Chapter 90 Endovascular Management of Dural Arteriovenous Fistulas
Dural arteriovenous fistulas (DAVFs), also sometimes referred to as dural arteriovenous malformations, represent approximately 10% to 15% of all intracranial arteriovenous malformations. These lesions can occur in the brain and the spine and are characterized by abnormal arteriovenous shunts located within dural leaflets. Cranial DAVFs are most commonly located in the vicinity of dural venous sinuses and spinal DAVFs in the region where the radiculomedullary artery enters the dural root sleeve. The etiology of DAVFs is unknown, but many are acquired and can occur after trauma (e.g., skull fracture), surgery, sinus thrombosis, and venous channel stenosis.1–4
The pathophysiology and clinical significance of DAVFs stem from the location of the fistula or shunt and its effect on and disruption of normal venous drainage. Various classification systems have been published in the literature, with the key feature in each being the pattern of venous flow.5–8 Borden et al. organized DAVFs into three groups: Type I DAVFs drain directly into meningeal veins or dural venous sinuses. Type II DAVFs also drain directly into dural sinuses or meningeal veins but have retrograde drainage into subarachnoid veins. Type III DAVFs do not have dural sinus or meningeal venous drainage; rather, they drain directly into subarachnoid veins.8 Cognard et al. proposed a classification scheme with five main types: Type I DAVFs drain directly into dural venous sinuses or meningeal veins, and all venous flow is anterograde (in the normal direction). Type II DAVFs drain into dural sinuses or meningeal veins but have retrograde flow into the associated sinus (type IIa), cortical veins (type IIb), or both (type IIa+b). Type III and IV DAVFs drain directly into cortical veins either with (type III) or without (type IV) venous ectasia. Type V DAVFs include spinal venous drainage. DAVFs without cortical venous reflux (CVR) (Cognard types I or IIa or Borden type I) are generally considered benign.7 Satomi et al. demonstrated that conservative management or palliative therapy is sufficient in 98% of cases of benign DAVFs; however, these patients have a 2% risk of developing CVR.9 DAVFs with persistent CVR (Cognard types IIb-V or Borden types II and III) are much more aggressive, with an annual mortality of 10.4% and annual risk of hemorrhage or nonhemorrhagic neurologic deficit of 8.1% and 6.9%, respectively.10 Symptomatic DAVFs are associated with variable clinical presentations, depending on the location of the shunt and the severity of venous hypertension secondary to retrograde leptomeningeal venous flow. Approximately 20% to 33% of symptomatic DAVFs present with intracranial hemorrhage.4,10 Other presentations include pulsatile tinnitus (or bruit), headaches, visual changes, alterations in mental status, seizure, myelopathy, cranial nerve palsies, and motor or sensory deficits.
DAVFs can be treated by surgical techniques, endovascular techniques, a combination of surgical and endovascular techniques, or radiation therapy. For endovascular management, embolization targets are selected based on a thorough understanding of the fistula anatomy. The key is to obliterate the fistulous connections while limiting adverse outcomes, such as inadvertent worsening of cortical venous flow, closing external to internal carotid artery anastomoses, and embolizing external carotid artery branches with important arterial supply to cranial nerves. Transarterial and transvenous approaches are available for endovascular treatment of DAVFs. Embolic materials used in such procedures include n-butyl-2-cyanoacrylate (n-BCA) glue (Trufill, Cordis Neurovascular, Miami Lakes, FL), Onyx (ev3 Endovascular, Irvine, CA), detachable microcoils, and particles of polyvinyl alcohol. Particles are rarely used as a sole agent for DAVF embolization because of their temporary effects. Instead, they are used in certain circumstances as an adjuvant to reduce flow in collateral vessels and promote thrombosis.11
Transarterial Embolization
Transarterial embolization is ideally used for high-grade DAVFs, such as those with direct cortical venous drainage, or in situations in which venous access is limited. Nelson et al. listed the following advantages of transarterial procedures for DAVFs: (1) the arteriovenous fistula transition can be occluded through a transarterial approach, decreasing the possibility of flow diversion into an alternate venous pathway; (2) treatment is not limited by venous access (e.g., stenotic or thrombosed venous sinuses); (3) fistula treatment does not require sacrificing a functional venous pathway; (4) de novo DAVFs can develop at a secondary site following transvenous embolization, possibly as a result of venous hypertension; and (5) complications specific to transvenous routes can be avoided (e.g., abducens nerve palsy from catheterization of the superior petrosal sinus).12
Cyanoacrylic Glue Techniques
Cyanoacrylate adhesives have been used extensively for the embolization of high-flow cerebrovascular lesions,13–15 including DAVFs.11,12,16 n-BCA, a cyanoacrylate ester, is a clear, colorless liquid with a strong odor that is insoluble in water. This agent polymerizes rapidly when in contact with ionic substances, including blood or tissue fluids. Rapid polymerization and excellent tensile strength make n-BCA a highly effective embolic agent for endovascular procedures. However, it must be handled with great care to avoid the high risk of unintentional embolization of normal tissue. n-BCA is diluted with Ethiodol (ethiodized oil) to make the mixture radiopaque. Tantalum or tungsten powder can also be added to increase radiographic visibility.17 The concentration of n-BCA in the mixture determines the migration, or penetration, of the embolic agent prior to polymerization. A high n-BCA–to–Ethiodol ratio (high concentration of glue) polymerizes more proximally in the arterial pedicle than a low n-BCA–to–Ethiodol ratio, which achieves more distal penetration. Glue concentrations of 25% to 33% are commonly used.
