available to be injected in case of aneurysm rupture. The usually dose is 10 mg of protamine per 1000 IU of heparin. The goal is to obtain an ACT of less than 150 seconds. Likewise, for patients with unruptured aneurysms who have been treated preoperatively with dual antiplatelet therapy, a five-pack of unpooled platelets are kept in preparation in case of rupture.
Patients who have not suffered a recent subarachnoid hemorrhage are preoperatively given 75 mg of clopidogrel (Plavix) and 81 mg of aspirin orally starting 7 days prior to the procedure to prevent thromboembolic complications. If emergent platelet inhibition is needed, the patient is loaded with a single dose of 600 mg of clopidogrel and 325 mg of aspirin. Full platelet inhibition occurs 2 hours afterward. If the procedure needs to be performed urgently, the patient may be given a glycoprotein IIb/IIIa inhibitor. A platelet inhibition assay is drawn for clopidogrel and aspirin prior to treatment, since approximately 25% of patients will have clopidogrel or aspirin resistance.9,10
A complete cerebral angiogram is performed prior to treatment with a 4- or 5-Fr catheter, including bilateral common carotid artery, internal carotid artery, and vertebral artery injections. Additional 3D rotational images are obtained to more accurately define the neck, dome, and size of the aneurysm. Once the diagnostic portion of the procedure is performed, the catheter is exchanged for a 6-Fr guide catheter, which is positioned as close as possible to the aneurysm. It is necessary to have a stable position of the guide catheter to be able to introduce a microcatheter safely into the aneurysm. Because of this, the guide catheter should be positioned as close to the aneurysm as possible. This gives optimal stability of the guide catheter and allows the operator to monitor the guide position on the same road map as the microcatheter during advancement of the coil into the aneurysm. New guide catheters that are more flexible, such as the Neuron (Penumbra, Alameda, CA), can be introduced farther into the intracranial circulation and can be routinely placed in the cavernous internal carotid artery or the basilar artery.11 This allows more stability in advancing devices into the intracranial circulation. This has been a major innovation in the endovascular treatment of aneurysms over the last 3 years. If additional stability is needed to treat an aneurysm, a triple coaxial system consisting of a long sheath introduced into the origin of the great vessel followed by a guide catheter through this may offer enhanced stability in advancing devices through tortuous anatomy for the treatment of intracranial aneurysms. If an aneurysm cannot be treated from a femoral artery approach, a brachial, radial, direct carotid, or vertebral artery puncture is another option.12–14
Coil Placement
Additional coils of various sizes and shapes are subsequently introduced into the aneurysm sac until the aneurysm sac is densely packed and no longer filling with contrast or until the microcatheter is pushed outside the aneurysm sac. The first coils used for treatment of intracranial aneurysms were made out of platinum, but there were aneurysm recurrences after treatment; therefore, bioactive coils were introduced with the goal of inducing an exuberant healing response and improved filling volume of the coiled aneurysm. The first bioactive coil was the Matrix (Boston Scientific Neurovascular, Fremont, CA), introduced in 2002. The U.S. FDA approved the Matrix coil based on equivalency with the conventional GDC coil. Four bioactive coils are now available for clinical use: the Matrix, HydroCoil (Microvention, Aliso Viejo, CA), Cerecyte (Micrus, Sunnyvale, CA), and Nexus. The newer coils are manufactured so that the aneurysm sac is filled in a Russian nesting doll manner, from the periphery toward the center.15 Filling coils are placed into the aneurysm sac after the placement of framing coils; once the aneurysm is nearly densely packed, the final coils usually placed into the aneurysm sac are finishing coils.16 These coils are very short and soft. Once the aneurysm is densely packed, the microcatheter is removed slowly from the aneurysm, and a post-treatment angiogram is performed to assess the degree of aneurysm occlusion, parent vessel, and patency of the distal vasculature.
The heparin is reversed after the procedure with protamine and manual pressure, or a closure device is utilized to obtain hemostasis at the femoral puncture site.17 Pressure is usually held on the puncture site for 20 to 30 minutes after removal of the sheath if manual compression is utilized, and the leg is immobilized for 6 hours to prevent groin complications. If a closure device is utilized, ambulation can occur as early as 2 hours after placement.18,19 If there is compromise of the parent vessel, protrusion of coils, or thrombus formation during the coiling procedure, the heparin may be continued overnight with the sheath left in place. Antiplatelet therapy may also be given.
Results of Endovascular Treatment for Ruptured Aneurysms
Two prospective randomized trials have compared outcomes of endovascular coiling versus surgical clipping. The first study was performed in Finland20 and randomized 109 patients with subarachnoid hemorrhage who were suitable for either surgery or endovascular coiling. Angiographic outcome in the posterior circulation was significantly better for endovascular coiling, whereas angiographic outcome in the anterior circulation was significantly better for surgery. Angiographic outcomes in the internal carotid artery and middle cerebral artery were similar in both groups. The Glasgow Outcome Scale was equivalent in both groups at 3 months. Mortality for technical reasons during surgery was twice that of the endovascular group (4% vs. 2%). One patient in the endovascular group suffered rebleeding following incomplete coiling of the aneurysm.