For transarterial glue embolization of DAVFs, the microcatheter should be positioned as close to the target fistula site as possible, because this increases the specificity of the injection and facilitates penetration of the embolic material to the fistula site. Wedging the microcatheter within a feeding artery creates a flow-arrest scenario that is thought to facilitate delivery of glue to the fistula site and its permeation into the extensive fistulous collateral network. In a series of 21 patients with 23 DAVFs, Nelson et al. demonstrated complete occlusion in all cases without complications using the wedge catheter technique.12 Wedge catheterization also allows for arterioarterial reflex with occlusion of multiple arterial feeders from a single pedicle injection.11
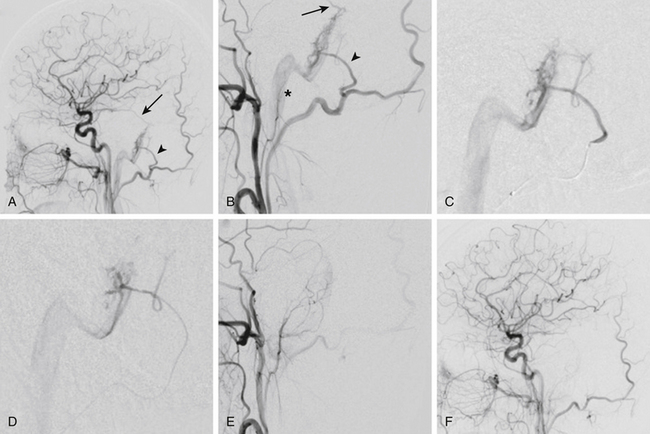
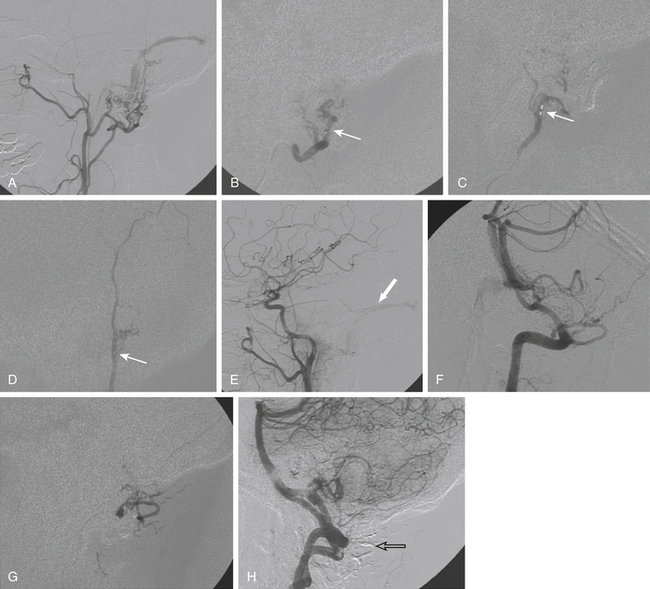
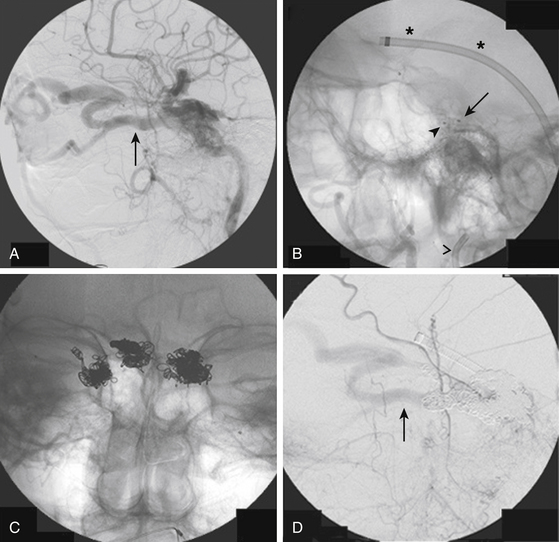
Once the optimal catheter position is obtained, microcatheter angiography and test injections are used to determine fistula flow characteristics and the appropriate glue concentration for the procedure. Prior to the actual glue injection, the microcatheter must be flushed thoroughly with a nonionic solution, such as 5% dextrose, to ensure that the glue does not polymerize within the delivery catheter. The glue is then injected under direct digital subtraction angiography (DSA) guidance either as a continuous column or as a bolus followed by a column of nonionic flush.17 If there is a suboptimal catheter position (e.g., the catheter is too proximal along the pedicle or the neurointerventionalist is unable to wedge the catheter tip), simultaneous injection of 5% dextrose through the guide catheter can improve distal migration of the glue toward its target.16 After adequate glue penetration, the microcatheter or the whole delivery system (microcatheter plus guide catheter) is rapidly removed from the patient. Communication among operators during a glue procedure is critical so that the catheter is pulled at the correct instant and not glued to the vessel. Examples of transarterial glue embolization of DAVFs are shown in Figs. 90-1 and 90-2.
Onyx Techniques
First reports describing the use of Onyx in vascular malformations were published in 1990.18,19 Onyx has been commercially available in Europe since 1999 and was approved by the U.S. Food and Drug Administration in July 2005.
Onyx is a liquid agent composed of a mixture of ethylene–vinyl alcohol copolymer suspended in the solvent dimethyl sulfoxide (DMSO). Tantalum powder is added to the compound for radiopacity. To obtain homogeneous radiopacity of the mixture, Onyx must be shaken for at least 20 minutes before use. The potential angiotoxic effects of DMSO are negligible if the recommended dose and infusion rate are followed.20–22 The polymer precipitates upon contact with aqueous solution, resulting in a soft, nonadherent material characterized by a “lavalike” flow pattern that is able to produce permanent vessel obliteration. Because of the presence of the solvent, all materials must be DMSO compatible, including syringes and microcatheters.
The therapeutic approach to cerebral DAVF mainly depends on the vascular drainage and CVR pattern of the malformation. In Cognard type II dural fistulas, with or without CVR, the best option, when feasible, remains the transvenous approach with coils or Onyx. However, this option entails the sacrifice of the sinus.23–27 In the same setting, transarterial embolization could have the advantage of preserving the sinus when still functional.