The second study was the ISAT,3,21 in which nearly 2000 patients predominantly from Europe and with subarachnoid hemorrhage were randomized to surgery or endovascular coiling based on judgment of the treating team. Outcome analysis on the basis of death or dependence at 2 months and 1 year based on the modified Rankin Scale score was the primary parameter of interest in the first publication in 2002. At 1-year postprocedure, 250 of 1063 (23.5%) of the endovascular patients were dead or dependent, while 326 of 1055 (30.9%) of surgical patients were dead. This represents an absolute risk reduction of 7.4% by those treated from an endovascular approach. Delayed rebleeding was more common in the endovascular group; however, several cases were due to incomplete treatments. Seizures were also less common in the endovascular group.
Results of Endovascular Treatment for Unruptured Aneurysms
The data from endovascular treatment versus surgical clipping for unruptured aneurysms do not show a clear benefit for one form of treatment versus the other. A review of modern large clipping and coiling trials for unruptured aneurysms was published in 2005.22 A majority of these trials were nonrandomized and retrospective. Adverse outcomes for endovascular coiling were estimated at 8.8% and for clipping were estimated at 17.8%. The International Study of Unruptured Intracranial Aneurysm2 adverse outcomes were less common with endovascular treatment (9.3%) than with surgery (13.7%); however, the study was nonrandomized, and the endovascular treatment group included a higher number of elderly patients, larger aneurysms, and aneurysms within the posterior circulation. Surgical adverse outcomes in this study correlated with patient age greater than 50 years, aneurysm size greater than 12 mm, location in the posterior circulation, previous ischemic cerebrovascular disease, and symptoms of mass effect from the aneurysm. Endovascular outcomes were less influenced by these factors. Additional unruptured aneurysm trials are needed.
Coiling of Wide-Neck Aneurysms
Balloon Remodeling Technique
Sidewall Wide-Neck Aneurysm
The main feature that limits the endovascular treatment of aneurysms is the width of the neck. Other features that may limit treatment include the shape of the aneurysm. In 1992, Moret introduced the balloon remodeling technique for treatment of wide-neck intracranial aneurysms.23,24 The technique involves placing a nondetachable balloon across the neck of the aneurysm during each coil placement. The coils remain molded around the balloon after deflation of the balloon, essentially “remodeling the arterial wall.” The technique has been improved over the last 17 years with better coils and balloons. It is routinely used today to treat wide-neck aneurysms, particularly in patients with subarachnoid hemorrhage, thus eliminating stent placement and the use of antiplatelet agents. Most interventionalists consider an aneurysm neck to be wide when the ratio between the maximum diameter of the aneurysm sac and the size of the neck is 1 or less.
Under the road map, the balloon first is advanced across the neck of the aneurysm. The microcatheter is then advanced into the aneurysm. The balloon is inflated across the neck of the aneurysm, causing temporary occlusion of the neck and parent vessel. The first coil is positioned within the aneurysm sac. The balloon is deflated to test the stability of the coil within the aneurysm sac. If no movement of the coil is observed, the balloon is reinflated and the coil is detached. The coil is not detached if coil movement (meaning that the coil is not well anchored in the sac) is detected after balloon deflation. An angiogram is then performed. This is repeated multiple times until the aneurysm no longer fills with contrast or has a dense coil mass within the confines of its lumen.
Results
Shapiro et al. in 2008 published a literature review with a meta-analysis of the safety and efficacy of adjunctive balloon remodeling during endovascular treatment of intracranial aneurysms.25 They concluded that there was no higher incidence of thromboembolic events or iatrogenic rupture with the use of adjunctive balloon remodeling compared with unassisted coiling. They also commented that balloon remodeling appears to result in a higher initial and follow-up aneurysm occlusion rates. Mu et al. successfully treated 40 wide-neck aneurysms with the HyperForm balloon remodeling technique, with only 2 failed cases, in 2008.26 Final results consisted of total occlusion in 34 cases (80.9%), subtotal occlusion in 4 cases (9.5%), and incomplete occlusion in 2 cases (4.8%). Nelson and Levy in 2001 treated 22 patients, with aneurysm occlusion on follow-up angiography at a mean of 19 months found in 17 of 20 patients.27 The other two patients died prior to follow-up. Layton et al. treated 73 of 221 aneurysms over a 3-year period with balloon-assisted coiling.28 They found no increased risk in thromboembolic complications when compared to simple coiling techniques. In conclusion, there appears to be no increased risk of complications with the balloon-remodeling technique. Aneurysm occlusion may be higher than with standard endovascular techniques; however, few studies report the results of this form of treatment.
Stent-Assisted Coiling
Stent Characteristics
A standard cerebral angiogram is obtained, along with a 3D angiogram of the vessel in question. The dimensions of the artery above and below the aneurysm are measured, along with the size of the aneurysm and length of the neck. The targeted landing zone of the stent is determined. The self-expandable stent is sized to a nominal diameter 0.5 to 1.0 mm greater than the parent vessel at the targeted landing zone. The stent length should be centered on the aneurysm neck. The stent length is chosen to provide at least 5 mm of coverage proximal and distal to the aneurysm neck. If the parent vessel is tortuous, an attempt is made to place the distal and proximal aspect of the stent within a straight segment of the parent vessel.