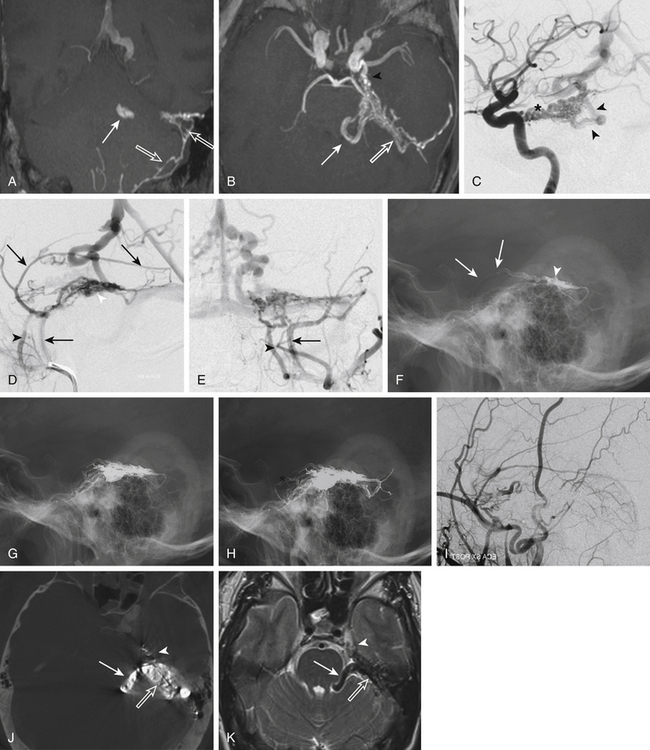
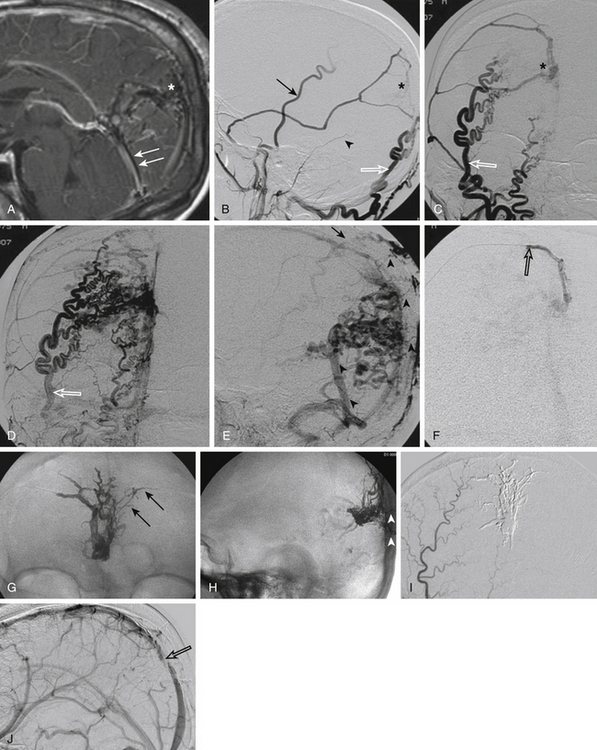
In cases of DAVF with direct CVR Cognard type III to V, the transarterial embolization is the most advantageous technique. Several reports in the recent literature highlight the benefit of Onyx in these cases.24,28–30 Compared to n-BCA glue injection, the Onyx technique has some technical advantages: it is less operator dependent, does not need a wedged microcatheter positioning, and has the capacity to occlude different feeders from a single pedicle with a single injection (Figs. 90-3 and 90-4). This last advantage is particularly relevant when the venous access is limited.28–31 Onyx may also be used in the treatment of cavernous DAVFs. However, in these cases, the transvenous approach is recommended to avoid the risk of cranial nerve damage and penetration into extra- or intracranial anastomoses.23,27
We prefer to perform the embolization procedure under general anesthesia and full heparinization monitored with activating clotting time.30 After a detailed diagnostic angiographic study, possibly including superselective injections of the vessels involved in the dural malformation, an accurate treatment strategy should be planned to select the cases that can benefit from intra-arterial Onyx injection. The strategy should be aimed at (1) locating the point, or points, of fistula; (2) recognizing the feeding arteries; (3) understanding the venous drainage pattern; and (4) selecting the most promising pedicles to be injected based on navigability, expected efficacy, and safety. Biplanar angiography is mandatory. Tridimensional DSA reconstructions and pseudo-CT scans may be useful adjuncts.
When the microcatheter is in the desired position, before injecting Onyx, an initial flushing of the microcatheter with about 5 ml of normal saline is required, followed by injection of DMSO to fill the dead space of the microcatheter. Subsequently, Onyx can be slowly injected in 40 seconds to replace the DMSO. The entire procedure must be performed under double road map fluoroscopy to recognize any premature leakage of Onyx.32 The injection speed can be adjusted according to vessel penetration and direction, as well as reflux conditions. If reflux is observed, however small, the procedure should be immediately stopped. It should be resumed within a few minutes to avoid plugging of the microcatheter with consequent risk of rupture. It is advisable to obtain a new road map prior to any injection to improve the visibility of the vessel penetration and reflux of Onyx. The control of the reflux is a crucial element for the success of the treatment; it is necessary to create a plug around the microcatheter that allows the Onyx to be pushed into the vascular malformation (Fig. 90-3F). On the other hand, excessive reflux can determine retrograde filling of the feeder where the microcatheter is positioned up to the origin of the parent artery, causing unwanted obliteration of normal vessels. An additional risk is entrapment of the microcatheter so that it becomes difficult to retrieve at the end of the procedure.
At the end of the procedure, the microcatheter must be gently retracted, maintaining continuous and regular traction for a few minutes. If the microcatheter remains entrapped by the Onyx, it can be cut and left in place without further complications.24 However, personal experience with two cases demonstrates that a microcatheter fixed to the arterial access can be safely retrieved 24 hours later.
Postembolization care includes medications for pain, which is caused by the dural involvement, and low-molecular-weight heparin for 1 to 4 weeks to prevent venous thrombosis. An immediate post-treatment CT scan is recommended, followed by an angiographic study 3 to 6 months after the procedure to control the stability of the results (Fig. 90-4).30
Transvenous Embolization
The goal of endovascular DAVF treatment using a transvenous approach is to embolize the venous outlet of the fistula. Several approaches for transvenous embolization of DAVFs have been described11,33: catheterization of the involved sinus via a venous approach,26,34 direct puncture of the affected sinus by a transcranial approach (used if endovascular navigation is not possible),30,35 and selective transvenous embolization of veins with CVR without occlusion of the sinus.36 The transvenous approach is favored when the main arterial feeders of a DAVF originate from the internal carotid artery or the vertebral artery, when sites of possible extracranial to intracranial anastomoses are involved, and when there is risk of compromising arterial supply to cranial nerves.37
Prior to transvenous occlusion of sinus for DAVF treatment, the venous drainage pathways of the normal brain must be clearly defined. Occlusion of a sinus that is necessary for normal venous drainage can lead to cerebral venous infarction and hemorrhage. Complete obliteration of the venous portion of the DAVF can lead to regression of the arterial feeders, which is in stark contrast to brain arteriovenous malformations, where occlusion of a draining vein can have serious consequences, including nidal rupture and bleeding.17 However, care must be taken not to iatrogenically isolate the sinus involved in a DAVF, because this may worsen the CVR and increase the risk of hemorrhage. Sinus occlusion is safe if the affected region of the sinus is isolated, there is significant CVR, and the sinus segment is not responsible for venous drainage of normal brain.
Transvenous approaches are commonly used for fistulas involving the cavernous sinus, such as carotid–cavernous fistulas (CCFs). CCFs are categorized into direct (Barrow type A) and indirect (Barrow types B-D) types.38 Indirect CCFs are dural fistulas with arterial feeders from the internal carotid artery (Barrow type B), external carotid artery (Barrow type C) (Fig. 90-5), or both (Barrow type D). For transvenous treatment of these lesions, numerous venous routes to the cavernous sinus are available: ipsilateral and contralateral inferior petrosal sinuses, basilar plexus, facial vein, superior ophthalmic vein (SOV), angular vein, and pterygoid plexus.33 Selection of a transvenous route depends on the specific venous anatomy of the patient and the dominant venous outflow of the fistula. In general, percutaneous transfemoral–transvenous approaches should be attempted prior to other transvenous or combined surgical–transvenous approaches, such as the combined surgical–endovascular approach to the cavernous sinus via the SOV.39 However, the latter techniques can be quite efficacious. The standard technique is to use retrograde catheterization along the internal jugular vein to approach the involved sinus.
A transvenous approach can be limited by sinus stenosis or occlusion, although recanalization is possible. Various techniques have been devised to accomplish transvenous embolization through an occluded sinus, such as embolization of a transverse sinus DAVF through an occluded sigmoid sinus. One method is recanalization of the occluded sinus with a stiff 0.035-inch guidewire, but perforation of the sinus is a potentially serious complication.40,41 Recanalization using a microwire and microcatheter has also been successful and is presumed safer than using a stiff guidewire.42 However, it is unknown whether true recanalization with a microwire versus navigation through existing small channels is being accomplished. Accessing the isolated sinus through a contralateral approach has also been described, but this technique is limited by the lengthy and tortuous route to the affected sinus.43,44
In cases where recanalization of a thrombosed sinus is unsuccessful, and the associated DAVF sinus is located close to the skin, a transcranial approach may be attempted.35,45 Houdart et al.35 reported a series of 10 patients who underwent craniectomy over the involved sinus in the operating room followed by sinus puncture with an 18-gauge angiocatheter, microcatheterization, and embolization. For the first case, catheterization was performed in the operating room immediately following the craniectomy; however, this case was complicated by a subdural hematoma. In all subsequent cases, the procedure was staged, with catheterization being done in the angiography suite under road map guidance 1 week after craniectomy. No further bleeding complications were reported. The authors note that a generous initial craniectomy is favorable, as 7 of the 10 patients in their series required enlargement of the craniectomy with repeat surgery. The craniectomy should allow distal sinus access to permit dense coil packing and an entry angle of 45 degrees for easy passage of the microcatheter.35 As an alternative to the transcranial approach, novel venous routes to the involved sinus, such as the percutaneous transvenous approach through a mastoid emissary vein, have been described.46
Transvenous embolization procedures, such as those of the cavernous sinus, have certain risks, including dural dissection leading to hemorrhage and cranial nerve injury. Injury to cranial nerves III, V, and VI as they course through the cavernous sinus can occur secondary to thrombosis, elevated pressure, or coil/balloon overpacking in the cavernous sinus.33 Similarly, navigation through the jugular foramen and inferior petrosal sinus could potentially result in damage to cranial nerves IX to XI. In addition, the SOV approach carries risks of visual loss, glaucoma, and acute exophthalmos.47,48
Follow-up angiography is recommended in patients following transvenous embolization of a DAVF, with resulting sinus occlusion to evaluate for recanalization of the treated DAVF and development of new DAVFs.33 Sinus occlusion with microcoils and thrombosis might activate angiogenic factors, resulting in recurrence or de novo fistula formation.49 Studies in rat models with experimentally induced venous hypertension have demonstrated increased dural angiogenesis and arteriovenous fistula formation, thought to be secondary to local cerebral hypoperfusion and ischemia.50–51
Outcomes of Transarterial Embolization with n-Butyl Cyanoacrylate
Transarterial embolization with n-BCA for DAVFs has a high cure rate, ranging from 64% to 100%, with some documented transient palsies but no permanent complications.11,52,53 Guedin et al. treated 38 patients with DAVFs with CVR with solely transarterial cyanoacrylate glue (Histoacryl, B. Braun, Melsungen, Germany) or Glubran 2 (GEM, Viareggio, Italy) embolization. A complete cure was achieved after a single session in 76% (29/38) of patients. On average, 1.37 sessions per patient to complete treatment were required and 2.37 feeding vessels were embolized. The mean duration of injection was less than 1 minute. A cure rate of 89.5% (34/38) was reported with 29 patients achieving immediate cure and 5 patients demonstrating postembolization secondary thrombosis. Two transient cases of cerebellar syndrome were reported, but both patients recovered rapidly. The authors noted no permanent complications as well as no endovascular therapy related mortality.11