Antiplatelet Treatment Regimen for Intracranial Stenting
A stent is a metallic foreign body; thus, introducing it into the cerebral vasculature may cause thrombus formation and platelet aggregation. To prevent this, it is important to administer antiplatelet agents prior to placement of an intracranial stent. This has been eloquently shown in the coronary literature, where placement of patients on a combination of aspirin and clopidogrel has been shown to prevent stent thrombosis and occlusion.29 The optimal dosage and treatment regimen, however, have not been determined. Given that the average life span of a platelet is 7 days, we administer aspirin (81 mg/day orally) and clopidogrel (75 mg/day orally) for 7 days prior to the procedure. If a stent needs to be placed emergently, we load the patient with clopidogrel (600 mg orally) and aspirin (325 mg orally), wait 2 hours, and then perform the procedure. If a stent needs to be placed during the course of an endovascular procedure and the patient has not been preoperative treated with antiplatelet medications, we load the patient with the glycoprotein IIb/IIIa inhibitor abciximab. The patient is loaded with the cardiac dose of 0.25 mg/kg and then started on a 12-hour intravenous infusion at 0.125 μg/kg/min. The patient is also started on aspirin and clopidogrel. Most endovascular specialists avoid placement of a stent in patients with an acutely ruptured broad-neck aneurysm. This is because the patient will need to be placed on antiplatelet medications and will most likely need a ventriculostomy, given the subarachnoid hemorrhage. Also, if the patient develops vasospasm in the subacute period, intracranial balloon angioplasty can be more difficult with a stent placed in the intracranial circulation.
Results
The use of the Enterprise stent was initially reported by Higashida et al.30 All 5 cases were technically successful. The stent was well visualized, deployed easily, could be repositioned if needed, and was accurately placed without technical difficulties. Weber et al. in 2007 described the use of the stent in 31 saccular wide-neck aneurysms.31 Follow-up angiography of 30 lesions after 6 months demonstrated 15 complete aneurysm occlusions, 8 aneurysm neck remnants, and 7 residual aneurysms. Two patients experienced possible device-related, serious adverse events. The Enterprise stent multicenter registry was published in 2009 and included 141 patients with 142 aneurysms who underwent 143 attempted stent deployments.32 The use of stent assistance with aneurysm coiling was associated with a 76% rate of at least 90% occlusion. The stent could not be navigated to the desired location in 3% of cases, and there was a 2% occurrence of inaccurate deployment. Procedure data demonstrated a 6% temporary morbidity, 2.8% permanent morbidity, and 2% mortality.
Kis et al. in 2006 described the successful deployment of the Leo stent in 24 of 25 aneurysms.33 There were 2 thromboembolic events related to the deployment of the Leo stent, 1 failure of stent deployment, difficulties in stent positioning in three cases, and 1 asymptomatic parent vessel occlusion after 7 months. Follow-up at an average of 5 months revealed aneurysm recurrence in 3 lesions, which were retreated.
The largest experience to date is with the Neuroform stent. This was the first intracranial stent available for the treatment of wide-neck aneurysms. The largest series to date is from China, reported by Liang et al. in 2009.34 Their series included 110 wide-necked aneurysms. In all cases, the Neuroform stent system was delivered and deployed accurately. Procedure-related morbidity was 5.6%, and procedural-related mortality was 0.9%. Angiographic follow-up in 51 aneurysms at an average of 37 months showed an overall recanalization rate of 13.7%. In 2005, Lylyk et al. reported on 46 patients with 48 intracranial aneurysms treated using the Neuroform stent.35 There was a 92% technical success rate. Approximately 19% of the stents were placed in a suboptimal location. In 31% of the cases, the stent was difficult to place. Procedure-related morbidity and mortality were 8.6% and 2.1%, respectively. Since this report, there have been at least two newer generations of the device, allowing easier placement of the stent. In 2007, Biondi et al. reported on 42 patients with 46 wide-neck aneurysms.36 The balloon remodeling technique was performed in 77% of patients prior to stent placement. The stent was successfully deployed in 94% of the cases. Angiographic and clinical follow-up was available in 31 patients with 33 aneurysms. In the 30 aneurysms treated with stent-assisted coiling, there were 17 aneurysm occlusions (57%), 7 neck remnants (23%), and 6 residual aneurysms (20%). In 3 recanalized aneurysms treated with stent alone, 2 neck remnants remained unchanged (67%), and 1 neck remnant decreased in size (33%).
TriSpan Device
Results
The largest experience using the TriSpan for treating intracranial aneurysms has been in Montreal. In 2001, Raymond et al. reported on 25 patients with 19 basilar bifurcation and 6 anterior circulation aneurysms.37 All aneurysms were wide neck except one. The procedure was successful in all patients, with complete obliteration of the aneurysm in 3 patients, residual necks in 13 patients, and a minimal sac in 7 patients. Follow-up angiogram in 16 patients reveal complete obliteration in 4 patients, a residual neck in 1 patient, a persistent residual neck in 4 patients, and recurrent aneurysm in 7 patients. De Keukeleire et al. in 2008 reported on 14 patients in whom 16 TriSpan devices were placed to assist coiling of wide-neck aneurysms in the anterior circulation.38 TriSpan-assisted embolization was successful in 15 of the 16 procedures (93.8%), with complete occlusion in 2 procedures (12.5%), near-complete occlusion in 10 procedures (62.5%), and incomplete occlusion in 3 procedures (18.75%).
Liquid Embolic Agents
Technique Onyx 500
Depending on the therapeutic strategy, coils or a liquid embolic agent is delivered first to occlude the major part of the aneurysm sac. The dead space of the microcatheter is first filled with the solvent DMSO to prevent precipitation of the liquid embolic agent within the lumen of the microcatheter. The aneurysm is then obliterated slowly by injecting 0.1 to 0.2 ml/min of Onyx 500 with a cadence syringe. During each injection, the balloon remains inflated across the neck of the aneurysm. The balloon is deflated after about 5 minutes, allowing the polymer to solidify. Angiography is performed to confirm the polymer’s location relative to the aneurysm, and this process is continued until the aneurysm is occluded. After the last injection, the material solidifies completely over a period of about 10 minutes, with diffusion of the solvent DMSO.
Results
Ev3 sponsored the Cerebral Aneurysm Multicenter European Onyx (CAMEO) trial in 20 centers.39 CAMEO was a prospective, observational trial that enrolled 119 consecutive patients with 123 aneurysms. Follow-up results were reported for 100 of these patients. CAMEO reported complete occlusion in 79% of aneurysms, subtotal occlusion in 13%, and incomplete occlusion in 8%. Delayed occlusion of the parent vessel occurred in 9 patients, 5 of whom were asymptomatic. Piske et al. in 2009 treated 69 patients with 84 aneurysms.40 All of the aneurysms had a wide neck. In this study, 50 aneurysms were small (less than 12 mm), 30 were large (12 to less than 25 mm), and 4 were giant. Angiographic follow-up was available for 65 of the 84 aneurysms at 6 months. Complete aneurysm occlusion was seen in 65.5% of aneurysms on immediate control and in 84.6% at 6 months. The rates of complete occlusion were 74% for small aneurysms and 80% for large aneurysms. Progression from incomplete to complete occlusion was seen in 68.2% of all aneurysms, with a higher percentage in small aneurysms (90.9%). Aneurysm recanalization was only observed in 3 patients (4.6%), with retreatment in 2 patients (3.3%).
Cekirge et al. presented the long-term clinical and angiographic follow-up results of 100 consecutive intracranial aneurysms treated with Onyx.41 Intracranial stenting was used adjunctively in 25 aneurysms, including 19 during initial treatment and 6 during retreatment. Of the 100 aneurysms, 35 were giant or large/wide neck, and 65 were small. Follow-up angiography was performed in all 91 surviving patients (96 aneurysms) at 3 and/or 6 months. Overall, aneurysm recanalization was observed in 12 of 96 aneurysms with follow-up angiography (12.5%). All 12 were large or giant aneurysms, resulting in a 36% recanalization rate in the large and giant aneurysm group. The authors found that 1 aneurysm out of 25 treated with the combination of a stent and Onyx showed recanalization.
Flow Diversion
Characteristics
A new generation of endovascular devices has appeared over the last 3 years. These devices are called flow divertors.42 They have been developed to treat aneurysms from an endolumial rather than an endosaccular approach. These stentlike devices are designed to reconstruct the parent vessel and to divert flow from the aneurysm lumen without the use of coils. The device promotes thrombus formation within the aneurysm by decreasing the hemodynamic effects within the aneurysm sac, thus promoting stasis. It addition, the device has tighter scaffolding than a conventional intracranial stent, providing a means over which endothelial cells can grow to seal off the neck of the aneurysm. Kallmes et al. have characterized the effects of the pipeline embolization device (PED) in an experimental rabbit aneurysm model.43,44 The PED (Ev3) is one of the first flow-diversion devices used in humans, and it was approved by the U.S. FDA for treatment of aneurysms on April 10, 2011. The PED is a cylindrical, stentlike construct composed of 48 braided strands of cobalt chromium and platinum. Its appearance is similar to the Wallstent; however, there is a denser wire mesh. The Silk stent (Balt Extrusion) is another device. It is a braided, self-expanding, high metal surface area coverage construct that has Conformité Européenne marking approval in Europe. Other devices were in development as this chapter was being written. The flow divertors are delivered and placed in a similar fashion to current conventional intracranial stents described previously.