Agid et al. reported 11 patients with anterior cranial fossa DAVFs who were treated by transarterial catheterization through the ophthalmic artery and subsequent injection of n-BCA. Of these patients, 7 (63.6%) were completely cured with no reported complications.52 Acrylic glue has proved effective and stable for use in endovascular procedures for more than 30 years. Some authors argue that glue compares advantageously, in terms of cost and long-term stability, to newer liquid agents such as Onyx.11
Outcomes of Transarterial Embolization with Onyx
Over the last several years, Onyx has been increasingly used in the endovascular treatment of DAVFs. Several large series have been published reporting cure rates between 61% and 91%, with complication rates between 0% and 16%. The volume of Onyx used per procedure ranged from 0.4 to 12.2 ml, and the duration of injection ranged from 7 to 100 minutes per pedicle.24,29–31,54–58 Some authors have reported 100% cure rates of transarterial Onyx in small series, with no complications reported.59,60
In one series, the treatment of DAVF with CVR and transarterial Onyx in 30 patients was prospectively analyzed. Complete angiographic cure was observed in 80% (24/30) of cases, with 83% (20/24) of these cures achieved after a single procedure. The authors reported two complications: one postprocedure hemorrhage secondary to venous outlet thrombosis and one temporary cranial nerve palsy.30
The role of transarterial Onyx in patients with Cognard type I and II DAVFs has also been studied. Anatomic cure was achieved in 50% (13/26) patients, and clinical cure was achieved in 65.4% (17/26) patients. All anatomic cures were achieved in a single procedure, and follow-up angiography showed no recurrence. Complications included two cardiac Onyx migrations, two reflexive bradycardia episodes, one transient visual hallucination, two transient fifth nerve palsies, and one permanent seventh nerve palsy.54 A rare side effect of Onyx embolization is trigeminocardiac reflex, which in one series had a reported rate of 10.7%.61
Outcomes of Transvenous Embolization of Indirect CCF
Since it was pioneered in the late 1970s by Mullan and Hosobuchi, the transvenous approach has become the preferred method of treatment of indirect CCFs.62–64 The two largest series to date have demonstrated complete obliteration in 87% to 91% patients with indirect CCFs treated with transvenous coils and a procedure-related permanent morbidity of 0% to 2.3%.65,66
Studies analyzing the long-term outcome in patients who underwent coil embolization of CCFs have found a 44% rate of persistent cranial nerve deficits with disturbance of oculomotor and visual functions. Bink et al. noted a significant correlation between coil volume and persistent diplopia and persistent cranial nerve VI paresis.67
A major disadvantage of solely using coils is difficulty obtaining complete occlusion.62 Wakhloo et al. evaluated the efficacy and safety of transvenous n-BCA alone or with coils in a series of 14 patients with indirect CCFs. Of the patients treated with n-BCA and coils, 88% (7/8) had complete angiographic obliteration of the CCF. Another 83% of patients (5/6) treated with n-BCA alone achieved immediate obliteration of the CCF. The remaining patient treated with n-BCA was noted to have thrombosed the residual internal carotid artery dural feeders at follow-up angiography 1 week later. One patient experienced a temporary worsening of clinical symptoms.68
Onyx has also been used as an adjuvant to coils in transvenous embolization of indirect CCFs. Complete angiographic cure rates ranged from 67% to 100% with complications between 0% and 33%.27,69–71 In one series, the authors prospectively studied eight patients with indirect CCFs who were treated with either transvenous Onyx alone (three patients) or a combination of Onyx and detachable coils (five patients). Complete fistula obliteration was achieved in all cases (100%) after a single session, as well as clinical resolution of the presenting symptoms in 100% of patients after 2 months. Some authors suggest the superiority of Onyx to coils alone or n-BCA because Onyx gradually permeates the sinus interstices and gradually precipitates from outside inward, allowing it to be injected more slowly and accurately. This allows interrupted injections, which in turn allow assessment of embolization patterns.69 In addition, the procedure time and cost decrease significantly when Onyx is used with detachable coils as opposed to coils alone.71
The SOV has been described as an excellent and definitive alterative treatment in patients for whom traditional endovenous routes have failed. A rate of 90% embolization of CCFs that were previously unsuccessfully attempted has been reported.72 Transorbital puncture has also been described in eight patients who underwent successful embolization of a CCF via the inferior ophthalmic vein.73
Agid R., Terbrugge K., Rodesch G., et al. Management strategies for anterior cranial fossa (ethmoidal) dural arteriovenous fistulas with an emphasis on endovascular treatment. J Neurosurg. 2009;110(1):79-84.
Arat A., Cekirge S., Saatci I., Ozgen B. Transvenous injection of Onyx for casting of the cavernous sinus for the treatment of a carotid–cavernous fistula. Neuroradiology. 2004;46:1012-1015.
Barrow D.L., Spector R.H., Braun I.F., et al. Classification and treatment of spontaneous carotid–cavernous sinus fistulas. J Neurosurg. 1985;62:248-256.
Bink A., Goller K., Luchtenberg M., et al. Long-term outcome after coil embolization of cavernous sinus arteriovenous fistulas. AJNR Am J Neuroradiol. 2010;31(7):1216-1221.
Borden J.A., Wu J.K., Shucart W.A. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82:166-179.
Cognard C., Gobin Y.P., Pierot L., et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671-680.
Cognard C., Januel A.C., Silva N.A.Jr., Tall P. Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: new management using Onyx. AJNR Am J Neuroradiol. 2008;29:235-241.
Goddard A.J., Khangure M.S. Multiple dural arteriovenous fistulas. Radiologic progression and endovascular cure. Case report. Interv Neuroradiol. 2002;8:183-191.
Guedin P., Gaillard S., Boulin A., et al. Therapeutic management of intracranial dural arteriovenous shunts with leptomeningeal venous drainage: report of 53 consecutive patients with emphasis on transarterial embolization with acrylic glue. J Neurosurg. 2010;112:603-610.
Houdart E., Saint-Maurice J.P., Chapot R., et al. Transcranial approach for venous embolization of dural arteriovenous fistulas. J Neurosurg. 2002;97:280-286.
Jiang C., Lv X., Li Y., et al. Endovascular treatment of high-risk tentorial dural arteriovenous fistulas: clinical outcomes. Neuroradiology. 2009;51:103-111.