Results
Lylyk et al. reported their single-center results for a series of 53 patients treated with the PED.45 No major procedure-related complications were reported in their series. On angiographic follow-up, there was a 93% and 95% rate of complete aneurysm occlusion observed at 6 and 12 months, respectively. Fiorella et al. reported two cases of fusiform vertebral artery aneurysms that were treated successfully with the PED.46 Kadziolka et al. reported on 6 patients with intracranial aneurysms treated with the Silk stent.47 The stent was deployed in all cases with no morbidity or mortality; however, the results were not commented upon in the abstract.
Complications of Endovascular Coiling and their Treatment
Coil Malposition
Deposition of a coil outside the aneurysm sac can occur. Most commonly, it is a single loop of coil protruding into the parent vessel. This seldom causes a thromboembolic complication. However, if thrombus forms around the loop of coil, a glycoprotein IIb/IIIa inhibitor can be given at the site of thrombus formation to dissolve the clot. The coil should then be removed with one of the retrieval devices. If this is not possible, then the single loop of coil should be pushed against the vessel wall by placement of a self-expanding intracranial stent. Malposition of a coil is infrequent and usually the result of a poor coil choice, poor catheter position, or poor technique. We commonly apply some minimal forward force on the microcatheter during coiling of the aneurysm in the late stages to prevent the microcatheter from being kicked out of the aneurysm and the coil loop being deposited outside of the aneurysm sac.
Rupture of Aneurysm
Aneurysm rupture is the most dreaded complication. This can occur spontaneously due to the fragile nature of the aneurysm. It may also occur iatrogenically from the cerebral angiogram or placement of a microcatheter or coil into the aneurysm.48 When this occurs, a rapid severe transient elevation of intracranial pressure causes severe elevation of the blood pressure. If electroencephalogram monitoring is being performed, electric activity will seize. The aneurysm needs to be secured quickly, since a ruptured aneurysm can easily cause death to the patient.49,50 If the patient is anticoagulated, this must be immediately reversed with protamine (i.e., 10 mg of protamine per 1000 IU of heparin given) so that the ACT is less than 150 seconds. If the patient has been premedicated with aspirin or clopidogrel, an attempt at reversal of platelet inhibition should be made with the infusion of platelets. We have a prepared, nonpooled, five-pack of platelets ready in the rooms prior to coil placement so as to be prepared for this event. The blood pressure should also be lower immediately by pharmaceutical means to try and prevent further extravasation of blood into the subarachnoid space. If a balloon is present within the parent vessel, this should be carefully inflated and the aneurysm should be rapidly packed with addition coils. If the microcatheter is within the subarachnoid space, this should not be removed, but coiling should be performed from the subarachnoid space into the aneurysm. Computed tomography of the head should be immediately performed after the aneurysm is secure to assess for hydrocephalus and the need for placement of a ventriculostomy catheter. Vasospasm should be treated by intra-arterial injection of a pharmacologic agent (verapamil, nicardipine, nimodipine, etc.) once the aneurysm is secure.
Rupture of Vessel
Perforation of a blood vessel by a microguidewire is rare. This may be self-limiting and seal or may lead to life-threatening hemorrhage. If life-threatening hemorrhage is identified, permanent occlusion of the vessel may be life saving. A liquid embolic agent or coils can be utilized.51 Rarely, rupture of a blood vessel after placement of a balloon-expandable stent can be managed conservatively with prolonged balloon inflation across the area of rupture.52
Procedural Thrombus Formation
A thromboembolic event is probably the most common complication related to endovascular management of an aneurysm. This has decreased over the last few years mainly due to better and more liberal use of antiplatelet agents. Patients are now routinely placed on clopidogrel and aspirin 7 days prior to treatment. This is based on the coronary intervention literature, which has shown a significant decrease in the risk of thromboembolic events in patients treated with this regimen. Furthermore, to prevent thrombus formation during the procedure, patients are fully anticoagulated so that the ACT range is between 250 and 300 seconds. Biliverdin is a direct thrombin inhibitor with a better anticoagulation profile and a shorter half-life when compared to heparin; however, it does not have a reversal agent, which limits its use in the cerebral vascular circulation. If thrombus forms on coils placed at the neck of the aneurysm, most interventionalists prefer local infusion of a glycoprotein IIb/IIIa receptor.53 This is administered intra-arterially through the microcatheter at a dosage of 4 to 10 mg over 10 to 20 minutes.54 This has also been shown to be effective in ruptured aneurysms with no increased risk of bleeding complications. Three glycoprotein IIb/IIIa receptor inhibitors are available on the market. The most common one utilized is abciximab, which is mainly used in our lab. However, eptifibatide (Integrilin) may be the preferred agent due to its shorter half-life and reversible binding to the glycoprotein IIb/IIIa receptor.
Biondi A., Janardhan V., Katz J.M., et al. Neuroform stent-assisted coil embolization of wide-neck intracranial aneurysms: strategies in stent deployment and midterm follow-up. Neurosurgery. 2007;61(3):460-468. discussion 468–469
Cekirge H.S., Saatci I., Ozturk M.H., et al. Late angiographic and clinical follow-up results of 100 consecutive aneurysms treated with Onyx reconstruction: largest single-center experience. Neuroradiology. 2006;48(2):113-126.