Kirsch M., Liebig T., Kühne D., Henkes H. Endovascular management of dural arteriovenous fistulas of the transverse and sigmoid sinus in 150 patients. Neuroradiology. 2009;51:477-483.
Klisch J., Huppertz H.J., Spetzger U., et al. Transvenous treatment of carotid–cavernous and dural arteriovenous fistulae: results for 31 patients and review of the literature. Neurosurgery. 2003;53:836-856. discussion 856-857
Lv X., Jiang C., Li Y., Wu Z. Results and complications of transarterial embolization of intracranial dural arteriovenous fistulas using Onyx-18. J Neurosurg. 2008;109:1083-1090.
Lv X., Jiang C., Li Y., et al. Intraarterial and intravenous treatment of transverse/sigmoid sinus dural arteriovenous fistulas. Interv Neuroradiol. 2009;15(3):291-300.
Macdonald J.H., Millar J.S., Barker C.S. Endovascular treatment of cranial dural arteriovenous fistulae: a single-centre, 14-year experience and the impact of Onyx on local practise. Neuroradiology. 2009 Nov. [in press]
McConnell K.A., Tjoumakaris S.I., Allen J., et al. Neuroendovascular management of dural arteriovenous malformations. Neurosurg Clin N Am. 2009;20:431-439.
Meyers P.M., Halbach V.V., Dowd C.F., et al. Dural carotid–cavernous fistula: definitive endovascular management and long-term follow-up. Am J Ophthalmol. 2002;134(1):85-92.
Moore C., Murphy K., Gailloud P. Improved distal distribution of n-butyl cyanoacrylate glue by simultaneous injection of dextrose 5% through the guiding catheter: technical note. Neuroradiology. 2006;48:327-332.
Morris P. Practical Neuroangiography. Philadelphia: Lippincott Williams & Wilkins; 2007.
Natarajan S.K., Ghodke B., Kim L.J., et al. Multimodality treatment of intracranial dural arteriovenous fistulas in the Onyx era: a single center experience. World Neurosurg. 2010;73:365-379.
Nelson P.K., Russell S.M., Woo H.H., et al. Use of a wedged microcatheter for curative transarterial embolization of complex intracranial dural arteriovenous fistulas: indications, endovascular technique, and outcome in 21 patients. J Neurosurg. 2003;98:498-506.
Nogueira R.G., Dabus G., Rabinov J.D., et al. Preliminary experience with Onyx embolization for the treatment of intracranial dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2008;29:91-97.
Stiefel M.F., Albuquerque F.C., Park M.S., et al. Endovascular treatment of intracranial dural arteriovenous fistulae using Onyx: a case series. Neurosurgery. 2009;65(Suppl 6):132-139. discussion 139-140
Vinuela F., Dion J.E., Duckwiler G., et al. Combined endovascular embolization and surgery in the management of cerebral arteriovenous malformations: experience with 101 cases. J Neurosurg. 1991;75:856-864.
1. Watanabe A., Takahara Y., Ibuchi Y., Mizukami K. Two cases of dural arteriovenous malformation occurring after intracranial surgery. Neuroradiology. 1984;26:375-380.
2. Nabors M.W., Azzam C.J., Albanna F.J., et al. Delayed postoperative dural arteriovenous malformations. Report of two cases. J Neurosurg. 1987;66:768-772.
3. Sakaki T., Morimoto T., Nakase H., et al. Dural arteriovenous fistula of the posterior fossa developing after surgical occlusion of the sigmoid sinus. Report of five cases. J Neurosurg. 1996;84:113-118.
4. McConnell K.A., Tjoumakaris S.I., Allen J., et al. Neuroendovascular management of dural arteriovenous malformations. Neurosurg Clin N Am. 2009;20:431-439.
5. Djindijian R., Merland J. Superselective Arteriography of the External Carotid Artery. Berlin: Springer-Verlag; 1978.
6. Lalwani A.K., Dowd C.F., Halbach V.V. Grading venous restrictive disease in patients with dural arteriovenous fistulas of the transverse/sigmoid sinus. J Neurosurg. 1993;79:11-15.
7. Cognard C., Gobin Y.P., Pierot L., et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671-680.
8. Borden J.A., Wu J.K., Shucart W.A. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82:166-179.
9. Satomi J., van Dijk J.M., Terbrugge K.G., et al. Benign cranial dural arteriovenous fistulas: outcome of conservative management based on the natural history of the lesion. J Neurosurg. 2002;97:767-770.
10. van Dijk J.M., terBrugge K.G., Willinsky R.A., Wallace M.C. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke. 2002;33:1233-1236.
11. Guedin P., Gaillard S., Boulin A., et al. Therapeutic management of intracranial dural arteriovenous shunts with leptomeningeal venous drainage: report of 53 consecutive patients with emphasis on transarterial embolization with acrylic glue. J Neurosurg. 2010;112:603-610.
12. Nelson P.K., Russell S.M., Woo H.H., et al. Use of a wedged microcatheter for curative transarterial embolization of complex intracranial dural arteriovenous fistulas: indications, endovascular technique, and outcome in 21 patients. J Neurosurg. 2003;98:498-506.
13. Debrun G., Vinuela F., Fox A., Drake C.G. Embolization of cerebral arteriovenous malformations with bucrylate. J Neurosurg. 1982;56:615-627.
14. Merland J.J., Rufenacht D., Laurent A., Guimaraens L. Endovascular treatment with isobutyl cyanoacrylate in patients with arteriovenous malformation of the brain. Indications, results and complications. Acta Radiol Suppl. 1986;369:621-622.
15. Vinuela F., Dion J.E., Duckwiler G., et al. Combined endovascular embolization and surgery in the management of cerebral arteriovenous malformations: experience with 101 cases. J Neurosurg. 1991;75:856-864.
16. Moore C., Murphy K., Gailloud P. Improved distal distribution of n-butyl cyanoacrylate glue by simultaneous injection of dextrose 5% through the guiding catheter: technical note. Neuroradiology. 2006;48:327-332.
17. Morris P. Practical Neuroangiography. Philadelphia: Lippincott Williams & Wilkins; 2007.
18. Taki W., Yonekawa Y., Iwata H., et al. A new liquid material for embolization of arteriovenous malformations. AJNR Am J Neuroradiol. 1990;11:163-168.
19. Terada T., Nakamura Y., Nakai K., et al. Embolization of arteriovenous malformations with peripheral aneurysms using ethylene–vinyl alcohol copolymer. Report of three cases. J Neurosurg. 1991;75:655-660.
20. Kirsch M., Liebig T., Kühne D., Henkes H. Endovascular management of dural arteriovenous fistulas of the transverse and sigmoid sinus in 150 patients. Neuroradiology. 2009;51:477-483.
21. Chaloupka J.C., Huddle D.C., Alderman J., et al. A reexamination of the angiotoxicity of superselective injection of DMSO in the swine rete mirabile embolization model. AJNR Am J Neuroradiol. 1992;20:401-410.
22. Murayama Y., Viñuela F., Ulhoa A., et al. Nonadhesive liquid embolic agent for cerebral arteriovenous malformations: preliminary histopathological studies in swine rete mirabile. Neurosurgery. 1998;43:1164-1175.
23. Arat A., Cekirge S., Saatci I., Ozgen B. Transvenous injection of Onyx for casting of the cavernous sinus for the treatment of a carotid–cavernous fistula. Neuroradiology. 2004;46:1012-1015.
24. Lv X., Jiang C., Li Y., Wu Z. Results and complications of transarterial embolization of intracranial dural arteriovenous fistulas using Onyx-18. J Neurosurg. 2008;109:1083-1090.
25. Dawson R.C.3rd, Joseph G.J., Owens D.S., Barrow D.L. Transvenous embolization as the primary therapy for arteriovenous fistulas of the lateral and sigmoid sinuses. AJNR Am J Neuroradiol. 1998;19:571-576.
26. Roy D., Raymond J. The role of transvenous embolization in the treatment of intracranial dural arteriovenous fistulas. Neurosurgery. 1997;40:1133-1141.
27. He H.W., Jiang C.H., Wu Z.X., et al. Transvenous embolization with a combination of detachable coils and Onyx for a complicated cavernous dural arteriovenous fistula. Chin Med J (Engl). 2008;121:1651-1655.
28. Nogueira R.G., Dabus G., Rabinov J.D., et al. Preliminary experience with Onyx embolization for the treatment of intracranial dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2008;29:91-97.
29. Jiang C., Lv X., Li Y., et al. Endovascular treatment of high-risk tentorial dural arteriovenous fistulas: clinical outcomes. Neuroradiology. 2009;51:103-111.
30. Cognard C., Januel A.C., Silva N.A.Jr, Tall P. Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: new management using Onyx. AJNR Am J Neuroradiol. 2008;29:235-241.
31. Macdonald J.H., Millar J.S., Barker C.S. Endovascular treatment of cranial dural arteriovenous fistulae: a single-centre, 14-year experience and the impact of Onyx on local practise. Neuroradiology. 2009 Nov. [in press]
32. Gao K., Yang X.J., Mu S.Q., et al. Embolization of brain arteriovenous malformations with ethylene–vinyl alcohol copolymer: technical aspects. Chin Med J (Engl). 2009;122:1851-1856.
33. Klisch J., Huppertz H.J., Spetzger U., et al. Transvenous treatment of carotid–cavernous and dural arteriovenous fistulae: results for 31 patients and review of the literature. Neurosurgery. 2003;53:836-856. discussion 856-857
34. Urtasun F., Biondi A., Casaco A., et al. Cerebral dural arteriovenous fistulas: percutaneous transvenous embolization. Radiology. 1996;199:209-217.
35. Houdart E., Saint-Maurice J.P., Chapot R., et al. Transcranial approach for venous embolization of dural arteriovenous fistulas. J Neurosurg. 2002;97:280-286.
36. Mironov A. Selective transvenous embolization of dural fistulas without occlusion of the dural sinus. AJNR Am J Neuroradiol. 1998;19:389-391.
37. Natarajan S.K., Ghodke B., Kim L.J., et al. Multimodality treatment of intracranial dural arteriovenous fistulas in the Onyx era: a single center experience. World Neurosurg. 2010;73:365-379.
38. Barrow D.L., Spector R.H., Braun I.F., et al. Classification and treatment of spontaneous carotid–cavernous sinus fistulas. J Neurosurg. 1985;62:248-256.
39. Miller N.R., Monsein L.H., Debrun G.M., et al. Treatment of carotid–cavernous sinus fistulas using a superior ophthalmic vein approach. J Neurosurg. 1995;83:838-842.
40. Gobin Y.P., Houdart E., Rogopoulos A., et al. Percutaneous transvenous embolization through the thrombosed sinus in transverse sinus dural fistula. AJNR Am J Neuroradiol. 1993;14:1102-1105.
41. Naito I., Iwai T., Shimaguchi H., et al. Percutaneous transvenous embolisation through the occluded sinus for transverse–sigmoid dural arteriovenous fistulas with sinus occlusion. Neuroradiology. 2001;43:672-676.
42. Wong G.K., Poon W.S., Yu S.C., Zhu C.X. Transvenous embolization for dural transverse sinus fistulas with occluded sigmoid sinus. Acta Neurochir (Wien). 2007;149:929-935. discussion 935-936
43. Halbach V.V., Higashida R.T., Hieshima G.B., et al. Transvenous embolization of dural fistulas involving the transverse and sigmoid sinuses. AJNR Am J Neuroradiol. 1989;10:385-392.
44. Komiyama M., Ishiguro T., Matsusaka Y., et al. Transfemoral, transvenous embolisation of dural arteriovenous fistula involving the isolated transverse–sigmoid sinus from the contralateral side. Acta Neurochir (Wien). 2002;144:1041-1046. discussion 1046
45. Endo S., Kuwayama N., Takaku A., Nishijima M. Direct packing of the isolated sinus in patients with dural arteriovenous fistulas of the transverse–sigmoid sinus. J Neurosurg. 1998;88:449-456.