Doerfler A., Wanke I., Egelhof T., et al. Aneurysmal rupture during embolization with Guglielmi detachable coils: causes, management, and outcome. AJNR Am J Neuroradiol. 2001;22(10):1825-1832.
Guglielmi G., Vinuela F., Dion J., Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: preliminary clinical experience. J Neurosurg. 1991;75(1):8-14.
Guglielmi G., Vinuela F., Sepetka I., Macellari V. Electrothrombosis of saccular aneurysms via endovascular approach. Part 1: electrochemical basis, technique, and experimental results. J Neurosurg. 1991;75(1):1-7.
Layton K.F., Cloft H.J., Gray L.A., et al. Balloon-assisted coiling of intracranial aneurysms: evaluation of local thrombus formation and symptomatic thromboembolic complications. AJNR Am J Neuroradiol. 2007;28(6):1172-1175.
Lee T., Baytion M., Sciacca R., et al. Aggregate analysis of the literature for unruptured intracranial aneurysm treatment. AJNR Am J Neuroradiol. 2005;26(8):1902-1908.
Le Roux P.D., Winn H.R., Newell D.W. Management of cerebral aneurysms. Philadelphia, Pa: Saunders; 2004.
Liang G., Gao X., Li Z., et al. Neuroform stent-assisted coiling of intracranial aneurysms: a 5 year single-center experience and follow-up. Neurol Res. 2010. 32(7):721-727
Lylyk P., Ferrario A., Pasbon B., et al. Buenos Aires experience with the Neuroform self-expanding stent for the treatment of intracranial aneurysms. J Neurosurg. 2005;102(2):235-241.
Lylyk P., Miranda C., Ceratto R., et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009;64(4):632-642. discussion 642-643; quiz N6
Mocco J., Snyder K.V., Albuquerque F.C., et al. Treatment of intracranial aneurysms with the Enterprise stent: a multicenter registry. J Neurosurg. 2009;110(1):35-39.
Molyneux A.J., Cekirge S., Saatci I., Gal G. Cerebral Aneurysm Multicenter European Onyx (CAMEO) trial: results of a prospective observational study in 20 European centers. AJNR Am J Neuroradiol. 2004;25(1):39-51.
Molyneux A.J., Kerr R., Stratton I., et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267-1274.
Molyneux A.J., Kerr R.S., Yu L.M., et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809-817.
Moret J., Cognard C., Weill A., et al. Reconstruction technique in the treatment of wide-neck intracranial aneurysms. Long-term angiographic and clinical results. Apropos of 56 cases. J Neuroradiol. 1997;24(1):30-44.
Mounayer C., Piotin M., Baldi S., et al. Intraarterial administration of abciximab for thromboembolic events occurring during aneurysm coil placement. AJNR Am J Neuroradiol. 2003;24(10):2039-2043.
Park M.S., Stiefel M.F., Fiorella D., et al. Intracranial placement of a new, compliant guide catheter: technical note. Neurosurgery. 2008;63(3):E616-E617. discussion E617
Piotin M., Iijima A., Wada H., Moret J. Increasing the packing of small aneurysms with complex-shaped coils: an in vitro study. AJNR Am J Neuroradiol. 2003;24(7):1446-1448.
Piotin M., Liebig T., Feste C.D., et al. Increasing the packing of small aneurysms with soft coils: an in vitro study. Neuroradiology. 2004;46(11):935-939.
Raymond J., Guilbert F., Roy D. Neck-bridge device for endovascular treatment of wide-neck bifurcation aneurysms: initial experience. Radiology. 2001;221(2):318-326.
Shapiro M., Babb J., Becske T., Nelson P.K. Safety and efficacy of adjunctive balloon remodeling during endovascular treatment of intracranial aneurysms: a literature review. AJNR Am J Neuroradiol. 2008;29(9):1777-1781.
Vanninen R., Koivisto T., Saari T., et al. Ruptured intracranial aneurysms: acute endovascular treatment with electrolytically detachable coils—a prospective randomized study. Radiology. 1999;211(2):325-336.
Wang T.H., Bhatt D.L., Topol E.J. Aspirin and clopidogrel resistance: an emerging clinical entity. Eur Heart J. 2006;27(6):647-654.
Wiebers D.O., Whisnant J.P., Huston J.3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103-110.
1. Le Roux P.D., Winn H.R., Newell D.W. Management of cerebral aneurysms. Philadelphia, Pa: Saunders; 2004.
2. Wiebers D.O., Whisnant J.P., Huston J.3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103-110.
3. Molyneux A.J., Kerr R., Stratton I., et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267-1274.
4. Guglielmi G., Vinuela F., Sepetka I., Macellari V. Electrothrombosis of saccular aneurysms via endovascular approach. Part 1: electrochemical basis, technique, and experimental results. J Neurosurg. 1991;75(1):1-7.
5. Guglielmi G., Vinuela F., Dion J., Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: preliminary clinical experience. J Neurosurg. 1991;75(1):8-14.