46. Rivet D.J., Goddard J.K.3rd, Rich K.M., Derdeyn C.P. Percutaneous transvenous embolization of a dural arteriovenous fistula through a mastoid emissary vein. Technical note. J Neurosurg. 2006;105:636-639.
47. Miller N.R. Severe vision loss and neovascular glaucoma complicating superior ophthalmic vein approach to carotid–cavernous sinus fistula. Am J Ophthalmol. 1998;125:883-884.
48. Devoto M.H., Egbert J.E., Tomsick T.A., Kulwin D.R. Acute exophthalmos during treatment of a cavernous sinus–dural fistula through the superior ophthalmic vein. Arch Ophthalmol. 1997;115:823-824.
49. Goddard A.J., Khangure M.S. Multiple dural arteriovenous fistulas. Radiologic progression and endovascular cure. Case report. Interv Neuroradiol. 2002;8:183-191.
50. Lawton M.T., Jacobowitz R., Spetzler R.F. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg. 1997;87:267-274.
51. Chen L., Mao Y., Zhou L.F. Local chronic hypoperfusion secondary to sinus high pressure seems to be mainly responsible for the formation of intracranial dural arteriovenous fistula. Neurosurgery. 2009;64:973-983. discussion 983
52. Agid R., Terbrugge K., Rodesch G., et al. Management strategies for anterior cranial fossa (ethmoidal) dural arteriovenous fistulas with an emphasis on endovascular treatment. J Neurosurg. 2009;110(1):79-84.
53. Shi Z.S., Ziegler J., Gonzalez N.R., et al. Transarterial embolization of clival dural arteriovenous fistulae using liquid embolic agents. Neurosurgery. 2008;62(2):408-415. discussion 415
54. Lv X., Jiang C., Li Y., et al. The limitations and risks of transarterial Onyx injections in the treatment of grade I and II DAVFs. Eur J Radiol. 2010.
55. Lv X., Jiang C., Li Y., et al. Intraarterial and intravenous treatment of transverse/sigmoid sinus dural arteriovenous fistulas. Interv Neuroradiol. 2009;15(3):291-300.
56. Panagiotopoulos V., Moller-Hartmann W., Asgari S., et al. Onyx embolization as a first line treatment for intracranial dural arteriovenous fistulas with cortical venous reflux. Rofo. 2009;181(2):129-138.
57. Saraf R., Shrivastava M., Kumar N., Limaye U. Embolization of cranial dural arteriovenous fistulae with Onyx: indications, techniques, and outcomes. Indian J Radiol Imaging. 2010;20(1):26-33.
58. Stiefel M.F., Albuquerque F.C., Park M.S., et al. Endovascular treatment of intracranial dural arteriovenous fistulae using Onyx: a case series. Neurosurgery. 2009;65(Suppl 6):132-139. discussion 139-140
59. van Rooij W.J., Sluzewski M. Curative embolization with Onyx of dural arteriovenous fistulas with cortical venous drainage. AJNR Am J Neuroradiol. 2010;31(8):1516-1520.
60. Trivelato F.P., Abud D.G., Ulhoa A.C., et al. Dural arteriovenous fistulas with direct cortical venous drainage treated with Onyx: a case series. Arq Neuropsiquiatr. 2010;68(4):613-618.
61. Ong C.K., Ong M.T., Le K., et al. The trigeminocardiac reflex in Onyx embolisation of intracranial dural arteriovenous fistula. J Clin Neurosci. 2010;17(10):1267-1270.
62. Gemmete J.J., Ansari S.A., Gandhi D.M. Endovascular techniques for treatment of carotid–cavernous fistula. J Neuroophthalmol. 2009;29(1):62-71.
63. Mullan S. Treatment of carotid–cavernous fistulas by cavernous sinus occlusion. J Neurosurg. 1979;50(2):131-144.
64. Hosobuchi Y. Electrothrombosis of carotid–cavernous fistula. J Neurosurg. 1975;42(1):76-85.
65. Kirsch M., Henkes H., Liebig T., et al. Endovascular management of dural carotid–cavernous sinus fistulas in 141 patients. Neuroradiology. 2006;48(7):486-490.
66. Meyers P.M., Halbach V.V., Dowd C.F., et al. Dural carotid–cavernous fistula: definitive endovascular management and long-term follow-up. Am J Ophthalmol. 2002;134(1):85-92.
67. Bink A., Goller K., Luchtenberg M., et al. Long-term outcome after coil embolization of cavernous sinus arteriovenous fistulas. AJNR Am J Neuroradiol. 2010;31(7):1216-1221.
68. Wakhloo A.K., Perlow A., Linfante I., et al. Transvenous n-butyl-cyanoacrylate infusion for complex dural carotid–cavernous fistulas: technical considerations and clinical outcome. AJNR Am J Neuroradiol. 2005;26(8):1888-1897.
69. Elhammady M.S., Peterson E.C., Aziz-Sultan M.A. Onyx embolization of a carotid–cavernous fistula via direct transorbital puncture. J Neurosurg. 2010 Feb 5. [Epud ahead of print]
70. Suzuki S., Lee D.W., Jahan R., et al. Transvenous treatment of spontaneous dural carotid–cavernous fistulas using a combination of detachable coils and Onyx. AJNR Am J Neuroradiol. 2006;27(6):1346-1349.
71. Lv X., Jiang C., Li Y., Wu Z. A promising adjuvant to detachable coils for cavernous packing: onyx. Interv Neuroradiol. 2009;15(2):145-152.
72. Wolfe S.Q., Cumberbatch N.M., Aziz-Sultan M.A., et al. Operative approach via the superior ophthalmic vein for the endovascular treatment of carotid–cavernous fistulas that fail traditional endovascular access. Neurosurgery. 2010;66(6 Suppl Operative):293-299. discussion 299
73. White J.B., Layton K.F., Evans A.J., et al. Transorbital puncture for the treatment of cavernous sinus dural arteriovenous fistula. AJNR Am J Neuroradiol. 2007;28(7):1415-1417.