6. Raymond J., Roy D. Safety and efficacy of endovascular treatment of acutely ruptured aneurysms. Neurosurgery. 1997;41(6):1235-1245. discussion 1245-1246
7. Roy D., Milot G., Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001;32(9):1998-2004.
8. Cognard C., Weill A., Castaings L., et al. Intracranial berry aneurysms: angiographic and clinical results after endovascular treatment. Radiology. 1998;206(2):499-510.
9. Wang T.H., Bhatt D.L., Topol E.J. Aspirin and clopidogrel resistance: an emerging clinical entity. Eur Heart J. 2006;27(6):647-654.
10. Tantry U.S., Bliden K.P., Gurbel P.A. Resistance to antiplatelet drugs: current status and future research. Expert Opin Pharmacother. 2005;6(12):2027-2045.
11. Park M.S., Stiefel M.F., Fiorella D., et al. Intracranial placement of a new, compliant guide catheter: technical note. Neurosurgery. 2008;63(3):E616-E617. discussion E617
12. Schonholz C., Nanda A., Rodriguez J., et al. Transradial approach to coil embolization of an intracranial aneurysm. J Endovasc Ther. 2004;11(4):411-413.
13. Nii K., Kazekawa K., Onizuka M., et al. Direct carotid puncture for the endovascular treatment of anterior circulation aneurysms. AJNR Am J Neuroradiol. 2006;27(7):1502-1504.
14. Blanc R., Piotin M., Mounayer C., et al. Direct cervical arterial access for intracranial endovascular treatment. Neuroradiology. 2006;48(12):925-929.
15. Piotin M., Iijima A., Wada H., Moret J. Increasing the packing of small aneurysms with complex-shaped coils: an in vitro study. AJNR Am J Neuroradiol. 2003;24(7):1446-1448.
16. Piotin M., Liebig T., Feste C.D., et al. Increasing the packing of small aneurysms with soft coils: an in vitro study. Neuroradiology. 2004;46(11):935-939.
17. Shoulders-Odom B. Management of patients after percutaneous coronary interventions. Crit Care Nurse. 2008;28(5):26-41. quiz 42
18. Khaghany K., Al-Ali F., Spigelmoyer T., et al. Efficacy and safety of the Perclose Closer S device after neurointerventional procedures: prospective study and literature review. AJNR Am J Neuroradiol. 2005;26(6):1420-1424.
19. Assali A.R., Sdringola S., Moustapha A., et al. Outcome of access site in patients treated with platelet glycoprotein IIb/IIIa inhibitors in the era of closure devices. Catheter Cardiovasc Interv. 2003;58(1):1-5.
20. Vanninen R., Koivisto T., Saari T., et al. Ruptured intracranial aneurysms: acute endovascular treatment with electrolytically detachable coils—a prospective randomized study. Radiology. 1999;211(2):325-336.
21. Molyneux A.J., Kerr R.S., Yu L.M., et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809-817.
22. Lee T., Baytion M., Sciacca R., et al. Aggregate analysis of the literature for unruptured intracranial aneurysm treatment. AJNR Am J Neuroradiol. 2005;26(8):1902-1908.
23. Moret J., Cognard C., Weill A., et al. [Reconstruction technique in the treatment of wide-neck intracranial aneurysms. Long-term angiographic and clinical results. Apropos of 56 cases]. J Neuroradiol. 1997;24(1):30-44.
24. Aletich V.A., Debrun G.M., Misra M., et al. The remodeling technique of balloon-assisted Guglielmi detachable coil placement in wide-necked aneurysms: experience at the University of Illinois at Chicago. J Neurosurg. 2000;93(3):388-396.
25. Shapiro M., Babb J., Becske T., Nelson P.K. Safety and efficacy of adjunctive balloon remodeling during endovascular treatment of intracranial aneurysms: a literature review. AJNR Am J Neuroradiol. 2008;29(9):1777-1781.
26. Mu S.Q., Yang X.J., Li Y.X., et al. Endovascular treatment of wide-necked intracranial aneurysms using of “remodeling technique” with the HyperForm balloon. Chin Med J (Engl). 2008;121(8):725-729.
27. Nelson P.K., Levy D.I. Balloon-assisted coil embolization of wide-necked aneurysms of the internal carotid artery: medium-term angiographic and clinical follow-up in 22 patients. AJNR Am J Neuroradiol. 2001;22(1):19-26.
28. Layton K.F., Cloft H.J., Gray L.A., et al. Balloon-assisted coiling of intracranial aneurysms: evaluation of local thrombus formation and symptomatic thromboembolic complications. AJNR Am J Neuroradiol. 2007;28(6):1172-1175.
29. Leon M.B., Baim D.S., Popma J.J., et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med. 1998;339(23):1665-1671.
30. Higashida R.T., Halbach V.V., Dowd C.F., et al. Initial clinical experience with a new self-expanding nitinol stent for the treatment of intracranial cerebral aneurysms: the Cordis Enterprise stent. AJNR Am J Neuroradiol. 2005;26(7):1751-1756.
31. Weber W., Bendszus M., Kis B., et al. A new self-expanding nitinol stent (Enterprise) for the treatment of wide-necked intracranial aneurysms: initial clinical and angiographic results in 31 aneurysms. Neuroradiology. 2007;49(7):555-561.
32. Mocco J., Snyder K.V., Albuquerque F.C., et al. Treatment of intracranial aneurysms with the Enterprise stent: a multicenter registry. J Neurosurg. 2009;110(1):35-39.
33. Kis B., Weber W., Berlit P., Kuhne D. Elective treatment of saccular and broad-necked intracranial aneurysms using a closed-cell nitinol stent (Leo). Neurosurgery. 2006;58(3):443-450. discussion 43-50
34. Liang G., Gao X., Li Z., et al. Neuroform stent-assisted coiling of intracranial aneurysms: a 5 year single-center experience and follow-up. Neurol Res. 2009. 2010;32(7):721-727
35. Lylyk P., Ferrario A., Pasbon B., et al. Buenos Aires experience with the Neuroform self-expanding stent for the treatment of intracranial aneurysms. J Neurosurg. 2005;102(2):235-241.
36. Biondi A., Janardhan V., Katz J.M., et al. Neuroform stent-assisted coil embolization of wide-neck intracranial aneurysms: strategies in stent deployment and midterm follow-up. Neurosurgery. 2007;61(3):460-468. discussion 468-469
37. Raymond J., Guilbert F., Roy D. Neck-bridge device for endovascular treatment of wide-neck bifurcation aneurysms: initial experience. Radiology. 2001;221(2):318-326.
38. De Keukeleire K., Vanlangenhove P., Defreyne L. Evaluation of a neck-bridge device to assist endovascular treatment of wide-neck aneurysms of the anterior circulation. AJNR Am J Neuroradiol. 2008;29(1):73-78.
39. Molyneux A.J., Cekirge S., Saatci I., Gal G. Cerebral Aneurysm Multicenter European Onyx (CAMEO) trial: results of a prospective observational study in 20 European centers. AJNR Am J Neuroradiol. 2004;25(1):39-51.
40. Piske R.L., Kanashiro L.H., Paschoal E., et al. Evaluation of Onyx HD-500 embolic system in the treatment of 84 wide-neck intracranial aneurysms. Neurosurgery. 2009;64(5):E865-E875. discussion E875
41. Cekirge H.S., Saatci I., Ozturk M.H., et al. Late angiographic and clinical follow-up results of 100 consecutive aneurysms treated with Onyx reconstruction: largest single-center experience. Neuroradiology. 2006;48(2):113-126.
42. Fiorella D., Kelly M.E., Albuquerque F.C., Nelson P.K. Curative reconstruction of a giant midbasilar trunk aneurysm with the pipeline embolization device. Neurosurgery. 2009;64(2):212-217. discussion 217
43. Kallmes D.F., Ding Y.H., Dai D., et al. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007;38(8):2346-2352.
44. Kallmes D.F., Ding Y.H., Dai D., et al. A second-generation, endoluminal, flow-disrupting device for treatment of saccular aneurysms. AJNR Am J Neuroradiol. 2009;30(6):1153-1158.
45. Lylyk P., Miranda C., Ceratto R., et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009;64(4):632-642. discussion 642-643; quiz N6
46. Fiorella D., Woo H.H., Albuquerque F.C., Nelson P.K. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2008;62(5):1115-1120. discussion 1120-1121
47. Kadziolka KDSEL, Estrade L., Leautaud A., Pierot L. Flow diverter treatment for tiny, uncoilable, ruptured intracranial aneurysms: silk stent experience. Neuroradiology. S38, 2009.
48. Doerfler A., Wanke I., Egelhof T., et al. Aneurysmal rupture during embolization with Guglielmi detachable coils: causes, management, and outcome. AJNR Am J Neuroradiol. 2001;22(10):1825-1832.
49. Hirai T., Suginohara K., Uemura S., et al. Management of aneurysm perforation during Guglielmi electrodetachable coil placement. AJNR Am J Neuroradiol. 2002;23(4):738-739.
50. Ricolfi F., Le Guerinel C., Blustajn J., et al. Rupture during treatment of recently ruptured aneurysms with Guglielmi electrodetachable coils. AJNR Am J Neuroradiol. 1998;19(9):1653-1658.
51. Halbach V.V., Higashida R.T., Dowd C.F., et al. Management of vascular perforations that occur during neurointerventional procedures. AJNR Am J Neuroradiol. 1991;12(2):319-327.
52. Wada H., Piotin M., Boissonnet H., et al. Carotid rupture during stent-assisted aneurysm treatment. AJNR Am J Neuroradiol. 2004;25(5):827-829.
53. Mounayer C., Piotin M., Baldi S., et al. Intraarterial administration of Abciximab for thromboembolic events occurring during aneurysm coil placement. AJNR Am J Neuroradiol. 2003;24(10):2039-2043.
54. Kwon O.K., Lee K.J., Han M.H., et al. Intraarterially administered abciximab as an adjuvant thrombolytic therapy: report of three cases. AJNR Am J Neuroradiol. 2002;23(3):447-451.